The Discoidin Domain Receptors (DDRs) are a unique class of receptor tyrosine kinases (RTKs) that bind to and are activated by collagen rather than soluble growth factors 1. Upon engagement with collagen, the receptor displays delayed and sustained tyrosine phosphorylation leading to the propagation of downstream signaling networks. DDR2 is one of two members of this class of RTKs that is commonly expressed in cells of mesenchymal origin and is activated by fibrillar collagens and collagen X 1, 2. DDR2 has been shown to play a role in cell invasion and collagen remodeling through the regulation of matrix metalloproteases and collagen fibrillogenesis 3-7. While much work has been done to elucidate the extracellular collagen binding properties of DDR2, there is very limited information about the intracellular interaction partners and signaling pathways activated by DDR2.
Crosstalk between RTKs mediate a large number of processes in human health and disease 8. This process is also critical for maintaining signal robustness in response to exogenous perturbations 9. The signaling pathways downstream of RTK crosstalk events are poorly characterized and in particular, the specific proteins where signal integration between RTKs occurs are largely unknown. Using HEK293 cells as a model system, a previous study has shed light on the molecular interactions between the insulin and epidermal growth factor (EGF) signaling networks and how these growth factor ligands act together to amplify mitogenic signaling 10.
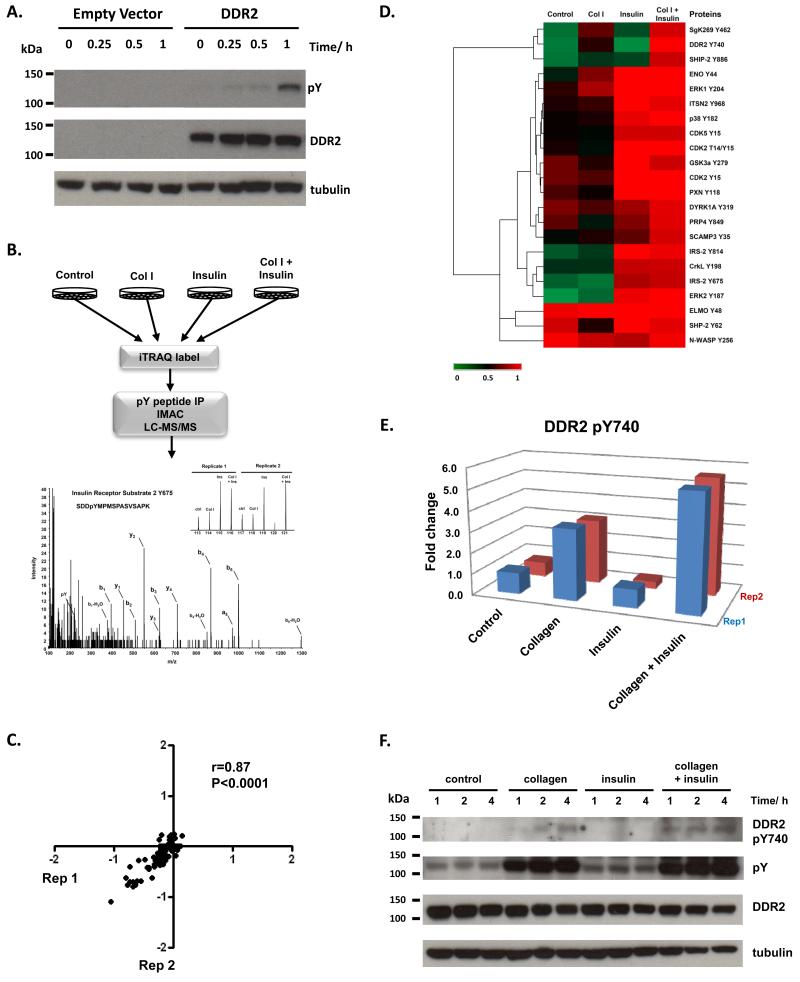
Vogel et al. has shown that DDR1 signals independently of the epidermal growth factor receptor (EGFR) and stimulation of cells with EGF does not induce DDR1 activation 11. In this study, we sought to determine if signaling crosstalk occurs between DDR2 and the insulin receptor (IR) by performing a phosphoproteomic survey of the signaling networks activated in cells co-stimulated with collagen I and insulin. HEK293 cells have previously been shown to endogenously express 9000 copies of the insulin receptor 12. HEK293-DDR2 cells were engineered as described in the methods and upon presentation with collagen I, showed robust receptor tyrosine phosphorylation at 1 hour (Figure 1A). These cells were serum starved for 16 hours prior to stimulation with 20μg/ml of acid-soluble collagen I and/or 150nM of insulin for 1 hour (Figure 1B). This time-point was chosen to maximize the crosstalk between the early activation of insulin signaling (minutes) and the delayed activation kinetics of DDR2 (hours) 13-15. As a control, HEK293-DDR2 cells were acid treated for 1 hour. Cells were lysed and subjected to stable isotope labeling with the 8-plex iTRAQ reagent before the tyrosine-phosphorylated peptides were immunoprecipitated with pan-specific anti-phosphotyrosine antibodies (See supplemental methods for details). The phosphotyrosine containing peptides were subjected to further enrichment using immobilized metal affinity chromatography (IMAC) prior to liquid chromatography tandem mass spectrometry (LC/MS/MS) analysis. In total, the profiles of 22 tyrosine phosphorylation sites across two biological replicates were generated (Supplemental table S1). Analysis of the phosphoproteomic data shows that there is good reproducibility between the two biological replicates with a Pearson correlation coefficient of 0.87 (Figure 1C).
Figure 1.
(A) Immunoblot of DDR2 activation from 0-60 minutes after stimulation with 20μg/ml of collagen I. HEK293 control cells do not endogenously express DDR2. Cells engineered to express DDR2 display robust tyrosine phosphorylation upon exposure to collagen I. (B) Schematic of phosphoproteomic experimental strategy. HEK293-DDR2 cells were stimulated with acid (control), collagen I or/and insulin. Cells were then lysed, proteins digested and the resultant peptides were labeled with iTRAQ 8-plex isobaric reagents. Labeled peptides were subjected to phosphotyrosine immunoprecipitation and IMAC enrichment prior to LC/MS/MS. Phosphopeptides were sequenced and quantified as described in the methods. Mass spectrum of IRS2 Y675 highlights the peak areas for the iTRAQ 8-plex marker ions which enable quantification of phosphorylation levels for each condition. Experiments were performed in two biological replicates. (C) Scatterplot to illustrate the reproducibility of biological replicate experiments with log10 iTRAQ ratios for the phosphoproteomic data. (D) Hierarchical clustering analysis of phosphoproteomic data comprising of 22 phosphorylation sites by Euclidean distance. Each column represents the relative phosphorylation levels in each of the four stimulation conditions (average of two biological replicates) normalized to the co-stimulation of collagen I and insulin. (E) Plot of the two biological replicates of DDR2 Y740 upon stimulation with collagen I or/and insulin. Measurements are expressed relative to the acid control. (F) Immunoblot of DDR2 phosphorylation in HEK293-DDR2 cells across 1 to 4 hours after stimulation with the indicated ligands show that phosphorylation of DDR2 Y740 and total receptor tyrosine phosphorylation levels (4G10 antibody) are increased when co-stimulated with collagen and insulin compared to collagen I alone.
To visualize the phosphotyrosine signaling networks modulated by the two stimuli, we subjected the data to hierarchical clustering analysis (Figure 1D). As expected, we observed a 3-fold increase in DDR2 phosphorylation at Y740 upon collagen stimulation. This is a site located on the DDR2 activation loop and is required for full activation of the receptor 16. Similarly, upon stimulation with insulin, we find a 3 to 5-fold increase in two sites (Y675 and Y814) on insulin receptor substrate 2 (IRS2), a well-characterized downstream substrate of the insulin receptor 17. The clustering analysis reveals a cluster of phosphorylation sites that are responsive to collagen I treatment but showed an enhancement in tyrosine phosphorylation upon co-treatment with insulin. Importantly, DDR2 Y740 was phosphorylated 5-fold when co-stimulated with collagen and insulin, compared to just 3-fold upon collagen treatment alone (figure 1E). This result indicates that the insulin signaling pathway promotes collagen I-mediated DDR2 phosphorylation at Y740. This enhanced phosphorylation required both collagen and insulin since insulin treatment alone was unable to induce DDR2 phosphorylation. To further validate this finding, we stimulated HEK293-DDR2 cells with collagen I and/or insulin for 1 to 4 hours and immunoblotted with a phospho-specific antibody directed against the activation loop phosphorylation site Y740 and the 4G10 pan-specific tyrosine phosphorylation antibody (Figure 1F). The immunoblot confirms our proteomic results and shows increased DDR2 Y740 and total receptor tyrosine phosphorylation at 1 hour with collagen I and insulin costimulation compared to collagen I stimulation alone. Interestingly, we find that this increased receptor phosphorylation is sustained from 1 to 4 hours, which suggests that insulin stimulation may promote DDR2 phosphorylation in a persistent manner.
Using quantitative phosphoproteomics, we show that consistent with previous reports of EGF and DDR1 11, DDR2 receptor phosphorylation is not induced by the addition of insulin alone (Figure 1E). However, upon co-stimulation with Collagen I, insulin has the capacity to enhance DDR2 tyrosine phosphorylation in its activation loop. Insulin and collagen signaling both have functional roles in cell growth and differentiation. DDR2 knockout mice exhibit dwarfism as a result of reduced chondrocyte proliferation 18. DDR2 is also critical for osteoblastic differentiation and genetic silencing of this receptor prevents differentiation in both in vitro and ex vivo models 19. Similarly, insulin signaling promotes chondrocyte and osteoblast proliferation and differentiation 20. Understanding how these two stimuli interact to modulate cellular responses will be important for developing targeted approaches to tackling diseases such as osteoarthritis. Interestingly, our clustering analysis also reveals that SHIP-2, an inositol phosphatase that regulates cell migration and adhesion 21, 22, displays the same phosphorylation profile as DDR2 Y740 (Figure 1D). A recent protein interactome analysis of DDR1 binders demonstrated that SHIP-2 binds with high affinity to the receptor 23. Similarly, SgK269 (also known as PEAK1), a cytoskeletal-associated kinase which controls cell spreading and migration clusters together with DDR2 phosphorylation 24. Taken together, our data suggests that SHIP-2 and SgK269 may be candidate substrates downstream of DDR2 which facilitate its functional role in cell migration. The mechanism by which insulin enhances DDR2 phosphorylation is an outstanding question. This enhancement may be the result of direct crosstalk between the insulin and DDR2 receptor signaling networks. Alternatively, it is possible that insulin alters the binding affinity of DDR2 to collagen which ultimately increases DDR2 receptor activation. It is envisioned that a higher resolution temporal analysis of collagen and insulin co-stimulation combined with DDR2 and IR depletion experiments will provide further insights into this mechanism. Elucidation of the crosstalk mechanisms between these two pathways will be key to designing novel therapeutic strategies to overcome DDR2-driven diseases such as osteoarthritis and cancer.
Supplementary Material
ACKNOWLEDGEMENTS
This research is supported by the Institute of Cancer Research and the Wellcome Trust (WT089028).
Abbreviations
- DDR2
Discoidin Domain Receptor 2
- IR
Insulin Receptor
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- IMAC
immobilized metal affinity chromatography
- RTK
receptor tyrosine kinase
REFERENCES
- 1.Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18:1108–16. doi: 10.1016/j.cellsig.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006;25:355–64. doi: 10.1016/j.matbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–78. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–55. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 6.Blissett AR, Garbellini D, Calomeni EP, Mihai C, Elton TS, Agarwal G. Regulation of collagen fibrillogenesis by cell-surface expression of kinase dead DDR2. J Mol Biol. 2009;385:902–11. doi: 10.1016/j.jmb.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihai C, Iscru DF, Druhan LJ, Elton TS, Agarwal G. Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1. J Mol Biol. 2006;361:864–76. doi: 10.1016/j.jmb.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 8.Xu AM, Huang PH. Receptor tyrosine kinase coactivation networks in cancer. Cancer Res. 2010;70:3857–60. doi: 10.1158/0008-5472.CAN-10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian T, Olson S, Whitacre JM, Harding A. The origins of cancer robustness and evolvability. Integr Biol (Camb) 3:17–30. doi: 10.1039/c0ib00046a. [DOI] [PubMed] [Google Scholar]
- 10.Borisov N, Aksamitiene E, Kiyatkin A, Legewie S, Berkhout J, Maiwald T, Kaimachnikov NP, Timmer J, Hoek JB, Kholodenko BN. Systems-level interactions between insulin-EGF networks amplify mitogenic signaling. Mol Syst Biol. 2009;5:256. doi: 10.1038/msb.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel W, Brakebusch C, Fassler R, Alves F, Ruggiero F, Pawson T. Discoidin domain receptor 1 is activated independently of beta(1) integrin. J Biol Chem. 2000;275:5779–84. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- 12.Beitner-Johnson D, LeRoith D. Insulin-like growth factor-I stimulates tyrosine phosphorylation of endogenous c-Crk. J Biol Chem. 1995;270:5187–90. doi: 10.1074/jbc.270.10.5187. [DOI] [PubMed] [Google Scholar]
- 13.Schmelzle K, Kane S, Gridley S, Lienhard GE, White FM. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes. 2006;55:2171–9. doi: 10.2337/db06-0148. [DOI] [PubMed] [Google Scholar]
- 14.Kruger M, Kratchmarova I, Blagoev B, Tseng YH, Kahn CR, Mann M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci U S A. 2008;105:2451–6. doi: 10.1073/pnas.0711713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang K, Kim JH, Kim HJ, Park IS, Kim IY, Yang BS. Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation. J Biol Chem. 2005;280:39058–66. doi: 10.1074/jbc.M506921200. [DOI] [PubMed] [Google Scholar]
- 17.Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MG, Jr., Glasheen E, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377:173–7. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 18.Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, Bruckner K, Goergen JL, Lemke G, Yancopoulos G, Angel P, Martinez C, Klein R. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2:446–52. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin KL, Chou CH, Hsieh SC, Hwa SY, Lee MT, Wang FF. Transcriptional upregulation of DDR2 by ATF4 facilitates osteoblastic differentiation through p38 MAPK-mediated Runx2 activation. J Bone Miner Res. 2010;25:2489–503. doi: 10.1002/jbmr.159. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Zhang X, Wang W, Liu J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem Funct. 2010;28:334–41. doi: 10.1002/cbf.1668. [DOI] [PubMed] [Google Scholar]
- 21.Kato K, Yazawa T, Taki K, Mori K, Wang S, Nishioka T, Hamaguchi T, Itoh T, Takenawa T, Kataoka C, Matsuura Y, Amano M, Murohara T, Kaibuchi K. The inositol 5-phosphatase SHIP2 is an effector of RhoA and is involved in cell polarity and migration. Mol Biol Cell. 2012;23:2593–604. doi: 10.1091/mbc.E11-11-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad N, Topping RS, Decker SJ. SH2-containing inositol 5′-phosphatase SHIP2 associates with the p130(Cas) adapter protein and regulates cellular adhesion and spreading. Mol Cell Biol. 2001;21:1416–28. doi: 10.1128/MCB.21.4.1416-1428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemeer S, Bluwstein A, Wu Z, Leberfinger J, Muller K, Kramer K, Kuster B. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. J Proteomics. 2012;75:3465–77. doi: 10.1016/j.jprot.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Kelber JA, Klemke RL. PEAK1, a novel kinase target in the fight against cancer. Oncotarget. 2010;1:219–23. doi: 10.18632/oncotarget.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.