Abstract
Background
Expert opinion is that about 20% of emergency stroke patients should receive thrombolysis. Currently 11-12% of patients in England and Wales receive thrombolysis, ranging from 2% to 24% between hospitals. The aim of this study was to assess how much variation is due to differences in local patient populations, and how much is due to differences in clinical decision making and stroke pathway performance, while estimating a realistic target thrombolysis use.
Methods
Anonymised data for 246,676 emergency stroke admissions to 132 acute stroke teams in England and Wales between 2016 and 2018 was obtained from the Sentinel Stroke National Audit Programme data. We used machine learning to learn decisions who to give thrombolysis to at each hospital. We used clinical pathway simulation to model effects of changing pathway performance. Qualitative research was used to assess clinician attitudes to these methods. Three changes were modelled: 1) arrival-to-treatment in 30 minutes, 2) proportion of patients with determined stroke onset times set to at least the national upper quartile, 3) thrombolysis decisions made based on majority vote of a benchmark set of hospitals.
Results
Of the modelled changes, any single change was predicted to increase national thrombolysis use from 11.6% to between 12.3% to 14.5% (clinical decision-making having the most effect). Combined, these changes would be expected to increase thrombolysis to 18.3%, but there would still be significant variation between hospitals depending on local patient population. Clinicians engaged well with the modelling, but those from hospitals with lower thrombolysis use were most cautious about the methods.
Conclusions
Machine learning and clinical pathway simulation may be applied at scale to national stroke audit data, allowing extended use and analysis of audit data. Stroke thrombolysis rates of at least 18% look achievable in England and Wales, but each hospital should have its own target.
Keywords: stroke, thrombolysis, alteplase, machine learning, simulation, health services research
Introduction
For ischaemic strokes, thrombolysis is an effective treatment for the management of acute stroke if given soon after stroke onset 1, and is recommended for use in many parts of the world including the US and Europe. In the 2019-2020 stroke national audit of England and Wales 2, thrombolysis use was 11.7% overall, with use by individual stroke teams ranging from 4.3 to 28.1%. Similar overall rates are observed in the US. Between 2012 and 2018, thrombolysis use in the US increased from 6.3% to 11.8% 3 and rates of about 11.5% have been maintained since, including through to June 2020 during the Covid pandemic 4.
The use of targets for thrombolysis varies across the world. The European Stroke Organisation have prepared a European Stroke Action Plan 5, and have suggested a European target of at least 15% thrombolysis, with median onset-to-needle (also known as onset-to-treatment) times of <120 minutes, noting that evidence suggests that achieving these targets may be aided by centralisation of stroke services 6,7. In the UK the NHS long term plan includes a target of 20% of emergency stroke admissions being treated with thrombolysis 8, and this is reflected by the NHS Sentinel Stroke National Audit Programme which monitors stroke care in the UK (excluding Scotland) 9. There are no overall targets for thrombolysis use in the US but the ‘Get with the guidelines – stroke’ programme 10 from the American Heart Association provides a quality improvement tool for hospital which includes monitoring tools for thrombolysis use and targets such as door-to-needle in 60 minutes. Though there are no specific targets for thrombolysis use in the US there have been considerable efforts to improve use and speed of thrombolysis, such as combining expert telemedicine consultation with the use of the Helsinki model for streaming the thrombolysis pathway 11.
An analysis of the IST-3 trial for thrombolysis concluded that 60% of ischaemic stroke patients arriving within 4 hours of known stroke onset were suitable for thrombolysis 12. Assuming 40% of patients arrive within 4 hours of known stroke onset, and assuming 85% of stroke is ischaemic, this gives a potential target of 20% thrombolysis (in 2016-18 in England and Wales, 37% of emergency stoke patients arrived within 4 hours of known stroke onset; see results section of this report).
There is therefore still a gap between clinical expert opinion and analysis on target use of thrombolysis, and actual use of thrombolysis.
In work described here we use machine learning and clinical pathway simulation to ask a series of ‘what if?’ questions about the thrombolysis pathway at each hospital – examining the effect of changing pathway speed or adopting the clinical decision-making of other hospitals. By examining these scenarios, we can produce a realistic target thrombolysis use, and resulting clinical benefit, for each hospital based on the hospital’s own emergency stroke admissions population. We also used qualitative research to understand the potential influence these modelling outputs can have on clinicians, with the aim to best support the maximal appropriate use of thrombolysis and reduce unnecessary variation. Though this work focusses on England and Wales, the methodology should be applicable to other geographies.
Methods
Original data for this project cannot be shared, but all analysis code and results may be found in an accompanying on-line book https://samuel-book.github.io/samuel-1 (DOI: 10.5281/zenodo.5078131).
The supplementary material contains more detail on data access (section 1), data fields (section 2), machine learning (section 3) and clinical pathway simulation (section 4). We followed the Turing Way 13 to produce detailed methodology, code, and all results, for this work, available in the accompanying on-line book. The supplementary material and accompanying on-line book adhere to the STRESS guidelines for reporting simulation studies14, and reporting of the machine learning models followed the TRIPOD guidelines15.
Data
Data was obtained from the Sentinel Stroke National Audit Programme (SSNAP), managed through the Healthcare Quality Improvement Partnership. SSNAP has near-complete coverage of all acute stroke admissions in the UK (outside Scotland). All hospitals admitting acute stroke participate in the audit, and year-on-year comparison with Hospital Episode Statistics confirms estimated case ascertainment of 95% of coded cases of acute stroke. The NHS Health Research Authority decision tool was used to confirm that ethical approval was not required to access the data. Data access was authorised by the UK Healthcare Quality Improvement Partnership (reference HQIP303).
Data was retrieved for 246,676 emergency stroke admissions to acute stroke teams in England and Wales between 2016 and 2018 (three full years). The 62 fields retrieved for each patient are given in the supplement.
Analysis environment
All analysis code was written in Python 3.8. Data manipulation, simulation, and general mathematical modelling was done using NumPy 16 v1.19 and Pandas 17 v1.2.0. Machine learning libraries used were Tensorflow 18 v2.2.6, Scikit-Learn 19 v0.23.2, Seriate v.1.1.2. All charts were produced with MatPlotLib 20 v3.3.2. All analyses were conducted in Jupyter-Lab 21 v 2.2.6.
Machine learning
Machine learning models were trained to predict whether a patient would receive thrombolysis or not at each hospital. Patients for machine learning prediction were restricted to those arriving within 4 hours of known stroke onset. Machine learning models were built either for individual hospitals, or were built to model all hospital simultaneously with hospital as a feature. Accuracy was measured using stratified k-fold validation. Two main accuracy measures are reported: % accuracy (the percentage of predictions that were correct), and Receiver Operator Characteristic (ROC) Area Under Curve (AUC). We also report the highest value of sensitivity and specificity that may be achieved together (the point where sensitivity and specificity curves cross each other as classification threshold is adjusted).
A total of 51 variables for pseudonymised patients were extracted from SSNAP for the machine learning. These covered a pseudonymised stroke team, patient characteristics (e.g. age band, gender ethnicity); pathway information (e.g onset to arrival minutes, onset known or unknown, mode of arrival, door to needle time); patient comorbidities (e.g. hypertension, pre-stroke diabetes mellitus, anticoagulant history); National Institutes of Health Stroke Scale; other clinical features (e.g. stroke type, transient ischemic attack in the last month); if thrombolysis was given; and reasons for not giving thrombolysis (e.g. age, co-morbidity, time, onset time unknown). No field directly informing whether thrombolysis was given (such as reasons for not giving thrombolysis) was used. The full list of variables and description can be found in the supplement.
We report on logistic regression, random forest, and neural network models, as described in more detail in the online supplement. Subsequent work uses hospital-based random forest models. We chose these models due to their good performance, easier explainability to clinicians, and strong hospital independence.
A benchmark set of hospitals was identified by passing the same cohort of 10k patients through all hospital models (this cohort was not used in training of the hospital models). The 30 hospitals with the highest predicted thrombolysis use in this cohort of patients were identified as benchmark hospitals. All hospitals’ patients were passed through this cohort of 30 hospitals to predict thrombolysis use (yes or no) at each benchmark hospital. A majority vote was used to classify a patient as ‘would receive thrombolysis’.
Clinical pathway simulation
Hyperacute stroke pathways are subject to variation in onset to arrival times, scanning, clinical decision making and other factors. We captured this variation using a Monte Carlo simulation model of the clinical pathway. The clinical pathway simulation is based on passing individual virtual patients through a stroke pathway. Each scenario passes approximately 8 million patients through the model (100 years of patients through each hospital). To attain the required speed the pathway simulation was coded using NumPy arrays in Python. Baseline process times (onset to arrival, time to scan, time from scan to treatment), and whether stroke onset time is determined were based on distributions fitted to each hospital’s data. The proportion of patients with known stroke onset was taken from the hospital’s own data. Likelihood to receive thrombolysis if scanned with time to treat in the baseline model was taken from the proportion of patients who were scanned within four hours of known stroke onset at each hospital.
Key process steps in the pathway are shown in Figure 1. Patients could leave the pathway at each step if their pathway durations exceed the permitted time limits, or they become ineligible for treatment. Only patients that satisfied all restrictions continued along the full length of the pathway and received thrombolysis. The outcome was then calculated as a probability of having a good outcome of modified Rankin Scale (mRS) score of 0-1. If the patient did not receive thrombolysis the probability of a good outcome was the baseline non-thrombolysed probability of a good outcome in the population age group (aged under 80 years, or aged 80+ years). If the patient received thrombolysis then the probability of a good outcome was based on age group and time to treatment as we have described in detail elsewhere 22.
Figure 1. Schematic representation of the stroke pathway as simplified for the simulation.
Three alternative ‘what if?’ scenarios were investigated for each hospital:
Base: Uses the hospitals’ recorded pathway statistics in SSNAP.
Speed: Sets 95% of patients having a scan within 4 hours of arrival, and all patients have 15 minutes arrival-to-scan time and 15 minutes scan-to-needle time.
Onset-known: Sets the proportion of patients with a known stroke onset time to the national upper quartile if currently less than the national upper quartile (leave any greater than the upper national quartile at their current level).
Benchmark: The benchmark thrombolysis rate takes the likelihood to give thrombolysis for patients scanned within 4 hours of onset from the majority vote of the 30 hospitals with the highest predicted thrombolysis use in a standard 10K cohort set of patients. These are from hospital-based random forest models.
Qualitative research
The overall objective of the qualitative research was to understand influence of modelling, including the use of machine learning techniques, in the context of the national audit, in order to support efforts to maximise the appropriate use of thrombolysis and reduce unnecessary variation. This was performed by a mixture of face-to-face or remote semi-structured interviews with 19 clinicians (18 medics and one specialist stroke nurse) either in groups or individually. Participants were chosen from hospitals with a range in current thrombolysis use (we sampled equally from tertiles of thrombolysis use: < 9.0%, 9.0 to 13.9%, and 14% and above. All data were anonymised. Ethical approval was provided by the UK Health Research Authority (HRA) and Health and Care Research Wales (HCRW) 19/HRA/5796. A framework analysis of the transcripts was performed with four broad objectives:
Explore current understanding and rationale for the use of thrombolysis for ischaemic stroke, in order to establish reasons for the variance in the use and speed of thrombolysis.
Understand physician perspectives on simulation and machine learning feedback, to influence how simulation can be incorporated into the Sentinel Stroke National Audi Programme (SSNAP) to have a positive impact on practice.
Identify potential routes for the implementation of machine learning feedback, to inform and improve future stroke management.
Explore how physicians interpret the potential consequences of following changes in pathway suggested by simulation.
(See supplement section 5 for further methodological details).
Results
Machine learning
Table 1 shows the accuracy of machine learning models. Models ranged from 81% to 86% accuracy depending on model type. The model with the highest accuracy, by a small margin, was a neural network using embedding layers for hospital ID, clinical features of the patients, and pathway timings. For the remaining experiments used hospital-level random forest models (with 84.3% accuracy). These models have slightly lower accuracy than other models, but have easier explainability and strong hospital-independence. Learning curves suggested that the accuracy of the hospital-level random forest models were limited a little by the data size available for each hospital. While overall accuracy was 81.4%, accuracy reached about 83% with a training set size of at least 500 cases per hospital and dropped below 80% with a training set size of fewer than 125 cases per hospital. 48% of stroke teams had a training set size of at least 500, and 97% had a training set size of at least 125.
Table 1.
Machine learning accuracy. Accuracy was assessed using stratified 5-fold stratified cross-validation. Single model fits encode hospital ID as one-hot features. Hospital-level models fitted a model to each hospital independently. Embedding neural nets encoded hospital id, pathway data, and clinical data into a single value vector each. [a] Receiver operator characteristic area under curve. Accuracy and ROC-AUC show mean results and 95% confidence limits. [b]. The maximum value of sensitivity and specificity that may be attained simultaneously
| Model | Accuracy % (95% CI) |
ROC-AUC (95% CI) [a] |
Max Sens=Spec (%) [b] |
|---|---|---|---|
| Logistic regression single model | 83.2 (0.2) | 0.904 (0.001) | 82.0 |
| Logistic regression hospital-level models | 80.6 (0.2) | 0.870 (0.001) | 78.9 |
| Random forest single model | 84.6 (0.2) | 0.914 (0.001) | 83.7 |
| Random forest hospital-level models | 84.3 (0.2) | 0.906 (0.001) | 83.2 |
| Fully-connected neural net single model | 84.4 (0.2) | 0.913 (0.001) | 83.3 |
| 1D Embedding neural net single model | 85.5 (0.2) | 0.921 (0.001) | 84.5 |
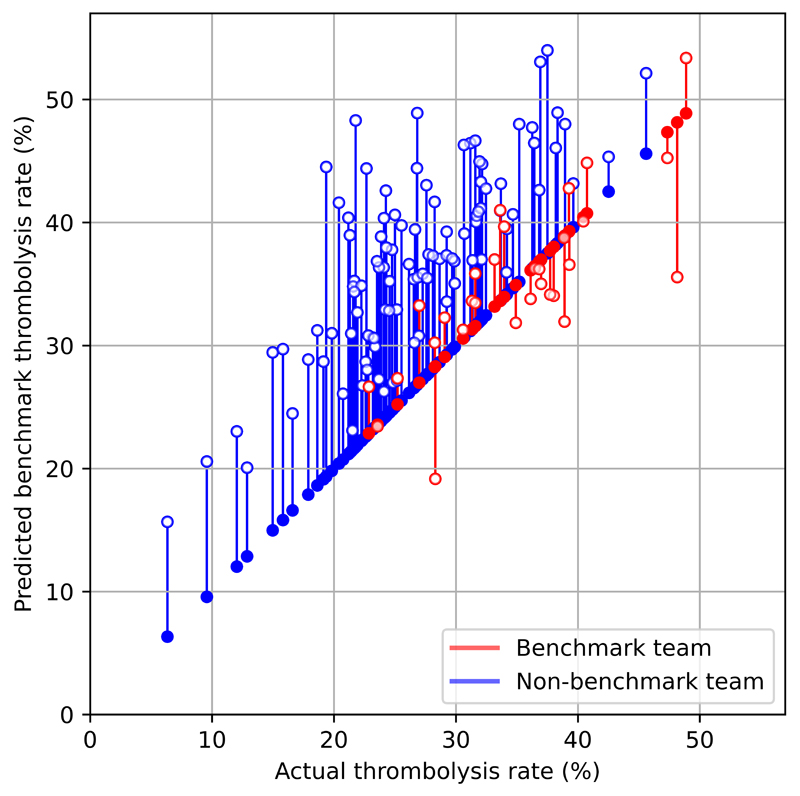
The predicted thrombolysis use at each hospital according to the majority vote of 30 benchmark hospital clinical decision models is shown in Figure 2. When results are weighted by the number of patients attending each hospital, using the benchmark hospital models to decide if a patient would receive thrombolysis, national thrombolysis use would increase from 29.5% to 36.9% of patients arriving within 4 hours of known stroke onset.
Figure 2.
A comparison of actual thrombolysis rate at each hospital and the predicted thrombolysis rate if decisions were made according to the majority vote of the 30 benchmark hospitals. Thrombolysis rate is predicted for patients arriving within 4 hours of known stroke onset. The solid circle shows the current thrombolysis use, and the open circle shows the thrombolysis use predicted by a majority vote of the benchmark hospitals. The red points are those hospitals that are in the top 30 thrombolysing hospitals (the benchmark set) when cohort thrombolysis use is predicted, with all other hospitals coloured blue.
When comparing decisions for the 10k cohort predicted by the model we found that overall, 78% of patients would have a treatment decision agreed by 80% of hospitals. However, there was more agreement around those not to give thrombolysis to than those who received thrombolysis: of those who were not given thrombolysis, 85% had agreement by 80% hospitals, whereas of those who were given thrombolysis, 60% had agreement by 80% hospitals.
Clinical pathway simulation
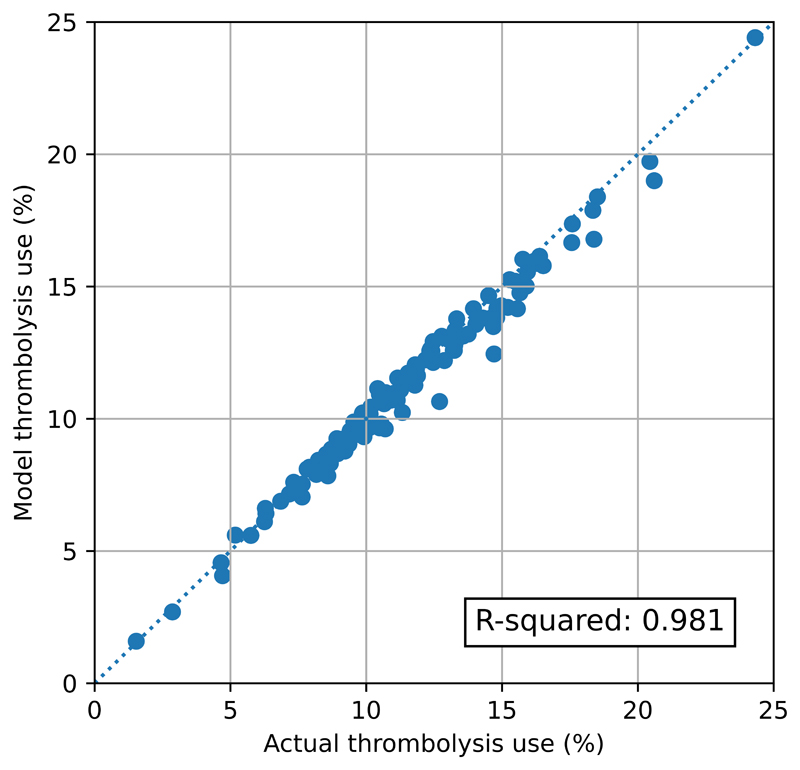
The pathway model reliably replicated the thrombolysis use in hospitals (Figure 3). Predicted thrombolysis use correlated with actual thrombolysis use with an R-squared of 0.979. The mean thrombolysis use (averaged at hospital-level, weighting all hospitals equally) was 11.45% in the observed data, and 11.23% in the pathway model output. The mean difference in thrombolysis use between predicted and actual was 0.22 percentage points. The mean absolute difference in thrombolysis use between predicted and actual was 0.52 percentage points.
Figure 3.
Validation of the stroke thrombolysis pathway model. The x-axis shows the actual thrombolysis use in each hospital (for patients with an out-of-hospital onset stroke), and the y-axis shows the thrombolysis use predicted from the pathway model. Model parameters were based on pathway statistics for each hospital. The dotted line shows a 1:1 correlation between actual and predicted values.
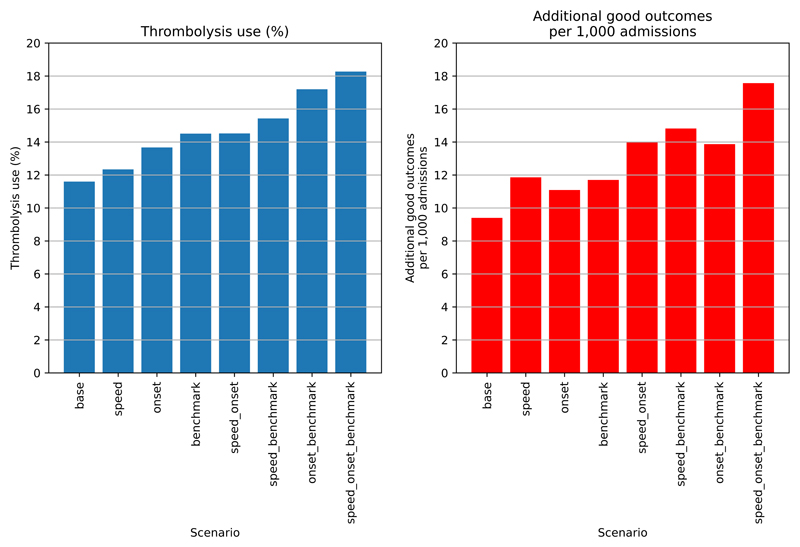
Figure 4 shows the overall net effect of separate and combined changes to the stroke pathway. The pathway simulation suggests that thrombolysis use could potentially be increased from 11.6% to 18.3% of all emergency admissions, and the clinical benefit increased from 9.4 to 17.6 additional good outcomes per 1K admissions. The main drivers in improvement in thrombolysis use are: benchmark decisions > determining stroke onset > speed, while the main drivers in improvement in outcomes are: speed > benchmark decisions > determining stroke onset.
Figure 4.
Net national changes in thrombolysis use (left) or clinical benefit defined as number of additional good outcomes (mRS0-1 at 3-6 months) per 1,000 emergency stroke admission (right) by changing aspects of the stroke pathway (speed of stoke pathway, determining stroke onset time, and using benchmark decisions). Results show effects across all 132 English stroke units, with averages weighted by admission numbers.
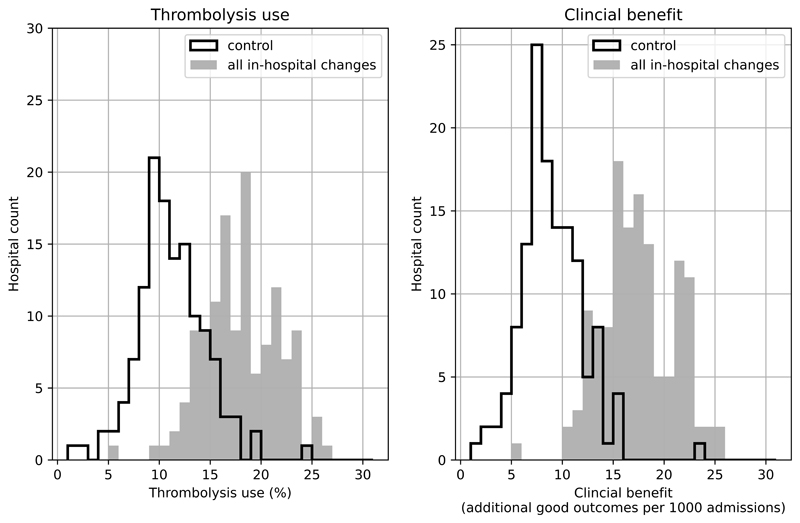
Figure 5 shows the distribution of use of, and benefit from, thrombolysis before and after all the modelled changes. It is noteworthy that there is still significant variation between hospitals, but that the distributions have been shifted the right.
Figure 5.
Histograms for changes in distribution in thrombolysis use (left) or clinical benefit defined as number of additional good outcomes (mRS0-1 at 3-6 months) per 1,000 emergency stroke admission (right) by combining changes to speed (95% of patients have 15 minutes arrival-to-scan and 15 minute scan-to-treatment, with other patients not being scanned within 4 hours of arrival), determining stroke onset time (to the national upper quartile if currently lower), and using benchmark decisions. The unshaded histogram shows the current base-case use of, and benefit from, thrombolysis, and the grey shaded histogram shows the predictions with all changes.
Qualitative research
Key findings from the qualitative research were:
Broadly, those units with higher thrombolysis use engaged more positively with the research, and those with lower thrombolysis use were more cautious.
Clinicians from lower thrombolysing units tended to emphasise differences in their patients as the reason for lower thrombolysis. Those in mid-rate thrombolysis units tended to emphasis access to specialist resources as being key in being able to deliver thrombolysis well. Those in higher thrombolysing units tended to emphasise the work and investment that had gone in to establishing a good thrombolysis pathway.
Clinicians wanted to see the machine learning models expanded to predict probability of good outcome and adverse effects of thrombolysis.
Despite this being a small study, physicians engaged with the machine learning process and outcomes, suggesting ways in which the outputs could be modified for feedback to stroke centres, and utilised to inform thrombolytic decision-making.
(See supplement section 5 for detailed qualitative research results).
Discussion
This work represents a novel approach to understanding the persisting variation that still exists in thrombolysis practice in England and Wales, and to developing new ways of supporting efforts to reduce that variation, and in so doing increase the clinical benefit to people with acute ischaemic stroke from thrombolysis. Though our study focuses on England and Wales, the approach should be applicable to other geographies. Not all countries currently collect the level of data that is collected in England and Wales, but we hope that by demonstrating how comprehensive national registry data may be put to use we may help encourage the broader collection of comprehensive clinical registry data sets.
Thrombolysis using alteplase was recommended in the UK in 2008 for acute ischaemic stroke within 4.5 hours of known onset, and initially usage increased rapidly to over 10% on average nationally 23. However since 2013, thrombolysis use has remained static at 11-12% 24. Furthermore, that national average conceals substantial variation in alteplase use between UK hospitals – as reported here, a five-fold variation in the overall thrombolysis rate.
Our aim was to use a national stroke clinical registry and the state-of-the-art tools of machine learning and clinical pathway simulation to gain a better understanding of what variation is due to processes and decision-making, and what a realistic target of thrombolysis use may be in England and Wales. One of our principal objectives was to develop, through the combination of pathway modelling and machine learning, bespoke outputs for individual hyperacute stroke centres that could be incorporated into routine reporting through national audit, moving away from a single identical target for thrombolysis use for all hospitals. We identified several clear and readily implementable changes to processes and decision-making in hyperacute stroke that together could achieve a 58% increase in the number of patients treated with alteplase in the UK, and result in a near-doubling of the clinical benefit from thrombolysis. Full implementation of the pathway changes identified in this study would go a long way towards achieving the stated ambition of the Long Term Plan for the NHS of bringing the UK up to among the best in Europe for reperfusion treatment for acute stroke 8.
Among the changes tested, clinical decision-making had the greatest single effect. Our work therefore confirms, and adds to, previous work from discrete choice experiments which showed that clinicians vary in their attitudes, regarding thrombolysis, to individual clinical features of patients 25.
Despite this, these machine learning techniques could not purport to replace clinical judgement in the decision about treating any individual patient, and could not be used to provide a definitive predictive model. The highest performing model, embedding neural networks, achieved 84% sensitivity and specificity simultaneously. The hospital-specific random forest model, which had 81% accuracy for the decision to thrombolyse (and could attain 78% sensitivity and specificity simultaneously), identifies agreement among 80% of hospitals in the decision to treat a patient in 60% of cases, and for the decision not to treat, agreement among a similar proportion of hospitals in the decision not to treat in 85% of cases. Apart from anything else, these observations confirm that unanimity in the decision to treat across all 132 hospitals contributing data is highly unusual, though it is easier to find agreement on who not to treat than who to treat. So the outputs from the machine learning can only ever be used probabilistically and to look for general patterns in thrombolysis use that may be used as a stimulus to scrutinise decision-making in audit at a local level. In this respect, it is more useful for the benchmarking process, in which the willingness to thrombolyse in an individual hospital is compared to other hospitals.
Pathway changes have been previously shown to have a significant effect on thrombolysis rates and door-to-needle time, for example by Meretoja and colleagues in a number of different settings 26,27. These projects would indicate that the 30-minute door-to-needle time proposed in our model is not an unrealistic or unachievable target.
A familiar pitfall when addressing clinical variation is that the phrase ‘if only all sites were as good as the best’ is, by definition, an oxymoron and lacks credibility with clinical teams who are far short of the best and/or struggling to improve. We have therefore sought to neutralise this pitfall through the use of a much more conservative approach – modelling based either on the typical clinical behaviour of just the ‘top 30’ hospitals or the top-quartile performance for the acquisition of a known onset time. This presents poorly-performing hospitals with a much more credible and achievable objective – you don’t need to be as good as the best, often regarded as unachievable, but merely match the performance of a better-than-average site, of which there are many.
Our method also addresses another familiar objection regarding high-performing centres – that such sites are needlessly thrombolysing mild strokes or even stroke mimics, although the latter are excluded from the SSNAP data used in this study. This issue arose in our linked qualitative work with low-thrombolysing sites. In our cohort of 10K standardised stroke patients presenting within 4 hours, half of the ‘top 30’ thrombolysing sites would have a lower thrombolysis rate after benchmarking. The moderating effect of a broad-based machine learning method removes extremes at both ends of the scale, but still contributes to a substantial increase in the overall thrombolysis rate for nearly all sites and a correspondingly greater population benefit. It would seem not unreasonable therefore, to anticipate an achievable national thrombolysis rate for patients presenting within 4 hours to be 36.9% compared to the current figure of 29.5%.
Limitations
A Limitation of the study is the amount of data available per individual. Though the current data is sufficient to predict use of thrombolysis with 85% accuracy there is sure to be factors influencing decision-making that are not included in the data set. This limitation does not negate the use of these models to explore patterns of thrombolysis use, but underly the importance that these tools should not be used for individual case decision-making.
Our predictions about differences in clinical decision-making are necessarily at site level. We pick up on general differences in attitude to thrombolysis between sites, but we cannot detect differences that exist between individual clinicians (as decision-making is the end-result of a process, and may be collective, it may be that decisions can never be fully assigned to an individual). For this work we were not able to identify the site and so could not look relationships with factor such as rurality, or organisation factors such as whether a stroke team employs a specialist stroke nurse to facilitate the emergency stroke pathway.
The machine learning model described only predicts use of thrombolysis and does not predict outcomes directly. In future work we plan to use machine learning also to predict outcomes (such as death, mRS on discharge, and thrombolysis-induced haemorrhage; such quality outcomes will also help assess the risk of treatment worsening outcomes in individual patients). We predict likelihood of a good outcome based on clinical trial meta-analysis on the relationship between time to thrombolysis (if given) and the probability of a ‘good’ outcome, measured as having a mRS of 0-1 at 3-6 months. It should be noted that this reflects an ‘excellent’ disability-free outcome, and does not incorporate all the benefit of thrombolysis (such as if a patient were improved from a mRS of 5 to 4).
The remaining limitations relate to the potential for implementation. Our qualitative sub-study identified the paradox that it was the confident thrombolysing physicians who were most open to the influence of machine learning and other methods of quality improvement, but who also needed it the least. Successfully engaging with a large and disparate group of middling-to-low thrombolysing sites and clinicians less open to these methods for improvement presents significant challenges and could blunt the impact and resultant benefits. Further research is likely to help address how the concerns of the lower thrombolysing units may be best addressed.
Implications for healthcare
Overall, our results suggest that England and Wales can get close to the target of 20% of emergency stroke admissions receiving thrombolysis, but this should not be seen as a single target for all hospitals. Realistically achievable thrombolysis use depends on local patient populations, so a universal target of 20% across all hospitals may over-estimate what is achievable at some hospitals, while under-estimating what is achievable at other hospitals. Local agreed targets may be more appropriate.
The tools developed here have the potential to add further depth of analysis to the national stroke audit outputs, providing each stroke team with more in-depth analysis of what an achievable use of thrombolysis may be in their hospital, and what changes to pathway or decision-making would help drive most improvement.
Conclusions
Machine learning and clinical pathway simulation may be applied at scale to national audit data, allowing extended use and analysis of audit data. These models may help hospitals identify what would most improve benefit from thrombolysis use (if improvement is needed), and identify realistic targets for hospitals given their own patient populations. We can identify patterns of differences in clinical decision-making between hospitals.
Our models have good accuracy. Decision-making can be predicted with 85% accuracy for those patients with a chance of receiving thrombolysis (arriving within four hours of stroke onset). This accuracy enables us to look for patterns in clinical decision-making in and between hospitals. Clinical pathway simulation predicts hospital thrombolysis use with an average absolute error of 0.5 percentage points.
Stroke thrombolysis rates of at least 18% look achievable in England and Wales, but each hospital should have its own target.
Supplementary Material
Acknowledgements
We thank our public and patient involvement representative, Leon Farmer and Penny Thompson, who were involved throughout the project and who helped the project develop deeper understanding and better ways of communicating results.
Sources of funding
This report is independent research funded by the National Institute for Health Research (NIHR) Applied Research Collaboration South West Peninsula and the National Institute for Health Research Health and Social Care Delivery Research (HSDR) Programme. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Footnotes
Disclosures
None.
Non-standard Abbreviations and Acronyms
None
References
- 1.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sentinel Stroke National Audit. Apr2019Mar2020-AnnualResultsPortfolio. [Accessed Jun1 1, 2021]. Available at https://www.strokeaudit.org/Documents/National/Clinical/Apr2019Mar2020/Apr2019Mar2020-AnnualResultsPortfolio.aspx .
- 3.Anand SK, Benjamin WJ, IV, Adapa AR, Park JV, Andrew Wilkinson D, Daou BJ, Burke JF, Pandey AS. Trends in Acute Ischemic Stroke Treatments and Mortality in the United States from 2012 to 2018. Neurosurg Focus. 2021;51:E2. doi: 10.3171/2021.4.FOCUS21117. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava PK, Zhang S, Xian Y, Xu H, Rutan C, Alger HM, Walchok JG, Williams JH, De Lemos JA, Decker-Palmer MR, et al. Treatment and Outcomes of Patients with Ischemic Stroke during COVID-19: An Analysis from Get with the Guidelines-Stroke. Stroke. 2021;52:3225–3232. doi: 10.1161/STROKEAHA.120.034414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norrving B, Barrick J, Davalos A, Dichgans M, Cordonnier C, Guekht A, Kutluk K, Mikulik R, Wardlaw J, Richard E, et al. Action Plan for Stroke in Europe 2018-2030. Eur Stroke J. 2018;3:309–336. doi: 10.1177/2396987318808719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray BD, Campbell J, Cloud GC, Hoffman A, Tyrrell PJ, Wolfe CDAA, Rudd AG. Bigger, faster?: Associations between hospital thrombolysis volume and speed of thrombolysis administration in acute ischemic stroke. Stroke. 2013;44:3129–3135. doi: 10.1161/STROKEAHA.113.001981. [DOI] [PubMed] [Google Scholar]
- 7.Lahr MMH, Luijckx GJ, Vroomen PCAJ, Van Der Zee DJ, Buskens E. Proportion of patients treated with thrombolysis in a centralized versus a decentralized acute stroke care setting. Stroke. 2012;43:1336–1340. doi: 10.1161/STROKEAHA.111.641795. [DOI] [PubMed] [Google Scholar]
- 8.NHS. The NHS Long Term Plan. [Accessed Jun1 1, 2021]. Available at https://www.longtermplan.nhs.uk/wpcontent/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf.
- 9.HQIP. Sentinel Stroke National Audit Programme - Annual Report 2019-20. [Accessed Jun1 1, 2021]. Available at https://www.hqip.org.uk/resource/sentinel-stroke-national-audit-programme-annual-report-2019-20/
- 10.American Heart Association. Get With The Guidelines®– Stroke. [Accessed February 2, 2022]. Available at https://www.heart.org/en/professional/quality-improvement/get-with-the-guidelines/get-with-the-guidelines-stroke.
- 11.Nguyen-Huynh MN, Klingman JG, Avins AL, Rao VA, Eaton A, Bhopale S, Kim AC, Morehouse JW, Flint AC. Novel telestroke program improves thrombolysis for acute stroke across 21 hospitals of an integrated healthcare system. Stroke. 2018;49:133–139. doi: 10.1161/STROKEAHA.117.018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bembenek J, Kobayashi A, Sandercock P, Czlonkowska A. How many patients might receive thrombolytic therapy in the light of the ECASS-3 and IST-3 data? Int J Stroke. 2010;5:430–431. doi: 10.1111/j.1747-4949.2010.00479.x. [DOI] [PubMed] [Google Scholar]
- 13.The Turing Way Community. The Turing Way: A handbook for reproducible data science. [Accessed Jun1 1, 2021]. https://the-turing-way.netlify.app/
- 14.Monks T, Currie CSM, Onggo BS, Robinson S, Kunc M, Taylor SJEP. Strengthening the reporting of empirical simulation studies: Introducing the STRESS guidelines. J Simul. 2019;13:55–67. [Google Scholar]
- 15.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350 doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 16.Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, et al. Array programming with NumPy. Nature. 2020;585:357–362. doi: 10.1038/s41586-020-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinney W. pandas: a foundational Python library for data analysis and statistics. Python High Perform Sci Comput. 2011;14 [Google Scholar]
- 18.Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, Citro C, Corrado GS, Davis A, Dean J, Devin M, Ghemawat S. Tensorflow: Large-scale machine learning on heterogeneous distributed systems. arXiv. 2016:1603.04467 [Google Scholar]
- 19.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, et al. Scikit-learn: Machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 20.Hunter JD. Matplotlib: A 2D graphics environment. Comput Sci & Eng. 2007;9:90–95. [Google Scholar]
- 21.Kluyver T, Ragan-Kelley B, Pérez F, Granger B, Bussonnier M, Frederic J, Kelley K, Hamrick J, Grout J, Corlay S, et al. In: Positioning and Power in Academic Publishing: Players, Agents and Agendas. Loizides F, Schmidt B, editors. 2016. Jupyter Notebooks -- a publishing format for reproducible computational workflows; pp. 87–90. [Google Scholar]
- 22.Allen M, Pearn K, Stein K, James M. Estimation of stroke outcomes based on time to thrombolysis and thrombectomy. medRxiv. 2020:2020.07.18.20156653 [Google Scholar]
- 23.Royal College of Physicians. How Good is Stroke Care? The First SSNAP Annual Report. 2014. [Accessed Jun1 1, 2021]. https://www.strokeaudit.org/Documents/National/Clinical/Apr2013Mar2014/Apr2013Mar2014-AnnualReport.aspx .
- 24.Sentinel Stroke National Audit Programme. Moving the Dial of Stroke Care: The 6th SSNAP National Report. 2019. [Accessed Jun1 1, 2021]. https://www.strokeaudit.org/Documents/National/Clinical/Apr2018Mar2019/Apr2018Mar2019-AnnualReport.aspx .
- 25.De Brún A, Flynn D, Ternent L, Price CI, Rodgers H, Ford GA, Rudd M, Lancsar E, Simpson S, Teah J, Thomson RG. Factors that influence clinicians’ decisions to offer intravenous alteplase in acute ischemic stroke patients with uncertain treatment indication: Results of a discrete choice experiment. Int J Stroke. 2018;13:74–82. doi: 10.1177/1747493017690755. [DOI] [PubMed] [Google Scholar]
- 26.Meretoja A, Weir L, Ugalde M, Yassi N, Yan B, Hand P, Truesdale M, Davis SM, Campbell BCV. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology. 2013;81:1071–1076. doi: 10.1212/WNL.0b013e3182a4a4d2. [DOI] [PubMed] [Google Scholar]
- 27.Wu TY, Coleman E, Wright SL, Mason DF, Reimers J, Duncan R, Griffiths M, Hurrell M, Dixon D, Weaver J, Meretoja A, et al. Helsinki stroke model is transferrable with "real-world" resources and reduced stroke thrombolysis delay to 34 min in Christchurch. Front Neurol. 2018;9:290. doi: 10.3389/fneur.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chollet F. Manning; 2018. Deep Learning with Python. [Google Scholar]
- 29.Guo C, Berkhahn F. Entity Embeddings of Categorical Variables. arXiv. 2016:160406737 [Google Scholar]
- 30.Crilly N, Blackwell AF, Clarkson PJ. Graphic elicitation: using research diagrams as interview stimuli. Qual Res. 2006;6:341–366. [Google Scholar]
- 31.Eakin JM, Gladstone B. "Value-adding" Analysis: Doing More With Qualitative Data. International Journal of Qualitative Methods. 2020:1609406920949333 [Google Scholar]
- 32.Archibald MM, Ambagtsheer RC, Casey MG, Lawless M. Using Zoom Videoconferencing for Qualitative Data Collection: Perceptions and Experiences of Researchers and Participants. Int J Qual Methods. 2020:1609406919874596 [Google Scholar]
- 33.Salmon J. Qualitative Online Interviews: Strategies, Design, and Skills. Sage; 2014. [Google Scholar]
- 34.Ritchie J, Spencer L. In: Analyzing qualitative data. Vol. 11: Qualitative data analysis for applied policy research. Burgess, Bryman, editors. London: Routledge; 1994. [Google Scholar]
- 35.Miles M, Huberman A, Saldana J. Qualitative data analysis: A sourcebook. Sage; 2014. [Google Scholar]
- 36.Mays N, Pope C. Qualitative Research: Rigour and qualitative research. BMJ. 1995;311:109–112. doi: 10.1136/bmj.311.6997.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.