Abstract
Multivalent glycosylated materials (polymers, surfaces, and particles) often show high affinity toward carbohydrate binding proteins (e.g., lectins) due to the nonlinear enhancement from the cluster glycoside effect. This affinity gain has potential in applications from diagnostics, biosensors, and targeted delivery to anti-infectives and in an understanding of basic glycobiology. This perspective highlights the question of selectivity, which is less often addressed due to the reductionist nature of glycomaterials and the promiscuity of many lectins. The use of macromolecular features, including architecture, heterogeneous ligand display, and the installation of non-natural glycans, to address this challenge is discussed, and examples of selectivity gains are given.
Keywords: glycopolymers, multivalency, nanoparticles, carbohydrates, chemical glycobiology
Carbohydrates are diverse (macro)molecules that coat cell surfaces and lipids (and even RNA1) and are present on >50% of human proteins, fulfilling functions including recognition, signal transduction, and fertilization and as sites for pathogen invasion.2,3 The huge structural diversity of glycans arises from the assembly of monosaccharides via different glycoside linkages, at different ring positions and with specific stereochemistry, resulting in the inherent complexity of the glycome.4 Proteins that interact or “read”5 carbohydrates include enzymes, anticarbohydrate antibodies, adhesins, and lectins.6 Hence, the development of probes, binders and inhibitors of carbohydrate-binding proteins has broad bio-technological and biomedical value. For material scientists, the motivation to incorporate glycans is to mimic their multivalent presentation found on cell surfaces. The actual binding affinity of a carbohydrate to its target lectin is typically weak (Kd = 10−3− 10−6 M) in comparison to antibody–antigen interactions, which can be <10−9 M. The presentation of multiple copies of the target carbohydrate on the cell surface gives rise to an increase in affinity greater than that of the linear sum of the individual sugars; this is known as the “cluster glycoside effect”.7–9 In short, polymers and particles bearing glycans can show affinity higher than that of a single “small molecule” of equal concentration, a concept that has been established now for around 40 years. In 1983, Lee et al.7 synthesized a series of oligosaccharides, based on N-acetyllactosamine-type glycans, and demonstrated their ability to inhibit the mammalian hepatic lectin binding to rabbit hepatocytes. This revealed inhibitory potency in the order tetraantennary > trianntenary ≫ biantennary ≫ monoan-tenneary, increasing from 1 mM to 1 nM, while only increasing the glycan concentration 3-fold. In 1996 Whitesides and co-workers showed that sialic acid-functional polyacrylamides could prevent influenza from agglutinating (i.e., stopping binding) erythrocytes, demonstrating the anti-infective potential of polymeric glycan mimetics.10 Kiessling showed nonlinear increases in affinity of well-defined ring opening metathesis polymerization (ROMP) derived mannosylated polymers toward Con A as a function of chain length.11 Of course, there are examples of medicinal chemistry approaches for small-molecule affinity, selectivity, and PK/PD profiles such as those developed for FimH inhibition.12,13 However, these are beyond the scope of this Perspective, which will focus on multivalent systems.
These (selected) early examples show the clear benefit of multivalent assemblies, which provide advantages over mono-valent assemblies.14 Multivalency enables spanning of multiple binding sites (on the same or different lectins), chelation, subsite binding, clustering, and statistical rebinding among others, and the mechanisms of these have been reviewed extensively.3,15,16 A vast range of multivalent architectures are known, which will not be reviewed in this Perspective but include dendrimers,17–25 peptides,26,27 polymers,28–33 particles,34–37 viruses,38 and presentations designed to specifically interact with the binding sites.39,40 Multivalent inhibitors for antiadhesion have also been covered previously,16,24,41–48 and this Perspective does not aim to re-review these.
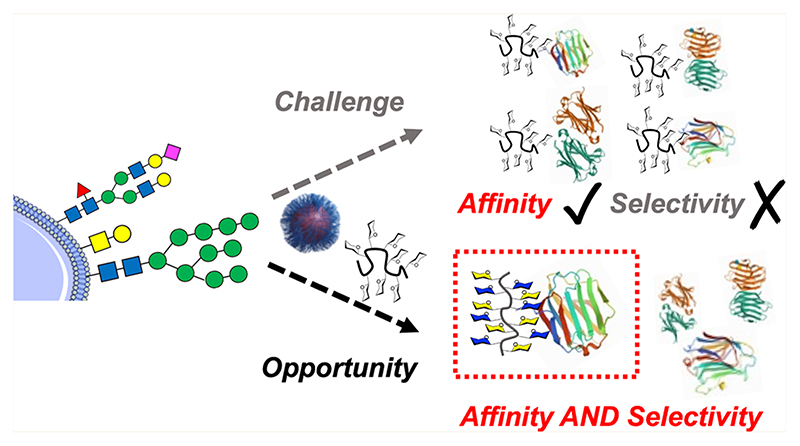
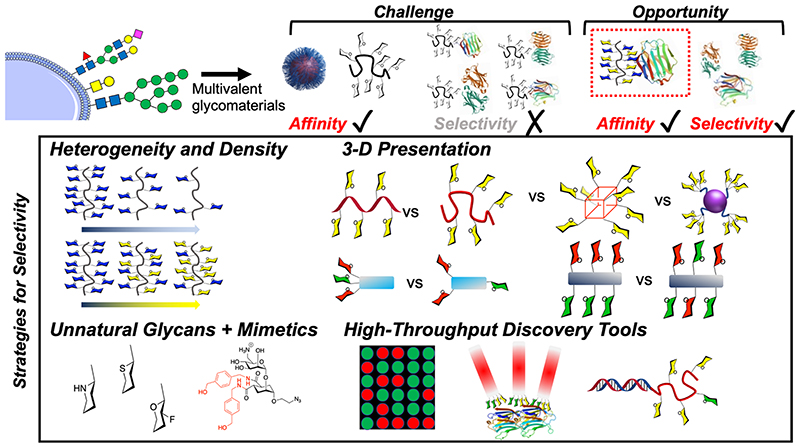
This Perspective aims to highlight potential macromolecular solutions to engineer selectivity into glycomaterials. Multivalent presentation almost always leads to an increase in affinity, but there is an exciting opportunity to develop macromolecular tools to increase selectivity. Figure 1 summarizes this challenge, and the approaches which are covered in this perspective will include glycan heterogeneity, control of 3D presentation, and the use of unnatural glycans. We also cover some emerging discovery approaches for the identification of selective binders.
Figure 1. Scope of the Perspective on moving from high affinity to high affinity and high selectivity glycomaterials. The lower panel schematic shows strategies that are discussed here.
Diversity of Interactions and Promiscuity
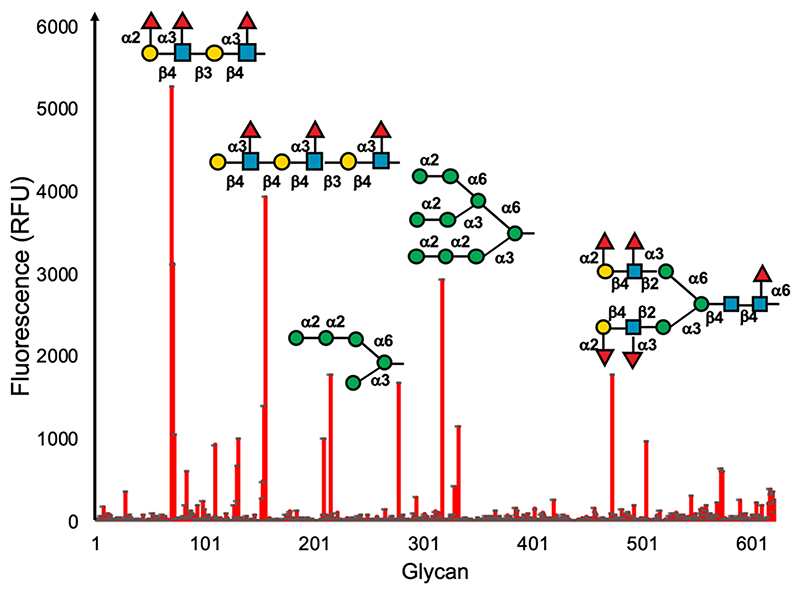
In any applications spanning delivery, sensing, or inhibition, ensuring selectivity is essential: not against all lectins (or e.g. antibodies), but against those likely to be in competition in the same environment. For example, respiratory viruses, including SARS-CoV-2, have affinities toward sialic acids,49 as does influenza,50 and hence any glycan-based sensor would require a strategy for selectivity. The selective targeting of DC-SIGN over other C-type lectins present on dendritic cells presents a challenge in glycomaterial design for therapy or immune modulation.51 Blocking DC-SIGN can reduce HIV viral infection, but another lectin, Langerin, is implicated in clearing viral particles.52 Both DC-SIGN and Langerin bind mannosy-lated glycans (with subtle differences in their profile53), and hence achieving selectivity between these two C-type lectins would be essential if a glycomaterial were to be used. Furthermore, mannosylated polymers can also activate the complement pathway, which may limit their translation.54 To highlight the diversity of glycan interactions, Figure 2 shows data from the Consortium for Functional Glycomics (CFG) glycan array55 versus DC-SIGN. Any one lectin can bind multiple different glycans, in this case including not just high mannose but also fucoyslated glycans. The branching pattern of the glycans also affects the observed extent of binding.
Figure 2.
Extract of glycan-array data for DC-SIGN from the Consortium for Functional Glycomics (primscreen_5273, Human DC-SIGN-AF488 200 μg mL−1). Increased fluorescence shows more protein binding to the immobilized glycans, highlighting how the same lectin can bind structurally diverse glycans. Selected high-binding glycans are indicated.
The galectin family of lectins plays a crucial role in human physiology, but all have affinity toward β-galactosides56 with subtle differences in their glycan binding profiles,57 which is also controlled by the architecture (chimeric, tandem repeat, or prototype).58 Therefore, if the aim is to selectively identify a galectin as a biomarker, for example, the cross-reactivity question is crucial. Finally, cross reactivity is context dependent—cross reactivity from blood biopsies will be distinct from a wastewater containing the galactose-binding cholera toxin.59 Unlike small molecules, materials chemistry solutions offer a huge opportunity to control the 3D presentation, density, heterogeneity, and nature of the glycans, and this Perspective introduces some approaches being taken to address this problem, hoping to show that this is an area that is ripe for innovation.
Glycan Density
Perhaps the simplest tool to tune the glycan/lectin interface is tuning the side chain density of glycans, which can be achieved by copolymerization or postpolymerization modification.60 While outside of the scope of this Perspective, the glycan array literature already makes extensive use of variable density surface display, where the differences in density can promote/inhibit inter-/intralectin binding interactions and have been reviewed.61 It is also crucial to note that density changes achieved by addition of another glycan (which introduces potential secondary binders) is covered later in this Perspective, as the effects from these similar concepts can be very distinct.
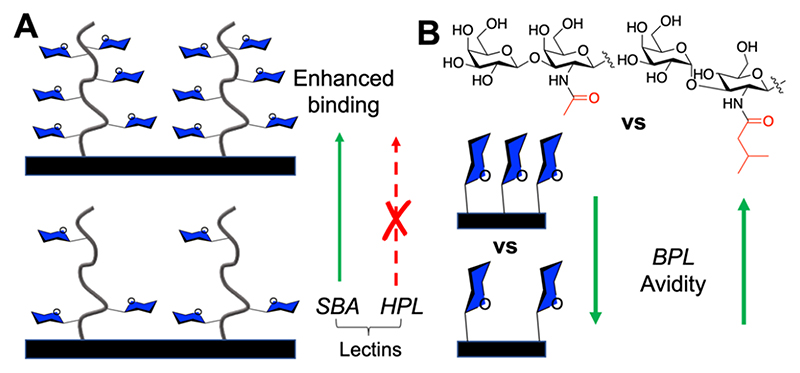
Godula et al. employed a microarray platform with immobilized synthetic glycopolymers to investigate how glycan valency and spatial separation affect the binding mode of a panel of four GalNAc specific lectins (Figure 3A).62 SBA (soybean agglutinin) showed the highest binding to the most dense arrays, whereas HPA (Helix pomatia agglutinin) showed the highest binding to the lowest density, even though they are both GalNAc binding lectins, with the key difference being the lectins’ ability to form interchain cross-links. Hence, simple density tuning, with careful consideration of the lectin architecture, introduces selectivity. This raises the question of how binding affinities/selectivities for isolated glycans scales with multivalent systems and that they are not always linear relationships.63
Figure 3.
Effect of glycan density on lectin binding. (A) Glycopolymer surfaces show differential responses to SBA and HPL as a function of GalNAc side density.62 (B) Monolayers containing two distinct glycans show differential responses to BPL binding.64
Whitesides and co-workers prepared self-assembled monolayers bearing galactose ligands (Figure 3B) and evaluated binding toward BPL (Bauhinia purpurea lectin).64 An unnatural glycan (with an N-valeryl group and α replacing a β linkage) showed increased avidity with higher density but the opposite effect for the natural glycan, showing that selectivity was possible. It should be noted that characterization of glycopolymer and other multivalent glycostructures is challenging, as spacing/clustering of glycans on these scaffolds is mostly unknown and will almost certainly influence both the affinity and selectivity. Kwon et al. synthesized 6′-sialyllactose presenting PAMAM-based dendrimers with well-defined ligand densities and spacing. The G4 dendrimer outperformed larger/ smaller dendrimers in an influenza inhibitory assay, with an estimated spacing of 3 nm between ligands estimated to be optimum.65 Smaller di- and trivalent ligands were also shown to be potent hemagglutinin inhibitors, enhancing >400-fold in comparison to monovalent ligands.66 The ligand density has been reported to be crucial for cholera toxin binding, with many studies reporting that low density (fewer galactose units) leads to maximum inhibition.67–69 On consideration of the inherent simplicity in changing density, this is a valuable tool for identifying selectivity (or preferentiality), whereby the lectin architecture, in addition to binding-site preference, can be exploited.
3D Presentation
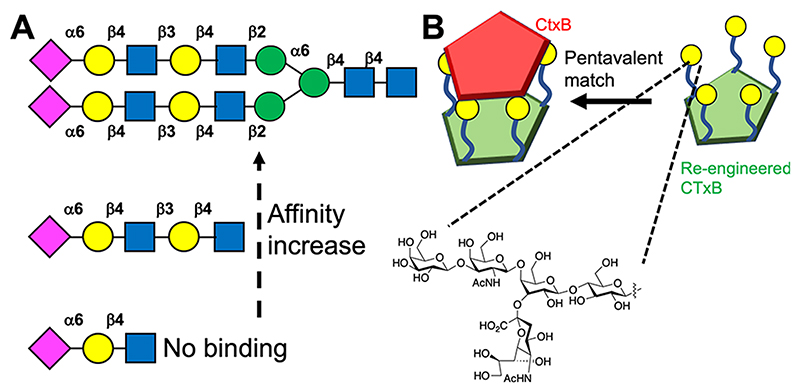
The reductionist nature of the glycan presentation in many materials does not (yet) recapitulate the precise 3D presentation and valency control which is found in oligosaccharides. Figure 4A shows selected data from a glycan microarray against human influenza hemagglutinins.70 Biantennary glycans led to significant binding, in comparison to monoantennary glycans, and all binding (or rather binding signal, in the particular assay) was removed when just a trisaccharide (with the same final three monosaccharide units) was displayed. Asymmetric linkages also prevented binding. While the interactions are complex, involving multiple contacts, this example shows how glycan selectivity in Nature is driven by the presentation as much as the chemical nature. Modeling has shown how the chemical nature of how glycans are presented on arrays can lead to false negatives.71 A glycan array strategy was again used to discover bivalent ligands capable of spanning binding sites in LecA (from Pseudomonas aeruginosa), with only ligands with a precise match leading to enhanced affinity and selectivity in comparison to a Shiga toxin.72 A synthetic biology solution to controlling the presentation was shown by Branson et al., who precisely displayed just five copies of the GM1-oligosaccharide onto cholera toxin proximal to its binding sites (Figure 4B), ensuring that the glycans were spatially located for optimal engagement with cholera toxin, leading to nM affinity,73 which has also been modeled showing that the size of the multivalent core must match the receptor unit display and valency.74
Figure 4.
3D presentation of glycans affects binding. (A) Neu5Ac terminated glycans versus hemagglutinins, showing branching and sequence-length dependent binding.70 (B) A pentameric glycosylated cholera toxin B subunit (CTxB) has a 3D match to CTxB for nM inhibition.73
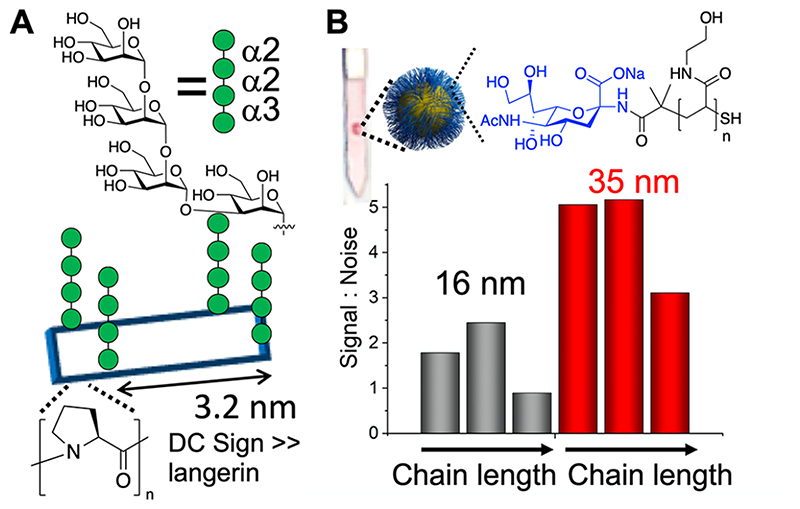
To tune the presentation of glycans, Kiessling and co-workers exploited ROMP to install cis or trans backbones, on otherwise identical polymers. The cis backbones led to an extended conformation, leading to stronger binding in comparison to trans backbones, mimicking native mucin presentation.69 Changing the side chain linker from an amide to an ester in glycopolymers, which in turn affects the flexibility of the glycan, was reported to dramatically alter the overall affinity of mannosylated glycopolymers.75 A proline macrocycle was used to control the presentation of mannose to discriminate between Langerin and DC-SIGN, increasing selectivity many thousand-fold. Both lectins bind to the mannose, but selectivity was achieved due to the spacing differences in the Langerin homotrimer, in comparison to the DC-SIGN homotetramer (Figure 5A).76 Bachem et al. used DNA-PNA scaffolds to precisely space and cluster glycans to selectively engage Langerin with 1150-fold increased affinity in comparison to the free ligand.77 In addition to precisely targeting 3D presentation to gain affinity, the presentation of glycans can affect a sensing outcome (which is not necessarily proportional to affinity). For example, SARS-CoV-2 spike protein binding in a flow-through assay was dependent on the length of polymeric linkers, connecting Neu5NAc to gold nanoparticles,49 and polymer chain length and chemistry tuned the outputs in gold nanoparticle aggregation assays (Figure 5B).34 The polymer architecture also gives rise to very different binding, with a linear sialic acid presenting polymer showing higher in vitro and in vivo activity for protection against influenza infection, attributed to the increased steric shielding by the linear polymer, in comparison to the compact dendrimer.78 Star branched polymannosides varying in only the number and length of arms showed differential responses to immobilized human lectins using SPR.79 These examples highlight how both precision and, more generally, macromolecular engineering could be easily exploited in the search for selectivity.
Figure 5.
Glycan presentation affects overall binding. (A) Cyclic proline scaffolds bearing Man4, with selective DC-SIGN binding, over Langerin, even though both bind the glycan individually.76 (B) Flowthrough detection of SARS-CoV-2 spike protein using polymer-tethered glyconanoparticles, with the signal controlled by diameter and chain length, with the same glycan. Image adapted from ref 49.
Glycan Heterogeneity and Targeting of Secondary Binding Sites
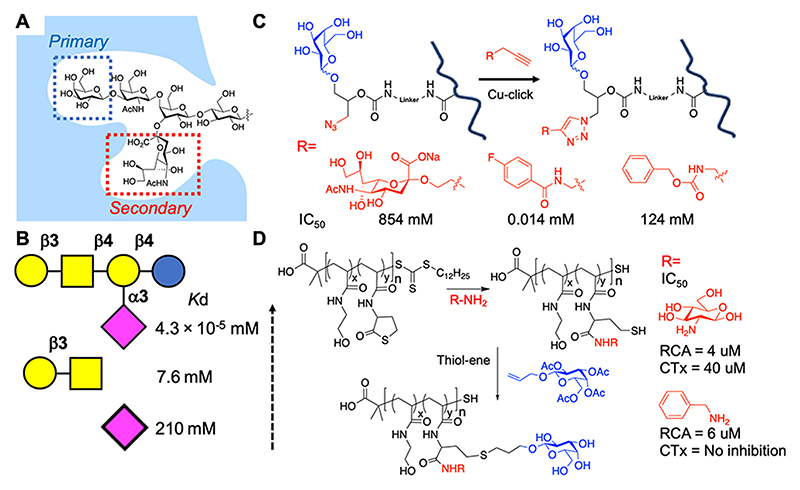
A classic description of lectins is also as “pattern recognition molecules”.80 Pathogens and the host share many glycans, and hence differentiating between these must be driven by a selection other than just their chemical identity. The primary binding site of a lectin is the subject of most focus, but allosteric (secondary) interactions can also be exploited, by incorporating multiple (smaller) glycans proximal to each other rather than as a single oligosaccharide, mimicking glycan branching. Hence, presenting multiple glycans can lead to selectivity gains (and is related to the previous section in that the 3D control of these can also matter).
Turnbull et al. probed the GM1 CTx interaction by ITC, showing that the sialic acid unit contributed 44% of the intrinsic binding energy, although the sialic acid ligand when used alone had no appreciable affinity (Figure 6A,B).81 This demonstrated that an approach to target secondary binding pockets, in addition to the primary β-galactose site, is a valid tool for gaining selectivity. Tran et al. used polymeric scaffolds bearing β-galactose as the primary ligand for CTx but also “clicked” (azide/alkyne) additional functional groups proximal to the galactose, to mimic the branched GM1 ganglioside.82 This approach led to a shift in IC50 from 584 μM (for sialic acid) to 0.014 μM for a fluorobenzyl derivative (Figure 6C). Gibson and co-workers showed that addition of aromatic secondary units (in addition to galactose) in a two-step postpolymerization strategy enabled the relative affinity (selectivity) of the glycopolymers toward CTx and PNA (peanut agglutinin) to be tuned by 20-fold.83,84 In contrast, using thiolactone chemistry (Figure 6D), a benzyl side chain reduced CTx inhibition but retained RCA120 inhibition, showing that the precise location and density of side chains has a significant effect.84 The density of side chains in CTx inhibition has also been reported in several studies, with lower galactose density often leading to increased inhibitory activity, showing that the “more is better” design principle is overly simplistic.27,68,69,85
Figure 6.
Targeting secondary binding sites in CTx. (A) Schematic of the GM1 glycan in the CTx binding site. (B) Affinity of glycans toward CTx from ITC.81 (C) Secondary binding site targeting via a click reaction proximal to the primary galactose unit and CTx inhibition.82 (D) Thiolactone ring opening to install secondary binding units and CTx/RCA120 inhibition.84
The importance of heterogeneity in biomimetics is in line with the complexity of the glycans in the glycocalyx,86 and the strongest binder may not be the only component essential for biological function. Worstell et al. used a nanocube-based sensing system to demonstrate that the addition of fucosyl GM1 into a mixture with GM2 led to enhanced binding affinity toward CTx, even though the fucosyl GM1 itself had minimal affinity.87 Similarly, galactose and fucose copolymers were more effective inhibitors of CTx binding to human enteroids than galacto- or fuco-polymers alone, due to the additional lower-affinity fucose-binding site.88,89 What is clear is that most materials strategies currently rely on trial and error to judge benefits (or not) from heterogeneity. The challenge of the polymer sequence for example, where extended sequences of one glycan may emerge, rather than a pure statistical distribution in a copolymer, makes quantification of the exact role of each component a major challenge.
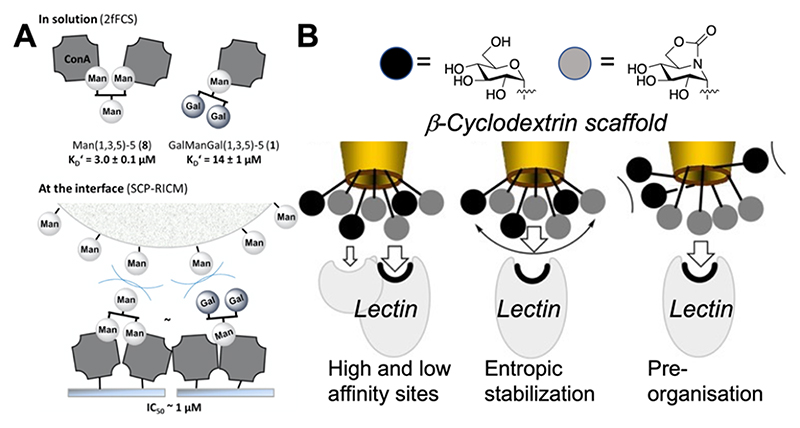
The above examples support mechanisms where the heterogeneity increases affinity/selectivity by targeting secondary sites, but that is not the sole mode of action. An alternative mechanism of action for heterogeneity gains (and hence potentially selectivity) is due to steric shielding, from a nonbinding partner. Hartmann and co-workers have shown that nonbinding galactose units on a sequence-defined oligomer enhance inhibitory activity but do not change the overall affinity (K d) due to a steric shielding effect, as shown by STD NMR (Figure 7A).90 Garcia-Fernandez and co-workers undertook an extremely detailed study using cyclodextrins as the scaffold for the heterogeneous display of glycan (Figure 7B).91 Selectivity between Con A and PNA was achieved not only by heterogeneity but also in their inhibitory action against glycosidases, tuning in selectivity toward maltase, isomalatase, and α-mannosidase. The data supported that sliding or steric shielding was again the mechanism for enhancement, rather than a secondary binding site (unlike the previous examples for CTx). Dendrimers bearing variable densities of mannose and galactose were immobilized on surfaces, and screening revealed “hot spots” where a specific presentation/ratio led to selectivity. The underpinning mechanism for this was not clear but showed the principle that heterogeneity could be deployed in a biosensor format.92 Otten et al. used mixtures of GalNAc and ManNAc in a gold nanoparticle biosensor format (for lectin aggregation). Nonlinear responses to glycan mixtures were seen, such that affinity for SBA could be retained but was significantly reduced toward RCA120 due to the addition of mannose.36
Figure 7.
Heterogeneous presentation of high- and low-affinity glycans can enhance binding. (A) Sequence-defined polymers bindingCon Ain solution and at the interface (competition experiment).90 Reprinted with permission from ref 90. Copyright 2014 American Chemical Society. (B) Proposed modes of affinity enhancement for heterogeneous glycoclusters based on cyclodextrin scaffolds.91 Reprinted with permission from ref 91. Copyright 2017 John Wiley and Sons.
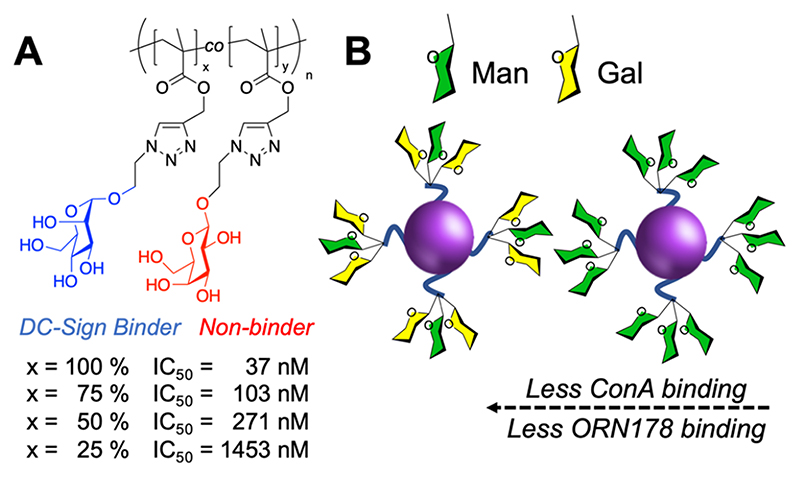
Variable-density glycopolymers have been immobilized onto glass slides.93 Using this strategy, individual glycan components from degraded heparin sulfate were screened for their ability to bind FGF2 (fibroblast growth factor 2).94 However, it is important to note that heterogeneity does not always lead to these increased gains and is material- and lectin-dependent. For example, dilution of an α-mannosylated glycopolymer with β-galactose side chains led to a decrease in DC-SIGN binding (measured by SPR; Figure 8A).33 Alternatively, the heterogeneity may play only a slight role, as seen for mannosylated gold nanoparticles for inhibiting FimH-driven adhesion (Figure 8B).95
Figure 8.
Glycan heterogeneity can reduce affinity. (A) Mannosylated glycopolymers show reduced inhibitory activity vs DC-SIGN on dilution with galactose.33 (B) Mannosylated glycoparticles with reduced affinity toward Con A and ORN178 (E. coli) as galactose is introduced.95
This subsection highlighted the inherent complexity (and huge potential) of heterogeneous and secondary-site targeting materials. A key observation is not all lectins respond to the dual effect of glycan dilution, showing that selectivity tuning is possible. The mechanism for selectivity is often subtle, covering steric shielding effects, targeting secondary sites, or allowing/ preventing inter-/intralectin binding site spanning. However, subtle differences between systems can have large effects and high-throughput screening-based approaches, based on sequentially modified scaffolds, could play a role in dissecting these interactions.
Non-Natural Glycans and Glycan Mimetics
To drive selectivity and affinity, a medicinal chemistry (e.g. not materials) approach would be to use non-natural glycans that have favorable binding and pharmacokinetic properties. Thiosugars (where the internal ring oxygen is substituted by a sulfur, rather than those with an anomeric thiol), iminosugars (NH replacement), and carbasugars (CH2) are established medicinal chemistry tools, especially as glycosidase inhibitors,96 and glycomimetic drugs have reached the clinic.97 The application of these approaches in multivalent systems is less common, as the synthetic burden may outweigh the intrinsic simplicity of many polymeric systems, but has already shown significant promise. Kiessling and co-workers developed a mannose mimetic that was displayed on BSA carrier proteins and functioned as a DC-SIGN agonist.98 The same group demonstrated that C-linked mannose glycopolymers were more potent binders of Con A than O-linked species30 and that selective positioning of sulfate groups on galactosylated polymers can tune the affinity between L- and P-selectins.99
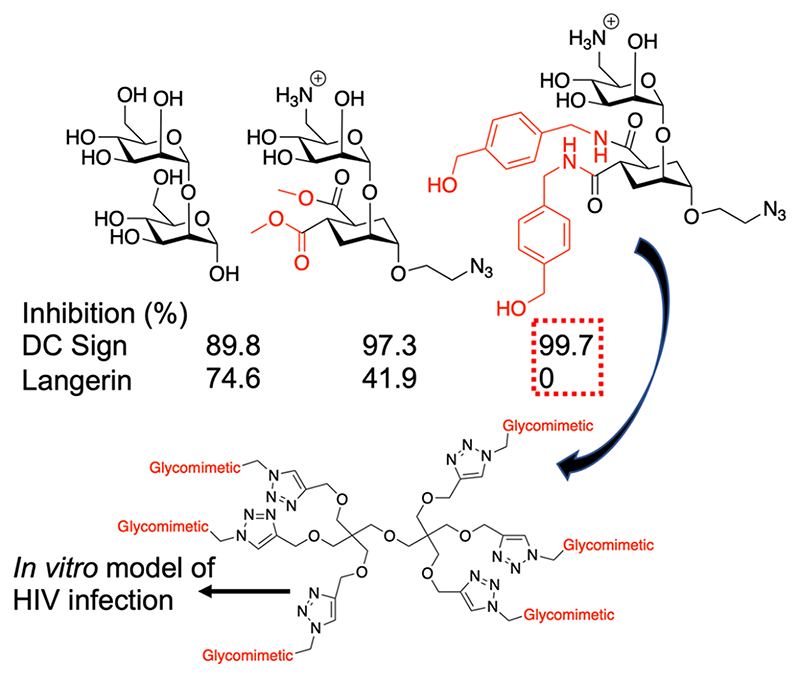
Fieschi and co-workers have explored the use of glycomi-mietics to tune selectivity. They observed that C-6 sulfation in GlcNAc derivatives led to selectivity toward Langerin. They developed this further to identify inhibitors (Figure 9A) which only bound DC-SIGN and not Langerin100 and were incorporated into dendritic structures, which selectively inhibited HIV infection in a model study.101
Figure 9. Glycomimetic strategy to identify selective DC-SIGN binders, with no inhibition of Langerin, and subsequent multivalent display.100,101 .
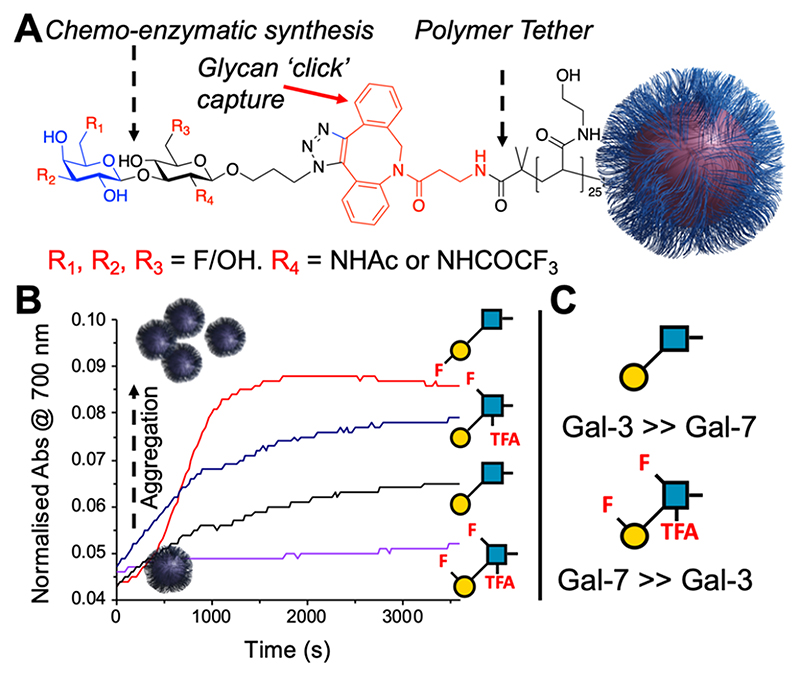
Fluorine is an appealing modification to glycans as a tool to modulate their pharmacokinetics, due to its small size and minimal effect on glycan conformation.102,103 Fluorine is not a hydrogen bond donor but is a weak acceptor, and hence the replacement of hydroxyls with fluorines can lead to significant changes in binding.104 Fluorinated phenyltriazolyl-thiogalacto-sides engaged with additional interactions with Galectin-3, in comparison to nonfluorinated species.105 Fluorination of the glycan portion of MUC-1 peptides resulted in differential antiserum responses.106 Site-specific fluorination at the terminal mannose C-6 in Man3GlcNAc was found to be crucial to Con A binding, but the branched mannose C-6 could tolerate fluorination, when it was displayed on a glycoprotein.107 Encouraged by this, Richards et al. employed a chemoenzymatic synthesis to obtain a library of fluorinated Lacto-N-biose derivatives, exploiting the promiscuity of the BiGalK and BiGalHexNAcP enzymes from Bifidobacterium infantis which tolerate fluorinated donors (Figure 10A). Incorporation of these onto multivalent gold nanoparticle platforms allowed the identification of specific fluorination sites to tune discrimination between Galectin-3 and Galectin-7 with the glycomaterials (Figure 10B, C).108
Figure 10.
Fluorinated Lacto-N-biose-functional gold nanoparticles to bind galectins.108 (A) Schematic of glyconanoparticle structure (B) Aggregation kinetics of selected glyconanoparticles with Galectin-3. (C) Glycans identified (in multivalent format only) with switched affinity. Reproduced from ref 108 with permission from the Royal Society of Chemistry.
Directed Evolution, High-Throughput, and Biochemical Panning Approaches
High-throughput discovery approaches offer an alternative (or complementary) tool for the discovery of high-affinity and selective ligands. For example, robotics and parallel synthesis have been used for polymeric109,110 and inorganic111 materials. Due to selection and amplification tools, protein and nucleic acid based materials can be screened by phage112 or apatamer/ SELEX113 technologies. However, for glycans, which are not template-directed and cannot be amplified, the discovery tools are fewer. Automated glycan synthesis is rapidly progressing but is still not a routine laboratory tool.114,115 It should be noted that glycan arrays are high-throughput, once the glycans are in hand,70 but have already been reviewed and are not covered here.116
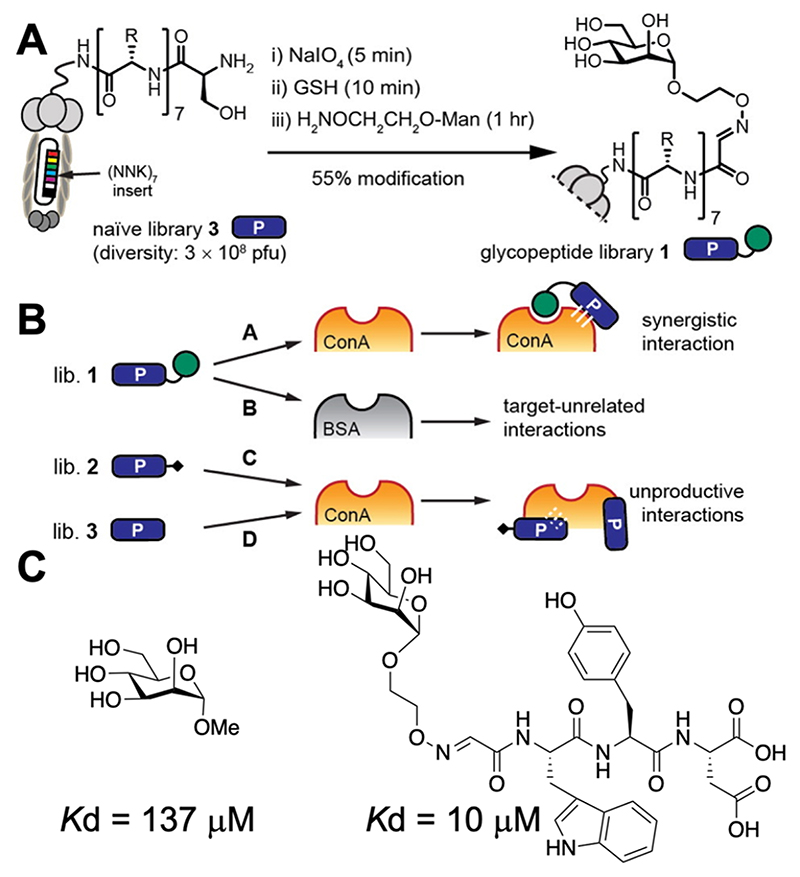
Krauss and co-workers made libraries of peptides containing non-natural amino acids (bearing an alkyne) connected to mRNA. This mRNA-encoded library could be glycosylated (using glycosyl azides), followed by selection and PCR amplification of the “winning” binders. Using this approach, a library of 1013 glycopeptides were screened and a picomolar binder to the HIV neutralizing antibody 2G12 was identified,117 with a chemical glycosylation step being essential during selection rounds. Ng et al. used a related strategy whereby phase display was employed, followed by oxidation of terminal serine residues to aldehydes to capture amino-oxy mannose (Figure 11A).118 Selection against Con A (positive) and BSA (negative) (Figure 11B) led to ligands with increased selectivity via modulation of the peptide linkage, with an example hit being shown in Figure 10C. Interestingly, the same ligand for Con A binding showed high affinity to DC-SIGN which also binds high-mannose, highlighting again the selectivity challenge. A method where the peptide is not varied but the glycan immobilized onto an M13 bacteriophage is, termed a “liquid glycan array”, has also been reported.119 Related approaches to identify selective binders using DNA-encoded glycans as microarray alternatives can be used to pan hundreds of glycans.120 These methods all show huge potential for true high-throughput screening and are especially suitable for positive/negative selection to introduce selectivity.
Figure 11.
Phage-based screening for glycosylated peptides to enhance mannose binding to Con A.118 (A) Schematic of glycosylation at the serine N-terminus of peptides on phage. (B) Selection and enrichment processes. (C) Example of a discovered peptide sequence with enhanced Con A affinity in comparison to methyl-mannoside. Reprinted (adapted) with permission from ref 118. Copyright 2015 American Chemical Society.
Outlook and Opportunities
The aim of this perspective is to highlight that macromolecular and materials science has a huge potential to have an effect on glycoscience and that not only is the selectivity challenge tractable but also there exists a diverse range of strategies to achieve it. This perspective is not intended to be comprehensive but to introduce the reader to some current strategies that show promise in this challenging area.
By drawing from detailed “small molecule” studies, significant gains in selectivity are possible by exploiting the benefits of multivalency, including the ability to present multiple different glycans on the same scaffold, use steric shielding effects, and tune the linker chemistry. However, moving from simple monosaccharides to oligo or non-natural glycans is essential to ensure that this large step is taken. Advances in high-throughput materials discovery is well placed to support this, as well as exciting macromolecular tools based upon, for example, sequence-controlled121 and folded polymers,122 which show early promise.90 Recent advances in structural biology, including cryo-electron microscopy123 and the new computation tools to predict protein structure,124 will inevitably feed into this as well. It is also crucial, if glycomaterials are to be used in biological environments, to understand how the media affect the performance. The protein corona, where proteins absorb to nanoparticle surfaces,125 has been shown to introduce additional glycoproteins126 and hence there is the potential for a highly selective binder in “pure” solutions to lose function in an application. We anticipate that the next generation of glycomaterials will move beyond using simple monosaccharides against plant lectins (which have obvious value still) to real targets under biomedically relevant conditions.
Acknowledgments
M.I.G. thanks the ERC for a Consolidator Grant (866056). BBSRC/Innovate are thanked for funding the Specialty Glycans project BB/M02878X/1. UoW, the EPSRC (EP/R511808/1), and the BBSRC (BB/S506783/1) impact acceleration accounts are acknowledged. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 814236, supporting the NanoCarb ITN network, which is thanked for useful discussions. The glycan array resources (DC-SIGN screening primscreen_5273) were provided by the Consortium for Functional Glycomics grant numbers GM62116 and GM098791.
Footnotes
Notes
The authors declare no competing financial interest.
References
- (1).Flynn RA, Pedram K, Malaker SA, Batista PJ, Smith BAH, Johnson AG, George BM, Majzoub K, Villalta PW, Carette JE, Bertozzi CR. Small RNAs Are Modified with N-Glycans and Displayed on the Surface of Living Cells. Cell. 2021;184(12):3109–3124. doi: 10.1016/j.cell.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Pilobello KT, Mahal LK. Deciphering the Glycocode: The Complexity and Analytical Challenge of Glycomics. Curr Opin Chem Biol. 2007;11(3):300–305. doi: 10.1016/j.cbpa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- (3).Bertozzi CR, Kiessling LL. Chemical Glycobiology. Science. 2001;291(5512):2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- (4).Adibekian A, Stallforth P, Hecht M-L, Werz DB, Gagneux P, Seeberger PH. Comparative Bioinformatics Analysis of the Mammalian and Bacterial Glycomes. Chem Sci. 2011;2(2):337–344. [Google Scholar]
- (5).Dedola S, Rugen MD, Young RJ, Field RA. Revisiting the Language of Glycoscience: Readers, Writers and Erasers in Carbohydrate Biochemistry. ChemBioChem. 2020;21(3):423–427. doi: 10.1002/cbic.201900377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lis H, Sharon N. Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition. Chem Rev. 1998;98(2):637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- (7).Lee YC, Townsend RR, Hardy MR, Lönngren J, Arnarp J, Haraldsson M, Lönn H. Binding of Synthetic Oligosaccharides to the Hepatic Gal/GalNAc Lectin. Dependence on Fine Structural Features. J Biol Chem. 1983;258(1):199–202. [PubMed] [Google Scholar]
- (8).Lee YC, Lee RT. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc Chem Res. 1995;28(8):321–327. [Google Scholar]
- (9).Lundquist JJ, Toone EJ. The Cluster Glycoside Effect. Chem Rev. 2002;102(2):555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- (10).Sigal GB, Mammen M, Dahmann G, Whitesides GM. Polyacrylamides Bearing Pendant α-Sialoside Groups Strongly Inhibit Agglutination of Erythrocytes by Influenza Virus: The Strong Inhibition Reflects Enhanced Binding through Cooperative Polyvalent Interactions. J Am Chem Soc. 1996;118(16):3789–3800. [Google Scholar]
- (11).Kanai M, Mortell KH, Kiessling LL. Varying the Size of Multivalent Ligands: The Dependence of Concanavalin A Binding on Neoglycopolymer Length. J Am Chem Soc. 1997;119(41):9931–9932. [Google Scholar]
- (12).Ernst B, Magnani JL. From Carbohydrate Leads to Glycomimetic Drugs. Nat Rev Drug Discovery. 2009;8(8):661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kleeb S, Pang L, Mayer K, Eris D, Sigl A, Preston RC, Zihlmann P, Sharpe T, Jakob RP, Abgottspon D, Hutter AS, et al. FimH Antagonists: Bioisosteres to Improve the in Vitro and in Vivo PK/PD Profile. J Med Chem. 2015;58(5):2221–2239. doi: 10.1021/jm501524q. [DOI] [PubMed] [Google Scholar]
- (14).Liang R, Yan L, Loebach J, Ge M, Uozumi Y, Sekanina K, Horan N, Gildersleeve J, Thompson C, Smith A, Biswas K, et al. Parallel Synthesis and Screening of a Solid Phase Carbohydrate Library. Science. 1996;274(5292):1520–1522. doi: 10.1126/science.274.5292.1520. [DOI] [PubMed] [Google Scholar]
- (15).Levine PM, Carberry TP, Holub JM, Kirshenbaum K. Crafting Precise Multivalent Architectures. MedChemComm. 2013;4(3):493–509. [Google Scholar]
- (16).Wittmann V, Pieters RJ. Bridging Lectin Binding Sites by Multivalent Carbohydrates. Chem Soc Rev. 2013;42(10):4492. doi: 10.1039/c3cs60089k. [DOI] [PubMed] [Google Scholar]
- (17).Thompson JP, Schengrund C-L. Oligosaccharide-Derivatized Dendrimers: Defined Multivalent Inhibitors of the Adherence of the Cholera Toxin B Subunit and the Heat Labile Enterotoxin of E. Coli to GM1. Glycoconjugate J. 1997;14(7):837–845. doi: 10.1023/a:1018590021762. [DOI] [PubMed] [Google Scholar]
- (18).Turnbull WB, Stoddart JF. Design and Synthesis of Glycodendrimers. Rev Mol Biotechnol. 2002;90(3-4):231–255. doi: 10.1016/s1389-0352(01)00062-9. [DOI] [PubMed] [Google Scholar]
- (19).Vrasidas I, De Mol NJ, Liskamp RMJ, Pieters RJ. Synthesis of Lactose Dendrimers and Multivalency Effects in Binding to the Cholera Toxin B Subunit. Eur J Org Chem. 2001;2001(24):4685–4692. [Google Scholar]
- (20).Arosio D, Vrasidas I, Valentini P, Liskamp RMJ, Pieters RJ, Bernardi A. Synthesis and Cholera Toxin Binding Properties of Multivalent GM1Mimics. Org Biomol Chem. 2004;2(14):2113. doi: 10.1039/b405344c. [DOI] [PubMed] [Google Scholar]
- (21).Branderhorst HM, Kooij R, Salminen A, Jongeneel LH, Arnusch CJ, Liskamp RMJ, Finne J, Pieters RJ. Synthesis of Multivalent Streptococcus Suis Adhesion Inhibitors by Enzymatic Cleavage of Polygalacturonic Acid and “click” Conjugation. Org Biomol Chem. 2008;6(8):1425–1434. doi: 10.1039/b800283e. [DOI] [PubMed] [Google Scholar]
- (22).Johansson EMV, Crusz SA, Kolomiets E, Buts L, Kadam RU, Cacciarini M, Bartels K-M, Diggle SP, Cámara M, Williams P, Loris R, et al. Inhibition and Dispersion of Pseudomonas Aeruginosa Biofilms by Glycopeptide Dendrimers Targeting the Fucose-Specific Lectin LecB. Chem Biol. 2008;15(12):1249–1257. doi: 10.1016/j.chembiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- (23).Kadam RU, Bergmann M, Hurley M, Garg D, Cacciarini M, Swiderska MA, Nativi C, Sattler M, Smyth AR, Williams P, Cámara M, et al. A Glycopeptide Dendrimer Inhibitor of the Galactose-Specific Lectin LecA and of Pseudomonas Aeruginosa Biofilms. Angew Chem, Int Ed. 2011;50(45):10631–10635. doi: 10.1002/anie.201104342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bernardi A, Jiménez-Barbero J, Casnati A, De Castro C, Darbre T, Fieschi F, Finne J, Funken H, Jaeger K-E, Lahmann M, Lindhorst TK, et al. Multivalent Glycoconjugates as Anti-Pathogenic Agents. Chem Soc Rev. 2013;42(11):4709–4727. doi: 10.1039/c2cs35408j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Reymond JL, Bergmann M, Darbrea T. Glycopeptide Dendrimers as Pseudomonas Aeruginosa Biofilm Inhibitors. Chem Soc Rev. 2013;42(11):4814–4822. doi: 10.1039/c3cs35504g. [DOI] [PubMed] [Google Scholar]
- (26).Wittmann V, Seeberger S. Spatial Screening of Cyclic Neoglycopeptides: Identification of Polyvalent Wheat-Germ Agglutinin Ligands. Angew Chem, Int Ed. 2004;43(7):900–903. doi: 10.1002/anie.200352055. [DOI] [PubMed] [Google Scholar]
- (27).Polizzotti BD, Kiick KL. Effects of Polymer Structure on the Inhibition of Cholera Toxin by Linear Polypeptide-Based Glycopolymers. Biomacromolecules. 2006;7(2):483–490. doi: 10.1021/bm050672n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Miura Y, Koketsu D, Kobayashi K. Synthesis and Properties of a Well-defined Glycopolymer via Living Radical Polymerization. Polym Adv Technol. 2007;18(8):647–651. [Google Scholar]
- (29).Baek M-G, Roy R. Relative Lectin Binding Properties of T-Antigen-Containing Glycopolymers: Copolymerization OfN-Acryloy-lated T-Antigen Monomer vs. Graft Conjugation of Aminated T-Antigen Ligands onto Poly(N-Acryloxysuccinimide) Macromol Biosci. 2001;1(7):305–311. [Google Scholar]
- (30).Mortell KH, Weatherman RV, Kiessling LL. Recognition Specificity of Neoglycopolymers Prepared by Ring-Opening Metathesis Polymerization. J Am Chem Soc. 1996;118(9):2297–2298. [Google Scholar]
- (31).Mammen M, Dahmann G, Whitesides GM. Effective Inhibitors of Hemagglutination by Influenza Virus Synthesized from Polymers Having Active Ester Groups. Insight into Mechanism of Inhibition. J Med Chem. 1995;38(21):4179–4190. doi: 10.1021/jm00021a007. [DOI] [PubMed] [Google Scholar]
- (32).Ladmiral V, Mantovani G, Clarkson GJ, Cauet S, Irwin JL, Haddleton DM. Synthesis of Neoglycopolymers by a Combination of “Click Chemistry” and Living Radical Polymerization. J Am Chem Soc. 2006;128(14):4823–4830. doi: 10.1021/ja058364k. [DOI] [PubMed] [Google Scholar]
- (33).Becer CRR, Gibson MIMI, Geng J, Ilyas R, Wallis R, Mitchell DADA, Haddleton DMDM. High-Affinity Glycopolymer Binding to Human DC-SIGN and Disruption of DC-SIGN Interactions with HIV Envelope Glycoprotein. J Am Chem Soc. 2010;132(43):15130–15132. doi: 10.1021/ja1056714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Georgiou PG, Baker AN, Richards S-J, Laezza A, Walker M, Gibson MI. Tuning Aggregative versus Non-Aggregative Lectin Binding with Glycosylated Nanoparticles by the Nature of the Polymer Ligand. J Mater Chem B. 2020;8(1):136–145. doi: 10.1039/c9tb02004g. [DOI] [PubMed] [Google Scholar]
- (35).Richards S-J, Baker AN, Walker M, Gibson MI. Polymer-Stabilized Sialylated Nanoparticles: Synthesis, Optimization, and Differential Binding to Influenza Hemagglutinins. Biomacromolecules. 2020;21(4):1604–1612. doi: 10.1021/acs.biomac.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Otten L, Vlachou D, Richards S-J, Gibson MI. Glycan Heterogeneity On Gold Nanoparticles Increases Lectin Discrimination Capacity in Label-Free Multiplexed Bioassays. Analyst. 2016;141(14):4305–4312. doi: 10.1039/c6an00549g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wang X, Ramström O, Yan M. A Photochemically Initiated Chemistry for Coupling Underivatized Carbohydrates to Gold Nanoparticles. J Mater Chem. 2009;19(47):8944–8949. doi: 10.1039/B917900C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Astronomo RD, Kaltgrad E, Udit AK, Wang SK, Doores KJ, Huang CY, Pantophlet R, Paulson JC, Wong CH, Finn MG, Burton DR. Defining Criteria for Oligomannose Immunogens for HIV Using Icosahedral Virus Capsid Scaffolds. Chem Biol. 2010;17(4):357–370. doi: 10.1016/j.chembiol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bundle DR, Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, Read RJ. Shiga-like Toxins Are Neutralized by Tailored Multivalent Carbohydrate. Nature. 2000;403(6770):669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- (40).Varner CT, Rosen T, Martin JT, Kane RS. Recent Advances in Engineering Polyvalent Biological Interactions. Biomacromolecules. 2015;16(1):43–55. doi: 10.1021/bm5014469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Galan MC, Dumy P, Renaudet O. Multivalent Glyco-(Cyclo)Peptides. Chem Soc Rev. 2013;42(11):4599–4612. doi: 10.1039/c2cs35413f. [DOI] [PubMed] [Google Scholar]
- (42).Jiménez Blanco JL, Ortiz Mellet C, García Fernández JM. Multivalency in Heterogeneous Glycoenvironments: Hetero-Glycoclusters, -Glycopolymers and -Glycoassemblies. Chem Soc Rev. 2013;42(11):4518–4531. doi: 10.1039/c2cs35219b. [DOI] [PubMed] [Google Scholar]
- (43).Kiessling LL, Gestwicki JE, Strong LE. Synthetic Multivalent Ligands as Probes of Signal Transduction. Angew Chem, Int Ed. 2006;45(15):2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Sharon N. Carbohydrates as Future Anti-Adhesion Drugs for Infectious Diseases. Biochim Biophys Acta, Gen Subj. 2006;1760(4):527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- (45).Spain SG, Cameron NR. A Spoonful of Sugar: The Application of Glycopolymers in Therapeutics. Polym Chem. 2011;2(1):60–68. [Google Scholar]
- (46).Branson TR, Turnbull WB. Bacterial Toxin Inhibitors Based on Multivalent Scaffolds. Chem Soc Rev. 2013;42(11):4613–4622. doi: 10.1039/c2cs35430f. [DOI] [PubMed] [Google Scholar]
- (47).Pieters RJ. Maximising Multivalency Effects in Protein-Carbohydrate Interactions. Org Biomol Chem. 2009;7(10):2013–2025. doi: 10.1039/b901828j. [DOI] [PubMed] [Google Scholar]
- (48).Ambrosi M, Cameron NR, Davis BG. Lectins: Tools for the Molecular Understanding of the Glycocode. Org Biomol Chem. 2005;3(9):1593–1608. doi: 10.1039/b414350g. [DOI] [PubMed] [Google Scholar]
- (49).Baker AN, Richards SJ, Guy CS, Congdon TR, Hasan M, Zwetsloot AJ, Gallo A, Lewandowski JR, Stansfeld PJ, Straube A, Walker M, et al. The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ACS Cent Sci. 2020;6(11):2046–2052. doi: 10.1021/acscentsci.0c00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Childs RA, Palma AS, Wharton S, Matrosovich T, Liu Y, Chai W, Campanero-Rhodes MA, Zhang Y, Eickmann M, Kiso M, Hay A, et al. Receptor-Binding Specificity of Pandemic Influenza A (H1N1) 2009 Virus Determined by Carbohydrate Microarray. Nat Biotechnol. 2009;27(9):797–799. doi: 10.1038/nbt0909-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Figdor CG, Van Kooyk Y, Adema GJ. C-Type Lectin Receptors on Dendritic Cells and Langerhans Cells. Nat Rev Immunol. 2002;2(2):77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- (52).de Witte L, Nabatov A, Geijtenbeek TBH. Distinct Roles for DC-SIGN+-Dendritic Cells and Langerhans Cells in HIV-1 Transmission. Trends Mol Med. 2008;14(1):12–19. doi: 10.1016/j.molmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- (53).Holla A, Skerra A. Comparative Analysis Reveals Selective Recognition of Glycans by the Dendritic Cell Receptors DC-SIGN and Langerin. Protein Eng, Des Sel. 2011;24(9):659–669. doi: 10.1093/protein/gzr016. [DOI] [PubMed] [Google Scholar]
- (54).Lin K, Kasko AM. Carbohydrate-Based Polymers for Immune Modulation. ACS Macro Lett. 2014;3(7):652–657. doi: 10.1021/mz5002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Consortium for Functional Glycomics
- (56).Rabinovich GA, Toscano MA. Turning “sweet” on Immunity: Galectin-Glycan Interactions in Immune Tolerance and Inflammation. Nat Rev Immunol. 2009;9(5):338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- (57).Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, et al. Oligosaccharide Specificity of Galectins: A Search by Frontal Affinity Chromatography. Biochim Biophys Acta, Gen Subj. 2002;1572(2-3):232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- (58).de Jong CGHM, Gabius H-J, Baron W. The Emerging Role of Galectins in (Re)Myelination and Its Potential for Developing New Approaches to Treat Multiple Sclerosis. Cell Mol Life Sci. 2020;77(7):1289–1317. doi: 10.1007/s00018-019-03327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Schofield CL, Field RA, Russell DA. Glyconanoparticles for the Colorimetric Detection of Cholera Toxin. Anal Chem. 2007;79(4):1356–1361. doi: 10.1021/ac061462j. [DOI] [PubMed] [Google Scholar]
- (60).Spain SG, Gibson MI, Cameron NR. Recent Advances in the Synthesis of Well-Defined Glycopolymers. J Polym Sci, Part A: Polym Chem. 2007;45(11):2059–2072. [Google Scholar]
- (61).Rillahan CD, Paulson JC. Glycan Microarrays for Decoding the Glycome. Annu Rev Biochem. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Godula K, Bertozzi CR. Density Variant Glycan Microarray for Evaluating Cross-Linking of Mucin-like Glycoconjugates by Lectins. J Am Chem Soc. 2012;134(38):15732–15742. doi: 10.1021/ja302193u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Liang R, Loebach J, Horan N, Ge M, Thompson C, Yan L, Kahne D. Polyvalent Binding to Carbohydrates Immobilized on an Insoluble Resin. Proc Natl Acad Sci U S A. 1997;94(20):10554–10559. doi: 10.1073/pnas.94.20.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Horan N, Yan L, Isobe H, Whitesides GM, Kahne D. Nonstatistical Binding of a Protein to Clustered Carbohydrates. Proc Natl Acad Sci U S A. 1999;96(21):11782–11786. doi: 10.1073/pnas.96.21.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Kwon SJ, Na DH, Kwak JH, Douaisi M, Zhang F, Park EJ, Park JH, Youn H, Song CS, Kane RS, Dordick JS, et al. Nanostructured Glycan Architecture Is Important in the Inhibition of Influenza A Virus Infection. Nat Nanotechnol. 2017;12(1):48–54. doi: 10.1038/nnano.2016.181. [DOI] [PubMed] [Google Scholar]
- (66).Lu W, Du W, Somovilla VJ, Yu G, Haksar D, de Vries E, Boons G-J, de Vries RP, de Haan CAM, Pieters RJ. Enhanced Inhibition of Influenza A Virus Adhesion by Di-and Trivalent Hemagglutinin Inhibitors. J Med Chem. 2019;62(13):6398–6404. doi: 10.1021/acs.jmedchem.9b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Liu S, Kiick KL. Architecture Effects on the Binding of Cholera Toxin by Helical Glycopolypeptides. Macromolecules. 2008;41(3):764–772. doi: 10.1021/ma702128a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Richards S-J, Jones MW, Hunaban M, Haddleton DM, Gibson MI. Probing Bacterial-Toxin Inhibition with Synthetic Glycopolymers Prepared by Tandem Post-Polymerization Modification: Role of Linker Length and Carbohydrate Density. Angew Chem, Int Ed. 2012;51(31):7812–7816. doi: 10.1002/anie.201202945. [DOI] [PubMed] [Google Scholar]
- (69).Kruger AG, Brucks SD, Yan T, Cárcarmo-Oyarce G, Wei Y, Wen DH, Carvalho DR, Hore MJA, Ribbeck K, Schrock RR, Kiessling LL. Stereochemical Control Yields Mucin Mimetic Polymers. ACS Cent Sci. 2021;7(4):624–630. doi: 10.1021/acscentsci.0c01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Wang Z, Chinoy ZS, Ambre SG, Peng W, McBride R, de Vries RP, Glushka J, Paulson JC, Boons G-J. A General Strategy for the Chemoenzymatic Synthesis of Asymmetrically Branched N-Glycans. Science. 2013;341(6144):379–383. doi: 10.1126/science.1236231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Grant OC, Smith HMK, Firsova D, Fadda E, Woods RJ. Presentation, Presentation, Presentation! Molecular-Level Insight into Linker Effects on Glycan Array Screening Data. Glycobiology. 2014;24(1):17–25. doi: 10.1093/glycob/cwt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Novoa A, Eierhoff T, Topin J, Varrot A, Barluenga S, Imberty A, Römer W, Winssinger N. A LecA Ligand Identified from a Galactoside-Conjugate Array Inhibits Host Cell Invasion by Pseudomonas Aeruginosa. Angew Chem, Int Ed. 2014;53(34):8885–8889. doi: 10.1002/anie.201402831. [DOI] [PubMed] [Google Scholar]
- (73).Branson TR, McAllister TE, Garcia-Hartjes J, Fascione MA, Ross JF, Warriner SL, Wennekes T, Zuilhof H, Turnbull WB. A Protein-Based Pentavalent Inhibitor of the Cholera Toxin B-Subunit. Angew Chem, Int Ed. 2014;53(32):8323–8327. doi: 10.1002/anie.201404397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Liese S, Netz RR. Quantitative Prediction of Multivalent Ligand-Receptor Binding Affinities for Influenza, Cholera, and Anthrax Inhibition. ACS Nano. 2018;12(5):4140–4147. doi: 10.1021/acsnano.7b08479. [DOI] [PubMed] [Google Scholar]
- (75).Nagao M, Kichize M, Hoshino Y, Miura Y. Influence of Monomer Structures for Polymeric Multivalent Ligands: Consideration of the Molecular Mobility of Glycopolymers. Biomacromolecules. 2021;22(7):3119–3127. doi: 10.1021/acs.biomac.1c00553. [DOI] [PubMed] [Google Scholar]
- (76).Wen HC, Lin CH, Huang JS, Tsai CL, Chen TF, Wang SK. Selective Targeting of DC-SIGN by Controlling the Oligomannose Pattern on a Polyproline Tetra-Helix Macrocycle Scaffold. Chem Commun. 2019;55(62):9124–9127. doi: 10.1039/c9cc03124c. [DOI] [PubMed] [Google Scholar]
- (77).Bachem G, Wamhoff E-C, Silberreis K, Kim D, Baukmann H, Fuchsberger F, Dernedde J, Rademacher C, Seitz O. Rational Design of a DNA-Scaffolded High-Affinity Binder for Langerin. Angew Chem, Int Ed. 2020;59(47):21016–21022. doi: 10.1002/anie.202006880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Bhatia S, Lauster D, Bardua M, Ludwig K, Angioletti-Uberti S, Popp N, Hoffmann U, Paulus F, Budt M, Stadtmüller M, Wolff T, et al. Linear Polysialoside Outperforms Dendritic Analogs for Inhibition of Influenza Virus Infection in Vitro and in Vivo. Biomaterials. 2017;138:22–34. doi: 10.1016/j.biomaterials.2017.05.028. [DOI] [PubMed] [Google Scholar]
- (79).Abdouni Y, Yilmaz G, Monaco A, Aksakal R, Becer CR. Effect of Arm Number and Length of Star-Shaped Glycopolymers on Binding to Dendritic and Langerhans Cell Lectins. Biomacromolecules. 2020;21(9):3756–3764. doi: 10.1021/acs.biomac.0c00856. [DOI] [PubMed] [Google Scholar]
- (80).Dam TK, Gerken TA, Brewer CF. Thermodynamics of Multivalent Carbohydrate-Lectin Cross-Linking Interactions: Importance of Entropy in the Bind and Jump Mechanism. Biochemistry. 2009;48(18):3822–3827. doi: 10.1021/bi9002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Turnbull WB, Precious BL, Homans SW. Dissecting the Cholera Toxin-Ganglioside GM1 Interaction by Isothermal Titration Calorimetry. J Am Chem Soc. 2004;126(4):1047–1054. doi: 10.1021/ja0378207. [DOI] [PubMed] [Google Scholar]
- (82).Tran H-A, Kitov PI, Paszkiewicz E, Sadowska JM, Bundle DR. Multifunctional Multivalency: A Focused Library of Polymeric Cholera Toxin Antagonists. Org Biomol Chem. 2011;9(10):3658–3671. doi: 10.1039/c0ob01089h. [DOI] [PubMed] [Google Scholar]
- (83).Jones MW, Otten L, Richards S-J, Lowery R, Phillips DJ, Haddleton DM, Gibson MI. Glycopolymers with Secondary Binding Motifs Mimic Glycan Branching and Display Bacterial Lectin Selectivity in Addition to Affinity. Chem Sci. 2014;5(4):1611–1616. [Google Scholar]
- (84).Wilkins LE, Badi N, Du Prez F, Gibson MI. Double-Modified Glycopolymers from Thiolactones to Modulate Lectin Selectivity and Affinity. ACS Macro Lett. 2018;7(12):1498–1502. doi: 10.1021/acsmacrolett.8b00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Martyn B, Biggs CI, Gibson MI. Comparison of Systematically Functionalized Heterogeneous and Homogenous Glycopolymers as Toxin Inhibitors. J Polym Sci, Part A: Polym Chem. 2019;57(1):40–47. [Google Scholar]
- (86).Moremen KW, Tiemeyer M, Nairn AV. Vertebrate Protein Glycosylation: Diversity, Synthesis and Function. Nat Rev Mol Cell Biol. 2012;13(7):448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Worstell NC, Krishnan P, Weatherston JD, Wu HJ. Binding Cooperativity Matters: A Gm1-like Ganglioside-Cholera Toxin b Subunit Binding Study Using a Nanocube-Based Lipid Bilayer Array. PLoS One. 2016;11(4):e0153265. doi: 10.1371/journal.pone.0153265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Wands AM, Cervin J, Huang H, Zhang Y, Youn G, Brautigam CA, Matson Dzebo M, Björklund P, Wallenius V, Bright DK, Bennett CS, et al. Fucosylated Molecules Competitively Interfere with Cholera Toxin Binding to Host Cells. ACS Infect Dis. 2018;4(5):758–770. doi: 10.1021/acsinfecdis.7b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Mandal PK, Branson TR, Hayes ED, Ross JF, Gavín JA, Daranas AH, Turnbull WB. Towards a Structural Basis for the Relationship between Blood Group and the Severity of El Tor Cholera. Angew Chem, Int Ed. 2012;51(21):5143–5146. doi: 10.1002/anie.201109068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Ponader D, Maffre P, Aretz J, Pussak D, Ninnemann NM, Schmidt S, Seeberger PH, Rademacher C, Nienhaus GU, Hartmann L. Carbohydrate-Lectin Recognition of Sequence-Defined Heteromultivalent Glycooligomers. J Am Chem Soc. 2014;136(5):2008–2016. doi: 10.1021/ja411582t. [DOI] [PubMed] [Google Scholar]
- (91).García-Moreno MI, Ortega-Caballero F, Rísquez-Cuadro R, Ortiz Mellet C, García Fernández JM. The Impact of Heteromultivalency in Lectin Recognition and Glycosidase Inhibition: An Integrated Mechanistic Study. Chem-Eur J. 2017;23(26):6295–6304. doi: 10.1002/chem.201700470. [DOI] [PubMed] [Google Scholar]
- (92).Gade M, Alex C, Ben-Arye SL, Monteiro JT, Yehuda S, Lepenies B, Padler-Karavani V, Kikkeri R. Microarray Analysis of Oligosaccharide-Mediated Multivalent Carbohydrate-Protein Interactions and Their Heterogeneity. ChemBioChem. 2018;19(11):1170–1177. doi: 10.1002/cbic.201800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Godula K, Bertozzi CR. Synthesis of Glycopolymers for Microarray Applications via Ligation of Reducing Sugars to a Poly(Acryloyl Hydrazide) Scaffold. J Am Chem Soc. 2010;132(29):9963–9965. doi: 10.1021/ja103009d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Huang ML, Smith RAA, Trieger GW, Godula K. Glycocalyx Remodeling with Proteoglycan Mimetics Promotes Neural Specification in Embryonic Stem Cells. J Am Chem Soc. 2014;136(30):10565–10568. doi: 10.1021/ja505012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Toraskar S, Gade M, Sangabathuni S, Thulasiram HV, Kikkeri R. Exploring the Influence of Shapes and Heterogeneity of Glyco-Gold Nanoparticles on Bacterial Binding for Preventing Infections. ChemMedChem. 2017;12(14):1116–1124. doi: 10.1002/cmdc.201700218. [DOI] [PubMed] [Google Scholar]
- (96).Gloster TM, Davies GJ. Glycosidase Inhibition: Assessing Mimicry of the Transition State. Org Biomol Chem. 2010;8(2):305–320. doi: 10.1039/b915870g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Smith BAH, Bertozzi CR. The Clinical Impact of Glycobiology: Targeting Selectins, Siglecs and Mammalian Glycans. Nat Rev Drug Discovery. 2021;20(3):217–243. doi: 10.1038/s41573-020-00093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Prost LR, Grim JC, Tonelli M, Kiessling LL. Noncarbohydrate Glycomimetics and Glycoprotein Surrogates as DC-SIGN Antagonists and Agonists. ACS Chem Biol. 2012;7(9):1603–1608. doi: 10.1021/cb300260p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Manning DD, Hu X, Beck P, Kiessling LL. Synthesis of Sulfated Neoglycopolymers: Selective P-Selectin Inhibitors. J Am Chem Soc. 1997;119(13):3161–3162. [Google Scholar]
- (100).Porkolab V, Chabrol E, Varga N, Ordanini S, Sutkevičiute I, Thépaut M, García-Jiménez MJ, Girard E, Nieto PM, Bernardi A, Fieschi F. Rational-Differential Design of Highly Specific Glycomimetic Ligands: Targeting DC-SIGN and Excluding Langerin Recognition. ACS Chem Biol. 2018;13(3):600–608. doi: 10.1021/acschembio.7b00958. [DOI] [PubMed] [Google Scholar]
- (101).Ordanini S, Varga N, Porkolab V, Thépaut M, Belvisi L, Bertaglia A, Palmioli A, Berzi A, Trabattoni D, Clerici M, Fieschi F, et al. Designing Nanomolar Antagonists of DC-SIGN-Mediated HIV Infection: Ligand Presentation Using Molecular Rods. Chem Commun. 2015;51(18):3816–3819. doi: 10.1039/c4cc09709b. [DOI] [PubMed] [Google Scholar]
- (102).Linclau B, Ardá A, Reichardt N-C, Sollogoub M, Unione L, Vincent SP, Jiménez-Barbero J. Fluorinated Carbohydrates as Chemical Probes for Molecular Recognition Studies. Current Status and Perspectives. Chem Soc Rev. 2020;49(12):3863–3888. doi: 10.1039/c9cs00099b. [DOI] [PubMed] [Google Scholar]
- (103).Linclau B, Golten S, Light M, Sebban M, Oulyadi H. The Conformation of Tetrafluorinated Methyl Galactoside Anomers: Crystallographic and NMR Studies. Carbohydr Res. 2011;346(9):1129–1139. doi: 10.1016/j.carres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- (104).Murray-Rust P, Stallings WC, Monti CT, Preston RK, Glusker JP. Intermolecular Interactions of the C-F Bond: The Crystallographic Environment of Fluorinated Carboxylic Acids and Related Structures. J Am Chem Soc. 1983;105(10):3206–3214. [Google Scholar]
- (105).Kumar R, Ignjatović MM, Peterson K, Olsson M, Leffler H, Ryde U, Nilsson UJ, Logan DT. Structure and Energetics of Ligand-Fluorine Interactions with Galectin-3 Backbone and Side-Chain Amides: Insight into Solvation Effects and Multipolar Interactions. ChemMedChem. 2019;14(16):1528–1536. doi: 10.1002/cmdc.201900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Oberbillig T, Mersch C, Wagner S, Hoffmann-Röder A. Antibody Recognition of Fluorinated MUC1 Glycopeptide Antigens. Chem Commun. 2012;48(10):1487–1489. doi: 10.1039/c1cc15139h. [DOI] [PubMed] [Google Scholar]
- (107).Orwenyo J, Huang W, Wang LX. Chemoenzymatic Synthesis and Lectin Recognition of a Selectively Fluorinated Glycoprotein. Bioorg Med Chem. 2013;21(16):4768–4777. doi: 10.1016/j.bmc.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Richards SJ, Keenan T, Vendeville JB, Wheatley DE, Chidwick H, Budhadev D, Council CE, Webster CS, Ledru H, Baker AN, Walker M, et al. Introducing Affinity and Selectivity into Galectin-Targeting Nanoparticles with Fluorinated Glycan Ligands. Chem Sci. 2021;12(3):905–910. doi: 10.1039/d0sc05360k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Hook AL, Chang CY, Yang J, Luckett J, Cockayne A, Atkinson S, Mei Y, Bayston R, Irvine DJ, Langer R, Anderson DG, et al. Combinatorial Discovery of Polymers Resistant to Bacterial Attachment. Nat Biotechnol. 2012;30(9):868–875. doi: 10.1038/nbt.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated Discovery of Synthetic Transfection Vectors: Parallel Synthesis and Screening of a Degradable Polymer Library [20] J Am Chem Soc. 2001;123(33):8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- (111).Collins C, Dyer MS, Pitcher MJ, Whitehead GFS, Zanella M, Mandal P, Claridge JB, Darling GR, Rosseinsky MJ. Accelerated Discovery of Two Crystal Structure Types in a Complex Inorganic Phase Field. Nature. 2017;546(7657):280–284. doi: 10.1038/nature22374. [DOI] [PubMed] [Google Scholar]
- (112).Pande J, Szewczyk MM, Grover AK. Phage Display: Concept, Innovations, Applications and Future. Biotechnol Adv. 2010;28(6):849–858. doi: 10.1016/j.biotechadv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- (113).Stoltenburg R, Reinemann C, Strehlitz B. SELEX-A (r)Evolutionary Method to Generate High-Affinity Nucleic Acid Ligands. Biomol Eng. 2007;24(4):381–403. doi: 10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- (114).Joseph AA, Pardo-Vargas A, Seeberger PH. Total Synthesis of Polysaccharides by Automated Glycan Assembly. J Am Chem Soc. 2020;142(19):8561–8564. doi: 10.1021/jacs.0c00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Li T, Liu L, Wei N, Yang JY, Chapla DG, Moremen KW, Boons GJ. An Automated Platform for the Enzyme-Mediated Assembly of Complex Oligosaccharides. Nat Chem. 2019;11(3):229–236. doi: 10.1038/s41557-019-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Oyelaran O, Gildersleeve JC. Glycan Arrays: Recent Advances and Future Challenges. Curr Opin Chem Biol. 2009;13(4):406–413. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Horiya S, Bailey JK, Temme JS, Guillen Schlippe YV, Krauss IJ. Directed Evolution of Multivalent Glycopeptides Tightly Recognized by HIV Antibody 2G12. J Am Chem Soc. 2014;136(14):5407–5415. doi: 10.1021/ja500678v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Ng S, Lin E, Kitov PI, Tjhung KF, Gerlits OO, Deng L, Kasper B, Sood A, Paschal BM, Zhang P, Ling CC, et al. Genetically Encoded Fragment-Based Discovery of Glycopeptide Ligands for Carbohydrate-Binding Proteins. J Am Chem Soc. 2015;137(16):5248–5251. doi: 10.1021/ja511237n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Sojitra M, Sarkar S, Maghera J, Rodrigues E, Carpenter EJ, Seth S, Ferrer Vinals D, Bennett NJ, Reddy R, Khalil A, Xue X, et al. Genetically Encoded Multivalent Liquid Glycan Array Displayed on M13 Bacteriophage. Nat Chem Biol. 2021;17(7):806–816. doi: 10.1038/s41589-021-00788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Yan M, Zhu Y, Liu X, Lasanajak Y, Xiong J, Lu J, Lin X, Ashline D, Reinhold V, Smith DF, Song X. Next-Generation Glycan Microarray Enabled by DNA-Coded Glycan Library and Next-Generation Sequencing Technology. Anal Chem. 2019;91(14):9221–9228. doi: 10.1021/acs.analchem.9b01988. [DOI] [PubMed] [Google Scholar]
- (121).Lutz J-F, Ouchi M, Liu DR, Sawamoto M. Sequence-Controlled Polymers. Science. 2013;341(6146):1238149. doi: 10.1126/science.1238149. [DOI] [PubMed] [Google Scholar]
- (122).Schmidt BVKJ, Fechler N, Falkenhagen J, Lutz J-F. Controlled Folding of Synthetic Polymer Chains through the Formation of Positionable Covalent Bridges. Nat Chem. 2011;3(3):234–238. doi: 10.1038/nchem.964. [DOI] [PubMed] [Google Scholar]
- (123).Kühlbrandt W. The Resolution Revolution. Science. 2014;343(6178):1443–1444. doi: 10.1126/science.1251652. [DOI] [PubMed] [Google Scholar]
- (124).Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the Cell “Sees” in Bionanoscience. J Am Chem Soc. 2010;132(16):5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- (126).Wan S, Kelly PM, Mahon E, Stöckmann H, Rudd PM, Caruso F, Dawson KA, Yan Y, Monopoli MP. The “Sweet” Side of the Protein Corona: Effects of Glycosylation on Nanoparticle-Cell Interactions. ACS Nano. 2015;9(2):2157–2166. doi: 10.1021/nn506060q. [DOI] [PubMed] [Google Scholar]