Abstract
Objective
To investigate if comorbidities are associated with change in health outcomes following an eight-week exercise and education program in knee and hip osteoarthritis (OA).
Methods
We included 24,513 individuals with knee or hip OA from the Good Life with osteoArthritis in Denmark (GLA:D®). GLA:D® consists of two patient education sessions and 12 supervised exercise sessions. Before the program, individuals self-reported having one or more of 11 common comorbidities. Physical function was assessed using the 40-m Fast-Paced Walk Test (FPWT, m/sec) before and immediately after the program. Pain intensity and health-related quality of life was self-reported before, immediately after, and at 12 months post-intervention using a visual analogue scale (VAS, 0–100) and the EQ-5D-5L index (-0.624 to 1.000), respectively. Associations of comorbidity combinations with change in outcomes immediately and at 12 months was estimated using mixed linear regression.
Results
Individuals with OA improved on average 0.12 m/sec (95%CI 0.12 to 0.13) in 40-m FPWT, -12.7 mm (95%CI -13.2 to -12.2) in VAS, and 0.039 (95%CI 0.036 to 0.041) in EQ-5D-5L from before to immediately after the intervention with minor additional improvements at 12 months. Despite that individuals with comorbidities had worse baseline scores in all outcomes than individuals without comorbidities, they had similar levels of improvement immediately and 12 months after the intervention.
Conclusion
Comorbidities are not associated with worse nor better health outcomes following an eight-week exercise and education program in individuals with OA, suggesting exercise as a viable treatment option for individuals with OA, irrespective of comorbidities.
Keywords: Knee, Hip, osteoarthritis, Co-Morbidity, Pain, Function, Quality of life, Hypertension, Depression, Diabetes, Heart disease, Respiratory disease
Introduction
Two thirds of individuals with knee or hip osteoarthritis (OA) have coexisting chronic conditions1, including cardiovascular and pulmonary diseases, diabetes, and depression2-4, and typically report worse pain intensity and physical function compared to those without multiple conditions1,5,6.
Individuals with knee or hip OA are commonly managed in primary care, and international guidelines recommend exercise therapy as part of the first line treatment7,8 supported by numerous randomised controlled trials (RCTs) demonstrating that exercise therapy is effective in reducing pain intensity and improving physical function and quality of life9,10. However, it is unclear, whether individuals with knee and hip OA and one or more comorbidities have similar improvements following exercise therapy as those without comorbidities. Unfortunately, the majority of published trials either excluded individuals with other conditions than OA or did not report results separately for those with comorbidities9,10.
It is important to determine if outcomes from exercise therapy are suboptimal in individuals with OA and specific comorbidities or combinations of comorbidities in order to optimize outcomes by tailoring treatment programs specific to the needs of different subgroups. Only one recent study, limited by including just six comorbidities and a relatively low number of individuals with some of the comorbidities, has investigated if the presence of comorbidities was associated with pain and function outcome after exercise therapy11. They found that obesity and anxiety/depression were predictive of both pain and function outcomes, whereas estimates for pulmonary conditions and diabetes were imprecise due to few cases, precluding any clear interpretations.
Therefore, the main aim of this study was to investigate if presence of the most prevalent comorbidities and combinations of comorbidities were associated with immediate improvements in physical function following an eight-week supervised exercise and patient education program in a large-scale cohort of individuals with knee and hip OA. Additionally, we wanted to investigate any association with immediate and one-year improvements in health-related quality of life and pain relief.
Method
Study design
This study is a comparative cohort study using registry-based data from the Good Life with osteoArthritis in Denmark (GLA:D®) program. GLA:D® is a university-based not-for-profit initiative to facilitate implementation of treatment guidelines and has been implemented nationwide in Denmark for individuals with knee or hip OA. It consists of two patient education sessions followed by 12 60-minutes sessions of neuromuscular exercise (twice weekly over six weeks) supervised by certified physical therapists. The program is evaluated using pre-defined and validated outcomes prior to, immediately after ((3 months), and 12 months after initiating the program. The content of GLA:D® with regard to treatment and outcomes has previously been published12, including details of the neuromuscular exercise program13. The Danish Data Protection Agency has previously approved the GLA:D® registry and all participants have consented to submitting their data to the registry. No ethics approval of GLA:D® is needed according to the local ethics committee of the North Denmark Region.
The reporting of this study conforms to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guideline14.
Participants
Individuals with knee or hip pain and/or functional impairments, resulting in contact with the health care system are assessed for eligibility in GLA:D®. Individuals are ineligible if they have other reasons for their joint symptoms than OA as evaluated by the physical therapist, e.g. inflammatory joint disease or patellar tendinopathy, if they have other symptoms that are more pronounced than the OA symptoms, e.g. chronic, generalized pain, or fibromyalgia, or if they do not understand Danish.
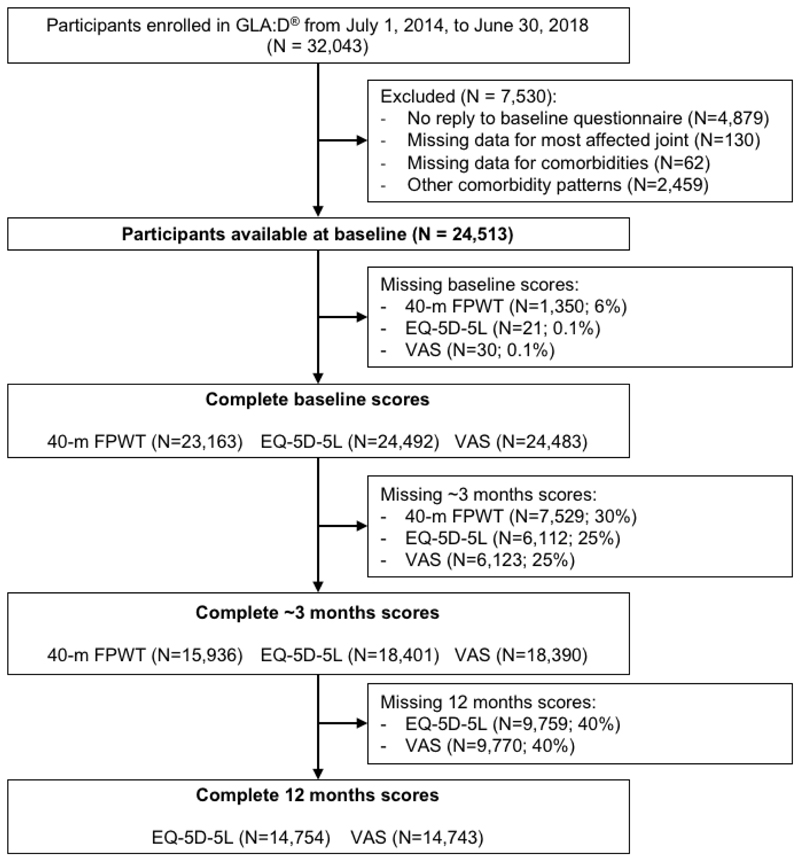
In the present study, only participants with complete baseline data for the most affected joint and comorbidities were included (Fig. 1). Furthermore, only data from participants enrolled in GLA:D® in the period from July 1, 2014 to June 30, 2018 were used as no information about comorbidities were included before this period.
Fig. 1. Flowchart of included participants.
Comorbidities (exposure)
The exposure of interest in the present study was the presence of one or more comorbidities. Before entering GLA:D®, participants were asked to indicate if they had any of the following comorbidities (yes/no), some of them with explanations to guide the participants when answering: ‘hypertension (HT)’, ‘heart diseases (HD) (e.g. narrowing of the heart’s blood vessels)’, ‘ulcer or other bowel diseases’, ‘respiratory diseases (e.g. chronic obstructive pulmonary disease (COPD))’, ‘diabetes mellitus (DM, type 1 or 2)’, ‘kidney or liver diseases’, ‘anemia (i.e., reduced number of red blood cells) or other blood diseases’, ‘cancer’, ‘depression’, ‘rheumatoid arthritis (RA) (NB not the same as osteoarthritis)’, or ‘neurological diseases (e.g. blood clots in the brain, Parkinson’s, or migraine)’. For analytic purposes, including having a sufficient number of participants in each comorbidity group, only the 20 most prevalent comorbidity combinations in GLA:D® were included in this study.
Outcomes
The main outcome was change in physical function from before to immediately after the treatment program assessed by the physical therapist (40-m Fast-Paced Walk Test (FPWT), (m/sec)). The FPWT is recommended by the Osteoarthritis Research Society International (OARSI) as a performance-based functional test for knee and hip OA15. A difference of minimum 0.20 m/sec was considered as clinically important16.
Secondary outcomes included change in quality of life and mean pain intensity from before to immediately after, and at 12 months after the program. Participants self-reported health-related quality of life using the EuroQol-5 dimensions 5 level index questionnaire (EQ-5D-5L), which is a generic quality of life measurement tool17. It was scored using the Danish crosswalk value set, and ranges from -0.624 to 1.000 corresponding to ‘worst’ and ‘best’ health, respectively. Change in self-reported mean pain intensity during the last month in the most affected knee was assessed on a 100-point visual analogue scale (VAS), which ranges from 0 corresponding to ‘no pain’ to 100 corresponding to ‘maximum pain’18. For EQ-5D-5L and VAS differences of minimum 0.05 and 15 mm, respectively, were considered clinically important17,19.
As an additional outcome, adherence to the supervised exercise sessions were reported by the physical therapist immediately after the program by replying to the question: ‘How many exercise sessions did the patient attend?’ with the reply options: ‘more than 12 sessions’, ‘10-12 sessions’, ‘7-9 sessions’, ‘1-6 sessions’, and ‘did not attend at all’. In the analyses, response options were dichotomized into ‘less than 10 sessions’ and ‘10 or more sessions’.
Statistical analyses
Multiple imputation
The proportion of missing data for the main outcome (40-m FPWT) at baseline and at follow-up (i.e. immediately after the GLA:D® program) was 6% and 30%, respectively, while the proportions for VAS at baseline, immediately after the program, and 12 months were 0.1%, 25% and 40%, respectively, which were the same for EQ-5D-5L (Fig. 1). For the additional outcome (adherence to exercise sessions) the proportion of missing data was 34% (n=8,247). Under the assumption of data being missing at random (MAR)20, missing values for the outcomes at all time points were imputed using multiple imputation with chained equations21. For the main and secondary outcomes, the multiple imputation model included all outcomes (i.e. 40-m FPWT, EQ-5D-5L, and VAS) at each time point, the exposure (i.e. comorbidity groups), confounders in the primary analysis (see under ‘main analyses’), and as auxiliary variable the most affected joint. The same confounders and auxiliary variables were included in the imputation model for adherence to exercise sessions, whereas only the baseline scores for 40-m FPWT, EQ-5D-5L, and VAS were included in this model. Linear regression was used to impute 40-m FPWT, while predictive mean matching (PMM) was used for EQ-5D-5L and VAS, and logistic regression for adherence to exercise. A total of 30 imputed data sets approximately equal to the proportion of missing observations for the main outcome were generated21.
Main analyses
The main outcome (between-group difference in 40-m FPWT change score from baseline to immediately after GLA:D®) was analyzed using linear mixed regression (restricted maximum likelihood estimation (REML)) with participants nested within clinics as random effects and group (no comorbidities vs. different comorbidities) and time (baseline and immediately after GLA:D®) as fixed effects. Both unadjusted and adjusted differences with 95% confidence intervals were estimated across imputed data sets using Rubin’s rules22. Included confounders in the adjusted analysis were age (continuous), sex (male/female), BMI (continuous), educational level (ordered categorical), not born in Denmark (yes/no – proxy of ethnicity), living alone (yes/no), pain in other joints (hip or knee – yes/no), use of analgesics within recent three months (yes/no), number of bodily pain sites (ordered categorical), and self-reported physical activity level (ordered categorical). A complete directed acyclic graph (DAG) of the assumed causal relationships is available in Supplementary Figure S1. The same analysis approach was used for pain intensity and health-related quality of life, with the addition of 12 months follow-up.
Because bodily pain, pain in the other knee than the most affected joint or hip joints, physical activity level, and use of analgesics may be considered intermediate factors (see DAG in Supplementary Figure S2), sensitivity analyses excluding these four variables as confounders were conducted. Furthermore, all analyses were repeated stratified for most affected joint (knee or hip), and lastly, the impact of missing data was explored by repeating all analyses using only participants with complete data at all time points (i.e. complete-case analysis). Assumptions underlying all models were examined using quantile-quantile plots and scatter plots of residuals and predicted values.
Additional analyses
As additional analyses, between group differences in change in all outcomes were analyzed using the same approach as for the main analyses, however substituting the group with number of comorbidities (i.e. 0 vs 1, 2, 3, or >3) instead of the specific comorbidity groups. Also, unadjusted and adjusted relative risks (RR) with 95% confidence interval for difference in risks of not adhering to exercise sessions between the different comorbidity groups were estimated from logistic regression23 across imputed data sets using Rubin’s rules22. Also, here the impact of missing data was explored by repeating the analyses using only participants with complete data at all time points (i.e. complete-case analysis).
All analyses were conducted using Stata V.16.1. Before any analyses commenced, the statistical analysis plan was uploaded to the Open Science Framework where the statistical codes used for the main analyses are also available (https://osf.io/8vdhq/).
Results
A total of 24,513 participants were included in the analyses, of whom completeness of the main outcome (40-m FPWT) was 70% at three months follow-up and for the secondary outcomes (EQ-5D-5L and VAS) 75% and 60% at three and 12 months, respectively (Fig. 1). Among included participants, approximately two thirds were women and reported the knee as the most affected joint, while the average age was 64.7 years (Table I). The most frequent comorbidities were HT alone (23%) and HT in combination with either DM or HD (6%), while the least frequent included anaemia and HT in combination with kidney/liver disease (<1%) (Table II and Supplementary Table S2). Participants with comorbidities were on average a little older and slightly more overweight than those with no comorbidities (Table I). Baseline characteristics in the specific comorbidity groups are available in Supplementary Table S2. Participants lost to follow-up had slightly poorer baseline scores in all outcome measures, while other characteristics were comparable to those with complete follow-up (Supplementary Table S1).
Table I. Baseline characteristics of participants with and without comorbidities.
|
All
(n = 24513) |
No comorbidities
(n = 12,128) |
Comorbidities
(n = 12,385) |
|
|---|---|---|---|
| Most affected joint, n (%) | |||
| Knee | 18,271 (75) | 8,923 (74) | 9,348 (75) |
| Hip | 6,242 (25) | 3,205 (26) | 3,037 (25) |
| Age, years (SD) | 64.7 (9.7) | 62.9 (10.1) | 66.5 (9.0) |
| Female, n (%) | 17,799 (73) | 9,052 (75) | 8,747 (71) |
| BMI, kg/m2 (SD)a | 28.2 (5.2) | 27.3 (4.9) | 29.1 (5.4) |
| Educational level, n (%)b | |||
| Primary school | 4,243 (17) | 1,738 (14) | 2,505 (20) |
| Secondary school | 2,736 (11) | 1,329 (11) | 1,407 (11) |
| Short-term educationc | 4,819 (20) | 2,373 (20) | 2,446 (20) |
| Middle-term educationd | 9,870 (40) | 5,145 (42) | 4,725 (38) |
| Long-term educatione | 2,834 (12) | 1,541 (13) | 1,293 (10) |
| Born outside Denmark, n (%)f | 917 (4) | 466 (4) | 451 (4) |
| Living alone, n (%)g | 6,216 (25) | 2,751 (23) | 3,465 (28) |
| Physical activity level, n (%)h | |||
| Inactive | 524 (2) | 200 (2) | 324 (3) |
| Low (e.g. walking and limited housework) | 7,114 (29) | 3,046 (25) | 4,068 (33) |
| Moderate (e.g. swimming and unlimited housework) | 8,104 (33) | 4,007 (33) | 4,097 (33) |
| High (e.g. prolonged biking and fitness) | 7,125 (29) | 3,870 (32) | 3,255 (26) |
| Very high (e.g. running, tennis and skiing) | 1,627 (7) | 995 (8) | 632 (5) |
| Pain in other knee or hip joints, n (%) | |||
| No | 10,535 (43) | 5,387 (44) | 5,148 (42) |
| Bilateral joint (from most affected joint) | 8,696 (35) | 4,294 (35) | 4,402 (36) |
| Hip or kneei | 4,200 (17) | 1,966 (16) | 2,234 (18) |
| Bilateral joint and hip or kneei | 1,082 (4) | 481 (4) | 601 (5) |
| Number of bodily pain areas, n (%) | |||
| 0 | 679 (3) | 356 (3) | 323 (3) |
| 1-2 | 10,966 (45) | 5,656 (47) | 5,310 (43) |
| 3-4 | 6,732 (27) | 3,290 (27) | 3,442 (28) |
| 5-6 | 2,838 (12) | 1,370 (11) | 1,468 (12) |
| 7 or more | 3,298 (13) | 1,456 (12) | 1,842 (15) |
| Use of analgesics within recent 3 monthsj | 15,415 (63) | 7,267 (60) | 8,148 (66) |
| Quality of life (EQ-5D-5L, -0.624 to 1.000), mean (SD)k | 0.714 (0.112) | 0.724 (0.107) | 0.703 (0.115) |
| Pain intensity (VAS, 0-100 mm), mean (SD)l | 47.4 (21.9) | 45.9 (21.8) | 48.9 (21.9) |
| 40m Fast-Paced Walk Test, m/s (SD)m | 1.50 (0.33) | 1.56 (0.33) | 1.43 (0.32) |
n: Number, SD: Standard Deviation, BMI: Body Mass Index (kg/m2), VAS: Visual Analogue Scale.
69 missing observations,
11 missing observations,
under 3 years after secondary school,
3-4 years after secondary school,
at least 5 years after secondary school.
7 missing observations,
7 missing observations,
19 missing observations,
hip if most affected joint is knee and vice versa,
at least one of the following: Acetaminophen, oral or topical Non-Steroidal Anti-inflammatory Drug (NSAID), Morphine, Tramadol, or Codeine,
21 missing observations,
30 missing observations,
1,350 missing observations.
Table II. 40-meter Fast-Paced Walk Test (40-m FPWT) differences at baseline, and changes immediately after exercise therapy and education between participants with and without comorbidities.
| Baseline 40-m FPWT Mean m/s (95% CI) |
3 mo. 40-m FPWT Mean m/s (95% CI) |
Change 3 mo. 40-m FPWT Mean m/s (95% CI) |
|
|---|---|---|---|
| No comorbidities, n=12,128 | 1.56 (1.56 to 1.57) | 1.69 (1.68 to 1.69) | 0.12 (0.12 to 0.13) |
| Difference at baseline | Difference in change at 3 months | ||
| Mean m/s (95% CI) |
Unadjusted mean m/s (95% CI) |
Adjusted* mean m/s (95% CI) |
|
| HT, n=5,716 | -0.12 (-0.13 to -0.11) | -0.00 (-0.01 to 0.01) | -0.00 (-0.01 to 0.01) |
| HT + DM, n=729 | -0.22 (-0.25 to -0.20) | -0.00 (-0.03 to 0.01) | 0.00 (-0.02 to 0.02) |
| HT + HD, n=630 | -0.17 (-0.19 to -0.14) | -0.01 (-0.03 to 0.01) | -0.01 (-0.03 to 0.01) |
| Resp, n=558 | -0.07 (-0.10 to -0.04) | -0.01 (-0.03 to 0.01) | -0.01 (-0.03 to 0.01) |
| HD, n=551 | -0.09 (-0.11 to -0.06) | -0.01 (-0.03 to 0.01) | -0.01 (-0.03 to 0.01) |
| Neu, n=508 | -0.05 (-0.08 to -0.03) | 0.00 (-0.02 to 0.02) | 0.00 (-0.02 to 0.02) |
| RA, n=501 | -0.06 (-0.09 to -0.04) | 0.02 (-0.00 to 0.04) | 0.02 (-0.00 to 0.04) |
| Ulcer, n=447 | -0.05 (-0.08 to -0.02) | -0.01 (-0.03 to 0.01) | -0.01 (-0.04 to 0.01) |
| Dep, n=408 | -0.09 (-0.12 to -0.06) | 0.01 (-0.02 to 0.04) | 0.01 (-0.02 to 0.04) |
| DM, n=358 | -0.15 (-0.18 to -0.11) | -0,01 (-0.04 to 0.01) | -0.01 (-0.04 to 0.01) |
| HT + Resp, n=323 | -0.20 (-0.24 to -0.17) | -0.02 (-0.05 to 0.00) | -0.03 (-0.05 to 0.00) |
| HT + RA, n=275 | -0.14 (-0.18 to -0.10) | -0.03 (-0.06 to 0.00) | -0.03 (-0.06 to 0.00) |
| HT + Neu, n=274 | -0.14 (-0.18 to -0.10) | 0.01 (-0.02 to 0.04) | 0.01 (-0.02 to 0.04) |
| HT + Ulcer, n=254 | -0.16 (-0.20 to -0.12) | 0.02 (-0.01 to 0.05) | 0.02 (-0.01 to 0.05) |
| Cancer, n=234 | -0.06 (-0.10 to -0.02) | 0.01 (-0.02 to 0.04) | 0.01 (-0.02 to 0.04) |
| HT + Dep, n=203 | -0.21 (-0.26 to -0.17) | 0.01 (-0.02 to 0.05) | 0.01 (-0.02 to 0.05) |
| HT + Cancer, n=137 | -0.16 (-0.22 to -0.11) | -0.01 (-0.05 to 0.04) | -0.01 (-0.05 to 0.04) |
| HT + HD + DM, n=117 | -0.26 (-0.32 to -0.20) | 0.01 (-0.04 to 0.06) | 0.01 (-0.04 to 0.06) |
| Anemia, n=83 | -0.04 (-0.11 to 0.03) | -0.06 (-0.11 to -0.1) | -0.06 (-0.11 to -0.01) |
| HT + Ren/Liv. n=79 | -0.09 (-0.16 to -0.02) | -0.03 (-0.08 to 0.02) | -0.03 (-0.08 to 0.02) |
| Number of comorbidities | |||
| 1 comorbidity, n=9,441 | -0.10 (-0.11 to -0.9) | -0.00 (-0.01 to 0.01) | -0.00 (-0.01 to 0.01) |
| 2 comorbidities, n=3,838 | -0.17 (-0.19 to -0.17) | -0.01 (-0.01 to 0.00) | -0.01 (-0.01 to 0.00) |
| 3 comorbidities, n=1,173 | -0.23 (-0.25 to -0.21) | -0.00 (-0.02 to 0.01) | -0.00 (-0.02 to 0.01) |
| ≥4 comorbidities, n=392 | -0.31 (-0.34 to -0.28) | 0.01 (-0.02 to 0.03) | 0.01 (-0.02 to 0.03) |
n: Number, CI: Confidence interval. HT: Hypertension, DM: Diabetes mellitus 1 or 2, Resp: Respiratory disease, HD: Heart disease, Neu: Neurologic disease, RA: Rheumatoid arthritis, Dep: Depression, Ren/Liv: Renal and/or liver disease.
Adjusted for age, sex, BMI, educational level, not born in Denmark, civil status, physical activity, problems in other joints, use of analgesics, and number of bodily pain areas. Estimates in bold reflect statistical significance (p < 0.05).
Main outcome (40-m FPWT)
Compared to participants without comorbidities, those with comorbidities had worse physical function (40-m FPWT) at baseline, which became more pronounced with increasing number of comorbidities (Table II). The average improvement from before to immediately after the treatment program was 0.12 m/sec (95% CI 0.12 to 0.13) in participants without comorbidities. For all comorbidities but one, the average improvement did not statistically significantly differ from those without comorbidities. Only individuals with anaemia had significantly less improvement, however, all differences in improvements were close to 0.00 m/sec with 95% confidence intervals excluding the predefined minimal clinically important difference of 0.20 m/sec (Table II).
Secondary outcomes (EQ-5D-5L & VAS)
Participants with comorbidities had worse pain intensity (VAS) and quality of life (EQ-5D-5L) at baseline than those without comorbidities (Tables III and IV). In participants without comorbidities, pain intensity declined on average -12.7 mm (95% CI -13.2 to -12.2) and -13.8 mm (95% CI-14.3 to -13.4) immediately after the treatment and at 12 months, respectively, while quality of life improved by 0.039 (95% CI 0.036 to 0.041) and 0.051 (95% CI 0.048 to 0.053). In both outcomes, only six out of twenty single or combined comorbidities, including HD and HT in combination with different other conditions, had a statistically significant difference in change at either 3 or 12 than those without comorbidities (Tables III and IV). Yet, for all comorbidities the point differences were only minor and all less than the predefined clinically important differences (15 mm for VAS, and 0.05 for EQ-5D-5L). Only for quality of life, participants with HT plus depression or neurologic disease, and participants with HT plus HD and DM confidence intervals included clinically important differences (Tables III and IV).
Table III. Knee and hip pain (VAS) differences at baseline, and changes immediately after, and 12 months after exercise therapy and education between participants with and without comorbidities.
| Baseline VAS Mean mm (95% CI) |
3 mo. VAS Mean mm (95% CI) |
Change 3 mo. VAS Mean mmm (95% CI) |
12 mo. VAS Mean mm (95% CI) |
Change 12 mo. VAS Mean mm (95% CI) |
|
|---|---|---|---|---|---|
| No comor, n=12,128 | 45.9 (45.5 to 46.3) | 33.2 (32.7 to 33.6) | -12.7 (-13.2 to -12.2) | 32.0 (31.6 to 32.5) | -13.8 (-14.3 to -13.4) |
| Difference at baseline | Difference in change at 3 months | Difference in change at 12 months | |||
| Mean mm (95% CI) |
Unadjusted mean mm (95% CI) |
Adjusted* mean mm (95% CI) |
Unadjusted mean mm (95% CI) | Adjusted* mean mm (95% CI) |
|
| HT, n=5,716 | 2.1 (1.4 to 2.9) | 0.3 (-0.6 to 1.1) | 0.3 (-0.6 to 1.1) | 1.2 (0.3 to 2.1) | 1.3 (0.4 to 2.2) |
| HT + DM, n=729 | 4.5 (2.8 to 6.2) | 1.5 (-0.5 to 3.5) | 1.5 (-0.5 to 3.5) | 2.5 (0.4 to 4.7) | 2.6 (0.4 to 4.8) |
| HT + HD, n=630 | 3.2 (1.4 to 5.0) | -0.0 (-2.1 to 2.1) | 0.0 (-2.1 to 2.1) | 1.2 (-1.2 to 3.6) | 1.2 (-1.2 to 3.6) |
| Resp, n=558 | 2.7 (0.8 to 4.6) | 0.2 (-2.1 to 2.5) | 0.2 (-2.1 to 2.5) | 1.5 (-1.0 to 4.1) | 1.6 (-1.0 to 4.1) |
| HD, n=551 | -0.6 (-2.5 to 1.4) | 3.5 (1.3 to 5.8) | 3.5 (1.3 to 5.8) | 4.0 (1.4 to 6.6) | 4.0 (1.4 to 6.6) |
| Neu, n=508 | 3.9 (1.9 to 5.9) | -1.5 (-3.8 to 0.9) | -1.3 (-3.6 to 1.1) | -0.6 (-3.8 to 0.9) | -0.6 (-3.2 to 2.0) |
| RA, n=501 | 3.5 (1.5 to 5.5) | -1.2 (-3.8 to 1.3) | -1.1 (-3.7 to 1.4) | -0.8 (-3.4 to 1.9) | -0.7 (-3.4 to 2.0) |
| Ulcer, n=447 | 5.7 (3.6 to 7.9) | -1.1 (-3.7 to 1.6) | -1.0 (-3.7 to 1.6) | -0.1 (-3.0 to 2.8) | -0.2 (-3.1 to 2.7) |
| Dep, n=408 | 6.6 (4.4 to 8.9) | 0.4 (-2.5 to 3.2) | 0.3 (-2.5 to 3.2) | 2.7 (-0.8 to 6.2) | 2.7 (-0.8 to 6.2) |
| DM, n=358 | 2.5 (0.2 to 4.9) | 0.3 (-2.5 to 3.2) | 0.3 (-2.5 to 3.2) | -0.6 (-3.9 to 2.8) | -0.5 (-3.9 to 2.8) |
| HT + Resp, n=323 | 3.0 (0.5 to 5.5) | 3.0 (0.0 to 5.9) | 3.0 (0.0 to 5.9) | 0.4 (-3.0 to 3.9) | 0.4 (-3.0 to 3.8) |
| HT + RA, n=275 | 3.9 (1.2 to 6.6) | 1.9 (-1.4 to 5.1) | 1.9 (-1.4 to 5.1) | 2.4 (-1.2 to 6.0) | 2.4 (-1.2 to 6.0) |
| HT + Neu, n=274 | 2.2 (-0.5 to 4.9) | 5.1 (1.8 to 8.4) | 5.0 (1.7 to 8.3) | 7.2 (3.7 to 10.7) | 7.1 (3.6 to 10.6) |
| HT + Ulcer, n=254 | 7.5 (4.7 to 10.3) | -0.5 (-4.0 to 3.0) | -0.3 (-3.8 to 3.3) | 2.7 (-1.1 to 6.6) | 2.9 (-0.9 to 6.8) |
| Cancer, n=234 | 1.4 (-1.5 to 4.3) | 2.5 (-1.1 to 6.0) | 2.5 (-1.1 to 6.0) | 2.9 (-0.8 to 6.7) | 2.9 (-0.8 to 6.7) |
| HT + Dep, n=203 | 7.7 (4.5 to 10.8) | -1.3 (-6.0 to 2.6) | -1.3 (-5.2 to 2.7) | 2.3 (-2.3 to 6.9) | 2.4 (-2.3 to 7.0) |
| HT + Cancer, n=137 | 1.0 (-2.8 to 4.8) | 5.3 (0.8 to 9.9) | 5.4 (0.9 to 10.0) | 0.2 (-4.9 to 5.3) | 0.3 (-4.8 to 5.4) |
| HT + HD + DM, n=117 | 6.6 (2.5 to 10.7) | 0.2 (-5.5 to 5.1) | 0.1 (-5.5 to 5.2) | -1.4 (-8.4 to 5.6) | -1.4 (-8.4 to 5.6) |
| Anemia, n=83 | 2.7 (-2.1 to 7.6) | -1.2 (-7.0 to 4.6) | -1.2 (-7.0 to 4.6) | -2.8 (-9.2 to 3.6) | -2.7 (-9.1 to 3.6) |
| HT + Ren/Liv. n=79 | 2.9 (-2.1 to 7.9) | -0.2 (-6.4 to 5.9) | -0.2 (-6.4 to 5.9) | 3.0 (-3.7 to 9.8) | 3.1 (-3.7 to 9.8) |
| Number of comorbidities | |||||
| 1 comorbidity, n=9,441 | 2.6 (1.9 to 3.2) | 0.2 (-0.5 to 0.0) | 0.2 (-0.5 to 0.9) | 1.1 (0.3 to 1.9) | 1.1 (0.4 to 1.9) |
| 2 comorbidities, n=3,838 | 4.5 (3.7 to 5.4) | 1.4 (0.4 to 2.3) | 1.4 (0.4 to 2.3) | 1.9 (0.9 to 2.9) | 1.9 (0.9 to 2.9) |
| 3 comorbidities, n=1,173 | 7.5 (6.1 to 8.8) | 0.5 (-1.1 to 2.0) | 0.5 (-1.1 to 2.1) | 1.3 (-0.6 to 3.1) | 1.3 (-0.6 to 3.1) |
| ≥4 comorbidities, n=392 | 11.8 (9.5 to 14.1) | 2.6 (-0.2 to 5.4) | 2.7 (-0.1 to 5.5) | 0.3 (-3.1 to 3.7) | 0.3 (-3.1 to 3.8) |
n: Number, CI: Confidence interval. Comor: Comorbidities, HT: Hypertension, DM: Diabetes mellitus 1 and 2, Resp: Respiratory disease, HD: Heart disease, Neu: Neurologic disease, RA: Rheumatoid arthritis, Dep: Depression, Ren/Liv: Renal and/or liver disease.
Adjusted for age, sex, BMI, educational level, not born in Denmark, civil status, physical activity, problems in other joints, use of analgesics, and number of bodily pain areas. Estimates in bold reflect statistical significance (p < 0.05).
Table IV. Quality of life (EQ-5D-5L) differences at baseline, and changes immediately after, and 12 months after exercise therapy and education between participants with and without comorbidities.
| Baseline EQ-5D-5L Mean (95% CI) |
3 mo. EQ-5D-5L Mean (95% CI) |
Change 3 mo. EQ-5D-5L Mean (95% CI) |
12 mo. EQ-5D-5L Mean (95% CI) |
Change 12 mo. EQ-5D-5L Mean (95% CI) |
|
|---|---|---|---|---|---|
| No comor, n=12,128 | 0.724 (0.722 to 0.726) | 0.763 (0.760 to 0.765) | 0.039 (0.036 to 0.041) | 0.775 (0.773 to 0.778) | 0.051 (0.048 to 0.053) |
| Difference at baseline | Difference in change at 3 months | Difference in change at 12 months | |||
| Mean (95% CI) |
Unadjusted mean (95% CI) |
Adjusted*
mean (95% CI) |
Unadjusted mean(95% CI) | Adjusted*
mean(95% CI) |
|
| HT, n=5,716 | -0.010 (-0013 to -0.006) | -0.004 (-0.008 to 0.001) | -0.004 (-0.008 to 0.001) | -0.008 (-0.012 to -0.003) | -0.008 (-0.012 to -0.003) |
| HT + DM, n=729 | -0.025 (-0.031 to -0.016) | -0.007 (-0.017 to 0.004) | -0.007 (-0.017 to 0.004) | -0.019 (-0.031 to -0.007) | -0.019 (-0.031 to -0.008) |
| HT + HD, n=630 | -0.022 (-0.031 to -0.012) | 0.000 (-0.011 to 0.011) | 0.000 (-0.012 to 0.011) | -0.023 (-0.037 to -0.009) | -0.023 (-0.038 to -0.009) |
| Resp, n=558 | -0.016 (-0.027 to -0.006) | 0.000 (-0.011 to 0.012) | 0.000 (-0.011 to 0.012) | -0.008 (-0.022 to 0.005) | -0.008 (-0.022 to 0.005) |
| HD, n=551 | -0.005 (-0.016 to 0.005) | - 0.014 (-0.025 to -0.003) | -0.014 (-0.025 to -0.003) | -0.016 (-0.029 to -0.002) | -0.015 (-0.029 to -0.002) |
| Neu, n=508 | -0.024 (-0.035 to -0.013) | 0.002 (-0.011 to 0.014) | 0.001 (-0.011 to 0.014) | -0.001 (-0.015 to .013) | -0.001 (-0.015 to 0.013) |
| RA, n=501 | -0.029 (-0.040 to -0.018) | 0.010 (-0.003 to 0.023) | 0.010(-0.003 to 0.022) | -0.001 (-0.015 to 0.013) | -0.001 (-0.015 to 0.013) |
| Ulcer, n=447 | -0.036 (-0.047 to -0.024) | 0.004 (-0.010 to 0.018) | 0.004 (-0.010 to 0.018) | -0.010 (-0.027 to 0.006) | -0.010 (-0.027 to 0.006) |
| Dep, n=408 | -0.112 (-0.124 to -0.100) | 0.014 (-0.001 to 0.029) | 0.014 (0.000 to 0.029) | 0.000 (-0.017 to 0.017) | 0.000 (-0.017 to 0.017) |
| DM, n=358 | -0.018 (-0.031 to -0.005) | 0.000 (-0.017 to 0.017) | -0.002 (-0.017 to 0.012) | 0.010 (-0.007 to 0.027) | 0.010 (-0.007 to 0.028) |
| HT + Resp, n=323 | -0.024 (-0.038 to -0.010) | -0.003 (-0.018 to 0.012) | -0.007 (-0.022 to 0.009) | -0.002 (-0.019 to 0.015) | -0.002 (-0.019 to 0.015) |
| HT + RA, n=275 | -0.024 (-0.039 to -0.010) | 0.010 (-0.007 to 0.027) | -0.002 (-0.019 to 0.015) | -0.008 (-0.027 to 0.011) | -0.008 (-0.028 to 0.011) |
| HT + Neu, n=274 | -0.019 (-0.034 to -0.005) | -0.007 (-0.022 to 0.009) | -0.012 (-0.030 to 0.005) | -0.032 (-0.051 to -0.012) | -0.032 (-0.051 to -0.012) |
| HT + Ulcer, n=254 | -0.030 (-0.046 to -0.015) | -0.002 (-0.019 to 0.015) | -0.001 (-0.019 to 0.016) | -0.015 (-0.034 to 0.005) | -0.014 (-0.034 to 0.005) |
| Cancer, n=234 | -0.014 (-0.030 to 0.002) | -0.003 (-0.020 to 0.014) | -0.008 (-0.026 to 0.010) | -0.015 (-0.033 to 0.004) | -0.015 (-0.033 to 0.004) |
| HT + Dep, n=203 | -0.109 (-0.126 to -0.092) | -0.008 (-0.027 to 0.011) | 0.014 (-0.008 to 0.036) | 0.024 (-0.004 to 0.053) | 0.024 (-0.005 to 0.053) |
| HT + Cancer, n=137 | -0.009 (-0.029 to 0.012) | -0.012 (-0.030 to 0.005) | -0.023 (-0.045 to 0.000) | -0.003 (-0.028 to 0.022) | -0.003 (-0.028 to 0.022) |
| HT + HD + DM, n=117 | -0.048 (-0.071 to -0.026) | -0.002 -0.019 to 0.016) | -0.012 (-0.039 to 0.016) | 0.022 (-0.011 to 0.054) | 0.022 (-0.010 to 0.054) |
| Anemia, n=83 | -0.003 (-0.024 to 0.029) | -0.008 (-0.026 to 0.010) | -0.002 (-0.032 to 0.029) | 0.010 (-0.023 to 0.043) | 0.010 (-0.023 to 0.043) |
| HT + Ren/Liv. n=79 | -0.018 (-0.045 to 0.009) | 0.015 (-0.007 to 0.037) | 0.002 (-0.030 to 0.034) | -0.001 (-0.036 to 0.035) | -0.001 (-0.036 to 0.035) |
| Number of comorbidities | |||||
| 1 comorbidity, n=9,441 | -0.017 (-0.021 to -0.014) | -0.002 (-0.005 to 0.002) | -0.002 (-0.005 to 0.002) | -0.006 (-0.011 to -0.002) | -0.006 (-0.011 to -0.002) |
| 2 comorbidities, n=3,838 | -0.036 (-0.040 to -0.031) | -0.002 (-0.007 to 0.003) | -0.002 (-0.007 to 0.003) | -0.011 (-0.017 to -0.006) | -0.011 (-0.017 to -0.006) |
| 3 comorbidities, n=1,173 | -0.060 (-0.068 to -0.053) | -0.006 (-0.014 to 0.003) | -0.006 (-0.015 to 0.003) | -0.013 (-0.024 to -0.003) | -0.014 (-0.024 to -0.003) |
| ≥4 comorbidities, n=392 | -0.111 (-0.124 to -0.098) | -0.005 (-0.011 to 0.022) | 0.005 (-0.011 to 0.022) | -0.004 (-0.024 to 0.017) | -0.004 (-0.024 to 0.017) |
n: Number, CI: Confidence interval. Comor: Comorbidities, HT: Hypertension, DM: Diabetes mellitus 1 and 2, Resp: Respiratory disease, HD: Heart disease, Neu: Neurologic disease, RA: Rheumatoid arthritis, Dep: Depression, Ren/Liv: Renal and/or liver disease.
Adjusted for age, sex, BMI, educational level, not born in Denmark, civil status, physical activity, problems in other joints, use of analgesics, and number of bodily pain areas. Estimates in bold reflect statistical significance (p < 0.05).
For both the main and secondary outcomes, results from sensitivity analyses did not alter the interpretation from the main analyses (Supplementary Tables S3 to S11).
Adherence to exercise sessions
The overall proportion of participants not attending 10 or more exercise sessions were 17% (95% CI 17% to 18%). Most comorbidity groups were not associated with adherence to exercise sessions (Table V). Participants with HT plus DM, and those with HT plus cancer were more likely to attend 10 or more sessions, while those with depression or DM alone, HT plus rheumatoid arthritis, HT plus neurologic disease, HT plus depression, and with two, four or more comorbidities were less likely to attend 10 or more exercise sessions than individuals without comorbidities. However, for most associations the lower or upper limit of the 95% confidence interval was close to 1.00 (i.e. no association) (Table V). In the complete-case analysis, all observed associations in the primary analysis diminished and the associations observed for depression, HT plus rheumatoid arthritis, HT plus neurologic disease, HT plus cancer, and those with two comorbidities became inconclusive with 95% confidence intervals that include 1.00 (Supplementary Table S12).
Table V. Relative risks (RR) for not adhering to ten or more exercise sessions during an exercise therapy and education program for individuals with comorbidities compared to individuals without comorbidities.
| Attending <10 exercise sessions, n (%) | Unadjusted RR (95% CI) | Adjusted* RR (95% CI) | |
|---|---|---|---|
| No comor, n=12,128 | 2,161 (18) | 1 (reference) | 1 (reference) |
| HT, n=5,716 | 925 (16) | 0.91 (0.85 to 0.97) | 1.02 (0.95 to 1.09) |
| HT + DM, n=729 | 85 (12) | 0.65 (0.53 to 0.80) | 0.74 (0.61 to 0.91) |
| HT + HD, n=630 | 100 (16) | 0.89 (0.74 to 1.07) | 1.06 (0.88 to 1.27) |
| Resp, n=558 | 94 (17) | 0.94 (0.78 to 1.14) | 1.02 (0.85 to 1.23) |
| HD, n=551 | 74 (13) | 0.75 (0.61 to 0.94) | 0.88 (0.71 to 1.09) |
| Neu, n=508 | 95 (19) | 1.05 (0.87 to 1.26) | 1.06 (0.88 to 1.28) |
| RA, n=501 | 95 (19) | 1.06 (0.88 to 1.28) | 1.09 (0.91 to 1.31) |
| Ulcer, n=447 | 82 (18) | 1.03 (0.85 to 1.326) | 1.03 (0.85 to 1.26) |
| Dep, n=408 | 106 (26) | 1.46 (1.23 to 1.73) | 1.32 (1.11 to 1.57) |
| DM, n=358 | 80 (22) | 1.26 (1.03 to 1.53) | 1.36 (1.12 to 1.65) |
| HT + Resp, n=323 | 55 (17) | 0.96 (0.75 to 1.22) | 1.16 (0.92 to 1.46) |
| HT + RA, n=275 | 56 (20) | 1.15 (0.90 to 1.45) | 1.27 (1.01 to 1.60) |
| HT + Neu, n=274 | 56 (20) | 1.14 (0.90 to 1.45) | 1.27 (1.00 to 1.60) |
| HT + Ulcer, n=254 | 34 (13) | 0.75 (0.55 to 1.03) | 0.89 (0.66 to 1.21) |
| Cancer, n=234 | 38 (16) | 0.91 (0.68 to 1.22) | 1.01 (0.76 to 1.35) |
| HT + Dep, n=203 | 61 (30) | 1.69 (1.37 to 2.09) | 1.75 (1.41 to 2.17) |
| HT + Cancer, n=137 | 9 (7) | 0.37 (0.20 to 0.70) | 0.45 (0.24 to 0.84) |
| HT + HD + DM, n=117 | 23 (20) | 1.10 (0.76 to 1.59) | 1.30 (0.92 to 1.86) |
| Anemia, n=83 | 15 (18) | 1.01 (0.64 to 1.60) | 1.09 (0.69 to 1.71) |
| HT + Ren/Liv. n=79 | 9 (11) | 0.64 (0.34 to 1.18) | 0.70 (0.38 to 1.29) |
| Number of comorbidities | |||
| 1 comorbidity, n=9,441 | 1,575 (17) | 0.93 (0.87 to 0.98) | 1.01 (0.95 to 1.08) |
| 2 comorbidities, n=3,838 | 665 (17) | 0.96 (0.89 to 1.04) | 1.09 (1.01 to 1.18) |
| 3 comorbidities, n=1,173 | 174 (15) | 0.82 (0.71 to 0.95) | 0.94 (0.81 to 1.08) |
| ≥4 comorbidities, n=392 | 79 (20) | 1.12 (0.91 to 1.36) | 1.24 (1.02 to 1.52) |
n: Number, CI: Confidence interval. Comor: Comorbidities, HT: Hypertension, DM: Diabetes mellitus 1 and 2, Resp: Respiratory disease, HD: Heart disease, Neu: Neurologic disease, RA: Rheumatoid arthritis, Dep: Depression, Ren/Liv: Renal and/or liver disease.
Adjusted for age, sex, BMI, educational level, not born in Denmark, civil status, physical activity, problems in other joints, use of analgesics, and number of bodily pain areas. Estimates in bold reflect statistical significance (p < 0.05).
Discussion
We found that individuals with knee and hip OA and comorbidities had worse function, pain, and quality of life prior to participating in an eight-week exercise and education, as compared to individuals without comorbidities. However, they experienced comparable improvements in walking speed, pain intensity and health-related quality of life and adhered to the exercise therapy program largely to the same extent as those without comorbidities. Our findings indicate that presence of comorbidities is disassociated with clinically important differences in outcomes after exercise therapy and suggest that individuals with knee or hip OA may be offered supervised exercise therapy and patient education regardless of having comorbidities or not.
To our knowledge, this is the first study to investigate the association of a large number of possible comorbidities with health outcomes after an exercise and education program for individuals with knee and hip OA. Only Legha et al. has recently made the attempt, however only included six different conditions with a smaller sample size and without investigating the association of different combinations of comorbidities11. They found an association between obesity and anxiety/depression with patient-reported pain and function after exercise therapy at six months follow-up, but no associations between diabetes, cardiac or pulmonary conditions, or pain in other body parts and the outcomes. Our results are generally in line with their findings, although presence of depression were disassociated with outcomes in the present study and that obesity was not included as a comorbidity, but a possible confounder for the association of other conditions with outcomes. The contrasting results may partly be explained by the deviating methods used for determining depression in Legha et al. and the present study. Where participants in GLA:D® were asked specifically with a binary reply option (yes/no) if they had depression, presence of depression in the previous study was assessed using a single item from the EQ-5D questionnaire, which is intended for assessing severity of anxiety/depression symptoms and not for diagnosing depression. The confidence in our findings is strengthened by similar immediate and one-year results and by the objectively measured physical function, which supported the findings in the patient-reported outcomes (pain and quality of life) between individuals with and without comorbidities.
None of the found differences in change in any of the outcomes in the present study between those with and without comorbidities reached the levels of the pre-defined clinically important differences, which partly may be explained by the overall relatively small observed improvements leaving less room for difference in change between the comorbidity groups. However, it should be noted that what constitutes a clinically important difference may not be a fixed value24. In individuals with OA differences of 0.2 to 0.3 in 40m-FPWT have been reported as clinically important16 and even lower (0.05 to 0.19) in other populations25,26. The same for VAS where values ranging from 7 to 37 mm, depending on the baseline pain intensity (low to high, respectively), have been reported as clinically important19. Still, taking the different levels of clinically important differences into account does not alter the overall interpretation of important differences between patients with and without comorbidities being absent in the present study.
Previous studies have suggested anxiety and depression as barriers for engagement and adherence to exercise in individuals with OA27,28, which is supported by our main results, as the proportion of individuals not adhering to 10 or more exercise sessions was highest among individuals with depression (Table V). However, the estimates were inconsistent in the sensitivity analysis with diminished RRs and wide confidence intervals that include 1.00, precluding any clear interpretation. Since adherence is important for the outcome of exercise therapy29,30, one would assume that anxiety and depression would be associated with outcomes after exercise therapy, if it had an effect on adherence to exercise therapy. However, this was not demonstrated in our study, as individuals with OA and depression had similar improvements in all outcomes as individuals without depression. A few comorbidity groups did show a small, statistically significant association with higher or lower risk of not attending 10 or more exercise sessions. Given the large number of statistical tests in the present study, caution should be taken when interpreting the importance of these findings, especially as the observed findings may be difficult to explain clinically and would need further investigation.
In the present study, individuals with comorbidities had worse baseline scores in all outcomes, which is similar to findings from other studies5, and also similar to a recent study from the GLA:D® cohort including a range of other health status outcomes6. This was especially evident for physical function and quality of life where particularly those with two or more comorbidities had poorer health status at baseline than individuals without comorbidities. Although it is positive that comorbidities seem not to be associated with worse or better outcomes after exercise therapy and education that also means that the poorer health status at baseline is retained after such a treatment. To reduce the absolute difference in health status between those with and without comorbidities after treatment, it may therefore still be important to screen for and target certain comorbidity groups with further or more individualized treatment. For instance, a recent Dutch trial found that such an individualized, comorbidity-adapted exercise program, taking into account any comorbidity specific restrictions for exercise, was effective in improving physical function and safe in patients with knee OA and severe comorbidities31, which supports that exercise therapy may be a viable option for individuals with OA and comorbidities. In relation to this, it should be stressed that the exercise sessions in GLA:D® mainly comprise neuromuscular exercises13, thus it is unknown if our results may differ if more focus on other exercise types, e.g. exercises focusing on aerobic capacity in individuals with OA and comorbidities such as diabetes and cardiovascular diseases32. In addition, our findings may only apply to individuals with OA and comorbidities in a less severe state, due to restrictions and contraindications for exercise therapy if more severe comorbidities (e.g. instable angina)33, and the lack of exercise adaptations and inclusion of those individuals in GLA:D®.
This study has some limitations. First, due to the observational single-arm design we are unable to assess if presence of comorbidities has an impact on the effect of exercise therapy (is a moderator) or simply is a prognostic factor regardless of treatment34. Second, the accuracy of self-reported comorbidities can be debated and has likely resulted in some misclassification. Also, we lack knowledge of the severity of reported comorbidities and were unable to distinguish between what specific conditions the comorbidity categories mainly comprised (e.g. which type of heart disease), however, some of the comorbidity questions included examples or explanations of specific conditions, which possibly have resulted in more uniform replies from participants. As there is no reason to suspect any misclassification to be associated with the outcomes, we believe that misclassifications are most likely to be non-differential. Second, the proportion of missing data for all outcomes was relatively large. However, as missingness and outcome scores were statistically significantly associated with baseline characteristics (data not shown), we assumed data to be missing at random (MAR)20 and imputed missing data using multiple imputation, which largely showed similar results as complete case analyses. Fourth, the analyses were based on clinical registry data reflecting wider variations in treatment protocols and collection of data than in clinical trials. However, the large sample of individuals with knee or hip OA from a nationwide clinical registry supports the generalizability of the findings to clinical practice. Still, selection bias cannot be ruled out as participants in GLA:D® is a selected group seeking physiotherapy for their OA symptoms where those with severe comorbidities may be detained by physicians from exercise therapy due to restrictions or contraindications for exercise therapy33, or referred to more suitable therapies, e.g. cardiac or pulmonary rehabilitation programs. This may partly be reflected by fewer individuals reporting having comorbidities and a much lower prevalence of comorbidities such as pulmonary disease and depression than previously reported from primary care in the United Kingdom2,11. Some of these discrepancies might be a consequence of different groupings and definitions of conditions but suggest that participants in GLA:D® overall are healthier than individuals with OA in general, which might explain the absence of associations between comorbidities and outcomes after exercise therapy in the present study.
In conclusion, we found that individuals with knee or hip OA having one or more comorbidities had worse baseline physical function, pain intensity and quality of life, but improved similarly in the same outcomes after an 8-week exercise therapy and patient education program as compared to individuals without comorbidities, indicating that presence of comorbidities is disassociated with clinically important differences in outcomes after exercise and education. This suggests that a combined exercise therapy and patient education program may be recommended as treatment to individuals with knee or hip OA irrespective of whether they have comorbidities or not.
Supplementary Material
Acknowledgements
The authors would like to thank the clinicians and participants involved in collecting data for GLA:D®. Furthermore, we would like to thank the MOBILIZE scientific advisory board consisting of Prof. Sallie Lamb, Prof. Alan Silman, Prof. Bente Klarlund Pedersen, Prof. Ewa M. Roos and Prof. Rod Taylor and the patient partners Gregers Aagaard, Margit Dybkjær and Tue Dybkjær.
Funding sources
The initiation phase of GLA:D® was partly funded by the Danish Physiotherapy Association’s fund for research, education and practice development; the Danish Rheumatism Association; and the Physiotherapy Practice Foundation.
The present study was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (MOBILIZE; grant agreement No 801790), Naestved, Slagelse and Ringsted Hospitals’ Research Fund and the Danish Physiotherapy Association’s fund for research, education and practice development.
Finally, STS is the recipient of an ongoing grant from Region Zealand (Exercise First, unrelated to the current study).
The funders did not have any role in the study other than to provide funding.
Footnotes
Author contributions
Conception and design: KP, EMR, RST, DTG and STS.
Acquisition of data: STS and EMR.
Analysis and interpretation of data: KP, EMR, RST, DTG and STS.
Drafting manuscript: KP and STS.
Revising manuscript and approving final version of manuscript: All authors.
Obtaining of funding: STS.
All authors take responsibility for the integrity of the data analyses.
Conflict of interests
EMR is deputy editor of Osteoarthritis and Cartilage, the developer of Knee Injury and Osteoarthritis Outcome Score (KOOS) and several other freely available patient-reported outcome measures and co-founder of Good Life with osteoArthritis in Denmark (GLA:D®), a non-for-profit initiative hosted at University of Southern Denmark aimed at implementing clinical guidelines for osteoarthritis in clinical practice.
STS is associate editor of the Journal of Orthopaedic & Sports Physical Therapy and has received a grant from The Lundbeck Foundation and personal fees from Munksgaard, all of which are outside the submitted work. Furthermore, he is co-founder of GLA:D®. The authors report no other conflicts of interests.
Contributor Information
Ewa M Roos, Email: eroos@health.sdu.dk.
Rod S Taylor, Email: rod.taylor@glasgow.ac.uk.
Dorte T Grønne, Email: dgronne@health.sdu.dk.
Søren T Skou, Email: stskou@health.sdu.dk.
References
- 1.Wesseling J, Welsing PM, Bierma-Zeinstra SM, Dekker J, Gorter KJ, Kloppenburg M, et al. Impact of self-reported comorbidity on physical and mental health status in early symptomatic osteoarthritis: the CHECK (Cohort Hip and Cohort Knee) study. Rheumatology (Oxford) 2013;52:180–188. doi: 10.1093/rheumatology/kes288. [DOI] [PubMed] [Google Scholar]
- 2.Kadam UT, Jordan K, Croft PR. Clinical comorbidity in patients with osteoarthritis: a case-control study of general practice consulters in England and Wales. Ann Rheum Dis. 2004;63:408–414. doi: 10.1136/ard.2003.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijk GM, Veenhof C, Schellevis F, Hulsmans H, Bakker JP, Arwert H, et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2008;9:95. doi: 10.1186/1471-2474-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer I, Kaduszkiewicz H, Wagner HO, Schon G, Scherer M, van den Bussche H. Reducing complexity: a visualisation of multimorbidity by combining disease clusters and triads. BMC Public Health. 2014;14:1285. doi: 10.1186/1471-2458-14-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calders P, Van Ginckel A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: A systematic review and meta-analysis. Semin Arthritis Rheum. 2018;47:805–813. doi: 10.1016/j.semarthrit.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Muckelt PE, Roos E, Stokes M, McDonough S, Grønne D, Ewings S, et al. Comorbidities and their link with individual health status: A cross-sectional analysis of 23,892 people with knee and hip osteoarthritis from primary care. Journal of Comorbidity. 2020;10 doi: 10.1177/2235042X20920456. 2235042X20920456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, Conaghan PG, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 8.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014:Cd007912. doi: 10.1002/14651858.CD007912.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49:1554–1557. doi: 10.1136/bjsports-2015-095424. [DOI] [PubMed] [Google Scholar]
- 11.Legha A, Burke DL, Foster NE, van der Windt DA, Quicke JG, Healey EL, et al. Do comorbidities predict pain and function in knee osteoarthritis following an exercise intervention, and do they moderate the effect of exercise? Analyses of data from three randomized controlled trials. Musculoskeletal Care. 2020;18:3–11. doi: 10.1002/msc.1425. [DOI] [PubMed] [Google Scholar]
- 12.Skou ST, Roos EM. Good Life with osteoArthritis in Denmark (GLA:D): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord. 2017;18:72. doi: 10.1186/s12891-017-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord. 2010;11:126. doi: 10.1186/1471-2474-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042–1052. doi: 10.1016/j.joca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319–327. doi: 10.2519/jospt.2011.3515. [DOI] [PubMed] [Google Scholar]
- 17.Bilbao A, Garcia-Perez L, Arenaza JC, Garcia I, Ariza-Cardiel G, Trujillo-Martin E, et al. Psychometric properties of the EQ-5D-5L in patients with hip or knee osteoarthritis: reliability, validity and responsiveness. Qual Life Res. 2018;27:2897–2908. doi: 10.1007/s11136-018-1929-x. [DOI] [PubMed] [Google Scholar]
- 18.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 19.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little RJA, Rubin DB. Statistical analysis with missing data. 2 Edition. Hoboken, N.J; Wiley: 2002. [Google Scholar]
- 21.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple imputation for nonresponse in surveys. New York: J. Wiley; 1987. [Google Scholar]
- 23.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata Journal. 2013;13:492–509. [Google Scholar]
- 24.King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11:171–184. doi: 10.1586/erp.11.9. [DOI] [PubMed] [Google Scholar]
- 25.Bennell K, Dobson F, Hinman R. Measures of physical performance assessments: Self-Paced Walk Test (SPWT), Stair Climb Test (SCT), Six-Minute Walk Test (6MWT), Chair Stand Test (CST), Timed Up & Go (TUG), Sock Test, Lift and Carry Test (LCT), and Car Task. Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S350–370. doi: 10.1002/acr.20538. [DOI] [PubMed] [Google Scholar]
- 26.Chui K, Hood E, Klima D. Meaningful Change in Walking Speed. Topics in Geriatric Rehabilitation. 2012;28:97–103. [Google Scholar]
- 27.Dobson F, Bennell KL, French SD, Nicolson PJ, Klaasman RN, Holden MA, et al. Barriers and Facilitators to Exercise Participation in People with Hip and/or Knee Osteoarthritis: Synthesis of the Literature Using Behavior Change Theory. Am J Phys Med Rehabil. 2016;95:372–389. doi: 10.1097/PHM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 28.Marks R. Knee osteoarthritis and exercise adherence: a review. Curr Aging Sci. 2012;5:72–83. doi: 10.2174/1874609811205010072. [DOI] [PubMed] [Google Scholar]
- 29.Pisters MF, Veenhof C, Schellevis FG, Twisk JW, Dekker J, De Bakker DH. Exercise adherence improving long-term patient outcome in patients with osteoarthritis of the hip and/or knee. Arthritis Care Res (Hoboken) 2010;62:1087–1094. doi: 10.1002/acr.20182. [DOI] [PubMed] [Google Scholar]
- 30.van Gool CH, Penninx BW, Kempen GI, Rejeski WJ, Miller GD, van Eijk JT, et al. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthritis Rheum. 2005;53:24–32. doi: 10.1002/art.20902. [DOI] [PubMed] [Google Scholar]
- 31.de Rooij M, van der Leeden M, Cheung J, van der Esch M, Hakkinen A, Haverkamp D, et al. Efficacy of Tailored Exercise Therapy on Physical Functioning in Patients With Knee Osteoarthritis and Comorbidity: A Randomized Controlled Trial. Arthritis Care Res (Hoboken) 2017;69:807–816. doi: 10.1002/acr.23013. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 33.de Rooij M, Steultjens MPM, Avezaat E, Häkkinen A, Klaver R, van der Leeden M, et al. Restrictions and contraindications for exercise therapy in patients with hip and knee osteoarthritis and comorbidity. Physical Therapy Reviews. 2013;18:101–111. [Google Scholar]
- 34.Hingorani AD, Windt DA, Riley RD, Abrams K, Moons KG, Steyerberg EW, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ. 2013;346:e5793. doi: 10.1136/bmj.e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.