Abstract
Background
Amodiaquine is a 4-aminoquinoline antimalarial used extensively for the treatment and prevention of malaria. Orally administered amodiaquine is largely converted to the active metabolite desethylamodiaquine. Oral amodiaquine can cause bradycardia, hypotension, and electrocardiograph (ECG) QT interval prolongation in animals and humans but the relationship of these changes to drug concentrations is not well characterised.
Methods
We conducted a secondary analysis of data from a pharmacokinetic study of the cardiac safety of amodiaquine (10mg/kg/day over 3 days) in 54 Kenyan adults (≥18 years) with uncomplicated malaria. Non-linear mixed effects modelling was used to assess amodiaquine and desethylamodiaquine concentration-effect relationships for vital sign (pulse rate, blood pressure) and ECG interval (QT, QRS, PR) outcomes. We also measured the spontaneous beating heart rate after cumulative dosing of amodiaquine and desethylamodiaquine in isolated mouse atrial preparations.
Findings
Amodiaquine and desethylamodiaquine caused concentration-dependent mean decreases in pulse rate (1.9beats/minute per 100nmol/L), supine systolic blood pressure (1.7mmHg per 100nmol/L), erect systolic blood pressure (1.5mmHg per 100nmol/L), and erect diastolic blood pressure (1.4mmHg per 100nmol/L). The mean QT interval prolongation was 1.4milliseconds per 100nmol/L irrespective of correction factor used after adjustment for residual heart rate dependency. There was no significant independent effect of drug concentration on postural change in blood pressure or PR and QRS intervals. In mouse atria, the spontaneous beating rate was significantly reduced by amodiaquine (n=7) and desethylamodiaquine (n=8) at 3μmol/litre (amodiaquine: 10% ± 2%; desethylamodiaquine: 12% ± 3%) and 10μmol/litre (amodiaquine: 50% ± 7%; desethylamodiaquine: 46% ± 6%) concentrations with no significant difference in potency between the two compounds.
Interpretation
Amodiaquine and desethylamodiaquine have concentration-dependent effects on heart rate, blood pressure, and ventricular repolarisation.
Introduction
Amodiaquine (AQ), a 4-aminoquinoline structurally similar to chloroquine, is an important antimalarial drug which has been deployed extensively in the treatment and prevention of malaria over the past 70 years. First synthesised by the United States World War II (WWII) antimalarial research programme1,2, it was initially sold as monotherapy under the trade name of Camoquin® by the American pharmaceutical company Parke-Davis2. In 2005, the World Health Organization (WHO) recommended that artemisinin-based combination therapy (ACT) should become the first-line treatment for all falciparum malaria. In 2008, artesunate-amodiaquine (ASAQ), the fixed-dose formulation of amodiaquine and artesunate (AS), became the first major development product of a public-private partnership, the Drugs for Neglected Diseases initiative (DNDi). ASAQ is now recommended by the WHO for the treatment of uncomplicated Plasmodium falciparum and P. vivax malaria3 and is the first-line oral antimalarial in more than 20 African countries4 where malaria is endemic. Since 2012, WHO has recommended use of seasonal malaria chemoprevention (SMC) for young children (aged 3-59 months) living in areas of seasonal high-intensity malaria transmission in the Sahel subregion of Africa. SMC comprises a single dose of sulfadoxine-pyrimethamine together with amodiaquine (SP + AQ) divided over three days monthly during the rainy season for up to four months annually. Millions of children are now protected with SMC every year5.
The cardiovascular effects of amodiaquine have been recognised from the earliest studies in animal models1. During its development, pulsus bigeminus was noted in anaesthetised dogs receiving high doses of parenteral amodiaquine1. Like chloroquine, amodiaquine exhibits anti-arrhythmic properties, terminating experimental atrial arrhythmias in both decentralised and innervated canine hearts6 but, unlike chloroquine, does not appear to protect against experimental ventricular arrhythmias7. Electrocardiograph QT interval prolongation7, bradycardia8, and hypotension8 have also been observed after parenteral amodiaquine administration to anaesthetised dogs and cats. We have reported recently that amodiaquine prolongs the QT interval less, but is more bradycardic and hypotensive than chloroquine at current standard oral malaria treatment doses when given to adolescents and adults9. The clinical significance of these cardiovascular effects with standard malaria treatment dosing is unclear9 although bradycardia and hypotension may contribute to the higher incidence of mild asthenia and asthenia-like reactions after amodiaquine compared to other antimalarials10–12. Direct multiple ion channel blockade of cardiac13–17 and vascular18 myocytes along with altered autonomic tone19,20 may both be relevant. There are few data on the cardiovascular pharmacology of amodiaquine and information on its main metabolite desethylamodiaquine is especially limited, as the metabolite desethylamodiaquine was only identified in the 1980s21. This was around the time reports of the fatal toxicity of amodiaquine in chemoprophylaxis22–24 led to its temporary withdrawal in 199025 from the list of WHO-recommended antimalarials.
We conducted a secondary analysis of a clinical pharmacokinetic study of ASAQ in adult malaria patients focusing on electrocardiographic interval (RR, QT, QRS, and PR) and cardiovascular vital sign (pulse rate and blood pressure) outcomes. This analysis of the clinical study was complemented by laboratory assessment of the concentration-heart rate response in murine atrial preparations with intact sino-atrial node (SAN).
Methods
Clinical
Trial Design
This was an open-label randomised controlled trial conducted between 2007 and 2008 in the Chulaimbo Sub-district Hospital of Kisumu, Kenya. The trial compared the fixed-dose ASAQ combination with loose (i.e. non-fixed dose) AS + AQ in adult patients (aged 18 to 60 years inclusive) presenting with acute uncomplicated P. falciparum monoinfection with an asexual parasitaemia of >1,000 parasites/μL and either a history of fever or a measured temperature of ≥37.5°C in the preceding 24 hours. Pregnant or lactating women, those with significant known comorbidities (including severe malnutrition or splenectomy), individuals with an ECG abnormality requiring urgent treatment, or those who had taken an artemisinin derivative and/or sulfadoxine-pyrimethamine in the previous three or seven days respectively were excluded. Further clinical details are reported in full elsewhere26,27.
Drug Regimen
Patients received either two fixed-dose ASAQ tablets (100/270mg, Sanofi-Aventis, France) daily for a total dose over three days of 600mg of AS and 1620mg of AQ, or four tablets each of non-fixed dose AS (Arsumax® 50mg, Sanofi-Aventis, France) and AQ (Flavoquine® 153mg, Sanofi-Aventis, France) daily for a total dose over three days of 600mg of AS and 1836mg of AQ. No concomitant food was given. All treatments were directly observed. Patients who vomited a dose within 1 hour after drug administration were retreated. Exact dosing times were recorded and used in modelling.
Clinical, Parasitological & ECG Procedures
Medical and drug histories were taken and physical examinations performed at baseline (D0). Basic clinical assessments including measurement of vital signs (pulse rate, supine and erect blood pressure, and axillary temperature) were conducted and malaria thick blood film slides were prepared at D0, D1, D2, D3, D7, D14, D21, and D28. A blood film was considered negative if no asexual parasites were seen after examination of 1000 white blood cells. Patients were asked about adverse events of headache, weakness, anorexia, nausea, abdominal pain, itching, vomiting, diarrhoea, rhinitis, cough, vertigo, and rash at each clinical assessment. Standard 12-lead ECGs were performed on D0 (pre-dose, +2 and +4 hours), D2 (+2 and +4 hours), and D28. ECGs were assessed centrally by cardiologists for arrhythmias and measurement of ECG intervals (RR, QT, QRS, and PR).
Blood Sampling Procedures
Full blood counts were performed on D0 (pre-dose), D7, and D28. Blood samples for drug concentration measurements were taken from all patients at fixed time points on D0 (pre-dose), D7, D14, D21, and D28. Each patient also had additional samples taken at random combinations of the following time points on D0 (+0.25, +0.5, +1, +1.5, +2, and +4 hours) and D2 (+0.25, +0.5, +1, +1.5, +2, and +4 hours). Exact sample times were recorded and used in the pharmacokinetic modelling.
Pharmacokinetic Methods
Venous plasma samples were analysed using liquid chromatography-mass spectrometry (LC-MS/MS) methods. Amodiaquine, desethylamodiaquine, and the internal standard (AST-D4) were analysed by reversed-phase liquid chromatography (X Terra C18 MS -3.5μm; 50mm x3mm id) and MS/MS (Sciex API3000) detection in the Turbo Ion Spray positive mode.
Ethics
The trial protocol was approved by the Kenya Medical Research Institute (KEMRI) Ethical Review Committee. Additional ethical approval for this secondary analysis of fully anonymised individual patient data was not deemed necessary in keeping with University of Oxford Central University Research Ethics Committee guidance.
Data Analysis
Trial data were standardised and checked according to a pre-specified data dictionary (Supplementary Appendix: Data Standardisation & Data Integrity Checks) for analysis. Measurements from fixed and non-fixed dose ASAQ arms were pooled as these dose formulations are known to be bioequivalent for amodiaquine and to not have an effect on the pharmacokinetic parameters of these drugs27,28. It is generally accepted that artesunate does not have a significant effect on the QT interval29.
In view of the inverse relationship between the QT interval and heart rate, measured QT intervals were adjusted for heart rate with the widely-used Bazett and Fridericia correction formulae. A study-specific correction formula was also applied with the correction exponent derived from log-log linear regression (Supplementary Appendix: Data Analysis – Study-Specific Heart Rate Correction). The QT interval was analysed as adjusted with the study-specific (QTcS), Fridericia (QTcF), and Bazett (QTcB) heart rate correction formulae.
All statistical analyses and data visualisation were done in R30 version 3.6.0, with linear mixed effects modelling conducted using the nlme31 package. Model fit was assessed by visual inspection of residuals while model discrimination was on the basis of likelihood ratio tests with p <0.05 as the threshold for statistical significance.
Pharmacokinetic Analysis
Observed amodiaquine and desethylamodiaquine concentrations, transformed into their natural logarithms, were analysed using non-linear mixed-effects modelling implemented in NONMEM version 7.432 using the first-order conditional estimation with interactions method. Concentrations below the lower limit of quantification of 1ng/ml were omitted. In addition to R, Pirana33 version 2.9.0 and Perl-speaks-NONMEM (PSN)34 version 4.8.0 were used for automation, model evaluation, and diagnostics during the modelling process. The NONMEM $PRIOR functionality was used to stabilise model performance.
The structural pharmacokinetic model of amodiaquine and desethylamodiaquine was based on a model developed from another study of amodiaquine monotherapy for treatment of P. vivax malaria in pregnant women with rich (i.e. more intensive) pharmacokinetic sampling35 (Supplementary Appendix: Figure 1). Amodiaquine concentrations were described by lagged first-order absorption with a two-compartment distribution model followed by a three-compartment distribution model of desethylamodiaquine. Amodiaquine was assumed to be metabolised completely into desethylamodiaquine as the drug-metabolite conversion fraction was not identifiable. The final population pharmacokinetic parameter and inter-individual variability estimates with their parameter uncertainties from the previous study35 were incorporated into the model developed for this study as prior estimates (Supplementary Appendix: Data Analysis – Pharmacokinetic Analysis).
Predicted amodiaquine and desethylamodiaquine concentrations for the time points at which cardiovascular vital signs (pulse rate and blood pressure) and electrocardiographic intervals (RR, QT, QRS, and PR) were measured were used in the concentration-effect analyses.
Concentration-Effect Analyses
Multivariable linear mixed effects modelling was performed with the corrected QT, QRS, and PR intervals as well as the change from baseline of pulse rate and blood pressure (systolic and diastolic in the supine and erect positions with their postural differences) as response variables (Supplementary Appendix: Data Analysis – Concentration-Effect Analyses). Individual patient was the random effect while fixed effect selection was based on directed acyclic graphs of proposed causal relationships among available variables (Supplementary Appendix Figures II-V) identified from literature review36 and expert consultation37. The drug effect was evaluated using the total predicted concentration of amodiaquine plus its metabolite desethylamodiaquine based on prior evidence that both amodiaquine6,7 and desethylamodiaquine38 affect cardiovascular physiology as well as lack of evidence for any substantial difference in their activities from descriptive analyses. In the ECG interval models, the other fixed effects were body temperature change, age, and sex, with the addition of RR interval change to adjust for residual heart rate changes. For cardiovascular vital signs measured at multiple time points after recovery from malaria, the malaria disease effect was incorporated as a binary categorical fixed effect variable present during days 0-2 of treatment, with the addition of body temperature change and sex for the change in the pulse rate model only.
Pre-clinical
Murine Atrial Studies
Adult male CD-1 mice (32-37 g, CD-1® IGS, Charles River Laboratories, UK) were housed maintained in a 12-hour light-dark cycle with ad libitum access to standard diet and sterilised water. Mice were culled by cervical dislocation in accordance with the United Kingdom Home Office Guidance on Animals (Scientific Procedures) Act 1986. The heart was excised rapidly and washed in heparin-containing Physiological Salt Solution (PSS, in millimoles: NaCl 125, NaHCO3 25, KCl 5.4, NaH2PO4 1.2, MgCl2 1, glucose 5.5, CaCl2 1.8, oxygenated with 95% O2/5% CO2). Ventricles were dissected away, the atria were separated at the atrial septum and the area adjacent to the SAN was cleared of connective tissue. The spontaneously-beating right atrial preparation was mounted in a 37°C organ bath containing PSS (continuously oxygenated with 95% O2/5% CO2) and connected to a force transducer (MLT0201 series, ADInstruments, New Zealand) with a resting tension of 0.2–0.3 g. The tension signal was low-pass filtered at 20Hz and the beating rate calculated from the time interval between contractions (LabChart, ADInstruments, New Zealand). After rate stabilisation in PSS (variation in average rate of a 10-second sample of no more than 2 beats/minute over a 10-minute period), cumulative concentrations of amodiaquine dihydrochloride (catalogue number 15314998, Acros Organics, Belgium) or N-desethylamodiaquine dihydrochloride solution (catalogue number D-039-1ML, Sigma Aldrich Co. Ltd., UK) were pipetted directly into the bath. Preparations were excluded if, under control conditions (PSS only), stabilised beating rate was less than 300 beats/minute or arrhythmic.
Data are presented as mean ± standard error of the mean (SEM). Repeated measures analysis of variance with Tukey’s or Dunnett’s correction as appropriate was used to assess the isolated heart findings.
Results
Clinical
54 patients aged between 18 and 60 years were randomised. Seven patients had received antimalarial pre-treatment: five patients chloroquine and two artemether-lumefantrine. None were receiving any cardiovascular concomitant medications. Baseline characteristics are presented in Table 1.
Table 1. Baseline Characteristics of Included Population (n = 54).
| Age (years) | |
| Median (IQR) | 24.0 (19.0-32.0) |
| 18-<35 | 43 (79.6%) |
| 35-<50 | 8 (14.8%) |
| ≥50 | 3 (5.6%) |
| Weight (kg) | |
| Median (IQR) | 59.0 (53.0-62.0) |
| Sex | |
| Female | 29 (53.7%) |
| Male | 25 (46.3%) |
| Temperature (°C) | |
| Median (IQR) | 37.7 (37.1-38.6) |
| ≥37.5 | 35 (64.8%) |
| Parasitaemia (parasites/μL) | |
| Median (IQR) | 17181 (5317-25876) |
| >10,000-50,000 | 24 (44.4%) |
| >50,000-100,000 | 6 (11.1%) |
| >100,000-250,000 | 1 (1.9%) |
| Haemoglobin (g/dL) | |
| Median (IQR) | 13.2 (11.9-14.8) |
| <8 | 0 |
| Heart Rate (beats/minute) | |
| Mean (SD) | 91.7 (15.7)a |
| ≥140 | 0 |
| 120-<140 | 2 (3.8%)a |
| 100-<120 | 17 (32.1%)a |
| 80-<100 | 21 (39.6%)a |
| 60-<80 | 13 (24.5%)a |
| <60 | 0 |
IQR = inter-quartile range, SD = standard deviation
1 participant had missing baseline heart rate
53 completed the full treatment course. One patient in the fixed-dose ASAQ arm withdrew consent. Four patients were subsequently lost to follow-up and censored in analyses at the time of drop-out. All patients recovered uneventfully. There were no serious cardiovascular events reported in any of the 54 patients.
Pharmacokinetic Analysis
A total of 363 post-dose venous plasma samples were collected from the 53 participants who received full treatment courses. Amodiaquine concentrations were measurable in 115 (31.7%) samples and 352 (97.0%) had measurable concentrations of desethylamodiaquine (Supplementary Appendix Figure VI).
Population pharmacokinetic parameter estimates were reliable, with small relative standard errors. Secondary pharmacokinetic parameters of maximum concentration, time to maximum concentration, terminal elimination half-life, and total exposure were also computed (Supplementary Appendix Table I). Goodness-of-fit diagnostics and the prediction-corrected visual predictive checks (Supplementary Appendix Figure VII-IX) demonstrated that the model described the observed data adequately.
Concentration-Effect Analyses – Cardiovascular Vital Signs
Plasma concentrations of amodiaquine and desethylamodiaquine were summed. The population mean maximum plasma total concentration (Cmax) of amodiaquine and desethylamodiaquine was approximately 750nmol/L (or 250ng/mL) (Supplementary Appendix Table I). This was associated with a mean decrease in pulse rate of 14.6 beats/minute (95% CI: 10.9 to 18.2). This effect was in addition to independent effects on pulse rate reduction following recovery from fever (5.7 beats/minute per 1°C decrease; 95% CI: 3.7 to 7.6) and from acute malaria (3.0 beats/minute; 95% CI: 0.5 to 5.5). Male sex was not associated with statistically significant effects on pulse rate compared to female sex at this sample size (Table 2).
Table 2. Multivariable Linear Mixed Effects Regression Analysis of Pulse Rate and Blood Pressure in Malaria Following Treatment with Amodiaquine.
| Pulse Rate (beats/minute) | Systolic Blood Pressure – Supine (mmHg) | Diastolic Blood Pressure – Erect (mmHg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Analyses | Multivariable Analyses | Univariable Analyses | Multivariable Analyses | Univariable Analyses | Multivariable Analyses | ||||||||
| Number of Observations | Crude Estimate (95% CI) | p value | Adjusted Estimate (95% CI) | p value | Crude Estimate (95% CI) | p value | Adjusted Estimate (95% CI) | p value | Crude Estimate (95% CI) | p value | Adjusted Estimate (95% CI) | p value | |
| Total plasma concentration of amodiaquine and desethylamodiaquine, per 750* nmol/litre increase | 362 | -19.51 (-23.10 to -15.92) | <0.0001 | -14.57 (-18.22 to -10.92) | <0.0001 | -18.09 (-20.99 to -15.20) | <0.0001 | -12.43 (-15.95 to -8.92) | <0.0001 | -10.64 (-13.03 to -8.25) | <0.0001 | -10.26 (-13.11 to -7.42) | <0.0001 |
| Body temperature change, per 1°C increase | 362 | 9.72 (8.32 to 11.12) | <0.0001 | 5.65 (3.74 to 7.56) | <0.0001 | 4.46 (3.14 to 5.79) | <0.0001 | 2.97 (1.94 to 4.00) | <0.0001 | ||||
| Malaria | 362 | ||||||||||||
| Yes (days 0-2) | 106 | -3.21 (-5.54 to -0.90) | 0.0066 | 2.98 (0.50 to 5.46) | 0.0186 | -9.41 (-11.52 to -7.30) | <0.0001 | -4.53 (-6.93 to -2.13) | 0.0002 | -1.13 (-2.84 to 0.58) | 0.1958 | 2.91 (0.97 to 4.85) | 0.0034 |
| No (days 3-28) | 256 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Sex | 362 | ||||||||||||
| Female | 193 | Reference | Reference | Reference | Reference | ||||||||
| Male | 169 | 3.06 (-4.81 to 10.93) | 0.4388 | 4.12 (-4.17 to 12.41) | 0.3232 | 2.52 (-3.74 to 8.78) | 0.4231 | 4.14 (-0.80 to 9.08) | 0.0985 | ||||
Mean maximum total plasma drug concentration (rounded) after a 3-day course of amodiaquine from pharmacokinetic analysis of same study
After adjusting for acute malaria effects, the total plasma concentration of amodiaquine plus desethylamodiaquine at Cmax was associated with a mean fall of 12.4mmHg (95% CI: 8.9 to 15.9) in supine systolic blood pressure and 11.0mmHg (95% CI: 7.4 to 14.7) in erect systolic blood pressure. Corresponding reductions in supine diastolic blood pressure were 4.7mmHg (95% CI: 1.9 to 7.4), and 10.3mmHg (95% CI: 7.4 to 13.1) in erect diastolic blood pressure (Table 2 & Supplementary Appendix Table III-IV). Total drug concentration effects on the postural change between supine and erect blood pressure measurements were small and of unclear significance (Supplementary Appendix Table III-IV).
Concentration-Effect Analyses – ECG Intervals
After adjustment for change in body temperature, age, sex, and change in RR interval, the mean corrected QT interval prolongation resulting from amodiaquine plus desethylamodiaquine at Cmax was very similar irrespective of the heart rate correction factor used (QTcS: 10.4 milliseconds, 95% CI: 5.9 to 15.0; QTcF: 10.7 milliseconds, 95% CI: 6.1 to 15.2; QTcB: 10.3 milliseconds, 95% CI: 5.7 to 14.8). Unlike the study-specific corrected QT interval, the Fridericia- and Bazett-corrected QT intervals retained clinically and statistically significant heart rate dependency after adjustment although these were in opposite directions (QTcF: 11.6 milliseconds per 300-millisecond increase in RR interval, 95% CI: 7.3 to 16.0; QTcB: -11.5 milliseconds; 95% CI: -15.8 to -7.1). Thus, without adjustment, use of the Fridericia heart rate correction overestimated amodiaquine-related QT prolongation from baseline while the Bazett correction underestimated it (Table 3).
Table 3. Multivariable Linear Mixed Effects Regression Analysis of the Corrected QT Interval in Malaria Following Treatment with Amodiaquine.
| QTcS – Study-specific Correction (milliseconds) | QTcF – Fridericia Correction (milliseconds) | QTcB – Bazett Correction (milliseconds) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Analyses | Multivariable Analyses | Univariable Analyses | Multivariable Analyses | Univariable Analyses | Multivariable Analyses | ||||||||
| Number of Observations | Crude Estimate (95% CI) | p value | Adjusted Estimate (95% CI) | p value | Crude Estimate (95% CI) | p value | Adjusted Estimate (95% CI) | p value | Crude Estimate (95% CI) | p value | Adjusted Estimate (95% CI) | p value | |
| Total plasma concentration of amodiaquine and desethylamodiaquine, per 750* nmol/litre increase | 356 | 12.98 (8.73 to 17.16) | <0.0001 | 10.43 (5.92 to 15.00) | <0.0001 | 20.35 (15.56 to 25.15) | <0.0001 | 10.67 (6.13 to 15.21) | <0.0001 | 6.10 (1.80 to 10.38) | 0.0055 | 10.26 (5.71 to 14.81) | <0.0001 |
| Body temperature change, per 1°C increase | 356 | -5.30 (-7.12 to -3.47) | <0.0001 | -3.97 (-6.49 to -1.46) | 0.0021 | -10.59 (-12.52 to -8.65) | <0.0001 | -4.04 (-6.57 to -1.52) | 0.0018 | -0.374 (-2.25 to 1.50) | 0.6948 | -3.90 (-6.43 to -1.37) | 0.0026 |
| Sex | 356 | ||||||||||||
| Female | 187 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Male | 169 | -16.24 (-25.48 to -7.00) | 0.0009 | -17.35 (-26.62 to -8.08) | 0.0004 | -12.72 (-22.11 to -3.34) | 0.0088 | -13.30 (-22.94 to -3.66) | 0.0078 | -19.54 (-29.00 to -10.08) | 0.0001 | -21.05 (-30.57 to -11.52) | <0.0001 |
| Age, per 10-year increase | 356 | 2.33 (-0.25 to 0.71) | 0.3339 | 4.16 (-0.17 to 8.49) | 0.0595 | 2.87 (-1.78 to 7.52) | 0.2209 | 4.47 (-0.30 to 8.98) | 0.0515 | 1.78 (-3.32 to 6.88) | 0.4859 | 3.87 (-0.57 to 8.32) | 0.0864 |
| RR interval change, per 300-millisecond increase | 356 | 7.50 (4.46 to 10.54) | <0.0001 | -0.42 (-4.80 to 3.95) | 0.8475 | 19.69 (16.65 to 22.74) | <0.0001 | 11.63 (7.25 to 16.01) | <0.0001 | -3.69 (-6.75 to -0.65) | 0.0177 | -11.45 (-15.84 to -7.06) | <0.0001 |
, where RR is in units of seconds
Mean maximum total plasma drug concentration (rounded) after a 3-day course of amodiaquine from pharmacokinetic analysis of same study
Mean change in RR interval from baseline (rounded) after last dose of amodiaquine treatment in this study
There was no significant effect of maximum plasma concentrations of amodiaquine plus desethylamodiaquine on the QRS and PR intervals once adjusted by change in body temperature, sex, age, and change in RR interval (QRS: -0.47 milliseconds, 95% CI: -2.51 to 1.57; PR: 2.01 milliseconds; 95% CI: -1.29 to 5.31) (Supplementary Appendix Table 5).
Pre-Clinical
Murine Atrial Studies
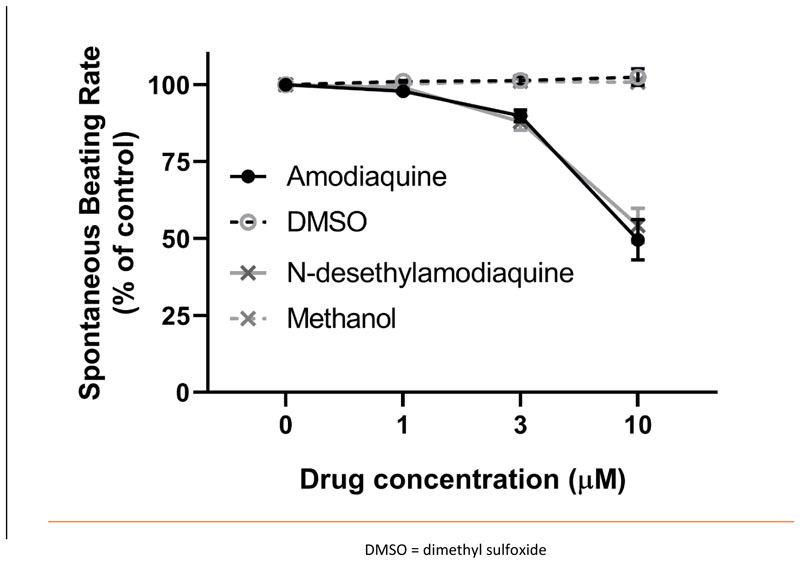
Spontaneously beating right atrial preparations contain the intact SAN pacemaker. They can be used to assess the effect of compounds on the intrinsic pacemaker of the heart by measurement of beating rate14. The application of cumulative doses of amodiaquine (n=6) and N-desethylamodiaquine (n=8) to spontaneously beating mouse atrial preparations produced concentration-dependent reductions in beating rate which were statistically significant (p < 0.05) at concentrations of 3 μmol/litre (amodiaquine: 10% ± 2%; N-desethylamodiaquine: 12% ± 3%) and 10 μmol/litre (amodiaquine: 50% ± 7%; N-desethylamodiaquine: 46% ± 6%) but not 1 μmol/litre (amodiaquine: 2% ± 1%; N-desethylamodiaquine: 1% ± 1%) compared to time-matched controls of the vehicles dimethyl sulfoxide (n=2) and methanol (n=2). There was no statistically significant difference between the response to amodiaquine and N-desethylamodiaquine at any concentration studied (Figure 1).
Figure 1.
Change (%) in Atrial Beating Rate During Cumulative Doses of 4-aminoquinoline Antimalarials Compared to Controls
Discussion
The 4-aminoquinoline antimalarial amodiaquine is a pro-drug converted rapidly after oral administration to its active metabolite desethylamodiaquine by cytochrome P450 isozyme 2C8 (CYP2C8)39. Thus desethylamodiaquine, with its higher concentration-time profile and longer terminal elimination half-life of 9-18 days40, contributes almost all of the antimalarial effect of this widely-deployed oral drug in the treatment and prevention of malaria.
Despite its widespread use, amodiaquine has been relatively little studied in recent years. To our knowledge, this is the most detailed investigation to date of the cardiovascular concentration-effect relationships of amodiaquine and its active metabolite desethylamodiaquine, incorporating both clinical data in malaria patients and a pre-clinical study of the cardiac pharmacology of desethylamodiaquine.
Cardiovascular Vital Signs
Bradycardia is a common cardiovascular effect after antimalarial treatment with amodiaquine and mefloquine41. It is observed more in adolescents and adults than in children9,42 for reasons which are not fully understood, although modulation of cardiac ion currents by sex hormones may play a role43.
Our murine atrial studies show that both amodiaquine and desethylamodiaquine have direct concentration-dependent bradycardic effects of similar potency. These effects are greater than that of hydroxychloroquine at the concentrations evaluated, measured using the same method in our previous study14. The amodiaquine-induced bradycardia in both innervated (malaria patients, anaesthetised dogs8) and decentralised (mouse) hearts supports a direct pharmacological effect on cardiac myocyte ion channels through modulation of the pacemaker If current at the SAN, as observed previously with hydroxychloroquine14. Autonomic tone may also be relevant as both amodiaquine and mefloquine are associated with reversible inhibition of human acetylcholinesterase (AChE), with amodiaquine having a much higher potency than mefloquine19,20.
The quinoline antimalarials quinine44 and chloroquine45 are known to cause lethal hypotension when injected rapidly but can be used safely with rate-controlled continuous intravenous infusion. As proposed for amodiaquine18, these hypotensive effects are likely due to vasodilation and negative inotropy from multiple ion channel blockade46. Orthostatic hypotension is a feature of acute malaria which is exacerbated by the quinoline antimalarials quinine and mefloquine47. However, in this study of uncomplicated malaria infections, there was no significant effect of the total plasma concentration of amodiaquine and desethylamodiaquine on postural changes in systolic or diastolic blood pressure after adjustment for malaria recovery.
Amodiaquine is the most bradycardic of the front-line antimalarials9. While amodiaquine and desethylamodiaquine cause concentration-dependent bradycardia and hypotension in adult malaria patients, the clinical impact of these effects appears to be mild26, although they may contribute to the commonly reported asthenia.
ECG Intervals
Drug-induced QT interval prolongation is the most widely-used surrogate marker of the risk of development of torsades de pointes (TdP), a polymorphic ventricular tachycardia that can degenerate in some cases into ventricular fibrillation and cause sudden cardiac death48. Despite their QT-prolonging potential, the frontline quinoline and structurally-related antimalarials recommended currently by the WHO all have excellent track records of cardiac safety. They have not been associated with increased risk of sudden cardiac death or cases of TdP in their extensive use at standard doses for the treatment or prevention of malaria over seven decades9,41,49.
The QT interval lengthens as heart rate decreases. Correction formulae modelling this inverse and non-linear relationship are used to attempt to minimise the heart rate dependency of measured QT intervals to allow for comparisons across different heart rates. However, the commonly used Bazett and Fridericia corrections are both known to retain significant heart rate dependency48,50, particularly in malaria where there is further confounding from disease recovery which occurs as antimalarial concentrations peak9,36. As before9, a study-specific correction provided the best heart rate correction of the QT interval (QTcS) in our analysis while use of the Fridericia (QTcF) and Bazett (QTcB) corrections respectively overestimated and underestimated amodiaquine-related QT prolongation in malaria patients. Once any residual heart rate dependency had been adjusted for the drug-attributable QT prolongation from amodiaquine and desethylamodiaquine was comparable regardless of correction factor used and consistent with QT prolongation similar to that observed with piperaquine51,52 but less than with chloroquine53 at standard malaria doses.
In contrast, we found no significant effect of total amodiaquine and desethylamodiaquine concentration on PR or QRS intervals after adjustment for demographic factors (age, sex) and malaria recovery (change in body temperature, change in heart rate). Small increases in unadjusted PR and QRS intervals after amodiaquine for malaria have been previously reported54,55 which may predominantly reflect recovery from malaria rather than a direct drug effect. This differs from chloroquine53 which prolongs both the PR and QRS intervals with over one quarter of the QT prolongation following chloroquine resulting from QRS widening.
Conclusion
The widely used 4-aminoquinoline amodiaquine, like other quinoline and quinoline-like drugs, has transient effects on cardiac and vascular physiology. We characterised the concentration-dependency of the bradycardic, hypotensive, and QT prolonging effects of amodiaquine and its main metabolite desethylamodiaquine providing further evidence of their causal role. Further characterisation of the cardiovascular effects of amodiaquine and desethylamodiaquine in adult healthy volunteers and other pre-clinical models may help to improve the tolerability of this important medicine in the treatment and prevention of malaria.
Supplementary Material
Acknowledgements
The authors thank all investigators and participants of the clinical study included in this analysis. We also thank Ilsa Haeusler, Jireh Tan, and Shu Kiat Chan for their kind assistance with data processing, Junjie Ding and Christopher L-H Huang of the University of Cambridge Physiological Laboratory for illuminating discussions, as well as Samuel Bose of the University of Oxford Department of Pharmacology for their technical input.
Funding
XHSC is an NIHR Academic Clinical Lecturer in Infectious Diseases at the University of Oxford supported by the Medical Research Council of the United Kingdom (MR/N013468/1) and the Jill and Herbert Hunt Travelling Scholarship of the University of Oxford. RAC is a post-doctoral scientist funded by the Wellcome Trust and Royal Society (109371/Z/15/Z). RABB is funded by a Sir Henry Dale Wellcome Trust and Royal Society Fellowship (109371/Z/15/Z) and acknowledges support from The Returning Carers’ Fund, Medical Sciences Division, University of Oxford as well as the Oxford British Heart Foundation Centre for Research Excellence. RABB is a Senior Research Fellow of Linacre College. NJW is a Wellcome Trust Principal Research Fellow (107886/Z/15/Z) and is a recipient of the Bill and Melinda Gates Foundation award (OPP1132628). The Mahidol-Oxford Tropical Medicine Research Unit research programme is supported by the Wellcome Trust (106698/Z/14/Z). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wiselogle FY. A Survey of Antimalarial Drugs 1941-1945. J.W. Edwards; Ann Arbor, Michigan: 1946. [Google Scholar]
- 2.Burckhalter JH, Tendick FH, et al. Aminoalkylphenols as antimalarials (heterocyclicamino)-alpha-amino-o-cresols; the synthesis of camoquin. J Am Chem Soc. 1948;70(4):1363–73. doi: 10.1021/ja01184a023. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Guidelines for the Treatment of Malaria. 3rd ed. Geneva, Switzerland: 2015. [Google Scholar]
- 4.World Health Organization. World Malaria Report 2019. Geneva, Switzerland: 2019. [Google Scholar]
- 5.World Health Organization. Seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in children: A field guide. Geneva, Switzerland: 2013. [Google Scholar]
- 6.Arora RB, Madan BR, Pathak RK. Antiarrhythmics. VIII. Chloroquine, amodiaquine, procaine amide and quinidine in experimental auricular arrhythmias simulating clinical disorders. Indian J Med Res. 1956;44(3):453–62. [PubMed] [Google Scholar]
- 7.Arora RB, Madan BR. Antiarrhythmics. II. Amodiaquin (camoquin) in cardiac arrhythmias. Indian J Med Res. 1956;44(1):99–106. [PubMed] [Google Scholar]
- 8.Arora RB, Lal A. Antimalarial Drugs on the Automaticity of Sino-Auricular and Atrio-Ventricular Nodes. Indian J Med Res. 1963;51:725–32. [PubMed] [Google Scholar]
- 9.Chan XHS, Haeusler IL, Win YN, et al. The cardiovascular effects of amodiaquine and structurally related antimalarials: An individual patient data meta-analysis. PLoS Med. 2021;18(9):e1003766. doi: 10.1371/journal.pmed.1003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assi SB, Aba YT, Yavo JC, et al. Safety of a fixed-dose combination of artesunate and amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in real-life conditions of use in Cote d’Ivoire. Malar J. 2017;16(1):8. doi: 10.1186/s12936-016-1655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodoo AN, Fogg C, Nartey ET, et al. Profile of adverse events in patients receiving treatment for malaria in urban Ghana: a cohort-event monitoring study. Drug Saf. 2014;37(6):433–48. doi: 10.1007/s40264-014-0164-9. [DOI] [PubMed] [Google Scholar]
- 12.Bassi PU, Osakwe AI, Isah A, et al. Safety of Artemisinin-Based Combination Therapies in Nigeria: A Cohort Event Monitoring Study. Drug Saf. 2013;36(9):747–56. doi: 10.1007/s40264-013-0044-8. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker DG, Capel RA, Hendrix M, et al. Cardiac TdP risk stratification modelling of anti-infective compounds including chloroquine and hydroxychloroquine. R Soc Open Sci. 2021;8(4):210235. doi: 10.1098/rsos.210235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capel RA, Herring N, Kalla M, et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: Novel electrophysiological insights and therapeutic potential. Heart Rhythm. 2015;12(10):2186–94. doi: 10.1016/j.hrthm.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borsini F, Crumb W, Pace S, et al. In vitro cardiovascular effects of dihydroartemisinin-piperaquine combination compared with other antimalarials. Antimicrob Agents Chemother. 2012;56(6):3261–70. doi: 10.1128/AAC.05688-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noujaim SF, Stuckey JA, Ponce-Balbuena D, et al. Structural bases for the different anti-fibrillatory effects of chloroquine and quinidine. Cardiovasc Res. 2011;89(4):862–9. doi: 10.1093/cvr/cvr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traebert M, Dumotier B, Meister L, Hoffmann P, Dominguez-Estevez M, Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484(1):41–8. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Adaramoye OA, Almeida MM. Vasorelaxation induced by amodiaquine in rat superior mesenteric arteries: in vivo and in vitro studies. Acta Pol Pharm. 2010;67(5):529–36. [PubMed] [Google Scholar]
- 19.Lim LY, Go ML. The anticholinesterase activity of mefloquine. Clin Exp Pharmacol Physiol. 1985;12(5):527–31. doi: 10.1111/j.1440-1681.1985.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 20.Go ML, Ngiam TL, Wan AS. Investigation of the anti-acetylcholinesterase activities of the antimalarial agent, amodiaquine, and related compounds. Southeast Asian J Trop Med Public Health. 1981;12(1):37–41. [PubMed] [Google Scholar]
- 21.Churchill FC, Patchen LC, Campbell CC, Schwartz IK, Nguyen-Dinh P, Dickinson CM. Amodiaquine as a prodrug: importance of metabolite(s) in the antimalarial effect of amodiaquine in humans. Life Sci. 1985;36(1):53–62. doi: 10.1016/0024-3205(85)90285-1. [DOI] [PubMed] [Google Scholar]
- 22.Hatton CS, Peto TE, Bunch C, et al. Frequency of severe neutropenia associated with amodiaquine prophylaxis against malaria. Lancet. 1986;1(8478):411–4. doi: 10.1016/s0140-6736(86)92371-8. [DOI] [PubMed] [Google Scholar]
- 23.Neftel KA, Woodtly W, Schmid M, Frick PG, Fehr J. Amodiaquine induced agranulocytosis and liver damage. Br Med J (Clin Res Ed) 1986;292(6522):721–3. doi: 10.1136/bmj.292.6522.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips-Howard PA, West LJ. Serious adverse drug reactions to pyrimethamine-sulphadoxine, pyrimethamine-dapsone and to amodiaquine in Britain. J R Soc Med. 1990;83(2):82–5. doi: 10.1177/014107689008300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Practical Chemotherapy of Malaria. Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1990. [PubMed] [Google Scholar]
- 26.Ogutu B, Juma E, Obonyo C, et al. Fixed dose artesunate amodiaquine-a phase IIb, randomized comparative trial with non-fixed artesunate amodiaquine. Malar J. 2014;13:498. doi: 10.1186/1475-2875-13-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jullien V, Ogutu B, Juma E, Carn G, Obonyo C, Kiechel JR. Population pharmacokinetics and pharmacodynamic considerations of amodiaquine and desethylamodiaquine in Kenyan adults with uncomplicated malaria receiving artesunate-amodiaquine combination therapy. Antimicrob Agents Chemother. 2010;54(6):2611–7. doi: 10.1128/AAC.01496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali AM, Penny MA, Smith TA, et al. Population Pharmacokinetics of the Antimalarial Amodiaquine: a Pooled Analysis To Optimize Dosing. Antimicrob Agents Chemother. 2018;62(10) doi: 10.1128/AAC.02193-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maude RJ, Plewes K, Faiz MA, et al. Does artesunate prolong the electrocardiograph QT interval in patients with severe malaria? Am J Trop Med Hyg. 2009;80(1):126–32. doi: 10.4269/ajtmh.2009.08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 31.Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 31-141 ed. 2019 [Google Scholar]
- 32.Bauer RJ. NONMEM Users Guide: Introduction to NONMEM 7.4.1. Gaithersburg, Maryland: ICON plc; 2017. [Google Scholar]
- 33.Keizer R, van Benten M, Beijnen J, Schellens J, Huitema A. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Computer Methods and Programs in Biomedicine. 2011;101(1):72–9. doi: 10.1016/j.cmpb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Lindbom L, Ribbing J, Jonsson E. Perl-speaks-NONMEM (PsN)--a Perl module for NONMEM related programming. Computer Methods and Programs in Biomedicine. 2004;75(2):85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Tarning J, Chotsiri P, Jullien V, et al. Population pharmacokinetic and pharmacodynamic modeling of amodiaquine and desethylamodiaquine in women with Plasmodium vivax malaria during and after pregnancy. Antimicrob Agents Chemother. 2012;56(11):5764–73. doi: 10.1128/AAC.01242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan XHS, Win YN, Haeusler IL, et al. Factors affecting the electrocardiographic QT interval in malaria: A systematic review and meta-analysis of individual patient data. PLoS Med. 2020;17(3):e1003040. doi: 10.1371/journal.pmed.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. WHO Evidence Review Group on the Cardiotoxicity of Antimalarial Medicines. Geneva, Switzerland: 2017. [Google Scholar]
- 38.Adjei GO, Oduro-Boatey C, Rodrigues OP, et al. Electrocardiographic study in Ghanaian children with uncomplicated malaria, treated with artesunate-amodiaquine or artemether-lumefantrine. Malar J. 2012;11:420. doi: 10.1186/1475-2875-11-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XQ, Bjorkman A, Andersson TB, Ridderstrom M, Masimirembwa CM. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther. 2002;300(2):399–407. doi: 10.1124/jpet.300.2.399. [DOI] [PubMed] [Google Scholar]
- 40.European Medicines Agency. Artesunate Amodiaquine Winthrop Tablets: Summary of Product Characteristics. 2014 [Google Scholar]
- 41.Haeusler IL, Chan XHS, Guerin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16(1):200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ter Kuile FO, Nosten F, Luxemburger C, et al. Mefloquine treatment of acute falciparum malaria: a prospective study of non-serious adverse effects in 3673 patients. Bull World Health Organ. 1995;73(5):631–42. [PMC free article] [PubMed] [Google Scholar]
- 43.Costa S, Saguner AM, Gasperetti A, Akdis D, Brunckhorst C, Duru F. The Link Between Sex Hormones and Susceptibility to Cardiac Arrhythmias: From Molecular Basis to Clinical Implications. Front Cardiovasc Med. 2021;8:644279. doi: 10.3389/fcvm.2021.644279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strahan JH. Quinine by continuous intravenous drip in the treatment of acute falciparum malaria. Trans R Soc Trop Med Hyg. 1948;41(5):669–76. doi: 10.1016/s0035-9203(48)90598-7. [DOI] [PubMed] [Google Scholar]
- 45.White NJ, Miller KD, Churchill FC, et al. Chloroquine treatment of severe malaria in children.Pharmacokinetics, toxicity, and new dosage recommendations. N Engl J Med. 1988;319(23):1493–500. doi: 10.1056/NEJM198812083192301. [DOI] [PubMed] [Google Scholar]
- 46.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–58. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 47.Supanaranond W, Davis TM, Pukrittayakamee S, Nagachinta B, White NJ. Abnormal circulatory control in falciparum malaria: the effects of antimalarial drugs. Eur J Clin Pharmacol. 1993;44(4):325–9. doi: 10.1007/BF00316467. [DOI] [PubMed] [Google Scholar]
- 48.ICH Harmonised Tripartite Guideline E14. The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. 2005 [Google Scholar]
- 49.Chan XHS, Win YN, Mawer LJ, Tan JY, Brugada J, White NJ. Risk of sudden unexplained death after use of dihydroartemisinin-piperaquine for malaria: a systematic review and Bayesian meta-analysis. Lancet Infect Dis. 2018;18(8):913–23. doi: 10.1016/S1473-3099(18)30297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabkin SW, Szefer E, Thompson DJS. A New QT Interval Correction Formulae to Adjust for Increases in Heart Rate. JACC Clin Electrophysiol. 2017;3(7):756–66. doi: 10.1016/j.jacep.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Wattanakul T, Ogutu B, Kabanywanyi AM, et al. Pooled Multicenter Analysis of Cardiovascular Safety and Population Pharmacokinetic Properties of Piperaquine in African Patients with Uncomplicated Falciparum Malaria. Antimicrob Agents Chemother. 2020;64(7) doi: 10.1128/AAC.01848-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chotsiri P, Wattanakul T, Hoglund RM, et al. Population pharmacokinetics and electrocardiographic effects of dihydroartemisinin-piperaquine in healthy volunteers. Br J Clin Pharmacol. 2017;83(12):2752–66. doi: 10.1111/bcp.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chotsiri P, Tarning J, Hoglund RM, Watson J, White NJ. Pharmacometric and electrocardiographic evaluation of chloroquine and azithromycin in healthy volunteers. Clin Pharmacol Ther. 2022 doi: 10.1002/cpt.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Funck-Brentano C, Ouologuem N, Duparc S, et al. Evaluation of the effects on the QT-interval of 4 artemisinin-based combination therapies with a correction-free and heart rate-free method. Sci Rep. 2019;9(1):883. doi: 10.1038/s41598-018-37113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngouesse B, Basco LK, Ringwald P, Keundjian A, Blackett KN. Cardiac effects of amodiaquine and sulfadoxine-pyrimethamine in malaria-infected African patients. Am J Trop Med Hyg. 2001;65(6):711–6. doi: 10.4269/ajtmh.2001.65.711. [DOI] [PubMed] [Google Scholar]
- 56.Chue AL, Moore RL, Cavey A, et al. Comparability of tympanic and oral mercury thermometers at high ambient temperatures. BMC Res Notes. 2012;5:356. doi: 10.1186/1756-0500-5-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dosne AG, Bergstrand M, Karlsson MO. An automated sampling importance resampling procedure for estimating parameter uncertainty. J Pharmacokinet Pharmacodyn. 2017;44(6):509–20. doi: 10.1007/s10928-017-9542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package dagitty. Int J Epidemiol. 2016;45(6):1887–94. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.