Abstract
The cytokine interleukin-21 (IL-21) is a potent immune modulator with diverse mechanisms of action on multiple cell types. IL-21 is in clinical use to promote tumour rejection and is an emerging target for neutralisation in the setting of autoimmunity. Despite its clinical potential, the biological actions of IL-21 are not yet fully understood and the full range of effects of this pleiotropic cytokine are still being uncovered. Here we identify a novel role for IL-21 as an inducer of the costimulatory ligand CD86 on B lymphocytes. CD86 provides critical signals through T cell-expressed CD28 that promote T cell activation in response to antigen engagement. Expression levels of CD86 are tightly regulated in vivo, being actively decreased by regulatory T cells and increased in response to pathogen-derived signals. Here we demonstrate that IL-21 can trigger potent and sustained CD86 upregulation through a STAT3 and PI3-kinase dependent mechanism. We show that elevated CD86 expression has functional consequences for the magnitude of CD4 T cell responses both in vitro and in vivo. These data pinpoint CD86 upregulation as an additional mechanism by which IL-21 can elicit immunomodulatory effects.
Introduction
IL-21 is known to influence multiple parameters of the immune response. The clinical importance of this pathway was first appreciated nearly a decade ago with the demonstration that IL-21 could augment anti-tumour immunity(1, 2), and this has since become an active area of research(3-5). In addition to augmenting immunity against tumours, IL-21 signalling can directly induce apoptotic pathways in chronic lymphocytic leukaemia (CLL) B cells(6, 7) and diffuse large B cell lymphoma(8).
The role of IL-21 in T cell-dependent B cell responses has been extensively documented. IL-21 critically regulates antibody production, partly in cooperation with IL-4(9), and promotes plasma cell differentiation in both mice(10) and humans(11). The intimate interaction between follicular helper T cells (TFH) and germinal centre B cells is also shaped by provision of IL-21; TFH-derived IL-21 directly targets germinal centre B cells, reinforcing their fate decision by sustaining bcl6 expression(12, 13).
Alongside effects on B cells, several studies have also reported that IL-21 promotes T cell activation. Pre-exposure to IL-21 has been shown to increase the antigen responsiveness of CD8 T cells(14) and permit triggering by weak TCR agonists(15). CD4 T cell responses can also be augmented by IL-21, in part due to its ability to counteract Treg suppression(16, 17). The mechanisms by which IL-21 directly or indirectly promotes T cell responses are not yet fully defined. In this study we identify a novel role for IL-21 in upregulating the expression of the costimulatory ligand CD86 on B cells. We show that this requires activation of the PI3-kinase pathway and is dependent on the PI3-kinase subunit p110δ, a molecule currently being targeted in the setting of several B cell malignancies (CLL, NHL)(18). The increased expression of CD86 on B cells is shown to have functional consequences for T cell expansion both in vitro and in vivo. Collectively these data suggest an additional mechanism by which IL-21 may augment adaptive immune responses and reveal a further level of T cell / B cell interaction directed by this cytokine.
Materials and Methods
Mice
DO11.10 TCR transgenic and BALB/c mice were purchased from The Jackson Laboratory. IL-21R−/− mice were provided by Manfred Kopf (ETH Zurich) and were bred with DO11.10 TCR transgenic mice to generate IL-21R−/− DO11.10 TCR transgenic progeny. p110δD910A mice were provided by K.O. Mice were housed at the University of Birmingham Biomedical Services Unit or at University College London and used according to Home Office and institutional guidelines.
Flow cytometry
Cells were stained with mAb against CD25 (PC61.5; eBioscience), CD4 (LT34; eBioscience), CD19 (1D3), CD86 (GL1; eBioscience), CD80 (16-10A1), pSTAT1 (14/P-STAT1), pSTAT3 (49/P-STAT3), pSTAT5 (47) and DO11.10 TCR (KJ1.26; eBioscience). All antibodies were purchased from BD Biosciences unless otherwise indicated. For pSTAT staining, cells were fixed in 4% paraformaldehyde for 10 minutes and permeabilized with 100% ice-cold methanol for 30 minutes. Statistics were performed using an unpaired 2-tailed t test with a 95% confidence interval.
Short-term splenocyte cultures
1×105 BALB/c splenocytes were cultured for 15-16 hours alone, with IL-21 at 25, 50, 100 or 200ng/ml (Peprotech), or with 1μg/ml LPS (Sigma). For time-course experiments, cells were harvested at 2, 4, 6, 8 or 15 hours.
Short-term B cell cultures
Magnetic separation (Miltenyi Biotec) was used to purify CD19+ B cells from BALB/c or p110δD910A spleen. 1×106 cells were cultured for 16 hours alone or in the presence of 200ng/ml IL-21 (Peprotech) or 10ng/ml IL-4 (Peprotech). For STAT3 inhibition experiments cultures were supplemented with 10, 50 or 100μM S3I-201 (Calbiochem) as indicated. For PI3K inhibition experiments cultures were supplemented with 10μM LY-294002 (Invitrogen) as indicated. For assessment of activated STAT proteins cells were cultured for 2 hours alone or in the presence of 200ng/ml IL-21 (Peprotech). For experiments to determine the target of IL-21 2.5×104 BALB/c or IL-21R−/− B cells were cultured with 2.5×104 magnetically separated (Miltenyi Biotec) CD4+CD25− T cells from BALB/c or IL-21R−/− lymph node for 16 hours alone or in the presence of 200ng/ml IL-21 (Peprotech).
Confocal microscopy
Magnetic separation (Miltenyi Biotec) was used to purify CD19+ B cells from BALB/c spleen. 1×106 cells were cultured for 6 hours alone, with 200ng/ml IL-21 (Peprotech), with 10μg/ml cycloheximide (Sigma) or both. Cells were stained with mAb against CD86 (GL1; eBioscience) and imaged using glass bottom culture dishes (MatTek). Imaging was carried out using a 100x oil immersion objective.
RT-PCR
Magnetic separation (Miltenyi Biotec) was used to purify CD19+ B cells from BALB/c spleen. mRNA was isolated at this point or after culture of 1×106 cells for 16 hours alone or in the presence of 200ng/ml IL-21 (Peprotech). Quantitative PCR was performed to assess the expression of β2 microglobulin (Eurofins MWG Operon) or CD86 (Taqman gene expression assay; Applied Biosystems).
In vitro proliferation assays
Magnetic separation (Miltenyi Biotec) was used to purify CD4+CD25− T cells from IL-21R−/− lymph node. 2.5×104 cells were cultured with 2.5×104 CD19+ B cells from BALB/c or IL-21R−/− spleen, with 0.8μg/ml anti-CD3 (BD Biosciences) alone or in the presence of 10μg/ml anti-CD86 (BioXcell). Triplicate wells were pooled and harvested at day 1 to assess CD86 expression or day 3 to determine cell counts by flow cytometry.
Adoptive transfers
CD19+ B cells from BALB/c or IL-21R−/− spleen were isolated by magnetic separation (Miltenyi Biotec) and cultured for 16 hours with 1μg/ml OVA peptide. 3×106 peptide pulsed cells were injected intravenously into IL-21R−/− recipients. Magnetic separation (Miltenyi Biotec) was used to purify CD4+ cells from IL-21R−/− DO11.10 TCR+ lymph node. 24 hours after B cell transfer a total of 2×106 CellTrace Violet labelled IL-21R−/− DO11.10 TCR+ T cells were injected intravenously and 1μg IL-21 (Peprotech) or PBS intraperitoneally. Where indicated recipients were also given 100μg anti-CD86 (BioXcell) or PBS intraperitoneally immediately after T cell transfer. Mice were culled at day 7 for analysis of inguinal lymph node and splenic cells.
Results
IL-21 upregulates CD86 expression on B cells
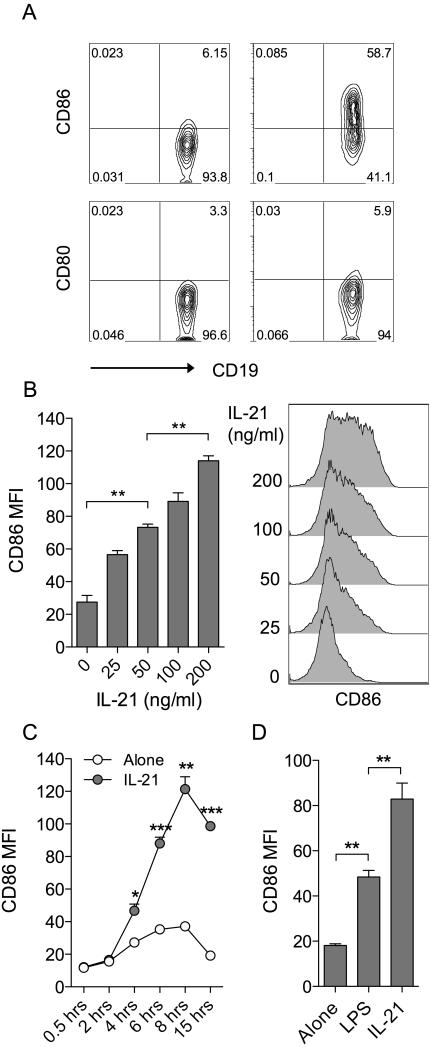
To explore the influence of IL-21 on costimulatory ligand expression, murine splenocytes were cultured in the presence or absence of IL-21 for 16h and the expression of CD86 and CD80 assessed by flow cytometry. We noted a marked elevation of CD86, but not CD80, on CD19+ B cells in the timeframe examined (Fig. 1A). Titration (Fig. 1B) and timecourse (Fig. 1C) data established that IL-21-mediated CD86 upregulation was dose-dependent and appeared maximal around 8h following stimulation. The magnitude of CD86 upregulation observed in response to IL-21 was comparable, if not greater, than that seen with a 1μg/ml dose of LPS, a TLR ligand known to drive CD86 upregulation (Fig. 1D). In contrast, splenic dendritic cells showed some CD86 expression at baseline and this was not further elevated by IL-21 under the conditions assessed (Supplemental Fig. 1).
Figure 1. IL-21 upregulates CD86 expression on B cells.
(A) 1×105 BALB/c splenocytes were cultured for 16 hours alone or in the presence of 200ng/ml IL-21. Plots show representative CD80 and CD86 expression for gated CD19+ B cells. (B) 1×105 BALB/c splenocytes were cultured for 16 hours alone or in the presence of the indicated doses of IL-21. Histograms show CD86 expression for gated CD19+ B cells and graph shows collated CD86 MFI data. (C) 1×105 BALB/c splenocytes were cultured alone or in the presence of 200ng/ml IL-21 and harvested at the indicated time points. Graph shows collated CD86 MFI data for gated CD19+ B cells. (D) 1×105 BALB/c splenocytes were cultured for 16 hours alone or in the presence of either 200ng/ml IL-21 or 1μg/ml LPS. Graph shows collated CD86 MFI data for gated CD19+ B cells. Data are representative of at least 3 independent experiments. * P < .05. ** P < .01. *** P < .001.
CD86 upregulation requires direct signalling to B cells and protein neosynthesis
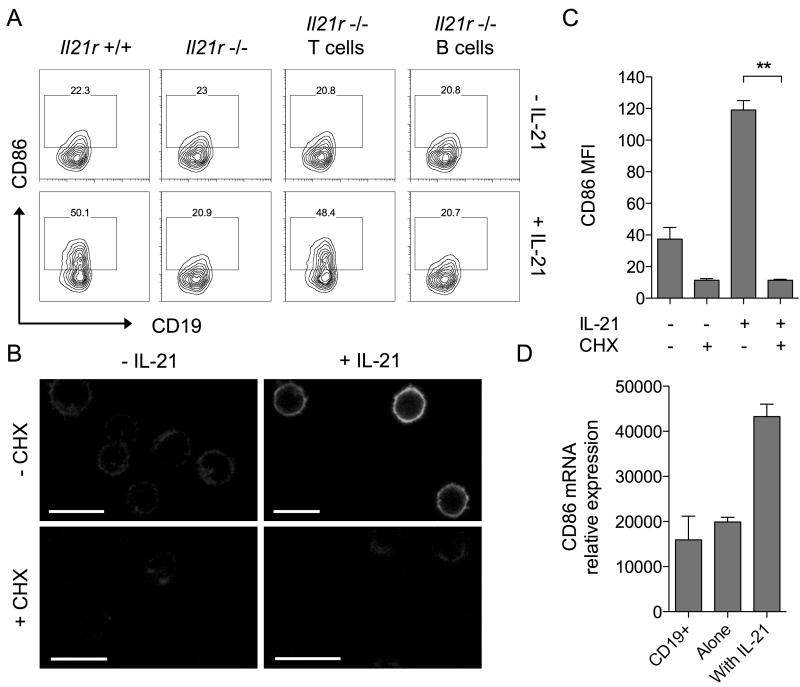
Since the above assays did not use purified B cells it remained possible that IL-21 was altering CD86 expression indirectly by acting on a different cell type. In this regard we have previously shown that conventional CD4 T cells express high levels of IL-21 receptor and can respond to IL-21 by acquiring resistance to Treg suppression(19). To dissect whether regulation of CD86 expression involved direct signalling to B cells or was an indirect consequence of IL-21 signalling to T cells, we took advantage of mice genetically deficient for the IL-21 receptor(20). Co-cultures of T cells and B cells were established in which the cells derived from either wildtype or IL-21 receptor deficient mice and these were incubated for 16h with or without IL-21. As expected, when both T and B cells expressed IL-21R, IL-21 upregulated CD86 expression (Fig. 2A, far left panels) and when neither cell type expressed IL-21R, CD86 was unchanged (Fig. 2A, second panels). Crucially, in situations where B cells were IL-21R+/+ but T cells were IL-21R−/−, addition of IL-21 retained the capacity to upregulate CD86 (Fig. 2A, third panels). Furthermore, preventing B cells alone from receiving IL-21 signals completely abrogated the ability of IL-21 to upregulate CD86 (Fig. 2A, far right panels). Collectively these data indicate that direct signalling of IL-21 to B cells is responsible for mediating CD86 upregulation.
Figure 2. Induction of CD86 by IL-21 requires direct signalling to B cells and is dependent on protein neosynthesis.
(A) 2.5×104 BALB/c CD4+CD25− T conv were cultured with 2.5×104 CD19+ B cells for 16 hours alone or in the presence of 200ng/ml IL-21. Cell populations were deficient for the IL-21R as indicated. Plots show CD86 expression for gated CD19+ B cells. (B) 1×106 BALB/c CD19+ B cells were cultured for 6 hours alone, with 200ng/ml IL-21, 10μg/ml cycloheximide or both. Representative confocal microscopy images show CD86 staining. Scale bars = 10μM. (C) Graph shows collated CD86 MFI for cells from B analysed by flow cytometry. Data are representative of 3 independent experiments. (D) 1×106 BALB/c CD19+ B cells were cultured for 16 hours alone or in the presence of 200ng/ml IL-21. Graph shows relative CD86 mRNA expression for cultured cells and freshly isolated CD19+ B cells. Data are representative of 3 independent experiments. ** P < .01.
The upregulation of CD86 by the cytokine IFNγ has been reported to be independent of protein neosynthesis(21). To examine whether upregulation of CD86 by IL-21 required protein neosynthesis we performed experiments using purified B cells in the presence or absence of cycloheximide. The ability of IL-21 to upregulate B cell CD86 expression was completely abrogated in the presence of cycloheximide, indicating a requirement for protein neosynthesis (Fig. 2B, 2C). We next assessed whether upregulation of CD86 by IL-21 was transcriptionally regulated; this revealed that mRNA for CD86 was strongly upregulated by exposure to IL-21 (Fig. 2D). Together these data indicate that de novo transcription and translation of CD86 mRNA is required for IL-21-mediated CD86 upregulation.
CD86 upregulation requires STAT3 and Pi3-kinase
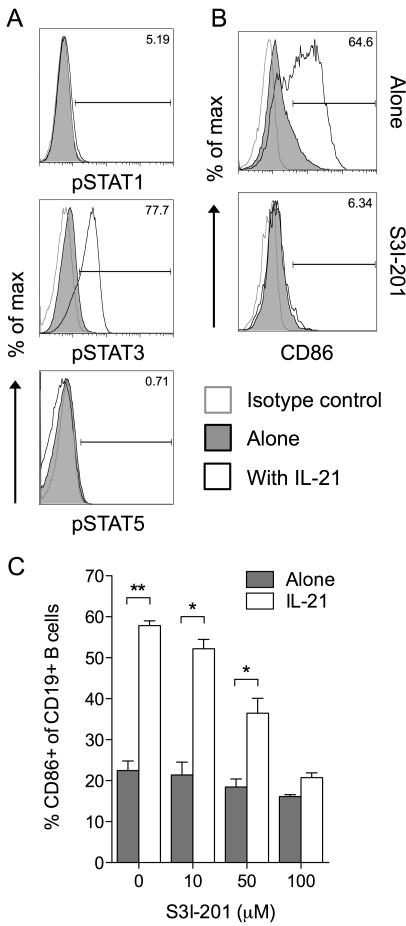
IL-21 signaling is known to involve STAT3 activation in both mouse(22) and human(23, 24) B cells, and the ability of STAT3 to mediate IL-21 effects has been demonstrated using B cells isolated from STAT3-deficient patients(25). We confirmed the ability of IL-21 to activate the STAT3 pathway in B cells (Fig. 3A) then sought to determine whether this pathway was involved in IL-21-mediated CD86 upregulation. In the presence of the STAT3 inhibitor S3I-201, IL-21 was no longer able to induce significant CD86 upregulation (Fig. 3B, 3C) suggesting a critical role for STAT3 in this process.
Figure 3. Induction of CD86 by IL-21 is dependent on STAT3 activation.
(A) 1×106 BALB/c CD19+ B cells were cultured for 2 hours alone or in the presence of 200ng/ml IL-21. Histograms show representative staining for phosphorylated STAT proteins as indicated. (B) 1×106 BALB/c CD19+ B cells were cultured for 16 hours alone or in the presence of 200ng/ml IL-21, 100μM S3I-201 or both. Histograms show representative CD86 expression for gated CD19+ B cells. (C) Graph shows collated CD86 expression data for cells from B when cultured with the indicated doses of S3I-201. Data are representative of 3 independent experiments. * P < .05. ** P < .01.
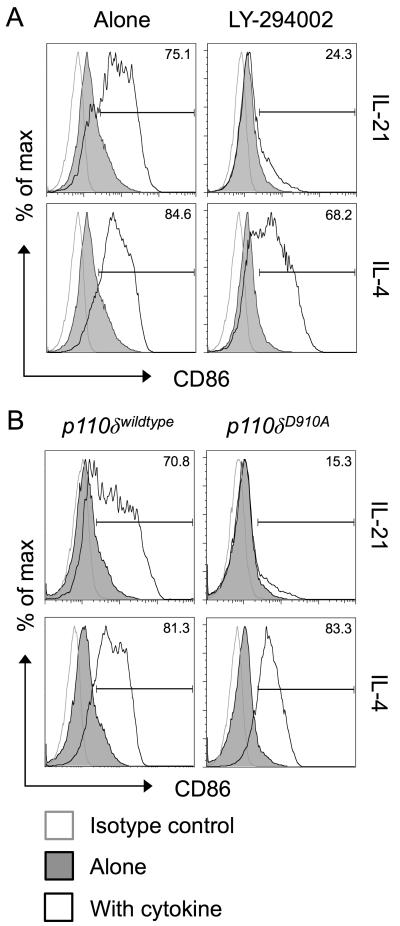
A second major pathway triggered by IL-21 is the activation of the enzyme phosphoinositide-3-kinase (PI3-kinase)(22, 26), and the ability of BCR engagement to upregulate CD86 is known to be dependent on PI3-kinase signalling(27). To determine whether IL-21 upregulates CD86 via a PI3-kinase-dependent pathway, we first employed the PI3-kinase inhibitor LY-294002. Strikingly, IL-21-mediated CD86 upregulation was completely abrogated in the presence of this inhibitor (Fig. 4A). The related cytokine IL-4 retained the ability to upregulate CD86 in the presence of the PI3-kinase inhibitor, consistent with previous studies(27). We next took advantage of mice expressing a catalytically inactive form of the PI3-kinase p110δ subunit (D910A mice)(28), since this subunit is known to be important in the B cell lineage(29, 30). Purified B cells from D910A animals failed to upregulate CD86 in response to IL-21, while their ability to upregulate CD86 in response to IL-4 remained intact (Fig. 4B). Collectively these data indicate that IL-21 uses STAT3 and PI3-kinase to drive CD86 upregulation and pinpoint p110δ as the critical PI3-kinase subunit responsible.
Figure 4. Induction of CD86 by IL-21 is dependent on PI3K p110δ.
(A) 1×106 BALB/c CD19+ B cells were cultured for 16 hours alone or in the presence of 200ng/ml IL-21 or 10ng/ml IL-4. Where indicated cultures also contained 10μM LY-294002. Histograms show CD86 expression for gated CD19+ B cells. (B) 1×106 BALB/c CD19+ B cells were cultured for 16 hours alone or in the presence of 200ng/ml IL-21 or 10ng/ml IL-4. Cells expressed the wildtype or mutant isoforms of PI3K P110δ as indicated. Histograms show CD86 expression for gated CD19+ B cells. Data are representative of 3 independent experiments.
IL-21 mediated CD86 upregulation has functional consequences in vitro
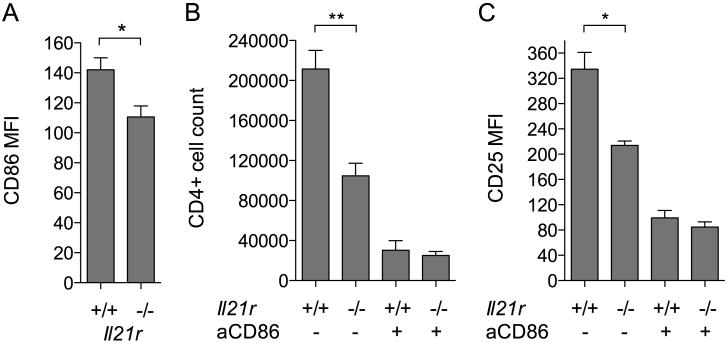
To test whether the upregulation of CD86 in response to IL-21 had functional consequences, in vitro T cell proliferation assays were performed. CD4+CD25− T cells from IL-21R−/− mice were used to preclude direct effects of IL-21 on the T cells themselves. IL-21R−/− T cells were activated by anti-CD3 in the presence of B cells that were either wildtype or IL-21R deficient; in this setting any IL-21 made by the T cells would potentially be able to upregulate CD86 on the wildtype B cells but not the IL-21R−/− ones. In line with this, CD86 was upregulated to a greater extent on the IL-21R+/+ B cells than the IL-21R−/− ones (Fig. 5A). IL-4 did not appear to contribute to CD86 upregulation in this setting as assessed by inclusion of blocking anti-IL-4 Ab (data not shown). The increased CD86 expression on IL-21R+/+ B cells was associated with significantly greater T cell proliferation (Fig. 5B) and activation marker expression (Fig. 5C and data not shown). T cell proliferation and activation marker expression was dependent on CD86 as demonstrated by antibody blockade of this pathway (Fig. 5B, 5C).
Figure 5. IL-21 signalling to B cells promotes CD86-dependent T cell proliferation.
2.5×104 IL-21R−/− CD4+CD25− T conv were cultured with 0.8μg/ml anti-CD3 and 2.5×104 IL-21R−/− or +/+ CD19+ B cells alone or in the presence of 10μg/ml anti-CD86. (A) Graph shows collated CD86 MFI data for gated CD19+ B cells harvested after 16 hours in the absence of anti-CD86 blocking antibody (B) Graph shows collated data for absolute CD4+ T conv counts after harvest at day 3 when cultured with IL-21R−/− or +/+ B cells as indicated. (C) Graph shows collated CD25 MFI data for CD4+ T conv from B. * P < .05. ** P < .01.
IL-21 mediated CD86 upregulation has functional consequences in vivo
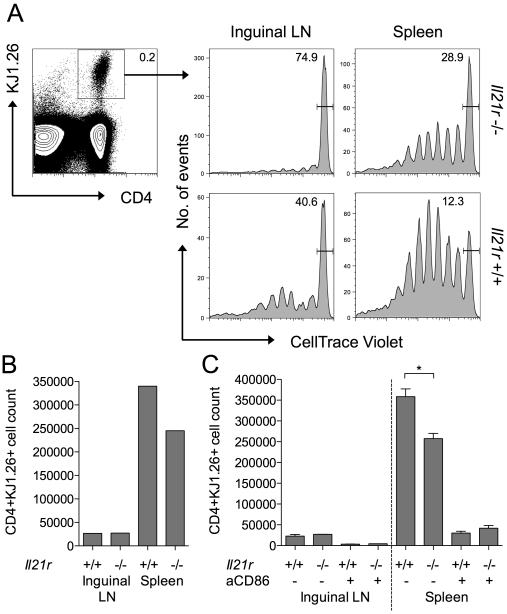
To extend the above observations to an in vivo setting, we established a system in which peptide-pulsed B cells were used to stimulate the proliferation of antigen-specific CD4 T cells. Wildtype or IL-21R−/− B cells were pulsed with OVA323-339 peptide and adoptively transferred into IL-21R−/− hosts. OVA-specific T cells (DO11) that were also IL-21R−/− were CellTrace labelled and injected into the same hosts, so that their response to the OVA-pulsed B cells could be tracked. To maximise the effects of IL-21 on B cell phenotype, recombinant IL-21 was injected i.p. where indicated. Ensuring that the host animals and the adoptively transferred T cells were IL-21R−/− allowed us to restrict the IL-21 effects to the B cell compartment. At d7, single cell suspensions from inguinal LN and spleen were stained by flow cytometry to identify the adoptively transferred antigen-specific T cells and assess their proliferation status (Fig. 6A). CellTrace profiles for gated antigen-specific T cells revealed greater proliferation in mice that had received IL-21R+/+ B cells compared with those that had received IL-21R−/− B cells. Proliferation was particularly evident in the spleen consistent with the likely trafficking of the adoptively transferred peptide-pulsed B cells to this site. The enhanced T cell response to IL-21R+/+ B cells was also reflected in higher absolute numbers of antigen-specific T cells (Fig. 6B). The ability of IL-21 signalling to B cells to augment the T cell response was dependent on CD86 as assessed by in vivo antibody blockade (Fig. 6C). Recovery of injected B cells at day 1 confirmed increased expression of CD86 on IL-21R+/+ B cells compared with IL-21R−/− B cells (Supplemental Fig. 2). Collectively these data demonstrate that IL-21 increases expression of CD86 on B cells and that this increased availability of costimulatory ligand has functional consequences for the magnitude of T cell responses in vitro and in vivo.
Figure 6. IL-21 signalling to B cells promotes CD86-dependent T cell proliferation in vivo.
(A) 3×106 OVA peptide loaded IL-21R−/− or +/+ CD19+ B cells were adoptively transferred into IL-21R−/− recipients. After 24 hours mice received 2×106 CellTrace labelled IL-21R−/− DO11+ CD4+ T cells intravenously and 1μg IL-21 intraperitoneally. Histograms show representative CellTrace dilution for gated CD4+ DO11+ T cells harvested from spleens or inguinal lymph nodes of IL-21R−/− or +/+ B cell recipients at day 7. (B) Graph shows representative absolute CD4+DO11+ T cell counts from A. (C) IL-21R−/− T cells were activated in the presence of OVA peptide loaded IL-21R−/− or +/+ CD19+ B cells (as above) and recipient mice received 100μg anti-CD86 or isotype control antibody intraperitoneally on day 1. Graphs show collated absolute CD4+DO11+ T cell counts. Data are representative of at least 2 independent experiments. * P < .05.
Discussion
IL-21 is recognised to exert multiple effects on the differentiation and function of B cells. While first recognised for its ability to promote human B cell proliferation(31), it can also trigger B cell apoptosis(32) with the outcome of IL-21R signalling most likely dictated by its context(10, 33). IL-21 has a well established role in the induction of plasma cell differentiation(10, 11) and this involves the cooperative binding of STAT3 and IRF4 to the IL-21 response element in the Prdm1 gene encoding Blimp-1(34). Intriguingly, IL-21 also promotes the expression of Bcl6 a key transcriptional regulator of germinal centre B cell differentiation(12, 13), despite the fact that Blimp-1 and Bcl6 have mutually antagonistic effects. Thus IL-21 acts at multiple stages to modulate B cell activation, differentiation and death. Our recent findings provide additional insight into the effects of IL-21 on B cells, demonstrating an upregulation of CD86 that has consequences for T cell dependent immune responses.
The role of IL-21 in DCs is less well studied and our side by side comparison of B cells and DCs suggested CD86 was not obviously upregulated by IL-21 in the latter population. While it has been shown that IL-21 can inhibit DC maturation(35, 36), other studies suggested IL-21 can enhance DC function(37), and recent analysis has identified a role for IL-21 in triggering DC migration in a virus-induced diabetes model(38). Interestingly, in the latter study, reduced migration of IL-21R−/− DC was accompanied by decreased CD86 expression. Thus, further work is required to fully elucidate the biological function of IL-21 in DCs.
The provision of CD28 costimulation is known to be of critical importance in thresholding T cell responses. As a consequence, stimuli that alter expression of the costimulatory ligands CD86 and CD80 are likely to have a significant impact on T cell immunity. This is particularly true of CD86, as it is thought to be the dominant ligand for driving T cell responses(39). Expression of CD86 and CD80 is tightly regulated, and the CD28 homologue CTLA-4 serves to limit their availability by competitive inhibition and ligand downregulation(40-42). Unregulated availability of ligands, in mice lacking CTLA-4, results in unfettered T cell responses that culminate in lethal autoimmunity(43, 44). Thus, restricting CD86 and CD80 expression represents a major control point for adaptive immunity. Counteracting the downregulation of ligand by CTLA-4, various inflammatory stimuli serve to enhance ligand expression. Accordingly TLR ligands and cytokines such as IFNγ have been shown to upregulate CD86 expression. Here we identify IL-21 as a potent upregulator of CD86 expression on B cells.
IL-21 is one of several cytokines associated with systemic autoimmunity(45) and has been linked with lupus pathogenesis in mice(10, 46) and humans(47). The capacity of IL-21 to upregulate CD86, and promote T cell costimulation, could conceivably contribute to its pathogenic effects. Consistent with this, B cell expression of the costimulatory ligands CD86 and CD80 has been shown to be essential for autoreactive T cell activation and development of joint pathology in the proteoglycan-induced arthritis model in mice(48).
A major cellular source of IL-21 is the follicular helper T cell (TFH) subset that provides crucial help to B cells during the germinal center (GC) reaction(49-51). Interestingly, IL-4 is also produced by TFH cells and is known to positively regulate the expression of CD86 during productive interactions with B cells. Since B cell-derived CD86 provides critical signals for TFH cell maintenance(52), the production of IL-21 and IL-4 provides an elegant mechanism for TFH to solicit their own survival signals.
We show that IL-21-dependent CD86 upregulation is strictly reliant on STAT3 phosphorylation and PI3-kinase, revealing unappreciated roles for these pathways in IL-21-mediated B cell responses. Using B cells expressing a catalytically inactive isoform of p110δ, we further identify this PI3-kinase subunit as essential for the promotion of CD86 by IL-21. In contrast, IL-4-driven CD86 upregulation is independent of PI3-kinase, consistent with previous studies that instead identified a role for STAT6(53). The precise relationship between PI3-kinase and STAT3 in orchestrating IL-21-dependent CD86 upregulation is unclear, however preliminary studies suggest that STAT3 activation is independent of the PI-3kinase pathway (Supplemental Fig. 3). The PI3-kinase pathway is an important mediator of lymphocyte survival(54) and inhibitors of the p110δ isoform are in clinical use for the treatment of CLL(55). Our data suggest that PI3-kinase inhibition may have a greater impact on the nature of the immune response than previously appreciated; in addition to blocking antigen-dependent proliferation and survival signals in B cells, abrogating PI3-kinase signalling may also influence the costimulatory profile of B cells and their ability to drive CD4 T cell responses.
IL-4 and IL-21 exhibit redundancy in their ability to support germinal centre formation, with deficiency in each pathway alone having only a minor impact(9). Likewise either IL-4 or IL-21 appears sufficient to support IgG secretion from mouse(56) or human(57) B cells in vitro with synergistic activity being seen in the presence of both. Redundant roles for IL-4 and IL-21 in B cell CD86 upregulation might explain why mice lacking the PI3-Kinase p110δ subunit in B cells retained the capacity to form germinal centers(58), since IL-4-mediated CD86 upregulation would be predicted to be intact in this setting. Although IL-21 and IL-4 are frequently co-expressed in germinal centre resident TFH cells(59, 60), the observation that Th17 cells can also provide cognate help to B cells(61, 62) suggests at least one scenario in which IL-21 provision may be uncoupled from that of IL-4.
Similar to IL-21, the cytokine IL-10 is well known as a plasma cell differentiation factor. Despite their largely overlapping roles in promoting plasma cell differentiation these cytokines have notably distinct effects on CD86 expression, with IL-10 being generally accepted to downregulate CD86. Intriguingly, the receptors for these cytokines appear to be subject to differential kinetic regulation during B cell differentiation, with IL-21 playing an earlier role than IL-10(63). This might imply a set window during which CD86 expression is amenable to IL-21-directed up-regulation. If late IL-10 signals downregulate CD86 this could suggest a requirement for appropriate curtailment of T cell costimulatory signals for optimal T cell / B cell collaboration. In keeping with the idea of active downregulation (as well as upregulation) of costimulatory ligands, Bcl6 is known to downregulate CD80 in germinal centre B cells (64). The fine tuning of CD86 and CD80 expression in germinal centre B cells, and the consequences for TFH homeostasis(52, 65) are incompletely understood and represent key areas for further investigation.
In summary we have shown that IL-21 upregulates CD86 expression on B cells in a manner depends on the PI3-Kinase p110δ subunit. This finding is likely to have important implications for T cell / B cell collaboration within the germinal center, and for T cell responses in the wider context of anti-tumor responses and autoimmunity.
Supplementary Material
Acknowledgements
We are grateful to Manfred Kopf for his kind gift of IL-21 receptor deficient mice.
References
- 1.Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 2.Ugai S, Shimozato O, Kawamura K, Wang YQ, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Expression of the interleukin-21 gene in murine colon carcinoma cells generates systemic immunity in the inoculated hosts. Cancer Gene Ther. 2003;10:187–192. doi: 10.1038/sj.cgt.7700552. [DOI] [PubMed] [Google Scholar]
- 3.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fewkes NM, Mackall CL. Novel gamma-chain cytokines as candidate immune modulators in immune therapies for cancer. Cancer J. 2010;16:392–398. doi: 10.1097/PPO.0b013e3181eacbc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–3519. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Totero D, Meazza R, Capaia M, Fabbi M, Azzarone B, Balleari E, Gobbi M, Cutrona G, Ferrarini M, Ferrini S. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008;111:517–524. doi: 10.1182/blood-2007-04-087882. [DOI] [PubMed] [Google Scholar]
- 7.Gowda A, Roda J, Hussain SR, Ramanunni A, Joshi T, Schmidt S, Zhang X, Lehman A, Jarjoura D, Carson WE, Kindsvogel W, Cheney C, Caligiuri MA, Tridandapani S, Muthusamy N, Byrd JC. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro. Blood. 2008;111:4723–4730. doi: 10.1182/blood-2007-07-099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarosiek KA, Malumbres R, Nechushtan H, Gentles AJ, Avisar E, Lossos IS. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B-cell lymphomas. Blood. 2010;115:570–580. doi: 10.1182/blood-2009-08-239996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 12.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon J, Chen XL, Forand-Boulerice M, Leblanc C, Raman C, Ramanathan S, Ilangumaran S. Increased antigen responsiveness of naive CD8 T cells exposed to IL-7 and IL-21 is associated with decreased CD5 expression. Immunology and cell biology. 2010;88:451–460. doi: 10.1038/icb.2009.109. [DOI] [PubMed] [Google Scholar]
- 15.Ramanathan S, Dubois S, Chen XL, Leblanc C, Ohashi PS, Ilangumaran S. Exposure to IL-15 and IL-21 enables autoreactive CD8 T cells to respond to weak antigens and cause disease in a mouse model of autoimmune diabetes. Journal of immunology. 2011;186:5131–5141. doi: 10.4049/jimmunol.1001221. [DOI] [PubMed] [Google Scholar]
- 16.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 17.Clough LE, Wang CJ, Schmidt EM, Booth G, Hou TZ, Ryan GA, Walker LS. Release from Regulatory T Cell-Mediated Suppression during the Onset of Tissue-Specific Autoimmunity Is Associated with Elevated IL-21. J Immunol. 2008;180:5393–5401. doi: 10.4049/jimmunol.180.8.5393. [DOI] [PubMed] [Google Scholar]
- 18.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012 doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attridge K, Wang CJ, Wardzinski L, Kenefeck R, Chamberlain JL, Manzotti C, Kopf M, Walker LS. IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood. 2012;119:4656–4664. doi: 10.1182/blood-2011-10-388546. [DOI] [PubMed] [Google Scholar]
- 20.Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, Kopf M. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 21.Curiel RE, Garcia CS, Rottschafer S, Bosco MC, Espinoza-Delgado I. Enhanced B7-2 gene expression by interferon-gamma in human monocytic cells is controlled through transcriptional and posttranscriptional mechanisms. Blood. 1999;94:1782–1789. [PubMed] [Google Scholar]
- 22.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenne AT, Ro TB, Waage A, Sundan A, Borset M, Hjorth-Hansen H. Interleukin-21 is a growth and survival factor for human myeloma cells. Blood. 2002;99:3756–3762. doi: 10.1182/blood.v99.10.3756. [DOI] [PubMed] [Google Scholar]
- 24.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 25.Avery DT, Ma CS, Bryant VL, Santner-Nanan B, Nanan R, Wong M, Fulcher DA, Cook MC, Tangye SG. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood. 2008;112:1784–1793. doi: 10.1182/blood-2008-02-142745. [DOI] [PubMed] [Google Scholar]
- 26.Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J Leukoc Biol. 2007;82:645–656. doi: 10.1189/jlb.0806494. [DOI] [PubMed] [Google Scholar]
- 27.Marshall-Clarke S, Tasker L, Heaton MP, Parkhouse RM. A differential requirement for phosphoinositide 3-kinase reveals two pathways for inducible upregulation of major histocompatibility complex class II molecules and CD86 expression by murine B lymphocytes. Immunology. 2003;109:102–108. doi: 10.1046/j.1365-2567.2003.01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 29.Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, Vanhaesebroeck B. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic interference with p110delta function in B cells. Blood. 2006;107:642–650. doi: 10.1182/blood-2005-07-3041. [DOI] [PubMed] [Google Scholar]
- 30.Beer-Hammer S, Zebedin E, von Holleben M, Alferink J, Reis B, Dresing P, Degrandi D, Scheu S, Hirsch E, Sexl V, Pfeffer K, Nurnberg B, Piekorz RP. The catalytic PI3K isoforms p110gamma and p110delta contribute to B cell development and maintenance, transformation, and proliferation. J Leukoc Biol. 2010;87:1083–1095. doi: 10.1189/jlb.0809585. [DOI] [PubMed] [Google Scholar]
- 31.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 32.Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 33.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 34.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, Ozato K, Levy DE, Nutt SL, Calame K, Leonard WJ. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 36.Strengell M, Lehtonen A, Matikainen S, Julkunen I. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. Journal of leukocyte biology. 2006;79:1279–1285. doi: 10.1189/jlb.0905503. [DOI] [PubMed] [Google Scholar]
- 37.Maeda M, Yanagawa Y, Iwabuchi K, Minami K, Nakamaru Y, Takagi D, Fukuda S, Onoe K. IL-21 enhances dendritic cell ability to induce interferon-gamma production by natural killer T cells. Immunobiology. 2007;212:537–547. doi: 10.1016/j.imbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Van Belle TL, Nierkens S, Arens R, von Herrath MG. Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity. 2012;36:1060–1072. doi: 10.1016/j.immuni.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 40.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 43.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 44.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 45.Moudgil KD, Choubey D. Cytokines in autoimmunity: role in induction, regulation, and treatment. J Interferon Cytokine Res. 2011;31:695–703. doi: 10.1089/jir.2011.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 47.Dolff S, Abdulahad WH, Westra J, Doornbos-van der Meer B, Limburg PC, Kallenberg CG, Bijl M. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R157. doi: 10.1186/ar3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 49.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 50.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 51.Luthje K, Kallies A, Shimohakamada Y, GT TB, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 52.Salek-Ardakani S, Choi YS, Rafii-El-Idrissi Benhnia M, Flynn R, Arens R, Shoenberger S, Crotty S, Croft M. B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. J Immunol. 2011;186:5294–5303. doi: 10.4049/jimmunol.1100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deszo EL, Brake DK, Kelley KW, Freund GG. IL-4-dependent CD86 expression requires JAK/STAT6 activation and is negatively regulated by PKCdelta. Cell Signal. 2004;16:271–280. doi: 10.1016/s0898-6568(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 54.Polak R, Buitenhuis M. The PI3K/PKB signaling module as key regulator of hematopoiesis: implications for therapeutic strategies in leukemia. Blood. 2012;119:911–923. doi: 10.1182/blood-2011-07-366203. [DOI] [PubMed] [Google Scholar]
- 55.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O’Brien S, Yu A, Miller LL, Lannutti BJ, Burger JA. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin H, Malek TR. Redundant and unique regulation of activated mouse B lymphocytes by IL-4 and IL-21. Journal of leukocyte biology. 2006;80:1416–1423. doi: 10.1189/jlb.0206096. [DOI] [PubMed] [Google Scholar]
- 57.Avery DT, Bryant VL, Ma CS, de Waal Malefyt R, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol. 2008;181:1767–1779. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- 58.Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, Santinelli S, Saunders T, Hebeis B, Killeen N, Okkenhaug K, Turner M. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. Journal of immunology. 2010;185:4042–4052. doi: 10.4049/jimmunol.1001730. [DOI] [PubMed] [Google Scholar]
- 59.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) Journal of immunology. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. The Journal of experimental medicine. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patakas A, Benson RA, Withers DR, Conigliaro P, McInnes IB, Brewer JM, Garside P. Th17 effector cells support B cell responses outside of germinal centres. PLoS One. 2012;7:e49715. doi: 10.1371/journal.pone.0049715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon SO, Zhang X, Berner P, Choi YS. IL-21 and IL-10 have redundant roles but differential capacities at different stages of Plasma Cell generation from human Germinal Center B cells. Journal of leukocyte biology. 2009;86:1311–1318. doi: 10.1189/jlb.0409268. [DOI] [PubMed] [Google Scholar]
- 64.Niu H, Cattoretti G, Dalla-Favera R. BCL6 controls the expression of the B7-1/CD80 costimulatory receptor in germinal center B cells. The Journal of experimental medicine. 2003;198:211–221. doi: 10.1084/jem.20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Good-Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. J Immunol. 2012;188:4217–4225. doi: 10.4049/jimmunol.1102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.