Summary
Background
The ISTH Bleeding Assessment Tool (BAT) was developed to record bleeding symptoms and to aid diagnosis in patients with a possible bleeding disorder.
Objectives
To investigate the utility of the ISTH BAT in predicting functional defects in platelet activation in participants with suspected inherited platelet function disorders.
Patients/Methods
Participants with clinical evidence of excessive bleeding and suspected inherited platelet function disorders and healthy volunteers were recruited to the Genotyping and Phenotyping of Platelets study (GAPP, ISRCTN 77951167). The ISTH BAT questionnaire was conducted by a trained investigator prior to lumiaggregometry.
Results
100 participants were included (n=79 with suspected inherited platelet function disorders and n=21 healthy volunteers). The ISTH BAT score in participants with suspected inherited platelet function disorders (median = 12; interquartile range [IQR] = 8 to 16) was significantly higher than in healthy volunteers (median 0; IQR 0 to 0). There was no difference between participants with suspected inherited platelet function disorders with a platelet defect detected by lumiaggregometry (median 11; IQR 8 to 16) and with normal platelet function (median 12; IQR 8 to 14) (p>0.05). The ISTH BAT score was not associated with a demonstrable platelet defect on platelet function testing (ROC curve AUC=0.501 [95% CI 0.372 to 0.630, p=0.98] and OR 1.01 [95% CI 0.93 – 1.09], p=0.91]).
Conclusions
The ISTH BAT is a powerful tool in documenting lifelong bleeding history. However, the score obtained is not predictive of the presence of a platelet defect on lumiaggregometry in patients with suspected inherited platelet function disorders.
Keywords: ISTH bleeding assessment tool, bleeding disorders, platelet function disorders, platelet phenotype, prediction
Introduction
Recent years have seen the development of many forms of bleeding assessment tools (BATs) with the aim of standardising the documentation of symptoms of excessive bleeding in patients, allowing comparison between patients and selecting patients in whom further investigations are appropriate. The evolution and validation of these tools along with the rapid progress in the field was covered in a recent comprehensive review [1]. Many of the tools developed have been specific to certain bleeding disorders, such as von Willebrand’s disease [2, 3] or have been targeted at certain populations, such as children [4-6] or women with menorrhagia [7, 8]. International collaboration has been recognised as being essential in further development of these tools due to the rarity of some inherited bleeding disorders.
The ISTH BAT was developed as a tool to accurately record bleeding symptoms in all haemorrhagic disorders and to aid diagnosis in patients referred with a possible bleeding disorder [9]. It was designed to capture the importance of recurrent minor bleeds in addition to more severe haemorrhage including life threatening episodes. It is also a potential research tool in studies that examine patients with excessive bleeding, allowing documentation of the severity of bleeding and comparison between patient groups across and within studies. A recent study has shown the ISTH BAT to be helpful in assessing children and parents in routine clinical practice, and found it to be equally discriminative as a BAT specifically designed for paediatric patients with regard to the prevalence of an underlying bleeding disorder [10]. However, it has not yet been validated in a large cohort of patients with suspected inherited platelet function disorders.
Materials and Methods
Participant selection
Participants with suspected inherited platelet function disorders were recruited to the Genotyping and Phenotyping of Platelets study (GAPP, ISRCTN 77951167) from October 2011 to December 2012 from UK Comprehensive Care Haemophilia Centres and were invited to participate in this study if they satisfied the following criteria:
Abnormal bleeding symptoms compatible with a possible inherited platelet function disorder (spontaneous mucocutaneous bleeding or abnormal bleeding at other sites following trauma or invasive procedures). Patients with existing diagnoses of Glanzmann’s thrombasthenia, Bernard-Soulier syndrome, May Hegglin anomaly or Hermansky-Pudlak syndrome were excluded.
Platelet count within the local laboratory reference range.
Results from coagulation factor tests within local laboratory reference intervals (minimum panel of prothrombin time, activated thromboplastin time, Clauss fibrinogen activity, von Willebrand factor antigen level, Ristocetin cofactor activity and FVIII:C activity)
Laboratory testing was deferred in participants exposed within two weeks to drugs known to affect platelet function.
Healthy volunteers aged 18 years or older were included in this study. Participants were considered healthy if they had not previously required clinical consultation or investigation for excessive bleeding,, did not require long-term medical therapy and had refrained from drugs known to influence platelet function in the previous 2 weeks.
This study was approved by the National Research Ethics Service Committee West Midlands – Edgbaston (REC reference: 06/MRE07/36) and all participants gave written informed consent in accordance with the Declaration of Helsinki.
Assessment of bleeding history
The ISTH bleeding assessment tool was used for assessment of the participants’ bleeding history. The questionnaire was carried out in patients with suspected inherited platelet function disorders (from UK Comprehensive Care Haemophilia Centres) and in healthy volunteers by trained personnel who regularly took bleeding histories in study participants. The bleeding assessment scores were compiled prior to undertaking platelet function testing so that investigators were not influenced by lumiaggregometry results when assessing and quantifying the bleeding history. The ISTH BAT score can range from 0 to 56 in women and 0-48 in men, based on a maximal score of 4 in fourteen separate categories assessing different types of bleeding.
Assessment of platelet function
Blood was drawn by venepuncture through a 21-gauge needle into evacuated tubes containing 3.8% sodium citrate. Platelet function was assessed using light transmission aggregometry as described previously [11]. Platelet-rich plasma (PRP) was prepared by centrifugation of whole blood at 200g for 20 min and autologous platelet-poor plasma (PPP) was obtained by further centrifugation at 1000g for 10 min. Platelet aggregation was measured with a dual-channel Chronolog lumiaggregometer (Model 460 VS, Havertown, PA, USA) in response to adenosine diphosphate (ADP) 3, 10 and 30 μM; adrenaline 10 and 30 μM; arachidonic acid 0.5 and 1 mM; collagen 1 and 3 μg/ml; PAR-1 receptor specific peptide (SFLLRN) 30 and 100 μM; and ristocetin 1.5 mg/ml. ATP secretion was assessed using the Luciferin-Luciferase reagent (Chronolume®) and an ATP standard solution. Results were classified as abnormal by reference to a bank of local healthy volunteers [11]. Platelet function testing operators were kept blind of the ISTH bleeding assessment tool score.
Statistical analyses
Normally distributed continuous variables are presented as mean ± standard deviation, non-normally distributed continuous variables as median (interquartile range, IQR), and categorical variables as frequencies (percentages). Variables were analyzed for a normal distribution with the Kolmogorov-Smirnov test. The association between the presence of platelet defects by platelet function testing and the ISTH BAT score was carried out by the non-parametric Kruskall-Wallis test, with Dunn’s adjustment for multiple comparisons. To evaluate the ISTH BAT’s ability to discriminate between patients with and without a demonstrable platelet defect on platelet function testing, a receiver operator characteristic (ROC) curve analysis was calculated and a simple logistic regression was used. A two-sided p-value < 0.05 was considered significant. Analyses were performed with SPSS 14.0 for Windows (SPSS Institute, Illinois, USA) and GraphPad Prism Software 5 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Participant characteristics
Of the 100 participants studied, 21 were healthy volunteers and 79 were recruited from UK Comprehensive Care Haemophilia Centres with suspected inherited platelet function disorders. The median age of participants with bleeding symptoms was 39 years (IQR from 31 to 53 years), while it was 32 years (IQR from 30 to 35 years) in healthy volunteers. The majority of recruited participants were female (81% among participants with bleeding symptoms and 67% among healthy volunteers).
Association of scores with platelet function testing results
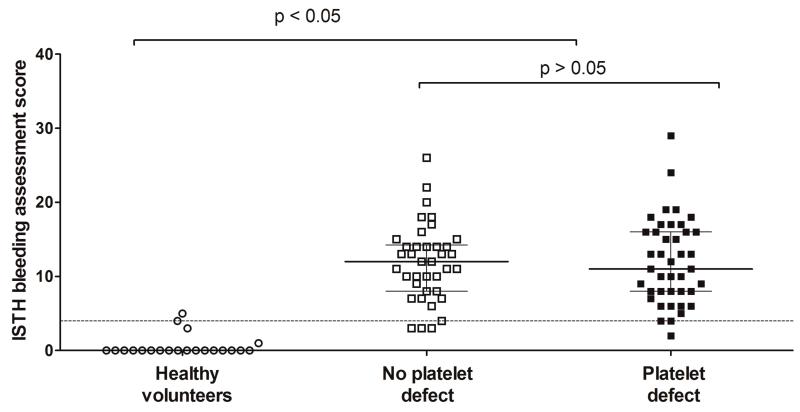
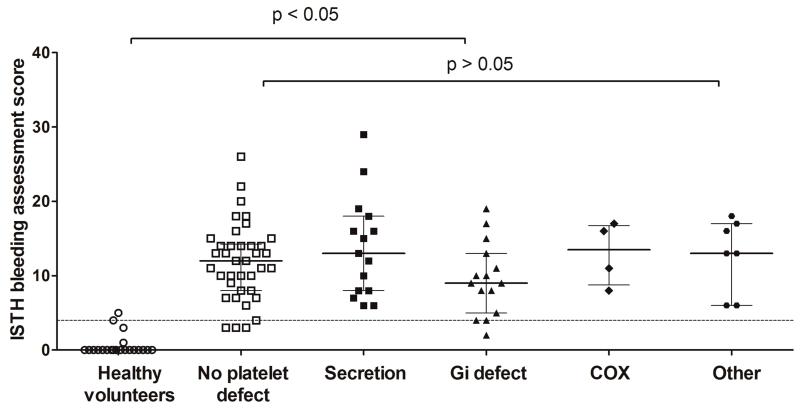
A platelet defect was found on lumiaggregometry in 52% of participants with bleeding symptoms, in accordance with our previously published findings [11]. The score obtained through the ISTH BAT in participants referred by UK Comprehensive Care Haemophilia Centres with bleeding symptoms (12; IQR 8 to 16) was significantly higher than in healthy volunteers (0; IQR 0 to 0), consistent with the clinical diagnosis of a bleeding disorder. However, there was no significant difference between participants in whom a platelet defect was detected by lumiaggregometry (11; IQR 8 to 16) and in whom platelet function was deemed normal (12; IQR 8 to 14) (Figure 1). There was no association between the type of platelet defect detected by lumiaggregometry (as defined in our recent study [11]) and the ISTH BAT score, although the numbers of patients in some diagnostic classification subgroups was relatively small (Figure 2).
Figure 1. Association between presence of a platelet function defect on lumiaggregometry and the ISTH bleeding assessment tool score.
95th percentile (score of 4) calculated from healthy volunteers and represented by horizontal dotted line. For the data points the line represents the median and the whiskers represent the interquartile range. Statistical analysis performed by the non-parametric Kruskall-Wallis test, with Dunn’s adjustment for multiple comparisons.
Figure 2. Association between type of platelet function defect on lumiaggregometry and the ISTH bleeding assessment tool score.
95th percentile (score of 4) calculated from healthy volunteers and represented by horizontal dotted line. For the data points the line represents the median and the whiskers represent the interquartile range. Statistical analysis performed by the non-parametric Kruskall-Wallis test, with Dunn’s adjustment for multiple comparisons. COX: cyclooxygenase. For description of types of defects, please refer to [11]
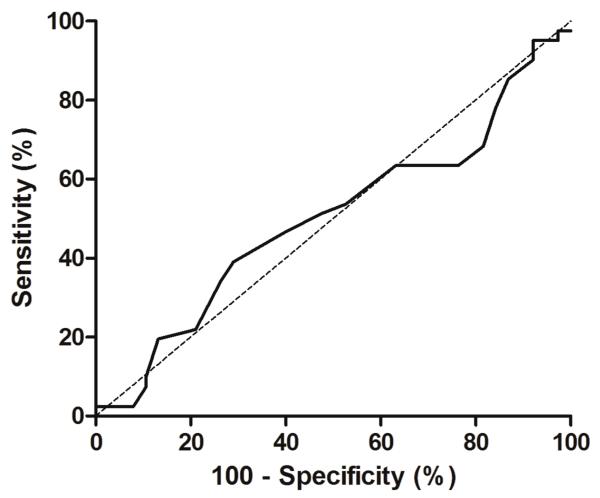
A ROC curve analysis was carried out to evaluate whether the ISTH bleeding assessment tool could discriminate between patients with and without a demonstrable platelet defect on lumiaggregometry (Figure 3). The area under the curve was 0.50 (95% CI 0.37 to 0.63, p=0.98) thus demonstrating a lack of discriminative ability. Moreover, the ISTH BAT score was not associated with a demonstrable platelet defect on platelet function testing in a simple logistic regression model (OR 1.01 [95% CI 0.93 – 1.09], p=0.91).
Figure 3. Receiver-operator curve for the presence of a platelet function defect on lumiaggregometry and the ISTH bleeding assessment tool score.
Area under the curve: 0.50 (95% CI 0.372 to 0.63), p=0.98
Subgroup analyses were performed to examine whether certain bleeding symptoms were predictive of the presence of a platelet defect on lumiaggregometry. ISTH BAT scores for epistaxis, cutaneous bleeding, post operative (including post dental work) bleeding and menorrhagia in women were assessed (Table 1). No statistical significance was found for any of these subgroups.
Table 1. Subgroup analyses for ISTH bleeding assessment tool score in patients with and without a platelet defect.
Symptoms assessed were epistaxis (maximum score 4), cutaneous bleeding (maximum score 4), post operative bleeding (including post dental work, maximum score 8) and menorrhagia (in female participants only, maximal score 4). Statistical analyses performed by the non-parametric Mann Whitney U test.
| Bleeding symptom | Patients with platelet defect (Median, Interquartile range) |
Patients without platelet defect (Median, Interquartile range) |
p value |
|---|---|---|---|
| Epistaxis | 1, 0-3 | 1, 0-2 | 0.67 |
| Cutaneous bleeding | 1, 0-2 | 1, 0-1 | 0.17 |
|
Post operative bleeding (including post dental work) |
4, 2-6 | 4, 2-6 | 0.89 |
| Menorrhagia | 3, 1-4 | 3, 2-4 | 0.27 |
Sensitivity, specificity, positive predictive value and negative predictive value of the ISTH BAT in predicting the presence of a platelet defect on lumiaggregometry was assessed (Table 2). A cut off value of 12 was used for the ISTH BAT score as this represented the 50th centile of ISTH BAT score in all patients. This analysis confirmed the absence of association between a high ISTH BAT score and the likelihood of having a platelet defect (κ=−0.04, [95% CI −0.26 to 0.18]).
Table 2. 2×2 analysis of the predictive value of a ISTH BAT score of 12 and over in predicting the presence of a platelet defect on lumiaggregometry.
ISTH BAT score of 12 represents the 50th centile in all patients studied. Sensitivity, specificity, positive predictive value and negative predictive value are shown. Kappa statistic for concordance = −0.04 (95% CI −0.26 to 0.18), demonstrating a lack of significance.
| Defect present on lumiaggregometry |
Defect absent on lumiaggregometry |
||
|---|---|---|---|
| BAT score ≥ 12 | 20 | 20 |
Positive predictive value = 50% |
| BAT score < 12 | 21 | 18 |
Negative predictive value = 54% |
| Sensitivity = 49% | Specificity = 53% |
Discussion
Our findings confirm that the ISTH BAT score is raised in patients with clinical evidence of excessive bleeding and a suspected platelet function disorder. We have also shown that healthy volunteers have a low score (≤4) in keeping with the absence of a history of bleeding episodes. However, the score is unable to discriminate between patients with clinically diagnosed bleeding disorders subsequently shown to have a platelet defect on lumiaggregometry and those in whom an underlying platelet defect was not detected. This suggests that the ISTH BAT is powerful in documenting bleeding symptoms, but that its level per se is not predictive of the likelihood of diagnosing an inherited platelet disorder in patients with excessive bleeding. It is possible that some patients classified as not having a platelet defect do have a mild defect which is below the limit of sensitivity of our current testing assays, or have a defect in other aspects of platelet function (such as procoagulant activity) which was not tested for. Given the history of excessive bleeding in all patients, abnormalities in other untested parts of the haemostatic pathway may account for the clinical presentation of a proportion of the patients in whom we did not detect a platelet defect.
The healthy volunteer group included in this study is small (n=21). The 95th centile for BAT score in this group is 4, although defining a more precise upper limit of normal range for the BAT score requires larger studies that ideally examine heterogenous populations. In addition, the median age in the healthy volunteer group was slightly lower than in the patient group. This may have reduced the BAT score in healthy volunteers due to fewer cumulative challenges to the haemostatic system such as surgery or pregnancy and childbirth in women. Previous work has shown that platelet aggregability slightly increases with age [12], meaning that the aggregation ranges observed in the healthy volunteer group may be lower than that expected in the patients if platelet function was normal. However, this would have biased the results in the patient group towards increased platelet reactivity and therefore should not have led to mislabeling of patients with normal responses as having a platelet defect.
The documentation of bleeding risk in mild bleeding disorders is challenging. Mucocutaneous bleeding symptoms are reported in a significant proportion of the healthy population, which makes determination of the threshold that constitutes excessive bleeding difficult [13]. Mild bleeding disorders are prevalent in the general population, reaching up to 1%, but both the clinical diagnosis and laboratory characterization remain complicated [14]. The presence of a laboratory phenotype does not necessarily lead to bleeding especially in the absence of a hemostatic challenge and as a consequence most mild bleeding disorders, particularly inherited platelet disorders, remain clinically and biochemically ill defined [14, 15]. There is an important clinical need for an assessment tool that would allow clinical characterization of this population in order to stratify which patients require further investigation.
Numerous scores for bleeding assessment have been proposed over the years (reviewed by Rodeghiero, Tosetto and Castaman [15] and by Rydz and James [1]). The strength of the ISTH BAT tool is to specifically capture the frequency of mild bleeding symptoms, which is often missed in traditional BATs [9]. As such, the ISTH BAT is particularly relevant to patients with recurrent and mild bleeding symptoms, which are a key feature of inherited platelet disorders [14, 15].
There is little prior experience with this tool in mild bleeding disorders. Using a previous version of a BAT based on the European MCMDM-1 VWD study [3], Tosetto et al. analyzed the BAT’s clinical utility in 215 patients with mild bleeding disorders [16]. The findings supported the use of the BAT instrument, as a low bleeding score could reasonably exclude the presence of a mild bleeding disorder in an unselected population, and the utility increased when the BAT score was used in conjunction with laboratory testing [16]. Our results agree with these findings, as patients with clinical evidence of excessive bleeding in our study did have significantly higher ISTH BAT scores than healthy volunteers. A further study by Bidlingmaier et al. looked at the clinical utility of the ISTH BAT questionnaire in a routine pediatric setting [10]. In this study on 100 children presenting with a mild bleeding diathesis, a low ISTH BAT score made a bleeding disorder diagnosis unlikely, consistent with our own findings and those of Tosetto et al. [10, 16]. However, only 5 children were diagnosed with a possible platelet disorder in this study, thus reducing the generalizability to a large cohort of patients with suspected inherited platelet function disorders. Also, given that the ISTH BAT assesses responses to hemostatic challenges, including menorrhagia, childbirth, dental procedures and surgery, the applicability of the scores obtained in children to an adult population is uncertain, as many children will not yet have been exposed to these challenges.
Our study is the first to report on the utility of the ISTH BAT in a large cohort of patients with suspected inherited platelet function disorders. Our findings support the use of the ISTH BAT to document recurrent bleeding symptoms in this population. However, the ISTH BAT score is not predictive of which patients have a platelet function defect by laboratory investigation using lumiaggregometry, which is the currently accepted gold standard of assessing platelet function.
Conclusion
Assessment of symptoms using BATs is likely to become more important in years to come as the laboratory investigation of individuals with excessive bleeding improves, and use of BATs will allow comparison between patient groups and treatment centres. The fact that the ISTH BAT documents recurrent minor bleeding episodes is important in the assessment of individuals with suspected inherited platelet function disorders.
Further work in this area should include international collaborations, such as the ISTH BAT repository [17], to allow further validation of the ISTH BAT and to obtain data on its performance in other hemorrhagic disorders.
Acknowledgements
The GAPP programme is chaired by Steve P. Watson and has principal investigators in Bristol (Andrew Mumford and Stuart Mundell), London (Paul Gissen) and Sheffield (Martina Daly). We would like to acknowledge the support of all of the GAPP team and Consultant Haematologists at our collaborating UK Haemophilia Comprehensive Care Centres.
This work was supported by the British Heart Foundation (RG/09/007/27917; PG/10/36/02 and the Wellcome Trust (093994). SPW holds a BHF chair (CH/03/003). ML is supported by the Canadian Institutes of Health Research (MFE-107592) and the British Heart Foundation (PG/11/31/28835). GL is supported by the Wellcome Trust (093994).
Footnotes
Declaration of interest
The authors have no relevant conflicts of interest to declare.
References
- 1.Rydz N, James PD. The Evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10:2223–9. doi: 10.1111/j.1538-7836.2012.04923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodeghiero F, Castaman G, Tosetto A, Batlle J, Baudo F, Cappelletti A, Casana P, De Bosch N, Eikenboom JC, Federici AB, Lethagen S, Linari S, Srivastava A. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. J Thromb Haemost. 2005;3:2619–26. doi: 10.1111/j.1538-7836.2005.01663.x. [DOI] [PubMed] [Google Scholar]
- 3.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, Meyer D, Fressinaud E, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Budde U, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill F, Peake I. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 4.Katsanis E, Luke KH, Hsu E, Li M, Lillicrap D. Prevalence and significance of mild bleeding disorders in children with recurrent epistaxis. J Pediatr. 1988;113:73–6. doi: 10.1016/s0022-3476(88)80532-8. [DOI] [PubMed] [Google Scholar]
- 5.Dean JA, Blanchette VS, Carcao MD, Stain AM, Sparling CR, Siekmann J, Turecek PL, Lillicrap D, Rand ML. von Willebrand disease in a pediatric-based population--comparison of type 1 diagnostic criteria and use of the PFA-100 and a von Willebrand factor/collagen-binding assay. Thromb Haemost. 2000;84:401–9. [PubMed] [Google Scholar]
- 6.Bowman M, Riddel J, Rand ML, Tosetto A, Silva M, James PD. Evaluation of the diagnostic utility for von Willebrand disease of a pediatric bleeding questionnaire. J Thromb Haemost. 2009;7:1418–21. doi: 10.1111/j.1538-7836.2009.03499.x. [DOI] [PubMed] [Google Scholar]
- 7.Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–9. doi: 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- 8.Philipp CS, Faiz A, Heit JA, Kouides PA, Lukes A, Stein SF, Byams V, Miller CH, Kulkarni R. Evaluation of a screening tool for bleeding disorders in a US multisite cohort of women with menorrhagia. Am J Obstet Gynecol. 2011;204:209 e1–7. doi: 10.1016/j.ajog.2010.10.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodeghiero F, Tosetto A, Abshire T, Arnold DM, Coller B, James P, Neunert C, Lillicrap D. ISTH/SSC Bleeding Assessment Tool: A Standardized Questionnaire and a Proposal for a New Bleeding Score for Inherited Bleeding Disorders. J Thromb Haemost. 2010;8:2063–5. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 10.Bidlingmaier C, Grote V, Budde U, Olivieri M, Kurnik K. Prospective evaluation of a pediatric bleeding questionnaire and the ISTH bleeding assessment tool in children and parents in routine clinical practice. J Thromb Haemost. 2012;10:1335–41. doi: 10.1111/j.1538-7836.2012.04775.x. [DOI] [PubMed] [Google Scholar]
- 11.Dawood BB, Lowe GC, Lordkipanidzé M, Bem D, Daly ME, Makris M, Mumford A, Wilde JT, Watson SP. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood. 2012;120:5041–9. doi: 10.1182/blood-2012-07-444281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meade TW, Vickers MV, Thompson SG, Stirling Y, Haines AP, Miller GJ. Epidemiological characteristics of platelet aggregability. Br Med J (Clin Res Ed) 1985;290:428–32. doi: 10.1136/bmj.290.6466.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodeghiero F, Castaman G. Congenital von Willebrand disease type I: definition, phenotypes, clinical and laboratory assessment. Best Pract Res Clin Haematol. 2001;14:321–35. doi: 10.1053/beha.2001.0136. [DOI] [PubMed] [Google Scholar]
- 14.Watson SP, Lowe GC, Lordkipanidze M, Morgan NV. Genotyping and phenotyping of platelet function disorders. J Thromb Haemost. 2013 doi: 10.1111/jth.12199. doi: 10.1111/jth.12199. [DOI] [PubMed] [Google Scholar]
- 15.Rodeghiero F, Tosetto A, Castaman G. How to estimate bleeding risk in mild bleeding disorders. J Thromb Haemost. 2007;5(Suppl 1):157–66. doi: 10.1111/j.1538-7836.2007.02520.x. [DOI] [PubMed] [Google Scholar]
- 16.Tosetto A, Castaman G, Plug I, Rodeghiero F, Eikenboom J. Prospective evaluation of the clinical utility of quantitative bleeding severity assessment in patients referred for hemostatic evaluation. J Thromb Haemost. 2011;9:1143–8. doi: 10.1111/j.1538-7836.2011.04265.x. [DOI] [PubMed] [Google Scholar]
- 17.International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH-BAT) [Accessed on 9 April 2013];ISTH-BATR. doi: 10.1111/ejh.13625. available from https://bh.rockefeller.edu/ISTH-BATR/ [DOI] [PubMed]