Abstract
WNT signalling comprises a diverse spectrum of receptor-mediated pathways activated by a large family of WNT ligands and influencing fundamental biological processes. WNT signalling includes the β-catenin (canonical) pathway and non-canonical pathways, namely the planar cell polarity and the calcium-dependent pathways. Advances over the past decade have linked non-canonical WNT signalling with key mechanisms of atherosclerosis, including oxidative stress, endothelial dysfunction, macrophage activation and vascular smooth muscle cell phenotype regulation. In addition, non-canonical WNT signalling is involved in crucial aspects of myocardial biology, from fibrosis to hypertrophy and oxidative stress. Importantly, non-canonical WNT signalling activation has complex effects in adipose tissue in the context of obesity, thereby potentially linking metabolic and vascular diseases. Tissue-specific targeting of non-canonical WNT signalling might be associated with substantial risks of off-target tumorigenesis, challenging its therapeutic potential. However, novel technologies such as monoclonal antibodies, recombinant decoy receptors, and tissue-specific gene silencing with small interfering RNAs and gene editing with CRISPR–Cas9 might enable a more efficient therapeutic targeting of WNT signalling in the cardiovascular system. This review summarises the components of non-canonical Wnt signalling, their links with principal mechanisms of atherosclerosis, heart failure and arrhythmias, as well as the rationale for targeting individual Wnt components to combat cardiovascular disease.
Introduction
Cardiovascular diseases, including atherosclerosis and myocardial disease, remain the leading cause of mortality worldwide1. Atherosclerosis is an inflammatory disease2–4 influenced by genetic and demographic risk factors5–7 and associated with complex phenotypic changes in endothelial cells, vascular smooth muscle cells (VSMCs) and macrophages8,9. Myocardial disease is often associated with the presence of coronary atherosclerosis and involves processes such as myocardial oxidative stress10,11, fibrosis and remodelling12, which can lead to diseases such as heart failure and arrhythmias11,13. Despite significant advances over the past decade, there is an unmet need to describe cardiovascular disease pathogenesis more accurately.
The WNT ligand family includes several secreted glycoproteins (19 in mammals) that activate an evolutionarily conserved spectrum of signalling pathways14,15. WNT signalling is implicated in embryogenesis and development via the regulation of cell motility and cell polarization. WNT signalling also regulates cell survival, growth and motility and, therefore, it has been pathophysiologically linked with tumorigenesis14,16 and cellular metabolism17. WNT signalling can be classified into two main modes of signalling: the canonical pathway, which was described first and involves a reduction of β-catenin degradation followed by the induction of the expression of β-catenin target genes, and the non-canonical pathway, which involves β-catenin-independent mechanisms, such as Ca2+ signalling and activation of small GTPases18. WNT ligands have varying degrees of selectivity towards the two pathways and can activate either of the two pathways depending on spatiotemporal parameters and receptor availability19. Certain WNT ligands, such as WNT5A and WNT11, seem to predominantly activate non-canonical WNT signalling15. Non-canonical WNT ligands have been implicated in multiple biological processes such as inflammation, cell motility and metabolism14. WNT5A and WNT11 have even been proposed as markers of respiratory distress syndrome severity in patients with coronavirus disease 2019 (COVID-19)22, which might be related to the capacity of these ligands to regulate cell migration, survival and apoptosis in lung injury. Additionally, non-canonical WNT signalling has emerged as a potential regulator of vascular disease pathogenesis via mechanistic links with inflammation, cell motility and oxidative stress23–25. Moreover, activation of non-canonical WNT signalling might be increased in obesity via increased secretion of non-canonical WNT ligands, such as WNT5A, by the adipose tissue15.
In this Review, we explore the complex roles of non-canonical WNT signalling in cardiovascular disease. First, we provide an overview of WNT signalling, followed by a comprehensive description of the role of non-canonical WNT signalling in cardiovascular disease pathogenesis. Finally, we explore the clinical potential of targeting non-canonical WNT signalling to treat atherosclerosis.
WNT pathways and molecular targets
The prototype WNT signalling, currently referred to as canonical WNT signalling, was first described in Drosophila, where it was shown to be involved in wing formation by signalling mediated via the glycoprotein product of the Wg gene26. This gene was later found to be homologous to the mammalian gene Int1; therefore, the nomenclature for this family of Wg/Int1 genes fused and became Wnt1, and more WNT ligands have been described since14,27. In mammals, the WNT family of ligands consists of 19 glycoproteins (Table 1), which undergo a variety of post-translational modifications including glycosylation and palmitoleic acid modifications15,27. As a result of these modifications, WNT ligands have moderate water solubility, which facilitates rapid protein-protein interactions and the propagation of local, paracrine signalling27.
Table 1. Overview of WNT ligands, receptors and associated pathways.
| Protein | Involvement in signalling pathway | |
|---|---|---|
| Canonical | Non-canonical | |
| WNT ligands | ||
| WNT1 | ++ | + |
| WNT2 | ++ | + |
| WNT2B | + | + |
| WNT3 | ++ | + |
| WNT3A | ++ | + |
| WNT4 | + | + |
| WNT5A | + | ++ |
| WNT5B | + | ++ |
| WNT6 | + | + |
| WNT7A | + | + |
| WNT7B | + | + |
| WNT8A | ++ | + |
| WNT8B | + | + |
| WNT9A | + | + |
| WNT9B | + | + |
| WNT10A | ++ | + |
| WNT10B | ++ | + |
| WNT11 | + | ++ |
| WNT16 | + | + |
| Receptors | ||
| FZD1 | ++ | + |
| FZD2 | + | ++ |
| FZD3 | + | + |
| FZD4 | + | + |
| FZD5 | + | + |
| FZD6 | + | ++ |
| FZD7 | + | + |
| FZD8 | + | + |
| FZD9 | + | ++ |
| FZD10 | + | ++ |
| LRP1, LRP5, LRP6 |
++ | + |
| ROR1 | + | ++ |
| ROR2 | + | ++ |
| RYK | + | ++ |
All ligands and WNT members can activate both pathways; assumed predominance of a given pathway is denoted as ++. ; FZD, Frizzled; LRP, LDL receptor-related protein; ROR, tyrosine-protein kinase transmembrane receptor ROR; RYK, receptor-like tyrosine kinase receptor
WNT ligands are ubiquitously secreted molecules, without exclusive tissue sources. Pathophysiologically important sources may include the adipose tissue15 and immune cells25. WNT ligands are also secreted by cardiovascular cells, including cardiomyocytes and the endothelium, although the baseline expression of WNT ligands in vascular cells may is lower than in adipose tissue15,28. Nonetheless, the relative contribution of each tissue to the systemic WNT ligand pool has been poorly described. For example, WNT ligand release is a complex process involving post-translational modifications and vesicle secretion and the upstream stimuli are unclear29. WNT ligands are often constitutively expressed, and certain processes, such as inflammation and obesity, can upregulate their expression via mediators such as adiponectin30.
WNT signalling is initiated after the binding of extracellular, secreted WNT ligands to various membrane receptors, mainly the Frizzled (FZD) family of G protein-coupled seven-transmembrane domain receptors14. The tyrosine-protein kinase transmembrane receptors ROR1 and ROR2 and the tyrosine-protein kinase RYK have also been shown to interact with WNT ligands, but these interactions are less well characterized than WNT-FZD interactions31. The WNT ligand-receptor interaction triggers an incompletely characterized chain of molecular events involving downstream protein interactions that lead to transcriptional regulation14,27. To date, two main pathways downstream of the WNT-receptor interaction have been described: the canonical WNT pathway and the non-canonical WNT pathway31. The canonical pathway prevents the degradation of β-catenin, thereby allowing for β-catenin-dependent transcriptional regulation to occur, which affects cell proliferation and survival32. The non-canonical pathway involves β-catenin-independent downstream signalling and can be broadly divided into the planar cell polarity (PCP) pathway and the Ca2+-dependent pathway31. WNT signalling is negatively regulated by the interaction of WNT ligands with secreted frizzled-related proteins (SFRP1-SFRP5 in humans), which are structurally similar to FZD receptors and thus have affinity to WNT ligands and act as decoy receptors31.
Of note, WNT ligands and receptors comprise an extremely complex canvas of interrelated pathways, and classification into canonical and non-canonical pathways and ligands is virtual and schematic. In reality, most WNT ligands might be able to activate both canonical or non-canonical WNT signalling, with primary effects on one of the pathways depending on context, spatiotemporal parameters and receptor availability19. Moreover, a negative interaction between the canonical and non-canonical pathways has been described, suggesting continuous crosstalk between the various pathways and ligands33,34.
Non-canonical WNT signalling
After the description of the canonical, β-catenin-dependent WNT signalling pathway, it became evident that certain WNT ligands exerted a wide range of biological effects in β-catenin-independent ways, namely the PCP and the Ca2+-dependent pathway35. These non-canonical pathways are not fully characterized; however, certain relevant downstream mediators and phenotypic consequences have been identified35 (FIG. 1).
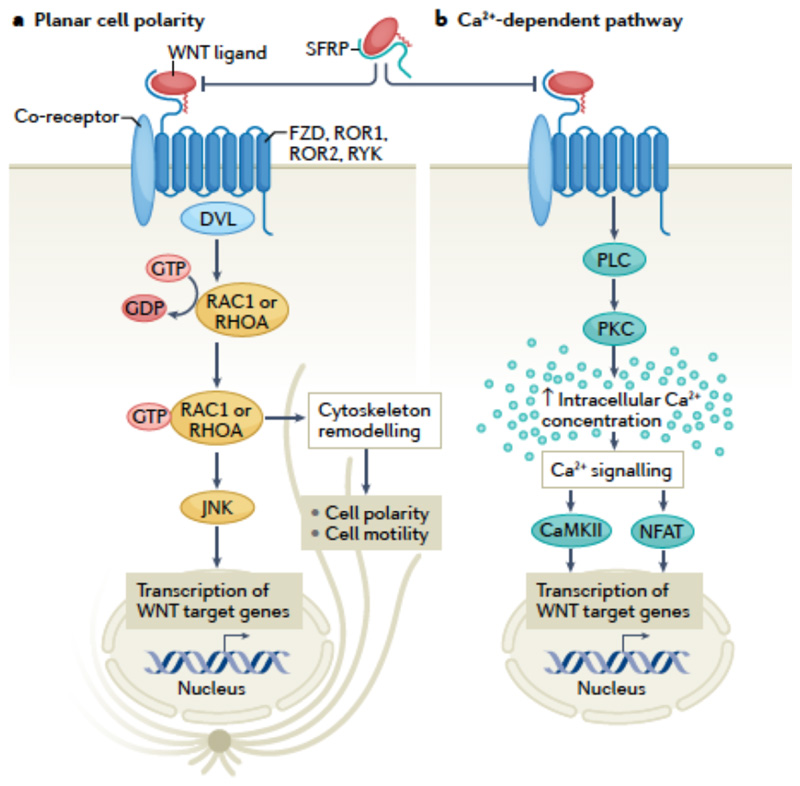
Fig 1. Overview of non-canonical WNT signalling pathways.
Non-canonical WNT signalling pathways involve the planar cell polarity pathway and the Ca2+-dependent pathway. Both pathways are initiated by binding of a WNT ligand to WNT receptors, such as Frizzled (FZD) receptors, tyrosine-protein kinase RYK and the tyrosine-protein kinase transmembrane receptors ROR1 and ROR2, which belong to the family of G protein-coupled receptors (also known as seven-transmembrane domain receptors), with the potential contribution of various co-receptors. Binding of WNT ligands to secreted frizzled-related proteins (SFRPs) blocks the WNT-receptor interaction. The downstream events involved in the planar cell polarity pathway are not well defined, but include Dishevelled (DVL) and lead to GTP-dependent activation of small GTPases, such as RHOA and RAC1, which in turn activate JUN N-terminal kinase (JNK), and ultimately regulate cell polarity and motility and gene transcription. The Ca2+-dependent pathway involves activation of phospholipase (PLC) and protein kinase C (PKC), which leads to increased intracellular Ca2+ concentration, triggering the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and the nuclear factor of activated T cells (NFAT) pathway, leading to transcriptional regulation.
The PCP pathway involves binding of a WNT ligand to FZD, ROR or RYK receptors35. This interaction leads to Dishevelled (DVL)-mediated GTP-dependent activation of small GTPases, such as RHOA and RAC1, although the exact intermediate events are not clear31. Activated RHOA and RAC1, in turn, stimulate the activation of JUN N-terminal kinase (JNK) via phosphorylation36. Given the involvement of RHOA and RAC1 in regulating cytoskeletal dynamics, changes in cell polarization and motility are the main phenotypes associated with activation of the PCP pathway20,35. In addition, WNT-mediated JNK activation has been linked with several other important pathophysiological processes, such as inflammation and insulin resistance36,37. RAC1 can also influence oxidative stress via activation of NADPH oxidases15, indicating that oxidative stress can be regulated by the PCP pathway.
WNT ligands can also trigger β-catenin-independent effects via regulation of intracellular Ca2+ concentration35. Indeed, WNT signalling acts in synergy with phospholipase C to increase intracellular Ca2+ levels38, prompting the activation of the Ca2+-sensitive kinases protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II (CaMKII)35. Ca2+-mediated WNT signalling activates the Ca2+-sensitive nuclear factor of activated T cells (NFAT) pathway35, which is important in immune response regulation39. Ca2+-dependent WNT signalling has mainly been explored in the context of embryonic development, and its clinical relevance in adults is less clear. However, considering the wide-ranging effects of its downstream targets PKC, CaMKII and NFAT, this mode of WNT signalling might contribute to several important biological processes20.
Importantly, non-canonical WNT signalling is negatively regulated by canonical WNT signalling. The extracellular domain of LDL receptor-related protein 6 (LRP6), a membrane co-receptor of the canonical WNT signalling pathway, has been shown to physically interact with WNT5A, acting as a decoy receptor for non-canonical Wnt signals40. In vivo, Lrp6−/−mice have congenital defects, which are rescued by Wnt5a deletion40, suggesting that this LRP6-related phenotype is mitigated by non-canonical WNT5A signalling. Similar defects were described in Xenopus embryos with knockdown of lrp5/lrp6 which were reversed by knockdown of the non-canonical WNT ligands wnt5a and wnt1140. WNT5A can activate or inhibit canonical WNT signalling in a spatiotemporal, tissue-specific manner41, which could be a result of spatially different receptor and related regulatory co-receptor profiles, similar to LRP5 and LRP6. Non-canonical WNT effects could therefore occur indirectly via changes in canonical WNT dynamics.
Non-canonical WNT signalling in vascular diseases
Non-canonical WNT signalling has been implicated in key contributing factors of atherosclerosis (Box 1), including oxidative stress, endothelial dysfunction, VSMC phenotypic switch and migration, and inflammation, as implied by observational association studies31 and mechanistic evidence15,25. Of note, WNT5A is the most well-studied non-canonical WNT ligand in cardiovascular disease to date, followed by other ligands such as WNT1125,31. Therefore, most of the relevant studies used WNT5A as a representative ligand that primarily activates non-canonical WNT signalling in the cardiovascular system. Nevertheless, the downstream pathways described in these studies be activated by any WNT ligand in the appropriate circumstances, and WNT5A itself can also activate canonical WNT signalling in vascular cells49,50.
Box 1. Fundamental mechanisms of atherosclerosis.
Atherosclerosis is a complex disease in terms of its underlying mechanisms. Disease initiation has long been believed to occur at endothelial sites that are subjected to dysregulated shear stress, such as the coronary arteries and other sites prone to developing turbulent flow (such as vascular bifurcations)8. Pulsatile, turbulent shear stress results in endothelial dysfunction and disruption of endothelial barrier integrity42, resulting in the deposition of lipids such LDL in the subendothelial space8,43. LDL can then be oxidized as a result of by various pro-oxidant stimuli, such as local inflammation (secondary to local tissue damage) 43. The oxidized LDL is internalized by macrophages, leading to macrophage activation and transformation into foam cells and further promoting inflammation and oxidative stress43. This vicious cycle establishes and promotes the formation and progression of atherosclerotic plaques.
Several pathogenic mechanisms are involved in promoting the atherogenic cycle, inducing further LDL oxidation, endothelial cell activation, macrophage recruitment and activation, and vascular smooth muscle cell phenotypic switch43,44, ultimately influencing atherosclerotic plaque characteristics and overall plaque burden45. Oxidative stress is one such mechanism, characterized by overproduction of reactive oxygen species by enzymes such as NADPH oxidases and uncoupled endothelial nitric oxide synthase6. Overproduction of reactive oxygen species in turn leads to reduced nitric oxide bioavailability and endothelial dysfunction6. Local inflammation and cellular insulin resistance at the level of the vascular wall are additional mechanisms contributing to atherosclerotic plaque burden via modulation of vascular smooth muscle cell phenotype and promotion of lipid oxidation and plaque necrotic core remodelling5,45–47.
Observational data
One of the first conclusive proofs for a causal link between WNT signalling and atherosclerosis came from a study published in 2007 on the genetic basis of the risk of early coronary artery disease (CAD) in a family with a high prevalence of CAD48. Genome-wide linkage analysis followed by direct DNA sequencing of the annotated genes and in vitro functional assessments demonstrated a link between the missense mutation R611C in LRP6 and the early CAD phenotype48, thereby causally linking WNT signalling with vascular disease. Further research has explored the mechanisms underlying this link, particularly regarding non-canonical WNT signalling.
Observational data indicate that high levels of circulating WNT5A are associated with the presence of atherosclerosis and related diseases such as obesity and diabetes mellitus 15,25,51. Work from our group has demonstrated that in humans, CAD is associated with elevated WNT5A bioavailability in the plasma independently of demographic risk factors15. In addition, in patients who underwent two CT scans for coronary Ca2+ score quantification, plasma WNT5A levels were positively associated with calcified plaque burden and new-onset calcification, independent of traditional risk factors15. Furthermore, WNT5A is locally expressed in mouse and human atherosclerotic plaques23. Levels of the non-canonical ligands WNT5A, WNT5b and WNT11 are also upregulated in human aortic calcified compared to non-calcified valves52. Given the link of these ligands with non-canonical activation of osteogenesis-related pathways in a variety of in vitro models53, non-canonical WNT might be hypothesized to contribute to valve or vascular wall calcification.
The aforementioned observational findings provide a strong proof-of-concept for a link between non-canonical WNT signalling (mainly WNT5A) and atherosclerosis54, but observational results do not confirm causality. However, consistent with the observational findings, several experimental studies have revealed underlying cellular hubs through which non-canonical WNT signalling causally interacts with key mechanisms of atherosclerosis (FIG. 2).
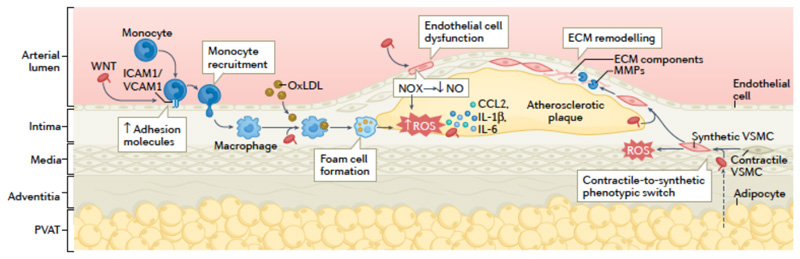
Fig 2. Non-canonical WNT signalling in atherosclerosis.
Non-canonical WNT ligands (WNT), such as WNT5A, can enter the vascular wall from the circulation and are also released by vascular macrophages and the perivascular adipose tissue (PVAT). In endothelial cells, binding of non-canonical WNT ligands to their receptors leads to the upregulation of the expression of intracellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1), which promotes monocyte recruitment to the arterial intima. Non-canonical WNT signalling in endothelial cells also induces the activation of NADPH oxidases (NOX), which leads to increased production of reactive oxygen species (ROS), activation of pro-inflammatory redox signalling, reduced nitric oxide (NO) bioavailability and endothelial dysfunction. In monocytes and macrophages, non-canonical WNT signalling promote pro-inflammatory activation, uptake of oxidized LDL (oxLDL) and foam cell formation, and production of pro-inflammatory cytokines, such as CCL2, IL-1β and IL-6. In vascular smooth muscle cells (VSMCs), non-canonical WNT signalling induces a switch from a contractile to a synthetic phenotype and increases cell migration via ROS signalling, thereby promoting the migration of synthetic VSMCs into the atherosclerotic plaque where they produce extracellular matrix (ECM) components and matrix metalloproteinases (MMPs). These mechanisms interact in complex ways to establish a vicious cycle, directly promoting atherogenesis and plaque instability.
Oxidative stress
WNT5A signalling increases the production of reactive oxygen species (ROS) in human he human granulosa-like tumour cell line KGN, which was speculated to be related to the induction of inflammation, considering that lipopolysaccharide stimulation had the same effect on ROS production in these cells 37. Despite the incomplete mechanistic data, this observation supports the notion that WNT5A might directly increase ROS production. By contrast, WNT5A has been shown to protect against oxidative stress-induced VSMC apoptosis, although this effect was achieved with a supraphysiological concentration of WNT5A that cross-activated the canonical pathway50. Therefore, this finding should be regarded as being mediated by canonical WNT signalling rather than a specific WNT5A-mediated effect.
Work from our group was the first to explore directly the role of WNT5A in the regulation of vascular oxidative stress in the context of atherosclerosis15. After demonstrating the specificity of physiological WNT5A concentrations towards non-canonical signalling, we showed that WNT5A directly increased NADPH oxidase activity in vitro in artery from patients with CAD15. This effect was mediated via increased GTP-dependent activation and membrane translocation of RAC1 (which is both a downstream target of the PCP pathway and a key subunit of the NOX2 and NOX2 isoforms of NADPH oxidases)15. This finding was further validated in vitro in WNT5A-treated primary VSMCs from patients with CAD as well as in aortic segments from mice overexpressing WNT5A15. Importantly, transcriptome analysis of WNT5A-treated human primary VSMCs revealed that WNT5A induces the differential expression of a large number of genes, and the expression profile of several of these genes was restored by administration of pegylated superoxide dismutase, suggesting that WNT5A is involved in redox-dependent transcriptional regulation in human vascular tissue15. Finally, we identified the ubiquitinating enzyme USP17 as a redox-sensitive downstream target of WNT5A that increases the GTP-dependent activation of RAC1 15. This study is the first to establish a causal role of WNT5A in increasing oxidative stress in human atherosclerosis, and identifies USP17 and RAC1 as the key mediators of WNT5A-induced oxidative stress.
In contrast to our study's findings, other work suggests that WNT5A inhibits hydrogen peroxide (H2O2)-induced apoptosis in VSMCs via CCN family member 4 (also known as WISP1), an effect mediated through canonical WNT signalling50. This finding could suggest that WNT5A has an antioxidant function; however, WNT5A was used at a supraphysiological concentration in this study, which might mean that this canonical WNT signalling effect on H2O2-induced apoptosis might not be physiological. Conversely, H2O2 is a more stable type of ROS than superoxide, and has more subtle signalling roles50. As such, WNT5A-mediated signalling might theoretically stimulate the production of both superoxide (which could be detrimental when in excess) and H2O2 (which could provide a more prolonged signalling effect), hinting towards a delicate WNT5A-regulated redox balance, which warrants further investigation.
Given that the activation of endothelial nitric oxide (NO) synthase (eNOS) is partly mediated by CaMKII55 and that both PKC and CaMKII interact with NAPDH oxidases56–58, it is highly plausible that non-canonical WNT Ca2+ signalling also regulates vascular oxidative stress and thereby contributes to atherosclerosis. However, this hypothesis remains to be studied.
Endothelial dysfunction
Although canonical WNT signalling has been linked to endothelial cell survival and angiogenesis59, the role of non-canonical WNT signalling in endothelial cells has only been explored during the past decade. A study in primary endothelial cells from patients with diabetes revealed that WNT5A signalling impairs AKT phosphorylation, eNOS activity, NO bioavailability and ultimately endothelial function in vitro, mediated by non-canonical JNK-mediated signalling60. Consistent with this finding, our group demonstrated that WNT5A-mediated signalling directly impairs NO bioavailability and endothelial function, evidenced by ex vivo endothelium-dependent vasorelaxation of WNT5A-incubated artery samples from patients with CAD15. This reduced NO bioavailability was secondary to NADPH oxidase activation by WNT5A, as explained in previous sections, which led to oxidative depletion of tetrahydrobiopterin15. The absence of tetrahydrobiopterin resulted in eNOS uncoupling and production of superoxide instead of NO15.
These findings provide solid proof for the causal role of WNT5A in propagating a dysfunctional endothelial phenotype in humans. However, the underlying mechanisms for this role have not been fully explored. In addition, as mentioned in the previous section, Ca2+-dependent WNT signalling potentially affects both eNOS coupling and activity via CaMKII and PKC55,61–63 and could, therefore, be hypothesised to contribute to endothelial dysfunction, which warrants further investigation.
VSMC function and phenotype
Cell motility, migration and polarity are the most well-studied phenotypes regulated by non-canonical WNT signalling, which has been demonstrated in a variety of embryonic development models and studies of tumours or cell types such as erythrocytes21,25,64,65. Canonical WNT signalling has been linked to VSMC biology, particularly cell migration50,66,67. However, evidence also suggests roles for non-canonical signalling in VSMCs.
After the demonstration of a link between the LPR6 R611C variant and CAD, further research has shed light into the underlying mechanisms with the use of VSMC in vitro assays and transgenic mouse models68. Indeed, loss of normal LRP6 activity as a result of the R611C variant is associated with increased non-canonical WNT signalling, evidenced by increased RHOA and JNK activity68. The activation of non-canonical WNT signalling resulted in the activation of the serine/threonine-protein kinase NLK followed by phosphorylation and subsequent ubiquitylation and degradation of transcription factor 7-like 2, a transcription factor that is associated with canonical WNT signalling68. These signalling events were associated with a phenotypic switch in VSMCs from a contractile to a synthetic phenotype, arterial media thickening and CAD promotion in mice68. Exogenous treatment with the canonical ligand WNT3A reversed the effects of the LRP6 R611C variant68. This finding is an elegant example of the crosstalk between the canonical and non-canonical pathways, with reciprocal effects on VSMC phenotypes.
Our group has shown that WNT5A induces redox-sensitive migration of human primary VSMCs without affecting their proliferation15. This effect could be partly mediated by RAC1 activation, but transcriptome analysis showed that WNT5A-mediated signalling also affected the expression of multiple genes related to migration in primary human VSMCs15. In addition, WNT5A can inhibit oxidative stress-induced apoptosis of VSMCs via the induction of WISP150. However, this effect was shown to be mediated by β-catenin signalling50 and might not be relevant in vivo, given that WNT5A at physiological concentrations is a selective ligand for non-canonical WNT signalling. Another non-canonical WNT ligand, WNT4, has been shown to stimulate VSMC proliferation, whereas its downregulation attenuates intima media thickening in mice69.
Beyond its effects on VSMC migration, non-canonical WNT signalling has been shown to induce a phenotypic switch in human primary VSMCs, characterized by the loss of the expression of the contractile markers aortic smooth muscle actin (also known as α2-SMA) and transgrelin, and an increased expression of matrix metalloproteinase 9 (MMP9)15. MMP9 has been shown to induce destabilization of atherosclerotic plaques in mouse models of atherosclerosis and in human genetic studies70–73. Taken together, these findings suggest that non-canonical WNT signalling might promote an 'aggressive' phenotype in VSMCs, characterized by an increased propensity for migration, the switch to a less differentiated phenotype and the upregulation of potentially plaque-destabilizing MMPs. WNT5A has been reported to be among the WNT ligands expressed by VSMCs isolated from artery samples from patients undergoing coronary artery bypass graft (CABG) surgery, which might be related to neointima formation74.
Non-canonical WNT signalling can also influence atherosclerotic plaque calcification by inducing changes in VSMC biology. WNT5A was found to stimulate the expression of genes related to chondrogenesis, in both mouse and human VSMCs75. WNT5A-mediated gene expression was inhibited by peroxisome proliferator-activated receptor-γ (PPARγ) signalling via SFRP2, suggesting that WNT5A promotes vascular calcification75. In addition, a correlation has been observed between the WNT5A–ROR2 pathway and the degree of VSMC calcification in vitro76. Canonical WNT signalling has also been shown to regulate vascular calcification in mouse aorta, via bone morphogenetic protein 2 signalling53. Interestingly, co-expression of the non-canonical WNT receptor FZD10 and the canonical co-receptors LRP5 or LRP6 in VSMCs leads to cross-inhibitory signals between the two pathways77. By contrast, LPR5 or LRP6 loss-of-function increased non-canonical WNT signalling and inhibited canonical β-catenin signalling, which was associated with a shift toward osteochondrogenic programming and calcification in VSMCs 77. These findings suggest a competition between the canonical and non-canonical pathways with regard to VSMC calcification, with LRP5 and LRP6 at the core.
Non-canonical WNT signalling can affect intracellular cholesterol accumulation. In an Apoe−/− mouse model of atherosclerosis, in vivo adenovirus-mediated delivery of small interfering RNA targeting Wnt5a to aortic tissues reduced atherosclerotic plaque lipid content without affecting blood lipid leves37. WNT5A has also been suggested to facilitate foam cell formation25. By contrast, another in vitro study indicated that WNT5A reduces intracellular cholesterol in VSMCs treated with oxidized LDL, mediated by stimulating the expression of ATP-binding cassette transporter (ABCA1)78, which is involved in reverse cholesterol transport. Overall, non-canonical WNT signalling seems to be involved in lipid handling in the atherosclerotic plaque cells, but the exact effects and the mechanisms involved are unclear.
Non-canonical WNT signalling might also affect neoangiogenesis. In a rat model of ischaemic myocardial injury, peri-infarct injection of conditioned medium from WNT11-overexpressing mesenchymal stem cells improved cardiac function and reduced infarct size, which was shown to involve non-canonical JNK–PKC signalling79. By contrast, in cultured mouse myeloid cells, independent mutations of Wnt5a and Wnt11 were associated with increased angiogenesis in mouse retinas, mediated by downstream suppression of Flt1 expression80, which encodes an inhibitor of vascular endothelial growth factor (VEGF). These findings suggest potentially complex roles for non-canonical WNT signalling in the regulation of angiogenesis.
Inflammation
Extensive evidence links non-canonical WNT signalling to inflammation and inflammatory conditions such as rheumatoid arthritis25,81, psoriasis81–83 and atherosclerosis25. Indeed, non-canonical WNT signalling influences key mechanisms of vascular inflammation via multiple effects on vascular wall cells, thereby contributing to atherosclerosis25.
WNT5A, but not WNT3A (a canonical WNT ligand), was shown to increase the expression of genes encoding pro-inflammatory factors, including cyclooxygenase 2, IL-6, IL-1α, Toll-like receptor 4, granulocyte colony-stimulating factor, granulocyte−macrophage colony-stimulating factor (GM-CSF), C-C motif ligand 2 (CCL2) and CCL8, in human aortic endothelial cells in vitro, via non-canonical Ca2+–PKC signalling24. These expression changes were associated with increased permeability of the endothelial cell monolayer24. WNT5A also activates pro-inflammatory nuclear factor-κB (NF-κB) signalling in endothelial cells24,37. Interestingly, activation of the Ca2+–NFAT pathway, a downstream target of non-canonical WNT signalling, has been shown to induce a pro-inflammatory phenotype in human coronary artery endothelial cells in vitro, characterized by increased expression of pro-inflammatory molecules 84. However, a direct link between non-canonical WNT signalling and NFAT signalling in endothelial cells has not been documented yet. By contrast, cardiac-specific overexpression of WNT11 in mice attenuated the inflammatory response to myocardial infarction and facilitated recovery after myocardial infarction in vivo85. This finding suggests an anti-inflammatory role for non-canonical WNT signalling in this context.
WNT5A can be secreted by circulating monocytes65, and is involved in innate responses in monocytes and macrophages, evidenced by the upregulation of WNT5A gene expression in pathogen-activated macrophages and during monocyte differentiation into macrophages in response to GM-CSF and IL-4 treatment in vitro86–88. Importantly, pro-inflammatory and pro-oxidant signals such as oxidized LDL, which are present in atherosclerotic plaques, upregulate the production of macrophage-derived WNT5A89. WNT5A, in turn, stimulates the secretion of pro-inflammatory cytokines (such as IL-1β, IL-6 and IL-8) from macrophages via Ca2+–CaMKII signalling and facilitates foam cell formation23,90,91. Evidence suggests that WNT5A promotes transforming growth factor-β (TGFβ)-mediated macrophage polarization, as demonstrated in the context of kidney fibrosis92. Extensive research in oncology suggests that WNT5A has a wide range of effects on macrophage activation, such as in establishing an NF-κB autocrine loop93,94. However, these WNT5A effects have not been fully investigated in atherosclerosis.
Several observational and mechanistic studies in in vivo models of atherosclerosis have further supported a role for non-canonical WNT signalling in promoting a vascular pro-inflammatory phenotype. In an Apoe−/− mouse model of atherosclerosis, Wnt5a knockdown inhibited lipid accumulation and inflammation in atherosclerotic plaques, which was suggested to involve ROR2 non-canonical WNT signalling and downstream nuclear translocation of NF-κB95. Furthermore, circulating WNT5A levels are increased in a variety of inflammatory processes, such as sepsis, based on experimental models, further supporting a connection between non-canonical WNT signalling and inflammation25,81. Despite the available strong evidence, further research is required to elucidate the full spectrum of the effects of non-canonical WNT ligands on cytokine production, inflammatory cell recruitment and activation, and overall lipid-driven and cytokine-driven vascular inflammatory responses.
Cellular insulin resistance
Observational data show that increased bioavailability of WNT5A in the circulati0n and high WNT5A expression in adipose tissue are both associated with systemic insulin resistance (defined as glucose intolerance), as first demonstrated by a team led by Kenneth Walsh, who were pioneers in the study of the metabolic and cardiovascular implications of non-canonical WNT signalling51,96–98. This association could be indirectly linked to atherosclerosis via the detrimental effects of hyperglycaemia on vascular function6. However, evidence suggests that WNT5A is associated with molecular insulin resistance in the vasculature, defined as abnormal vascular insulin signalling36,60. Indeed, ex vivo insulin-mediated vasorelaxation of arterioles from visceral adipose tissue isolated from individuals with obesity was impaired compared with that of arterioles from subcutaneous adipose tissue from the same individuals36. JNK activation and Wnt5a expression was increased in visceral adipose tissue compared with subcutaneous fat36. Furthermore, in endothelial cells isolated from adipose tissue, treatment with recombinant WNT5A stimulated JNK activation and induced insulin resistance, demonstrated by a reduction in downstream AKT and eNOS phosphorylation36. Consistently, the capacity of WNT5A to abolish insulin-induced AKT and eNOS phosphorylation and NO production has been replicated in studies in primary endothelial cells from patients with diabetes60.
Given the well-described link between JNK activity and molecular insulin resistance99, the link between non-canonical WNT signalling, particularly the PCP pathway, and induction of vascular insulin resistance is not surprising. Inflammatory factors, such as NF-κB and tumour necrosis factor, which are induced by non-canonical WNT signalling, can also interfere with molecular insulin signalling100–103, providing indirect links between non-canonical WNT signalling and vascular insulin resistance. Finally, Ca2+–PKC non-canonical WNT signalling might also contribute to vascular insulin resistance, given that certain PKC isoforms directly induce cellular insulin resistance6.
Adipose tissue secretome
The adipose tissue is a dynamic organ that interacts with the vascular wall in paracrine and endocrine manners through the secretion of biologically active molecules5,104,105. Metabolic diseases such as visceral obesity and diabetes are associated with a pro-inflammatory phenotype of visceral adipose tissue, which secretes molecules that can directly reach the vascular wall via the bloodstream and exert biological effects106,107. Epicardial adipose tissue is a multifaceted, dynamic marker of cardiometabolic disease that is affected by pleiotropic pharmacological therapies and influences the heart in multiple paracrine ways104,108–111 Perivascular adipose tissue (PVAT) can exert paracrine effects on the vasculature owing to its close proximity to the vascular wall, and can also act as a receiver of biological signals from the vascular wall, thereby establishing a bidirectional crosstalk with the vasculature112. PVAT senses signals of adjacent coronary inflammation, changing its phenotype and lipid content116. This phenomenon can be captured by an imaging biomarker of coronary inflammation derived from coronary CT angiography, which has been standardized and is used in clinical practice113–116.
Our group has shown that WNT5A is the predominant WNT ligand expressed by the PVAT surrounding internal mammary arteries from patients undergoing CABG surgery15. WNT5A expression in PVAT is upregulated in the context of obesity and is independently associated with the activity of NADPH oxidases in the underlying vessels15. Furthermore, human primary VSMCs co-cultured with human adipocytes with Wnt5a knockdown produced less NADPH oxidase-derived superoxide than VSMCs co-cultured with control adipocytes15. This finding confirms the concept that adipocyte-derived WNT5A might have paracrine pro-oxidant effects on vascular cells in humans. These findings are in agreement with a study reporting elevated levels of WNT5A and reduced levels of SFRP5 in the plasma of patients with peripheral occlusive arterial disease compared with healthy individuals117. Therefore, non-canonical WNT signalling, based on findings from the paradigm ligand WNT5A, is a novel paracrine link between obesity and vascular disease pathogenesis (FIG. 3).
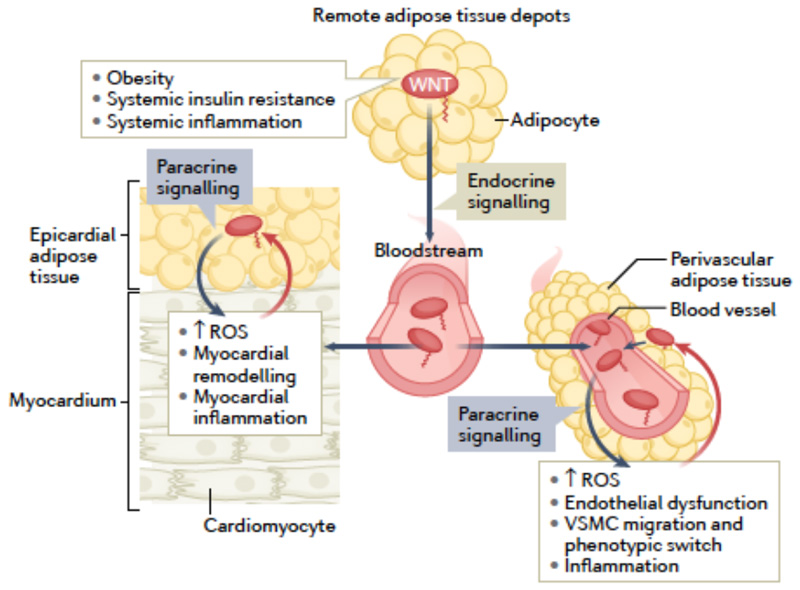
Fig 3. Non-canonical WNT signalling and bidirectional interactions between adipose tissue depots and the cardiovascular system.
Obesity, systemic insulin resistance and systemic inflammation induce systemic upregulation of non-canonical WNT ligands, partly via upregulation of WNT secretion from adipose tissue depots. WNT ligands can reach the heart and blood vessels via the systemic circulation and from adjacent adipose tissue depots, such as the epicardial adipose tissue and perivascular adipose tissue, respectively. Regardless of the source, WNT ligands exert multiple effects on the cardiovascular system, including the induction of reactive oxygen species (ROS) generation, myocardial remodelling, endothelial dysfunction, vascular smooth muscle cell (VSMC) migration and phenotypic switch, and inflammation. These changes in turn stimulate the release of signals that influence WNT expression in epicardial adipose tissue and perivascular adipose tissue, thereby establishing potential paracrine interaction loops between the cardiovascular system and adipose tissues.
Obesity and diabetes mellitus
Non-canonical WNT signalling has multiple broad roles related to the pathogenesis of metabolic diseases, such as obesity and diabetes. These roles include multifaceted effects on adipose tissue, the liver and the pancreas, ranging from the regulation of energy storage and handling, to adipogenesis and apoptosis97,118,119. Work by Fuster, Walsh and colleagues strongly suggests a causal association between obesity and increased bioavailability of WNT5A in the circulation and visceral adipose tissue in animal models and humans15,96,97,120. Furthermore, WNT5A overexpression in mouse myeloid cells augmented adipose tissue inflammation in vitro, and WNT5A directly induced JNK signalling and molecular insulin resistance in adipocytes of visceral adipose tissue in obese mice97. All these effects might influence obesity-related adipose tissue function and, indirectly, cardiovascular biology via regulation of the adipose tissue secretome.
Non-canonical WNT signalling influences adipose tissue biology in the context of obesity and diabetes, in terms of adipose tissue volume, distribution and secretome and, therefore, also influences the interactions between the adipose tissue and the cardiovascular system. Non-canonical WNT signalling, mediated by WNT10b and WNT5A, is believed to have an anti-adipogenic effect via FZD and LRP receptors, leading to reduced adipocyte size, and also regulates the adipose tissue secretome and overall insulin sensitivity121. Non-canonical WNT signalling also induces adipose tissue inflammation independently of expansion97. In summary, non-canonical WNT signalling can contribute to the formation of small adipocytes, with low lipid content and increased inflammation, which are all hallmarks of visceral adipose tissue. We must note that WNT ligands create a complicated, cross-interacting canvas in the adipose tissue, which makes deciphering the integrated effects extremely challenging and prone to inaccuracies122.
Non-canonical WNT signalling in cardiac diseases
Cardiac diseases, such as heart failure and arrhythmias, are caused by different pathogenic mechanisms, such as arrhtymogenesis123, dysregulated cardiac biomechanics124, structural remodelling125, abnormal energetics126,127 and oxidative stress128. Observational evidence suggests that non-canonical WNT signalling is linked to cardiac diseases. For example, circulating levels of WNT5A were elevated in patients with heart failure compared with individuals without heart failure and were associated with haemodynamic markers of heart failure, such as ejection fraction and filling pressures135. In a cohort of patients with dilated cardiomyopathy, higher plasma WNT5A levels were associated with increased right ventricular filling pressures and decreased right ventricular ejection fraction, and WNT5A expression was elevated in the right ventricle compared with the left ventricle136. Importantly, several experimental studies suggest that non-canonical WNT signalling is a causal regulator of cardiac disease (FIG. 4).
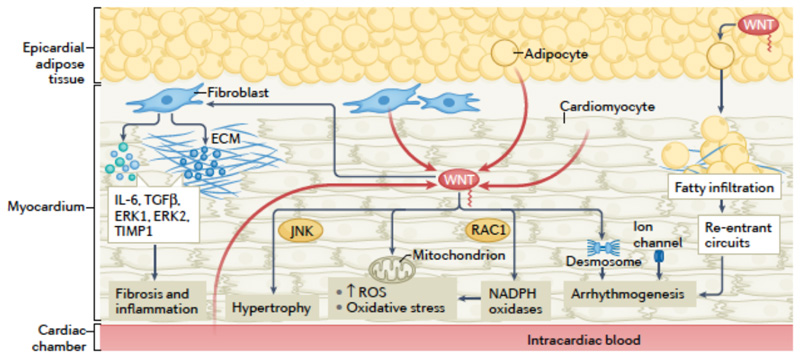
Fig 4. Non-canonical WNT signalling in myocardial disease.
Summary of established and putative mechanisms linking non-canonical WNT signalling and myocardial disease. In the myocardium, sources of non-canonical WNT ligands, such as WNT5A, include cardiomyocytes, the microcirculation, the blood in the cardiac cavities and the adjacent epicardial adipose tissue. Non-canonical WNT signalling can stimulate cardiac fibroblasts, inducing the upregulation of the expression of IL-6, transforming growth factor-β (TGFβ), extracellular signal-regulated kinase 1 (ERK1) and ERK2 and the metalloproteinase inhibitor TIMP1, thereby potentially promoting fibrosis and inflammation. Non-canonical WNT signalling can induce cardiac hypertrophy via JUN N-terminal kinase (JNK) activation. Non-canonical WNT signalling can promote oxidative stress through activation of NAPDH oxidases mediated by the small GTPase RAC1. WNT might be linked to mitochondrial biology, regulating mitochondrial aggregation and the production of mitochondrial reactive oxygen species (ROS), although the direction of this interplay is unclear. WNT can also regulate desmosome and ion channel function, potentially promoting arrhythmogenesis. WNT secreted by epicardial adipose tissue can cause adipose tissue expansion and myocardial fatty infiltration, facilitating the development of re-entrant circuits and arrhythmias such as atrial fibrillation.
Arrhythmogenesis
Arrhythmias are caused by abnormal electrical impulse generation or conduction123,129. Abnormal impulses rise from increased automaticity, early afterdepolarizations complicating slow action potential repolarization or by late diastolic depolarizations cause by sarcoplasmic reticulum Ca2+leakage129. Abnormal conduction includes accessory pathways and re-entry circuits (often caused by a structural substrate, such as regional fibrosis) 129. Triggers include myocardial ischaemia, electrolyte disorders, medications and genetic channelopathies123.
A large number of experimental studies have linked canonical WNT signalling to arrhythmogenic conditions such as arrhythmogenic cardiomyopathy, which is not surprising given the crucial role of β-catenin in the regulation of desmosomal intercellular junctions137. Furthermore, canonical WNT signalling regulates myocardial fibrosis, as shown in mouse models, thereby interfering with the formation of re-entry substrates, such as in atrial fibrillation138,139. Both canonical β-catenin WNT signalling and non-canonical RHO-mediated WNT signalling have been associated with the pathophysiology of arrhythmogenic right ventricular cardiomyopathy (ARVC) in in silico models, which showed that the inactivation of the aforementioned pathways is linked with increased PPARγ expression and ARVC pathogenesis140.
By contrast, the mechanistic role of non-canonical WNT signalling in human arrhythmogenesis has not been adequately explored. In a study in patients with rheumatic valve disease undergoing valve surgery, the presence of atrial fibrillation was associated with elevated expression of a transcription factor SNAIL1 and several WNT ligands, including the non-canonical WNT ligands WNT5A and WNT11, in the right atrium141. WNT5A has been associated with the upregulation of Snail1 protein expression in melanoma cells via non-canonical PKC activation, which was associated with epithelial-to-mesenchymal transition (EMT) and metastasis potential142. Furthermore, WNT5A has been linked to TGFβ-dependent fibrosis and EMT in a variety of tissues such as the liver138,143. Atrial fibrillation is tightly linked to processes such as EMT and fibrosis144. Therefore, these findings might imply a potentially causal role for non-canonical WNT signalling in arrhythmogenesis by facilitating the development of re-entrant circuits.
Cardiac remodelling
Cardiac remodelling involves structural changes caused by myocardial wall stress (as a result of for example hypertension or valvular disease) and inflammation (such as after ischaemic myocardial injury)125. At the cellular level, remodelling is caused by cardiomyocyte hypertrophy and collagen deposition in the extracellular matrix caused by pro-inflammatory and redox signalling and metabolic signals125,128,130. These changes can induce arrhythmogenic substrates or impair contractile efficiency127.
Non-canonical WNT signalling might contribute to cardiac fibrosis. For example, WNT5A was shown to stimulate human primary cardiac fibroblasts in vitro, inducing the production of IL-6 and tissue inhibitor of metalloproteinase 1 (TIMP1) and the activation of ERK1/ERK2 signalling135. This finding suggests that WNT5A can potentially promote inflammation and fibrosis in vivo. WNT5A also induces a reduction in glycogen synthase kinase 3β (GSK3β) levels in human cardiac fibroblasts in vitro, which promoted fibrosis partly via transactivation of TGFβ, although this effect seemed to be predominantly mediated by canonical WNT signalling145. Non-canonical, Ca2+-dependent WNT signalling in human ventricles stimulates the NFAT–calcineurin pathway, a pathway that contributes to cardiac fibrosis based on experimental models.
Non-canonical WNT signalling has been shown to stimulate cardiomyocyte hypertrophy138. More specifically, WNT5A activated PCP signalling mediated by Dapper 1 and led to downstream activation of JNK to promote hypertrophy in human cardiomyocytes in vitro, assessed by microscopy and cardiomyocyte surface area146. Interestingly, in a mouse model of left ventricular hypertrophy induced by aortic constriction, both neutrophil depletion and myeloid-specific knockdown of Wnt5a reduced neutrophil infiltration in the myocardium and cardiac hypertrophy147. This finding suggests that WNT5A might also regulate cardiac hypertrophy in indirect ways involving neutrophils and local inflammatory responses in the heart.
Fatty infiltration of the myocardium is found in a spectrum of myocardial disease phenotypes ranging from arrhythmogenesis to contractile dysfunction148. Although suppression of canonical WNT signalling has been linked to fatty infiltration of the myocardium, for example, in animal models of ARVC149, limited evidence exists on non-canonical WNT signalling and fatty remodelling of the myocardium. Non-canonical WNT signalling has been implicated in fatty infiltration in other organs150,151, such as in a mouse model of non-alcoholic fatty liver disease, which was rescued by canonical WNT3a signalling152.
Myocardial energetics
Under physiological conditions, fatty acid oxidation is the major source of ATP in the heart, followed by glycolysis and lactate metabolism127. Different myocardial diseases are associated with different metabolic profiles, but most eventually lead to ATP depletion, reducing contractile efficiency and altering a wide number of downstream signalling events131. Several types of myocardial dysfunction are characterized by a shift towards glycolysis and ketone body oxidation to meet the increasing energy demands of the failing heart under stress153. Medications such as SGLT2 inhibitors might exert some of their beneficial effects by regulating ketone body metabolism, glycolysis substrate inflow and fatty acid oxidation154, which highlights the concept that metabolic pathway balance and fuel shifts might be crucial for modifying myocardial performance153.
Evidence suggests that WNT signalling mediates metabolic pathway reprogramming in a variety of cell types, although this action mainly involves canonical signalling155,156. Non-canonical WNT signalling attenuates mitochondrial aggregation in the HEK93 cell line157, and protects mitochondria from fission–fusion alterations and prevents mitochondrial loss in neurons158, both via PKC and regulation of intracellular Ca2+ dynamics. Overexpression of the non-canonical WNT ligand WNT11 preserves mitochondrial membrane potential and protects cardiomyocytes against hypoxia in vitro, via mechanisms involving insulin-like growth factor 1 and VEGF159. Non-canonical WNT signalling also accelerates glucose oxidative metabolism in the liver, which leads to steatosis via de novo lipogenesis160. Although these findings provide putative links to myocardial metabolism, the role of non-canonical WNT signalling in myocardial energetics has not been directly explored.
Oxidative stress
Oxidative stress has a key pathophysiological role in myocardial diseases, such as in ischaemia–reperfusion injury after myocardial infarction161 and through redox signalling in heart failure and arrhythmia162. Oxidative stress results from ischaemia–reperfusion injury133, inflammation-mediated stimulation of NADPH oxidases128,132, and disturbed mitochondrial energetics127,134. Myocardial ROS in turn can induce hypertrophy, apoptosis, autophagy, metabolic enzyme dysregulation and impaired contractility, thereby drastically contributing to myocardial disease11,128,133,134.
Ischaemia–reperfusion injury is characterized by sudden oxygen abundance in a stunned myocardium, in which free radicals are produced because of an imbalance between pro-oxidant and anti-oxidant enzymes133,161. The excess free radicals induce cardiomyocyte death via multiple intracellular pathways, including proteolysis, caspase activation and mitochondrial regulation133,161. Co-expression of AKT1 and the non-canonical ligand WNT11 stimulates proliferation and cardiomyocyte differentiation of mesenchymal stem cells and attenuates hypoxia–reperfusion injury163. By contrast, a study in mice showed that blockade of non-canonical WNT signalling attenuates myocardial ischaemia–reperfusion injury165. NADPH oxidases have been implicated in atrial fibrillation and heart failure via a multitude of redox-sensitive intracellular transcription pathways132,164. Non-canonical WNT signalling is linked to NADPH oxidases via RAC1, as shown in VSMCs in vitro15. Whether these findings are relevant to the human myocardium remains to be proven.
Targeting non-canonical WNT signalling
The studies discussed above indicate a strong mechanistic link between non-canonical WNT signalling and cardiovascular disease. Therefore, successful targeting of this pathway could have multiple beneficial effects in patients, especially in the context of obesity, when the bioavailability of the principal non-canonical WNT ligand, WNT5A, is increased15,25. Interestingly, the downstream mediators of non-canonical WNT signalling (PLC-CaMKII, RAC1, RHOA and JNK) are converging points for multiple non-WNT pathways, such as the catecholamine and renin-angiotensin-aldosterone pathways47,166,167. The exact contribution of WNT ligands to cardiovascular pathophysiology in this context is unknown. However, targeting WNT signalling might offer an advantage over targeting downstream molecules, which would also influence the outputs of multiple other, non-WNT pathways.
Non-canonical WNT signalling is a conserved pathway affecting virtually all cell types and governing fundamental biological processes18,168. Consequently, its targeting is prone to nonspecific, off-target adverse effects (with tumorigenesis being the most important), and it is challenging when embryonic development is relevant (for example, in pregnant women)18,168. The interconnected network of WNT ligands and receptors further complicates targeting a single ligand18,168.
So far, early attempts have mainly focused on targeting the canonical WNT signalling pathway, especially in the context of cancer18,168,169. By contrast, targeting of non-canonical WNT signalling has not been explored. Current advances in biotechnology, gene editing and drug delivery, coupled with a better understanding of the downstream mediators of non-canonical WNT signalling, might help towards this end.
Targeting strategies
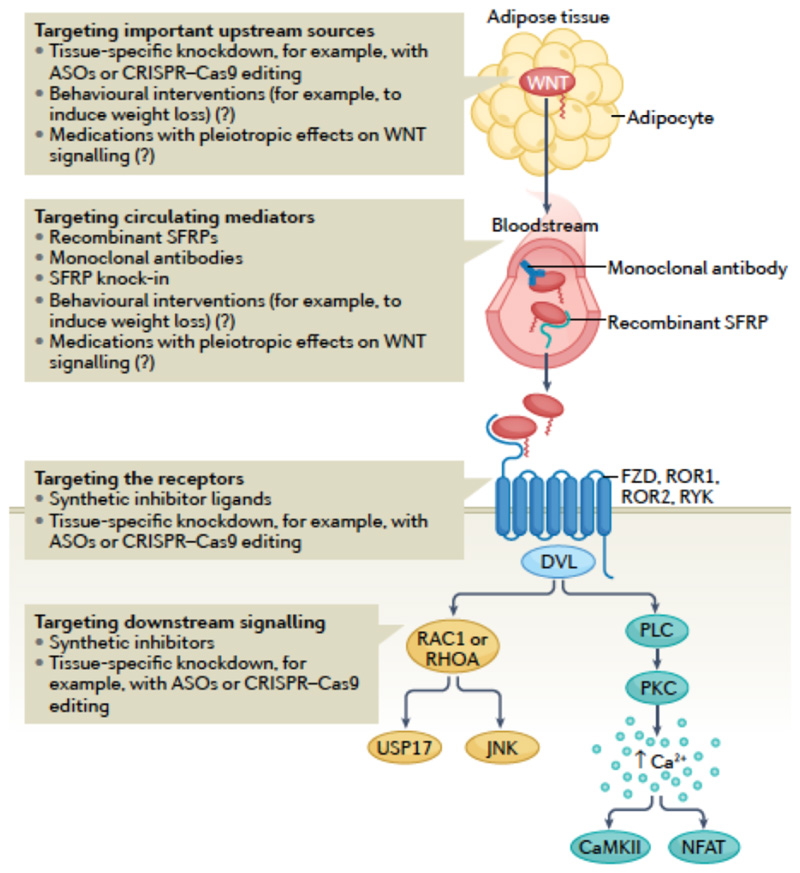
Targeting non-canonical WNT signalling might be theoretically achieved with the use of chemical inhibitors of, for example, SFRPs or RAC115,18. The use of monoclonal antibodies, antisense oligonucleotides (ASOs) and gene editing methods (such as CRISPR–Cas9) can allow for a more efficient targeting of non-canonical WNT signalling than previously thought170–173. More efficient drug delivery to target tissues could also be achieved with the use of nanoparticles170,174,175. The main challenge of using monoclonal antibodies is the systemic, whole-body delivery, posing the risk of substantial adverse effects resulting from systemic WNT inhibition in other tissues in addition to the cardiovascular system. This issue might be bypassed by using tissue-specific targeting of WNT elements with tools such as ASOs and CRISPR–Cas9 editing. However, these strategies are not clinically feasible at present, and require extensive validation and optimization. Nanoparticle technology could be used to guide drugs and vectors to sites of interest, via systemic or local administration of nanoparticles with affinity to particular molecules, such as endothelial markers (for example, vascular cells adhesion molecules). This approach, in combination with molecular techniques (CRISPR-Cas9 or ASOs), could increase local efficacy and tissue specificity of WNT targeting, as demonstrated with the use of macrophage-specific, promoter-driven plasmids contained in lipid nanoparticles to target inflammatory cells such as macrophages176. The characteristics of these strategies are summarized in Figure 5.
Fig 5. Potential strategies for targeting non-canonical WNT signalling therapeutically.
Modulating WNT effects can be achieved by targeting the main sources of WNT ligands, such as the adipose tissue, with the use of tissue-specific knockdown technologies, for example, antisense oligonucleotides (ASOs) and CRISPR–Cas9 methods. However, these approaches require considerable research before their eventual translation into the clinic. The balance between different circulating WNT ligands can be targeted by using recombinant decoy receptors of WNT ligands (such as secreted frizzled-related proteins (SFRPs)) or monoclonal antibodies against WNT ligands. Overall, targeting of WNT signalling systemically might be associated with off-target adverse effects. WNT signalling can also be targeted by blocking or decreasing WNT receptors, with the added theoretical benefit of tissue specificity when using knockdown methods. Finally, downstream signalling could be targeted by developing chemical inhibitors or knockdown technologies. Targeting individual downstream molecules instead of the WNT ligands would provide a theoretical benefit of more specifically targeting individual subphenotypes caused by WNT signalling activation. Given that obesity is associated with increased WNT bioavailability, behavioural interventions, such as interventions to induce weight loss, and the use of medications with known metabolic effects might have pleiotropic effects on WNT signalling, although the effect of these approaches on WNT signalling is unclear.
Potential targets
Non-canonical WNT signalling comprises a complex network of receptors and downstream molecules. Therefore, one could in theory interfere with non-canonical WNT signalling at different levels by using the techniques mentioned in the previous section. Importantly, we should keep in mind that the canonical and non-canonical pathways cross-regulated each other; therefore, any intervention on non-canonical WNT signalling is expected to have effects on canonical WNT signalling168,177.
WNTligands andSFPR inhibitors
Targeting non-canonical WNT ligands would be the most obvious way to inhibit all downstream signalling. However, simultaneous targeting of all non-canonical WNT ligands would not be feasible. WNT5A is a paradigm non-canonical WNT ligand, having been the focus of most research on non-canonical WNT signalling; therefore, WNT5A would seem to be an appropriate therapeutic target in this context15,69. Systemic WNT5A targeting (for example, with monoclonal antibodies or exogenously administered recombinant forms of SFPRs) might be the most obvious way forward, but this approach would pose the aforementioned risks of global inhibition. Considering the pathophysiological mechanisms evaluated in the previous sections, tissue-specific (for example, adipose tissue-specific) targeting would be more efficient, although still not feasible at present.
WNT receptors
Downregulation of WNT receptors such as FZD2 and FZD5 could markedly attenuate non-canonical WNT signalling. This strategy could be important given that FZD2 and FZD5 have been shown to be upregulated in internal mammary arteries from individuals with obesity, suggesting a higher sensitivity to WNT ligands15. Downregulation of WNT receptors could be achieved by ASO-mediated silencing or CRISPR-Cas9 gene editing, in which the use of tissue-specific promoters would be ideal. However, as mentioned, these strategies remain hypothetical at present.
Downstream signal transduction molecules
Non-canonical WNT signalling converges on a number of downstream molecules, including RAC1, JNK, CaMKII and PKC, as well as USP17, a newly described WNT5A target15,59. Targeting of these downstream targets might reverse the detrimental effects of non-canonical WNT signalling. However, many non-WNT pathways also converge on these downstream molecules, which would make their targeting prone to non-specific effects.
Studies in mouse models of RAC1 depletion have reported beneficial effects, such as reduced endoplasmic reticulum stress and reduced cardiac oxidative stress178. In addition, several allosteric inhibitors of RAC1 have been developed. Several JNK inhibitors have been used in animal models of diseases such as neurodegeneration179 and in a preliminary pulmonary fibrosis clinical trial180, supporting the clinical relevance of targeting JNK. Similarly, CaMKII inhibition in vivo is associated with decreased atherosclerotic plaque burden in Apoe-/- mice181. Administration of the chemical CaMKII inhibitor KN93 in a mouse model of heart failure induced by pressure overload had beneficial effects182. PKC inhibition in vivo is more challenging, given the large number of PKC isoforms6. Finally, USP17 is a newly identified link between WNT signalling, RAC1 activation and downstream redox signalling, which might warrant further investigation as a potential therapeutic target15.
Conclusion
Non-canonical WNT signalling, acting via receptor-mediated pathways and through activation of second messengers such as RAC1, JNK and Ca2+-mediated CaMKII and PKC, is causally linked to vascular and myocardial disease in animal and human experimental models. In particular, non-canonical WNT signalling induces vascular oxidative stress via NADPH oxidase activation, promotes endothelial dysfunction and insulin resistance via JNK signalling and oxidative eNOS uncoupling, increases vascular inflammation in endothelial cells and macrophages, and triggers a VSMC contractile-to-synthetic phenotypic switch that might promote atherosclerotic plaque instability. Importantly, non-canonical WNT signalling is upregulated in adipose tissue, including PVAT, in obesity, exerting paracrine and endocrine vascular effects. Non-canonical WNT signalling has putative links to a wide spectrum of cardiac disease phenotypes via regulation of myocardial metabolism, fibrosis and adipogenesis pathways and redox signalling mediators such as NADPH oxidases and NF-kB, potentially contributing to myocardial remodelling, arrhythmogenic substrate formation and contractile dysfunction.
Non-canonical WNT signalling is thus a multifaceted causal mediator of atherosclerosis and a link between obesity and vascular disease. Advances in biotechnology have opened up new ways to potentially modulate non-canonical WNT signalling through targeting specific tissues, proteins or genes related to WNT ligands, receptors or the downstream signalling network, which warrant further investigation.
Key points.
Cardiovascular disease is a major cause of morbidity and mortality worldwide, prompting the need for a better understanding of the underlying pathogenic mechanisms.
Non-canonical WNT signalling is an evolutionarily conserved and ubiquitous range of pathways affecting fundamental processes, such as inflammation, metabolism, cell motility, oxidative stress and homeostasis.
Non-canonical WNT signalling is a promising target in vascular disease, influencing vascular oxidative stress, endothelial dysfunction, inflammation, vascular smooth muscle cell phenotypes and cellular insulin resistance, all of which can affect atherosclerosis progression and plaque stability.
Non-canonical WNT signalling has putative links to cardiac disease by influencing myocardial oxidative stress, inflammation, repair capacity, energetics and remodelling, including fibrotic or adipose infiltration of the myocardium, all of which can generate the substrate for contractile dysfunction and arrhythmogenic potential.
Non-canonical WNT ligands are secreted by adipose tissue and are upregulated in obesity, acting as an endocrine and paracrine link between obesity and cardiovascular complications via an adipose tissue–cardiovascular system crosstalk.
Therapeutic targeting of non-canonical WNT signalling can be done at multiple levels and could involve several state-of-the-art technologies; however, more research in this area is required.
Footnotes
Author contributions
I.A. researched data for the article and wrote the manuscript. M.P. researched data for the article. I.A. and C.A. contributed to the discussion of content. All authors reviewed and/or edited the manuscript before submission.
Competing interests
C.A. is founder, shareholder, and director of Caristo Diagnostics, a CT image analysis company. The other authors declare no competing interests.
References
- 1.Manemann SM, et al. Recent trends in cardiovascular disease deaths: a state specific perspective. BMC Public Health. 2021;21:1–7. doi: 10.1186/s12889-021-11072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson K. The heart of immunology: immune mechanisms in cardiovascular medicine. Cardiovasc Res. 2021:166–168. doi: 10.1093/cvr/cvab314. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 2021;117:2525–2536. doi: 10.1093/cvr/cvab303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredman G, MacNamara KC. Atherosclerosis is a major human killer and non-resolving inflammation is a prime suspect. Cardiovasc Res. 2021;117:2563–2574. doi: 10.1093/cvr/cvab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akoumianakis I, Antoniades C. The interplay between adipose tissue and the cardiovascular system: Is fat always bad? Cardiovascular Research. 2017 doi: 10.1093/cvr/cvx111. [DOI] [PubMed] [Google Scholar]
- 6.Akoumianakis I, Antoniades C. Impaired Vascular Redox Signaling in the Vascular Complications of Obesity and Diabetes Mellitus. Antioxid Redox Signal. 2019;30:333–353. doi: 10.1089/ars.2017.7421. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, Bennett M. Aging and Atherosclerosis. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis — An Inflammatory Disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, et al. NOX1 mediates metabolic heart disease in mice and is upregulated in monocytes of humans with diastolic dysfunction. Cardiovasc Res. 2021 doi: 10.1093/cvr/cvab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol - Hear Circ Physiol. 2011 doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 12.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: The fibroblast awakens. Circulation Research. 2016 doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazbanov IV, Ten Tusscher KHWJ, Panfilov AV. Effects of Heterogeneous Diffuse Fibrosis on Arrhythmia Dynamics and Mechanism. Sci Rep. 2016 doi: 10.1038/srep20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan CY, Nusse R. THE WNT SIGNALING PATHWAY IN DEVELOPMENT AND DISEASE. Annu Rev Cell Dev Biol. 2004 doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 15.Akoumianakis I, et al. Adipose tissue–derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci Transl Med. 2019;11:eaav5055. doi: 10.1126/scitranslmed.aav5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laudes M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. Journal of Molecular Endocrinology. 2011 doi: 10.1530/JME-10-0169. [DOI] [PubMed] [Google Scholar]
- 17.Sethi JK, Vidal-puig A. Wnt signalling and the control of cellular metabolism. 2015;427:1–17. doi: 10.1042/BJ20091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman ZF, Moon RT, Chien AJ. Targeting Wnt Pathways in Disease. Cold Spring Harb Perspect Biol. 2012;4:a008086. doi: 10.1101/cshperspect.a008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171:1195–209. doi: 10.1111/bph.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenov MV, Habas R, MacDonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 131:1378.e1–1378.e2. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Orte E, Sáenz-Narciso B, Moreno S, Cabello J. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 2013;29:545–553. doi: 10.1016/j.tig.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Choi EY, et al. Wnt5a and Wnt11 as acute respiratory distress syndrome biomarkers for severe acute respiratory syndrome coronavirus 2 patients. Eur Respir J. 2020;56:2001531. doi: 10.1183/13993003.01531-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christman Ma, et al. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010 doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt PM, Malgor R. Wnt5a: A player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis. 2014 doi: 10.1016/j.atherosclerosis.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 27.Wiese KE, Nusse R, van Amerongen R. Wnt signalling: conquering complexity. Development. 2018 doi: 10.1242/dev.165902. [DOI] [PubMed] [Google Scholar]
- 28.Foulquier S, et al. WNT Signaling in Cardiac and Vascular Disease. Pharmacol Rev. 2018;70:68–141. doi: 10.1124/pr.117.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–8. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 30.Wada N, et al. Selective modulation of Wnt ligands and their receptors in adipose tissue by chronic hyperadiponectinemia. PLoS One. 2013;8:e67712. doi: 10.1371/journal.pone.0067712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinou K, Christodoulides C, Antoniades C, Koutsilieris M. Wnt signaling in cardiovascular physiology. Trends Endocrinol Metab. 2012 doi: 10.1016/j.tem.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012 doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Wook-Jin Chae, Bothwell ALM. Canonical and Non-Canonical Wnt Signaling in Immune Cells Wook-Jin. Physiol Behav. 2016;176:100–106. [Google Scholar]
- 34.Baksh D, Boland GM, Tuan RS. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem. 2007;101:1109–24. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- 35.James RG, Conrad WH, Moon RT. β-Catenin-independent Wnt pathways: Signals, core proteins, and effectors. Methods Mol Biol. 2008 doi: 10.1007/978-1-59745-249-6_10. [DOI] [PubMed] [Google Scholar]
- 36.Farb MG, et al. WNT5A-JNK regulation of vascular insulin resistance in human obesity. Vasc Med. 2016;21:489–496. doi: 10.1177/1358863X16666693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, et al. Wnt5a Promotes Inflammatory Responses via Nuclear Factor κB (NF-κB) and Mitogen-activated Protein Kinase (MAPK) Pathways in Human Dental Pulp Cells. J Biol Chem. 2014 doi: 10.1074/jbc.M113.546523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997 doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 39.Crabtree GR, Olson EN. NFAT signaling: Choreographing the social lives of cells. Cell. 2002 doi: 10.1016/S0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 40.Bryja Vitezslav, Andersson Emma R, Schambony Alexandra, E M, Bryjova Lenka, Biris Kristin K, Hall Anita C, Kraft Bianca, C L, Yamaguchi Terry P, Buckingham Margaret, et al. The Extracellular Domain of Lrp5/6 Inhibits Noncanonical Wnt Signaling In Vivo. Mol Biol Cell. 2009;20:924–936. doi: 10.1091/mbc.E08-07-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas G, et al. A key role for the novel coronary artery disease gene JCAD in atherosclerosis via shear stress mechanotransduction. Cardiovasc Res. 2019 doi: 10.1093/cvr/cvz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nature Medicine. 2011 doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 44.Pirillo A, Bonacina F, Norata GD, Catapano AL. The Interplay of Lipids, Lipoproteins, and Immunity in Atherosclerosis. Curr Atheroscler Rep. 2018;20:12. doi: 10.1007/s11883-018-0715-0. [DOI] [PubMed] [Google Scholar]
- 45.Silvestre-Roig C, et al. Atherosclerotic Plaque Destabilization. Circ Res. 2014;114:214–226. doi: 10.1161/CIRCRESAHA.114.302355. [DOI] [PubMed] [Google Scholar]
- 46.Hansson GK. Inflammation, atherosclerosis and coronary artery disease. N Engl J Med. 2005 doi: 10.1056/NEJM199408183310709. [DOI] [PubMed] [Google Scholar]
- 47.Akoumianakis I, et al. Insulin-induced vascular redox dysregulation in human atherosclerosis is ameliorated by dipeptidyl peptidase 4 inhibition. Sci Transl Med. 2020;12:eaav8824. doi: 10.1126/scitranslmed.aav8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mani A, et al. LRP6 Mutation in a Family with Early Coronary Disease and Metabolic Risk Factors. 2010;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu H-D, et al. Wnt5a mediated canonical Wnt signaling pathway activation in orthodontic tooth movement: possible role in the tension force-induced bone formation. J Mol Histol. 2016;47:455–66. doi: 10.1007/s10735-016-9687-y. [DOI] [PubMed] [Google Scholar]
- 50.Mill C, et al. Wnt5a-Induced Wnt1-Inducible secreted protein-1 suppresses vascular smooth muscle cell apoptosis induced by oxidative stress. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303922. [DOI] [PubMed] [Google Scholar]
- 51.Lu YC, et al. Circulating secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2013 doi: 10.1002/dmrr.2426. [DOI] [PubMed] [Google Scholar]
- 52.Albanese I, et al. Role of Noncanonical Wnt Signaling Pathway in Human Aortic Valve Calcification. Arterioscler Thromb Vasc Biol. 2017;37:543–552. doi: 10.1161/ATVBAHA.116.308394. [DOI] [PubMed] [Google Scholar]
- 53.Albanese I, Khan K, Barratt B, Al-Kindi H, Schwertani A. Atherosclerotic calcification: Wnt is the hint. J Am Heart Assoc. 2018 doi: 10.1161/JAHA.117.007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badimon L, Borrell-Pages M. Wnt signaling in the vessel wall. Curr Opin Hematol. 2017;24:230–239. doi: 10.1097/MOH.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 55.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. European Heart Journal. 2012 doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubiella U, et al. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci. 2013;110:8744–8749. doi: 10.1073/pnas.1221294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook-Mills JM, et al. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fontayne A, Dang PM-C, Gougerot-Pocidalo M-A, El Benna J. Phosphorylation of p47 p hox Sites by PKC α, βll δ, and ζ: Effect on Binding to p22 p hox and on NADPH Oxidase Activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 59.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319:1317–1323. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 60.Bretón-Romero R, et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.115.306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiraishi H, et al. cGMP Inhibits GTP Cyclohydrolase I Activity and Biosynthesis of Tetrahydrobiopterin in Human Umbilical Vein Endothelial Cells. J Pharmacol Sci. 2003;93:265–271. doi: 10.1254/jphs.93.265. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi S, Mendelsohn ME. Synergistic Activation of Endothelial Nitric-oxide Synthase (eNOS) by HSP90 and Akt. J Biol Chem. 2003;278:30821–30827. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- 63.Murthy S, et al. Endothelial CaMKII as a regulator of eNOS activity and NO-mediated vasoreactivity. PLoS One. 2017;12:e0186311. doi: 10.1371/journal.pone.0186311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masckauchán TNH, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006 doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siman-Tov R, et al. Circulating Wnt Ligands Activate the Wnt Signaling Pathway in Mature Erythrocytes. Arterioscler Thromb Vasc Biol. 2021:E243–E264. doi: 10.1161/ATVBAHA.120.315413. [DOI] [PubMed] [Google Scholar]
- 66.Hulin-Curtis S, Williams H, Wadey KS, Sala-Newby GB, George SJ. Targeting Wnt/β-Catenin Activated Cells with Dominant-Negative N-cadherin to Reduce Neointima Formation. Mol Ther - Methods Clin Dev. 2017;5:191–199. doi: 10.1016/j.omtm.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown BA, et al. Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells. Aging Cell. 2019;18:e12844. doi: 10.1111/acel.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava R, et al. Impaired LRP6-TCF7L2 Activity Enhances Smooth Muscle Cell Plasticity and Causes Coronary Artery Disease. Cell Rep. 2015;13:746–759. doi: 10.1016/j.celrep.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsaousi A, Mill C, George SJ. The Wnt pathways in vascular disease. Curr Opin Lipidol. 2011;22:350–357. doi: 10.1097/MOL.0b013e32834aa701. [DOI] [PubMed] [Google Scholar]
- 70.San José G, et al. Insulin-induced NADPH oxidase activation promotes proliferation and matrix metalloproteinase activation in monocytes/macrophages. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Kong Y-Z, et al. Macrophage migration inhibitory factor induces MMP-9 expression: implications for destabilization of human atherosclerotic plaques. Atherosclerosis. 2005;178:207–215. doi: 10.1016/j.atherosclerosis.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 72.Fiotti N, et al. MMP-9 Microsatellite Polymorphism and Susceptibility to Carotid Arteries Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1330–1336. doi: 10.1161/01.ATV.0000219233.31702.c9. [DOI] [PubMed] [Google Scholar]
- 73.Fiotti N, et al. MMP-9 microsatellite polymorphism: Association with the progression of intima-media thickening and constrictive remodeling of carotid atherosclerotic plaques. Atherosclerosis. 2005;182:287–292. doi: 10.1016/j.atherosclerosis.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Pandey S, Chandravati Wnt signaling cascade in restenosis: a potential therapeutic target of public health relevance in a North American cohort of Nebraska State. Mol Biol Rep. 2014;41:4549–4554. doi: 10.1007/s11033-014-3325-0. [DOI] [PubMed] [Google Scholar]
- 75.Woldt E, et al. The nuclear hormone receptor PPARγ counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun. 2012 doi: 10.1038/ncomms2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xin H, Xin F, Zhou S, Guan S. The Wnt5a/Ror2 pathway is associated with determination of the differentiation fate of bone marrow mesenchymal stem cells in vascular calcification. Int J Mol Med. 2013;31:583–588. doi: 10.3892/ijmm.2013.1242. [DOI] [PubMed] [Google Scholar]
- 77.Cheng S-L, et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR-/- mice by restraining noncanonical Wnt signals. Circ Res. 2015;117:142–56. doi: 10.1161/CIRCRESAHA.117.306712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin L, et al. The Novel Role and Underlying Mechanism of Wnt5a in Regulating Cellular Cholesterol Accumulation. Clin Exp Pharmacol Physiol. 2014 doi: 10.1111/1440-1681.12258. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 79.Wang Jingcai, Gong Min, Zuo Shi, Xu Jie, Paul Chris, Li Hongxia, Liu Min, Wang Yi-Gang, Muhammad Ashraf MX. WNT11-conditioned medium promotes angiogenesis through the activation of non-canonical WNT-PKC-JNK signaling pathway. Genes (Basel) 2020:1–17. doi: 10.3390/genes11111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stefater JA, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–5. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pashirzad M, et al. Role of Wnt5a in the Pathogenesis of Inflammatory Diseases. J Cell Physiol. 2017;232:1611–1616. doi: 10.1002/jcp.25687. [DOI] [PubMed] [Google Scholar]
- 82.Tian F, Mauro TM, Li Z. The pathological role of Wnt5a in psoriasis and psoriatic arthritis. J Cell Mol Med. 2019;23:5876–5883. doi: 10.1111/jcmm.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fiechter RH, et al. IL-12p40/IL-23p40 Blockade With Ustekinumab Decreases the Synovial Inflammatory Infiltrate Through Modulation of Multiple Signaling Pathways Including MAPK-ERK and Wnt. Front Immunol. 2021;12:1–18. doi: 10.3389/fimmu.2021.611656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, et al. The role of Ca2+/NFAT in Dysfunction and Inflammation of Human Coronary Endothelial Cells induced by Sera from patients with Kawasaki disease. Sci Rep. 2020;10:4706. doi: 10.1038/s41598-020-61667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morishita Y, et al. Wnt11 Gene Therapy with Adeno-associated Virus 9 Improves Recovery from Myocardial Infarction by Modulating the Inflammatory Response. Sci Rep. 2016;6:21705. doi: 10.1038/srep21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaussabel D, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 87.Nau GJ, et al. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehtonen A, et al. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J Leukoc Biol. 2007;82:710–720. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- 89.Shao Y, et al. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget. 2016;7:67674–67684. doi: 10.18632/oncotarget.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ackers I, et al. Blocking Wnt5a signaling decreases CD36 expression and foam cell formation in atherosclerosis. Cardiovasc Pathol. 2018;34:1–8. doi: 10.1016/j.carpath.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 92.Feng Y, et al. The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J Biol Chem. 2018;293:19290–19302. doi: 10.1074/jbc.RA118.005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Naskar D, et al. Wnt5a-Rac1-NF-κB Homeostatic Circuitry Sustains Innate Immune Functions in Macrophages. J Immunol. 2014;192:4386–4397. doi: 10.4049/jimmunol.1302817. [DOI] [PubMed] [Google Scholar]
- 94.Barbero G, et al. An Autocrine Wnt5a Loop Promotes NF-κB Pathway Activation and Cytokine/Chemokine Secretion in Melanoma. Cells. 2019;8:1060. doi: 10.3390/cells8091060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang C-J, et al. Wnt5a/Ror2 pathway contributes to the regulation of cholesterol homeostasis and inflammatory response in atherosclerosis. Biochim Biophys Acta - Mol Cell Biol Lipids. 2020;1865:158547. doi: 10.1016/j.bbalip.2019.158547. [DOI] [PubMed] [Google Scholar]
- 96.Ouchi N, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science (80-. ) 2010 doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuster JJ, et al. Noncanonical Wnt Signaling Promotes Obesity-Induced Adipose Tissue Inflammation and Metabolic Dysfunction Independent of Adipose Tissue Expansion. Diabetes. 2015 doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghila L, et al. Chronically elevated exogenous glucose elicits antipodal effects on the proteome signature of differentiating human ipsc-derived pancreatic progenitors. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22073698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 100.Hotamisligil GS, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science (80-. ) 1996 doi: 10.1126/SCIENCE.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 101.da Costa RM, et al. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc Diabetol. 2016;15:119. doi: 10.1186/s12933-016-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hotamisligil G. Mechanisms of TNF-α-induced insulin resistance. Exp Clin Endocrinol Diabetes. 2009;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 103.Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int J Obes. 2003;27:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 104.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nature Reviews Endocrinology. 2015 doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 105.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16:83–99. doi: 10.1038/s41569-018-0097-6. [DOI] [PubMed] [Google Scholar]
- 106.Van Gaal LF. Mechanisms linking obesity with cardiovascular disease. Diabetes Obes Metab. 2010 doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 107.Camarena V, et al. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr Metab Cardiovasc Dis. 2017;27:739–750. doi: 10.1016/j.numecd.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Antonopoulos AS, et al. Mutual Regulation of Epicardial Adipose Tissue and Myocardial Redox State by PPAR-γ/Adiponectin Signalling. Circ Res. 2016;118:842–55. doi: 10.1161/CIRCRESAHA.115.307856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iacobellis G, Baroni MG. Cardiovascular risk reduction throughout GLP-1 receptor agonist and SGLT2 inhibitor modulation of epicardial fat. J Endocrinol Invest. 2021 doi: 10.1007/s40618-021-01687-1. [DOI] [PubMed] [Google Scholar]
- 110.Malavazos AE, Goldberger JJ, Iacobellis G. Does epicardial fat contribute to COVID-19 myocardial inflammation? . Eur Heart J. 2020;41:2333. doi: 10.1093/eurheartj/ehaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Villasante Fricke AC, Iacobellis G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int J Mol Sci. 2019;20:5989. doi: 10.3390/ijms20235989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Akoumianakis I, Tarun A, Antoniades C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis: identifying novel therapeutic targets. Br J Pharmacol. 2016 doi: 10.1111/bph.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oikonomou EK, et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc Res. 2021:2677–2689. doi: 10.1093/cvr/cvab286. [DOI] [PubMed] [Google Scholar]
- 114.Oikonomou EK, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kotanidis CP, Antoniades C. Perivascular fat imaging by computed tomography (CT): a virtual guide. Br J Pharmacol. 2021;178:4270–4290. doi: 10.1111/bph.15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Antonopoulos AS, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 117.Wang B, et al. Sfrp5/Wnt5a and leptin/adiponectin levels in the serum and the periarterial adipose tissue of patients with peripheral arterial occlusive disease. Clin Bio chem. 2021;87:46–51. doi: 10.1016/j.clinbiochem.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 118.Catalán V, et al. Activation of noncanonical wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-1191. [DOI] [PubMed] [Google Scholar]
- 119.Ackers I, Malgor R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diabetes Vasc Dis Res. 2018;15:3–13. doi: 10.1177/1479164117738442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends in Endocrinology and Metabolism. 2009 doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Christodoulides C, Lagathu C, Sethi JK, Vidal- A. Adipogenesis and WNT signalling. 2015;20:16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tse G. Mechanisms of cardiac arrhythmias. Journal of Arrhythmia. 2016 doi: 10.1016/j.joa.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Voorhees AP, Han HC. Biomechanics of cardiac function. Compr Physiol. 2015 doi: 10.1002/cphy.c140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Azevedo PS, Polegato BF, Minicucci MF, Paiva SAR, Zornoff LAM. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arquivos brasileiros de cardiologia. 2016 doi: 10.5935/abc.20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lopez R, et al. Impaired Myocardial Energetics Causes Mechanical Dysfunction in Decompensated Failing Hearts. Function. 2020 doi: 10.1093/function/zqaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: Implications beyond atp production. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.D'Oria R, et al. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid Med Cell Longev. 2020:1–29. doi: 10.1155/2020/5732956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dumotier BM. A straightforward guide to the basic science behind arrhythmogenesis. Heart. 2014 doi: 10.1136/heartjnl-2014-305647. [DOI] [PubMed] [Google Scholar]
- 130.Hinderer S, Schenke-Layland K. Cardiac fibrosis – A short review of causes and therapeutic strategies. Advanced Drug Delivery Reviews. 2019 doi: 10.1016/j.addr.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 131.Tuomainen T, Tavi P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Experimental Cell Research. 2017 doi: 10.1016/j.yexcr.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 132.Iwata K, et al. Up-regulation of NOX1/NADPH oxidase following drug-induced myocardial injury promotes cardiac dysfunction and fibrosis. Free Radic Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.03.053. [DOI] [PubMed] [Google Scholar]
- 133.Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxidative Medicine and Cellular Longevity. 2016 doi: 10.1155/2016/1656450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scolletta S, Biagioli B. Energetic myocardial metabolism and oxidative stress: Let’s make them our friends in the fight against heart failure. Biomedicine and Pharmacotherapy. 2010 doi: 10.1016/j.biopha.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 135.Abraityte A, et al. Wnt5a is elevated in heart failure and affects cardiac fibroblast function. J Mol Med. 2017 doi: 10.1007/s00109-017-1529-1. [DOI] [PubMed] [Google Scholar]
- 136.Abraityte A, et al. Wnt5a is associated with right ventricular dysfunction and adverse outcome in dilated cardiomyopathy. Sci Rep. 2017 doi: 10.1038/s41598-017-03625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Austin KM, et al. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nature Reviews Cardiology. 2019 doi: 10.1038/s41569-019-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dawson K, Aflaki M, Nattel S. Role of the Wnt-Frizzled system in cardiac pathophysiology: A rapidly developing, poorly understood area with enormous potential. Journal of Physiology. 2013 doi: 10.1113/jphysiol.2012.235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lv X, et al. Overexpression of miR-27b-3p targeting Wnt3a regulates the signaling pathway of Wnt/β-catenin and attenuates atrial fibrosis in rats with atrial fibrillation. Oxid Med Cell Longev. 2019 doi: 10.1155/2019/5703764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parrotta EI, et al. Deciphering the role of wnt and rho signaling pathway in ipsc-derived arvc cardiomyocytes by in silico mathematical modeling. Int J Mol Sci. 2021;22:1–19. doi: 10.3390/ijms22042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo F, Yi X, Li M, Fu J, Li S. Snail1 is positively correlated with atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease. Exp Ther Med. 2017 doi: 10.3892/etm.2017.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dissanayake SK, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007 doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beljaars L, Daliri S, Dijkhuizen C, Poelstra K, Gosens R. WNT-5A regulates TGF-β-related activities in liver fibrosis. Am J Physiol - Gastrointest Liver Physiol. 2017 doi: 10.1152/ajpgi.00160.2016. [DOI] [PubMed] [Google Scholar]
- 144.Kato T, et al. Endothelial-mesenchymal transition in human atrial fibrillation. J Cardiol. 2017 doi: 10.1016/j.jjcc.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 145.Działo E, et al. WNT3a and WNT5a transported by exosomes activate WNT signaling pathways in human cardiac fibroblasts. Int J Mol Sci. 2019 doi: 10.3390/ijms20061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hagenmueller M, et al. Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett. 2014 doi: 10.1016/j.febslet.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, et al. Wnt5a-Mediated Neutrophil Recruitment Has an Obligatory Role in Pressure Overload-Induced Cardiac Dysfunction. Circulation. 2019 doi: 10.1161/CIRCULATIONAHA.118.038820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Anumonwo JMB, Herron T. Fatty infiltration of the myocardium and arrhythmogenesis: Potential cellular and molecular mechanisms. Frontiers in Physiology. 2018 doi: 10.3389/fphys.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lorenzon A, et al. Wnt/β-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget. 2017;8:60640–60655. doi: 10.18632/oncotarget.17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abou Ziki MD, Mani A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr Res. 2019;70:18–25. doi: 10.1016/j.nutres.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang J, et al. Vibration and β-hydroxy-β-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J Cachexia Sarcopenia Muscle. 2020;11:564–577. doi: 10.1002/jcsm.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S, et al. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 2015;29:3436–3445. doi: 10.1096/fj.15-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac Energy Metabolism in Heart Failure. Circ Res. 2021:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X, et al. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail Rev. 2021 doi: 10.1007/s10741-021-10096-8. [DOI] [PubMed] [Google Scholar]
- 155.Moorer MC, Riddle RC. Regulation of Osteoblast Metabolism by Wnt Signaling. Endocrinol Metab. 2018;33:318–330. doi: 10.3803/EnM.2018.33.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mo Y, et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J Cancer. 2019;10:3789–3797. doi: 10.7150/jca.31166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Serrat R, et al. The Non-Canonical Wnt/PKC Pathway Regulates Mitochondrial Dynamics through Degradation of the Arm-Like Domain-Containing Protein Alex3. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0067773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arrázola MS, Silva-Alvarez C, Inestrosa NC. How the Wnt signaling pathway protects from neurodegeneration: The mitochondrial scenario. Front Cell Neurosci. 2015;9:1–13. doi: 10.3389/fncel.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li H-X, Lin J, Jiang B, Yang X-J. Wnt11 preserves mitochondrial membrane potential and protects cardiomyocytes against hypoxia through paracrine signaling. J Cell Biochem. 2020;121:1144–1155. doi: 10.1002/jcb.29349. [DOI] [PubMed] [Google Scholar]
- 160.Zhu L, et al. Upregulation of non-canonical Wnt ligands and oxidative glucose metabolism in NASH induced by methionine-choline deficient diet. 2019:47–56. [PMC free article] [PubMed] [Google Scholar]
- 161.Heusch G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 162.van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen B, et al. Co-expression of Akt1 and Wnt11 promotes the proliferation and cardiac differentiation of mesenchymal stem cells and attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Biomed Pharmacother. 2018;108:508–514. doi: 10.1016/j.biopha.2018.09.047. [DOI] [PubMed] [Google Scholar]
- 164.Matsushima S, Tsutsui H, Sadoshima J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends in Cardiovascular Medicine. 2014 doi: 10.1016/j.tcm.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nakamura K, et al. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J Biol Chem. 2016 doi: 10.1074/jbc.M115.693937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang P. CaMKII: The molecular villain that aggravates cardiovascular disease. Exp Ther Med. 2017;13:815–820. doi: 10.3892/etm.2017.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 168.Blagodatski A, Poteryaev D, Katanaev VL. Targeting the Wnt pathways for therapies. Mol Cell Ther. 2014;2:28. doi: 10.1186/2052-8426-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 170.Naik P, Marwah M, Venkataraman M, Rajawat GS, Nagarsenker M. Drug Delivery Approaches and Nanosystems, Volume 2. Drug Delivery Approaches and Nanosystems: Volume 2 Drug Targeting Aspects of Nanotechnology. Apple Academic Press; 2017. [DOI] [Google Scholar]
- 171.Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40–R46. doi: 10.1093/hmg/ddu125. [DOI] [PubMed] [Google Scholar]
- 172.Crooke ST. Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid Ther. 2017;27:70–77. doi: 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reichert J. Monoclonal Antibodies as Innovative Therapeutics. Curr Pharm Biotechnol. 2008;9:423–430. doi: 10.2174/138920108786786358. [DOI] [PubMed] [Google Scholar]
- 174.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 176.Luo Y-L, et al. Macrophage-Specific in Vivo Gene Editing Using Cationic Lipid-Assisted Polymeric Nanoparticles. ACS Nano. 2018;12:994–1005. doi: 10.1021/acsnano.7b07874. [DOI] [PubMed] [Google Scholar]
- 177.Schulte G, Bryja V. WNT signalling: mechanisms and therapeutic opportunities. Br J Pharmacol. 2017 doi: 10.1111/bph.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li J, et al. Deficiency of Rac1 Blocks NADPH Oxidase Activation, Inhibits Endoplasmic Reticulum Stress, and Reduces Myocardial Remodeling in a Mouse Model of Type 1 Diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Porte B, Marguerit G, Thomasseau S, Paquet C, Hugon J. Dose-dependent neuroprotective effect of the JNK inhibitor Brimapitide in 5xFAD transgenic mice. Brain Res. 2020;1727:146587. doi: 10.1016/j.brainres.2019.146587. [DOI] [PubMed] [Google Scholar]
- 180.Greenberg S, et al. Diffuse Parenchymal Lung Disease. European Respiratory Society; 2017. Late Breaking Abstract - Evaluation of the JNK inhibitor, CC-90001, in a phase 1b pulmonary fibrosis trial; OA474 [DOI] [Google Scholar]
- 181.Ebenebe O, Heather A, Erickson J. The Role of CaMKII in Atherosclerotic Plaque Calcification in the ApoE-null Mouse. Hear Lung Circ. 2017;26:S65. [Google Scholar]
- 182.He Q, Cheng J, Wang Y. Chronic CaMKII inhibition reverses cardiac function and cardiac reserve in HF mice. Life Sci. 2019;219:122–128. doi: 10.1016/j.lfs.2019.01.010. [DOI] [PubMed] [Google Scholar]