Abstract
Background
Most previous studies support a direct link between total parasite load and the clinical severity of Plasmodium falciparum malaria infections.
Methods
We estimated P falciparum parasite loads in 3 groups of children with malaria infections of differing severity: (1) children with World Health Organization–defined severe malaria (n = 1544), (2) children admitted with malaria but without features of severity (n = 200), and (3) children in the community with asymptomatic parasitemia (n = 33).
Results
Peripheral parasitemias were highest in those with uncomplicated malaria (geometric mean [GM] parasite count, 111 064/μL; 95% confidence interval, CI, 86 798–141 819/μL), almost 3 times higher than in those with severe malaria (39 588/μL; 34 990–44 791/μL) and >100 times higher than in those with asymptomatic malaria (1092/μL; 523–2280/μL). However, the GM P. falciparum histidine-rich protein 2 (PfHRP2) values (95% CI) increased with severity, being 7 (4–12) ng/mL in asymptomatic malaria, 843 (655–1084) ng/mL in uncomplicated malaria, and 1369 (1244–1506) ng/mL in severe malaria. PfHRP2 concentrations were markedly lower in the subgroup of patients with severe malaria and concomitant invasive bacterial infections of blood or cerebrospinal fluid (GM concentration, 312 ng/mL; 95% CI, 175–557 ng/mL; P < .001) than in those without such infections (1439 ng/mL; 1307–1584; P < .001).
Conclusions
The clinical severity of malaria infections related strongly to the total burden of P. falciparum parasites. A quantitative test for plasma concentrations of PfHRP2 could be useful in identifying children at the greatest clinical risk and identifying critically ill children in whom malaria is not the primary cause.
Keywords: malaria, Plasmodium falciparum histidine-rich protein-2, PfHRP2, parasite biomass, sequestration
Plasmodium falciparum histidine-rich protein 2 (PfHRP2) is a 30-kDa molecule that is produced by most strains of P. falciparum malaria parasites [1] and is involved in the conversion of the toxic molecule heme to the more neutral malaria pigment hemozoin [2]. About 5 fg of PfHRP2 is released from the cytoplasm of P falciparum–infected red blood cells during schizont rupture [3, 4], and PfHRP2 can therefore be detected in the plasma of individuals with P. falciparum infection by means of enzyme-linked immunosorbent assay or rapid diagnostic tests [5, 6]. Previous studies have shown that plasma PfHRP2 provides a more accurate reflection of the total P falciparum load in individuals than parasite counts in peripheral blood because, unlike the latter, PfHRP2 levels provide information about the burden of mature-phase parasites that are sequestered in the deep vasculature [7].
Positive associations have been reported between both plasma PfHRP2 and disease severity in a number of previous studies [7–12]. However, this has not been seen universally [13, 14], prompting some to question the causal link between sequestration and severe malaria [15]. We have estimated the P. falciparum parasite loads in children with malaria infections of differing severity in Kilifi, Kenya, with the aim of contributing to this debate.
Methods
Study Design and Participants
Our study involved 3 groups of children <14 years of age who were residents of Kilifi County, Kenya: (1) children admitted to the High Dependency Unit at Kilifi County Hospital with ≥1 feature of severe falciparum malaria; (2) children admitted to the general pediatric ward with uncomplicated malaria; and (3) children from the surrounding area with asymptomatic malaria infections.
Clinical and Laboratory Data
Data and samples from children in groups 1 and 2 were captured through a routine surveillance system that has operated at Kilifi County Hospital since 1989, as described in detail elsewhere [16]. Severe malaria was defined as P. falciparum blood-film positivity with ≥1 of the following clinical or laboratory features: cerebral malaria (Blantyre coma scale score <3), respiratory distress (abnormal deep breathing), severe malarial anemia (hemogloblin level <5 g/dL), or other features of severity, as described elsewhere [17, 18]. Children in group 1 were admitted between 1998 and 2010 and those in group 2 between 2004 and 2005; children in group 3 were recruited during cross-sectional community surveys conducted in 2010-2011 [19], based on the availability of archived samples.
All laboratory tests were conducted at the KEMRI-Wellcome Trust Research laboratories, which are accredited according to Good Clinical Laboratory Practice standards (assessment by Qualogy). Parasitemia was determined in real time from blood films using standard methods [20], and PfHRP2 was batch-analyzed by enzyme-linked immunosorbent assay in plasma samples archived at −80°C since the time of collection [10]. Hemoglobin S (HbS) and α-thalassemia were genotyped by polymerase chain reaction as described elsewhere [21, 22].
Parasite Burden
The total number of circulating parasites (P circ), the whole-body total parasite load (P tot), and the sequestration index (SI), an indication of the proportion of parasites that are hidden from detection through sequestration in deep vascular beds, were estimated for each participant individually using published formulas [7, 10], as follows:
-
(1)
P circ = Parasites/μL × 106 × circulating blood volume [0.08 L/kg] × body weight [kg],
-
(2)
P tot = 7.3 × PfHRP2 [g/L] × (1 – hematocrit) × body weight [kg] × 1013,
and
-
(3)
SI = P tot/Pcirc.
Statistical Analysis
Continuous data were compared using Student’s t or Mann-Whitney tests as appropriate, and proportions were compared using the χ2 test. Nonnormal data were log-transformed before analysis. Linear regression was performed both with and without adjustment for ethnicity, HbS phenotype and α-thalassemia genotype. Children displaying multiple features of severe malaria were included in multiple categories. All data were analyzed using Stata software, version 15.1 (StataCorp).
Ethics
Written informed consent was provided by the parents of all participants. Ethical approval was granted by the Kenya Medical Research Institute/National Ethical Review Committee in Nairobi, Kenya, and the Oxford Tropical Research Ethics Committee in Oxford, United Kingdom.
Results
The study flow is summarized in Supplementary Figure 1. Clinical and laboratory data and plasma samples from a total of 1544 children with severe malaria, 200 with uncomplicated malaria, and 33 with asymptomatic malaria were retrieved for this study. PfHRP2 was undetectable in 30 (1.7%) of these samples (23 from patients with severe, 2 from patients with uncomplicated, and 5 from patients with asymptomatic malaria), leaving 1747 contributing data to the final analysis.
Demographic and Clinical Features
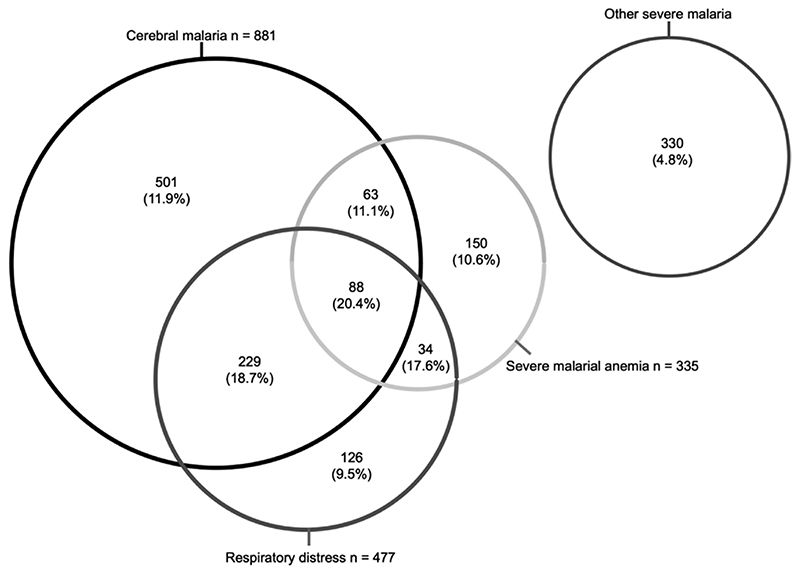
The demographic and clinical characteristics of participants are summarized in Table 1. Children with severe malaria were significantly younger than those with uncomplicated malaria, and those with asymptomatic malaria were significantly older. Overall, the in-hospital mortality rate was 11.7% in children admitted with severe malaria but 0% in those admitted with uncomplicated malaria. Many children with severe malaria presented with >1 severity feature (Figure 1). Mortality rates were similar (9.5%-11.1%) across the 3 main groups (cerebral malaria, respiratory distress, and severe malarial anemia) but were higher in children with 2 features and higher still (20.5%) in those with all 3 (P < .001). Conversely, the mortality rate was comparatively lower (4.8%) in those with none of the main features but who instead displayed other features such as prostration, hypoglycemia, and hyperparasitemia (Table 1).
Table 1. Demographic and Clinical Characteristics of Children with Malaria, Stratified by Severity Grouping.
| Severe Malariaa | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | All severe malaria patients | CM | SMA | RD | Other | Uncomplicated Malaria | P Valueb | Asymptomatic Malaria | P Valuec |
| Total, no. (%) | 1521 (100) | 881 (58.0) | 335 (22.0) | 477 (31.4) | 330 (21.7) | 198 (100) | NA | 28 (100) | NA |
| Female sex, no. (%) | 753 (49.5) | 442 (50.2) | 171 (51.0) | 232 (48.6) | 154 (46.7) | 92 (46.5) | <.001 | 7 (35.0) | <.001 |
| Age, (median, IQR), y | 2.4 (1.4–3.7) | 2.3 (1.4–3.6) | 1.7 (0.8–2.5) | 2.2 (1.3–3.3) | 2.8 (1.8–4.7) | 3.1 (1.9–4.3) | <.001 | 7.6 (6.5–9.5) | <.001 |
| WAZ score, mean (SD) | –1.9 (1.3) | –1.9 (1.3) | –2.1 (1.2) | –1.9 (1.4) | –1.7 (1.2) | –1.7 (0.9) | .12 | NA | NA |
| HAZ score, mean (SD) | –1.4 (1.8) | –1.3 (1.7) | –1.5 (1.8) | –1.4 (1.9) | –1.4 (1.7) | –1.5 (1.2) | .53 | NA | NA |
| Prostration (BCS score, 3 or 4), no. (%) | 271/1484 (18.3) | 0/881 (0) | 36/317 (11.4) | 89/472 (18.9) | 169/315 (53.7) | 0(0) | <.001 | NA | NA |
| Coma (BCS score <3), no. (%) | 881/1484 (59.4) | 881/881 (100) | 151/317 (47.6) | 317/472 (67.2) | 0/315 (0) | 0(0) | <.001 | NA | NA |
| Hemoglobin, mean (SD), g/dl_ | 7.0 (2.5) | 7.2 (2.4) | 3.7 (0.9) | 6.8 (2.6) | 7.8 (2.0) | 8.9 (1.6) | <.001 | 11.3 (1.2) | <.001 |
| Hematocrit, mean (SD), % | 22.1 (7.8) | 23.0 (7.5) | 11.8 (2.8) | 21.4 (8.0) | 24.6 (6.3) | 27.5 (4.7) | <.001 | NA | NA |
| Severe anemia (Hb <5 g/dl_), no. (%) | 335 (22.0) | 151 (17.1) | 335 (100) | 122 (25.3) | 0(0) | 0(0) | <.001 | NA | NA |
| RD, no. (%) | 477 (31.5) | 317 (36.2) | 122 (36.4) | 477 (100) | 0(0) | 50 (25.1) | <.001 | NA | NA |
| Hypoglycemia, no. (%) | 187 (12.3) | 126 (14.3) | 59 (17.6) | 95 (19.9) | 13 (3.9) | 8 (4.0) | <.001 | NA | NA |
| Base deficit, mean (SD), mmol/L | –10.5 (6.2) | –10.6 (5.9) | –13.1 (7.0) | –13.4 (6.4) | –7.5 (5.4) | –4.1 (4.1) | <.001 | NA | NA |
| Severe acidosis (BD <8 mmol/L), no (%) | 766/1238 (61.8) | 471/720 (65.4) | 196/260 (75.3) | 319/400 (79.7) | 111/275 (40.3) | 24/176 (13.6) | <.001 | NA | NA |
| IBI, no. (%)d | 50 (3.3) | 26 (2.9) | 15 (4.5) | 10 (2.1) | 12 (3.6) | 1 (0.5) | .02 | NA | NA |
| Hyperparasitemia, no. (%) | 454 (29.8) | 266 (30.0) | 80 (23.8) | 169 (35.4) | 93 (28.1) | 79 (39.9) | .004 | NA | NA |
| In-hospital death, no. (%) | 178 (11.7) | 128 (14.5) | 47 (14.0) | 79 (16.5) | 16 (4.8) | 0(0) | <.001 | NA | NA |
Abbreviations: BCS, Blantyre coma scale; CM, cerebral malaria; HAZ score, height-for-age z score; Hb, hemoglobin; I Bl, invasive bacterial infection; IQR, interquartile range; NA, not available or not applicable; RD, respiratory distress; SD, standard deviation; SMA, severe malarial anemia; WAZ score, weight-for-age z score.
Some children with severe malaria manifest >1 clinical feature of severity.
Between uncomplicated and all severe malaria.
Between asymptomatic and all severe malaria.
The following organisms grew in cultures from patients with severe malaria: Enterobacter cloacae (n = 1), Escherichia coll (n = 4), Haemophilus influenzae (n = 3), Neisseria species (¤ = 1), Pseudomonas species (¤ =4), Shigella sonnet (n = 1), nontyphoidal Salmonella species (n = 11), Staphylococcus aureus (n = 4), group A Streptococcus (n = 4), Streptococcus pneumoniae (n = 16), and Streptococcus viridans (n = 1). S. viridanswas cultured from a patient with uncomplicated malaria (n = 1). All IBI organisms were grown from blood cultures, except for a single S. pneumoniae infection that was detected in cerebrospinal fluid only.
Figure 1. Distribution of clinical syndromes among patients with severe malaria. Numbers represent the number of children in each group, with in-hospital mortality rates in parentheses.
Markers of Parasite Biomass
Overall, parasite densities in peripheral blood were lowest in children with asymptomatic, highest in those with uncomplicated, and intermediate in those with severe malaria (Table 2). By contrast, a stepwise increase in plasma PfHRP2 levels was seen between these 3 severity classes, with the highest (geometric mean [GM], 1369 ng/mL; 95% confidence interval [CI], 1244–1506 ng/mL) seen in those with severe malaria (Table 2).
Table 2. Quantitative Markers of Parasite Load Stratified by Severity Groupings.
| Geometric Mean (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
| Parasite Biomass, Parasites per Child | ||||||
| Patient Group | Parasite Density, Parasites/μL | PfHRP2, ng/ml | Total | Circulating | Sequestered | SI |
| Group 1: all severe malaria (n = 1521) | 39 588 (34 990–44 791) | 1369 (1244–1506) | 1.2 × 1012 (1.1 × 1012 to 1.4 × 1012) | 3.1 × 1010 (2.8 × 1010 to 3.6 × 1010) | 1.2 × 1012 (1.1 × 1012 to 1.4 × 1012) | 40.6 (35.7–46.0) |
| Group 2: uncomplicated malaria (n = 198) | 111 064 (86 798–141 819) | 843 (655–1084) | 5.2 × 1011 (4.0 × 1011 to 6.8 × 1011) | 1 1.0 × 1011 (8.2 × 1010 to 1.3 × 1011) | 6.0 × 1011 (4.3 × 1011 to 8.4 × 1011) | 4.9 (3.5–6.8) |
| Group 3: asymptomatic malaria (n = 28) | 1092 (523–2280) | 7 (4–12) | 6.4 × 109(3.9 x 109to 1.0 × 1010) | 1.5 × 109 (7.4 x 108 to 3.4 × 109) | 4.3 × 109 (1.9 × 109to9.4× 109) | 4.0 (1.8–8.8) |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Severe malaria | ||||||
| Patients who survived (n = 1343) | 43 840 (38 463–19 967) | 1401 (1268–1547) | 1.3 × 1012 (1.2 × 1012 to 1.5 × 1012) | 3.5 × l010(3.0× 1010 to 3.9 × 1010) | 1.3 × 1012 (1.1 × 1012 to 1.4 × 1012) | 38.8 (33.9–14.4) |
| Patients who died (n = 178) | 25 737 (17 495–37 843) | 1146 (833–1577) | 9.4 × 1011 (6.7 × 1011 to 1.3 × 1012) | 1.7 × 1010 (1.1 × 1010 to 2.5 × 1010) | 1.0 × 1012 (7.2 × 1011 to 1.4 × 1012) | 56.9 (39.4–82.3) |
| P value | .01 | .33 | .052 | <.001 | .10 | .01 |
Abbreviations: P/HRP2, Plasmodium falciparum histidine-rich protein 2; SI, sequestration index.
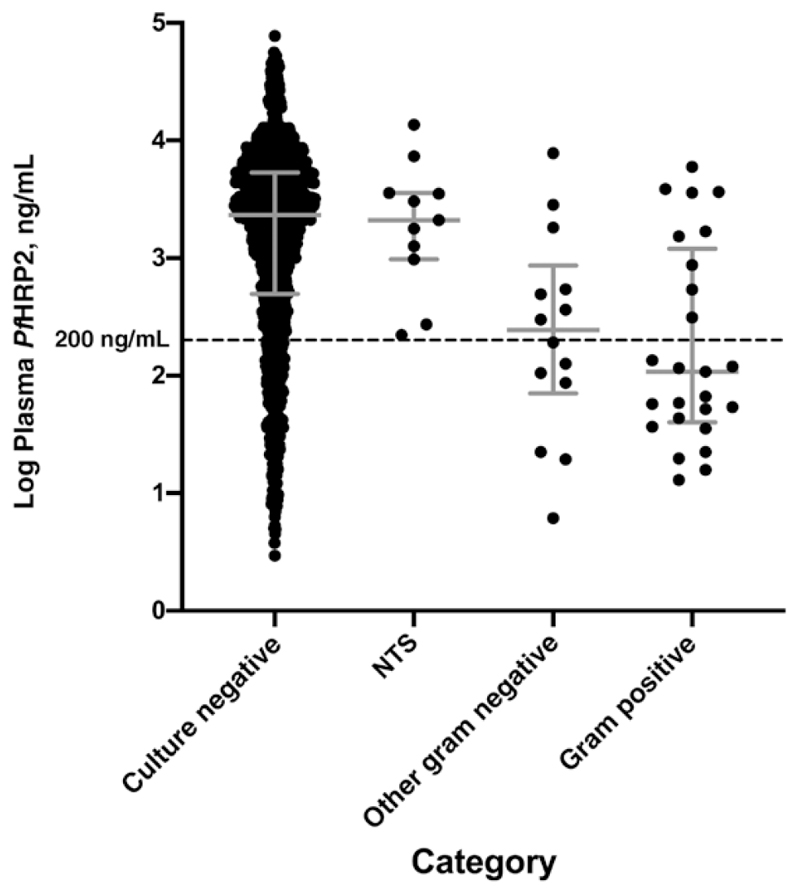
One child with uncomplicated malaria and 50 with severe malaria had concomitant invasive bacterial infections (IBIs) diagnosed by culture of blood or cerebrospinal fluid (see Table 1 footnote for organisms and origins). Among patients with severe malaria, PfHRP2 values were markedly lower in those with IBIs (GM, 312 ng/mL; 95% CI, 175–557 ng/mL) than in those without (1439 ng/mL; 1307–1584 ng/mL; P < .001). This difference was not seen in the subset of 11 infections with nontyphoidal Salmonella species (GM, 1883 ng/mL; 95% CI, 811–4371 ng/mL; P > .99), being confined to the 39 other gram-negative and gram-positive infections (188 ng/mL; 101-352 ng/mL; P < .001) (Figure 2 and Table 1). Of these 39 patients with non-Salmonella IBIs, 23 had a plasma PfHRP2 value <200 ng/mL (a previously proposed cutoff for identifying patients at high risk of an alternative diagnosis), along with 240 patients without IBI. Hence 23 of 263 patients (8.7%) with a plasma PfHRP2 level <200 ng/mL had an IBI, compared with only 16 of 1258 (1.3%) with levels above this threshold (an approximately 7-fold difference).
Figure 2.
Plasma Plasmodium falciparum histidine-rich protein 2 (P/HRP2) levels in patients with severe malaria according to category of invasive bacterial infection. Error bars represents medians with interguartile range. Categories include nontyphoidal Salmonella species (NTS; n = 11); other gram-negative bacteria, including Enterobactercloacae(n = 1), Escherichia coli(n = 4), Haemophilus influenzae (n = 3), Neisseria species (n = 1), Pseudomonas species (n = 4) Shigella sonnei (n = 1); and gram-positive bacteria, including Staphylococcus aureus (n = 4), group A Streptococcus (n = 4), Streptococcus pneumoniae (n = 16), and Streptococcus viridans (n = 1).
Plasma PfHRP2 levels exceeded 1000 ng/mL in 1007 of 1521 children (66.2%) with severe malaria and 104 of 198 (52.5%) with uncomplicated malaria but was <1000 ng/mL (range, 0.9-91.3 ng/mL) in all children with asymptomatic malaria. The parameter that most markedly differed between groups was the SI. The GM SI (95% CI) was only 4.0 (1.8-8.8) and 4.9 (3.5-6.8) in children with asymptomatic or uncomplicated malaria, respectively, but was 40.6 (35.7-46.0) in those with severe malaria (P < .001). Overall, after adjustment for HbS, α-thalassemia, and ethnicity, the GM SI was 8.3 (95% CI, 5.6-12.3) times higher in children admitted with severe malaria than in those admitted with uncomplicated malaria. Within those with severe malaria only, parasite densities were significantly lower in the subgroup who died in the hospital, although a trend was seen toward an increased SI (Table 2).
Markers of Parasite Biomass in Children With Different Phenotypes of Severe Malaria
Finally, we calculated the various measures of parasite biomass in children with specific severe malaria phenotypes and compared them with values from children in the uncomplicated group (Table 3). Among the children with severe malaria, peripheral parasite densities were lowest in those with none of the 3 major features and highest in those with respiratory distress. The most notable differences in PfHRP2-based values related to children with severe malarial anemia, in whom GM plasma concentrations were 3.2 (95% CI, 2.3–4.5; P < .001) times higher and the SI 18.2 (11.8–28.2; P < .001) times higher than in children with uncomplicated malaria.
Table 3. Linear Regression Analysis of Markers of Parasite Biomass Among Patients With Severe Malaria.
| Adjusted Multivariate Linear Regressiona | ||||
|---|---|---|---|---|
| Marker by Malaria Group | Patients, No. | GM Value (95% CI) | Exponentiated Coefficient (95% CI) | P Value |
| Parasite density, parasites/μL | ||||
| UM | 198 | 111 064 (86 798–141 819) | Reference | |
| CM | 871 | 39 046 (33 121–46 030) | 0.38 (.26–.56) | <.001 |
| SMA | 331 | 40 901 (32 193–51 964) | 0.35 (.24–.52) | <.001 |
| RD | 473 | 57 158 (46 172–70 757) | 0.54 (.36–.78) | . 001 |
| Other SM | 320 | 32 587 (24 756–42 896) | 0.28 (.19–.43) | <.001 |
| PfHRP2 (ng/ml) | ||||
| UM | 198 | 842 (655–1084) | Reference | |
| CM | 881 | 1297 (1143–1472) | 1.5 (1.1–2.0) | .006 |
| SMA | 335 | 2565 (2137–3078) | 3.2 (2.3–4.5) | <.001 |
| RD | 477 | 1728 (1466–2037) | 2.1 (1.5–2.8) | <.001 |
| Other SM | 330 | 1026 (829–1270) | 1.1 (.9–1.6 | .46 |
| Total biomass parasites/child | ||||
| UM | 191 | 5.2 × 1011 (4.0 × 1011 to 6.8 × 1011) | Reference | |
| CM | 876 | 1.2 × 1012 (1.0 × 1012 to 1.4 × 1012) | 2.2 (1.6–3.0) | <.001 |
| SMA | 335 | 2.3 × 1012 (1.9 × 1012 to 2.8 × 1012) | 4.7 (3.3–6.5) | <.001 |
| RD | 473 | 1.5 × 1012 (1.3 × 1012 to 1.8 × 1012) | 2.9 (2.1–4.1) | <.001 |
| Other SM | 328 | 1.1 × 1012 (8.5 × 1011 to 1.3 × 1012) | 1.9 (1.3–2.7) | .001 |
| SI | ||||
| UM | 191 | 4.9 (3.5–6.8) | Reference | |
| CM | 875 | 38.7 (32.6–46.0) | 72 (4.8–11.0) | <.001 |
| SMA | 335 | 82.4 (64.5–105) | 18.2 (11.8–28.2) | <.001 |
| RD | 473 | 36.1 (28.6–45.5) | 74 (4.8–11.2) | <.001 |
| Other SM | 327 | 35.8 (27.4–46.7) | 76 (4.9–12.0) | <.001 |
Abbreviations: CI, confidence interval; CM, cerebral malaria; GM, geometric mean; PfHRP2, Plasmodium falciparum histidine-rich protein 2; RD, respiratory distress; SI, sequestration index; SM, severe malaria; SMA, severe malarial anemia; UM, uncomplicated malaria.
Linear regression analysis was adjusted for Hemoglobin S genotype (HbS), α-thalassemia genotype, and ethnicity. Values show the exponentiated coefficients of the log10-transformed data.
Discussion
We have estimated both the peripheral and sequestered burdens of P. falciparum parasites in children with malaria of differing severity in Kilifi County, Kenya. We found no significant differences in the SI between those with asymptomatic and uncomplicated P falciparum malaria infections, but the SI was approximately 8 times higher in those with severe malaria, being particularly high in those with severe malarial anemia. Our study supports the hypothesis that malaria severity is proportionate to total parasite load, an observation that could be helpful in directing care to those at greatest need [5, 10].
A direct relationship between parasite load and malaria severity was first suggested in the late 19th century [23], and most studies that have investigated this question more recently through PfHRP2-based methods have supported this general conclusion. In the first study of that kind, a strong relationship was seen with total parasite load in adults admitted to hospital on the Thai-Burmese border [7]. Loads were approximately 6 times higher in patients with severe than in those with nonsevere malaria and were especially high in those who later died. Similar results were found in a second study, in Indonesian adults [8].
Children presenting in coma with a positive malaria blood film result in Africa are generally assumed to have cerebral malaria. However, asymptomatic parasitemia is common during childhood, and one postmortem study showed that many such children have an alternative diagnosis [24]. Subsequently, Seydel and colleagues [25] demonstrated the potential utility of plasma PfHRP2 concentration as a marker of intracerebral parasite sequestration, identifying children with cerebral malaria confirmed by either autopsy or malarial retinopathy. Later studies have confirmed the strong relationship between total parasite burden and malaria severity in African children [10, 26]. First, within the multinational AQUAMAT trial, high PfHRP2 concentrations were found in the majority of children while levels were significantly higher in those who died than in those who survived. Of particular interest, a U-shaped association was seen between PfHRP2 concentration and death, potentially explained by the misclassification to malaria of the subgroup with the lowest concentrations [10].
In a subsequent study conducted in Tanzanian children, the plasma PfHRP2 level was 19 (15–23) ng/mL in asymptomatic carriers, 163 (137–194) ng/mL in children with uncomplicated malaria, detected in the community, and 1510 (1180–1933) and 1746 (1577–1934) ng/mL among children with severe malaria admitted during 2 different time periods [26]. A concentration of <200 ng/mL was found to indicate severe febrile illness caused by an alternative diagnosis in >10% of patients. Our data relating to IBIs extend these findings and exemplify the potential for plasma levels to contribute to clinical diagnosis. PfHRP2 levels were substantially lower in the subgroup of children with severe malaria who also had a concomitant IBI.
As expected, this was not the case for the subset whose cultures were positive for nontyphoidal Salmonella species, because previous studies have shown that IBIs due to Salmonella can complicate malaria via a mechanism involving gut barrier dysfunction [27]. Compared with children without IBIs, plasma PfHRP2 levels were approximately 7.5 times lower in children with other gram-negative as well as gram-positive bloodstream infections, indicating that these IBIs were likely to have been the main agents of severe illness in many of these children. In our study, plasma PfHRP2 levels were <200 ng/mL in only one-sixth of severe cases but identified 23 of the 39 children with non-Salmonella IBIs: that is, approximately 9% of children with these low PfHRP2 levels had an IBI. The 16 other children with such IBIs were among 1258 with a PfHRP2 level above this cutoff (1.3%). Hence, the 200-ng/mL threshold enriched for children with non–malaria-associated IBIs by approximately 7-fold.
These findings are similar to those of Hendriksen and colleagues [26], although in their study the relationship between plasma PfHRP2 concentration and distinct categories of IBI was less clear cut than in the current study. PfHRP2 levels have also been correlated with childhood cerebral malaria in a number of smaller studies conducted in Tanzania [9], Malawi [11], and Uganda [12], and with various forms of adult malaria among imported cases in France [28]. At present, there are no point-of-care methods for quantifying PfHRP2. The development of such tests in the future would be of major potential benefit.
The pathogenesis of severe falciparum malaria is complex, but a compromised microcirculation in vital organs caused by the sequestration of cytoadhered parasitized red blood cells to the vascular endothelium is central, compounded by endothelial dysfunction, reduced red blood cell deformability [29], and rosetting [30]. Other complications of the disease might relate to a disordered inflammatory response or oxidative damage caused by plasma free hemoglobin [31]. Moreover, the likelihood that any particular P falciparum infection will progress to become severe or fatal probably depends on a wide range of genetic, immunological, physiological, and behavioral characteristics of both the host and the infecting parasite [30].
With specific reference to our current study, the central role played by sequestration is a question that has been long debated. Although florid sequestration of parasites in the small vessels of multiple organs is a consistent feature of postmortem studies and is supported by observations of microvascular blockage in the retina and rectal mucosa in living patients with severe falciparum malaria [32], it is likely that other pathophysiological processes also play a major role. However, the results of our current study, which mirror those from the majority of previous studies, support the conclusion that the most common and dangerous clinical complications of P. falciparum are directly related to the sequestered parasite load.
Most previous studies of severe malaria have been too small to investigate differences in sequestration between children with different clinical phenotypes. However, the relatively large sample size allowed this in our current study. We found that the SI was high across a range of different phenotypes but particularly high in severe malarial anemia. As with most complications, the etiology of severe anemia is multifactorial, involving both hemolysis and an inappropriately low erythropoietic response [33]. Nevertheless, our findings suggest that such processes may also be related to parasite load.
The children with “uncomplicated” malaria who we recruited to our current study were significantly sicker than those in many previous studies, including the Tanzanian study described above [26], because we enrolled children from a hospital as opposed to an outpatient setting. This may explain why a surprising proportion had a PfHRP2 value of >1000 ng/mL, a value that has been proposed as a threshold for identifying children with “true” severe malaria [6, 26]. The study by Rubach et al [9] also found that a substantial proportion of children with uncomplicated malaria had a plasma PfHRP2 of >1000 ng/mL, again perhaps reflecting a sicker population, although the median level was still less than half that in children with severe malaria.
In our current study there was considerable overlap in plasma PfHRP2 concentrations between the uncomplicated and severe malaria groups, but the SI was more discriminatory, being approximately 8-fold higher in the severe than in the uncomplicated malaria group, in which the SI was similar to that in the group with asymptomatic malaria. The SI of approximately 40 is close to estimates based on the postmortem examination of brain tissue from Southeast Asian patients with cerebral malaria [34, 35]. The low predictive value of peripheral parasite densities is a consistent finding in previous studies [10, 26]. Also of relevance is the proportion of mature-stage parasites, consistent with the hypothesis that this also reflects the total parasite biomass and thus the severity of the disease [36]. Unfortunately, peripheral blood parasites were not staged in the current study.
Although a positive correlation between plasma PfHRP2 and malaria severity has been found in the majority of studies, this has not been universal. In one study from Papua New Guinea, no significant difference in PfHRP2 was found between children with severe versus uncomplicated malaria [13]. In agreement with observations from elsewhere in the Pacific [37], case fatality was very low in that study, and PfHRP2 concentrations in children defined as having severe malaria were considerably lower than in other studies. The same profile of low mortality rate and low PfHRP2 concentrations was reported in a second study, conducted in the Gambia [14], in which the authors also found no correlation between plasma PfHRP2 and severity. The lack of agreement between these studies and our own might thus be explained by different definitions for severe malaria.
There are 2 potential caveats to the use of PfHRP2 levels for predicting prognosis and directing care to those at greatest risk. First is the recent recognition that deletions in the genes encoding pfhrp2 (and its homolog pfhrp3) mean that some clones of P. falciparum parasites do not express PfHRP2 [38]. Although the existence of such parasites is now well established, at present they have only been found in high proportions in Latin America and the horn of Africa [39, 40]; rates of >40% have been detected in a number of studies in the Peruvian Amazon. Rates >2% are rare among studies from sub-Saharan Africa [38] (eg, Ghana [41] and Rwanda [42]). This increasing trend toward the presence of pfhrp2 deletions could undermine the utility of quantitative PfHRP2 levels going forward. Furthermore, our observations cannot be extended to nonfalciparum infections, which are common in parts of Ethiopia and Eritrea [40].
The second caveat relates to the recognition that some individuals produce antibodies to PfHRP2 that could potentially reduce the levels measurable in plasma [43]. Although such antibodies are common in some settings, the degree to which they suppress plasma levels is yet to be determined [43]. In the current study, PfHRP2 was undetectable despite the presence of P. falciparum parasites in peripheral blood in only 1.7% of the children, suggesting that despite the above caveats, PfHRP2-based risk-assessment methods remain currently useful.
In summary, we have found that the P. falciparum parasite load, as estimated through measurement of plasma PfHRP2, is strongly related to the severity of clinical malaria in children on the Kenyan coast, and that a low plasma PfHRP2 level suggests an alternative pathological mechanism. This does not mean that severely ill children with a positive blood film result but a low PfHRP2 level should be denied appropriate antimalarial treatment, nor that antibiotics should be withheld from those with a high PfHRP2 level. Both should be treated empirically, as currently recommended in the World Health Organization guidelines [18], but clinicians should be alert to the potential for an alternative diagnosis. Our observation adds weight to the hypothesis that treatments that reduce sequestration, such as the heparinlike molecule sevuparin [44], might be useful in mitigating or reversing disease severity in patients infected with P falciparum parasites.
Supplementary Material
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank Emily Nyatichi and Metrine Tendwa for laboratory support, the staff of Kilifi County Hospital and the KEMRI-Wellcome Trust Research Programme, Kilifi, for their help with data and sample collection, and the study participants and their parents for participating in this study. This paper is published with permission from the director of the Kenya Medical Research Institute (KEMRI).
Financial support
This work was supported by the Wellcome Trust (grants 203077/Z/16/Z to the KEMRI-Wellcome Trust Research Programme in Kilifi, Kenya, 209265/Z/17/Z to A. M. D. and K. M., and 089275/Z/09/Z to A. M. D.; and senior research fellowships 202800/Z/16/Z and 091758/Z/10 to T N. W.). S. U. was funded through the DELTAS Africa Initiative (DEL-15-003), an independent funding scheme of the African Academy of Sciences’ Alliance for Accelerating Excellence in Science in Africa that is supported by the New Partnership for Africa’s Development Planning and Coordinating Agency with funding from the Wellcome Trust (grant 203077/Z/16/Z) and the UK government.
Footnotes
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Stahl HD, Kemp DJ, Crewther PE, et al. Sequence of a cDNA encoding a small polymorphic histidine- and alanine-rich protein from Plasmodium falciparum . Nucleic Acids Res. 1985;13:7837–46. doi: 10.1093/nar/13.21.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan DJ, Jr, Gluzman IY, Goldberg DE. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science. 1996;271:219–22. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]
- 3.Parra ME, Evans CB, Taylor DW. Identification of Plasmodium falciparum histidine-rich protein 2 in the plasma of humans with malaria. J Clin Microbiol. 1991;29:1629–34. doi: 10.1128/jcm.29.8.1629-1634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desakorn V, Dondorp AM, Silamut K, et al. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum . Trans R Soc Trop Med Hyg. 2005;99:517–24. doi: 10.1016/j.trstmh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Sinha I, Ekapirat N, Dondorp AM, Woodrow CJ. Use of a rapid test to assess plasma Plasmodium falciparum HRP2 and guide management of severe febrile illness. Malar J. 2015;14:362. doi: 10.1186/s12936-015-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol. 2020;36:112–26. doi: 10.1016/j.pt.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo TW, Lampah DA, Gitawati R, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A. 2008;105:17097–102. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubach MP, Mukemba J, Florence S, et al. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian children. PLoS One. 2012;7:e35985. doi: 10.1371/journal.pone.0035985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L, et al. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma Pf HRP2 measurement. PLoS Med. 2012;9:e1001297. doi: 10.1371/journal.pmed.1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox LL, Taylor TE, Pensulo P, et al. Histidine-rich protein 2 plasma levels predict progression to cerebral malaria in Malawian children with Plasmodium falciparum infection. J Infect Dis. 2013;208:500–3. doi: 10.1093/infdis/jit176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park GS, Opoka RO, Shabani E, Wypyszynski A, Hanisch B, John CC. Plasmodium falciparum histidine-rich protein-2 plasma concentrations are higher in retinopathy-negative cerebral malaria than in severe malarial anemia. Open Forum Infect Dis. 2017;4:ofx151. doi: 10.1093/ofid/ofx151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manning L, Laman M, Stanisic D, et al. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations do not reflect severity of malaria in Papua New Guinean children. Clin Infect Dis. 2011;52:440–6. doi: 10.1093/cid/ciq105. [DOI] [PubMed] [Google Scholar]
- 14.Cunnington AJ, Bretscher MT, Nogaro SI, Riley EM, Walther M. Comparison of parasite sequestration in uncomplicated and severe childhood Plasmodium falciparum malaria. J Infect. 2013;67:220–30. doi: 10.1016/j.jinf.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunnington AJ, Riley EM, Walther M. Stuck in a rut? reconsidering the role of parasite sequestration in severe malaria syndromes. Trends Parasitol. 2013;29:585–92. doi: 10.1016/j.pt.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyoga S, Ndila CM, Macharia AW, et al. MalariaGEN Consortium. Glucose-6-phosphate dehydrogenase deficiency and the risk of malaria and other diseases in children in Kenya: a case-control and a cohort study. Lancet Haematol. 2015;2:e437-44. doi: 10.1016/S2352-3026(15)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Severe malaria. Trop Med Int Health. 2014;19(suppl):7–131. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- 19.Olotu A, Fegan G, Williams TN, et al. Defining clinical malaria: the specificity and incidence of endpoints from active and passive surveillance of children in rural Kenya. PLoS One. 2010;5:e15569. doi: 10.1371/journal.pone.0015569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams TN, Mwangi TW, Wambua S, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–86. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams TN, Uyoga S, Macharia A, et al. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet. 2009;374:1364–70. doi: 10.1016/S0140-6736(09)61374-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams TN, Wambua S, Uyoga S, et al. Both heterozygous and homozygous alpha+thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–71. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 23.Marchiafava E, Bignami A. In: Two monographs on malaria and the parasites of malarial fevers. Marchiafava E, editor. New Sydenham Society; London: 1894. On summer-autumn malarial fevers; pp. 1–232. (translated from the first Italian edition by JH Thompson) [Google Scholar]
- 24.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 25.Seydel KB, Fox LL, Glover SJ, et al. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J Infect Dis. 2012;206:309–18. doi: 10.1093/infdis/jis371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendriksen IC, White LJ, Veenemans J, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis. 2013;207:351–61. doi: 10.1093/infdis/jis675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med. 2014;12:31. doi: 10.1186/1741-7015-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argy N, Kendjo E, Augé-Courtoi C, et al. CNRP study group. Influence of host factors and parasite biomass on the severity of imported Plasmodium falciparum malaria. PLoS One. 2017;12:e0175328. doi: 10.1371/journal.pone.0175328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishioka H, Ghose A, Charunwatthana P, et al. Sequestration and red cell deformability as determinants of hyperlactatemia in falciparum malaria. J Infect Dis. 2016;213:788–93. doi: 10.1093/infdis/jiv502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahlgren M, Goel S, Akhouri RR. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat Rev Microbiol. 2017;15:479–91. doi: 10.1038/nrmicro.2017.47. [DOI] [PubMed] [Google Scholar]
- 31.Plewes K, Turner GDH, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31:69–77. doi: 10.1097/QCO.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White NJ, Turner GD, Day NP, Dondorp AM. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis. 2013;208:192–8. doi: 10.1093/infdis/jit116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casals-Pascual C, Kai O, Cheung JO, et al. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108:2569–77. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 34.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvas-cular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pongponratn E, Turner GD, Day NP, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–59. [PubMed] [Google Scholar]
- 36.Silamut K, White NJ. Relation of the stage of parasite development in the peripheral blood to prognosis in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1993;87:436–43. doi: 10.1016/0035-9203(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 37.Maitland K, Williams TN, Peto TE, et al. Absence of malaria-specific mortality in children in an area of hyperendemic malaria. Trans R Soc Trop Med Hyg. 1997;91:562–6. doi: 10.1016/s0035-9203(97)90026-2. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Q, Gatton ML, Barnwell J, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J. 2014;13:283. doi: 10.1186/1475-2875-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akinyi Okoth S, Abdallah JF, Ceron N, et al. Variation in Plasmodium falciparum histidine-rich protein 2 (Pfhrp2) and Plasmodium falciparum histidine-rich protein 3 (Pfhrp3) gene deletions in Guyana and Suriname. PLoS One. 2015;10:e0126805. doi: 10.1371/journal.pone.0126805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berhane A, Anderson K, Mihreteab S, et al. Major threat to malaria control programs by Plasmodium falciparum lacking histidine-rich protein 2, Eritrea. Emerg Infect Dis. 2018;24:462–70. doi: 10.3201/eid2403.171723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owusu EDA, Djonor SK, Brown CA, Grobusch MP, Mens PF. Plasmodiumfalciparum diagnostic tools in HIV-positive under-5-year-olds in two ART clinics in Ghana: are there missed infections? Malar J. 2018;17:92. doi: 10.1186/s12936-018-2231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozycki CT, Umulisa N, Rulisa S, et al. False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission. Malar J. 2017;16:123. doi: 10.1186/s12936-017-1768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho MF, Baker J, Lee N, et al. Circulating antibodies against Plasmodium falciparum histidine-rich proteins 2 interfere with antigen detection by rapid diagnostic tests. Malar J. 2014;13:480. doi: 10.1186/1475-2875-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saiwaew S, Sritabal J, Piaraksa N, et al. Effects of sevuparin on rosette formation and cytoadherence of Plasmodium falciparum infected erythrocytes. PLoS One. 2017;12:e0172718. doi: 10.1371/journal.pone.0172718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.