Abstract
In breast cancer, humoral immune responses may contribute to clinical outcomes, especially in more immunogenic subtypes. Here we investigated B lymphocyte subsets, immunoglobulin expression, and clonal features in breast tumors, focusing on aggressive triple-negative breast cancers (TNBC). In samples from TNBC patients and healthy volunteers, circulating and tumor-infiltrating B lymphocyte (TIL-B) were evaluated. CD20+CD27+IgD- isotype-switched B lymphocytes were increased in tumors, compared with matched blood. TIL-B frequently formed stromal clusters with T lymphocytes and engaged in bidirectional functional crosstalk, consistent with gene signatures associated with lymphoid assembly, co-stimulation, cytokine-cytokine receptor interactions, cytotoxic T cell activation, and T cell-dependent B cell activation. TIL-B upregulated B cell receptor (BCR) pathway molecules FOS and JUN, germinal center chemokine regulator RGS1, activation marker CD69, and TNFα signal transduction via NFκB, suggesting BCR-immune complex formation. Expression of genes associated with B lymphocyte recruitment and lymphoid assembly, including CXCL13, CXCR4, DC-LAMP, was elevated in TNBC compared with other subtypes and normal breast. TIL-B-rich tumors showed expansion of IgG but not IgA isotypes, and IgG isotype-switching positively associated with survival outcomes in TNBC. Clonal expansion was biased towards IgG, showing expansive clonal families with specific variable region gene combinations and narrow repertoires. Stronger positive selection pressure was present in the complementary determining regions (CDRs) of IgG compared to their clonally related IgA in tumor samples. Overall, class-switched B lymphocyte lineage traits were conspicuous in TNBC, associated with improved clinical outcomes, and conferred IgG-biased, clonally expanded, and likely antigen-driven humoral responses.
Keywords: B lymphocyte, Memory B lymphocyte, IgG isotype-switching, Breast cancer, Triple-negative breast cancer, Tumor-infiltrating lymphocytes, Immunoglobulin repertoire, Clonal expansion, Tumor microenvironment
Introduction
Initiation of effective adaptive immunity may contribute to tumor growth restriction through specific antigen-directed responses. The T lymphocyte component of anti-tumor immunity has received significant attention (1). In contrast, B lymphocytes, especially the memory and isotype-switched B lymphocyte compartments, and their expressed antibody profiles remain only partially elucidated. Emerging findings suggest that aspects of humoral immune responses may correlate with improved clinical outcomes via B lymphocyte tumor-infiltration and expression of antibodies in lesions or in the circulation (2, 3). These could differ across tumor types, potentially offering opportunities for stratification and for guiding therapy options.
Breast cancer is one of the most frequently-diagnosed malignancies, divided into biological, and differential therapy-associated subtypes based on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, with specific prognostic and predictive biomarker implications. Triple-negative breast cancers (TNBC) do not express any of these markers and demonstrate the least-favorable prognosis due to both an aggressive phenotype and limited targeted therapies (4). Although breast cancer has not traditionally been regarded as a typical immunogenic malignancy, emerging studies report the presence and potential clinical significance of tumor-infiltrating immune cells for clinical outcomes (5). Paradoxically, despite an overall poor prognosis of patients with TNBC, immune infiltration is more pronounced compared with other breast cancer types. Consistent with an immunogenic tumor microenvironment (TME), some TNBC patients may benefit from anti-programmed death-ligand 1 (PD-L1) and anti-PD-1 immunotherapy with atezolizumab in combination with chemotherapy (6). TNBCs are characterized by immunologically-variable and compartmentalized tumors with structural features in the tumor-immune interphase and large variability across individuals, mandating the need for patient stratification for therapy selection (7). The most thoroughly-studied effector cells within the breast cancer setting are CD8+ cytotoxic T lymphocytes and natural killer (NK) cells (8). However, tumor-infiltrating B lymphocytes (TIL-Bs) aggregating within tertiary lymphoid structures (TLS) (9) may have an antigen-educated phenotype (10) and autoantibodies are thought to trigger tumor cell clearance (11). TIL-Bs might also serve as antigen-presenting cells to promote anti-tumor Th responses (12). Therefore, it is increasingly-recognized that humoral immune responses may be important contributors to breast cancer outcome, especially in more immunogenic TNBCs.
The interaction between the immune system and malignant cells therefore constitutes a major focus of current translational and clinical investigation (13). Recent studies have provided evidence of TIL-Bs and tumor-reactive immunoglobulin in several solid tumors, including in breast cancer, and TIL-Bs have been reported to respond to B cell receptor stimulation and produce immunoglobulins ex vivo (14–16).
Here, in peripheral blood and cancer lesions of patients with breast cancer, specifically in individuals with TNBC, we performed cytofluorimetric, transcriptomic, immunofluorescence, single-cell RNA-seq and long-read immunoglobulin repertoire studies to evaluate isotype-switched and memory B lymphocyte subsets, immunoglobulin (Ig) isotype distribution, and clonal expansion profiles.
Materials and Methods
Clinical sample collection and cohort descriptions
A collection of internal and external cohorts of healthy volunteer (HV) and patient samples, including the unique accession numbers, are summarized in Suppl. Table 1 and Suppl. Materials and Methods. All internal King’s College London (KCL) samples were collected with informed written consent, in accordance with the Helsinki Declaration (study design was approved by the Guy’s Research Ethics Committee (REC No. 07/H0804/131), Guy’s and St. Thomas’ NHS Foundation Trust). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque PLUS density gradient centrifugation, and single cells from breast tissues were isolated as described in Suppl. Materials and Methods.
Gene expression (GEx) profiling of lymphocyte and lymphoid assembly markers
Gene expression levels were analyzed from internal Guy’s Hospital and TCGA Breast cohorts (KCL and TCGA GEx cohorts), and compared between PAM50 (Basal-like, HER2, luminal A, luminal B and normal-like) and TNBC-subtypes (basal-like 1/2, immunomodulatory, mesenchymal, mesenchymal stem–like, luminal androgen receptor) according to classification described in Brasó-Maristany et al. (17). A B lymphocyte metagene signature was analysed from published NanoString data of primary and metastatic breast cancers (GSE102818) (18). Kaplan–Meier (KM) plotter tool was utilized to generate survival plots (19). CIBERSORT was applied to evaluate naïve B cells, plasma cells and memory B cells identified among 22 immune subsets in the KCL GEx cohort (20). Univariate Cox proportional hazards regression models were used to investigate the prognostic importance of these immune subsets in high and low TIL infiltrated tumors (semi-quantitative TIL quantification was performed by a trained histopathologist using tissue microarrays (Suppl. Materials and Methods)). B lymphocyte function-associated gene sets were identified from gene ontology (GO) database (Suppl. Materials and Methods). A lymphoid assembly-associated gene signature was compiled from a set of known markers (21).
Single-cell RNA-sequencing (scRNA-seq) analysis
Analyses were performed on a published scRNA-seq dataset (GSE114725) (22) (Single cell cohort) using R package Seurat (23). Dimensionality reduction was performed using Uniform Manifold Approximation and Projection (UMAP) and cells were clustered using the Louvain algorithm (23). Ig isotypes were detected based upon heavy chain gene expression. Differentially expressed genes identified by Seurat were used to perform gene set enrichment analysis (GSEA) using the fgsea package. Gene sets were obtained from Broad Institute Molecular Signature Database using R package msigdbr (24). CellPhoneDB v2.0 was used to analyze B cell-T cell interactions (25).
Immunohistochemical/Immunofluorescence (IHC/IF) evaluations of TIL-B distribution and surface Ig expression
Three sections per tissue sample were stained with fluorescently-labelled antibodies conferring three panels: TIL classification (DAPI/CD20/CD3/PanCK), naïve B lymphocyte identification (DAPI/CD20/IgD), Ig isotype expression (DAPI/CD27/IgG/IgA/IgM). Antibodies used are detailed in Suppl. Table. 2. TIL-B structural features were evaluated (Suppl. Materials and Methods) following TIL working group guidelines (26), guided by trained pathologists. Olympus VS120-S5 or Nikon TE 2000-U microscopes were used for imaging.
Long-read Ig repertoire analysis
Immunoglobulin repertoire analysis was performed from cDNA synthesized from breast tissues with the 5’ RACE template switch method (Suppl. Materials and Methods). Full-length Ig cDNA were PCR-amplified with primers containing unique molecular barcodes. Purified DNA samples were sequenced using PacBio Single Molecule, Real-Time (SMRT) Sequencing platform (27). Redundant sequences with identical molecular barcodes were removed. Ig genes and complementary determining region (CDR)3 sequences were determined using IMGT/HighV-QUEST (28). Relatedness among sequences were estimated using BRepertoire webserver (29) “Clonotype clustering” function, after partitioning all CDR3 DNA sequences by the sample and the V gene family used (30). Selection pressure analysis was performed using R package shazam (31).
Statistical analyses
GraphPad Prism and R were used for statistical analyses of paired and unpaired data sets. Data are presented as mean ± standard error mean (SEM). P-values reported as: P<0.05 (*), P<0.01 (**), P<0.001 (***), P<0.0001 (****) and all tests were two-sided.
Further details can be found in Suppl. Materials and Methods.
Results
Collapse in circulating memory B lymphocytes contrasts with amplification of intratumoral class-switched memory B lymphocyte compartment
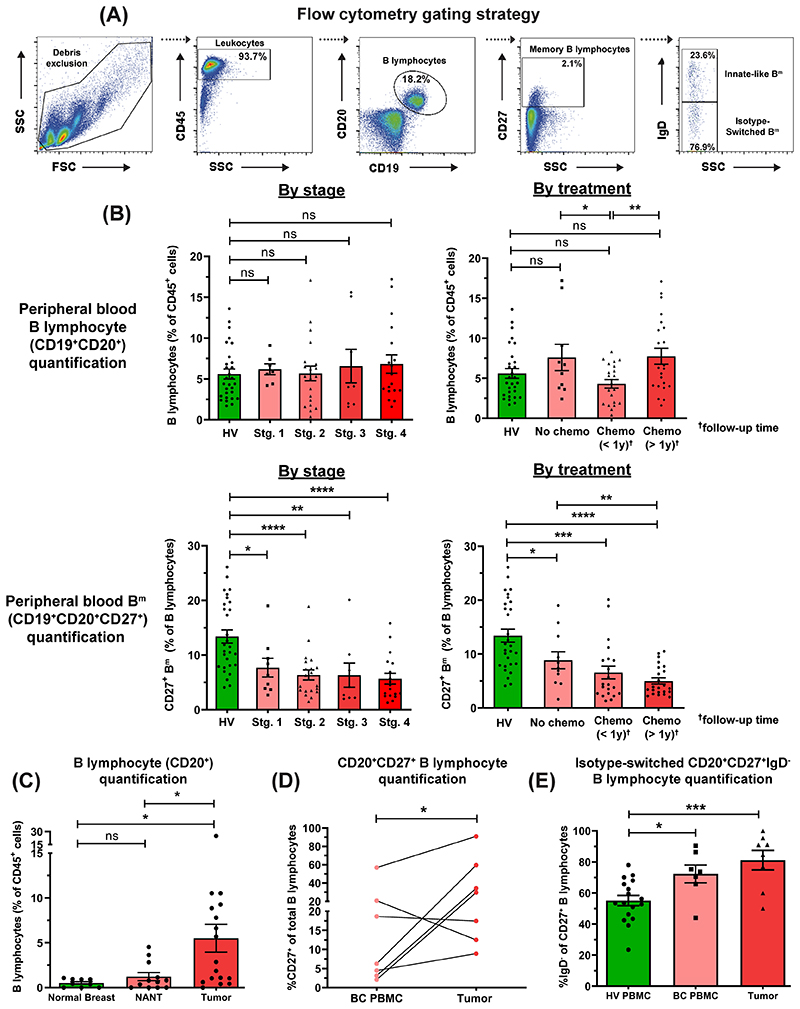
We compared B lymphocyte subsets between breast cancer patient blood and tumors (KCL flow cohort; see Suppl. Table 1 for information of all cohorts used). We quantified percentages of B lymphocytes (CD19+CD20+) and memory (CD19+CD20+CD27+) B lymphocytes (Bm) in patients (N=55) and healthy subjects (N=48) by flow cytometry (Fig. 1A–B). Consistent with a report in melanoma (32), we identified a fall in peripheral Bm in patients compared to healthy volunteers. This was independent of disease stage or chemotherapy treatment status. We observed significantly-lower proportions of B lymphocytes (of CD45+ cells) in the circulation of patients with recent chemotherapy compared to those without treatment (Fig. 1B), alongside reduced serum immunoglobulin titres in chemotherapy-treated individuals (Suppl. Fig. 1A–B, KCL Luminex cohort). Baseline serum immunoglobulin isotype titers in patients were not predictive of breast cancer-specific death (Suppl. Fig. 2, Apolipoprotein Mortality Risk (AMORIS) cohort (33)).
Figure 1. Flow cytometric analyses reveal reduced circulating CD20+CD27+ memory and amplification of tumor-infiltrating CD20+CD27+IgD- class-switched subsets among B lymphocytes.
(A) Gating strategy for identification of B lymphocytes and memory (Bm) lymphocyte derived from PBMC (example patient PBMC shown). (B) Quantification of total circulating-B cells [top] and Bm cells [bottom] as % of CD45+ cells in HV (N=48) and patient (N=55) peripheral blood (KCL flow cohort; Supp. Table 1 for patient information), stratified according to stage and treatment status. (C) Quantification of B lymphocytes (CD20+) from single cell suspensions of normal breast (N=9), NANT (N=12) and cancer tissue (N=17) samples. (D) Quantification of matched patient circulating- and tumor-infiltrating CD20+CD27+ B cells (matched samples of 7 patients). (E) Quantification of CD20+CD27+IgD- B cells in HV (N=17), patient peripheral blood (N=7) and cancers (N=8) of total CD20+CD27+ B cells. Statistical significance was determined using the Student’s t-test.
In contrast, using tumor single-cell suspensions (N=17), we identified a higher B lymphocyte infiltration compared to non-adjacent, non-tumor (NANT) (N=12) and normal breast (N=9) tissues (Fig. 1C). In matched blood and tumor samples (N=7), tumors contained higher CD20+CD27+ B cell proportion (Fig. 1D). Both peripheral and intratumoral CD20+CD27+ B cell populations showed a bias towards the loss of IgD expression (N=32) (Fig. 1E). Additional quantitative single B lymphocyte gene expression analyses (Single cell cohort) also confirmed larger proportions of CD20+CD27+ and CD20+CD27+IgD- B lymphocytes in tumors compared to blood (Suppl. Fig. 3A–B). Furthermore, isotype-switched TIL-B populations comprise memory, germinal center (GC) B cells and plasma cells in non-TNBC and TNBC (IHC/IF, KCL IHC cohort and Single cell cohort) (Suppl. Fig. 3A–E).
These findings reveal a reduced peripheral Bm population in patients compared to healthy volunteers, independently of disease stage or treatment, in parallel with an enriched tumor-infiltrating class-switched B cell compartment in the TME compared to matched patient blood.
Elevated TIL-B signatures and assembly within stromal clusters in TNBC
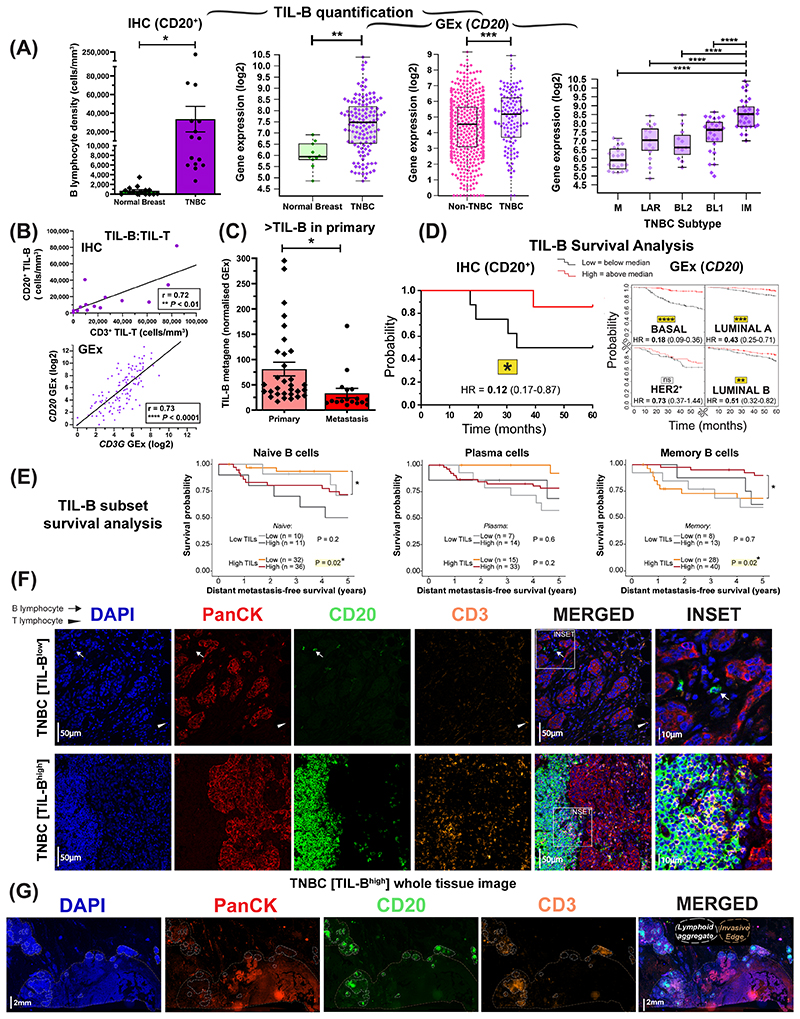
We asked whether TNBC demonstrate increased immune cell infiltration, particularly concentrating on the B cell population, compared with normal breast or other breast cancer types. Quantitative IHC/IF evaluations (Bart’s IHC cohort) and GEx analyses (KCL and TCGA GEx cohorts) revealed an expansion of CD20+ TIL-B within TNBC lesions compared with normal tissues (Fig. 2A), and compared to non-TNBC carcinomas, expression of B lymphocyte (CD20 and BCR complex CD79A) and T lymphocyte (CD3D and CD3G) markers were elevated in TNBC. Within different TNBC subtypes, these markers were elevated especially in the Lehmann’s immunomodulatory (IM) molecular TNBC subtype, known to be enriched in core immune signal transduction pathways and cytokine signaling (N=122) (34) (Fig. 2A, Suppl. Fig. 4A–B). TIL-B density and CD20 gene expression positively correlated with tumor-infiltrating T lymphocyte (TIL-T) density and CD3 gene expression respectively (Fig. 2B). The expression of a B lymphocyte metagene signature (NanoString cohort) was significantly-higher in primary cancers compared to patient-matched metastatic sites (N=31 vs 17), suggesting a diminished immune TME with advanced disease (Fig. 2C).
Figure 2. B lymphocyte infiltration and its positive prognostic value in TNBC.
(A) TIL-B density comparison by IHC within normal breasts and TNBC (N=15 each, Bart’s IHC cohort), and by GEx (normal breast vs TNBC (N=10 vs 131, KCL GEx cohort); non-TNBC vs TNBC (N=515 vs 123, TCGA GEx cohort); TNBC subtypes (mesenchymal (M), luminal androgen receptor (LAR), basal-like 1 and 2 (BL1 and 2) and immunomodulatory (IM) (N=122, KCL GEx cohort). Mann-Whitney test was used for statistical significance (B) TNBC TIL-B correlation with TIL-T by IHC (r=0.72, Bart’s IHC cohort) and by GEx (r=0.73, KCL GEx cohort). Linear regression analysis was used to calculate correlation coefficients (r) and p-values. (C) B lymphocyte metagene NanoString GEx data comparing B lymphocytes in primary tumors with metastatic sites (N=31 vs 17) (NanoString cohort). (D) High (above median) TIL-B densities by IHC were associated with better overall survival in TNBC (N=15) (Bart’s IHC cohort), and in the basal-like subtype by high CD20 GEx (N=241) (KM plotter cohort) (log rank test used to assess statistical significance). (E) Kaplan–Meier survival curves display DMFS for naïve B cells, plasma cells and memory B cells in TNBC (KCL GEx cohort) using CIBERSORT (20). Data were divided into four groups based on B lymphocyte subset and TIL levels stratified by semi-quantitative TIL classification. Statistical significance was assessed using univariate Cox proportional hazards regression models. (F) Representative IHC/IF images (Bart’s IHC cohort) depicting nucleated cells (DAPI), epithelial cells (PanCK), B lymphocytes (CD20) and T lymphocytes (CD3) within normal breast and TNBC. Scale bar=50μm. (G) Representative TNBC images highlighting numerous lymphoid aggregates (within white lines) consisting of B lymphocytes assembled adjacent to a T lymphocyte zone. Brown dash lines indicate carcinoma edge. Scale bar=2mm.
Both quantitative IHC/IF (N=15) and KM-plotter survival analysis (N=241) demonstrated TIL-B in primary tumors were significantly-associated with more favorable overall survival, most prominently in basal-like/TNBC (Fig. 2D, Suppl. Fig. 4C). Additional Kaplan-Meier analyses using CIBERSORT (from KCL GEx cohort, see Suppl. Materials and Methods) (20) investigated the associations between distant metastasis-free survival (DMFS) and naïve, plasma and memory B cell phenotypes in TNBC (N=89). In tumors with high TIL infiltrates (classified by using tissue microarrays), memory B cells were associated with a more-favorable DMFS, while a negative prognostic value was shown for naïve B cells in this cohort (Fig. 2E). IHC/IF staining analyses (Bart’s IHC cohort) confirmed the heterogeneity of TIL-T and TIL-B (Fig. 2F), with evidence of expansive cluster formation (demarcated in white (>30 TIL-B and >30 TIL-T aggregated), typically of a B lymphocyte assembly adjacent to a T lymphocyte zone) (Fig. 2G). This highlight close B-T lymphocyte interactions within the TME.
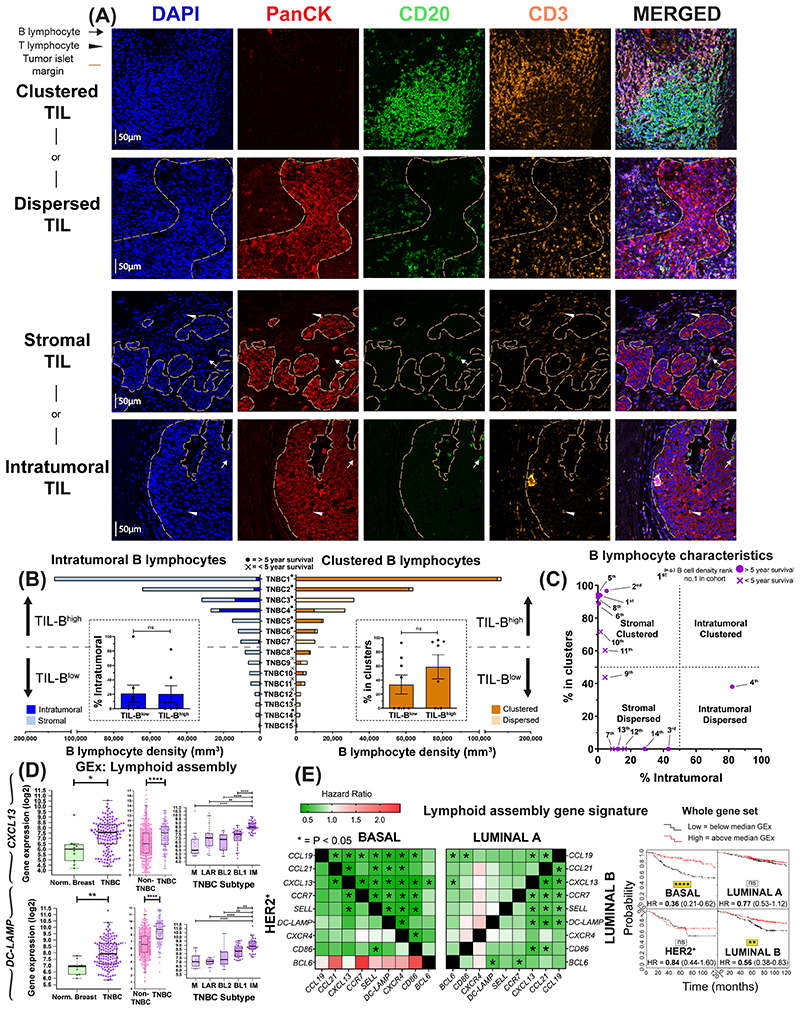
Since we found B-T lymphocyte clusters in a large proportion (9 of 15) of TNBCs (Bart’s IHC cohort), we next evaluated TIL-B spatial and structural characteristics. We characterized TIL-B as either stromal or intratumoral, according to penetration within PanCK+ tumor islets. Cells were further categorized as forming either clusters or dispersions, according to the extent of aggregation along with CD3+ TIL-T (Fig. 3A). Tumors were split according to into TIL-Bhigh and TIL-Blow groups, as defined by median TIL-B density. B lymphocytes typically formed within stromal clusters irrespective of overall TIL-B density (Fig. 3B). Five individuals featuring highly-clustered TIL-B characteristics showed a >5 year survival post-surgery (Fig. 3C). Consistent with the B-T lymphocyte cluster formation observed, we found elevated expression of B lymphocyte recruitment and lymphoid assembly marker genes (CXCL13, CXCR4 and DC-LAMP) in TNBC compared to both non-TNBC and normal breast, and within the TNBC cohort the highest expression of these genes was detected in IM tumors (Fig. 3D, Suppl. Fig. 4D, KCL and TCGA GEx cohorts). Higher expression of these signatures were also associated with significantly-improved odds of overall survival (10-year follow-up) in basal-like/TNBC (Fig. 3E, KM plotter cohort).
Figure 3. Occurrence of B lymphocytes in stromal clusters.
(A) Representative IHC/IF images (Bart’s IHC cohort), highlighting key TIL characteristics: clustered TIL versus dispersed TIL and stromal TIL (outside tumor nests) vs intratumoral TIL (within tumor nests). Scale bar=50μm. (B) Quantitative assessment of TIL-B spatial and structural characteristics within TNBC (N=15): [left] intratumoral vs stromal; [right] clustered versus non-clustered. Patients ranked according to CD20+ TIL-B density (TNBC1=highest), and overall survival data indicated. [Inset] Patients samples split at median density into high and low TIL-B groups and the % of intratumoral (left) or clustered (right) B lymphocytes were analyzed. Statistical significance was determined using Student’s t-test. (C) Characterization of TNBC TIL-B profile as stromal clustered, intratumoral clustered, stromal dispersed, or intratumoral dispersed. Overall survival data are indicated. (D) GEx data for lymphoid assembly marker genes CXCL13 and DC-LAMP ([left] normal breast vs TNBC (N=10 vs 131, KCL GEx cohort); non-TNBC vs TNBC ([middle] N=515 vs 123, TCGA GEx cohort); TNBC subtypes ([right] N=122, KCL GEx cohort). (E) Survival analysis in KM Plotter of determined ER- HER2-/basal surrogate, HER2+, luminal A, luminal B subtype KM plotter surrogate subgroups (19) (KM plotter cohort). These indicate that expression of lymphoid cell assembly genes carries positive prognostic value in TNBC/basal-like and luminal B subtype ([left] Individual genes were evaluated in combination with each other gene, and [right] gene set as a whole).
Therefore, substantial TIL-B densities in TNBC, typically form clusters along with T lymphocytes. TIL-B marker (CD20 and CD79A) and GEx features conferring B lymphocyte recruitment and lymphoid assembly were elevated compared to non-TNBC and normal breast tissues, and associated with more favorable survival in basal-like/TNBC subtype.
Tumor-infiltrating B lymphocytes are activated via the B Cell Receptor
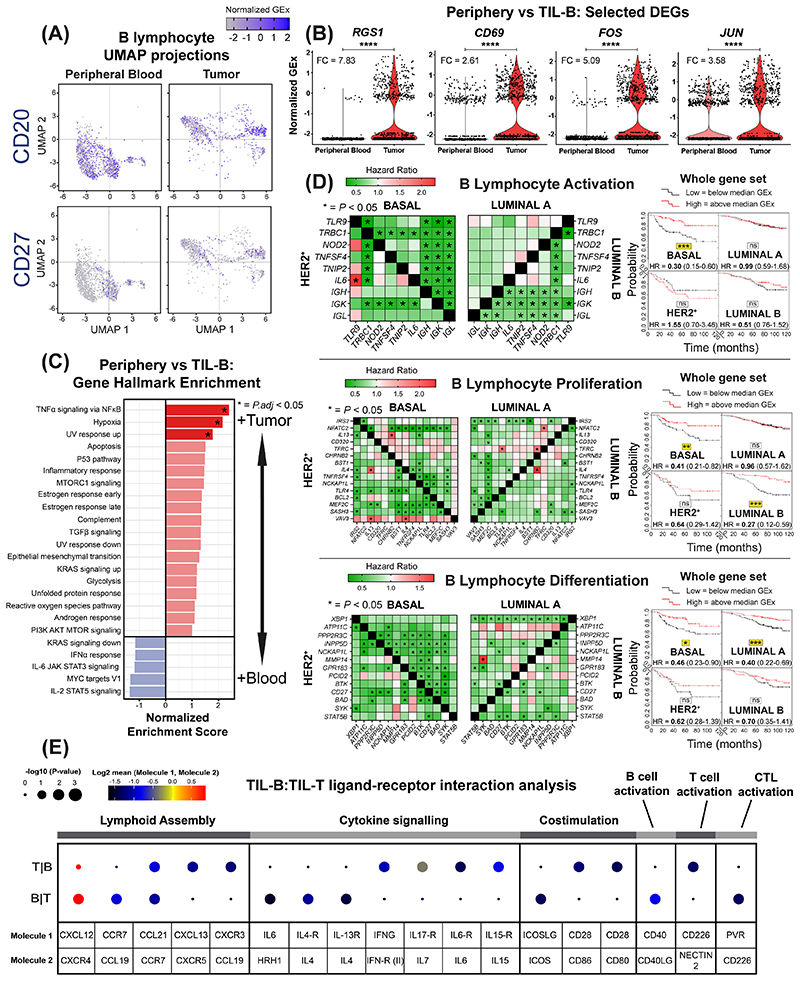
We sought direct evidence of active roles for B lymphocytes in the TME, using a previously-published dataset (Single cell cohort). We applied dimensionality reduction (UMAP) to single-cell B lymphocyte populations and revealed distinct CD20+ and CD27+ TIL-B populations compared to those in the circulation (blood (N=1,476 B cells) and tumors (N=1,021 B cells) of eight patients) (Fig. 4A). Downstream differential expression gene (DEG) analysis identified several upregulated genes in TIL-B: FOS and JUN, molecules downstream of BCR complex pathway (35); RGS1, germinal center B lymphocyte regulator of chemokine receptor signaling (36); and the lymphocyte activation marker CD69, triggered through crosslinking of surface Ig (37) (Fig. 4B). Hallmark enrichment analysis (38) revealed significantly-enhanced expression among TIL-B for genes controlling TNFα signaling via NFκB, hypoxia and UV response pathways, in comparison with circulating-B lymphocytes (Fig. 4C). Several genes, known to positively regulate B lymphocyte activation, proliferation and differentiation functions were upregulated in TNBC compared to normal breast (Suppl. Fig. 5, KCL and TCGA GEx cohorts). Survival analysis of these gene signatures revealed positive associations with overall survival (10-year follow up), most pronounced in basal-like/TNBC (Fig. 4D, KM plotter cohort). We next evaluated B cell-T cell interactions in the tumor microenvironment (Single cell cohort) using CellPhoneDB (25). We identified cell communication pathways associated with lymphoid assembly (CXCL12, CCL19, CCL21 and CXCL13), cytokine signaling (IL-4, IL-6, IL-13, IL-15, IL-17 and IFNγ), co-stimulation (CD28 and ICOS) and immune activation (CD40 and CD226). These findings support a bi-directional functional crosstalk between tumor-infiltrating B and T lymphocytes (Fig. 4E).
Figure 4. scRNA-seq analysis reveals BCR-driven TIL-B activatory signatures and B lymphocyte lineage gene markers predict positive survival outcome.
(A) UMAP visualization according to global GEx of single B lymphocytes pooled from the peripheral blood (1,476 cells) and tumors (1,021 cells) of eight patients (Single cell cohort), colored by relative normalized gene expression levels for CD20 and CD27. (B) The detection of differentially expressed genes (DEGs, on cells originally annotated by Azizi et al. (22) as B cells) demonstrates elevated expression of FOS, JUN, RGS1 and CD69, indicated by fold change (FC) (determined using Wilcoxon Rank Sum test). (C) Gene set enrichment analysis of TIL-B relative to circulating B lymphocytes using hallmark gene sets. Red color indicates significant upregulation of normalized enrichment scores in TIL-B. (D) Survival analysis in KM Plotter of determined ER-HER2-/basal surrogate, HER2+, luminal A, luminal B subtype KM plotter surrogate subgroups (19) (KM plotter cohort) for expression of gene signatures positively regulating key B lymphocyte properties (activation, proliferation and differentiation). Representative genes listed for B lymphocyte proliferation (44 total in set). Signatures from all three functions carry positive prognostic value in the basal-like cancer subtype ([left] Individual genes were evaluated in combination with each other gene, and [right] gene set as a whole). (E) CellPhoneDB (25) was applied to analyze B cell-T cell interactions (Single cell cohort). After false discovery rate (FDR<0.001) correction, communication pathways identified included lymphoid assembly, cytokine signaling, co-stimulation, T-cell dependent B cell activation, and cytotoxic T lymphocyte (CTL) activation. Circle sizes indicate p-value while color-coding represents the average expression level of interacting molecule 1 in cluster 1 and interacting molecule 2 in cluster 2.
These findings indicate the presence of distinct B lymphocyte populations between patient blood and cancers and evidence of TIL-B antigen-Ig complex, BCR pathway stimulation and evidence of B cell-T cell crosstalk. Key functional attributes for the initiation of B lymphocyte responses are associated with improved patient outcomes, especially in TNBC.
IgG+ B lymphocyte densities are elevated in tumors, and IgG isotype-switching predicts positive survival outcomes in TNBC
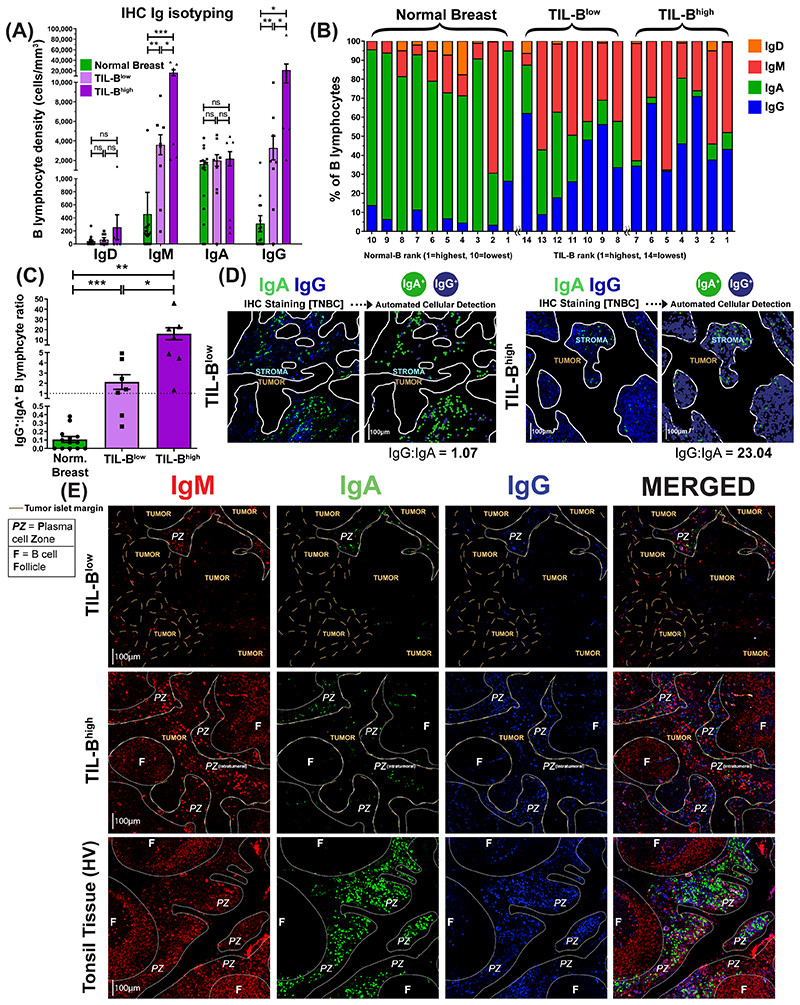
We studied B lymphocyte densities according to Ig isotype expression in primary breast cancers by quantitative IHC/IF evaluations of IgD, IgM, IgA and IgG isotype-expressing B lymphocytes (Bart’s IHC cohort) (normal breast tissue (N=10), TNBC (N=14)). Naïve (IgD+) B lymphocyte densities were found at low densities in normal breast, TIL-Blow and TIL-Bhigh tumors (Suppl. Fig. 6A). Higher IgG+ and IgM+ B lymphocyte densities were found in TIL-Bhigh and, to a lesser extent, in TIL-Blow cancers compared with normal breast (Fig. 5A–B). In contrast, IgA+ B lymphocyte densities were consistent among tissue compartments, highlighting an overall bias towards increased IgG+:IgA+ B lymphocyte ratio within cancers compared to normal breast, and within TIL-Bhigh compared to TIL-Blow cancers (Fig. 5C–D).
Figure 5. Quantitative fluorescence IHC reveals elevated IgG+:IgA+ ratio within high TIL-B tumors, implicating expansion of IgG+ B lymphocytes within TNBC tumor microenvironment.
(A) Comparison of surface immunoglobulin-expressing B lymphocyte density in normal breast and TNBC with low TIL-B density (below median CD20+) and high TIL-B density (above median CD20+). (B) Quantitative IHC analysis profiling the proportions of B lymphocytes present within the microenvironment of normal breast tissue (N=10) and TNBC (N=14) expressing each Ig isotype. (C) Enumeration of IgG+:IgA+ B lymphocyte ratios. (D) [Right] Example images illustrating IgG+ and IgA+ B lymphocytes in a typical TIL-B low individual and a TIL-B high individual. Automated cellular detection identifies IgA (green) and IgG (blue) B lymphocytes. Scale bar=100μm. (E) Representative images depicting typical Ig isotype expression among B lymphocytes: IgM+ (red), IgA+ (green), and IgG+ (blue). Images from Bart’s IHC cohort. Brown dash lines indicate margin of the cancer. White lines separate distinct regions of B lymphocyte compartments (PZ=plasma cell zone; F=B lymphocyte follicle). Scale bar=100μm. Statistical significance was determined using the Student’s t-test.
IHC/IF showed the dominating IgA+ B lymphocyte profile present in normal breast is contained within normal lobules and their periphery (Suppl. Fig. 6B). While TIL-Blow cancers lacked large B lymphocyte follicles and contained small IgM/IgA/IgG plasma cell zones, TIL-Bhigh cancers typically contained denser IgM/IgA/IgG plasma cell zones surrounding expansive IgM+ follicular B lymphocyte clusters with defined germinal centers (Fig. 5E). The resulting B lymphocyte assembly in these TIL-Bhigh tumors shares some structural similarities with those in tonsil tissues. IgG and IgA expression were accompanied with CD27 upregulation, validating the differentiated, isotype-switched status of these cells (Suppl. Fig. 7A–C). We further confirmed that intratumoral IgG+ cells comprised both CD20+ as well as CD138+ (plasma) cell populations, the latter being low or negative for CD20 expression (Suppl. Fig. 7D, Suppl. Fig. 3C).
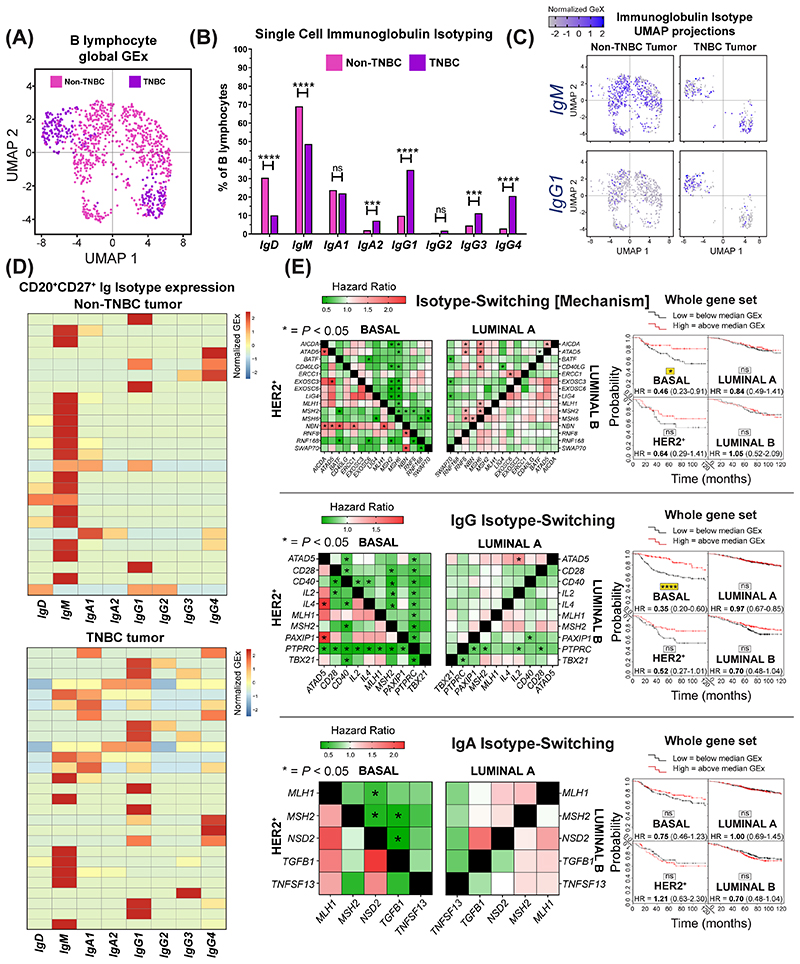
Since TNBCs feature high levels of IgG-expressing B lymphocytes, we studied B immunoglobulin isotypes (Ig heavy chain) using a published scRNA-seq dataset (Single cell cohort; N=1,021 TIL-B across eight patients). UMAP applied to single-cell B lymphocyte populations revealed distinct TIL-B populations in TNBC compared with non-TNBC samples. This analysis also revealed enhanced isotype-switching to IgG1, IgG3, IgG4 and IgA2 subclasses in TNBC (Fig. 6A–B). UMAP confirmed differential isotype-expressing B lymphocyte compartments in TNBC compared with non-TNBC for IgM and IgG1 based on clustering (Fig. 6C). Heatmaps of single CD20+CD27+ B lymphocyte IgCH expression showed a propensity towards non-switched IgM transcripts in blood in contrast to high levels of isotype-switched IgG and IgA B lymphocytes in tumor samples (Suppl. Fig. 8), and higher levels of isotype-switching in TNBC compared to non-TNBC tumor samples (Fig. 6D). These support an active tumor-resident humoral response, different to the equivalent response in the circulation, and likely driven by inflammatory and possibly antigenic signals which may support Ig class-switch recombination in the breast cancer microenvironment, especially in TNBC.
Figure 6. Single cell RNA-seq data analysis reveals favor of IgG isotypes in TNBC and isotype-switching gene markers predict positive survival outcome.
(A) UMAP visualization of B lymphocyte populations in non-TNBC vs TNBC (Single cell cohort). (B) Percentage of each Ig isotype based upon raw data of Ig heavy chain (Student’s t-test) (C) UMAP visualization for IgM and IgG1 isotypes colored by relative normalized gene expression levels (N=1,021 cells). (D) IgCH switch transcripts of single B lymphocytes (CD19+CD27+/CD20+CD27+/CD22+CD27+ single cells) were analyzed in non-TNBC and TNBC tissues and demonstrated more Ig isotype-switching events in the TNBC samples. (E) Survival analysis in KM Plotter of determined ER-HER2-/basal surrogate, HER2+, luminal A, luminal B subtype KM plotter surrogate subgroups (19) (KM plotter cohort) for expression of gene signatures positively regulating isotype-switching (total Ig, IgG and IgA isotype-switching). Signatures from all three functions carry positive prognostic value in basal-like cancer ([left] Individual genes were evaluated in combination with each other gene, and [right] the gene set as a whole).
Survival analysis of isotype-switching gene signatures revealed a positive association with 10-year overall survival for IgG, but not for IgA, isotype-switching, most pronounced in basal-like/TNBC (Fig. 6E, KM plotter cohort). Moreover, as expected, several genes involved in the mechanism of and/or positively regulating isotype-switching were upregulated in TNBC compared to normal breast (Suppl. Fig. 9, KCL and TCGA GEx cohorts).
These findings indicate a shift in favor of IgG+ B lymphocytes in TIL-Bhigh TNBC and point to IgG isotype-switching as a contributor to the overall positive role of B lymphocyte responses to breast cancers.
IgG-biased, clonally-expanded, immunoglobulin repertoires in breast cancer
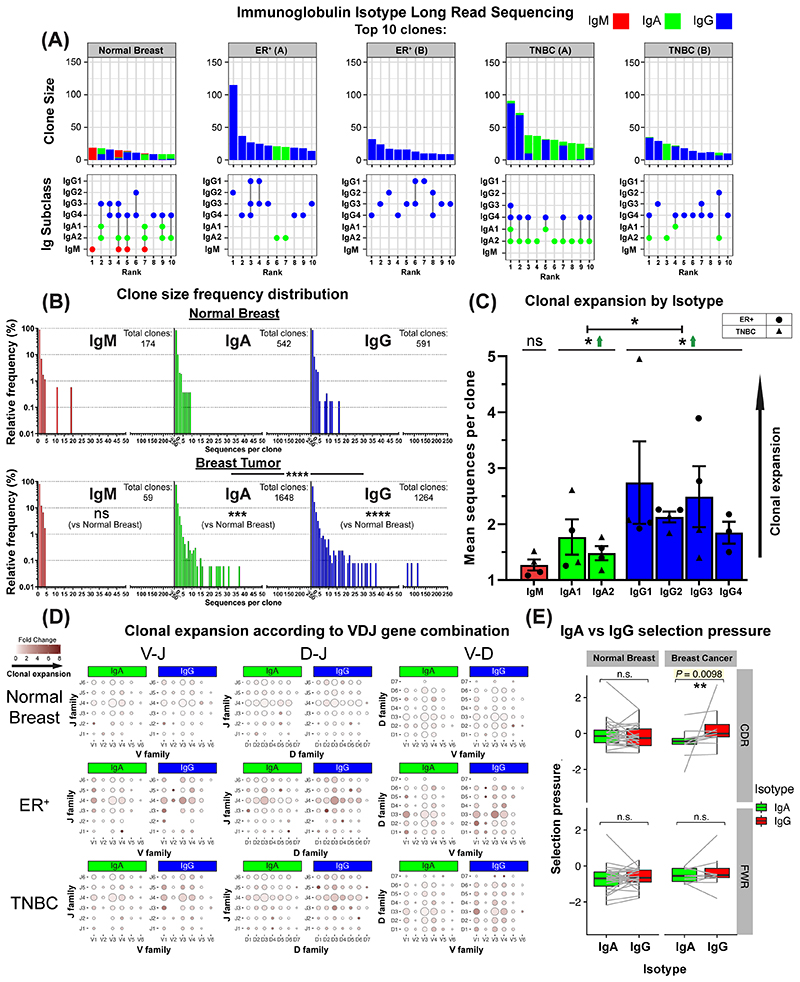
Using long-read sequencing we next generated a dataset of Ig heavy chain repertoires (N=7,670) from two TNBCs, two ER+ cancers and one normal breast tissue (KCL sequencing cohort) and performed sequence clustering analyses to define clonotypes and compare the distributions of Ig isotypes following B lymphocyte clonal expansion. We observed consistently-larger clonal family sizes within the 10 largest clones of cancers compared to normal breast (Fig. 7A, top panel). B lymphocyte sequences from ER+ cancers featured a heterogeneous IgG subclass expansion with few IgA clones. In contrast, TNBCs showed clones with co-existing IgG1 and IgA1 subclasses, suggested intra-clone isotype-switching (Fig. 7A, bottom panel). Kolmogorov-Smirnov analyses revealed that IgG and IgA clonal family frequency distributions were significantly-different between carcinomas and normal breast (Fig. 7B). Accordingly, IgG and IgA were clonally-expanded within cancers (Suppl. Fig. 10A–B), and on average IgG+ B lymphocytes belonged to larger clonal families than IgA+ cells (Fig. 7C). These data point to an inherent bias towards the preferential clonal expansion of IgG isotypes within breast cancers. When we compared variable region gene usage, we found specific V(D)J genes whose combined usage was overrepresented in clonally-expanded IgG and IgA sequences in cancers. Specific combinatorial gene usages appeared to expand in TNBC but not ER+ lesions, and vice versa, suggestive of clonally-restricted, likely antigen-focused, immunoglobulin repertoires (Fig. 7D, Suppl. Fig. 10C). Furthermore, immunoglobulin light chain repertoire transcripts from single CD20+CD27+ B lymphocytes from two matched blood and tumor samples (Single cell cohort, Suppl. Figure 10D) showed similarities in kappa and lambda chain V genes (IGKV, IGLV) and J genes (IGKJ, IGLJ), pointing to potential common clonal origins. Furthermore, we observed a stronger positive selection pressure in the CDRs of IgG compared to their clonally related IgA in tumor samples, while such a relationship was absent in the FWR, and in BCRs from normal breast. (Fig. 7E). This hints at different aspects of B lymphocyte responses where IgA expression may act as an early response and IgG+ B cells may confer higher affinity antigen-driven responses.
Figure 7. B lymphocyte repertoire analyses of immunoglobulin isotype-switching and clonal expansion in breast cancers.
(A) 7,670 immunoglobulin heavy chain sequences were analyzed (KCL sequencing cohort). Top 10 clones determined by B lymphocyte repertoire long read data analyses. Clonotypes were estimated via clustering CDR3 sequences. [Top] Bars depict sizes of clones and their breakdown by isotypes. [Bottom] Isotypes present in each clone are indicated by dots. Vertical lines signify co-occurrence of isotypes in the same clone. (B) Clone size frequency distribution of IgM/IgA/IgG sequences in normal breast (1,771 sequences) and breast cancer (5,899 sequences). Kolmogorov-Smirnov analysis highlights significant differences in clone size frequency distributions. (C) Mean sequences per clone of IgM/IgA/IgG isotypes. IgG and to a lesser extent, IgA isotypes are clonally expanded, while on average IgG isotypes have significantly-larger clone sizes than IgA. (D) Comparisons of IgA and IgG variable usage of V-J, D-J and V-D genes extracted from normal breast, ER+ cancer and TNBC. For each gene usage combination, dot size is proportional to the frequency prior to clonal expansion. Dot colors correspond to fold change in the number of sequences following clonal expansion, indicating the preference of B lymphocytes with that specific VDJ combination to be clonally expanded. (E) Selection pressure in clonally related IgA and IgG. Clonally related sequences are represented as paired observations (grey lines), and selection pressure is considered separately for the Complementarity Determining Regions (CDR) and Framework Regions (FWR). Sequences are grouped into normal breast and breast cancer (BC) (containing two TNBC and two ER+ samples). Paired Wilcoxon tests were conducted, and p-values were corrected (Benjamini-Hochberg) for multiple comparisons.
Large clonal families, isotype-switching within clonally-expanded TIL-B, and a bias for IgG subclasses accumulating mutations on specific variable region gene combinations, together suggest a mature humoral immune response driven towards specific antigenic stimuli in breast cancer. Further understanding of these features may reveal therapeutic targets, prognostic biomarkers and patient subpopulations upon whom to focus adaptive immune response enhancing therapies.
Discussion
Clinical outcomes in cancer patients may be influenced by the initiation of effective anti-tumoral adaptive T lymphocyte responses, but these are likely to be significantly more effective when launched in combination with humoral immunity, including induction of isotype-switched B lymphocytes and secretion of antibodies. We undertook flow cytometric, IHC/IF, bulk GEx, scRNA-seq and long-read immunoglobulin sequencing analyses to investigate activated, memory and isotype-switched B lymphocytes in breast cancers, including the more-aggressive and more-immunogenic TNBC subtypes. Consistent with previous reports (39, 40), the TIL-B compartment in tumor stroma is typically organized into clusters of lymphocytes including isotype-switched memory, GC B cells, and plasma cells. Enhanced isotype-switched B lymphocytes, with an IgG-isotype bias may be part of the humoral clonal expansion mechanism, especially pronounced in TIL-Bhigh TNBC. The accompanied narrow mature immunoglobulin variable region repertoires and enhanced BCR signaling in TIL-B strongly signify antigen-driven responses. This dynamic humoral immune profile is especially associated with immunogenic TNBC and indicative of more favorable patient outcomes.
We found a depleted memory CD19+CD20+CD27+ B lymphocyte repertoire in patients’ peripheral blood regardless of stage or treatment history for their carcinoma. Moreover, this effect appears to be exacerbated in breast cancer patients who have undergone chemotherapy, possibly owing to the depletion of B lymphocytes during treatment, which may irreversibly diminish long-lived memory populations. Accordingly, patients receiving chemotherapy have lower total serum immunoglobulin titers, indicative of a depleted circulating antibody-secreting B lymphocyte population. In contrast, our flow cytometric analyses revealed an upregulated CD20+CD27+IgD- B cell compartment among TIL-B. In agreement, single-cell transcriptomic analyses point to a bias towards non-switched IgM transcripts in blood memory CD20+CD27+ B lymphocytes and higher levels of isotype-switched IgG and IgA CD20+CD27+ B lymphocytes in tumor samples. Consistent with an immunogenic signature in TNBCs, we found higher levels of isotype-switching shown in single CD20+CD27+ B lymphocyte transcripts in TNBC samples compared to non-TNBC tumors. These may be driven by a combination of antigen exposure and inflammation in the breast cancer microenvironment which promotes Ig class-switch recombination.
The TIL-B population is largely assembled in clusters (>30 TIL-B and >30 TIL-T aggregated) and positively associates with overall survival. Whilst evident across breast cancer types, transcriptomic analysis suggests that TIL-B infiltration is more pronounced in TNBC compared to non-TNBC. TNBC, especially the IM molecular subtype, featured elevated expression of B lymphocyte recruitment and lymphoid cell assembly (CXCL13, CXCR4, DC-LAMP) genes, compared to normal breast. Enhanced local expression of CXCL13 in arthritic synovial fluids can draw circulating B lymphocytes to inflammation sites (41) and B lymphocytes may traffic from the blood towards lymphoid tissues via CXCR4 stimulation (42). The evident expression of these signals within the TME may recruit B lymphocytes, including Bm, from the periphery to cancer lesions. Consistent with this, we report reduced circulating and enhanced intratumoral CD20+CD27+ B cell compartments in patients. Such chemoattractant signals may also promote local B lymphocyte assembly into B-T clusters, in line with our observations of close proximity and strong correlation between tumor-infiltrating B and T lymphocytes. Whilst the B-T clusters we describe are not entirely equivalent to TLSs identified in routine histology (43), within these clusters, B-T lymphocytic crosstalk can lead to B lymphocyte activation, immunoglobulin isotype-switching and local clonal expansion (39, 44).
Alongside immunohistochemical evidence of spatial B-T association, an active and functional B lymphocyte compartment is also indicated by BCR signaling and isotype-switching. Analyses of scRNA-seq data demonstrated that TIL-B are phenotypically distinct to those in blood, featuring upregulated BCR complex pathway molecules FOS and JUN, germinal center chemokine regulator RGS1, lymphocyte activation marker CD69 and TNFα signaling via NFκB. These implicate active BCR engagement by immune complexes. In concordance, Ig repertoire analyses revealed IgG-skewed clonal family expansion with clonally-restricted immunoglobulin variable regions. We detected significantly-larger IgG and to a lesser extent IgA, clones with narrow variable region repertoires in cancers compared to normal breast, indicative of antigen-focused clonal expansion. Together, these suggest a dynamic expanded IgG-biased humoral response focused towards a small repertoire of antigenic stimuli in the TME the greater understanding of which has the potential to inform therapeutic and biomarker strategies in patients.
Several studies reported that TIL-B carry positive prognostic value (5, 26), while others found no significant effect (45), or even poorer survival with CD138+ plasma cell infiltrates (46). However, the extent to which B lymphocyte activity may correlate with prognosis has not been addressed. In our TNBC cohort, we report positive prognostic associations of memory, but not of naïve or plasma, B lymphocyte infiltration in high-TIL tumors. This may point to positive contributions of memory B lymphocytes as part of the humoral response in immunogenic breast cancers and especially in TNBC. In concordance, our findings indicate that genes which positively regulate B lymphocyte functions, particularly those involved in activation, proliferation, differentiation and isotype-switching, and especially genes associated with isotype-switching to IgG, may carry positive prognostic value. Furthermore, evidence of a bi-directional functional crosstalk between B and T cells, reveals expression of gene pairs associated with lymphoid assembly, co-stimulation, cytokine-cytokine receptor interactions, cytotoxic T cell activation and T cell-dependent B cell activation. These interactions between B and T cells may have functional relevance in driving B cell stimulation and maturation. Positive associations with prognosis may stem from the observed crosstalk between B and T lymphocytes within TLS (47), where antigen presentation and antibody affinity maturation may occur. B lymphocyte-mediated T-helper lymphocyte activation may contribute to immunotherapy response in TNBC with high mutation burden (48) and local antigen presentation could amplify tumor antigen-specific immune responses (12). Our findings also provide support for the involvement of TIL-B in tumor immune surveillance through secretion of cytokines such as TNFα, which may promote differentiation of Th1 cells and polarize immune effector cells towards classically-activated phenotypes (49). Tumor-associated B lymphocytes may therefore receive, trigger and respond to significant T lymphocyte-mediated innate and antigenic signals including BCR-immune complex formation and co-stimulation. These may induce Ig class-switch recombination and affinity maturation, especially in TNBC.
We identified an IgG-dominated clonally-expanded B lymphocyte response in those breast cancers which were highly-infiltrated by immune cells, and positive associations between IgG class regulator signatures and patient outcome, especially in TNBC. The immunoglobulin isotypes produced in the TME may be critical for containing tumor growth. Antibodies can directly block cancer cell signaling, and if expressed of the IgG class, and specifically IgG1, they engender immune-mediated clearance of cancer cells via complement activation and engagement of Fc receptor-expressing monocytes, macrophages and NK cells (50). In cancer lesions we observed a higher mutation load in the CDRs of IgG compared to their clonally-related IgA, absent in the FWR, and in BCRs from normal breast. This stronger positive selection pressure on IgG+ B lymphocytes may represent different aspects of humoral immunity, likely arising from a common B cell precursor, with IgA+ and IgG+ B lymphocytes driven to generate highly-specific but divergent BCRs. Our work suggests significant implications regarding the effectiveness of anti-tumor B lymphocyte responses within highly-infiltrated cancers, where a higher IgG+:IgA+ ratio among the expansive B lymphocyte infiltrate could engender potent anti-tumor responses through the increased relative proportion of IgG isotypes, featuring increased capacity to trigger antibody-dependent cytotoxicity by NK cells and macrophages. Future therapeutic interventions may facilitate or take advantage of IgG+ memory and plasma cell infiltrates and thus influence the balance in favor of immune-activatory immunoglobulin isotypes in tumors.
Collectively, our findings indicate a highly-activated B lymphocyte compartment in breast cancers. Intratumoral B lymphocytes are spatially-associated with T lymphocytes within large stromal clusters, isotype-switch, expand into large clonal families featuring a bias towards IgG subclasses, and carry specific variable region gene combinations with narrow repertoires, all suggesting targeted humoral responses to specific antigenic stimuli. Clonally-restricted IgG expressing B cells may include in situ generated germinal center and activated memory B cells and terminally differentiated plasma cells. Together, these may contribute to humoral immune responses in breast cancer, likely more prominent in TNBC. These expansive and clonally-skewed immunoglobulin repertoires, and in particular those switched to IgG isotypes, may be associated with more-favorable patient survival. Although found across breast cancer types, these dynamic B lymphocyte traits are highly-prominent in TNBC. Despite the poor prognosis and aggressive nature of TNBC in many, this analysis implicates considerable biological and associated prognostic heterogeneity that extends to the TME. Expansive and active B lymphocyte cancer infiltrates, in a proportion of individuals, provide a degree of anti-tumor activity that may, in combination with other immune responses, confer a survival benefit and may be exploited with immunotherapies to aid in tumor clearance. Elucidating the microenvironmental conditions required to initiate, sustain and enhance these beneficial anti-tumor responses may be key to developing novel treatments.
Supplementary Material
Significance.
Tumor-infiltrating B lymphocytes assemble in clusters, undergoing B cell receptor-driven activation, proliferation, and isotype-switching. Clonally expanded, IgG isotype-biased humoral immunity associates with favorable prognosis primarily in triple-negative breast cancers.
Acknowledgments
The authors acknowledge support by Breast Cancer Now (147; KCL-BCN-Q3); the Cancer Research UK King’s Health Partners Centre at King’s College London (C604/A25135); Cancer Research UK (C30122/A11527; C30122/A15774); the Medical Research Council (MR/L023091/1); CR UK//NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Centre (C10355/A15587). The research was supported by the National Institute for Health Research Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London (IS-BRC-1215-20006). The authors are solely responsible for study design, data collection, analysis, decision to publish, and preparation of the manuscript. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors acknowledge the Breast Cancer Now Tissue Bank in collecting and making available samples used in the generation of this publication. We acknowledge the Biomedical Research Centre Immune Monitoring Core Facility at Guy’s and St Thomas’ NHS Foundation Trust and the Nikon Imaging Centre at Kings College London for assistance.
Abbreviations
- BCR
B cell Receptor
- Bm
Memory B lymphocyte
- CDR
complementary determining region
- CTL
cytotoxic T lymphocyte
- DMFS
distant metastasis-free survival
- ER
Estrogen receptor
- FWR
framework region
- GC
Germinal center
- GEx
Gene expression
- GSEA
gene set enrichment analysis
- HER2
Human epidermal growth factor receptor 2
- IF
Immunofluorescence
- Ig
Immunoglobulin
- IHC
Immunohistochemical
- IM
Immunomodulatory
- NANT
Non-adjacent, non-tumor
- NK
Natural killer
- PD-L1
Programmed death-ligand 1
- PR
Progesterone receptor
- scRNA-seq
Single-cell RNA-sequencing
- TIL
Tumor-infiltrating lymphocytes
- TLS
Tertiary lymphoid structure
- TNBC
Triple-negative breast cancers
- UMAP
Uniform Manifold Approximation and Projection
Footnotes
Disclosure of Potential Conflicts of Interest
S. N. Karagiannis and J. F. Spicer are founders and shareholders of Epsilogen Ltd. All other authors have declared that no conflict of interest exists.
Author Contributions
ANJT, SNK conceived and guided the project. RJH, AC, JCFN, RL, AMC, MF, DDR, JR, DL, EA, JQ, AS carried out the experiments and analyzed the data. RJH, AC, DL, SI collected clinical samples and the data. SC, FL, SP, CG, NH, MVH, DKDW, FF, JFS, KEL, ST, AG discussed the data, provided critical ideas and assistance. RJH, AC, JCFN, RL, SNK wrote the manuscript. All the authors have read and approved the manuscript.
Data availability
The R code used to analyze scRNA-seq data from GSE114725 (22) can be accessed from https://codeocean.com/capsule/8562693. The R code to analyze BCR Repertoire data collected from breast tissues can accessed at https://codeocean.com/capsule/8594411.
References
- 1.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012 Mar 1;72(5):1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladanyi A, Kiss J, Mohos A, Somlai B, Liszkay G, Gilde K, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011 Dec;60(12):1729–38. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garaud S, Zayakin P, Buisseret L, Rulle U, Silina K, de Wind A, et al. Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in situ by Tumor-Infiltrating B Cells in Breast Cancer. Front Immunol. 2018;9:2660. doi: 10.3389/fimmu.2018.02660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann BD, Pietenpol JA, Tan AR. Triple-negative breast cancer: molecular subtypes and new targets for therapy. Am Soc Clin Oncol Educ Book. 2015:e31–9. doi: 10.14694/EdBook_AM.2015.35.e31. [DOI] [PubMed] [Google Scholar]
- 5.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014 Aug;25(8):1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 6.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020 Jan;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 7.Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S, et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell. 2018 Sep 6;174(6):1373–87.:e19. doi: 10.1016/j.cell.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010 Apr;120(4):1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seow DYB, Yeong JPS, Lim JX, Chia N, Lim JCT, Ong CCH, et al. Tertiary lymphoid structures and associated plasma cells play an important role in the biology of triple-negative breast cancers. Breast Cancer Res Treat. 2020 Apr;180(2):369–77. doi: 10.1007/s10549-020-05548-y. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Al-Eryani G, Carswell S, Ferguson JM, Blackburn J, Barton K, et al. High-throughput targeted long-read single cell sequencing reveals the clonal and transcriptional landscape of lymphocytes. Nat Commun. 2019 Jul 16;10(1):3120. doi: 10.1038/s41467-019-11049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012 Mar 22;12(4):278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 12.Rossetti RAM, Lorenzi NPC, Yokochi K, Rosa M, Benevides L, Margarido PFR, et al. B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumor responses. PLoS One. 2018;13(7):e0199034. doi: 10.1371/journal.pone.0199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015 Apr 3;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Zhang J, Wang J, Fu J, Li T, Zheng X, et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat Genet. 2019 Mar;51(3):560–7. doi: 10.1038/s41588-018-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020 Jan;577(7791):549–55. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019 Aug 13;5 doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braso-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, et al. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med. 2016 Nov;22(11):1303–13. doi: 10.1038/nm.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szekely B, Bossuyt V, Li X, Wali VB, Patwardhan GA, Frederick C, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018 Nov 1;29(11):2232–9. doi: 10.1093/annonc/mdy399. [DOI] [PubMed] [Google Scholar]
- 19.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010 Oct;123(3):725–31. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 20.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015 May;12(5):453–7. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014 Nov;35(11):571–80. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell. 2018 Aug 23;174(5):1293–308.:e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018 Jun;36(5):411–20. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat Protoc. 2020 Apr;15(4):1484–506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 26.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015 Feb;26(2):259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu YC, Kipling D, Dunn-Walters D. Assessment of B Cell Repertoire in Humans. Methods Mol Biol. 2015;1343:199–218. doi: 10.1007/978-1-4939-2963-4_16. [DOI] [PubMed] [Google Scholar]
- 28.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008 Jul 1;36:W503-8. doi: 10.1093/nar/gkn316. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margreitter C, Lu HC, Townsend C, Stewart A, Dunn-Walters DK, Fraternali F. BRepertoire: a user-friendly web server for analysing antibody repertoire data. Nucleic Acids Res. 2018 Jul 2;46(W1):W264–W70. doi: 10.1093/nar/gky276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsend CL, Laffy JM, Wu YB, Silva O’Hare J, Martin V, Kipling D, et al. Significant Differences in Physicochemical Properties of Human Immunoglobulin Kappa and Lambda CDR3 Regions. Front Immunol. 2016;7:388. doi: 10.3389/fimmu.2016.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaari G, Uduman M, Kleinstein SH. Quantifying selection in high-throughput Immunoglobulin sequencing data sets. Nucleic Acids Res. 2012 Sep 1;40(17):e134. doi: 10.1093/nar/gks457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter EL, Mick R, Rech AJ, Beatty GL, Colligon TA, Rosenfeld MR, et al. Collapse of the CD27+ B-cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin Cancer Res. 2009 Jul 1;15(13):4277–87. doi: 10.1158/1078-0432.CCR-09-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walldius G, Malmstrom H, Jungner I, de Faire U, Lambe M, Van Hemelrijck M, et al. Cohort Profile: The AMORIS cohort. Int J Epidemiol. 2017 Aug 1;46(4):1103-i. doi: 10.1093/ije/dyw333. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011 Jul;121(7):2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin Q, Wang X, McBride J, Fewell C, Flemington E. B-cell receptor activation induces BIC/miR-155 expression through a conserved AP-1 element. J Biol Chem. 2008 Feb 1;283(5):2654–62. doi: 10.1074/jbc.M708218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moratz C, Kang VH, Druey KM, Shi CS, Scheschonka A, Murphy PM, et al. Regulator of G protein signaling 1 (RGS1) markedly impairs Gi alpha signaling responses of B lymphocytes. J Immunol. 2000 Feb 15;164(4):1829–38. doi: 10.4049/jimmunol.164.4.1829. [DOI] [PubMed] [Google Scholar]
- 37.D’Arena G, Musto P, Nunziata G, Cascavilla N, Savino L, Pistolese G. CD69 expression in B-cell chronic lymphocytic leukemia: a new prognostic marker? Haematologica. 2001 Sep;86(9):995–6. [PubMed] [Google Scholar]
- 38.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015 Dec 23;1(6):417–25. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maletzki C, Jahnke A, Ostwald C, Klar E, Prall F, Linnebacher M. Ex-vivo clonally expanded B lymphocytes infiltrating colorectal carcinoma are of mature immunophenotype and produce functional IgG. PLoS One. 2012;7(2):e32639. doi: 10.1371/journal.pone.0032639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silina K, Rulle U, Kalnina Z, Line A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother. 2014 Jul;63(7):643–62. doi: 10.1007/s00262-014-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armas-Gonzalez E, Dominguez-Luis MJ, Diaz-Martin A, Arce-Franco M, Castro-Hernandez J, Danelon G, et al. Role of CXCL13 and CCL20 in the recruitment of B cells to inflammatory foci in chronic arthritis. Arthritis Res Ther. 2018 Jun 7;20(1):114. doi: 10.1186/s13075-018-1611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, et al. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002 Jul 1;196(1):65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavoni E, Monteriu G, Santapaola D, Petronzelli F, Anastasi AM, Pelliccia A, et al. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 2007 Oct 18;7:70. doi: 10.1186/1472-6750-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buisseret L, Desmedt C, Garaud S, Fornili M, Wang X, Van den Eyden G, et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol. 2017 Sep;30(9):1204–12. doi: 10.1038/modpathol.2017.43. [DOI] [PubMed] [Google Scholar]
- 45.Song IH, Heo SH, Bang WS, Park HS, Park IA, Kim YA, et al. Predictive Value of Tertiary Lymphoid Structures Assessed by High Endothelial Venule Counts in the Neoadjuvant Setting of Triple-Negative Breast Cancer. Cancer Res Treat. 2017 Apr;49(2):399–407. doi: 10.4143/crt.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohammed ZM, Going JJ, Edwards J, Elsberger B, McMillan DC. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2013 Sep 17;109(6):1676–84. doi: 10.1038/bjc.2013.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017 Feb;66(2):342–51. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell. 2019 Nov 14;179(5):1191–206.:e21. doi: 10.1016/j.cell.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008 Jun;20(3):332–8. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung A, Opzoomer J, Ilieva KM, Gazinska P, Hoffmann RM, Mirza H, et al. Anti-Folate Receptor Alpha-Directed Antibody Therapies Restrict the Growth of Triple-negative Breast Cancer. Clin Cancer Res. 2018 Oct 15;24(20):5098–111. doi: 10.1158/1078-0432.CCR-18-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R code used to analyze scRNA-seq data from GSE114725 (22) can be accessed from https://codeocean.com/capsule/8562693. The R code to analyze BCR Repertoire data collected from breast tissues can accessed at https://codeocean.com/capsule/8594411.