Abstract
Freud-1/CC2D1A represses the gene transcription of serotonin-1A (5-HT1A) autoreceptors, which negatively regulate 5-HT tone. To test the role of Freud-1 in vivo, we generated mice with adulthood conditional knockout of Freud-1 in 5-HT neurons (cF1ko). In cF1ko mice, 5-HT1A autoreceptor protein, binding and hypothermia response were increased, with reduced 5-HT content and neuronal activity in the dorsal raphe. The cF1ko mice displayed increased anxiety- and depression-like behavior that was resistant to chronic antidepressant (fluoxetine) treatment. Using conditional Freud-1/5-HT1A double knockout (cF1/1A dko) to disrupt both Freud-1 and 5-HT1A genes in 5-HT neurons, no increase in anxiety- or depression-like behaviour was seen upon knockout of Freud-1 on the 5-HT1A autoreceptor-negative background, rather a reduction in depression-like behaviour emerged. These studies implicate transcriptional dys-regulation of 5-HT1A autoreceptors by the repressor Freud-1 in anxiety and depression and provide a clinically relevant genetic model of antidepressant resistance. Targeting specific transcription factors like Freud-1 to restore transcriptional balance may augment response to antidepressant treatment.
INTRODUCTION
Major depression and anxiety disorders are highly prevalent and often comorbid lifelong diseases (Kessler and Bromet, 2013; Whiteford et al., 2013) that involve reductions in the activity of monoaminergic systems, particularly the serotonin (5-HT) system (Jans et al., 2007; Krishnan and Nestler, 2008; Booij et al., 2015). The chronic course of depression and antidepressant action implicates long-term dys-regulation of 5-HT system. A critical node regulating 5-HT activity is the 5-HT1A receptor gene (HTR1A), that is expressed as a somatodendritic autoreceptor to inhibit the firing of 5-HT neurons, and as a heteroreceptor at targets of the 5-HT system implicated in mood, emotion, stress response and antidepressant action (Albert and Lemonde, 2004; Albert et al., 2014; Garcia-Garcia et al., 2014). Increases in 5-HT1A autoreceptors, which would tend to reduce 5-HT neurotransmission, are found in depressed and attempted suicide subjects and in depressed suicide post-mortem tissue (Albert et al., 2011; Hesselgrave and Parsey, 2013; Sullivan et al., 2015), and the latency to respond to antidepressant treatment has been attributed in part to the time required to desensitize 5-HT1A autoreceptors (Albert and Lemonde, 2004; Blier and El Mansari, 2013). However, the transcriptional mechanisms that dictate long-term receptor expression and their roles in anxiety, depression and response to antidepressants remain unclear.
The HTR1A gene contains a series of repressor elements upstream of the promoter that suppress its expression (Albert and Fiori, 2014). Within the repressor region, a strong dual repressor element (DRE) binds to Freud-1/Cc2d1a, which represses HTR1A transcription in neuronal and non-neuronal cells (Ou et al., 2000; Ou et al., 2003; Lemonde et al., 2004a; Rogaeva and Albert, 2007). In adult mice, Freud-1 is co-expressed with 5-HT1A receptors throughout the brain (Ou et al., 2003). In the raphe, Freud-1 is co-expressed with 5-HT1A autoreceptors, unlike its homologue Freud-2/Cc2d1b, which is weakly detected in the raphe (Hadjighassem et al., 2009; Szewczyk et al., 2010). Knockdown of Freud-1 results in derepression of 5-HT1A transcription in raphe cells (Ou et al., 2003), and thus we hypothesized that Freud-1 functions as a key repressor of 5-HT1A autoreceptor expression in vivo, that might affect 5-HT regulation, and depression and anxiety behavior, and have generated mice with knockout of Freud-1 in adult 5-HT neurons to address this hypothesis.
Materials and Methods
Experimental Design
Mouse Models: All animal studies were done in accordance with the University of Ottawa Animal Care Committee guidelines. Animals were maintained on a 12-hour light/dark cycle (7:00 AM to 7:00 PM) with ad libitum access to food and water. Both sexes were used and the proportion of male/female did not differ among groups; since no differences between male and female were observed in the tests conducted the data were pooled. The Cc2d1a (Freud-1)flx/flx mice (Oaks et al., 2016) were crossed with TPH2-CreERT2 mice (stock#016584, C57BL/6N background, Jackson Labs, https://www.jax.org/strain/016584; RRID:IMSR_JAX:016584) to generate heterozygous TPH2-CreERT2-Freud-1wt/flx mice, which were interbred to generate homozygous TPH2-CreERT2-Freud-1flx/flx (cF1ko) and TPH2-CreERT2-Freud-1wt/wt (WT) littermates (Fig. 1A). At 8 weeks of age, mice were administered tamoxifen (Sigma, cat#T5648, 180 mg/day, approximately 3 mg/kg, i.p.) once/day for 5 consecutive days to activate CreERT2-induced recombination. To detect Cre-induced recombination, cF1ko mice were crossed into a ROSA26-flxSTOP-GFP C57BL/6J background (obtained from Dr. Diane Lagace, Univ. of Ottawa). The TPH2-Cre-ERT2/Freud-1wt/flx were crossed with fl1A mice (C57BL/6 × 129Sv × ROSA-Flpe background) (Szewczyk et al., 2014; Samuels et al., 2015) and bred to obtain TPH2-CreERT2-Freud-1flx/flx/1Aflx/flx mice (cF1/1Adko) (Fig. 1A). To obtain Freud-1 WT mice on the 5-HT1AautoKO background, cFreud-1wt/wt:1Awt/flx were crossed with cFreud1wt/wt:1Awt/flx. To obtain mice on the corresponding 5-HT1A wild-type background TPH2-CreERT2-Freud-1wt/flx/1Awt/wt mice were bred to generate F1WT-1AWT mice.
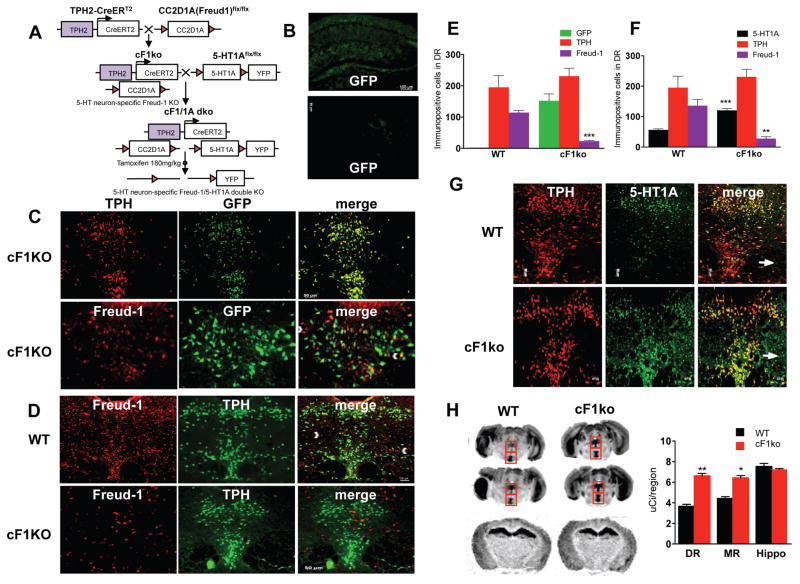
Figure 1. Loss of Freud-1 in 5-HT neurons increases 5-HT1A autoreceptors.
A. Conditional Knockout Strategy. To delete Freud-1 in 5-HT neurons, the cF1ko mouse was generated by crossing CC2D1A(Freud-1)flx/flx mice with TPH2-CreERT2 mice. At 8 weeks of age, mice were administered tamoxifen to activate CreERT2-induced recombination. To delete both Freud-1 and 5-HT1A autoreceptors in 5-HT neurons, the cF1ko mice were mated to the 5-HT1Aflx/flx mice, in which the 5-HT1A gene is flanked by LoxP sites and a YFP cassette to generate the Freud-1/5-HT1A double knockout mice following tamoxifen administration. B. Tamoxifen-induced recombination specificity. Hippocampal and prefrontal cortex sections from tamoxifen-treated conditional Freud-1 knockout/ROSA-GFP mice (cF1KO) show background GFP staining. C. Tamoxifen-induced recombination and loss of Freud-1 in dorsal raphe. DR sections from tamoxifen-treated cF1KO mice were stained for GFP and either TPH or Freud-1 (10× magnification, scale bar = 20 μm; inset, 20x). GFP was present in 92% of TPH+ cells, while 88% of Freud-1 was in GFP-cells in cF1KO sections (n=4). D. Loss of Freud-1 in 5-HT neurons. Freud-1/TPH-labeled cells in DR were almost absent in cF1ko compared to WT. By contrast, Freud-1+/TPH-cells remained (white arrowheads) (n=4). E. Quantification of GFP-, TPH-and Freud-1-stained cells in dorsal raphe of cF1KO and WT mice (n=4), shown as mean ± S.E. (p <0.001). F. Quantification of 5-HT1A-, TPH- and Freud-1-stained cells in dorsal raphe of cF1ko (non-ROSA-GFP) and WT mice (n=4), shown as mean ± S.E. (p <0.001). G. Loss of Freud-1 and increased 5-HT1A-positive cells in dorsal raphe of cF1ko mice. DR sections from tamoxifen-treated cF1ko vs. F1wt (WT) mice (scale=50 μm, n=4) were stained for TPH and 5-HT1A receptors (arrow, 5-HT1A in TPH-cells); 5-HT1A receptors were increased in TPH+ cells. H. Increased 5-HT1A binding in raphe of cF1ko mice. At left are representative images of 125I-MPPI autoradiography of sections from cF1ko and WT mice in dorsal and median raphe (boxes) at two levels (Bregma −4.60 and −4.72 mm) and hippocampus (Bregma −1.70). At right is quantification of 125I-MPPI binding. Data represent mean ± SEM (n=4/group), *p < 0.05; **p < 0.01. 5-HT1A binding was increased in raphe of cF1ko mice; DR (unpaired two-tailed Student’s t-test, DF=6, t=9.129, **p<0.001), MR (unpaired two-tailed Student’s t test, DF=6, t=6.635 *p<0.01).
Genotyping
Ear tissue samples were taken at 3 weeks of age and DNA extracted using the REDExtract-N-Amp Tissue PCR kit (Sigma). PCR was done using the following primers and conditions: Cc2d1Aflx/flx: 5′-TAG AAA CAC TTA CCC TCC ACA TTG-3′ and 5′-TAG GAA GTG CCC ACC CAG A-3′. The PCR conditions were: 94°C for 4min; 15 cycles at 94°C for 30s, 70°C for 30s, 0.5°C/cycle, 72°C for 30s; 20 cycles at 94°C for 30s, 62°C for 30s, 72°C for 30s; 62°C for 30s; 72°C for 10min, 10°C. This protocol results in 202-bp (wild-type) and 382-bp (floxed) products.
TPH2-CreERT2: TPH2-11679 5′-GCT GAG AAA GAA AAT TAC ATC G-3′, CRE-125235′-TGG CTT GCA GGT ACA GGA GG-3′, OIMR8744 5′-CAA ATG TTG CTT GTC TGG TG-3′ and OIMR8745 5′-GCT AGT CGA GTG CAC AGT TT-3′. The PCR conditions were: 94°C for 1min; 35 cycles at 94°C for 15s, 57°C for 20s, 72°C for 10s; 94°C for 15s, 72°C for 2min, 10°C. This protocol results in 200-bp (wild-type) and 300-bp (transgenic) products.
ROSA-YFP: OIMR4982 5′-AAG ACC GCG AAG AGT TTG TC-3′, OIMR8545 5′-AAA GTC GCT CTG AGT TCT TAT-3′, OIMR8546 5′-GGA GCG GGA GAA ATG GAT ATG-3′. The PCR conditions were: 94°C for 3min, 94°C for 30s, 58°C for 1min, 72°C for 1min, 35 cycles: 94°C for 30s, 72°C for 10min, 10°C. This protocol results in 600-bp (wild-type) and 320-bp (transgenic) products.
1Aflx/flx: 5′-GGG CGT CCT CTT CAC GTA G-3′ and 5′-CAG GGA CGT TGT GGT GTT GT-3′. The PCR conditions were: 94°C for 2min, 15 cycles at 94°C for 30s, 68°C for 30s -0.5°C/cycle, 68°C for 20s; 20 cycles at 94°C for 30s, 60°C for 30s, 68°C for 20s; 60°C for 30s, 68°C for 5 min, 10°C. This protocol results in 254-bp (wild-type) and 292-bp (floxed) products.
Immunofluorescence
Mice were anesthetized by lethal injection (0.01 ml/g, i.p.) of sodium pentobarbital (Somnitol; MTC Pharmaceuticals, Cambridge, ON, Canada) and perfused by cardiac infusion of 30 ml PBS, then 25 ml 4% paraformaldehyde. Whole brains were isolated, cryo-protected overnight in 20% sucrose and frozen at −80°C. Coronal brain slices (20 μm) were prepared using the following coordinates: Dorsal raphe: Bregma 4.36 to 4.72 mm (Paxinos and Franklin, 2001). Slices were thaw-mounted on Superfrost slides (Thermo-Fisher) and kept at −80°C. The sections were washed 3 × in PBS, blocked 1 h in PBS with 1% BSA, 10% NDS, 0.1% Triton X-100 (or 0.3% Tween 20 for 5-HT1A antibody), followed by overnight incubation at 22°C with chicken anti-GFP, (Abcam, ab13970, 1:500; RRID:AB_300798), sheep anti-TPH, (Millipore, ab1541 1:100; RRID:AB_90754), rabbit anti-5-HT1A receptor (custom made primary antibody raised to the i2 loop of the 5-HT1A receptor sequence; Cedarlane, Hornby, ON, Canada, 1:50, (Czesak et al., 2012)); rabbit anti-Freud-1, 1:1000 (Rogaeva and Albert, 2007); goat anti-5-HT (Abcam, ab66047, 1:500; RRID:AB_1142794) and rabbit anti-FosB (Santa Cruz, sc-48, 1:500; RRID:AB_631515). The sections were then washed three times in PBS and incubated for 1h in secondary antibody at 22°C. The secondary antibodies were as follows: Alexa Fluor 488 anti-chicken (Jackson, 103-545-155, 1:250; RRID:AB_2337390), anti-sheep Cy3 (Jackson, 713-165-003, 1:200; RRID:AB_2340727), Alexa Fluor 488 anti-rabbit (ThermoFisher, A-21206, 1:1000; RRID:AB_2535792), Alexa Fluor 594 anti-rabbit (ThermoFisher, A-21207, 1:200; RRID:AB_141637), Alexa Fluor 647 anti-goat (ThermoFisher, A-21447, 1:200; RRID: AB_2535864) in blocking solution. Images of dorsal raphe were acquired with the Axiovision imaging software (RRID:SCR_002677) on a Zeiss Axio Observer D1 microscope under 10X and 20X magnification (n=4/group). Positive-stained cells were manually counted within a standardized template using ImageJ 1.48v software; RRID:SCR_003070.
5-HT1A autoradiography
For autoradiography, mice were sacrificed by cervical dislocation and decapitation. Extracted brains were frozen immediately on dry ice (-75°C) and maintained at −80°C until sectioning. Brains were cryo-sectioned at a thickness of 25 μm and mounted sections were maintained at −80°C until processing. Mounted sections were processed for 125I-MPPI (Perkin Elmer, Boston, MA) autoradiography as described (Donaldson et al., 2014; Luckhart et al., 2016). Sections were exposed to Kodak BioMax MR film (VWR) for 24 h. Films were digitized at 1200-dpi resolution using an Epson Perfection V500 Photo Scanner, and signal density was measured using the mean luminosity function in ImageJ (1.49). Levels of 5-HT1A binding (μCi) were quantified by analyzing a standardized template outlining the region-of-interest, and adjacent background lacking specific binding subtracted. For raphe, data from sections at Bregma −4.36, −4.48, −4.60, −4.72 cm were averaged; for hippocampus, Bregma; −1.82 cm was used. Signals were within the linear range of the film and quantified based on standard curve using ARC146-F 14C standard (American Radiochemicals Inc, St. Louis, MO).
High Performance Liquid Chromatography (HPLC) Analysis
Levels of 5-HT and 5-HIAA were quantified in extracts of dissected tissues by HPLC (Czesak et al., 2012). For HPLC, cF1ko and matched WT littermate mice (n=4, 11 wks old) were sacrificed by cervical dislocation and decapitation. The entire dorsal raphe, hippocampus and prefrontal cortex were dissected, pooled, frozen immediately on dry ice and maintained at −80°C until homogenization and analysis (Czesak et al., 2012). In brief, 300 μL of homogenization solution (0.3 M monochloroacetic acid, 0.1 mM EDTA, 10% methanol and internal standard) was added to each sample followed by sonication. Following sonication, 100 μL was aliquoted and frozen for protein concentration determination (Pierce™ Coomassie (Bradford) Protein Assay). The remaining 200 μL was centrifuged and the supernatant analyzed for 5-HT and 5-HIAA content using HPLC (Agilent Technologies, Walbronn, Germany). A 10-μL volume of supernatant was injected via an autoinjector (1100 series Autosampler; Agilent, Walbronn, Germany) into the HPLC system equipped with an electrochemical detector (VT-03 flow cell, Intro detector; Antec Leyden, Montreal, Quebec, Canada) with an applied potential of 500 mV, a filter of 1 s, and a range of 100 nA/V. Separation of these analytes was achieved by their passage through a reverse phase analytical column (Phenomenex Kinetex® 2.6 μm C-18, 100 × 4.6 mm). The column was equilibrated at a flow rate of 0.5 ml/min with mobile phase consisting of the following (in mM): 90 NaH2PO4, 1.7 1-octane sulfonic acid (sodium salt), 50 citric acid (monohydrate), 5 KCl, 50 EDTA, and 14% acetonitrile, final pH 3.0. The quantification of the analytes was performed by comparing their area under the curve to those of known external standards using computerized Agilent ChemStation chromatography data acquisition system (Agilent).
DPAT-Induced Hypothermia
The hypothermia procedure was performed from 9–11 AM. Mice were weighed and internal temperature was taken using a rectal thermometer every 10 minutes for 40 minutes (4 baseline measurements). Animals were administered 8OH-DPAT (0.75 mg/kg, i.p., Sigma) followed by 3 measurements of basal body temperature at 10-min intervals (Martin et al, 1992). For analysis purposes, the first baseline temperature was discarded. The remaining three baseline values were averaged and the difference between the average baseline and recorded temperature was plotted across time.
Whole-cell Electrophysiology
Brainstem slices (300-μm) containing the DRN were prepared from 10–11 week old mice as previously described (Geddes et al., 2016). In brief, mice were anesthetized and sacrificed by decapitation. Once the brain was removed, coronal slices were made from a block of brain tissue while immersed in ice-cold cutting solution (in mM): 119 choline-Cl, 2.5 KCl, 1 CaCl2, 4.3 MgSO4-7H2O, 1 NaH2PO4, 1.30 sodium L-ascorbate, 26.20 NaHCO3, 11 glucose at 37°C and equilibrated with 95% O2, 5% CO2. Slices were then transferred to a recovery chamber containing standard Ringer’s solution (in mM): 119 NaCl, 2.5 CaCl2, 1.3 MgSO4-7H2O, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose at 37°C, bubbled with 95/5% O2/CO2 and left to recover for > 1 h and equilibrated to room temperature (~25°C) prior to recordings. DRN neurons were visualized using an upright microscope (Examiner D1; Zeiss, Oberkochen, Germany) equipped with Dodt-gradient-contrast (40x/0.75NA objective). 5-HT neurons were identified by morphological and biophysical characteristics as previously established (Geddes et al., 2015). Whole-cell recordings carried out at room temperature in standard Ringer’s solution using borosilicate glass patch electrodes (3–6 MΩ; World Precision Instruments). 5-HT1A receptor-mediated currents were elicited by bath applying the 5-HT1A receptor agonist 5-carboxamidotryptamine (5-CT; 10 nM; Tocris) and holding current was monitored at 0.1 Hz (Vm = −55 mV. These recordings were carried out using an internal solution of the following composition (in mM): 115 potassium gluconate, 20 KCl, 10 sodium phosphocreatine, 10 HEPES, 4 Mg2+-ATP, and 0.5 GTP (pH 7.25 adjusted with KOH; osmolarity, 280–290 mOsmol/L). Access resistance was continuously monitored by applying a 125 ms, 2 mV hyperpolarizing pulse every 10 s, and recordings were discarded if the access resistance changed by >30%.
Behavioral assays
Behavioral tests were conducted in littermates starting 2 weeks after the last tamoxifen injection, at 11 weeks of age. Mice were housed under normal light conditions and tests were performed beginning at 10:00 AM, after at least 1 h of habituation to the testing room. Testing was performed under white light illumination with the exception of the forced swim test (FST), which was performed under red light. All animals were of the same age at the start of testing and all tests were done in the order below and completed within 10 days. Each cohort included 10–32 mice/group. Throughout testing and behavioral analyses, experimenter was blind to the mouse genotype.
Elevated plus maze (EPM) test
The mice were placed in the center of an elevated two-arm plus maze, measuring ~ 20-cm high, ~ 6-cm wide and ~ 75-cm long (Noldus, The Netherlands; RRID:SCR_004074). The arms of the maze are crossed with one arm having an open platform, the other arm having a closed platform with walls that are ~ 20-cm tall with overhead illumination (100–110 Lux) and camera. Mice were placed in the centre of the maze with the head toward the closed arm of maze and allowed to explore the maze for 10 min. The mouse movements were videotaped and the time spent in closed and open arms was determined (Ethovision 10, Noldus IT; RRID:SCR_000441).
Open field (OF) test
The mice were placed in a corner of the arena (45-cm long in each side and 45-cm high) and allowed to explore the new environment for a total of 10 min at light levels of 300 Lux. Mouse movements were videotaped and the time spent in the outside of a center (24 × 24 cm) of the OF arena was analyzed (Ethovision 10, Noldus IT).
Tail suspension (TS) test
The tail of the mouse was secured with tape to a horizontal bar and the animals were suspended for 6 min in mouse TS boxes (Med Associates). An automated detection device (ENV-505TS Load-Cell Amplifier) was used to determine mobility and immobility time through Med Associates software (Ethovision XT, Noldus IT).
Forced Swim Test (FST)
Each mouse was placed into clear plastic cylinder 22-cm in diameter and 37-cm deep filled with 4 l of water (24°C). The mouse was videotaped from the side of the cylinder for 6 min under red light illumination and the duration of immobility time was quantified using an automated video-tracking software from Med Associates (Ethovision XT).
Novelty-suppressed feeding (NSF) test
The NSF test was used to assess anxiety-related behaviors (Santarelli et al., 2003). Briefly, animals were food deprived for 16 h. After 3 min of habituation they were placed in a new cage. Animals were individually placed in an arena (45-cm long in each side and 45-cm high; 300 lux) with a food pellet placed in the center. The latency of the mice to begin eating food was recorded manually and immediately after mice approached the food or after 10 min had expired for the trial, the mice were removed from the arena and placed in their home cage and the latency to approach the food and the amount of food consumed in 5 min was measured.
Beam Break (BBK) test
Mice were placed into a novel home cage for 30 min. The home cage locomotor activity was measured by recording number of breaks of an invisible infrared light beams located on a frame surrounding the cage (Crawley, 2008) (Omnitech Electronics, Columbus, OH, USA).
Chronic SSRI treatment
For SSRI treatment, a separate cohort of mice, including conditional Freud-1 knockout and WT littermates (11 wk old, n=10), was single housed and received 18 mg/kg/day fluoxetine hydrochloride (Santarelli et al., 2003; Samuels et al., 2015) (Enzo life science, cat#BML-NS140-0250, Farmingdale, NY, USA) in drinking water for 3–4 weeks using opaque bottles to protect the SSRI from light. Then, several behavioral tests were done as described above, with ongoing SSRI treatment, following the timeline shown in Figure 4. The consumption of fluoxetine was measured accurately by weighing to determine the amount of drinking water consumed every 3 days (~3 ml/day) and did not differ between groups.
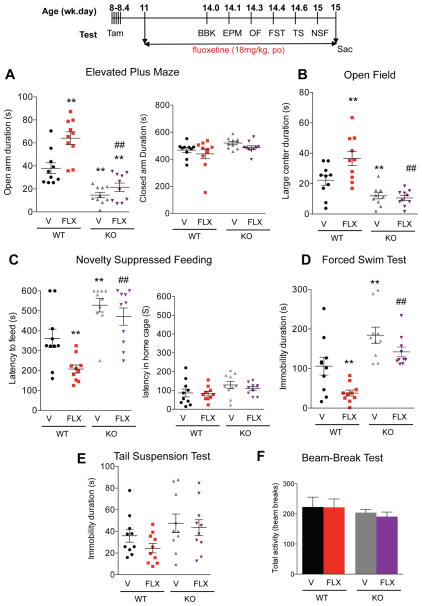
Figure 4. Resistance to chronic SSRI treatment in cF1ko mice.
Wild-type (WT) or cF1ko (KO) mice were treated with fluoxetine (FLX) or vehicle (V) for 3 weeks and throughout the behavioural assays (timeline). A–C. Anxiety phenotype: chronic FLX reduced anxiety in WT mice, but did not affect the increased anxiety seen in cF1KO mice. A. EPM, significant changes in time spent in open arms in EPM test: one-way ANOVA treatment X genotype interaction, F3.36=27.01, p<0.01; post-hoc Tukey **p < 0.01 vs WT-Veh, ##p < 0.01 vs WT-FLX. B. OF, changes in distance travelled in large center: one-way ANOVA treatment X genotype interaction, F3.041 =14.53, p<0.01; post-hoc Tukey **p < 0.01 vs WT-Veh, ##p < 0.01 vs WT-FLX. C. NSF, latency to approach food in novel arena: one-way ANOVA treatment X genotype interaction, F3.36 =14.16, p<0.01; post-hoc Tukey **p < 0.01 vs WT-Veh, ##p < 0.01 vs WT-FLX. D–E. Depression phenotype. D. FST. Depression-like behavior in cF1ko (veh) compared to F1wt (veh) was indicated by increased immobility in the FST. Chronic FLX significantly reduced immobility in F1wt, but not in cF1ko mice FST, one-way ANOVA treatment X genotype interaction, F3.36 =13.62, p<0.01; post-hoc Tukey **p < 0.01 vs WT-Veh, ##p < 0.01 vs WT-FLX. E. TST, no significant changes were observed. F. Beam break. No differences in 30-min activity in novel arena were observed. Data represent individual animals with mean ± SEM, n=10/group; **p < 0.01 vs. WT-Veh, ##p < 0.01 vs WT-FLX.
Statistical Analyses
All analyses were done using the Statistical Package for the Social Sciences (GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA, USA; www.graphpad.com; RRID:SCR_002798). Data are expressed as mean ± SEM; p ≤ 0.05 was used as the threshold for significance. Data comparing KO and WT littermates on one outcome measure were analyzed using unpaired t-test. One-way analysis of variance followed by Tukey’s post-test was performed for multiple comparisons.
RESULTS
Adult knockout of Freud-1 in 5-HT neurons induces 5-HT1A autoreceptor upregulation
The cF1ko mice were generated for inducible knockout of Freud-1 in adult 5-HT neurons by crossing tamoxifen-inducible TPH2-CreERT2 and Cc2d1a (Freud-1)flx/flx mice (Oaks et al., 2016)(Figure 1A). Initially, to assess recombination specificity, cF1ko mice were bred on a ROSA26-flxSTOP-GFP background (cF1KO). Tamoxifen injection in adulthood revealed that recombination to produce GFP was not detected in non-serotonergic regions that express 5-HT1A receptors, including the PFC and hippocampus (Figure 1B). However, GFP was present in 92% of TPH2-positive neurons in the raphe nuclei, and 100% of GFP-positive neurons were co-localized with TPH (Figure 1C), indicating 5-HT neuron-specific recombination. Importantly, tamoxifen treatment of cF1KO compared to cF1wt mice strongly reduced the number of Freud-1-positive cells (114 WT vs. 22.5 cF1KO, t test, DF=6, t=11.566 ***p=0.0022), with ~90% of GFP- or TPH-positive raphe cells lacking Freud-1 (Figure 1C–F). Freud-1 protein remained present in TPH-negative cells in the raphe (Figure 1C, white arrowheads). In addition, there was no change in the number of TPH-positive cells, indicating that Freud-1 knockout did not alter the number of 5-HT neurons (Figure 1E). Thus, tamoxifen efficiently induces Freud-1 knockout specifically in 5-HT neurons of the cF1ko mice.
To identify changes in 5-HT1A autoreceptors upon knockout of Freud-1 in 5-HT neurons, co-staining of 5-HT1A receptors and TPH in the dorsal raphe was examined (Figure 1G). A significant increase in 5-HT1A-stained cells was observed in cF1ko compared to cF1wt sections (t test, DF=6, t=8.813 ***p=0.0001) with no change in total TPH-positive cells (Figure 1F). There was a significant increase in 5-HT1A/TPH co-stained cells (WT, 49.50±6.752 vs cF1ko, 132.3±10.10 cells/template; t test, DF=6, t=6.810 ***p=0.0005), with no change in 5-HT1A/TPH-negative cell count (Fig. 1G, arrows), indicating a specific up-regulation of 5-HT1A autoreceptors upon Freud-1 knockout in 5-HT neurons. In the cF1ko raphe, we confirmed the reduction of Freud-1-positive cells (Fig. 1F, WT, 135.8±20.95; cFko, 27.50±6.538 cells/template; t test, DF=6, t=4.933 **p=0.0026). To further quantify the levels of 5-HT1A binding sites, autoradiography was performed using the selective 5-HT1A antagonist 125I-MPPI (Donaldson et al., 2014) and revealed a significant >1.5-fold increase in 5-HT1A binding in the dorsal and median raphe nuclei of cF1ko vs. cF1wt mice), but similar levels of 5-HT1A binding in hippocampus (Figure 1H). The 5-HT1A binding (uCi/region) was: DR: wt, 3.644±0.214 vs. cF1ko, 6.609±0.244, t test, DF=6, t=9.129, ***p=0.0001; MR: wt, 4.406±0.1895 vs. ko, 6.411±0.235, t test, DF=6, t=6.635, **p=0.0006; hippocampus, wt, 7.509±0.318 vs ko, 7.245±0.086, t test, DF=6, t=0.801, p=0.45. In summary, consistent with its repressor function, loss of Freud-1 in 5-HT neurons results in a significant up-regulation of 5-HT1A autoreceptors, while 5-HT1A heteroreceptor levels remain unchanged.
Enhanced 5-HT1A function and reduced raphe 5-HT levels in cF1ko mice
In order to determine the effect of Freud-1 deficiency on 5-HT1A autoreceptor function in vivo, we measured hypothermia in response to acute administration of the 5-HT1A agonist 8OH-DPAT, which in mice is dependent on 5-HT1A autoreceptor levels (Albert et al., 2014). Within 30 min, 0.75 mg/kg 8OH-DPAT induced a maximal reduction in body temperature in the cF1ko mice that was significantly greater than that in the cF1wt mice and persisted for 70 min, indicating an enhanced 5-HT1A autoreceptor response to the 5-HT1A agonist (Figure 2A). To assess 5-HT1A autoreceptor function in single neurons, we performed whole-cell voltage-clamp recordings from dorsal raphe slices and bath applied the 5-HT1A receptor agonist 5-CT (10 nM). The magnitude of 5-HT1A-mediated outward currents was similar in cF1ko and F1wt mice (Figure 2B) and similar results were obtained using 100 nM 5-CT (data not shown). Limitations of the slice preparation, including a lack of 5-HT auto-inhibition or increased 5-CT induced internalization (Bouaziz et al., 2014; Andrade et al., 2015) could obscure the effect of 5-CT to produce increased response in the presence of excess 5-HT1A receptors.
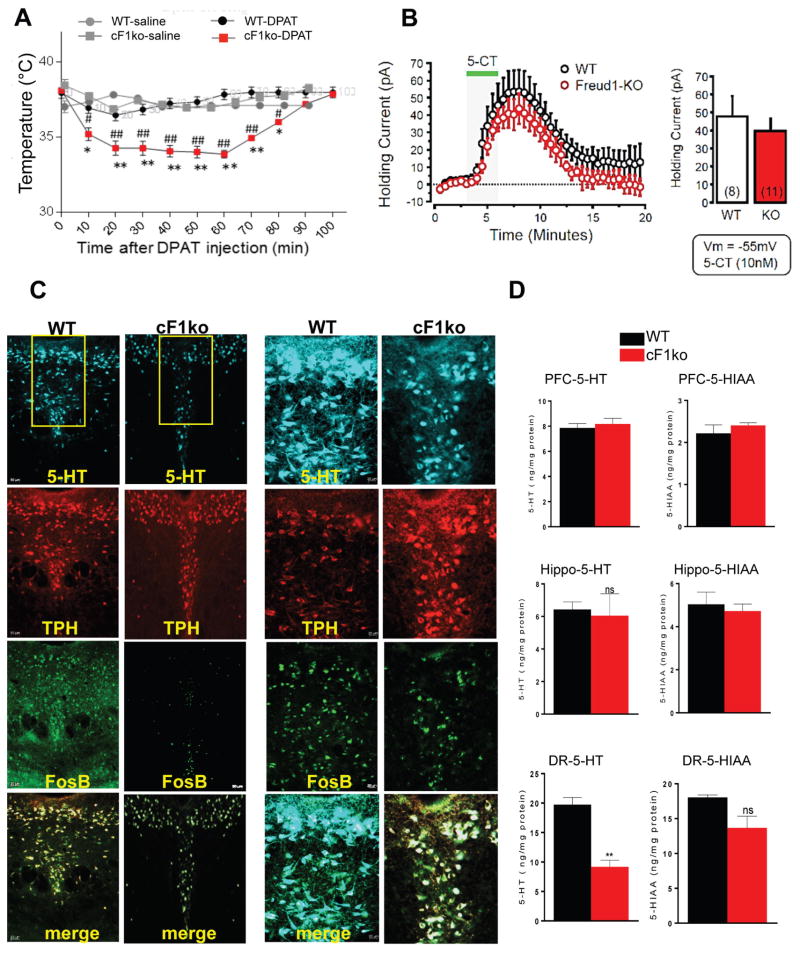
Figure 2. Loss of Freud-1 augments 5-HT1A autoreceptor function and reduces 5-HT neuron activity and 5-HT levels.
A. 5-HT1A-induced hypothermia. 8-OH-DPAT (0.75 mg/kg, i.p.) induced a greater body temperature reduction in cF1ko compared to F1wt (WT). Data represent mean±SEM (n=3/group); *p<0.05 vs. WT-saline, **p<0.001 vs. wild-type-DPAT, #p<0.05 vs. cF1ko-saline, ##p<0.001 vs. cF1ko-DPAT. B. Whole-cell voltage-clamp recordings (Vm = −55 mV) of 5-HT neurons in slices of dorsal raphe in vitro, from cF1ko or F1wt mice (n = 4) in response to 5-CT (10 nM). No significant difference in 5-HT1A receptor-induced outward current was observed. C. Reduced 5-HT- and FosB-stained cells in cF1ko raphe. DR sections of cF1ko or F1wt (WT) mice stained for 5-HT, TPH, and FosB shown at 10× (left, scale=50 μm) or 20× magnification of boxed region (right, scale=20 μm). D. Reduced raphe 5-HT content in cF1ko mice. Tissue 5-HT and 5-HIAA content was quantified by HPLC for dorsal raphe, hippocampus (Hippo) and prefrontal cortex (PFC) of cF1ko vs. WT mice. Data represent mean ± SEM, n=3/group; reduced raphe 5-HT content in cF1ko vs. WT mice (unpaired two-tailed Student’s t test, DF=4, t=6.675 **p<0.01).
Because 5-HT1A autoreceptors exert inhibitory tone on 5-HT neurons, we determined whether 5-HT levels are altered in cF1ko mice. In cF1ko compared to cF1wt dorsal raphe, there was a significant decrease (~50%) in 5-HT-positive cells from 68±10.5 to 35±8.2 cells/template (t test, DF=6 t=5.635, **p=0.0013), with no change in the number of TPH-positive cells (Figure 2C). Similarly, quantitative measurement of 5-HT and its major metabolite 5-HIAA revealed a ~50% reduction in raphe 5-HT content in Freud-1 knockout vs. wild-type mice (t test, DF=4 t=6.375, **p=0.0031), with no significant change in raphe 5-HIAA or in hippocampal or PFC 5-HT or 5-HIAA levels (Figure. 2D). To address whether the activity of 5-HT neurons was changed, FosB staining was used as a marker for acute and chronic cellular activation. There was a ~50% reduction in FosB/TPH stained cells (49±11 vs. 25±7.9 cells/template, t test, DF=6 t=4.035, **p=0.0023) in the dorsal raphe of cF1ko compared to F1wt mice, suggesting a chronic reduction of 5-HT neuronal activity. Taken together, these data indicate an up-regulation of 5-HT1A autoreceptor expression and function upon loss of Freud-1 associated with increased auto-inhibition of 5-HT neuron activity and reduced raphe 5-HT levels.
Fluoxetine-resistant anxiety- and depression-like behavior in cF1ko mice
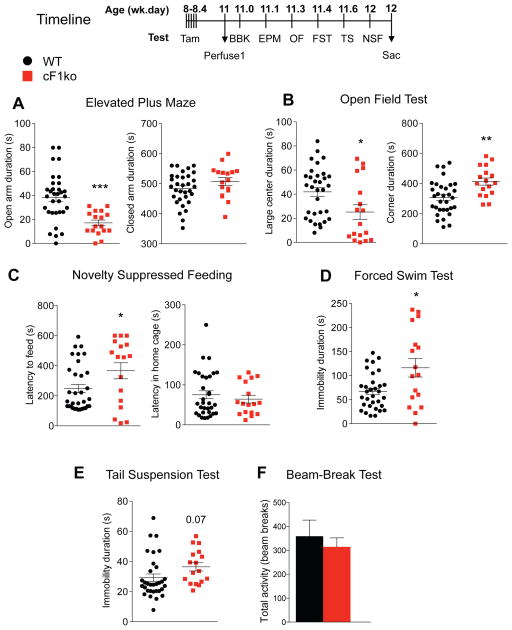
The behavioural phenotype of adult cF1ko mice was assessed using multiple validated tests (Figure 3, timeline). In contrast, compared to F1wt littermates, cF1ko mice displayed robust anxiety-like behavior in the elevated plus maze (EPM), open field (OF) and novelty suppressed feeding (NSF) tests (Figure 3A–C). In the EPM test, cF1ko mice had a significant 50% reduction in time spent in the open arms, with no difference in total distance or closed arm time (Figure 3A). In the OF test, cF1ko mice spent significantly less time in the center of the arena, and more time in the corners (Figure 3B). In the NSF test, the cF1ko mice displayed significantly greater latency to feed in the novel cage, while no difference was observed in latency for home cage food consumption (Figure 3C). A depression-like phenotype was also detected in the cF1ko mice, with increased immobility in the forced swim (FST) (Figure 3D) and a trend (p=0.07) in the tail suspension (TS) test (Figure 3E). No difference in open field locomotor activity was detected between cF1ko and F1wt littermates in the beam break (BBK) test (Figure 3F). These results were confirmed in a second independent cohort of mice (data not shown). These data indicate that adult knockout of Freud-1 in 5-HT neurons confers both anxiety- and depression-like phenotypes.
Figure 3. Increased anxiety- and depression-like behavior in cF1ko mice.
The cF1ko and WT littermates underwent the indicated behavioural tests or assays according to the timeline shown, 2 weeks after the last tamoxifen injection (11-wk of age). A–C. Increased anxiety in cF1ko mice. A. EPM test. Compared to WT, cF1ko mice spent less time in open arms (unpaired two-tailed Student’s t-test, DF=47, t=4.104**p<0.01), with no difference detected in closed arm time. B. OF. cF1ko mice displayed significantly reduced distance travelled in large center (unpaired two-tailed Student’s t-test, DF=47, t=2.486 *p=0.0165), with no change in total distance (not shown). C. NSF test. cF1ko mice showed greater latency to approach food in the novel arena (unpaired two-tailed Student’s t test, DF=48, t=2.221 *p=0.0311), but no difference in the home cage. D–E. Depression-like behaviour in cF1ko mice. D. FST. The cF1ko showed significant greater immobility duration in the FST compared to F1wt mice (unpaired two-tailed Student’s t-test, DF=47, t=2.962 *p<0.05). E. TST. No difference in immobility duration between cF1ko and WT was seen. F. Locomotion test. Results from the beam break (BBK) test showed no difference in total 30-min activity comparing cF1ko and F1wt (WT) mice. Data represent mean ± SEM in cF1ko mice (n=17) vs. F1wt (WT) (n=32).
Increase in 5-HT1A autoreceptors is thought to reduce responsiveness to chronic SSRI treatment (Albert and Le Francois, 2010; Richardson-Jones et al., 2010). The behavioral response to fluoxetine (FLX) was tested in singly housed cF1ko and F1wt littermates (11 wk old, n=10/group) treated or not with FLX in drinking water for 3–4 weeks using the above tests. In each test, the effect of conditional Freud-1 knockout to increase anxiety/depression-like behavior was replicated. In the anxiety tests (EPM, OF and NSF), chronic fluoxetine treatment reduced anxiety-like behaviors in the cF1wt mice, but did not alter the anxiety phenotype in cF1ko mice, compared to the vehicle-treated group (Figure. 4A–C), with no changes in control measures. Furthermore, the cF1ko mice had increased immobility but showed no significant response to FLX in the FST, while the F1wt showed reduced immobility in FLX-treated vs. vehicle (Figure 4D). Neither cF1ko nor F1wt mice showed any differences or response to FLX in the TS (Fig. 4E), nor in the beam break test (Figure 4F). These results indicate that the anxiety/depression-like phenotype seen in the cF1ko mice and associated with overexpression of 5-HT1A autoreceptors is resistant to chronic fluoxetine treatment.
Requirement of 5-HT1A autoreceptors for Freud-1-dependent behavioral effects
To address whether the behavioral phenotype induced by loss of Freud-1 is dependent on the increased level of 5-HT1A autoreceptors, the cF1ko mice were mated to the flx-1A mice (Samuels et al., 2015), in which the 5-HT1A gene is flanked by LoxP sites and a YFP cassette (Figure 1A). Tamoxifen-induced recombination in 5-HT neurons in cF1/1Adko mice was verified by co-staining for YFP and TPH (Figure 5A). Staining for 5-HT1A receptors revealed a strong reduction in the number of 5-HT1A/TPH co-labeled cells in cF1/1Adko compared to cF1ko/1Awt littermates (Figure 5B, cF1ko/1Awt, 120.5±6.19; cF1ko/1Ako: 16± 2.48 cells; t test, DF=6, t=15.650 ***p=0.0001). The presence of weak 5-HT1A staining in TPH-negative cells (Figure 5B, white arrows) may represent 5-HT1A heteroreceptors in non-5-HT neurons of the raphe (Calizo et al., 2011). The extent of loss of 5-HT1A receptors in the cF1/1Adko mice was determined by autoradiography using the selective 5-HT1A antagonist 125I-MPPI. The results show significant reduction of 5-HT1A binding in the dorsal (6.327±0.40 vs 1.339±0.1770 uCi; t test, DF=4, t=11.24 ***p=0.0004) and median (5.015±0.326 vs. 1.779±0.5248 uCi; t test, DF=4, t=5.235 **p=0.0064) raphe of cF1/1Adko (Figure 5C). No difference in post-synaptic 5-HT1A receptor levels was observed in hippocampus of cF1/1Adko compared to cF1ko/1Awt littermates. Thus, the cF1/1Adko mice show a significant reduction in the number of 5-HT1A autoreceptors.
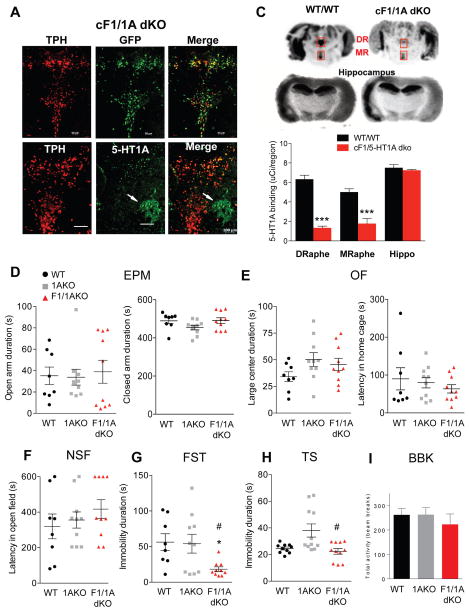
Figure 5. Reversal of anxiety- and depression-like phenotypes upon loss of Freud-1 in the absence of 5-HT1A autoreceptors.
Tissues from mice with conditional knockout of Freud-1 and 5-HT1A receptor in adult serotonin neurons (cF1/1A dKO) were compared to 5-HT1A conditional knockout (c1AKO) or wild-type littermates (WT/WT). A. Tamoxifen-induced recombination. Co-staining for YFP and TPH in the dorsal raphe. Recombination occurred exclusively in 5-HT cells; 92% of TPH-positive cells were YFP-positive (scale bar = 100 μm). B. Loss of 5-HT1A autoreceptors. Immunostaining for 5-HT1A and TPH showed significant loss of 5-HT1A immunostaining in TPH-positive cells of cF1/1A dKO mice. White arrows indicate the presence of 5-HT1A receptors in non-TPH positive cells in dorsal raphe (scale=100 μm). C. 5-HT1A autoradiography. 5-HT1A receptor autoradiography using 125I-MPPI of representative midbrain sections from WT/WT and cF1/1A dKO mice, including dorsal (DR) and median (MR) raphe (boxes) and hippocampal sections. DR and MR showed significant decreases in 5-HT1A autoreceptor binding: DR (unpaired two-tailed Student’s t-test, DF=4, t=11.24, ***p<0.001), MR (unpaired two-tailed Student’s t-test, DF=4, t=5.235, *p<0.01). D–H. Behavioral studies were done in either wild-type (WT) or conditional knockout (KO) for Freud-1 (F1) and/or 5-HT1A receptor (1A) in littermate mice. D. EPM. No change in anxiety-like behavior (open arm time) was observed. E. OF. No difference among groups was observed in time spent in the large centre. F. NSF. The latency to feed was not altered. G. FST. Immobility duration was reduced in F1/1AKO vs. WT and 1AKO mice (one-way ANOVA genotype X genotype interaction, F2,25=4.463, p=0.0067; post-hoc Tukey *p<0.05 vs. WT, #p<0.05 vs. 1AKO). H. TS. Immobility time was reduced in F1/1AKO compared to WT or 1AKO (one-way ANOVA genotype X genotype interaction, F2,29=6.998, p=0.0033; post-hoc Tukey *p<0.05 vs. WT, #p<0.05 vs. F1/1AKO). I. Beam break test. No difference in 30-min activity in novel arena was observed. Data points represent individual mice, with mean ± SEM: WT, n=10; F1WT/1AKO, n=11; F1/1AKO, n=11.
The effect of Freud-1 knockout on a 5-HT1A autoreceptor-negative background was examined using the cF1/1A dko mice. In Freud-1 wild-type mice, knockout of the 5-HT1A autoreceptor in adults (comparing cF1wt/1Ako to cF1wt/1Awt) did not alter anxiety or depression behavior (Figure 5D–H), consistent with previous results (Richardson-Jones et al., 2010). In mice lacking 5-HT1A autoreceptors, there was no difference in anxiety behaviors between Freud-1 knockout (cF1/1Adko) and wild-type (cF1wt/1Ako) mice in the EPM, OF or NSF tests, nor in control measures (Figure 5D–F). Surprisingly, although the knockout of Freud-1 in the 5-HT1A wild-type background increased immobility in the FST, knockout of Freud-1 in the 5-HT1A-knockout background induced a stress resilient phenotype with reduced immobility in FST and TS compared to control groups (cF1wt/1Ako vs. cF1/1Adko, Figure 5G–H). Thus, the absence of 5-HT1A autoreceptors not only blocked the pro-depressant effect of Freud-1 deletion but unmasked an antidepressant-like effect. No significant change in locomotor activity among genotypes was observed in the beam-break test (Figure 5I). Taken together, these results indicate that the anxiety/depression phenotype observed upon conditional knockout of Freud-1 in 5-HT neurons depend on the presence of 5-HT1A autoreceptors.
DISCUSSION
Freud-1 represses 5-HT1A autoreceptors to regulate 5-HT, anxiety and depression
A reduction in 5-HT neurotransmission implicated in anxiety and depression (Mann, 1999; Jans et al., 2007) has been associated with increased 5-HT1A autoreceptor expression in depressed subjects (Parsey et al., 2010) and depressed suicides (Stockmeier et al., 1998; Boldrini et al., 2008). Thus, the transcriptional “set point” of 5-HT1A autoreceptor expression may be elevated in depression, suggesting altered function of transcription factors (Albert et al., 2011). Here we show that loss of Freud-1 in adult 5-HT neurons leads to up-regulation of 5-HT1A autoreceptors (Figure. 1D–G), and is correlated with increased 5-HT1A response to DPAT-induced hypothermia (Figure 2A), reduced raphe 5-HT levels (Figure 2C–D), and anxiety/depression-like behaviors (Figure 3) that are resistant to chronic SSRI treatment (Figure 4). These findings implicate a key role for the endogenous repressor Freud-1 in 5-HT1A autoreceptor expression leading to reduced raphe 5-HT and antidepressant-resistant anxiety and depression. In contrast, global knockout of the repressor Deaf1 resulted in up-regulation of 5-HT1A autoreceptor expression, reduced raphe 5-HT, but only a mild anxiety phenotype (Czesak et al., 2012; Luckhart et al., 2016), consistent with a stronger effect of Freud-1 to repress the human 5-HT1A gene in raphe cells (Lemonde et al., 2003; Ou et al., 2003).
Previous studies have indirectly implicated transcriptional dys-regulation of the human HTR1A gene in psychopathology and treatment resistance (Albert et al., 2011). For example, within the human HTR1A repressor region, a C(-1019)G polymorphism that prevents binding and repression by Deaf1/NUDR (Lemonde et al., 2003) has been associated with major depression and bipolar depression (Kishi et al., 2013) and with SSRI resistance (Le Francois et al., 2008; Kato et al., 2015; Takekita et al., 2015) and with increased 5-HT1A autoreceptors levels in depressed subjects (Hesselgrave and Parsey, 2013). Allele-specific RT-PCR analysis has revealed that this polymorphism leads to lifelong alterations in 5-HT1A RNA levels in human PFC, which is attenuated in depressed subjects (Donaldson et al., 2016). Our finding of a major role for Freud-1 in repressing 5-HT1A autoreceptor expression and in SSRI-resistant anxiety/depression phenotype in mice are consistent with a key role for Freud-1-mediated repression in human anxiety and depression.
Importantly, we demonstrate that the behavioral effect of Freud-1 deletion is dependent on the presence of 5-HT1A autoreceptors in adult mice (Fig. 5). Previously, a mild (30%) knock-down of the 5-HT1A autoreceptor in adulthood resulted in no change in anxiety or depression behaviors, but improved stress-coping (Richardson-Jones et al., 2010), a phenotype that we did not assess in these studies. The anti-depressed phenotype seen upon Freud-1 deletion suggests that Freud-1 has a pro-depressant effect that is revealed when 5-HT1A autoreceptors are absent. Loss of Freud-1 in 5-HT1A-negative 5-HT neurons could alter the transcription of other genes, including de-repressing dopamine-D2 receptors (Rogaeva et al., 2007), which have been shown to increase 5-HT neuron activity (Aman et al., 2007), which in turn could lead to an antidepressant effect. We did not address whether other gene targets of Freud-1 contribute to behavioral outcomes in cF1ko mice, apart from increased 5-HT1A autoreceptor expression. In addition to 5-HT1A and dopamine-D2 gene repression, Freud-1 induces NF-κB expression (Matsuda et al., 2003; Zhao et al., 2010). Gene deletion of Freud-1 reduces NF-κB signaling to synaptic plasticity in cortical development, leading to abnormal cortical dendrite organization and reduced dendritic spine density (Manzini et al., 2014). However, these effects of Freud-1 deletion appear to be developmental, and deletion of Freud-1 in adulthood did not appear to affect neuronal organization (Oaks et al., 2016).
SSRI resistance in Freud-1 conditional knockout mice
The three-week latency for clinical efficacy of SSRI treatment is thought to involve desensitization of 5-HT1A autoreceptors to release 5-HT neurons from recurrent inhibition (Pineyro and Blier, 1999; Albert et al., 2011). Our results suggest that loss of Freud-1 may render 5-HT neurons resistant to desensitization by chronic SSRI treatment. In human depression, increased 5-HT1A autoreceptors are correlated with resistance to SSRI treatment (Parsey et al., 2010). Furthermore, the G(-1019) allele associates with resistance to SSRI treatment (Lemonde et al., 2004b; Le Francois et al., 2008; Parsey et al., 2010). The G-allele is also associated with resistance of negative symptoms to treatment with atypical antipsychotics that act in part by targeting 5-HT1A receptors (Reynolds et al., 2006; Newman-Tancredi and Albert, 2012). Taken together, these results implicate the increase in 5-HT1A autoreceptors upon loss of Freud-1 in resistance to chronic SSRI treatment. This resistance could suggest a role for activation of Freud-1 in desensitizing 5-HT1A autoreceptors in response to antidepressants. 5-HT1A receptors couple to inhibition of intracellular calcium levels (Albert et al., 1990; Penington et al., 1991), which could activate Freud-1 DNA binding and repression (Ou et al., 2003), leading to 5-HT1A autoreceptor desensitization. The loss of Freud-1 would prevent this process, leading to SSRI resistance. Alternately, the elevated levels of 5-HT1A autoreceptors may require high SSRI doses or longer times to fully desensitize. In this regard, 5-HT1A partial agonists or allosteric modulators that enhance Freud-1 signaling to desensitize presynaptic 5-HT1A receptors may prove useful to augment SSRI response in treatment resistant subjects (Trivedi et al., 2006). The cF1KO mouse may provide a useful model to test novel antidepressants like ketamine or deep brain stimulation for their dependence on 5-HT auto-regulation, which has previously been examined using 5-HT depletion (Hamani et al., 2010; du Jardin et al., 2016).
Roles of Freud-1 in vivo
Our data suggest that susceptibility to anxiety and depression due to transcriptional dys-regulation of the 5-HT1A receptor by Freud-1 can be induced in adulthood and leads to reduced raphe 5-HT/5-HIAA level, suggesting a chronic reduction of 5-HT activity. Acute tryptophan depletion leads to relapse in recovered depressed but not control subjects, suggesting that depression confers increased susceptibility to 5-HT depletion that could be due to persistent transcriptional dys-regulation in recovered depressed subjects as exemplified by the cF1KO mice. Furthermore, patients that relapse often become more resistant to SSRI treatments, consistent with the SSRI-resistant phenotype of the cF1KO mice, and suggesting a role for loss of Freud-1 function in the development of this resistance.
Disruption of the CC2D1A/Freud-1 gene in humans is linked to NSID and autism (Basel-Vanagaite et al., 2006; Manzini et al., 2014), conditions often associated with reduced social interaction. Knockout of Freud-1 in the postnatal mouse forebrain results in impaired cortical neuronal development, reduced cognitive function and social interaction, as well as anxiety-like behavior in the OF test (Oaks et al., 2016). This suggests that post-synaptic Freud-1 is also important for normal behavioral development. Consistent with this, Freud-1 levels are reduced in PFC following chronic stress in rats (Iyo et al., 2009), while both Freud-1 and 5-HT1A protein levels were reduced in PFC of young depressed subjects (Szewczyk et al., 2010), suggesting that early impairments in cortical Freud-1 levels may predispose to depression. The importance of presynaptic Freud-1 in 5-HT1A autoreceptor regulation and anxiety/depression behavior reported here, and its complementary role in the forebrain in anxiety and cognitive function suggest that enhancing Freud-1 expression or function may provide a useful target, particularly in treatment-resistant forms of these diseases.
In summary, this study provides evidence that loss of Freud-1 in 5-HT neurons causes over-expression of 5-HT1A autoreceptors, associated with SSRI-resistant and 5-HT1A autoreceptor-dependent anxiety/depression phenotypes and a 5-HT1A autoreceptor-independent anti-depressed phenotype, implicating Freud-1 as a key transcriptional regulator in 5-HT function and behaviour.
SIGNIFICANCE STATEMENT.
Altered regulation of the 5-HT1A autoreceptor has been implicated in human anxiety, major depression, suicide and resistance to antidepressants. This study uniquely identifies a single transcription factor, Freud-1, as crucial for 5-HT1A autoreceptor expression in vivo. Disruption of Freud-1 in serotonin neurons in mice links up-regulation of 5-HT1A autoreceptors to anxiety/depression-like behavior and provides a new model of antidepressant resistance. Treatment strategies to re-establish transcriptional regulation of 5-HT1A autoreceptors could provide more robust and sustained antidepressant response.
Acknowledgments
We thank Dr. C. A. Walsh (Harvard University) for providing the Cc2d1a (Freud-1)flx/flx mice, Drs. K. Nautiyal and Z. Donaldson, Columbia University for helpful discussions, and Dr. D. C. Lagace and the UOttawa Behavioral Core for helpful advice and technical assistance. This research was supported by grants from the Canadian Institutes for Health Research (MOP-115098 and MOP-123426 to P.R.A.) and trainee support to F.V.-A. from the Canadian Partnership for Stroke Recovery. These agencies had no role in the design of the study, collection and analysis of data or decision to publish.
Footnotes
F.V.-A., S.D.G., J.-C.B., J.S.J., Z.M., and P.R.A. designed research; F.V.-A., M.D., S.D.G., and J.S.J. performed research; F.V.-A., M.D., S.D.G., J.-C.B., J.S.J., Z.M., and P.R.A. analyzed data; F.V.-A., M.C.M., R.H., S.D.G., J.-C.B., and P.R.A. wrote the paper; M.C.M., K.F.T., R.H., and P.R.A. contributed unpublished reagents/analytic tools.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
References
- Albert PR, Lemonde S. 5-HT1A Receptors, Gene Repression, and Depression: Guilt by Association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Albert PR, Francois BL. Modifying 5-HT1A receptor gene expression as a new target for antidepressant therapy. Front Neurosci. 2010;4:35. doi: 10.3389/fnins.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Fiori LM. Transcriptional dys-regulation in anxiety and major depression: 5-HT1A gene promoter architecture as a therapeutic opportunity. Curr Pharm Des. 2014;20:3738–3750. doi: 10.2174/13816128113196660740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Le Francois B, Millar AM. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain. 2011;4:21. doi: 10.1186/1756-6606-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. Journal of Biological Chemistry. 1990;265:5825–5832. [PubMed] [Google Scholar]
- Aman TK, Shen RY, Haj-Dahmane S. D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J Pharmacol Exp Ther. 2007;320:376–385. doi: 10.1124/jpet.106.111690. [DOI] [PubMed] [Google Scholar]
- Andrade R, Huereca D, Lyons JG, Andrade EM, McGregor KM. 5-HT1A Receptor-Mediated Autoinhibition and the Control of Serotonergic Cell Firing. ACS Chem Neurosci. 2015;6:1110–1115. doi: 10.1021/acschemneuro.5b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Attia R, Yahav M, Ferland RJ, Anteki L, Walsh CA, Olender T, Straussberg R, Magal N, Taub E, Drasinover V, Alkelai A, Bercovich D, Rechavi G, Simon AJ, Shohat M. The CC2D1A, a member of a new gene family with C2 domains, is involved in autosomal recessive non-syndromic mental retardation. J Med Genet. 2006;43:203–210. doi: 10.1136/jmg.2005.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120536. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Tremblay RE, Szyf M, Benkelfat C. Genetic and early environmental influences on the serotonin system: consequences for brain development and risk for psychopathology. J Psychiatry Neurosci. 2015;40:5–18. doi: 10.1503/jpn.140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz E, Emerit MB, Vodjdani G, Gautheron V, Hamon M, Darmon M, Masson J. Neuronal Phenotype Dependency of Agonist-Induced Internalization of the 5-HT1A Serotonin Receptor. J Neurosci. 2014;34:282–294. doi: 10.1523/JNEUROSCI.0186-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, Pan YZ, Lemos JC, Craige C, Heemstra LA, Beck SG. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Czesak M, Le Francois B, Millar AM, Deria M, Daigle M, Visvader JE, Anisman H, Albert PR. Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice. J Biol Chem. 2012;287:6615–6627. doi: 10.1074/jbc.M111.293027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Piel DA, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG, Champagne FA, Hen R. Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology. 2014;39:291–302. doi: 10.1038/npp.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, le Francois B, Santos TL, Almli LM, Boldrini M, Champagne FA, Arango V, Mann JJ, Stockmeier CA, Galfalvy H, Albert PR, Ressler KJ, Hen R. The functional serotonin 1a receptor promoter polymorphism, rs6295, is associated with psychiatric illness and differences in transcription. Transl Psychiatry. 2016;6:e746. doi: 10.1038/tp.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Jardin KG, Muller HK, Elfving B, Dale E, Wegener G, Sanchez C. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27–38. doi: 10.1016/j.pnpbp.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl) 2014;231:623–636. doi: 10.1007/s00213-013-3389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes SD, Assadzada S, Sokolovski A, Bergeron R, Haj-Dahmane S, Beique JC. Time-dependent modulation of glutamate synapses onto 5-HT neurons by antidepressant treatment. Neuropharmacology. 2015;95:130–143. doi: 10.1016/j.neuropharm.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Geddes SD, Assadzada S, Lemelin D, Sokolovski A, Bergeron R, Haj-Dahmane S, Beique JC. Target-specific modulation of the descending prefrontal cortex inputs to the dorsal raphe nucleus by cannabinoids. Proc Natl Acad Sci U S A. 2016;113:5429–5434. doi: 10.1073/pnas.1522754113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjighassem MR, Austin MC, Szewczyk B, Daigle M, Stockmeier CA, Albert PR. Human Freud-2/CC2D1B: a novel repressor of postsynaptic serotonin-1A receptor expression. Biol Psychiatry. 2009;66:214–222. doi: 10.1016/j.biopsych.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hesselgrave N, Parsey RV. Imaging the serotonin 1A receptor using [11C]WAY100635 in healthy controls and major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120004. doi: 10.1098/rstb.2012.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo AH, Kieran N, Chandran A, Albert PR, Wicks I, Bissette G, Austin MC. Differential regulation of the serotonin 1 A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience. 2009;163:1119–1127. doi: 10.1016/j.neuroscience.2009.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Kato M, Serretti A, Nonen S, Takekita Y, Wakeno M, Azuma J, Kinoshita T. Genetic variants in combination with early partial improvement as a clinical utility predictor of treatment outcome in major depressive disorder: the result of two pooled RCTs. Transl Psychiatry. 2015;5:e513. doi: 10.1038/tp.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Fukuo Y, Okochi T, Matsunaga S, Umene-Nakano W, Nakamura J, Serretti A, Correll CU, Kane JM, Iwata N. The serotonin 1A receptor gene confer susceptibility to mood disorders: results from an extended meta-analysis of patients with major depression and bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2013;263:105–118. doi: 10.1007/s00406-012-0337-4. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Rogaeva A, Albert PR. Cell type-dependent recruitment of trichostatin A-sensitive repression of the human 5-HT1A receptor gene. J Neurochem. 2004a;88:857–868. doi: 10.1046/j.1471-4159.2003.02223.x. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol. 2004b;7:501–506. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du LS, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart C, Philippe TJ, Le Francois B, Vahid-Ansari F, Geddes SD, Beique JC, Lagace DC, Daigle M, Albert PR. Sex-dependent adaptive changes in serotonin-1A autoreceptor function and anxiety in Deaf1-deficient mice. Mol Brain. 2016;9:77. doi: 10.1186/s13041-016-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Manzini MC, Xiong L, Shaheen R, Tambunan DE, Di Costanzo S, Mitisalis V, Tischfield DJ, Cinquino A, Ghaziuddin M, Christian M, Jiang Q, Laurent S, Nanjiani ZA, Rasheed S, Hill RS, Lizarraga SB, Gleason D, Sabbagh D, Salih MA, Alkuraya FS, Walsh CA. CC2D1A regulates human intellectual and social function as well as NF-kappaB signaling homeostasis. Cell Rep. 2014;8:647–655. doi: 10.1016/j.celrep.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, Hayashi H, Sugano S. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22:3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Albert PR. Gene polymorphism at serotonin 5-HT1A receptors: moving towards personalized medicine for psychosis and mood deficits. In: Sumiyoshi T, editor. Schizophrenia Research: Recent Advances. New York, NY: Nova Publishers; 2012. pp. 337–358. [Google Scholar]
- Oaks AW, Zamarbide M, Tambunan DE, Santini E, Di Costanzo S, Pond HL, Johnson MW, Lin J, Gonzalez DM, Boehler JF, Wu GK, Klann E, Walsh CA, Manzini MC. Cc2d1a Loss of Function Disrupts Functional and Morphological Development in Forebrain Neurons Leading to Cognitive and Social Deficits. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Jafar-Nejad H, Storring JM, Meng JH, Lemonde S, Albert PR. Novel dual repressor elements for neuronal cell-specific transcription of the rat 5-HT1A receptor gene. J Biol Chem. 2000;275:8161–8168. doi: 10.1074/jbc.275.11.8161. [DOI] [PubMed] [Google Scholar]
- Ou XM, Lemonde S, Jafar-Nejad H, Bown CD, Goto A, Rogaeva A, Albert PR. Freud-1: A novel calcium-regulated repressor of the 5-HT1A receptor gene. J Neuroscience. 2003;23:7415–7425. doi: 10.1523/JNEUROSCI.23-19-07415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, Oquendo MA, Mann JJ. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. A study of the mechanism of Ca2+ current inhibition produced by serotonin in rat dorsal raphe neurons. Journal of Neuroscience. 1991;11:3594–3609. doi: 10.1523/JNEUROSCI.11-11-03594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- Reynolds GP, Arranz B, Templeman LA, Fertuzinhos S, San L. Effect of 5-HT1A receptor gene polymorphism on negative and depressive symptom response to antipsychotic treatment of drug-naive psychotic patients. Am J Psychiatry. 2006;163:1826–1829. doi: 10.1176/ajp.2006.163.10.1826. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT(1A) Autoreceptor Levels Determine Vulnerability to Stress and Response to Antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva A, Albert PR. The mental retardation gene CC2D1A/Freud-1 encodes a long isoform that binds conserved DNA elements to repress gene transcription. Eur J Neurosci. 2007;26:965–974. doi: 10.1111/j.1460-9568.2007.05727.x. [DOI] [PubMed] [Google Scholar]
- Rogaeva A, Ou XM, Jafar-Nejad H, Lemonde S, Albert PR. Differential repression by Freud-1/CC2D1A at a polymorphic site in the dopamine-D2 receptor gene. J Biol Chem. 2007;282:20897–20905. doi: 10.1074/jbc.M610038200. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, Madronal N, Donaldson ZR, Drew LJ, Dranovsky A, Gross CT, Tanaka KF, Hen R. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 2015;18:1606–1616. doi: 10.1038/nn.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Milak M, Miller JM, Burke A, Ogden RT, Parsey RV, Mann JJ. Positron Emission Tomography Quantification of Serotonin1A Receptor Binding in Suicide Attempters With Major Depressive Disorder. JAMA Psychiatry. 2015;72:169–178. doi: 10.1001/jamapsychiatry.2014.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Kotarska K, Daigle M, Misztak P, Sowa-Kucma M, Rafalo A, Curzytek K, Kubera M, Basta-Kaim A, Nowak G, Albert PR. Stress-induced alterations in 5-HT1A receptor transcriptional modulators NUDR and Freud-1. Int J Neuropsychopharmacol. 2014;17:1763–1775. doi: 10.1017/S146114571400100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Rogaeva A, Fitzgibbon H, May WL, Rajkowska G, Miguel-Hidalgo JJ, Stockmeier CA, Woolverton WL, Kyle PB, Wang Z, Austin MC. Decreased expression of Freud-1/CC2D1A, a transcriptional repressor of the 5-HT1A receptor, in the prefrontal cortex of subjects with major depression. Int J Neuropsychopharmacol. 2010;13:1089–1101. doi: 10.1017/S1461145710000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekita Y, Fabbri C, Kato M, Nonen S, Sakai S, Sunada N, Koshikawa Y, Wakeno M, Okugawa G, Kinoshita T, Serretti A. HTR1A Gene Polymorphisms and 5-HT1A Receptor Partial Agonist Antipsychotics Efficacy in Schizophrenia. J Clin Psychopharmacol. 2015;35:220–227. doi: 10.1097/JCP.0000000000000304. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Zhao M, Li XD, Chen Z. CC2D1A, a DM14 and C2 domain protein, activates NF-kappaB through the canonical pathway. J Biol Chem. 2010;285:24372–24380. doi: 10.1074/jbc.M109.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]