Abstract
Procollagen type I N-propeptide (PINP) and the C-terminal telopeptide of type I collagen (β-CTX) in blood have been designated as reference bone turnover markers in osteoporosis by the International Osteoporosis Foundation (IOF) and International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). The IFCC Committee on Bone Metabolism (C-BM) has examined current commercial assays and performed a multicentre study to examine the agreement between assays for PINP and β-CTX in serum and plasma. The results of these studies will inform our work towards the harmonization of PINP assays and the standardization of β-CTX assays in blood, with the development of common calibrators and reference measurement procedures in collaboration with the reagent manufacturing industry. Successful achievement of these goals will help develop universally acceptable practice guidelines for the management of osteoporosis with the inclusion of common reference intervals and treatment targets for PINP and β-CTX.
Keywords: Procollagen type I N-propeptide, PINP, C-terminal telopeptide of type I collagen, β-CTX, bone turnover markers, bone formation, bone resorption, standardization, harmonization
Introduction
Osteoporosis is a disease characterized by low bone mass and micro-architectural deterioration of bone tissue, leading to an increased risk of fracture with associated morbidity and mortality [1]. It constitutes a major global public health burden with increasing prevalence and morbidity worldwide [2]. Methods for the identification of at-risk patients and the diagnosis of osteoporosis are widely recognized, and effective treatments are available to reduce the risk of fractures [3–5]. Bone turnover markers in blood and urine are useful tools in monitoring treatment effects and may be useful for improving treatment adherence, and therefore, outcomes [6–8]. The IOF-IFCC Joint Working Group on Bone Marker Standards (WG-BMS) recommended PINP and β-CTX be used as blood reference markers for bone formation and bone resorption, respectively, in osteoporosis [9]. However, it was also recognized that due to inter-method variation, the standardization or harmonization of commercial assays would need to be achieved in order for these assays to be widely used and interchangeably employed in clinical practice as well as in research studies [9]. This narrative review describes the current status of assays for PINP and β-CTX in blood, as well as the plans for and progress towards the achievement of harmonization or standardization of commercial assays for PINP and β-CTX undertaken by IFCC C-BM.
Structures of PINP and β-CTX molecules
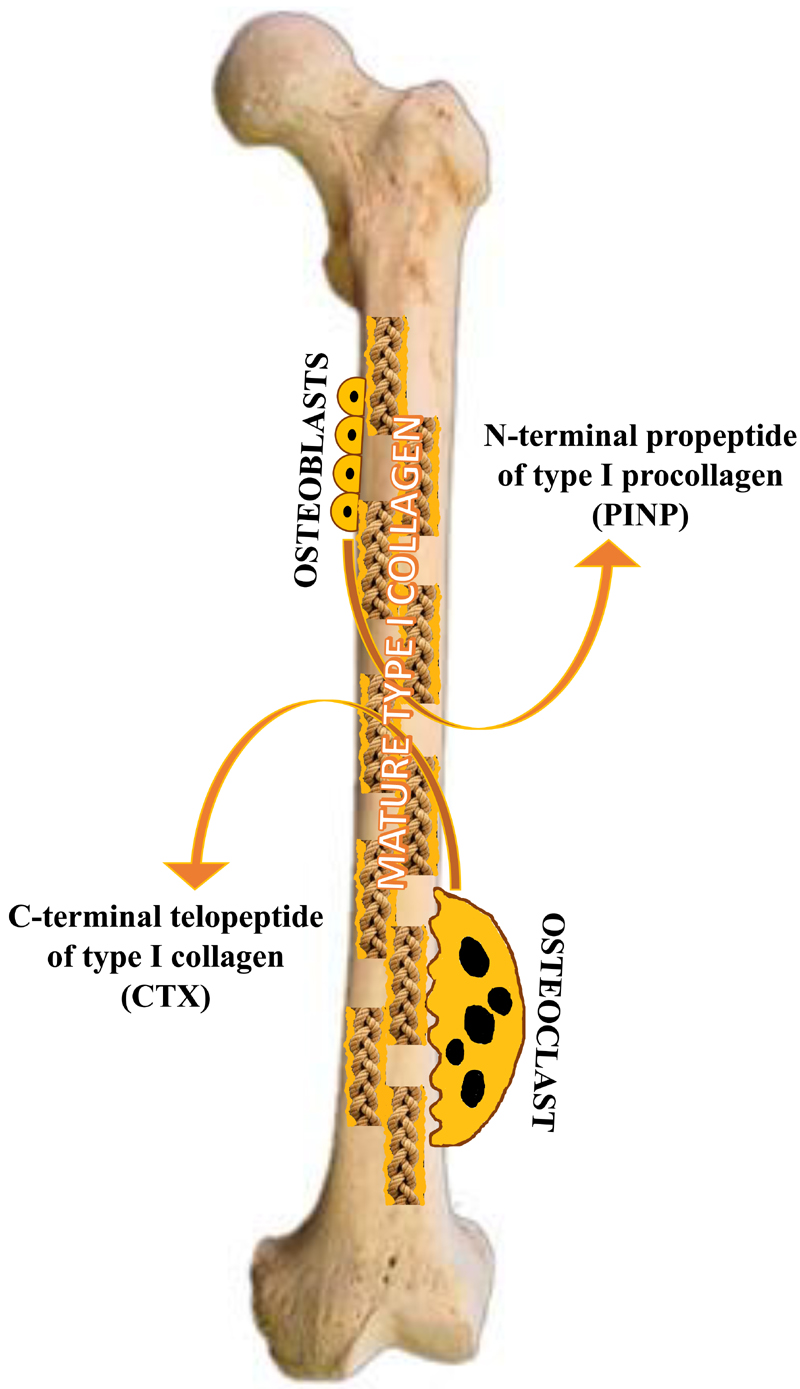
The organic compartment of the bone is constituted mainly by osteoblast-produced collagen, the main structural protein being type I collagen. Collagens are characterized by repeating sequences of glycine-proline-hydroxyproline in domains of triple-helical conformation, with type I collagen having two α1 and one α2 chains. Intracellularly, the translated polypeptide, i.e., the pre-pro-α-chain contains a signal sequence and amino (N)- and carboxyl (C)-terminal propeptide extensions [10]. Once secreted, the N- and C-terminal propeptides are cleaved by specific peptidases, and these enter the circulation [11].
During bone resorption, osteoclasts resorb bone by the secretion of acid and proteases and digest mature type I collagen into fragments which may contain pyridinium cross-links or the N- and C-telopeptide domains [12]. These fragments enter the circulation and are excreted in urine (Figure 1).
Figure 1.
Schematic diagram illustrating the secretion of procollagen type I N-propeptide (PINP) and the C-terminal telopeptide of type I collagen (CTX) into the circulation.
The two bone markers designated as reference markers in osteoporosis as mentioned above are the N-terminal propeptide (PINP), which is cleaved and released during bone formation, and the C-telopeptide (β-CTX), which is released during bone resorption [9].
Current commercial assays and their descriptions and performance characteristics
PINP assays
PINP was first isolated from amniotic fluid and was referred to as fetal antigen 2, prior to its identification as a homomer of α1 chains of PINP [13]. Polyclonal antibodies formed the basis for the first PINP enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA) [14–17]. The anti-PINP antibodies recognize two molecular forms of PINP in this ELISA, but only the high molecular weight form is recognized by the 125I-labelled anti-PINP antibodies in this RIA [18]. Pertaining to assay technology, it has since been recognized that the thermal stability of soluble collagen is low, and the high molecular weight form of PINP are the intact α1 chains in a trimeric moiety and the low molecular weight form the monomer [19]. Assays that measure the trimeric form are named intact PINP assays and those that measure trimeric and monomeric forms are termed total PINP assays.
Presently there are four commercially available assays for the measurement of serum PINP. Table 1 summarizes the key characteristics of the four commercially available assays for serum PINP and table 2 shows some analytical performance data of these methods.
Table 1.
Key characteristics of four commercially available assays for serum N-terminal propeptide of type I collagen (PINP).
| Manufacturer | Methodology | Measurand | Analytics |
|---|---|---|---|
| Orion Diagnostica, Finland | Radioimmunoassay | Intact PINP | Manual |
| Immunodiagnostics Systems (IDS), UK | Chemiluminescence immunoassay | Intact PINP | Automated |
| Roche Diagnostics, Germany | Electrochemiluminescence immunoassay | Total PINP | Automated |
| Uscn, Life Science, China | Enzyme-linked immunosorbent assay | Total PINP | Manual |
Table 2.
Performance specifications of the four commercially available assays for serum N-terminal propeptide of type I collagen (PINP) [15, 20–22].
| PINP assay | Measuring range | Lower limit of detection | Intra-assay CV | Inter-assay CV |
|---|---|---|---|---|
| Orion Diagnostica, Finland | 5 – 250 μg/L | 2.3 μg/L | 2.3 – 3.5% | 2.7 – 6.1% |
| iSYS (IDS), UK | 2 – 230 μg/L | 2.0 μg/L | 2.6 – 3.0% | 4.2 – 5.3% |
| Roche Diagnostics, Germany | 5 – 1200 μg/L | 5.0 μg/L | 1.4 – 2.3% | 2.1 – 4.5%. |
| Uscn, Life Science, China | 0.78 – 6.2 μg/L | 0.041 μg/L | 2.9 – 4.9% | 4.6 – 5.3% |
In 1995, Orion Diagnostica developed a manual radioimmunoassay (RIA) for the intact N-terminal propeptide of type I procollagen and it was validated by Tahtela et al. in 1997 [20]. The Orion Diagnostica UniQ™ PINP RIA (Espoo, Finland) was approved by the US Food and Drug Administration (FDA) in 2005. A manual total PINP ELISA assay was developed by Uscn Life Science Inc., China in 1996 and subsequently validated by Orum et al [15]. The measuring range, and hence the values, reported by the latter assay are an order of magnitude lower than other commercial assays. There are no published comparison studies for this assay with other commercial assays. To our knowledge, this assay has not been used in any publications of clinical trials
The automated intact PINP chemiluminescence immunoassay (CLIA) assay was launched in 2009 by IDS for the iSYS instrument (IDS, Boldon, UK) and validated in 2010 by Koivula et al. [21]. The automated total PINP electrochemiluminescence immunoassay (ECLIA) was developed by Roche Diagnostics, Mannheim, Germany and validated by Garnero et al. in 2008 [22].
β-CTX assays
All β-CTX assay methods employ the CrossLaps antibodies (Osteometer BioTech A/S, Denmark), which recognize an 8 glu-lys-ala-his-asp-gly-gly-arg amino acid sequence (EKAHD-β-GGR) in the C-terminal telopeptide region of the α1 chain [23,24]. Among other reasons, including a large diurnal variation, a need for urine volume correction by creatinine, an inability for quantification in very dilute urine samples, the cumbersome nature of 24-hour urine collection, and other preanalytical factors the use and development of assays for β-CTX in blood have been favored [9]. EDTA plasma is the preferred matrix for β-CTX measurement in blood due to better stability [25]. A fasted sample is necessary and early morning sampling is preferred to minimize the effects of circadian variation [25]
The first β-CTX assay in blood was a competitive polyclonal antibody ELISA [26]. Subsequently, a one-step ELISA using two monoclonal antibodies was developed [27] and then recalibrated using a synthetic cross-linked polypeptide containing two copies of the EKAHD-β-GGR sequence [28]. The manual ELISA assay has been available commercially as CrossLaps® [29]. The CrossLaps® ELISA is FDA approved and is currently marketed by IDS. Both IDS (UK) and Roche Diagnostics, (Germany) have adapted the one-step ELISA to develop automated assays for β-CTX. Table 3 summarizes the key characteristics of the commercially available assays for CTX in blood.
Table 3.
Key characteristics of commercially available assays for C-terminal telopeptide of type I collagen (β-CTX) in blood.
| Manufacturer | Methodology | Measurand | Analytics |
|---|---|---|---|
| IDS, UK. | Chemiluminescence immunoassay | β-CTX | Automated |
| Roche Diagnostics, Germany | Electrochemiluminescence immunoassay | β-CTX | Automated |
| IDS, UK | Enzyme-linked immunosorbent assay | β-CTX | Manual |
The Roche ECLIA assay was evaluated by Garnero et al. in 2001 [30] and the CLIA serum β-CTX assay on the IDS-iSYS analyser was assessed by Seres et al. in 2010 [31]. Performance specifications of the three commercial assays are detailed in Table 4.
Table 4.
Performance specifications of the two automated assays for serum C-terminal telopeptide of type I collagen (CTX) [references 27–29].
| β-CTX assay | Measuring range | Lower limit of detection | Intra-assay CV | Inter-assay CV |
|---|---|---|---|---|
| iSYS, IDS, UK | 50-6000 ng/L | 20 ng/L | 2.7-3.7% | 2.5-5.2% |
| Roche Diagnostics, Germany | 10-6000 ng/L | 10 ng/L | 1.2-4.1% | <5.7% |
| CrossLaps®, IDS, UK | 20-3380 ng/mL | 20 ng/L | <2.5% | 2.2-5.5% |
Comparison studies of current commercial assays for PINP and β-CTX in blood
PINP
IFCC C-BM conducted a multi-centre study of β-CTX and PINP in serum and EDTA plasma with the participation of four laboratories in Europe. The results for PINP have been published elsewhere [32] and the results for β-CTX are currently being analyzed for publication. Herein, we have summarized the published results. PINP was measured by three commercial assays in both serum and EDTA plasma samples taken from 796 osteoporosis clinic patients with eGFR > 30 ml/min/1,73 m2). Each assay gave similar results for serum and EDTA plasma, leading us to conclude that both matrices were acceptable and the results from either matrix could be used interchangeably for each assay [32].
The automated methods for PINP (Roche Cobas and IDS iSYS) gave similar results (see Table 5) [32]. The Bland-Altman plots and the V-shaped models showed that 87.4% and 86.1% of the samples were within the V-shape limits for serum and plasma, respectively. The median difference observed between the two methods was 0.25 μg/L, which was not significant [32]. In contrast, a significant proportional bias was observed between the Orion RIA and the two automated assays [32]. The Passing-Bablok regression for Roche Elecsys against the Orion RIA was Elecsys = 1.22 × Orion-0.0 in serum and Elecsys = 1.24 × Orion RIA-0.2 in plasma. The Bland-Altman plots showed a significant proportional bias and the V-shaped model showed 61.1% and 60.3% of serum and plasma samples, respectively, were within limits. The Passing-Bablok regression for the IDS iSYS method against the Orion RIA was iSYS=1.35 x Orion RIA–3.2 in serum and iSYS = 1.45 × Orion RIA-3.7 in plasma. The Bland-Altman plots showed a significant proportional bias, and the V-shaped model showed 57.4% of serum samples and 49.1% of plasma samples to be within limits. Results from other studies of the three commercially available assays broadly reflect our findings [33,34].
Table 5.
The Passing-Bablok regression equations for the Roche assay and IDS iSYS assay for PINP (references 32, 34–37)
| Subjects | Regression equation | Slope 95% CI | Intercept 95% CI |
|---|---|---|---|
| Osteoporosis patients32 | Cobas = 0.91 iSYS + 2.6 | 0.90, 0.92 | 2.2, 3.1 |
| Healthy blood donors34 | Elecsys = 0.94 iSYS – 3.6 | 0.80, 1.15 | – 18.4, 3.6 |
| Healthy subjects, patients with osteoporosis35 | iSYS = 1.05 Cobas – 1.4 | 1.04, 1.06 | – 1.9, – 0.8 |
| Healthy volunteers, patients with rheumatoid arthritis37 | iSYS = 0.98 Elecsys – 1.42 | 0.94, 1.03 | – 2.86, 0.08 |
| Haemodialysis patients34 | Elecsys = 5.74 iSYS – 95.6 | 4.56, 8.57 | – 240.9, – 31.9 |
| Patients with CKD36 | iSYS = 0.74 Cobas + 3.7 | 0.67, 0.81 | 1.2, 5.8 |
| Bedridden elderly patients34 | Elecsys = 1.57 iSYS – 12.0 | 1.43, 1.73 | – 19.0, – 5.7 |
Jørgensen et al. in a large study of 2308 individuals with eGFR > 30 mL/min/1.73m2 (1250 males and 1058 females, age range: 24–76 years) measured serum PINP using IDS-iSYS and Roche Cobas analyzers [33]. They found an R2 of 0.8573 (p < 0.001) for PINP measured by the two assays. The mean difference was -3 μg/L, which was statistically significant. The variability of the difference increased as the mean values increased. The reference intervals for PINP (calculated as a central 95%) did not differ greatly between the two assays for men or women [33].
Koivula et al. examined the relationships between the Roche Elecsys PINP assay and the Orion RIA in the serum of 34 apparently healthy blood donors, 39 patients with chronic renal failure, and 173 bedridden elderly in-patients > 65 years [34]. The PINP results by the two assays were similar for the healthy blood donors, but the Elecsys assay gave significantly higher results in haemodialysis patients and bedridden elderly patients [34]. The Passing-Bablok regression equations are shown in Table 5.
Morovat et al. compared serum PINP results by Roche Elecsys and IDS iSYS in 828 healthy subjects, including children and osteoporotic patients [35]. The relationship between the two assays was non-linear. In addition, the iSYS results were significantly higher than those obtained by Elecsys, except at low and high PINP concentrations of < 100 μg/L and > 670 μg/L, respectively, where iSYS gave lower values than Elecsys. Cavalier et al. compared serum PINP by Roche Elecsys and IDS iSYS in two CKD populations: 157 patients in stage 3–5 CKD and 125 patients in stage 5D [36]. The authors found that the two assays produced the most discrepant results when eGFR was < 30 mL/min/1.73 m2. However, some discrepancy was apparent even for eGFR values between 30 and 60 mL/min/1.73 m2, which raises the question whether the two assays can be harmonized only in subjects with eGFR >60 mL/min/1.73 m2.
Wheater et al. examined serum PINP results from Roche Elecsys and IDS iSYS in 127 subjects with eGFR > 30 mL/min, 72 self-reported healthy volunteers (56 < 50 years and 16 > 50 years) with no known bone disease, and 55 rheumatoid arthritis (RA) patients (5 < 50 years and 50 > 50 years) [37]. The median difference for PINP values between the two systems was 2.0 μg/L (95% CI 1.3, 2.8 μg/L), which was statistically significant [37].
Overall, these results suggest that the two automated PINP assays (Roche Diagnostics and IDS iSYS) provide values that were very similar in healthy subjects with eGFR >30 mL/min/1.73 m2, but that a significant proportional bias (albeit with good correlation) exists between the Orion RIA and the two automated assays. Further work to standardize or harmonize the three commercial assays for PINP is required. Since the molecular structures of the PINP molecule and the different peptides/fragments recognized by the intact and total PINP assays have not been clearly ascertained, the synthesis of a reference standard and the development of a reference measurement procedure required for the standardization of these assays will be problematic. Therefore, the IFCC/IOF Joint Committee on Bone Metabolism is of the view that the harmonization of PINP assays is a more realistic goal [38]. Universal harmonization of PINP assays needs to be achieved in order for international multicentre trials to be conducted using any commercially available assays, and will be undertaken in collaboration with the commercial reagent manufacturers. This is also a requirement for the development of clinical guidelines with uniform reference intervals and treatment targets. Given the results of the studies summarized above, the harmonization (if not styandardization) of commercially available assays should be possible by the use of common calibrators and the development of a reference method for PINP for subjects with a GFR > 60mL/min/1.73m2 [38]. The drawback of this approach it that only international multicenter studies with a reasonable kidney function can benefit. Any new commercial assay developed in the future should be traceable to the new developed calibrators and reference method.
β-CTX
As stated above, the results for β-CTX from the IFCC C-BM multi-centre study of four laboratories are yet to be published. Previous studies have examined the relationship between commercial assays for β-CTX in blood.
Jørgensen et al. in their study of 2308 individuals with eGFR > 30 mL/min/1.73m2 (1250 males and 1058 females, age range: 24–76 years) measured serum β-CTX using IDS-iSYS and Roche Cobas analyzers. They found an R2 of 0.8924 (p < .001) for β-measured by the two assays [33]. The mean difference was 13 ng/L, which was statistically significant. In addition, they found a complex systematic bias between the methods with Cobas giving a higher value at low concentrations and iSYS giving a higher value at higher concentrations. The upper reference limit for serum β-CTX (calculated from the central 95%) measured with the iSYS assay was higher than that by the Cobas assay in both men and women. The authors concluded that the complex correlation between the two methods meant that no simple conversion factor could be applied between the methods, thus making the potential for harmonization more complicated [33].
Chubb et al. examined β-CTX measured in EDTA plasma from 161 patients [119 females and 42 males, median age: 65 (inter-quartile range 57–76) years] with metabolic bone disease by the two automated assays (Roche Elecsys and IDS iSYS) as well as the manual ELISA assay marketed by IDS [39]. They found significant proportional and systematic biases when the iSYS assay was compared to both the ELISA and Elecsys methods. They also confirmed the findings of Jørgensen et al. that the iSYS assay gave lower β-CTX values in the lower range and higher values in the higher range compared to the Elecsys assay [39].
Wheater et al. in their study of 127 subjects also found a significant negative systematic bias and positive proportional bias when results from the iSYS were compared to the Elecsys assay [37]. The median difference for the β-CTX values between the two systems was -30 ng/L (95% CI -45, -21 ng/L), which was statistically significant. However, this single estimate of bias was inadequate to describe the variation in bias across the full range due to the complex relationship between the values given by the two assays [37].
In summary, the results of the above studies show poor agreement in results given by the current commercial assays for β-CTX in blood. Since the structure of the β-CTX molecule (EKAHD-β-GGR), the measurand, is well characterised, the synthesis of a reference standard preparation is possible. The development of a reference measurement procedure would allow for the standardization of commercial assays for β-CTX in blood. It is the goal of the IFCC/IOF Joint Committee on Bone Metabolism to achieve standardization of all commercial assays for β-CTX in blood.
Next steps
IFCC C-BM in collaboration with commercial reagent manufacturers plan to prepare commutable international reference materials and develop common measurement procedures for PINP and β-CTX in blood. Once harmonization or standardization, as appropriate, is achieved, regulatory authorization of these modified assays will be sought. At a clinical level, common reference intervals and universally-acceptable decision limits and treatment targets for β-CTX and PINP will be developed in collaboration with IOF. Further studies are also needed to determine fracture prediction strength of the reference bone turnover markers.
Table 6.
The Passing-Bablok regression equations for the Roche automated assay and IDS iSYS automated assay for β-CTX (references 37 and 39)
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service and the US Department of Health and Human Services.
Footnotes
Disclosures
EC is a consultant for Diasorin, IDS, Fujirebio, bioMerieux, Menarini and Nittobo. RE receives consultancy funding from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Lilly, Sandoz, Nittobo, Abbvie, Samsung, Haoma Medica, CL Bio, and Viking and grant funding from Nittobo, IDS, Roche, Amgen and Alexion. NRJ has received free reagents for research use from Roche Diagnostics and IDS. JAK reports grant support from Amgen, Lilly and Radius Health. CC has received lecture fees and honoraria from Amgen, Danone, Eli Lilly, GSK, Kyowa Kirin, Medtronic, Merck, Nestlé, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB outside of the submitted work. SLS has received grant support from Roche, consulting funding from Amgen, Pfizer, Lilly, Radius and is a speaker for Amgen and Radius.
References
- 1.Am J Med; Consensus Development Conference: Diagnosis, prophylaxis, and treatment of osteoporosis; 1993. pp. 646–650. [DOI] [PubMed] [Google Scholar]
- 2.Cheng SY, Levy AR, Lefaivre KA, Guy P, Kuramoto L, Sobolev B. Geographic trends in incidence of hip fractures: a comprehensive literature review. Osteoporos Int. 2011;22:2575–2586. doi: 10.1007/s00198-011-1596-z. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42:467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 4.McCloskey EV, Johansson H, Oden A, Kanis JA. From relative risk to absolute fracture risk calculation: the FRAX algorithm. Curr Osteoporos Rep. 2009;7:77–83. doi: 10.1007/s11914-009-0013-4. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. doi: 10.1016/S2213-8587(17)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NF. Diagnosis of endocrine disease: Bone turnover markers: are they clinically useful? Eur J Endocrinol. 2018;178:R19–R31. doi: 10.1530/EJE-17-0585. [DOI] [PubMed] [Google Scholar]
- 7.Diez-Perez A, Naylor K, Abrahamsen B, Agnusdei D, Brandi ML, Cooper c, Dennion E, Eriksen EF, Gold DT, Guañabens N, Hadji P, et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int. 2017;28:767–774. doi: 10.1007/s00198-017-3906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorentzon M, Branco J, Brandi ML, Bruyère O, Chapurlat R, Cooper C, Cortet B, Diez-Perez A, Ferrari S, Gasparik A, Herrmann M, et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv Ther. 2019;36:2811–2824. doi: 10.1007/s12325-019-01063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 10.Robey PG, Fisher LW, Young MF, Termine JD. The biochemistry of bone. In: Riggs BL, Melton LJ III, editors. Osteoporosis: Etiology, Diagnosis and Management. Raven Press; New York: 1988. pp. 95–109. [Google Scholar]
- 11.Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996;17:333–338. doi: 10.1210/edrv-17-4-333. [DOI] [PubMed] [Google Scholar]
- 12.Eyre DR. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 13.Teisner B, Rasmussen HB, Højrup P, Yde-Andersen E, Skjødt K. Fetal antigen 2: an amniotic protein identified as the aminopropeptide of the alpha 1 chain of human procollagen type I. APMIS. 1992;100:1106–1114. doi: 10.1111/j.1699-0463.1992.tb04047.x. [DOI] [PubMed] [Google Scholar]
- 14.Fay TN, Jacobs I, Teisner B, Poulsen O, Chapman MG, Stabile I, et al. Two fetal antigens (FA-1 and FA-2) and endometrial proteins (PP12 and PP14) isolated from amniotic fluid; preliminary observations in fetal and maternal tissues. Eur J Obstet Gynecol Reprod Biol. 1988;29:73–85. doi: 10.1016/0028-2243(88)90167-0. [DOI] [PubMed] [Google Scholar]
- 15.Orum O, Hansen M, Jensen CH, et al. Procollagen type I N-terminal propeptide (PINP) as an indicator of type I collagen metabolism: ELISA development, reference interval, and hypovitaminosis D induced hyperparathyroidism. Bone. 1996;19:157–163. doi: 10.1016/8756-3282(96)00165-2. [DOI] [PubMed] [Google Scholar]
- 16.Price KM, Silman R, Armstrong P, Grudzinskas JG. Development of a radioimmunoassay for fetal antigen 2. Clin Chim Acta. 1994;224:95–102. doi: 10.1016/0009-8981(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 17.Melkko J, Kauppila S, Niemi S, Risteli L, Haukipuro K, Jukkola A, et al. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clin Chem. 1996;42:947–954. [PubMed] [Google Scholar]
- 18.Jensen CH, Hansen M, Brandt J, Rasmussen HB, Jensen PB, Teisner B. Quantification of the N-terminal propeptide of human procollagen type I (PINP): comparison of ELISA and RIA with respect to different molecular forms. Clin Chim Acta. 1998;269:31–41. doi: 10.1016/s0009-8981(97)00182-4. [DOI] [PubMed] [Google Scholar]
- 19.Brandt J, Krogh TN, Jensen CH, Frederiksen JK, Teisner B. Thermal instability of the trimeric structure of the N-terminal propeptide of human procollagen type I in relation to assay technology. Clin Chem. 1999;45:47–53. [PubMed] [Google Scholar]
- 20.Tahtela R, Turpeinen M, Sorva R, Karonen SL. The aminoterminal propeptide of type I procollagen: evaluation of a commercial radioimmunoassay kit and values in healthy subjects. Clin Biochem. 1997;30:35–40. doi: 10.1016/s0009-9120(96)00134-8. [DOI] [PubMed] [Google Scholar]
- 21.Koivula MK, Richardson J, Leino A, Valleala H, Griffiths K, Barnes A, et al. Validation of an automated intact N-terminal propeptide of type I procollagen (PINP) assay. Clin Biochem. 2010;43:1453–1457. doi: 10.1016/j.clinbiochem.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Garnero P, Vergnaud P, Hoyle N. Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem. 2008;54:188–196. doi: 10.1373/clinchem.2007.094953. [DOI] [PubMed] [Google Scholar]
- 23.Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C. Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin Chem. 1994;40:2022–2225. [PubMed] [Google Scholar]
- 24.Rosenquist C, Fledelius C, Christgau S, Pedersen BJ, Bonde M, Qvist P, et al. Serum CrossLaps One Step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem. 1998;44:2281–2289. [PubMed] [Google Scholar]
- 25.Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET, National Bone Health Alliance Bone Turnover Marker Project Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28:2541–2556. doi: 10.1007/s00198-017-4082-4. [DOI] [PubMed] [Google Scholar]
- 26.Bonde M, Garnero P, Fledelius C, Qvist P, Delmas PD, Christiansen C. Measurement of bone degradation products in serum using antibodies reactive with an isomerized form of an 8 amino acid sequence of the C-telopeptide of type I collagen. J Bone Miner Res. 1997;12:1028–1034. doi: 10.1359/jbmr.1997.12.7.1028. [DOI] [PubMed] [Google Scholar]
- 27.Rosenquist C, Fledelius C, Christgau S, Pedersen BJ, Bonde M, Qvist P, et al. Serum CrossLaps One Step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem. 1998;44:2281–2289. [PubMed] [Google Scholar]
- 28.Crofton PM, Evans N, Taylor MR, Holland CV. Serum CrossLaps: pediatric reference intervals from birth to 19 years of age. Clin Chem. 2002;48:671–673. [PubMed] [Google Scholar]
- 29.Rogers A, Glover SJ, Eastell R. A randomised, double-blinded, placebo-controlled, trial to determine the individual response in bone turnover markers to lasofoxifene therapy. Bone. 2009;45:1044–52. doi: 10.1016/j.bone.2009.07.089. [DOI] [PubMed] [Google Scholar]
- 30.Garnero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. 2001;47:694–702. [PubMed] [Google Scholar]
- 31.Seres Z, Dixon C, Middlemist S, Wynn C, Laurie D, Fox C, Barnes A, Garrity M. A new β-crosslaps® (CTX-I) assay on the IDS-ISYS automated analyser. Bone. 2010;47:S143. [Google Scholar]
- 32.Cavalier E, Eastell R, Rye Jørgensen N, Makris K, Tournis S, Vasikaran S, et al. IFCC-IOF Joint Committee for Bone Metabolism (C-BM) A multicenter study to evaluate harmonization of assays for N-terminal propeptide of type I procollagen (PINP): a report from the IFCC-IOF Joint Committee for Bone Metabolism. Clin Chem Lab Med. 2019;57:1546–1555. doi: 10.1515/cclm-2019-0174. [DOI] [PubMed] [Google Scholar]
- 33.Jørgensen NR, Møllehave LT, Hansen YBL, Quardon N, Lylloff L, Linneberg A. Comparison of two automated assays of BTM (CTX and PINP) and reference intervals in a Danish population. Osteoporos Int. 2017;28:2103–2113. doi: 10.1007/s00198-017-4026-z. [DOI] [PubMed] [Google Scholar]
- 34.Koivula MK, Ruotsalainen V, Björkman M, Nurmenniemi S, Ikäheimo R, Savolainen, et al. Difference between total and intact assays for N-terminalpropeptide of type I procollagen reflects degradation of pN-collagen rather than denaturation of intact propeptide. Ann Clin Biochem. 2010;47:67–71. doi: 10.1258/acb.2009.009110. [DOI] [PubMed] [Google Scholar]
- 35.Morovat A, Catchpole A, Meurisse A, Carlisi A, Bekaert AC, Rousselle O, Paddon M, James T, Cavalier E. IDS iSYS automated intact procollagen-1-N-terminus propeptide assay: method evaluation and reference intervals in adults and children. Clin Chem Lab Med. 2013;51:2009–2018. doi: 10.1515/cclm-2012-0531. [DOI] [PubMed] [Google Scholar]
- 36.Cavalier E, Lukas P, Carlisi A, Gadisseur R, Delanaye P. Aminoterminal propeptide of type I procollagen (PINP) in chronic kidney disease patients: the assay matters. Clin Chim Acta. 2013;425:117–118. doi: 10.1016/j.cca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Wheater G, Goodrum C, Tuck SP, Datta HK, van Laar JM. Method-specific differences in beta-isomerised carboxy-terminal cross-linking telopeptide of type I collagen and procollagen type I amino-terminal propeptide using two fully automated immunoassays. Clin Chem Lab Med. 2014;52:e135–138. doi: 10.1515/cclm-2013-0934. [DOI] [PubMed] [Google Scholar]
- 38.Vasikaran SD, Bhattoa HP, Eastell R, Heijboer AC, Jørgensen NR, Makris K, Ulmer C, Kanis JA, Cooper C, Silverman S, Cavalier E. Harmonization of commercial assays for PINP; the way forward. Osteoporos Int. 2020;31:409–412. doi: 10.1007/s00198-020-05310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chubb SA, Mandelt CD, Vasikaran SD. Comparison of results from commercial assays for plasma CTX: The need for harmonization. Clin Biochem. 2015;48:519–524. doi: 10.1016/j.clinbiochem.2015.03.002. [DOI] [PubMed] [Google Scholar]