Abstract
Background
Rapid drug susceptibility testing (DST) is crucial to confirm eligibility for new tuberculosis (TB) regimens. Genotype MTBDRsl is a widely-deployed World Health Organization (WHO)-endorsed assay yet programmatic performance data, including non-actionable results from smear-negative sputum, are scarce.
Methods
Sputa from Xpert MTB/RIF-rifampicin resistant individuals (n=951) were tested by Genotype MTBDRplus and MTBDRsl (both v2) in a routine laboratory. Phenotypic DST was the second-line drug reference standard. Discrepant results underwent Sanger sequencing.
Findings
89% (849/951) individuals were culture-positive [56% (476/849) smear-negative]. MTBDRplus had at least one non-actionable result (control and/or TB-detection bands absent or invalid, precluding resistance reporting) in 19% (92/476) of smear-negatives and, for MTBDRsl, 40% (171/427) were non-actionable [28% (120/427) false-negative TB, 17% (51/427) indeterminate]. In smear-negatives, MTBDRsl sensitivity for fluoroquinolones was 84% (95% CI 67-93), 81% (54-95) for second-line injectables, and 57% (28-82) for both. Specificities were 93% (89-98), 88% (81-93), and 97% (91-99), respectively. 23% (172/746) of Xpert rifampicin-resistant specimens were MTBDRplus isoniazid-susceptible. Days-to-second-line-susceptibility reporting with the programmatic advent of MTBDRsl improved [6 (5-7) vs. 37 (35-46); p<0.001].
Conclusion
MTBDRsl did not generate a result in almost half of smear-negative individuals (4/10 failed), resulting in substantial missed resistance. However, if MTBDRsl generates an actionable result, that result is accurate in ruling-in second-line resistance. Isoniazid susceptibility testing remains crucial. This study provides, in the context of WHO guidance, real-world direct second-line susceptibility testing performance data on non-actionable results (which, if unaccounted for, result in an overestimation of test utility), accuracy, and care cascade impact.
Keywords: Genotype MTBDRplus, Genotype MTBDRsl, smear-negative, TB, resistance
Introduction
Drug-resistant tuberculosis (DR-TB) is a leading cause of death. Globally, there were half a million rifampicin-resistant (RR) TB cases in 2019; 78% were estimated to be multidrug-resistant (MDR) [1] and only 59% of RR-MDR individuals started on treatment in 2018 were treated successfully [2], partly due to the underdiagnosis of resistance to drugs other rifampicin (RIF) like isoniazid (INH) and the fluoroquinolones (FQs) [3, 4].
The Genotype MTBDRplus (Hain LifeSciences, Germany) and MTBDRsl (Hain LifeSciences, Germany) molecular line probe assays (LPAs) are globally used for rapid DR-TB detection. Both are World Health Organization (WHO)-endorsed and commercially-available [5]. According to the Western Cape Province Department of Health (DoH) TB guidelines [6], MTBDRplus is done after Xpert MTB/RIF (Xpert) to check for Xpert-detected false-positive rifampicin-resistance and confirm MDR [7]. MTBDRsl is subsequently done to detect second-line resistance. One underappreciated yet important component of these workflows is that, even when an individual is confirmed as TB-positive using Xpert, the downstream reflex test must itself successfully amplify Mycobacterium tuberculosis complex (Mtb) DNA (for LPAs Mtb detection is reported as TUB-band positivity). This applies to many reflex technologies and not just LPAs, including new drug susceptibility tests (DSTs) like Xpert MTB/XDR [8, 9], which is yet to be available at scale.
As frontline TB test performance improves, it can outstrip reflex tests’ ability to detect TB and do DST (e.g., Xpert MTB/RIF is almost always done before the LPAs, despite LPAs being an older technology) [10]. Both MTBDRplus and MTBDRsl can hence generate non-actionable results (indeterminate or invalid results) that are critical to report to quantify the overall number of drug-resistant cases missed (i.e., not just due to imperfect sensitivity for resistance, but also due to a failure of the test to detect TB). Such performance data that includes non-actionable results are scarce and a major limitation of the current literature. Despite increased demand for DST due to new oral regimens for RR-MDR TB (with the possibility of new fluoroquinolone-based first-line regimens), MTBDRsl is one of only two WHO-endorsed rapid tests that can be used to confirm eligibility for these regimens.
The WHO recommend MTBDRplus is used on smear-positive sputum (direct testing) and on culture isolates (indirect testing) for smear-negatives [11]. In contrast, MTBDRsl version 2 is recommended for direct smear-negative testing, however, evidence is of “low certainty” [5, 12] and meta-analyses had insufficient data to create summary point estimates [13-16]. This uncertainty in performance is one reason why LPA uptake for the direct testing is suboptimal: in a global survey of 32 LPA-using laboratories, 66% and 50% tested smear-negative specimens with MTBDRplus and MTBDRsl, respectively [17]; despite the positive WHO recommendation. Critically, more data are therefore needed.
Our overarching aim was to therefore evaluate MTBDRplus and MTBDRsl v2.0 performance, including in smear-negative specimens, and by describing the non-actionable result rate. Importantly, we did this in a programmatic context that relies on affordable existing diagnostic tools to help guide therapeutic decisions. This approach enabled us to evaluate the association between the expansion of direct second-line DST and time-to-treatment and compare this to the period prior to the advent of direct second-line DST. Our intention was to provide data for laboratories and clinicians diagnosing and treating drug-resistant TB in resource-constrained settings where programmatic laboratory decisions and policies related to rapid diagnostic testing follow WHO guidance.
Materials and methods
Study design
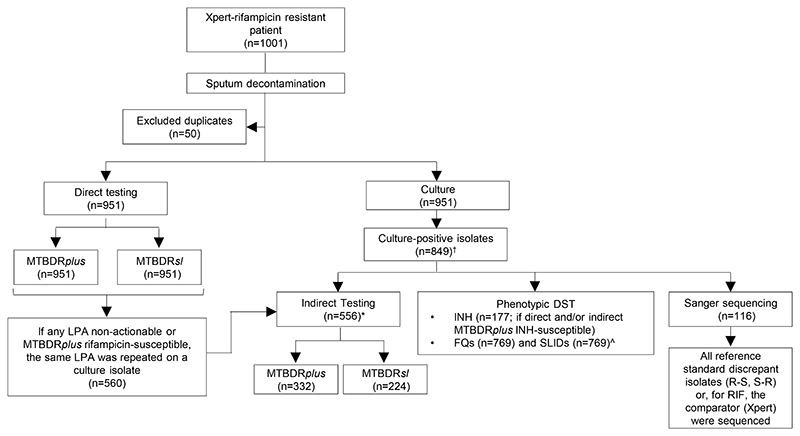
This study was performed in a programmatic context following the TB diagnostic algorithm in the Western Cape, South Africa (Figure 1). Direct testing was performed initially using MTBDRplus and MTBDRsl on sputum consecutively tested with no study-specific criteria between 1 June 2016-30 September 2019.
Figure 1.
Testing flow diagram showing direct and indirect testing using MTBDRplus and MTBDRsl and the use of reference standard phenotypic testing for second-line drugs, irrespective of the LPA result. Prior to the study, the flow of tests was the same except MTBDRsl was not used and MTBDRplus was only done directly if the specimen was smear-positive.
*4 direct non-actionables were culture-negative and unable to be test indirectly
†102 Xpert-positives were not culture-positive and hence did not have an isolate available ^80 isolates were contaminated upon regrowth for FQ and SLID pDST
Abbreviations: DST-drug susceptibility testing, R-resistant, S-susceptible, n-number, INH-isoniazid, MDR-multi drug resistant, Xpert-Xpert MTB/RIF, FQs-fluoroquinolones, SLID-second-line injectables drugs, n-number, LPA-line probe assay.
MTBDRplus was performed on specimens of all smear status, defined below as the “after period”. All valid results were reported and reflexed for MTBDRsl testing. All TUB-band negative, indeterminate for one or both drugs were reported as invalid (MTBDRplus/ MTBDRsl), rifampicin-susceptible results were reported as discrepant and reflexed for indirect testing using a confirmed culture-positive isolate. All culture isolate results, except Sanger sequencing, formed part of indirect diagnostic workflows including Genotype MTBDRplus, MTBDRsl and pDST and all valid results were reported immediately. Phenotypic DST was done on specimens with valid direct and indirect LPA results. All discrepant results for MTBDRplus/ MTBDRsl with reference standard pDST, were resolved with repeat testing on the cultured isolate. For discrepancies which remained even after repeat testing sequencing was performed (Figure 1).
Ethics
This study was done in accordance with relevant guidelines and regulations, approved by the Health Research Ethics Committee of Stellenbosch University (N16/04/045) and the Western Cape Province Department of Health (2016RP18 637). Permission was granted to access anonymised residual specimens collected as part of routine diagnostic practice and informed consent waived.
Sputum collection and preparation
In the Western Cape Province, two sputum samples were collected upfront for screening of presumptive TB per local guidelines [6]. Sputum processing and testing was done at the National Health Laboratory Service (NHLS) Green Point reference laboratory in Cape Town, South Africa. Pre-treatment individuals whom were first tested using Xpert MTB/RIF (version 4.3; Xpert) formed part of the then standard-of-care algorithm[18]. A paired sputum specimen from Xpert-rifampicin resistant individuals (n=1001) was decontaminated using N-acetyl-L-cysteine-sodium hydroxide (NaOH-NALC; final concentration 1%) and the sediment resuspended in 2ml phosphate buffer [19]. Auramine microscopy was performed. From decontaminated sputum 0.5ml was inoculated into a MGIT (Mycobacteria Growth Indicator Tube; Becton Dickinson, United States) and incubated in a BACTEC MGIT960 instrument for ≤35 days (our programmatic standard-of-care due to space limitations).
DNA extraction and line probe assay testing
DNA extracted per manufacturer’s guidelines [20, 21] from resuspended sputum sediments was tested directly with MTBDRplus and MTBDRsl (version 2 of both) in parallel by a single operator irrespective of smear status. The GT blot (Hain Lifesciences) and Genoscan software (GS-001, Hain Lifesciences) were used to analyse results followed by operator visual confirmation. All invalid tests (direct testing) were repeated as recommended (the repeat result was reported in analyses). For specimens (direct testing) TB-negative per LPAs (i.e., TUB-band negative), indeterminate for at least one locus, or with an LPA DST result discrepant with phenotypic drug susceptibility testing (pDST), the corresponding isolate was tested using the same LPA (indirect testing). 332 and 224 isolates were tested using MTBDRplus and MTBDRsl, respectively. The manufacturer-recommended 2.2°C/s ramp rate [17, 22] and ISO15189 standards were used. Results were interpreted per Supplementary Table 1.
TB and phenotypic drug susceptibility testing reference standards
MGIT960 culture positivity with MTBDRplus TUB-positivity was used for the detection of TB. Rifampicin pDST was not done. pDST was done programmatically for isoniazid, fluoroquinolones and second-line injectables and, per the algorithm, only MTBDRplus rifampicin-resistant, isoniazid-susceptible isolates received isoniazid pDST to ensure resistance was not excluded (we are hence unable to calculate MTBDRplus’s sensitivity, specificity, and PPV for isoniazid resistance). If direct MTBDRplus was non-actionable or isoniazid susceptible, indirect MTBDRplus testing was done and only based on this result, was isoniazid pDST done (hence only the NPV of indirect MTBDRplus for resistance was calculable). Supplementary Methods for more information.
Discrepant analysis
Sanger sequencing was used as the composite reference standard to resolve discrepancies involving LPAs, pDST, and Xpert rifampicin-resistant and MTBDRplus-rifampicin susceptible specimens (Supplementary Methods and Supplementary Table 6).
Implementation and effect of programmatic MTBDRplus and MTBDRsl testing
We compared the diagnostic care cascade in the “before algorithm” (2 January 2012 - 30 December 2015) vs “after algorithm” periods (1 June 2016- 30 September 2019). In the “before algorithm” period, programmatic DST for isoniazid, fluoroquinolones and amikacin was done phenotypically. MTBDRplus (includes v1) was done routinely for both rifampicin and isoniazid directly in smear-positives or on culture isolates. In the “after algorithm” period, MTBDRplus and MTBDRsl (both v2) was implemented programmatically and reported for potential patient management (Supplementary Methods for more detail on these periods).
Statistical analyses
GraphPad Prism (version 6; GraphPad Software, USA) and STATA (version 14.0; Statacorp, USA; two sample proportion test and McNemar’s) were used. P-values ≤0.05 were significant.
Results
Cohort characteristics
Of 1001 Xpert rifampicin-resistant sputa, 95% (951) were from unique patients, 89% (849) were confirmed culture-positive (93 were culture-negative and 10 culture-contaminated), 81% (769) had a usable second-line pDST result [8% (80) contaminated] (Figure 1). Most individuals were male with smear-negative TB (Supplementary Table 2) and, in individuals with a known HIV status, 50% (203/404) were HIV-positive. HIV-positives were more likely to be sputum smear-negative than HIV-negatives [59% (120/203) vs. 48% (110/230); p=0.018].
Smear microscopy, culture, and phenotypic DST results
Amongst culture-positives, 44% (373/849) and 56% (476/849) were sputum smear-positive and smear-negative, respectively. Using MTBDRplus, 21% (177/849) and 60% (509/849) were classified as rifampicin-monoresistant and MDR (Figure 2). Using MTBDRsl, 5% (42/769), 1% (11/769), and 2% (19/769) were FQ-resistant, SLID-resistant, or both FQ and SLID resistant, respectively (Figure 3).
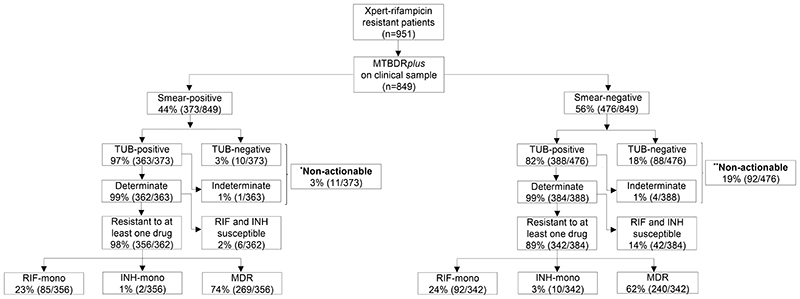
Figure 2.
Direct MTBDRplus testing of sputum is successful in almost all smear-positives and most smear-negatives, however, it fails to generate a susceptibility result in a significant minority of smear-negatives (one in five), indicating that a failure to detect TB is the primary cause of drug-resistance being missed (i.e., non-actionable results). Furthermore, a significant minority of Xpert rifampicin-resistant patients do not have MDR per MTBDRplus, suggesting a continued role for isoniazid DST. Importantly, in patients with actionable MTBDRplus results, sensitivity and specificity for resistance did not differ by smear status. Resistance classifications on bottom two rows of boxes are per direct MTBDRplus
Of the 951 Xpert rifampicin-resistant patients only 849 were confirmed culture-positive.
*Indirect smear-positive MTBDRplus results: MDR (n=7), Rif-mono (n=0), INH-mono (n=1), fully-susceptible (n=3), and non-actionable (n=0).
**Indirect smear-negative MTBDRplus results: MDR (n=69), Rif-mono (n=0), INH-mono (n=3), fully-susceptible (n=20), and non-actionable (n=0).
Abbreviations: RIF-rifampicin, INH-isoniazid, mono-mono-resistant, MDR-multi–drug resistant, TUB-TUB-band, n-number, Xpert-Xpert MTB/RIF.
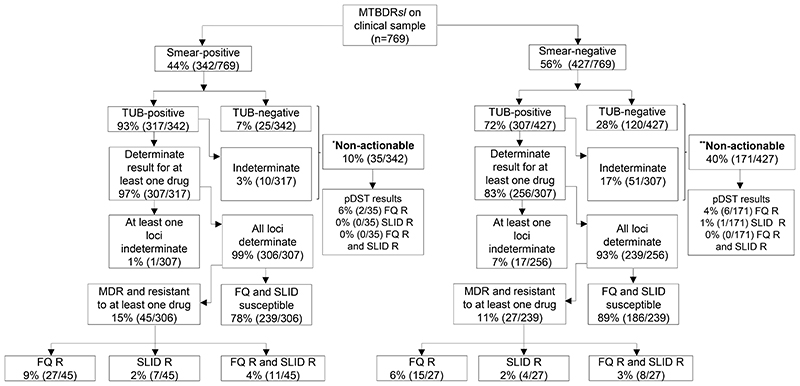
Figure 3.
Although direct MTBDRsl testing of sputum is successful in most patients, it results in relatively high proportions of non-actionable results in smear-positives and especially in smear-negatives. MTBDRsl failed in four out of 10 smear-negative patients with Xpert-diagnosed rifampicin-resistance. As seen for MTBDRplus, a failure to generate an actionable result on smear-negatives was the primarily cause of resistance missed (as opposed to a false-negative susceptible result).
Resistance classifications on bottom two rows of boxes are per direct MTBDRsl
Of the 849 culture-positive patients only 769 had usable pDST (80-contaminated).
*Indirect smear-positive MTBDRsl results: FQ-R (n=3), SLID-R (n=0), FQ-R and SLID-R (n=0), fully susceptible (n=33), and non-actionable (n=0).
**Indirect smear-negative MTBDRsl results: FQ-R (n=7), SLID-R (n=4), FQ-R and SLID-R (n=2), fully susceptible (n=175), and non-actionable (n=0)
Abbreviations: FQ-fluoroquinolones, SLID-second line injectable drug, R-resistant, n-number, TUB-TUB-band, pDST-phenotypic drug susceptibility testing.
MTBDRplus
Non-actionables: 3% (11/373) and 19% (92/476) of sputum smear-positives and - negatives had non-actionable results, respectively and, of these, 70% (521/746) were phenotypically isoniazid resistant (Figure 2). Of the sputum smear-negative non-actionables, 18% (88/476) were due to a false-negative TB result and 1% (4/476) were due to an indeterminate call (Figure 2). Non-actionable results from indirect testing are in Supplementary Figure 1. No MTBDRplus invalid results occurred.
Mtb
MTBDRplus’s sensitivity was 97% (363/373) and 82% (388/476; p<0.001) for sputum smear-positives and -negative TB, respectively (Table 1).
Table 1. Accuracy of direct MTBDRplus and MTBDRsl testing for TB and phenotypic second-line drug resistance in sputum of Xpert-positive rifampicin-resistant patients. Data are % (n/N) 95% CI.
| Overall | Smear-positive | Smear-negative | |||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | ||
| MTBDRplus | TB | 88 (751/849) 86-90 |
43 (40/93) 32-53 |
97 (363/373) 94-98 |
36 (4/11) 10-69 |
82 (388/476) 77-84 *p<0.001 |
44 (36/82) 32-54 *p=0.635 |
| MTBDRsl | TB | 82 (696/849) 79-84 †p<0.001 |
51 (47/93) 32-54 †p=0.303 |
93 (347/373) 90-95 †p=0.006 |
73 (8/11) 39-93 †p=0.086 |
73 (349/476) 69-77 *p<0.001 †p=0.002 |
48 (39/82) 36-58 *p=0.117 †p<0.001 |
| Fluoroquinolones | 87 (71/82) 77-93 |
93 (297/321) 90-96 |
89 (40/45) 75-96 |
92 (180/195) 88-96 |
84 (31/37) 67-93 *p=0.105 |
93 (117/126) 89-98 *p=0.855 |
|
| Second-line injectables drugs | 84 (32/38) 68-93 **p=0.720 |
94 (317/339) 90-95 **p=0.820 |
86 (19/22) 65-97 **p=0.001 |
97 (205/212) 93-98 **p=0.108 |
81 (13/16) 54-95 *p=0.011 **p=0.821 |
88 (112/127) 81-93 *p=0.002 **p=0.052 |
|
| Fluoroquinolone and second-line injectable drugs | 70 (19/27) 69-98 |
97 (257/264) 94-98 |
85 (11/13) 54-98 |
97(165/169) 94-99 |
57 (8/14) 28-82 *p=0.118 |
97 (92/95) 91-99 *p=0.701 |
|
Within row comparisons between smear statuses
Within column comparisons for second-line injectables vs. fluoroquinolones
Within column comparisons for MTBDRsl vs. MTBDRplus
Rifampicin
91% (686/746) of the Xpert rifampicin-resistant patients whose direct MTBDRplus was actionable were MTBDRplus rifampicin-resistant [24% (177/746) had MTBDRplus-defined rifampicin-monoresistance]. In a head-to-head comparison of direct MTBDRplus and Xpert actionable results, 8% (60/746) were Xpert-resistant MTBDRplus-susceptible, with most discrepants in smear-negatives TB rather than - positives (Figure 2). Overall, of the discrepants successfully sequenced (nine culture-contaminated, three non-amplifiable), 85% (22/26) resolved in favour of Xpert (Table 2). Indirect MTBDRplus results are in Supplementary Figure 1.
Table 2.
Sequencing of MTBDRplus targets (rpoB, katG, inhA promoter region) done to resolve discrepant results either between MTBDRplus and Xpert (rifampicin) or MTBDRplus and phenotype (isoniazid). Sequencing suggested Xpert is more sensitive for rifampicin resistance than MTBDRplus. MTBDRplus detected mutations known to cause isoniazid resistance better than pDST. See Supplementary Methods for how LPA results were categorised as discrepant.
| Sequencing | ||||||||
|---|---|---|---|---|---|---|---|---|
| Locus | MTBDRplus | Comparator result | Mutation | No. isolates | No. with HR | Susceptibility result | Resolved in favour of LPA or comparator | |
| Rifampicin | rpoB*(n=29) | S | R | S531L | 8 | 1 | R | Xpert |
| H526Y | 2 | 0 | R | Xpert | ||||
| D516V | 3 | 1 | R | Xpert | ||||
| Q513P | 1 | 0 | R | Xpert | ||||
| L511P** | 8 (1 double mutant with D485N) | 1 | R | Xpert | ||||
| WT | 4 | 0 | S | MTBDRplus | ||||
| NR | 3 | |||||||
| Discrepant resolution by sequencing |
85% (22/26) resistant (resolved in favour of Xpert)
15%(4/26) susceptible (resolved in favour of MTBDRplus) |
|||||||
| Isoniazid | katG^(n=24) | S | R | G312C | 1 | R | pDST | |
| S315T | 3 | R | pDST | |||||
| WT | 19 | S | MTBDRplus | |||||
| NR | 1 | |||||||
| inhA promoter^(n=24) | S | R | -8 T/C | 1 | R | pDST | ||
| WT | 23 | S | MTBDRplus | |||||
| Discrepant resolution by sequencing |
21% (5/24) resistant (resolved in favour of pDST)
79% (19/24) susceptible (resolved in favour of MTBDRplus) |
|||||||
Only Xpert rifampicin-resistant and MTBDRplus rifampicin-susceptible discrepant sputa were sequenced from the isolate
Discrepant isolates sequenced included only MTBDRplus-susceptible that were phenotypic resistant (due to contemporaneous programmatic algorithm).
L511P is considered borderline by “WHO” who recommend people found with this mutation be classified as resistant [28].
Abbreviation: R-resistant, S-susceptible, HR-heteroresistance, WT-wild-type, NR-Not reportable (did not amplify for sequencing), LPA-line probe assay, n-number, pDST-phenotypic drug susceptibility testing, Xpert-Xpert MTB/RIF
Isoniazid
68% (509/746) of Xpert rifampicin-resistant patients whose direct MTBDRplus was actionable had, per MTBDRplus, MDR and 2% (12/746) isoniazid mono-resistance (remainder rifampicin-monoresistant). 328 received indirect MTBDRplus testing and 53% (177/328) were MTBDRplus rifampicin-resistant, isoniazid susceptible (Supplementary Figure 1). 17% (30/177) were phenotypically resistant. We can only calculate MTBDRplus’s NPV for isoniazid resistance when done indirectly, which was 83% (147/177). When discrepant isoniazid results [indirect MTBDRplus-susceptible, pDST-resistant (n=30)] were analysed, 80% (24/30) had usable sequences. 79% (19/24), all of which were sequencing wildtype, resolved in favour of MTBDRplus (Table 2), resulting in NPV increasing to 97% (166/171).
MTBDRsl
Non-actionable
When done directly, 10% (35/342) of sputum smear-positives and 40% (171/427) of smear-negatives were non-actionable (Figure 3). 4% (8/206), 0% (1/206), and 0% (0/206) of non-actionables were phenotypically resistant to the fluoroquinolones, SLIDs, or both fluoroquinolones and SLIDs, respectively. Like MTBDRplus on sputum smear-negatives, most MTBDRsl smear-negative results were non-actionable due to a false-negative TB result [28% (120/427)] or an indeterminate result [17% (51/427)] (Figure 3). 28 MTBDRsl results were initially invalid prior pDST [1% (2/373) vs. 5% (26/476) for sputum smear-positives and - negatives respectively; p<0.001] (Supplementary Table 3) but all resolved upon retesting (and were hence ultimately not non-actionable). No indirect non-actionable results occurred (Supplementary Figure 2).
Mtb
Sensitivity was 93% (347/373) and 73% (349/476; p<0.001) for sputum smear-positive and -negative specimens, respectively (Table 1), and less than MTBDRplus in the same individuals (97% [95% CI 94-98] vs 93% [90-95]; p<0.001) for sputum smear-positives and (82% [77-84] vs 73% [69-77]; p<0.001) for smear-negatives.
Fluoroquinolones
For direct sputum smear-positive and -negative testing, sensitivities were 89% (40/45) and 84% (31/37; p=0.105) and specificities 92% (180/195) and 93% (117/126; p=0.855), respectively (Table 1; Figure 4). For indirect testing, sensitivity was 92% (12/13) and specificity 100% (211/211; Supplementary Table 4). When discrepant FQ results from direct testing were analysed [MTBDRsl-resistant pDST-susceptible (n=24); MTBDRsl-susceptible pDST-resistant (n=11)], 83% (29/35) generated usable sequences. 69% (20/29) of discrepancies were in favour of MTBDRsl and 31% (9/29) favoured pDST (Table 3). MTBDRsl falsely reported two specimens with gyrA S95T natural polymorphisms [23] as resistant through the absence of a wild-type band (WT3, MUT3C). After following discrepant analysis reclassification, sensitivities and specificities increased (Figure 4; Supplementary Table 5).
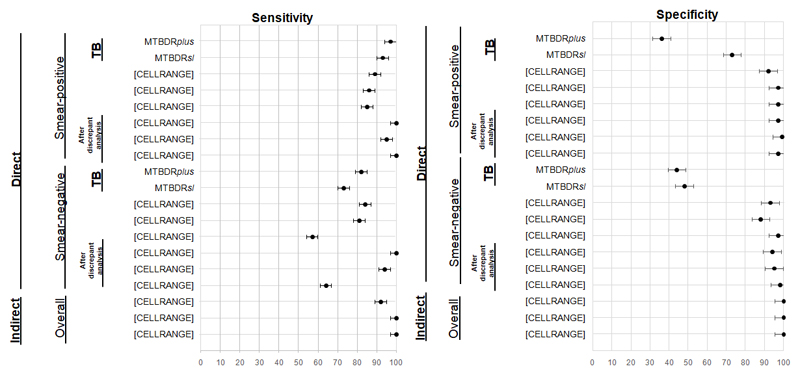
Figure 4.
Selected summary forest plots showing sensitivity and specificity estimates (with 95% CI) for MTBDRplus and MTBDRsl. Importantly, only patients first detected as TB positive (top two rows) can generate an actionable LPA DST result. Estimates for smear-negatives were lower than smear-positives and, overall, estimates for SLIDs were lower than for FQs. All estimates improved in favour of LPAs after discrepant resolution.
Abbreviations: TB-tuberculosis, FQ-fluoroquinolones, SLID-second line injectable drugs
Table 3. Sequencing of MTBDRsl targets (gyrA, rrs) to resolve results discrepant with pDST. Most fluoroquinolone discrepants resolved in favour of MTBDRsl whereas most SLIDs discrepants resolved in favour of pDST.
| Sequencing | |||||||
|---|---|---|---|---|---|---|---|
| Locus | MTBDRsl | pDST | Mutation | No. isolates | Susceptibility result | Resolved in favour of LPA or pDST | |
| Fluoroquinolones | gyrA(n=11) | S | R | G81C* | 1 | S | MTBDRsl |
| A88T | 1 | R | pDST | ||||
| WT | 9 | S | MTBDRsl | ||||
| (n=24) | R | S | A88T | 1 | R | MTBDRsl | |
| C86T | 1 | R | MTBDRsl | ||||
| D89N | 1 | R | MTBDRsl | ||||
| A90V | 4 | R | MTBDRsl | ||||
| S91P | 1 | R | MTBDRsl | ||||
| D94G | 2 | R | MTBDRsl | ||||
| S95T^ | 2 | S | pDST | ||||
| WT | 6 | S | pDST | ||||
| NR | 6 | ||||||
| Discrepant resolution by sequencing |
69% (20/29) in favour of MTBDRsl
31% (9/29) in favour of pDST |
||||||
| Second-line injectables | rrs(n=6) | S | R | WT | 3 | S | MTBDRsl |
| NR | 3 | ||||||
| (n=22) | R | S | WT | 8 | S | pDST | |
| A1401G | 1 | R | MTBDRsl | ||||
| NR | 13 | ||||||
| Discrepant resolution by sequencing |
33% (4/12) in favour of MTBDRsl
67% (8/12) in favour of pDST |
||||||
Second-line injectable drugs
For direct testing in sputum smear-positive and - negatives, sensitivities were 86% (19/22) and 81% (13/16; p=0.011), respectively and specificities 97% (205/212) and 88% (112/127; p=0.002), respectively (Table 1; Figure 3). For indirect testing, sensitivity was 100% (6/6) and specificity 100% (218/218) (Supplementary Table 4; Figure 4). When direct MTBDRsl-pDST discrepant results [MTBDRsl-resistant pDST-susceptible (n=22), MTBDRsl-susceptible pDST-resistant (n=6)] were analysed, 43% (12/28) had sequencable isolate DNA. In contrast to fluoroquinolones, most discrepancies [67% (8/12)] resolved in favour of pDST (Table 3; Supplementary Table 7). Following reclassification, sensitivity and specificity increased (Figure 4; Supplementary Table 5).
Joint FQ and SLID resistance
For sputum smear-positives and smear-negatives, direct sensitivity was 85% (11/13) and 57% (8/14; p=0.118), respectively, and specificities 97% (165/169) and 97% (92/95; p=0.701), respectively (Table 1; Figure 3). Indirect testing sensitivity and specificity were very high (Supplementary Table 4; Figure 4). Like that observed for the individual drug classes, after discrepancy resolution, MTBDRsl sensitivity and specificity increased (Supplementary Table 5).
Diagnosis care cascade gaps in “before” and “after” periods
We compared of programmatic data from period immediately preceding the study (“before period”; when MTBDRplus was the only LPA done directly - only on sputum smear-positives - and the only second-line testing was pDST) to a similar period after the start of study testing ("after period"; both LPAs were done, at a minimum, directly and reported for routine patient management). With MTBDRsl implementation, the proportion of individuals on treatment without second-line DST results decreased from 23% (668/2938) to 5% (40/799; p<0.001) (Table 4), and second-line DST results were available quicker [33 (29-38) to 16 (13-22) days for smear-positives, 42 (36-50) to 22 (18-27) days for smear-negatives], even after factoring in many smear-negatives with direct non-actionables result that required sub-culture for further testing compared to smear-positives [37% (143/383) vs 9% (36/416); p<0.001] (Table 4).
Table 4. Comparison of key care cascade gaps for the diagnosis of drug-resistance before and after the implementation of improved molecular diagnostics for resistance beyond rifampicin.
Implementation of first-line MTBDRplus testing on Xpert rifampicin-resistant sputum to include smear-negatives and MTBDRsl testing on all sputum resulted in a greater proportion of patients receiving second-line DST, reduced reliance on culture, and reduced turnaround time. The Supplementary Methods contains more information on these periods. Data are median (IQR) or % (n/N).
| Retrospective period MTBDRplus only on smear-positives Second-line DST by pDST only |
Prospective period MTBDRplus and MTBDRsl irrespective of smear-status Second-line pDST still done |
|||||
|---|---|---|---|---|---|---|
| Overall (n=2938) | Smear-positive (n=1674) | Smear-negative (n=1264) | Overall (n=799) | Smear-positive (n=416) | Smear-negative (n=383) | |
| On treatment without receiving any second-line DST | 23 (668/2938) | 21 (357/1674) | 25 (311/1264) *p=0.358 |
5 (40/799) ^p<0.001 |
2 (7/416) ^p<0.001 |
9 (33/383) *p<0.001 ^p<0.001 |
| MTBDRplus direct testing | N/A | 100 (1674/1674) | N/A | 100 (799/799) | 100 (416/416) | 100 (383/383) |
| With an actionable result | N/A | 79 (1317/1674) | N/A | 99 (797/799) | 100 (416/416) | 99 (381/383) *p=0.140 |
| Without an actionable result | N/A | 21 (357/1674) | N/A | 0 (2/799) | 0 (0/416) | 1 (2/383) |
| MTBDRsl direct testing | N/A | N/A | N/A | 100 (799/799) | 100 (416/416) | 100 (383/383) |
| With an actionable result | N/A | N/A | N/A | 78 (622/799) | 91 (380/416) | 63 (242/383) *p<0.001 |
| Without an actionable result | N/A | N/A | N/A | 22 (177/799) | 9 (36/416) | 37 (141/383) *p<0.001 |
| Days-to-result (actionable or non-actionable) | N/A | N/A | N/A | 6 (5-7) | 6 (5-7) | 6 (5-7) *p<0.001 |
| MTBDRsl indirect testing | N/A | N/A | N/A | 22 (177/177) | 9 (36/36) | 37 (141/141) |
| With an actionable result | N/A | N/A | N/A | 22 (177/177) | 9 (36/36) | 37 (141/141) |
| Without an actionable result | N/A | N/A | N/A | 0 | 0 | 0 |
| Days-to-result (actionable or non-actionable) | N/A | N/A | N/A | 22 (16-26) | 16 (13-22) | 22 (18-27) *p=0.081 |
| pDST | 77 (2270/2938) | 79 (1317/1674) | 75 (953/1264) *p=0.358 | 94 (750/799) ^p<0.001 | 96 (400/416) ^p<0.001 | 91 (350/383) *p=0.500 ^p<0.001 |
| Days-to-result (IQR) | 37 (35-46) | 33 (29-38) | 42 (36-50) *p<0.001 | 30 (27-36) ^p<0.001 | 28 (25-35) ^p<0.001 | 34 (30-40) *p<0.001 ^p<0.001 |
| Overall, second-line DST | ||||||
| Patients who required second-line DST on isolates (indirect MTBDRsl or pDST) when direct MTBDRsl was non-actionable | 0 | 0 | 0 | 22 (177/799) | 9 (36/416) | 37 (141/383) *p<0.001 |
| Days-to-first actionable second line DST result (direct MTBDRsl, indirect MTBDRsl, or pDST) | 37 (35-46) | 33 (29-38) | 42 (36-50) *p<0.001 | 6 (5-7) | 6 (5-7) | 6 (5-7) *p<0.001 |
Comparisons within rows in “retrospective” vs “prospective” periods
Comparisons within rows and between columns by same smear status
Abbreviations and definitions: pDST-phenotypic drug susceptibility testing, IQR-interquartile range, n-number, N/A-non applicable, DST-drug susceptibility testing.
Discussion
There are limited data on non-actionable results, accuracy, and effect of rapid molecular assays for the diagnosis of resistance beyond rifampicin, especially on smear-negative sputum. To address this, we performed a large-scale evaluation of the newest-generation LPAs in a routine programmatic setting, did comprehensive reference standard testing, and compared care cascade data before and after. Definitive data on MTBDRsl’s performance on smear-negative specimens is essential as the need for fluoroquinolone susceptibility testing increases and new tools like Xpert MTB/XDR remain expensive (cost per cartridge $19.80, at least $3860 to upgrade existing modules [24]).
Our key findings include: 1) 19% and 40% of smear-negative individuals tested by MTBDRplus and MTBDRsl were non-actionable, respectively; resulting in many individuals with resistance missed, 2) About 25% of Xpert rifampicin-resistant patients have MTBDRplus-defined isoniazid susceptibility, and 3) deployment of direct LPA testing was associated with improvements in days-to-diagnosis, more individuals receiving DST, and reduced culture reliance.
MTBDRsl had almost double the non-actionable result rate of MTBDRplus in smear-negatives for TB detection, causing diagnostic and treatment delays. Our data highlights the suboptimal ability of reflex DSTs to detect TB even in individuals already identified as TB-positive by frontline tests. This information loss will persist as the limit of detection of new frontline tests outstrips that of reflex tests (Xpert MTB/RIF Ultra vs. Xpert MTB/XDR). We recommend all studies that evaluate reflex test report this key metric (non-actionable results).
In Xpert rifampicin-resistant specimens that were MTBDRplus rifampicin-susceptible, Xpert was correct more frequently than MTBDRplus [25, 26]. Possible reasons include heteroresistance and variants not included in MTBDRplus. These findings question diagnostic algorithms that use MTBDRplus to confirm Xpert-detected rifampicin resistance [7, 26, 27].
Importantly, MTBDRplus has value for isoniazid susceptibility detection: our data suggest isoniazid is likely effective in 25% of Xpert rifampicin-resistant individuals and, in agreement with that observed in the Democratic Republic of the Congo [28], and Iran [29], we recommend rifampicin-resistant TB is not automatically assumed to be MDR and all Xpert rifampicin-resistant individuals receive isoniazid DST (which should be done anyway as INH resistance prevalence is globally in excess that of rifampicin [30]).
Fluoroquinolones are key components of new regimens and second-line injectable drugs like amikacin remain important. Although important new tools Xpert MTB/XDR are emerging [31], MTBDRsl, is already established in many laboratories worldwide. The sensitivity and specificity for FQ on smear-negatives were 84% and 93%, respectively. High MTBDRsl sensitivity (81%) was observed on smear-negatives for SLID, however, specificity was less (88%); both improving after discrepant analysis. Importantly, in contrast to fluoroquinolones, most SLID MTBDRsl-pDST discrepant results resolved in favoured of pDST-confirmed susceptibility.
In the “after period” we found significant improvements in the proportion of people that had any second-line DST results (such individuals are thus more likely to start effective treatment) and time-to-result. Such real-world data regarding the programmatic impact of TB diagnostics is scarce but important. With the scale-up of second-line LPAs, individual with smear-negative TB still suffered from unacceptably long times-to-diagnosis. This subset of individuals should be targeted for interventions to accelerate treatment initiation, such as new expensive new assays like Xpert MTB/XDR or Deeplex Myc-TB (Genoscreen) [8, 32].
A strength and limitation is the programmatic context of the study, permitting it to be large and the results reported for potential patient management within the South African care cascade. However, this meant the study was constrained by contemporary diagnostic algorithms, which affected specimen and meta-data availability given the suboptimal quality-of-care common in high-volume resource-scarce settings.
Time-to-DST results associated with LPA scale-up may vary across other provinces within South Africa as, unlike in the Western Cape, only one specimen is collected initially for presumptive TB and a second-sputum specimen is dependent on an individual returning to clinic (this may affect generalisability). We were unable to do pDST for rifampicin and isoniazid, however, our primary objective was to evaluate LPA performance for second-line drugs. We also did targeted rather than whole genome sequencing and discrepant analyses may have missed rare non-canonical variants, however, WHO-recommended second-line pDST was done in all isolates [33].
LPA use in our programmatic laboratory was associated with improvements in the care cascade and patient-important outcomes remained suboptimal. Until next generation reflex DSTs are widely available, expanded LPA testing remains key to the successful scale-up of new regimens, despite important paucibacillary specimen performance caveats.
Supplementary Material
Summary.
MTBDRsl assay fails to generate a result in almost half of smear-negatives, resulting in substantial missed resistance, but has high rule-in value. Many rifampicin-resistant individuals would likely benefit from isoniazid. Programmatic direct testing was nevertheless associated with care cascade improvements. 40/40
Footnotes
Author Contributions
S.P., M.dV., G.T. and R.W. conceptualized the experiments. T.D., S.P., B.D., R.V. assisted with data curation. S.P performed formal analysis, methodology and writing original draft. A.D.S, L.A.S, and E.S. assisted S.P. with the investigation. All authors reviewed and edited the manuscript.
Conflict of interests: All authors have declared that no potential conflict of interest.
Contributor Information
M de Vos, Email: Margaretha.DeVos@finddx.org.
B Derendinger, Email: brigitta@sun.ac.za.
E Streicher, Email: lizma@sun.ac.za.
T Dolby, Email: Tania.Dolby@nhls.ac.za.
LA Scott, Email: lascott@sun.ac.za.
AD Steinhobel, Email: amys@sun.ac.za.
RM Warren, Email: rw1@sun.ac.za.
Acknowledgements
The authors thank the National Health Laboratory and Hain Lifesciences. The authors thank Dr Rouxjeane Venter for help with patient data collection.
Funding
This work was supported by Stellenbosch University Faculty of Health Sciences, the National Research Foundation and Harry Crossley. RMW acknowledges funding from the South African Medical Research Council. GT acknowledges funding from the EDCTP2 programme supported by the European Union [grant # SF1401, OPTIMAL DIAGNOSIS] and the National Institute of Allergy and Infection Diseases of the National Institutes of Health [grant # U01AI152087]. Hain Lifesciences donated MTBDRsl kits but had no role in study design or results interpretation.
References
- 1.World Health Organization. Global Tuberculosis Report. 2020
- 2.World Health Organization. Global Tuberculosis Report. 2021
- 3.Naidoo P, Theron G, Rangaka MX, et al. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. The Journal of Infectious Diseases. 2017;216(suppl_7):S702–S13. doi: 10.1093/infdis/jix335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox H, Dickson-Hall L, Ndjeka N, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS medicine. 2017;14(2):e1002238. doi: 10.1371/journal.pmed.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs: policy guidance. Report No.: 9241510560. 2016
- 6.Director AC, Kruger MJ. Clinical Guidelines & Standard Operating Procedure for the Implementation of the Short & Long DR-TB regimens for Adults, Adolescents and Children.
- 7.Beylis N, Ghebrekristos Y, Nicol M. Management of false-positive rifampicin resistant Xpert MTB/RIF. The Lancet Microbe. 2020;1(6):e238. doi: 10.1016/S2666-5247(20)30123-3. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. World Health Organization consolidated guidelines on tuberculosis: module 3: diagnosis-rapid diagnostics for tuberculosis detection: web annex 4: evidence synthesis and analysis. 2021.
- 9.Penn-Nicholson A, Georghiou SB, Ciobanu N, et al. Detection of isoniazid, fluoroquinolone, ethionamide, amikacin, kanamycin, and capreomycin resistance by the Xpert MTB/XDR assay: a cross-sectional multicentre diagnostic accuracy study. The Lancet Infectious Diseases. 2021 doi: 10.1016/S1473-3099(21)00452-7. [DOI] [PubMed] [Google Scholar]
- 10.García-Basteiro AL, DiNardo A, Saavedra B, et al. Point of care diagnostics for tuberculosis. 2018;24(2):73–85. doi: 10.1016/j.rppnen.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Samra Z, Kaufman L, Bechor J, Bahar J. Comparative study of three culture systems for optimal recovery of mycobacteria from different clinical specimens. European Journal of Clinical Microbiology and Infectious Diseases. 2000;19(10):750–4. doi: 10.1007/s100960000369. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: 2016. [PubMed] [Google Scholar]
- 13.Theron G, Peter J, Richardson M, et al. The diagnostic accuracy of the GenoType® MTBDRsl assay for the detection of resistance to second-line anti-tuberculosis drugs. 2014. [DOI] [PMC free article] [PubMed]
- 14.Bai YWY, Shao C, Hao Y, Jin Y. GenoType MTBDRplus Assay for Rapid Detection of Multidrug Resistance in Mycobacterium tuberculosis: A Meta-Analysis. PLoS One. 2016;11(3):e0150321. doi: 10.1371/journal.pone.0150321. journalpone0150321 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drobniewski FCM, Jordan J. Health Technology Assessment. 34. Vol. 19. NIHR Journals Library; Southampton (UK): 2015. May, Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Chapter 3, Systematic review. 2015 Available from https://www.ncbi.nlm.nih.gov/books/NBK293793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theron G, Peter J, Richardson M, Warren R, Dheda K, Steingart KR. GenoType® MTBDRsl assay for resistance to second-line anti-tuberculosis drugs. The Cochrane Library; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derendinger B, De Vos M, Nathavitharana R, et al. Widespread use of incorrect PCR ramp rate negatively impacts multidrug-resistant tuberculosis diagnosis (MTBDR plus) 2018;8(1):3206. doi: 10.1038/s41598-018-21458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Communicable Diseases Division of National Health Laboratory Service. South African Tuberculosis Drug Resistant Survey. 2012-2014. 2016
- 19.Kent PT. Public health mycobacteriology: a guide for the level III laboratory. US department of health and human services, public health service, Centers; 1985. [Google Scholar]
- 20.Hain Lifescience. Genotype MTBDRplus version 2.0: instructions manual. GmbH; Nehren, Germany: 2012. [Google Scholar]
- 21.Hain Lifescience GmbH. GenoType MTBDRsl Ver2.0: instruction manual. Hain Lifescience GmbH; Nehren, Germany: 2015. [Google Scholar]
- 22.Derendinger B, de Vos M, Pillay S, et al. Frequent suboptimal thermocycler ramp rate usage negatively impacts MTBDRsl performance for second-line drug resistant tuberculosis diagnosis. 2021. [DOI] [PMC free article] [PubMed]
- 23.Miotto P, Tessema B, Tagliani E, et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. 2017;50(6) doi: 10.1183/13993003.01354-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naidoo K, Dookie N. Can the GeneXpert MTB/XDR deliver on the promise of expanded, near-patient tuberculosis drug-susceptibility testing? The Lancet Infectious Diseases. 2022 doi: 10.1016/S1473-3099(21)00613-7. [DOI] [PubMed] [Google Scholar]
- 25.Van Rie A, Whitfield MG, De Vos E, et al. Discordances between molecular assays for rifampicin resistance in Mycobacterium tuberculosis: frequency, mechanisms and clinical impact. 2020;75(5):1123–9. doi: 10.1093/jac/dkz564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngabonziza JCS, Decroo T, Migambi P, et al. Prevalence and drivers of false-positive rifampicin-resistant Xpert MTB/RIF results: a prospective observational study in Rwanda. The Lancet Microbe. 2020;1(2):e74–e83. doi: 10.1016/S2666-5247(20)30007-0. [DOI] [PubMed] [Google Scholar]
- 27.Ghebrekristos Y. Characterization of Mycobacterium tuberculosis isolates with discordant rifampicin susceptibility test results. University of Cape Town; 2018. [Google Scholar]
- 28.Bisimwa BC, Nachega JB, Warren RM, et al. Xpert MTB/RIF-detected Rifampicin Resistance is a Sub-Optimal Surrogate for Multidrug Resistant Tuberculosis in Eastern Democratic Republic of the Congo: Diagnostic and Clinical Implications. 2020 doi: 10.1093/cid/ciaa873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasiri M, Zamani S, Pormohammad A, et al. The reliability of rifampicin resistance as a proxy for multidrug-resistant tuberculosis: a systematic review of studies from Iran. 2018;37(1):9–14. doi: 10.1007/s10096-017-3079-4. [DOI] [PubMed] [Google Scholar]
- 30.Dean AS, Zignol M, Cabibbe AM, et al. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: A multicountry analysis of cross-sectional data. PLoS medicine. 2020;17(1):e1003008. doi: 10.1371/journal.pmed.1003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bainomugisa A, Gilpin C, Coulter C, Marais BJ. New xpert MTB/XDR: Added value and future in the field. Eur Respiratory Soc. 2020 doi: 10.1183/13993003.03616-2020. [DOI] [PubMed] [Google Scholar]
- 32.Feuerriegel S, Kohl TA, Utpatel C, et al. Rapid genomic first-and second-line drug resistance prediction from clinical Mycobacterium tuberculosis specimens using Deeplex-MycTB. European Respiratory Journal. 2021;57(1) doi: 10.1183/13993003.01796-2020. [DOI] [PubMed] [Google Scholar]
- 33.Georghiou SB, Schumacher SG, Rodwell TC, et al. Guidance for studies evaluating the accuracy of rapid tuberculosis drug-susceptibility tests. The Journal of Infectious Diseases. 2019;220(Supplement_3):S126–S35. doi: 10.1093/infdis/jiz106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.