Abstract
Perineuronal nets are extracellular matrix structures surrounding cortical neuronal cell bodies and proximal dendrites, and are involved in the control of brain plasticity and the closure of critical periods. Expression of the link protein Crtl1/Hapln1 in neurons has recently been identified as the key event triggering the formation of perineuronal nets. Here we show that the genetic attenuation of perineuronal nets in adult brain Crtl1 knockout mice enhances long term object recognition memory and facilitates long-term depression in the perirhinal cortex, a neural correlate of object recognition memory. Identical prolongation of memory follows localised digestion of perineuronal nets with chondroitinase ABC, an enzyme that degrades the chondroitin sulphate proteoglycans (CSPGs) components of PNNs. The memory-enhancing effect of chondroitinase ABC treatment attenuated over time, suggesting that regeneration of PNNs gradually restored control plasticity levels. Our findings indicate that perineuronal nets regulate both memory and experience-driven synaptic plasticity in adulthood.
Introduction
Recent studies show that specialized structures of condensed and stable extracellular matrix known as perineuronal nets (PNNs), which form around the synapses on the cell soma and proximal dendrites of neurons (Hartig et al., 1992; Carulli et al., 2007; Carulli et al., 2010; Dityatev et al., 2010), play a key role in the control of plasticity in the CNS (Berardi et al., 2000; Kwok et al., 2011). For example, degradation of chondroitin sulphate proteoglycans (CSPGs), major components of PNNs, with chondroitinase ABC (chABC) can re-open the juvenile critical period for ocular dominance plasticity in adult rats (Pizzorusso et al., 2002). Furthermore, treatment with chABC facilitates recovery from spinal cord injury and other forms of damage to the nervous system (Kwok et al., 2011; Bartus et al., 2012). Similar results have been obtained with adult knockout mice lacking the Crtl1/Hapln1 gene in the CNS (Ctrl1 ko), which encodes a link protein essential for PNN formation, thus leading to attenuated PNNs in these animals (Czipri et al., 2003; Carulli et al., 2010).
Experience-driven changes in synaptic strength are not only a driving factor for sensory development, but are essential for learning and memory (Bliss and Collingridge, 1993; Martin and Morris, 2002; Griffiths et al., 2008). The increase in plasticity following attenuation of PNNs in the visual cortex and elsewhere, together with the observation that chondroitinase digestion in the amygdala enhances fear erasure (Gogolla et al., 2009), raises the intriguing possibility that a similar approach could be used to enhance the forms of plasticity thought to underlie declarative/explicit learning and memory (Squire and Zola-Morgan, 1988).
In animals, this type of memory is commonly modelled using the spontaneous object recognition task (Ennaceur and Delacour, 1988; Manns and Squire, 1999; Forwood et al., 2005; Bussey and Saksida, 2007; Eichenbaum et al., 2007; Winters et al., 2008; Saksida, 2009; Bartko et al., 2011). In the present study we used this test, along with two methods of attenuating PNNs, to explore whether a reduction of PNNs in adult animals can enhance recognition memory. In addition, we assessed synaptic function and plasticity with extracellular field recordings in the perirhinal cortex, a structure critical for object recognition memory.
We found that either genetic reduction of PNNs in Crtl1 ko mice, or the less specific but more localized and reversible enzymatic degradation of PNN structures with chABC, prolonged long-term recognition memory. Furthermore, genetic or pharmacological reduction of PNNs enhanced basal synaptic transmission and long-term depression (LTD) in the perirhinal cortex, a form of synaptic plasticity thought by many to be the principal physiological mechanism underlying object recognition memory (Brown et al., 1987; Zhu et al., 1996; Xiang and Brown, 1998; Wan et al., 2004; Griffiths et al., 2008; Massey et al., 2008; Kealy and Commins, 2011).
Materials and Methods
Animal housing and conducting of animal experiments
All mice were housed in groups of 2 or 3, in a room with a 12 h light/dark cycle (lights off at 7:00 P.M.). Food and water were available ad libitum throughout the experiment. All experimentation was conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act (1986).
Genetic mouse model of PNN reduction
Postnatal neuronal expression of Crtl1 is the key event triggering formation of PNNs (Carulli et al., 2010). For this study, we used mice lacking the Crtl1/Hapln1 gene in the CNS, but not cartilage, which leads to attenuated PNNs in the adult cortex (Ctrl1 ko; Czipri et al., 2003). Since the Crtl1 product is essential for cartilage, Crtl1 was disrupted globally (Crtl1−/−), and then reintroduced under the control of the type II collagen cartilage-specific promotor by crossbreeding with a second transgenic mouseline (Crtl1-Tg). Resulting Crtl1−/−/Crtl1-Tg mice were on a BALB/C background. For behavioural and electrophysiological experiments, they were backcrossed into a 129sV background for >7 generations. Testing cohorts of male, 3 month old homozygous Crtl1−/−/Crtl1-Tg and wild type (Crtl1+/+) littermates were obtained by pairing Crtl1+/−/Crtl1-Tg females with Crtl1+/− males. Although a cartilage-specific promoter was used to express the Crtl1-Tg, and Crtl-1 was absent from brain in Crtl1-Tg mice (Czipri et al., 2003), we initially used a second control group of wild type mice expressing the Crtl-Tg (Crtl1+/+/Crtl-Tg). However, no apparent behavioural differences were found between wild type mice and wild type mice expressing the Crtl1-Tg in cartilage (Figure 2 CD, Table 1 A). Therefore, wild type mice were used as control group in all following experiments. Genotypes were identified by PCR with primers for wt Crtl1, disrupted Crtl1 and the Crtl1 transgene expressed in cartilage (Czipri et al., 2003).
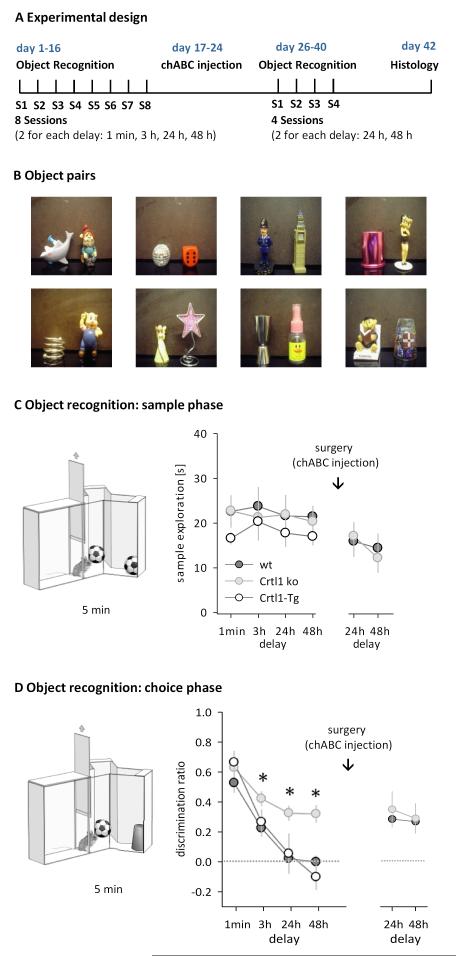
Figure 2. Mice deficient in brain Crtl1 show persistent memory on an object recognition paradigm.
A Schematic diagram of the experimental design. B Photographs of 8 representative object pairs used for object recognition experiments (out of 12 pairs used in total). C Sample phase. Illustration of the procedure (left panel) and sample exploration times before and after chABC treatment, plotted separately for each subsequent delay (right panel). D Choice phase. Illustration of the procedure (left panel) and discrimination rates after each delay, before and after chABC treatment (right panel). Data are presented as mean +/− sem, * simple main effect, p < 0.05.
Table 1.
RM ANOVA results for Crtl1 ko mice vs Crtl1-Tg and wt mice
| Main effect or interaction |
F | p | simple main effects | |
|---|---|---|---|---|
| A Discrimination ratio with delays of 1 min, 3 h, 24 h, 48 h | ||||
| genotype | F2,25 = 32.7 |
< 0.001 | Crtl1 ko vs. wt: F1,21 = 21.34, p < 0.001 Crtl1 ko vs. Crtl1-Tg: F1,16 = 18.2, p < 0.001 Crtl1-Tg vs. wt: F > 1, p = 0.842 |
|
| delay | F3,75 = 31.1 |
< 0.001 | ||
| genotype × delay |
F6,75 = 9.2 |
= 0.003 |
Crtl1 ko vs. wt: 1 min: F < 1, p = 0.121 3 h: F1,21 = 4.1, p = 0.014 24 h: F1,21 = 4.5, p = 0.030 48 h: F1,21 = 10.2, p < 0.001 |
Crtl1-Tg vs wt: 1 min: F < 1, p = 0.778 3 h: F < 1, p = 0.971 24 h: F < 1, p = 0.882 48 h: F < 1, p = 0.612 |
| B Discrimination ratio with delays of 24 h and 48 h before and after chABC treatment | ||||
| genotype | F1,9 = 9.1 |
= 0.015 |
Crtl1 ko vs. wt: Before chABC: F1,9 = 18.9, p = 0.009; After chABC: F < 1, p = 0.751 |
|
| delay | F < 1 | = 0.410 | ||
| chABC | F1,9 = 2.1 |
= 0.179 |
Before vs. after treatment: wt: F1,9 = 5.7, p = 0.041; Crtl1 ko: F < 1, p = 0.664 |
|
| chABC × genotype |
F1,9= 4.3 |
= 0.043 | ||
| chABC × genotype × delay |
F < 1 | = 0.757 | ||
Pharmacological mouse model of PNN reduction
PNNs have a structure similar to cartilage, in which link proteins (Crtl1/Hapln1 and Bral2/Hapln4) stabilise the binding of various chondroitin sulphate proteoglycans (CSPGs) to hyaluronan (Morgelin et al., 1994; Watanabe et al., 1998; Carulli et al., 2007; Kwok et al., 2011). Digestion of CSPGs with the enzyme chABC leads to a marked reduction of PNNs around proximal dendrites in the cortex, although it also affects the diffuse ECM around neurons and glia (Pizzorusso et al., 2002; Gogolla et al., 2009). Protease-free chondroitinase ABC (Seikagaku Kogyo) or penicillinase (Sigma, P0389) were dissolved to 50 U/ml in 0.1 % BSA and filtered through a 0.2micron filter. 6 injections (3 per hemisphere, 0.2 μl each at 0.1 μl per minute) were performed stereotaxically under isoflurane anesthesia with a 1 μl Hamilton syringe and a 33 gauge needle at the following sites in the perirhinal cortex (in mm from bregma and the surface of the dura mater): 1. anteriorposterior (AP): −1.755; lateral (L): ± 4.6; ventral (V): −4.4. 2. AP: −2.88; L: ± 4.8; V: −4.25. 3. AP: −3.88; L: ± 4.8; V: −3.75. After each injection, the needle was left in situ for another 4 minutes before being withdrawn. For post-operative pain management, animals received intraperitoneal injections of Meloxicam (Metacam, 0.1 ml /10g).
Spontaneous Object Recognition
The task was conducted in a Y-shaped apparatus (Figure 2 C,D) as previously described for rats (Ennaceur and Delacour, 1988; Winters et al., 2004) and mice (Bartko et al., 2011). The Y-apparatus had high, homogenous white walls constructed from Perspex. One arm was used as the start arm, and the other two arms were used to display the objects (randomly shaped junk objects, dimensions approximately 10 cm × 4cm × 4 cm, Figure 2 B). All walls were 30 cm high, and each arm was 16 cm in length and 8 cm wide. A lamp illuminated the apparatus, and a video camera was mounted 50 cm above the apparatus to record trials. All mice were habituated in two consecutive daily sessions in which they were to explore the empty Y-apparatus for 5 min. The following test sessions were separated by a minimum of 48 h. Each test session consisted of a sample phase and a choice phase. In the sample phase, two identical objects A were placed at the end of each arm. The animal was left to explore the objects for 5 minutes (Figure 2 C, left panel). The choice phase followed after a delay of either 1 min, 3 h, 24 h or 48 h, which the animal spent in the home cage. The choice phase was procedurally identical to the sample phase, except that one arm contained a novel object B, whereas the other arm contained the familiar object A (Figure 2 D, left panel). Each animal received 2 test sessions for each delay. A different object pair was used for each session for a given animal, and the order of exposure to object pairs as well as the designated sample and novel objects for each pair were counterbalanced within and across groups. The time spent exploring objects was assessed from video recordings of the sample and choice phases. Exploratory bouts were scored using a personal computer running a custom made program written in Visual Basic 6.0 (Microsoft). Times where an animal climbed or sat on an object were not counted. For the choice phase, a discrimination score was calculated by dividing the difference in exploration of the novel and familiar objects by the total object exploration time. Therefore, a score of 1 corresponded to exploration of the novel object only, whereas a score of 0 corresponded to the mouse equally exploring the novel and the familiar object. The mean discrimination score across the two test sessions was calculated for each animal. Group means were compared by ANOVA with a significance level of p < 0.05, using SPSS V. 10.
Immunohistochemistry
16 days after chABC or Pnase injections (if applicable), animals were anesthetized by intraperitoneal injections of 0.5ml Euthalal (Rhône Mérieux) and perfused transcardially with 50 ml 0.1 M phosphate buffered saline (PBS, ph 7.4) followed by 50 ml 4 % paraformaldehyde. The brains were removed and cryoprotected in 25 % sucrose in PBS overnight. Coronal sections (20 μm) were cut on a cryostat. The N-acetylgalactosamine-binding lectin Wisteria floribunda agglutinin was used to visualise PNNs. Labelling was performed by first rinsing tissue with 0.1 M PBS followed by quenching of endogenous peroxidase activity with 10 % methanol, 2 % H2O2, and 0.3% Triton X-100 in PBS for 10 min at RT. Tissue was rinsed in triplicate in PBS and was subsequently blocked with 3% NGS, 0.3 % Triton X-100 in PBS for one hour at room temperature (RT). Tissue was then incubated with biotinylated wisteria floribunda agglutinin (20 μg/ml, Sigma Aldrich) overnight at 4°C. The following day tissue was rinsed in triplicate in PBS and followed by incubation with ABC solution (ABC Elite Kit, Vector Labs) for one hour at RT. After the tissue was rinsed in triplicate with tris non-saline (TNS), tissue was incubated with diaminobenzidine (0.5 mg/ml in TNS with 0.3 μl H2O2, Sigma) for 5-10 min at RT. Tissue was rinsed thoroughly with TNS before coverslips were applied using DPX mounting medium. Imaging was performed using a Leica DM6000 microscope for brightfield. For quantification of perineuronal nets after Pnase or chABC treatment, 3 brain sections per animal in the range of bregma − 1.8 mm to − 3.8 mm were photographed under x25 magnification using a digital camera attached to the microscope. All images were taken at the same settings for light and exposure. The staining intensity in the perirhinal cortex (region of interest) was analyzed using ImageJ (version 1.43u, NIH, USA).
Electrophysiology
Animals were anesthetized with isoflurane and decapitated. The brain was rapidly removed and placed in ice-cold cutting solution bubbled with 95% O2/5% CO2 which comprised the following: 132.8 mM NaCl, 3.1 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 1 mM K2HPO4, 4 mM NaHCO3, 5 mM D-glucose, 0.01 mM glycine, 1 mM Ascorbic Acid, 0.5 mM MyoInositol, 2 mM pyruvate, 10 mM HEPES, adjusted to pH 7.35. A midsagittal section was made, and the rostral part of one hemisphere was cut at 45° to the dorsoventral axis (Cho et al., 2000). The cerebellum was removed from the brain with a further caudal cut along the dorsoventral axis. The hemisphere was glued by its rostral end to a vibratome stage (Leica VT 1000s). Slices (350 μm) of perirhinal cortex were taken in the region −2.5 mm to −4 mm rostral from bregma. Slices were stored submerged in bubbled, artificial CSF (20-25 °C, same composition as cutting solution, except 2 mM CaCl2, 1 mM MgCl2) for 2h before the onset of recordings. For extracellular field recordings, a single slice was placed in an interface recording chamber superfused by artificial CSF (30°C, flow rate 2 ml/ min). Evoked field EPSPs (fEPSP) were recorded from layers II/III from directly below the rhinal sulcus (area 35). A stimulation electrode was placed in layer II/III on the temporal side (0.5 mm, area 36) of the recording electrode (Cho et al., 2000). Stimuli (0.1 ms duration) were delivered to the stimulation electrode at 0.1 Hz. Input/Output curves were produced with stimulation intensities from 50 μA to 500 μA, in steps of 50 μA. For monitoring baseline synaptic transmission before LTD induction, fEPSPs were reduced to 50 – 60 % of the maximum amplitude and recorded for at least 30 minutes, or until responses were stable (less than 20 % amplitude change over 30 min). For LTD induction, 900 stimulus pairs (1 ms each, 20 ms inter-stimulus interval) were delivered at 2 Hz. Subsequently, fEPSPs elicited by 0.1 Hz stimulation were recorded for further 60 min. Before completion of the experiment, 10 paired pulses were delivered at 0.5 Hz, 5 pairs with an interval of 20 ms and 5 pairs with an interval of 30 ms. Field potentials were amplified with a CyberAmp 320 (Axon Instruments) and recorded and analyzed with custom made software written in LabView (National Instruments). For offline LTD analysis, fEPSPs were averaged across one minute and the peak amplitude of the mean fEPSP was expressed relative to the preconditioning baseline. Paired-pulse plasticity was expressed as the mean ratio of 2nd and 1st fEPSP amplitude in %.The significance of group means was established using either paired or unpaired t-tests or repeated measures (RM) ANOVA, followed by tests of simple main effects, if applicable.
Results
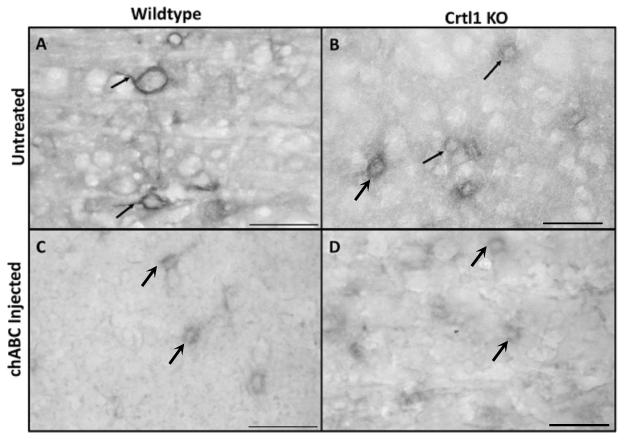
Object recognition is regarded as a rodent test for declarative memory (Gaffan, 1974; Mishkin, 1978; Ennaceur and Delacour, 1988) and depends, in the present version, on the perirhinal cortex (Baxter 2010; Winters et al., 2004). We visualized PNNs in this cortical region of 4 month old Crtl1 ko mice by post-mortem Wisteria floribunda agglutinin (WFA) staining (Hartig et al., 1992). In wild type mice, PNN structures were clearly visible both around the cell bodies and proximal dendrites (Figure 1 A). Consistent with our previous observations in other cortical regions (Carulli et al., 2010), PNNs in the perirhinal cortex of Crtl1 ko mice were vestigial around the somata, and absent around the dendrites (Figure 1 B).
Figure 1. Depletion of brain Crtl1 and chABC-injection reduce PNNs in the perirhinal cortex.
Perineuronal nets in the perirhinal cortex in wildtype (A, C) and Crtl1 knockout mice (B, D) before (A, B) and after (C, D) chABC injection into the perirhinal cortex. Wisteria floribunda agglutinin (WFA) labeling at high magnification indicates that PNNs are complete in wild type untreated animals with PNNs covering cell bodies and dendrites (arrows in A). WFA labeling in Crtl1 KO animals exhibit reduced PNNs with an absence of labeling around the dendrites (arrows in B). After chABC injection, PNNs and diffuse ECM are greatly diminished in both the wildtype and Crtl1 KO perirhinal cortex (C, D). Scale bar = 50μm.
In order to address whether the reduction of PNNs altered recognition memory, we tested 4 month-old Crtl1 ko mice (Crtl1−/−/Crtl1-Tg) and two litter mate control groups – wild type overexpressing Crtl1 in cartilage (Crtl1-Tg) and wild type mice- (Figure 2 A, cohort 1, Crtl1 ko n = 11; Crtl1-Tg n = 6; wt n = 11) on a spontaneous object recognition paradigm highly sensitive to perirhinal cortex lesions (Ennaceur et al., 1996; Murray and Bussey, 1999; Winters et al., 2004). A sample object was presented to the animals (Figure 2 C), and after a delay of either 1 min, 3h, 24 h or 48 h, recognition memory was tested by measuring exploration times of a novel object and the previously presented familiar object (Figure 2 D). Crtl1 ko mice performed differently from both control groups on the choice phase of the task, which assesses the persistence of memory for the sample object (Figure 2 D, Table 1 A). Whereas all groups showed a similar preference for the novel object when the delay between sample and choice phase was short, i.e. the mnemonic demand was low, Crtl1 ko mice showed enhanced object recognition after longer delays (Figure 2 D, Table 1 A). Strikingly, Crtl1 ko mice continued to treat sample objects as familiar even after 48 h, whereas Crtl1-Tg and wild type mice showed no memory of the familiar object after 24 h (One-sample t-test comparing to chance performance (0): LP ko 24 h: t = 7.3, p = 0.004, 48 h: t = 5.3, p = 0.005; Crtl1-Tg 24 h: t = 0.5; p = 0.782; 48 h: t = 0.3; p = 0.877; wt 24 h: t = 0.1, p = 0.921; 48h: t = 0.5, p = 0.788). Because we found no differences in object recognition between the two control groups (Crtl1-Tg and wild type, Figure 2 C,D, Table 1 A), we continued the following experiments with Crtl1-ko and wild type controls. Prolonged object recognition in Crtl1-ko mice was not due to enhanced exploration of the sample object, since sample phase exploration times were similar in all groups (Figure 2 C, RM ANOVA, no main effect of genotype, delay or delay x genotype interaction, all F < 1). However, we cannot entirely exclude the possibility that more subtle changes to anxiety levels, in particular towards novelty, have contributed to the results. Nevertheless, the large memory-enhancing effect in the absence of major differences in sample exploration, and the fact that choice phase performance was similar between groups at shorter delays, suggest that such changes to novelty-related behaviour are minor and not the main reason for the effects observed.
If PNNs in the perirhinal cortex were causally related to the enhancement of object recognition memory, we hypothesized that it should be possible to convert the object recognition phenotype of wild type mice into the phenotype of Crtl1 ko mice by acutely removing PNNs in the perirhinal cortex. To test this, we injected the CSPG-degrading enzyme chABC into the perirhinal cortex of Crtl1 ko and wild type mice that had previously performed the object recognition task (cohort 1, n = 6/5). This led to a long-term depletion of PNNs in the perirhinal cortex in the wild type mice (Figure 1 C,D; Figure 3; Figure 4). ChABC digests both the 2% of CSPGs found in PNNs as well as the remainder found in the diffuse extracellular matrix (Deepa et al., 2006). If the prolongation of memory that we observed is due only to the CSPGs in PNNs, digestion of the diffuse extracellular matrix in Crtl1 ko mice should have no effect.
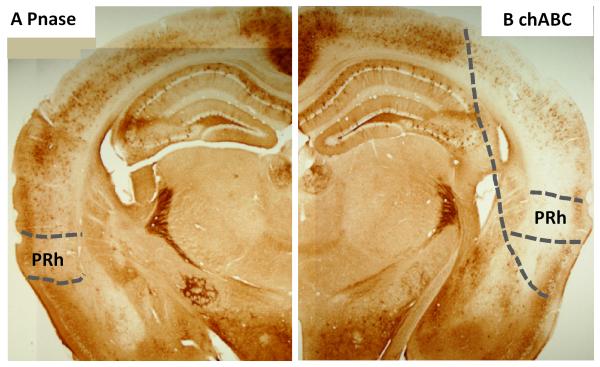
Figure 3. Effect of enzyme injection into the perirhinal cortex.
A Pnase injections into the perirhinal cortex had no effect. B chABC injections attenuated PNNs in the perirhinal cortex and adjacent cortical areas. The area of chondroitinase digestion and PNN depletion is shown by the dotted line.
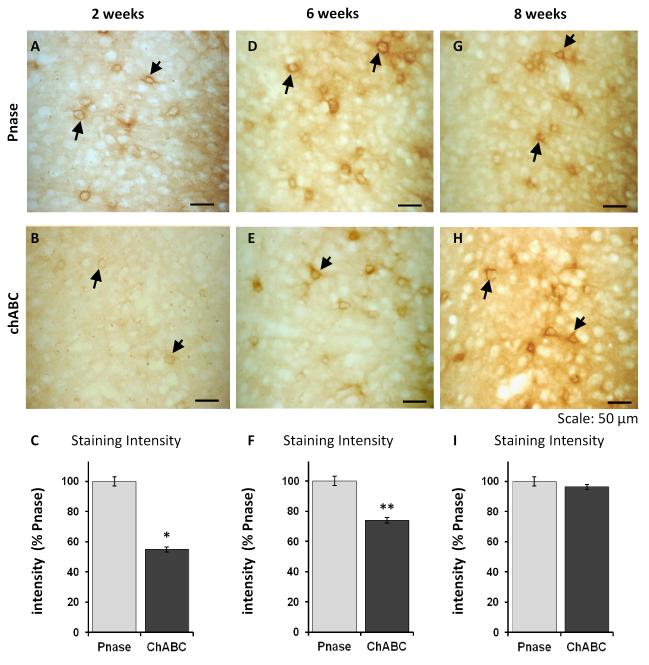
Figure 4. PNNs gradually repair after chABC treatment.
Wisteria floribunda agglutinin labelling of coronal sections at different time points after Pnase/chABC injections demonstrates the gradual restoration of PNNs over the course of 8 weeks. Two weeks after enzyme treatment, PNNs were still visible in Pnase treated wild type animals (A), but depleted in chABC-treated animals (B). Staining intensity measures of the perirhinal cortex with ImageJ showed a profound reduction of labelling in chABC treated mice (C, n = 4 per group; t-test, 2 weeks: t = 3.2). D,E 6 weeks after enzyme injections, PNNs started to re-occur in chABC-treated slices, but were still significantly reduced compared to Pnase-treated animals (F ,n = 4 per group; t-test , t = 5.3). 8 weeks after enzyme injections, prominent PNNs were visible in Pnase (G) and chABC treated animals (H). Staining intensity measures of the perirhinal cortex indicated no significant difference of labelling between Pnase and chABC-treated mice (I, n = 4 per group; t-test, t = 0.8). Data are presented as mean +/− sem; * p < 0.05, **p < 0.005.
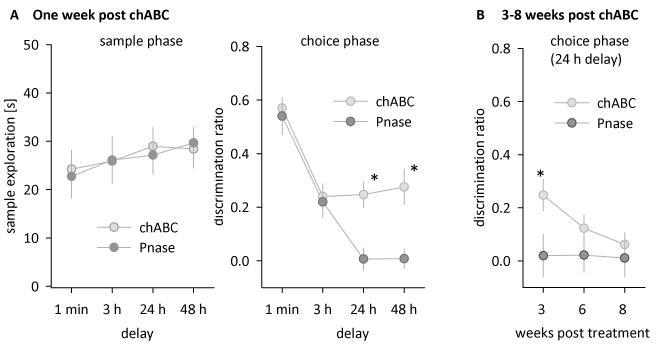
7 days after chABC treatment, mice were re-tested on the same object recognition task with delays of 24 h and 48 h. In the choice phase 24 h or 48 h later, wild type mice with chABC now showed the same memory prolongation as Crtl1 ko mice, and ChABC treatment had no effect on Crtl1 ko mice (Figure 2 D, Table 1 B). Thus, the attenuation of PNNs in the perirhinal cortex with chABC significantly prolonged object recognition memory in wild type mice, but did not further enhance memory in Crtl1 ko mice (Table 1 B). As before, wild type and Crtl1 ko mice spent a similar amount of time exploring the sample objects (Figure 2 C, RM ANOVA, no main effects or interactions, all F < 1).
In order to rule out that the enhancement of object recognition memory in chABC-treated wild type mice was due to non-specific effects of surgery and enzyme application, we repeated the above experiment with two new cohorts (cohort 2 and 3, see below) of wild type mice that received either chABC or the control enzyme penicillinase (Pnase). Pnase treatment had no effect on PNNs, whereas chABC treatment significantly altered appearance and quantity of PNNs around perirhinal neurons (Figure 3, 4 A-C). After a recovery period of one week, chABC/Pnase-treated mice were tested on the object recognition task with delays of 1 min, 3 h (cohort 2, n = 7/8 for chABC/Pnase), 24 h and 48 h (cohort 3, n = 8/9 for chABC/Pnase). On the choice phase, Pnase controls performed similarly to wild type mice in the previous experiments, showing no memory of the familiar object by 24 hours (Figure 5 A, right panel). In contrast, chABC-treated animals performed significantly better than Pnase-treated animals at longer delays and showed robust memory at 24 and 48 hrs (Figure 5 A, right panel, Table 2 AB), similar to chABC-treated wild type mice and Crtl1 ko mice in the previous experiments. These results, in particular the lack of an effect in Pnase-treated animals, further supports the conclusion that the memory-enhancement seen in the first cohort after chABC treatment was due to its digestion of CSPGs. Overall, depletion of brain Crtl1 and chABC treatment to the perirhinal cortex both enhanced recognition memory to the same extent, supporting the hypothesis that enhanced recognition memory is related to the lack of PNNs. Sample exploration times during the sample phase were similar in all groups (Figure 5 A, left panel, RM ANOVAs, no main effects or interactions, all F < 1).
Figure 5. Enzymatic degradation of PNNs in the perirhinal cortex prolongs object recognition.
A Sample exploration times of wild type mice after chABC or Pnase treatment (left panel) and object preference on the choice phase after chABC or Pnase treatment (right panel, 1 min and 3 h delay: chABC n = 7, Pnase n = 8; 24 h and 48 h delay: chABC n = 8, Pnase n = 9). B Object recognition with a 24 h delay 3,6 and 8 weeks after chABC or Pnase treatment (chABC: n = 5; Pnase: n = 5). Data are presented as mean +/− sem, * simple main effect, p < 0.05.
Table 2.
RM ANOVA results chABC vs Pnase treatment
| Main effect or interaction |
F | p | simple main effects |
|---|---|---|---|
| A Discrimination ratio of chABC vs Pnase treated wild type mice: 1 min and 3 h delay | |||
| treatment | F1,13=0.002 | = 0.967 | |
| delay | F1,13 = 16.4 | = 0.001 | |
| treatment × delay | F1,13 = 0.8 | = 0.776 | |
| B Discrimination ratio of chABC vs Pnase treated wild type mice: 24 h and 48 h delay | |||
| treatment | F1,15=21.5 | < 0.001 | 24 h: F1,15 = 14.9, p = 0.002 48 h: F1,15 = 13.3, p = 0.002 |
| delay | F1,15 = 0.14 | = 0.714 | |
| treatment × delay | F1,15 = 0.12 | = 0.738 | |
| C Discrimination ratio of chABC vs Pnase treated wild type mice: 3, 6 and 8 weeks post treatment (24 h delay) | |||
| treatment | F1,8=3.5 | = 0.097 | |
| time | F1,8 = 7.5 | = 0.026 | |
| treatment × time | F1,8 = 6.1 | = 0.038 | 3 weeks: F1,8 = 6.7, p = 0.033; 6 weeks: F1,8 = 1.6, p = 0.246; 8 weeks: F1,8 = 0.4, p = 0.555 |
Histology revealed that chABC-induced PNN degradation gradually recovered over the course of two months (Figure 4). Therefore, we hypothesized that memory of chABC-treated animals should eventually return to control levels. In order to test this, we repeatedly tested 24 h object recognition performance of the same animals (cohort 4, n = 5 for each treatment) 3, 6 and 8 weeks after chABC or Pnase treatment. As predicted, the memory-enhancing effect of chABC gradually decreased with time (Figure 5 B). Consistent with the gradual re-appearance of PNNs (Figure 4), chABC-treated mice performed similarly to Pnase treated animals 6 and 8 weeks after treatment (Figure 5 B, Table 2 C).
Object recognition memory is associated with long-term reductions in neuronal responsiveness in perirhinal cortex (Brown et al., 1987; Zhu et al., 1996; Xiang and Brown, 1998) and requires the expression of perirhinal LTD (Griffiths et al., 2008). In order to gain insights into the physiological mechanisms responsible for enhanced object recognition in Crtl1 ko mice, we measured perirhinal synaptic transmission, paired-pulse facilitation and LTD in coronal slices from 4 month old Crtl1 ko and wild type mice (cohort 5, Figure 6 A). Stimulation of temporal cortex input into layer II/III of the perirhinal cortex with different stimulus intensities revealed that basal fEPSPs in Crtl1 ko mice were greater than in wild type mice (Figure 6 B, RM ANOVA, intensity x genotype interaction, F9,171 = 4.0, p < 0.001; trend of main effect of genotype: F9,171 = 3.9, p = 0.065). Furthermore, Crtl1 ko mice, in contrast to wild type mice, showed no paired-pulse facilitation (Figure 6 C, RM ANOVA, main effect of genotype, F1,14 = 14.7, p < 0.005; no further effects or interactions, all F < 1). Moreover, LTD induction was greatly facilitated in Crtl1 ko mice, which expressed significantly smaller fEPSPs than wild type mice 50 min after low frequency stimulation (LFS, Figure 6 D, wt 81 +/− 4 %; Crtl1 ko 63 +/− 6 %; One-Way ANOVA, main effect of genotype, F1,9 = 6.6, p < 0.05).
Figure 6. Altered synaptic transmission and plasticity in the perirhinal cortex of Crtl1 ko mice.
A Setup for extracellular field recordings from perirhinal cortex in brain slices. B Layer II/III fEPSP amplitudes (normalized to the maximal amplitude) after stimulation of temporal Layer II/III input with different stimulus intensities, n = 9 for both genotypes. C Paired-pulse facilitation, wt: n = 10; Crtl1 ko: n = 6. D LTD induced by low frequency stimulation (LFS), n = 6 for both genotypes. Data are presented as mean +/− s.e.m. *simple main effects, p < 0.05.
Similar results were found in wild type mice treated with chABC (cohort 6). Injections of chABC into the perirhinal cortex increased basal synaptic transmission (Figure 7 A, RM ANOVA, intensity x chABC interaction, F9,216 = 2.1, p < 0.05), reduced paired-pulse facilitation (Figure 7 B, t-test, t32 = 2.3, p < 0.05) and led to a significant increase of LTD at layer II/III synapses (Figure 7 C, 50 min after LFS: control 82.4 +/− 0.4 %; chABC 67 +/− 0.4 %; One-Way ANOVA, main effect of chABC, F1,12 = 28.8, p < 0.001). The similarity of results obtained after genetic or pharmacological reduction of PNNs provides converging evidence that the removal of PNNs facilitates the induction of perirhinal LTD and increases synaptic transmission, providing a potential physiological correlate to superior object recognition in Crtl1 ko and chABC-treated animals.
Figure 7. Altered synaptic transmission and plasticity in the perirhinal cortex of chABC-treated mice.
A Layer II/III fEPSP amplitudes (normalized to the maximal amplitude) after stimulation of temporal Layer II/III input with different stimulus intensities (n = 14 for Pnase-treated mice; n = 12 for chABC-treated mice). B Paired-pulse facilitation, control: n = 19; chABC: N = 15. C LTD induced by low frequency stimulation (LFS), control: n = 7; chABC: n = 6. Data are presented as mean +/− s.e.m. *simple main effects, p < 0.05.
Discussion
Attenuation of PNNs in the visual system has been shown to re-open the critical window for visual system plasticity (Pizzorusso et al., 2002; Carulli et al., 2010). Here, we demonstrate that PNNs also regulate recognition memory in adulthood: attenuation of PNNs either globally by genetic deletion of the Crtl1 gene, or by localized application of chABC in the perirhinal cortex, produced a profound enhancement of object recognition memory. Moreover, depletion of PNNs led to enhanced perirhinal LTD, thought to be the major synaptic mechanism underlying object recognition memory. The chABC-induced enhancement of object recognition was reversible: over the course of 8 weeks, memory gradually returned to control levels, suggesting that re-occurring PNNs gradually restored control plasticity levels.
Although previous evidence that more general perturbations of the extracellular matrix (ECM) can affect learning and memory, these earlier studies led to inconsistent results regarding the nature of the effect. For example, Morrellini and colleagues reported that mice with deletion of the ECM protein tenascin-R showed normal hippocampus-dependent spatial memory, but were superior on subsequent reversal learning and working memory paradigms (Morellini et al., 2010). In contrast, deletion of the ECM protein tenascin-C produced a deficit in hippocampus-dependent contextual memory (Strekalova et al., 2002). Gogolla and colleagues report that chABC infused into the amygdala prior to fear conditioning had no effect on fear learning, but affected subsequent extinction, reinstatement and renewal (Gogolla et al., 2009). Explanations for these diverse effects and controversies may partly be found in the different aspects of memory studied; furthermore, the range of treatments and genetic manipulations used affect not only PNNs but also alter the composition of the ECM surrounding many types of neurons and glia. In our study, we therefore used a combined approach to address the function specifically of PNNs: the same improvement of memory was found after chABC treatment, which is regionally and temporally controlled but also has profound effects on other ECM structures including digestion of the 98% of CSPGs not contained in PNNs, and after Crtl1 knock out which attenuates PNNs but does not alter the overall levels of CSPGs or their pattern of sulphation (Deepa et al., 2006; Carulli et al., 2010). Taken together with recent evidence that Crtl1 expression is the key molecular event triggering PNN formation (Carulli et al., 2010; Kwok et al., 2010), the similarity of our results from both approaches gives us confidence that the clear improvement of recognition memory is related to compromised PNNs, rather than a consequence of general alterations to the ECM.
The principal physiological mechanisms thought to support object recognition memory are LTD-like processes in the perirhinal cortex (Zhu et al., 1996; Xiang and Brown, 1998; Warburton et al., 2003; Wan et al., 2004; Warburton et al., 2005; Barker et al., 2006; Griffiths et al., 2008; Massey et al., 2008; Kealy and Commins, 2011). We found that the Crtl1 ko mice and chABC-treated animals showed a facilitation of perirhinal LTD, which may be sufficient to explain superior familiarity detection. We also observed reduced paired-pulse facilitation, and an increase in basal synaptic transmission. Taken together, the pattern of results suggests that the depletion of brain Crtl1/PNNs may alter the induction or expression rules for synaptic plasticity: high basal synaptic transmission levels or other mechanisms facilitate long-term depression, but reduce the potential for paired-pulse facilitation.
How PNNs alter synaptic plasticity in the perirhinal cortex is unclear. Given the pattern of enhanced excitatory synaptic transmission and facilitated LTD in the perirhinal cortex of Crtl1/chABC-treated mice, PNNs might in some way regulate the influence of GABA-mediated inhibition (but see also (Frischknecht et al., 2009) for direct actions of PNNs on AMPA receptor mobility). This hypothesis seems particularly intriguing, since an increase in intracortical inhibition mediated by parvalbumin-containing neurons contributes to the closure of the critical period in the visual cortex (Huang et al., 1999; Berardi et al., 2000; Hensch, 2004), and PNNs are mostly, but not exclusively, located around such perisomatic GABAergic interneurons (Celio, 1993; Alpar et al., 2006). Not mutually exclusive of alterations in GABAergic transmission, depletion of PNNs may also shift the relative contributions of multiple co-existing LTD induction mechanisms (Cho et al., 2000; Cho and Bashir, 2002; Harris et al., 2004; Park et al., 2006). In particular, the lack of PNNs may have preserved/re-instated juvenile LTD-induction mechanisms characteristic for early postnatal development (Jo et al., 2006).
Overall, our data suggest that the removal of PNNs not only promotes plasticity and regeneration of sensory/motor systems (Bradbury et al., 2002; Carulli et al., 2010), but also facilitates cognitive abilities in adults by altering the rules of underlying, experience-driven synaptic plasticity. Our findings in mice do not have direct clinical implications, but altering the sensitivity to synaptic plasticity by manipulation of PNNs may provide a clinically relevant opportunity for the symptomatic enhancement of recognition memory in aging as well as Alzheimer’s Disease and other disorders of cognition. Although the focus of the current study was on the effect of PNNs on experience-driven learning, our findings might also be informative on how ECM modification enhances recovery after lesions: previous studies in which chABC has enhanced recovery from spinal cord injury and other conditions have focused on the increase in sprouting of corticospinal and other axons in treated animals, and suggested that the formation of new connections through sprouting is the main mechanism of recovery. Our current results suggest that changes in synaptic function could be equally important, and may also be relevant to the ability of chABC treatment to enhance the effectiveness of rehabilitation (Garcia-Alias et al., 2009; Bartus et al., 2012; Sharma et al., 2012).
Acknowledgements
This work was supported by the Wellcome Trust, the Medical Research Council, the Alzheimer’s Research Trust (UK), the NIHR Cambridge Biomedical Research Centre, the Internationale Stiftung fur Forschung in Paraplegie, and the EU 7th Framework program [[FP2007-2013] under grant agreements no 223326, 223524, IEF 254801, the EXTRAPLAST IIT project, project Plasticise and ERC project ECMneuro. JWF is a paid consultant for Acorda Therapeutics, which is involved in the commercial development of chondroitinase.
Footnotes
disclosures The other authors declare no competing financial interests.
References
- Alpar A, Gartner U, Hartig W, Bruckner G. Distribution of pyramidal cells associated with perineuronal nets in the neocortex of rat. Brain Res. 2006;1120:13–22. doi: 10.1016/j.brainres.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Barker GR, Warburton EC, Koder T, Dolman NP, More JC, Aggleton JP, Bashir ZI, Auberson YP, Jane DE, Brown MW. The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: implications for underlying plasticity mechanisms. J Neurosci. 2006;26:3561–3566. doi: 10.1523/JNEUROSCI.3154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Romberg C, White B, Wess J, Bussey TJ, Saksida LM. Intact attentional processing but abnormal responding in M(1) muscarinic receptor-deficient mice using an automated touchscreen method. Neuropharmacology. 2011;61:1366–1378. doi: 10.1016/j.neuropharm.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2012;235:5–17. doi: 10.1016/j.expneurol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Baxter MG. “I’ve seen it all before”: explaining age-related impairments in object recognition. Theoretical comment on Burke et al. Behav Neurosci. 2010;124:706–709. doi: 10.1037/a0021029. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brown MW, Wilson FA, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol. 2007;501:83–94. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Celio MR. Perineuronal nets of extracellular matrix around parvalbumin-containing neurons of the hippocampus. Hippocampus. 1993;3 Spec No:55-60. [PubMed] [Google Scholar]
- Cho K, Bashir ZI. Cooperation between mglu receptors: a depressing mechanism? Trends Neurosci. 2002;25:405–411. doi: 10.1016/s0166-2236(02)02228-2. [DOI] [PubMed] [Google Scholar]
- Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Czipri M, Otto JM, Cs-Szabo G, Kamath RV, Vermes C, Firneisz G, Kolman KJ, Watanabe H, Li Y, Roughley PJ, Yamada Y, Olsen BR, Glant TT. Genetic rescue of chondrodysplasia and the perinatal lethal effect of cartilage link protein deficiency. J Biol Chem. 2003;278:39214–39223. doi: 10.1074/jbc.M303329200. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Recognition impaired and association intact in the memory of monkeys after transection of the fornix. J Comp Physiol Psychol. 1974;86:1100–1109. doi: 10.1037/h0037649. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, Uney J, Brown MW, Warburton EC, Bashir ZI. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–194. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Harris SL, Cho K, Bashir ZI, Molnar E. Metabotropic glutamate receptor signalling in perirhinal cortical neurons. Mol Cell Neurosci. 2004;25:275–287. doi: 10.1016/j.mcn.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Hartig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3:869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Jo J, Ball SM, Seok H, Oh SB, Massey PV, Molnar E, Bashir ZI, Cho K. Experience-dependent modification of mechanisms of long-term depression. Nat Neurosci. 2006;9:170–172. doi: 10.1038/nn1637. [DOI] [PubMed] [Google Scholar]
- Kealy J, Commins S. The rat perirhinal cortex: A review of anatomy, physiology, plasticity, and function. Prog Neurobiol. 2011;93:522–548. doi: 10.1016/j.pneurobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Carulli D, Fawcett JW. In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J Neurochem. 2010;114:1447–1459. doi: 10.1111/j.1471-4159.2010.06878.x. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011 doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR. Impaired recognition memory on the Doors and People Test after damage limited to the hippocampal region. Hippocampus. 1999;9:495–499. doi: 10.1002/(SICI)1098-1063(1999)9:5<495::AID-HIPO2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RG. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- Massey PV, Phythian D, Narduzzo K, Warburton EC, Brown MW, Bashir ZI. Learning-specific changes in long-term depression in adult perirhinal cortex. J Neurosci. 2008;28:7548–7554. doi: 10.1523/JNEUROSCI.1935-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Morellini F, Sivukhina E, Stoenica L, Oulianova E, Bukalo O, Jakovcevski I, Dityatev A, Irintchev A, Schachner M. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex. 2010;20:2712–2727. doi: 10.1093/cercor/bhq017. [DOI] [PubMed] [Google Scholar]
- Morgelin M, Heinegard D, Engel J, Paulsson M. The cartilage proteoglycan aggregate: assembly through combined protein-carbohydrate and protein-protein interactions. Biophys Chem. 1994;50:113–128. doi: 10.1016/0301-4622(94)85024-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Park Y, Jo J, Isaac JT, Cho K. Long-term depression of kainate receptor-mediated synaptic transmission. Neuron. 2006;49:95–106. doi: 10.1016/j.neuron.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Saksida LM. Neuroscience. Remembering outside the box. Science. 2009;325:40–41. doi: 10.1126/science.1177156. [DOI] [PubMed] [Google Scholar]
- Sharma H, Alilain WJ, Sadhu A, Silver J. Treatments to restore respiratory function after spinal cord injury and their implications for regeneration, plasticity and adaptation. Exp Neurol. 2012;235:18–25. doi: 10.1016/j.expneurol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. Memory: brain systems and behavior. Trends Neurosci. 1988;11:170–175. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Sun M, Sibbe M, Evers M, Dityatev A, Gass P, Schachner M. Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol Cell Neurosci. 2002;21:173–187. doi: 10.1006/mcne.2002.1172. [DOI] [PubMed] [Google Scholar]
- Wan H, Warburton EC, Zhu XO, Koder TJ, Park Y, Aggleton JP, Cho K, Bashir ZI, Brown MW. Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. Eur J Neurosci. 2004;20:2214–2224. doi: 10.1111/j.1460-9568.2004.03688.x. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Glover CP, Massey PV, Wan H, Johnson B, Bienemann A, Deuschle U, Kew JN, Aggleton JP, Bashir ZI, Uney J, Brown MW. cAMP responsive element-binding protein phosphorylation is necessary for perirhinal long-term potentiation and recognition memory. J Neurosci. 2005;25:6296–6303. doi: 10.1523/JNEUROSCI.0506-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Yamada Y, Kimata K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J Biochem. 1998;124:687–693. doi: 10.1093/oxfordjournals.jbchem.a022166. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Mapping visual recognition memory through expression of the immediate early gene c-fos. Neuroreport. 1996;7:1871–1875. doi: 10.1097/00001756-199607290-00037. [DOI] [PubMed] [Google Scholar]