Abstract
Background
The utility of MRI brain in patients with transient or minor neurological symptoms (TMNS) is uncertain. We sought to determine the proportion of participants with TMNS at different clinical probabilities of TIA or minor stroke who had MRI evidence of acute ischemia.
Methods
Cohort of TMNS participants from emergency and outpatient settings. Clinicians at different levels of training gave each participant a diagnostic probability (‘probable’ when TIA/stroke was the most likely differential diagnosis; ‘possible’ when TIA/stroke was not the most likely differential diagnosis; or ‘uncertain’ when diagnostic probability could not be given) before 1.5 or 3T brain MRI ≤5 days from onset. Post-hoc, each clinical syndrome was defined blind to MRI findings as: NINDS criteria TIA/stroke; International Headache Society criteria migraine aura; non-TIA focal symptoms, or non-focal symptoms. MRI evidence of acute ischemia was defined by 2 reads of MRI. We measured the occurrence of stroke for at least 90 days and up to 18 months after recruitment.
Results
272 participated (47% female, mean age 60, SD 14), 58% with MRI ≤2 days of onset. Most (92%) reported focal symptoms. MR evidence of acute ischemia was found, for stroke/TIA clinical probabilities of: probable 23/75 (31%, 95%CI:21-42%); possible 26/151 (17%,12-24%); and uncertain 9/43, (20%, 10-36%). MRI evidence of acute ischemia was found in: NINDS criteria TIA/stroke 40/95 (42%, 32-53%); migraine aura 4/38 (11%, 3-25%); non-TIA focal symptoms 16/99 (16%, 10-25%); and no focal features 1/29 (3%, 0-18%). After MRI, a further 14 (5%, 95%CI: 3-8) would be treated with an antiplatelet drug compared with treatment plan before MRI. By 18 months, a new ischemic stroke occurred in 9/61 (18%) patients with MRI evidence of acute ischemia and 2/211 (1%) without (age adjusted HR 13, 95%CI: 3-62;p<0.0001).
Conclusions
MRI evidence of acute brain ischemia was found in about 1 in 6 TMNS patients with a non-stroke/TIA initial diagnosis or uncertain diagnosis. Methods to determine the clinical and cost-effectiveness of MRI are needed in this population.
Non-Standard Abbreviations And Acronyms
- CT
computerized tomography
- DWI
diffusion weighted imaging
- IHS
International Headache Society
- MRI
magnetic resonance imaging
- MI
myocardial infarction
- NICE
National Institute of Health and Care Excellence
- NIHSS
National Institutes of Health Stroke Scale
- NINDS
National Institute of Neurological Disorders and Stroke
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology.
- TMNS
Transient or minor neurological symptoms
- WMH
white matter hyperintensities
Introduction
Neurologists and stroke physicians find it difficult to rule in or out a diagnosis of minor stroke or transient ischemic attack (TIA) in patients with transient or minor neurological symptoms (TMNS). In these patients, the best initial investigation strategy is still uncertain.
One strategy is to use magnetic resonance imaging (MRI) of the head for every patient with a TMNS. MRI of the brain may detect diffusion weighted imaging (DWI) lesions that are typical of acute brain ischemia or (rarely) may make a positive diagnosis of an alternative cause for symptoms such as multiple sclerosis or brain tumor. Where the diagnosis of TIA or stroke is secure, the pattern of ischemia seen on MRI may help to identify the underlying cause, such as multi-vascular-territory ischemia in cardio-embolism. However, a MRI brain without signs of acute ischemia is the commonest finding in patients with a clinical diagnosis of TIA or minor stroke, and may be falsely reassuring.1,2 This is particularly likely in patients with brainstem TIA or minor stroke, or with delayed presentation, where normal MRI brain imaging found more frequently 2.
To measure the benefits of an MRI strategy that scans all patients rather than a clinically targeted strategy, we need an estimate of the proportion of patients with positive DWI at different levels of clinical probability of a diagnosis of a TIA or minor stroke. For example, if the great majority of patients with a high clinical probability of TIA or stroke had DWI changes, or the great majority of patients with a low clinical probability had a negative MRI, then MRI could be targeted at those with intermediate clinical suspicion. However, if clinical probability did not identify a very high or low probability of DWI lesions, then a policy to scan all patients might be preferred.
We therefore sought to recruit a cohort of participants from clinical practice with transient or minor symptoms where TIA or stroke was suspected but not confirmed, and to determine the proportion of patients with MRI evidence of acute ischemia at different clinically predicted risks of TIA or minor stroke.
Methods
Data from this study is available on request from the corresponding author
We invited adults ≥18 years to participate within 5 days of TMNS where TIA or minor stroke was suspected by an assessing clinician. Participants in a major acute teaching hospital were referred to the study team by stroke physicians in the emergency department, acute medical wards and a TIA clinic (which dealt with emergency department and general practitioner referrals). Symptoms were minor when participants had an NIH stroke scale (NIHSS) ≤5 at assessment and were expected to be discharged or spend no more than one night in hospital. Predefined, relevant symptoms included: diplopia, vertigo, cognitive complaints of sudden onset, isolated speech disturbance (dysphasia or dysarthria), transient or mild weakness, heaviness or clumsiness of a limb, isolated limb sensory disturbance, hemi-visual field disturbance, migraine aura with no prior history of migraine aura ≥50 years of age, or a combination of these symptoms. We excluded potential participants: with a definite clinical diagnosis of TIA or stroke (i.e. where the assessing clinician diagnosed TIA or stroke with no differential diagnosis); with monocular symptoms; who had been considered for thrombolysis or thrombectomy; with a contraindication to MRI scanning; who were pregnant; who were not resident in Lothian UK; or who could not be easily followed-up (e.g., no address or telephone number). At presentation, a study nurse collected age, sex, baseline medical and demographic variables, and patients answered a pre-specified questionnaire about their symptoms.
Each assessing clinician gave their clinical diagnosis prior to imaging as ‘probable’ or ‘possible’ TIA or stroke, or an ‘uncertain’ diagnosis. Clinicians made: a ‘probable’ diagnosis where stroke or TIA was the most likely of a number of differential diagnoses; a ‘possible diagnosis’ where a non-cerebrovascular diagnosis was the most likely and stroke or TIA less likely; and an ‘uncertain’ diagnosis where a diagnostic probability could not be assigned on a proforma with diagnostic reminder. Each clinician gave their alternative non-cerebrovascular diagnoses, and planned treatment if MRI imaging was not available.
Post-hoc, with review of all pre-imaging clinical records and blind to MRI results, participants’ symptoms were classified by a stroke neurologist into: a TIA or stroke with National Institute of Neurological Disorders and Stroke (NINDS) criteria3; a typical migraine aura with or without headache with International Headache Society Criteria (IHS)4; an attack with focal features (i.e. could be attributed to dysfunction in one brain area) not in NIH or IHS criteria; or an attack with no focal features. An ABCD2 score was calculated for each participant.5
Each participant had an MRI of the brain within five days of symptom onset with 3T or 1.5T scanner with T1, T2, FLAIR, blood sensitive and DWI sequences. Each MRI was reported by a clinical radiologist with symptoms and separately by a masked research radiologist using a standardized proforma. The presence of acute ischemia was defined primarily by the presence of a DWI lesion, but also from the appearance of T2 and FLAIR sequences. We also recorded deep and subcortical white matter hyperintensities (WMH) using the Fazekas scale,6 the number of old lacunes, microbleeds, old cortical, large subcortical and likely small subcortical or posterior fossa infarcts, old hemorrhages, cortical siderosis, superficial and deep brain atrophy. Other structural lesions likely to have caused the symptoms were also recorded (tumors, subdural hematoma, etc.). Where there was disagreement between the clinical and research radiologist, a third radiologist reviewed the imaging and came to a final decision.
At 90 days post event, we contacted each participant by email or letter depending on preference, and by telephone for those who did not respond. We asked each participant whether they had had further symptoms or a stroke or myocardial infarction (MI) participants’ modified Rankin scale, and Euroquol 5-level score. We further assessed the occurrence of further stroke, MI or death by reading each participant’s hospital based electronic health records up to 19th May 2020 (i.e. when the last participant had 90 days of follow-up).
We analyzed data with SAS (V 9.4 SAS Institute Inc., Cary, NC, USA). We compared the characteristics of groups of participants with and without MRI evidence of acute ischemia, using chi-squared, Fisher’s exact or t-tests as appropriate. We calculated 95% confidence intervals of proportions. To compare the incidence of stroke during follow up in participants with and without MRI evidence of acute ischemia, we calculated a hazard ratio adjusting for age (with few events, we included only one covariate in this model)7. We calculated an area under the receiver operator characteristic curve for a logistic regression model predicting MRI evidence of acute ischemia, using the predictors defined in a previous publication8: age, sex, motor or speech symptoms, ongoing symptoms, abnormal neurological examination, and no prior identical symptomatic event. The report is consistent with the STROBE guidelines (Table S1, Figure S1).9
All participants gave written consent. The East of England (Essex) Research Ethics Committee gave approval for the study (18/EE/0157).
Results
Between 18th August 2018 and 19th February 2020, 286 people consented to take part; 14 were unable to have an MRI scan (4 claustrophobia, 1 contraindication, 3 too large for scanner, and 6 for >1 reason).
Of the 272 participants, most had an MRI brain ≤2 days of symptom onset (157, 58%). Participants were mostly male (145, 53%); had a mean age of 60 (SD 14); had only one episode of neurological symptoms (205, 75%); and had mild or no neurological signs (NIHSS=0: 247, 91%), which had either not resolved at presentation (118, 43%) or had resolved but lasted for ≥1 hour (94, 35%). In most participants, symptoms were mono-symptomatic or all began simultaneously (156, 57%). Common non-focal symptoms were tiredness or fatigue (54%), headache (43%), and nausea or sickness (40%). (Table 1,2)
Table 1. Characteristics of participants at recruitment by presence or absence of acute ischemia on MRI head.
| Acute ischemia on MRI (N,%) | Total (N,%) | P-value | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| Number of cases | n=61 | n=211 | n=272 | ||
| Sex | Male | 41 (67) | 104 (49) | 145 (53) | 0.014 |
| Female | 20 (33) | 107 (51) | 127 (47) | ||
| Age group | <60 | 20 (33) | 122 (58) | 142 (52) | 0.002 |
| 60 to <70 | 15 (25) | 39 (18) | 54 (20) | ||
| ≥70 | 26 (43) | 50 (24) | 76 (28) | ||
| Recruited from | TIA clinic | 28 (46) | 105 (50) | 133 (49) | |
| Emergency Department | 19 (31) | 66 (31) | 85 (31) | 0.596 | |
| Admissions ward | 13 (21) | 32 (15) | 45 (17) | ||
| Other | 1 (2) | 8 (4) | 9 (3) | ||
| Smoker | Never | 36 (59) | 105 (50) | 141 (52) | |
| Ex-smoker > one year | 14 (23) | 71 (34) | 85 (31) | ||
| Ex-smoker < one year | 4 (7) | 7 (3) | 11 (4) | 0.277 | |
| Current smoker | 7 (11) | 28 (13) | 35 (13) | ||
| Weekly alcohol intake | Never | 10 (16) | 40 (19) | 50 (18) | |
| < 14 units a week | 40 (66) | 135 (64) | 175 (64) | 0.898* | |
| > 14 unit a week | 11 (18) | 34 (16) | 45 (17) | ||
| Alcohol dependent | 0 | 1 (0) | 1 (0) | ||
| Prior history | Diabetes | 9 (15) | 20 (9) | 29 (11) | 0.240 |
| Hypertension | 22 (36) | 72 (34) | 94 (35) | 0.779 | |
| Myocardial infarction or angina | 8 (13) | 18 (9) | 26 (10) | 0.284 | |
| Stroke or TIA | 4 (7) | 27 (13) | 31 (11) | 0.177 | |
| Atrial fibrillation | 5 (8) | 8 (4) | 13 (5) | 0.3085 | |
| Anxiety or depression | 18 (30) | 76 (36) | 94 (35) | 0.346 | |
| Epilepsy | 2 (3) | 3 (1) | 5 (2) | 0.313* | |
| Migraine or other headache | 24 (39) | 76 (36) | 100 (37) | 0.635 | |
| Hearing loss or wears hearing aid | 11 (18) | 32 (15) | 43 (16) | 0.589 | |
| Non-refractive visual impairment | 9 (15) | 20 (9) | 29 (11) | 0.240 | |
| Medication at presentation | Antiplatelet | 16 (26) | 44 (21) | 60 (22) | 0.372 |
| Antihypertensive | 25 (41) | 66 (31) | 91 (33) | 0.157 | |
| Cholesterollowering medication | 20 (33) | 62 (29) | 82 (30) | 0.610 | |
| MoCA | missing | 1 (2) | 3(1) | 4(1) | 0.898 |
| <20 | 3 (5) | 8 (4) | 11 (4) | ||
| 20-24 | 17 (28) | 63 (30) | 80 (29) | ||
| 25-30 | 40 (66) | 137 (65) | 177 (65) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (years) | 65 (14) | 59 (14) | 60 (14) | 0.002 | |
| Systolic BP (mmHg) | 148 (27) | 142 (19) | 143 (21) | 0.088 | |
| Diastolic BP (mmHg) | 82 (13) | 84 (12) | 84 (13) | 0.395 | |
| Total MoCA | 25 (3) | 25 (3) | 25 (3) | 0.658 | |
p-value from Fishers exact test due to small expected counts.
Table 2. Characteristics of symptom onset at recruitment by presence or absence of acute ischemia on MRI head.
| Acute ischemia on MRI (N,%) | Total (N,%) | P-value | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| Number of cases | n=61 | n=211 | n=272 | ||
| NIHSS | 0 | 56 (92) | 191 (91) | 247 (91) | 0.760 |
| 1-4 | 5 (8) | 20 (9) | 25 (9) | ||
| Missing | 0 | 2 (1) | 2 (1) | ||
| Symptom duration | <10 minutes | 3 (5) | 14 (7) | 17 (6) | |
| 10-<60 minutes | 10 (16) | 31 (15) | 41 (15) | 0.914* | |
| 60 mins - 24hrs | 19 (31) | 75 (36) | 94 (35) | ||
| Not resolved | 29 (48) | 89 (42) | 118 (43) | ||
| Symptom onset | Simultaneous (or one symptom) | 45 (74) | 111 (53) | 156 (57) | |
| Gradual symptoms development | 16 (26) | 85 (40) | 101 (37) | 0.017# | |
| Don’t know (patient uncertain) | 0 | 8 (4) | 8 (3) | ||
| Unknown | 0 | 7 (3) | 7 (3) | ||
| Focal | Any focal symptom | 58 (95) | 192 (91) | 250 (92) | 0.426 * |
| Unilateral tingling | 25 (41) | 77 (36) | 102 (38) | 0.523 | |
| Unilateral numbness | 32 (52) | 79 (37) | 111 (41) | 0.036 | |
| Unilateral weakness | 17 (28) | 53 (25) | 70 (26) | 0.665 | |
| Unilateral loss of vision | 1 (2) | 4 (2) | 5 (2) | 1.00* | |
| Unilateral positive visual | 0 | 21 (10) | 21 (8) | 0.006* | |
| Slurred speech | 25 (41) | 45 (21) | 70 (26) | 0.002 | |
| Speaking nonsense | 8 (13) | 42 (20) | 50 (18) | 0.228 | |
| Difficulty with writing or typing | 12 (20) | 26 (12) | 38 (14) | 0.145 | |
| Vertigo | 17 (28) | 90 (43) | 107 (39) | 0.037 | |
| Non focal | Any non-focal symptom | 43 (70) | 185 (88) | 228 (84) | 0.001 |
| Headache | 22 (36) | 94 (45) | 116 (43) | 0.238 | |
| Pain in arms or legs | 9 (15) | 25 (12) | 34 (13) | 0.546 | |
| Chest pain | 0 | 21 (10) | 21 (8) | 0.006* | |
| Nausea or sickness | 16 (26) | 94 (45) | 110 (40) | 0.010 | |
| Palpitations | 7 (11) | 48 (23) | 55 (20) | 0.054 | |
| Hot flush or sweating | 13 (21) | 71 (34) | 84 (31) | 0.066 | |
| Breathlessness | 4 (7) | 36 (17) | 40 (15) | 0.041 | |
| Loss of awareness | 5 (8) | 34 (16) | 39 (14) | 0.120 | |
| Tiredness or fatigue | 25 (41) | 122 (58) | 147 (54) | 0.020 | |
| Time of onset to MRI (days) | ≤2 days | 36 (60) | 121 (57) | 157 (58) | 0.811* |
| >2 to ≤5 days | 25 (40) | 90 (43) | 115 (42) | ||
| N episode in previous month | Unknown | 2 (3) | 6 (3) | 8 (3) | |
| 0 | 44 (72) | 161 (76) | 205 (75) | ||
| 1 | 8 (13) | 18 (9) | 26 (10) | ||
| 2 | 4 (7) | 10 (5) | 14 (5) | 0.553* | |
| >=3 | 3 (5) | 16 (8) | 19 (7) | ||
| ABCD2 Score | 0 | 1 (2) | 16 (8) | 17 (6) | |
| 1-2 | 21 (34) | 69 (33) | 90 (33) | 0.252 | |
| 3-4 | 27 (44) | 98 (46) | 125 (46) | ||
| ≥5 | 12 (20) | 28 (13) | 40 (15) | ||
p-value from Fishers exact test due to small expected counts.
Chi-squared excludes missing data
MRI evidence of acute brain ischemia was present in 61 (22%) participants. Of these 61 participants, 16 (26%) had one cortical infarct, 16 (26%) had one subcortical lacunar infarct, 11 (18%) had one cerebellar or brainstem infarct, and 18 (30%) had more than one infarct. The infarcts were all thought to be relevant to the participant’s symptoms.
The absolute differences in clinical characteristics between participants with and without MR evidence of acute ischemia were modest: 33% versus 51% female (p=0.0135) and mean age 65 years versus 59 years (p=0.002) respectively. Focal symptoms were frequent in both groups (95% versus 91%, p=0.4264), although some symptoms were more frequent in participants with MRI evidence of acute brain ischemia: simultaneous onset of more than one symptom or one symptom type (74% versus 53%, p=0.017); unilateral numbness (52% vs 37%, p=0.0356); and slurred speech (41% versus 21%, p=0.002). Vertigo was less frequent in participants with an acute infarct on imaging (28% versus 43%, p=0.0373). Non-focal symptoms were frequent in participants with and without MRI evidence of acute ischemia (70% versus 80% p=0.001), but individual non-focal symptoms were less frequent in participants with MRI evidence of acute ischemia: nausea or sickness (26% vs 45%, p=0.010), breathlessness (7% versus 17%, p=0.041); and tiredness or fatigue (41% vs 58%, p=0.020). (Table 1,2)
Participants with an acute infarct on imaging had more evidence of cerebral small vessel disease and brain atrophy. They had more WMH that were deep (p=0.0307) or periventricular (p=0.0104); atrophy that was deep (p=0.0015) or superficial (p=0.0199); old infarction (52% versus 25%, p<0.0001) and more lacunes. (Table 3)
Table 3. Other MRI findings by presence of MRI detected acute ischemia.
| Acute ischemia on MRI (n,%) | Total | P-value | |||
|---|---|---|---|---|---|
| Number of cases | Yes n=61 | No n=211 | No n=272 | ||
| Fazekas scale (white matter hyperintensities) | |||||
| Deep | 0 | 11 (18) | 63 (30) | 74 (27) | 0.031 |
| 1 | 28 (46) | 105 (50) | 133 (49) | ||
| 2 | 13 (21) | 31 (15) | 44 (16) | ||
| 3 | 9 (15) | 12 (6) | 21 (8) | ||
| Periventricular | 0 | 1 (2) | 4 (2) | 5 (2) | 0.011* |
| 1 | 30 (49) | 139 (66) | 169 (62) | ||
| 2 | 17 (28) | 53 (25) | 70 (25) | ||
| 3 | 13 (21) | 15 (7) | 28 (10) | ||
| Old infarct | No | 29 (48) | 159 (75) | 188 (69) | <0.0001 |
| Yes | 32 (52) | 25 (25) | 84 (31) | ||
| Deep atrophy | 1 | 13 (21) | 98 (46) | 111 (41) | 0.002 |
| 2 | 15 (25) | 42 (20) | 57 (21) | ||
| 3+ | 33 (54) | 71 (34) | 104 (38) | ||
| Superficial atrophy | 1 | 12 (20) | 81 (38) | 93 (34) | 0.002 |
| 2 | 12 (20) | 55 (26) | 67 (25) | ||
| 3+ | 37 (61) | 75 (36) | 112 (41) | ||
| Number of lacunes | 0 | 34 (56) | 170 (81) | 204 (75) | 0.001* |
| 1 | 16 (26) | 29 (14) | 45 (17) | ||
| 2 | 7 (11) | 7 (3) | 14 (5) | ||
| 3+ | 2 (3) | 4 (2) | 6 (2) | ||
Assessing clinicians gave their clinical suspicion of stroke or TIA prior to imaging: consultant 104, 38%, trainee 166, 61%; and stroke medicine 158, 58%, geriatrics 43, 16% and neurology 62, 23%. Before brain imaging, diagnoses were: uncertain (16%); definite TIA or stroke (1%); probable TIA or stroke (28%); or possible TIA or stroke (55%). The differential diagnoses were: migraine (23%); unknown (21%); vertigo (15%); functional neurological disorder (10%); neuropathy (5%); seizure (4%); anxiety (2%); and other diagnoses (21%).
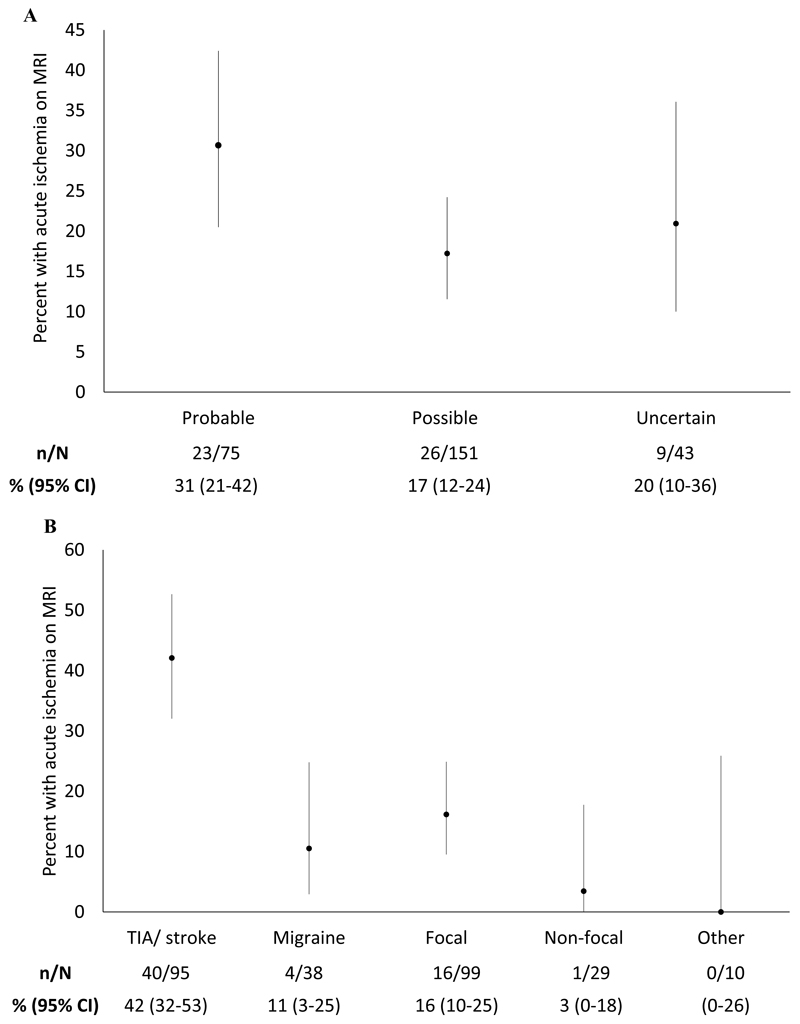
The proportion of participants with MRI evidence of acute ischemia in each diagnostic group was for: probable stroke or TIA, 31% (95% CI 21-42%); possible stroke or TIA 17%, (95% CI 12-24%); and uncertain, 20% (95% CI 10-36%) [χ2p=0.07]. (Figure 1a) Three participants were recruited in error with a definite diagnosis of TIA or stroke, all of whom had MR evidence of acute ischemia.
Figure 1.
(a) Percentage of participants with MRI evidence of acute ischemia with a prospectively provided clinical diagnosis of probable, possible or uncertain stroke or TIA (χ2p=0.07). (b) Percentage of participants with MRI evidence of acute ischemia with a retrospective diagnosis of TIA or stroke by NINDS definition; migraine; focal non-NINDS attacks; non focal symptoms; or other diagnosis.
Post-hoc, the diagnosis of symptoms after review of records blind to imaging were: TIA /stroke by NIH criteria (95, 35%) non-NIH focal symptoms (99, 36%); migraine by IHS criteria (38, 14%); no focal features (29, 11%); and other diagnosis (10, 4%). The proportion of participants with MR evidence of acute ischemia in each group was: NIH criteria TIA or stroke, 42% (32-53%); migraine by IHS criteria, 11% (3-25%); NIH focal symptoms, 16% (10-25%); and non-focal features, 3% (0-18%) and other diagnosis 0%. (Figure 1b) The distribution of the ABCD2 score was similar in participants with and without MRI evidence of acute ischemia (p=0.252). A logistic regression model with covariates (age, sex, motor/speech symptoms, ongoing symptoms, abnormal initial neurological exam, prior identical symptomatic event) from a previous study8 had only modest discrimination (c-statistic 0.70, 95%CI: 0.62-0.77).
Before assessment, 60 (22%) of participants were taking an antiplatelet. Before clinicians had access to MRI scanning, they said they would treat a further 142 (52%) with an antiplatelet for at least a month, whatever the MRI results. If all participants with MRI evidence of acute ischemia would be treated with an antiplatelet, a further 14 (5% 95%CI:3-8) would be treated with an antiplatelet after MRI.
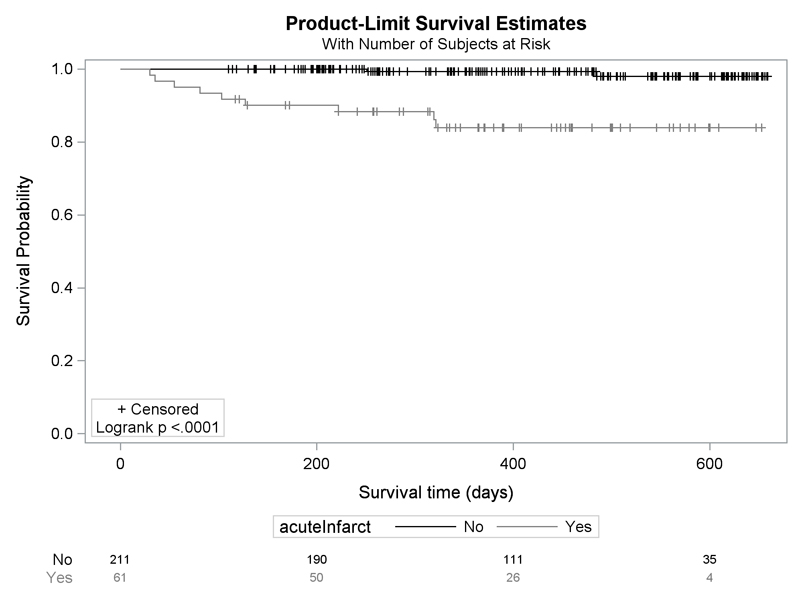
We followed 264 (97%) participants up to 90 days by email, post or telephone. By 90 days, 4 participants, all with MRI evidence of acute ischemia at baseline, had a subsequent ischaemic stroke. By 19th May 2020, 9 participants with MRI evidence of acute ischemia and 2 without (age adjusted HR 13, 95%CI: 3 to 63) had a new ischemic stroke. (Figure 2). Symptoms were equally common at 90 days between participants with and without MRI evidence of acute ischemia, and both groups had similar disability (mRS 0-1 acute ischemia: 82%; no acute ischemia: 85% p=0.368) and quality of life (EuroQOL 0.94 vs 1.00, p=0.6038). (Table 4)
Figure 2.
Kaplan-Meier survival plot for the incidence of stroke during follow up after MR brain images, in participants with and without MR evidence of acute ischemia (age adjusted HR 13, 95%CI: 3 to 63)
Table 4. Follow up at 90 days by letter, email or telephone.
| Acute ischemia on MRI (n,%) | All, n(%) | P-value | ||
|---|---|---|---|---|
| Total Number | Yes n=61 | No n=211 | n=272 | |
| Symptoms | ||||
| No persistent symptoms | 42 (69) | 152 (72) | 194 (71) | 0.63 |
| Any new symptom | 16 (26) | 59 (28) | 75 (28) | 0.79 |
| New headache | 1 (2) | 16 (8) | 17 (6) | 0.13 |
| New unilateral numbness, weakness, visual loss or speech loss at 90 days | 5 (8) | 14 (7) | 19 (7) | 0.69 |
| Other symptoms at 90 days | 9 (15) | 33 (16) | 42 (15) | 0.86 |
| modified Rankin scale | ||||
| mRS | ||||
| 0-1 | 50 (83) | 180 (88) | 230 (87) | 0.368 |
| 2-6 | 10 (17) | 25 (12) | 35 (13) | |
| Health related quality of life | ||||
| EuroQOL EQ-5d-5L Median (q1, q3) | 0.94 (0.84, 1.00) | 1.00 (0.85, 1.00) | 0.95 (0.84, 1.00) | 0.604 |
p-value from Mann-Whitney test.
Discussion
In this study, MRI evidence of acute ischemia was seen in 17% (95%CI:3-25%) of participants with possible stroke or TIA (where another diagnosis was thought more likely) and 16% (95%CI:10-25%) participants with focal symptoms that were not within the NINDS definition of TIA. Using the prescription of anti-platelets as a proxy for secondary prevention, we estimated that MRI would have led to a modest increase (5%, 95%CI: 3-8%) in the number of people who would have been prescribed an antiplatelet, compared with treatment plans without MRI.
The findings from this study are consistent with other literature. In a study of 1,028 participants from multiple countries with minor or transient symptoms, 12% of participants with a diagnosis of transient focal neurological events had a MRI DWI finding.8 In other smaller studies, DWI positive lesions were present in 23% (I3/56)10 of patients with transient neurological attacks, and 35% (21/59) patients with neurological symptoms and normal brain CT presenting to an emergency department11.
Clinical diagnosis in transient and minor neurological symptoms relies on a history which may be poorly interpreted by a clinician or remembered with difficulty because of acute or chronic cognitive problems. Therefore, doctors often disagree about whether a patient’s symptoms are due to a TIA when comparing: general practitioners with neurologists12, emergency department (ED) doctors with neurologists12, neurologists with neurologists13–15, or even stroke-trained neurologists with stroke-trained neurologists 16. Despite this, clinical diagnosis of TIA or stroke is moderately predictive of stroke recurrence in patients presenting to TIA services, even in the absence of brain imaging 17. This could improve with a structured proforma for focal symptoms, given that the proportion of people with a DWI lesion was higher in patients with a NINDS defined TIA or minor stroke in this study.
The lack of a gold standard diagnosis is a problem in all studies of TIA or minor stroke. People with TMNS without a DWI lesion have a higher risk of future stroke than controls. These people may have a normal MRI brain because they are more resilient to brain ischaemia, or smaller infarcts that resolve more quickly.18
Strengths
Our study had several strengths. External validity was supported because clinical diagnosis was given by doctors from a range of clinical backgrounds and experience, and participants were recruited from several clinical locations. Internal validity was supported by the small number of participants who did not have an MRI or were lost to follow-up; the recording of a clinical diagnosis prior to imaging; dual reading of images; and the use of standard proforma to prospectively collect data on symptoms, diagnosis and demographics.
Limitations
Our study has some limitations. First, because this was a research study, we could only include participants who consented to take part, which may have led us to recruiting a population with a different risk profile to a population-based cohort, despite our efforts to recruit all patients who presented to our service. Compared with all patients arriving at the clinic, these participants were younger (60 versus 69 years) although the distribution of sex and symptoms were similar.17 Second, we did not follow every participant up to 24 hours after their onset of symptoms to determine the speed of symptom resolution, and so we could not make a reliable time-defined TIA diagnosis. Third, the sample size was modest, and limited by the cost of MRI scanning for research. Fourth, there may have been a Hawthorne effect through observing the diagnostic process, which may have biased pre-imaging diagnostic probability. Fifth, if we had recruited from a larger number of sites, our findings would have been more generalizable. We did not assess further non-consenting patients. Sixth, the investigation of stroke cause was clinically driven, and so not all participants were investigated with CT or MR angiography, echocardiography or prolonged ECG recording. Finally, we were unable to re-approach clinicians to ask whether the results of a scan would have changed their practice. However, the assumption that a positive DWI would change their diagnosis from uncertain to definite is reasonable.
Implication for research
The utility of MRI scanning in clinical practice is uncertain, and there have been calls for randomised comparisons of imaging strategies to reduce recurrent stroke risk.19–21 However, the sample size for such a study, given the relatively low event rate of stroke in all patients with suspected stroke or TIA, and the modest plausible effect of scanning on recurrent stroke (only acting through additional secondary prevention in the smaller proportion who would not have been diagnosed with a standard strategy), would make any study very large and potentially undeliverable.
Implications for practice
Clinical guidelines differ in their recommendations for imaging in patients with suspected TIA or stroke. The National Institute of Health and Care Excellence (NICE) in the UK recommends avoiding CT scanning and to consider MRI after specialist assessment to look for alternative pathologies or the territory of ischemia21. The Canadian Stroke Best Practices recommend CT or MRI imaging of brain and major vessels, with the recommended timing of the imaging dependent on clinical symptoms; for patients with focal transient symptoms atypical for ischemia, MRI is recommended within 7 days of symptoms 22. The European Stroke Organisation guideline considered there was insufficient evidence to make a recommendation on imaging strategy in TIA or minor stoke.20 Lastly, the American Heart Association guidelines recommend CT or MRI of the brain in patients with suspected stroke or TIA, and with a lower quality of evidence, follow up MRI or CT if the initial imaging is normal.23
Health economic studies disagree whether MR scanning is cost effective. One study concluded MRI was cost effective, estimated with an MRI sensitivity of 94% and specificity of 100% (this is an overestimate because only 2/3 of minor stroke have DWI findings) and an estimate of an 80% reduction in risk of recurrent stroke with secondary prevention in the longer term, which may also be an overestimate. In this study, the 30 years costs of an MRI strategy for patients with normal CT were estimated to be $26,304 compared with costs of $27,109 with a CT only strategy.24 However, in a more extensive study, which modelled most of the care pathway, MRI was not cost effective compared with any other imaging modality (with incremental costs of between £63 and £407).19
Countries or regions where access to MRI is not constrained could implement a policy of MRI scanning for all patients with transient or minor neurological symptoms, and use the results of MRI to determine subsequent treatment. However, even in locations with less resource limitation, only 40% of patients with suspected TIA or stroke receive an MRI within two days.25. For regions which have limited access to MRI, developing a targeting strategy would be sensible to guide the best use of this resource.
Conclusion
MRI scanning in all patients presenting with transient or minor neurological symptoms is likely to identify some patients who would otherwise not receive a diagnosis of stroke or TIA, because they were otherwise thought to be at low risk. However, whether this strategy is cost effective, or how MRI scanning could best be targeted, is unclear.
Supplementary Material
Footnotes
Disclosures
None
Sources Of Funding
The project was funded by the Chief Scientist’s Office of the Scottish Government (CSO TCS/17/08). YW was supported by State Scholarship Fund to pursue study in the United Kingdom as a visiting scholar, Fund Number: 201808535094. WW was supported by a Scottish Senior Clinical Fellowship (CAF/17/01). MR is supported by an National health Service Research Scotland Career Researcher Clinician award. CG reports grants from the British Heart Foundation. JMW reports grants from Wellcome Trust; grants from Medical Research Council; grants from Fondation Leducq; grants from Dunhill Medical Trust; and grants from Chief Scientist Office. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to an Author Accepted Manuscript version arising from this submission
References
- 1.Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis M, Sandercock PAG, Muir K, Wardlaw JM. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann Neurol. 2014;75:67–76. doi: 10.1002/ana.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makin SDJ, Doubal FN, Dennis MS, Wardlaw JM. Clinically Confirmed Stroke With Negative Diffusion-Weighted Imaging Magnetic Resonance Imaging: Longitudinal Study of Clinical Outcomes, Stroke Recurrence, and Systematic Review. Stroke. 2015;46:3142–8. doi: 10.1161/STROKEAHA.115.010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Advisory Council for the National Institute of Neurological and Communicative Disorders and Stroke. A Classification and Outline of Cerebrovascular Diseases II. Stroke. 1975;6:564–616. doi: 10.1161/01.str.6.5.564. [DOI] [PubMed] [Google Scholar]
- 4.The International Classification of Headache Disorders. (3rd) Copyright. [Google Scholar]
- 5.Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 6.Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 7.Harrell F. Regression modelling strategies: with applications to linear models, logistic regression and survival analysis. 1st. Springer Verlag; New York: 2001. [Google Scholar]
- 8.Coutts SB, Moreau F, Asdaghi N, Boulanger J-M, Camden M-C, Campbell BCV, Demchuk AM, Field TS, Goyal M, Krause M, et al. Rate and Prognosis of Brain Ischemia in Patients With Lower-Risk Transient or Persistent Minor Neurologic Events. JAMA Neurol. 2019;76:1439. doi: 10.1001/jamaneurol.2019.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Van Rooij FG, Vermeer SE, Gõraj BM, Koudstaal PJ, Richard E, De Leeuw FE, Van Dijk EJ. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol. 2015;78:1005–1010. doi: 10.1002/ana.24539. [DOI] [PubMed] [Google Scholar]
- 11.Kazmierczak PM, Dührsen M, Forbrig R, Patzig M, Klein M, Pomschar A, Kunz WG, Puhr-Westerheide D, Ricke J, Solyanik O, et al. Ultrafast Brain Magnetic Resonance Imaging in Acute Neurological Emergencies: Diagnostic Accuracy and Impact on Patient Management. Invest Radiol. 2020;55:181–189. doi: 10.1097/RLI.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 12.Ferro JM, Falcao I, Rodrigues G, Canhao P, Melo TP, Oliveira V, Pinto AN, Crespo M, Salgado AV. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke. 1996;27:2225–2229. doi: 10.1161/01.str.27.12.2225. [DOI] [PubMed] [Google Scholar]
- 13.Kraaijeveld CL, van Gijn J, Schouten HJ, Staal A. Interobserver agreement for the diagnosis of transient ischemic attacks. Stroke. 1984;15:723–725. doi: 10.1161/01.str.15.4.723. [DOI] [PubMed] [Google Scholar]
- 14.Tomasello F, Mariani F, Fieschi C, Argentino C, Bono G, De Zanche L, Inzitari D, Martini A, Perrone P, Sangiovanni G. Assessment of inter-observer differences in the Italian multicenter study on reversible cerebral ischemia. Stroke. 1982;13:32–35. doi: 10.1161/01.str.13.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Koudstaal PJ, van Gijn J, Staal A, Duivenvoorden HJ, Gerritsma JG, Kraaijeveld CL. Diagnosis of transient ischemic attacks: improvement of interobserver agreement by a check-list in ordinary language. Stroke. 1986;17:723–728. doi: 10.1161/01.str.17.4.723. [DOI] [PubMed] [Google Scholar]
- 16.Castle J, Mlynash M, Lee K, Caulfield AF, Wolford C, Kemp S, Hamilton S, Albers GW, Olivot J-MM. Agreement Regarding Diagnosis of Transient Ischemic Attack Fairly Low Among Stroke-Trained Neurologists. Stroke. 2010;41:1367–1370. doi: 10.1161/STROKEAHA.109.577650. [DOI] [PubMed] [Google Scholar]
- 17.Graham C, Bailey D, Hart S, Hutchison A, Sandercock P, Doubal F, Sudlow C, Farrall A, Wardlaw J, Dennis M, et al. Clinical diagnosis of TIA or minor stroke and prognosis in patients with neurological symptoms: A rapid access clinic cohort. PLoS One. 2019;14:e0210452. doi: 10.1371/journal.pone.0210452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteley WN, Thompson D, Sandercock P. Response to Letter Regarding Article, “Targeting Recombinant Tissue-Type Plasminogen Activator in Acute Ischemic Stroke Based on Risk of Intracranial Hemorrhage or Poor Functional Outcome. Stroke. 2014;45:1000–1006. doi: 10.1161/STROKEAHA.113.004362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardlaw J, Brazzelli M, Miranda H, Chappell F, McNamee P, Scotland G, Quayyum Z, Martin D, Shuler K, Sandercock P, et al. An assessment of the cost-effectiveness of magnetic resonance, including diffusion-weighted imaging, in patients with transient ischaemic attack and minor stroke: a systematic review, meta-analysis and economic evaluation. Heal Technol Assess. 2014;18 doi: 10.3310/hta18270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonseca AC, Merwick Á, Dennis M, Ferrari J, Ferro JM, Kelly P, Lal A, Ois A, Olivot JM, Purroy F. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur stroke J. 2021;6:CLXIII–CLXXXVI. doi: 10.1177/2396987321992905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Health Care and Clinical Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management | Guidance | NICE. NICE; 2019. [Internet] [PubMed] [Google Scholar]
- 22.Canadian Stroke Best Practices. Triage and Initial Diagnostic Evaluation of Transient Ischemic Attack and Non-Disabling Stroke. 2020 [Internet] [Google Scholar]
- 23.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:E364–E467. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 24.Puhr-Westerheide D, Froelich MF, Solyanik O, Gresser E, Reidler P, Fabritius MP, Klein M, Dimitriadis K, Ricke J, Cyran CC, et al. Cost-effectiveness of short-protocol emergency brain MRI after negative non-contrast CT for minor stroke detection. Eur Radiol. 2021;2021:1–10. doi: 10.1007/s00330-021-08222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaturvedi S, Ofner S, Baye F, Myers LJ, Phipps M, Sico JJ, Damush T, Miech E, Reeves M, Johanning J, et al. Have clinicians adopted the use of brain MRI for patients with TIA and minor stroke? Neurology. 2017;88:237–244. doi: 10.1212/WNL.0000000000003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.