Abstract
Regenerative medicine is aimed at restoring normal tissue function and can benefit from the application of tissue engineering and nano-therapeutics. In order for regenerative therapies to be effective, the spatiotemporal integration of tissue engineered scaffolds by the native tissue, and the binding/release of therapeutic payloads by nano-materials, must be tightly controlled at the nanoscale in order to direct cell fate. However, due to a lack of insight regarding cell-material interactions at the nanoscale and subsequent downstream signaling, the clinical translation of many regenerative therapies is limited due to poor material integration, rapid clearance and complications such as graft-versus-host disease. This review paper is intended to outline our current understanding of cell-material interactions with the aim of highlighting potential areas for knowledge advancement or application in the field of regenerative medicine. This is achieved by reviewing the nanoscale organization of key cell surface receptors, the current techniques used to control the presentation of cell-interactive molecules on material surfaces, as well as the most advanced techniques for characterizing the interactions that occur between cell surface receptors and materials intended for use in regenerative medicine.
Keywords: Bio-mimicry, bio-instructive, biomaterials, nanoscale ligand spacing, functionalization, cell adhesion, integrins, receptor clustering
1. Introduction
The goal of regenerative medicine is to restore normal tissue function by combining molecular biology and material science [1]. The translational research that underpins regenerative medicine often employs a ‘biomaterial’ that is implanted to actively augment existing biological processes and facilitate repair. Such biomaterials range in scale from tissue engineered scaffolds intended for whole organ replacement, to nano-materials intended for targeted therapeutic drug delivery.
In order for regenerative therapies to be effective, the spatiotemporal integration of tissue engineered scaffolds by the native tissue, and the binding/release of therapeutic payloads by nano-materials, must be tightly controlled using our understanding of cell-material signaling interactions. However, due to a lack of insight regarding cell-material signaling interactions at the nanoscale, the majority of implanted biomaterials are either rejected by the host or rapidly cleared from the tissue, thus limiting the current clinical translation status of many regenerative therapies. Improved biomaterial fabrication and characterization at the nanoscale could advance our understanding of the complex presentation of nanoscale material-based molecules to cells, and the subsequent signaling reaction at the single cell level, and therefore aid in directing therapies towards effective regenerative outcomes [2].
Here, we outline our current understanding of cell-material signaling interactions by reviewing the organization of key cell surface receptors, the current techniques used to control the presentation of cell-interactive molecules on biomaterial surfaces, as well as presenting several of the most important and advanced techniques for probing the interactions that occur between cell surface receptors and biomaterials intended for use in regenerative medicine.
2. Nanoscale cell surface receptor regulation for bio-instructive therapeutic design
Complex biophysical regulation of cell signaling occurs at the nanoscale and governs the processes of tissue development, maturation, homeostasis and repair. Physical and chemical stimuli from other cells, the extracellular matrix (ECM) or soluble signaling molecules cause specific, controlled downstream signaling cascades. In this way, cells can both sense environmental cues and respond by modifying their behavior, or altering the synthesis/breakdown of ECM in their immediate surroundings. Errors in signaling and processing of cellular information can result in disease, with cells no longer able to control their microenvironment or react to pathological changes. Recent technological advances, such as those in super-resolution microscopy (see Section 4), now enable visualization and quantification of cell surface receptor number, clustering and subsequent signaling at the nanoscale. Adhesion receptors enable cells to bind, sense and respond to their environment through nanoscale organization of their surface presentation. There are four adhesion receptor superfamilies; integrins, cadherins, selectins and immunoglobulin (Ig) cell adhesion molecules (CAMs). Here, we focus on integrins (the major cell-matrix adhesion receptors) and cadherins (prevalent cell-cell adhesion receptors) (Fig. 1), and how they provide important targets in regenerative medicine to ensure that the interactions between implanted biomaterials and the surrounding cells lead to effective regenerative outcomes.
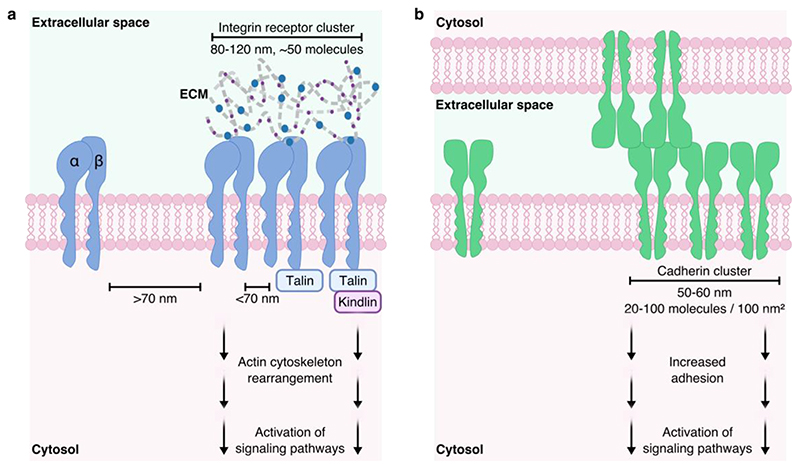
Fig. 1. Cell surface adhesion receptor spatial presentation.
(a) Integrin clustering occurs when integrins are closer than 70 nm. Integrin receptor clusters are highly controlled, with cluster sizes found conserved at 80-120 nm in diameter and containing around 50 molecules. Integrins additionally bind to intracellular adaptor proteins, such as talin and kindlin, further stabilizing the clusters. These adaptor proteins additionally bind the actin cytoskeleton, transmitting the forces generated by integrins that bind to the extracellular matrix (ECM), into migratory cell behavior. (b) Cadherin cluster size is highly conserved at around 50-60 nm in diameter, however molecular density varies in range from 20-100 molecules per 100 nm2. Cadherins form cis-homodimers, which laterally associate into clusters and trans-dimerize with cadherins in neighboring cell membranes forming cell-cell adhesions.
2.1. Role of integrins in health, disease and regenerative medicine
Integrins are a superfamily of 24 known transmembrane heterodimeric adhesion receptors, formed from the non-covalent interaction between an alpha (18 subtypes) and a beta (8 subtypes) subunit [3], and are around 12 nm in size [4]. They are involved in integrating chemical and mechanical signals in a bidirectional manner across the plasma membrane, enabling cells to sense and respond to their extracellular environment through coordination with intracellular pathways [5]. Integrins are almost ubiquitously expressed across all cell types, and bind a wide range of ligands, with several integrins capable of binding the same ligand [6]. Specifically, many integrins bind components of the ECM, such as collagen (α1β1, α2βl, α10β1, α11β1) [7], laminin (α3β1, α6β1, α6β4, α7β1) [8] and fibronectin (α5β1, αvβ3) [4], [9], making them vital for cell adhesion and migration. The conformation of the integrin receptor provides further regulation of ligand binding, with inactive integrins assuming a bent conformation. Inactive integrins can then be activated through force generation or intracellular biochemical interactions, leading to an extended conformation which induces an increase in affinity for its’ ligand and strong adhesion to the ECM [10]. Importantly, additional signaling mechanisms arise due to integrin clustering at the plasma membrane following ligand binding. Using a rigid template of gold nanodots functionalized with the binding peptide RGD, it was demonstrated that cells recognize integrins as being clustered when the receptors are less than 70 nm apart [11]. This clustering directly influenced cell adhesion and spreading on the substrate. Despite the large number of different integrins and variation in their ligands and biological purpose, individual integrin clusters have been shown to remain around 80-120 nm in diameter (Fig. 1), and contain around 50 molecules [12]. This conserved integrin cluster size was exhibited by cells interfacing with substrates of widely varying rigidities, and thus can be considered a mechanism of cell adhesion applicable to most tissues of the body. Integrin clusters form a key component of the cellular adhesome [13], ranging from early nascent adhesions, with <1 μm assemblies of clusters of active integrins, to mature focal adhesions, 1-5 μm in size, linking the ECM to the cell cytoskeleton via a complex of intracellular adaptor proteins, including talins and kindlins [14]–[17]. Constant recycling and endocytosis of integrins at the membrane facilitates cell migration through generation of new adhesion sites of active integrin clusters [18].
Integrin clustering is crucial to the correct functioning of immune cells. There are several leukocyte specific integrins that are specialized for immune regulatory functions and tissue repair [19]–[21]. The T cell specific integrin, αLβ2 (LFA-1), is activated at sites of inflammation and induces its extended conformation to enable the T cell to bind and cross the endothelium and reach sites of injury [21]–[23]. It was understood that clustering of αLβ2 at the immune synapse enables communicaton between T cells and antigen presenting cells (APCs) [24], but the signaling mechanisms remain unclear. Consequently, several material approaches have been exploited to further understand the effect of ligand spatial clustering on communication between T cells and APCs. These include the use of micropatterning of costimulatory ligands [25], supported lipid bilayers presenting tethered proteins [26] and biofunctionalized gold nanoarrays [27]. The strength of T cell response on the CD-3 functionalized gold nanoarrays decreased with increased ligand spacing, with 69 nm spacing generating only a background T cell response [27]. Thus ligand spacing at the nanoscale can dramatically affect the immune response.
Due to the role of integrins in cell adhesion and migration, many integrins are implicated in pathologies such as infection, inflammation and cancers. Various infectious agents have been shown to exploit integrin binding to enable their cellular internalization, while changes in integrin presentation and clustering are known to be implicated in virus and bacterial entry into cells. Staphylococcus aureus binds fibronectin which mediates an interaction with integrin αIIbβ3 [28], Papilloma virus binds α6β4 directly [29], and Ebola virus binds α5β1 [30]. Blocking the interaction between specific viruses and integrin clusters on the host cells is being explored as a promising route to anti-viral therapies. Tumor growth and invasion partially involves ECM binding [31] and several integrins have been identified as key players in carcinogenesis of various tissues, including α5β1 [32]–[34] and αvβ3 [35]. Many nanotherapeutics are therefore aimed toward blocking integrin signaling. PEGylated titanium dioxide nanoparticles were shown to inhibit cancer cell migration via decreasing the cell surface expression of β1 integrins [36]. Gold nanoparticles targeted to integrin αvβ3 inhibited the integrin-dependent melanoma tumor cell adhesion to vitronectin, with very low non-specific background binding, making them a highly selective diagnostic probe and therapy [37]. Recently a study quantified the density of integrin αvβ3 on glioblastoma cells and found that the density of receptors dictated the cell response to inhibitor molecules [38]. Cell viability and invasion following the inhibitor treatment correlated with the density of the integrin receptor at the surface.
Several tissue engineering studies have recently tried to incorporate the precise positioning of ligands into their material designs to better understand how ligand density can affect cell-material interactions, particularly through integrin clustering. Functionalizing titanium implants with polymer brushes coated with clusters of different fibronectin domains, improved implant-tissue integration in vivo through enhanced integrin α5β1 clustering and binding [39]. The interplay between spacing of RGD within nanoarrays (46 and 95 nm) and the size of the arrays (35 and 65 μm length) showed a complex relationship between the length scales on differentiation of mesenchymal stem cells down adipogenic and osteogenic lineages [40]. Using elastin-like electrospun fabric one study showed clustering of ligands enhanced integrin-dependent clustering and subsequent signaling as a function of the global ligand density [41]. Furthermore, they determined that clustered ligands enhanced cell proliferation and increased the number of focal adhesions. Most recently, integrin-specific hydrogels enhanced the survival and osteo-reparative functions of MSCs by modulating their cytokine production and gene expression of factors associated with bone formation and immunomodulation [42]. Much remains to be understood regarding the downstream signaling of clustered integrins and regulation of receptor availability at the cell surface. However, cell-material interaction studies have enabled a better understanding of how integrin clustering at the nanoscale affects cell behavior at the microscale. Future studies should incorporate precise ligand positioning on the material surface to ensure that biomaterials intended for regenerative medicine applications are integrated effectively into the host.
2.2. Role of cadherins in health, disease and regenerative medicine
Cadherins are a superfamily of membrane-spanning adhesion molecules, formed of homodimers with the extracellular portion measuring 20 nm in length [43], that associate into macromolecular complexes at the cell surface. In humans, over 80 different types of cadherins have been sequenced [44]. Cadherins are present in almost all cell types and are involved in cell-cell junctions, cell polarity and hence structural integrity of tissues [45]. There are several cadherin subtypes, classified primarily by the location in which they are found, e.g. neural (N)-cadherin and epithelial (E)-cadherin [46]. The biological function of cadherins is regulated at the molecular level via their organization into lateral clusters at the cell surface, which are distributed extensively throughout cell-cell junctions. Cadherin nanoclusters have been demonstrated, using super-resolution microscopy, to maintain a diameter of 50-60 nm (Fig. 1), with cadherin molecule densities varying in magnitude between 20-100 /100 nm2 [47]–[49]. Larger microclusters of 1-2 μm form from aggregates of ligand-bound nanoclusters [50]. The cell-cell adhesion of cadherins is driven by cis/trans dimerization of the homomers [43], [51]. Cadherins in the same plasma membrane form cis dimers (parallel), which interact with cadherins in the plasma membrane of the adjacent cell to form trans dimers [52]. Accordingly, close spatial presentation of cadherins is implicated in their effective function. The clustering of cadherins is believed to strengthen adhesion, with adhesive strength correlating with the number of microclusters [50], [53]. Lateral clustering also provides a signaling hub through interactions with other proteins, such as catenins, as well as a mechanical link to the actin cytoskeleton [54]. Using colloidal lithography, a threshold of 173 nm diameter patterns was estimated to be necessary for epithelial cell attachment to E-cadherin [55]. In order to understand the relationship between receptor density and the adhesive forces of cadherins, one study used self-assembled monolayers of thiols, to which they bound extracellular fragments of E- cadherin, and measured cell binding using single molecule force spectroscopy (SMFS) [56]. They found a lateral distance of 5-11 nm was optimal for E-cadherin function. Similar to integrins, cadherins are involved in cellular migration, immune surveillance and wound healing. During development, cadherins assist in the positioning of cells [57]–[59] through a process termed epithelial-to-mesenchymal transition (EMT) whereby epithelial cells lose their cell-cell connections (through regulated decreased expression of cadherins), reorganize their cytoskeleton and acquire migratory behavior [60]. However, EMT is also a mechanism associated with pathologies involving dysregulation of wound healing. This includes fibrosis [61] and cancer [62]. Fibrosis is a major hurdle in regenerative medicine, as introducing foreign materials into the body can induce fibrosis, negatively impacting the biochemical and mechanical properties of the regenerated tissue [63], [64]. Recent studies using materials aimed at clustering cadherins are exploring their use in improved tissue implantation without inducing fibrosis. EMT causes significant problems for the use of vascular implants, such as stents, with stiff substrates causing endothelial cells to lose their phenotype and undergo EMT. This phenomenon was studied on poly-L-lysine/hyaluronate acid multilayer films with controlled stiffness [65]. Cadherin mimetic peptides immobilized on material surfaces were shown to induce increased cadherin surface expression and clustering which in turn increased epithelial cell adhesion [66]. Percutaneous titanium implants, functionalized with E-cadherin, demonstrated increased epidermal adhesion with limited fibroblast attachment, thereby providing a promising approach to skin grafts with improved implant integration and decreased fibrotic scarring [67]. The EMT phenotype is also associated with invasion and migration of cancer cells and material properties are now being studied for their effect on tissue stiffness and the induction of EMT phenotypes as a tool to study tumorigenesis as well as a basis for chemotherapeutics [65], [68], [69]. Chitosan-hyaluronan membranes were used to study 3D tumor spheroids and found hyaluronan concentration scaled with increasing tumor size and higher EMT phenotype, including increased expression of cadherins and tumor invasiveness [68]. Nanoparticle based delivery systems aimed at upregulation of E-cadherin are thought to be promising approach to inhibiting the progression of certain cancers. Unmodified gold nanoparticles were shown to upregulate E-cadherin expression and reverse EMT, thereby inhibiting tumor growth in two models of ovarian cancer [70].
Biomaterial exploitation of cadherin clustering, and therefore the functioning of cell-cell adhesions, is less widely investigated compared to integrin clustering, and research in this area is likely to increase in the coming years. The information presented in the preceding section, regarding how spatial presentation of ligands influences cell fate, could be readily employed to overcome many of the current problems facing the clinical translation of biomaterials and nano-materials, such as rejection by the host or rapid clearance from the tissue.
3. Material based techniques for studying cell surface receptors and fabricating cell instructive biomaterials
The material-based research that underpins regenerative medicine encompasses the fields of tissue engineering and nano-therapeutics. Tissue engineering aims to develop treatments for specific tissue defects by providing a scaffold that replicates the structural and spatiotemporal signaling complexity of the tissue microenvironment, thereby providing a platform that supports cell integration and ECM formation. While, nano-therapeutics utilize nano-particles, decorated with functional groups, binding domains or growth factors, to detect and target specific cell surfaces or molecules to induce local responses [71]–[74].
Tissue engineering and nano-therapeutics both rely on the precision guidance of cell fate to replicate or exploit tissue architecture and function. Utilizing our understanding of the cellular interactions that influence tissue development, immunity and repair, along with exact surface receptor spacing, is essential to triggering specific material-cell signaling responses. Here we discuss the recent advances in material fabrication techniques that are used to incorporate bioactive molecules onto the surfaces of materials in order to improve our understanding of material-cell signaling (Fig. 2).
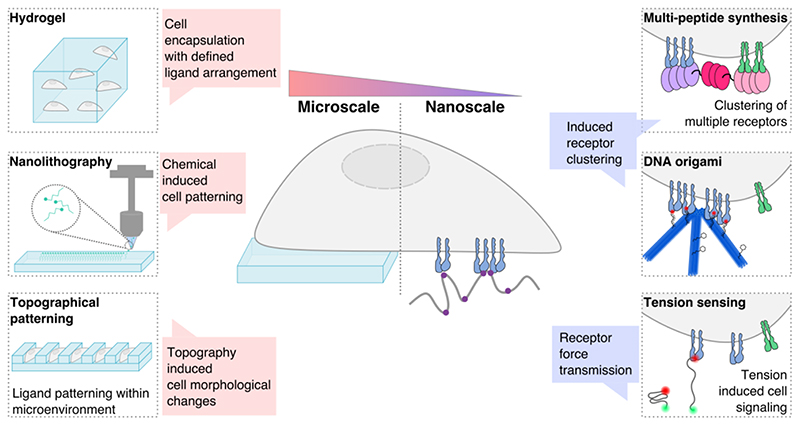
Fig. 2. Material techniques for studying cell-material interactions.
Fabrication techniques range from the micro to the nanoscale, thereby influencing cell fate across multiple length scales. Cells can be encapsulated in hydrogels at the macroscale, with ligands arranged for cell binding. Nanolithography enables precise patterning of ligands onto material surfaces for the study of cell receptor spacing and cell-material interactions. Topography can be introduced on material surfaces, alongside biochemical ligand patterning, thereby defining the microenvironment. Engineering multi-peptide complexes enables binding of several receptors, bringing them in close association. DNA origami provides a tool for precise ligand presentation and hence the study of receptor clustering. Tension sensors enable the study of the downstream signaling that is associated with force generation following receptor binding. All of these techniques enable the study of the nanoscale receptor spatial organization, and the downstream effects on cell fate when interfaced with a biomaterial.
3.1. Hydrogels
Hydrogels are comprised of hydrophilic polymer chains, connected by physical or chemical cross-links, dispersed in a liquid medium. Although not a method of functionalization, hydrogels represent a class of highly tunable materials that have facilitated numerous studies of cell-material interactions. Hydrogels can be functionalized with specific cell-binding and proteolytic sites via chemical conjugation of peptides using a variety of coupling procedures [75]. The cell adhesion peptide arginyl-glycyl-aspartic acid (RGD), derived from fibronectin, is most commonly used to impart or improve the cell binding properties of hydrogels [76]. While collagenase [77] or MMP [78]–[81] cleavable peptides are most commonly added to impart or increase proteolytic sites in hydrogels. The ability to functionalize hydrogels in a controlled manner has led to a number of findings that have advanced our understanding of cell-material interactions. Generally, the inclusion of RGD peptides has been shown to influence cell viability [82], [83], differentiation [84] and expression [85], while the inclusion of MMP peptides has been shown to promote outward cell migration [80]. Specifically, stretchable PNAGA-based hydrogel systems have been used to alter ligand spacing in single direction, and demonstrate that osteoblasts and fibroblasts are unable to stably adhere to hydrogel surfaces when the distance between neighboring adhesion ligands is >70 nm in one direction even if the ligand spacing between neighboring ligands in the opposite direction is ≤70 nm [86]. PEGDA hydrogels, functionalized with PEG spacers of increasing length, have been used to show that increasing the distance of RGD peptides from the hydrogel surface decreases the concentration of RGD required to support corneal epithelial cell attachment and spreading [87]. Furthermore, by varying both the surface density and spatial distribution of RGD on the surface of PEO-based hydrogel, it has been shown that fibroblast migration speed is a function of surface ligand density, and that clustering ligands reduces the ligand density required to support cell migration [88].
3.2. Chemical patterning
Cell signaling is dictated by chemical mediators and the topographical environment, both of which can be exploited in studying and manipulating cell signaling. Microscale chemical patterning has been in use since the 1990s. Micro-contact printing can be used to stamp specific molecules of defined shapes and sizes onto a surface, and therefore facilitates the study and control of cell binding and spreading [89]. The use of this technique has demonstrated that geometry can affect cell survival and differentiation [90]–[92]. Thus, shape is an important factor to consider when designing cell-instructive materials that support cell proliferation and differentiation.
Soon after the introduction of micro-contact printing, dip-pen nanolithography was developed, enabling multiplexed deposition of molecules via an atomic force microscopy tip with nanoscale precision in a positive printing mode [93]. This technique has also been used to measure single molecular interactions, e.g. between integrin αvβ3 and vitronectin [94].
3.3. Topographical patterning
Material topography is an additional characteristic that has also been exploited to direct cell signaling in numerous studies. Chemical etching of silicon wafers can form precise relief patterns, reverse masks, or can be used to produce nanoscale structures that are directly seeded with cells. The use of a silicon mask to produce grooves in a PDMS mold has demonstrated that constraining cells within grooves alters their epigenetic markers, enabling reprogramming of cells [95], [96]. In another instance, porous silicon nanoneedle structures have delivered multiple payloads (nanoparticles, proteins and nucleic acids) [97]–[99], in addition to monitoring the tissue pH environment, thereby enabling both delivery and diagnostics [100].
Combining specific surface topography with chemical functionalization can improve the biocompatibility of materials for clinical applications. One such study combined reactive chemistry with surface micro-patterning by spincoating a cyanoacrylate tissue glue on to PCL patches that were previously patterned via hot embossing onto an ion etched silicon wafer. Results showed that the quantity of cyanoacrylate tissue glue that is required to achieve tissue adhesion is reduced on micro-patterned patches compared to flat patches [101]. Taking into account nanoscale spatiotemporal chemical profiles, such as cell surface receptor spacing, along with the microenvironment topography could aid the fine-tuning materials to minimize off- target effects on cell signaling.
3.4. Peptide self-assembly
Self-assembly enables the organization of complex biological structures and is used to precisely synthesize stimuli-responsive, complex nano-materials for more precise drug delivery and tissue repair. Nanostructures comprised of multiple binding peptides can be used to induce clustering of receptors and hence modulate cellular activity. These structures are designed bio-mimetically and can incorporate several different peptides together in a complex that is capable of simultaneously clustering multiple proteins or temporally releasing factors [102]. Different types of self-assembling peptides have been used for regenerative medicine applications. Fiber-forming coiled-coil-based peptides that assemble to display carbohydrate and ligands have been used for antigen ligand display [103]. The peptide RADA16-I-BMHP1 has been mixed with PLGA nano-fibers for functional nerve regeneration [104]. The self-assembling peptide hydrogel SPG-178-Gel has been used for bone regeneration [105], and an arginine-rich peptide has been used for the delivery of nucleic acid therapeutics [106]. This approach allows materials to interact with protein complexes on the cell surface, and elucidate the cell dependence on precise spatiotemporal presentation.
3.5. DNA origami
DNA origami is a method used to control receptor positioning, whereby DNA is built in 2D or 3D and functionalized with chemical moieties at defined locations. This approach enables a high degree of spatial control, up to 5 nm [107], and is therefore effective in controlling the presentation of ligands to cells. Ligand nano-calipers have been used to arrange DNA origami modified with ephrin ligands to define EphA2 receptor spatial distribution and receptor-mediated signaling [108]. Precise nano-patterning of antigens using DNA origami has demonstrated that the binding affinities of antibodies change with antigen distances, with a distinct preference observed for antigens separated by approximately 16 nm [109]. These antigen patterns have implications for directing immune responses. DNA origami has also been used to fabricate biomimetic nano-arrays enabling multivalent analysis of ligand-receptor interactions with nanoscale spatial resolution [110]. In this way, DNA origami can be utilized as a tool to probe the effects of spacing on receptor signaling. DNA origami could also be incorporated into nanoparticle fabrication so that chemical moieties are presented to cells at defined distances.
3.6. Tension mediated sensing
Functionalizing material surfaces with sensors that measure tension or allow movement of molecules, permits mechanical studies at the molecular level to elucidate how tension mediated signals are experienced by the cell. Many tension sensors have a FRET-based read-out (determined by the proximity of two fluorescent molecules), which is reversed upon higher tension. DNA nanoparticle tension sensors have also been utilized to measure integrin receptor tension during cell adhesion [111] and also to demonstrate that T-cell receptors transmit defined forces to their antigens, thus showing that the cells can optimize their specificity to defined ligands [112]. Receptor spacing and affinity can therefore alter in response to certain forces and the subsequent signaling pathways can be fine-tuned.
4. Imaging based techniques for understanding cell surface receptor organization and characterizing cell-material interactions
The application of precisely defined fabrication techniques has revolutionized our ability to engineer biomaterials that replicate the nanoscale organization and presentation of various adhesive ligands. Traditionally, the distribution of adhesive ligands on biomaterials was often not characterized [113]. Rather ligand display was inferred from the design of surface modifications and monitoring of the cell response including adhesion, cell spreading and the formation of focal adhesions [41], [114]. However, advances made in nanoscale and label-free imaging over the last decade enable better understanding of material ligand spacing, cell adhesion receptors and the behavior that their interactions trigger at the cell-material interface. In this section, we critically discuss several applications of key imaging characterization tools. Furthermore, we will compare the relative strengths and weaknesses in these analytical tools in Table 1 and provide some prospective with regards to how these advanced tools could aid the efficacy of next-generation nano-engineered biomaterials.
Table 1.
| Sample Type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells | Material | Cell-Material | ||||||||||
| Diffraction Limited | Confocal Microscopy | ✓ | ✓ | ✓ | 200 nm (XY) 500 nm (Z) | 200 μm | Semi (Diffraction limited) | ✓ | × | -Photo-damage to cells, particularly when high laser power is required in weakly labeled samples. -Poor light penetration can limit observation of cell-material responses particularly in dense, opaque or thick biomaterial systems. Multi-photon and light sheet microscopy have better optical sectioning capabilities than confocal microscopy. |
-Fluorescence cross correlation spectroscopy (FCCS) and image correlation spectroscopy (ICS) have been used to study the molecular dynamics of fluorescently labeled proteins in living cells. Future applications could apply these powerful approaches to develop further understanding of the molecular dynamics of these adhesion proteins and proteins associated with their assembly. | [40], [41], [115], [159–[164] |
| SuperResolution Fluorescence Microscopy | Single Molecule Localization Microscopy (SMLM) | ✓ | ✓ | × | 20-50 nm(XY) 100 nm (Z) | 1-5 μm | ✓ | ✓ | × | -Both STORM and PALM require specific fluorophores. Photo-switching tags for STORM (e.g. Alexa Fluor 647, Cy3, Cy5). While PALM requires photo- switchable or photo-activatable proteins (e.g. photo-activatable GFP). -STORM and PALM require extensive optimization; including high labeling density (to obey Nyquist-Shannon Theorem) and the use of oxygen scavenging buffers in STORM. |
-SMLM approaches are yet to be optimized to evaluate adhesive functionalized hydrogels and the interactions which exist at the cell-material interface. | [12], [113], [165]–[168] |
| Structured Illumination Microscopy (SIM) | ✓ | ✓ | ✓ | 100 nm(XY) 250 nm (Z) | <15 μm | ✓ | ✓ | × | -To exploit the Moiré effect for super-resolution imaging at least three rotating wide-field images per image slice in the Z-stack. Dyes must therefore demonstrate good photostability throughout image acquisition in order for effective image reconstruction without artifacts. | -Thick or dense biomaterials scatter light extensively. These may present challenges to imaging. Newer techniques including instant two-photon SIM or light-sheet SIM are being reported but they are yet to be applied specifically to study the cell-material interface. | [38], [120],[131], [169],[170] | |
| Electron Microscopy | SEM and FIB-SEM | ✓ | ✓ | ✓ | <5 nm (XY) 50 nm (Z) | × | × | × | -Very time and labor-intensive sample preparation. Can be subject to artifacts due to sample dehydration, processing and staining. -More difficult to investigate dynamic processes which may be possible to observe using live cell imaging techniques as are available using fluorescence imaging or Raman spectroscopy. |
-Correlative light and electron microscopy or correlation of EM techniques to nanoscale secondary ion mass spectrometry (NanoSIMS)imaging could reveal new insights when combined together in the context of the cell-material interface. | [134] | |
| Physical | Atomic Force Microscopy | ✓ | ✓ | ✓ | <1 nm(XY) | <20 nm | ✓ | ✓ | ✓ | -Single scan images achievable are around 150 μm2, which is more restrictive than EM techniques. -Thermal drift can be problematic, particularly for long imaging times. |
-Combining AFM with optical fibre nanospectroscopy (e.g. Tip enhanced Raman Spectroscopy) enables precise spectroscopic assessments of samples enabling important spatial and compositional information to be resolved. | [135], [138],[171], [172] |
| Secondary Ion Mass | ToF-SIMS | ✓ | ✓ | ✓ | 200 nm(XY) <1 nm (Z) | Static -<5 nm | × | × | ✓ | -Static SIMS is capable of analyzing the surface of biomaterials and therefore ideal for monitoring surface treatments. Depth profiling is possible with dynamic SIMS but the depth of penetration is challenging to control. -Material analysis conducted under ultra-high vacuum. |
-Further enhancements in the mass resolving power with the OrbiTrap mass analyser in Orbi- SIMS offers new potential to study in detail the metabolomic influences that functionalized biomaterials may have on cell response and behaviour. | [158], [173],[174] |
| NanoSIMS | ✓ | × | × | 50 nm (XY) <1 nm (Z) | <5 nm | × | × | ✓ | -Very limited number of instruments available globally. -Unlike ToF-SIMS, NanoSIMSis limited to the detection of elements or small fragments such as CN-. Isotope labeling of biomolecules is therefore key to detecting biomolecules of interest with NanoSIMS. |
-NanoSIMS imaging could be combined with other SMLM optical approaches in order to validate the resolution of the technique. Significant need to expand NanoSIMS to characterize material functionalization. | [175] | |
| Vibrational Spectroscopy | Raman | ✓ | ✓ | ✓ | 250 nm(XY) | 50 μm | Semi | ✓ | ✓ | -Raman scattering is inefficient, requiring prolonged image acquisition times and excessive sample exposure to laser power which can induce photo-toxic effects in cells. Newer Raman spectroscopy approaches may reduce acquisition times including Coherent Anti-stokes Raman Spectroscopy (CARS) or Surface Enhanced Raman Spectroscopy (SERS) by boosting signal intensities. | -Given that Raman Spectroscopy can be conducted on live cell samples there remains a need to characterize spatiotemporal interactions of cells with functionalized biomaterial systems using this technique. | [145], [176] |
4.1. Fluorescent imaging
Fluorescence imaging methods have been extensively utilized to investigate cell adhesion receptors and the responses generated through cell mediated interactions with these adhesive biomaterial systems. Confocal fluorescence can monitor vital features of the cellular response to 3D patterned hydrogels including cell adhesion, cytoskeletal organization, cell viability and cell traction force [41], [115], [116]. The variety of fluorophores and versatile array of antibodies which can mediate affinity targeting to adhesion receptors or other protein-based targets involved in the downstream signaling from these receptors upon activation have certainly aided the development of fluorescence imaging as a characterization tool for studying the cell-biomaterial interface.
However, due to the Abbe/Rayleigh limit of light diffraction, conventional fluorescence microscopes have a resolution limit of 200 nm. Recently, super-resolution microscopy, a collective term for imaging techniques that achieve resolution below the diffraction limit, has revolutionized fluorescent imaging and has proven instrumental in guiding our current understanding of fundamental aspects of cell surface receptor clustering and function. Single molecule localization microscopy (SMLM) techniques, such as stochastic optical reconstruction microscopy (STORM) [117] and photo-activated localization microscopy (PALM) [118] have enabled visualization and quantification of subcellular biomolecule localization, morphology and interactions [119]–[122].
SMLM has been a key approach that has informed much of our current understanding of the clustering of integrins and cadherins at the cell membrane [12], [48], as well as integrin receptor dynamics and their subsequent downstream signaling [123], [124]. Recent advancements to these imaging technologies include live tracking of fluorescent proteins [125] or quantum dots [126]. Furthermore, spatio-temporal monitoring of integrin receptors is now possible by genetically fusing target proteins with fluorescent proteins or self-labeling tag systems [127], [128]. In addition, to unravel cell phenotype and function, SMLM imaging techniques can be combined with in-situ hybridization to spatially resolve single cell transcriptomics in 3D space [129].
Besides studying cellular proteins, the scope of SMLM imaging has now also been successfully extended to analyze material properties [130], and improve the characterization of functionalized biomaterial surfaces. Previously, STORM imaging confirmed the presentation of RGD ligand nanodomains from thin films assembled from co-polymers of polystyrene and poly(ethylene oxide). Importantly, STORM verified that domains of ligands were arranged 52 nm apart which is critical to enable their interaction with clusters of cell adhesion receptors [113]. Despite much progress, SMLM remains yet to be implemented in the study of nanoscale interactions of cells at the cell-material interface. So far, alternative super-resolution fluorescence imaging techniques including 3D structured illumination microscopy (3D-SIM) have been more widely implemented. 3D-SIM achieves its resolution enhancements through software mediated extraction of high-frequency information from rotating widefield fluorescence images [120]. 3D-SIM has the advantage over SMLM given that it requires significantly less fluorophore labeling optimization and does not require use of specific imaging buffers. The trade-off being that 3D-SIM fails to resolve single molecules and is limited to imaging protein clusters at best with maximal lateral resolution of 100 nm. Despite this, 3D-SIM has previously related ligand patterning to cellular protein distributions. More specifically, live imaging of the spatial correlation between RGD ligand cluster growth and movement with cellular focal adhesion formation was investigated [12], [131]. 3D-SIM has thus been proven as a powerful technique to elucidate interactions between functionalized materials and the cell response. Future investigations are needed to implement SMLM imaging in a similar manner. Undoubtedly, by achieving single molecule accuracy, SMLM could permit biomaterial adhesive ligand presentation to be more specifically linked to organization of cell surface receptors in a similar way to other nanoscopic techniques including atomic force microscopy (AFM) (see section 4.3).
4.2. Electron microscopy
Electron microscopy (EM) can provide nanometer level resolution, which is particularly valuable for the characterization of cell ultrastructure, complex biomaterials and for evaluating the physical interactions that exist at the cell-material interface. The scope of EM imaging has now also been extended since it can be used to characterize cellular protein spatial localization to complement high resolution structural and morphological insight within cell as they interact with engineered material platforms. Immunogold labeling may also unlock details of the spatial distributions of cellular proteins [132]. Gold immunolabeling demonstrated an enhanced redistribution of the αvβ3 and αvβ1 integrin receptors within MSCs when the cells were seeded onto gold nanorods of varying aspect ratio and randomly coated with adhesive RGD peptide [133]. Furthermore, combining immunogold with the 3D reconstruction capabilities of focused ion beam- scanning electron microscopy (FIB-SEM) has enabled much greater appreciation of the effects that material physical topography has on biochemical signaling. In one such work, cells seeded on microgroove substrates were evaluated using immunogold FIB-SEM to correlate the morphological changes in the cell to the redistribution of histone marker H3K9me3 to the nuclear laminar and periphery of the cell [134].
4.3. Atomic force microscopy
Since its first discovery over 30 years ago, AFM has made significant contributions to the characterization of bio-interfaces. Today the variety of different AFM modes available make it possible to spatiotemporally map topographical, mechanical, electrostatic and binding site functionality present on the surfaces of materials and cells with unprecedented atomic length scale resolution [135].
AFM reveals nano-topographical biomaterial features and is frequently used in combination with scanning electron microscopy to characterize the presentation of material topology either for ligand presentation or for the activation of cell mechano-transduction, which may play a role in the control of cell fate [135]. Different AFM modes can be used to explore material topography, with the most widely applied including (1) contact-mode AFM, where the cantilever deflection is kept constant by adjusting the distance between the stylus and sample, or (2) dynamic mode AFM, where the cantilever is oscillated and dynamically interacts with the surface of a material. So far, AFM imaging has been used to spatially resolve the organization of integrin binding nanopatterns formed by DNA origami [110], gold nanoparticles [136] and dendrimers [137].
Further adaptations to the AFM instrumentation allow the quantification of substantially more complex interactions residing between cells and at the cell-material interface [138]. In single cell force spectroscopy (SCFS) single cells are attached to tip-less AFM cantilevers, which have been coated with positively charged cell-adhesive polymers, such as poly-L-lysine. Under physiological conditions, single cells can be brought into close contact with the other cells or materials for a specified time and then removed while time and force curves are generated to quantify adhesive interactions [138].
This pioneering approach was first used to quantify cell-cell adhesion between trophoblasts and uterine epithelial cells [139]. Since then substantial enhancements to SCFS have been developed, with availability of commercial AFM instruments capable of enhanced pulling ranges (>100 μm), precision (0.1 nm) and force sensitivity (5 pN) [140]. These optimized setups enable SCFS to probe adhesion interactions over a much broader range of detachment forces. As a result, more complex interactions can be investigated. Recently SCFS proved that human neural stem cell de-adhesion was largely driven by the discrete unbinding of integrin-RGD complexes as opposed to elastic restoration of gelatin methacrylate (GelMA) chains [141]. While early co-operation between α2β1-mediated adhesion receptors on nanopatterned collagen type I matrices was critical in order to form higher order adhesion structures [142].
4.4. Confocal Raman spectroscopy
Confocal Raman spectroscopy is an optical characterization strategy, which non- invasively reveals chemical compositional information by detecting the vibrations of specific chemical bonds based on their inelastic scattering of light. Confocal Raman spectroscopy has a spatial resolution of a few hundred nanometers, and can penetrate up to 50 micrometers. As a result, it is suitable to investigate cellular biomolecule distributions within 3D biomaterials in a label-free manner. Recently Raman spectroscopy has been used in biomaterial development to validate the cellular response to patterned bioactive molecules. Polarized distributions of the bone minerals (β-tricalcium phosphate and hydroxyapatite) secreted by human mesenchymal stem cells (MSC) differentiated within a scaffold functionalized with a gradient of bone morphogenic growth factor (BMP-2) have been identified [143], [144]. Moreover, quantitative 3D volumetric assessments are also possible with Raman spectroscopy being used to investigate human MSC response to RGD chemically functionalized into bio-inert PEG hydrogels [145]. The human MSCs within these RGD gels displayed filopodia extension and spreading, while cells in the unfunctionalized gels remained spherical. Non-invasive and label-free discovery of the influence of that ligand patterning has on cell phenotype, behavior and biological secretions are key advantages for Raman spectroscopy [144]. These properties make this technique of significant value for evaluating the spatiotemporal interactions between functionalized 3D biomaterials and living cells without the need for fluorescent labels. However, slow image acquisition and complex data processing may continue to limit applications in this area.
4.5. Secondary Ion Mass Spectrometry
Another label-free imaging strategy to chemically map the presentation of biomaterial surface functionalization and its influence on cell behavior is secondary ion mass spectrometry. Mass Spectrometry Imaging (MSI) measures chemical composition of materials and can therefore elucidate the presence of effective surface patterning and the spatial characterization of receptor clustering. Time of Flight Secondary Ion Mass Spectrometry (ToF-SIMS) offers high sensitivity verification of biomaterial surface chemistries. ToF-SIMS has been used in the spatial mapping of bioactive moieties ranging in molecular mass from simple small molecule chemical ligands [146], polymers [147], peptides [148] to proteins [149] at very low and biologically meaningful analytical concentrations. During ToF-SIMS analysis pulsed ion beams interact with deposited biomaterials to generate positively and negatively charged secondary molecular ions and fragments generated at the material surface. ToF- SIMS can therefore provide unique insights regarding protein identity, conformation [150] and orientation [151], which could evaluate biomaterials or cells patterned with bioactive molecules. Fundamentally, this information would ensure that bioactive portions of proteins readily orientate to enable more precise and efficacious interactions with binding partners [152]. Previously, ToF-SIMS chemical analyses and multivariate partial least squares models revealed that surfaces functionalized with high acrylate contents were particularly effective at engaging integrin αvβ3 and αvβ5 via adsorbed vitronectin [153]. Multivariate statistical analyses could also distinguish the complex natural compositions of decellularized ECM matrices from primary mouse osteoblasts exposed to different cytokine challenges [154].
Enhancements to the resolution possible with mass spectrometry imaging has been achieved using nanoscale secondary ion mass spectrometry (NanoSIMS). Nanoscopic resolution of protein distributions in cells were obtained by NanoSIMS through the detection of fluorinated nanobodies [155]. Importantly, these nanobodies were smaller and less prone to aggregation than gold labeled antibodies for EM analysis [155]. NanoSIMS imaging has been shown to reveal T-cell receptor aggregation in response to MHC complexes. T-cell receptors were found to form clusters of between 60 to 150 nm at the plasma membrane [156]. Unlike other nanoscale imaging modalities, such as fluorescence based super-resolution microscopy or electron microscopy that operate with nanoscale resolution, NanoSIMS can provide unique compositional insights regarding the presence of isotopically labeled metabolites. This has been used to study the redistribution of isotopically labeled structural molecules and their regulation of cell surface receptor organization [157].
Overall secondary ion mass imaging offers a unique strategy to spatially resolve biomolecule distributions in biological specimens, however complex spectra can present great analytical challenges. This can make it challenging to distinguish molecules with certainty if their mass-to-charge ratios are similar. The recent development of 3D-OrbiSIMS, which provides greater mass resolving power, enables accurate measurements of molecular ions and fragments [158]. The application of 3D-OrbiSIMS in combination with new advanced cell instructive biomaterials design could shed new light regarding the metabolomic status of cells adhering to functionalized biomaterial surfaces.
5. Future perspectives
This review paper highlights a number of important insights that we hope will be useful for the field of regenerative medicine. Firstly, our understanding of how cells interpret differences in ligand presentation must be improved in order for subtle differences to be exploited in precision medicines. Secondly, a variety of material-based techniques are available to better understand the molecular biology underlying ligand positioning in biological systems. Thirdly, characterization of the interface between biomaterials and cells at the nanoscale must be performed, with the techniques capable of achieving this resolution discussed herein. The techniques discussed here are by no means exhaustive, but indicate a large breadth of methods that currently exist to probe various nanoscale cellular mechanisms and functionalize biomaterial surfaces. This combined effort could lead to more precise regenerative medicine strategies that can be effective at lower doses with decreased off-target effects.
Lay Summary.
The combination of biology, chemistry, materials science, and imaging technology, affords exciting opportunities to better diagnose and treat a wide range of diseases. Recent advances in imaging technologies have enabled better understanding of the specific interactions that occur between human cells and their immediate surroundings in both health and disease. This biological understanding can be used to design smart therapies and tissue replacements that better mimic native tissue. Here, we discuss the recent advances in molecular biology and technologies that can be employed to functionalize materials and characterize their interaction with biological entities to facilitate the design of more sophisticated medical therapies.
9. Funding
S.A.M. was funded by a PhD studentship in Biomedicine and Bioengineering in Osteoarthritis, Imperial College London. C.W.W. was funded by the Biotechnology and Biological Sciences Research Council Doctoral Training Partnership (BB/N503952/1). M.M.S. and E.M.C. were funded by a grant from the UK Regenerative Medicine Platform “Acellular / Smart Materials - 3D Architecture” (MR/R015651/1).
Footnotes
Author Contributions
S.A.M. wrote the manuscript with contribution from C.W.W. to the imaging techniques and E.M.C. to the material fabrication. M.M.S. revised the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Langer R, Vacanti J. Advances in Tissue Engineering. J Pediatr Surg. 2016 Jan;51(1):8. doi: 10.1016/j.jpedsurg.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011 Jan;6(1):13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- [4].Xiong J-P, et al. Crystal structure of the extracellular segment of integrin alphaVbeta 3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296(5565):151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4(4):E65–8. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- [6].Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006 Oct;119(19):3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin α2β1. Cell. 2000 Mar;101(1):47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- [8].Ramovs V, te Molder L, Sonnenberg A. Matrix Biology. 57-58. Elsevier BV; 2017. Jan 01, The opposing roles of laminin-binding integrins in cancer; pp. 213–243. [DOI] [PubMed] [Google Scholar]
- [9].Takagi J, Strokovich K, Springer TA, Walz T. Structure of integrin α5β1 in complex with fibronectin. EMBO J. 2003;22(18):4607–4615. doi: 10.1093/emboj/cdg445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110(5):599–11. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- [11].Arnold M, et al. Activation of integrin function by nanopatterned adhesive interfaces. Chem Phys Chem. 2004;5(3):383–8. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- [12].Changede R, Xu X, Margadant F, Sheetz M. Nascent integrin adhesions form on all matrix rigidities after integrin activation. Dev Cell. 2015;35(5):614–621. doi: 10.1016/j.devcel.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [13].Horton ER, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015 Nov;17(12):1577–1587. doi: 10.1038/ncb3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013 Aug;14(8):503–17. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hohenester E. Signalling complexes at the cell-matrix interface. Curr Opin Struct Biol. 2014;29C:10–16. doi: 10.1016/j.sbi.2014.08.009. [DOI] [PubMed] [Google Scholar]
- [16].Sun Z, Costell M, Fässler R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol. 2019 Jan;21(1):25–31. doi: 10.1038/s41556-018-0234-9. [DOI] [PubMed] [Google Scholar]
- [17].Kanchanawong P, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468(7323):580–4. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Paul NR, Jacquemet G, Caswell PT. Current Biology. 22. Vol. 25. Cell Press; 2015. Nov 16, Endocytic Trafficking of Integrins in Cell Migration; pp. R1092–R1105. [DOI] [PubMed] [Google Scholar]
- [19].Shamri R, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6(5):497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- [20].Constantin G, et al. Chemokines Trigger Immediate β2 Integrin Affinity and Mobility Changes. Immunity. 2000 Dec;13(6):759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- [21].Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- [22].Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Activation of Leukocyte β2 Integrins by Conversion from Bent to Extended Conformations. Immunity. 2006;25(4):583–594. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- [23].Ren G, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cairo CW, Mirchev R, Golan DEE. Cytoskeletal Regulation Couples LFA-1 Conformational Changes to Receptor Lateral Mobility and Clustering. Immunity. 2006 Aug;25(2):297–308. doi: 10.1016/j.immuni.2006.06.012. [DOI] [PubMed] [Google Scholar]
- [25].Shen K, Thomas VK, Dustin ML, Kam LC. Micropatterning of costimulatory ligands enhances CD4+ T cell function. Proc Natl Acad Sci U S A. 2008 Jun;105(22):7791–7796. doi: 10.1073/pnas.0710295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shen K, Tsai J, Shi P, Kam LC. Self-aligned supported lipid bilayers for patterning the cell-substrate interface. J Am Chem Soc. 2009 Sep;131(37):13204–13205. doi: 10.1021/ja904721h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Delcassian D, et al. Nanoscale ligand spacing influences receptor triggering in T cells and NK cells. Nano Lett. 2013 Nov;13(11):5608–5614. doi: 10.1021/nl403252x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fitzgerald JR, et al. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol Microbiol. 2006;59(1):212–230. doi: 10.1111/j.1365-2958.2005.04922.x. [DOI] [PubMed] [Google Scholar]
- [29].Yoon C-S, Kim K-D, Park S-N, Cheong S-W. α6 integrin is the main receptor of Human Papillomavirus type 16 VLP. Biochem on Biophys Res Commun. 2001;283(3):668–673. doi: 10.1006/bbrc.2001.4838. [DOI] [PubMed] [Google Scholar]
- [30].Schornberg KL, et al. α5β1-Integrin controls ebola virus entry by regulating endosomal cathepsins. Proc Natl Acad Sci. 2009;106(19):8003–8008. doi: 10.1073/pnas.0807578106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tian YF, et al. Integrin-specific hydrogels as adaptable tumor organoids for malignant B and T cells. Biomaterials. 2015 Dec;73:110–119. doi: 10.1016/j.biomaterials.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schaffner F, Ray A, Dontenwill M. Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers (Basel) 2013 Jan;5(1):27–47. doi: 10.3390/cancers5010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Roman J, Ritzenthaler JD, Roser-Page S, Sun X, Han S. alpha5beta1 -integrin expression is essential for tumor progression in experimental lung cancer. Am J Respir Cell Mol Biol. 2010 Dec;43(6):684–91. doi: 10.1165/rcmb.2009-0375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin 5 1 facilitates cancer cell invasion through enhanced contractile forces. J Cell Sci. 2011 Feb;124(3):369–383. doi: 10.1242/jcs.071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hersey P, et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alphaVbeta3, ± dacarbazine in patients with stage IV metastatic melanoma. Cancer. 2010;116(6):1526–1534. doi: 10.1002/cncr.24821. [DOI] [PubMed] [Google Scholar]
- [36].Sun Q, Kanehira K, Taniguchi A. PEGylated TiO2 nanoparticles mediated inhibition of cell migration via integrin beta 1. Sci Technol Adv Mater. 2018 Dec;19(1):271–281. doi: 10.1080/14686996.2018.1444318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maggi V, et al. Gold Nanoparticles Functionalized with RGD-Semipeptides: A Simple yet Highly Effective Targeting System for α v β 3 Integrins. Chem - A Eur J. 2018 Aug;24(46):12093–12100. doi: 10.1002/chem.201801823. [DOI] [PubMed] [Google Scholar]
- [38].Wang T, et al. Quantitative profiling of integrin αvβ3 on single cells with quantum dot labeling to reveal the phenotypic heterogeneity of glioblastoma. Nanoscale. 2019 Oct;11(39):18224–18231. doi: 10.1039/c9nr01105f. [DOI] [PubMed] [Google Scholar]
- [39].Petrie TA, et al. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci Transl Med. 2010 Aug;2(45):45ra60. doi: 10.1126/scitranslmed.3001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang X, Li S, Yan C, Liu P, Ding J. Fabrication of RGD micro/nanopattern and corresponding study of stem cell differentiation. Nano Lett. 2015 Mar;15(3):1457–1467. doi: 10.1021/nl5049862. [DOI] [PubMed] [Google Scholar]
- [41].Benitez PL, Mascharak S, Proctor AC, Heilshorn SC. Use of protein-engineered fabrics to identify design rules for integrin ligand clustering in biomaterials. Integr Biol (United Kingdom) 2016 Jan;8(1):50–61. doi: 10.1039/c5ib00258c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Clark AY, et al. Integrin-specific hydrogels modulate transplanted human bone marrow-derived mesenchymal stem cell survival, Q⋂ engraftment, and reparative activities. Nat Commun. 2020 Dec;11(1):1–14. doi: 10.1038/s41467-019-14000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shapiro L, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995 Mar;374(6520):327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- [44].Tepass U, Truong K, Godt D, Ikura M, Peifer M. Nature Reviews Molecular Cell Biology. 2. Vol. 1. European Association for Cardio-Thoracic Surgery; 2000. Cadherins in embryonic and neural morphogenesis; pp. 91–100. [DOI] [PubMed] [Google Scholar]
- [45].Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005 Aug;6(8):622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- [46].Hulpiau P, van Roy F. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol. 2009 Feb;41(2):349–369. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- [47].Wu Y, Kanchanawong P, Zaidel-Bar R. Actin-Delimited Adhesion-Independent Clustering of E-Cadherin Forms the Nanoscale Building Blocks of Adherens Junctions. Dev Cell. 2015 Jan;32(2):139–154. doi: 10.1016/j.devcel.2014.12.003. [DOI] [PubMed] [Google Scholar]
- [48].Changede R, Sheetz M. Integrin and cadherin clusters: A robust way to organize adhesions for cell mechanics. BioEssays. 2017;391(1):e201600123. doi: 10.1002/bies.201600123. Chang. [DOI] [PubMed] [Google Scholar]
- [49].Bertocchi C, et al. Nanoscale architecture of cadherin-based cell adhesions. Nat Cell Biol. 2017 Jan;19(1):28–37. doi: 10.1038/ncb3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: A fundamental determinant of cadherin function. Curr Biol. 1997 May;7(5):308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- [51].Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996 Mar;380(6572):360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- [52].Tomschy A, Fauser C, Landwehr R, Engel J. Homophilic adhesion of E-cadherin occurs by a co-operative two-step interaction of N-terminal domains. EMBO J. 1996 Jul;15(14):3507–3514. [PMC free article] [PubMed] [Google Scholar]
- [53].Smutny M, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010 Jul;12(7):696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Strale PO, et al. The formation of ordered nanoclusters controls cadherin anchoring to actin and cell-cell contact fluidity. J Cell Biol. 2015 Jul;210(2):333–346. doi: 10.1083/jcb.201410111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kristensen SH, Pedersen GA, Nejsum LN, Sutherland DS. Nanoscale E-cadherin ligand patterns show threshold size for cellular adhesion and adherence junction formation. Nano Lett. 2012 Apr;12(4):2129–2133. doi: 10.1021/nl300514v. [DOI] [PubMed] [Google Scholar]
- [56].Fichtner D, et al. Covalent and Density-Controlled Surface Immobilization of E-Cadherin for Adhesion Force Spectroscopy. PLoS One. 2014 Mar;9(3):e93123. doi: 10.1371/journal.pone.0093123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Paulson AF, Prasad MS, Thuringer AH, Manzerra P. Regulation of cadherin expression in nervous system development. Cell Adh Migr. 2014 Jan;8(1):19–28. doi: 10.4161/cam.27839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dianati E, Poiraud J, Weber-Ouellette A, Plante I. Connexins, E-cadherin, Claudin-7 and β-catenin transiently form junctional nexuses during the post-natal mammary gland development. Dev Biol. 2016 Aug;416(1):52–68. doi: 10.1016/j.ydbio.2016.06.011. [DOI] [PubMed] [Google Scholar]
- [59].Killen AC, Barber M, Paulin JJW, Ranscht B, Parnavelas JG, Andrews WD. Protective role of Cadherin 13 in interneuron development. Brain Struct Funct. 2017 Nov;222(8):3567–3585. doi: 10.1007/s00429-017-1418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gheldof A, Berx G. Cadherins and Epithelial-to-Mesenchymal Transition. Progress in molecular biology and translational science. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- [61].Stone RC, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016 Sep;365(3):495–506. doi: 10.1007/s00441-016-2464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol. 2017 Dec;232(12):3261–3272. doi: 10.1002/jcp.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sola A, et al. Microencapsulated macrophages releases conditioned medium able to prevent epithelial to mesenchymal transition. Drug Deliv. 2018 Jan;25(1):91–101. doi: 10.1080/10717544.2017.1413449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Horejs C-M, et al. Preventing tissue fibrosis by local biomaterials interfacing of specific cryptic extracellular matrix information. Nat Commun. 2017 Jun;8:15509. doi: 10.1038/ncomms15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang H, et al. Effect of Polyelectrolyte Film Stiffness on Endothelial Cells During Endothelial-to-Mesenchymal Transition. Biomacromolecules. 2015 Nov;16(11):3584–3593. doi: 10.1021/acs.biomac.5b01057. [DOI] [PubMed] [Google Scholar]
- [66].Li J, Di Russo J, Hua X, Chu Z, Spatz JP, Wei Q. Surface Immobilized E-Cadherin Mimetic Peptide Regulates the Adhesion and Clustering of Epithelial Cells. Adv Healthc Mater. 2019 Apr;8(8):1801384. doi: 10.1002/adhm.201801384. [DOI] [PubMed] [Google Scholar]
- [67].Dehli J, Karlsson C, Bizelli-Silveira C, Jiang X, Kraft D, Foss M. E-cadherin mediated cell-biomaterial interaction reduces migration of keratinocytes in-vitro. Colloids Surfaces B Biointerfaces. 2019 Aug;180:326–333. doi: 10.1016/j.colsurfb.2019.04.010. [DOI] [PubMed] [Google Scholar]
- [68].Huang Y-J, Hsu S. Acquisition of epithelial–mesenchymal transition and cancer stem-like phenotypes within chitosan-hyaluronan membrane-derived 3D tumor spheroids. Biomaterials. 2014 Dec;35(38):10070–10079. doi: 10.1016/j.biomaterials.2014.09.010. [DOI] [PubMed] [Google Scholar]
- [69].Agarwalla P, Mukherjee S, Sreedhar B, Banerjee R. Glucocorticoid receptor-mediated delivery of nano gold–withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomedicine. 2016 Oct;11(19):2529–2546. doi: 10.2217/nnm-2016-0224. [DOI] [PubMed] [Google Scholar]
- [70].Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R, Mukherjee P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc Natl Acad Sci U S A. 2013 Apr;110(17):6700–6705. doi: 10.1073/pnas.1214547110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010 Sep;10(9):3223. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zandi N, et al. Biomimetic proteoglycan nanoparticles for growth factor immobilization and delivery. Biomater Sci. 2019 Aug; doi: 10.1039/c9bm00668k. [DOI] [PubMed] [Google Scholar]
- [73].Yamamoto S, et al. Epidermal growth factor-nanoparticle conjugates change the activity from anti-apoptotic to pro-apoptotic at membrane rafts. Acta Biomater. 2019 Apr;88:383–391. doi: 10.1016/j.actbio.2019.02.026. [DOI] [PubMed] [Google Scholar]
- [74].Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle Technologies for Cancer Therapy. Handbook of experimental pharmacology. 2010;(197):55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- [75].Neves SC, Pereira RF, Araújo M, Barrias CC. Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair. Elsevier Inc; 2018. Bioengineered peptide-functionalized hydrogels for tissue regeneration and repair; pp. 101–125. [Google Scholar]
- [76].Hersel U, Dahmen C, Kessler H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- [77].Valdez J, et al. On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials. 2017 Jun;130:90–103. doi: 10.1016/j.biomaterials.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fonseca KB, et al. Enzymatic, physicochemical and biological properties of MMP-sensitive alginate hydrogels. Soft Matter. 2013 Mar;9(12):3283–3292. [Google Scholar]
- [79].Fonseca KB, Bidarra SJ, Oliveira MJ, Granja PL, Barrias CC. Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater. 2011 Apr;7(4):1674–1682. doi: 10.1016/j.actbio.2010.12.029. [DOI] [PubMed] [Google Scholar]
- [80].Fonseca KB, et al. Injectable MMP-sensitive alginate hydrogels as hMSC delivery systems. Biomacromolecules. 2014 Jan;15(1):380–390. doi: 10.1021/bm4016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tsurkan MV, et al. Defined polymer-peptide conjugates to form cell-instructive starpeg-heparin matrices in situ. Adv Mater. 2013 May;25(18):2606–2610. doi: 10.1002/adma.201300691. [DOI] [PubMed] [Google Scholar]
- [82].Neves SC, et al. Biofunctionalized pectin hydrogels as 3D cellular microenvironments. J Mater Chem B. 2015 Mar;3(10):2096–2108. doi: 10.1039/c4tb00885e. [DOI] [PubMed] [Google Scholar]
- [83].Bidarra SJ, Oliveira P, Rocha S, Saraiva DP, Oliveira C, Barrias CC. A 3D in vitro model to explore the inter-conversion between epithelial and mesenchymal states during EMT and its reversion. Sci Rep. 2016 Jun;6 doi: 10.1038/srep27072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bidarra SJ, Barrias CC, Fonseca KB, Barbosa MA, Soares RA, Granja PL. Injectable in situ crosslinkable RGD-modified alginate matrix for endothelial cells delivery. Biomaterials. 2011 Nov;32(31):7897–7904. doi: 10.1016/j.biomaterials.2011.07.013. [DOI] [PubMed] [Google Scholar]
- [85].Xie J, et al. Substrate stiffness-regulated matrix metalloproteinase output in myocardial cells and cardiac fibroblasts: Implications for myocardial fibrosis. Acta Biomater. 2014;10(6):2463–2472. doi: 10.1016/j.actbio.2014.01.031. [DOI] [PubMed] [Google Scholar]
- [86].Deng J, Zhao C, Spatz JP, Wei Q. Nanopatterned Adhesive, Stretchable Hydrogel to Control Ligand Spacing and Regulate Cell Spreading and Migration. ACS Nano. 2017 Aug;11(8):8282–8291. doi: 10.1021/acsnano.7b03449. [DOI] [PubMed] [Google Scholar]
- [87].Wilson MJ, Liliensiek SJ, Murphy CJ, Murphy WL, Nealey PF. Hydrogels with well-defined peptide-hydrogel spacing and concentration: Impact on epithelial cell behavior. Soft Matter. 2012 Jan;8(2):390–398. doi: 10.1039/C1SM06589K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(Pt 1):1677–86. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- [89].Kumar A, Whitesides GM. Features of gold having micrometer to centimeter dimensions can be formed through a combination of stamping with an elastomeric stamp and an alkanethiol ‘ink’ followed by chemical etchin. Appl Phys Lett. 1993;63(14):2002–2004. [Google Scholar]
- [90].Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric Control of Cell Life and Death. Science (80-) 1997 May;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- [91].Lee J, Abdeen AA, Huang TH, Kilian KA. Controlling cell geometry on substrates of variable stiffness can tune the degree of osteogenesis in human mesenchymal stem cells. Journal of the Mechanical Behavior of Biomedical Materials. 2014 doi: 10.1016/j.jmbbm.2014.01.009. [DOI] [PubMed] [Google Scholar]
- [92].von Erlach TC, et al. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat Mater. 2018;17(3):237–242. doi: 10.1038/s41563-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Piner RD, Zhu J, Xu F, Hong S, Mirkin CA. ‘Dip-Pen’ nanolithography. Science. 1999 Jan;283(5402):661–3. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- [94].Lee M, et al. Protein nanoarray on Prolinker™ surface constructed by atomic force microscopy dip-pen nanolithography for analysis of protein interaction. Proteomics. 2006 Feb;6(4):1094–1103. doi: 10.1002/pmic.200500392. [DOI] [PubMed] [Google Scholar]
- [95].Downing TL, et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater. 2013 Dec;12(12):1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Morez C, Noseda M, Paiva MA, Belian E, Schneider MD, Stevens MM. Enhanced efficiency of genetic programming toward cardiomyocyte creation through topographical cues. Biomaterials. 2015 Nov;70:94. doi: 10.1016/j.biomaterials.2015.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gopal S, et al. Porous Silicon Nanoneedles Modulate Endocytosis to Deliver Biological Payloads. Adv Mater. 2019 Mar;31(12):1806788. doi: 10.1002/adma.201806788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chiappini C, Liu X, Fakhoury JR, Ferrari M. Biodegradable porous silicon barcode nanowires with defined geometry. Adv Funct Mater. 2010;20(14):2231–2239. doi: 10.1002/adfm.201000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chiappini C, Martinez JO, De Rosa E, Almeida CS, Tasciotti E, Stevens MM. Biodegradable Nanoneedles for Localized Delivery of Nanoparticles in Vivo: Exploring the Biointerface. ACS Nano. 2015 May;9(5):5500. doi: 10.1021/acsnano.5b01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chiappini C, et al. Mapping Local Cytosolic Enzymatic Activity in Human Esophageal Mucosa with Porous Silicon Nanoneedles. Adv Mater. 2015 Sep;27(35):5147–5152. doi: 10.1002/adma.201501304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Pereira MJN, et al. Combined Surface Micropatterning and Reactive Chemistry Maximizes Tissue Adhesion with Minimal Inflammation. Adv Healthc Mater. 2014 Apr;3(4):565–571. doi: 10.1002/adhm.201300264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bruggeman KF, Rodriguez AL, Parish CL, Williams RJ, Nisbet DR. Temporally controlled release of multiple growth factors from a self-assembling peptide hydrogel. Nanotechnology. 2016 Sep;27(38):385102. doi: 10.1088/0957-4484/27/38/385102. [DOI] [PubMed] [Google Scholar]
- [103].Zacco E, et al. A Self-Assembling Peptide Scaffold for the Multivalent Presentation of Antigens. Biomacromolecules. 2015 Jul;16(7):2188–2197. doi: 10.1021/acs.biomac.5b00572. [DOI] [PubMed] [Google Scholar]
- [104].Nune M, Subramanian A, Krishnan UM, Kaimal SS, Sethuraman S. Self-assembling peptide nanostructures on aligned poly(lactide-co-glycolide) nanofibers for the functional regeneration of sciatic nerve. Nanomedicine. 2017 Feb;12(3):219–235. doi: 10.2217/nnm-2016-0323. [DOI] [PubMed] [Google Scholar]
- [105].Tsukamoto J, et al. Efficacy of a Self-Assembling Peptide Hydrogel, SPG-178-Gel, for Bone Regeneration and Three-Dimensional Osteogenic Induction of Dental Pulp Stem Cells. Tissue Eng Part A. 2017 Dec;23(23-24):1394–1402. doi: 10.1089/ten.TEA.2017.0025. [DOI] [PubMed] [Google Scholar]
- [106].Kang JH, Battogtokh G, Ko YT. Self-Assembling Lipid–Peptide Hybrid Nanoparticles of Phospholipid–Nonaarginine Conjugates for Enhanced Delivery of Nucleic Acid Therapeutics. Biomacromolecules. 2017 Nov;18(11):3733–3741. doi: 10.1021/acs.biomac.7b01084. [DOI] [PubMed] [Google Scholar]
- [107].Dai M, Jungmann R, Yin P. Optical imaging of individual biomolecules in densely packed clusters. Nat Nanotechnol. 2016 Sep;11(9):798–807. doi: 10.1038/nnano.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Shaw A, et al. Spatial control of membrane receptor function using ligand nanocalipers. Nat Methods. 2014 Aug;11(8):841–6. doi: 10.1038/nmeth.3025. [DOI] [PubMed] [Google Scholar]
- [109].Shaw A, et al. Binding to nanopatterned antigens is dominated by the spatial tolerance of antibodies. Nat Nanotechnol. 2019 Feb;14(2):184–190. doi: 10.1038/s41565-018-0336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Huang D, Patel K, Perez-Garrido S, Marshall JF, Palma M. DNA Origami Nanoarrays for Multivalent Investigations of Cancer Cell Spreading with Nanoscale Spatial Resolution and Single-Molecule Control. ACS Nano. 2019 Jan;13(1):728–736. doi: 10.1021/acsnano.8b08010. [DOI] [PubMed] [Google Scholar]
- [111].Zhang Y, Ge C, Zhu C, Salaita K. DNA-based digital tension probes reveal integrin forces during early cell adhesion. Nat Commun. 2014 Dec;5(1):5167. doi: 10.1038/ncomms6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Liu Y, et al. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proc Natl Acad Sci U S A. 2016 May;113(20):5610–5. doi: 10.1073/pnas.1600163113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mollazade M, et al. Can single molecule localization microscopy be used to map closely spaced RGD nanodomains? PLoS One. 2017 Jul;12(7):e0180871. doi: 10.1371/journal.pone.0180871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: A combined computational and experimental approach. Biomaterials. 2007 Oct;28(30):4409–4417. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- [115].Maskarinec SA, Franck C, Tirrell DA, Ravichandran G. Quantifying cellular traction forces in three dimensions. Proc Natl Acad Sci U S A. 2009 Dec;106(52):22108–22113. doi: 10.1073/pnas.0904565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gallagher LB, et al. Pre-culture of mesenchymal stem cells within RGD-modified hyaluronic acid hydrogel improves their resilience to ischaemic conditions. Acta Biomater. 2020 Apr;107:78–90. doi: 10.1016/j.actbio.2020.02.043. [DOI] [PubMed] [Google Scholar]
- [117].Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3(10):793–5. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- [119].Gould TJ, Verkhusha VV, Hess ST. Imaging biological structures with fluorescence photoactivation localization microscopy. Nat Protoc. 2009;4(3):291–308. doi: 10.1038/nprot.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Schermelleh L, et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320(5881):1332–6. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Shroff H, Galbraith CG, Galbraith JA, Betzig E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat Methods. 2008;5(5):417–23. doi: 10.1038/nmeth.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Xu K, Babcock HP, Zhuang X. Dual-objective STORM reveals three-dimensional filament organization in the actin cytoskeleton. Nat Methods. 2012;9(2):185–8. doi: 10.1038/nmeth.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J Cell Biol. 2005;171(2):383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153(7):1427–40. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Manley S, et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008 Feb;5(2):155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- [126].Dahan M, Lévi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003 Oct;302(5644):442–5. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- [127].Huet-Calderwood C, Rivera-Molina F, Iwamoto DV, Kromann EB, Toomre D, Calderwood DA. Novel ecto-tagged integrins reveal their trafficking in live cells. Nat Commun. 2017 Dec;8(1):570. doi: 10.1038/s41467-017-00646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Soto-Ribeiro M, et al. β1D integrin splice variant stabilizes integrin dynamics and reduces integrin signaling by limiting paxillin recruitment. J Cell Sci. 2019 Apr;132(8) doi: 10.1242/jcs.224493. [DOI] [PubMed] [Google Scholar]
- [129].Moffitt JR, Hao J, Wang G, Chen KH, Babcock HP, Zhuang X. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc Natl Acad Sci U S A. 2016 Sep;113(39):11046–51. doi: 10.1073/pnas.1612826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Feng J, et al. Imaging of Optically Active Defects with Nanometer Resolution. Nano Lett. 2018 Mar;18(3):1739–1744. doi: 10.1021/acs.nanolett.7b04819. [DOI] [PubMed] [Google Scholar]
- [131].Slater JH, et al. Modulation of endothelial cell migration via manipulation of adhesion site growth using nanopatterned surfaces. ACS Appl Mater Interfaces. 2015 Feb;7(7):4390–4400. doi: 10.1021/am508906f. [DOI] [PubMed] [Google Scholar]
- [132].Lansky Z, et al. 3D mapping of native extracellular matrix reveals cellular responses to the microenvironment. J Struct Biol X. 2019 Jan;1:100002. doi: 10.1016/j.yjsbx.2018.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wong SHD, et al. Anisotropic Nanoscale Presentation of Cell Adhesion Ligand Enhances the Recruitment of Diverse Integrins in Q7 Adhesion Structures and Mechanosensing-Dependent Differentiation of Stem Cells. Adv Funct Mater. 2019 Feb;29(8):1806822 [Google Scholar]
- [134].Gopal S, et al. Immunogold FIB-SEM: Combining Volumetric Ultrastructure Visualization with 3D Biomolecular Analysis to Dissect Cell-Environment Interactions. Adv Mater. 2019 Aug;31(32):1900488. doi: 10.1002/adma.201900488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Müller DJ, Dufrêne YF. Nature Nanotechnology. 5. Vol. 3. Nature Publishing Group; 2008. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology; pp. 261–269. [DOI] [PubMed] [Google Scholar]
- [136].Huang J, et al. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009;9(3):1111–6. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Lagunas A, et al. Large-scale dendrimer-based uneven nanopatterns for the study of local arginine-glycine-aspartic acid (RGD) density effects on cell adhesion. Nano Res. 2014 Jan;7(3):399–409. [Google Scholar]
- [138].Helenius J, Heisenberg CP, Gaub HE, Muller DJ. Single-cell force spectroscopy. J Cell Sci. 2008 Jun;121(11):1785–1791. doi: 10.1242/jcs.030999. [DOI] [PubMed] [Google Scholar]
- [139].Thie M, et al. Interactions Between Trophoblast and Uterine Epithelium: Monitoring of Adhesive Forces. Hum Reprod. 1998;13(11):3211–9. doi: 10.1093/humrep/13.11.3211. [DOI] [PubMed] [Google Scholar]
- [140].Puech PH, Poole K, Knebel D, Muller DJ. A new technical approach to quantify cell-cell adhesion forces by AFM. Ultramicroscopy. 2006 Jun;106(8-9):637–644. doi: 10.1016/j.ultramic.2005.08.003. [DOI] [PubMed] [Google Scholar]
- [141].Puckert C, Tomaskovic-Crook E, Gambhir S, Wallace GG, Crook JM, Higgins MJ. Molecular interactions and forces of adhesion between single human neural stem cells and gelatin methacrylate hydrogels of varying stiffness. Acta Biomater. 2020 Apr;106:156–169. doi: 10.1016/j.actbio.2020.02.023. [DOI] [PubMed] [Google Scholar]
- [142].Taubenberger A, Cisneros DA, Friedrichs J, Puech PH, Muller DJ, Franz CM. Revealing early steps of α2β1 integrin-mediated adhesion to collagen type I by using single-cell force spectroscopy. Mol Biol Cell. 2007 May;18(5):1634–1644. doi: 10.1091/mbc.E06-09-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Li C, et al. Buoyancy-Driven Gradients for Biomaterial Fabrication and Tissue Engineering. Adv Mater. 2019 Apr;31(17):1900291. doi: 10.1002/adma.201900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Li C, et al. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials. 2018 Sep;176:24–33. doi: 10.1016/j.biomaterials.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Kallepitis C, et al. Quantitative volumetric Raman imaging of three dimensional cell cultures. Nat Commun. 2017 Apr;8(1):14843. doi: 10.1038/ncomms14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Goor OJGM, et al. Efficient Functionalization of Additives at OQ Supramolecular Material Surfaces. Adv Mater. 2017 Feb;29(5):1604652. doi: 10.1002/adma.201604652. [DOI] [PubMed] [Google Scholar]
- [147].Roberts JN, et al. Dynamic Surfaces for the Study of Mesenchymal Stem Cell Growth through Adhesion Regulation. ACS Nano. 2016 Jul;10(7):6667–6679. doi: 10.1021/acsnano.6b01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zelzer M, McNamara LE, Scurr DJ, Alexander MR, Dalby MJ, Ulijn RV. Phosphatase responsive peptide surfaces. J Mater Chem. 2012 May;22(24):12229. [Google Scholar]
- [149].Yang J, et al. Polymer surface functionalities that control human embryoid body cell adhesion revealed by high throughput surface characterization of combinatorial material microarrays. Biomaterials. 2010 Dec;31(34):8827–38. doi: 10.1016/j.biomaterials.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Henry M, Dupont-Gillain C, Bertrand P. Conformation Change of Albumin Adsorbed on Polycarbonate Membranes as Revealed by ToF-SIMS. 2003.
- [151].Liu F, Dubey M, Takahashi H, Castner DG, Grainger DW. Immobilized antibody orientation analysis using secondary ion mass spectrometry and fluorescence imaging of affinity-generated patterns. Anal Chem. 2010 Apr;82(7):2947–58. doi: 10.1021/ac902964q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Welch NG, et al. Surface immobilized antibody orientation determined using ToF-SIMS and multivariate analysis. Acta Biomater. 2017 Jun;55:172–182. doi: 10.1016/j.actbio.2017.03.038. [DOI] [PubMed] [Google Scholar]
- [153].Saha K, et al. Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions. Proc Natl Acad Sci U S A. 2011 Nov;108(46):18714–9. doi: 10.1073/pnas.1114854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Taylor AJ, Ratner BD, Buttery LDK, Alexander MR. Revealing cytokine-induced changes in the extracellular matrix with secondary ion mass spectrometry. Acta Biomater. 2015 Mar;14:70–83. doi: 10.1016/j.actbio.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kabatas S, et al. Fluorinated nanobodies for targeted molecular imaging of biological samples using nanoscale secondary ion mass spectrometry. J Anal At Spectrom. 2019 Jun;34(6):1083–1087. [Google Scholar]
- [156].Wang F, Lozano M, Lillemeier B, Boxer S, Davis M. Lateral distribution of the T cell receptor and cholesterol detected by high-resolution secondary ion mass spectrometry (P3370) J Immunol. 2013;190(1) [Google Scholar]
- [157].Kraft ML. Sphingolipid Organization in the Plasma Membrane and the Mechanisms That Influence It. Front cell Dev Biol. 2016 Jan;4:154. doi: 10.3389/fcell.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Passarelli MK, et al. The 3D OrbiSIMS—label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat Methods. 2017 Dec;14(12):1175–1183. doi: 10.1038/nmeth.4504. [DOI] [PubMed] [Google Scholar]
- [159].Enemchukwu NO, et al. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. J Cell Biol. 2016 Jan;212(1):113–124. doi: 10.1083/jcb.201506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sameni M, Dosecu J, Yamada KM, Sloane BF, Cavallo-Medved D. Functional Live-Cell Imaging Demonstrates That beta1-integrin Promotes Type IV Collagen Degradation by Breast and Prostate Cancer Cells - PubMed. Mol Imaging. 2008;7(5):199–213. [PMC free article] [PubMed] [Google Scholar]
- [161].Li S, et al. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat Mater. 2017 Sep;16(9):953–961. doi: 10.1038/nmat4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Biswas S, Wang X, Morales AR, Ahn HY, Belfield KD. Integrin-targeting block copolymer probes for two-photon fluorescence bioimaging. Biomacromolecules. 2011 Feb;12(2):441–449. doi: 10.1021/bm1012212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wiseman PW, et al. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J Cell Sci. 2004;117(23):5521–5534. doi: 10.1242/jcs.01416. [DOI] [PubMed] [Google Scholar]
- [164].Son S, Moroney GJ, Butler PJ. β1 -Integrin-Mediated Adhesion Is Lipid-Bilayer Dependent. Biophys J. 2017 Sep;113(5):1080–1092. doi: 10.1016/j.bpj.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Chien FC, Kuo CW, Yang ZH, Chueh DY, Chen P. Exploring the formation of focal adhesions on patterned surfaces using super-resolution imaging. Small. 2011 Oct;7(20):2906–2913. doi: 10.1002/smll.201100753. [DOI] [PubMed] [Google Scholar]
- [166].Endesfelder U, Heilemann M. Direct stochastic optical reconstruction microscopy (Dstorm) Methods Mol Biol. 2014;1251:263–276. doi: 10.1007/978-1-4939-2080-8_14. [DOI] [PubMed] [Google Scholar]
- [167].Lippincott-Schwartz J, Patterson GH. Trends in Cell Biology. 11. Vol. 19. Elsevier Current Trends; 2009. Nov 01, Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging; pp. 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Whelan DR, Bell TDM. Image artifacts in single molecule localization microscopy: why optimization of sample preparation protocols matters. Sci Rep. 2015;5:7924. doi: 10.1038/srep07924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Colin-York H, et al. Spatiotemporally Super-Resolved Volumetric Traction Force Microscopy. Nano Lett. 2019 Jul;19(7):4427–4434. doi: 10.1021/acs.nanolett.9b01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Digman MA, Brown CM, Horwitz AR, Mantulin WW, Gratton E. Paxillin dynamics measured during adhesion assembly and disassembly by correlation spectroscopy. Biophys J. 2008;94(7):2819–2831. doi: 10.1529/biophysj.107.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Müller DJ, Helenius J, Alsteens D, Dufrne YF. Nature Chemical Biology. 6. Vol. 5. Nature Publishing Group; 2009. May 15, Force probing surfaces of living cells to molecular resolution; pp. 383–390. [DOI] [PubMed] [Google Scholar]
- [172].Xiao L, Schultz ZD. Targeted-TERS detection of integrin receptors on human cancer cells. Cancer cell Microenviron. 2016;3(4) [PMC free article] [PubMed] [Google Scholar]
- [173].Fearn S. Materials Science and Technology (United Kingdom) 2. Vol. 31. Maney Publishing; 2015. Jan 01, Characterisation of biological material with ToF-SIMS: A review; pp. 148–161. [Google Scholar]
- [174].Vanbellingen QP, Elie N, Eller MJ, Della-Negra S, Touboul D, Brunelle A. Time-of-flight secondary ion mass spectrometry imaging of biological samples with delayed extraction for high mass and high spatial resolutions. Rapid Commun Mass Spectrom. 2015 Jul;29(13):1187–1195. doi: 10.1002/rcm.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Nuñez J, Renslow R, Cliff JB, Anderton CR. NanoSIMS for biological applications: Current practices and analyses. Biointerphases. 2018 Jun;13(3):03B301. doi: 10.1116/1.4993628. [DOI] [PubMed] [Google Scholar]
- [176].Bergholt MS, et al. Fiberoptic Confocal Raman Spectroscopy for Real-Time In Vivo Diagnosis of Dysplasia in Barrett’s Esophagus. Gastroenterology. 2014 Jan;146(1):27–32. doi: 10.1053/j.gastro.2013.11.002. [DOI] [PubMed] [Google Scholar]