Abstract
Study question
To determine the incidence of defects in Ca2+ signalling pathways mediating hyperactivation (calcium influx and store mobilisation) among donors and sub fertile patients and to ascertain if these are functionally significant i.e. related to fertilisation success at IVF.
Summary answer
This study identifies, for the first time, the incidence of Ca2+store defects among research donors, IVF and ICSI patients and highlights the biological role and importance of Ca2+-signalling (Ca2+ store mobilisation) for fertilisation at IVF.
What is known already
Sperm motility and hyperactivation (HA) are important for fertility, mice with sperm incapable of HA are sterile. Recently there has been significant progress in our knowledge of the factors controlling these events, in particular the generation and regulation of calcium signals. Both pH-regulated membrane Ca2+channels (CatSper) and Ca2+ stores (potentially activating store-operated Ca2+ channels) have been implicated in controlling HA.
Study design, size, and duration
This was a prospective study examining a panel of 68 donors and 181 sub fertile patients attending the Assisted Conception Unit, Ninewells Hospital Dundee for IVF and ICSI. 21 of the donors gave a second sample (~4 weeks later) to confirm consistency/reliability of the recorded responses. Ca2+ signaling was manipulated using three agonists, NH4Cl (activates CatSper via pH), progesterone (direct activation of CatSper channels, potentially enhancing mobilisation of stored Ca2+ by CICR) and 4-AP (effect on pH equivalent to NH4Cl and mobilises stored Ca2+). IBMX, a potent activator of HA was also used for comparison. For patient samples, an aliquot surplus to requirements for IVF/ICSI treatment was examined, allowing direct comparison of Ca2+-signalling and motility data with functional competence of the sperm.
Materials, setting, methods
The donors and sub fertile patients were screened for HA (using CASA) and changes in intracellular Ca2+ were assessed by loading with fura and measuring fluorescence using a plate reader (FluoStar).
Main results and the role of chance
Relative efficacy of the stimuli in inducing HA was 4-AP>>IBMX>progesterone. NH4Cl increased [Ca2+]i similarly to 4-AP and progesterone but did not induce a significant increase in HA. Failure of samples to generate HA (no significant increase in response to stimulation with 4-AP) was seen in just 2% of research donors but occurred in 10% of IVF patients (P= 0.025). All donor samples generated a significant [Ca2+]i increase when stimulated with 4-AP but 3.3% of IVF and 25% of ICSI patients failed to respond. Amplitudes of HA and [Ca2+]i responses to 4-AP were correlated with fertilisation rate at IVF (P= 0.029; P=0.031 respectively). Progesterone reliably induced [Ca2+]i responses (97% of donors, 100% of IVF patients) but was significantly less effective than 4-AP in inducing HA. 25% of ICSI patients failed to generate a [Ca2+]i response to progesterone (P=0.035). Progesterone-induced [Ca2+]i responses were correlated with fertilisation rate at IVF (P= 0.037) but induction of HA was not. In donor samples examined on more than one occasion consistent responses for 4-AP-induced [Ca2+]i (R2 = 0.97) and HA (R2=0.579) were obtained.
In summary, the data indicate that defects in Ca2+ leading to poor HA do occur and that ability to undergo Ca2+ -induced HA affects IVF fertilising capacity. The data also confirm that release of stored Ca2+ is the crucial component of Ca2+signals leading to HA and that Ca2+ store defects may therefore underlie HA failure.
Limitations, reasons for caution
This is an in vitro study of sperm function. Whilst the repeatability of the [Ca2+]i and HA responses in samples from the same donor were confirmed, data for patients were from 1 assessment and thus the robustness of the failed responses in patients’ needs to be established. The focus of this study was on using 4AP, which mobilizes stored Ca2+ and is a potent inducer of HA. The n values for other agonists, especially calcium assessments, are smaller.
Wider implications of the findings
Previous studies have shown a significant relationship between basal levels of HA, calcium responses to progesterone and IVF fertilisation rates. Here we have systematically investigated the ability/failure of human sperm to generate Ca2+ signals and HA in response to targeted pharmacological challenge and, related defects in these responses to IVF success. [Ca2+]i signalling is fundamental for sperm motility and data from this study will lead to assessment of the nature of these defects using techniques such as single cell imaging and patch clamping.
Study funding/competing interest(s)
Resources from a Welcome Trust Project Grant (# 086470, Publicover and Barratt PI) primarily funded the study. The authors have no competing interests.
Keywords: calcium signalling, sperm, male fertility, hyperactivation, sperm motility, IVF
Introduction
Sperm dysfunction (lacking ‘normal’ function) has consistently been identified as the single most common cause of male infertility (Hull et al., 1985; Irvine., 1998). Men can produce sperm which are dysfunctional even when their semen parameters are ‘normal’ (Aitken et al.,1991). Currently there are no drugs a man can take, or add to his spermatozoa in vitro, to treat sperm dysfunction. The only option is ART (Assisted Reproductive Technology), which comprises a range of treatments, all of which are invasive. The particular treatment selected depends on the severity of the condition, i.e. IUI (Intra Uterine Insemination) for mild, IVF (in vitro Fertilisation) for moderate and, ICSI (Intra cytoplasmic sperm injection) for men with severe sperm dysfunction. The development of non-invasive, pharmacological treatment alternatives has been severely hampered by our limited understanding of the cellular and molecular workings of the mature spermatozoon (reviews Aitken and Henkel, 2011, Barratt et al.,2011; Barratt 2011). There are, however, some areas where progress has been made. One that has received considerable attention, ignited by the creation and characterisation of CatSper knockout mice, concerns the generation and regulation of calcium signals that control sperm motility, in particular hyperactivation (see below and Publicover et al.,2007; Costello et al.,2009; Publicover and Barratt, 2011, Barratt and Publicover, 2012; Strünker et al.,2011; Lishko et al.,2011).
Hyperactivation (HA) is critical to sperm function, playing a key role in the ability of sperm to successfully ascend the female reproductive tract and reach the site of fertilisation. For example, HA may facilitate sperm migration through the highly visco-elastic oviductal mucus and enable penetration of the layers surrounding the oocyte (Suarez et al.,1991; Suarez and Dai, 1992; Ren et al.,2001; Carlson et al., 2003; Quill et al.,2003; Stauss et al.,1995). Additionally, experimental studies show that HA may be required to detach sperm from the oviduct epithelium in animals (Demott and Suarez, 1992; Gwathmey et al.,2003) and in humans (Pacey et al.,1995). Although data is limited in humans, clinical studies on HA generally suggest that (1) the percentage of hyperactivated sperm correlates with fertilisation rate in vitro (e.g. Sukcharoen et al.,1995) and (2) there are significant differences in the proportion of hyperactivated cells (spontaneous and in response to physiological or artificial stimulants) between men with normal semen parameters and sub fertile patients (Burkman., 1984; Tesarik et al.,1992; Peedicayil et al.,1997; Munire et al.,2004).
As the spermatozoon ascends the female tract, its motility must be finely regulated by cues from the female tract and cumulus-oocyte complex, in order that the cell can deploy behaviour appropriate to its environment (Olson et al., 2011). The central regulator is Ca2+, elevated [Ca2+]i being required both for the initiation and maintenance of hyperactivated motility. There are at least two sources of Ca2+ that contribute to HA in mammalian sperm: Firstly, entry of Ca2+ via pH-dependent CatSper channels in the plasma membrane of the flagellar principal piece. Sperm from mice null for CatSper are motile but do not hyperactivate, rendering them unable to migrate to or within the oviduct and unable to fertilise oocytes even by IVF (Ren et al.,2001; Carlson et al 2003; Quill et al.,2003; Ho et al., 2009). In bovine sperm elevation of pHi with NH4Cl, to activate CatSper, induces HA and similar results have been reported in mouse sperm (Marquez and Suarez 2007; Chang and Suarez, 2011). Secondly, induction of HA has been reported in bovine, mouse and human cells upon mobilisation of calcium stored in the neck/midpiece region (Ho and Suarez., 2001; Ho and Suarez., 2003; Marquez et al.,2007; Costello et al.,2009). In human sperm thimerosal (which activates intracellular Ca2+ channels, releasing stored Ca2+) potently induces HA (Alasmari et al., 2013) whereas stimulation of CatSper with progesterone (Strünker et al.,2011; Lishko et al.,2011) or by raising pHi have little effect (Alasmari et al., 2013). 4-AP, a particularly potent inducer of HA (Bedu-Addo et al.,2008; review Costello et al.,2009), both stimulates release of stored Ca2+ and raises pHi (Ishida et al, 1993; Grimaldi et al, 2001; Navarro et al., 2007; Bhaskar et al, 2008; Chang and Suarez, 2011; Alasmari et al., 2013). However, equivalent cytoplasmic alkalinisation induced with NH4Cl or trimethylamine hydrochloride fails to cause HA and pharmacological block of CatSper does not inhibit 4-AP-induced HA (Alasmari et al., 2013). Suffice it to say, 4-AP stimulates human sperm HA primarily or fully through its action on stored Ca2+.
A key question therefore is: do functional defects occur in sperm Ca2+ signalling apparatus that prevent regulation of HA and cause sperm dysfunction? To investigate this a panel of donors and sub fertile patients were screened for HA and changes in intracellular Ca2+ in response to targeted agonists namely (i) NH4Cl (pH-induced activation of flagellar CatSper channels); (ii) progesterone (direct activation of flagellar CatSper channels, potentially enhancing mobilisation of stored Ca2+ by CICR Harper et al, 2004) and (iii) 4-AP (raises pHi [similarly to NH4Cl] and mobilises stored Ca2+ in the neck/midpiece of human sperm). IBMX was used to stimulate HA via the cAMP pathway which does not induce an immediate calcium influx in human sperm cells (Strunker et al.,2011). Additionally, the relationship between HA (spontaneous and induced), intracellular stimulus-induced Ca2+ responses and fertilization rates at IVF were investigated. The primary aims of this study were to examine (1) the incidence of defects in the Ca2+-signalling pathways that mediate HA in sperm from donors and from sub fertile patients; (2) if these defects (assessed by measuring HA/calcium signaling) were related to IVF success.
Materials and Methods
Reagents
4-aminopyridine (4-AP) (Sigma Aldrich, Catalog number 275875-5G, UK), progesterone (Sigma Aldrich, Catalog number P8783-5G, UK), Isobutyl-1-methylxanthine (IBMX) (Calbiochem, Catalog number 410957, UK) and ammonium chloride (NH4Cl) (Sigma Aldrich, Catalog number 4316230J, UK) were dissolved in distilled water, ethanol, DMSO and distilled water respectively. Aliquots were diluted and added to the sperm suspensions to achieve a final concentration of 2 mM, 3.6 μM, 100 μM and 25 mM respectively. Fura-2 acetoxymethyl ester (Fura-2/AM) (Molecular Probes, Invitrogen, Oregon, USA) was dissolved in DMSO and used at a final concentration of 1 μM.
Media used for donor samples
Synthetic tubal fluid (based upon Mortimer, 1986) was used as capacitating media (CM) for donor samples. It consisted of 4.7 mM KCl, 3 mM CaCl2, 1 mM MgSO4.7H2O, 106 mM NaCl, 5.6 mM D-Glucose, 1.5 mM NaH2PO4, 1 mM Na pyruvate, 41.8 mM Na lactate, 25 mM NaHCO3, 1.33 mM Glycine, 0.68 mM Glutamine, 0.07 mM Taurine, Non-essential amino acids (1: 100 dilution in STF) and 30mg/ml BSA. A non capacitating HEPES-buffered medium (NCM) adapted from the above but lacking in both albumin and bicarbonate (5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4.7H2O, 116.4 mM NaCl, 5.6 mM D-Glucose, 1.0 mM NaH2PO4, 2.7 mM Na pyruvate, 41.8 mM Na lactate and 25 mM HEPES) was also used.
Ethical approval
Written consent was obtained from each patient in accordance with the Human Fertilisation and Embryology Authority (HFEA) Code of Practice (version 8) under local ethical approval (08/S1402/6) from the Tayside Committee of Medical Research Ethics B. Similarly, volunteer sperm donors were recruited in accordance with the HFEA Code of Practice (version 8) under the same ethical approval.
Study subjects
Semen samples were obtained from three groups: 100 semen samples from 68 young healthy research donors (mainly students with no known fertility problems, aged 20-35 and with a normal sperm concentration and motility according to WHO criteria 1999), 181 sub fertile patients who underwent IVF (170 patients), or ICSI (11 patients) treatment at the Assisted Conception Unit (ACU), Ninewells Hospital, Dundee, Scotland between April 2009 and August 2011.
Semen samples
Semen samples from donors and patients were collected by masturbation into a sterile plastic container after 2-3 days of sexual abstinence. The samples were used for analysis after liquefaction of the semen at 37°C for approximately 30 minutes and within 1 hour of production. Semen samples obtained from patients were assessed for the semen profile by clinical embryologists. Patients were selected for IVF or ICSI according to clinical indications and semen quality. For the latter, although it wasn’t always the case, men with approximately 1×106 progressively motile cells post preparation were allocated to IVF and below this limit to ICSI.
With respect to the patient samples, to eliminate inter ejaculate-variation, the surplus of the clinical sample used in the IVF or ICSI treatment process was taken for analysis of HA and where possible intracellular Ca2+. IVF fertilisation rates were obtained in order to examine the potential functional relationship between stimulation with agonists known to act on different components of the Ca2+ signalling system and IVF.
Density gradient centrifugation (DGC)
For the donors, spermatozoa were isolated from seminal plasma by DGC using PureSperm (Nidacon, Molndal, Sweden) buffered with NCM. After centrifugation, the supernatant was discarded and pellet was resuspended in CM, and incubated for ~2 hours (hrs) at 37°C in a 5% CO2 incubator. This incubation period in CM was chosen because in general, no further notable change was observed in the percentage of spontaneous/induced HA (data not presented) or in agonist stimulated intracellular Ca2+ level. In the Assisted Conception Unit commercially available media was used for sperm preparation. The spermatozoa were separated from semen by DGC using PureSperm diluted with Cook Sydney IVF Gamete Buffer, a HEPES-buffered solution (Cook Sydney IVF Limited, National Technology Park, Ireland, UK). After centrifugation, the pellet was washed by centrifugation at 500g for 10 minutes in 4 ml of Cook Gamete Buffer. If the samples were assigned for IVF, following centrifugation, the supernatant was discarded and pellet resuspended in Cook Sydney IVF Fertilisation medium (FM), a bicarbonate buffered medium similar to STF, containing 25 mM NaHCO3 (Moseley et al.,2005; data not presented). If the sample was allocated for ICSI, the cells were washed in Cook Gamete Buffer. Following this, IVF samples were gassed with CO2 and kept at room temperature (up to 4 hrs) until one hour before the insemination, at which point the sample was incubated at 37°C in a 5% CO2 incubator. The ICSI samples were kept at room temperature (up to 4 hrs) until the time of the injection procedure. Following insemination or injection the remaining portion of the sample was analysed for basal and agonist induced HA and if there was a sufficient yield of sperm available, for intracellular Ca2+ (see below). ICSI samples were prepared in the IVF clinic (in Cook Gamete Buffer™ which does not support the completion of capacitation - Moseley et al., 2005). These samples were only assessed for intracellular Ca2+ and were re-suspended in CM as part of the fura loading protocol (see below).
Assessment of basal level of HA and sperm motion characteristics by CASA
A Hamilton Thorn CEROS machine (version 12) attached to an external microscope was used to assess motion characteristics. The concentration of prepared spermatozoa from donors or patients was adjusted to between 5 and 25 ×106/ml with CM. Samples with a concentration ≤2 ×106/ml were generally excluded from the study as the number of cells was insufficient to obtain data comparable to the other samples. The samples were mixed to ensure a homogenous concentration of spermatozoa. Hamilton-Thorn 2X-Cel chambers (20μm depth) (Dual Sided Sperm Analysis Chamber, Hamilton Thorn Biosciences, Beverly, MA, USA) were pre warmed at 37°C on a heated stage of an Olympus CX41 microscope (Olympus Corporation, Tokyo, Japan) after which 4μl of sperm suspension was placed onto each slide chamber and then covered by a pre warmed cover slip (22mm × 22mm). Slides were maintained at 37°C for ~2 minutes prior to the start of data acquisition. For hyperactivation (HA) and motion characteristics were assessed under a negative phase contrast objective (×10) at a final magnification of ×100. Four different aliquots were assessed for each sample and at least 200 motile cells were counted on randomly selected fields in each aliquot with approximately 800 motile cells as a minimum total count. The percentage of hyperactivated cells was assessed using standard criteria to identify hyperactivation, namely VCL ≥ 150 μm/s, linearity ≤ 50%, and ALH ≥ 7 (Mortimer et al.,1998).
Assessment of hyperactivated motility in response to different agonists
Agonist stimulation was achieved by adding 1 μl of agonist to 99 μl of sperm suspension, giving final concentrations of 2 mM 4-AP, 100 μM IBMX, 3.6 μM progesterone or 25 mM NH4Cl. Sperm HA and other kinematic parameters were then assessed as described above.
21 of the 68 donors produced more than one sample. The basal and 4-AP induced HA for donors who produced two samples is presented in Supplementary Figure 2. Importantly, the HA response to 4-AP was consistent between the assessments in all 21 donors with 20/21 donors showing a normal significant response in both assessments and 1/21 showing a poor response in both samples (Supplementary Figure 1a).
Fertilisation rate (FR) at IVF
Oocytes were considered normally fertilised when two pronuclei (2PN) and two distinct or fragmented polar bodies were observed. In IVF, the fertilisation rate was calculated from the number of oocytes normally fertilised divided by the total number of inseminated oocytes. In order to reduce the influence of minimal egg numbers the data used for fertilization rates in this analysis are only those cases where at least 4 mature eggs were inseminated for IVF (n=145). Fertilisation rates where ICSI was the designated treatment were not taken into account as ICSI bypasses any functional requirement needed for a sperm to bind and penetrate the egg vestments. The median female age of IVF patients (n=145) was 34 (31-37 25 and 75th centile respectively). The median number of eggs recovered in the IVF patients (n=145) was 10.5 (8-14; 25 and 75th centile respectively).
Measurement of intracellular Ca2+
After sperm preparation (either in the research laboratory for healthy donors or at the IVF laboratory for patients), 500 μl aliquots of sperm suspension (concentration adjusted to ~8-20 ×106/ml with CM), were loaded with 1 μl of Fura-2/AM (1 μM) for 12 min at 37°C and 5% CO2 in the dark, then centrifuged at 500g for 15 min. The supernatant was removed and the pellet resuspended in 100μl of medium. Since loading with fura required a centrifugation step followed by resuspension, samples from all three groups (donors, IVF, ICSI) could be resuspended in the same medium (CM). The intracellular Ca2+ response is rapidly developed with capacitation (Baldi et al.,1991; Bedu-Addo et al.,2005). Fluorescence measurements were carried out on a FLUOstar Omega (BMG Labtech Offenburg, Germany) using alternating excitation wavelengths of 340nm and 380nm and recording emission at 510 nm. Stimulus-induced increments in the ratio of emission intensities (at 340 and 380 excitation) were used to quantify changes in [Ca2+]i concentration. Aliquots of 50 μl were pipetted into a 96 well plate and 100 seconds of control data (20 readings) were acquired (resting level (R)). 5 μl of agonist (4-AP or progesterone) was then added and the response was recorded. Usually, a minimum of ~2 million cells per well (50 μl) were required for robust results. In a number of cases (primarily those involving ICSI samples) this could not be achieved and no measurement of intracellular Ca2+ response was reported.
To examine the consistency of different samples from the same donors, 8 donors were tested on 2 occasions with an interval of at least one month between donations. Responses to progesterone were very similar between samples (R2= 0.972) (Supplementary Figure 1b). One donor who showed a poor response was tested on several different occasions with consistent results (Supplementary Figure 1b).
Definition of failed HA and Ca2+ responses among donors and sub fertile patients
A failed HA response was recorded when agonist stimulation did not induce a significant change in the % of hyperactivated cells compared to control (basal) level (assessed by one way ANOVA and non parametric analysis of variance on ranks Kruskal-Wallis test, p<0.05).
To define failed Ca2+ responses a normal range was determined from the distribution of response amplitudes (agonist-induced increments in the 340/380 ratio) in donor populations stimulated with 4-AP and progesterone (Supplementary Figure 2). Upper and lower limits were set to include 99% of the distribution (mean ± 2.58 × SD; see statistical analysis section below for normalising procedure). Using this approach, the cut-off values for a failed Ca2+ response were increments in the 340/380 ratio of ≤ 0.1 upon addition of 4-AP or progesterone.
Statistical analysis
Normality of data was assessed according to the Kolmogorov-Smirnov test. Results are expressed as the mean ± SD (standard deviation), median and range for HA. Statistical comparisons were made using the analysis of variance (ANOVA) if the data were either originally normally distributed or normalised after transformation by square root. However, some HA and intracellular Ca2+ data were not normalised by transformation. Thus, the statistical comparisons for these data were examined by non-parametric analysis of variance Kruskal – Wallis one way analysis of variance on ranks. The correlation between HA and intracellular Ca2+ in response to agonists (4-AP and progesterone) was examined using Pearson’s correlation coefficient. The correlations between HA and intracellular Ca2+ in the basal level and in response to agonists with IVF fertilisation rates were examined by Spearmans correlation coefficient (because the data were not normalised after transformation) and Pearson’s correlation coefficient, respectively.
To define a cut-off value for a failed Ca2+ response, the data was log transformed and cut-off values were calculated based on mean and SD. The differences in proportions of failed responders between the populations (donors and IVF patients) and between agonists were examined using a Chi-squared test. P<0.05 was considered significant. All statistical analyses were performed using the SigmaStat 10 statistical package (Systat software inc., Chicago, IL, USA)
Results
Induction of HA in donor and IVF patient samples
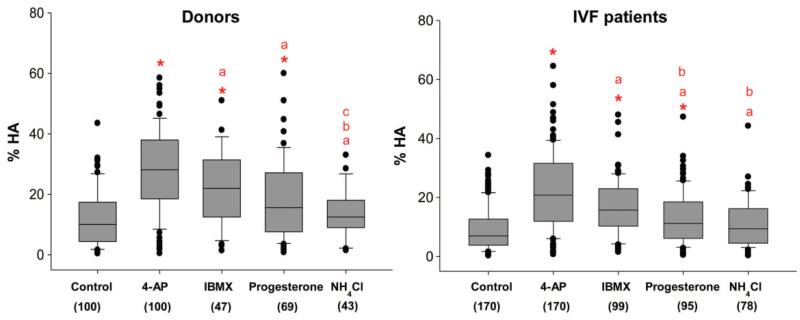
Of the 4 agonists used (4-AP, NH4Cl, progesterone, IBMX), 4-AP was the most potent inducer of HA in sperm from donors, the magnitude of the effect being significantly greater than that of all other agonists (P<0.001; Figure 1). IBMX was the next most effective agonist, followed by progesterone (Figure 1). As reported in detail elsewhere (Alasmari et al., 2013), 25 mM NH4Cl was not an effective inducer of HA (NS; Figure 1) though in a minority of samples, primarily those with low basal HA (≤10% HA), there was a detectable response (P <0.05).
Figure 1.
Comparison of four different agonists on HA) in two populations (donors and IVF patients). Box and whisker plots illustrating the data distribution for HA in the baseline (control) and samples treated with 4-AP, IBMX, progesterone and NH4Cl from donors and IVF patients. The boxes represent the interquartile range and lines within them are the medians. The number in brackets is the sample size. *Highlights that the agonist-induced HA is significantly different to baseline, (a) highlights a significant difference between responses to 4-AP and all other agonists (b) highlights a significant difference between responses to IBMX compared with progesterone and NH4Cl and (c) highlights a significant difference between responses to progesterone and NH4Cl. Significance was considered as P, 0.05 assessed by one-way ANOVA and non-parametric ANOVA on ranks Kruskal–Wallis test. Not all samples were tested with each of the agonists.
The relative efficacy of the 4 different agonists on IVF patient samples was similar to that seen with donor samples (Figure 1 compare left and right panels), but for the three effective stimuli (4-AP, IBMX and progesterone) the percentage of hyperactivated cells after treatment was significantly lower in IVF patients (P<0.05) (Figure 1 and Supplementary Figure 3). The basal level of HA was significantly lower in IVF patients compared to donors (P<0.05).
Intracellular calcium responses to 4-AP and progesterone in donors and patients
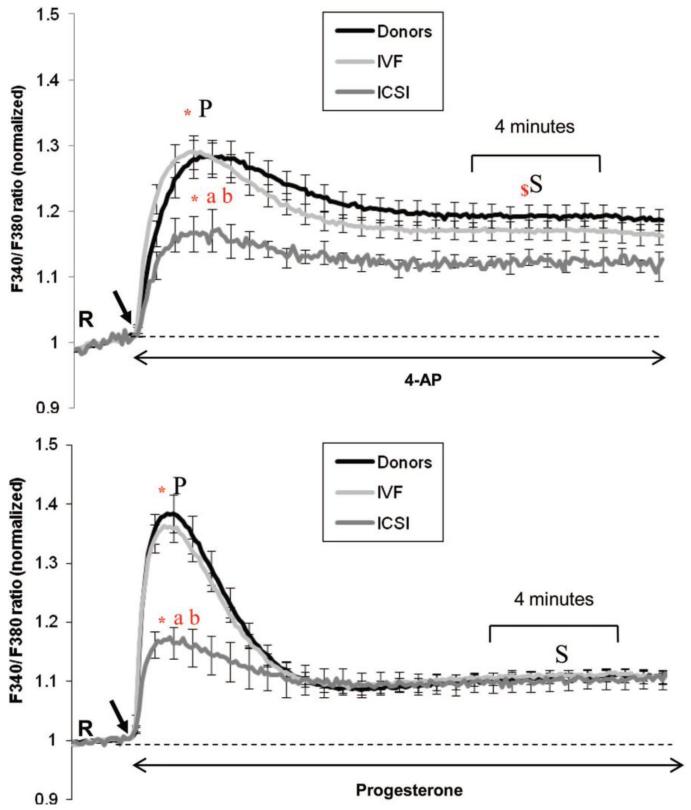
Spermatozoa from 37 donors, 68 IVF and 11 ICSI patients were assessed for their [Ca2+]i responses to 4-AP and progesterone. The 340/380 ratio for fluorescence of fura-2 (R) in resting cells differed between individuals. To facilitate comparison the data from each individual were normalised to the pre-stimulus value (Figure 2). Descriptive statistics of the raw 340/380 ratio data are presented in Supplementary Table I. The basal Ca2+ was significantly lower in spermatozoa from ICSI patients, compared to research donors and IVF patients (P<0.05) (Supplementary Table 1).
Figure 2.
Ca2+ ratio in response to 4-AP and progesterone in three populations (donors, IVF and ICSI patients). Intracellular Ca2+ responses induced by 4-AP (upper panel) and progesterone (lower panel) in donors (4-AP n = 36, progesterone n = 37), IVF (4-AP n = 61, progesterone n = 68) and ICSI patients (4-AP n = 7, progesterone n = 11). Each trace shows mean of n fluorimetric (population) responses+SE. Agonists were added (indicated by black arrow) at 100 s after acquisition of 20 readings at resting level (R). The data for each sample were normalized to pre-stimulus (R) level to facilitate comparison. * Significant difference between the ratio at the peak (P) and the initial resting level (R), (a) significant difference of ratio at peak between donor and ICSI patients, (b) significant difference of ratio at peak between IVF and ICSI patients. $ Significant difference between the ratio at the sustained phase (S) between donor and ICSI patients (P<0.001) and between IVF and ICSI patients (P = 0.007). There was no significant difference in the sustained response with progesterone between the groups. Significance was considered as P <0.05 assessed
Both 4-AP and progesterone-induced a biphasic elevation of intracellular Ca2+ in donor sperm, comprising a [Ca2+]i transient followed by a sustained phase. However the shape of these responses was clearly different, the transient induced by progesterone typically being larger than that induced by 4-AP, whereas 4-AP induced a greater sustained [Ca2+]i increase (Figure 2; Supplementary Figure 4). Both agonists were effective in raising [Ca2+]i in sperm from the two patient groups, but whereas responses in IVF patients were similar to those in donor sperm, the magnitude of the response was much smaller in ICSI samples induced by 4AP (Figure 2, P<0.05).
Failed HA and Ca2+ responses among donors and sub-fertile patients
To further characterise the differences between donor and patient groups we assessed the occurrence of HA and [Ca2+]i signal ‘failures’ (as defined in the methods section). In response to stimulation with 4-AP only 2% of the donor samples gave a ‘failed’ HA response, whereas HA failure occurred in ≈10% of the IVF patients (χ2= 7.9, P=0.025). Due to practical and technical limitations it was not possible to assess intracellular Ca2+ for all patients but of those who could be tested a failed response was recorded in 2/61 (~3%) and 2/7 (~25%) of IVF and ICSI patients respectively (P=0.048). In contrast none of 37 donor responses fell below the threshold defining failure (see methods). The two IVF patients who showed a failed intracellular Ca2+ response to 4-AP did not hyperactivate in response to 4AP and had poor IVF fertilisation rates (39%, 6%).
Stimulation with progesterone induced a significant [Ca2+]i response in >97% of samples from donors and all IVF patients. However, as with 4-AP, ICSI patients showed a higher failure rate (3/11 men, 27.3%; P=0.035 cf. donors). Failure of significant HA was much more common in response to stimulation with progesterone compared to 4-AP, 51% of donors and 62% of IVF samples failing to respond. In some of the samples where there was no significant induction of HA there were still detectable effects on motility. Changes in one or more kinematic parameters (VCL, ALH or LIN) were observed in 56% (19/34 samples) of the donors who failed to hyperactivate and 40% (23/57) of the IVF patients. 20/65 (31%) of IVF samples screened for both HA and intracellular Ca2+ showed a normal Ca2+ response to progesterone but no significant change in HA or kinematics as measured by CASA.
In most cases failure of HA was not stimulus-specific, the majority of samples that showed a failed HA response to 4-AP showing no significant response to IBMX, progesterone or NH4Cl. (Supplementary Table II)..
Correlation between HA and intracellular [Ca2+]i
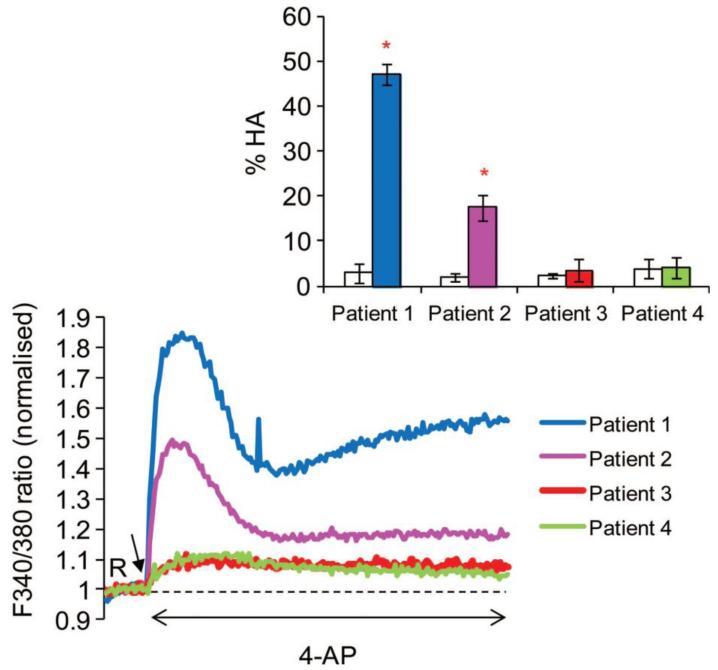
To determine whether or not there was a relationship between the magnitude of agonist-induced intracellular Ca2+ elevation (absolute increase in 340/380 ratio) and HA (absolute increase % cells), we examined responses in samples from IVF patients to 4-AP (n=57) and progesterone (n=65). There was a significant correlation for responses induced by 4-AP (R=0.35, P=0.009). Figure 3 shows data from four representative IVF patient samples upon stimulation with 4-AP. There was no significant relationship between the Ca2+ and HA responses induced by progesterone (Rs=−0.002, P=0.98), an observation consistent with the great disparity in [Ca 2+]i and HA responses seen with this agonist.
Figure 3.
Intracellular Ca2+ in response to 4-AP from four IVF patients including two with a failed Ca2+ response. Inset shows % HA from the same patients labelled with the same colours; white bars show % HA in the baseline (control) and coloured bars show % HA in samples treated with 4-AP. Patients 3 and 4 showed a failed intracellular Ca2+ and HA responses to 4-AP (IVF fertilization rates for patients 3 and 4–39% and 6% respectively). 4-AP was added to suspensions at 100 s after acquisition of 20 readings at resting level (R) indicated by black arrow. The HA data (inset) are the mean+SD. *Values are significantly different to baseline (P, 0.05). Ca2+ store mobilization and hyperactivation 7
Relationship between agonist-induced HA, [Ca2+]i and IVF fertilisation rates
To assess the clinical significance of [Ca2+]i and HA responses, basal and 4-AP-induced [Ca2+]i and HA in cells from IVF patients were examined in relation to fertilization rates. Analysis of [Ca2+]i data showed a significant relationship between basal intracellular Ca2+ (340/380 ratio before stimulation) and fertilisation rates (R=0.3, P=0.025). Additionally there was a significant relationship between the increment in [Ca2+]i induced by 4-AP (52 patients) and progesterone (57 patients) and fertilisation rates (R=0.28, P=0.047, R=0.280, P=0.037 respectively).
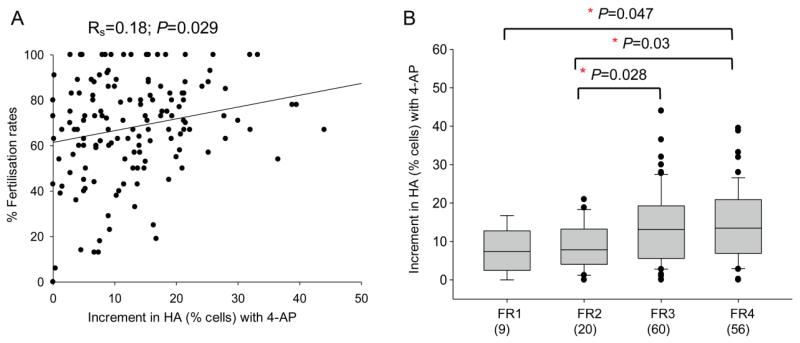
Fertilisation rate was significantly related both to the basal level of HA (Rs=0.19; P=0.02) and to the 4-AP-induced increment in HA (Rs=0.18, P=0.029) (Figure 4A). When the data were separated into four groups according to the fertilisation rates achieved: FR1 ≤25%, FR2 25%-50%, FR3 50-75%, FR4 >75%, it was clear that sperm samples giving the highest fertilisation rate also gave the greatest increment in HA when stimulated with 4-AP (Figure 4B). Incidence of failure to respond to 4-AP was similarly related to fertilisation success. There was no significant relationship between fertilisation rates and increment in HA in response to progesterone (n=84) or to the other agonists IBMX (n=89) and NH4Cl (n=68) (data not shown).
Figure 4.
Relationship between increment in HA (% cells) stimulated by 4-AP and IVF fertilization rate. (A) 4-AP-induced increment in HA (% cells) was significantly correlated to fertilization rate (Rs = 0.18; P = 0.031, n = 145). (B) Expression of these data in four defined groups according to fertilization rate: FR 1 ≤25%, FR2 .25–≤50%, FR3 .50–≤75%, FR4 .75%. Box and whisker plots show the 4-AP-induced increment in HA for the samples from patients in each group. The boxes represent the inter-quartile range and lines within them are the medians. The number in brackets is the sample size. Significance was assessed by one-way ANOVA.
Discussion
The primary aims of this study were to examine the incidence of defects in Ca2+ signalling pathways mediating HA among donors and sub fertile patients and the significance of such defects for IVF success. The most effective inducer of HA, in both donors and IVF patients, was 4-AP and this compound was therefore the focus of this study. Failure of HA in response to stimulation with 4-AP was significantly more common in IVF patients than in donors and failure of Ca2+ signalling was a common observation in ICSI patients. Importantly, both 4-AP-induced intracellular Ca2+ responses and consequent induction of HA were significantly related to IVF fertilisation rate. It has been established previously that there are differences in HA (spontaneous and in response to physiological or artificial stimulants) between men with proven fertility and sub-fertile patients (Burkman., 1984; Tesarik et al.,1992; Peedicayil et al.,1997; Munire et al.,2004). The data reported here document, for the first time, the biological significance of responses to direct, targeted manipulation of the Ca2+ signalling apparatus in human spermatozoa.
This study not only employed a large sample size (compared to other clinical studies on HA and [Ca2+]i signalling) but responses to agonist stimulation were assessed in samples that were used for IVF treatment, permitting direct comparison of functional responses with fertilisation success. In achieving this it was necessary to analyse the IVF samples in keeping with the clinical protocols. We believe this had a minimal effect on the results as (1) the donors and IVF samples were prepared using similar techniques and in comparable media supporting capacitation (data not presented, Moseley et al., 2005) and (2) though IVF samples were incubated in capacitating media for longer than donor samples, preliminary experiments showed that 4-AP-induced HA was not modified by varying incubation time (2-4 h) under capacitating conditions (data not presented). (3) Though ICSI samples were prepared and incubated in non capacitating conditions in the IVF laboratory, transfer to capacitating conditions restores [Ca2+]i responses within minutes (Bedu-Addo et al.,2005) and as such calcium assessments are robust and allow comparison between donors, IVF and ICSI patients. The robust nature of the data reported here is illustrated by the consistency in agonist-induced [Ca2+]i and HA responses between ejaculates (Supplementary Figure 1).
Differing potency of the agonists used
4-AP was the most effective inducer of HA in both donor and IVF cells. Cytoplasmic alkalinisation by 25 mM NH4Cl, is equivalent to the effect on pHi of 2 mM 4-AP (Alasmari et al., 2013). NH4Cl, induced Ca2+ influx under both capacitating and non capacitating conditions (data not presented) yet there was negligible effect on HA. These data confirm the pivotal role of stored Ca2+ in HA of human sperm and demonstrate that Ca2+-influx induced by elevated pHi alone is not sufficient to induce robust levels of HA (Figure 1). Progesterone induced an intracellular calcium response in >97% of donors and all IVF patients yet HA was weak. It has been suggested that the large progesterone-induced [Ca2+]i transient (up to 40 seconds) is accompanied by a burst of HA in some cells (Gakamsky et al., 2009, Servin-Vences et al., 2012). This brief effect is hard to detect using standard CASA and in our study HA was not significantly different between 1 and 5 minutes of treatment with progesterone (data not presented). Thus Ca2+ influx (through CatSper) induced by progesterone, similarly to cytoplasmic alkalinisation, was not sufficient to induce sustained changes in kinetic parameters and consistently reach the threshold criteria to identify HA. Whilst CatSper channels are essential for HA in mice (Ren et al.,2001; Carlson et al., 2003; Quill et al.,2003), their role in humans remains largely unknown (Brenker et al., 2012). Genetic studies have indicated that mutations in human CatSper channels are significant in rare cases of male infertility (Avidan et al., 2003; Avenarius et al., 2009), but HA was not examined. Our observations clearly do not preclude involvement of CatSper in human HA. CatSper may become activated during capacitation, supporting spontaneous HA (Alasmari et al., 2013) and when strongly activated (as occurs during the progesterone transient) CatSper may trigger activation of Ca2+ stores or store-operated channels (Lefievre et al., 2012). It is also possible that more sophisticated sperm function tests may be required to determine the effect of direct CatSper activation such as penetration into a viscous media (Ivic et al., 2002) or more sophisticated analysis of flagella motion - 3-dimensional tracking (Su et al., 2012) and high-speed videomicroscopy (Kirkman Brown & Smith 2011; Curtis et al., 2012)
IBMX was used to stimulate HA by strong activation of the cAMP pathway, which does not induce an immediate Ca2+ influx in human sperm cells (Strunker et al., 2011). The majority of the samples from donors and IVF patients responded to IBMX with a significant increase in HA, consistent with other studies on human sperm, which have reported a specific influence of phosphodiesterase inhibitors such as pentoxyfilline on the kinematic parameters defining HA (Tesarik et al.,1992; Kay et al.,1993; Tournaye et al.,1994). These observations are in contrast to other studies on bull sperm which have documented that HA required only the elevation of intracellular Ca2+ and did not depend on the AC/cAMP/PKA signalling pathway (Ho et al.,2002; Marquez and Suarez, 2004; Marquez and Suarez, 2008). The difference in findings between studies on human and bull sperm may simply reflect species variation in the requirements for induction of HA.
Differences in HA and calcium between donor and patient groups
4-AP-induced HA was correlated with the amplitude of the [Ca2+]i response, confirming that 4-AP-induced HA was mediated by this pathway. The proportion of samples in which 4-AP failed to induce normal HA and [Ca2+]i responses was significantly greater in IVF samples than in donors showing that malfunction of the Ca2+-signalling pathway activated by 4-AP is likely to be responsible for a proportion of cases of male-factor sub-fertility. Additionally, there was a clear difference in [Ca2+] iresponses between ICSI patients and other populations (donors and IVF patients) demonstrating increased incidence of [Ca2+] i abnormalities in the former. Interestingly, the basal Ca2+ was significantly lower in spermatozoa from ICSI patients (Supplementary Table 1; P<0.05). This suggests that these cells have a reduced ability to initiate the Ca2+ signalling cascade. The reasons for this are unclear but may be due to (1) low expression or abnormalities in CatSper leading to reduction in Ca2+ influx across the plasma membrane and consequently poor recruitment of intracellular Ca2+ stores and/or (2) the sperm cytoplasm does not become sufficiently alkalinised to activate CatSper due to abnormality in the expression or regulation of HV1; and/ or (3) defects in internal store channels such as RyRs, IP3R or SOCs.
Samples which gave a failed HA response to 4-AP usually failed to respond to IBMX, progesterone or NH4Cl. Though the lower efficacy of these stimuli as inducers of HA (particularly progesterone and NH4Cl) makes interpretation complex, it appears many HA failures involve a signalling node or late stage in a cascade such that alternative stimulation (such as increasing cAMP) does not bypass the lesion (new Figure 5). HA failure may reflect either failure of Ca2+ signalling itself or of events downstream of Ca2+, independent of the [Ca2+]i signal amplitude. Analysis of HA and [Ca2+]i in 4-AP-stimulated cells showed that the amplitudes of the two responses were significantly correlated and that in those samples where [Ca2+]i signal failure occurred (≈3%), there was also a failure of HA. Thus it is likely that failed HA can reflect failure of Ca2+ signalling. Potential defects include impaired capacitation, which may affect activity of CatSper channels (Lishko et al.,2011; Strunker et al.,2011), resulting in a low resting [Ca2+]i and consequent effects on emptying and filling of the internal stores. It has been suggested that Src kinase located in the neck/midpiece and in the post-acrosomal region of human sperm head regulates Ca2+ store mobilisation during capacitation (Varano et al.,2008). Further investigation of Ca2+ store filling and mobilisation in sperm is required to clarify this.
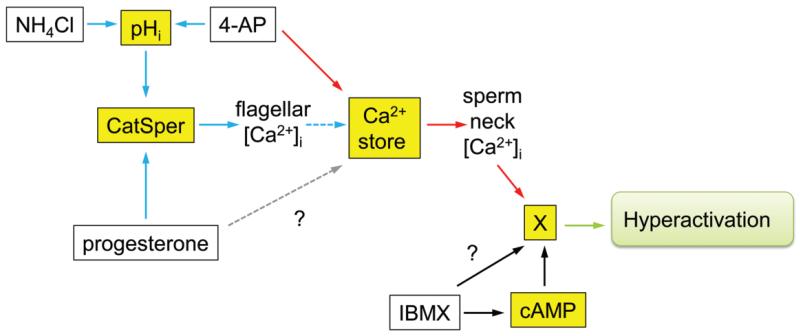
Figure 5.
Model of the calcium-signalling cascade in the human spermatozoon: key points in the pathway affected in sub-fertile men (adapted from Barratt and Publicover, 2012). White boxes show compounds used to manipulate pHi (NH4Cl, 4AP), stored calcium (4AP) and cAMP (IBMX). Yellow boxes show signalling components activated by these compounds (pHi, Ca2+, cAMP). Blue and red arrows show Ca2+-signalling pathways involving CatSper and the calcium store, respectively. The Ca2+-store pathway may involve activation of SOCs (not shown; Lefievre et al., 2012). Dashed blue arrow shows the mobilization of stored Ca2+ downstream of CatSper activation by CICR. This occurs in a minority of the cells, is sensitive to modulation (e.g. by capacitation) and is responsible for CatSper-mediated hyperactivation. Dashed grey arrow shows possible modulation of Ca2+-store mobilization by progesterone through CatSper-independent mechanisms (Sagare-Patil et al., 2012). X represents unknown target mechanism/pathway whereby following mobilization of stored Ca2+ hyperactivation is stimulated.? represents potential pathway requiring further experimentation. The amplitude of the calcium transient (stimulated by both 4AP and progesterone) and the hyperactivation response (to 4AP) were significantly related to IVF fertilization rates suggesting the occurrence of important abnormalities in the Ca2+-signalling pathways mediated by CatSper (blue) and the calcium store (red). The calcium signal (4AP and progesterone) in the ICSI patients was significantly lower than in the donors or the IVF patients (≈25%; Fig. 2), providing further evidence of abnormalities in the CatSper functioning/operating complex and calcium store in male infertility. Examination of individual cases demonstrated ≈10% of men undergoing IVF had defective calcium hyperactivation. Although the data are limited, a number of these men did not show a hyperactivation response to IBMX (probably through cAMP, black arrows,?). Previous studies have indicated that, in humans, increases in cAMP do not lead to changes in [Ca2+]i (Brenker et al., 2012), thus these men may suffer from a specific defect in hyperactivation as opposed to a Ca2+-signalling deficit (Supplementary data, Table SII).
Stimulus-induced [Ca2+]i elevation, HA and fertilisation success
Fertilisation rate of the IVF samples was correlated with basal HA, a finding that is concordant with data from other studies (Burkman., 1991; Wang et al.,1993; Sukcharoen et al., 1995). More significantly, the ability of 4-AP to raise [Ca2+]i and to induce HA was correlated significantly with IVF outcomes, providing clear evidence of a biological role of the sperm Ca2+ signalling apparatus (almost certainly store mobilisation; see above) in human sperm (Figures 4). Amplitude of the [Ca2+] itransient induced by progesterone, probably reflecting activation of CatSper (Strünker et al.,2011; Lishko et al.,2011), was similarly correlated to fertilisation rates at IVF, consistent with other studies (Krausz et al., 1996). However, progesterone was not a potent inducer of HA (Figure 1) and targeted activation of CatSper is not sufficient to induce HA in human sperm (Alasmari, 2013), thus there was no significant correlation between progesterone induced HA and IVF success. Defective Ca2+ responses to progesterone were not seen in IVF patients and occurred in one donor and three ICSI patients. This low incidence of functionally significant CatSper defects, as manifested by failed [Ca2+]i responses, suggests that such failures are relatively rare. Patch clamping studies will be required to confirm abnormalities in CatSper function (Kirichok and Lishko, 2011, Lefievre et al., 2012).
In summary, this study highlights the biological role and importance of Ca2+-signalling (and neck/midpiece Ca2+ store mobilisation) for fertilisation at IVF and identifies for the first time, the incidence of store defects among donors, IVF and ICSI patients. Further studies are required to investigate the nature of these defects. Such studies will act as a platform for a potential drug based screening program to augment/modulate calcium mobilisation as a possible rational treatment for sperm dysfunction.
Supplementary Material
Acknowledgements
Work in the authors’ laboratories is funded by The Welcome Trust, TENOVUS (Scotland), University of Dundee, MRC (Developmental Pathway Funding Scheme), Ministry of Higher Education-Kingdom of Saudi Arabia (PhD studentship to Wardah Alasmari), NHS Tayside and Scottish Enterprise. Resources from a Welcome Trust Project Grant (Principal investigators C. Barratt and S. Publicover [grant # 086470]) primarily funded the data presented in this study.
The authors are very grateful to all members of the Assisted Conception Unit at Ninewells Hospital for their invaluable assistance in obtaining donor and patient samples for research and training purposes, in particular the embryologists (Ellen, Sylvia, Anne and Philip) and nurses. We are also grateful to all the patients and donors who took part in this study. The authors acknowledge other members of the laboratory, in particular Dr Lindsay Tulloch, Dr Steve Tardif and Steven Mansell for their continual helpful advice and comments, and Evelyn Barratt for assisting with the recruitment of patients and donors. We also acknowledge the statistical support of Professor Steve Hubbard, Professor C De Jonge for reading and commenting on the manuscript and Timo Strunker for advice regarding FLUOstar.
Footnotes
Further information.
The Excel spread sheets with all the primary data is available from c.barratt@dundee.ac.uk
References
- Aitken RJ, Henkel RR. Sperm cell biology: current perspectives and future prospects. Asian J Androl. 2011;13:3–5. doi: 10.1038/aja.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Irvine DS, Wu FC. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol. 1991;164:542–51. doi: 10.1016/s0002-9378(11)80017-7. [DOI] [PubMed] [Google Scholar]
- Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L, Kirkman-Brown J, Michelangeli M, Publicover S, Barratt CLR. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviours in human sperm. J Biol Chem. 2013;288:6248–6258. doi: 10.1074/jbc.M112.439356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LL, Kahrizi K, Najmabadi H, Smith RJ. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet. 2009;84:505–10. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, Thulliez M, Borot N, Moati L, Barthelme A, Shalmon L, Krasnov T, Ben-Asher E, Olender T, Khen M, Yaniv I, Zaizov R, Shalev H, Delaunay J, Fellous M, Lancet D, Beckmann JS. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet. 2003;11:497–502. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl. 1991;12:323–330. [PubMed] [Google Scholar]
- Barratt C, Mansell S, Beaton C, Tardif S, Oxenham S. Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl. 2011;13:53–58. doi: 10.1038/aja.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt CL, Publicover SJ. Sperm are promiscuous and CatSper is to blame. EMBO J. 2012;31:1624–6. doi: 10.1038/emboj.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedu-Addo K, Costello S, Harper C, Machado-Oliveira G, Lefievre L, Ford C, Barratt CL, Publicover S. Mobilisation of stored calcium in the neck region of human sperm--a mechanism for regulation of flagellar activity. Int J Dev Biol. 2008;52:615–26. doi: 10.1387/ijdb.072535kb. [DOI] [PubMed] [Google Scholar]
- Bedu-Addo K, Lefièvre L, Moseley FL, Barratt CL, Publicover SJ. Bicarbonate and bovine serum albumin reversibly ‘switch’ Bicarbonate and bovine serum albumin reversibly ‘switch’ capacitation-induced events in human spermatozoa. Mol Hum Reprod. 2005;11:683–691. doi: 10.1093/molehr/gah226. [DOI] [PubMed] [Google Scholar]
- Bhaskar A, Subbanna PK, Arasan S, Rajapathy J, Rao JP, Subramani S. 4-aminopyridine-induced contracture in frog ventricle is due to calcium released from intracellular stores. Indian J Physiol Pharmacol. 2008;52:366–74. [PubMed] [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar D, Naruse M, Krahling M, Muller A, Kaupp B, Strunker T. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J. 2012;31:1654–65. doi: 10.1038/emboj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkman LJ. Characterization of hyperactivated motility by human spermatozoa during capacitation: comparison of fertile and oligozoospermic sperm populations. Arch Androl. 1984;13:153–65. doi: 10.3109/01485018408987514. [DOI] [PubMed] [Google Scholar]
- Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A. 2003;100:14864–8. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Suarez SS. Two Distinct Ca2+ Signaling Pathways Modulate Sperm Flagellar Beating Patterns in Mice. Biol Reprod. 2011;85:296–305. doi: 10.1095/biolreprod.110.089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, Michelangeli F, Nash K, Lefievre L, Morris J, Machado-Oliveira G, Barratt C, Kirkman-Brown J, Publicover S. Ca2+-stores in sperm: their identities and functions. Reprod. 2009;138:425–37. doi: 10.1530/REP-09-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MP, Kirkman-Brown JC, Connolly TJ, Gaffney EA. Modelling a tethered mammalian sperm cell undergoing hyperactivation. J Theor Biol. 2012;309:1–10. doi: 10.1016/j.jtbi.2012.05.035. [DOI] [PubMed] [Google Scholar]
- DeMott RP, Suarez SS. Hyperactivated sperm progress in the mouse oviduct. Biol Reprod. 1992;46:779–785. doi: 10.1095/biolreprod46.5.779. [DOI] [PubMed] [Google Scholar]
- Falsetti C, Baldi E, Krausz C, Casano R, Failli P, Forti G. Decreased responsiveness to progesterone of spermatozoa in oligozoospermic patients. J Androl. 1993;14:17–22. [PubMed] [Google Scholar]
- Gakamsky A, Armon L, Eisenbach M. Behavioral response of human spermatozoa to a concentration jump of chemoattractants or intracellular cyclic nucleotides. Hum Reprod. 2009;24:1152–1163. doi: 10.1093/humrep/den409. [DOI] [PubMed] [Google Scholar]
- Grimaldi M, Atzori M, Ray P, Alkon DL. Mobilization of calcium from intracellular stores potentiation of neurotransmitter-induced calcium transients and capacitative calcium entry by 4-aminopyridine. J Neurosci. 2001;21:3135–43. doi: 10.1523/JNEUROSCI.21-09-03135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Kirkman-Brown JC, Korchev Y, Barratt CL, Publicover SJ. Multi-state, 4-aminopyridine-sensitive ion channels in human spermatozoa. Dev Biol. 2004;274:308–317. doi: 10.1016/j.ydbio.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Gwathmey TM, Ignotz GG, Suarez SS. PDC-109 (BSP-A1/A2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biol Reprod. 2003;69:809–815. doi: 10.1095/biolreprod.102.010827. [DOI] [PubMed] [Google Scholar]
- Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca2+](i) oscillations and cyclical transitions in flagellar beating. J Biol Chem. 2004;279:46315–25. doi: 10.1074/jbc.M401194200. [DOI] [PubMed] [Google Scholar]
- Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol. 2002;250:208–17. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- Ho K, Wolff CA, Suarez SS. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod Fertil Dev. 2009;21:345–50. doi: 10.1071/rd08183. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65:1606–15. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod. 2003;68:1590–6. doi: 10.1095/biolreprod.102.011320. [DOI] [PubMed] [Google Scholar]
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;29:1693–7. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13(Suppl 1):33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Honda H. Inhibitory action of 4-aminopyridine on Ca(2+)-ATPase of the mammalian sarcoplasmic reticulum. J Biol Chem. 1993;268:4021–4024. [PubMed] [Google Scholar]
- Ivic A, Onyeaka H, Girling A, Brewis IA, Ola B, Hammadieh N, Papaioannou S, Barratt C. Critical evaluation of methylcellulose as an alternative medium in sperm migration tests. Hum Reprod. 2002;17:143–9. doi: 10.1093/humrep/17.1.143. [DOI] [PubMed] [Google Scholar]
- Kay VJ, Coutts JR, Robertson L. Pentoxifylline stimulates hyperactivation in human spermatozoa. Hum Reprod. 1993;8:727–31. doi: 10.1093/oxfordjournals.humrep.a138129. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Lishko PV. Rediscovering sperm ion channels with the patch-clamp technique. Mol Hum Reprod. 2011;17:478–99. doi: 10.1093/molehr/gar044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Smith DJ. Sperm motility: is viscosity fundamental to progress? Mol Hum Reprod. 2011;17:539–44. doi: 10.1093/molehr/gar043. [DOI] [PubMed] [Google Scholar]
- Krausz C, Bonaccorsi L, Maggio P, Luconi M, Criscuoli L, Fuzzi B, Pellegrini S, Forti G, Baldi E. Two functional assays of sperm responsiveness to progesterone and their predictive values in in-vitro fertilization. Hum Reprod. 1996;11:1661–7. doi: 10.1093/oxfordjournals.humrep.a019466. [DOI] [PubMed] [Google Scholar]
- Lefievre L, Nash K, Mansell S, Costello S, Punt E, Correia J, Morris J, Kirkman-Brown J, Wilson SM, Barratt CL, Publicover S. 2-APB-potentiated channels amplify CatSper-induced Ca2+ signals in human sperm. Biochem J. 2012 Sep 3; doi: 10.1042/BJ20120339. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–91. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev Biol. 2007;303:214–21. doi: 10.1016/j.ydbio.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez B, Suarez SS. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol Reprod. 2007;76:660–5. doi: 10.1095/biolreprod.106.055038. [DOI] [PubMed] [Google Scholar]
- Marquez B, Suarez SS. Different signaling pathways in bovine sperm regulate capacitation and hyperactivation. Biol Reprod. 2004;70:1626–33. doi: 10.1095/biolreprod.103.026476. [DOI] [PubMed] [Google Scholar]
- Marquez B, Suarez SS. Soluble adenylyl cyclase is required for activation of sperm but does not have a direct effect on hyperactivation. Reprod Fertil Dev. 2008;20:247–52. doi: 10.1071/rd07146. [DOI] [PubMed] [Google Scholar]
- Mortimer D. Elaboration of a new culture medium for physiological studies on human sperm motility and capacitation. Hum Reprod. 1986;1:247–50. doi: 10.1093/oxfordjournals.humrep.a136394. [DOI] [PubMed] [Google Scholar]
- Mortimer ST, Swan MA, Mortimer D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum Reprod. 1998;13:2139–2146. doi: 10.1093/humrep/13.8.2139. [DOI] [PubMed] [Google Scholar]
- Moseley FLC, Jha KN, Björndahl L, Brewis IA, Publicover SJ, Barratt CL, Lefièvre L. Induction of human sperm capacitation varies between incubation media; an effect that is not associated with protein kinase A activation. Mol Hum Reprod. 2005;11:523–529. doi: 10.1093/molehr/gah188. [DOI] [PubMed] [Google Scholar]
- Munire M, Shimizu Y, Sakata Y, Minaguchi R, Aso T. Impaired hyperactivation of human sperm in patients with infertility. J Med Dent Sci. 2004;51:99–104. [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA. 2007;104:7688–92. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SD, Fauci LJ, Suarez SS. Mathematical modeling of calcium signaling during sperm hyperactivation. Mol Hum Reprod. 2011;17:500–10. doi: 10.1093/molehr/gar040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey AA, Davies N, Warren MA, Barratt CL, Cooke ID. Hyperactivation may assist human spermatozoa to detach from intimate association with the endosalpinx. Hum Reprod. 1995;10:2603–9. doi: 10.1093/oxfordjournals.humrep.a135754. [DOI] [PubMed] [Google Scholar]
- Park KH, Kim BJ, Kang J, Nam TS, Lim JM, Kim HT, Park JK, Kim YG, Chae SW, Kim UH. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci Signal. 2011;4(173):ra31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- Peedicayil J, Deendayal M, Sadasivan G, Shivaji S. Assessment of hyperactivation, acrosome reaction and motility characteristics of spermatozoa from semen of men of proven fertility and unexplained infertility. Andrologia. 1997;29:209–18. doi: 10.1111/j.1439-0272.1997.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Publicover SJ, Barratt CL. Sperm motility: things are moving in the lab! Mol Hum Reprod. 2011;17:453–6. doi: 10.1093/molehr/gar048. [DOI] [PubMed] [Google Scholar]
- Publicover S, Barratt C. Reproductive biology: Progesterone’s gateway into sperm. Nature. 2011;471:313–4. doi: 10.1038/471313a. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm--making the most of what you’ve got. Nat Cell Biol. 2007;9:235–42. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci U S A. 2003;100:14869–74. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren DNB, Perez G, Jackson Ac, Hsu S, Shi Q, Tilly Jl, Clapham De. A sperm ion channel required for sperm motility and male fertiliy. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Boron WF, Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sagare-Patil V, Galvankar M, Satiya M, Bhandari B, Gupta SK, Modi D. Differential concentration and time dependent effects of progesterone on kinase activity, hyperactivation and acrosome reaction in human spermatozoa. Int J Androl. 2012;35:633–44. doi: 10.1111/j.1365-2605.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- Servin-Vences R, Tatsu Y, Ando H, Guerrero A, Yumoto N, Darszon A, Nishigaki T. A caged progesterone analog alters intracellular Ca2+ and flagellar bending in human sperm. Reproduction. 2012;144:101–9. doi: 10.1530/REP-11-0268. [DOI] [PubMed] [Google Scholar]
- Stauss CR, Votta TJ, Suarez SS. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod. 1995;53:1280–5. doi: 10.1095/biolreprod53.6.1280. [DOI] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–6. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Dai X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol Reprod. 1992;46:686–91. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Katz DF, Owen DH, Andrew JB, Powell RL. Evidence for the function of hyperactivated motility in sperm. Biol Reprod. 1991;44:375–81. doi: 10.1095/biolreprod44.2.375. [DOI] [PubMed] [Google Scholar]
- Su TW, Xue L, Ozcan A. High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories. Proc Natl Acad Sci U S A. 2012;109:16018–22. doi: 10.1073/pnas.1212506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukcharoen N, Keith J, Irvine DS, Aitken RJ. Predicting the fertilizing potential of human sperm suspensions in vitro: importance of sperm morphology and leukocyte contamination. Fertil Steril. 1995;63:1293–300. doi: 10.1016/s0015-0282(16)57614-6. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Mendoza C. Defective function of a nongenomic progesterone receptor as a sole sperm anomaly in infertile patients. Fertil Steril. 1992;58:793–7. doi: 10.1016/s0015-0282(16)55329-1. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Thebault A, Testart J. Effect of pentoxifylline on sperm movement characteristics in normozoospermic and asthenozoospermic specimens. Hum Reprod. 1992;7:1257–63. doi: 10.1093/oxfordjournals.humrep.a137837. [DOI] [PubMed] [Google Scholar]
- Tournaye H, Wieme P, Janssens R, Verheyen G, Devroey P, Van Steirteghem A. Incubation of spermatozoa from asthenozoospermic semen samples with pentoxifylline and 2-deoxyadenosine: variability in hyperactivation and acrosome reaction rates. Hum Reprod. 1994;9:2038–43. doi: 10.1093/oxfordjournals.humrep.a138390. [DOI] [PubMed] [Google Scholar]
- Varano G, Lombardi A, Cantini G, Forti G, Baldi E, Luconi M. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod. 2008;23:2652–2662. doi: 10.1093/humrep/den314. [DOI] [PubMed] [Google Scholar]
- Wang C, Lee GS, Leung A, Surrey ES, Chan SY. Human sperm hyperactivation and acrosome reaction and their relationships to human in vitro fertilization. Fertil Steril. 1993;59:1221–7. [PubMed] [Google Scholar]
- World Health Organization . WHO laboratory manual for the examination of human semen and sperm-cervical mucus interactions. 4th edn Cambridge University Press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.