Abstract
Activation of the hippocampus is required in order to encode memories for new events (or episodes). Observations from animal studies suggest that for these memories to persist beyond 4 to 6 hours, a release of dopamine generated by strong hippocampal activation is needed. This predicts that dopaminergic enhancement should improve human episodic memory persistence also for events encoded with weak hippocampal activation. Here, using pharmacological fMRI in an elderly population where there is a loss of dopamine neurons as part of normal aging, we show this very effect. The dopamine precursor levodopa led to a dose-dependent (inverted U-shape) persistent episodic memory benefit for images of scenes when tested after 6 hours, independent of whether encoding-related hippocampal fMRI activity was weak or strong (U-shaped dose-response relationship). This lasting improvement even for weakly encoded events supports a role for dopamine in human episodic memory consolidation albeit operating within a narrow dose range.
Introduction
Converging evidence from animal studies suggests that dopamine critically contributes to the cellular consolidation of hippocampal-dependent memories by inducing protein-synthesis in hippocampal neurons (Frey and Morris, 1997; O’Carroll et al., 2006). Behavioural evidence from episodic-like memory paradigms in animals show that the availability of dopamine within the hippocampus during encoding is necessary for long-lasting memories (4 to 6 hours and longer), but does not influence memory across short delays (30 minutes) (Bethus et al., 2010). Consequently, weakly encoded events not leading to dopamine release in the hippocampus can be recollected after short-delays but are forgotten after delays of six hours and longer (O’Carroll et al., 2006; Bethus et al., 2010). Testing for a similar role in human episodic memory requires manipulation of dopamine levels at encoding with subsequent testing of memory after both short and long retention intervals (Wang and Morris, 2010; Lisman et al., 2011).
A key prerequisite for characterizing the role of dopamine in human episodic memory consolidation is to relate the effects of dopamine to the strength of hippocampal activation at encoding. This is because the hippocampal release of dopamine can be enhanced by strong hippocampal activation (for a review see (Lisman et al., 2011)). If increasing dopamine levels in humans improves memory performance after long delays through similar mechanisms to that seen in animal studies (O’Carroll et al., 2006; Bethus et al., 2010), dopamine administration should improve long-term memory for events that elicit only weak hippocampal activity at encoding, events that normally would be associated with low levels of hippocampal dopamine. This predicts that increasing availability of dopamine would decrease the influence of hippocampal activation at encoding, measured using functional MRI (fMRI), on delayed memory.
Here we tested these hypotheses amongst healthy older adults in a pharmacological fMRI study. We chose an elderly population as our target group for two reasons. Firstly, understanding the role of dopamine in episodic memory is of particular relevance in old age where a decline in episodic memory is well recognised (Light, 1991; Hedden and Gabrieli, 2004; Randy L, 2004). Secondly, there is known age-dependent degeneration of substantia nigra/ventral tegmental area (SN/VTA) dopamine neurons (Fearnley and Lees, 1991; Bäckman et al., 2006). Consequently, we recruited 32 healthy older adults who participated in a double-blind crossover study with the dopamine-precursor levodopa (L-DOPA) and placebo. Participants performed an fMRI encoding task in which they viewed indoor and outdoor scenes and episodic memory for these scenes was tested after two hours (‘early test’) or six hours (‘delayed test’) (Figure 1). We used an additional manipulation of reward as a way of manipulating endogenous mechanisms of dopamine-related memory enhancement and to compare its effect on memory consolidation to the exogenous manipulation through the administration of L-DOPA. Thus half the stimuli were probabilistically associated with reward and half with no reward. In order to account for the possibility that the effect of L-DOPA may depend on the structural integrity of the SN/VTA in our older participants, we used a semi-quantitative MR imaging technique called magnetization transfer (MT) imaging (Wolff and Balaban, 1989; Helms et al., 2009). It is known that the cognitive effects of dopamine are dose-dependent (Knecht et al., 2004), often showing a non-linear dose-response curve (Goldman-Rakic et al., 2000; Cools et al., 2001; Li and Sikström, 2002; Takahashi et al., 2008). To account for such a possibility also in episodic memory, we conducted planned correlations between body-weight adjusted relative doses of L-DOPA and our behavioural and encoding-related functional outcomes measures.
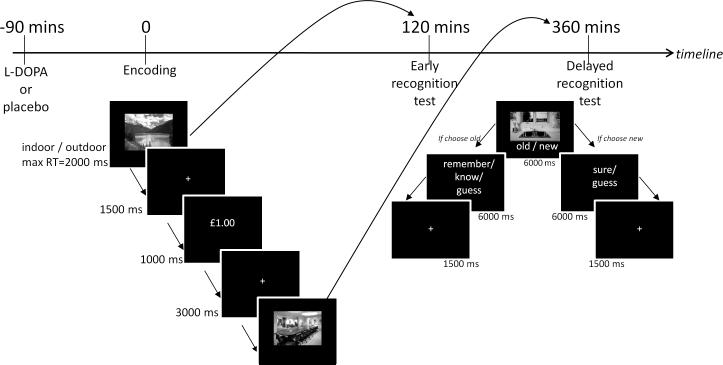
Figure 1. Study timeline and tasks.
In the fMRI scanner, participants viewed randomly presented images of scenes and were required to indicate whether they were indoor or outdoor scenes with a button press. 80% of correct responses for one category were followed by a reward (£1.00) and for the other category was followed by a neutral outcome (£0.00), thereby the images served as reward-predicting and neutral cues respectively. Following this outside the scanner, memory for half the scenes were tested two hours after encoding (‘early’) and the remaining scenes six hours after encoding (‘delayed’) using a remember/know paradigm.
Material and methods
Participants
We recruited participants via our departmental website, advertisement in local public buildings and word of mouth. To ensure participants were healthy, volunteers were initially screened by telephone and excluded if they had any of the following: current or past history of neurological, psychiatric or endocrinological disorders (including diabetes mellitus and thyroid dysfunction), metallic implants, tinnitus, major visual impairment, history of drug addiction. To control for vascular risk factors, individuals known to have had a stroke or transient ischemic attack, myocardial infarction or require more than one anti-hypertensive medication were not eligible for participation. All participants had a Mini-Mental State Examination score ≥28 and a Geriatric Depression Scale score ≤7 (a score >11 would indicate depression). All participants performed within 1.5 SD of age-related norms on a range of neuropsychological tests, ensuring they were cognitively intact as follows: Rey Auditory and Verbal Learning Test (RAVLT) trials 1-5 (mean 50.2, SD 8.3), RAVLT trial 7 (mean 9.5, SD 2.3), D2 cancellation test of attention (mean 152.3, SD 33.5), Digit Span Forward (median 8, range 4 – 9), Digit Span Backward (median 5, range 3 – 8), Controlled Oral Word Association test (COWA) phonemic fluency (mean 58.0, SD 14.0), COWA semantic fluency (mean 26.5, SD 6.6) and Visual and Object Space Perception number location (median 10, range 8-10). All subjects had a normal neurological examination (performed by a neurologist R.C.) ensuring participants did not have concurrent undiagnosed neurological conditions. Written informed consent was obtained from all participants. The study received ethical approval from the North West London Research Ethics Committee 2.
Participants in the current study (n = 32) were selected from a larger sample of 42 healthy older adults aged 65 – 75 years who had participated in a previous study within the preceding six months. Preselection was based on an assessment of magnetization transfer (MT) values of the SN/VTA. MT imaging is a semi-quantitative MR imaging technique that reflects structural integrity (Wolff and Balaban, 1989) where lower MT values suggest less structural integrity (Eckert et al., 2004; Düzel et al., 2008). 10 individuals with MT values of the SN/VTA scattered around the mean MT values of the group were excluded to increase the variance in the sample, resulting in 16 participants with higher MT values (‘high integrity’ group) and 16 with lower MT values (‘low integrity’ group). Note that the current cohort still had a normal distribution of midbrain integrity in both the final subset of participants for the behavioural analysis (n=29: Kolmogorov-Smirnov test statistic = 0.11, p = .200) and the fMRI analysis (n=23: Kolmogorov-Smirnov test statistic = 0.093, p = .200). The two MT groups were matched for age (independent t-test, p = .208) and closely matched for gender (low group 12 females, high group 9 females; Mann Whitney U test, p = .272).
Three subjects were excluded from all analyses. Of these, two were excluded due to poor performance in the encoding task (<60% correct indoor/outdoor judgement) consequent upon side-effects of L-DOPA (vomiting during the encoding task) or misunderstanding the task instructions. One other participant misunderstood the instructions for the first remember/know test and was excluded from all analyses. With regards to side-effects of L-DOPA, four subjects vomited of whom one was excluded as noted above. For the other three participants, this brief side-effect occurred after the encoding task had been completed and their encoding performance was >98% correct, thus we did not exclude them from our analyses.
Study procedure
We used a double-blind placebo controlled crossover design. Participants attended on two occasions, one week apart and performed the same task on both days, 90 minutes after ingestion of either levodopa (150mg levodopa + 37.5mg benserazide mixed in orange juice; L-DOPA) or placebo (orange juice alone), the order of which was counterbalanced. Benserazide promotes higher levels of dopamine in the brain whilst minimising peripheral side-effects such as nausea and vomiting. We chose a dose of 150mg as a previous study has shown that although 100mg can improve verbal learning in younger adults, those with a lower body-weight who effectively received higher doses showed a greater effect (Knecht et al., 2004). To achieve comparable drug absorption across individuals, subjects were instructed not to eat for up to two hours before commencing the study. Repeated physiological measurements were recorded on both days before and after the pharmacological manipulation, which showed a reduction in blood pressure from baseline to 90 minutes after L-DOPA (from average 148/82 to 142/80; paired t-tests systolic t = 3.12, p = .004; diastolic t = 2.46, p = .020) but no change in heart rate. Subjective mood rating scales were also recorded. This series of 16 visual analogue scales collapsed down to measure three factors: how alert, content and calm participants felt (Bond and Lader, 1974). When baseline levels were taken into account, there was no significant difference in these subjective mood rating scales at encoding after receiving L-DOPA compared to placebo. For the early and delayed memory tests, the only significant difference was at early test where participants felt more content on the day they received L-DOPA compared to placebo (mean difference in scores 5.06, SD 11.1, paired t-test t = 2.37, p = .027).
fMRI encoding task
We presented participants with 120 grey-scale images of indoor (n=60) and outdoor (n=60) scenes in a randomised fashion (Figure 1). Different images were used on each of the two test days. Participants were required to indicate with a button press whether the image was of an indoor or outdoor scene (response required within 2s). After a brief delay (fixation cross, 1.5s), this was followed by either a reward (indicated by £1.00) or neutral outcome (indicated by £0.00) (outcome, 1s) and finally a further fixation cross (3s). 80% of correct responses for each category of scenes predicted either a reward or no reward. For each participant, which category predicted reward (indoor or outdoor) was different on the two test days and this order was counterbalanced across subjects.
Prior to pharmacological administration, participants were familiarized with the encoding task through ten practice trials using different images. During this practice task the outcome was not probabilistic. Participants were told which category (indoor or outdoor) would be rewarded, with this category remaining the same for the study task. The purpose of this was to ensure by the time participants performed the task in the scanner, they would anticipate a reward when they saw images in the rewarded category (thus serving as reward predicting cues) and vice versa for the unrewarded category (serving as neutral cues).
Participants were given written and verbal task instructions that included the following: ‘pay attention to all the images presented as we may return to some of them later in the day’. This same instruction was given on both days without being explicitly told on the second day that they would be performing memory recognition tasks again to minimise practice effects. After completion of the encoding task, participants immediately went on to have further scanning to acquire diffusion tensor images on one day (not reported here) or were asked to sit alone in a room for an equivalent amount of time and not perform any activities on the other day. All participants also completed a brief unrelated decision-making task prior to performing the encoding task on both days (not reported here).
Behavioural recognition tasks
We tested memory for scenes shown during the fMRI encoding task behaviourally two hours and six hours after encoding (henceforth referred to as the early and delayed test respectively) using a remember/know paradigm. For the early test, participants were shown a random selection of half of the scenes they had previously viewed during the encoding task (30 indoor and 30 outdoor scenes) and 30 new distractor scenes (15 indoor and 15 outdoor). Memory for the remaining scenes intermixed with another set of 30 new distractor images was tested at the delayed test. For both tests, whilst the scene was displayed participants indicated with a button press whether the image was old (seen before during the encoding task) or new (never seen before) (maximum reaction time 6s). We also asked for a second decision in relation to the stimuli (maximum reaction time 6s): if they chose ‘old’ then participants had to commit to one of three further options: remember, know or guess, where ‘remember’ indicated they could recollect something specific about the study episode. If the image was confidently familiar, but they had no recollective experience they were instructed to choose ‘know’. Guess responses were given when unsure that the image was old. If participants indicated the picture was ‘new’ then a further decision was made of whether they were sure or had guessed. Remember responses are thought to reflect hippocampal-dependent episodic memory recollection, whereas know responses index familiarity (Yonelinas et al., 2002).
We chose to perform the early test of memory two hours after encoding (3.5 hours after L-DOPA/placebo administration) to minimise the potential confounding factor of persisting drug effects at early but not delayed test. Thus we were able to test participants’ memory on two occasions after a short and long interval, ensuring they were not under the peak effects of L-DOPA on both occasions, whilst still allowing a sufficient time window between the tests to investigate our hypothesis that L-DOPA modulated consolidation (four to six hours post-encoding). We acknowledge that by performing the early test of memory after a two hour delay, the consolidation process may have already begun, but we would expect the effects to be less pronounced than at delayed test.
Behavioural analysis
For the encoding task we calculated the percentage of correct indoor and outdoor responses and reaction times for these responses. For the early and delayed memory tests, hit rates were calculated as a proportion of correctly encoded items for the condition of interest. Corrected hits were calculated as correct old responses minus old responses for new items (false alarms). We then separated this into corrected remember hits (remember responses for correct old items minus remember responses for false alarms) and corrected know hits (know responses for correct old items minus know responses for false alarms). Corrected hit rates were calculated for the following conditions: reward-predicting scenes at early test on placebo, neutral scenes at early test on placebo, reward-predicting scenes at early test on L-DOPA, neutral scenes at early test on L-DOPA, reward-predicting scenes at delayed test on placebo, neutral scenes at delayed test on placebo, reward-predicting scenes at delayed test on L-DOPA and neutral scenes at delayed test on L-DOPA. Although all analyses were performed using corrected hit rates, we also report uncorrected hit rates and false alarms, d’ and response bias for completeness. D’ and response bias were calculated using standard Excel formula (Stanislaw H and N., 1999). We analysed hit rates using a 2 × 2 × 2 mixed ANOVA with drug (L-DOPA/placebo), time of test (early/delay) and reward (reward-predicting scenes/neutral scenes) as the within-subjects factors, and SN/VTA integrity group indexed by MT values (low integrity/high integrity) as a between-subjects factor.
Given evidence of an inverted U-shape relationship between dopamine and working memory, we hypothesised a similar relationship for episodic memory. Since the effective dose of L-DOPA is dependent on body-weight (Zappia et al., 2002), we calculated the weight-adjusted dose for each participant (150/body-weight, mg/kg). We then performed regression analyses using both linear and quadratic models, where corrected remember and know hit rates were the dependent variable and the weight-adjusted dose of L-DOPA was the independent variable.
We also grouped participants into three ‘dose groups’ (referred to as low, middle and high dose groups). We note that within these groupings, there were no significant differences in health-related variables or neuropsychological test performance as follows: years of education (one-way ANOVA, F = 0.364, p = .698), blood pressure (F = 2.120, p = .139), smoking status (F = 0.225, p = .800), cholesterol status (F = 1.465, p = .250), IQ (F = 0.316, p = .732), RAVLT trials 1-5 (F = 0.352, p = .707), RAVLT trial 7 (F = 1.302, p = .290), D2 cancellation test of attention (F = 0.738, p = .488), Forward Digit Span (F = 3.17, p = .066), Backward Digit Span (F = 2.096, p = .143), phonemic fluency (F = 0.045, p = .956), semantic fluency (F = 0.023, p = .977) and Visual and Object Space Perception (F = 0.379, p = .689).
We report results significant at the threshold p<0.05. All significance tests are two-tailed.
Neuroimaging
All imaging was acquired using a 3.0T Trio MRI scanner (Siemens) with a 32-channel head coil.
Anatomical MRI data acquisition
A structural multi-parameter map protocol employing a 3D multi-echo fast low angle shot (FLASH) sequence at 1mm isotropic resolution (Helms et al., 2008b) was used to acquire MT weighted images (echo time, TE, 2.2-14.70ms, repetition time, TR, 23.7ms, flip angle, FA, 6 degrees), T1 weighted images (TE 2.2-14.7ms, TR 18.7ms, FA 20 degrees) and proton density weighted images (TE 2.2-19.7ms, TR 23.7ms, FA 6 degrees) (Helms et al., 2008b). B1 mapping (TE 37.06 and 55.59ms, TR 500ms, FA 230:-10:130 degrees, 4mm3 isotropic resolution) was acquired to correct the T1 maps for inhomogeneities in the transmit radiofrequency field (Lutti et al., 2010). A double-echo FLASH sequence (TE1 10ms, TE2 12.46ms, 3 × 3 × 2 mm resolution and 1mm gap) was used to measure local field inhomogeneities and correct for the image distortions in the B1 mapping data. Using in-house code, quantitative MT maps were extracted for each subject (Helms et al., 2008a).
fMRI data acquisition
Functional data was acquired for each subject on both test days. On each day, two fMRI time series were acquired containing 148 volumes (matrix size = 64 × 74; 48 slices; image resolution= 3 × 3 × 3mm; FOV=192 × 222mm; TR=70ms; TE=30ms). The fMRI acquisition protocol was optimised to minimise susceptibility-induced BOLD signal losses in inferior frontal and temporal lobe regions (Weiskopf et al., 2006). Six additional volumes at the beginning of each series were acquired to allow for steady state magnetization and were subsequently discarded. Individual field maps were recorded using a double echo FLASH sequence (matrix size = 64 × 64; 64 slices; spatial resolution = 3 × 3 × 3 mm; gap = 1 mm; short TE=10 ms; long TE=12.46 ms; TR=1020 ms) for distortion correction of the acquired EPI images. Using the FieldMap toolbox, field maps were estimated from the phase difference between the images acquired at the short and long TE.
fMRI data preprocessing
Data were analysed using Statistical Parametric Mapping software (SPM8, Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm). Bias correction (part of the Segmentation step in SPM8) was performed as fMRI data acquired with a 32-channel head coil may be prone to strong intensity inhomogeneities. Pre-processing then included realignment, unwarping using individual fieldmaps, co-registration and spatial normalization to the Montreal Neurology Institute (MNI) space. For normalisation, we used unified segmentation to classify anatomical T1w images into grey matter, white matter and cerebrospinal fluid (Ashburner and Friston, 2005), followed by the diffeomorphic registration algorithm (DARTEL) to generate flowfields to warp EPI images to MNI space (Ashburner, 2007). Finally, data were smoothed with a 6mm FWHM Gaussian kernel. The fMRI time series data were high-pass filtered (cut-off = 128 s) and whitened using an AR (1)-model. For each subject a statistical model was computed by applying a canonical hemodynamic response function (HRF) combined with time and dispersion derivatives.
fMRI data analysis
Statistical analysis was performed using the general linear model (GLM) approach. We used a subsequent memory analysis whereby neural activity at encoding was contrasted for items that were subsequently remembered or forgotten. We built two different GLMs at the first level, the first of which was to determine the overall subsequent memory effect for all ‘recognised’ items and a second model, more specific to our behavioural results, to determine the subsequent memory effect for items later ‘remembered’, in particular for neutral items. We collapsed together responses at early and delayed recall to increase statistical power. For the same reason we excluded participants who had less than 10% of trials in either the remember plus know, remember or forget categories to ensure analyses were statistically robust. Thus whilst GLM 1 involved all 29 participants, GLM 2 included data from 23 participants (note the order of L-DOPA/placebo administration remained counterbalanced for this subset, where 11 participants received L-DOPA on day one and 12 participants on L-DOPA on day two). At the first level analysis, separate design matrices for L-DOPA and placebo were constructed.
GLM 1: Recognised (remember plus know) versus forgotten
At the first level, the design matrix consisted of regressors at the onset of the image presentation for rewardpredicting scenes and neutral scenes. These encoding-related responses were modelled separately for items subsequently recognised (correct ‘old’ responses, thus collapsing both remember and know responses together) and forgotten (incorrect ‘new’ responses). Thus the following were the main regressors of interest:
Correct old response for neutral scenes (‘recognised’)
Correct old response for reward-predicting scenes (‘recognised’)
Incorrect new response for neutral scenes (‘forgotten’)
Incorrect new response for reward-predicting scenes (‘forgotten’)
Separate regressors at the time of the outcome for a win (where participants saw £1.00 on the screen) and no win (where participants saw £0.00) outcomes were included. We included a regressor of no interest for errors when participants failed to press the correct button to indicate whether the image was of an indoor or outdoor scene. To capture residual movement-related artefacts, six covariates (the three rigid-body translation and three rotations resulting from realignment) were also included as regressors of no interest. Finally we also included 18 regressors for cardiac and respiratory phases in order to correct for physiological noise (Glover et al., 2000; Birn et al., 2006). At the first level, we implemented a contrast for the main effect of memory (recognised > forgotten).
GLM 2: Remember versus forgotten
Our behavioural results identified a specific effect of L-DOPA on episodic memory indexed by remember responses. On this basis we performed another analysis where we divided correct ‘old’ responses into ‘remember’ and ‘know’ responses and modelled them as separate regressors.
Thus, the following constituted the main regressors of interest:
Correct remember response for neutral scenes (‘remember’)
Correct remember response for reward-predicting scenes (‘remember’)
Correct know response for neutral scenes (‘know’)
Correct know response for reward-predicting scenes (‘know’)
Incorrect new response for neutral scenes (‘forgotten’)
Incorrect new response for reward-predicting scenes (‘forgotten’)
All other regressors of no interest used in the previous design were also included in this model. We were then able to contrast remember > forgotten responses at the first level for both rewarded and neutral items together and for neutral items alone.
Since we were predominantly interested in the effect of L-DOPA on memory, for both GLM 1 and 2, contrast images were entered into a paired t-test design at the second level. We were then able to examine the main effect of memory and the interaction between memory and drug (L-DOPA > placebo, and placebo > L-DOPA) using T-contrasts.
Based on an a priori hypothesis that dopamine promotes hippocampal consolidation, we built a functional ROI that included those voxels within the hippocampus that were more active for remembered when compared to forgotten items. We extracted parameter estimates within these voxels using the MarsBaR toolbox (Brett, 2002) and entered them in a two (remember/forget) by two (L-DOPA/placebo) ANOVA. With this approach we were able to test for the effects of drug on the selected voxels. Note that the drug effects are orthogonal to the main effect of memory used to define the functional ROI.
Anatomical masks
We created anatomical masks averaged across our study participants to use for small volume correction and to anatomically constrain functional ROIs from which parameter estimates were extracted. Our motivation for this was to account for age-related structural brain changes to try to lend greater accuracy to our analyses. Freesurfer’s (version 4.5.0, http://surfer.nmr.mgh.harvard.edu/) automated recon-all pipeline was used to parcellate the hippocampus (Fischl et al., 2004). The high level of grey/white matter contrast on MT images was exploited to manually segment the SN/VTA for each subject, performed by a trained individual (R.C) as per Düzel et al (Duzel et al., 2008) using MRIcro (Rorden C, 2000). Individual subjects’ hippocampal and SN/VTA masks were warped to MNI space using DARTEL as previously described. A group-averaged mask was made from these warped images. For the bilateral hippocampus, we used 22 subjects’ masks, where subjects were excluded due to preprocessing errors (n=6) or inaccurate segmentation after visual inspection (n=1). For the bilateral SN/VTA, all subjects’ masks were used.
Statistics
Clusters were defined using a threshold of p<0.001 and >10 voxels. We report results corrected for multiple comparisons across the whole brain or after small volume correction (SVC) for the bilateral hippocampus and bilateral SN/VTA using family-wise error correction (FWE) at a threshold of p<0.05. We selected the hippocampus and SN/VTA a priori for small volume correction given evidence for a functional SN/VTA-hippocampal circuit underlying the dopaminergic modulation of episodic memory (Lisman and Grace, 2005; Lisman et al., 2011). Imaging results are overlaid on a group-average MT image as the high level of grey/white matter allows good visualisation of both of our main regions of interest (hippocampus and SN/VTA).
Non-linear modulation of neural activity by dopamine
We tested if neural activity was also modulated in a non-linear (i.e. quadratic) manner by L-DOPA. We performed regression analyses using the parameter estimates from functionally activated clusters found in the hippocampus. In these models, parameter estimates were the dependent variable and the weight-adjusted dose of L-DOPA was the independent variable. A similar regression analysis was performed using MT values of the SN/VTA rather than L-DOPA dose.
Results
Encoding performance
Demographic data for 29 of the 32 healthy older adults who participated are shown in Table 1. Accuracy for button presses for indoor and outdoor images during encoding was high (mean correct responses 97.6%, SD 2.99). Accuracy was not affected by L-DOPA and reward (main effect of reward: F(1,29)=3.67, p=.07; main effect of drug: F(1,29) = 3.62, p=.07; reward*drug interaction: F(1,29) = 0.15, p=.71), nor was reaction time (main effect of reward: F(1,29) = 2.44, p=.13; main effect of drug: F(1,29) = 0.58p=.45; reward*drug interaction: F(1,29) = 0.012, p=.91).
Table 1.
Demographic details for participants
| Age (yrs) | 70.31 (3.22) |
|---|---|
| Gender M:F | 10: 19 |
| Education (yrs) | 16.00 (2.63) |
| National Adults Reading Test IQ | 121.38 (6.58) |
| Body mass index | 26.8(4.45) |
| Non-smoker | 28 (96.6%) |
| Normotensive | 27 (93.1%) |
| Mini-Mental State Examination | 30 (28-30) |
| Geriatric Depression Scale | 1 (0-7) |
Results are mean (SD), number (%) or median (range).
Dose-dependent enhancement of memory by L-DOPA
All analyses were performed using corrected hit rates (hits minus false alarms as a proportion of correctly encoded trials, for the condition of interest). A summary of uncorrected hit rates and false alarms, d’ and response bias are provided in Tables 2 and 3 respectively.
Table 2.
Mean hit rates and false alarms for the conditions of interest (SD in parentheses).
| Remember | Know | |||||||
|---|---|---|---|---|---|---|---|---|
| reward hits |
neutral hits |
reward false alarms |
neutral false alarms |
reward hits |
neutral hits |
reward false alarms |
neutral false alarms |
|
| All (n=29 ) | ||||||||
| Placebo | ||||||||
| early | 0.32(0.23) | 0.27(0.22) | 0.12(0.15) | 0.07(0.10) | 0.19(0.17) | 0.14(0.15) | 0.13(0.11) | 0.08(0.13) |
| delay | 0.22(0.17) | 0.24(0.17) | 0.06(0.08) | 0.08(0.12) | 0.16(0.14) | 0.15(0.13) | 0.12(0.13) | 0.08(0.09) |
| L-DOPA | ||||||||
| early | 0.36(0.22) | 0.31(0.19) | 0.10(0.13) | 0.11(0.11) | 0.18(0.14) | 0.20(0.16) | 0.14(0.15) | 0.11(0.12) |
| delay | 0.23(0.23) | 0.23(0.16) | 0.06(0.09) | 0.07(0.11) | 0.16(0.13) | 0.17(0.13) | 0.14(0.13) | 0.11(0.11) |
|
| ||||||||
| Low dose-group (n = 10 ) | ||||||||
| Placebo | ||||||||
| early | 0.22(0.16) | 0.25(0.24) | 0.10(0.16) | 0.07(0.11) | 0.28(0.18) | 0.19(0.21) | 0.17(0.15) | 0.15(0.18) |
| delay | 0.15(0.13) | 0.19(0.12) | 0.04(0.06) | 0.04(0.05) | 0.22(0.19) | 0.20(0.17) | 0.13(0.16) | 0.11(0.09) |
| L-DOPA | ||||||||
| early | 0.29(0.24) | 0.18(0.16) | 0.09(0.09) | 0.15(0.14) | 0.20(0.12) | 0.25(0.16) | 0.19(0.18) | 0.15(0.13) |
| delay | 0.18(0.20) | 0.17(0.15) | 0.03(0.03) | 0.08(0.13) | 0.19(0.15) | 0.17(0.11) | 0.13(0.09) | 0.10(0.08) |
|
| ||||||||
| Middle dose-group (n = 9) | ||||||||
| Placebo | ||||||||
| early | 0.41(0.27) | 0.30(0.22) | 0.17(0.15) | 0.09(0.12) | 0.13(0.13) | 0.12(0.13) | 0.13(0.12) | 0.02(0.03) |
| delay | 0.24(0.14) | 0.24(0.16) | 0.09(0.09) | 0.11(0.13) | 0.12(0.10) | 0.06(0.06) | 0.11(0.12) | 0.07(0.11) |
| L-DOPA | ||||||||
| early | 0.45(0.18) | 0.43(0.15) | 0.16(0.15) | 0.11(0.11) | 0.16(0.11) | 0.18(0.19) | 0.14(0.15) | 0.12(0.15) |
| delay | 0.35(0.30) | 0.37(0.18) | 0.10(0.13) | 0.08(0.13) | 0.13(0.10) | 0.17(0.16) | 0.11(0.10) | 0.13(0.09) |
|
| ||||||||
| High dose-group (n = 10) | ||||||||
| Placebo | ||||||||
| early | 0.35(0.22) | 0.27(0.21) | 0.09(0.13) | 0.05(0.08) | 0.16(0.16) | 0.13(0.08) | 0.09(0.34) | 0.07(0.09) |
| delay | 0.27(0.22) | 0.29(0.22) | 0.05(0.07) | 0.09(0.15) | 0.13(0.09) | 0.16(0.12) | 0.11(0.09) | 0.05(0.06) |
| L-DOPA | ||||||||
| early | 0.34(0.23) | 0.31(0.17) | 0.06(0.12) | 0.06(0.07) | 0.18(0.18) | 0.17(0.13) | 0.10(0.12) | 0.07(0.05) |
| delay | 0.17(0.16) | 0.18(0.08) | 0.07(0.08) | 0.05(0.04) | 0.16(0.15) | 0.15(0.11) | 0.19(0.17) | 0.11(0.15) |
Table 3.
Mean d’ and response bias (SD in parentheses)
| d’ | Response bias | |||
|---|---|---|---|---|
| reward | neutral | reward | neutral | |
| All (n=29) | ||||
| Placebo | ||||
| early | 0.77 (0.62) | 0.88 (0.70) | 1.41 (1.48) | 1.83 (2.19) |
| delay | 0.66 (0.63) | 0.83 (0.68) | 1.29 (0.45) | 1.86 (2.19) |
| L-DOPA | ||||
| early | 0.77 (0.71) | 0.81 (0.60) | 1.45 (1.68) | 1.39 (1.61) |
| delay | 0.56 (0.48) | 0.62 (0.59) | 1.27 (0.50) | 1.49 (1.60) |
|
| ||||
| Low dose-group (n=10) | ||||
| Placebo | ||||
| early | 0.73 (0.82) | 0.55 (0.56) | 2.08 (2.43) | 1.04 (0.26) |
| delay | 0.79 (0.63) | 0.75 (0.78) | 1.17 (0.29) | 1.25 (0.68) |
| L-DOPA | ||||
| early | 0.43 (0.66) | 0.43 (0.61) | 0.96 (0.24) | 1.15 (0.33) |
| delay | 0.56 (0.41) | 0.39 (0.48) | 1.15 (0.30) | 1.18 (0.57) |
|
| ||||
| Middle dose-group (n=9) | ||||
| Placebo | ||||
| early | 0.69 (0.58) | 1.19 (0.82) | 1.05 (0.29) | 3.21 (3.62) |
| delay | 0.68 (0.46) | 0.75 (0.71) | 1.37 (0.36) | 2.41 (2.71) |
| L-DOPA | ||||
| early | 0.98 (0.64) | 1.04 (0.60) | 1.29 (0.84) | 1.96 (2.89) |
| delay | 0.87 (0.35) | 1.01 (0.70) | 1.37 (0.76) | 1.96 (2.81) |
|
| ||||
| High dose-group (n=10) | ||||
| Placebo | ||||
| early | 0.89 (0.47) | 0.92 (0.64) | 1.10 (0.34) | 1.38 (0.59) |
| delay | 0.52 (0.77) | 0.99 (0.60) | 1.34 (0.64) | 1.97 (2.69) |
| L-DOPA | ||||
| early | 0.91 (0.77) | 0.97 (0.44) | 2.09 (2.71) | 1.12 (0.27) |
| delay | 0.28 (0.50) | 0.50 (0.43) | 1.30 (0.38) | 1.39 (0.58) |
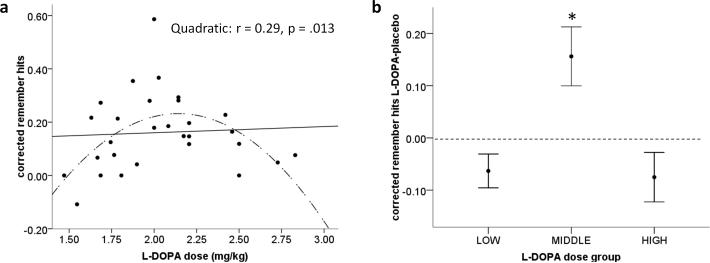
The ability to recognize old (i.e. previously encoded) items and reject new items was better at early compared to delayed test as shown by a main effect of time (F(1,27) = 9.505, p = .005) in a two (drug: L-DOPA/placebo) by two (time of test: early/delay) by two (reward: reward-predicting scenes/neutral scenes) repeated measures ANOVA with structural integrity of the SN/VTA (low integrity/ high integrity) as a between-subjects factor. There was also a time by reward interaction (F(1,27) = 48.289 p = .000). Here post hoc tests showed that for reward-predicting images, corrected hit rates were higher at early compared to delayed test (paired t-test, t(28)=7.178, p=.000) with no difference evident for neutral items (t(28) = 1.201, p=.240). There was no main effect of drug (p=.530) and no interactions with drug (drug*time: p=.797; drug*reward: p=.250; drug*time*reward: p=.254). Furthermore there were no significant interactions with SN/VTA structural integrity (integrity group*drug: p=.169; integrity group*reward p=.780; integrity group*time of test p=.664). We next performed a planned assessment of the potential dose-dependent effect of L-DOPA using regression analyses with the weight-adjusted dose of L-DOPA (150 mg / body weight, mg/kg) and corrected remember and know hit rates for each condition separately (Table 4). Remember hit rates for neutral images at early test showed a significant quadratic and linear regression, explaining 28% (p = .014) and 22% (p = .011) of the variance respectively. At delayed test there was a significant quadratic regression alone, where the weight-adjusted dose of L-DOPA explained 29% (p = .013) of variance in an inverted ‘U-shape’ pattern (Figure 2a). In contrast, L-DOPA dose did not predict remember hit rates for the rewarded scene category, nor did it predict know hit rates for any condition. This points to a modulatory effect of L-DOPA on episodic memory for neutral items.
Table 4.
Regression analyses between remember and know responses and weight-adjusted dose of L-DOPA
| Quadratic | Linear | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hit rate | F | p | R2 | F | p | R2 |
| Early test | ||||||
| Remember Reward | 0.587 | .563 | .043 | 0.170 | .683 | .006 |
| Remember No Reward |
5.028 | .014* | .279 | 7.463 | .011* | .217 |
| Know Reward | 1.498 | .242 | .103 | 2.986 | .095 | .100 |
| Know No Reward | 0.652 | .529 | .048 | 0.950 | .338 | .034 |
| Delayed test | ||||||
| Remember Reward | 0.365 | .698 | .027 | 0.620 | .438 | .022 |
| Remember No Reward |
5.205 | .013* | .286 | 0.088 | .769 | .003 |
| Know Reward | 0.330 | .722 | .025 | 0.663 | .423 | .024 |
| Know No Reward | 1.490 | .244 | .103 | 0.094 | .762 | .003 |
There was a significant quadratic relationship between dose and early and delayed remember responses for neutral items. A linear relationship was also seen for early remember responses for neutral items.
p<0.05
Figure 2. Delayed test results for recollection of neutral items.
(a) Significant quadratic correlation (indicated by the dashed inverted U-shape line) between the weight-adjusted dose of L-DOPA and corrected remember hits for neutral items at delayed test.
(b) Corrected remember hits on L-DOPA minus placebo for participants divided into three groups based on the amount of L-DOPA they received. Performance was significantly different between all three groups and better recollection on L-DOPA than placebo (indicated by performance above the dashed line) was seen for participants receiving the middle dose (average 2mg/kg). Bars represent mean ± 1 SEM. * two-tailed p<0.05
To further assess whether L-DOPA enhanced memory recollection for neutral scenes, we subtracted hit rates on placebo from those on L-DOPA. To visualise this memory performance difference between L-DOPA and placebo, and to quantify the dose of L-DOPA that boosted memory, we ranked subjects based on the weight-adjusted L-DOPA dose they received and divided our cohort into three groups as follows: ‘low’ dose group (n=10), who received an average of 1.7 mg/kg L-DOPA, ‘middle’ dose group (n=9), who received an average of 2.0mg/kg and the ‘high’ dose group (n=10) who received an average of 2.5mg/kg. Here we saw that for delayed recall of neutral items, memory performance in the middle dose group was higher on L-DOPA than on placebo (one-sample t-test, t(8)=2.767, p=.024) (Figure 2b). Performance in the low and high dose groups did not differ between drug or placebo (p = .083& p = .147 respectively). Performance was significantly different between all three groups (one-way ANOVA F(2,26) = 7.803, p = .002; low versus middle group, p=.007; middle versus high group p=.005). This demonstrates that within the ‘inverted U-shape’ relationship between L-DOPA and episodic memory, L-DOPA significantly enhanced memory performance within a relatively narrow dose range. We note that individual drug-minus-placebo difference scores did not show a significant quadratic correlation with L-DOPA weight-adjusted dose (F(2,26)=2.518, p=.100), presumably because the difference scores were more noisy than the behavioural scores under the drug.
In contrast, at early test performance did not differ between L-DOPA and placebo for the middle and high dose-groups and participants in the low dose group performed better on placebo than L-DOPA (one-sample t-test, two-tailed: low t(9) = −3.097, p = .013; middle t(8) = 1.356, p=.212; high t(9) = 0.429, p=.678). There was no significant between-group differences either (one-way ANOVA F(2,28) = 3.027, p=.066). Overall these results demonstrate that the beneficial effect of L-DOPA on episodic memory was more robust at delayed than early testing.
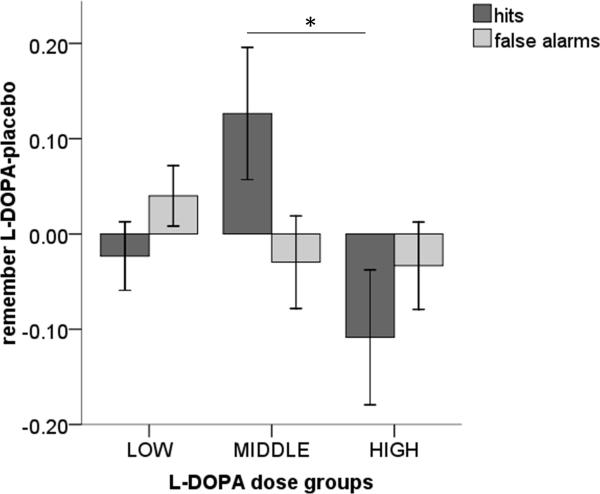
Modulation of memory performance for neutral items at delayed test by L-DOPA was due to an effect on hits rather than false alarms. This was demonstrated by a significant 3-way interaction between drug (L-DOPA/placebo), memory performance (remember hits/false alarms) and dose group (low/middle/high) (F(1,26) = 7.80, p = .002). As shown in Figure 3, post hoc tests showed a selective increase in remember hits in the middle dose group compared to the high dose group (t = 2.36, p = .031) and a trends towards increased remember hits in the middle dose group compared to the low dose group (t = 1.97, p = .065), with no difference in false alarm rates between groups.
Figure 3. Difference between L-DOPA and placebo remember hits and false alarms for neutral items at delayed test.
The behavioural enhancement of memory by L-DOPA compared to placebo in the middle dose group was due to an increase in remember hits with no change in false alarms. Bars represent mean ± 1 SEM. * p<0.05
Order effects
To assess for order effects, we performed a repeated measures ANOVA across all responses (remember plus know corrected hit rates) with drug (L-DOPA/placebo), time of test (early/delayed), reward (reward-predicting scenes/neutral scenes) as within-subjects factors, and L-DOPA dose-group (low/middle/high) and order (L-DOPA day 1/L-DOPA day 2) as between-subject factors. This showed no significant interactions with order (drug*order p = .183, drug*dose-group*order p = .975, time*order p = .248, time*dose-group*order p = .519, reward*dose*group*order p = .962, drug*time*order p = .270, drug*time*dose-group*order p = .709, drug*reward*order p = .723, drug*reward*dose-group*order p = .438, drug*time*reward*order p = .642, drug*time*reward*dose-group*order p = .585). As a follow-up, to determine if an order effect interacted with our key observation (that L-DOPA modulated corrected remember hits for neutral items), we performed a post hoc ANOVA for these responses only. This also showed no interactions with drug order (drug*time of test*dose-group*order F = 1.121, p = .343), confirming order effects were not present.
Subsequent memory effects on fMRI data
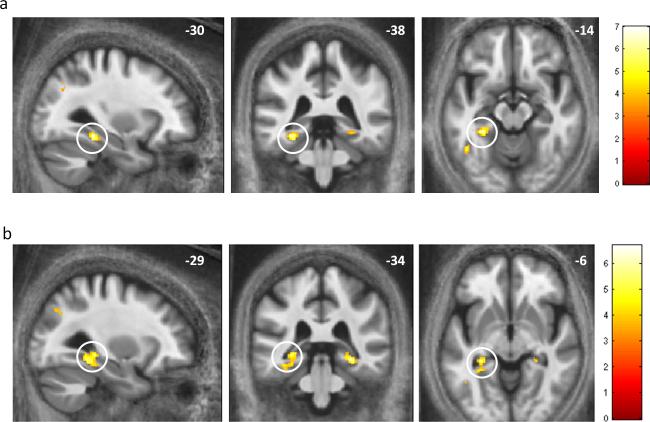
To analyse subsequent memory effects of the fMRI data acquired during encoding, we contrasted activation for items at encoding that were subsequently recognized (both remember and know responses) with items that were subsequently forgotten (classified as new during test). We collapsed over early and delayed tests and excluded participants with less than 10% of trials in a category of interest to ensure analyses were statistically robust (where participants are excluded, we report the number of subjects for that particular analysis). The contrast ‘remember and know > forget’ revealed subsequent memory activation in the left parahippocampal gyrus [Montreal Neurological Institute (MNI) space coordinates (x,y,z) −30,−38,−14; peak Z = 5.02; p<0.05 whole brain FWE] and left mid-occipital gyrus [MNI −44,−72,26; peak Z = 5.27; p<0.05 whole brain FWE] (Figure 4a). This contrast did not reveal activation in the hippocampus or SN/VTA, nor was activity seen in these regions when examining for an interaction between memory and pharmacological manipulation (results at the uncorrected threshold p<0.001 are available on request).
Figure 4. Medial temporal lobe activation for subsequent memory.
(a) Difference in activation for subsequently recognised (remember and know) versus forgotten items in the left parahippocampal gyrus (n=29)
(b) Activation in both left and right hippocampi, extending into parahippocampal gyri for remembered versus forgotten items (n=23). Displayed at the level of peak activation in the left hippocampus (circled).
Images displayed at the uncorrected threshold p<0.001 on a group-averaged magnetization transfer image.
The behavioural effect we demonstrated of L-DOPA on episodic memory (indexed by remember responses) was restricted to neutral items. This motivated a further analysis to identify areas of activation that showed a subsequent memory effect for recollection of neutral items alone (n=23 participants that fulfilled the 10% correct criteria described above). This revealed activation in a cluster extending into the left hippocampus [MNI −26,−33,−11; peak Z = 4.01, p<0.05 FWE SVC for bilateral hippocampus ROI] (Figure 5a; see Table 5 for all uncorrected results). Here there was no main effect of drug using a functional ROI approach (F(1,22) = 1.952, p = .176) (Figure 5b). For this contrast, we found an interaction between memory (remember > forget) and drug (L-DOPA > placebo) in the SN/VTA [MNI 5,−18,−18; peak Z = 4.15, p<0.01 FWE SVC for bilateral SN/VTA ROI], whereby greater activation for forgotten compared to remembered neutral scenes on placebo was reversed by L-DOPA (see Table 6 for full uncorrected results). Another cluster with a peak in the right ventricle [MNI 20,−37,9; peak Z = 5.07, p<0.05 whole brain FWE; 214 voxels], of which only a small part extended into the right hippocampus [MNI 18,−36,7; peak Z = 4.01, p<0.05 FWE SVC for bilateral hippocampus ROI; 16 voxels] will not be considered further. We did not find any regions of activation for the interaction between memory and placebo > L-DOPA.
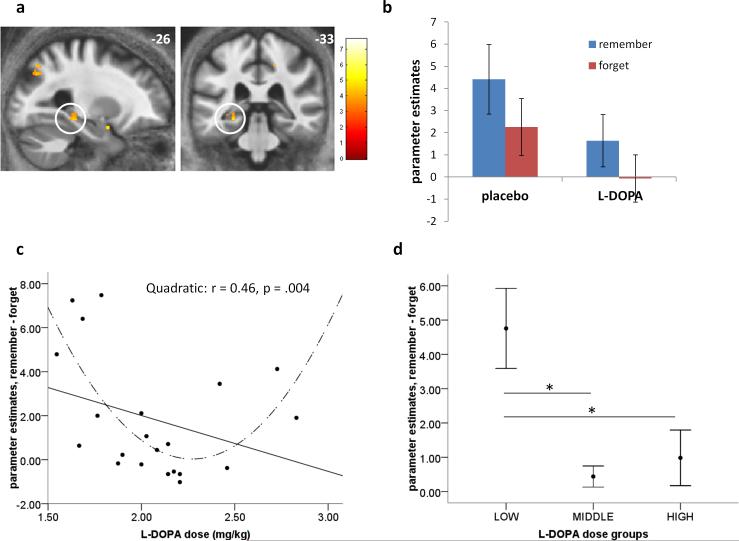
Figure 5. Dose-dependent ‘U-shaped’ modulation of hippocampal activity by L-DOPA.
Left hippocampal activity for neutral items that were remembered compared to those that were forgotten (a,b).
(c) Parameter estimates from this left hippocampal region (n=21) for remembered minus forgotten items showed a non-linear (‘U-shape’) modulation by the weight-adjusted dose of L-DOPA (dashed line), indicating no difference in encoding-related activity for subsequently remembered and forgotten items. Thus hippocampal activity at encoding did not predict an improvement in recollection seen in participants receiving the middle dose of L-DOPA (d). Bars represent mean ± 1 SEM. *p < 0.05
Table 5.
Remembered versus forgotten neutral items
| No. voxel s |
T | Z | x | y | z | L/R | region |
|---|---|---|---|---|---|---|---|
| 728 | 7.64 | 5.29 | −34.5 | −82.5 | 30 | L | mid occipital* |
| 41 | 6.03 | 4.58 | 18 | −31.5 | 48 | R | mid cingulum |
| 58 | 5.41 | 4.27 | 30 | −37.5 | −12 | R | fusiform |
| 32 | 5.31 | 4.22 | −42 | −54 | −10.5 | L | fusiform |
| 68 | 5.31 | 4.22 | 34.5 | −73.5 | 34.5 | R | mid occipital |
| 217 | 5.05 | 4.07 | −34.5 | −39 | −10.5 | L | hippocampal/ parahippocampal |
| 31 | 4.93 | 4.00 | −24 | 1.5 | −19.5 | L | amygdala |
| 64 | 4.91 | 3.99 | 13.5 | −54 | 21 | R | precuneus |
| 84 | 4.65 | 3.84 | −48 | −54 | −1.5 | L | mid temporal |
| 22 | 4.31 | 3.63 | 0 | −37.5 | −21 | cerebellar vermis | |
| 50 | 4.23 | 3.58 | −9 | −52.5 | 18 | L | precuneus |
| 13 | 4.17 | 3.54 | 24 | −60 | 45 | R | sup occipital |
| 17 | 3.98 | 3.42 | −19.5 | −60 | 49.5 | L | sup parietal |
| 12 | 3.78 | 3.28 | 30 | −79.5 | 12 | R | mid occipital |
Uncorrected results p<0.001, >10 voxels (whole brain FWE-p<0.05)
Table 6.
Interaction between memory for neutral items (remember > forgotten) and drug
| No. voxel s |
T | Z | x | y | z | L/R | region |
|---|---|---|---|---|---|---|---|
| L-DOPA > placebo | |||||||
| 214 | 7.10 | 5.07 | 19.5 | −37.5 | 9 | R | ventricle / hippocampus |
| 107 | 5.84 | 4.49 | 54 | −25.5 | 37.5 | R | supramarginal |
| 55 | 5.82 | 4.48 | 4.5 | −18 | −19.5 | R | SN/VTA |
| 135 | 5.76 | 4.45 | 6 | −60 | 48 | R | precuneus |
| 143 | 5.44 | 4.29 | −9 | −66 | 54 | L | precuneus |
| 200 | 5.44 | 4.28 | −4.5 | −55.5 | −4.5 | L | cerebellum |
| 65 | 5.43 | 4.28 | 21 | −70.5 | 55.5 | R | superior parietal |
| 89 | 5.32 | 4.22 | 9 | −7.5 | 9 | R | thalamus |
| 23 | 4.81 | 3.94 | −31.5 | 42 | 39 | L | midfrontal |
| 112 | 4.65 | 3.84 | 37.5 | −49.5 | 40.5 | R | inferior parietal |
| 26 | 4.63 | 3.83 | 19.5 | −58.5 | 69 | R | superior parietal |
| 106 | 4.56 | 3.78 | 31.5 | 43.5 | 31.5 | R | midfrontal |
| 77 | 4.44 | 3.71 | −1.5 | 28.5 | 21 | L | anterior cingulum |
| 14 | 4.44 | 3.71 | 0 | −66 | 15 | L | calcarine |
| 48 | 4.32 | 3.63 | 3 | 21 | 49.5 | L | supplementary motor area |
| 82 | 4.30 | 3.63 | −39 | 12 | 1.5 | L | insula |
| 39 | 4.28 | 3.61 | −33 | 33 | 31.5 | L | mid frontal |
| 26 | 4.15 | 3.53 | 24 | −51 | −18 | R | cerebellum |
| 21 | 4.12 | 3.51 | 0 | −70.5 | 28.5 | L | cuneus |
| 39 | 4.11 | 3.50 | −4.5 | −40.5 | 51 | L | mid cingulum |
| 13 | 4.07 | 3.47 | −1.5 | −69 | −12 | vermis | |
| 31 | 4.02 | 3.45 | −3 | −25.5 | 43.5 | L | mid cingulum |
| 14 | 3.95 | 3.40 | 15 | −79.5 | 40.5 | R | cuneus |
| 12 | 3.95 | 3.39 | 1.5 | −33 | 40.5 | R | mid cingulum |
| 11 | 3.93 | 3.38 | −7.5 | −78 | 51 | L | precuneus |
| 17 | 3.93 | 3.38 | −16.5 | −48 | −15 | L | cerebellum |
| 14 | 3.92 | 3.38 | 25.5 | 51 | 30 | R | mid frontal |
| 20 | 3.91 | 3.37 | −43.5 | −40.5 | 40.5 | L | inferior parietal |
| 15 | 3.82 | 3.31 | −12 | −13.5 | 9 | L | thalamus |
| placebo > L-DOPA | |||||||
| 15 | 5.02 | 4.06 | 15 | −31.5 | 49.5 | R | paracentral |
| 88 | 5.73 | 3.89 | −40.5 | −28.5 | 46.5 | L | postcentral |
Uncorrected results p<0.001, >10 voxels (no regions whole brain FWE-p<0.05)
Dose-dependent modulation of encoding activity
Given the dose-dependent non-linear effect of L-DOPA on episodic memory performance for neutral events, we extracted parameter estimates from the functional hippocampal cluster for subsequent memory (remember minus forgotten responses for neutral events) on L-DOPA and used this measure in a regression analysis with the weight-adjusted dose of L-DOPA as the independent variable (as used for the behavioural regression analyses).
We found that L-DOPA modulated hippocampal activation for subsequent episodic memory for neutral items in a non-linear ‘U-shape’ pattern (regression with L-DOPA dose for n=21; quadratic: F(2,18) = 7.68, p = .004, R2 = .46; linear regression: F(1,19) = 2.27, p = .152, R2 = .11) (Figure 5c). Next, we explored how these parameter estimates related to the previously denoted weight-adjusted dose groups, where we previously identified behavioural enhancement of memory by L-DOPA in those who received the middle dose. Within this cohort of 21 subjects, six participants were from the ‘low’ dose group, eight from the ‘middle’ group and seven from the ‘high’ dose group. Hippocampal parameter estimates significantly differed between the three groups (one-way between-group ANOVA: F(2,20) = 8.767, p = .002; mean difference between low and middle dose groups = 4.32, p = .003; mean difference between low and high dose groups = 3.77, p = .01). Figure 5d illustrates that whilst the low dose group showed what could be considered a ‘standard’ pattern of activation (more activity for subsequently remembered than forgotten items), both the middle and high dose groups showed no difference in encoding-related activity. Thus, we show a dose-dependent reduction of the subsequent memory effect by L-DOPA, evident in participants whose memory improved on L-DOPA (i.e. the middle dose group).
We found activation in both the left hippocampus [MNI −29,−34,−6; peak Z = 4.38, p<0.05 FWE SVC] and right hippocampus [MNI 29,−33,−11; peak Z = 4.27, p<0.05 FWE SVC] for items remembered more than forgotten when collapsing across both rewarded and neutral items (Figure 4b; uncorrected results available on request). However these parameter estimates (n = 21) were not robustly modulated by the weight-adjusted dose of L-DOPA (left hippocampus: quadratic model F=3.01, p=.074, linear model F=1.79, p=.197; right hippocampus: quadratic model F=0.071, p=.932, linear model F=0.071, p =.793), which, in keeping with our behavioural results, indicated a high degree of specificity of our neural findings for neutral items.
Discussion
We show that in healthy older adults, dopamine enhances recollection of neutral scenes in a dose-dependent inverted ‘U-shape’ pattern, where a dose of approximately 2 mg/kg bodyweight improved recollection in contrast to higher and lower doses which were ineffective. This pattern was not explained by encoding-related activity in the hippocampus, supporting a view that dopamine modulates a post-encoding consolidation process. In fact our data fit neatly with an influential model of molecular consolidation in the hippocampus, where encoding only leads to a short-lasting strengthening of synaptic connections. Dopamine-dependent protein synthesis is then necessary to stabilize and maintain these connections (Lisman et al., 2011). Our behavioural data align with these findings in our demonstration that L-DOPA in comparison to placebo impacts primarily on delayed, but not early, recollection performance.
Our neuroimaging data reveal whether the benefits of L-DOPA can be attributed to an enhancement in the hippocampal contribution to encoding. Molecular consolidation invokes effects on long-term plasticity as evident in animal models of long-term potentiation (LTP) (Frey and Morris, 1997; Smith et al., 2005; Bethus et al., 2010). There is behavioural evidence in rodents that dopamine antagonists at encoding do not impair hippocampus-dependent memories tested after short delays, but do cause an impairment after long delays (Bethus, Tse et al. 2010). According to the ‘synaptic-tagging and capture model’, the benefit of dopamine arises out of an effect on protein synthesis linked to consolidation (Frey and Morris 1997; Morris 2003). In fact, this post-encoding benefit of dopamine predicts that items which engender low levels of hippocampal activation at encoding, that may be classed as know or forgotten, should be ‘rescued’ by subsequent protein-synthesis. This ability to later remember also weakly encoded events should lead to a decrease of encoding-related hippocampal subsequent memory activation under L-DOPA. Our data show this to be the case where there is a tight dose-response relationship between L-DOPA dose, behaviour and fMRI effects. An fMRI subsequent memory effect in the hippocampus was modulated in a dose-dependent non-linear ‘U-shape’ manner, whereby it was entirely abolished under an optimal dose of L-DOPA. Note that the combination of behavioural assessment after long-retention intervals and fMRI data from the time of encoding is a key strength of our study allowing, for the first time in humans, identification of a post-encoding mechanism that accounts for improved memory recollection following L-DOPA.
Evidence from molecular imaging studies using PET link dopamine receptor density to cognitive performance, whereby dopamine binding in the striatum and hippocampus correlate with standard neuropsychological measures of immediate recall (Takahashi et al., 2007; Cervenka et al., 2008). Importantly, dopamine loss with age of both D2 receptors and dopamine transporter mediate age-related episodic memory deficits (Bäckman et al., 2000; Erixon-Lindroth et al., 2005). Such studies have used immediate recall as a measure of episodic memory, which suggests that dopamine can modulate encoding processes. Our study expands on this empirical molecular imaging evidence by using a functional measure of encoding in relation to subsequent memory tested after long retention intervals to infer dopaminergic modulation of post-encoding consolidation processes.
An inverted U-shape effect of dopamine on working memory performance, which is dependent on dopamine effects within the prefrontal cortex, is well recognised (Williams and Goldman-Rakic, 1995; Goldman-Rakic et al., 2000; Cools et al., 2001). In this model, an optimal dose of dopamine enhances function but higher doses are detrimental. Our results show the same effect of dopamine on episodic memory performance, as well as a dose-dependent modulation of hippocampal activity. We suggest that the memory improvement from the optimal dose of L-DOPA results from increased hippocampal protein synthesis. Whilst higher doses of dopamine may increase protein synthesis in the hippocampus, other mechanisms are likely to account for a lack of improvement in recollection. At a molecular level, excess dopamine can induce a long-term depression through inhibition of NMDA receptors, thereby inhibiting memory consolidation (Thirugnanasambandam et al., 2011). At a systems level, a model that explains the physiology underlying the inverted U-shape phenomenon in working memory invokes moderate amounts of dopamine enhancing excitatory inputs to pyramidal cells, with higher levels associated with greater interneuron activity leading to inhibition of pyramidal cells and thus impaired cognitive performance (Goldman-Rakic et al., 2000). It should be noted that our fMRI data rule out an enhanced excitatory input to pyramidal cells as the mode of action through which dopamine boosted late memory under optimal doses. Such a mechanism would have been associated with increased hippocampal activation for subsequently recollected events under the optimal dose of L-DOPA. Finally, although we report the subsequent memory effects of L-DOPA in the SN/VTA for completeness, we did not entertain any specific hypothesis regarding the direction of effect of L-DOPA for this contrast due to the potential complexity of the effect dopamine exerts in this region (for example, the effect of D2 autoreceptors on the firing rate of neurons as a consequence of manipulating the availability of dopamine). To determine if there is an optimal dose of L-DOPA for boosting the long-term persistence of episodic memories, future studies could combine our paradigm with a wide range of different dosages coupled with a measure of underlying dopamine reserve using molecular imaging methods (e.g. PET).
The dose-dependent effects of L-DOPA on both recollection and hippocampal activity were restricted to neutral items. SN/VTA activation in response to novelty has been previously demonstrated (Bunzeck and Duzel, 2006), suggesting that dopamine neurons are responsive to novelty even in the absence of apparent reward (Duzel et al., 2009; Krebs et al., 2009; Krebs et al., 2011) . We anticipated L-DOPA would improve memory recollection for neutral items since a novelty induced dopamine release in response to these items would be expected. This is indeed what we found.
It is a well established observation that dopamine release is increased by reward-prediction (Schultz et al., 1997). This effect of reward has been associated with improved long-term memory in younger adults, where recollection was better for reward-predicting compared to neutral items when tested after a delay of three weeks (Wittmann et al., 2005). We included a reward component in our task as a way of manipulating endogenous dopamine release, so as to compare its effect to the exogenous manipulation through administration of L-DOPA. Our hypothesis was that reward would shift the dose-response relationship between memory performance at the delayed test and L-DOPA to the left. However, we found no effect of reward on recollection at delayed test or any interaction with L-DOPA. One possible reason for this is that reward-prediction in our task failed to elicit increased phasic dopamine release. Impaired reward processing, particularly in tasks with probabilistic reward, has been reported with increasing age (Marschner et al., 2005; Schott et al., 2007; Mell et al., 2009; Eppinger et al., 2011) and therefore the effects of reward-predicting cues in our task, both alone and in combination with L-DOPA, may have been more variable. We speculate that an interesting implication of our data is that L-DOPA administration in older adults does not restitute the known effects of reward-anticipation, i.e. even under L-DOPA there is no benefit of reward on memory suggesting that a lack of dopamine cannot account for the lack of a reward-related memory enhancement of memory in old age.
We took advantage of the inter-individual variation in body weight to determine relative dose-dependent effects of L-DOPA, since the effective dose of L-DOPA is dependent on body weight (Zappia et al., 2002). The enhancement of memory in the middle dose group at delayed test on L-DOPA compared to placebo suggests that memory performance differences were due to the drug rather than body weight or other variables associated with body weight. Furthermore, health-related measures and general cognitive performance did not differ between the three body-weight dose groups and therefore were unlikely to account for differences in memory performance. However we acknowledge a limitation of this study is that other unmeasured variables associated with body weight may have influenced memory performance across participants (Volkow et al., 2012).
Since dopamine loss varies across older individuals, we cannot be certain that all participants responded to L-DOPA in a similar manner. Although we did not have a true measure of intrinsic dopamine signalling, we obtained MT values of the SN/VTA. This demonstrated inter-individual variability in the structural integrity of dopaminergic midbrain but importantly, did not relate to differences in memory performance or body-weight. Thus MT is one measure that illustrates that although there was variable integrity of the dopaminergic midbrain amongst our cohort of older adults, this did not modulate memory performance or interact with L-DOPA. Hence, we do not have a strong reason to believe that our participants showed markedly different physiological responses to L-DOPA. Molecular imaging methods such as PET would be required to characterize body-weight related responsivity to L-DOPA more fully.
The possibility of selectively influencing consolidation in humans, whilst not affecting encoding, has remained largely theoretical (with the exception of sleep-related studies) and to our knowledge our study is the first demonstration of this in conjunction with a measure of encoding activity. By combining behavioural and fMRI data with a pharmacological manipulation, we have identified a specific effect of dopamine on consolidation rather than encoding and could characterize its narrow dose-range. This effect of dopamine on consolidation depends not only on the weight-adjusted dose, but also to some extent on the underlying inter-individual variability in the structural integrity of the dopaminergic midbrain. The research we report has wider implications given that an episodic memory decline with increasing age is both common and distressing. Thus far, research into post-encoding consolidation processes in old age has been largely neglected. Our findings indicate that this may be an important area for research because by enhancing post-encoding consolidation, memory for weakly encoded events can be rescued. Hence an exogenous modification of consolidation can potentially compensate for hippocampal deficits in encoding, thereby providing a new therapeutic perspective to memory dysfunction.
Acknowledgments
RC is supported by a Wellcome Trust Research Training Fellowship [WT088286MA]. NB is supported by Hamburg state cluster of excellence. RD is supported by the Wellcome Trust [078865/Z/05/Z]. The Wellcome Trust Centre for Neuroimaging is supported by core funding from the Wellcome Trust [091593/Z/10/Z]. The authors would like to thank Laura Sasse and Jasmine Medhora for assistance with data collection, Professor Stephen Jackson for assistance with recruitment, Christian Lambert for assistance with creating anatomical masks and all participants.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare
References
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S-C, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience & Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA, Wahlin T-BR, Wahlin Å , Halldin C, Farde L. Age-Related Cognitive Deficits Mediated by Changes in the Striatal Dopamine System. Am J Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Bethus I, Tse D, Morris RGM. Dopamine and Memory: Modulation of the Persistence of Memory for Novel Hippocampal NMDA Receptor-Dependent Paired Associates. J Neurosci. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. British Journal of Medical Psychology. 1974;47:211–218. [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cervenka S, Backman L, Cselenyi Z, Halldin C, Farde L. Associations between dopamine D2-receptor binding and cognitive performance indicate functional compartmentalization of the human striatum. Neuroimage. 2008;40:1287–1295. doi: 10.1016/j.neuroimage.2007.12.063. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or Impaired Cognitive Function in Parkinson’s Disease as a Function of Dopaminergic Medication and Task Demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Düzel S, Schutze H, Stallforth S, Kaufmann J, Bodammer N, Bunzeck N, Munte TF, Lindenberger U, Heinze HJ, Duzel E. A close relationship between verbal memory and SN/VTA integrity in young and older adults. Neuropsychologia. 2008;46:3042–3052. doi: 10.1016/j.neuropsychologia.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Duzel E, Bunzeck N, Guitart-Masip M, Duzel S. NOvelty-related Motivation of Anticipation and exploration by Dopamine (NOMAD): Implications for healthy aging. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Duzel S, Schutze H, Stallforth S, Kaufmann J, Bodammer N, Bunzeck N, Munte TF, Lindenberger U, Heinze HJ, Duzel E. A close relationship between verbal memory and SN/VTA integrity in young and older adults. Neuropsychologia. 2008;46:3042–3052. doi: 10.1016/j.neuropsychologia.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Eckert T, Sailer M, Kaufmann J, Schrader C, Peschel T, Bodammer N. Differentiation of idiopathic Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage. 2004;21:229–235. doi: 10.1016/j.neuroimage.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Hämmerer D, Li S-C. Neuromodulation of reward-based learning and decision making in human aging. Annals of the New York Academy of Sciences. 2011;1235:1–17. doi: 10.1111/j.1749-6632.2011.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erixon-Lindroth N, Farde L, Robins Wahlin T-B, Sovago J, Halldin C, Bäckman L. The role of the striatal dopamine transporter in cognitive aging. Psychiatry Research: Neuroimaging. 2005;138:1–12. doi: 10.1016/j.pscychresns.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s Disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically Parcellating the Human Cerebral Cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly IEC, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Research Reviews. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Helms G, Dathe H, Dechent P. Quantitative FLASH MRI at 3T using a rational approximation of the Ernst equation. Magnetic Resonance in Medicine. 2008a;59:667–672. doi: 10.1002/mrm.21542. [DOI] [PubMed] [Google Scholar]
- Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magnetic Resonance in Medicine. 2008b;60:1396–1407. doi: 10.1002/mrm.21732. [DOI] [PubMed] [Google Scholar]
- Helms G, Draganski B, Frackowiak R, Ashburner J, Weiskopf N. Improved segmentation of deep brain grey matter structures using magnetization transfer (MT) parameter maps. Neuroimage. 2009;47:194–198. doi: 10.1016/j.neuroimage.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Breitenstein C, Bushuven S, Wailke S, Kamping S, Flöel A, Zwitserlood P, Ringelstein EB. Levodopa: Faster and better word learning in normal humans. Annals of Neurology. 2004;56:20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Duzel E. Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol Psychiatry. 2009;65:103–110. doi: 10.1016/j.biopsych.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Heipertz D, Schuetze H, Duzel E. Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: Evidence from high-resolution fMRI. Neuroimage. 2011;58:647–655. doi: 10.1016/j.neuroimage.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Li S-C, Sikström S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neuroscience & Biobehavioral Reviews. 2002;26:795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and Aging: Four Hypotheses in Search of Data. Annual Review of Psychology. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends in Neurosciences. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The Hippocampal-VTA Loop: Controlling the Entry of Information into Long-Term Memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lutti A, Hutton C, Finsterbusch J, Helms G, Weiskopf N. Optimization and validation of methods for mapping of the radiofrequency transmit field at 3T. Magnetic Resonance in Medicine. 2010;64:229–238. doi: 10.1002/mrm.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR. Reward-based decision-making and aging. Brain Research Bulletin. 2005;67:382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR. Altered function of ventral striatum during reward-based decision making in old age. Frontiers in Human Neuroscience. 2009;3 doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RGM. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learning & Memory. 2006;13:760–769. doi: 10.1101/lm.321006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randy LB. Memory and Executive Function in Aging and AD: Multiple Factors that Cause Decline and Reserve Factors that Compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rorden CBM. Stereotaxic display of brain lesions. Behavioral Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, Duzel E. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130:2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic Stimulation of Local Protein Synthesis Enhances Surface Expression of GluR1 and Synaptic Transmission in Hippocampal Neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, N. T. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Hayashi M, Okubo Y, Takano A, Ito H, Suhara T. Memory and frontal lobe functions; possible relations with dopamine D2 receptors in the hippocampus. Neuroimage. 2007;34:1643–1649. doi: 10.1016/j.neuroimage.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Takano H, Arakawa R, Okumura M, Otsuka T, Kodaka F, Hayashi M, Okubo Y, Ito H, Suhara T. Differential Contributions of Prefrontal and Hippocampal Dopamine D1 and D2 Receptors in Human Cognitive Functions. The Journal of Neuroscience. 2008;28:12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Grundey J, Paulus W, Nitsche MA. Dose-Dependent Nonlinear Effect of l-DOPA on Paired Associative Stimulation-Induced Neuroplasticity in Humans. The Journal of Neuroscience. 2011;31:5294–5299. doi: 10.1523/JNEUROSCI.6258-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and Drug Reward: Overlapping Circuits in Human Obesity and Addiction. Current Topics in Behavioral Neurosciences. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Morris RGM. Hippocampal-Neocortical Interactions in Memory Formation, Consolidation, and Reconsolidation. Annual Review of Psychology. 2010;61:49–79. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: A whole-brain analysis at 3 T and 1.5 T. Neuroimage. 2006;33:493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine Dl receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magnetic Resonance in Medicine. 1989;10:135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Quamme JR, Lazzara MM, Sauve M-J, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Zappia M, Crescibene L, Arabia G, Nicoletti G, Bagala A, Bastone L, Caracciolo M, Bonavita S, Di Costanzo A, Scornaienchi M, Gambardella A, Quattrone A. Body Weight Influences Pharmacokinetics of Levodopa in Parkinson’s Disease. Clinical Neuropharmacology. 2002 Mar-Apr;25:79–82. doi: 10.1097/00002826-200203000-00004. [DOI] [PubMed] [Google Scholar]