Abstract
Objectives
Morning stiffness (MS) is characteristic for Rheumatoid Arthritis (RA) and associates with markers of systemic and local inflammation in RA-patients. In patients with arthralgia, MS is a cardinal symptom to recognize arthralgia at-risk for RA-development (i.e. clinically suspect arthralgia, CSA). In CSA, MS is also assumed to reflect inflammation, but this has never been studied. Therefore we aimed to study whether MS in CSA-patients is associated with systemic- and subclinical joint-inflammation.
Methods
575 patients presenting with CSA underwent laboratory investigations and contrast-enhanced 1.5T-MRI of hand and forefoot (scored according to the RAMRIS-method). Associations of MS (duration ≥60 minutes) with presence of subclinical joint-inflammation (synovitis, tenosynovitis and osteitis) and increased-CRP (≥ 5mg/L) were determined with logistic regression. Additionally, the effect of MS-duration (≥30, ≥60, ≥120 minutes) was studied.
Results
195 (34%) CSA-patients experienced MS. These patients more often had subclinical synovitis (34% versus 21%, OR 1.95 (95%CI 1.32-2.87)), subclinical tenosynovitis (36% versus 26%, OR 1.59 (1.10-2.31)) and increased-CRP (31% versus 19%, OR 1.93 (1.30-2.88)) than patients without MS. In multivariable analyses, subclinical synovitis (OR 1.77 (1.16-2.69)) and CRP (OR 1.78 (1.17-2.69)) remained independently associated with MS. In CSA-patients who later developed RA, and thus in retrospect were ‘pre-RA’ at time of CSA, MS was more strongly associated with subclinical synovitis (OR 2.56 (1.04-6.52)) and CRP (OR 3.86 (1.45-10.24)). Furthermore, associations increased with longer MS-durations.
Conclusion
Inflammation indeed associates with MS, already in the CSA-phase that preceded clinical arthritis. These results increase understanding of MS when assessing arthralgia in clinical practice.
Keywords: Morning Stiffness, Clinically Suspected Arthralgia, Inflammation, MRI, Rheumatoid Arthritis
Introduction
Morning stiffness (MS) is a hallmark of rheumatoid arthritis (RA). Until the past decade it was included in the classification criteria for RA and it still is a pivotal symptom for diagnosis.(1,2) In patients without clinical arthritis, but with arthralgia, MS is a cardinal symptom to clinically recognize arthralgia patients that are at increased risk to develop RA (i.e. clinically suspect arthralgia, CSA). This is also reflected by its inclusion in the EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis.(3)
MS is generally considered as a sign of inflammation. Indeed, in established-RA and early arthritis, MS is shown to associate with local joint-inflammation, as well as disease activity and markers of systemic inflammation, such as acute phase reactants (e.g. C-reactive protein (CRP)) and cytokines (e.g. IFNγ, TNFα, and IL-6).(2,4–6) Although it is presumed that MS in arthralgia is also related to inflammation, this has never been studied.
We therefore hypothesized that in patients with CSA, MS is associated with local subclinical inflammation and systemic inflammation. To investigate this, we studied the association of MS with MRI-detected subclinical synovitis, tenosynovitis, osteitis and CRP using data of more than 500 CSA-patients.
Methods
Patient population
We studied 575 consecutive CSA-patients that were included in the Leiden CSA-cohort between April-2012 and February-2019 (supplementary figure 1 and supplementary table 1). This is a population-based inception cohort of patients with recent-onset (<1 year) small-joint arthralgia, that is suspected for progression to RA, according to the treating rheumatologist, based on clinical expertise and pattern recognition. Per definition, patients were not included in the cohort when arthritis was detected upon physical examination or when a different explanation for the joint pain (e.g. osteoarthritis, fibromyalgia) was more likely than imminent RA, as both conditions preclude the presence of CSA. At inclusion, questionnaires were filled, laboratory investigations were done and an MRI-scan was made. CSA-patients were followed during two years for the development of clinical arthritis (determined at physical joint examination by the treating rheumatologist). During follow-up, treatment with disease-modifying anti-rheumatic drugs (DMARDs, including steroids) was not allowed. Only after a patient developed arthritis and therefore had left the CSA-cohort, DMARD-therapy could be initiated. The study-population is further described in the supplementary methods and elsewhere.(7)
Written informed consent was obtained from all patients. The study was approved by the local Medical Ethics committee Leiden.
Morning stiffness
At inclusion the duration of MS was assessed by asking the patient about the presence of MS (“are your joints stiff in the morning: yes/no”), secondly on the duration of MS (“how long does it take until you MS improves?”). Patients could choose the answer on the second question from the following categories: none, 1-29 min, 30-59 min, 60-119 min, 120 min-239min, ≥240 min. The primary outcome in the current study was the dichotomized duration of MS into ≥60minutes or <60 minutes.(3) Patients without MS (zero minutes) fell into the category of patients with <60 minutes of MS.
CRP
Baseline CRP-levels were measured and dichotomized into increased (≥5mg/L) or normal (<5mg/L). This cut-off equals the reference value as used by the Leiden University Medical Centre and is based on an international standard work.(8)
Subclinical joint inflammation
A gadolinium-enhanced MRI of metacarpophalangeal (MCP), wrist and metatarsophalangeal (MTP) joints of the most painful side, or the dominant side in case of symmetrically severe symptoms, was performed between 8.00h and 16.00h. Patients were asked not to use non-steroidal antiinflammatory drugs (NSAIDs) 24h prior to the MRI. The MRI-protocol can be found in the supplementary methods.
MRIs were evaluated for osteitis, synovitis and tenosynovitis, according to the Outcome Measures in Rheumatology Clinical Trials (OMERACT) RAMRIS(9), and for tenosynovitis, as described by Haavardsholm et al.(10) Two independent trained readers scored the MRIs, blinded to clinical data. Average-scores of the two readers were dichotomized into presence or absence of an inflammatory feature: a feature was considered present when scored by both readers and present at the same location in <5% of age-matched healthy volunteers. This cut-off was based on a previous study by Mangnus et al. that studied the prevalence of MRI-detected inflammation in 196 healthy controls.(11) Mangnus et al. developed age-matched and location-specific reference values based on this symptom-free population. The use of this reference was shown to reduce false-positive MRI results compared to using no ‘reference of normality’.(12) Presence of any subclinical inflammation was defined as presence of ≥1 inflammatory feature (osteitis, synovitis or tenosynovitis). Number of locations with subclinical inflammation was assessed as a measure of the severity of subclinical inflammation. This was calculated as the sum of bones, joints or tendons with an inflammatory feature present (corrected for findings in healthy individuals as described above).
RA development
Patients were followed on the development of RA, which was defined as clinical arthritis with a clinical diagnosis of RA and either fulfilling the 1987- or 2010-criteria for RA(1,13) or starting with DMARD-treatment. 1987-criteria were used in addition to 2010-criteria, as autoantibody negative patients have difficulties with fulfilling the 2010-criteria as >10 involved joints are required.(14) Start of DMARD-treatment was used as well to capture patients with a clinical RA-diagnosis in whom fulfillment of classification criteria was prevented by early treatment-initiation.
Statistical analysis
Associations between MS and local subclinical- and systemic inflammation were tested with univariable and multivariable logistic regression, with and without adjustment for age and gender. The explained variance of the multivariable model was assessed by the Nagelkerke R2. The association between MS and the number of locations with inflammatory features was analyzed with logistic regression. Additionally, the effect of MS-duration(≥30, ≥60, ≥120 minutes) was studied. Furthermore, analyses were repeated in the subgroup of patients who progressed to RA during follow-up. The univariable association of MS for development of RA was tested with Cox regression. Patients were censored at time of last visit. Data on the development of RA were all-encompassing, since our outpatient clinic is the only referral center in a healthcare region of approximately 400.000 inhabitants and patients (especially those participating to clinical studies) have very easy access to our outpatient clinic. In addition, we questioned if there was a mediating role of MS on the association of CRP or MRI-detected subclinical inflammation and RA development. This analysis is described in detail in a supplementary file.
IBM SPSS version 25 was used. P values <0.05 were considered significant.
Results
Associations of inflammation with MS
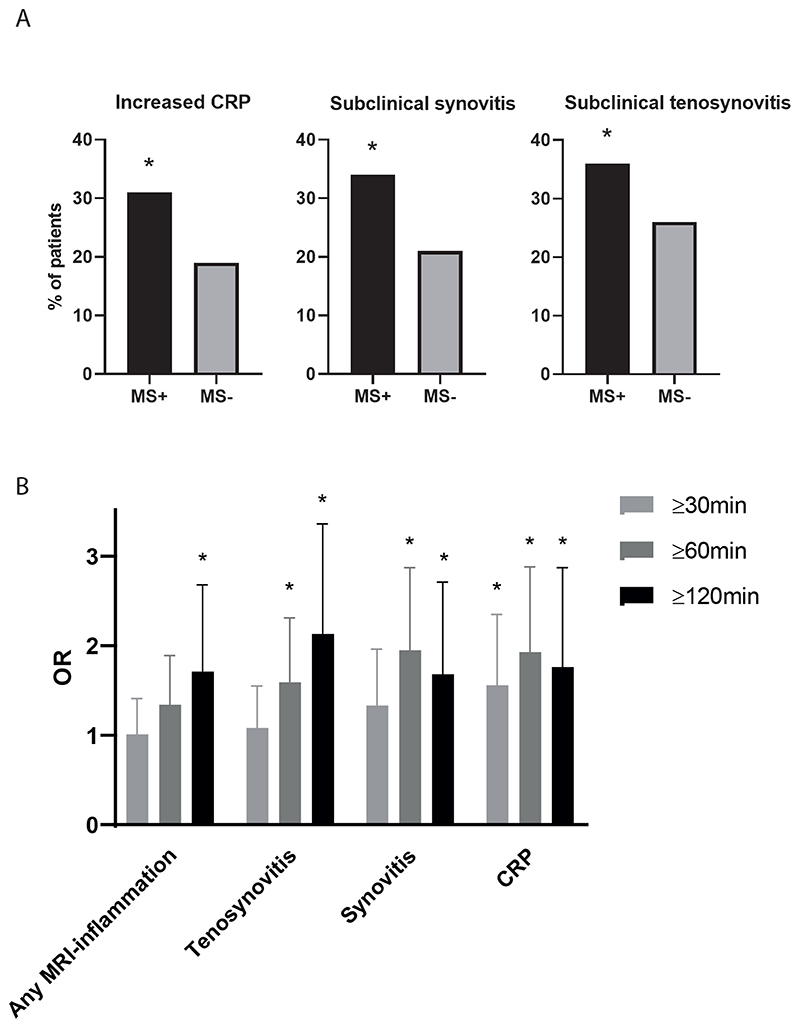
The mean age of the study-population was 44 years (SD 13), 439 patients (76%) were female, median tender joint count (TJC68) was 5 (IQR 2-10), and 79 (14%) patients were ACPA-positive.(Supplemental table 2) MS was present in 195 CSA-patients (34%). These patients more often had subclinical synovitis, subclinical tenosynovitis and increased CRP compared to patients without MS.(Figure 1A, Table1A)
Figure 1. Inflammatory measures in CSA-patients with and without MS (A) and associations for different MS-cut-offs (B).
CRP: C-reactive protein (increased if ≥5 mg/L), MS+: presence of MS with a duration ≥60 minutes, +: presence of an MRI-feature
Any subclinical inflammation: presence of ≥1 inflammatory feature (osteitis, synovitis and tenosynovitis)
* marks statistically significant associations (CI not including 1).
A. Increased CRP levels were more often found in CSA-patients with MS (31% versus 19%). Likewise, subclinical synovitis was more often present in CSA-patients with MS compared to patients without MS (34% versus 21%). Also, subclinical tenosynovitis was more frequently present in patients with MS (36% versus 26%).
B. Evaluating the univariable association for different MS-durations (≥30, ≥60, ≥120minutes), showed a “dose-response” relation, with a step-wise increase for the OR for any subclinical MRI inflammation and subclinical tenosynovitis. For subclinical synovitis and CRP an increase for ≥60 minutes compared to ≥30 minutes was observed, but no further increase for ≥120minutes. Vertical error bars represent the 95% confidence interval (CI).
Table 1. MS and inflammatory measures within the CSA-cohort (A) and patients who progressed to RA (B).
| (A) Complete cohort (n=575) OR (95%CI) | (B) RA-subgroup (n=76) OR (95%CI) | |
|---|---|---|
| Univariable: | ||
| Increased CRP | 1.93 (1.30-2.88) | 3.86 (1.45-10.24) |
| Any subclinical inflammation + | 1.34 (0.95-1.89) | 5.00 (0.99-24.41) |
| Subclinical synovitis + | 1.95 (1.32-2.87) | 2.56 (1.04-6.52) |
| Subclinical tenosynovitis + | 1.59 (1.10-2.31) | 3.09 (0.99-9.60) |
| Subclinical osteitis + | 1.14 (0.76-1.72) | 1.50 (0.59-3.84) |
| Multivariable #: | ||
| Increased CRP | 1.78 (1.17-2.69) | 3.24 (1.13-9.25) |
| Subclinical synovitis + | 1.77 (1.16-2.69) | 2.07 (0.73-5.87) |
| Subclinical tenosynovitis + | 1.13 (0.75-1.72) | 1.47 (0.40-5.49) |
| Multivariable *: | ||
| Increased CRP | 1.79 (1.18-2.72) | 10.57 (2.27-49.17) |
| Subclinical synovitis + | 1.69 (1.10-2.58) | 1.63 (0.52-5.09) |
| Subclinical tenosynovitis + | 1.23 (0.80-1.91) | 1.76 (0.42-7.35) |
Increased CRP: C-reactive protein (increased if ≥5 mg/L), +: presence of an MRI-feature, ACPA: anti-citrullinated peptide antibody (anti-CCP2, positive if ≥7U/mL), 95% CI: 95% confidence interval
Independent variables: increased CRP, subclinical synovitis + and subclinical tenosynovitis +.
Independent variables: increased CRP, subclinical synovitis + and subclinical tenosynovitis +, age and gender.
Explained variance (Nagelkerke R2) of the multivariable model (#) in the complete cohort was 5% and increased to 18% in the RA-subgroup.
Multivariable analysis including these three inflammatory features revealed that subclinical synovitis (OR 1.78 (95%-CI 1.17-2.69)) and increased CRP (1.77 (1.16-2.69)) were independently associated with MS. The explained variance of the multivariable model was 5%.(Figure 1A, Table 1A) Results remained similar after also adjusting for age and gender.(Table 1)
Then the number of locations with inflammatory features was studied as marker of the severity of subclinical inflammation. This showed that an increase in severity was associated with an higher odds of having MS (OR 1.06 (1.00-1.20) per increase in location with subclinical inflammation).
Assessment of MS-duration
Evaluating the association of different MS-duration(≥30, ≥60, ≥120 minutes), a ’dose-response‘ relation was found, as analyses showed a step-wise increase in effect sizes for subclinical tenosynovitis and any subclinical MRI inflammation in relation to MS. The effect sizes of subclinical synovitis and CRP, increased for MS ≥60 minutes compared to ≥30 minutes, but did not further increase further for MS ≥120 minutes. In line with the ‘dose-response’ relation, associations with MRI inflammation were not significant for the 30-minutes outcome.(Figure 1B) The finding that only tenosynovitis was statistically significant in the multivariable analysis for 120-minutes, is not completely consistent with the ‘dose-response’ trend, but is maybe due to lower statistical power for this less frequent outcome.(supplemental table 3)
MS and the development of RA
During a median follow-up of 773 days, 76 participants progressed to RA during follow-up (;31 patients fulfilled both the 1987- and 2010-RA criteria, 25 patients fulfilled the 2010-criteria, 8 patients fulfilled the 1987-criteria and 12 patients were prescribed DMARD-therapy while not yet fulfilling the 1987- or 2010-criteria). CSA-patients with MS (duration ≥60 minutes) progressed more often to RA (HR 1.56 (0.99-2.45)). Noteworthy, MS did not predict the onset of RA independently of CRP or MRI-detected subclinical inflammation (i.e. after adjusting for these variables in the Cox model). This is consistent with the associations between MS and inflammation. A mediating role of MS in the path of inflammation and RA development was not found.(supplementary file and table 4)
Analyses between MS and the inflammatory measures were repeated in patients who developed RA and thus, in retrospect, were truly ‘pre-RA’ when presenting with arthralgia.(supplemental table 5) We hypothesized that associations in this subgroup would be stronger. Indeed, somewhat higher OR were observed, although statistical significance was lost in some associations due to decreased power. The explained variance of the multivariable model in this subgroup increased to 18%, which was 5% in all CSA-patients.(Table 1B)
Discussion
Inquiring on MS is standard practice in the clinical appraisal of arthralgia patients. In patients with clinical arthritis, MS is a known hallmark of RA which associates with inflammation, both local and systemic.(2,4–6) In the differential diagnosis of patients with arthralgia, MS is a key factor for considering patients as having CSA or inflammatory-type arthralgia.(3) However, so far it was unknown whether MS in this phase also reflected inflammation. This prompted us to perform the current study. We observed that MS indeed associated with both subclinical joint inflammation detected on MRI and acute phase reactants. In addition, patients with more subclinical inflammation more often had MS. With respect to subclinical joint inflammation, the association was strongest for subclinical synovitis. These results suggest that inflammation indeed contributes to MS already in the phase that precedes clinical arthritis.
Our finding that MS associates with local subclinical inflammation in CSA-patients is in line with previous ultrasound- and MRI-studies in early arthritis and RA-patients. Previous studies in RA reported that MS was independently associated with both synovitis and tenosynovitis.(4,5) In the phase however, a setting with less inflammation than in RA, only subclinical synovitis was independently associated with MS. The involvement of synovial tissue in MS is in line with a recent histological study in 176 RA-patients, showing that MS may be related to impaired fibrinolysis of neutrophil-enmeshed fibrin deposits along the synovial membrane.(15)
This study had some limitations. Although we focused on the duration of MS, which is the most frequently used measure to define MS, a uniform definition of MS does not exist.(16) Reassuringly, the observed “dose-response” relationship for duration of MS and inflammation supports the robustness of this outcome. Interestingly, there appears to be a ceiling to the “dose-response” effect for synovitis and CRP, whilst this was not observed for tenosynovitis. Notwithstanding, for the associations found, the explained variance was relatively small. This may indicate that the inflammatory measures studied here were incomplete proxies for inflammation. Especially CRP may have been an insufficient reflection of underlying systemic inflammation. It is know that many cytokines with distinct circadian rhythms (e.g. IFNγ, TNFα, and IL-6) are increased in RA, yet these were not measured in the current study.(2) Future research could confirm the relationship between MS and systemic inflammation in CSA by measuring pro-inflammatory cytokines, ideally in 24-hour levels. Alternatively, the small explained variance may suggest that factors other than inflammation are important.
Finally, analyses were conducted within a selection of arthralgia patients that were identified as having an increased risk of RA, namely CSA, and in whom MS may have contributed to this identification. In this selected group we observed an association of MS with RA-development. In clinical practice, MS is also used to differentiate CSA from other arthralgia patients. In our study patients were selected based on clinical symptoms reflecting the ‘inflammatory nature’ of the arthralgia. Thereby some selection on the presence of subclinical inflammation may have occurred, resulting in a higher prevalence of subclinical inflammation than in a more unselected arthralgiapopulation. Consequently, there may be a reduction in variation in MS and subclinical inflammation, possibly resulting in lower effect sizes, compared to a more unselected arthralgia-population. The association of MS with RA development may therefore be stronger in a more unselected arthralgia-population. This is also a subject for further research.
A strength of the current study is its relatively large sample-size and the use of MRI to sensitively detect subclinical inflammation. The used measures of local inflammation (that is subclinical MRI-inflammation) and systemic inflammation (i.e. CRP) are known to remain stable during the day, minimizing interference of the timing of these investigations with their relationship with MS.(17,18)
In conclusion, MS precedes the development of rheumatoid arthritis in patients with CSA, and is associated with subclinical synovitis and increased CRP-levels. This confirms the clinical assumption that MS already reflects inflammation in the phase that precedes clinical arthritis. These results increase understanding of MS when used in the clinically assessing arthralgia.
Supplementary Material
Key messages.
In arthralgia-patients, morning stiffness is a cardinal symptom to recognize patients at-risk for RA-development.
Until now, it was unknown if morning stiffness in arthralgia-patients also associates with inflammation.
This study showed that, in the arthralgia-phase preceding arthritis development, morning stiffness already reflects systemic and subclinical joint inflammation.
Footnotes
Competing interests: The authors declare that they have no competing interests
Authors’ contributors:
All authors were involved in drafting the article or revising it critically.
Study conception and design: Krijbolder, Wouters, van Mulligen, van der Helm-van Mil
Acquisition of data: Krijbolder, Wouters
Analysis and interpretation of data: Krijbolder, van Mulligen, van der Helm-van Mil
Provenance and peer review:
Not commissioned; externally peer reviewed.
Ethics approval and consent to participate:
This study was carried out in compliance with the Helsinki declaration and all participating patients provided written informed consent. The study was approved by the medical ethical committee of the Leiden University Medical Centre (LUMC) (B19.008)
Acknowledgements
Not applicable
Funding
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Starting grant, agreement No 714312), and the Dutch Arthritis Society.
Availability of data and materials
The datasets analyzed during the current study available from the corresponding author on reasonable request.
References
- 1.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 2.Sierakowski S, Cutolo M. Morning symptoms in rheumatoid arthritis: a defining characteristic and marker of active disease. Scandinavian journal of rheumatology supplement. 2011;125:1–5. doi: 10.3109/03009742.2011.566433. [DOI] [PubMed] [Google Scholar]
- 3.van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJ, Brouwer E, Codreanu C, Combe B, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Annals of the rheumatic diseases. 2017;76(3):491–6. doi: 10.1136/annrheumdis-2016-209846. [DOI] [PubMed] [Google Scholar]
- 4.Bellis E, Scirè CA, Carrara G, Adinolfi A, Batticciotto A, Bortoluzzi A, et al. Ultrasound-detected tenosynovitis independently associates with patient-reported flare in patients with rheumatoid arthritis in clinical remission: results from the observational study STARTER of the Italian Society for Rheumatology. Rheumatology (Oxford, England) 2016;55(10):1826–36. doi: 10.1093/rheumatology/kew258. [DOI] [PubMed] [Google Scholar]
- 5.Boer AC, Boeters DM, Niemantsverdriet E, van der Helm-van Mil A. Contribution of tenosynovitis of small joints to the symptom morning stiffness in patients presenting with undifferentiated and rheumatoid arthritis. Scandinavian journal of rheumatology. 2020:1–5. doi: 10.1080/03009742.2019.1696404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boers M, Buttgereit F, Saag K, Alten R, Grahn A, Storey D, et al. What Is the Relationship Between Morning Symptoms and Measures of Disease Activity in Patients With Rheumatoid Arthritis? Arthritis care & research. 2015;67(9):1202–9. doi: 10.1002/acr.22592. [DOI] [PubMed] [Google Scholar]
- 7.van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TW, van der Helm-van Mil AH. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Annals of the rheumatic diseases. 2016;75(10):1824–30. doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- 8.Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed. Elsevier Saunders; St. Louis, Missouri, USA: 2012. p. 2142. [Google Scholar]
- 9.Ostergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B, et al. An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Annals of the rheumatic diseases. 2005;64(Suppl 1):i3–7. doi: 10.1136/ard.2004.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haavardsholm EA, Ostergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Annals of the rheumatic diseases. 2007;66(9):1216–20. doi: 10.1136/ard.2006.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AH. Magnetic Resonance Imaging-Detected Features of Inflammation and Erosions in Symptom-Free Persons From the General Population. Arthritis & rheumatology (Hoboken, NJ) 2016;68(11):2593–602. doi: 10.1002/art.39749. [DOI] [PubMed] [Google Scholar]
- 12.Boer AC, Burgers LE, Mangnus L, Ten Brinck RM, Nieuwenhuis WP, van Steenbergen HW, et al. Using a reference when defining an abnormal MRI reduces false-positive MRI results-a longitudinal study in two cohorts at risk for rheumatoid arthritis. Rheumatology (Oxford, England) 2017;56(10):1700–6. doi: 10.1093/rheumatology/kex235. [DOI] [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the rheumatic diseases. 2010;69(9):1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 14.Boeters DM, Gaujoux-Viala C, Constantin A, van der Helm-van Mil AHM. The 2010 ACR/EULAR criteria are not sufficiently accurate in the early identification of autoantibody-negative rheumatoid arthritis: Results from the Leiden-EAC and ESPOIR cohorts. Seminars in arthritis and rheumatism. 2017;47(2):170–4. doi: 10.1016/j.semarthrit.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Orange DE, Blachere NE, DiCarlo EF, Mirza S, Pannellini T, Jiang CS, et al. Rheumatoid Arthritis Morning Stiffness Is Associated With Synovial Fibrin and Neutrophils. Arthritis & rheumatology (Hoboken, NJ) 2020;72(4):557–64. doi: 10.1002/art.41141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halls S, Sinnathurai P, Hewlett S, Mackie SL, March L, Bartlett SJ, et al. Stiffness Is the Cardinal Symptom of Inflammatory Musculoskeletal Diseases, Yet Still Variably Measured: Report from the OMERACT 2016 Stiffness Special Interest Group. The Journal of rheumatology. 2017;44(12):1904–10. doi: 10.3899/jrheum.161073. [DOI] [PubMed] [Google Scholar]
- 17.Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Frontiers in immunology. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hug C, Huber H, Terrier F, Häuselmann HJ, Aue W, Vock P, et al. Detection of flexor tenosynovitis by magnetic resonance imaging: its relationship to diurnal variation of symptoms. The Journal of rheumatology. 1991;18(7):1055–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study available from the corresponding author on reasonable request.