Abstract
The 5-HT5A receptor is the least understood serotonin (5-HT) receptor. Here, we electrophysiologically identify and characterize a native 5-HT5A receptor current in acute ex vivo brain slices of adult rodent prefrontal cortex. In the presence of antagonists for the previously characterized 5-HT1A and 5-HT2 receptors, a proportion of layer V pyramidal neurons continue to show 5-HT-elicited outward currents in both rats and mice. These 5-HT currents are suppressed by the selective 5-HT5A antagonist, SB-699551, and are not observed in 5-HT5A receptor knock-out mice. Further characterization reveals that the 5-HT5A current is activated by submicromolar concentrations of 5-HT, is inwardly rectifying with a reversal potential near the equilibrium potential for K+ ions, and is suppressed by blockers of Kir3 channels. Finally, we observe that genetic deletion of the inhibitory 5-HT5A receptor results in an unexpected, large increase in the inhibitory 5-HT1A receptor currents. The presence of functional prefrontal 5-HT5A receptors in normal rodents along with compensatory plasticity in 5-HT5A receptor knock-out mice testifies to the significance of this receptor in the healthy prefrontal cortex.
Introduction
Serotonin (5-HT) receptors control a number of physiological processes, most notably emotional behaviors. The 5-HT5A receptor subtype is the least understood, despite its widespread expression in the human and rodent brains (Pasqualetti et al., 1998; Kinsey et al., 2001). To date, there has been limited functional evidence of the 5-HT5A receptor in the brain (Sprouse et al., 2004; Thomas et al., 2006), and its endogenous channel effector(s) remain uncertain (Grailhe et al., 2001; Noda et al., 2003). Given this lack of functional characterization in the native brain tissue, the 5-HT5A receptor remains only provisionally classified within the 5-HT receptor family (IUPHAR database) (Hannon and Hoyer, 2008).
The recent development of the selective 5-HT5A antagonist (SB-699551) (Corbett et al., 2005) and the generation of 5-HT5A knock-out mice (Grailhe et al., 1999) have now made it possible to examine functional 5-HT5A receptors within native ex vivo brain tissue. Here, for the first time, we identify and characterize functional 5-HT5A receptor currents in cortical neurons and investigate the consequence of genetic deletion of the 5-HT5A receptor on postsynaptic serotonin receptor signaling.
Materials and Methods
Experimental animals
Sprague Dawley rats, Sv129 mice, and C57BL/6 mice were obtained from Charles River. Serotonin 5-HT5A receptor (htr5A) transgenic mice on an Sv129 background (Grailhe et al., 1999) were bred at the University of Toronto. We used male adolescent and adult rats [postnatal day (P) 46 ± 3; n = 22 rats] and adult mice (Sv129: P110 ± 7; n = 45 mice; C57BL/6: P223 ± 41; n = 3 mice).
Genotyping
To genotype sibling 5-HT5A+/+ and 5-HT5A−/− mice for our experiments, the following PCR protocol was used: 95°C for 3 min, 35 cycles of (94°C for 45 s, 52°C for 45 s, and 72°C for 1 min), and 72°C for 10 min. The following primers were added to the PCR to amplify the 5-HT5A wild-type allele: forward primer 5′-TTTCTAGCTGCGGCCACATTCACT-3′ and reverse primer 5′-TCATCACATTGGAGACACGCTT GC-3′. The following primers were added to the PCR to amplify the 5-HT5A knock-out allele: forward primer 5′-ATTCGGCTATGACTGGGCACAACA-3′ and reverse primer 5′-GTAAAGCACGAGGAGGAAGC GGTCAGC-3′. The expected sizes of the PCR products were 340 bp and 676 bp for the wild-type and knock-out alleles, respectively.
Brain slice preparation
In brief, coronal slices (400 μm thick) were made from prefrontal cortex (4.20–2.52 mm from bregma for rats; 2.46–1.34 mm for mice). Excised brains were rapidly cooled with 4°C oxygenated sucrose ACSF (254 mM sucrose was substituted for NaCl), cut on a Dosaka Linear Slicer (SciMedia) and transferred to 30°C oxygenated ACSF (128 mM NaCl, 10 mM D-glucose, 26 mM NaHCO3, 2 mM CaCl2, 2 mM MgSO4, 3 mM KCl, 1.25 mM NaH2PO4; pH 7.4). Slices were allowed to recover for at least 1–2 h, then were placed in a superfusion chamber on the stage of a BX50WI microscope (Olympus). Regular bubbled ACSF (95% oxygen and 5% carbon dioxide; 31–33°C) flowed at a rate of 3–4 ml/min.
Electrophysiology
Whole-cell recording electrodes (3–4 MΩ) containing 120 mM potassium gluconate, 5 mM KCl, 2 mM MgCl2, 4 mM K2-ATP, 0.4 mM Na2-GTP, 10 mM Na2-phosphocreatine, and 10 mM HEPES buffer (adjusted to pH 7.3 with KOH) were used to patch layer V pyramidal neurons in medial prefrontal cortex under visual control. Currents were recorded using a Multiclamp 700b under continuous single-electrode voltage-clamp mode at a holding potential of −75 mV (Molecular Devices). Current–voltage (IV) relationships were obtained using 15 mV/s voltage ramps from −120 to −60 mV. The IV curve obtained at baseline was subtracted from the IV curve obtained during 5-HT agonist application. All data were acquired at 20 kHz (reduced to 1 kHz for illustrations) and low-pass filtered at 3 kHz, using pClamp10.2/Digidata1440 software (Molecular Devices).
Rat layer V neurons (n = 149) had a resting potential of −80.5 ± 0.4 mV, spike amplitude of 87.2 ±0.5 mV, and input resistance of 92.1 ±3.0 MΩ. For mouse neurons, there were no significant differences in the neuronal properties by breeding location or htr5A genotype. Combined, Sv129 layer V neurons (n = 329) had a resting potential of −86.4 ± 0.4 mV, spike amplitude of 84.6 ± 0.3 mV, and input resistance of 170.5 ± 3.4 MΩ. Combined, Sv129 layer II/III neurons (n = 55) had a resting potential of −92.2 ± 0.9 mV, spike amplitude of 83.9 ± 0.9 mV, and input resistance of 144.3 ± 8.6 MΩ.
A current step (500 ms) twice the amplitude of the rheobase current was used to elicit a spike train. The firing frequency (f) of the first (f1), second (f2), and last (fL) interspike intervals were then used to calculate the burst index (f1/f2), adaptation index (fL/f2), and maximum frequency (Otsuka and Kawaguchi, 2008). Layer V neurons from5-HT5A +/+ and 5-HT5A−/− mice displayed no difference in the bursting index (p = 0.9), maximum spike frequency (p = 0.9), adaptation index (p = 0.2), or mean interspike interval (p = 0.7). Moreover, the proportions of slow-adapting, slow-adapting with an initial doublet, and fast-adapting neurons did not differ between the genotypes (p = 0.3).
Pharmacology
Serotonergic currents were probed by adding serotonin (5-HT; 30 s) to the bath after a baseline period. Other drugs were also added to the bath: 2 μM tetrodotoxin (TTX), 3 μM baclofen, 1 mM barium chloride (BaCl2), 10–300 nM WAY-100635, 10 μM caboxamindotryptamine maleate (5-CT), 1–2 μM ketanserin, 10 μM SB-699551, 10 μM (R)-(+)-hydroxy-DPAT hydrobromide (8-OH-DPAT). All compounds were obtained from Sigma, Tocris Bioscience, or Alomone and stored in stock solutions at −20°C.
Western blot
Prefrontal cortical brain tissue was collected from 5-HT5A+/+ and 5-HT5A−/− mice (n = 6 per genotype), as described above. Medial sections were dissected and processed to extract total protein (Millipore). Equal amounts of denatured protein extracts (20 μg) were separated by SDS-PAGE on 12% gels and transferred to nitrocellulose membranes. Membranes were incubated overnight at 4°C with an anti-5-HT1A receptor polyclonal primary antibody (1:4000, AB15350; Millipore) (Jacobsen et al., 2011), incubated for 1 h with a peroxidase-conjugated secondary antibody (1:7000; Jackson Immunoresearch), and visualized using chemiluminescence. Band intensities were quantified using ImageJ and normalized to β-actin.
Statistical analysis
The peak amplitude of the serotonergic current was measured using Clampfit software (Molecular Devices). This measurement was obtained by subtracting the 1 s averaged holding current at the peak of the 5-HT response from holding current at the baseline. Statistical comparisons for within-cell responses to either one or several pharmacological agents were determined using Student’s two-tailed paired t tests or repeated-measures ANOVA, respectively. To evaluate between-cell responses, we used Student’s two-tailed unpaired t tests. We used Fisher’s exact tests to compare the differences in proportions of neurons displaying a response of interest. IV curves were statistically analyzed using a comparison of fits between a straight line and a second-order polynomial. Data are expressed as mean ±SE and statistical comparisons evaluated at a significance level of 0.05.
Results
Evidence that the 5-HT5A receptor mediates an unidentified 5-HT current in cortex
The 5-HT5A receptor is found in the rodent cerebral cortex (Grailhe et al., 1999; Kinsey et al., 2001) and expressed preferentially in layer V neurons (Belgard et al., 2011), together with the more extensively studied 5-HT1A and 5-HT2 receptors. To examine the 5-HT5A receptor current, the latter receptors were blocked with 10–30 nM WAY-100635 and 1–2 μM ketanserin; higher concentrations were used for rapid blockade (10 min), followed by continued application of the lower concentrations. These concentrations were selected based on previous studies (Béïque et al., 2004; Goodfellow et al., 2009).
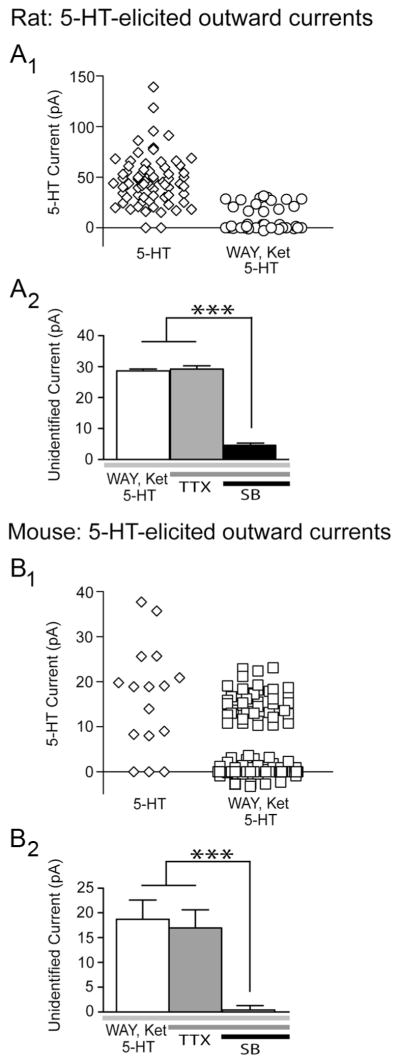
In the presence of these antagonists, we found that 5-HT (10 μM; 30 s) continued to elicit unidentified outward currents, which exceeded 3 times root mean square baseline noise and persisted for at least 60 s. These currents were observed in a proportion of layer V pyramidal neurons in the prefrontal cortex of Sprague Dawley rats (14/42 neurons; 33%; 24.8 ± 1.4 pA; Fig. 1A), Sv129 mice (42/114 neurons; 37%; 15.4 ±0.5 pA), as well as C57BL/6 mice (5/10 neurons; 50%; 14.8 ± 1.8 pA). These findings are consistent with previous reports of unidentified inhibitory effects of 5-HT in the rodent cortex (Amargós-Bosch et al., 2004; Villalobos et al., 2005; Zhong and Yan, 2011). Subsequent within-cell experiments showed that the unidentified 5-HT currents were resistant to TTX (2 μM, 20 min; rat, n =4; mouse, n = 5; Fig. 1B,D) and to antagonists of the glutamate and GABA receptors (100.2 ± 13.7% of baseline unidentified 5-HT current, n = 6, p = 0.9; Fig. 2A). In contrast, they were significantly suppressed by the 5-HT5A receptor antagonist, SB-699551 (10 μM, 20 min; Fig. 1B,D). These findings suggest the presence of functional 5-HT5A receptors in layer V neurons of the prefrontal cortex.
Figure 1.
An unidentified 5-HT current in prefrontal cortex of rat and mouse: evidence of functional 5-HT5A receptors. In the rat (A1) and mouse (B1) prefrontal cortex, bath application of 5-HT (10 μM ; 30 s) elicits an unidentified outward current in the presence of the 5-HT1A and 5-HT2 antagonists, WAY-100635 (WAY) and ketanserin (Ket). The bar graphs summarize within-cell paired experiments from rats (A2) and mice (B2), showing that the baseline unidentified 5-HT current is a postsynaptic current that does not change in the presence of TTX but is significantly suppressed by the 5-HT5A antagonist, SB-699551 (SB) (repeated-measures ANOVA; rat, ***p =0.0001; mouse, ***p <0.0001).
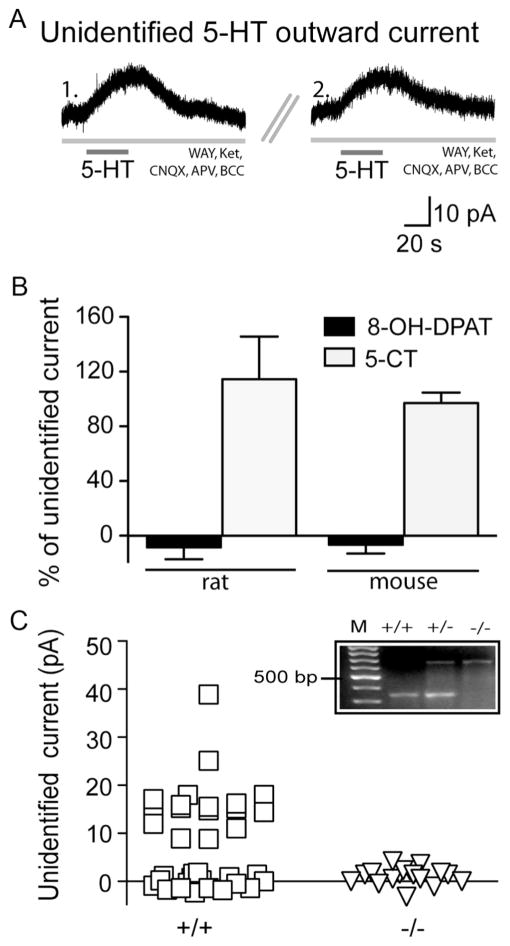
Figure 2.
Pharmacological and transgenic confirmation that the 5-HT5A receptor mediates the unidentified 5-HT current. A, Voltage-clamp traces showing the unidentified 5-HT current in the presence of WAY-100635 (WAY) and ketanserin (Ket) (1.) can be re-elicited upon repeat application of 5-HT following sufficient washout (5 min) (2.). B, Under these conditions, the unidentified 5-HT outward current was not elicited by the 5-HT1A agonist, 8-OH-DPAT (paired t test; rat, p =0.0007; mice, p <0.0001), but was elicited by the mixed 5-HT receptor agonist, 5-CT (paired t test; rat, p =0.7; mice, p =0.4). C, In 5-HT5A+/+ mice, a substantial proportion of layer V neurons display an unidentified 5-HT current (squares). In sibling 5-HT5A−/− mice, however, layer V neurons do not display this current (triangles; Fisher’s exact test, p =0.0003). Inset, PCR products derived from 5-HT5A wild-type (+/+), heterozygous knock-out (+/−), and homozygous knock-out (−/−) mice. Lane M corresponds to a 100 bp DNA ladder with the 500 bp marker labeled.
Control experiments using pharmacological tools and 5-HT5A−/− transgenic mice
Since the prefrontal cortex also expresses receptors from the inhibitory 5-HT1 receptor subfamily (Bruinvels et al., 1994; Amargós-Bosch et al., 2004), we performed a series of additional control experiments. First, we investigated whether the unidentified 5-HT current resulted from an incomplete blockade of the 5-HT1A receptor. The unidentified 5-HT current was not elicited by the 5-HT1A agonist, 8-OH-DPAT (10 μM; 5 min; rats, n = 5; mice, n = 7; Fig. 2B) and persisted following bath application of a higher concentration of the 5-HT1A antagonist, WAY-100635 (300 nM; 104 ±10.2% of baseline unidentified 5-HT current, n = 6, p = 0.6). Moreover, we did not observe an unidentified 5-HT current in layer II/III neurons (0 of 15 neurons; p = 0.003), cells with functional 5-HT1A receptors (Goodfellow et al., 2009) that do not express 5-HT5A receptors (Belgard et al., 2011). Second, additional experiments in layer V revealed that the unidentified 5-HT current was not blocked by the selective 5-HT1B antagonist, SB-224289 (2 μM; 10 min; 106.3 ± 10.8% of baseline unidentified current, n = 5, p = 0.7) and could not be elicited by the potent 5-HT1E/1F agonist, BRL54443 (1 μM, 3 min; n = 4). Finally, we found that the unidentified 5-HT current could, however, be mimicked by 5-CT (10 μM, 30 s; rats, n = 6; mice, n = 17; Fig. 2B), a mixed 5-HT receptor agonist with high affinity for the 5-HT5A receptor (Matthes et al., 1993). Together, these findings suggest that the unidentified 5-HT current is not mediated by a member of the 5-HT1 receptor family and further support the involvement of the 5-HT5A receptor.
To test the hypothesis that the 5-HT5A receptor mediates the unidentified 5-HT current, we recorded from mice with the deletion of the htr5A gene (5-HT5A−/−) and their littermate wild-type siblings (5-HT5A+/+) (Grailhe et al., 1999). As illustrated in Figure 2C, a substantial proportion of layer V neurons in 5-HT5A+/+ mice display unidentified 5-HT currents in the presence of the 5-HT1A and 5-HT2 receptor antagonists (17/36 neurons, 47%; 16.8 ± 1.6 pA). In contrast, recordings made in layer V of 5-HT5A−/− mice under identical conditions did not reveal an unidentified 5-HT current (0/17 neurons, p = 0.0003). Examination of the spike firing patterns in 5-HT5A+/+ and 5-HT5A−/− mice suggests that similar populations of neurons were recorded in both genotypes (see Materials and Methods, above).
Characterization of native 5-HT5A receptor currents in adult prefrontal cortex
Next, we characterized the 5-HT5A receptor currents in normal rodents. The 5-HT5A current had a compelling influence on the excitability of pyramidal neurons in mice (Fig. 3A). When layer V neurons were injected with positive current to induce sustained action potential firing (2.5 ± 0.3 Hz, n = 6), stimulation of the 5-HT5A current eliminated their firing (0 ± 0 Hz, n = 6, p = 0.001). This inhibitory influence of the 5-HT5A current on neuronal excitability is likely enhanced by its reduction of the input resistance in layer V neurons (−30.4 ± 8.8 MΩ from baseline; n =9; p =0.009). Concentration-response analyses revealed that the 5-HT5A receptor is activated by submicromolar levels of 5-HT (rat EC50: 0.6 μM, 95% CI: 0.3–1.2 μM, n = 5; mouse EC50: 0.9 μM, 95% CI: 0.4–1.9 μM, n = 5; Fig. 3B). Current–voltage analysis showed that the 5-HT5A current is inwardly rectifying (4 of 4 neurons; comparison of fits, p <0.0001) with a reversal potential (−98 mV, 95% CI: −98 to −99 mV) near the calculated equilibrium potential for K+ ions (Fig. 3C). Extending this finding, the 5-HT5A current can be suppressed by blockers of G-protein-linked inwardly rectifying K+ (Kir3) channels: Ba2+ ions (1 mM, 10 min; n = 5) and Tertiapin-Q (0.1 μM, 20–40 min; n = 5; Fig. 3D). Together, these results demonstrate that in ex vivo brain slice, the 5-HT5A receptor has relatively high affinity for 5-HT and elicits a K+ current through activation of Kir3 channels.
Figure 3.

Characterization of the 5-HT5A current in the normal adult rodent cortex. A, Current-clamp trace illustrates that the 5-HT5A current can inhibit neuronal excitability resulting from a constant depolarizing current. B, The concentration–response curves demonstrate that the 5-HT5A receptor has relatively high affinity for applied 5-HT in both the rat and mouse prefrontal cortex. C, Current–voltage graph illustrates that the 5-HT5A response is inwardly rectifying and reverses near the calculated equilibrium potential for K+ ions. D, Bar graph shows that the 5-HT5A current is suppressed by the Kir3 channel blockers: Ba2+ ions (paired t test; **p = 0.002) and Tertiapin-Q (paired t test; ***p = 0.001). WAY: WAY-100635; Ket: ketanserin.
To examine whether 5-HT5A currents are enriched in a particular population of layer V neurons, we compared the spike firing characteristics (Otsuka and Kawaguchi, 2008) between wild-type mouse neurons with and without a 5-HT5A current response. For this analysis, we used the wild-type 5-HT5A+/+ neurons from Figures 1B1 and 2C, for which we had assessed the spiking pattern in response to an injection of twice the rheobase current (n = 144 neurons). We found that an unexpectedly high proportion of initial-doublet neurons (burst index > 2.7) had 5-HT5A current responses (19 of 26 neurons; 73%; p = 0.0001). Since neurons with similar firing patterns tend to project to the same brain region (Hattox and Nelson, 2007), 5-HT5A receptors may suppress preferentially a specific type of prefrontal cortical output mediated by this class of neuron.
Genetic deletion of the 5-HT5A receptor increases 5-HT1A receptor currents
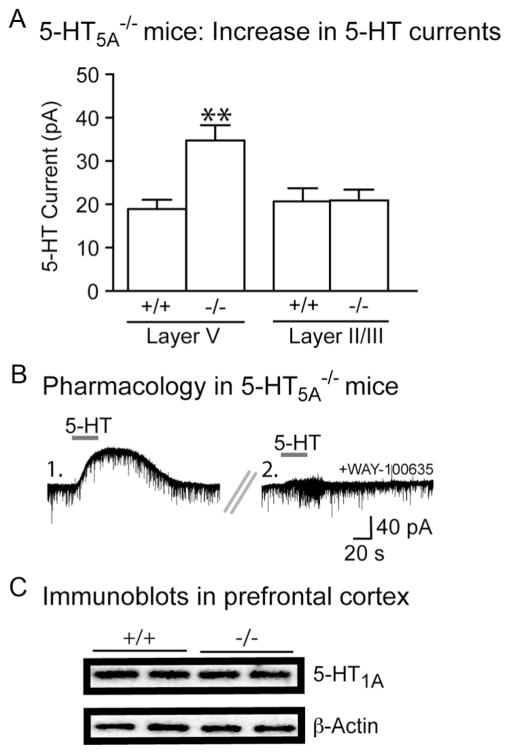
Since 5-HT modulates prefrontal cortex through several 5-HT receptors (Amargós-Bosch et al., 2004; Béïque et al., 2004), we investigated whether genetic deletion of htr5A gene altered the overall neuronal response to 5-HT. Recording in the absence of antagonists, we observed that the loss of the inhibitory 5-HT5A receptor paradoxically increased 5-HT-elicited inhibitory outward currents in layer V neurons (5-HT5A+/+ neurons, n = 36; 5-HT5A −/− neurons, n = 35; p = 0.0003; Fig. 4A). This supra-compensatory plasticity in 5-HT5A −/− mice appeared to be mediated by an increase in 5-HT1A receptor currents (baseline 5-HT current: 51.0 ±7.5 pA; after 30 nM WAY-100635: −1.8 ±1.8 pA; n =6; p =0.002; Fig. 3B). Interestingly, we detected no difference in medial prefrontal 5-HT1A receptor protein content between 5-HT5A+/+ (0.52 ± 0.03 arbitrary units, n = 6) and 5-HT5A−/− mice (0.47 ± 0.02 arbitrary units, n =6; p =0.2; Fig. 4C). To test the specificity of the electrophysiological effect for the 5-HT1A receptor, we examined the magnitude of another Gαi/o-mediated current using a selective GABAB agonist (baclofen; 3 μM, 30 s). In contrast, the GABAB outward currents were similar in 5-HT5A +/+ (67.4 ±3.7 pA, n =20) and 5-HT5A −/− mice (73.6 ± 4.8 pA, n = 20; p = 0.3). Next, we examined whether the increased 5-HT1A receptor currents in 5-HT5A −/− mice were restricted to the cortical layer with functional 5-HT5A receptors (see Results, above). To this end, we examined the 5-HT1A-mediated outward currents in layer II/III neurons in the absence of any antagonists (5-HT5A+/+ neurons, n = 20; 5-HT5A −/− neurons, n = 20). A two-way ANOVA revealed a significant interaction between htr5A genotype and the prefrontal cortical layer (Fig. 4A). Together, these experiments suggest that genetic deletion of the 5-HT5A receptor triggers a specific upregulation of 5-HT1A outward currents selectively in layer V output neurons of the prefrontal cortex.
Figure 4.
5-HT5A−/− mice display a selective upregulation of layer V 5-HT1A currents, but not of prefrontal 5-HT1A protein content. A, In the absence of antagonists, the amplitude of the5-HT outward current is significantly larger in layer V neurons from 5-HT5A−/− mice compared with layer V neurons from 5-HT5A+/+ mice or layer II/III neurons from either 5-HT5A+/+ or 5-HT5A−/− mice (two-way ANOVA, significant interaction; **p = 0.01). B, Voltage clamp traces illustrate that the larger 5-HT outward current observed in 5-HT5A−/− mice (1.) is completely suppressed by the selective 5-HT1A receptor antagonist, WAY-100635 (2.). C, Representative immunolabeling from two 5-HT5A+/+ and two 5-HT5A−/− mice illustrating that prefrontal 5-HT1A protein content is not significantly affected by genotype.
Discussion
In the present study, we provide direct evidence of functional, native 5-HT5A receptors in cortical neurons of both rats and mice. We find that these receptors produce a small, inwardly rectifying K+ current through Kir3 channels in a subpopulation of neurons, and this 5-HT current is absent in the cortex of 5-HT5A receptor knock-out mice. Finally, we show that loss of the htr5A gene is sufficient to trigger the upregulation of another inhibitory 5-HT current mediated by the 5-HT1A receptor. These results, to our knowledge, are the first to characterize functionally the 5-HT5A receptor in ex vivo cortical brain tissue and to establish a previously unknown interaction between the 5-HT5A receptor and the therapeutically relevant 5-HT1A receptor.
Serotonergic inhibition of the prefrontal cortex is important for coordinating emotional behaviors (Puig and Gulledge, 2011). To date, this inhibition has been attributed entirely to 5-HT1A receptors, despite evidence suggesting the presence of an additional, unidentified, inhibitory 5-HT effect (Amargós-Bosch et al., 2004, Villalobos et al., 2005; Zhong and Yan, 2011). Specifically, prefrontal 5-HT1A receptors are thought to regulate emotional responses by inhibiting the major output neurons of the prefrontal cortex. Our findings, however, demonstrate a previously unappreciated role of 5-HT5A receptors in modulating prefrontal neurons. Notably, the 5-HT5A receptor and 5-HT1A receptor display similar coupling to effectors (for 5-HT1A, see Raymond et al., 1999; for 5-HT5A, see Grailhe et al., 2001; present study) and efficacy for the 5-HT ligand (for 5-HT1A, see Okuhara and Beck, 1998; for 5-HT5A, see present study). Moreover, like the 5-HT1A receptor, the 5-HT5A receptor is expressed in a number of limbic regions, including the hippocampus and cortex (Grailhe et al., 1999; Kinsey et al., 2001). Despite these similarities, the 5-HT5A knock-out mice do not display the anxiety phenotype observed in 5-HT1A knock-out mice (Ramboz et al., 1998), but rather exhibit altered LSD-mediated explorative behaviors (Grailhe et al., 1999). It is tempting to speculate that the presence of the closely related 5-HT1A and 5-HT5A receptors in the same neuronal cells may serve as a biological safeguard, such that disruption of one receptor may induce compensatory up-regulation of the other receptor (Gingrich and Hen, 2000). In support of this hypothesis, we show that loss of htr5A gene strongly upregulates 5-HT1A receptor-mediated currents in the prefrontal cortex. This interaction may have clinical implications since 5-HT1A receptor agonists have been used in the treatment of mood disorders (Blier and Ward, 2003). Development of selective 5-HT5A ligands is critical to improving our understanding the physiological relevance of this relatively unknown 5-HT receptor as well as elucidating its interactions with other members of the 5-HT receptor family.
Acknowledgments
This work was supported by an NSERC Discovery Grant (E.K.L.), the Scottish Rite Charitable Foundation (E.K.L.), and the Canada Research Chairs program (E.K.L.). N.M.G. was supported by a Margaret Santalo fellowship and CIHR Banting and Best Doctoral award. We thank Dr. Rene Hen of Columbia University for the generous gift of the transgenic mice and the knock-out primer sequences, and Dr. Jeff Muller for facilitating the transfer of these mice. We thank Dr. Beverley Orser of the University of Toronto for the use of the imaging facility and Drs. Milton Charlton, Sabine Cordes, and Paul Fletcher for constructive suggestions on the manuscript.
Footnotes
Author contributions: N.M.G., C.D.C.B., and E.K.L. designed research; N.M.G. performed research; N.M.G., C.D.C.B., and E.K.L. analyzed data; N.M.G., C.D.C.B., and E.K.L. wrote the paper.
References
- Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1a and serotonin2a receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgard TG, Marques AC, Oliver PL, Abaan HO, Sirey TM, Hoerder-Suabedissen A, García-Moreno F, Molnár Z, Margulies EH, Ponting CP. A transcriptomic atlas of mouse neocortical layers. Neuron. 2011;71:605–616. doi: 10.1016/j.neuron.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biological Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios MJ. Localization of 5-HT 1 B, 5-HT 1 Dα, 5-HT 1 E and 5-HT 1 F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Corbett DF, Heightman TD, Moss SF, Bromidge SM, Coggon SA, Longley MJ, Roa AM, Williams JA, Thomas DR. Discovery of a potent and selective 5-ht5A receptor antagonist by high-throughput chemistry. Bioorg Med Chem Lett. 2005;15:4014–4018. doi: 10.1016/j.bmcl.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. The broken mouse: the role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr Opin Neurobiol. 2000;10:146–152. doi: 10.1016/s0959-4388(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Goodfellow NM, Benekareddy M, Vaidya VA, Lambe EK. Layer II/III of the prefrontal cortex: inhibition by the serotonin 5-HT1A receptor in development and stress. J Neurosci. 2009;29:10094–10103. doi: 10.1523/JNEUROSCI.1960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron. 1999;22:581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Grabtree GW, Hen R. Human 5-HT(5) receptors: the 5-HT(5A) receptor is functional but the 5-HT(5B) receptor was lost during mammalian evolution. Eur J Pharmacol. 2001;418:157–167. doi: 10.1016/s0014-2999(01)00933-5. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- Jacobsen KX, Czesak M, Deria M, Le François B, Albert PR. Region-specific regulation of 5-HT1A receptor expression by Pet-1-dependent mechanisms in vivo. J Neurochem. 2011;116:1066–1076. doi: 10.1111/j.1471-4159.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey AM, Wainwright A, Heavens R, Sirinathsinghji DJ, Oliver KR. Distribution of 5-ht(5A), 5-ht(5B), 5-ht(6) and 5-HT(7) receptor mR-NAs in the rat brain. Brain Res Mol Brain Res. 2001;88:194–198. doi: 10.1016/s0169-328x(01)00034-1. [DOI] [PubMed] [Google Scholar]
- Matthes H, Boschert U, Amlaiky N, Grailhe R, Plassat JL, Muscatelli F, Mattei MG, Hen R. Mouse 5-hydroxytryptamine5A and 5-hydroxytryptamine5B receptors define a new family of serotonin receptors: cloning, functional expression, and chromosomal localization. Mol Pharmacol. 1993;43:313–319. [PubMed] [Google Scholar]
- Noda M, Yasuda S, Okada M, Higashida H, Shimada A, Iwata N, Ozaki N, Nishikawa K, Shirasawa S, Uchida M, Aoki S, Wada K. Recombinant human serotonin 5A receptors stably expressed in C6 glioma cells couple to multiple signal transduction pathways. J Neurochem. 2003;84:222–232. doi: 10.1046/j.1471-4159.2003.01518.x. [DOI] [PubMed] [Google Scholar]
- Okuhara DY, Beck SG. Corticosteroids alter 5-hydroxytryptamine1A receptor-effector pathway in hippocampal subfield CA3 pyramidal cells. J Pharmacol Exp Ther. 1998;284:1227–1233. [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Firing-pattern-dependent specificity of cortical excitatory feed-forward subnetworks. J Neurosci. 2008;28:11186–11195. doi: 10.1523/JNEUROSCI.1921-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Marazziti D, Castagna M, Nardi I. Distribution of 5-HT2c and 5-ht5a receptor mRNA in human brain. Ann N Y Acad Sci. 1998;861:245. doi: 10.1111/j.1749-6632.1998.tb10202.x. [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gettys TW, Garnovskaya MN. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br J Pharmacol. 1999;127:1751–1764. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Braselton J, Schmidt A. Serotonin-induced phase advances of SCN neuronal firing in vitro: a possible role for 5-HT5A receptors? Synapse. 2004;54:111–118. doi: 10.1002/syn.20070. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Soffin EM, Roberts C, Kew JN, de la Flor RM, Dawson LA, Fry VA, Coggon SA, Faedo S, Hayes PD, Corbett DF, Davies CH, Hagan JJ. SB-699551-A (3-cyclopentyl-N-[2-(dimethylamino)ethyl]-N-[(4′-{[(2-phenylethyl)amino]methyl}-4-biphenylyl)methyl]propanamide dihydrochloride), a novel 5-ht5A receptor-selective antagonist, enhances 5-HT neuronal function: evidence for an autoreceptor role for the 5-ht5A receptor in guinea pig brain. Neuropharmacology. 2006;51:566–577. doi: 10.1016/j.neuropharm.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Beique JC, Gingrich JA, Andrade R. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur J Neurosci. 2005;22:1120–1126. doi: 10.1111/j.1460-9568.2005.04307.x. [DOI] [PubMed] [Google Scholar]
- Zhong P, Yan Z. Differential regulation of the excitability of prefrontal cortical fast-spiking interneurons and pyramidal neurons by serotonin and fluoxetine. PLoS One. 2011;6:e16970. doi: 10.1371/journal.pone.0016970. [DOI] [PMC free article] [PubMed] [Google Scholar]