Abstract
OBJECTIVES
Two studies reported an increased risk of autistic disorder in children conceived less than 12 months after a previous birth. Our objective was to examine the association between the interpregnancy interval (IPI) and autism spectrum disorder (ASD) in a Canadian cohort.
METHODS
Using administrative datasets housed at the Manitoba Centre for Health Policy, we identified pairs of first- and second-born singleton siblings born between 1988 and 2005. Diagnoses of ASD were ascertained by searching physician billing claims, hospital discharge abstracts, education data, and a database containing information on individuals identified for a 2002–2007 ASD surveillance program in Manitoba. Logistic regression models were fit to examine the association between the IPI and ASD in 41,050 second-born siblings where the first-borns did not have ASD, using IPIs of ≥36 months as the reference category and specifying three case groups. Case Group 1 included individuals with at least one ASD code (n = 490); Case Group 2 included those with two or more ASD codes (n = 375); and Case Group 3 comprised individuals with a record in the ASD surveillance program database (n = 141).
RESULTS
The adjusted odds ratios (ORs) for IPIs shorter than 12 months ranged from 1.22 (95% CI: 0.91–1.63) for Case Group 1 to 1.72 (95% CI: 0.96–3.06) for Case Group 3. When the case groups were restricted to individuals with more severe ASD, the ORs increased and were significant for Case Groups 1 and 2.
CONCLUSION
Our findings also support an association between short IPIs and more severe ASD.
Keywords: Autism, interpregnancy interval, secondary analysis, administrative data, record linkage, Manitoba Centre for Health Policy
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder. Twin studies provide evidence of moderate-to-high heritability,1,2 but environmental factors also influence risk.2 The increasing prevalence3,4 and substantial economic burden5 of ASD form a strong rationale for investigating potentially modifiable risk factors.
Two studies6,7 have reported an increased risk of autism* associated with short interpregnancy intervals (IPIs, defined as the length of time between the end of one pregnancy and the beginning of subsequent gestation). In the first of these studies,6 researchers identified pairs of first and second singletons born to the same parents in California from 1992 through 2002. Case status was assigned by searching for codes corresponding to “autism, full syndrome” or “autism, residual state” in California’s Department of Developmental Services electronic file. An inverse association between the IPI and autism was observed among 662,730 second-born children, with the highest risk reported for intervals shorter than 12 months as compared to 36 months or more. More recently, investigators analyzed data from a Norwegian birth cohort of first- and second-born singleton full siblings.7 ASD diagnoses were ascertained through the Norwegian Patient Registry. IPIs shorter than 12 months were associated with an increased risk of autistic disorder among 223,476 second-born children.
Prenatal nutritional status has been linked to brain development in the offspring,8–11 which supports a potential biologic basis for the association between short IPIs – which may contribute to maternal nutritional depletion12 – and autism/ASD. If this or some other biologic mechanism underlies the observed associations, rather than confounding or chance, it would open up opportunities for modifying risk. To add to the emerging epidemiologic evidence, this retrospective cohort study examined the association between the IPI and ASD in a Manitoba birth cohort.
METHODS
This study was approved by research ethics boards at Queen’s University and the University of Manitoba, and Manitoba’s Health Information Privacy Committee. Approvals were also granted by Manitoba Education and Manitoba Family Services for the use of their data.
Data sources
The Population Health Research Data Repository at the Manitoba Centre for Health Policy (MCHP) houses linkable databases that contain health and other information collected for administrative purposes. A unique encrypted personal health identification number is attached to each record in the Repository databases; this system maintains privacy and confidentiality while ensuring the accurate matching of records across databases.13
Five Repository databases were used for this study: 1) Medical Services (physician claims, which are submitted by fee-for-service physicians and by those compensated through alternative payment mechanisms; one “most responsible” diagnosis is recorded to the third digit of the ICD-9-CM); 2) Hospital Discharge Abstracts (records related to admissions to acute and chronic care facilities, and out-patient surgeries provided in a hospital setting; a maximum of 16 diagnoses are coded to the fifth digit of the ICD-9-CM for encounters up to March 31, 2004, and a maximum of 25 diagnoses are coded to the fifth digit of the ICD-10 thereafter); 3) Education (enrollment and assessment information from 1995 onwards for kindergarten through Grade 12 students, including those who attend private schools or are home-schooled; a nominal variable indicates whether a child received funding under a special needs category); 4) Social Assistance Management Information Network (data from 1995 onwards to indicate whether an individual received income assistance in a particular month and year); and 5) Manitoba Health Insurance Registry (dates of birth, death, and moves within and to and from the province for all individuals covered under Manitoba’s health plan). We also obtained permission to use a sixth data source, the Children’s Special Services dataset, which was created and brought into the Repository for another project led by one of the investigators (H. Ouellette-Kuntz). That dataset contained information on children and youth who, as part of a larger Canadian epidemiologic study14 (www.nedsac.ca), were identified for an ASD surveillance program in Manitoba from 2002–2007 through Children’s Special Services, a provincial government program that supports children with special needs throughout Manitoba (with the exception of those living on First Nations reserves). Proof of a diagnosis made by a qualified health professional (e.g., developmental pediatrician, psychologist, psychiatrist) is required to access program services related to ASD.14
Study cohort and case definitions
Individuals born in Manitoba between January 1, 1988 and March 31, 2005 were identified from hospital records, and the Manitoba Health Insurance Registry was used to identify first and second births to the same mother (i.e., first- and second-born sibling pairs). We ascertained ASD case status by searching up to March 31, 2011 for relevant diagnostic codes in the Medical Services (ICD-9 code 299) and Hospital Discharge Abstracts (ICD-9 codes 299.0, 299.8, 299.9; ICD-10 codes F84.0, F84.1, F84.5, F84.8, F84.9) databases, or an “ASD” special needs designation in the Education data. Individuals were also considered to have ASD if they had a record in the Children’s Special Services dataset (collectively, these criteria are referred to as “ASD codes”). The IPI was calculated as the interval between the birthdates of the two siblings minus the younger sibling’s gestational age at birth in completed weeks.
The sensitivity and specificity of the codes used to identify cases of ASD in Manitoba’s administrative health and education data have not been evaluated. Accordingly, we applied three case definitions to better interpret the findings. Case Group 1 included any individual for whom at least one ASD code was found. The other two case groups were subsets of Case Group 1. Case Group 2 included individuals with two or more ASD codes (at least two codes in the Medical Services, Hospital Discharge Abstracts, or Education data, or at least one code in two or more databases, including the Children’s Special Services dataset). Case Group 3 included individuals with a record in the Children’s Special Services dataset. As previously described, these individuals were identified for an ASD surveillance program in Manitoba. Our assumption was that the proportion of true positives (i.e., individuals with ASD) would differ in a predictable manner across the groups. Based on algorithms that have been tested to identify other conditions using administrative data,15 we assumed that Case Group 2 would have a higher proportion of true positives than Case Group 1, and that all individuals in Case Group 3 would have ASD. If the IPI is associated with ASD, we would therefore expect to see the weakest effect in Case Group 1 and the strongest in Case Group 3.
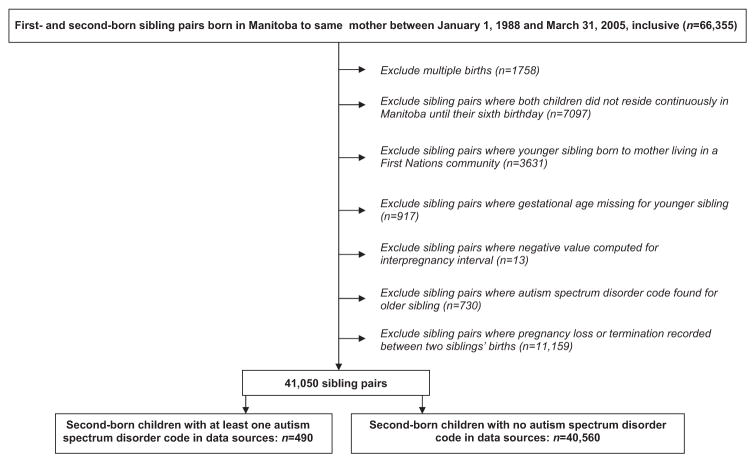
Multiple births were excluded from the study cohort, as were sibling pairs where both children did not reside continuously in Manitoba until at least their sixth birthday (to ensure diagnoses were not missed because they were made outside Manitoba, and because 12% of children with an administrative diagnosis of ASD are only captured in the Education data16). Sibling pairs were also excluded if the mother was living in a First Nations community when the second child was born. This was to ensure comparability of the findings for the three case groups; the Children’s Special Services dataset did not capture cases of ASD among children living on reserves. Other exclusion criteria included sibling pairs where the gestational age was missing for the younger sibling or where the calculated IPI had a negative value, and sibling pairs where an ASD code was found for the first-born child. We restricted the cohort for the main analysis to sibling pairs where there was no record of a pregnancy loss or termination between the two births, as an association between the IPI and ASD may be mediated by maternal physiologic parameters during pregnancy.6,17 Since our calculation of the IPI was based on live births, the impact of intervals of equal length may not be comparable if a pregnancy loss or termination occurred between some births.
Analysis
The data were analyzed at MCHP using SAS 9.3 (SAS Institute Inc., Cary, NC). The IPI was converted into months and similar categories to those analyzed in the California and Norwegian studies were specified (<12, 12–23, 24–35, ≥36 (reference)).6,7 Separate logistic regression models were fit for each case group to estimate crude and adjusted odds ratios (ORs) for ASD in the second-born children, using the same comparison group in each model. A priori, we decided to include the following covariates in the models due to previously documented associations with ASD: child’s sex, birth year (we mean-centered this variable and created a quadratic term to account for birth year’s non-linear association with ASD in our cohort), and presence of an intellectual disability (defined as a “Multiple Handicaps” special needs code in the Education data, or the presence of various diagnostic codes in the Medical Services or Hospital Discharge Abstracts databases18), as well as maternal age at delivery (<30, 30–34, ≥35 years19–21) and whether the mother had ever received income assistance. In a supplementary analysis, we added product terms to the regression models to explore whether certain factors interacted with the IPI to modify the association. Those factors included the child’s sex, whether he or she had an intellectual disability, or was born in 1998 or earlier (Canada instituted a mandatory folic acid fortification program for white flour and enriched grain products in 1998), and maternal age.
We performed several sensitivity analyses to assess whether methodological factors might explain any difference between our findings and those of the California6 and Norwegian7 studies. In the main analysis, we excluded sibling pairs where a pregnancy loss or termination was reported between the two births; this was not done in the other studies. In the first sensitivity analysis (“Sensitivity Analysis 1”), we included second-born children from these sibling pairs.
The California study examined the association between the IPI and autism.6 The Norwegian cohort included cases across the spectrum, but their main outcome was autistic disorder.7 There was no direct measure of ASD severity for many of our cases (the Education data indicate whether a child was approved for Level 2 support for “moderate ASD” or Level 3 support for “severe to profound ASD,”22 but only about 60% of cases were identified in that source (data not shown)). A previous study reported that the median age at diagnosis of autistic disorder in Manitoba was 42 months, compared to 77 months for pervasive developmental disorder, not otherwise specified (PDD-NOS) and 101 months for Asperger disorder.23 As a proxy measure of greater severity then, we restricted the case groups to individuals diagnosed before 42 months of age, or to those with an “ASD” code in the Education data who were approved for Special Needs Level 2 or Level 3 funding (“Sensitivity Analysis 2”). These individuals are referred to as “more severe” cases throughout the remainder of this paper, with the quotation marks retained to indicate the proxy nature of this designation.
All references to significance are based on a p-value of <0.05.
RESULTS
A total of 66,355 sibling pairs were initially identified. After the exclusion criteria were applied, 41,050 pairs remained for the main analysis (Figure 1). Of those, 40,560 were pairs where the younger sibling had no record of an ASD diagnosis. There were 490 cases in Case Group 1; 375 in Case Group 2; and 141 in Case Group 3.
Figure 1.
Selection of study cohort for main analysis
Table 1 shows the distribution of the IPI and characteristics of the second-born children in the study cohort. All three case groups had a higher proportion of boys and individuals with an intellectual disability than the comparison group. The IPI distribution was fairly similar across all groups.
Table 1.
Characteristics of second-born children from sibling pairs born to the same mother in Manitoba between January 1, 1988 and March 31, 2005, where no record of autism spectrum disorder was found for first-born siblings
| Second-born children with at least one ASD code
|
Second-born children with no ASD code (n = 40,560) | |||
|---|---|---|---|---|
| Case Group 1* (n = 490) | Case Group 2† (n = 375) | Case Group 3‡ (n = 141) | ||
| Interpregnancy interval in months, n (%) | ||||
| <12 | 125 (25.5) | 99 (26.4) | 38 (27.0) | 9545 (23.5) |
| 12–23 | 179 (36.5) | 141 (37.6) | 59 (41.8) | 15,571 (38.4) |
| 24–35 | 95 (19.4) | 70 (18.7) | 24 (17.0) | 7968 (19.6) |
| ≥36 | 91 (18.6) | 65 (17.3) | 20 (14.2) | 7476 (18.4) |
| Boy, n (%) | 380 (77.6) | 303 (80.8) | 114 (80.9) | 20,599 (50.8) |
| Year of birth, n (%) | ||||
| 1988–1992 | 80 (16.3) | 63 (16.8) | 29 (20.6) | 7910 (19.5) |
| 1993–1996 | 152 (31.0) | 126 (33.6) | 53 (37.6) | 11,858 (29.2) |
| 1997–2000 | 136 (27.8) | 98 (26.1) | 35 (24.8) | 10,474 (25.8) |
| 2001–2005 | 122 (24.9) | 88 (23.5) | 24 (17.0) | 10,318 (25.4) |
| Intellectual disability (child), n (%) | 120 (24.5) | 97 (25.9) | 54 (38.3) | 464 (1.1) |
| Maternal age at delivery,§ years, n (%) | ||||
| ≥35 | 61 (12.4) | 46 (12.3) | 25 (17.7) | 4074 (10.0) |
| 30–34 | 167 (34.1) | 136 (36.3) | 57 (40.4) | 12,173 (30.0) |
| <30 | 262 (53.5) | 193 (51.5) | 59 (41.8) | 24,312 (59.9) |
| Ever receipt of income assistance (mother), n (%) | 105 (21.4) | 82 (21.9) | 24 (17.0) | 6220 (15.3) |
| “More severe” cases, || n (%) | 338 (69.0) | 310 (82.7) | 132 (93.6) | – |
ASD: Autism spectrum disorder.
At least one ASD code in any of the following databases: Medical Services, Hospital Discharge Abstracts, Education, or Children’s Special Services dataset.
At least two ASD codes in one of the following databases: Medical Services, Hospital Discharge Abstracts, or Education; or at least one ASD code in two or more of the following databases: Medical Services, Hospital Discharge Abstracts, Education, or Children’s Special Services dataset.
Record in the Children’s Special Services dataset.
One value missing for the group of second-born children with no ASD code.
Case groups restricted to individuals diagnosed before 42 months of age or with an “ASD” code in the Education data who were approved for Special Needs Level 2 (“moderate ASD”) or Level 3 (“severe to profound ASD”) funding.
Table 2 provides crude and adjusted ORs for the association between the IPI and ASD in the second-born children. While a test for linear trend approached significance for Case Groups 2 and 3, we did not additionally model the IPI as a continuous variable as there was no evidence of a stronger association for shorter intervals within each IPI category (data not shown).
Table 2.
Crude and adjusted odds ratios for the interpregnancy interval and autism spectrum disorder in second-born children from sibling pairs born in Manitoba to the same mother between January 1, 1988 and March 31, 2005, where no record of autism spectrum disorder was found for first-born siblings
| Interpregnancy interval, months | Case Group 1*
|
Case Group 2†
|
Case Group 3‡
|
|||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted§ OR (95% CI) | Crude OR (95% CI) | Adjusted§ OR (95% CI) | Crude OR (95% CI) | Adjusted§ OR (95% CI) | |
| <12 | 1.07 (0.82–1.41) | 1.22 (0.91–1.63) | 1.19 (0.87–1.63) | 1.36 (0.97–1.90) | 1.49 (0.87–2.56) | 1.72 (0.96–3.06) |
| 12–23 | 0.94 (0.73–1.22) | 1.08 (0.83–1.41) | 1.04 (0.78–1.40) | 1.19 (0.87–1.62) | 1.42 (0.85–2.35) | 1.59 (0.93–2.71) |
| 24–35 | 0.98 (0.73–1.31) | 1.08 (0.80–1.45) | 1.01 (0.72–1.42) | 1.10 (0.78–1.56) | 1.13 (0.62–2.04) | 1.29 (0.70–2.38) |
| ≥36 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| p-value (test for linear trend) | – | 0.20 | – | 0.07 | – | 0.05 |
OR: Odds ratio; CI: Confidence interval.
At least one autism spectrum disorder (ASD) code in any of the following databases: Medical Services, Hospital Discharge Abstracts, Education, or Children’s Special Services dataset.
At least two ASD codes in one of the following databases: Medical Services, Hospital Discharge Abstracts, or Education; or at least one ASD code in two or more of the following databases: Medical Services, Hospital Discharge Abstracts, Education, or Children’s Special Services dataset.
Record in the Children’s Special Services dataset.
Models adjusted for child’s sex, mean-centered birth year, mean-centered birth year squared, intellectual disability (child), maternal age at delivery, and whether mother ever received income assistance.
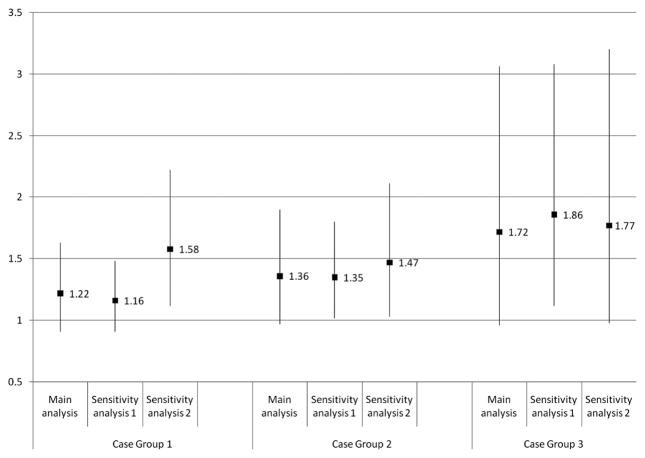
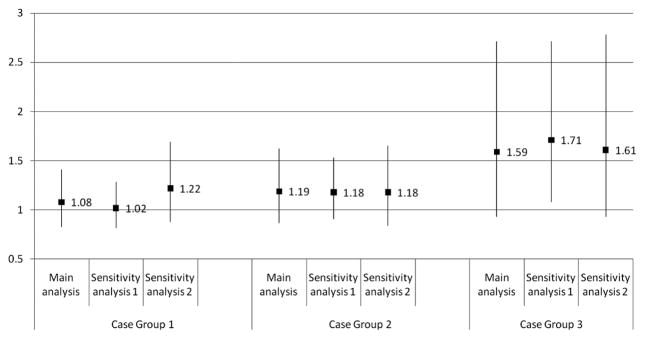
Figures 2 and 3 illustrate the results of the sensitivity analyses (shown for IPIs of <12 and 12–23 months only). There was little change in the ORs for Case Groups 1 and 2 when all second-born children were included in the analysis, regardless of whether their mothers had experienced a pregnancy loss or termination after the birth of the older sibling (Sensitivity Analysis 1), while the effect sizes for both IPI categories increased and attained significance for Case Group 3. The ORs increased for intervals shorter than 12 months when the case groups were restricted to “more severe” cases (Figure 2, Sensitivity Analysis 2). The magnitude of that increase was greatest for Case Group 1 and negligible for Case Group 3.
Figure 2.
Results of sensitivity analyses (for interpregnancy intervals shorter than 12 months)
NOTE: The numbers shown in Figures 2 and 3 are odds ratios from logistic regression models, adjusted for child’s sex, mean-centered birth year, mean-centered birth year squared, intellectual disability (child), maternal age at delivery, and whether the mother ever received income assistance. The bars represent 95% confidence intervals.
Case Group 1: At least one autism spectrum disorder (ASD) code in any of the following databases: Medical Services, Hospital Discharge Abstracts, Education, or Children’s Special Services dataset.
Case Group 2: At least two ASD codes in one of the following databases: Medical Services, Hospital Discharge Abstracts, or Education; or at least one ASD code in two or more of the following databases: Medical Services, Hospital Discharge Abstracts, Education, or Children’s Special Services dataset.
Case Group 3: Record in the Children’s Special Services dataset.
Sensitivity Analysis 1: Includes second-born children from sibling pairs where a pregnancy loss or termination was reported between the older and younger siblings’ births.
Sensitivity Analysis 2: Case groups restricted to individuals diagnosed before 42 months of age, or with an “ASD” code in the Education data and approved for Special Needs Level 2 (“moderate ASD”) or Level 3 (“severe to profound ASD”) funding.
Figure 3.
Results of sensitivity analyses (for interpregnancy intervals of 12–23 months)
See notes under Figure 2.
While the overall tests of interaction were generally not significant, some of the stratum-specific associations were (see Supplementary Table).
DISCUSSION
Our discussion focuses on the findings for IPIs shorter than 12 months (“short IPIs”), as these were associated with an increased risk of autism in both the California6 and the Norwegian7 studies. (In contrast, significant associations for IPIs of 12–23 and 24–35 months were also reported in the former6 but not the latter7 study.) Despite the non-significant findings from the main analysis, our study does support an association between short IPIs and autism: when we restricted the case groups to “more severe” cases, the ORs increased and attained significance for Case Groups 1 and 2 (Figure 2, Sensitivity Analysis 2). While the ORs did not follow the expected pattern in this second sensitivity analysis (i.e., lowest in Case Group 1, highest in Case Group 3), the strongest effect was observed for Case Group 3 (OR = 1.77; 95% CI: 0.98–3.20), as predicted. The lack of a significant association for the latter group may have been due to its small number of cases (n = 132 for Sensitivity Analysis 2). Our a priori power calculations indicated we would have sufficient power to detect the ORs reported in the California study for IPIs shorter than 12 months, but those calculations were based on the estimated number of cases with at least one ASD code (i.e., Case Group 1).
The association between short IPIs and PDD-NOS and Asperger disorder combined was not significant in the Norwegian study.7 The lower ORs for Case Groups 1 and 2 in the main analysis, compared to Case Group 3 (Table 2), could be attributable to a higher proportion of individuals on the milder end of the spectrum in those two groups, as evidenced by their lower proportions of “more severe” cases (Table 1). It is important to note, however, that higher proportions of false positives in those groups may also have attenuated the associations.
When the outcome was restricted to “more severe” cases versus no ASD (Figure 2, Sensitivity Analysis 2), the associations we observed more closely supported the findings from the Norwegian study,7 where the ORs for autistic disorder were 1.71 (95% CI: 1.07–2.64) for intervals of 9–11 months and 2.18 (95% CI: 1.42–3.26) for intervals of less than 9 months. The association for short IPIs was stronger in the California study (OR = 3.39; 95% CI: 3.00–3.82).6 The latter investigation reported significant heterogeneity in the IPI–autism association across maternal and paternal age strata, and stratified analyses by maternal race and use of California’s Medicare system as payment for delivery yielded p-values approaching significance. To our knowledge, these factors were not considered in terms of potential effect modification in the Norwegian study.7 However, the investigators did examine whether there was an interaction between maternal folate use and the IPI. They noted that, while not statistically significant, the effect of short IPIs was stronger in children whose mothers had not taken folic acid supplements prior to or during pregnancy (and that larger studies with more precise measures of folic acid intake are needed). If certain factors interact with the IPI, and those factors are distributed differently across study populations, it could explain some of the disparity in terms of the strength of the associations observed. Our study likely lacked sufficient power to detect significant interactions, but we conducted a supplementary analysis to provide preliminary data for those wishing to examine potential effect modifiers in future studies (see Supplementary Table).
Our study cohort was selected using hospital records and the Manitoba Health Insurance Registry. It should have captured most of the target population, as fewer than 1% of births in Manitoba take place in a non-hospital setting.24 This, together with the fact that the health and education data capture information on almost all residents of Manitoba, means that our cohort was truly population-based. While this was a great strength of the study, we acknowledge the limitations imposed by the use of primarily administrative data to define the outcome of ASD, rather than “gold standard” case definitions based on developmental histories and observational assessments. We used three case groups to mitigate this limitation.
A further limitation was our inability to restrict the analysis to full-sibling pairs, as our dataset did not include information on paternity. A cohort of full-sibling pairs, where the older children do not have ASD, would provide greater assurance of minimizing any genetic contribution to ASD in the younger siblings. If some of the cases included in our analysis have an older paternal half-sibling with ASD, and if the IPIs for those second-born children tended to be longer, it may have attenuated the true effect of short IPIs on the risk of autism.
While the findings from three studies now support an association between short IPIs and autism, other questions remain. First, is the association stronger in certain groups, such as those defined by maternal age or ethnicity? Second, does folic acid intake prior to or during pregnancy modify the strength of the association? Third, are IPIs of 12 months or more also associated with an increased risk of autism, compared to longer IPIs (or some “ideal” interval)? Fourth, does the association persist in children from higher-parity births? The answers to these questions will help inform public health initiatives aimed at reducing the occurrence of autism.
Supplementary Material
Acknowledgments
This work was supported by a Secondary Analysis of Databases Operating Grant from the Canadian Institutes of Health Research (SEC 117119). We are indebted to Manitoba Education and Advanced Learning, Manitoba Family Services, and Manitoba Jobs and the Economy, as well as Health Information Management, Manitoba Health, Healthy Living and Seniors, for provision of data. The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository under project # HIPC 2011/2012-37.
Footnotes
In this paper, “autism” is a generic term for a more severe form of ASD, including autistic disorder, while “ASD” refers to the entire autism spectrum.
Disclaimer: The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Healthy Living and Seniors, or other data providers is intended or should be inferred.
Conflict of Interest: None to declare.
References
- 1.Posthuma D, Polderman TJ. What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Curr Opin Neurol. 2013;26(2):111–21. doi: 10.1097/WCO.0b013e32835f19c3. [DOI] [PubMed] [Google Scholar]
- 2.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years –Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. Morb Mortal Wkly Rep Surveill Summ. 2014;63(2):1–21. [PubMed] [Google Scholar]
- 4.Ouellette-Kuntz H, Coo H, Lam M, Breitenbach MM, Hennessey PE, Jackman PD, et al. The changing prevalence of autism in three regions of Canada. J Autism Dev Disord. 2014;44(1):120–36. doi: 10.1007/s10803-013-1856-1. [DOI] [PubMed] [Google Scholar]
- 5.Lavelle TA, Weinstein MC, Newhouse JP, Munir K, Kuhlthau KA, Prosser LA. Economic burden of childhood autism spectrum disorders. Pediatrics. 2014;133(3):e520–e529. doi: 10.1542/peds.2013-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics. 2011;127(2):246–53. doi: 10.1542/peds.2010-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunnes N, Suren P, Bresnahan M, Hornig M, Lie KK, Lipkin WI, et al. Interpregnancy interval and risk of autistic disorder. Epidemiology. 2013;24(6):906–12. doi: 10.1097/01.ede.0000434435.52506.f5. [DOI] [PubMed] [Google Scholar]
- 8.Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry. 1995;166(5):601–6. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- 9.St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294(5):557–62. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22(4):476–85. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suren P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309(6):570–77. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr. 2003;133(5 Suppl 2):1732S–36S. doi: 10.1093/jn/133.5.1732S. [DOI] [PubMed] [Google Scholar]
- 13.Roos LL, Brownell M, Lix L, Roos NP, Walld R, MacWilliam L. From health research to social research: Privacy, methods, approaches. Soc Sci Med. 2008;66(1):117–29. doi: 10.1016/j.socscimed.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Ouellette-Kuntz H, Coo H, Yu CT, Lewis ME, Dewey D, Hennessey PE, et al. Status Report – National Epidemiologic Database for the Study of Autism in Canada (NEDSAC) Chronic Dis Inj Can. 2012;32(2):84–89. [PubMed] [Google Scholar]
- 15.Lix L, Yogendran M, Mann J. An Update with ICD-10-CA. Winnipeg, MB: Manitoba Centre for Health Policy; 2008. Defining and Validating Chronic Diseases: An Administrative Data Approach. [Google Scholar]
- 16.Ouellette-Kuntz H, Coo H, Cobigo V, Chledowski B. Enhancing Surveillance of Autism Spectrum Disorders in Canada. Queen’s University; Jun 30, 2011. [Accessed January 21, 2014]. Available at: http://www.nedsac.ca/Publications/FamilyUpdates/Enhancing_Surveillance_ASD_Canada_PHAC_RPT_2011_06_30.pdf. [Google Scholar]
- 17.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: A meta-analysis. JAMA. 2006;295(15):1809–23. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- 18.Manitoba Centre for Health Policy. [Accessed November 3, 2014];Concept: Intellectual Disability (Mental Retardation)/Developmental Disability. Available at: http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1365.
- 19.Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161(4):334–40. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- 20.Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161(10):916–25. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- 21.Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168(11):1268–76. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manitoba Education. [Accessed November 29, 2013];Special Needs Categorical Funding Criteria Levels 2 and 3. Available at: http://www.edu.gov.mb.ca/k12/specedu/funding/level2-3.html.
- 23.Ouellette-Kuntz HM, Coo H, Lam M, Yu CT, Breitenbach MM, Hennessey PE, et al. Age at diagnosis of autism spectrum disorders in four regions of Canada. Can J Public Health. 2009;100(4):268–73. doi: 10.1007/BF03403945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zierler A. Giving Birth in Manitoba. Winnipeg, MB: Manitoba Centre for Health Policy; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.