Abstract
Objective
Findings from animal studies indicate that the early gut bacteriome is a potential mechanism linking maternal prenatal stress with health trajectories in offspring. However, clinical studies are scarce and the associations of maternal psychological profiles with the early infant faecal bacteriome are unknown. This study aimed to investigate the associations of prenatal stressors and distress with early infant faecal bacterial profiles in a South African birth cohort study.
Methods
Associations between prenatal symptoms of depression, distress, intimate partner violence (IPV) and posttraumatic stress disorder (PTSD) and faecal bacterial profiles were evaluated in meconium and subsequent stool specimens from 84 mothers and 101 infants at birth, and longitudinally from a subset of 69 and 36 infants at 4–12 and 20–28 weeks of age, respectively, in a South African birth cohort study.
Results
Infants born to mothers that were exposed to high levels of IPV had significantly higher proportions of Citrobacter and three unclassified genera, all of which belonging to the family Enterobacteriaceae detected at birth. Proportions of these Enterobacteriaceae remained significantly increased over time (birth to 20–28 weeks of life) in infants born to mothers with high levels of IPV exposure compared to infants from mothers with no/low IPV exposure. Infants born to mothers exposed to IPV also had higher proportions of the genus Weissella at 4–12 weeks compared to infants from mothers with no/low IPV exposure. Faecal specimens from mothers exposed to IPV had higher proportions of the family Lactobacillaceae and lower proportions of Peptostreptococcaceae at birth. Maternal psychological distress was associated with decreased proportions of the family Veillonellaceae in infants at 20–28 weeks and a slower decline in Gammaproteobacteria over time. No changes in beta diversity were apparent for maternal or infant faecal bacterial profiles in relation to any of the prenatal measures for psychological adversities.
Conclusion
Maternal lifetime IPV and antenatal psychological distress are associated with altered bacterial profiles in infant and maternal faecal bacteria. These findings may provide insights in the involvement of the gut bacteria linking maternal psychological adversity and the maturing infant brain.
Keywords: microbiota, meconium, domestic violence, intimate partner violence, maternal antenatal stress
Introduction
The prevalence of maternal prenatal stressors and distress is particularly high in rural South Africa. Previous studies reported that 30–40% of pregnant mothers have depression (Rochat et al., 2006; Hartley et al., 2011; Vythilingum et al., 2012), 11–19% have posttraumatic stress disorder (PTSD) (Koen et al., 2016; van Heyningen et al., 2017) and 21–32% experienced intimate partner violence (IPV) within the past year (Koen et al., 2016; Bernstein et al., 2016). Psychological stressors and distress during pregnancy may have a range of negative consequences on the development and health of the foetus and infant, including premature delivery, impaired cognitive development, poor infant growth and dysregulated behaviour of infants (Stein et al., 2014). The biological mechanisms responsible for the intergenerational transmission of the effects of maternal psychological stress during pregnancy are, however, still unclear. Studies in mice (Gur et al., 2017) and monkeys (Bailey et al., 2004) have found that exposure to psychological stressors during pregnancy alters the profile of the maternal faecal microbiota, which is then transferred to the offspring. There are, however, few human studies. A Dutch human study (Zijlmans et al., 2015) found that a higher self-reported stress during pregnancy was associated with temporal differences in infants’ faecal bacterial profiles, starting 7 days after delivery. Furthermore, infant faecal bacterial profiles from mothers reporting higher stress levels were characterised by higher relative abundances of Proteobacteria (including the Enterobacteriaceae Escherichia, Serratia and Enterobacter) and a lower relative abundances of lactic acid bacteria and Bifidobacteria (Zijlmans et al., 2015). A recent study examined the associations of maternal prenatal anxiety, depression and stress during pregnancy with changes to the microbiome in meconium of 75 newborns (Hu et al., 2019). Higher measures of pregnancy-related anxiety were associated with lower abundances of an unidentified genus in the family Enterobacteriaceae (Hu et al., 2019). However, the association of other types of psychological stressors and distress during pregnancy with the temporal changes in the infant faecal microbiome has not yet been studied.
The acquired faecal microbiota may contribute to child neuro-development and health trajectories (Jašarević et al., 2015; O’Mahony et al., 2017; Sordillo et al., 2019). A delayed or altered establishment of a mature intestinal microbiota in childhood (termed microbiota immaturity) has been associated with various diseases, including diarrhoea, malnutrition, atopic conditions, inflammatory bowel disease, obesity, diabetes, neurological conditions and impaired neurodevelopment (Arrieta et al., 2014; Carlson et al., 2018). Studies in mice have demonstrated that the gut microbiome may function as mechanistic link between maternal prenatal stress and reduction in social behavioural and neurobiological changes, that is, neuroinflammation, in the offspring (Gur et al., 2017; Gur et al., 2019). The early establishment of the gut bacterial environment may have long-lasting effects on health and brain functioning. In this respect, a study in germ-free mice showed that colonisation of the gut directly after birth was essential for the development of the hypothalamic–pituitary–adrenocortical axis (Sudo et al., 2004). A study in humans also showed that changes in gut bacteria at 1 year of age were associated with with lower cognition, including visual reception and expressive language skills in infants at 2 years of age (Carlson et al., 2018). However, evidence for the association between maternal prenatal psychological adversities with changes to the infant gut microbiome in utero, that is, meconium, is still limited.
Aims of the study
The primary aim of this study was to determine the association between different profiles of maternal prenatal stressors and distress (including IPV, psychological distress, depression, and PTSD) experienced by South African mothers and the development of infants’ faecal microbiome early in life. The secondary aim included the investigation of the relationship of these prenatal psychological adversities with maternal faecal bacterial profiles at delivery.
Materials and methods
Study participants
Mother–infant dyads were evaluated in a subsample from the Drakenstein Child Health Study (DCHS). The DCHS is multidisciplinary birth cohort study investigating determinants of child health over time. Further details of participant recruitment, data collection and the setting of the DCHS have been provided previously (Zar et al., 2015; Stein et al., 2015). Pregnant women were recruited from two low socio-economic communities, TC Newman and Mbekweni in a peri-urban area outside Cape Town, South Africa. Enrolment of pregnant women took place at 20–28 weeks of gestation at routine antenatal visits to health care facilities. Mothers provided informed written consent for enrolment of their infants at the time of delivery and follow-up until 5 years of age. Inclusion criteria for this substudy were longitudinal collection of faecal specimens and availability of maternal prenatal psychological measurements. Exclusion criteria for this substudy were maternal age < 18 years, residence outside of the Drakenstein subdistrict and intention to move out of the region within 2 years of giving birth.
This study and parent study (DCHS) both received ethical approval from the Faculty of Health Sciences, Human Research Ethics Committee of the University of Cape Town (401/2009 and 742/2013, respectively) (Zar et al., 2015).
Measurements of maternal prenatal psychological stress and distress
Locally validated and reliable psychological measures of stress and distress were performed in pregnant mothers when enrolled at the antenatal clinic as previously described (Stein et al., 2015; Zar et al., 2015; Koen et al., 2016). The measures for maternal prenatal stressors and distress in this substudy were evaluated at a gestational age of 27.4 ± 4.2 weeks by field workers in the absence of the partner/husband. On-site female fieldworkers administered a battery of self-report measures described below. Female field-workers were selected based on criteria previously found to affect women’s willingness to divulge intimate information, including measures of IPV exposure (Jansen et al., 2004). All female fieldworkers had at least a Grade 12 certificate and had prior experience in psychiatric/psychological research. They were fluent both in English and Afrikaans or isiXhosa and were therefore able to administer questionnaires in the participants’ preferred language. Further, fieldworkers received extensive in-service training on all aspects of Good Clinical Practice (WHO, 2002).
The self-reporting questionnaire 20-item (SRQ-20) (Harding et al., 1980), a WHO-endorsed measure of symptoms of psychological distress, with good face validity and reliability, has been used widely in international studies and in South African settings (Mari & Williams, 1986; Rumble et al., 1996). Symptoms of depression were measured by the Beck Depression Inventory-II (BDI-II), a widely used and reliable measure of depressive symptoms (Beck et al., 1988; Beck et al., 1996). The modified PTSD Symptom Scale (MPSS) is a 17-item self-report scale with excellent internal consistency, high test–retest reliability and concurrent validity to diagnose PTSD consistent with Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria as defined by DSM-IV (Falsetti et al., 1993; Foa & Tolin, 2000). The validity of the MPSS was established in a South African student sample with a Cronbach’s alpha of 0.92, in relation to a diagnosis by psychiatrists based on clinical interviews for PTSD, for which kappa was found to be 0.68 (McGowan & Kagee, 2013).The IPV questionnaire used in this study was adapted from the WHO multi-country study (Jewkes, 2002) and the Women’s Health Study in Zimbabwe (Shamu et al., 2011). The IPV questionnaire assesses lifetime and recent (past year) exposure to emotional, physical and sexual IPV. Each category of violence was assessed across multiple items measuring the frequency of a number of specified violent acts. Each item is scored using a frequency scale from 1 (‘never’) to 4 (‘many times’). Bivariable analyses were performed on scoring guidelines based on prior work in South Africa (Dunkle et al., 2004). Psychological assessments of IPV were previously validated and optimised in local studies of maternal mental health for their use in the South African setting (Stein et al., 2009).
Faecal bacteria analyses
Details of the study methods related to the analyses of faecal bacteria have previously been reported (Claassen-Weitz et al., 2018). In short, faecal specimens were collected using sterile spatulas and faecal screw cap containers. Study staff collected faecal specimens from mothers and infants prior to hospital discharge or during visits to clinics. All specimens collected at hospital or visits to clinics were immediately stored at −20°C. Mothers were instructed to collect and immediately store infant faecal specimens at −20°C in the event where faecal specimens were not passed at hospital or during scheduled visits to clinics. All home collections were transported to clinics using ice boxes and were delivered to study staff within 24 h of collection. Transport of faecal specimens between the study site and laboratory was performed under controlled conditions using ice boxes. Upon arrival at the laboratory, faecal specimens were stored at −80°C until further processing. Faecal specimens included in this study were selected based on the availability of faecal specimens from mother–infant dyads at birth and at follow-up visits. Specimens were selected to include the maximum number of longitudinal sample sets at the time of study.
Nucleic acid was extracted from each of the faecal specimens using approximately 50 mg of starting material as described previously (Claassen et al., 2013; Claassen-Weitz et al., 2018). A detailed description of the amplicon library preparation, sequencing and bioinformatics steps was published previously, including access to the raw sequence files supporting the findings of this manuscript (Claassen-Weitz et al., 2018). Briefly, amplicon library preparation was performed by amplifying the V4 hyper-variable region of the 16S ribosomal ribonucleic acid (rRNA) gene (Claassen-Weitz et al., 2018). Sequencing was carried out using the Illumina® MiSeq™ platform and the MiSeq Reagent Kit v3, 600 cycles (Illumina, CA, USA) (Claassen-Weitz et al., 2018). Quality filtering steps of raw sequences, removal of potential contaminants, de-replication of sequences occurring more than twice, clustering of sequences into operational taxonomic units (OTUs), removal of chimeras and taxonomic assignment was performed using an in-house bioinformatics pipeline incorporating various software tools (Claassen-Weitz et al., 2018).
Covariates
Data on maternal demographics (residential area, education) and health (HIV status, smoking) were obtained at enrolment. Maternal body mass index (BMI) was determined at 6–10 weeks post-partum. Data for mode of delivery, gestational age, birth weight and length, infant sex, antibiotic use and household members were obtained at the time of delivery at Paarl Hospital, where all births took place. Information on feeding practices was obtained at infant follow-up visits at 4–12 and 20–28 weeks of age (Zar et al., 2015). The covariates included in this study were chosen based on their potential effect on faecal bacterial profiles as shown previously (Claassen-Weitz et al., 2018). Variables that were associated with prenatal psychological measures; or bacterial taxa at phylum, class, order, family or genus level at each of the time points under study; or diversity indices at each of the time points under study were included as covariates and referenced in the results section. Measures for other covariates, for example, maternal diet at birth, that may affect the associations investigated in this study, were not assessed in this study.
Statistical analyses
R software version 3.1.1 (R_Development_Core_Team, 2014) together with RStudio software version 0.98.50751 was used for all statistical analyses as well as graphical representations of the data. Count data (Anders & Huber, 2010) were transformed to compositional data by calculating the relative abundance of each OTU per specimen (McMurdie & Holmes, 2014; Fernandes et al., 2014). Alpha diversities were determined using the Shannon diversity (H′) index (Shannon, 1948; Morgan & Huttenhower, 2012) using the vegan R package (Oksanen et al., 2013). Beta diversities were computed based on the ‘w’ metric as decribed in Koleff et al. (2003) with the vegan R package and represented in a SMACOF (Scaling by MAjorizing a COmplicated Function) multidimensional scaling map (Borg and Groenen, 2005). Maternal prenatal psychological measures in this study included symptoms of depression, psychological distress, measures for IPV and PTSD. Measures for psychological distress (SRQ-20) and symptoms of depression (BDI-II) were used as continuous variables. Dichotomised measures for IPV were used and PTSD was categorised according to ‘trauma-exposed PTSD’, ‘suspected PTSD’ and ‘no exposure to PTSD’. A multivariate approach was followed, testing simultaneously which covariates significantly influenced the microbiome composition by performing Permutational multivariate analysis of variance (PERMANOVA) (Anderson, 2001) on the Bray–Curtis dissimilarity matrix (calculated using the [vegdist] function from the R package vegan) (Bray & Curtis, 1957; Faith et al., 1987; Clarke and Warwick, 2001; Morgan & Huttenhower, 2012). For those covariates identified as significant, generalised linear models (GLMs) with a log link function and a quasi-Poisson family allowing for overdispersion were used to evaluate the association of prenatal psychological measures with each taxon and alpha diversity at each of the time points under study (Claassen-Weitz et al., 2018). Similarly, generalised linear mixed models (GLMMs) were used to investigate covariate effects across time points using a subset of 36 infants with complete longitudinal data sets. Hypothesis testing was performed at a 5% significance level and since the GLM and GLMM models were fitted for one taxon at a time, multiple testing necessitated controlling the false discovery rate with the Benjamini–Hochberg correction (Benjamini and Hochberg, 1995).
Results
Study characteristics
Faecal specimens of 90 mothers, 107 infants at birth, 72 infants at 4–12 weeks and 36 infants at 20–28 weeks were collected and successfully sequenced (Claassen-Weitz et al., 2018). Maternal prenatal psychological measures were not collected from six of the mothers. These mothers and their infants (six infants at birth and three infants at 4–12 weeks) were excluded from this study. Associations between prenatal distress measures and infant faecal bacterial profiles were therefore investigated from 101 infant meconium specimens, 69 faecal specimens collected at 4–12 weeks and 36 faecal specimens collected at 20–28 weeks. Associations between prenatal distress measures and maternal faecal bacterial profiles were analysed from 84 maternal faecal specimens collected at the time of delivery. A median of 5465 [interquartile range (IQR): 3159–9877)] post-filtered reads per specimen were obtained following the removal of potential contaminant reads. Infant meconium specimens produced the highest number of post-filtered reads (median: 10 002; IQR: 5065–14 830), followed by infant faecal specimens collected at 4–12 weeks (median: 6407; IQR: 4069–9042) and 20–28 weeks (median: 5636; IQR: 4228–7084) (Claassen-Weitz et al., 2018). Maternal faecal specimens sampled at birth produced the lowest number of post-filtered reads (median: 3155; IQR: 2104–4355) (Claassen-Weitz et al., 2018). All specimen types produced sufficient sequencing depth for calculating Shannon diversity indices in our study (Supplementary Fig. 1).
Demographic and clinical data of the mothers and infants are reported in Table 1. The median (IQR) measures for psychological distress (SRQ-20) and for symptoms of depression (BDI) from mothers with available data (n = 101) were 4.0 (1–7) and 13 (7–12), respectively. Trauma-associated PTSD was diagnosed among 3% (3/101) of mothers. A total of 45% (45/101) of the mothers were exposed to any form of IPV, of which 32% (34/101) experienced emotional abuse, 29% (31/101) physical abuse and 13% (14/101) sexual abuse. Furthermore, 30% (32/101) of the mothers were recently exposed to IPV (during the past 12 months), and 45 % (45/101) had lifetime IPV exposure.
Table 1.
Demographic and clinical characteristics of mothers and infants included in the study
| Characteristics | Mothers at delivery (n = 84) | Infants at birth (n = 101) | Infants at 4–12 weeks (n = 69) | Infants at 20–28 weeks (n = 36) |
|---|---|---|---|---|
| Age at which specimens were collected, median (IQR) | 25.3 (21.9–31.24) years | 12.4 (4.2–24.6) h | 7.8 (6.9–9.3) weeks | 24.1 (23.9–26.4) weeks |
| Residential area: | ||||
| Mbekweni, n (%) | 37 (44.0) | 54 (53.5) | 39 (56.5) | 20 (55.6) |
| TC Newman n (%) | 47 (56.0) | 47 (46.5) | 30 (43.5) | 16 (44.4) |
| Maternal education: | ||||
| Primary level, n (%) | 8 (9.5) | 11 (10.9) | 8 (11.6) | 7 (19.4) |
| Secondary level, n (%) | 70 (83.4) | 82 (81.2) | 57 (82.6) | 26 (72.2) |
| Tertiary level, n (%) | 6 (7.1) | 8 (7.9) | 4 (5.8) | 3 (8.3) |
| Maternal prenatal distress | ||||
| SRQ-20, median (IQR) | 4.0 (2–6) | 4.0 (1–7) | 5 (1–8) | 4.5 (1.3–8) |
| BDI, median (IQR) | 10 (6–18) | 13 (7–22) | 13 (7–23) | 13 (7–22.5) |
| PTSD | ||||
| Trauma-exposed, n (%) | 3 (3.6) | 3 (3.0) | 2 (2.9) | 0 (0) |
| Suspected PTSD, n (%) | 0 (0) | 1 (1.0) | 1 (1.4) | 1 (2.8) |
| No exposure, n (%) | 81 (96.4) | 97 (96.0) | 66 (95.7) | 35 (97. 2) |
| IPV | ||||
| IPV emotional, n (%) | 29 (34.5) | 34 (33.7) | 24 (34.8) | 13 (36.1) |
| IPV physical, n (%) | 26 (31.0) | 31 (30.7) | 23 (33.3) | 10 (27.8) |
| IPV sexual, n (%) | 10 (11.9) | 14 (13.9) | 11 (15.9) | 7 (19.4) |
| IPV any, n (%) | 36 (42.9) | 45 (44.6) | 32 (46.4) | 18 (50. 0) |
| IPV any recent, n (%) | 27 (32.1) | 32 (31.7) | 25 (36.2) | 15 (41.7) |
| IPV any lifetime, n (%) | 36 (42.9) | 45 (44.6) | 32 (46.4) | 18 (50.0) |
| Maternal HIV status: | ||||
| HIV infected, n (%) | 18 (21.4) | 26 (25.7) | 19 (27.5) | 10 (27.8) |
| Maternal BMI at 6–10 weeks postnatal, median (IQR) | 25.4 (23.2–29.2)† | 25.1 (23.0–28.8)† | 25.8 (22.9–29.7)† | 25.1 (22.4–30.4)† |
| Maternal smoking status:* | ||||
| Active smoker (cotinine levels > 500), n (%) | 28 (33.3) | 28 (27.7) | 21 (30.4) | 13 (36.1) |
| Passive smoker (cotinine levels > 10 < 500), n (%) | 36 (42.9) | 36 (35.6) | 19 (27.5) | 9 (25.0) |
| Non-smoker (cotinine levels < 10), n (%) | 20 (23.8) | 20 (19.8) | 12 (17.4) | 6 (16.7) |
| Mode of delivery: | ||||
| Vaginal delivery, n (%) | 69 (82.1) | 81 (80.2) | 57 (82.6) | 29 (80.6) |
| Elective caesarean section delivery, n (%) | 3 (3.6) | 5 (5.0) | 4 (5.8) | 3 (8.3) |
| Emergency caesarean section delivery, n (%) | 12 (14.3) | 15 (14.9) | 8 (11.6) | 4 (11.1) |
| Gestational age (weeks), median (IQR) | 39 (37.3–40.0) | 39.0 (38.0–40.0) | 39.0 (38.0–40.0) | 39.0 (38.3–40.0) |
| Birth weight (kg), median (IQR) | 3.0 (2.7–3.3) | 3.0 (2.7–3.4) | 3.1 (2.7–3.5) | 3.0 (2.6–3.4) |
| Birth length (cm), median (IQR) | 50.5 (48.0– 52.8) | 50 (48.0–53.0) | 51 (48.5–53.0) | 50.5 (49–53) |
| Sex: | ||||
| Male, n (%) | 36 (42.9) | 43 (42.6) | 30 (43.5) | 18 (50) |
| Milk feeding: | ||||
| Exclusive breastfeeding, n (%) | 71 (84.5)‡$ | 81 (80.2)‡$ | 32 (46.4)‡‖ | 6 (16.7)‡¶ |
| Exclusive formula feeding, n (%) | 9 (10.7)‡$ | 16 (15.8)‡$ | 11 (15.9)‡‖ | 7 (19.4)‡¶ |
| Mixed feeding**, n (%) | – | – | 25 (36.2)‡‖ | 23 (63.9)‡¶ |
| Antibiotic use, n (%) | 9 (10.7) | 15 (14.9) | 9 (13.0) | 3 (8.3) |
| Household members, median (IQR) | 5 (3–7) | 4 (3–6) | 4 (3–6) | 4 (3–6) |
IQR, Interquartile range; SRQ-20, psychological distress; BDI, Beck depression inventory; PTSD, Posttraumatic stress disorder; IPV, Intimate partner violence; BMI, Body mass index.
Number of observations recorded: Mothers at delivery: 84; Infants at birth: 84; Infants at 4–12 weeks: 52; Infants at 20–28 weeks: 28.
Number of observations recorded: Mothers at delivery: 52; Infants at birth: 65; Infants at 4–12 weeks: 51; Infants at 20–28 weeks: 27.
Number of observations recorded: Mothers at birth: 86; Infants at birth: 103; Infants at 4–12 weeks: 71; Infants at 20–28 weeks: 36.
Feeding recorded prior to discharge from hospital.
Feeding recorded at 4–12 weeks.
Feeding recorded at 20–28 weeks.
Breast milk, formula milk and/or solids.
Association of maternal prenatal psychological measures with infant faecal bacterial profiles
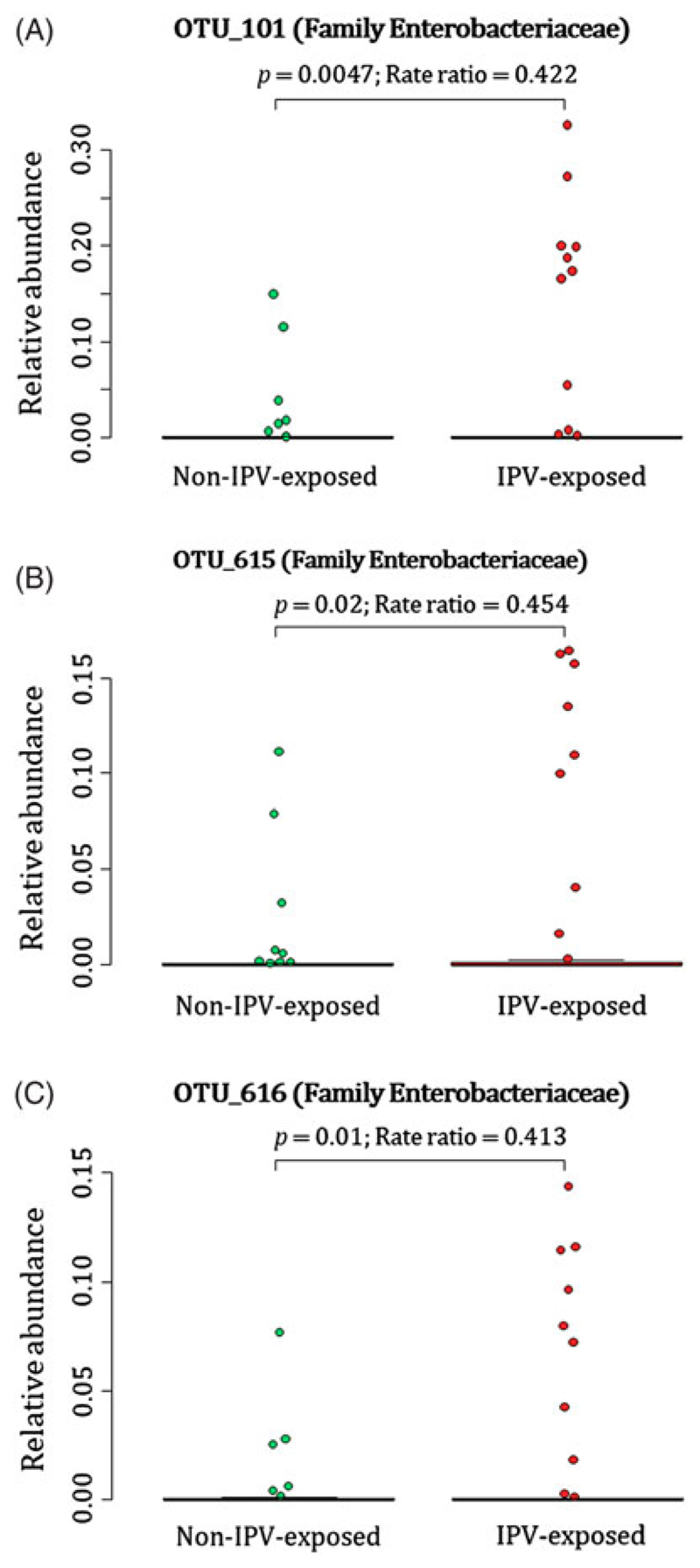
Infants born to mothers with exposure to lifetime IPV had higher proportions of unclassified genera within the family Enterobacteriaceae [OTU 101 (p < 0.01); OTU 615 (p = 0.02); OTU 616 (p = 0.01)] measured from meconium (Fig. 1(A)–(C)) (covariates significantly associated with prenatal psychological measures or bacterial taxa that were included in the statistical model: maternal BMI and breastfeeding).
Fig. 1.
Relationship between maternal lifetime exposure to IPV and the infant meconium bacterial profiles. The number of participants represented in this figure was infants from which we detected the respective OTUs at proportions > 0. (A) Differences in proportions of OTU_101 detected from 88 infant meconium specimens; (B) differences in proportions of OTU_615 detected from 73 infant meconium specimens; and (C) differences in proportions of OTU_616 detected from 71 infant meconium specimens at proportions > 0. IPV = intimate partner violence.
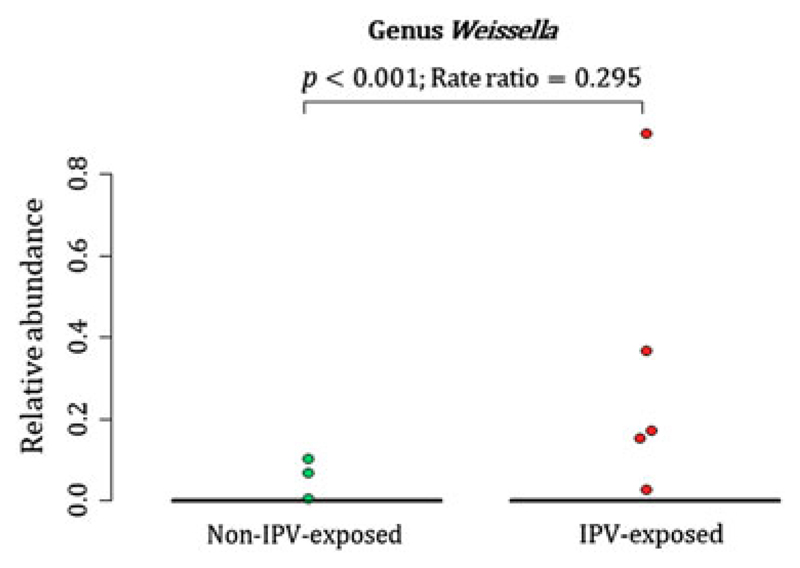
A total of 88 participants at birth had OTU 101, 73 infants had OTU 615 and 71 infants had OTU 616 detected at proportions > 0 from their meconium (Fig. 1(A)–(C)). At 4–12 weeks of age, infants born to mothers with exposure to lifetime IPV had higher proportions of the genus Weissella (Phylum Firmicutes; Class Bacilli; Order Lactobacillales; Family Leuconostocaceae) (p < 0.001) when compared to infants born to mothers who were not exposed to lifetime IPV (Fig. 2) (covariates significantly associated with prenatal psychological measures, bacterial taxa or diversity that were included in the statistical model: area and maternal HIV status). A total of 44 infants at 4–12 weeks of age had Weissella detected at proportions > 0 from their faecal specimens (Fig. 2). This association was not found to be significant at birth or at 20–28 weeks (Supplementary Fig. 2).
Fig. 2.
Relationship between maternal lifetime exposure to IPV and the infant faecal bacteria at 4–12 weeks. The figure presents differences in proportions of genus Weissella that was detected from 44 infant faecal specimens at proportions > 0. IPV = intimate partner violence.
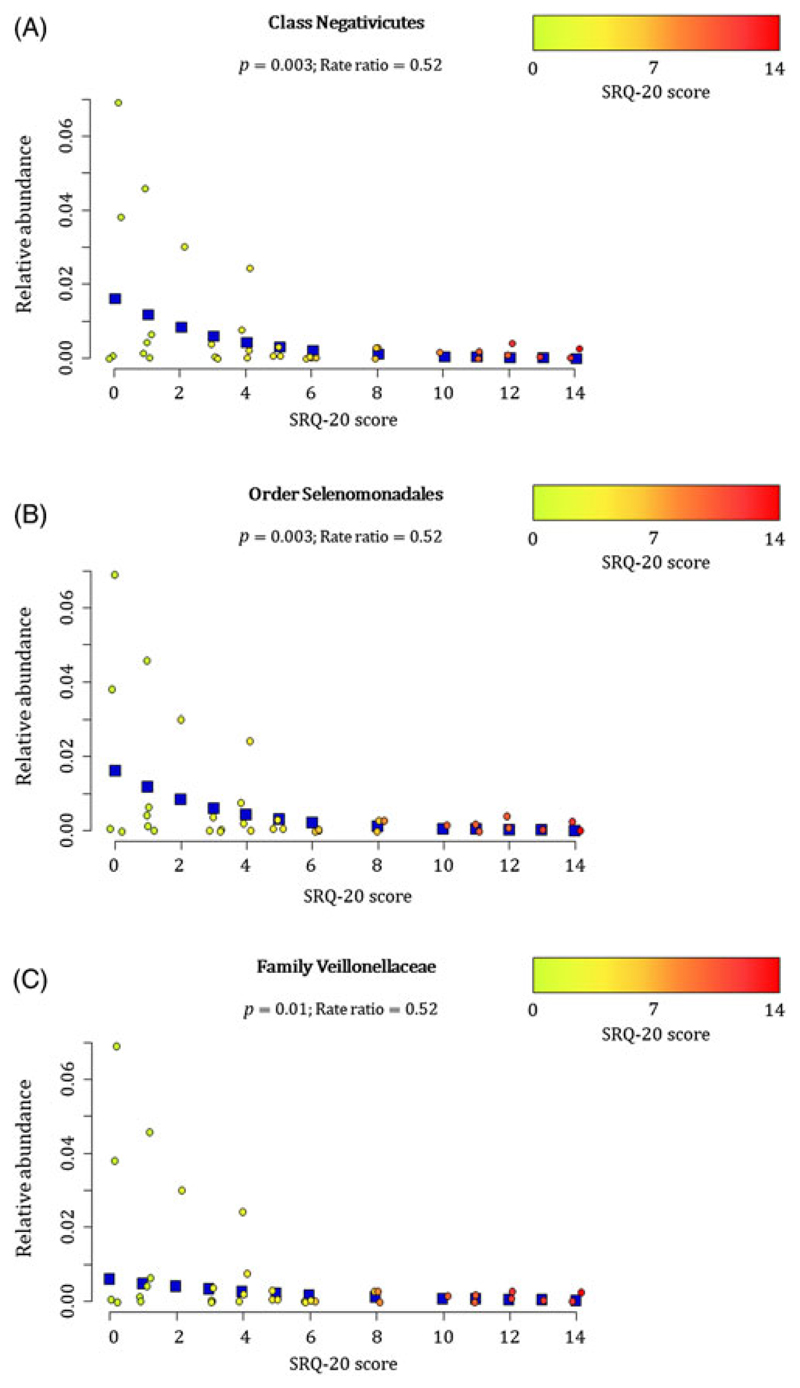
At 20–28 weeks, increased measures of maternal psychological distress (SRQ-20) were significantly associated with lower proportions of the family Veillonellaceae (Phylum Firmicutes, Class Negativicutes (p = 0.003), Order Selenomondales (p = 0.003)) (p = 0.01) (covariates significantly associated with psychological measures, bacterial taxa or diversity which were included in the statistical model: area, maternal HIV status, maternal education and gender of infant) (Fig. 3(A)–(C)). This association was only significant at 20–28 weeks (Supplementary Fig. 3).
Fig. 3.
Relationship between maternal prenatal psychological distress (SRQ-20) and infant faecal bacterial profiles at 20–28 weeks. Proportions of the (A) class Negativicutes, (B) order Selenomonadales and (C) family Veillonellaceae observed from faecal specimens collected at 20–28 weeks were inversely correlated with maternal psychological distress (SRQ-20) (p < 0.01). Individual maternal SRQ-20 scores for the 36 infants investigated at 20–28 weeks are represented by circles using a gradient of colours ranging from green (low SRQ-20 scores) to red (high SRQ-20 scores). Blue squares represent the non-parametric regression smoother used to show the trend in the observations.
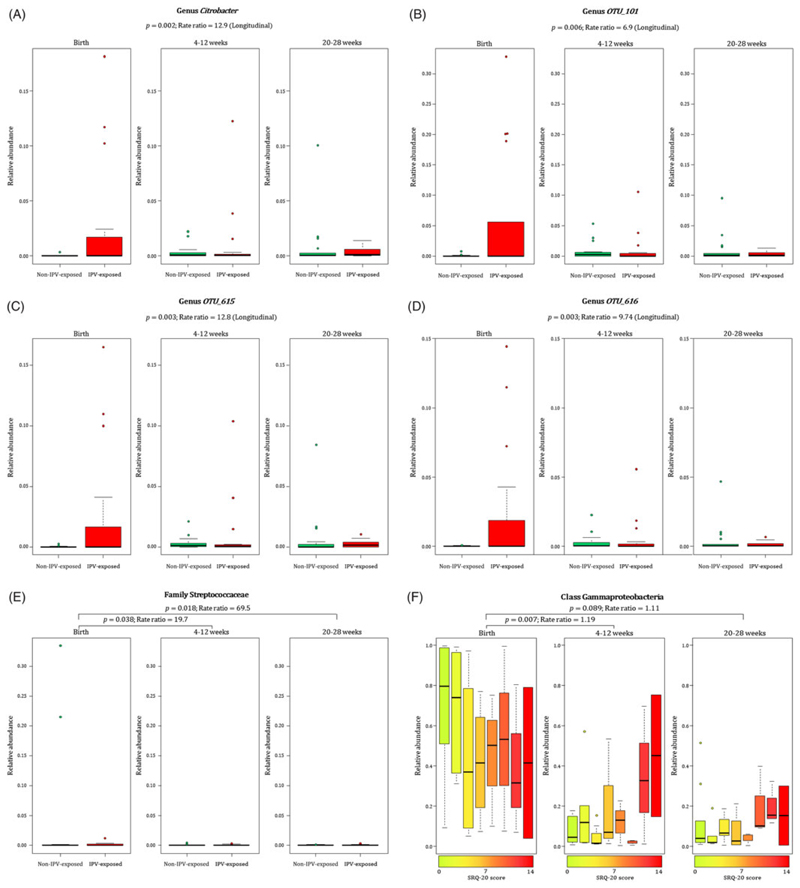
When measuring the effect of maternal prenatal stressors and distress on n = 36 infant faecal bacterial profiles over time, we found that infants from mothers with exposure to lifetime IPV had increased proportions of taxa within the family Enterobacteriaceae [genus Citrobacter (p = 0.002) (Fig. 4(A)) and unclassified genera OTU 101 (p = 0.006) (Fig. 4(B)), OTU 615 (p = 0.003) (Fig. 4(C)) and OTU 616 (p = 0.003) (Fig. 4(D))] at birth through to 20–28 weeks when compared to infants from mothers with no/low IPV exposure (covariates significantly associated with psychological measures or bacterial taxa that were included in the statistical model: gender, area and maternal HIV status). No interaction for age and bacterial proportions with reference to the four genera within the family Enterobacteriaceae from IPV-exposed or non-IPV-exposed infants was found, and longitudinal analyses were therefore performed for results presented in Fig. 4(A)–(D). A significant interaction effect between age and IPV exposure was found for the family Streptococcaceae (p = 0.046) and analysis was therefore stratified according to the age groups under investigation (Fig. 4(E)). Higher proportions of the Streptococcaceae family was found at birth in infants born to mothers with IPV versus infants from IPV-unexposed mothers when compared to these infants at 4–12 weeks (p = 0.038) and 20–28 weeks (p = 0.018) of age (Fig. 4(E)). A significant interaction effect (p = 0.003) was found between age and SRQ for the class Gammaproteobacteria as shown in Fig. 4(F). Overall, lower proportions of the class Gammaproteobacteria was observed at age groups 4–12 weeks and 20–28 weeks compared to the proportions detected at birth (Fig. 4(F)). This decrease was more pronounced in infants born to mothers with low prenatal psychological distress at 4–12 weeks (p = 0.007), and a similar non-significant trend was observed at 20–28 weeks (p = 0.089) of age, compared to these infants at birth (covariates significantly associated with psychological measures or bacterial taxa that were included in the statistical model: gender, area and maternal HIV status) (Fig. 4(F)).
Fig. 4.
Relationship between maternal exposure to lifetime IPV, psychological distress and temporal changes with infant faecal bacterial profiles. Longitudinal changes to the proportions of (A) Genus Citrobacter and unclassified genera within the family Enterobacteriaceae (B–D) [OTU_101 (p = 0.006); OTU_615 (p = 0.003); OTU_616 (p = 0.003)] in the infants born to mothers exposed to lifetime IPV compared to infants from mothers exposed to no/low lifetime IPV. (E) Differential decrease in proportions of the family Streptococcaceae from birth to ages 4–12 weeks (p = 0.038) and from birth to 20–28 weeks (p = 0.018) with a more pronounced decline in Streptococcaceae for infants from mothers with no/low lifetime IPV exposure compared to infants from mothers exposed to lifetime IPV. (F) Association of continuous measures of maternal prenatal psychological distress (SRQ-20) with faecal Gammaproteobacteria in infants at birth compared to the infants at ages 4–12 weeks (p = 0.007) and 20–28 weeks (p = 0.089). Individual maternal SRQ-20 scores for the 36 infants with faecal specimens collected at all three time points are represented by a gradient of colours ranging from green (low SRQ-20 scores) to red (high SRQ-20 scores). IPV = intimate partner violence.
No significant associations with infant faecal bacterial profiles were found when assessing symptoms of depression or PTSD. Supplementary Figs. 4–8 show different measures for maternal prenatal stressors and distress with the infant microbiota at genus level measured over time. No significant associations were found between any maternal prenatal psychological measures and infant faecal bacterial diversity indices (data not shown).
Prenatal psychological measures and maternal faecal bacterial profiles at delivery
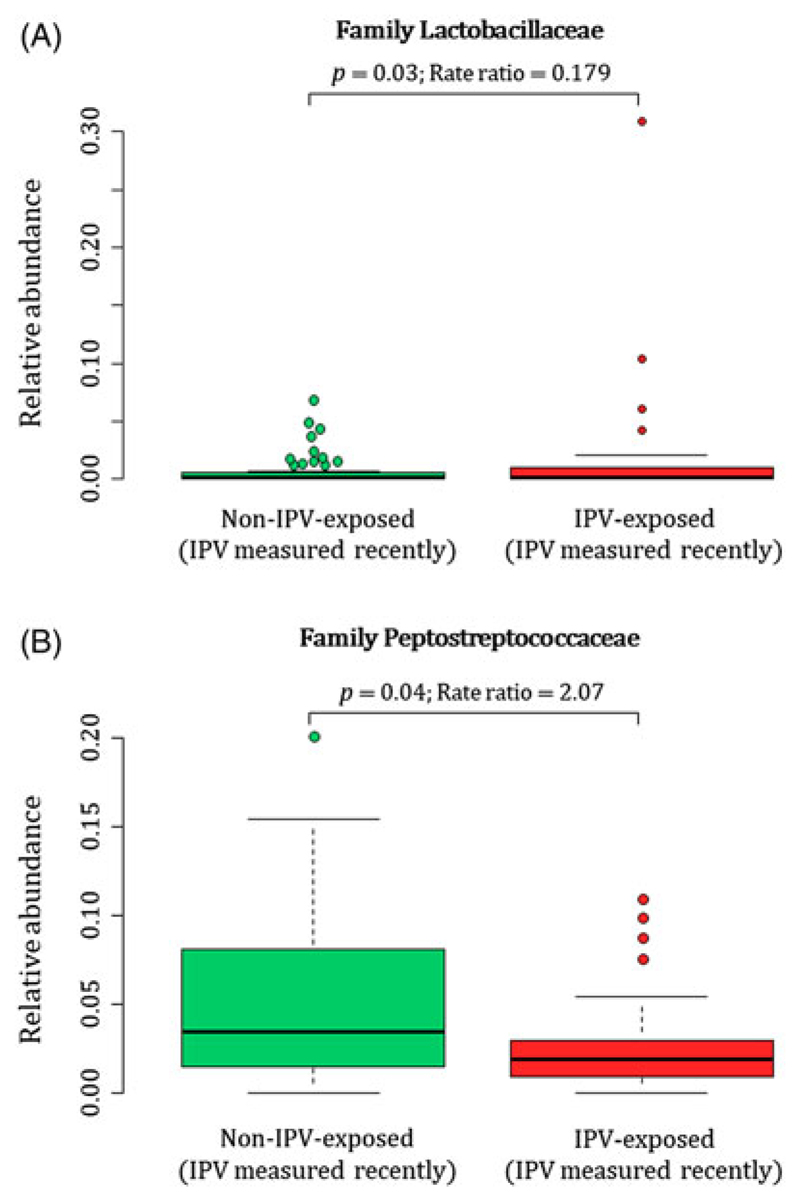
Maternal exposure to recent IPV (past year) was associated with higher proportions of the family Lactobacillaceae (p = 0.03) (phylum Firmicutes, class Bacilli, order Lactobacillales) and lower proportions of the family Peptostreptococcaceae (p = 0.04) (phylum Firmicutes, class Clostridia, order Clostridiales) in maternal faecal specimens at delivery (covariates significantly associated with psychological measures or bacterial taxa that were included in the statistical model: mode of delivery) (Fig. 5(A) and (B)). None of the prenatal psychological measures were associated with maternal faecal bacterial diversity indices (data not shown).
Fig. 5.
Relationship between maternal exposure to recent IPV (past year) during pregnancy and maternal faecal bacterial profiles at delivery. IPV = intimate partner violence.
Beta diversity and measures for maternal psychological adversities
No clustering patterns were apparent for maternal or infant faecal bacterial profiles in relation to any of the prenatal measures for psychological adversities based on the ‘w’ metric for beta diversities (results not shown).
Discussion
This study characterised the association of maternal prenatal stressors and distress with early-life infant faecal and maternal bacterial profiles. Our results demonstrate that maternal exposure to lifetime IPV and psychological distress were significantly associated with early infant faecal bacterial profiles.
Until recently, the foetus has been considered to exist in a sterile environment with initial microbial colonisation of the newborn taking place during birth (D’Argenio, 2018). Recent studies have reported the detection of microbes from amniotic fluid, placenta and meconium, suggesting that microbial colonisation may begin in utero (Willyard, 2018; Stinson et al., 2019). In addition, a number of studies have reported bacterial communities from meconium specimens (Seferovic et al., 2018). Dysbiosis of faecal bacterial profiles early in life has been associated with a number of neonatal diseases, including asthma, allergic diseases and obesity (D’Argenio, 2018), which further emphasises the importance of in utero colonisation on the health of newborns.
With cross-sectional analysis, we detected higher abundances of unclassified taxa (OTU 101, OTU 615 and OTU 616) within the family Enterobacteriaceae from meconium specimens of infants born to mothers exposed to IPV. Analysis of longitudinal data showed that these findings persisted over the first 6 months after birth in this study, as higher proportions of these unclassified Enterobacteriaceae taxa, as well as the genus Citrobacter (also a member of the family Enterobacteriaceae), were detected from infants born to IPV-exposed mothers as compared to infants from non-IPV-exposed mothers. Our findings in relation to the family Enterobacteriaceae is of interest, given previous findings. A higher cumulative score of self-reported maternal stress during pregnancy has previously been associated with increased relative abundances of Proteobacteria in infant faecal specimens over time (Zijlmans et al., 2015). The family Enterobacteriaceae (within the phylum Proteobacteria) has also been associated with depression in an adult population (Jiang et al., 2015), and leakage of lipopolysaccharide from Enterobacteriaceae into the circulation has been associated with major depression. Increased faecal Enterobacteriaceae in infants at 3–6 months of age has also been associated with impaired follow-up fine motor skills in the children at 3 years (Sordillo et al., 2019). Our findings on the other hand differ from a study by Hu and colleagues that showed an association of higher scores for pregnancy-related anxiety with lower abundances of an unclassified genus in the family Enterobacteriaceae from meconium of 75 infants (Hu et al., 2019). This discrepancy may be due to differences of the psychological measures and possibly the larger proportion of mothers (27% vs. 11%) that used antibiotics prior to delivery compared to our study.
We also found that infants born to mothers with IPV exposure had higher proportions of the genus Weissella (phylum Firmicutes) at 4–12 weeks of age. The relative abundance of Weissella increased in all infants with age. However, this increase occurred earlier for infants born to mothers with IPV exposure and subsequently reached the same level for all infants at 20–28 weeks of age. To the best of our knowledge, no prior associations have been reported between psychological stressors or distress and the genus Weissella. Furthermore, premature delivery has been associated with distinct infant faecal bacterial profiles, including higher abundances of Weissella (Arboleya et al., 2012). However, the infants assessed in this study sample had a median gestational age of 39 weeks (IQR: 38–40) (Claassen-Weitz et al., 2018). Moreover, there were no significant age differences (p > 0.5) between infants from mothers that were IPV-exposed versus non-IPV-exposed at any of the time points at which specimens were collected.
Our results further show that proportions of Gammaproteobacteria detected from stool decreased at 4–12 and 20–28 weeks compared to profiles detected from infants at birth. This decrease was less pronounced at 4–12 and 20–28 weeks after birth for infants born to mothers with a higher ratio of psychological distress (SRQ-20). Our results therefore indicate that infants from mothers with higher measures of psychological distress (SRQ-20) had a slower decrease in Gammaproteobacteria during the first 6 months after birth. The class Gamma-proteobacteria (and family Enterobacteriaceae in particular) are the most abundant faecal bacteria in the first month of life, subsequently substituted by anaerobes (Bokulich et al., 2016). Slower reduction rates of these bacteria over time may be an indication of impaired maturation of the infant’s gut bacteriome (Bokulich et al., 2016). Furthermore, higher measures of maternal prenatal psychological distress were associated with lower relative abundance of Veillonellaceae (phylum Firmicutes) in infant faecal specimens at 20–28 weeks. The median relative abundance of Veillonellaceae increased with age overall amongst infants in this study. However, at the age of 4–12 weeks, this abundance was relatively lower (not significant) in infants from mothers with higher scores for psychological distress and more pronounced and significantly lower in these infants at the age of 20–28 weeks. Veillonellaceae are lactate-utilising bacteria that are abundant in infant faecal specimens up to 6 months and subsequently decrease during the transition to toddler and then remaining at stable levels throughout healthy adulthood (Laursen et al., 2016; Pannaraj et al., 2017; Drell et al., 2017). Decreased abundances of Veillonellaceae have been found in children with autism (Kang et al., 2013), as well as in patients with depression (Jiang et al., 2015). A recent Belgian population cohort showed that the genus Dialister (within the family Veillonellaceae) was significantly deceased in depression, a finding that was further validated in a large Dutch cohort (Valles-Colomer et al., 2019). These findings suggest a consistent association between decreased abundance of Veillonellaceae and neurodevelopmental impairment or psychological distress.
Overall, our results illustrate that the associations of different psychological profiles during pregnancy on faecal bacteria may vary. Exposure to lifetime IPV, in particular, was associated with differences in the infant and maternal faecal bacteria. IPV is a traumatic stressor that often occurs over prolonged and repeated episodes (Thompson et al., 2006) and IPV during pregnancy often persists post-partum (Charles & Perreira, 2007; Groves et al., 2015; Barnett et al., 2018). It should be noted that other effects of IPV which were not controlled for in this study may contribute to its association with changes to infant faecal bacterial composition. For example, mothers who have experienced IPV are more likely to have weakened bonding with their infant (Zeitlin et al., 1999; Barnett et al., 2018), which may in turn affect the infant faecal bacterial composition. Supporting evidence from animal work has showed that male rat pups that were stressed by maternal separation had elevated faecal levels of Enterobacteria and Bacteroides during adulthood (Garcia-Rodenas et al., 2006). Another animal study found that maternal separation of pups resulted in a reduced ratio of Firmicutes to Bacteroides in the adult gut of these female rats (Pusceddu et al., 2015). The effect of IPV on child feeding may also contribute to faecal bacterial changes. Studies have showed that maternal IPV can contribute to infant malnutrition in low to middle-income countries (Rico et al., 2011; Misch & Yount, 2014; Barnett et al., 2018). Malnutrition has been associated with marked changes in infants’ faecal bacterial composition (Kane et al., 2015). Therefore, other measures that accompany IPV post-partum may play a role in the development of infant faecal bacterial composition.
Several limitations to our study deserve emphasis. First, there were missing faecal specimens over time, due mainly to the inability to collect stool at the scheduled study visits (stool was only collected if the infant was able to produce a stool sample during the study visit). Therefore, our results should be interpreted with caution, especially at the 20–28 weeks interval. Second, given the limited number of mothers with PTSD, our analysis has insufficient statistical power to draw definitive conclusions about the possible association between PTSD and an altered faecal bacteriome. Third, the cross-sectional analysis for the individual time points does not allow for definitive conclusions about their longitudinal changes in the faecal bacterial profiles. Further work is needed to understand whether IPV is a proxy for other key variables that may affect the associations found with the infants’ faecal gut bacteria.
In summary, maternal prenatal psychological stressors and distress, particularly lifetime IPV, are associated with early changes in infant faecal bacterial profiles in a semi-rural South African area. Further studies are needed to verify our findings, and to determine whether these changes to the gut bacteriome due to maternal prenatal psychological stressors and distress are associated with neurodevelopmental impairments in children over time. In addition, it would be of interest to examine if preventative strategies to reduce the risk of prenatal psychological stressors and distress can prevent longitudinal changes to the infant’s gut bacteria and associated health risks. Such work may provide further insights into understanding relevant mechanisms and identifying appropriate targets, with the ultimate aim of preventing the negative effects of intergenerational transmission of psychopathology on the developing brain.
Supplementary Material
Significant outcomes
This is the first study to report an association between maternal prenatal stressors and distress with an altered bacterial profile in their infants’ meconium and longitudinal stool specimens.
Maternal prenatal exposure to intimate partner violence (IPV) is associated with differences in faecal bacterial profiles of mothers at delivery (Lactobacillaceae and Peptostreptococcaceae) and of infants at birth (family Enterobacteriaceae) and 4–12 weeks of age (genus Weissella). These infants had increased proportions of taxa within the family Enterobacteriaceae (Citrobacter; OTU 101; OTU 615; OTU 616) during the first 6 months after birth compared to infants from mothers with no/low IPV exposure.
Higher psychological distress during pregnancy is associated with lower infant faecal bacterial profiles of the family Veillonellaceae at 20–28 weeks.
Limitations
Missing faecal specimens over time.
Limited number of mothers with posttraumatic stress disorder.
Acknowledgements
We would like to thank the families and their children that participated in this study. We thank the study staff, administrative staff and clinical staff of the Western Cape Government Health Department at TC Newman and Mbekweni clinics, and Paarl Hospital for their support of the study. Petrus Naudé, Shantelle Claassen-Weitz, Mamadou Kaba, Heather Zar, Mark Nicol and Dan Stein contributed to the concept and design of the study. Shantelle Claassen-Weitz, Sugnet Gardner-Lubbe, Gerrit Botha and Mamadou Kaba contributed to the acquisition and analysis of data. All mentioned authors contributed to the interpretation of the results presented in this manuscript. Petrus Naudé and Shantelle Claassen-Weitz drafted the manuscript. The critical revisions by Sugnet Gardner-Lubbe, Gerrit Botha, Mamadou Kaba, Heather Zar, Mark Nicol and Dan Stein were essential for the intellectual content of this manuscript. All listed authors provided final approval of the version to be published.
Financial support
The DCHS is funded by Bill and Melinda Gates Foundation (OPP1017641; OPP1017579). HJZ and DJS are supported by the South African Medical Research Council. This study was also supported by an H3Africa U01 award from the National Institutes of Health of the USA to MPN and HJZ (1U01AI110466-01A1). MK is a recipient of Carnegie Corporation of New York (USA) early-career fellowship, Wellcome Trust Training Fellowship, United Kingdom (102429/Z/13/Z) and CTN International fellowship (Canada). PJWN is supported by the National Alliance for Research on Schizophrenia and Depression Young Investigator Grant (No. 25199) and Scott-Gentle Foundation.
Footnotes
Conflict of interest. None
Ethical standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11:R106–R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ. Anew method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. [Google Scholar]
- Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, de los Reyes-Gavilán CG, Gueimonde M. Establishment and development of intestinal microbiota in preterm neo-nates. FEMS Microbiology Ecology. 2012;79:763–772. doi: 10.1111/j.1574-6941.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Frontiers in Immunology. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. Journal of Pediatric Gastroenterology and Nutrition. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Barnett W, Halligan S, Heron J, Fraser A, Koen N, Zar HJ, Donald KA, Stein DJ. Maltreatment in childhood and intimate partner violence: a latent class growth analysis in a South African pregnancy cohort. Child Abuse and Neglect. 2018;86:336–348. doi: 10.1016/j.chiabu.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric propertiesof the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodology) 1995;57:289–300. [Google Scholar]
- Bernstein M, Phillips T, Zerbe A, McIntyre JA, Brittain K, Petro G, Abrams EJ, Myer L. Intimate partner violence experienced by HIV-infected pregnant women in South Africa: a cross-sectional study. BMJ Open. 2016;6:e011999. doi: 10.1136/bmjopen-2016-011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, D Lieber A, Wu F, Perez-Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez-Bello MG, Blaser MJ. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Science Translational Medicine. 2016;8 doi: 10.1126/scitranslmed.aad7121. 343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg I, Groenen PJF. Modern Multidimensional Scaling: Theory and Applications. New York: Springer Science & Business Media; 2005. [Google Scholar]
- Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs. 1957;27:325–349. [Google Scholar]
- Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, Thompson AL, Geng X, Gilmore JH, Knickmeyer RC. Infant gut microbiome associated with cognitive development. Biol Psychiatry. 2018;83:148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P, Perreira KM. Intimate partner violence during pregnancy and 1-year post-partum. Journal of Family Violence. 2007;22:609–619. [Google Scholar]
- Claassen S, du Toit E, Kaba M, Moodley C, Zar HJ, Nicol MP. A comparison of the efficiency of five different commercial DNA extraction kits for extraction of DNA from faecal samples. Journal of Microbiological Methods. 2013;94:103–110. doi: 10.1016/j.mimet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen-Weitz S, Gardner-Lubbe S, Nicol P, Botha G, Mounaud S, Shankar J, Nierman WC, Mulder N, Budree S, Zar HJ, Nicol MP, Kaba M. HIV-exposure, early life feeding practices and delivery mode impacts on faecal bacterial profiles in a South African birth cohort. Scientific Reports. 2018;8:5078. doi: 10.1038/s41598-018-22244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RK, Warwick RM. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. Plymouth, UK: Primer-E Ltd; 2001. [Google Scholar]
- D’Argenio V. The prenatal microbiome: a new player for human health. High Throughput. 2018;7:38. doi: 10.3390/ht7040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drell T, Štšepetova J, Simm J, Rull K, Aleksejeva A, Antson A, Tillmann V, Metsis M, Sepp E, Salumets A, Mändar R. The influence of different maternal microbial communities on the development of infant gut and oral microbiota. Scientific reports. 2017;7:9940–9940. doi: 10.1038/s41598-017-09278-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk ofHIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363:1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- Faith DP, Minchin PR, Belbin L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio. 1987;69:57–68. [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick D. The modified PTSD symptom scale: a brief self-report measure of posttraumatic stress disorder. The Behavioral Therapist. 1993;16:161–162. [Google Scholar]
- Fernandes AD, Reid JNS, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. Unifying the analysis of high-throughput sequencing data-sets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome. 2014;2:15. doi: 10.1186/2049-2618-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Tolin DF. Comparison of the PTSD symptom scale–interview version and the clinician-administered PTSD scale. Journal of Traumatic Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, Ornstein K, Rochat F, Corthesy-Theulaz I. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. Journal of Pediatric Gastroenterology and Nutrition. 2006;43:16–24. doi: 10.1097/01.mpg.0000226376.95623.9f. [DOI] [PubMed] [Google Scholar]
- Groves AK, Moodley D, McNaughton-Reyes L, Martin SL, Foshee V, Maman S. Prevalence, rates and correlates of intimate partner violence among South African women during pregnancy and the postpartum period. Maternal and Child Health Journal. 2015;19:487–495. doi: 10.1007/s10995-014-1528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur TL, Palkar AV, Rajasekera T, Allen J, Niraula A, Godbout J, Bailey MT. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behavioural Brain Research. 2019;359:886–894. doi: 10.1016/j.bbr.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur TL, Shay L, Palkar AV, Fisher S, Varaljay VA, Dowd S, Bailey MT. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain, Behavior, and Immunity. 2017;64:50–58. doi: 10.1016/j.bbi.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Harding TW, de Arango MV, Baltazar J, Climent CE, Ibrahim HH, Ladrido-Ignacio L, Murthy RS, Wig NN. Mental disorders in primary health care: a study of their frequency and diagnosis in four developing countries. Psychological Medicine. 1980;10:231–241. doi: 10.1017/s0033291700043993. [DOI] [PubMed] [Google Scholar]
- Hartley M, Tomlinson M, Greco E, Comulada WS, Stewart J, le Roux I, Mbewu N, Rotheram-Borus MJ. Depressed mood in pregnancy: prevalence and correlates in two Cape Town peri-urban settlements. Reproductive Health. 2011;8:9. doi: 10.1186/1742-4755-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ly J, Zhang W, Huang Y, Glover V, Peter I, Hurd YL, Nomura Y. Microbiota of newborn meconium is associated with maternal anxiety experienced during pregnancy. Developmental Psychobiology. 2019;61:640–649. doi: 10.1002/dev.21837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen HAFM, Watts C, Ellsberg M, Heise L, Garda-Moreno C. Interviewer training in the WHO multi-country study on women’s health and domestic violence. Violence Against Women. 2004;10:831–849. [Google Scholar]
- Jašarević E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelop-ment. Neurobiology of Stress. 2015;1:81–88. doi: 10.1016/j.ynstr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewkes R. Intimate partner violence: causes and prevention. Lancet. 2002;359:1423–1429. doi: 10.1016/S0140-6736(02)08357-5. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kane AV, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatric Research. 2015;77:256–262. doi: 10.1038/pr.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-W, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of prevotella and other fer-menters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen N, Brittain K, Donald KA, Barnett W, Koopowitz S, Maré K, Zar HJ, Stein DJ. Psychological trauma and posttraumatic stress disorder: risk factors and associations with birth outcomes in the Drakenstein Child Health Study. European Journal of Psychotraumatology. 2016;7 doi: 10.3402/ejpt.v7.28720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleff P, Gaston KJ, Lennon JJ. Measuring beta diversity for presence-absence data. Journal of Animal Ecology. 2003;72:367–382. [Google Scholar]
- Laursen ME, Andersen LBB, Michaelsen KF, Mølgaard C, Trolle E, Bahl MI, Licht TR. Infant Gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere. 2016;1 doi: 10.1128/mSphere.00069-15. e00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari JJ, Williams P. A validity study of a psychiatric screening questionnaire (SRQ-20) in primary care in the city of Sao Paulo. The British Journal of Psychiatry. 1986;148:23–26. doi: 10.1192/bjp.148.1.23. [DOI] [PubMed] [Google Scholar]
- McGowan TC, Kagee A. Exposure to traumatic events and symptoms of post-traumatic stress among South African university students. South African Journal of Psychology. 2013;43:327–339. [Google Scholar]
- McMurdie PJ, Holmes S. Waste not, want not: why rarefying microbiome data is inadmissible. PLOS Computational Biology. 2014;10:e1003531. doi: 10.1371/journal.pcbi.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misch ES, Yount KM. Intimate partner violence and breastfeeding in Africa. Maternal and Child Health Journal. 2014;18:688–697. doi: 10.1007/s10995-013-1294-x. [DOI] [PubMed] [Google Scholar]
- Morgan XC, Huttenhower C. Chapter 12: Human microbiome analysis. PLoS Computer Biology. 2012;8:e1002808. doi: 10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Dinan TG, Cryan JF. Early life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H. Package ‘vegan’. Community Ecology Package, version 2. 2013 [Google Scholar]
- Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, Bailey A, Bushman FD, Sleasman JW, Aldrovandi GM. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatrics. 2017;171:647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu MM, El Aidy S, Crispie F, O’Sullivan O, Cotter P, Stanton C, Kelly P, Cryan JF, Dinan TG. N-3 Polyunsaturated Fatty Acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS One. 2015;10:e0139721. doi: 10.1371/journal.pone.0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. 2014. R Foundation for Statistical Computing. [Google Scholar]
- Rico E, Fenn B, Abramsky T, Watts C. Associations between maternal experiences of intimate partner violence and child nutrition and mortality: findings from Demographic and Health Surveys in Egypt, Honduras, Kenya, Malawi and Rwanda. Journal of Epidemiology and Community Health. 2011;65:360–367. doi: 10.1136/jech.2008.081810. [DOI] [PubMed] [Google Scholar]
- Rochat TJ, Richter LM, Doll HA, Buthelezi NP, Tomkins A, Stein A. Depression among pregnant rural South African women undergoing HIV testing. JAMA. 2006;295:1376–1378. doi: 10.1001/jama.295.12.1376. [DOI] [PubMed] [Google Scholar]
- Rumble S, Swartz L, Parry C, Zwarenstein M. Prevalence of psychiatric morbidity in the adult population of a rural South African village. Psychological Medicine. 1996;26:997–1007. doi: 10.1017/s0033291700035327. [DOI] [PubMed] [Google Scholar]
- Seferovic MD, Belfort B, Major A, Chu D, Racusin D, Castro E, Carroll M, Stewart C, Versalovic J, Aagaard K. 695: visualization of intact placental microbes in both term and preterm births. American Journal of Obstetrics and Gynecology. 2018;218:S417–S418. doi: 10.1016/j.ajog.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu S, Abrahams N, Temmerman M, Musekiwa A, Zarowsky C. A systematic review of African studies on intimate partner violence against pregnant women: prevalence and risk factors. PLoS One. 2011;6:e17591. doi: 10.1371/journal.pone.0017591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. Bell System Technical Journal. 1948;27:379–423. [Google Scholar]
- Sordillo JE, Korrick S, Laranjo N, Carey V, Weinstock GM, Gold DR, O’Connor G, Sandel M, Bacharier LB, Beigelman A, Zeiger R, Litonjua AA, Weiss ST. Association ofthe infant gut microbiome with early childhood neurodevelopmental outcomes: an ancillary study to the VDAART randomized clinical trial. JAMA Network Open. 2019;2:e190905. doi: 10.1001/jamanetworkopen.2019.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM. Effects of perinatal mental disorders on the fetus and child. The Lancet. 2014;384:1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Koen N, Donald KA, Adnams CM, Koopowitz S, Lund C, Marais A, Myers B, Roos A, Sorsdahl K, Stern M, Tomlinson M, van der Westhuizen C, Vythilingum B, Myer L, Barnett W, Brittain K, Zar HJ. Investigating the psychosocial determinants of child health in Africa: the Drakenstein Child Health Study. Journal of Neuroscience Methods. 2015;252:27–35. doi: 10.1016/j.jneumeth.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Williams DR, Kessler RC. The South African Stress and Health (SASH) study:a scientific base for mental health policy. South African Medical Journal. 2009;99:337. [PubMed] [Google Scholar]
- Stinson LF, Boyce MC, Payne MS, Keelan JA. The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Frontiers in Microbiology. 2019;10:1124. doi: 10.3389/fmicb.2019.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RS, Bonomi AE, Anderson M, Reid RJ, Dimer JA, Carrell D, Rivara FP. Intimate partner violence: prevalence, types, and chronicity in adult women. American Journal of Preventive Medicine. 2006;30:447–457. doi: 10.1016/j.amepre.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J. The neuro-active potential of the human gut microbiota in quality of life and depression. Nature Microbiology. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- van Heyningen T, Honikman S, Myer L, Onah MN, FieldSandTomlinson M. Prevalence and predictors of anxiety disorders amongst low-income pregnant women in urban South Africa: a cross-sectional study. Archives of Women’s Mental Health. 2017;20:765–775. doi: 10.1007/s00737-017-0768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingum B, Roos A, Faure SC, Geerts L, Stein DJ. Risk factors for substance use in pregnant women in South Africa. South African Medical Journal. 2012;102:851–854. doi: 10.7196/samj.5019. [DOI] [PubMed] [Google Scholar]
- Willyard C. Could baby’s first bacteria take root before birth? Nature. 2018;553:264–266. doi: 10.1038/d41586-018-00664-8. [DOI] [PubMed] [Google Scholar]
- World Heatlh Organization (WHO) Handbook for Good Clinical Research Practice (GCP) - Guidance for Implementation. [accessed December 2013];2002 http://apps.who.int/prequal/info_general/documents/GCP/gcp1.pdf.
- Zar HJ, Barnett W, Myer L, Stein DJ, Nicol MP. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax. 2015;70:592–594. doi: 10.1136/thoraxjnl-2014-206242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin D, Dhanjal T, Colmsee M. Maternal-foetal bonding: the impact of domestic violence on the bonding process between a mother and child. Archives of Women’s Mental Health. 1999;2:183–189. [Google Scholar]
- Zijlmans MAC, Korpela K, Riksen-Walraven JM, de VosWManddeWeerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.