Abstract
Tonic γ-aminobutyric acid (GABA)A receptor-mediated signalling controls neuronal network excitability in the hippocampus. Although the extracellular concentration of GABA (e[GABA]) is critical in determining tonic conductances, knowledge on how e[GABA] is regulated by different GABA transporters (GATs) in vivo is limited. Therefore, we studied the role of GATs in the regulation of hippocampal e[GABA] using in vivo microdialysis in freely moving rats. Here we show that GAT-1, which is predominantly presynaptically located, is the major GABA transporter under baseline, quiescent conditions. Furthermore, a significant contribution of GAT-3 in regulating e[GABA] was revealed by administration of the GAT-3 inhibitor SNAP-5114 during simultaneous blockade of GAT-1 by NNC-711. Thus, the GABA transporting activity of GAT-3 (the expression of which is confined to astrocytes) is apparent under conditions in which GAT-1 is blocked. However, sustained neuronal activation by K+-induced depolarization caused a profound spillover of GABA into the extrasynaptic space and this increase in e[GABA] was significantly potentiated by sole blockade of GAT-3 (i.e. even when uptake of GAT-1 is intact). Furthermore, experiments using tetrodotoxin to block action potentials revealed that GAT-3 regulates extrasynaptic GABA levels from action potential-independent sources when GAT-1 is blocked. Importantly, changes in e[GABA] resulting from both GAT-1 and GAT-3 inhibition directly precipitate changes in tonic conductances in dentate granule cells as measured by whole-cell patch-clamp recording. Thus, astrocytic GAT-3 contributes to the regulation of e[GABA] in the hippocampus in vivo and may play an important role in controlling the excitability of hippocampal cells when network activity is increased.
Introduction
Sustained responses to γ-aminobutyric acid (GABA) mediated by high-affinity, slowly desensitizing, extrasynaptic GABAA receptors occur in many brain regions, including the hippocampus (Nusser & Mody, 2002; Yeung et al. 2003; Semyanov et al. 2004; Farrant & Nusser, 2005). This form of tonic GABA conductance is of particular interest because extrasynaptic GABAA receptor signalling is implicated in a range of neurological and psychiatric disorders (Walker & Semyanov, 2008; Brickley & Mody, 2012; Hines et al. 2012; Pavlov & Walker, 2012). The magnitude of tonic conductance is a dynamic parameter which can influence the ultimate effect of this form of signalling on cell excitability (Song et al. 2011). Thus, the factors which regulate the magnitude of tonic conductance may be critical for hippocampal function. One such factor is the extracellular concentration of GABA (e[GABA]). However, understanding how e[GABA] is regulated in vivo and how it responds to various physiological and psychological challenges is still limited. We have demonstrated that hippocampal e[GABA] is responsive to stress (De Groote & Linthorst, 2007). This effect is stressor-dependent, with increases observed after mild psychological stress and decreases after strong combined psychological/physical stress. These observations are of significance as the hippocampus is a key area in the coordination of the cognitive and neuroendocrine aspects of the stress response (Trollope et al. 2012).
Extracellular GABA originates from different sources (Semyanov et al. 2004). Microdialysis studies administering the sodium channel blocker tetrodotoxin (TTX) into the hippocampus have shown that the contribution of GABA derived from action potential-dependent neuronal release is by far the largest (Rowley et al. 1995; De Groote & Linthorst, 2007). On the other hand, these studies have also revealed that, in freely moving rats, a significant proportion (~30%) of hippocampal extracellular GABA is independent of action potential-dependent release and may represent non-vesicular neuronal and astrocytic release (Rossi et al. 2003; Semyanov et al. 2004).
During normal behaviour, hippocampal e[GABA] appears to be fairly constant (De Groote & Linthorst, 2007), probably due to a tight regulation by plasma membrane GABA transporters (GATs). Four different transporters (GAT-1/-2/-3 and betaine-GABA transporter-1) have been isolated, with GAT-1 representing the most abundant transporter in the forebrain (Borden 1996). Ex vivo pharmacological and mutant mouse studies have shown that blockade or deletion of GAT-1 results in increased hippocampal tonic current (Nusser & Mody, 2002; Jensen et al. 2003; Semyanov et al. 2003), suggesting a direct correlation between GAT-1 activity and neuronal excitability. Surprisingly, while hippocampal expression has been shown (Borden 1996; Ribak et al. 1996; Heja et al. 2009), the exact role of GAT-3 in the regulation of e[GABA], and thus tonic conductance, has not yet been clarified. Furthermore, although reverse activity of GATs has been demonstrated in cell cultures and hippocampal slices (Wu et al. 2007; Heja et al. 2012), whether this readily occurs in vivo is unclear. Therefore, we determined: (i) whether GAT-1 and GAT-3 differentially contribute to the regulation of hippocampal e[GABA] originating from action potential-dependent and -independent release in freely moving rats, and (ii) whether such regulation affects tonic conductances in dentate granule cells.
Here, we provide the first evidence in freely moving animals that GAT-3 can contribute to the transport of GABA originating from both action potential-dependent and -independent release in the hippocampus in vivo. Therefore, both GAT-1 and GAT-3 are of functional significance as they synergistically modulate the level of tonic conductances in hippocampal neurons.
Methods
Ethical approval
All procedures and animal care were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and were approved by the local Ethical Review Groups of the University of Bristol and University College London, and by the Home Office, UK. All efforts were taken to minimize animal numbers.
Animals
For immunofluorescence, Western blot and microdialysis experiments male Sprague–Dawley rats (Harlan, Loughborough, UK) were housed three per cage under standard housing conditions (lights on between 05:00 and 19:00 h, 21–22°C, 50–60% relative humidity) with free access to food pellets and drinking water. For the immunofluorescence and Western blot studies, rats were handled daily (approximately 5 min per rat) until the day of the experiment, at which they weighted approximately 240–260 g (8–9 weeks old). For the microdialysis experiments, rats were handled daily starting 1 week before surgery and continuing until the day of the insertion of the microdialysis probe. At the time of surgery, rats weighted about 240–260 g. For electrophysiology experiments, hippocampal slices were prepared from 3- to 4-week-old male Sprague–Dawley rats (see below).
Western blot and immunofluorescence studies
For description of methods see Supplemental material file.
In vivo microdialysis experiments
GABA transporter inhibitors
To investigate the involvement of the different GABA transporters in modulating in vivo e[GABA] and in vitro tonic currents (see below) in the CA3-dentate gyrus of the hippocampus, we used two selective GABA transporter inhibitors. 1,2,5,6-Tetrahydro-1-[2-[[(diphenylmethylene)amino] oxy]ethyl]-3-pyridinecarboxylic acid hydrochloride (NNC-711) is a potent and selective GAT-1 inhibitor with an IC50 of 0.38 μm (ability to inhibit uptake of radiolabelled GABA via cloned GABA transporters expressed in COS-7 cells; IC50 for GAT-2 and GAT-3 are 729 and 349 μm, respectively (Borden et al. 1995)). SNAP-5114 is a selective GAT-2 and GAT-3 inhibitor with an IC50 of 21 and 5 μm for these transporters, respectively (ability to inhibit uptake of radiolabelled GABA via cloned GABA transporters expressed in COS-7 cells; IC50 for GAT-1 is 388 μm (Borden et al. 1994)). Given that GAT-2 is mainly expressed in the leptomeninges but not in neurons and glial cells (Durkin et al. 1995), any effect of SNAP-5114 observed in this study is probably due to selective blockade of GAT-3. Both NNC-711 and SNAP-5114 were purchased from Tocris Bioscience (Bristol, UK).
Surgical and microdialysis procedures
Surgical procedures were performed under isoflurane (2.5–2.8%; Merial Animal Health Ltd, Harlow, UK) anaesthesia. Rats received Carprofen (Rimadyl, 4mg kg−1, S.C.; Pfizer, Sandwich, UK) for post-operative pain relief. Nine days before the start of the experiment, rats were surgically prepared for microdialysis of the hippocampus by stereotaxic implantation of a guide cannula (MAB 6.14.IC; Microbiotech/se AB, Stockholm, Sweden) as previously described (Droste et al. 2008). After surgery, rats were housed individually in Plexiglas cages (length × width × height = 27 × 27 × 35 cm) under similar housing conditions as described above.
Seven days after surgery, a microdialysis probe (polyethersulfone membrane, length 4 mm, 15 kDa cut-off, outer diameter 0.6 mm, MAB 6.14.4; Microbiotech/se AB) was inserted via the guide cannula into the hippocampus under short-lasting isoflurane anaesthesia. The localization of the probe was such that dialysis is essentially restricted to the CA3 and dentate gyrus subregions of the hippocampus (Linthorst et al. 1994). Rats were connected to a liquid swivel and a counterbalance arm (Microbiotech/se AB) allowing free movement in all directions. Fluorethylenepolymer tubing with a dead volume of 1.2 μl per 100mm length (Microbiotech) was used for all connections. Dead volumes were accounted for during the experiment. Microdialysis probes were perfused with sterile, pyrogen-free Ringer’s solution (Delta Pharma, Pfüllingen, Germany) at a flow rate of 2 μl min−1 using a microinfusion pump (KDS220; KD Scientific, Holliston, MA, USA). Experiments were performed 2 days after insertion of the microdialysis probe on six rats simultaneously. Microdialysis samples were collected using automated refrigerated sample collectors (Microsampler 820; Univentor, Malta). The samples were stored at −80°C for later determination of the concentration of GABA.
Experimental protocols
For all experiments, six 10-min baseline samples were collected between 10:00 and 11:00 h. At 11:00 h, infusion of drug(s) into the hippocampus via reverse dialysis commenced and continued for up to 90 min (see protocols below), followed by a wash-out period during which Ringer’s solution was perfused. Drugs were dissolved in Ringer’s solution or in Ringer’s solution maximally containing 1% DMSO by volume (NNC-711 and SNAP-5114, see below). The following treatment regimes were used:
Protocol A
To investigate the role of GAT-1 and GAT-3 in the regulation of e[GABA], microdialysis probes were perfused for 60 min with vehicle, the GAT-1 inhibitor NNC-711 (0.1–10 μm), the GAT-3 inhibitor SNAP-5114 (100 μm) or with NNC-711 (1 μm) and SNAP-5114 (100 μm) simultaneously. A 2-h washout period with Ringer’s solution followed all drug infusions. The sampling interval was 10 min.
Protocol B
To investigate GAT-1 and GAT-3 regulation of e[GABA] under conditions of increased neuronal activation, microdialysis probes were perfused with 10 μm NNC-711 or 100 μm SNAP-5114 for 90 min in total. Thirty minutes after the start of perfusion with NNC-711 or SNAP-5114, sustained increased neuronal activation was induced by perfusing the microdialysis probes with modified Ringer’s solution containing 50 mm KCl (in the presence of the respective GAT inhibitor) for 30 min. After completion of the treatment, a 90-min washout period followed. The sampling interval was 10 min with the exception of the KCl perfusion period during which six 5-min samples were collected.
Protocol C
To establish the proportion of e[GABA] independent of action potential-evoked release, microdialysis probes were perfused with 1 μm TTX (Tocris Bioscience) (De Groote & Linthorst, 2007) for 180 min. Next, to elucidate whether GAT-1 and/or GAT-3 regulate the action potential-independent fraction of e[GABA], 1 μm TTX was perfused for 180 min. Sixty minutes after the start of the perfusion with TTX, the microdialysis probes were perfused with 10 μm NNC-711, 100 μm SNAP-5114 or NNC-711 together with SNAP-5114 (all in the presence of TTX) for 30 min. TTX perfusion was maintained for a further 90 min after this 30-min drug infusion period. The sampling interval was 10 min with the exception of the GAT-1 and GAT-3 inhibitor perfusion period during which six 5-min samples were collected.
Histology
At the end of the experiment, rats were killed via an overdose of pentobarbital (Euthatal, 200 mg kg−1 body weight i.p.; Merial Animal Health Ltd) and the brains were removed and stored in 4% buffered paraformaldehyde solution. Histological examination was performed as previously described (Linthorst et al. 1994; Droste et al. 2008). Only data from rats with correctly placed microdialysis probes were included in the analyses.
Measurement of GABA
High-pressure liquid chromatography (HPLC) with electrochemical detection was used to measure GABA in the microdialysates based upon previously described protocols (De Groote & Linthorst, 2007). Briefly, GABA was separated on a TARGA C18 10 cm × 1 mm column (particle size 3 μm; Higgins Analytical, Mountain View, CA, USA) using filtered and degassed mobile phase (16% methanol, 0.1 m NaH2PO4, 0.2 mm EDTA, pH 4.46 with o-phosphoric acid) pumped at 50 μl min−1 by an Alexys LC-100 pump (Antec Leyden BV, Zoeterwoude, The Netherlands). Standards and samples were injected via a thermostatically controlled (8°C) Alexys AS-100 autosampler (Antec Leyden BV). Before injection, 13 μl standard or sample was derivatized with 2 μl o-phthaldialdehyde/sulfite solution (1.6 mm o-phthaldialdehyde, 0.5% methanol, 1.88 mm Na2SO3, 11.25 mm Na2B4O7) for 4 min to make GABA electrochemically active. Next, 10 μl of the derivatized mixture was injected onto the column and GABA detected using a VT-03 electrochemical flow cell (Antec Leyden BV) set at +850 mV against an Ag/AgCl reference electrode. Both column and detector were housed in a Faraday-shielded oven (DECADE II; Antec Leyden BV) thermostatically controlled at 38°C. Chromatograms were recorded and analysed using Alexys chromatography software (Antec Leyden BV). The detection limit for GABA at a signal-to-noise ratio of 3:1 was 11–15 fmol per injection on column.
Statistical analysis
Baseline GABA concentrations were calculated by averaging the values of the six baseline samples, and dialysate levels of GABA were expressed as percentage of baseline. Analysis of variance (ANOVA) with repeated measures was performed with ‘treatment’ as the between-subject factor and ‘time’ as the within-subject factor (SPSS version 16.0; IBM Corp., Armonk, NY, USA). The levels of the factor ‘time’ included the mean baseline value and the subsequent time points. Bonferroni or Dunnett post hoc tests for multiple comparisons or paired or unpaired t tests were used to locate the exact differences between factor levels where appropriate. For the post hoc analyses, to reduce the risk of type 1 errors, the number of levels of the factor time was reduced by averaging measurements (for each animal individually) over distinct time periods (30 min or 1 h intervals). The level of significance was set at P < 0.05 (adjusted by Bonferroni correction if appropriate). For clarity, only the results of the post hoc analysis are depicted in the figures. The effect of the factors and their interactions, as assessed by ANOVA, are described in the text. All data are expressed as mean±SEM.
To determine the EC50 and Emax of NNC-711, data of the concentration–response curve (Fig. 1C) were analysed by non-linear regression using GraphPad Prism (version 5.0; La Jolla, CA, USA).
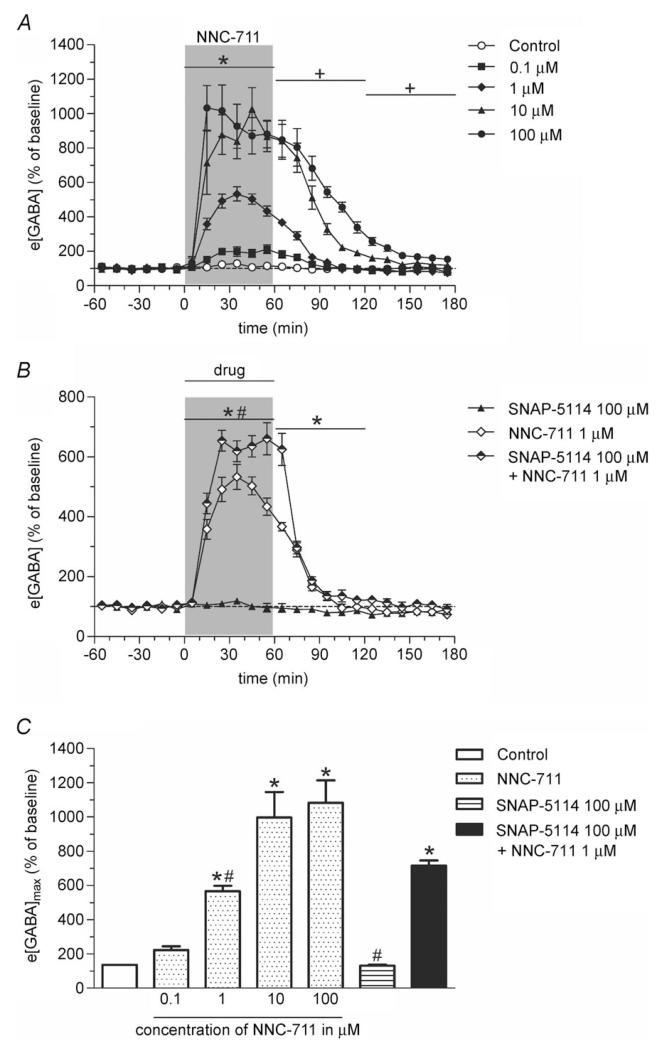
Figure 1. The GAT-1 inhibitor NNC-711 and the GAT-3 inhibitor SNAP-5114 increase e[GABA] synergistically in the rat hippocampus.
A, reverse dialysis of NNC-711 causes a dose-dependent increase in hippocampal e[GABA]. After 1 h of baseline sampling, NNC-711 (0.1, 1, 10 or 100 μm; n = 4–5; filled symbols) or 1% DMSO in Ringer’s solution (control; n = 3; open symbols) was perfused for 60 min through the microdialysis probe (shaded bar) followed by a 2-h washout period with Ringer’s solution. Samples were collected every 10 min. Significance: *1, 10 and 100 μm NNC-711 significantly different from control; +10 and 100 μm NNC-711 significantly different from control (both P < 0.001; Dunnett post hoc test). B, perfusion (shaded bar) of SNAP-5114 (100 μm; n = 5; filled symbols) for 60 min has no effect on hippocampal e[GABA]. Co-perfusion of SNAP-5114 with 1 μm NNC-711 (n = 5; half-filled symbols) caused a significantly larger increase in e[GABA] as compared with infusion of NNC-711 alone (n = 4; open symbols). *NNC-711 and NNC-711 + SNAP-5114 significantly different from SNAP-5114; #NNC-711 + SNAP-5114 significantly different from NNC-711 (both P < 0.001, Bonferroni post hoc test). C, maximal increase in e[GABA] (e[GABA]max; as derived from data in A and B). Co-perfusion of the GAT-1 and GAT-3 inhibitor causes a significantly larger maximum increase in e[GABA] as compared with perfusion of either inhibitor alone. *Significantly different from control (P < 0.05, Dunnett post hoc test); #significantly different from NNC-711 + SNAP-5114 (P < 0.01, Bonferroni post hoc test). All values are expressed as percentage of baseline (mean ± SEM).
In vitro electrophysiology experiments
In vitro electrophysiological recordings were performed using 350 μm-thick hippocampal slices prepared from 3- to 4-week-old rats. After decapitation brains were rapidly removed, hippocampi were dissected and cut with a VT1200S vibratome (Leica, Wetzlar, Germany) in an ice-cold sucrose-based solution containing (in mm): sucrose (70), NaCl (80), KCl (2.5), MgCl2 (7), CaCl2 (0.5), NaHCO3 (25), NaH2PO4 (1.25) and glucose (22), bubbled continuously with 95% O2 + 5% CO2 to yield a pH of 7.4. The slices were then allowed to recover in a sucrose-free solution (in mM): NaCl (119), KCl (2.5), MgSO4 (1.3), CaCl2 (2.5), NaHCO3 (26.2), NaH2PO4 (1) and glucose (22), bubbled with 95% O2 and 5% CO2 in an interface chamber for at least 1 h at room temperature before being transferred to a submerged recording chamber, and perfused with the same solution.
Visualized whole-cell patch-clamp recordings were performed from dentate gyrus granule cells using an infrared differential interference contrast imaging system. All recordings were done at 32°C. Recording pipettes were filled with caesium-based internal solution containing (in mm): CsCl (120), Hepes (10), EGTA (2), NaCl (8), Mg-ATP (2), Na-GTP (0.3), QX-314 bromide salt (5), pH 7.2, 290 mOsm. Pipettes had a tip resistance of 4–5 MΩ. All recordings were performed in the presence of NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline; 25 μm), d-AP5 (d(–)-2-amino-5-phosphonopentanoic acid; 50 μm), CGP55845 (1 μm) and TTX (1 μm) to block AMPA/kainate, N-methyl-d-aspartate (NMDA) and GABAB receptors, as well as action potentials. Dentate granule cells were voltage clamped at −70 mV. Tonic GABAA receptor-mediated currents were measured as changes in holding current (Ihold) following application of GABA transporter inhibitors (NNC-711, 1 μm; SNAP-5114, 100 μm) and the GABAA receptor blocker picrotoxin (100 μm). Root mean square (RMS) noise values were calculated for 50 ms epochs free of synaptic events. The change of RMS noise was calculated as the difference between the values before and 7–10 min after drug application. A separate set of experiments was carried out under the same conditions as described above but without the addition of TTX (see Supplemental material file). Furthermore, to test the effects of the GAT inhibitors on the kinetics of the miniature inhibitory postsynaptic currents (mIPSCs), single exponential decay time constants (τd) were calculated. Receptor antagonists were either from Tocris Bioscience or from Ascent Scientific (Bristol, UK).
Recordings were obtained using a MultiClamp 700B amplifier (Axon Instruments, Foster City, CA, USA), filtered at 4 kHz, digitized at 10 kHz and stored on a personal computer. Series resistance was monitored throughout experiments using a −5mV step command. Cells showing a >20% change in series resistance, or a series resistance >20M or an unstable holding current were rejected. Data acquisition and off-line analysis were performed using Strathclyde Electrophysiology Windows Electrophysiology Disk Recorder (WinEDR v3.0.1; Dr J. Dempster, University of Strathclyde, Glasgow, UK) and Clampfit 10.0 (Molecular Devices Corp., Sunnyvale, CA, USA) software. The effects of the GABA transport inhibitors and picrotoxin on Ihold and RMS noise were analysed by one-way ANOVA followed by paired and unpaired t tests with the level of significance set at P <0.05 (lowered by Bonferroni correction if appropriate). All data are expressed as mean±SEM.
Results
Simultaneous blockade of GAT-1 and GAT-3 reveals GAT-3 modulation of e[GABA] under basal conditions
Western blot analysis showed that both GAT-1 and GAT-3 are expressed within the different subregions of the rat hippocampus (CA1, CA3 and dentate gyrus (DG); Fig. S1A). We also confirmed GAT-1 and GAT-3 localization in the CA3 and dentate gyrus using confocal imaging (Fig. S1B). We focused on the CA3 and dentate gyrus as these were the subregions studied in the microdialysis experiments described below. GAT-1 immunoreactivity was localized throughout the granule cell layer of the dentate gyrus (Fig. S1B-1 and S1B-2, left panel) and showed co-localization with the presynaptic marker synaptophysin (Fig. S1B-1, right panel) but not with glial fibrillary acidic protein (GFAP), a specific astrocytic marker (Fig. S1B-2, right panel). We also found GAT-3 immunolabelling in the dentate gyrus (Fig. S1B-3 and S1B-4, left panel) which showed clear co-localization with GFAP (Fig. S1B-4, right panel), but not with synaptophysin (Fig. S1B-3, right panel), suggesting that this transporter is predominantly localized in astrocytes. Similar results were obtained in the CA3 region, with expression of GAT-1/synaptophysin in the principal cell layer and localization of GAT-3 in astrocytes (data not shown).
To assess the role of GAT-1 and GAT-3 in the regulation of e[GABA] we performed in vivo microdialysis in freely behaving rats and administered selective GAT inhibitors locally into the hippocampus via reverse dialysis (Protocol A). Local administration of the GAT-1 inhibitor NNC-711 (0.1–100 μm) caused a marked and concentration-dependent increase in e[GABA] (effect of time F18,270 = 69.78, P≤0.0005, interaction between time and concentration F72,270 = 11.76, P≤0.0005, repeated-measures ANOVA; Fig. 1A and C). Post hoc analysis revealed significant differences between control and NNC-711 treatment during both the drug infusion period (1, 10 and 100 μm) and the washout period (10 and 100 μm) (P <0.05, Dunnett post hoc test; Fig. 1A). The EC50 and Emax of NNC-711, as calculated from the data shown in Fig. 1C, were 1.1 μm and 1093±24% of baseline, respectively. While GAT-3 is expressed in the hippocampus, local infusion of a concentration of SNAP-5114 (100 μm) known to be effective in other brain structures (Dalby 2000; Bhattarai et al. 2011) did not significantly change hippocampal e[GABA] during the infusion and the first hour of the washout period (Fig. 1B). However, co-infusion of SNAP-5114 with NNC-711 (100 and 1 μm, respectively) markedly potentiated the NNC-711-induced increase in e[GABA] (interaction between time and treatment F36,180 = 36.3, P ≤ 0.0005, repeated-measures ANOVA; Fig. 1B). A supra-additive effect of simultaneous blockade of GAT-1 and GAT-3 on e[GABA] was found during both the drug infusion period and the first hour of washout (P < 0.05, Bonferroni post hoc test; Fig. 1B; e[GABA]max: 566 ± 32% and 716 ± 30% of baseline for GAT-1 and GAT-1+GAT-3 blockade, respectively; P < 0.01, Bonferroni post hoc test; Fig. 1C). These data therefore indicate that GAT-3 is an active transporter of extracellular GABA but that its activity is masked by highly efficient re-uptake of GABA via GAT-1 (see also below).
GAT-1 and GAT-3 regulate e[GABA] during potassium-evoked release of GABA
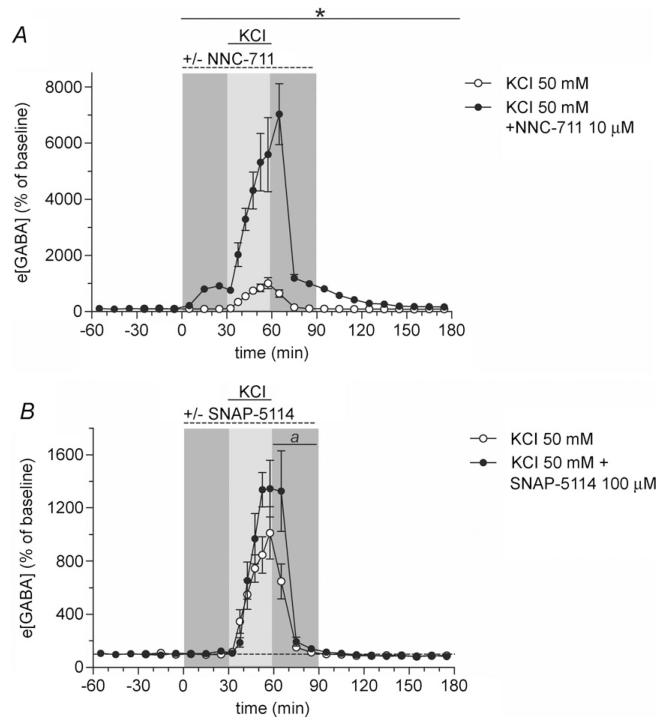
After having identified that not only GAT-1 but also GAT-3 can participate in controlling e[GABA] in vivo, we next wanted to find out how these transporters regulate extracellular GABA originating from the spillover of synaptically released neurotransmitter. Thereto, we induced a substantial and sustained increase in synaptic release by depolarizing neurons with increased extracellular K+. Microdialysis probes were perfused with Ringer’s solution containing 50 mm KCl for 30 min in the absence or presence of selective GAT inhibitors (Protocol B). We used 50 mm KCl for perfusion to achieve K+ concentrations in the tissue which would cause sustained neuronal spiking; K+ in the tissue will in reality be much lower than 50 mm because of the recovery rate of the microdialysis probe (~25% for small molecules at a flow rate of 2 μl min−1) and tissue diffusion. In line with our earlier observations (de Groote & Linthorst, 2007), 50 mm KCl resulted in a profound increase in e[GABA], reaching a maximum of 1079 ± 200% of baseline (effect of time F21,147 = 20.5, P ≤ 0.0005, repeated-measures ANOVA; Fig. 2A). Co-infusion of NNC-711 (10 μm) with KCl resulted in a seven-fold higher increase in e[GABA] (e[GABA]max is 7529 ± 975% of baseline, P ≤ 0.0005, t test) in comparison with infusion of KCl alone (interaction between time and treatment F21,231 = 32.4, P ≤ 0.0005; Fig. 2A). The dramatic K+-evoked increase in e[GABA] during concomitant blockade of GAT-1 reveals the vast capacity of this GABA transporter. Furthermore, we observed that infusion of SNAP-5114 (100 μm) significantly augmented the K+-evoked rise in e[GABA] (interaction between time and treatment F21,273 = 2.9, P ≤ 0.0005, repeated-measures ANOVA; in addition, a trend towards a higher maximum increase in e[GABA] was found: e[GABA]max is 1733 ± 268% of baseline, P = 0.068, t test; Fig. 2B). Thus, these observations show that in the hippocampus not only GAT-1 but also GAT-3, albeit to a lesser extent, regulate the levels of extracellular GABA that originate from synaptic spillover during enhanced neuronal activity.
Figure 2. Selective blockade of GAT-1 or GAT-3 potentiates the rise in e[GABA] induced by neuronal depolarization.
A, potassium chloride (KCl, 50 mm, 30 min, full horizontal line) was perfused either in the absence of (open symbols, n = 8) or during concomitant perfusion of the microdialysis probe with NNC-711 (10 μm, 90 min, dashed horizontal line) (filled symbols, n = 5). Samples were collected every 10 min or, during KCl perfusion, every 5 min. The effect of KCl on e[GABA] was approximately 7-fold higher in the presence of NNC-711 as compared with the effect of KCl under control conditions. *Significant difference between NNC-711- and vehicle-perfused rats (P < 0.001, t test). B, KCl (50 mm, 30 min, full horizontal line) was infused either in the absence (open symbols, n = 8) or the presence of SNAP-5114 (100 μm, 90 min, dashed horizontal line) (filled symbols, n = 7). Samples were collected every 10 min except during KCl perfusion when 5-min samples were obtained. Repeated-measures ANOVA identified a significantly more pronounced effect of KCl in rats co-perfused with SNAP-5114 than in those perfused with KCl under control conditions (see Results). a, P < 0.05 for KCl + SNAP-5114 as compared with KCl alone (t test). All values are expressed as percentage of baseline (mean ± SEM).
GAT-1 and GAT-3 regulate extracellular GABA originating from action potential-independent release
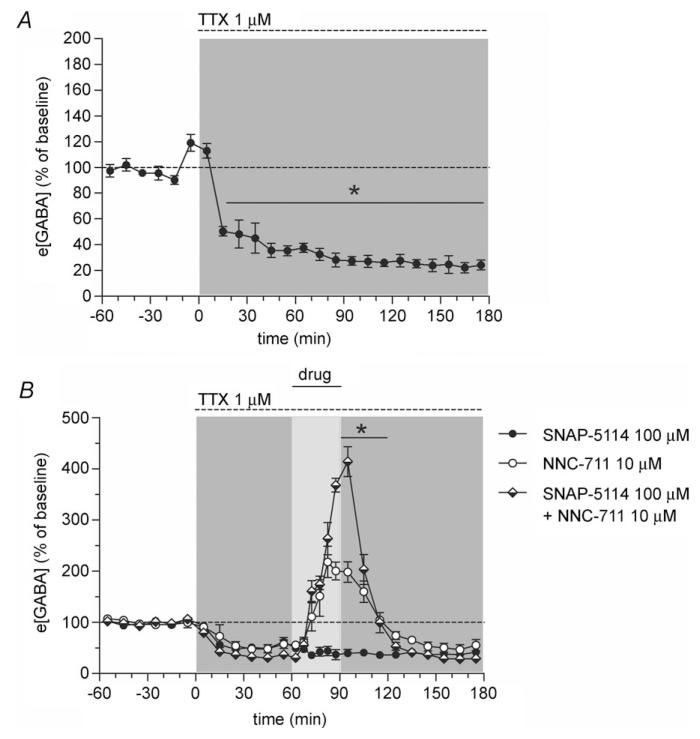
Blockade of action potentials by local administration of the voltage-gated sodium channel inhibitor TTX (Protocol C) decreased e[GABA] to approximately 25% of baseline (effect of time F18,72 = 74.7, P ≤ 0.0005, repeated-measures ANOVA; Fig. 3A), which is in accordance with earlier reports by our group and others (Rowley et al. 1995; De Groote & Linthorst, 2007). So far, nothing is known about the regulation of the TTX-insensitive pool (i.e. the action potential-independent fraction) of e[GABA] by GABA transporters. This is an important question given that this pool of GABA forms a significant proportion (~25%) of e[GABA] in vivo. Astrocytes are capable of both uptake and release of GABA and astrocytic GABA may therefore, together with non-vesicular neuronal release and action potential-independent vesicular release, account for the TTX-insensitive fraction. To assess the effects of GAT-1 and GAT-3 on action potential-independent extracellular GABA, NNC-711, SNAP-5114 or NNC-711 + SNAP-5114 were co-infused with TTX for 30 min (Protocol C). Given the low e[GABA] under TTX conditions, 10 μm NNC-711 was used in this protocol to ensure maximum blockade of GAT-1 (Fig. 1A). Before the co-treatment started, TTX was perfused for 60 min to block action potential-dependent release of GABA (see also Fig. 3A). Combined administration of NNC-711 and TTX, but not of SNAP-5114 or TTX, resulted in a marked increase in e[GABA] (Fig. 3B) with an e[GABA]max of 226 ± 27% relative to the pre-treatment baseline. However, the combined administration of SNAP-5114 and NNC-711 further enhanced the NNC-711-induced increase in e[GABA] (effect of treatment F2,16 = 27.9, P ≤ 0.0005; interaction between time and treatment F42,336 = 43.0, P ≤ 0.0005, repeated-measures ANOVA; Fig. 3B). The co-infusion of both GAT inhibitors resulted in a two-fold higher e[GABA] in comparison to infusion of NNC-711 alone (e[GABA]max: 419 ± 25 and 226 ± 27% of pre-treatment baseline for NNC-711 + SNAP-5114 and NNC-711, respectively (P ≤ 0.0005, Bonferroni post hoc test)). Thus, similar to baseline non-TTX conditions (see above), the finding that infusion of SNAP-5114 further increases e[GABA] when GAT-1 is blocked suggests that both GAT-1 and GAT-3 can also contribute to the uptake of action potential-independent GABA from the extracellular space.
Figure 3. Simultaneous inhibition of GAT-1 and GAT-3 results in a supra-additive increase in e[GABA] under tetrodotoxin (TTX) conditions.
A, local infusion of TTX (1 μm, 180 min, dashed horizontal line at top) markedly decreased e[GABA] in the hippocampus (n = 5, filled symbols). However, approximately 25% of e[GABA] is TTX-insensitive (revealing an action potential-independent pool of e[GABA]). *Significantly different from baseline (P < 0.001, paired t test). B, TTX was perfused for 180 min (1 μm, dashed horizontal line at top). SNAP-5114 (100 μm, filled symbols, n = 9), NNC-711 (10 μm, open symbols, n = 5) or the combination of these two inhibitors (half-filled symbols, n = 5) were co-perfused with TTX for 30 min commencing 60 min after the start of the TTX perfusion (‘drug’, full horizontal line). *NNC-711 + SNAP-5114 significantly different from NNC-711 (P < 0.001, Bonferroni post hoc test). For sake of clarity, significant post hoc differences versus SNAP-5114 alone have not been included in the figure. All values are expressed as percentage of baseline (mean ± SEM).
Changes in e[GABA] affect tonic conductances in the dentate gyrus
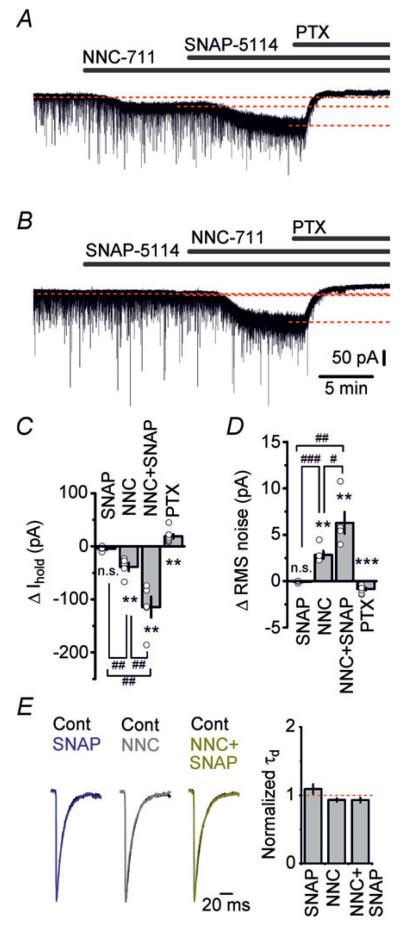
Even low concentrations of ambient GABA continuously activate extrasynaptic high-affinity GABAA receptors. We therefore investigated whether the changes in e[GABA] following inhibition of GABA transport observed in our microdialysis experiments have a functional impact on individual neurons. To test this we recorded tonic GABAA receptor-mediated currents in dentate granule cells in acute hippocampal slices. Whole-cell patch-clamp recordings were performed in the presence of NBQX, d-AP5 and CGP55845 to block ionotropic glutamate receptors and GABAB receptors, and TTX to block spontaneous action potentials. A high internal Cl− concentration was used, and neurons were voltage clamped at −70 mV. Tonic currents were measured as shifts in the holding current upon application of GABA transporter inhibitors, or the GABAA receptor blocker picrotoxin. Inhibition of GAT-1 by NNC-711 (1 μm) produced a persistent inward current and significantly increased the RMS noise in the granule cells (P < 0.01, paired t test; Fig. 4A, C and D). Unlike NNC-711, application of SNAP-5114 (100 μm) did not change the holding current or the background noise, indicating that under these conditions GAT-3 does not contribute to setting the overall concentration of extracellular GABA, and therefore has no impact on tonic conductances (Fig. 4B–D). In contrast, adding SNAP-5114 to the perfusion solution in the presence of NNC-711 resulted in a profound increase of the inward current and the RMS noise (Fig. 4A, C and D). In the presence of the two drugs the change in the holding current was more than double of that produced by NNC-711 alone (P < 0.01, t test). Similar effects of the GAT inhibitors on tonic current and RMS noise were obtained in the absence of TTX (see Supplemental material Fig. S2). Tonic currents and changes in RMS noise induced by NNC-711 and SNAP-5114 were completely blocked by picrotoxin, confirming that they were mediated by the activation of GABAA receptors. NNC-711 and/or SNAP-5114 had no significant effect on mIPSC decay kinetics (Fig. 4E; P > 0.05 paired t test), suggesting that GABA transporters do not determine the duration of unitary synaptic events. The in vitro electrophysiological results agree with our in vivo microdialysis data which showed that, under both baseline and TTX conditions, simultaneous blockade of GAT-1 and GAT-3 caused a profoundly higher increase in e[GABA] as compared with the increase brought about by blockade of GAT-1 alone. Taken together these results indicate that alterations in tonic conductances represent a direct functional correlate of manipulations of e[GABA] (as induced by blockade of GABA transporters).
Figure 4. Blockade of GAT-3 by SNAP-5114 markedly increases the tonic GABAA receptor-mediated current in dentate granule cells when GABA uptake by GAT-1 is inhibited.
A, representative trace showing changes in holding current (Ihold) in a dentate granule cell following consecutive application of NNC-711 (1 μm), SNAP-5114 (100 μm) and picrotoxin (PTX, 100 μm). In the presence of NNC-711, application of SNAP-5114 increases tonic GABAA receptor-mediated current. B, representative trace showing changes in holding current in a dentate granule cell following consecutive application of SNAP-5114 (100 μm), NNC-711 (1 μm) and PTX (100 μm). Blockade of GAT-3 by SNAP-5114 does not affect holding current. C and D, summary graphs showing changes in holding currents (C) and RMS noise (D) following application of SNAP-5114 (n = 4) and NNC-711 (n = 5), in the presence of both drugs (n = 5), and after a blockade of GABAA receptors by PTX (n = 8). One-way ANOVA holding current F3,18 = 34.68, P < 0.001; one way ANOVA RMS noise F3,18 = 33.38, P < 0.001. n.s., non significant; **P < 0.01 and ***P < 0.001, compared with the baseline condition (paired t test); #P < 0.05, ##P < 0.01 and ###P < 0.001 (unpaired t test). ΔIhold and ΔRMS noise values represent changes from the baseline condition. E, left: superimposed normalized averaged mIPSC traces in control (Cont) conditions and in the presence of SNAP-5114 (100 μm) and/or NNC-711 (1 μm). Right: summary graph of mIPSC decay time constants (τd; single-exponential fit of the mIPSC decay kinetics) in the presence of SNAP-5114 (n = 4), NNC-711 (n = 5) and their combination (n = 5) normalized to control values (P > 0.05, paired t test).
Discussion
Western blot analysis demonstrated the presence of both GAT-1 and GAT-3 in the CA3 and dentate gyrus, which are the hippocampal subregions dialysed in the microdialysis experiments. These results agree with the reported expression of GAT-1 and GAT-3 mRNA in the hippocampus (Durkin et al. 1995; Sperk et al. 2003). Double immunofluorescence showed neuronal expression of GAT-1 in the principal cell layer of the CA3 and in the granular cell layer of the dentate gyrus but no expression in astrocytes. Co-localization of GAT-1 with synaptophysin demonstrates the presence of the transporter in presynaptic terminals. However, GAT-1-immunopositive/synaptophysin-immunonegative cells were also observed, indicating an additional, putatively perisynaptic, localization of GAT-1. The expression of GAT-3 appeared to be confined to astrocytes. Hippocampal expression of GAT-1 and of GAT-3 have been reported previously (Ribak et al. 1996; Heja et al. 2009). Using electron microscopy, Ribak et al. (1996) demonstrated that GAT-1 is localized in both neurons and in glial processes with the highest concentration of GAT-1 immunoreactivity found in axon terminals which form symmetric synapses. They also reported that GAT-3 is only found in astrocytic processes. Therefore, the overall conclusion based on our and previous studies is that in the rat hippocampus the predominant localization of GAT-1 is neuronal and that of GAT-3 is astrocytic.
Given its synaptic localization, GAT-1 is uniquely positioned to modulate GABA uptake immediately after release, thereby also regulating synaptic spillover and thus ultimately impacting on e[GABA]. Reverse dialysis of the GAT-1 blocker NNC-711 resulted in a dose-dependent long-lasting increase in e[GABA] in the hippocampus reaching maximum levels of almost 1100%. The observed EC50 of 1.1 μm is in accordance with the previously described IC50 found for uptake of radiolabelled GABA in COS-7 cells transfected with GAT-1 (Borden et al. 1995). Our data are in line with the increased levels of hippocampal extracellular GABA found after administration of the GAT-1-specific inhibitor tiagabine in anaesthetized rats (Dalby 2000). K+ stimulation revealed a vast capacity of GAT-1. The K+-induced increase in e[GABA] reached a maximum level of approximately 1100%. However, simultaneous NNC-711 perfusion produced e[GABA] levels of up to 7500% of baseline. High concentrations of K+ can reverse neurotransmitter transporters (Richerson & Wu, 2003). However, in our experiments K+-induced increase in e[GABA] in vivo was not blocked by GAT inhibitors, arguing against this possibility (see also Rossi et al. 2003). The vast increase demonstrates the prominent role of GAT-1 in the regulation of extracellular GABA originating from synaptic release. In this respect it is of interest that thalamic GAT-1 functioning is compromised in genetic mouse models of absence seizures (Cope et al. 2009). Furthermore, hippocampal GAT-1 mRNA expression shows a transient increase followed by a decrease after kainic acid-induced seizures (Sperk et al. 2003), suggesting an essential role of GAT-1 in maintaining e[GABA] within a physiological range.
While GAT-3 expression was found in the hippocampus, local infusion of the GAT-3 inhibitor SNAP-5114 did not alter e[GABA], in agreement with prior observations in anaesthetized rats (Dalby 2000). Importantly, microdialysis studies have shown that 100 μm SNAP-5114 is sufficient to increase extracellular GABA in the rat thalamus (Dalby 2000), and in the primate globus pallidus (Galvan et al. 2005), arguing against the possibility that the concentration of GAT-3 inhibitor was too low in our study. Given the low expression of GAT-3 in the hippocampus (which contrasts with a higher expression in the thalamus and other brain regions (Durkin et al. 1995)), the lack of effect of GAT-3 blockade may be the result of a compensatory uptake via the very effective GAT-1. This hypothesis was tested in experiments in which SNAP-5114 was infused during simultaneous blockade of GAT-1 by NNC-711. Combined infusion of NNC-711 and SNAP-5114 synergistically increased e[GABA], which was approximately 150% higher than after the sole administration of NNC-711. Interestingly, blockade of a small fraction of GAT-1 transporters using a low dose of NNC-711 (0.1 μm) was not sufficient to reveal an effect of SNAP-5114 (data not shown), indicating that a substantial blockade of GAT-1 is required to unmask hippocampal GAT-3 activity. Alternatively, at present it cannot be excluded that inhibition of GAT-1 indirectly, via changes in network activity, increases the activity of GAT-3. Nevertheless, our observations clearly underscore the concept that GAT-3 can act as an active hippocampal GABA transporter in vivo.
GAT-3 is expressed in astrocytes in the hippocampus and is thus localized at a distance from the synapse. We therefore postulated that GAT-3 regulates e[GABA] by uptake of extrasynaptic GABA; this in contrast to GAT-1, which would also be in the position to transport GABA in, or in close vicinity of, the synapse. As a consequence, GAT-3 would only regulate extracellular GABA when the concentrations of e[GABA] become very high, such as after increased spillover from the synapse during episodes of sustained neuronal activity (Kinney & Spain, 2002). To test this hypothesis, e[GABA] was measured in the presence of a high concentration of K+ and of 100 μm SNAP-5114 in the perfusion fluid. Inhibition of GAT-3 indeed caused a further elevation of the K+-induced increase in e[GABA] by approximately 600%. These data clearly support the importance of astrocytic uptake of GABA via GAT-3 in the regulation of e[GABA] in the face of increased neuronal activity.
Apart from GABA uptake via astrocytic GAT-3, there is also evidence for GABA release from glial cells in different brain regions (Angulo et al. 2008), for which various mechanisms including a debated role for bestrophin-1 anion channels (Lee et al. 2010; Diaz et al. 2012) and glutamate/GABA exchange and reversal of GAT-3 (Heja et al. 2012) have been proposed. Based on its predominant astrocytic localization, we hypothesized that GAT-3 may be involved in particular in the regulation of extracellular GABA released via action potential-independent mechanisms, to which astrocytic release may represent an important contributor. We studied this possibility by blocking GABA transporters during simultaneous inhibition of action potential-dependent release by TTX. TTX lowered the levels of extracellular GABA to 25% of baseline, demonstrating a considerable action potential-independent pool of extracellular GABA. Blockade of GAT-1 still increased e[GABA] under TTX conditions, albeit to a somewhat smaller extent than under normal conditions. Also under TTX conditions, infusion of SNAP-5114 had no effect on e[GABA]. However, when SNAP-5114 was co-infused with NNC-711 a supra-additive (doubling) effect of the two GABA transporter blockers was found, demonstrating that under these conditions GAT-3 plays a marked role in regulating e[GABA]. Interestingly, a recent report has provided evidence that astrocytes are indeed able to increase extracellular GABA and interneuron mIPSCs via a TRPA1 channel-mediated decrease of GABA uptake by astrocytic GAT-3 (Shigetomi et al. 2012). Notably, although it has been hypothesized that reverse transporter uptake could contribute to extracellular GABA concentrations, we found no evidence of this in vivo. Thus, no decreases in e[GABA] have been observed after blockade of GAT-1 and/or GAT-3 under diverse in vivo conditions.
To study the functional consequences of e[GABA] regulation by GATs we measured tonic conductances in dentate granule cells while blocking GAT-1 and GAT-3. In line with previous studies in the CA1 and dentate gyrus (Nusser & Mody, 2002; Semyanov et al. 2003), elevation of e[GABA] by NNC-711 produced a persistent inward current and increased the RMS noise in dentate granule cells; this effect could be completely blocked by picrotoxin, confirming that these tonic currents were mediated by the activation of GABAA receptors. In the hippocampus and other brain areas such as the neocortex, genetic deletion of GAT-1 causes an increase in tonic current (Jensen et al. 2003; Bragina et al. 2008), suggesting an important role of GAT-1 in regulating tonic signalling. In full agreement with the microdialysis studies, perfusion of hippocampal slices with SNAP-5114 had no effect on tonic current. However, combined administration of SNAP-5114 and NNC-711 amplified GABAergic tonic currents and RMS noise. These data revealed the contribution of GAT-3 to the modulation of tonic conductance in dentate granule cells and provided further support to the supra-additive action of the two GATs observed in vivo.
In conclusion, we have demonstrated that both GAT-1 and GAT-3 can contribute to the regulation of hippocampal e[GABA] in vivo. As indicated by our in vitro results, fluctuations in hippocampal e[GABA] are expected to have direct functional consequences as they lead to changes in GABAergic tonic conductances. Importantly, the in vivo microdialysis data together with the immunofluorescence and electrophysiological findings highlight the role of astrocytes in the regulation of tonic GABA-mediated signalling in the hippocampus. Tonic GABA conductance is maintained in various models of epilepsy (e.g. Zhang et al. 2007; Pavlov et al. 2011) and may represent an important target for post-stroke treatment (Clarkson et al. 2010). Also hippocampal GABA plays an important role in responses to fear and stress (De Groote & Linthorst, 2007; Makkar et al. 2012). Synergistic action of GAT-1 and GAT-3 inhibitors therefore could have future therapeutic potential for neurological and psychiatric disorders.
Supplementary Material
Key points.
The extracellular concentration of the neurotransmitter γ-aminobutyric acid (GABA) is critical in determining GABAA receptor-mediated tonic conductance in the hippocampus.
Two GABA transporters (GAT-1 and GAT-3) are present in the CA3 and dentate gyrus of the hippocampus. The expression of GAT-3 is confined to astrocytes and its role in the regulation of GABAergic neurotransmission is unclear.
Using microdialysis and specific GAT uptake inhibitors we show that not only GAT-1 but also GAT-3 contributes to the regulation of hippocampal extracellular concentrations of GABA in rats under in vivo conditions.
We further found that changes in extracellular concentrations of GABA resulting from both GAT-1 and GAT-3 inhibition precipitate supra-additive changes in tonic conductance in dentate granule cells in vitro.
These results help us to understand the mechanisms underlying the regulation of GABAergic tonic conductance in the hippocampus and can help to develop improved therapeutic strategies for neurological and psychiatric disorders.
Acknowledgements
This study was supported by a Wellcome Trust project grant to M.C.W., A.S. and A.C.E.L. (083163/B/07/Z), an Epilepsy Research UK Fellowship (A0832) to I.P. and a Pewterers’ Research Fellowship to I.P.
Abbreviations
- d-AP5
d(−)-2-amino-5-phosphonopentanoic acid
- e[GABA]
extracellular concentration of GABA
- GABA
γ-aminobutyric acid
- GAT
GABA transporter
- HPLC
high-performance liquid chromatography
- Ihold
holding current
- mIPSC
miniature inhibitory postsynaptic current
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline
- PTX
picrotoxin
- RMS
root mean square
- τd
decay time constant
- TTX
tetrodotoxin
References
- Angulo MC, Le MK, Kozlov AS, Charpak S, Audinat E. GABA, a forgotten gliotransmitter. Prog Neurobiol. 2008;86:297–303. doi: 10.1016/j.pneurobio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Bhattarai JP, Park SA, Park JB, Lee SY, Herbison AE, Ryu PD, Han SK. Tonic extrasynaptic GABAA receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology. 2011;152:1551–1561. doi: 10.1210/en.2010-1191. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Borden LA, Dhar TG, Smith KE, Branchek TA, Gluchowski C, Weinshank RL. Cloning of the human homologue of the GABA transporter GAT-3 and identification of a novel inhibitor with selectivity for this site. Receptors Channels. 1994;2:207–213. [PubMed] [Google Scholar]
- Borden LA, Smith KE, Vaysse PJ, Gustafson EL, Weinshank RL, Branchek TA. Re-evaluation of GABA transport in neuronal and glial cell cultures: correlation of pharmacology and mRNA localization. Receptors Channels. 1995;3:129–146. [PubMed] [Google Scholar]
- Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABAA receptor-mediated inhibition in the cerebral cortex. J Neurochem. 2008;105:1781–1793. doi: 10.1111/j.1471-4159.2008.05273.x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Di GG, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO. GABA-level increasing and anticonvulsant effects of three different GABA uptake inhibitors. Neuropharmacology. 2000;39:2399–2407. doi: 10.1016/s0028-3908(00)00075-7. [DOI] [PubMed] [Google Scholar]
- De Groote L, Linthorst ACE. Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience. 2007;148:794–805. doi: 10.1016/j.neuroscience.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Wadleigh A, Hughes BA, Woodward JJ, Valenzuela CF. Bestrophin1 channels are insensitive to ethanol and do not mediate tonic GABAergic currents in cerebellar granule cells. Front Neurosci. 2012;5:148. doi: 10.3389/fnins.2011.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, De Groote L, Atkinson HC, Lightman SL, Reul JMHM, Linthorst ACE. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–3253. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Mol Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidment NT, Ackerson LC, Smith Y, Wichmann T. GABAergic modulation of the activity of globus pallidus neurons in primates: in vivo analysis of the functions of GABA receptors and GABA transporters. J Neurophysiol. 2005;94:990–1000. doi: 10.1152/jn.00068.2005. [DOI] [PubMed] [Google Scholar]
- Heja L, Barabas P, Nyitrai G, Kekesi KA, Lasztoczi B, Toke O, Tarkanyi G, Madsen K, Schousboe A, Dobolyi A, Palkovits M, Kardos J. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS One. 2009;4:e7153. doi: 10.1371/journal.pone.0007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heja L, Nyitrai G, Kekesi O, Dobolyi A, Szabo P, Fiath R, Ulbert I, Pal-Szenthe B, Palkovits M, Kardos J. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol. 2012;10:26. doi: 10.1186/1741-7007-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012;22:552–558. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Spain WJ. Synaptically evoked GABA transporter currents in neocortical glia. J Neurophysiol. 2002;88:2899–2908. doi: 10.1152/jn.00037.2002. [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Linthorst ACE, Flachskamm C, Holsboer F, Reul JMHM. Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenocortical axis activity, and body temperature. Endocrinology. 1994;135:520–532. doi: 10.1210/endo.135.2.7518383. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2012;37:1793–1794. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Huusko N, Drexel M, Kirchmair E, Sperk G, Pitkanen A, Walker MC. Progressive loss of phasic, but not tonic, GABAA receptor-mediated inhibition in dentate granule cells in a model of post-traumatic epilepsy in rats. Neuroscience. 2011;194:208–219. doi: 10.1016/j.neuroscience.2011.07.074. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Walker MC. Tonic GABAA receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.04.003. in press. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WM, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley HL, Martin KF, Marsden CA. Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods. 1995;57:93–99. doi: 10.1016/0165-0270(94)00132-z. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Savtchenko L, Semyanov A. Tonic excitation or inhibition is set by GABAA conductance in hippocampal interneurons. Nat Commun. 2011;2:376. doi: 10.1038/ncomms1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Heilman J, Furtinger S, Reimer RJ, Edwards RH, Nelson N. Expression of plasma membrane GABA transporters but not of the vesicular GABA transporter in dentate granule cells after kainic acid seizures. Hippocampus. 2003;13:806–815. doi: 10.1002/hipo.10133. [DOI] [PubMed] [Google Scholar]
- Trollope AF, Gutierrez-Mecinas M, Mifsud KR, Collins A, Saunderson EA, Reul JMHM. Stress, epigenetic control of gene expression and memory formation. Exp Neurol. 2012;233:3–11. doi: 10.1016/j.expneurol.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABAA receptors. Results Probl Cell Differ. 2008;44:29–48. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.