Abstract
Adolescence is a critical period of brain development during which maturation of areas involved in cognitive functioning, such as the medial prefrontal cortex (mPFC), is still ongoing. Tobacco smoking during this age can compromise the normal course of prefrontal development and lead to cognitive impairments in later life. Recently, we reported that nicotine exposure during adolescence results in a short-term increase and lasting reduction in synaptic mGluR2 levels in the rat mPFC, causing attention deficits during adulthood. It is unknown how changed synaptic mGluR2 levels after adolescent nicotine exposure affect the ability of mPFC synapses to undergo long-term synaptic plasticity. Here, we addressed this question. To model nicotine exposure, adolescent (P34–P43) or adult (P60–P69) rats were treated with nicotine injections three times per day for 10 d. We found that, both during acute activation of nicotinic receptors in the adolescent mPFC as well as immediately following nicotine treatment during adolescence, long-term plasticity in response to timed presynaptic and postsynaptic activity (tLTP) was strongly reduced. In contrast, in the mPFC of adult rats 5 weeks after they received nicotine treatment during adolescence, but not during adulthood, tLTP was increased. Short-and long-term adaptation of mPFC synaptic plasticity after adolescent nicotine exposure could be explained by changed mGluR2 signaling. Blocking mGluR2s augmented tLTP, whereas activating mGluR2s reduced tLTP. Our findings suggest neuronal mechanisms by which exposure to nicotine during adolescence alters the rules for spike timing-dependent plasticity in prefrontal networks that may explain the observed deficits in cognitive performance in later life.
Introduction
Novelty-seeking and risk-taking behavior during adolescence has been linked to late development of brain areas involved in cognitive control, such as the medial prefrontal cortex (mPFC) (Gogtay et al., 2004; Casey et al., 2005). Adolescence also marks a period of increased vulnerability to initiation and subsequent abuse of drugs, including tobacco smoking (Chassin et al., 2000). Indeed, 70% of adolescents report experimenting with smoking cigarettes (Escobedo et al., 1993; Currie et al., 2008). This early exposure of prefrontal brain areas to nicotine can compromise normal development and have long-lasting consequences for cognitive performance. In adolescents, chronic smoking is associated with disturbances in working memory and attention as well as reduced PFC activation (Jacobsen et al., 2005, 2007; Musso et al., 2007). In rats, adolescent nicotine treatment results in a lasting impairment in attention and enhanced impulsive behavior during adulthood, 5 weeks after exposure (Counotte et al., 2009).
The acute effects of nicotine on cognition may be explained by the action of nicotine on excitatory synapses in the PFC (Counotte et al., 2011). Activation of nicotinic acetylcholine receptors (nAChRs) in the PFC modulates excitatory, inhibitory GABAergic and dopaminergic transmission (Gioanni et al., 1999; Lambe et al., 2003; Couey et al., 2007; Livingstone et al., 2009). Thereby it interferes with endogenous acetylcholinergic signaling in the PFC, which is crucial for attention (Parikh and Sarter, 2008). Exposure to nicotine during adolescence causes lasting adaptations in the PFC network, secondary to nAChR activation, which persist in later life. These molecular adaptations involve a short-term upregulation, followed by a long-term downregulation of the inhibitory metabotropic glutamate receptor 2 (mGluR2) (Counotte et al., 2011). Restoring mGluR2 activity in vivo by intra-PFC infusion of mGluR2 agonist in adult rats that received nicotine as adolescents rescues attention disturbances (Counotte et al., 2011). Although these molecular changes caused by nicotine underlie the consequent cognitive deficits at later age, it is still unclear how complex information processing, such as activity-dependent plasticity in PFC, is affected as a result of nicotine exposure during adolescence.
The ability of synapses to change their strength in response to timed presynaptic and postsynaptic activity called spike timing-dependent plasticity (STDP) is thought to play an important role in cortical development and underlie some forms of learning (Feldman et al., 1999; Feldman and Brecht, 2005; Letzkus et al., 2007). In juvenile mice, acute nicotine application can change the rules for plasticity at excitatory synapses (Mansvelder and McGehee, 2000; Couey et al., 2007) and thereby affect prefrontal network development and function. Metabotropic GluR2 receptors modulate excitatory transmission by inhibiting glutamate release and were also implicated in plasticity (Yokoi et al., 1996; Nicholls et al., 2006; Bellone et al., 2008). Thus, changes in synaptic levels of mGluR2s as observed after nicotine exposure during adolescence may affect long-term plasticity, but that has not been tested. Here, we address this issue and find that long-term effects of nicotine exposure during adolescence on STDP are opposite to the acute and short-term effects of nicotine exposure during adolescence.
Materials and Methods
Animals
Timed-pregnant female Wistar rats arrived at 5 d of gestation. At postnatal day 21 (P21), litters were weaned and housed four per cage. Only males were used in these experiments. For a small number of control experiments, male Wistar rats were obtained directly from the breeder (Harlan or Charles River). All experiments were approved by the animal ethical committee of Vrije Universiteit (Amsterdam, The Netherlands). In total, 47 saline-treated rats, 40 nicotine-treated rats, and 7 control untreated rats were killed. The numbers of rats per experimental group are reported in the figure legends. To avoid litter effects, pups from the same mother were always divided between the experimental groups.
Nicotine exposure
To test the long-term effects of nicotine, a rat model of nicotine exposure during adolescence was used (see Figs. 2a, 3a, 4a). Rats were injected subcutaneously with either nicotine [0.4 mg/kg; calculated as a base (–) nicotine hydrogen tartrate salt; Sigma-Aldrich] or saline three times a day (at 10:00 A.M., 1:00 P.M., and 4:00 P.M.) for 10 d. Nicotine/saline injections were administered during adolescence (P34–P43; see Fig. 2a) and for the control group of adult nicotine exposure during adulthood (P60–P69; see Fig. 4a). After the 10 d treatment, the injections stopped, and either 1–4 d or 5 weeks later the electrophysiological experiments were performed.
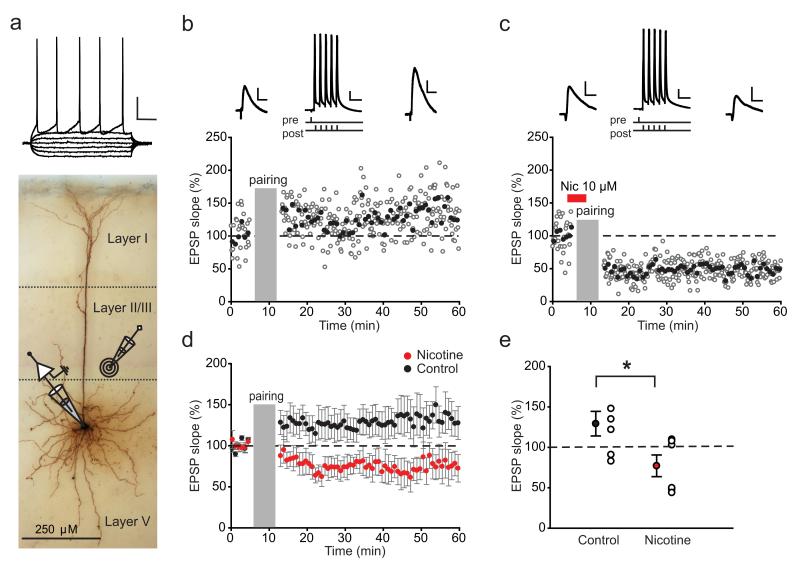
Figure 1.
Nicotine bath application to PFC slices of adolescent rats strongly reduces tLTP. a, Biocytin-filled layer V pyramidal cell in rat PFC; the position of the recording and stimulating electrodes is schematically shown. Membrane potential in response to current injections is shown above. Calibration: 20 mV, 100 ms. Examples of STDP in a control cell (b), showing LTP, and in a cell in which nicotine was bath applied (c), showing LTD. Here and further, the open circles represent individual EPSP slopes; the filled circles show an average of five EPSPs. Duration of nicotine application is indicated by the red bar; the insets above show example EPSP traces (calibration here and further: 2 mV, 20 ms) and the induction protocol with an AP trace (calibration here and further: 20 mV, 20 ms). d, Average STDP in control cells (black circles; n=7 cells from 4 rats) and cells in which nicotine was bath applied (red circles; n = 6 cells from 3 rats); data points represent bins of five EPSPs. e, Summary of STDP results shown in d; the filled circles represent average EPSP slopes within a 10 min window 35–45 min after pairing; the open circles show individual experiments. *p < 0.05. Data are mean ± SEM.
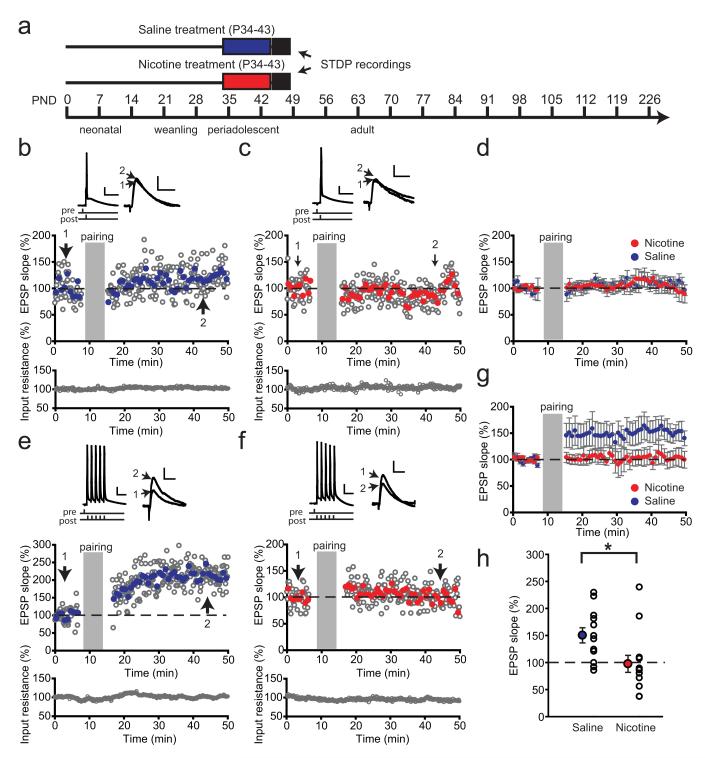
Figure 2.
Nicotine exposure during adolescence leads to a short-term decrease in tLTP. a, Schematic representation of the experimental setup. Rats were treated with nicotine (red bar)/saline (blue bar) during adolescence, and the STDP recordings were performed within 1–4 d after treatment. b–d, STDP induction using pairing of a single AP to EPSP with 5 ms timing interval did not result in LTP in PFC layer V pyramidal neurons from rats after saline/nicotine treatment during adolescence. b, c, Example experiments recorded from rats with saline (b) or nicotine (c) during adolescence. The gray open circles represent individual EPSP slopes; blue (nicotine)- and red (saline)-filled circles show an average of five EPSPs. Input resistance was monitored during the experiments and stayed stable (bottom panel). Pairing protocol with an AP trace (calibration: 20 mV, 20 ms) and example EPSP traces before (1) and after pairing (2) are shown above (calibration: 2 mV, 20ms). d, Average STDP in saline-treated rats (blue circles; n = 9 cells from 4 rats) and nicotine-treated rats (red circles; n = 9 cells from 3 rats); data represent bins of five EPSPs. e–h, STDP induction by pairing a burst of five APs to EPSPs led to LTP in control rats but failed to induce plasticity in rats treated with nicotine during adolescence. e, f, Example experiments recorded from an animal treated with saline (e) and nicotine (f); symbols, traces, and calibration are as in b and c. g, Average STDP in saline-treated rats (blue circles; n = 12 cells from 3 rats) and nicotine-treated rats (red circles; n = 13 cells from 3 rats); data represent bins of five EPSPs. h, Summary of STDP results shown in g. The filled circles represent average EPSP slopes within a 10 min window 40 min after pairing; the open circles show individual experiments.*p<0.05. Data are mean ± SEM.
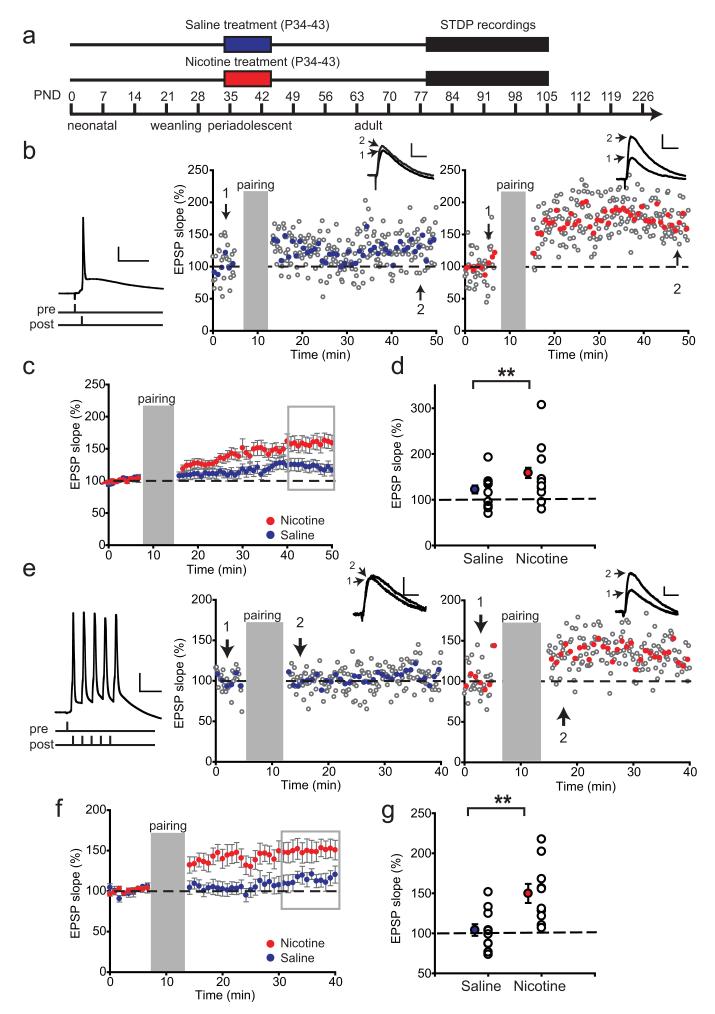
Figure 3.
Nicotine exposure during adolescence leads to a lasting increase in LTP in adult rats. a, Schematic representation of the experimental setup. Rats were treated with nicotine (red bar)/saline (blue bar) during adolescence, and the electrophysiological recordings took place starting 5 weeks after treatment. b, Example experiments showing STDP recorded from rats 5 weeks after treatment with saline (left panel) or nicotine (right panel) during adolescence using an induction protocol with single AP pairings. Induction protocol is shown left. The insets show example EPSP traces before (1) and after pairing (2); symbols and calibration are as in Figure 2. c, Average STDP in saline-treated rats (blue circles; n = 23 cells from 11 rats) and nicotine-treated rats (red circles; n = 22 from 10 rats). d, Summary of STDP results shown in c. The filled circles represent average EPSP slopes of last 10 min of the recordings (shown in gray rectangle); the open circles show individual experiments. **p < 0.01. Data are mean ± SEM. e, Example experiments showing STDP recorded from rats 5 weeks after treatment with saline (left panel) or nicotine (right panel) during adolescence using an induction protocol with a burst of five APs (left). f, Average STDP in saline-treated rats (blue circles; n = 11 cells from 5 rats) and nicotine-treated rats (red circles; n = 10 cells from 5 rats). g, Summary of STDP results shown in f. The filled circles represent average EPSP slopes of last 10 min of the recordings (gray rectangle); the open circles show individual experiments. **p < 0.01. Data are mean ± SEM.
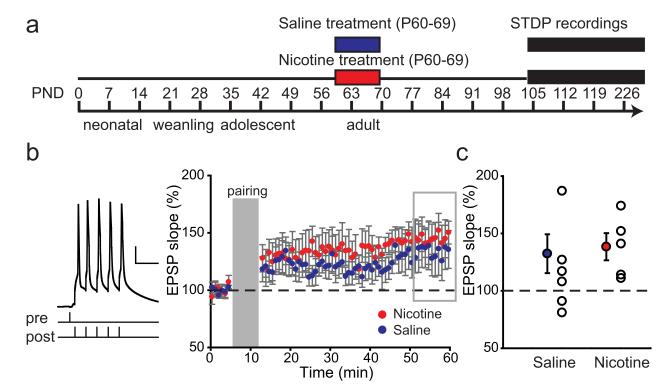
Figure 4.
Nicotine exposure during adulthood does not lead to lasting changes in LTP. a, Schematic representation of the experimental setup. Rats were treated with nicotine (red bar)/saline (blue bar) during adulthood, and the electrophysiological recordings took place starting 5 weeks after treatment. b, Average STDP recorded in PFC layer V pyramidal neurons from saline-treated rats (blue circles; n = 8 cells from 6 rats) and nicotine-treated rats (red circles; n = 5 cells from 5 rats) recorded 5 weeks after treatment. For pairing, a burst of five APs was used (example trace and pairing protocol are shown left; calibration and symbols are as in Fig.2). c, Summary of STDP results shown in b. The filled circles represent average EPSP slopes of last 10 min of the recordings (gray rectangle); the open circles show individual experiments.
Slice preparation
Either 1–4 d (P44–P48) or 5 weeks after nicotine or saline exposure (starting at P78 for rats exposed during adolescence and at P104 for rats exposed during adulthood), rats were decapitated and their brains were rapidly removed. Coronal mPFC slices of 300 μm thickness were prepared in ice-cold artificial CSF (ACSF) consisting of 125 mm NaCl, 3 mm KCl, 1.2 mm NaH2PO4, 7 mm MgSO4, 0.5 mm CaCl2, 26 mm NaHCO3, and 10 mm d-glucose. Slices were transferred to holding chambers with ACSF containing 125 mm NaCl, 3 mm KCl, 1.2 mm Na2PO4, 1 mm MgSO4, 2 mm CaCl2, 26 mm NaHCO3, and 10 mm glucose, bubbled with carbogen gas (95% O2 and 5% CO2).
Electrophysiology
Whole-cell patch-clamp recordings were made from layer V pyramidal cells in prelimbic area of mPFC under visual guidance by differential interference contrast microscopy. Slices were kept in a submerged recording chamber at 32–35°C. Pipettes were made from standard borosilicate capillary glass tubing with resistance between 2.5 and 4 MΩ resistance and filled with intracellular solution containing the following: 110 mm K-gluconate, 10 mm KCl, 10 mm HEPES, 10 mm K2-phosphocreatine, 4 mm ATP-Mg, 0.4 mm GTP, 5 mg/ml biocytin, pH adjusted with KOH to 7.3, 290–300 mOsm. Series resistance was monitored but not compensated for. Pyramidal cells were identified based on their morphology and spiking profile. Cells were filled with biocytin (0.5%) and then processed for post hoc cell identification. All experiments were performed in the absence of blockers of GABAergic synaptic transmission. Recordings were made using Multiclamp 700B amplifier (Molecular Devices) sampling at 10 kHz, filtered at 3 kHz, and later analyzed off-line using custom-written MATLAB scripts (MathWorks). Whole-cell current injections and extracellular stimulation (both timing and levels) were controlled with a Master-8 stimulator (A.M.P.I.) triggered by the data acquisition software.
Spike timing-dependent plasticity
EPSPs were evoked every 10 s using an extracellular stimulation electrode positioned at ~200 μm along the somatodendritic axis of the cell (see Fig. 1a). During the pairing period, presynaptic extracellular stimulations were paired to a single postsynaptic action potential (AP) (50 times at 0.1 Hz) or a burst of five action potentials at 100 Hz (50 times at 0.1 Hz). During the induction protocol pairing intervals of 5 ms were used (pre-before-post), timed from the onset of the evoked EPSP to the onset of the action potential. The slope of the initial 2 ms of the EPSP was analyzed to ensure that the data reflected only the monosynaptic component of each EPSP (Couey et al., 2007). Synaptic gain was measured as the percentage change in EPSP slope: the average EPSP slope during 10 min in the period 35–45 min (where a different time window was used, it is specified in the figure legends) after pairing compared with the average baseline EPSP slope. Mean baseline EPSP slopes were averaged from at least 30 sweeps. Cell input resistance was monitored by applying a –100 pA, 200–500 ms hyperpolarizing pulse at the end of each sweep. Cells in which input resistance change during the experiment was >30% were discarded from the analysis. No blockers of GABAergic transmission were used during the STDP recordings.
Pharmacology
For experiments in adolescent mPFC (see Fig. 1), nicotine (Sigma-Aldrich; 10 μm) was bath applied for 5 min (2 min prior and 3 min during pairing). To measure mGluR2-dependent inhibition (see Fig. 5), EPSCs were recorded in ACSF containing gabazine (1 μm). Stimulation intensity–response amplitude curves were taken for each cell before the recording. Baseline EPSCs were recorded for 10 min; mGluR2-specific agonist (1S,2R,5R,6R)-2-amino-4-oxabicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY379268) (Tocris; 5 μm) was bath applied for 7 min (the last 10 EPSCs were averaged for each cell and used as estimation of mGluR2 effect on EPSC amplitude) and then washed out for 25 min. For estimation of mGluR2 involvement in STDP after nicotine/saline exposure during adolescence, mGluR2 agonist LY379268 (Tocris; 1 μm) or antagonist (RS)-α-methyl-4-phosphonophenylglycine (MPPG) (Tocris; 100 μm) were bath applied 5 min prior and for the whole duration of the recordings.
Figure 5.
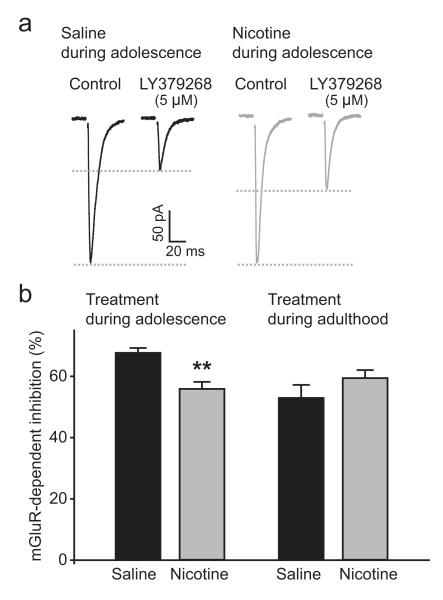
mGluR2 signaling is reduced in adult rats after nicotine exposure during adolescence. a, EPSC traces recorded in PFC layer V pyramidal neurons 5 weeks after saline (black) and nicotine (gray) treatment during adolescence. mGluR2 agonist LY379268 leads to a less robust decrease in EPSC amplitude after nicotine treatment. b, mGluR2-dependent decrease in EPSC amplitude is impaired as a result of nicotine treatment during adolescence (saline, n = 39 from 14 rats; nicotine, n = 47 from 13 rats) with no difference in rats treated as adults (saline, n = 22 from 8 rats; nicotine, n = 26 from 7 rats). **p < 0.01. Data are mean ± SEM.
Statistics
GraphPad Instat software was used to assess statistical significance (GraphPad Software). Data were first tested for deviations from Gaussian distribution using the Kolmogorov–Smirnov normality test. For normally distributed data, Student’s t tests were applied.
To determine significance in EPSP slope change for individual cells, the EPSP slopes within a 10 min window 35–45 min after pairing were compared with baseline EPSP slopes using Student’s t test. On the basis of this test, the experiments were divided into three categories: timing-dependent long-term potentiation (tLTP) (significant increase in EPSP slope), timing-dependent long-term depression (tLTD) (significant decrease in EPSP slope), and neurons with no change in plasticity (nonsignificant EPSP slope change). To assess the effects of nicotine treatment on STDP, all cells recorded within the treatment group were compared with all cell recorded from saline-treated controls (individual data points are shown in summary plots in Figs. 1–4, 6). In the experiment in which MPPG was applied in nicotine-treated rats (see Fig. 6b–d), statistical significance was assessed using the χ2 test of independence (SPSS software) to compare the proportion of cells with tLTP, tLTD, and no change in presence and absence of MPPG.
Figure 6.
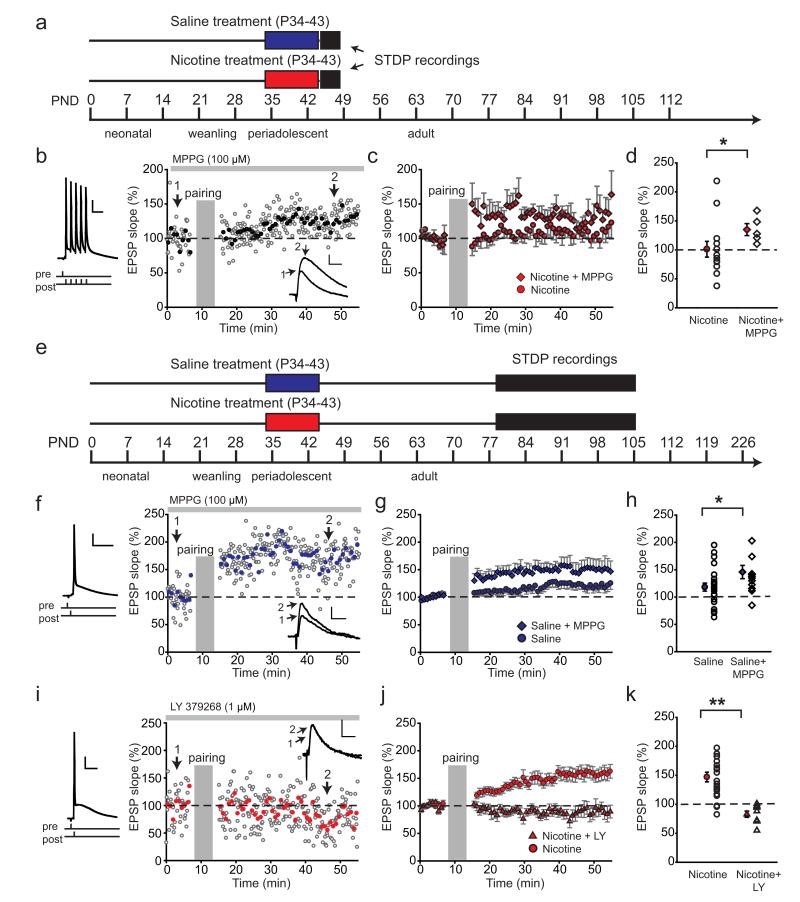
Impaired mGluR2 signaling is responsible for short- and long-term changes in LTP after nicotine exposure during adolescence. a, Schematic representation of the experimental setup for data shown in b–d. Rats were treated with nicotine (red bar)/saline (blue bar) during adolescence, and the electrophysiological recordings took place within 1–4 d after treatment. b, Example experiment showing LTP in nicotine-treated rats after blocking mGluR2 with MPPG. MPPG (100 μm) was washed in continuously during the experiment (gray bar above). Pairing protocol is shown left; the inset shows example EPSP traces before (1) and after pairing (2). The calibration and symbols are as in Figure 2. c, Blocking mGluR2 with MPPG restores LTP in nicotine-treated rats. Average STDP in nicotine-treated rats (red circles; n = 9 cells from 3 rats) and in presence of MPPG (100 μm; red diamonds; n = 5 cells from 1 animal). d, Summary of STDP results shown in c; the filled symbols represent average EPSP slopes within a 10 min window 40 min after pairing; the open circles are individual experiments in nicotine-treated rats; the open diamonds are individual experiments in nicotine-treated rats in the presence of MPPG. *p < 0.05. Data are mean ± SEM. e, Schematic representation of the experimental setup for data shown in f–k. Rats were treated with nicotine (red bar)/saline (blue bar) during adolescence, and the electrophysiological recordings took place starting 5 weeks after treatment. f, Example experiment showing LTP in saline-treated rats after blocking mGluR2 with MPPG. MPPG (100 μm) was washed 5 min before and for the duration of the experiment (gray bar above). Pairing protocol is shown left; the inset shows example EPSP traces before (1) and after pairing (2). Calibration and symbols are as in Figure 2. g, Blocking mGluR2 with MPPG leads to LTP in nicotine-treated rats. Average STDP in nicotine-treated rats (blue circles; n = 23 cells from 11 rats) and in presence of MPPG (100 μm; blue diamonds; n = 13 cells from 6 animals). h, Summary of STDP results shown in g; the filled symbols represent average EPSP slope after pairing; the open circles are individual experiments in saline-treated rats; the open diamonds are individual experiments in saline-treated rats in the presence of MPPG. *p< 0.05. Data are mean ± SEM. i, Example experiment showing failure to induce spike-timing-dependent plasticity in nicotine-treated rats after activating mGluR2 with agonist LY379268. LY379268 (1 μm) was washed 5 min before and for the duration of the experiment (gray bar above). Pairing protocol is shown left; the inset shows example EPSP traces before (1) and after (2) pairing. Calibration and symbols are as in Figure 2. j, Activating mGluR2 with LY379268 abolishes LTP in nicotine-treated rats. Average STDP in nicotine-treated rats recorded in control conditions (red circles; n = 22 from 10 rats) and in presence of LY379268 (1 μm; red triangles; n = 9 cells from 3 rats). k, Summary of STDP results shown in j; the filled symbols represent average EPSP slope after pairing. **p < 0.01. Data are mean ± SEM.
Results
Acute nicotine reduces tLTP in adolescent PFC
Both human and rodent synapses can increase their strength when presynaptic activity precedes postsynaptic spiking within a window of tens of milliseconds (Markram et al., 1997; Caporale and Dan, 2008; Testa-Silva et al., 2010). This type of plasticity, STDP, is thought to be a neural substrate of some forms of learning (Letzkus et al., 2007) and plays an important role in cortical maturation (Feldman and Brecht, 2005). In the juvenile mouse PFC, acute nicotine application increases the threshold for synaptic spike timing-dependent potentiation due to increased inhibitory transmission to pyramidal neurons (Couey et al., 2007). We asked how nicotine receptor activation affects STDP in the medial PFC of adolescent rats. To test this, we made whole-cell recordings from layer V pyramidal neurons from rats of 34–43 d of age and evoked EPSPs in the recorded cell by extracellular stimulation (Fig. 1a). After recording 5 min of EPSP baseline at 0.1 Hz, EPSPs were repeatedly paired to a burst of five postsynaptic APs (100 Hz), with 5 ms interval between presynaptic and postsynaptic stimulation (50 at 0.1 Hz), since presynaptic stimulation paired with single postsynaptic action potentials was less effective at inducing LTP at this age in hippocampus (Meredith et al., 2003). Pairing of single postsynaptic APs with EPSPs did not produce tLTP in PFC pyramidal neurons of adolescent rats (Fig. 2b–d). After pairing, EPSPs were monitored for 45 min (Fig. 1b). During the entire recording, other basic electrophysiological parameters such as resting membrane potential and input resistance did not change significantly (see Materials and Methods) (Fig. 2b,c,e,f). In control cells, this protocol resulted in the increase of synaptic strength of 130 ± 15% 35–45 min after tLTP induction. Bath application of nicotine for 5 min (2 min prior and 3 min during the pairing protocol) completely abolished tLTP (Fig. 1c– e; t(11)=2.51, p=0.028). Instead, in three of six cells, the same induction protocol resulted in long-term depression (tLTD), in one cell no significant EPSP slope change was observed, and in two cells the EPSP slope increased <10% (Fig. 1e). Thus, consistent with the results in juvenile mice (Couey et al., 2007), acute nicotine in adolescent mPFC reduces the ability of excitatory synapses to undergo tLTP.
Nicotine exposure during adolescence leads to a short-term decrease in LTP
Since nicotine receptor activation can directly affect STDP in the PFC of adolescent rats, repeated exposure to nicotine during adolescence may lead to changes in STDP rules. To test this, we induced tLTP in layer V pyramidal neurons in adolescent rats (P44–P47) within the first 4 d following the cessation of treatment (Fig. 2a). We first applied a modest induction protocol (single AP pairing; Fig. 2b–d) to ensure that any differences in LTP would not be overridden by the stronger induction protocol (five AP bursts). However, single AP pairing did not result in significant increases in EPSP slope in either of the groups (Fig. 2b– d) and no effect of treatment was observed. We next applied the more robust induction protocol using five AP bursts to cells recorded from a separate group of rats. This protocol resulted in significant tLTP in saline-treated rats (Fig. 2e,g), whereas in rats following nicotine treatment tLTD was observed in 10 of 13 cells (1 cell showed no change in EPSP slope, 2 showed significant tLTP; Fig. 2g). Thus, nicotine treatment during adolescence resulted in reduced tLTP within 4 d after treatment (Fig. 2e; t(23)=2.49, p=0.02). These results indicate that short-term effects of nicotine exposure during adolescence on STDP are similar to direct nicotine receptor stimulation in naive adolescent rats (Fig. 1).
STDP is enhanced in adult rats exposed to nicotine during adolescence
We hypothesized that repeated exposure to nicotine during adolescence would also lead to lasting adaptations in STDP rules. To test this, we induced tLTP in layer V pyramidal neurons in adult rats (>P79) that were exposed to nicotine or saline during adolescence (P34–P43; Fig. 3a). We first used the single AP protocol to induce plasticity (Fig. 3b). In contrast to adolescent rats (Fig. 1b–d), single AP pairing in adult rats resulted in modest but significant tLTP in saline-treated rats (Fig. 3c,d). Surprisingly, in nicotine-treated rats the same pairing protocol led to substantially higher potentiation compared with saline-treated controls (increase in EPSP slope in nicotine, 163 ± 10%; saline, 121 ± 9%, t(44)=2.94, p=0.005; Fig. 3c,d). These results were reproduced in a separate group of rats, in which nicotine treatment showed a similar increase in tLTP (data not shown; t(19)=2.27, p=0.03). Next, we used the stronger induction protocol of five AP bursts (Fig. 3e–g) in cells from an independent group of saline- or nicotine-treated rats. Also here tLTP was increased in adult rats treated with nicotine during adolescence with respect to saline-treated rats (Fig. 3f,g; nicotine, 150 ± 12%; saline, 104 ± 7%; t(19)=3.35, p=0.003). Remarkably, the stronger protocol did not result in more potentiation in both saline and nicotine-treated rats compared with single AP pairing (Fig. 3c,d), rather the time course of tLTP was changed. Already in the first 10 min of pairing there was significantly higher tLTP in nicotine-treated rats compared with saline controls (t(19)=2.91; p=0.009).
Thus, nicotine treatment during adolescence has lasting effects on the mechanisms of STDP and leads to augmented tLTP in adult rats. Nicotine treatment induced opposite effects on the ability of synapses to undergo plasticity depending on the time after exposure. After direct nicotine receptor activation in adolescent PFC and within 4 d after nicotine exposure, tLTP is decreased. In contrast, nicotine treatment during adolescence resulted in augmented tLTP in adult rats. Thus, nicotine exposure during adolescence leads to persistent synaptic alterations that increase tLTP.
STDP is not altered after nicotine exposure during adulthood
Adolescence is a period when PFC networks are still actively developing, which may underlie the fact that nicotine exposure at this age results in lasting changes at the cellular as well as at the behavioral level (Counotte et al., 2011). In contrast, nicotine exposure during adulthood does not cause lasting effects on PFC functioning such as impaired attention performance (Counotte et al., 2009, 2011). We tested whether the observed change in STDP rules were specific to nicotine exposure during adolescence or could also be caused by nicotine exposure during adulthood. To this end, we recorded STDP in mPFC layer 5 pyramidal cells of rats that were treated with nicotine or saline during adulthood (P60–P69), and 5 weeks later STDP recordings were performed (at >P95; Fig. 4a). Single presynaptic stimulations were paired with postsynaptic bursts of five APs (Fig. 4b). Unlike with treatment during adolescence, saline and nicotine treatment during adulthood did not induce any difference in tLTP (Fig. 4b,c). Thus, nicotine exposure during adulthood does not lead to lasting changes in STDP, suggesting that adolescence is a period of vulnerability for lasting effects of nicotine on STDP.
Reduced mGluR2 signaling underlies short- and long-term changes in STDP in rats treated with nicotine during adolescence
What is the mechanism underlying the effects of nicotine exposure during adolescence on STDP? In a previous study, we showed that nicotine exposure during adolescence has lasting effects on synaptic mGluR2 levels in mPFC that affect synapse function (Counotte et al., 2011). We hypothesized that altered levels of mGluR2 receptors can explain the lasting effects of nicotine on STDP. Presynaptic terminals of mPFC glutamatergic synapses are rich in mGluR2 receptors, which are activated by glutamate spillover and inhibit further glutamate release (Mateo and Porter, 2007). Nicotine exposure during adolescence leads to an initial increase in protein levels of this receptor in mPFC synaptic membranes on the first day of withdrawal and is followed by a lasting decrease in mGluR2 levels in adult rats 5 weeks after nicotine exposure during adolescence (Counotte et al., 2011). We first tested whether mGluR2 signaling was also reduced in adult rats after nicotine exposure during adolescence as we showed previously (Counotte et al., 2011). To this end, we recorded EPSCs evoked by extracellular stimulation (experimental set up the same as in Fig. 2a,b). In this experiment, GABAergic transmission was blocked by gabazine (1 μm) to isolate the glutamatergic currents. When mGluR2 receptors were activated by bath application of mGluR2 agonist LY379268, EPSC amplitude significantly decreased. The decrease in EPSC amplitude in nicotine-treated rats was less prominent than in saline-treated controls (Fig. 5a,b). Metabotropic GluR2-dependent inhibition was unaltered in rats treated as adults (Fig. 5b). These results indicate that, as in our previous study (Counotte et al., 2011), mGluR2 receptors are downregulated in adult mPFC glutamatergic synapses after nicotine exposure during adolescence.
Changed mGluR2 functionality after nicotine exposure during adolescence may contribute to changes in STDP. To test this possibility, we manipulated the level of mGluR2 activation by using the mGluR2 agonist LY379268 and mGluR2 antagonist MPPG while inducing STDP in adolescent or adult rats treated with nicotine or saline during adolescence. Bath application of either the agonist or antagonist started 5 min before the experiment and lasted for the duration of the experiment (Fig. 6b,f,i).
We first tested whether the short-term decrease in tLTP induced by nicotine treatment during adolescence could be caused by an increase in levels of mGluR2s during the first four days of withdrawal (Counotte et al., 2011). Using the more robust induction protocol with bursts of five postsynaptic APs (Fig. 6a), all cells of nicotine-treated rats showed tLTP in the presence of the mGluR2 blocker MPPG (Fig. 6b–d). This was significantly more cells compared with the nicotine-treated control group in the absence of MPPG, in which only 2 of 13 cells showed tLTP; 10 of 13 cells showed tLTD, and 1 cell showed no change (χ22=9.22; p=0.01). On average, the amount of slope change in the nicotine-treated groups was not significantly different in the absence or presence of MPPG (t(16)=1.65; p=0.12) due to the large variation in plasticity in the nicotine group (Fig. 6d). Nevertheless, blocking mGluR2s resulted in a higher proportion of cells showing tLTP in rats that were treated with nicotine during adolescence, similarly to saline-treated rats (Fig. 2h).
Reduced mGluR2 signaling in adult rats after nicotine exposure (Counotte et al., 2011) may contribute to the decreased plasticity we observed. To test this, we pharmacologically manipulated the level of mGluR2 activation while inducing STDP in adult rats treated with nicotine or saline during adolescence (Fig. 6e). In saline-treated control rats, the level of tLTP could be modulated using the mGluR2 agonist LY379268 and the antagonist MPPG. Enhancing mGluR2 activity with LY379268 abolished tLTP in saline-treated rats (slope change, 74%; saline plus LY, n=9 from 5 rats; data not shown). Blocking mGluR2 receptors with MPPG resulted in increased tLTP comparable with levels observed in adult rats treated with nicotine during adolescence (Fig. 6f–h; t(34)=2.072; p=0.046). In nicotine-treated rats, in which the synaptic mGluR2 receptor levels are reduced (Fig. 5) (Counotte et al., 2011), enhancing mGluR2 activity by applying mGluR2 agonist LY379268 completely abolished tLTP (Fig. 6i–k; t(29)=4.19; p=0.0002). These findings show that mGluR2 signaling can bidirectionally influence tLTP: reducing mGluR2-dependent inhibition leads to increased tLTP, while enhancing mGluR2 activation blocked LTP. Together, these data suggest that, immediately following adolescent nicotine exposure, increased levels of mGluR2s may be responsible for reduced tLTP induction, and 5 weeks following adolescent nicotine exposure, the lasting reduction in mGluR2 signaling can explain the increased tLTP in the adult mPFC.
Discussion
In this study, we addressed the question whether nicotine exposure during adolescence has lasting consequences for STDP in the adult mPFC. Activation of nicotinic receptors in the adolescent mPFC decreases the ability of layer V pyramidal neurons to undergo tLTP. Similarly, shortly (1–4 d) following cessation of nicotine treatment during adolescence, a decrease in tLTP was observed. In contrast, 5 weeks following nicotine exposure during adolescence, tLTP was increased. Short- and long-term changes in mGluR2 signaling in mPFC synapses resulting from nicotine exposure during adolescence can explain altered plasticity. These changes did not occur with nicotine exposure during adulthood, emphasizing that adolescence is a critical period of vulnerability for long-term nicotine effects on the PFC.
In humans, the PFC shows delayed development with respect to other cortical areas during adolescence with delayed thinning of cortical gray matter, most likely reflecting fine-tuning of synaptic contacts (Gogtay et al., 2004; Sowell et al., 2004; Casey et al., 2005). Rearrangement of local inhibitory inputs and decreases in synaptic densities and branch points of excitatory connections between pyramidal neurons occur within the developing PFC (Woo et al., 1997; Cruz et al., 2003). Spike timing-dependent modifications are likely to be important for cortical development, map plasticity, and functioning of neural networks: correlated inputs lead to strengthening of connections (LTP), while uncorrelated inputs lead to weakening (LTD) and pruning of unused synapses (Bi and Poo, 2001; Song and Abbott, 2001; Feldman and Brecht, 2005). Our results show that acute nicotinic receptor activation in adolescent PFC abolishes tLTP. Thus, repeated exposure to nicotine during adolescence may thereby affect the way the prefrontal circuitry matures and lead to changes in the prefrontal network that may last for a lifetime.
Short-term effects of nicotine exposure during adolescence
In the PFC, nAChRs enhance the activity of both excitatory and inhibitory inputs to pyramidal cells (Gioanni et al., 1999; Couey et al., 2007). In PFC layer V, the increase in inhibition dominates the effects of nicotine on synaptic plasticity (Couey et al., 2007). Nicotine-stimulated inhibition reduces dendritic calcium signaling and renders postsynaptic activity in layer V pyramidal cells insufficient to induce potentiation (Couey et al., 2007). In layer II/III, the layer in which the synaptic inputs were stimulated in present work, nAChR activation results predominantly in activation of interneurons (Poorthuis et al., 2012). We find that, also in adolescent rats, nicotine application to mPFC slices reduces STDP.
Recently, we demonstrated that nicotine exposure during adolescence leads to a transient increase in the expression levels of high-affinity nAChRs with functional consequences for GABAergic inhibition in PFC layers II/III (Counotte et al., 2012). In that study, we found that GABAergic transmission by itself is not affected by nicotine treatment during adolescence, and as such, it cannot explain the reduced STDP shortly following nicotine treatment during adolescence we describe here (Counotte et al., 2012). We did find that acute nicotine application to the PFC slice induced a stronger amplitude increase of IPSCs received by PFC pyramidal neurons following nicotine treatment during adolescence (Counotte et al., 2012). Since in the present experiments assessing STDP immediately following nicotine treatment during adolescence, nicotine was not applied, this can also not explain the reduced STDP we observed.
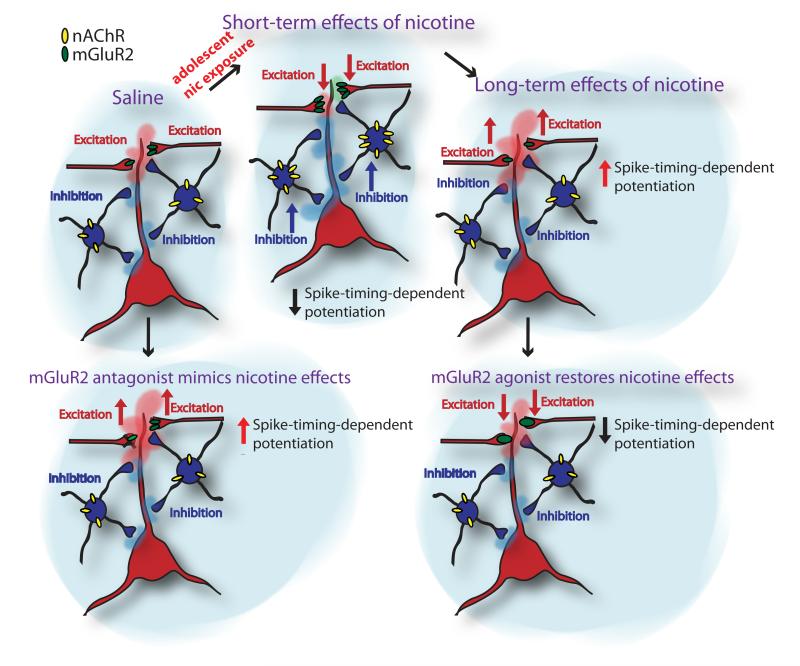
However, the decrease in tLTP can be explained by a short-term increase in the level of synaptic mGluR2 (Counotte et al., 2011). Metabotropic GluR2s modulate cortical processing by inhibiting glutamate release from excitatory synapses in PFC. This inhibition provides a feedback mechanism for preventing excessive excitation of neurons (Shen and Johnson, 2003; Poisik et al., 2005; Mateo and Porter, 2007). Under conditions of repeated activation of deep layer pyramidal cells by nicotine (Kassam et al., 2008; Poorthuis et al., 2012), the elevated expression of this receptor in PFC might suppress nicotine-induced excitation and lead to reduced plasticity. We show that pharmacological blockade of mGluR2s in nicotine-treated rats significantly increases the proportion of synapses that show potentiation, which suggests that changes in mGluR2 levels induced by nicotine exposure during adolescence can alter STDP (Fig. 7 shows a model for nicotine effects in PFC).
Figure 7.
A model for short- and long-term consequences of nicotine exposure during adolescence in PFC network.
Lasting effects of nicotine exposure during adolescence
Few studies have investigated whether adolescent nicotine exposure produces changes in neural functioning that persist during adulthood. We previously showed that adolescent, but not adult, nicotine treatment reduces attention performance and impulse control (Counotte et al., 2009). Similar nicotine-induced deficits have been found in a serial pattern learning paradigm (Fountain et al., 2008). In a recent study, chronic nicotine exposure during adolescence produced long-lasting impairments in contextual learning that were observed during adulthood, whereas adult chronic nicotine exposure did not (Portugal et al., 2012). We find that one of the long-term adaptations on the synaptic level in mPFC is the increased ability of prefrontal synapses to undergo spike-timing-dependent LTP (Fig. 7). STDP depends on the precise timing of the synaptic input and the postsynaptic action potential and this temporal relationship resembles typical features of associative learning (Letzkus et al., 2007). Although STDP has not been directly linked to attention performance, the ability to associate goal-relevant information is crucial for any cognitive behavior. In nicotine-treated rats, the same amount of presynaptic and postsynaptic activity leads to more synaptic potentiation. This may suggest that the PFC network would even associate irrelevant stimuli.
In addition to enhanced tLTP after adolescent nicotine, we recently reported that short-term depression during trains of stimulation is reduced as a result of reduced mGluR2 function after nicotine exposure during adolescence (Counotte et al., 2011). Thus, mGluR2 activation filters information transfer at mPFC synapses. In other cortical areas, this type of synaptic filtering can give rise to phenomena such as sensory and behavioral adaptation or habituation to a stimulus (Zucker, 1989). In rat somatosensory cortex, in vivo whole-cell recordings in cortical neurons during whisker deflection directly demonstrated that synaptic depression of thalamic input to the cortex contributes to rapid adaptation of sensory responses (Chung et al., 2002). Selective attention, the ability of an organism to filter out relevant information in the face of distractors, may also build upon these types of synaptic processes. Reduced short-term plasticity after nicotine exposure may thereby compromise the ability of prefrontal neurons to efficiently filter out irrelevant information. Therefore, both increased long-term potentiation and reduced short-term plasticity contribute to reduced signal-to-noise ratio. These impaired plasticity mechanisms that depend on mGluR2 signaling may explain the concomitant reduced attention performance (Counotte et al., 2009, 2011).
mGluRs, addiction, and cognition
Lasting changes in mGluR2 signaling have been implicated in the long-term effects of drug exposure (Kenny and Markou, 2004). mGluR2/3 receptors negatively regulate brain reward function (Kenny and Markou, 2004). Following nicotine exposure, altered function of mGluR2/3 is also found in other brain areas outside the mPFC, such as ventral tegmental area and the nucleus accumbens. There, altered mGluR2 transmission is involved in the rewarding effects of nicotine (Helton et al., 1997; Kenny et al., 2003; Kenny and Markou, 2004; Liechti et al., 2007). In these brain areas, mGluR2 receptors are similarly positioned at presynaptic glutamatergic terminals and can be activated by glutamate spillover, thereby inhibiting glutamate release. The mGluR2/3 receptors play an important role in the development of drug dependence and the expression of the negative affective state observed during withdrawal (Kenny and Markou, 2004). However, the role of mGluR2 receptors in withdrawal appears complex and most likely depends on multiple brain areas.
We previously found that preventing the long-term reduction in mGluR2 levels in the medial PFC was sufficient to restore attention performance (Counotte et al., 2011). Enhanced glutamate release in PFC is associated with attention deficit and loss of impulse control (Pozzi et al., 2011). Since mGluR2 has an important role in autoinhibition of excitatory transmission, its influence on attention performance would be most prominent in conditions of increased excitation. Indeed, mGluR2 agonists are effective in improving cognitive deficits if enhanced glutamate release is caused by a NMDA receptor antagonist (Pozzi et al., 2011). On the behavioral level, blocking mGluR2 transmission by injection of MPPG into PFC causes deficits in attention performance, while stimulating already reduced mGluR2 signaling by agonist in nicotine-treated rats restores attention (Counotte et al., 2011). Impairments in mGluR2 transmission caused by other factors than nicotine exposure, such as rearing in impoverished environment or pharmacological treatment, were also shown to cause cognitive deficits that can be restored by mGluR2 agonists (Melendez et al., 2004; Pozzi et al., 2011). Furthermore, altered mGluR2 levels in PFC are linked to brain disorders such as depression and schizophrenia and activation of this receptor was proposed as a novel target for treatment (Gupta et al., 2005; Palucha and Pilc, 2005; Pilc et al., 2008; Conn et al., 2009). Thus, mGluR2 signaling shapes cognitive behavior. A lasting reduction in mGluR2 signaling after nicotine exposure can result in increased LTP and therefore potentially to a less efficient ability to filter information, thereby hampering cognitive performance.
Acknowledgments
This work was supported by The Netherlands Organization for Scientific Research Grants 917.76.360 and 912.06.148, ERCStG “BrainSignals,” the Dutch Fund for Economic Structure Reinforcement (0908 “NeuroBasic PharmaPhenomics Project”), European Union Seventh Framework Programme (HEALTH-F2-2009-242167 “SynSys”), and Vrije Universiteit (Amsterdam, The Netherlands). We thank Jaap Timmerman and Hans Lodder for technical assistance.
Footnotes
Author contributions: N.A.G. and H.D.M. designed research; N.A.G. performed research; N.A.G. analyzed data; N.A.G. and H.D.M. wrote the paper.
The authors declare no competing financial interests.
References
- Bellone C, Lüscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol. 2000;19:223–231. [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011;14:417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Moretti M, Smoluch MT, Irth H, Clementi F, Schoffelmeer AN, Mansvelder HD, Smit AB, Gotti C, Spijker S. Adolescent nicotine exposure transiently increases high-affinity nicotinic receptors and modulates inhibitory synaptic transmission in rat medial prefrontal cortex. FASEB J. 2012;26:1810–1820. doi: 10.1096/fj.11-198994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. Postnatal development of preand postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Currie C, Nic Gabhainn S, Godeau E, Roberts C, Smith R, Currie D, Pickett W, Richter M, Morgan A, Barnekow V, editors. Health Policy for Children and Adolescents. No. 5. WHO Regional Office for Europe; Copenhagen: 2008. Inequalities in young people’s health: HBSC international report from the 2005/06 Survey. [Google Scholar]
- Escobedo LG, Marcus SE, Holtzman D, Giovino GA. Sports participation, age at smoking initiation, and the risk of smoking among US high school students. JAMA. 1993;269:1391–1395. [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Exp Brain Res. 2008;187:651–656. doi: 10.1007/s00221-008-1346-4. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepousé C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse. 2005;57:123–131. doi: 10.1002/syn.20164. [DOI] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. LY354740: a metabotropic glutamate receptor agonist which ameliorates symptoms of nicotine withdrawal in rats. Neuropharmacology. 1997;36:1511–1516. doi: 10.1016/s0028-3908(97)00170-6. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Kassam SM, Herman PM, Goodfellow NM, Alves NC, Lambe EK. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci. 2008;28:8756–8764. doi: 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Does spike timing-dependent synaptic plasticity underlie memory formation? Clin Exp Pharmacol Physiol. 2007;34:1070–1076. doi: 10.1111/j.1440-1681.2007.04724.x. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S. alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci. 2009;29:539–550. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Mateo Z, Porter JT. Group II metabotropic glutamate receptors inhibit glutamate release at thalamocortical synapses in the developing somatosensory cortex. Neuroscience. 2007;146:1062–1072. doi: 10.1016/j.neuroscience.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Floyer-Lea AM, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus: role of GABAergic inhibition. J Neurosci. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso F, Bettermann F, Vucurevic G, Stoeter P, Konrad A, Winterer G. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology (Berl) 2007;191:159–169. doi: 10.1007/s00213-006-0499-8. [DOI] [PubMed] [Google Scholar]
- Nicholls RE, Zhang XL, Bailey CP, Conklin BR, Kandel ER, Stanton PK. mGluR2 acts through inhibitory Gα subunits to regulate transmission and long-term plasticity at hippocampal mossy fiber-CA3 synapses. Proc Natl Acad Sci U S A. 2006;103:6380–6385. doi: 10.1073/pnas.0601267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha A, Pilc A. The involvement of glutamate in the pathophysiology of depression. Drug News Perspect. 2005;18:262–268. doi: 10.1358/dnp.2005.18.4.908661. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Poisik O, Raju DV, Verreault M, Rodriguez A, Abeniyi OA, Conn PJ, Smith Y. Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus. Neuropharmacology. 2005;49(Suppl 1):57–69. doi: 10.1016/j.neuropharm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CP, Mansvelder HD. [Retrieved June 20, 2012];Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr390. Advance online publication. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012;97:482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L, Baviera M, Sacchetti G, Calcagno E, Balducci C, Invernizzi RW, Carli M. Attention deficit induced by blockade of N-methyl-d-aspartate receptors in the prefrontal cortex is associated with enhanced glutamate release and cAMP response element binding protein phosphorylation: role of metabotropic glutamate receptors 2/3. Neuroscience. 2011;176:336–348. doi: 10.1016/j.neuroscience.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Group II metabotropic glutamate receptor modulation of excitatory transmission in rat subthalamic nucleus. J Physiol. 2003;553:489–496. doi: 10.1113/jphysiol.2003.052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32:339–350. doi: 10.1016/s0896-6273(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa-Silva G, Verhoog MB, Goriounova NA, Loebel A, Hjorth J, Baayen JC, de Kock CP, Mansvelder HD. Human synapses show a wide temporal window for spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010;2:12. doi: 10.3389/fnsyn.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]