Abstract
Objective
Patients with clinically suspect arthralgia (CSA) are at risk for developing rheumatoid arthritis (RA). These patients often report joint swelling while this is not objectified by physical examination. To explore the value of patient-reported swelling in CSA, we aimed to determine its association with subclinical joint inflammation on imaging and RA development.
Methods
In two independent, similarly designed CSA cohorts from the Netherlands, symptomatic patients at risk for RA were studied. At baseline, patients indicated whether they had experienced swelling in hand joints. Subclinical joint inflammation was assessed with MRI or US. Patients were followed for inflammatory arthritis development.
Results
In total, 534 CSA patients from two independent cohorts were studied, and patient-reported swelling was present in 57% in cohort 1 and in 43% in cohort 2. In both cohorts patient-reported swelling was associated with subclinical joint inflammation. Using MRI, it associated specifically with tenosynovitis (odds ratio [OR] 3.7 [95% CI: 2.0, 6.9]) and when using US with synovitis (OR 2.3 [95% CI: 1.04, 5.3]). CSA patients with self-reported swelling at baseline developed arthritis more often, with hazard ratios of 3.7 (95% CI: 2.0, 6.9) and 3.4 (95% CI: 1.4, 8.4) in cohort 1 and 2, respectively. This was independent of clinical predictors (e.g. morning stiffness), autoantibody positivity and US-detected subclinical joint inflammation. However, when corrected for MRI-detected subclinical joint inflammation, self-reported swelling was no longer an independent predictor.
Conclusion
Patient-reported joint swelling in CSA relates to subclinical joint inflammation and is an independent risk factor for RA development, but it is less predictive than the presence of MRI-detected subclinical joint inflammation.
Keywords: clinically suspect arthralgia, rheumatoid arthritis, patient-reported joint swelling, magnetic resonance imaging, ultrasound
Introduction
Patients with clinically suspect arthralgia (CSA) are at risk to develop rheumatoid arthritis (RA), but do not yet have clinical arthritis. However, symptoms and functional limitations in the CSA phase can be as serious as in RA patients [1–4]. In addition to generally reported symptoms such as pain, morning stiffness and functional impairments, CSA patients often report previously experienced joint swelling without having clinical apparent arthritis at physical examination by a rheumatologist [5, 6].
Previous research in early and established RA patients showed a relation between self-reported joint swelling and clinical arthritis, which could be helpful in, for example, telemonitoring, though the agreement at joint level between the patients and rheumatologists was moderate [7–9]. In addition, the concordance between self-reported joint swelling and ultrasound (US)-detected joint inflammation has been shown to be poor in established RA patients [10]. In patients at risk for RA, little research is performed on the value of patient-reported swelling. For the development of the EULAR definition of CSA, which used clinical expertise as a reference, patient-reported swelling was evaluated as one of the criteria. Here, patient-reported swelling was of importance for the rheumatologist, but it did not make it to the final criteria. Based on expertise of a rheumatologist as a reference, self-reported joint swelling was not considered as an independent contributor to the concept of CSA [6]. One study investigated the predictive value of patient-reported swelling in ACPA positive arthralgia patients, which showed that patient-reported joint swelling was associated with an increased risk of inflammatory arthritis (IA) development [5]. To our knowledge, no other studies have evaluated the association of patient-reported joint swelling with subclinical joint inflammation in CSA patients.
We aimed to explore the value of patient-reported swelling in CSA patients. We hypothesized that CSA patients might experience swelling due to the presence of subclinical joint inflammation, which could increase the probability to develop clinical arthritis. Therefore, we aimed to determine the association between patient-reported swelling and the presence of subclinical joint inflammation, assessed with MRI or US, and to determine the risk of IA development if patients reported joint swelling.
Methods
Cohort design
For this study two independent, but similarly designed CSA cohorts were used (the CSA Leiden and CSA Rotterdam cohorts). Both CSA cohorts have been described earlier [11, 12]. Inclusion and exclusion criteria were completely similar: patients could be included if they had arthralgia of the small joints (for <1 year) and the symptoms were, according to the clinical expertise of the rheumatologist, suspected to progress to RA over time. CSA was not considered if clinical arthritis was already present or the arthralgia could be explained by another disease. Patients were followed for 2 years for development of IA, confirmed with joint swelling at physical examination by the rheumatologist. During follow-up, patients were not treated with DMARDs or glucocorticoids. Follow-up visits were scheduled at 4, 12 and 24 months, with additional visits in between if patients experienced an increase in symptoms to assess whether they had developed IA. If patients developed IA, the follow-up ended and patients were subsequently treated with DMARDs following the treatment strategy of the rheumatologist. All patients provided written informed consent prior to inclusion. The study was conducted in compliance with the Declaration of Helsinki. The research protocol for the Leiden CSA cohort (P11.210) was approved by the local Medical Ethical Committee of Leiden Den Haag Delft. The research protocol for the Rotterdam CSA cohort (MEC-2017-028) was approved by the local Medical Ethical Committee of the Erasmus Medical Center (EMC).
Patients
For the current study, we included a total of 472 consecutive CSA Leiden patients who were enrolled between April 2015 and May 2022. Patients included between 2012 and 2015 were not studied, since self-reported swelling was only included in the questionnaires from 2015 onwards. From the 472 included patients, 39 (8%) did not answer the question.
In the Rotterdam CSA cohort a total of 132 patients were included from May 2017 onwards. Thirty-one patients (23%) did not answer the question about self-reported swelling.
Sensitivity analysis showed no clinically relevant differences between patients who did or did not answer the questionnaire for both cohorts (Supplementary Table S1, available at Rheumatology online).
Self-reported joint swelling
In both cohorts, patients filled out online questionnaires concerning their symptoms at baseline. One of the questions was: ‘Have you ever experienced joint swelling in one of the hand joints?’ This was a question to the patient and was thus independent of the physical examination by the rheumatologist.
Detection of subclinical joint inflammation
Subclinical joint inflammation at baseline was assessed with MRI in the CSA Leiden cohort and with US in the CSA Rotterdam cohort.
MRI
All patients, included between 2015 and June 2021, underwent a unilateral contrast-enhanced 1.5 T MRI of the hand (metacarpophalangeal [MCP] joints 2–5 and wrist) with the most symptoms, or the dominant side if symptom severity was symmetrical. In total 352 MRI scans were made. Details on MRI scanning and scoring are present in Supplementary Data S1 and S2, available at Rheumatology online. MRIs were evaluated for synovitis, tenosynovitis and osteitis according to the method of the Rheumatoid Arthritis MRI scoring system (RAMRIS) and Haavardsholm and summed as total MRI inflammation score [13, 14]. All MRIs were scored blinded for clinical data. If there were any inflamed tissues present, the MRI was positive for subclinical joint inflammation. Synovitis, osteitis or tenosynovitis were only present if they were scored in a severity that was present at the same location in <5% of age-matched healthy controls [15, 16]. Inter- and intrareader intraclass correlation coefficients were ≥0.90, as published earlier [17].
US
At baseline, 100 patients underwent an US of the joints of both hands (MCP joints 2–5, proximal interphalangeal [PIP] joints 2–5 and wrist). The presence of subclinical synovitis and tenosynovitis (grey scale [GS] and/or power Doppler [PD]) was scored according to the latest developed EULAR-OMERACT-scoring method and ranged from 0 to 3 [18, 19]. Subclinical joint inflammation was defined as GS ≥2 and/or PD ≥1 for the presence of synovitis and/or tenosynovitis. Details on US performance and scoring are available in Supplementary Data S3, available at Rheumatology online.
Statistical analyses
The association between subclinical joint inflammation and self-reported swelling was studied cross-sectionally using univariable logistic regression models. Univariable models, which included binary variables for the presence of subclinical joint inflammation, were used to detect a possible association. For MRI, this was the sum of synovitis, tenosynovitis and osteitis (only positive if scored in a severity that was present in <5% of the age-matched healthy controls in that specific location, see Supplementary Data S2). For US, this was the sum of synovitis scores (GS and/or PD) and tenosynovitis scores (GS and/or PD) (corrected and dichotomized as above described). Patient-reported swelling could have different associations with the different subclinically inflamed tissues. To evaluate independency, additional multivariable analyses were performed. For US, also GS and PD were studied separately as it is known from earlier research that PD and GS can be differently associated with IA development [20, 21]. As a sensitivity analysis, we repeated the abovementioned analyses including subclinical joint inflammation from only the hand joints (MCP joints [MRI] and MCP and PIP joints [US]), but not the wrist joints, as patients were only asked about swelling of the hand joint (without further specification).
The risk of self-reported swelling and IA development was studied with Cox proportional hazard regression analyses. Time to IA was the time from inclusion to the date of first detection of IA. Patients who did not develop IA were censored at their last study visit or after 2 years’ follow-up. In addition, hazard ratios (HRs) were also calculated for the risk of RA development. RA was defined as fulfilling the 1987 and/or 2010 criteria [22, 23].
To assess the value of patient-reported swelling in respect to current predictors for IA development in the CSA phase, patient-reported swelling was tested in multivariable Cox regression models. Thus we aimed to study the prognostic value of patient-reported swelling, but we did not aim to identify or develop and validate a prediction model. In order to prevent overfitting of the multivariable model (by adding too many variables relative to the number of events), we aimed to restrict the selection of variables. Therefore, we analysed whether associated variables, known from previous research, were independently predictive for IA development, namely age, sex, morning stiffness, positive squeeze test, onset of symptoms in small joints, tender joint count (TJC), ACPA status, CRP levels, RF status and subclinical joint inflammation detected with MRI and US [5, 24, 25]. To make implementation possible in different outpatient clinic settings, the independency of patient-reported swelling was presented in three different models (‘clinical’, ‘additional laboratory’ and ‘additional imaging’ models) using a stepwise forward algorithm. The predictors were applied in the multivariable models only when there was an association in the univariable analysis. These models were constructed based on the CSA Leiden cohort and subsequently used in the CSA Rotterdam cohort, but without sex and age to avoid overfitting in the CSA Rotterdam models.
The number of patients varied per analysis due to less availability of the MRI scanner due to logistical reasons. Number of patients are indicated in each table and figure. P-values <0.05 were considered statistically significant. All statistical analyses were performed using Stata 17.0 (StataCorp, College Station, TX, USA).
Results
Patients
In the CSA Leiden cohort, 79% of the patients were female with a mean age of 44 years and a median tender joint count of 4. These baseline characteristics were comparable in the CSA Rotterdam cohort (Table 1). In total, 245 (57%) patients from the CSA Leiden and 43 (43%) from the CSA Rotterdam cohort reported swelling in one of the hand joints at baseline.
Table 1. Baseline characteristics of the CSA Leiden cohort and CSA Rotterdam cohort.
| CSA Leiden (n = 433) |
CSA Rotterdam (n = 101) |
|
|---|---|---|
| Female sex, n (%) | 340 (79) | 78 (77) |
| Age, mean (s.d.), years | 44 (13) | 45 (12) |
| Symptom duration, median (IQR), weeks | 19 (10–48) | 19 (12–32) |
| Positive squeeze test hand, n (%) | 177 (42) | 34 (34) |
| Morning stiffness ≥60 min, n (%) | 65 (18) | 19 (22) |
| Start of symptoms in small joints, n (%) | 391 (93) | 85 (93) |
| TJC68, median (IQR) | 4 (2–9) | 3 (0–7) |
| CRP, median (IQR) | 3.0 (1.9–4.1) | 3.0 (1.0–4.0) |
| RF positive, n (%) | 81 (19) | 25 (26) |
| ACPA positive, n (%) | 58 (14) | 25 (25) |
CSA: clinically suspect arthralgia; IQR: interquartile range; TJC68: tender joint count of 68 joints.
Self-reported swelling and subclinical joint inflammation
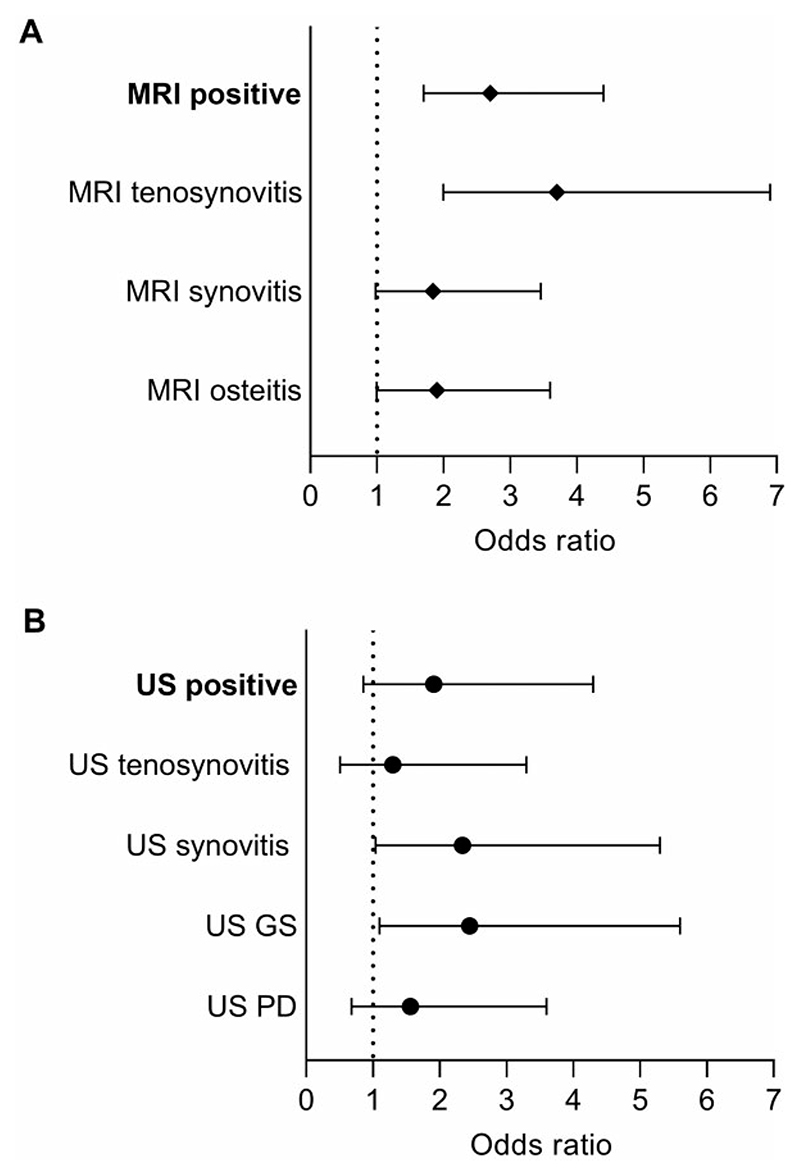
In the CSA Leiden cohort, 117 patients (33%) had an MRI scan positive for subclinical joint inflammation. Patient-reported swelling was associated with the presence of subclinical joint inflammation detected with MRI (OR 2.7 [95% CI: 1.7, 4.4]; Fig. 1A). Of the separate inflamed joints tissues, tenosynovitis had the strongest association (OR 3.7 [95% CI: 2.0, 6.9]; Fig. 1A). After correction for synovitis and osteitis, only MRI-detected tenosynovitis was independently associated with self-reported swelling (OR 3.3 [95% CI: 1.7, 6.5]; Table 2). Absolute numbers of patients with and without reported joint swelling in the presence of subclinical MRI-detected joint inflammation are presented in Supplementary Table S2, available at Rheumatology online.
Figure 1. Univariable odds ratios for the presence of subclinical joint inflammation in patients with reported joint swelling.
(A) Patients evaluated with MRI from the CSA Leiden cohort (n = 352). (B) patients evaluated with US from the CSA Rotterdam cohort (n = 100). MRI was scored positive only if <5% of the age-matched healthy volunteers had inflammation on a specific joint/tissue. US was scored positive if GS was ≥2 or PD was ≥1. US GS indicates whether a patient had only GS ≥ 2 in at least one joint; US PD indicates whether a patients had only PD ≥ 1 in at least one joint. Odd Ratios based on univariate analysis (also see Supplementary Table S3, available at Rheumatology online). GS: grey scale; PD: power Doppler
Table 2. Multivariable odds ratios for patient-reported swelling and subclinical inflamed tissues detected with MRI (A) or US (B).
| Odd ratio (95% CI) | |
|---|---|
| A, MRI | |
| Tenosynovitis | 3.3 (1.7, 6.5) |
| Synovitis | 1.1 (0.57, 2.3) |
| Osteitis | 1.4 (0.70, 2.8) |
| B, ultrasound | |
| Model 1 | |
| Synovitis | 2.4 (1.02, 5.9) |
| Tenosynovitis | 0.87 (0.31, 2.4) |
| Model 2 | |
| GS | 2.4 (0.94, 6.1) |
| PD | 1.04 (0.41, 2.7) |
(A) CSA Leiden (n = 352); (B) CSA Rotterdam (n = 100). US model 1: synovitis (GS and/or PD) and tenosynovitis (GS and/or PD); US model 2: any GS and any PD. Statistically significant associations are displayed in bold. GS: grey scale; PD: power Doppler.
Of all Rotterdam CSA patients, 49 (49%) had a positive US for one of the joints or tendons assessed. Although statistically non-significant, there was a positive association between patient-reported swelling and subclinical joint inflammation detected with US (OR 1.9 [95% CI: 0.9, 4.3]; Fig. 1B). This correlation was the strongest and statistically significant for US positivity for GS (OR 2.4 [95% CI: 1.1, 5.6]) and US-detected synovitis (OR 2.3 [95% CI: 1.04, 5.3]; Fig. 1B). The correlation of patient-reported swelling with GS remained present after correction for PD (OR 2.4 [95% CI: 0.94, 6.1]), and the correlation of patient-reported swelling US-detected synovitis remained significant after correction for tenosynovitis (OR 2.4 [95% CI: 1.0, 5.9]) (Table 2). Absolute numbers of patients with and without reported joint swelling in the presence of sub-clinical US-detected joint inflammation are presented in Supplementary Table S2, available at Rheumatology online.
In addition, analyses were repeated with only MCP-joints for MRI and with MCP and PIP joints for US. In the Leiden CSA cohort, the relation between patient-reported swelling in the hand joints was somewhat stronger if the wrist was incorporated (OR 2.7 [95% CI: 1.7, 4.4] vs 2.0 [95% CI: 1.2, 3.5] without the wrist). In the Rotterdam cohort, the relation between patient-reported swelling and subclinical joint inflammation detected with US was slightly lower with the wrist included (OR 1.9 [95% CI: 0.86, 4.3] vs 2.7 [95% CI: 1.2, 6.1]; Supplementary Table S3, available at Rheumatology online).
Patient-reported swelling and IA development
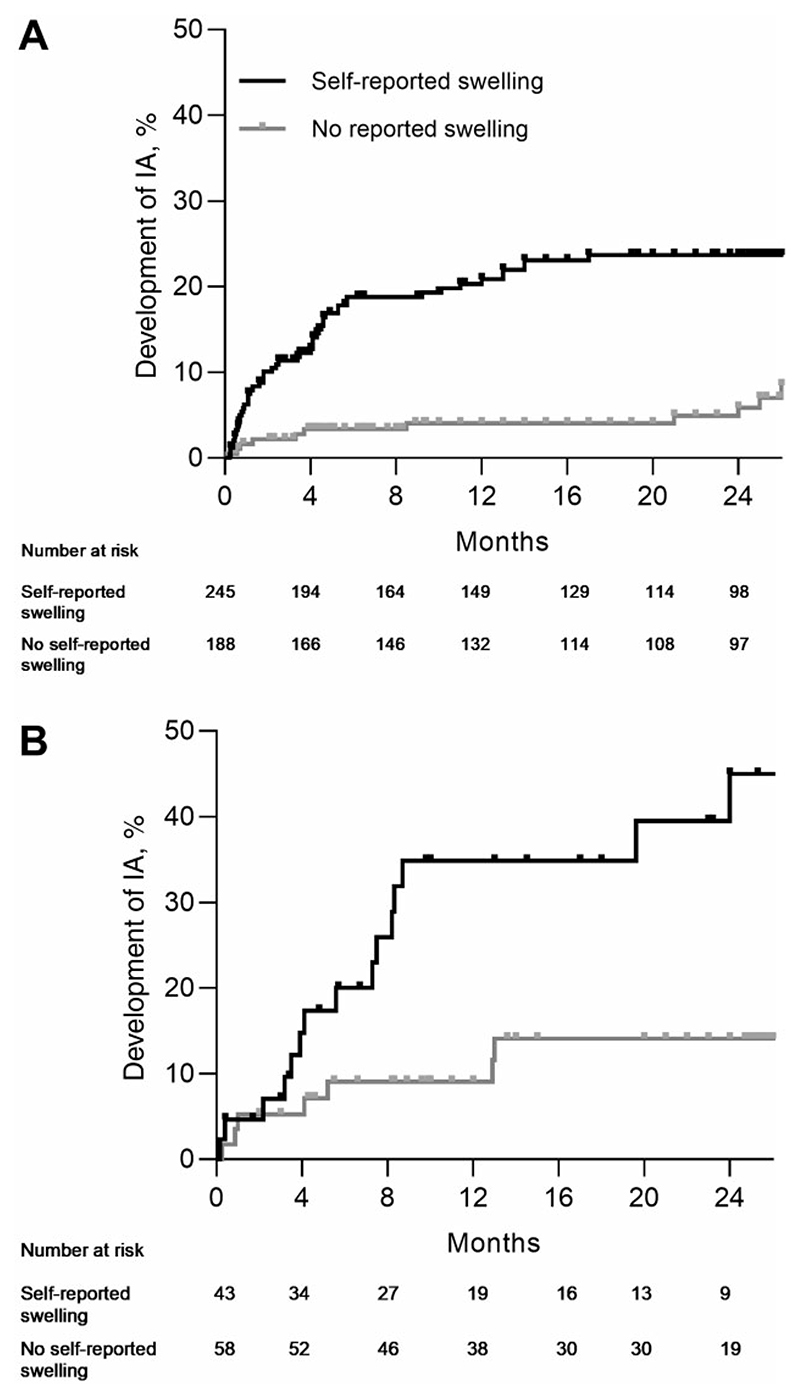
In the Leiden CSA cohort, median follow-up duration was 21 months (interquartile range [IQR] 5.7–26). IA developed in 66 patients and patient-reported swelling associated with IA development with a HR of 3.7 (95% CI: 2.0, 6.9) (Fig. 2A).
Figure 2. Development of inflammatory arthritis in CSA patients for both cohorts for patients with and without patient-reported swelling in the hand joints at baseline.
(A) CSA Leiden (n = 433). (B) CSA Rotterdam (n = 101). CSA: clinically suspect arthralgia; IA: inflammatory arthritis
Also in the Rotterdam CSA cohort patient-reported swelling associated with IA development (Fig. 2B). Median follow-up duration was 14 months (IQR 6.6–24). The HR for patient-reported swelling was 3.4 (95% CI: 1.4, 8.4).
In both cohorts, patient-reported swelling also associated with development of RA (HR 3.2 [95% CI: 1.6, 6.5] and 4.7 [95% CI: 1.3, 17] for the Leiden and Rotterdam cohorts, respectively; Supplementary Fig. S1, available at Rheumatology online). Absolute numbers of patients who developed IA and/ or RA after 2 years and corresponding positive predictive values are represented in Supplementary Tables S4 and S5, available at Rheumatology online.
Value of self-reported swelling in the clinical setting
Other known predictors were studied in our cohorts to apply in the multivariable models. Female sex, age (in years) and morning stiffness (≥60min) were associated with IA development and therefore included in the multivariable ‘clinical model’ (HRs in Supplementary Table S6, available at Rheumatology online). In addition, autoantibody positivity (RF and/or presence of ACPA) and elevated CRP (dichotomized ≥5 mg/l) were associated with IA development (autoantibody positivity HR 5.5 [95% CI: 3.3, 9.0] and elevated CRP HR 2.6 [95% CI: 1.5, 4.3] for the Leiden CSA cohort), and therefore included in the multivariable ‘additional laboratory model’. In the Rotterdam CSA cohort, antibody positivity was significantly associated with IA development (Supplementary Table S6, available at Rheumatology online).
Then the different multivariable models were studied, starting first with only clinical variables. After correction for age, female sex and morning stiffness (≥60 min) a strong independent association of patient-reported swelling and arthritis development remained in the ‘clinical model’ (HR for the Leiden cohort 3.1 [95% CI: 1.5, 6.2]; Table 3). After correction for auto-antibody positivity and elevated CRP in the ‘additional laboratory model’, patient-reported swelling remained independently predictive for IA (HR for CSA Leiden 2.7 [95% CI: 1.3, 5.5]). In addition, also in the CSA Rotterdam cohort, patient-reported swelling remained predictive after correction for autoantibody positivity and morning stiffness (HR 3.2 [95% CI: 1.3, 8.0]; Table 3). Then the imaging results were added. Patient-reported swelling did not remain an independent predictor after correction for subclinical joint inflammation detected with MRI, but was still independently predictive in the multivariable model including subclinical joint inflammation detected with US (HR 2.8 [95% CI: 1.1, 7.1]; Table 3).
Table 3. The added value of patient-reported swelling step-wise adjusted for other predictors within multivariable Cox regression analyses.
| HR (95%CI) | |||
|---|---|---|---|
| Clinical | Additional lab | Additional imaging | |
| CSA Leiden | |||
| Age, per year | 1.04 (1.0, 1.1) | 1.03 (1.0, 1.1) | 1.04 (1.0, 1.1) |
| Female sex | 1.0 (0.53, 2.0) | — | — |
| Patient-reported swelling | 3.1 (1.5,6.2) | 2.7 (1.3, 5.5) | 1.9 (0.89,3.9) |
| Morning stiffness ≥60 min | 2.2 (1.2, 4.0) | 2.1 (1.2, 3.9) | 2.7 (1.4, 5.2) |
| Auto-antibody positive | — | 4.0 (2.2, 7.1) | 4.4 (2.3, 8.2) |
| Elevated CRP (≥5 mg/l) | — | 1.7 (0.96, 3.3) | — |
| MRI positivea | — | — | 6.1 (2.7, 14) |
| CSA Rotterdam | |||
| Patient-reported swelling | 3.3 (1.3, 8.7) | 3.2 (1.3, 8.0) | 2.8 (1.1, 7.1) |
| Morning stiffness ≥60 min | 1.1 (0.35, 3.2) | — | — |
| Auto-antibody positive | — | 4.5 (1.8, 11) | 3.8 (1.5, 9.6) |
| US positiveb | — | — | 2.4 (0.88,6.3) |
Presented are HRs of multivariable analyses towards inflammatory arthritis including 433 patients in CSA Leiden cohort and 101 in the CSA Rotterdam cohort. In every column variables are added to the multivariable model. In the ‘clinical model’ only anamnestic variables were added. In addition, in the ‘laboratory model’ autoantibody positivity (ACPA and/or RF positivity) and CRP were added to the model (without female sex). In the ‘imaging model’ both imaging results are added. Statistically significant values are presented in bold.
352 patients.
100 patients. CSA: clinically suspect arthralgia; HR: hazard ratio.
Discussion
In the outpatient clinic, patients with arthralgia at risk for RA often report joint swelling [5, 6]. We aimed to evaluate the association of patient-reported swelling with the presence of subclinical joint inflammation and the risk of RA development. Our study showed that in the setting of CSA, patients who experience joint swelling more often have subclinical joint inflammation and are more likely to develop RA during follow-up.
The concordance of joint swelling reported by the patients and observed by the rheumatologist at physical examination in an early arthritis setting was moderate and therefore considered of little value to replace physical joint examination [7–10, 26]. In the setting of CSA, where clinical arthritis is per definition absent, patient-reported swelling was associated with the presence of subclinical joint inflammation and showed its value also on top of other common predictors. Thus although clinical arthritis was absent and patients and rheumatologist were discordant in this respect, patient-reported swelling did have a prognostic value.
Furthermore, our study showed the value of patient-reported swelling as an independent predictor for IA development. However, the positive predictive values for IA development were 22% (CSA Leiden) and 35% (CSA Rotterdam), indicating that a certain amount of false-positive outcomes are present. This means that patients reported joint swelling, but did not develop arthritis. The clinical impact could still be that patients with self-reported swelling will be followed more closely, or an MRI scan could be made to verify whether subclinical joint inflammation is present. As MRI in CSA patients is not standardized in the daily outpatient clinics, this easy question is could still be valuable.
Interestingly, at the time of developing the EULAR definition of CSA, experts did not identify patient-reported swelling as most relevant for the construct of CSA [6]. The current data, however, showed the importance of patient-reported swelling within CSA for predicting IA or RA. Our findings are in line with previous research in ACPA positive arthralgia, which also identified patient-reported swelling as risk factor for RA development [5].
For subclinical joint inflammation detected with MRI, tenosynovitis was independently associated with patient-reported joint swelling (and not with synovitis or osteitis). This association with tenosynovitis was not observed when US was used. This can be explained by the fact that US is less sensitive in detecting tenosynovitis and, compared with MRI, up to 80% of tenosynovitis lesions are missed when using US [25, 27, 28]. This difference in sensitivity to detect tenosynovitis may explain the difference in related subclinically inflamed joint tissue when using US or MRI.
Furthermore, for our definition of a positive MRI—for subclinical joint inflammation—age- and joint-matched data from almost 200 healthy individuals were used. Such a reference increases the specificity for MRI without affecting the sensitivity [16]. For US, such a reference was not available. Therefore, more age-related, false-positive signs of subclinical joint inflammation could be still present in the US data, while true signs of subclinical joint inflammation could be lost. We tried to overcome this issue by using strict cut-offs for determining US positivity. Nonetheless, MRI and US results should not be directly compared. In addition, for US bilateral scanning was used, while MRI included unilateral scanning. Also, PIP joints were incorporated in the US protocol, but were not evaluated in the MRI protocol. Lastly, the Rotterdam cohort is smaller, making the outcomes more uncertain. However, despite some differences in imaging modalities and imaging protocol, patient-reported swelling associated with subclinical joint inflammation using both modalities.
Since patient-reported swelling is clinical information that is easily obtained in clinical practice, the independent value in addition to other variables was studied in a stepwise approach. It is of independent added value to other clinical predictors and also to known serological predictors. It was not of added value to MRI, but this is less feasible for clinical practice. It was, however, independently predictable from US, which is an imaging mode more frequent used in clinical practice.
A possible limitation is that with imaging we assessed hand and wrist joints, but patients were only asked about swelling of the hand joints without further defining which joints. This could possibly lead to misclassification bias leading to an under-representation of the association between subclinical joint inflammation and self-reported joint swelling. We performed a sensitivity analysis that revealed no strong increase in the relation between patient-reported swelling and MRI-detected subclinical joint inflammation if only the hand joints (MCP/PIP) were studied in relation to patient-reported swelling of hand joints.
A strength of the current study is the use of two similar, but independent cohorts, which allowed us to validate our results. Also, we had the possibility to correlate patient-reported joint swelling with inflammation detected by two different imaging modalities, resulting in a better applicability to clinical practice.
In conclusion, we showed that patients with CSA who report joint swelling have subclinical joint inflammation more frequently. Patient-reported joint swelling is an independent risk factor for RA development, but it is not an independent risk factor when MRI for the detection of subclinical joint inflammation is available. Asking for experienced joint swelling is easy to implement in the outpatient clinic without additional costs and therefore of value in daily practice.
Supplementary Material
Supplementary material is available at Rheumatology online.
Rheumatology key messages.
Patient-reported joint swelling in CSA patients is associated with the presence of subclinical joint inflammation detected with MRI or ultrasound.
CSA patients who experience joint swelling are more likely to develop arthritis during follow-up.
Although patients with CSA have per definition no clinical arthritis, patient-experienced joint swelling is of prognostic relevance.
Funding
This work was supported by the Dutch Arthritis Foundation and The European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (starting grant, agreement no. 714312).
Footnotes
Disclosure statement: The authors have declared no conflicts of interest.
Data availability
The data underlying this article are available from the corresponding author upon reasonable request.
References
- 1.Ten Brinck RM, van Steenbergen HW, Mangnus L, et al. Functional limitations in the phase of clinically suspect arthralgia are as serious as in early clinical arthritis; a longitudinal study. RMD Open. 2017;3:e000419. doi: 10.1136/rmdopen-2016-000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Tuyl LH, Stack RJ, Sloots M, et al. Impact of symptoms on daily life in people at risk of rheumatoid arthritis. Musculoskelet Care. 2016;14:169–73. doi: 10.1002/msc.1127. [DOI] [PubMed] [Google Scholar]
- 3.Khidir SJH, Wouters F, van der Helm-van Mil AHM, van Mulligen E. The course of fatigue during the development of rheumatoid arthritis and its relation with inflammation: a longitudinal study. Joint Bone Spine. 2022;89:105432. doi: 10.1016/j.jbspin.2022.105432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krijbolder DI, Khidir SJH, Matthijssen XME, et al. Hand function is already reduced before RA development and reflects subclinical tenosynovitis. RMD Open. 2023;9:e002885. doi: 10.1136/rmdopen-2022-002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis. 2013;72:1920–6. doi: 10.1136/annrheumdis-2012-202127. [DOI] [PubMed] [Google Scholar]
- 6.van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJJ, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis. 2017;76:491–6. doi: 10.1136/annrheumdis-2016-209846. [DOI] [PubMed] [Google Scholar]
- 7.Rogier C, van Dijk BT, Brouwer E, de Jong PHP, van der Helm-van Mil AHM. Realising early recognition of arthritis in times of increased telemedicine: the value of patient-reported swollen joints. Ann Rheum Dis. 2021;80:668–9. doi: 10.1136/annrheumdis-2020-219513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barton JL, Criswell LA, Kaiser R, Chen YH, Schillinger D. Systematic review and metaanalysis of patient self-report versus trained assessor joint counts in rheumatoid arthritis. J Rheumatol. 2009;36:2635–41. doi: 10.3899/jrheum.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rampes S, Patel V, Bosworth A, et al. Systematic review and metaanalysis of the reproducibility of patient self-reported joint counts in rheumatoid arthritis. J Rheumatol. 2021;48:1784–92. doi: 10.3899/jrheum.201439. [DOI] [PubMed] [Google Scholar]
- 10.Hirata A, Ogura T, Hayashi N, et al. Concordance of Patient-Reported Joint Symptoms, Physician-Examined Arthritic Signs, and Ultrasound-Detected Synovitis in Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2017;69:801–6. doi: 10.1002/acr.23006. [DOI] [PubMed] [Google Scholar]
- 11.van Steenbergen HW, van Nies JAB, Huizinga TWJ, et al. Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRI. Ann Rheum Dis. 2015;74:1225–32. doi: 10.1136/annrheumdis-2014-205522. [DOI] [PubMed] [Google Scholar]
- 12.Rogier C, Frazzei G, Kortekaas MC, et al. An ultrasound negative for subclinical synovitis in arthralgia patients: is it helpful in identifying those not developing arthritis? Rheumatology (Oxford) 2022;61:4892–7. doi: 10.1093/rheumatology/keac239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostergaard M, Peterfy C, Conaghan P, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6. [PubMed] [Google Scholar]
- 14.Haavardsholm EA, Ostergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66:1216–20. doi: 10.1136/ard.2006.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangnus L, van Steenbergen HW, Reijnierse M, van der Helm-van Mil AH. Magnetic resonance imaging-detected features of inflammation and erosions in symptom-free persons from the general population. Arthritis Rheumatol. 2016;68:2593–602. doi: 10.1002/art.39749. [DOI] [PubMed] [Google Scholar]
- 16.Boer AC, Burgers LE, Mangnus L, et al. Using a reference when defining an abnormal MRI reduces false-positive MRI results-a longitudinal study in two cohorts at risk for rheumatoid arthritis. Rheumatology (Oxford) 2017;56:1700–6. doi: 10.1093/rheumatology/kex235. [DOI] [PubMed] [Google Scholar]
- 17.Dakkak YJ, Matthijssen XME, van der Heijde D, Reijnierse M, van der Helm-van Mil AHM. Reliability of Magnetic Resonance Imaging (MRI) scoring of the metatarsophalangeal joints of the foot according to the rheumatoid arthritis MRI score. J Rheumatol. 2020;47:1165–73. doi: 10.3899/jrheum.190258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino MA, Terslev L, Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open. 2017;3:e000428. doi: 10.1136/rmdopen-2016-000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naredo E, D’Agostino MA, Wakefield RJ, et al. OMERACT Ultrasound Task Force. Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann Rheum Dis. 2013;72:1328–34. doi: 10.1136/annrheumdis-2012-202092. [DOI] [PubMed] [Google Scholar]
- 20.Molina Collada J, López Gloria K, Castrejón I, et al. Ultrasound in clinically suspect arthralgia: the role of power Doppler to predict rheumatoid arthritis development. Arthritis Res Ther. 2021;23:299. doi: 10.1186/s13075-021-02685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Beers-Tas MH, Blanken AB, Nielen MMJ, et al. The value of joint ultrasonography in predicting arthritis in seropositive patients with arthralgia: a prospective cohort study. Arthritis Res Ther. 2018;20:279. doi: 10.1186/s13075-018-1767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TW, van der Helm-van Mil AH. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis. 2016;75:1824–30. doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- 25.Matthijssen XME, Wouters F, Boeters DM, et al. A search to the target tissue in which RA-specific inflammation starts: a detailed MRI study to improve identification of RA-specific features in the phase of clinically suspect arthralgia. Arthritis Res Ther. 2019;21:249. doi: 10.1186/s13075-019-2002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radner H, Grisar J, Smolen JS, Stamm T, Aletaha D. Value of self-performed joint counts in rheumatoid arthritis patients near remission. Arthritis Res Ther. 2012;14:R61. doi: 10.1186/ar3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Berg R, Ohrndorf S, Kortekaas MC, van der Helm-van Mil AHM. What is the value of musculoskeletal ultrasound in patients presenting with arthralgia to predict inflammatory arthritis development? A systematic literature review. Arthritis Res Ther. 2018;20:228. doi: 10.1186/s13075-018-1715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohrndorf S, Boer AC, Boeters DM, et al. Do musculoskeletal ultrasound and magnetic resonance imaging identify synovitis and tenosynovitis at the same joints and tendons? A comparative study in early inflammatory arthritis and clinically suspect arthralgia. Arthritis Res Ther. 2019;21:59. doi: 10.1186/s13075-019-1824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available from the corresponding author upon reasonable request.