Abstract
Background and purpose
Seeking consent rapidly in acute stroke trials is crucial as interventions are time-sensitive. We explored the association between consent pathways and time to enrolment in Tranexamic acid in IntraCerebral Haemorrhage-2 randomised controlled trial.
Methods
Consent was provided by patients or by a relative or an independent doctor in incapacitated patients, using a one-stage (full written consent) or two-stage (initial brief consent followed by full written consent post-randomisation) approach. The CT-to-randomisation time according to consent pathways were compared using Kruskal-Wallis test. Multivariable logistic regression was performed to identify variables associated with onset-to-randomisation time of ≤3 hours.
Results
Of 2325 patients, 817 (35%) gave self-consent using one-stage (557, 68%) or two-stage consent (260, 32%). For 1507 (65%), consent was provided by a relative (one-stage 996, 66%; two-stage 323, 21%) or a doctor (all two-stage, 188, 12%). One patient did not record pre-randomisation consent, with written consent obtained subsequently. The median [IQR] CT-to-randomisation time was 55 [38, 93] minutes for doctor consent, 55 [37, 95] minutes for two-stage patient, 69 [43, 110] minutes for two-stage relative, 75 [48, 124] minutes for one-stage patient, and 90 [56, 155] minutes for one-stage relative consents (p<0.001). Two-stage consent was associated with onset-to-randomisation time of ≤3 hours compared to one-stage consent (aOR 1.9; 95%CI 1.5-2.4). Doctor consent increased the odds (aOR 2.3, 1.5-3.5) while relative consent reduced the odds of randomisation ≤3 hours (aOR 0.10; 0.03-0.34) compared with patient consent. Only 2/771 patients (0.3%) in the two-stage pathways withdrew consent when full consent was sought later. Two-stage consent process did not result in higher withdrawal rates or lost to follow-up.
Conclusions
The use of initial brief consent was associated with shorter times to enrolment, while maintaining good participant retention. Seeking written consent from relatives was associated with significant delays.
Non-standard Abbreviations and Acronyms
- Non
standard Abbreviations and Acronyms
- aOR
adjusted odds ratio
- ICH
intracerebral hemorrhage
- NIHR
National Institute for Health Research
- NIHSS
National Institutes of Health Stroke Scale
- HTA
Health Technology Assessment
- TICH-2
Tranexamic acid in IntraCerebral Hemorrhage-2
Introduction
Obtaining consent in a timely manner is a major challenge for investigators in hyper-acute stroke trials 1, 2 . In these studies, the intervention has to be delivered in a very short therapeutic time window to be effective. On the other hand, obtaining consent is difficult when patients lack capacity and no relatives or persons with power of attorney are available to provide consent on their behalf 1, 2 . The COVID-19 pandemic has further complicated the consent process due to physical distancing precautions. Furthermore, patients or their representatives may also be overwhelmed by the acute scenario and unable to comprehend the information provided nor the rationale for taking part in a clinical trial 2 . Simplifying the consent process by providing concise but pertinent information to patients and their relatives may improve and shorten time to recruitment in acute stroke trials.
Tranexamic acid in IntraCerebral Haemorrhage-2 (TICH-2) endeavoured to minimise time to enrolment by using a two-stage pathway, where consent was sought initially from patients or their legal representatives using a brief information sheet followed by a full consent after randomisation. We aimed to explore the characteristics of patients enrolled using one- and two-stage consent, the acceptability and the effects on time to randomisation. We hypothesised that the use of brief information sheets would reduce time to randomisation.
Methods
Definitions
A personal legal representative is a person acting as legal representative to an incapacitated patient by virtue of their relationship with the patient. A personal legal representative is usually a relative, but could be a close friend who may be aware of the patients likely wishes if a relative was not available. A professional legal representative is a doctor or nominated healthcare professional unconnected to trial who acts as a patient’s legal representative to provide consent. A professional legal representative must not be involved in the trial management, be an investigator or part of the trial team, or be under the direction of the trial investigator 8 . Proxy consent is a consent given by a personal or professional legal representative. For brevity purpose in this article, personal legal representative is referred to as a relative and professional legal representative a doctor.
Consent pathways
The consent pathways consisted of one-stage patient and relative consent, two-stage patient and relative consent, as well as two-stage doctor consent.
One-stage patient consent
In the one-stage patient consent pathway, eligible patients were given the full version patient information sheet. This was a four-page information sheet (2474 words, Supplemental Methods III) which explained the condition, the purpose of the trial, the drug being studied, randomisation, blinding, follow-up assessments, possible benefit and harm, alternative treatments, withdrawal from the trial, data confidentiality and governance, dissemination of trial results and complaint procedures. Patients were then required to provide full written informed consent by writing their initials adjacent to seven relevant statements on the consent form and provide a full signature, name and date. Patients with capacity were required to sign the form themselves. If handwriting was not possible or legible due to arm weakness or use of the non-dominant hand, a third person acted as a witness by signing the form.
One-stage relative consent
For incapacitated patients, a relative was approached for proxy consent using the full version legal representative information sheet (2668 words) and consent form with similar details as the full patient version detailed above (Supplemental Methods IV).
Two-stage patient consent
When it was deemed not feasible to seek full written informed consent due to a short therapeutic time window, patients provided initial consent by signing a brief information sheet, followed by full written informed consent at the earliest subsequent opportunity after enrolment. The brief information sheet consisted of only one page of information (288 words, Supplemental Methods V) which explained the condition (ICH), treatment (tranexamic acid or placebo), blinding, and that there would be an additional CT scan after 24 hours. The patients were required to sign only once and did not need to initial the statements as in full consent.
Two-stage relative consent
A two-stage process using an initial brief legal representative information sheet (one-page, 290 words, Supplemental Methods VI) with similar details as the brief patient version, could be used in seeking consent in incapacitated patients, followed later by full consent.
Two-stage doctor consent
When the patient lacked capacity and there was no relative available, an independent doctor was approached to provide proxy consent. The independent doctor was part of the clinical team caring for the patient and should not have received prior trial-related training. Information relating to the trial was provided to the doctor, and if he/she agreed for the patient’s inclusion in the trial a brief information sheet was signed. Subsequently, a full written informed consent was sought from patients or their relatives as soon as practicable. Notably, doctors did not provide full consent.
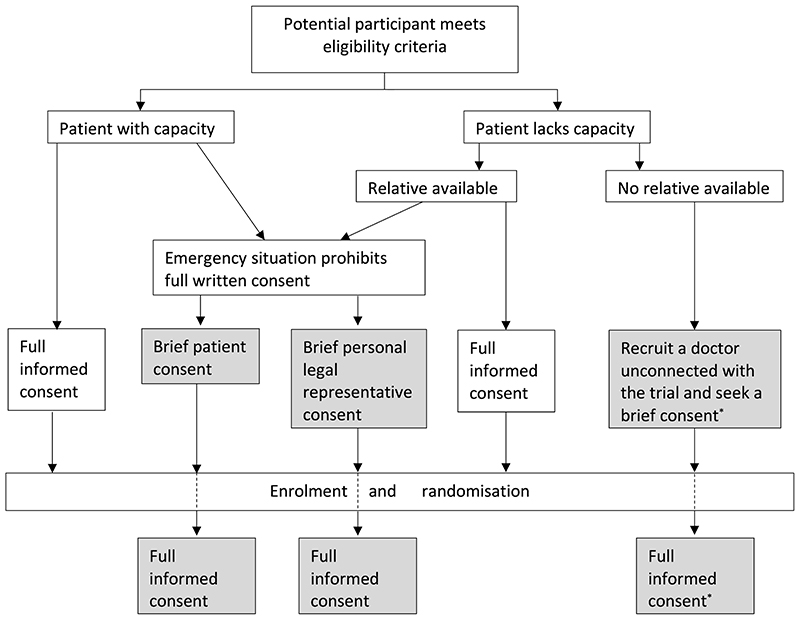
Figure 1 describes the consent pathways in TICH-2. Different countries had slightly different consent pathways, versions of documents in different languages. All countries permitted the one-stage patient consent, while there were variations in permission to use the other consent pathways (Table 1)
Figure 1. Consent process in the TICH-2 trial.
*Patient who lacked capacity with no relatives present was discussed with an independent doctor who acted as a professional legal representative. Full consent was sought later from the patient (if regained capacity) or a relative, if available. Two-stage pathways are marked grey.
Table 1. Types of initial consent in each country.
| No. of participants (n=2324) | One-stage patient (n=557, 24%) | Two-stage patient (n=260, 11.2%) | One-stage relative (n=996, 42.8%) | Two-stage relative (n=323, 13.9%) | Two-stage doctor (n=188, 8.1%) | |
|---|---|---|---|---|---|---|
| United Kingdom* | 1910 | 410 (21.5%) | 245 (12.8%) | 797 (41.7%) | 313 (16.5%) | 144 (7.5%) |
| Ireland | 17 | 3 (17.6%) | 4 (23.5%) | 6 (35.3%) | 4 (23.5%) | 0(0%) |
| Italy | 96 | 70 (72.9%) | 9 (9.4%) | 13 (13.5%) | 1 (1.0%) | 3 (3.1%) |
| Switzerland | 46 | 25 (54.3%) | 0(0%) | 12 (26.1%) | 0(0%) | 9 (19.6%) |
| Turkey | 9 | 1 (11.1%) | 0(0%) | 7 (77.8%) | 1 (11.1%) | NP |
| Malaysia | 46 | 18 (39.1%) | 0(0%) | 24 (52.2%) | 4 (8.7%) | NP |
| Spain | 1 | 0(0%) | 0(0%) | 1 (100%) | 0(0%) | NP |
| Georgia | 141 | 14 (9.9%) | NP | 125 (88.7%) | NP | 2 (1.4%) |
| Denmark | 39 | 6 (15.4%) | NP | 3 (7.7%) | NP | 30 (76.9%) |
| Hungary | 9 | 1 (11.1%) | NP | 8 (88.9%) | NP | NP |
| Sweden | 8 | 6 (75%) | 2 (25%) | NP | NP | NP |
| Poland | 3 | 3 (100%) | NP | NP | NP | NP |
NP=not permitted in the specified country.
Due to a protocol violation initial consent was not obtained in one patient from UK before randomisation. Full written consent from the patient was then subsequently obtained.
We retrospectively calculated the ‘fog index’, which assesses readability and estimates the level of education needed to understand the text on the first reading 9 . The fog indices of the brief patient and legal representative information sheets were 7.8 and 8.0, respectively, and 11.4 and 12.3 for full patient and legal representative information sheet. A fog index of 8.0 indicates that a 13- or 14-year-old would be able to understand at first reading, whilst text with a fog index of 12.0 can be understood by a 17- or 18-year-old.
Outcomes
We explored the time to enrolment, defined as CT-to-randomisation time, according to different consent pathways. We specifically explored if consent pathways were associated with onset-to-randomisation time of ≤3 hours. This time window was chosen as a meta-analysis of over 40,000 patients with traumatic haemorrhage and post-partum haemorrhage suggested that tranexamic acid is only beneficial when given within 3 hours 10 . Furthermore, most haematoma growth occurs within 3 hours 11 . We compared follow-up completion and withdrawal rates according to types of initial consent as a surrogate marker for acceptability.
Statistics
Baseline characteristics and outcomes were compared using Chi-squared tests for categorical variables, Mann-Whitney U and t-Student tests for continuous variables as appropriate. Time to enrolment for different consent pathways were compared using Chi-squared tests for categorical variables and Kruskal-Wallis tests for medians. Multivariable logistic regression, with adjustment of variables with p <0.1 on univariate analysis, was performed to identify factors associated with onset-to-randomisation times of ≤3 hours. In addition, we performed sensitivity analyses including only countries using two-stage consent. A p-value of <0.05 was considered statistically significant, and 95% confidence intervals (CI) are given. Analyses were performed using Statistical Package for the Social Sciences version 26 (IBM, Armonk, NY).
Results
2325 patients were recruited from 12 countries between March 2013 and September 2017. Only 817 patients (35.1%) gave self-consent by using one-stage (557, 24%) or two-stage consents (260, 11.2%). The majority of patients (1507, 64.8%) were enrolled by proxy consent provided by a relative (one-stage consent in 996, 42.8% and two-stage consent in 323, 13.9%) or by a doctor (188, 8.1%). For one patient from the UK, written consent was not given at time of randomisation, and this was reported as a protocol violation; full consent was subsequently given by the patient.
Of the 771 patients who provided a brief consent initially (260 patient, 323 relative and 188 doctor consents), follow-on full consent was given in all but 105 patients (13.6%) prior to hospital discharge. Reasons for not obtaining follow-on consent were: death (n=38), patient lacked capacity and no relative available (n=38), discharged (n=8), repatriated (n=13), no reason given (n=6) (Supplemental Table I). Only two patients, for whom a relative provided initial brief consent, declined to give further consent and withdrew from the trial prior to discharge. A further two patients who provided follow-on full consent withdrew before the day 90 follow-up. There was no significant difference in the number of patients lost to follow up and in withdrawals at day 90 between the different consent pathways (Supplemental Table II).
Patients who were recruited following proxy consent were older, more likely to be female and had higher National Institutes of Health Stroke Scale (NIHSS) scores (median 16 vs 7), lower Glasgow Coma Scale scores (median 14 vs 15), severe aphasia (42.1% vs 2.9%), intraventricular haemorrhage (36.2% vs 24.2%), and larger haematoma volumes (mean 30.2 mL vs 12.7 mL), but there was no significant difference in onset-to-CT time (Table 2). Patients who were recruited via proxy consent had more haematoma expansion (30.7% vs 21.9%), higher mortality (14.5% vs 2.7%) at day 7, and more death and disability (67.6% vs 30.5%) at day 90.
Table 2. Characteristics and outcome of patients according to consent type.
| Baseline variable | Self-consent | Proxy consent | P | One-stage | Two-stage | P |
|---|---|---|---|---|---|---|
| Patients randomised | 817 (35.1%) | 1507 (64.8%) | - | 1553 (66.8%) | 771 (33.2%) | - |
| Age (years) | 66.23 (13.39) | 70.34 (13.83) | <0.001 | 68.5 (13.7) | 69.8 (13.9) | 0.027 |
| Sex (male) | 523 (64.0%) | 778 (51.6%) | <0.001 | 902 (58.1%) | 399 (51.8%) | 0.004 |
| Pre-stroke modified Rankin Scale (/5) | 0.0 [0.0, 0.0] | 0.0 [0.0, 1.0] | <0.001 | 0.0 [0.0, 1.0] | 0.0 [0.0, 1.0] | 0.16 |
| Prior antiplatelet therapy | 180 (22.0%) | 431 (28.6%) | 0.001 | 403 (25.9%) | 208 (27.0%) | 0.58 |
| Glasgow coma scale (/15) | 15 [15, 15] | 14 [11, 15] | <0.001 | 15 [12, 15] | 14 [12, 15] | 0.27 |
| NIHSS score [/42] | 7.0 [4.0, 11.0] | 16.0 [10.0, 21.0] | <0.001 | 12.0 [6.0, 18.0] | 14.0 [8.0, 20.0] | <0.001 |
| No aphasia | 706 (86.4%) | 619 (41.1%) | <0.001 | 909 (58.5%) | 416 (54.0%) | 0.036 |
| Aphasia-mild to moderate | 87 (10.6%) | 254 (16.9%) | <0.001 | 227 (14.6%) | 114 (14.8%) | 0.91 |
| Aphasia-severe or mute | 24 (2.9%) | 634 (42.1%) | <0.001 | 417 (26.9%) | 241 (31.3%) | 0.026 |
| Right-sided limb weakness | 273 (33.4%) | 891 (59.1%) | <0.001 | 775 (49.9%) | 389 (50.5%) | 0.80 |
| Systolic blood pressure (mmHg) | 175.6 (30.2) | 174.4 (29.6) | 0.36 | 173.4 (29.2) | 177.6 (30.8) | 0.001 |
| Haematoma volume (mL) | 12.7 (15.7) | 30.2 (30.0) | <0.001 | 21.7 (25.6) | 28.6 (29.6) | <0.001 |
| Intraventricular haemorrhage | 167 (20.7%) | 540 (36.3%) | <0.001 | 445 (29.1%) | 262 (34.2%) | 0.012 |
| Onset-to-CT time (minutes) | 118 [85, 180] | 114 [80, 168] | 0.054 | 116 [83, 171] | 112 [80, 170] | 0.27 |
| UK sites | 655 (80.2%) | 1254 (83.2%) | 0.068 | 1207 (77.7%) | 702 (91.1%) | <0.001 |
| Haematoma expansion | 168 (21.9%) | 402 (30.7%) | <0.001 | 371 (26.6%) | 199 (29.2%) | 0.21 |
| Outcomes -Day 7 | ||||||
| Do not attempt resuscitation | 56 (6.5%) | 453 (30.1%) | <0.001 | 286 (18.5%) | 223 (29.1%) | <0.001 |
| Death | 20 (2.4%) | 204 (13.5%) | <0.001 | 122 (7.9%) | 102 (13.2%) | <0.001 |
| Day 90 | ||||||
| Death | 69 (8.5%) | 430 (28.7%) | <0.001 | 293 (19.0%) | 206 (27.0%) | <0.001 |
| Modified Rankin Scale 4-6 | 247 (30.5%) | 1012 (67.6%) | <0.001 | 792 (51.3%) | 467 (61.3%) | <0.001 |
Data are n (%), mean (standard deviation), or median [interquartile range]. Analyses are Chi-squared test for categorical variables, Mann-Whitney U test for median and t-Student test for mean comparison. NIHSS=National Institutes of Health Stroke Scale. One-stage consent includes one-stage patient and relative consent. Two-stage consents include two-stage patient, relative and doctor consent. Proxy consent includes one- and two-stage relative consent and doctor consent.
Similarly, patients who were recruited using two-stage consent were older, more likely to be female, had higher NIHSS (median 14 vs 12), more likely to have severe aphasia (31.3% vs 26.9%), intraventricular haemorrhage (34.2% vs 29.1%), larger haematoma volume (mean 28.6 mL vs 21.7 mL), and were more likely to be recruited in the UK (Table 2). Patients recruited using two-stage consent had higher mortality at day 7 (27% vs 19%), and more death and disability at day 90 (61.3% vs 51.3%).
Using two-stage doctor and the two-stage patient consent resulted in the shortest enrolment time (median CT-to-randomisation time 55 minutes for both) followed by the two-stage relative (69 [43, 110] minutes), the one-stage patient (75 [48, 124] minutes) and the one-stage relative consent (90 [56, 155]; Kruskal-Wallis test p<0.001; Table 3). The onset-to-treatment time was shortest with the two-stage doctor consent (200 [149, 259] minutes) compared to 267 [193, 364] minutes for the one-stage relative consent (the longest time to treatment). There was no significant difference in time from randomisation to treatment between the consent pathways.
Table 3. Enrolment time of patients according to consent pathways.
| All | Two-stage doctor | Two-stage patient | Two-stage relative | One-stage patient | One-stage relative | P | |
|---|---|---|---|---|---|---|---|
| Onset-to-CT time (minutes) | 115 [81, 171] | 99 [70, 150] | 117 [80, 173] | 119 [85, 175] | 119 [85, 182] | 116 [80, 168] | 0.003 |
| CT-to-randomisation (minutes) | 75 [47, 128] | 55 [38, 93] | 55 [37, 95] | 69 [43, 110] | 75 [48,124] | 90 [56, 155] | <0.001 |
| Randomisation to first dose of IMP (minutes) | 20 [12, 31] | 17 [10, 28] | 18 [11, 28] | 19 [11, 28] | 18 [11, 30] | 22 [13, 36] | 0.614 |
| Onset-to-randomisation (mean, SD, minutes) | 237 (102) | 195 (89) | 215 (98) | 225 (91) | 238 (108) | 253 (103) | <0.001 |
| Onset-to-randomisation (median, IQR, minutes) | 217 [155, 302] | 174 [122, 236] | 193 [142, 270] | 211 [156, 278] | 222 [155, 305] | 236 [167, 332] | <0.001 |
| ≤60 minutes | 2 (0.1%) | 2 (1.1%) | 0 | 0 | 0 | 0 | - |
| ≤120 minutes | 232 (10.0%) | 40 (21.3%) | 41 (15.8%) | 35 (10.8%) | 59 (10.6%) | 57 (5.7%) | <0.001 |
| ≤180 minute | 833 (35.8%) | 100 (53.2%) | 114 (43.8%) | 129 (39.9%) | 186 (33.4%) | 303 (30.4%) | <0.001 |
| Onset to first dose of IMP (minutes) | 244 [180, 330] | 200 [149, 259] | 215 [163, 299] | 229 [175, 304] | 247 [180, 330] | 267 [193, 364] | <0.001 |
Data are n (%), median [IQR, interquartile range] or mean (SD, standard deviation). Analyses are Chi-squared test for categorical variables and Kruskal-Wallis test for median or one-way ANOVA for mean comparison.
Apart from consent pathways, other factors associated with an onset-to-randomisation time of ≤3 hours include higher NIHSS, higher systolic blood pressure, shorter onset-to-CT time and recruitment from the UK (Supplemental Table III). Multivariable logistic regression showed that factors independently associated with onset-to-randomisation time of ≤ 3 hours include higher NIHSS, higher SBP, shorter onset-to-CT time, recruitment from the UK and use of two-stage patient and relative consent (aOR 1.89; 95%CI 1.49-2.39; p<0.001; Table 4, Model 1) and two-stage doctor consent (aOR 2.29, 1.52-3.47; p<0.001; Table 4, Model 2). Conversely, relative consent reduced the odds ratio of randomisation ≤3 hours of onset (aOR 0.10, 95%CI 0.03-0.34; p<0.001; Table 4, Model 3). Sensitivity analysis excluding countries which did not recruit participants using two-stage consent (Spain and Hungary, n=10) yielded similar results (Supplementary Table IV).
Table 4. Multivariable logistic regression of factors associated with time-to-randomisation of ≤3 hours.
| Variables | Model 1, aOR (95%CI) | P | Model 2, aOR (95%CI) | P | Model 3, aOR (95%CI) | P |
|---|---|---|---|---|---|---|
| Age (years) | 1.01 (0.99-1.01) | 0.26 | 1.01 (0.99-1.02) | 0.25 | 1.00 (0.98-1.01) | 0.55 |
| Sex (male) | 1.23 (0.98-1.54) | 0.074 | 1.22 (0.94-1.59) | 0.14 | 1.11 (0.88-1.40) | 0.39 |
| Systolic BP (mmHg) | 1.01 (1.00-1.01) | 0.001 | 1.01 (1.00-1.01) | <0.001 | 1.01 (1.00-1.01) | 0.001 |
| NIHSS (/42) | 1.02 (1.00-1.03) | 0.046 | 1.02 (1.00-1.04) | 0.050 | 1.04 (1.02-1.06) | <0.001 |
| Onset-to-CT time (minutes) | 0.97 (0.96-0.97) | <0.001 | 0.97 (0.96-0.97) | <0.001 | 0.97 (0.96-0.97) | <0.001 |
| Recruitment from UK | 2.68 (1.97-3.65) | <0.001 | 3.10 (2.24-4.31) | <0.001 | 3.29 (2.37-4.58) | <0.001 |
| Consent type* | ||||||
| 2-stage vs 1-stage (reference) | 1.89 (1.49-2.39) | <0.001 | - | - | - | |
| Doctor vs Self (reference) | - | - | 2.29 (1.52-3.47) | <0.001 | - | - |
| Relative vs Self (reference) | - | - | - | - | 0.10 (0.03-0.34) | <0.001 |
aOR=adjusted odds ratio; NIHSS=National Institutes of Health Stroke Scale.
3 different models used as there was overlap of consent types: one-stage consent includes one-stage patient and relative consent; two-stage consents include two-stage patient, relative and doctor consent; relative consent includes one- and two-stage relative consent; self-consent includes one- and two-stage patient consent.
Discussion and conclusion
In this post-hoc analysis, we found that two-stage consent using brief information sheets reduced the time to randomisation by approximately 20 minutes. CT-to-randomisation time was shortest with the two-stage doctor or patient consent, and longest for consent given by relatives. Apart from shortening onset-to-CT time with improvement of local stroke pathways, the use of the rapid consent pathway may be one of the most important approaches to improving time to enrolment for trials of treatments for acute ICH, as well as more generally in all acute stroke trials.
The brief information sheet, developed in consultation with our patient, carer and public representatives, was concise but contained pertinent information, which allowed patients to decide to proceed with the trial while more information was given later. The shorter text and better readability reduced reading time and enabled easier understanding. In addition, time used to sign the consent form was shortened with the brief consent, as only one signature was required instead of seven sets of initials and one signature in the full consent. Our approach in developing and utilising brief information sheet was similar to those that had been used in other large clinical trials such as Third International Stroke Trial (IST-3) 12 .
Most importantly, the use of brief consent appeared acceptable to patients and relatives, as very few (2 of 771, 0.3%) withdrew their consent when full consent was requested later. The use of brief consent did not result in higher withdrawal rates from trial or loss to follow up. The Cord Pilot study which employed a similar two-stage brief consent reported positive feedback with participants satisfied with the information received while having sufficient time to make their decision 13 . Brief consent was also acceptable to clinicians seeking consent 14 .
It is also noteworthy that a larger proportion of patients recruited via two-stage process were female compared to one-stage process. While the reasons for this is not known and needs to be further explored, the use of two-stage consent could potentially improve the enrolment of female participants, who are frequently under-represented in acute stroke trials 15 .
Similar to previous reports 16, 17 , patients recruited via proxy consent were older, had more severe strokes, and would have been excluded from the trial if legal representatives’ consent had not been permitted. Exclusion of incapacitated patients does not only unfairly deprive such patients from receiving potentially beneficial treatment, but it also introduces selection bias, as only patients with milder stroke can be recruited. In this respect, the option to include doctors as a professional legal representatives enabled patients who lacked capacity to participate in the trial when no relatives were available. Prior knowledge of the natural history of ICH and the risks and benefits of tranexamic acid combined with the use of an initial brief consent enabled professional legal representative to make enrolment decisions rapidly on behalf of incapacitated patients. However, it needs to be noted that this consent pathway was not permitted in all participating countries.
Proxy consent by a relative was associated with significant delays and markedly reduced odds of randomisation within 3 hours of onset compared to self-consent. This supports previous findings that relatives might not always be suitable surrogate decision makers 18, 19 . Furthermore, relatives may not be physically present at the bedside, especially in the COVID-19 era 20 , and additional time is spent looking for the relatives in a busy emergency department. The use of digital technology via telemedicine, videotelephony and electronic forms to seek consent from relatives may be an alternative to conventional face-to-face consultations 21–23 . However, the patient’s relatives may be stressed and distracted and needed more time to consider their decisions or may be unable to make decisions 2, 18, 24 . Results of a focus group consultation suggest that stroke survivors are worried about the additional stress the consent process imposes on an already distressed relative 25 .
Uncertainties about the patient’s wishes, the complexities of the patient’s condition and intervention, and the use medical terminology may lead to relatives requiring longer time for consent 26, 27 . Although it was reassuring that only two patients in this study who regained capacity disagreed with their relatives’ decision and withdrew consent, it remains unclear how much time should be allowed for the consent process in an emergency setting and how much information should be provided.
Current European Union regulations, approved in 2014 after the initiation of TICH-2 trial, allow recruitment of patients in emergency clinical trials without prior consent, if the patient lacks capacity and it is not possible to obtain informed consent from a legal representative within the therapeutic window 28 . This directive defined the concept that expert clinicians and ethics committee, based on rigorous review of study protocol, are in a better position to make decisions on whether the trial was designed to the patient’s best interest 29 . Furthermore, community consultation during the ethics review process ensures opinions and concerns of the study population are taken into consideration 22 . Whilst deferral or waiver of consent is permissible in emergency situations and may be a preferable option, many researchers do not utilise this approach. A deferral or waiver of consent was also recommended by the Hemorrhagic Stroke Academia Industry (HEADS) Roundtable and the European Stroke Organisation Trials Network Committee as one approach of reduce time to treatment in stroke trials 21, 30 . Such deferral or waiver of consent can be appropriate when the condition studied is acute, rapidly deteriorating, with poor outcome and the intervention studied has good safety profile 22 . Some trialists suggest that seeking consent is unethical if it delays the initiation of trial treatment leading to reduced treatment effects. This is especially so if the trial intervention constitutes the only possibility for an improved outcome 22,31,32 . Waiver of consent has been successfully applied in several emergency trials, such as FASTEST (FVIIa for Acute Hemorrhagic Stroke Administered at Earliest Time Trial; https://www.clinicaltrials.gov; unique identifier: NCT03496883) and ESETT (Established Status Epilepticus Treatment Trial) 33 .
One limitation of this study is that we have not surveyed patients, relatives or doctors regarding the consent process. Future trials could explore the implications of two-stage consent on participants’ experience, including if they felt that they were appropriately involved, the quality of interaction with researchers, what they felt was important at the time of decision-making, their perception, understanding of their contribution to research as study participants, recall of the consent process at a later date, and post-enrolment discussion. Although withdrawal after initial brief consent was rare, we have not captured data of potentially eligible patients who declined participation when first approached. As TICH-2 had a short enrolment period of only 8 hours, it is uncertain if a two-stage consent is appropriate and effective for trials with longer recruitment windows. Doctor consent was only used in a minority (8%) of patients despite a shorter time to enrolment. As it was not a requirement to appoint professional legal representative a priori, it may be difficult to establish in an emergency whether a doctor is truly “unconnected” to the trial. Brief and doctor consents were not permitted in some countries due to regulatory requirements, limiting the generalisability of their use.
In conclusion, offering a two-stage consent process and engaging doctors as professional legal representatives should be considered in emergency stroke trials with tight recruitment time windows. Both processes appeared acceptable with good follow-up completion and the possibility to recruit more patients with severe ICH.
Supplementary Material
Acknowledgements
We thank the patients, investigators and research staff at the participating sites and members of the Independent Data Monitoring Committee, for their involvement in and support for, the study.
Funding
TICH-2 was funded by the NIHR Health Technology Assessment (NIHR HTA project code 11_129_109).
Disclosures
Dr Bath is Stroke Association Professor of Stroke Medicine and a NIHR Senior Investigator. He has received consulting fees from DiaMedica, Moleac, Nestle, Phagenesis and Sanofi; he is an unpaid advisor to Platelet Solutions. Dr Robinson is a NIHR Senior Investigator. Dr Collins has accepted speaker’s honoraria from Bayer, Daichii Sankyo and Pfizer. Dr Werring has received honoraria from Bayer, Alnylam, Portola and NovNordisk. Dr Al-Shahi Salman has received grants from NIHR during the conduct of the study and grants from British Heart Foundation outside the submitted work. Dr Appleton, Dr Dineen, Dr Sprigg, Dr Roffe and Dr England report grants from NIHR HTA during the conduct of the study. Dr Lyrer has received advisory board compensation from Bayer, Switzerland, non-financial support, advisory board compensation and travel grant from Boehringer Ingelheim, advisory board compensation and travel grant from Pfizer, research grants from University Hospital Basel, Neurology Clinic, research grants from Swiss National Foundation SNF, research grants from ACTICOR France, advisory board compensation from Biogen, Switzerland, investigator fees from Alexion, and grants from Bayer, Germany outside the submitted work. Dr Karlinski has received personal fees from Boehringer Ingelheim, Pfizer, Bayer and Medtronic outside the submitted work. Dr Christensen has received national lead fees to institutional research account from Bayer and Portola, personal fees from BMS and Bayer outside the submitted work. Dr Duley was the chief investigator of a perinatal trial which developed a similar brief consent process. This was adapted for use in acute stroke, as reported in the current study.
Registration-URL:https://www.isrctn.com; Unique identifier:ISRCTN93732214
Data availability statement
The trial data can be shared, upon reasonable request to the corresponding author and the trial steering committee.
TICH-2 was an international prospective multicentre double-blind randomised placebo-controlled trial testing the efficacy and safety of intravenous tranexamic acid in patients with acute spontaneous intracerebral haemorrhage (ICH) within eight hours of symptom onset. Details of the trial were previously published 3–5 . The consent-related procedures were developed in partnership with stroke survivors, some of whom were members of the trial steering committee. The information sheets and consent forms were designed according the principles outlined in the Medicines for Human Use (Clinical Trials Regulations) 2004 and European Clinical Trials Directive (EC2001/20) and based on templates provided by the United Kingdom (UK)’s Health Research Authority 6 . The consent procedures were approved by each participating country or centre’s ethics review committee.
This analysis is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (Supplemental Method I-checklist and Supplemental Method II-flow diagram) 7 .
References
- 1.Rose DZ, Kasner SE. Informed consent: the rate-limiting step in acute stroke trials. Front Neurol. 2011;2:65. doi: 10.3389/fneur.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kompanje EJO, van Dijck J, Chalos V, van den Berg SA, Janssen PM, Nederkoorn PJ, van der Jagt M, Citerio G, Stocchetti N, Dippel DWJ, et al. Informed consent procedures for emergency interventional research in patients with traumatic brain injury and ischaemic stroke. Lancet Neurol. 2020;19:1033–1042. doi: 10.1016/S1474-4422(20)30276-3. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty K, Bath PM, Dineen R, Law Z, Scutt P, Pocock S, Sprigg N. Statistical analysis plan for the ‘Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage’ (TICH-2) trial. Trials. 2017;18:607. doi: 10.1186/s13063-017-2341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprigg N, Robson K, Bath P, Dineen R, Roberts I, Robinson T, Roffe C, Werring D, Al-Shahi Salman R, Pocock S, et al. Intravenous tranexamic acid for hyperacute primary intracerebral hemorrhage: Protocol for a randomized, placebo-controlled trial. Int J Stroke. 2016;11:683–694. doi: 10.1177/1747493016641960. [DOI] [PubMed] [Google Scholar]
- 5.Sprigg N, Flaherty K, Appleton JP, Salman RA-S, Bereczki D, Beridze M, Ciccone A, Collins R, Dineen RA, Duley L, et al. Tranexamic acid to improve functional status in adults with spontaneous intracerebral haemorrhage: the TICH-2 RCT. Health Technol Assess. 2019;23:1–48. doi: 10.3310/hta23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Health Research Authority. Consent and participant information guidance. [Date accessed 1 July 2020]. http://www.hra-decisiontools.org.uk/consent/
- 7.PLOS Medicine Editors. Observational studies: getting clear about transparency. PLoS Med. 2014;11:e1001711. doi: 10.1371/journal.pmed.1001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coats TJ, Shakur H. Consent in emergency research: new regulations. Emerg Med J. 2005;22:683–685. doi: 10.1136/emj.2005.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunning Robert. The Technique of Clear Writing. McGraw-Hill; 1952. pp. 36–37. [Google Scholar]
- 10.Gayet-Ageron A, Prieto-Merino D, Ker K, Shakur H, Ageron FX, Roberts I. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018;391:125–132. doi: 10.1016/S0140-6736(17)32455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Koops L, Lindley RI. Thrombolysis for acute ischaemic stroke: consumer involvement in design of new randomised controlled trial. BMJ. 2002;325:415. doi: 10.1136/bmj.325.7361.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawyer A, Chhoa C, Ayers S, Pushpa-Rajah A, Duley L. Women’s views and experiences of two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials. 2017;18:422. doi: 10.1186/s13063-017-2149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhoa CY, Sawyer A, Ayers S, Pushpa-Rajah A, Duley L. Clinicians’ views and experiences of offering two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials. 2017;18:196. doi: 10.1186/s13063-017-1940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Secondary service connection for diagnosable illnesses associated with traumatic brain injury. Final rule. Federal register. 2013;78:76196–76209. [PubMed] [Google Scholar]
- 16.Thomalla G, Boutitie F, Fiebach JB, Simonsen CZ, Nighoghossian N, Pedraza S, Lemmens R, Roy P, Muir KW, Heesen C, et al. Effect of informed consent on patient characteristics in a stroke thrombolysis trial. Neurology. 2017;89:1400–1407. doi: 10.1212/WNL.0000000000004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demarquay G, Derex L, Nighoghossian N, Adeleine P, Philippeau F, Honnorat J, Trouillas P. Ethical issues of informed consent in acute stroke. Analysis of the modalities of consent in 56 patients enrolled in urgent therapeutic trials. Cerebrovasc Dis. 2005;19:65–68. doi: 10.1159/000083250. [DOI] [PubMed] [Google Scholar]
- 18.Barrett KA, Ferguson ND, Athaide V, Cook DJ, Friedrich JO, McDonald E, Pinto R, Smith OM, Stevenson J, Scales DC. Surrogate decision makers’ attitudes towards research decision making for critically ill patients. Intensive Care Med. 2012;38:1616–1623. doi: 10.1007/s00134-012-2625-x. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd V, Hood K, Sheehan M, Griffith R, Wood F. ‘It’s a tough decision’: a qualitative study of proxy decision-making for research involving adults who lack capacity to consent in UK. Age Ageing. 2019;48:903–909. doi: 10.1093/ageing/afz115. [DOI] [PubMed] [Google Scholar]
- 20.North CM, Dougan ML, Sacks CA. Improving Clinical Trial Enrollment - In the Covid-19 Era and Beyond. N Engl J Med. 2020;383:1406–1408. doi: 10.1056/NEJMp2019989. [DOI] [PubMed] [Google Scholar]
- 21.Hemorrhagic Stroke Academia Industry (HEADS) Roundtable Participants. Recommendations for Clinical Trials in ICH: The Second Hemorrhagic Stroke Academia Industry Roundtable. Stroke. 2020;51:1333–1338. doi: 10.1161/STROKEAHA.119.027882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal M, Ospel JM, Ganesh A, Marko M, Fisher M. Rethinking Consent for Stroke Trials in Time-Sensitive Situations: Insights From the COVID-19 Pandemic. Stroke. 2021;52:1527–1531. doi: 10.1161/STROKEAHA.120.031976. [DOI] [PubMed] [Google Scholar]
- 23.Haussen DC, Doppelheuer S, Schindler K, Grossberg JA, Bouslama M, Schultz M, Perez H, Hall A, Frankel M, Nogueira RG. Utilization of a Smartphone Platform for Electronic Informed Consent in Acute Stroke Trials. Stroke. 2017;48:3156–3160. doi: 10.1161/STROKEAHA.117.018380. [DOI] [PubMed] [Google Scholar]
- 24.Ciccone A. Consent to thrombolysis in acute ischaemic stroke: from trial to practice. Lancet Neurol. 2003;2:375–378. doi: 10.1016/s1474-4422(03)00412-5. [DOI] [PubMed] [Google Scholar]
- 25.Ali K, Roffe C, Crome P. What patients want: consumer involvement in the design of a randomized controlled trial of routine oxygen supplementation after acute stroke. Stroke. 2006;37:865–871. doi: 10.1161/01.STR.0000204053.36966.80. [DOI] [PubMed] [Google Scholar]
- 26.Burns KE, Prats CJ, Maione M, Lanceta M, Zubrinich C, Jeffs L, Smith OM. The Experience of Surrogate Decision Makers on Being Approached for Consent for Patient Participation in Research. A Multicenter Study. Ann Am Thorac Soc. 2017;14:238–245. doi: 10.1513/AnnalsATS.201606-425OC. [DOI] [PubMed] [Google Scholar]
- 27.Long B, Clark L, Cook P. Surrogate decision making for patients with severe traumatic brain injury. J Trauma Nurs. 2011;18:204–212. doi: 10.1097/JTN.0b013e31823a453a. [DOI] [PubMed] [Google Scholar]
- 28.Parliament E, Union tCotE. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. Off J Eur Union. 2014;57:L58-51-5820176 [Google Scholar]
- 29.Savonitto S, Coppola T, Braglia P, Ciccone A. [Informed consent for clinical investigation in the critically ill patient. An introduction to the regulation 536/2014/EC on clinical investigation of medicinal products for human use, repealing Directive 2001/20/EC] G Ital Cardiol (Rome) 2016;17:326–334. doi: 10.1714/2252.24252. [DOI] [PubMed] [Google Scholar]
- 30.Berge E, Al-Shahi Salman R, van der Worp HB, Stapf C, Sandercock P, Sprigg N, Macleod MR, Kelly PJ, Nederkoorn PJ, Ford GA. Increasing value and reducing waste in stroke research. Lancet Neurol. 2017;16:399–408. doi: 10.1016/S1474-4422(17)30078-9. [DOI] [PubMed] [Google Scholar]
- 31.Roberts I, Prieto-Merino D, Shakur H, Chalmers I, Nicholl J. Effect of consent rituals on mortality in emergency care research. Lancet. 2011;377:1071–1072. doi: 10.1016/S0140-6736(11)60317-6. [DOI] [PubMed] [Google Scholar]
- 32.Research in emergency situations: with or without relatives consent. Emerg Med J. 2004;21:703. doi: 10.1136/emj.2002.004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, Rogers A, Barsan W, Cloyd J, Lowenstein D, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395:1217–1224. doi: 10.1016/S0140-6736(20)30611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The trial data can be shared, upon reasonable request to the corresponding author and the trial steering committee.
TICH-2 was an international prospective multicentre double-blind randomised placebo-controlled trial testing the efficacy and safety of intravenous tranexamic acid in patients with acute spontaneous intracerebral haemorrhage (ICH) within eight hours of symptom onset. Details of the trial were previously published 3–5 . The consent-related procedures were developed in partnership with stroke survivors, some of whom were members of the trial steering committee. The information sheets and consent forms were designed according the principles outlined in the Medicines for Human Use (Clinical Trials Regulations) 2004 and European Clinical Trials Directive (EC2001/20) and based on templates provided by the United Kingdom (UK)’s Health Research Authority 6 . The consent procedures were approved by each participating country or centre’s ethics review committee.
This analysis is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (Supplemental Method I-checklist and Supplemental Method II-flow diagram) 7 .