Abstract
Background
Concerns regarding the safety of transfused blood have prompted reconsideration of the use of allogeneic (from an unrelated donor) red blood cell (RBC) transfusion, and a range of techniques to minimise transfusion requirements.
Objectives
To examine the evidence for the efficacy of cell salvage in reducing allogeneic blood transfusion and the evidence for any effect on clinical outcomes.
Search methods
We identified studies by searching CENTRAL (The Cochrane Library 2009, Issue 2), MEDLINE (1950 to June 2009), EMBASE (1980 to June 2009), the internet (to August 2009) and bibliographies of published articles.
Selection criteria
Randomised controlled trials with a concurrent control group in which adult patients, scheduled for non-urgent surgery, were randomised to cell salvage (autotransfusion) or to a control group who did not receive the intervention.
Data collection and analysis
Data were independently extracted and the risk of bias assessed. Relative risks (RR) and weighted mean differences (WMD) with 95% confidence intervals (CIs) were calculated. Data were pooled using a random-effects model. The primary outcomes were the number of patients exposed to allogeneic red cell transfusion and the amount of blood transfused. Other clinical outcomes are detailed in the review.
Main results
A total of 75 trials were included. Overall, the use of cell salvage reduced the rate of exposure to allogeneic RBC transfusion by a relative 38% (RR 0.62; 95% CI 0.55 to 0.70). The absolute reduction in risk (ARR) of receiving an allogeneic RBC transfusion was 21% (95% CI 15% to 26%). In orthopaedic procedures the RR of exposure to RBC transfusion was 0.46 (95% CI 0.37 to 0.57) compared to 0.77 (95% CI 0.69 to 0.86) for cardiac procedures. The use of cell salvage resulted in an average saving of 0.68 units of allogeneic RBC per patient (WMD −0.68; 95% CI −0.88 to −0.49). Cell salvage did not appear to impact adversely on clinical outcomes.
Authors’ conclusions
The results suggest cell salvage is efficacious in reducing the need for allogeneic red cell transfusion in adult elective cardiac and orthopaedic surgery. The use of cell salvage did not appear to impact adversely on clinical outcomes. However, the methodological quality of trials was poor. As the trials were unblinded and lacked adequate concealment of treatment allocation, transfusion practices may have been influenced by knowledge of the patients’ treatment status potentially biasing the results in favour of cell salvage.
Medical Subject Headings (MeSH): *Blood Transfusion, Autologous; *Erythrocyte Transfusion; Blood Specimen Collection [methods]; Randomized Controlled Trials as Topic; Surgical Procedures, Elective
MeSH check words: Adult, Humans
BACKGROUND
Concerns regarding the safety of transfused blood, have prompted reconsideration of the use of allogeneic (blood from an unrelated donor) red cell transfusion. The risks associated with volunteer donor blood transfusion (allogeneic blood) that has been screened by a competently managed modern laboratory are generally considered minimal, with the risks of human immunodeficiency virus (HIV) and hepatitis C (HCV) being extremely low (Glynn 2000;Whyte 1997). However, of great concern is that in many developing countries there is a high prevalence of such infections and trans-fusion services are inadequately equipped to conduct universal antibody screening (Lackritz 1998; McFarland 1997). Meanwhile, in developed countries, although the risks of acquiring a transfusion-transmitted disease (TTD) are considered low, the costs associated with providing a safe and reliable blood product are escalating (Hadjianastassiou 2002).
Recent concerns that variant Creutzfeldt-Jakob disease (vCJD) could be transmitted by blood transfusion (Brown 2000; Houston 2000) have prompted blood transfusion services worldwide to adopt more stringent donor selection procedures and the deferral of current donors who may have been exposed to vCJD (Oliver 2002). The ramifications of such actions have been, in some cases, the elimination of a sizable proportion of blood donors from an already declining volunteer donor pool. Blood is now, more than ever, an incredibly scarce resource.
Concerns regarding blood safety, continual blood shortages, and spiraling health costs associated with blood bank operations, have collectively generated considerable enthusiasm for the use of technologies intended to reduce the use of allogeneic blood (Forgie 1998). However, some of the alternatives to allogeneic blood have their own risks and are highly expensive (Coyle 1999; Fergusson 1999a).
With early reports suggesting that between 60% and 70% of all red blood cell units are transfused in the surgical setting (Cook 1991;Lenfant 1992; Surgenor 1990; Wallace 1993) and more recently, that half of all the blood transfused in the United Kingdom is to surgical patients (Regan 2002), it is of no surprise that considerable interest has been shown in a range of interventions designed to reduce perioperative allogeneic red cell transfusion. Generally, such interventions fall into three groups: (1) the administration of agents to diminish blood loss (e.g. aprotinin, tranexamic acid, epsilon aminocaproic acid, fibrin sealant), (2) agents that promote red blood cell production (e.g. erythropoietin), and (3) techniques for re-infusing a patient’s own blood (e.g. pre-operative autologous donation, acute normovolaemic haemodilution, cell salvage).
Cell salvage is one technique that has been used extensively in the surgical setting. Cell salvage (CS), alternatively known as ‘auto-transfusion’, is a term that covers a range of techniques that scavenge blood from operative fields or wound sites, and re-infuse the blood back into the patient. Cell salvage can be performed during the intra- and/or postoperative periods. To remove non-cellular matter prior to reinfusion, some of the devices use centrifugal washing of the salvaged blood (Huet 1999).
This review builds on the systematic review published by Huet et al (Huet 1999). It examines the evidence for the efficacy of cell salvage in reducing the need for perioperative allogeneic red blood cell transfusion in adult elective surgery and whether there is a greater reduction in allogeneic blood transfusion demonstrated in identifiable patient sub-groups. This review employs methods developed by the International Study of Perioperative Transfusion (ISPOT) study group (a ten country study of evidence, attitudes and practices relating to the use of alternatives to allogeneic blood transfusion) (Fergusson 1999b).
OBJECTIVES
To examine the effects of cell salvage in minimising perioperative allogeneic red blood cell transfusion and on other clinical outcomes.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials with a concurrent control group.
Types of participants
The study participants were adults (over 18 years). The surgery being conducted was elective or non-urgent.
Types of interventions
The intervention considered was cell salvage (CS). Studies with a combination of active comparisons were included if both the intervention and control groups were equally exposed to the active treatment (active plus cell salvage versus active comparisons).
Types of outcome measures
Primary outcomes
The number of patients who were transfused with allogeneic or autologous blood, or both
The amounts of allogeneic and/or autologous blood transfused
Secondary outcomes
Re-operation for bleeding
Post-operative complications (thrombosis, infection, renal failure, non-fatal myocardial infarction)
Mortality
Length of hospital stay (LOS)
Search methods for identification of studies
Electronic searches
This review drew on the literature searches that were constructed as part of the International Study of Perioperative Transfusion (ISPOT) (Huet 1999). The searches were last updated in June 2009.
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 2);
MEDLINE (1950 to June (Week 3) 2009);
EMBASE (1980 to Week 26, 2009);
Current Contents (ISI Web of Knowledge) (to June 2009).
The searches were based on the MEDLINE strategy shown in Appendix 1, and were modified as appropriate to the specifications of each database.
In MEDLINE and EMBASE two search filters were used to restrict electronic searches and improve the specificity of the searches. Firstly, the ISPOT filter (Laupacis 1997), which identifies blood transfusion trials, and secondly, a modified version of the Cochrane Collaboration filter (Dickersin 1996) which identifies randomised controlled trials. These search filters were coupled with the MeSH headings and relevant text-word terms for cell salvage.
Searching other resources
The internet site of the International Network of Agencies of Health Technology Assessment (INAHTA) was searched to June 2009. Reference lists of relevant reviews and identified articles were searched for additional studies. Contact was made with experts in the field to identify reports or projects in progress, relevant to the review. In addition, authors were contacted to identify any additional published or unpublished data.
Data collection and analysis
Selection of studies
The titles and/or abstracts of identified studies were screened by two independent authors. To be eligible for inclusion, studies had to include adult patients, scheduled for elective surgery, who were randomised to cell salvage or to a control group that did not receive cell salvage. Study reports had to provide data on the number of patients transfused with red cells, or the volume of blood transfused. Two authors independently selected trials that met the defined inclusion criteria with disagreements resolved by consensus.
Data extraction and management
Two authors independently extracted study characteristics and outcomes using an article extraction form. The extraction form was used to record information regarding; randomisation criteria, trial methodology, the presence of a transfusion protocol, the type of surgery involved, treatment outcomes, and general comments. Information regarding demographics (age, sex), the type of surgery, the presence or absence of a transfusion protocol, the timing of cell salvage, and the type of cell salvage (washed or unwashed) was also recorded. Data were extracted for allogeneic blood transfusion if it were expressed as whole blood or packed red cells. Transfusion data expressed in millilitres were converted to units by dividing by 300.
Data on the following outcomes were recorded:
the number of patients transfused allogeneic and/or autologous blood;
the volume of red cells transfused;
post-operative complications (thrombosis, infection, haemorrhage, non-fatal myocardial infarction, renal failure);
mortality;
length of hospital stay (LOS).
Data were also recorded for blood loss and the number of patients requiring re-operation for bleeding.
Authors were contacted to provide missing data where possible.
Assessment of risk of bias in included studies
Studies were assessed for methodological quality using the Cochrane Collaboration’s tool for assessing risk of bias presented in Higgins 2009. Disagreements were resolved by consensus.
The following domains were assessed for each study:
sequence generation,
allocation concealment,
blinding.
We completed a risk of bias table for each study, incorporating a description of the study’s performance against each of the above domains and our overall judgement of the risk of bias for each entry as follows; ‘Yes’ indicates a low risk of bias, ‘Unclear’ indicates unclear or unknown risk of bias, and ‘No’ indicates a high risk of bias.
Assessment of reporting biases
Funnel plots were inspected for evidence of publication bias.
Data synthesis
Data were extracted and then entered into Review Manager by one author. Articles identified as duplicate publications were combined to obtain one set of data. Dichotomous and continuous data werepooled across trials using a random effects model. If the standard deviation (SD) or the standard error of the mean (SEM) were not reported for continuous data the study was not included in the meta-analysis.
Subgroup analysis and investigation of heterogeneity
Statistical heterogeneity was examined by both the I-squared and chi-squared tests. The I-squared test describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, whereas values >50% indicates substantial heterogeneity (Higgins 2009). The Q statistic, which has an approximate Chi2 distribution with degrees of freedom equal to the number of studies minus one, was also used to assess heterogeneity of treatment effect (Der Simonian 1986). A P value less than or equal to 0.1 was used to define statistically significant heterogeneity.
Analysis of a priori subgroups was performed to determine whether effect sizes varied according to factors such as:
the type of surgery;
the use of transfusion protocols;
the type of salvaged blood retransfused (washed or unwashed);
the timing of cell salvage (intra- or post-operative, or both);
trial methodological quality.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Seventy-five studies fulfilled the inclusion criteria. The majority of trials included in the analysis were conducted in the United Kingdom (n = 23) and the United States (n = 14). The remaining trials were conducted across a range of countries: the Netherlands (n = 6), Sweden (n = 5), China (n = 3), France (n = 3), Germany (n = 3), India (n = 3), Australia (n = 2), Canada (n = 2), Denmark (n = 2), Greece (n = 2), Croatia (n = 1), Hong Kong (n = 1), Italy (n = 1), Lithuania (n = 1), Norway (n = 1), Russia (n = 1) and Turkey (n = 1). Studies were published between 1978 and 2008. Five studies (Fragnito 1995; Lorentz 1991; Menges 1992;Rosencher 1994; Zhang 2008) were published in languages other than English. These studies were translated before being included in the analysis. The majority of trials were small with less than 100 patients in each arm of the trial. Only four trials included more than 100 patients in each trial arm (Gannon 1991; Klein 2008; McGill 2002; Ritter 1994). Of the 75 trials included in the analysis, 36 trials involved patients undergoing orthopaedic procedures, 33 involved patients undergoing cardiac procedures, and six involved vascular surgery.
Methods of cell savage
Of the 75 included trials, 48 studied cell salvage during the postoperative period, 16 studied intra-operative cell salvage, and 11 studied both intra-operative and post-operative cell salvage. One trial (Sait 1999) failed to describe the timing of cell salvage. Twenty-six trials studied cell salvage systems that reinfused washed salvaged blood, and 44 trials studied cell salvage systems that re-infused unwashed filtered salvaged blood. One trial (Rollo 1995) studied both washed and unwashed cell salvage (four-arm trial) and provided two comparisons of cell salvage (Rollo 1995a; Rollo 1995b). One trial (Klein 2008) studied intra-operative washed and post-operative unwashed cell salvage. For three trials (Mercer 2004; Sait 1999; Zhang 2008) the method used to process salvaged autologous blood prior to re-transfusion was unclear.
Types of cell salvage devices
Various types of cell salvage (autotransfusion) systems were studied. The following is a list of those systems used.
ABTrans autologous re-transfusion system
Atrium 2050
Atrium 2550 in-line autotransfusion drainage system
Autovac postoperative orthopaedic autotransfusion canister
Bard cardiotomy reservoir
Bellovac ABT autotransfusion system
Beijing PerMed Biomedical Engineering Company
Bentley Catr hard shell cardiotomy reservoir
BIODREN autotransfusion system
BRAT-2 Cell Saver
CATR 3500 cardiotomy reservoir
Cell Trans system (Summit Medical)
ConstaVac CBC system
ConstaVac CBCII system
COBE Bayler rapid autotransfusion system
Dideco Compact
Dideco Electra system
Dideco 742 cardiotomy reservoir
Dideco 797 reinfusion system (Sorin Biomedical)
DONOR system (Van Straten Medical)
Electromedic Autotrans AT-100
Electromedics BT-795
Flow-Gard 6200 (Baxter)
Frensenius continuous autotransfusion system (C.A.T.S)
Gish Orthofuser Biomedical autotransfusion system
Haemonetics Cell Saver
Haemonetics Cell Saver 3
Haemonetics Cell Saver 3 Plus
Haemonetics Cell Saver 4
Haemonetics Cell Saver 5
Haemonetics Haemolite cell washer
Haemonetics Haemolite-2
Medtronic Autolog system
Ortho-Evac system
Pleur-evac autotransfusion system
Shiley hardshell cardiotomy reservoir
Solcotrans Cell Saver
Solcotrans Orthopedic Plus system
Solcotrans Orthopedic system
Sorenson ATS (autotransfusion system)
Terumo TE-171 system (Terumo)
Transfusion ‘triggers’ or thresholds
Of the 75 included trials, 60 reported the use of a transfusion protocol. Of these, 59 trials included a transfusion ‘trigger’ value, that being the haemoglobin (Hb) or haematocrit (Hct) value at which point a transfusion of red blood cells, was considered appropriate. However, there was significant variation between trials in the transfusion ‘trigger’ value used. The post-operative transfusion trigger for haemoglobin (Hb) ranged from 7.0 g/dL to 10.0 g/dL, whereas the intra-operative Hb transfusion trigger value ranged from 5.6 to 10.0 g/dL.
Of the 55 trials that reported a post-operative transfusion threshold, 15 trials reported a Hb transfusion threshold of 10.0 g/dL, 15 trials reported a transfusion threshold of between 9.0 g/dL and 9.5 g/dL, 21 trials reported a transfusion threshold of between 8.0 g/dL and 8.9g/dL, two trials reported a transfusion threshold of 7.0 g/dL, one trial reported a transfusion threshold of 5.0 mmol/L and one trial transfused patients when the haematocrit value was less than 30%. Of the 21 trials that reported the use of an intra-operative transfusion threshold, the haemoglobin ‘trigger’ value ranged from as low as 5.6 g/dL to as high as 10.0 g/dL.
Risk of bias in included studies
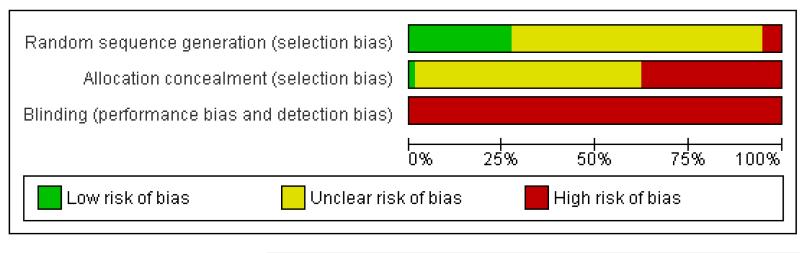
The performance of the included trials against each domain is summarised in the ‘Risk of bias’ tables (Figure 1; Figure 2).
Figure 1.
Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Figure 2.
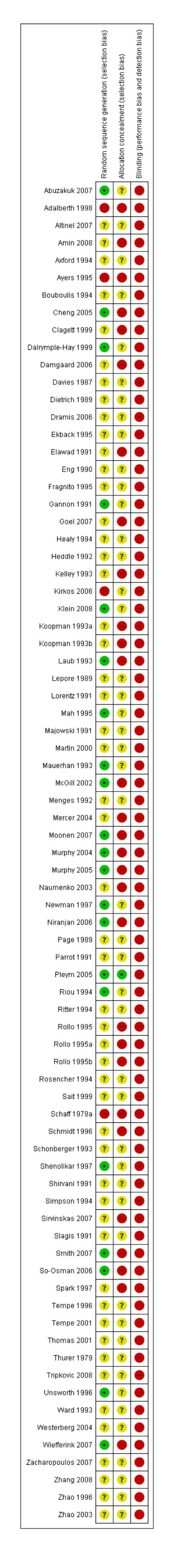
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Adequate sequence generation
The risk of bias for this item was judged to be low for 21 trials. For four trials the method of sequence generation was judged to be inadequate. The remaining 50 trials presented no information regarding the method of sequence generation and were rated unclear.
Allocation concealment
The risk of bias for this item was judged to be low in one trial (Pleym 2005) which used central randomisation (off-site, computer-generated randomisation). For 27 trials the method used to conceal treatment allocation was judged to be inadequate (for example sealed envelopes). The remaining 47 trials presented no information regarding the method of allocation concealment and were rated unclear.
Blinding
None of the 75 included trials were judged to be double-blind. Given the nature of the intervention double-blinding is accepted as being problematic.
Effects of interventions
Aggregated analysis
Sixty-seven trials of cell salvage (autotransfusion) reported data on the number of subjects exposed to allogeneic blood transfusion. These trials included a total of 6025 patients of whom 3048 were randomised to cell salvage. Overall, the use of cell salvage reduced the rate of exposure to allogeneic RBC transfusion by a relative 38% (RR 0.61; 95% CI 0.55 to 0.70). Heterogeneity between these trials was statistically significant (P<0.00001, I2=81%). The absolute reduction in risk (ARR) of exposure to allogeneic blood transfusion was 21% (risk difference (RD) −0.21; 95% CI −0.26 to −0.15) and, on average, 4.8 patients would need to undergo cell salvage so that one would avoid an allogeneic RBC transfusion (number needed to treat (NNT)).
Transfusion protocol
Of the 67 trials that reported data on the number of subjects exposed to allogeneic blood transfusion, 52 reported the use of trans-fusion protocols. These trials included a total of 4755 patients, of whom 2377 were randomised to cell salvage. The relative risk of exposure to allogeneic RBC transfusion in those patients treated with cell salvage was 0.62 (95% CI 0.54 to 0.71). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 81%). For the 15 trials that did not report the use of transfusion protocols, the relative risk of exposure to allogeneic RBC trans-fusion was 0.58 (95% CI 0.41 to 0.82). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 77%).
Type of surgery
Of the trials that reported data on the number of patients exposed to allogeneic RBC transfusion, 32 involved orthopaedic surgery, 31 involved cardiac procedures and four involved vascular surgery. A larger relative risk reduction (RRR) was observed in orthopaedic trials (RRR 54%; 95% CI 43% to 63%) than in cardiac trials (RRR 23%; 95% CI 14% to 31%). For those four trials that involved vascular surgery, the relative risk of exposure to allogeneic blood transfusion was not statistically significant (RR 0.63; 95% CI 0.34 to 1.15). For each of these three subgroup analyses, heterogeneity was statistically significant (I2 = 72%, I2 = 62%, and I2 = 81%; respectively).
Type of cell salvage - washed versus unwashed
Twenty-seven trials studied washed cell salvage whilst 40 trials investigated unwashed cell salvage. One trial (Rollo 1995) studied both washed and unwashed cell salvage (four-arm trial) and provided two comparisons of cell salvage (Rollo 1995a; Rollo 1995b). One trial (Sait 1999) did not describe the method of cell salvage investigated.
Overall, when cell salvage was conducted with devices that washed salvaged blood, the relative risk of exposure to red cell transfusion was only slightly lower than that with unwashed cell salvage. For those trials that used washed cell salvage the relative risk of exposure to allogeneic RBC transfusion was 0.60 (95% CI 0.51 to 0.70) compared to 0.66 (95% CI 0.57 to 0.77) for those trials that used unwashed cell salvage. For both these subgroup analyses, heterogeneity was statistically significant (I2 = 68% and I2 = 81%; respectively).
Timing of cell salvage
Eleven trials reported the use of intra-operative cell salvage. These trials included a total of 805 patients, of whom 402 were randomised to intra-operative cell salvage. The relative risk of exposure to allogeneic blood transfusion was 0.59 (95% CI 0.46 to 0.76). Heterogeneity between these trials was statistically significant (P = 0.002, I2 = 64%). Forty-six trials reported the use of post-operative cell salvage. These trials included a total of 4361 patients, of whom 2209 were randomised to post-operative cell salvage. The risk of exposure to allogeneic blood transfusion was reduced on average by a relative 37% in those patients treated with post-operative cell salvage compared to control (RR =0.63; 95% CI 0.54 to 0.74). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 83%). Nine trials reported the use of both intra- and post-operative cell salvage. These trials included a total of 737 patients of whom 357 were randomised to intra- and post-operative cell salvage. The use of intra- and post-operative cell salvage decreased exposure to allogeneic red cell transfusion by a relative 30% (RR 0.70; 95% CI 0.54 to 0.92). Heterogeneity between these trials was statistically significant (P < 0.005, I2 = 64%).
Volume of blood transfused
Thirty-two trials provided data for the volume of allogeneic RBC transfused. These trials included a total of 2321 patients, of whom 1172 were randomised to cell salvage. On average, the use of cell salvage reduced the volume of red cells transfused by 0.68 units per patient (WMD −0.68 units; 95% CI −0.88 to −0.49 units). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 75%). For those 27 trials that reported the use of a transfusion protocol the use of cell salvage reduced the amount of allogeneic blood transfused by an average of 0.69 units per patient (WMD −0.69 units; 95% CI −0.90 to −0.49 units). For those five trials that did not report the use of a transfusion protocol the use of cell salvage did not statistically significantly reduce the amount of allogeneic blood transfused (WMD −0.64 units; 95% CI −1.30 to 0.01 units). For both these subgroup analyses, heterogeneity between trials was statistically significant (P < 0.00001 and P < 0.0001, respectively).
When data were stratified by the type of surgery performed, greater reductions in the volume of allogeneic RBC transfused per patient were observed in trials that involved orthopaedic surgery (WMD −0.81 units; 95% CI −1.22 to −0.39 units) compared to cardiac surgery (WMD −0.67 units; 95% CI −0.89 to −0.44 units). For both these subgroup analyses, heterogeneity between trials was statistically significant (P < 0.00001 and P < 0.0001, respectively). Similar statistically significant reductions in the volume of RBC transfused was not observed in trials involving vascular surgery (WMD 0.02 units; 95% CI −0.34 to 0.38 units).
Type of cell salvage - washed versus unwashed - type of surgery
When the type of cell salvage was stratified by the type of surgery, the results indicated that the use of washed cell salvage in cardiac surgery was associated with an average 34% relative risk reduction in exposure to allogeneic red cell transfusion (RR 0.66; 95% CI = 0.55 to 0.80). Heterogeneity between these trials was statistically significant (P = 0.002, I2 = 61%). Reduced exposure to red cell transfusion was also observed in those trials that used unwashed cell salvage in cardiac surgery, (RR 0.85; 95% CI 0.76 to 0.95), heterogeneity between these trials was statistically significant (P = 0.0001, I2 = 65%). For orthopaedic trials both types of cell salvage were associated with significant reductions in transfusion exposure rates. For washed cell salvage the relative risk of exposure to red cell transfusion was reduced on average by 52% (RR 0.48; 95% CI 0.36 to 0.64) and for unwashed cell salvage there was a relative 53% reduction in risk of exposure (RR 0.47; 95% CI 0.36 to 0.63). For these subgroup analyses, heterogeneity was statistically significant (P = 0.03 and P = 0.0001, respectively). All four trials conducted in the setting of vascular surgery used washed cell salvage. For these trials, the relative risk of exposure to allogeneic red cell transfusion was not statistically significant (RR 0.63; 95% CI 0.34 to 1.15). Heterogeneity between these trials was statistically significant (P = 0.001, I2 = 81%).
Blood loss
Thirty-two trials of cell salvage reported data for total blood loss. These trials included a total of 2311 patients of whom 1158 were randomised to cell salvage. The use of cell salvage did not appear to adversely impact on blood loss volumes (WMD −39.02 mls; 95% CI −85.10 to 7.05 mls). Heterogeneity between these trials was statistically significant (P = 0.01, I2 = 41%).
Active versus control
Forty-three trials compared cell salvage alone to a control group who did not receive cell salvage (autotransfusion) or any other form of active treatment. These trials included a total of 3666 patients, of whom 1854 were randomised to cell salvage. The relative risk of receiving an allogeneic RBC transfusion was 0.61 (95% CI 0.52 to 0.71). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 83%).
Transfusion protocol
Thirty-four trials of cell salvage reported the use of transfusion protocols. These trials included a total of 3030 patients of whom 1525 were randomised to cell salvage. For those trials that used a transfusion protocol, the risk of receiving an allogeneic red cell transfusion was reduced on average by 39% (RR 0.61; 95% CI 0.51 to 0.73). For the nine trials that did not report the use of a transfusion protocol, the relative risk of receiving an allogeneic red cell transfusion was 0.56 (95% CI 0.35 to 0.89). For both these subgroup analyses, heterogeneity was statistically significant (P < 0.00001).
Type of surgery
Of the 43 trials that provided data for the number of patients exposed to allogeneic blood transfusion, 18 involved cardiac surgery, 21 involved orthopaedic surgery, and four involved vascular surgery. When cell salvage was used in orthopaedic surgery, the risk of exposure to red cell transfusion was reduced by a relative 55% (RR 0.45; 95%CI 0.34 to 0.60) compared to 22% (RR 0.78; 95% CI 0.68 to 0.91) in the case of cardiac surgery. For the four trials involving vascular surgery, the relative risk of receiving an allogeneic RBC transfusion was not statistically significant (RR 0.63; 95% CI 0.34 to 1.15). Heterogeneity was statistically significant for each of the subgroups analysed (P < 0.001).
Type of cell salvage - washed versus unwashed
When washed cell salvage was used, the relative risk of receiving an allogeneic RBC transfusion was 0.57 (95% CI 0.45 to 0.72) compared to 0.67 (95% CI 0.56 to 0.81) for unwashed cell salvage. Heterogeneity was statistically significant in the two subgroups analysed (P < 0.00001).
Timing of cell salvage
Eight trials of intra-operative cell salvage provided data for the number of patients exposed to allogeneic RBC transfusion. These trials included a total of 564 patients, of whom 281 were randomised to cell salvage. Intra-operative cell salvage was associated with a relative reduction in the risk of receiving an allogeneic RBC transfusion of 36% (RR 0.64; 95% CI 0.50 to 0.83). Heterogeneity between these trials was statistically significant (P = 0.07, I2 = 46%). Twenty-nine trials of post-operative cell salvage provided data for the number of patients exposed to allogeneic RBC transfusion. These trials included a total of 2852 patients, of whom 1452 were randomised to cell salvage. The use of post-operative cell salvage reduced the risk of exposure to allogeneic red cell transfusion by a relative 41% (RR 0.59; 95% CI 0.48 to 0.73). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 87%). Six trials studied both intra- and post-operative cell salvage. These trials included a total of 250 patients of whom 121 were randomised to cell salvage. with The combined use of intra- and post-operative cell salvage reduced exposure to allogeneic RBC transfusion by a relative 42% (RR 0.58; 95% CI 0.35 to 0.95). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 84%).
Volume of blood transfused
Twenty-three trials reported data for the volume of allogeneic RBC transfused. These trials included a total of 1608 patients, of whom 818 were randomised to cell salvage. Overall, the use of cell salvage was associated with a modest reduction in the volume of red cells transfused. In those patients treated with cell salvage, there was an average saving of 0.81 units of RBC per patient (WMD −0.81 units; 95% CI −1.08 to −0.54 units). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 80%).
Volume of blood transfused - transfusion protocol
Stratifying the volume of blood transfused by the presence of a transfusion protocol, showed that greater reductions in the volume of red cells transfused were observed in those trials that reported the use of transfusion protocols (WMD −86; 95% CI −1.17 to −0.55 units) compared to those trials that did not use a transfusion protocol to guide transfusion practice (WMD −0.68 units; 95% CI −1.63 to 0.27 units). For both these subgroup analyses, heterogeneity was statistically significant (P < 0.00001).
Volume of blood transfused - type of surgery
There were 13 cardiac trials, including a total of 995 patients, that reported data for the volume of RBC transfused. The use of cell salvage in cardiac surgery provided, on average, a saving of around one unit of blood per patient (WMD −0.93 units; 95% CI −1.27 to −0.59 units). Heterogeneity between these trials was statistically significant (P < 0.0001, I2 = 71%). There were seven orthopaedic trials, including a total of 427 patients, that reported data for the volume of blood transfused. For those patients randomised to cell salvage, there was an average saving of 0.82 units of RBC per patient (WMD −0.82 units; 95% CI −1.36 to −0.27 units). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 86%). For the three trials involving vascular surgery, the weighted mean difference in the volume of RBC transfused was not statistically significant (WMD 0.02 units; 95% CI −0.34 to 0.38 units).
Blood loss
Twenty-four trials of cell salvage reported data for total blood loss. These trials included a total of 1570 patients, of whom 789 were randomised to cell salvage. The use of cell salvage did not appear to adversely impact on total blood loss (WMD −38.98 mls; 95% CI −99.91 to 21.95 mls). Heterogeneity between these trials was statistically significant (P = 0.01, I2 = 44%).
Active versus active
There were 25 trials that compared cell salvage, combined with another form of active treatment (blood conservation intervention), to a control group who received the same active treatment but did not receive cell salvage (autotransfusion). For these 25 trials the relative risk of exposure to allogeneic RBC transfusion was 0.63 (95% CI 0.53 to 0.76). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 76%).
Transfusion protocol
There were 19 trials of cell salvage that reported the use of trans-fusion protocols. For these trials, the relative risk of exposure to allogeneic blood transfusion was 0.64 (95% CI 0.52 to 0.77). Heterogeneity between these trials was statistically significant (P < 0.00001, I2 = 79%). For those six trials that did not report the use of a transfusion protocol, the relative risk of exposure to allogeneic red cells was 0.59 (95% CI 0.35 to 1.00).
Type of surgery
Fourteen trials involving cardiac surgery provided data for the number of patients receiving allogeneic red cell transfusion. These trials included a total of 1304 patients, of whom 641 were randomised to cell salvage. For these trials, the risk of receiving an allogeneic red cell transfusion was reduced on average by a relative 27% (RR 0.73; 95% CI 0.61 to 0.87). Heterogeneity between these trials was statistically significant (P < 0.0001, I2 = 72%). For the 11 trials involving orthopaedic surgery, the risk of receiving an allogeneic red cell transfusion was reduced by a relative 54% (RR 0.46; 95% CI 0.33 to 0.65). Heterogeneity between these trials was statistically significant (P = 0.03, I2 = 50%).
Type of cell salvage - washed versus unwashed
When washed cell salvage was used, the relative risk of receiving an allogeneic red cell transfusion was 0.61 (95% CI 0.48 to 0.78) compared to 0.68 (95% CI 0.52 to 0.89) for unwashed cell salvage. For both of these subgroup analyses, heterogeneity was statistically significant (P = 0.01 and P < 0.00001, respectively).
Timing of cell salvage
Four trials of intra-operative cell salvage provided data on the number of patients receiving allogeneic red cell transfusion. These trials included a total of 281 patients, of whom 141 were randomised to cell salvage. Intra-operative cell salvage reduced the rate of exposure to allogeneic red cell transfusion by a relative 46% (RR 0.54; 95% CI 0.35 to 0.84). The 18 trials of post-operative cell salvage included a total of 1629 patients, of whom 817 were randomised to cell salvage. For these trials, the risk of receiving an allogeneic red cell transfusion was reduced on average by a relative 35% (RR 70; 95% CI 0.50 to 0.85). Heterogeneity for both these subgroup analyses was statistically significant (P = 0.005 and P < 0.00001, respectively). For the four trials involving intra- and post-operative cell salvage, the relative risk of receiving an allogeneic RBC transfusion was 0.76 (95% CI 0.59 to 0.98; I2 = 27%).
Volume of blood transfused
Eight trials of cell salvage reported data for the volume of allogeneic red cells transfused. On average there was a saving of 0.66 units of RBC per patient (WMD −0.66; 95% CI −1.02 to −0.30) when cell salvage was combined with another form of active treatment. Of these eight trials, six involved cardiac surgery and seven reported the use of transfusion protocols. For these two subgroup analyses, the volume of allogeneic red cells transfused was reduced on average by 0.39 units per patient (WMD −0.39; 95% CI −0.67 to −0.12) and by 0.67 units per patient (WMD −0.67; 95% CI −1.09 to −0.25), respectively. In the case of the two orthopaedic trials, the use of cell salvage reduced the amount of allogeneic red blood cells transfused on average by 1.1 units per patient (WMD −1.10; 95% CI −1.91 to −0.29; I2 = 79%).
Blood loss
Eight trials of cell salvage reported data for total blood loss. These trials included a total of 741 patients of whom 369 were randomised to cell salvage. The use of cell salvage did not appear to adversely impact on total blood loss (WMD −48.32 mls; 95% CI −116.38 to 19.74 mls).
Adverse events and other outcomes
Mortality
In aggregate, 22 trials of cell salvage reported data for mortality. These trials included a total of 1788 patients, of whom 902 were randomised to cell salvage. For eight trials there were no reported deaths for either the intervention or control groups; therefore it was not possible to estimate the relative risks for these trials. Overall, the use of cell salvage did not statistically significantly impact on mortality (RR 0.96; 95% CI 0.49 to 1.88; I2 = 0%). Similar non-significant results were observed for those 16 trials that compared cell salvage alone to untreated controls (RR 1.41; 95% CI 0.66 to 3.05; I2 = 0%). It should be noted that two trials provided over 22% of the information in the analysis and six of the 16 trials reported no deaths in either the intervention or control groups. There were six trials of active versus active comparisons (cell salvage combined with another form of active treatment compared to an actively treated control group) that provided mortality data. These trials included a total of 656 patients, of whom 326 were randomised to cell salvage. For these trials there were nine recorded deaths; eight of which occurred in the control arms. The use of cell salvage in this subgroup of trials did not appear to impact significantly on the rate of mortality (RR 0.27; 95% CI 0.07 to 1.08; I2 = 0%).
Re-operation for bleeding
In aggregate, 19 trials of cell salvage provided data for re-operation due to bleeding. These trials included a total of 1683 patients, of whom 841 were randomised to cell salvage. The use of cell salvage did not statistically significantly impact on the rates of re-operation for bleeding (RR 0.90; 95% CI 0.53 to 1.53; I2 = 0%). Ten trials, comparing cell salvage to untreated controls (active versus control comparisons), reported data for re-operation due to bleeding. These trials included a total of 688 patients, of whom 349 were randomised to cell salvage. The relative risk of requiring a re-operation due to bleeding was not statistically significant (RR 1.00; 95% CI 0.45 to 2.24; I2 = 0%). There were nine trials of active versus active comparisons (cell salvage combined with another form of active treatment compared to an actively treated control group) that provided data for re-operation due to bleeding. Cell salvage did not appear to statistically significantly impact on the rates of re-operation for bleeding in this subset of trials (RR 0.83; 95% CI 0.41 to 1.68; I2 = 0%).
Any infection
In aggregate, 23 trials reported data for infection of any type. These trials included a total of 2892 patients, of whom 1474 were randomised to cell salvage. The use of cell salvage appeared to be associated with a slight decrease in the rate of infection compared to control (RR 0.68; 95% CI 0.46 to 0.99; I2 = 0%). Sixteen trials, comparing cell salvage to untreated controls, reported data for infection of any type. These trials included a total of 1860 patients, of whom 942 were randomised to cell salvage. A statistically significant reduction in the rate of infection in those patients treated with cell salvage was observed (RR 0.63; 95% CI 0.41 to 0.96; I2 = 1%). For the seven trials that investigated active versus active comparisons the relative risk of developing an infection was not statistically significant (RR 0.88; 95% CI 0.37 to 2.07; I2=0%).
Wound complications
In aggregate, 16 trials of cell salvage reported data for a wound complication (for example haematoma, infection). These trials included a total of 1962 patients, of whom 1011 were randomised to cell salvage. The use of cell salvage did not statistically significantly impact on the rates of wound complication (RR 0.94; 95% CI 0.57 to 1.55; I2 = 0%). Similar results were observed for the twelve trials that compared cell salvage alone to an untreated control group (RR 0.88; 95% CI 0.49 to 1.57; I2 = 0%). For the four trials of active versus active comparisons, the relative risk of developing a wound complication was 1.13 (95% CI 0.43 to 2.99; I2 = 0%).
Any thrombosis
In aggregate, 11 trials reported data for thrombosis of any type. These trials included a total of 925 patients, of whom 476 were randomised to cell salvage. For five of the 11 trials, there were no reported events of thrombosis in either the intervention or control groups. The relative risk of developing any thrombosis in those patients treated with cell salvage compared to control was not statistically significant (RR 1.13; 95% CI 0.48 to 2.66; I2 = 0%). Of the 11 trials that reported data for this outcome 10 involved active versus untreated control comparisons.
Stroke
In aggregate, seven trials reported data for stroke. These trials included a total of 696 patients, of whom 347 were randomised to cell salvage. The relative risk of developing a stroke in those patients treated with cell salvage compared to control was not statistically significant (RR 0.65; 95% CI 0.21 to 1.98; I2=0%).
Non-fatal myocardial infarction
In aggregate, 11 trials reported data for non-fatal myocardial infarction. These trials included a total of 951 patients, of whom 471 were randomised to cell salvage. The relative risk of non-fatal myocardial infarction in those patients treated with cell salvage compared to control was not statistically significant (RR 0.80; 95% CI 0.43 to 1.46; I2 = 0%). Similar results were observed for the six trials that compared cell salvage alone to an untreated control group (RR 0.65; 95% CI 0.32 to 1.31; I2 = 0%). For those five trials that investigated active versus active comparisons, the relative risk of non-fatal myocardial infarction was not statistically significant (RR 1.46; 95% CI 0.43 to 4.88; I2 = 0%).
Deep vein thrombosis
Seven trials reported data for deep venous thrombosis. These trials included a total of 645 patients, of whom 320 were randomised to cell salvage. The relative risk of developing deep venous thrombosis in those subjects treated with cell salvage compared to control was not statistically significant (RR 0.83; 95% CI 0.31 to 2.23; I2 = 0%).
Hospital length of stay
Ten trials reported data for hospital length of stay. These trials included a total of 772 patients, of whom 399 were randomised to cell salvage. In those patients treated with cell salvage hospital length of stay was not statistically significantly reduced compared to control (WMD −1.38 days; 95% CI −2.79 to 0.03 days; I2 = 83%).
DISCUSSION
Principal findings
We identified 75 randomised trials of cell salvage, carried out over a 29-year period (1979 to 2008). Overall, the results of the meta-analysis indicated that the use of cell salvage reduced peri-operative allogeneic RBC transfusion exposure by a relative 38% (RR 0.62; 95% CI 0.55 to 0.70). The average absolute reduction in risk (ARR) of exposure to allogeneic red cell transfusion was 21% (RD −0.21; 95% CI −0.26 to −0.15), equating to a number needed to treat (NNT) of 4.8. The efficacy of cell salvage in reducing the need for allogeneic red cell transfusion appeared to be greatest in the setting of orthopaedic surgery. In this setting, cell salvage reduced the risk of exposure to allogeneic blood transfusion by a relative 54% compared to 23% in cardiac surgery. In orthopaedic surgery very little difference in treatment effects was observed between washed cell salvage (RR 0.48; 95% CI 0.36 to 0.64) and unwashed cell salvage (RR 0.47; 95% CI 0.36 to 0.63). This was not the case in cardiac surgery, where there were clear differences between washed cell salvage, which showed significant reductions in allogeneic red cell transfusion rates (RR 0.66; 95% CI 0.55 to 0.80), and unwashed cell salvage, which appeared to be only marginally effective (RR 0.85; 95% CI 0.76 to 0.95). However, significant variation in treatment effects was observed for all of the main study outcomes.
In the case of exposure to allogeneic blood transfusion, a relative risk reduction of 37% was observed when cell salvage was combined with another active intervention (for example pre-operative autologous donation (PAD), acute normovolaemic haemodilution (ANH), aprotinin) and compared with that intervention on its own. When cell salvage was compared to a non-active intervention (for example standard untreated control), a relative risk reduction of 39% was observed.
The use of cell salvage was also associated with only slight reductions in the volume of red cells transfused. Overall, in those patients treated with cell salvage, there was an average saving of 0.68 units of RBC per patient (WMD −0.68 units; 95% CI −0.88 to −0.49 units). When cell salvage was combined with another form of active intervention and compared with that intervention on its own, the reduction in the volume of RBC transfused was around 0.66 units of RBC per patient (WMD −0.66 units; 95% CI −1.02 to −0.30 units). Such a result may well have been expected as both groups were actively treated with some form of blood sparing intervention.
Sources of heterogeneity
The observed variation in treatment effects was in terms of both the size and direction of effect with relative risk point estimates for red cell transfusion exposure for the individual trials, ranging from 0.03 to 5.64. Of the 67 trials that provided data for the number of patients exposed to allogeneic red cell transfusion, more than half of these trials (n = 36) found that cell salvage did not statistically significantly reduce the risk of receiving a red cell transfusion. None of the subgroup analyses performed established a clear reason for the observed variability in treatment effect. Statistically significant heterogeneity was observed for all of the subgroup analyses performed.
The impact that trial methodological quality had on treatment effects was difficult to determine, as the majority of trials were of poor quality. The most concerning feature of the trials reviewed here is that only one trial reported a method of concealing treatment allocation that was judged to be adequate. A lack of allocation concealment has been shown to significantly influence the estimate of treatment effect (Schulz 1995). When trials that reported data for the number of patients transfused allogeneic red blood cells were stratified by methodological quality (adequate allocation concealment: yes, unclear, or no), the relative risks of exposure to allogeneic blood transfusion varied only slightly. For those trials (n = 24) that were assessed as providing inadequate concealment of treatment allocation the relative risk of exposure to allogeneic red cell transfusion was 0.62 (95% CI 0.51 to 0.75), whereas for those trials (n=42) that did not report the method used to conceal treatment allocation or it was unclear what method was used to conceal treatment allocation, the relative risk was 0.62 (95% CI 0.53 to 0.72).
Sources of bias
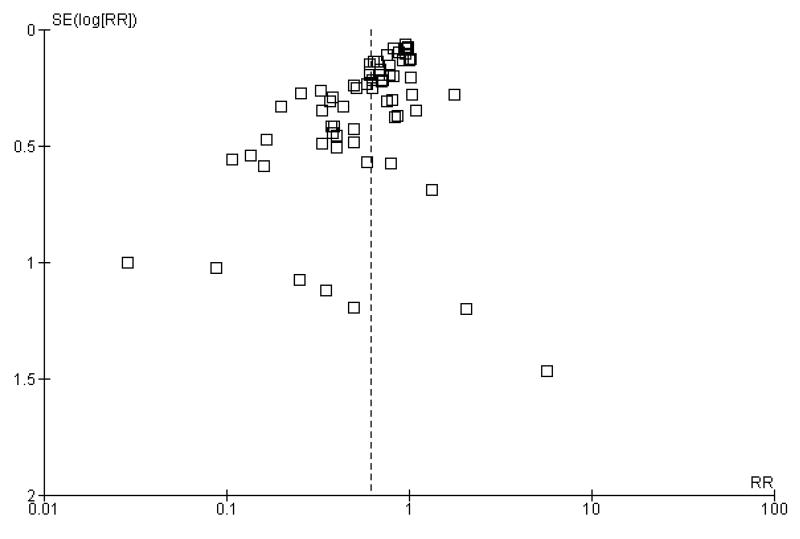
The majority of trials reviewed here were small with less than 60 participants in each trial arm. Reliance on small trials raises concerns about the effects of publication bias. Funnel plot assessment revealed some evidence of this in the form of a ‘missing’ population of small negative studies (Figure 3). Although there is a clustering of trials around the null (RR = 1), there were very few trials that showed an overall negative result for cell salvage for allogeneic red cell transfusion exposure.
Figure 3.
Funnel plot of comparison: 1 Cell Salvage - Blood Transfused (All Studies), outcome: 1.1 No. exposed to allogeneic blood (All Studies).
Although this review did not exclude studies on the basis of language, only six non-English studies fulfilled the inclusion criteria (Fragnito 1995; Lorentz 1991; Menges 1992; Naumenko 2003;Rosencher 1994; Zhang 2008). For those studies published in English language, the relative risk of exposure to allogeneic red cell transfusion was 0.61 (95% CI 0.54 to 0.70) compared to 0.63 (95% CI 0.50 to 0.79) for those studies published in languages other than English. Due to the lack of non-English language studies it is difficult to interpret these results with any degree of confidence. However, it is of interest to note, that although the heterogeneity in treatment effect for the English language studies was statistically significant (P < 0.00001, I2 = 83%) this was not the case with those studies published in non-English languages (P = 0.89, I2 = 0%).
The main study outcome used in these trials was a practice variable (the decision to transfuse a patient with allogeneic red cells) and, as such, may have been a major source of bias. The decision to transfuse a patient requires a degree of subjectivity on the part of the clinicians, and as all the trials reviewed here were unblinded and lacked adequate concealment of treatment allocation. This is a particularly important source of bias that may have potentially influenced the results in favour of cell salvage.
Adverse events and other outcomes
Mortality, re-operation for bleeding, infection, wound complication, non-fatal myocardial infarction, thrombosis, stroke, and hospital length of stay did not appear to be adversely affected by the use of cell salvage. Even though one of the known risks associated with cell salvage is infection (due to contamination of the autologous product during the salvaging and reinfusion process), fewer cell salvage patients experienced infection (RR 0.68; 95% CI 0.46 to 0.99). However, cell salvage did not appear to be associated with any significant reductions in wound complications (RR 0.94; 95% CI 0.57 to 1.55).
It should be noted that the event rates were small ranging for 1.2% in the case of stroke to 5.1% in the case of any infection. Therefore it is difficult to draw firm conclusions regarding the impact of cell salvage on important clinical outcomes. There were only two outcomes (any infection and non-fatal myocardial infarction) where the event rates in the control groups were greater than 3.5% (5.1% and 4.8%, respectively). From the very limited data, it appears that the potential benefit of cell salvage in reducing exposure to allogeneic blood transfusion, is not offset by serious adverse effects.
Clinical significance of the results
In an attempt to avoid allogeneic blood transfusion during the perioperative period, technologies such as cell salvage have been introduced without firm evidence to support their use. Although cell salvage provides peace of mind, knowing a patient’s own blood will be transfused should it be needed, cell salvage is not without its own risks and costs (Forgie 1998). The risks associated with cell salvage are well documented, and include, bacterial contamination of the salvaged blood, air embolism, nephrotoxicity, and coagulation disorders (Faught 1998; Huet 1999; Semmens 2000;Spahn 2000). In its most simplistic form, unwashed cell salvage merely represents a very laborious means of obtaining an autologous volume expander, which is not necessarily advocated due to the potential serious side effects (Huet 1999). Although washed cell salvage provides a better quality blood product, the overall cost of this technology is rather substantial. However, a recent cost-effectiveness analysis indicated that cell salvage was cost-effective compared with all other transfusion strategies except ANH (Davies 2006). This study indicated that the net benefit of cell salvage was between £112 and £359 per person, compared with the allogeneic blood transfusion strategy, PAD, PAD plus erythropoietin (EPO), fibrin sealants, antifibrinolytics, and EPO. This study claimed that the use of cell salvage could result in net reductions in the volume of allogeneic blood transfused of between 6500 and 320,000 units per year, translating into annual savings to the National Health Service (NHS) of £0.73 million to £36 million (Davies 2006).
Any intervention that forms part of a blood conservation strategy needs to be critically examined in respect to its cost-effectiveness. On the basis of cost alone, cell salvage may appear to be an attractive alternative to the other currently available technologies, in particular aprotinin. However, as highlighted by Fergusson 1999a, in many instances neither the cost of the technologists needed to operate the device nor the cost of the cell saver device itself are considered in the overall calculations of cost. Further to this,Fergusson 1999a propose that the conclusions of studies that suggest cell salvage is cost saving should be interpreted with caution. The true value of avoiding allogeneic blood transfusion remains debatable. Those concerned with the risks of transfusion transmitted disease (TTD) will be more interested in avoiding blood transfusion completely, rather than reducing the volume of blood transfused. However, the importance of avoiding transfusion depends on the probability of avoiding disease transmission, or other adverse effects that have been attributed to blood transfusion, such as alloimmunisation or febrile non-haemolytic transfusion reaction (FNHTR). The rate of HIV or viral hepatitis transmission in most developed countries is very low, due to the presence of quality blood screening programmes (Coyle 1999; Whyte 1997). However, this assumption does not equally apply to developing countries where allogeneic blood is frequently administered without adequate screening in an environment where there is a high prevalence of viral pathogens amongst donors (Kimball 1995;McFarland 1997). In these settings there may be much greater clinical value in a range of interventions that diminish or avoid the need for allogeneic blood. However, the costs associated with such interventions may be prohibitive in developing countries, a situation that may well apply to cell salvage.
Most of the data have been collected in the context of major cardiac and orthopaedic surgery, where blood loss is often substantial. Consequently the applicability of the results to clinical settings where blood loss is minor is questionable. This review has highlighted the fact that cell salvage is frequently used alongside other interventions designed to minimise the need for allogeneic blood transfusion. This is particularly evident in the area of cardiac surgery, where over the last 10-20 years there has been a steady increase in the use of anti-fibrinolytic drugs (for example aprotinin, tranexamic acid, epsilon aminocaproic acid), acute normovolaemic haemodilution (ANH), and pre-operative autologous donation (PAD). A meta-analysis (Henry 2007) of the aforementioned anti-fibrinolytic drugs showed that both aprotinin and tranexamic acid were highly efficacious in reducing surgical blood loss and allogeneic blood transfusion in cardiac surgery. However, the findings of a Canadian study (Fergusson 2008), which reported an increased risk of death in cardiac surgery patients treated with aprotinin compared with the lysine analogues (tranexamic acid and epsilon aminocaproic acid), led to the market suspension of aprotinin on November 5, 2007. The loss of aprotinin from the armamentarium of the cardiac surgeon has lead to a re-exploration of alternative approaches to haemostasis management (Baker 2009).
The evidence on the efficacy and safety of ANH and PAD has been reviewed by the International Study of perioperative Trans-fusion (ISPOT) group (Bryson 1998; Forgie 1998). The literature regarding these interventions is generally viewed as being of indifferent quality because of inadequate randomisation and lack of blinding of outcomes assessment. However, these techniques have been shown to have modest blood sparing effects. This and the growing evidence on the efficacy of transfusion triggers indicates that a more conservative approach to blood transfusion is generally desirable in patients without cardiovascular risk factors, such as ischaemic heart disease or cerebral vascular disease (Carson 1998; Hebert 1999). This conservative approach, combined with the use of anti-fibrinolytic drugs may well offer the best approach for managing the transfusion requirements of patients in high-risk settings such as cardiac surgery. However, in settings other than cardiac, such as vascular and orthopaedic surgery, the choice of intervention that best minimises patient exposure to allogeneic blood transfusion is not that clear cut, although a more conservative approach to transfusion practice has been shown to be efficacious across a range of clinical domains (Carson 2002; Hill 2003). The decision to use any of the current available technologies as an alternative to allogeneic blood transfusion, including cell salvage, will be primarily based on availability, cost, and surgeon preference. To delineate the efficacy of the various technologies used in non-cardiac settings is rather difficult as the current available evidence is equivocal.
AUTHORS’ CONCLUSIONS
Implications for practice
The use of washed cell salvage appears justified in orthopaedic surgery. However, in situations where there are concerns about the safety of the blood supply, the use of unwashed, filtered cell salvage may well be justified. Although the use of washed or unwashed cell salvage in cardiac surgery may well be justified on the basis of the evidence reviewed here, due consideration needs to be given to those technologies (that is anti-fibrinolytic agents) that, unlike cell salvage, have been shown to significantly reduce peri-operative blood loss and re-operation due to bleeding in the context of cardiac surgery (Henry 2007). Re-operation alone is associated with substantial additional costs due to additional surgery and costs associated with increased length hospital stay (Ray 1999).
Implications for research
There is no need for further small randomised controlled trials of cell salvage in orthopaedic and cardiac surgery. The principal need is for large, methodologically rigorous, comparative trials to assess the relative efficacy, safety, and cost-effectiveness of cell salvage in different surgical procedures.
PLAIN LANGUAGE SUMMARY.
Cell salvage (collecting a patient’s own blood during surgery) for reducing transfusions with donated blood
Some patients who undergo surgery require blood transfusions to compensate for the blood loss that occurs during the procedure. Often the blood used for the transfusion has been donated by a volunteer. The risks associated with receiving volunteer donor blood that has been screened by a competently managed modern laboratory are considered minimal, with the risk of contracting diseases such as HIV and hepatitis C being extremely low. However there is concern in many developing countries, where there is a high prevalence of such infections and transfusion services are inadequately equipped to screen donor blood as thoroughly. Although in developed countries the risks of acquiring a disease from transfused blood are low, the financial costs associated with providing a safe and reliable blood product are escalating. Therefore there is much attention being placed on alternative strategies to minimise the need for transfusions of donor blood.
’Cell salvage’ or ‘autotransfusion’ is one technique designed to reduce the use of such transfusions. It involves the collection of a patient’s own blood from surgical sites which can be transfused back into the same person during or after surgery, as required.
The authors undertook this systematic review to examine the evidence for the effectiveness of cell salvage in reducing the need for blood transfusions of donor blood in adults (over 18 years) undergoing surgery.
The authors found 75 studies investigating the effectiveness of cell salvage in orthopaedic (36 studies), cardiac (33 studies), and vascular (6 studies) surgery. Overall, the findings show that cell salvage reduces the need for transfusions of donated blood. The authors conclude that there appears to be sufficient evidence to support the use of cell salvage in cardiac and orthopaedic surgery. Cell salvage does not appear to cause any adverse clinical outcomes.
As the methodological quality of the trials was poor, the findings may be biased in favour of cell salvage. Large trials of high methodological quality that assess the relative effectiveness, safety, and cost-effectiveness of cell salvage in different surgical procedures should be the focus of future research in this area.
Acknowledgments
SOURCES OF SUPPORT
Internal sources
Research Management Committee, The University of Newcastle, NSW, Australia. Special purpose grant, Hunter Area Pathology Services, NSW, Australia.
External sources
Australian Health Ministers’ Advisory Committee. National Health and Medical Research Council of Australia, Australia.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Patients were randomised to receive an autologous reinfusion drain or a standard suction drain using the computer program MINIM. The method used to conceal treatment allocation was not described | |
| Participants | 104 consecutive patients undergoing primary cemented total knee arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, blood loss, hospital length of stay, Hb & Hct levels, wound problems, knee range of motion | |
| Notes | Transfusion threshold: allogeneic blood transfusion was given if the haemoglobin level was less than 9.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The software program MINIM was used to randomise patients to intervention or control |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Concealment of treatment allocation was by use of sealed envelopes. Method of generating allocation sequences was not described | |
| Participants | 90 patients undergoing primary total knee arthroplasty were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, blood loss, hospital length of stay, Hb and Hct levels | |
| Notes | Transfusion threshold: allogeneic blood transfusion was given if the haemoglobin level was less than 9.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was carried out with sealed envelopes, opened just before closure of the wound. Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Sealed envelopes were used to conceal treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Patients undergoing bi- or tri-compartmental total knee arthroplasties with a diagnosis of primary osteoarthritis were included in the study | |
| Participants | 32 patients undergoing total knee arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, blood loss, hospital length of stay, adverse events | |
| Notes | Transfusion threshold: allogeneic blood transfusion was given if the haemoglobin level was less than 9.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Between May 2005 and December 2005, 178 patients were entered into the study. All patients over 55 years with osteoarthritis and/or inflammatory arthritis of the knee, and awaiting total knee replacement (TKR), were considered for the study. In a pre-assessment clinic patients were randomly assigned into two groups by sealed envelopes | |
| Participants | 178 patients undergoing total knee replacement were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, hospital length of stay, adverse events | |
| Notes | Transfusion threshold: allogeneic blood transfusion was given if the haemoglobin level was less than 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Sealed envelopes were used to conceal treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Between June 1988 and August 1989, 103 patients who gave informed consent to participate in the study underwent cardiopulmonary bypass. Of the initial 103 patients, 71 were excluded from the study. Method of randomisation and allocation concealment was not described | |
| Participants | 32 patients undergoing cardiac surgery requiring cardiopulmonary bypass were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, amount of autologous blood transfused, number of patients transfused autologous and/or allogeneic blood, complications, bleeding times, plasma protein variables, post transfusion febrile reactions | |
| Notes | Transfusion threshold: the decision to transfuse a patient post-operatively was made by the clinician who was responsible for the patient’s post-operative care, and who was not involved in the study. The clinical criteria used to determine the need for transfusion consisted of the following: systolic BP less than 80mmHg; mean arterial pressure less than 50mmHg; central venous pressure (CVP) less than 5mmHg; pulmonary capillary wedge pressure (PCWP) less than 5mmHg; cardiac index (CI) less than 2.0L/min/m2; evidence of inadequate end-organ perfusion (ie: urine output less than 20ml/hr), or anaemia (Hct less than 25%). Any patient who bled more than 400ml in the first 4 hours post-operatively and who met any of these criteria underwent transfusion | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to concealment treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | The study was conducted between October 15, 1991 through to January 1, 1993. The patients included 125 women and 107 men who were 20-89 years of age (mean age = 72 years). All patients were advised to donate blood pre-operatively. The 156 patients (67%) who were scheduled to have a primary procedure were advised to donate 2 units of autologous blood, and the 76 patients (33%) who were scheduled to have a revision procedure were a advised to donate 4 units of autologous blood | |
| Participants | 232 patients undergoing total hip arthroplasties were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic and./or autologous blood, blood loss, Hb levels | |
| Notes |
Transfusion threshold: Transfusion protocol not reported. All revision patients were exposed to cell salvage intra-operatively. 85% of Group 1 patients pre-deposited blood pre-operatively (PAD). 77% of Group 2 patients pre-deposited blood pre-operatively (PAD) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randomly assigned on the basis of their hospital record number |
| Allocation concealment (selection bias) | High risk | Inadequate allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Study was conducted between January 1993 and May 1993. Consecutive patients underwent elective or urgent coronary artery bypass surgery. All procedures were performed by the same cardiac surgeon. Method of randomisation and allocation concealment was not described | |
| Participants | 75 consecutive patients undergoing coronary artery bypass graft surgery were randomised into one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of blood retransfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, complications, wound infection, re-operation for bleeding, hospital length of stay, fever, mortality | |
| Notes | Transfusion threshold: allogeneic packed cells were transfused intra-operatively or postoperatively when the haematocrit fell below 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Between June 2002 and May 2004, 60 patients undergoing unilateral total knee arthroplasty (TKA) were enrolled in this prospective randomised trial. Randomisation was by sealed opaque envelopes which were mixed by independent personnel and consecutively assigned a case number from 1 to 60. All surgeries were performed by specialists of the joint and reconstruction team using an identical surgical approach and technique. Near the end of the operation the corresponding envelope was opened and the surgeon was informed at the time of drain insertion to achieve a single-blind effect | |
| Participants | 60 patients undergoing unilateral total knee arthroplasty (TKA) were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, febrile complications, adverse events, blood loss | |
| Notes | Transfusion threshold: allogeneic blood transfusion was given if the haemoglobin level was less than 9.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | High risk | Sealed opaque envelopes were used to conceal treatment allocation |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Single-blind design. |
| Methods | Patients undergoing elective abdominal aortic aneurysm (AAA) repair or aortofemoral bypass (AFB) for occlusive disease were eligible for entrance into the study. Randomisation was carried out in blocks of 10 stratified for AAA repair or AFB. Patients were randomised by means of drawing sealed envelopes that contained prescriptions for either intra-operative autotransfusion (IAT) or control therapy. The study was unblinded | |
| Participants | 100 patients undergoing aortic surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, blood loss, hospital length of stay, ICU length of stay, adverse events | |
| Notes | Transfusion threshold: patients were transfused allogeneic RBCs intra-operatively if the haemoglobin level was less than 10.0g/dL. Post-operatively patients were transfused allogeneic RBCs if the haemoglobin level was less than 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Treatment allocation was inadequately concealed. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Patients undergoing either coronary artery bypass grafting, valve replacement/repair operations or a combination of the two were randomised pre-operatively into two groups using a binary random number table. Method used to conceal treatment allocation was not described | |
| Participants | 112 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, mortality, re-operation for bleeding, blood loss, coagulopathy, Hb levels | |
| Notes | Transfusion threshold: patients were transfused allogeneic RBCs intra-operatively if the haemoglobin level was less than 7.0g/dL. Post-operatively patients were transfused allogeneic RBCs if the haemoglobin level was less than 10.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | The study was conducted between September 2003 to October 2004. Patients admitted for elective or sub-acute coronary bypass surgery without the use of the cardiopulmonary bypass (CPB) machine were included. If the CPB machine became necessary during the operation the patient was excluded. Patients were randomised to intervention or control by means of sealed opaque envelopes numbered in sequence | |
| Participants | 60 patients undergoing ‘off-pump’ coronary artery bypass surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, blood loss, Hb levels, adverse events, costs | |
| Notes | Transfusion threshold: patients were transfused allogeneic RBCs if the haematocrit level was less than 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Fifty patients having aortic surgery for either abdominal aortic aneurysm or aorto-iliac occlusive disease were selected for study. Method of randomisation and allocation concealment was not described | |
| Participants | 50 patients undergoing aortic surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, mortality, reoperation for bleeding, haemodialysis, blood loss, coagulopathy, Hb levels, organisms cultured from autologous vs allogeneic blood | |
| Notes | Transfusion threshold: patients received allogeneic RBC transfusion if the haematocrit level fell below 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | The efficacy of four different blood conservation techniques in decreasing allogeneic blood transfusion in different cardiac operations were studied in 100 patients undergoing myocardial revascularisation. Method of randomisation and allocation concealment was not described | |
| Participants | 100 patients undergoing myocardial revascularisation were randomly assigned to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, complications, mortality, ICU length of stay, blood loss, re-exploration for bleeding, operation time, haematological variables, Hct levels | |
| Notes | Transfusion threshold: in all patients signs of hypovolaemia and haematocrit values below 30% were indications for allogeneic blood transfusion | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Patients undergoing primary unilateral total knee arthroplasty over a consecutive 30-day period were studied to assess the efficacy and financial cost of post-operative reperfusion of drained blood | |
| Participants | 49 patients undergoing primary unilateral total knee arthroplasty were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, Hb levels, cost | |
| Notes | Transfusion threshold: the trigger for transfusing allogeneic blood was a post-operative haemoglobin level of less than 9.0g/dL or clinical symptoms of anaemia | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Patients with severe hip arthrosis undergoing total hip arthroplasty were studied to evaluate the efficacy of different peri-operative blood saving techniques to reduce allogeneic blood transfusion. Method of randomisation and allocation concealment was not described | |
| Participants | 45 patients undergoing total hip arthroplasty were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, amount of autologous blood transfused, number of patients transfused allogeneic blood, complications, adverse events | |
| Notes | Transfusion threshold: patients were transfused allogeneic blood to maintain the erythrocyte volume fraction (EVF) >27% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised trial to study the quality and effect of blood produced by the cell saver compared with allogeneic blood in primary total hip arthroplasty | |
| Participants | 40 patients undergoing primary total hip arthroplasty were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic units transfused, number of patients receiving allogeneic blood, complications, blood loss, haematological variables | |
| Notes | Transfusion threshold: a transfusion of allogeneic blood was given if the haemoglobin was less than 8.5g/dL or if there were symptoms of anaemia | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Prospective randomised trial to investigate the safety and efficacy of post-operative autologous blood transfusion carried out in two matched groups of twenty patients undergoing elective coronary artery bypass surgery. Method of randomisation and allocation concealment were not described | |
| Participants | 40 patients (33 males and 7 females) undergoing elective coronary artery bypass surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, hospital length of stay, mortality, blood loss, haematological variables, adverse events | |
| Notes | Transfusion threshold: allogeneic blood transfusion was used only when the haematocrit fell below 25%, haemoglobin below 9.0g/dL or the blood loss exceeded 500mls in the first 4 hours | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | To determine if autotransfusion of unwashed shed mediastinal blood led to a reduction in post-operative banked blood requirements a prospective randomised study of 82 patients undergoing myocardial revascularisation was conducted in 1994 at the Cardiovascular Center of Parma. Method of randomisation and allocation concealment were not described [Italian article] | |
| Participants | 82 patients undergoing myocardial revascularisation were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, number of patients transfused allogeneic blood, amount of allogeneic blood transfused, blood loss, mortality | |
| Notes | Transfusion threshold: allogeneic blood transfusion was given during surgery if the haemoglobin level fell below 7.5g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Consecutive patients undergoing total hip or total knee replacement procedures between January 1989 and April 1989 were included in this study. A computer-generated random number list was used to pre-operatively assign patients to intervention or control groups. Method used to conceal treatment allocation was not described | |
| Participants | 239 consecutive patients undergoing total knee replacement procedures were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, number of patients transfused allogeneic blood, adverse events | |
| Notes | Transfusion threshold: All patients whose post-operative haemoglobin value was less than 9.0g/dL were transfused allogeneic blood. The decision to transfuse patients with haemoglobin values greater than 9.0g/dL was made by the internist on the basis of each patient’s medical condition | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer-generated random number list was used to pre-operatively assign patients to either intervention or control |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Between March 2004 and June 2004, all patients admitted for elective or urgent first-time coronary artery bypass grafting (CABG) were enrolled in the study | |
| Participants | 50 patients undergoing ‘off-pump’ first-time coronary artery bypass grafting (CABG) were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, volume of blood re-transfused from the cell saver, blood loss, adverse events | |
| Notes | Transfusion threshold: the indication for allogeneic blood transfusion in the intraoperative period was a haemoglobin level less than 9.0g/dL or a haematocrit level less than 27%. In the autotransfusion group, all the processed red blood cells collected during surgery were re-transfused as required. Banked allogeneic blood was used only if the haemoglobin level remained less than 9.0g/dL despite autotransfusion | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Sealed envelopes were used to conceal treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | The efficacy of autologous shed blood in reducing allogeneic blood transfusion was evaluated at four medical centres in a prospective randomised study. Method of randomisation and allocation concealment was not described | |
| Participants | 128 patients undergoing total hip arthroplasty, total knee arthroplasty, or spine fusion were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of blood retransfused from the cell saver, number of patients transfused allogeneic blood, amount of allogeneic blood transfused, adverse events | |
| Notes | Transfusion threshold: transfusion protocol was not reported. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Consecutive patients undergoing elective knee arthroplasty at two institutions were enrolled in the study. Method of randomisation and allocation concealment was not described | |
| Participants | 81 patients undergoing elective knee arthroplasty were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, blood loss, coagulation variables, venogram tests | |
| Notes | Transfusion thresholds: on postoperative Day 2 through to Day 5, the criteria for allogeneic red cell transfusions were identical for both groups. Patients were given one unit of red cell concentrate if their haemoglobin was within the range of 8.0 to 8.9g/dL, two units when the value was from 7.0 to 7.9g/dL, three units when the value was from 6.0 to 6.9g/dL, and four units if the value was from 5.0 to 5.9g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | This study was prospectively performed on the cases of a single vascular surgeon operating at two institutions from January 1989 to January 1990. Patients undergoing elective infrarenal abdominal aortic bypass for either occlusive or aneurysmal disease were included in the study sample. Patients were randomised on an alternating basis to either intervention or control. Method of randomisation was not described | |
| Participants | 36 patients undergoing aortobifemoral or aortobi-iliac bypass for occlusive disease were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, adverse events, hospital length of stay, blood loss, haemoglobin levels | |
| Notes | Transfusion threshold: after the operation allogeneic red cell transfusions were not given to patients who were haemodynamically stable, and had haemoglobin values greater than 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | This prospective randomised trial evaluated the safety and efficacy of post-operative blood retrieval and re-infusion in patients undergoing total knee arthroplasty for primary knee osteoarthritis during 2002. Method of randomisation and allocation concealment was not described | |
| Participants | 155 patients undergoing total knee arthroplasty were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, days with fever, fever, volume of blood re-transfused, haemoglobin levels | |
| Notes | Transfusion threshold: patients were transfused allogeneic blood if the haemoglobin level fell to less than 10.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Study allocated patients to intervention or control on an alternating basis. The first patient to participate in the study was classified in Group B, the second patient in Group A, and so on. If a Group B patient was discarded from the study during the operation the next patient to participate in the study was again classified in Group B |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | All patients scheduled for non-emergency first-time coronary artery bypass graft surgery (CABG), valve surgery or combined CABG and valve procedures requiring cardiopulmonary bypass (CPB) were eligible for enrolment. Operations associated with a high risk of transfusion, such as transplantation and operations on the thoracic aorta were excluded | |
| Participants | 213 patients undergoing first-time CABG and/or cardiac valve surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, number of patients transfused fresh frozen plasma, number of patients transfused platelets, blood loss, adverse events, re-operation for bleeding | |
| Notes | Transfusion threshold: patients were transfused allogeneic blood when the haemoglobin level fell below 7.0g/dL during surgery, and fell below 8.0g/dL post-operatively | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated to intervention or control by simple randomisation generated by an independent statistician using a computer random number function, stratified by type of surgery |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A randomised controlled trial was undertaken to investigate the efficacy of cell salvage during cardiac surgery at the University Hospital Sint Radboud, Nijmegen. Patients undergoing elective coronary bypass graft (CABG) surgery allocated on an alternating basis to one of two groups. The method of randomisation was not described | |
| Participants | 40 patients undergoing elective coronary artery bypass graft surgery (CABG) were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of blood retransfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, blood loss, Hb & Hct levels | |
| Notes | Transfusion threshold: allogeneic packed cells were transfused to maintain an Hct at 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Patients undergoing total hip arthroplasty or dorsal lumbo-sacral spinal fusion (implantation of a H-frame) were entered into this randomised study. Each patient was allocated on an alternating basis to one of two groups. The method of randomisation was not described | |
| Participants | 60 patients undergoing total hip arthroplasty or dorsal lumbo-sacral fusion surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of blood retransfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, blood loss, Hb & Hct levels | |
| Notes | Transfusion threshold: allogeneic packed cells were transfused to maintain an Hct at 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Patients undergoing isolated primary coronary revascularisation between July and December 1989 were enrolled in this randomised control trial. Patients were randomised by coded instruction packets which specified the processing and administration of the patient’s salvaged intra-operative blood. Sealed instruction packets were randomised using a shuffle deck procedure, serially numbered, and assigned sequentially to patients in order of enrolment. The sealed instruction packets were sent with the patients to the operating room | |
| Participants | 50 patients undergoing primary coronary revascularisation were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, number of patients transfused allogeneic blood, amount of allogeneic blood transfused, amount of any blood product transfused | |
| Notes | Transfusion threshold: packed red blood cell transfusions were given if the patients haemoglobin was less than 7.0g/dL or if the patient was haemodynamically unstable due to volume loss | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Single-blinded. |
| Methods | Randomised controlled trial of 135 adults undergoing primary cardiac surgery. Method of randomisation and allocation concealment was not described | |
| Participants | 135 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, mortality, blood loss | |
| Notes | Transfusion threshold: Transfusion protocol was not reported. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | The efficiency of pre-operative deposit, pre-operative haemodilution, and intra- and post-operative autotransfusion in reducing allogeneic blood transfusions was studied in this randomised trial. Method of randomisation and allocation concealment was not clear [German article] | |
| Participants | 64 patients scheduled for total hip arthroplasty were randomly divided into one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, blood loss | |
| Notes | Transfusion threshold: polygeline was used for volume resuscitation. If the Hb concentration fell below 9.0g/dL in the operating room and the intensive care unit or below 10.0g/dL in the general ward, autologous or allogeneic packed red cells were transfused | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Patients admitted for elective primary joint replacement surgery were enrolled in this randomised controlled trial. Patients were randomised using a computer-generated randomisation table. Method of allocation concealment was not described | |
| Participants | 99 patients undergoing elective primary total knee replacement surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, blood loss. | |
| Notes | Transfusion threshold: allogeneic blood transfusions were used intra/post-operatively to maintain a safe blood volume and a haemoglobin level around 10.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using a computer-generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A series of 40 patients undergoing primary unilateral total knee arthroplasty were entered into this randomised controlled trial to assess the safety and efficacy of post-operative autologous blood salvage and reinfusion. Method of randomisation and allocation concealment were not described | |
| Participants | 40 patients undergoing primary unilateral total knee arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of blood retransfused from the cell saver, amount of allogeneic blood transfused, adverse events, haematological variables | |
| Notes | Transfusion threshold: allogeneic blood was given to patients if the haemoglobin level fell below 9.5g/dL or if indicated haemodynamically | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective randomised clinical trial was undertaken to compare the current approach of mediastinal drainage without reinfusion to a system specifically designed for reinfusion. From September 1998 to January 1999, patients admitted for coronary artery bypass grafting operations, valvular replacement, or both procedures under cardiopulmonary bypass (CPB) were offered the option to participate in the study. Method of randomisation and allocation concealment were not described | |
| Participants | 198 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, blood loss, adverse events | |
| Notes | Transfusion threshold: during CPB allogeneic red blood cells were transfused for haemoglobin concentrations below 6.0g/dL. In the post-operative period the threshold for allogeneic red blood cell transfusion was Hb<8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective randomised study was undertaken to quantify the effect of reinfusion of post-operative shed blood drainage on the haemoglobin levels in patients undergoing elective primary total hip arthroplasty (THA) and total knee arthroplasty (TKA). Patients were enrolled between December 1990 and August 1991. Randomisation was performed using a random number table. Allocation concealment was not described | |
| Participants | 111 patients undergoing elective primary total hip arthroplasty and total knee arthroplasty were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic or autologous blood, postoperative drainage, Hb levels | |
| Notes | Transfusion threshold: intra-operative blood transfusion was left to the discretion of the operating surgeon. No transfusion threshold or trigger was reported | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A randomised controlled trial was conducted to assess the effectiveness of two mechanical methods of blood conservation in reducing the need for allogeneic red blood cells or coagulation products during cardiac surgery. Patient allocations were generated from random number tables by an independent observer and concealed in sealed opaque envelopes | |
| Participants | 256 patients undergoing elective coronary artery bypass surgery were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, number of patients receiving any blood product, amount of allogeneic blood transfused, blood loss, reoperation for bleeding, hospital length of stay, infection, stroke, renal failure, myocardial infarction | |
| Notes | Transfusion threshold: allogeneic red blood cells were transfused in all groups when the haemoglobin level fell below 9.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patient allocations were generated from random number tables by an independent observer |
| Allocation concealment (selection bias) | High risk | Allocation concealment was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | The influence of two different methods of autologous transfusion were investigated in a prospective randomised controlled trial. Method of randomisation and allocation concealment was not described [German article] | |
| Participants | 42 patients undergoing total hip surgery and pre-operative plasmapheresis (Abbott Autotrans) were randomised to one of three groups:
|
|
| Interventions |
NB: Study investigated the influence of two different methods of autotransfusion on the intravascular haemostatic system |
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, number of patients transfused allogeneic blood, blood loss, Hb & Hct levels, clotting status (PT/TT/PTT/ATIII) | |
| Notes | Transfusion threshold: patients were transfused if haemoglobin fell below 9.0g/dL or haematocrit fell below 28% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | This was a single-centre randomised clinical trial of intra-operative autotransfusion (IAT) in surgery for abdominal aortic aneurysm (AAA). Patients were randomised using sealed envelopes. Patients were blinded to the transfusion group allocation. Members of the operating surgical team were responsible for the continuing care of patients, decision to use blood transfusion and investigation of post-operative complications. They were independent of the research team, but were not blinded to the use of IAT | |
| Participants | 81 patients undergoing elective repair of infrarenal abdominal aortic aneurysm were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, blood loss, adverse events, mortality, hospital length of stay | |
| Notes | Transfusion threshold: patients received allogeneic blood transfusion to maintain haemoglobin levels above 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Single-blind. |
| Methods | This randomised clinical trial was designed to evaluate the clinical efficacy of retransfusion of filtered shed blood in patients undergoing consecutively scheduled primary total knee arthroplasty (TKA) or total hip arthroplasty (THA). The use of alternatives other than post-operative cell salvage (autotransfusion) to reduce allogeneic blood transfusions were excluded. A treatment allocation schedule was randomly generated and then concealed in sealed envelopes that were labelled with a consecutive case number from 1 to 160. Blocking and stratification were not used | |
| Participants | 160 patients undergoing elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, volume of blood re-transfused, adverse events | |
| Notes | Transfusion threshold: after surgery the anaesthesiologist determined the Hb transfusion trigger, that is, 8.1, 8.9, or 9.7g/dL, depending on comorbidity classified in the ASA (American Society of Anesthesiologists) classification and according to hospital policy. When the Hb level dropped below this trigger an allogeneic blood transfusion was given | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | This randomised controlled trial was designed to ascertain whether cell salvage and autotransfusion after first time elective coronary artery bypass grafting (CABG) is associated with a significant reduction in the use of allogeneic blood, a clinically significant derangement of post-operative clotting profiles, or an increased risk of post-operative bleeding. Between March 2002 and January 2003, patients admitted for CABG operations utilising cardiopulmonary bypass (CPB) were enrolled in the study | |
| Participants | 200 patients undergoing first time elective coronary artery bypass grafting (CABG) were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, number of patients transfused fresh frozen plasma (FFP), number of patients transfused platelets, volume of blood autotransfused, blood loss, adverse events, mortality, hospital length of stay | |
| Notes | Transfusion threshold: patients were transfused allogeneic red blood cells when the haemoglobin level fell below 7.0g/dL or if clinically indicated in patients with excessive blood loss and cardiovascular instability at the discretion of intensive care staff | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to the two treatment arms in a 1:1 ratio by using a block randomisation procedure. Allocations were generated by card system |
| Allocation concealment (selection bias) | High risk | Allocations were concealed in sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A randomised controlled trial was conducted to evaluate the safety and effectiveness of intra-operative cell salvage and autotransfusion of washed salvaged red blood cells after first-time coronary artery bypass grafting (CABG) performed on the beating heart. Patients were assigned to one of two randomised group, in a 1:1 ratio by using block randomisation. Allocations were generated by a card system and concealed in sealed opaque envelopes. Patients who had given consent were randomised immediately before surgery | |
| Participants | 61 patients undergoing cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, volume of blood collected by the cell saver, volume of blood re-transfused from the cell saver, number of patients transfused fresh frozen plasma (FFP), number of patients transfused platelets, blood loss, mortality, adverse events | |
| Notes | Transfusion threshold: the threshold for transfusion of allogeneic blood was a haemoglobin level less than 8.0g/dL or a haematocrit less than 0.23. In patients with excessive blood loss and cardiovascular instability, blood was given at the discretion of anaesthetic or intensive care unit staff | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Method of randomisation was not reported and allocation concealment was unclear. Baseline comparability was unclear. However the study reported that, “no significant difference between groups were detected at any stage of the study.” Participants were not blind to treatment allocation and blinding of the outcome assessor was unclear [Russian article] | |
| Participants | 66 patients undergoing elective coronary artery bypass surgery (CABG) were randomly allocated to one of two groups:
Inclusion criteria: patients with an uneventful postoperative period (discharge of less than 800mls through draining tubes during first 8 hours post operation) |
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood. | |
| Notes | Transfusion threshold: transfusion protocol not reported. Russian study. English abstract only | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not used. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised controlled trial of consecutive osteoarthritic patients undergoing unilateral total knee replacement was conducted. Randomisation was by random number tables. Method of allocation concealment was not described | |
| Participants | 70 consecutive patients undergoing unilateral total knee replacement with a cruciate-sparing Kinemax Plus prosthesis were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from cell saver, amount of allogenic blood transfused, number of patients transfused allogeneic blood, adverse events, hospital length of stay | |
| Notes | Transfusion threshold: transfusion protocol was not reported. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Consecutive patients undergoing first-time coronary artery bypass grafting (CABG) requiring at least three bypass grafts with moderate-good left ventricular function were invited to participate in the randomised trial. Randomisation was achieved by mixing non-transparent envelopes containing cards marked with the code of each group. Randomisation was done the day before surgery | |
| Participants | 80 patients undergoing first-time isolated CABG surgery were randomly allocated to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, volume of blood collected by the cell saver, blood loss, mortality, hospital length of stay, adverse events | |
| Notes | Transfusion threshold: allogeneic blood was only transfused if the haemoglobin concentration was than 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Single-blind. |
| Methods | Consecutive patients having elective coronary artery or valvular operations were enrolled in a prospective, randomised controlled trial comparing allogeneic blood consumption between conventional mediastinal drainage and reinfusion of shed mediastinal blood using a hard-shell cardiotomy reservoir. Method of randomisation and allocation concealment was not described | |
| Participants | 100 consecutive patients undergoing elective coronary artery or valvular operations were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, re-exploration for bleeding | |
| Notes | Transfusion threshold: allogeneic blood or hetastarch was infused to maintain cardiovascular stability and a haematocrit of 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised controlled trial was undertaken to evaluate blood salvage using an intraoperative blood recovery system and amediastinal drainage blood recovery system during and after cardiac surgery. Method of randomisation and allocation concealment was not described | |
| Participants | 66 patients undergoing aortocoronary bypass surgery were randomly assigned to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, mortality, blood loss, Hct levels | |
| Notes | Transfusion threshold: allogeneic blood transfusions were given if the haematocrit dropped below 20% during bypass, 28% at the end of the procedure, 30% within 24 hours, or if the haemoglobin level was less than 10.0g/dL while on the cardiac surgery ward (8 to 10 days) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Prospective randomised controlled trial of autotransfusion of mediastinal shed blood in patients with unstable angina pectoris scheduled for first-time coronary artery bypass graft (CABG) surgery. The Unit for Applied Clinical Research at the Norwegian University of Science and Technology randomised patients into two groups by means of a computer program. At the end of uneventful surgery, the Unit for Applied Clinical Research was contacted by telephone and the randomisation was done | |
| Participants | 50 patients scheduled for first-time CABG surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, volume of blood re-transfused from the cell saver, amount of fresh frozen plasma and platelets transfused, blood loss, adverse events, mortality | |
| Notes | Transfusion threshold: transfusion protocol was not reported. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of generating allocation sequences was adequate. |
| Allocation concealment (selection bias) | Low risk | Method used to conceal treatment allocation was adequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Prospective, randomised controlled study was performed to determine the haematological and biochemical changes, and clinical safety of post-operative autotransfusion in patients undergoing elective, non-emergency spinal surgery. A random number table was used to assign patients in equal numbers to two groups. Method of allocation concealment was not described | |
| Participants | 50 patients undergoing elective spinal surgery were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, haematological variables | |
| Notes | Transfusion threshold: blood transfusion (allogeneic and/or autologous) was given if the haematocrit level was below 25% during the peri-operative period | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A randomised, prospective study of patients undergoing primary total hip or total knee replacement over a six-month period. Method of randomisation and allocation concealment were not described | |
| Participants | 415 patients undergoing primary total hip or total knee replacement were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of transfused blood, adverse events, knee flexion | |
| Notes | Transfusion threshold: allogeneic blood was transfused if the haemoglobin level fell below 9.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A controlled, randomised, prospective study was performed evaluating the need for perioperative blood salvage for primary total hip arthroplasty patients who had donated autologous blood before surgery. Patients were randomised by month of birth into four groups | |
| Participants | 153 patients undergoing primary total hip arthroplasty were randomised to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic and/or autologous blood transfused, number of patients transfused allogeneic blood, adverse events, Hb & Hct levels, thigh circumference measures, wound drainage | |
| Notes | Transfusion threshold: all decisions for allogeneic blood transfusion were based on the clinical condition of the patient. The absolute value of the haemglobin or haematocrit was not considered in isolation. Patients who were able to donate at least 2 units of autologous blood pre-operatively were included in the study | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A controlled, randomised, prospective study was performed evaluating the need for perioperative blood salvage for primary total hip arthroplasty patients who had donated autologous blood before surgery. Patients were randomised by month of birth into four groups | |
| Participants | 153 patients undergoing primary total hip arthroplasty were randomised to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic and/or autologous blood transfused, number of patients transfused allogeneic blood, adverse events, Hb & Hct levels, thigh circumference measures, wound drainage | |
| Notes |
Transfusion threshold: all decisions for allogeneic blood transfusion were based on the clinical condition of the patient. The absolute value of the haemglobin or haematocrit was not considered in isolation. Patients who were able to donate at least 2 units of autologous blood pre-operatively were included in the study NB: Data from Rollo 1995 has been used to formulate Rollo 1995/a which represents a comparison of Haemonetics Cell-Saver group vs No drainage group (Control) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A controlled, randomised, prospective study was performed evaluating the need for perioperative blood salvage for primary total hip arthroplasty patients who had donated autologous blood before surgery. Patients were randomised by month of birth into four groups | |
| Participants | 153 patients undergoing primary total hip arthroplasty were randomised to one of four groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic and/or autologous blood transfused, number of patients transfused allogeneic blood, adverse events, Hb & Hct levels, thigh circumference measures, wound drainage | |
| Notes |
Transfusion threshold: all decisions for allogeneic blood transfusion were based on the clinical condition of the patient. The absolute value of the haemglobin or haematocrit was not considered in isolation. Patients who were able to donate at least 2 units of autologous blood pre-operatively were included in the study NB: Data from Rollo 1995 has been used to formulate Rollo 1995/b which represents a comparison of the Solcotrans group vs No drainage group (Control) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised controlled trial of autotransfusion devices in patients undergoing knee-joint replacement surgery. Method of randomisation and allocation concealment was not described. [French article] | |
| Participants | 30 patients undergoing total knee arthroplasty were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, number of patients transfused allogeneic blood, composition of drainage blood | |
| Notes | Transfusion threshold: allogeneic blood was transfused to maintain a haematocrit of 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised study of consecutive total knee arthroplasties was carried out over a period of 2 years. Method of randomisation and allocation concealment was not described. [Abstract] | |
| Participants | 120 patients undergoing total knee arthroplasty were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood. | |
| Notes | Transfusion threshold: transfusion protocol was not reported. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | During a 3-month period from January 1977 to April 1977, adult patients undergoing cardiac surgery at the John Hopkins Hospital were randomised by odd or even history numbers to receive in the post-operative period either conventional blood bank transfusion therapy or autotransfusion of shed mediastinal blood | |
| Participants | 114 patients undergoing cardiac surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, total blood and blood component replacement, mediastinal blood loss, haematological variables, adverse events | |
| Notes | Transfusion threshold: if Hct values were below 35% and left ventricular filling was judged to be adequate, whole blood and/or packed red blood cells were infused to restore intravascular volume. With higher haematocrit values and with low left ventricular filling pressures, patients received an infusion of colloid solution or crystalloid solution (Ringer’s lactate) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method used to generate allocation sequences was inadequate. |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Between November 1992 and October 1993, adult patients undergoing primary elective coronary artery bypass grafting entered the prospective, randomised, controlled study. Method of randomisation was not described. Allocation concealment was by means of sealed envelopes | |
| Participants | 120 adult patients undergoing primary elective coronary artery bypass grafting were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, sternal infections, myocardial infarction, sepsis, mortality, blood loss | |
| Notes | Transfusion threshold: patients were transfused allogeneic blood if the haemoglobin concentration was less than 5.0mmol/L in the intensive care unit and less than 5.5mmol/L during the rest of the hospital stay | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised study evaluated the effect of autotransfusion of shed blood on the reduction and avoidance of donor blood requirements in patients undergoing internal mammary artery bypass (IMA) surgery and treatment with low-dose aprotinin (2 million KIU). Method of randomisation and allocation concealment was not described | |
| Participants | 40 patients undergoing elective primary unilateral internal mammary (IMA) artery bypass grafting were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, re-exploration for bleeding, blood loss | |
| Notes | Transfusion threshold: allogeneic packed red cells were transfused when the post-operative Hct fell below 25% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised study to assess the impact of cell salvage autotransfusion on the requirements for allogeneic blood in patients undergoing a total knee replacement was conducted. Patients were allocated to groups according to a computer generated randomisation schedule. Method of allocation concealment was not described | |
| Participants | 100 consecutive patients undergoing total knee replacement were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, hospital length of stay | |
| Notes | Transfusion threshold: allogeneic blood was given in the post-operative period when the haemoglobin fell below 9.0g/dL. Routine procedure of crossmatching two units of packed cells was performed for all patients in the study | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised controlled trial was conducted to evaluate the advantages and disadvantages of post-operative autotranfusion in patients undergoing first-time coronary artery bypass grafting (CABG). Method of randomisation and allocation concealment were not described | |
| Participants | 40 patient undergoing first-time coronary artery bypass graft sugery were randomly divided into one of two groups. The two groups were further subdivided according to whether the patients received aspirin preoperatively or not:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, re-operation for bleeding, blood loss | |
| Notes | Transfusion threshold: the indication for allogeneic blood transfusion was the maintenance of a haematocrit (Hct) level of 30% to 35% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Consecutive patients scheduled to undergo elective primary total joint arthroplasty was entered into a randomised controlled trial. Method of randomisation and allocation concealment was not described | |
| Participants | 24 patients undergoing elective total joint arthroplasty were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, average collection times, blood loss, Hb & Hct levels, coagulation variables | |
| Notes | Transfusion threshold: post-operative transfusions were given when the haemoglobin level was less than 10.0g/dL or the haematocrit was less than 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised controlled trial was conducted from 2005 to 2006 to evaluate the efficacy of re-infusion of autologous shed mediastinal blood in patients undergoing either coronary artery bypass graft surgery, valve replacement/repair operations or a combination of both, in the Clinic of Cardiothoracic and Vascular Surgery, Kaunas University of Medicine | |
| Participants | 90 patients undergoing cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, blood loss, hospital length of stay, adverse events | |
| Notes | Transfusion threshold: post-operatively the indication for allogeneic blood transfusion was a haemoglobin level of less than 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised, clinical trial was undertaken to determine whether postoperative blood salvage in patients undergoing total hip or knee arthroplasty decreased the need for transfusion with banked blood. The groups included all patients undergoing hip or knee arthroplasty at the University of Arizona Medical Centre between August 1, 1988 and June 1, 1989. Method of randomisation and allocation concealment were not described | |
| Participants | 109 patients undergoing total hip or knee arthroplasty were randomly assigned to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of blood retransfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, coagulopathy, blood loss, costs | |
| Notes | Transfusion threshold: transfusion protocol was not reported. Patients who were transfused only one unit of blood received only pre-banked autologous blood | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised study was conducted to analyse differences in post-operative haemoglobin levels and allogeneic blood requirements in patients undergoing primary total hip replacement (THR). Between December 2003 and December 2005, consecutive patients undergoing elective primary THR for arthritis at Weston General Hospital were enrolled. The patients were block randomised (computer-generated) to one of two groups from sealed envelopes opened by a nurse after reduction of the prosthesis | |
| Participants | 190 patients undergoing elective primary total hip replacement were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, volume of blood re-transfused from the cell saver, hospital length of stay, adverse events | |
| Notes | Transfusion threshold: the individual orthopaedic team decided whether to give allogeneic blood transfusion. Local practice was to give two units if the post-operative Hb was less than 8.0g/dL or if patients were symptomatic with Hb in the range of 8.0g/dL to 10.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | In 2003, patients ≥18 years of age who were scheduled for a primary or revision total hip replacement or total knee replacement at the Leiden University Medical Centre were included in this randomised controlled trial. All patients were of American Society of Anaesthesiologists (ASA) 2 or 3 category. A randomisation list was generated by a statistical software package. Sealed envelopes were made which contained the randomisation group. Pre-operatively, the patient was allocated to one of the groups by opening a sealed envelope | |
| Participants | 70 patients undergoing a primary or revision total hip replacement or total knee replacement were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, volume of blood re-transfused from the cell saver, blood loss, hospital length of stay, adverse events | |
| Notes | Transfusion threshold: during the study, a restrictive transfusion trigger according to the Dutch guidelines was used (CBO consensus guidelines, 2004) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised study was conducted to determine if cell-salvaged autologous blood can serve as an alternative to allogeneic blood in patients undergoing elective infra-renal abdominal aortic surgery. Method of randomisation not described. Allocation concealment was by sealed envelopes | |
| Participants | 50 patients undergoing elective infrarenal abdominal aortic aneurysm surgery were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, hospital length of stay, blood loss, mortality | |
| Notes | Transfusion threshold: patients were transfused allogeneic blood if the Hct fell below 25% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised study was performed in a New Delhi tertiary care hospital involving consecutive patients undergoing elective cardiac valve surgery using cardiopulmonary bypass. Method of randomisation and allocation concealment were not described | |
| Participants | 150 consecutive patients undergoing elective valve surgery using cardiopulmonary bypass were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, re-exploration for bleeding, blood loss, Hct levels | |
| Notes | Transfusion threshold: bank blood (whole blood) was used in all groups if the haematocrit was less than 25% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised study was performed in a tertiary care hospital involving adult patients undergoing elective cardiac valve surgery using cardiopulmonary bypass. Method of randomisation and allocation concealment were not described | |
| Participants | 60 patients undergoing cardiac valve surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of allogeneic blood transfused, re-exploration for bleeding | |
| Notes | Transfusion threshold: bank blood (whole blood) was used in all groups if the haemoglobin level fell below 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A single-centre, randomised controlled study was conducted of patients undergoing total knee replacement (TKR). Method of randomisation and allocation concealment were not described | |
| Participants | 231 patients undergoing elective total knee replacement surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, adverse events | |
| Notes | Transfusion threshold: allogeneic blood was transfused if the haemoglobin level fell below 9.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A randomised controlled trial was conducted to evaluate the safety and effectiveness of the collection and re-transfusion of post-operatively shed mediastinal blood in patients undergoing cardiac surgery. Method of randomisation and allocation concealment were not described | |
| Participants | 113 consecutive adult patients undergoing cardiac surgical procedures requiring cardiopulmonary bypass were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood collected by the cell saver, amount of blood retransfused from the cell saver, amount of allogeneic blood transfused, adverse events, myocardial infarction, mortality, post-operative infections, renal function impariment, fluid replacement, blood loss | |
| Notes | Transfusion threshold: intra-operative blood replacement was left to the discretion of the staff surgeon and anaesthesiologist. In patients who were unstable haemodynamically and in those patients whom complete revascularisation was not possible the haematocrit was raised to 30% or higher | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A prospective, randomised controlled study was conducted to analyse the effect of postoperative autotransfusion on the need for allogeneic transfusion and to determine the quality of post-operatively collected drainage blood and to compare it with other blood sources in patients undergoing primary total hip replacement. Method of randomisation and allocation concealment were not described | |
| Participants | 60 patients undergoing primary total hip replacement were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, blood loss, haematological variables | |
| Notes | Transfusion threshold: patients received allogeneic blood to maintain a haemoglobin level of 10.0g/dL or haematocrit level of 30% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A randomised controlled trial was conducted between January 1993 and June 1993 of patients undergoing primary elective coronary artery bypass graft surgery. Patients were randomised on the day before surgery using a computer randomisation programme. Method of allocation concealment was not described | |
| Participants | 105 patients undergoing primary elective coronary artery bypass graft surgery were randomised to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients receiving allogeneic blood, adverse events, re-exploration for bleeding, blood loss, mortality, haematological variables, coagulation variables | |
| Notes | Transfusion threshold: allogeneic blood was transfused to maintain the haematocrit level greater than 25% | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | A randomised controlled trial was conducted to study the effectiveness of autotransfusion of shed mediastinal blood in decreasing the need for allogeneic blood transfusion in routine cardiac surgery. Method of randomisation and allocation concealment were not described. The operative team was blinded to the randomisation until the patient arrived in the surgical intensive care unit. No patient in either group donated autologous blood | |
| Participants | 35 consecutive male patients undergoing elective myocardial revascularisation or valve replacement were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, number of patients transfused allogeneic blood, adverse events, re-operation for bleeding, blood loss, mortality, myocardial infarction, wound infection | |
| Notes | Transfusion threshold: patients in both groups received transfusions intra-operatively and post-operatively with packed red blood cells when the haemoglobin level fell to less than 8.0g/dL | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Prospective, randomised controlled trial of patients undergoing coronary artery surgery to compare inflammatory response, myocardial injury, and post-operative bleeding when cardiotomy suction blood and mediastinal shed blood were either discarded or re-transfused. Method of randomisation and allocation concealment were not described | |
| Participants | 35 patients undergoing cardiac surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, volume of shed mediastinal blood, blood loss | |
| Notes | Transfusion threshold: transfusion protocol was not reported. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised controlled trial was conducted to investigate the influence of processing both shed mediastinal blood and residual cardiopulmonary bypass (CPB) blood in patients undergoing isolated primary elective myocardial re-vascularisation. Patients were randomly allocated to intervention or control using sealed, opaque, sequentially numbered envelopes. The sequence of allocations was obtained from a computer-generated random number list. Clinicians in the Intensive Care Unit were blinded to the group | |
| Participants | 30 patients undergoing isolated primary elective myocardial re-vascularisation were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, plasma D-dimer levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method used to generate allocation sequences was adequate. |
| Allocation concealment (selection bias) | High risk | Method used to conceal treatment allocation was inadequate. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Single-blind. |
| Methods | Prospective, randomised controlled trial was conducted to determine the effectiveness of a post-operative autologous blood re-infusion system as an alternative to allogeneic, banked blood in patients undergoing unilateral total knee replacement between January 2002 to November 2004 | |
| Participants | 60 patients undergoing unilateral total knee replacement were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, amount of allogeneic blood transfused, volume of blood re-transfused from the cell saver, blood loss, Hb & Hct levels | |
| Notes | Transfusion threshold: the criteria for allogeneic blood transfusion post-operatively were the values of haemoglobin (lower than 9.0g/dL) in correlation with the clinical signs of the patient | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised controlled trial was conducted to investigate the effectiveness of pre-operative plateletpheresis combined with intra-operative autotransfusion on the blood coagulation of orthopaedic patients.[Chinese] | |
| Participants | 60 patients undergoing orthopaedic procedures were randomly allocated to one of three groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, blood loss. | |
| Notes | Transfusion threshold: uncertain. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was unclear. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Prospective, randomised controlled trial was conducted to determine whether the autotransfusion of shed mediastinal blood after open heart surgery is safe and effective. Method of randomisation and allocation concealment were not described | |
| Participants | 42 patients undergoing cardiac operations were randomised to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: amount of blood re-transfused from the cell saver, amount of allogeneic blood transfused, blood loss, Hb levels | |
| Notes | Transfusion threshold: transfusion protocol was not reported. English abstract only | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
| Methods | Randomised controlled trial was conducted to determine the safety and effectiveness of autotransfusion of shed mediastinal blood after open heart surgery. Between January 2000 and October 2000, patients undergoing primary elective coronary artery bypass graft (CABG) surgery were enrolled in this randomised controlled trial. Method of randomisation and allocation concealment were unclear. Participants were not blind to treatment allocation and blinding of the outcome assessor was unclear | |
| Participants | 60 patients undergoing elective primary coronary artery bypass graft surgery were randomly allocated to one of two groups:
|
|
| Interventions |
|
|
| Outcomes | Outcomes reported: number of patients transfused allogeneic blood, volume of allogeneic blood transfused, number of patients transfused autologous blood, volume of autologous blood transfused, blood loss | |
| Notes | Transfusion threshold: transfusion protocol for allogeneic blood transfusion was not reported | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate allocation sequences was not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to conceal treatment allocation was not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adan 1988 | Insufficient data. |
| Bartels 1996 | Compared two active interventions. No control group. |
| Bell 1992 | Insufficient data. |
| Breakwell 2000 | Insufficient data. |
| Dalrymple-Hay 2001 | Duplicate article. |
| Deramoudt 1991 | Insufficient data. |
| Elawad 1992 | Inappropriate control group. |
| Farrer 1997 | Duplicate article. |
| Jacobi 1997 | Insufficient data. |
| Kristensen 1992 | Insufficient data. |
| Mac 1993 | Insufficient data. |
| Mayer 1985 | Insufficient data. |
| McShane 1987 | Insufficient data. |
| Schaff 1978b | Duplicate article. |
| Schmidt 1997a | Duplicate article. |
| Schmidt 1997b | Duplicate article. |
| Skoura 1997 | Insufficient data. |
| Thompson 1990 | Insufficient data. |
| Trubel 1995 | Compared two active interventions. No control group. |
| Vertrees 1996 | Inappropriate control group. |
DATA AND ANALYSES
Comparison 1.
Cell salvage - blood transfused (all studies)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. exposed to allogeneic blood (All Studies) | 67 | 6025 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.55, 0.70] |
| 2 No. exposed to allogeneic blood (Transfusion Protocol) | 67 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Transfusion Protocol | 52 | 4755 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.54, 0.71] |
| 2.2 No Transfusion Protocol | 15 | 1270 | Risk Ratio (M-H, Random, 95% CI) | 0.58 [0.41, 0.82] |
| 3 No. exposed to allogeneic blood (Type of Surgery) | 67 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Cardiac | 31 | 2518 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.69, 0.86] |
| 3.2 Orthopaedic | 32 | 3240 | Risk Ratio (M-H, Random, 95% CI) | 0.46 [0.37, 0.57] |
| 3.3 Vascular | 4 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.34, 1.15] |
| 4 No. exposed to allogeneic blood - (Washed vs Unwashed) | 67 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Washed | 27 | 2225 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.51, 0.70] |
| 4.2 Unwashed | 40 | 3717 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.57, 0.77] |
| 5 No. exposed to allogeneic blood (Timing) | 66 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 Intra-operative cell salvage | 11 | 805 | Risk Ratio (M-H, Random, 95% CI) | 0.59 [0.46, 0.76] |
| 5.2 Post-operative cell salvage | 46 | 4361 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.54, 0.74] |
| 5.3 Intra & post-operative cell salvage | 9 | 737 | Risk Ratio (M-H, Random, 95% CI) | 0.70 [0.54, 0.92] |
| 6 Units of allogeneic blood transfused (All Studies) | 32 | 2321 | Mean Difference (IV, Random, 95% CI) | −0.68 [−0.88, −0.49] |
| 7 Units of allogeneic blood transfused (Transfusion Protocol) | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Transfusion Protocol | 27 | 1950 | Mean Difference (IV, Random, 95% CI) | −0.69 [−0.90, −0.49] |
| 7.2 No Transfusion Protocol | 5 | 371 | Mean Difference (IV, Random, 95% CI) | −0.64 [−1.30, 0.01] |
| 8 Units of allogeneic blood transfused (Type of Surgery) | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Cardiac | 19 | 1497 | Mean Difference (IV, Random, 95% CI) | −0.67 [−0.89, −0.44] |
| 8.2 Orthopaedic | 10 | 638 | Mean Difference (IV, Random, 95% CI) | −0.81 [−1.22, −0.39] |
| 8.3 Vascular | 3 | 186 | Mean Difference (IV, Random, 95% CI) | 0.02 [−0.34, 0.38] |
Comparison 2.
Cell salvage - blood transfused (washed versus unwashed)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. exposed to allogeneic blood (Cardiac) | 31 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Washed | 13 | 1158 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.55, 0.80] |
| 1.2 Unwashed | 18 | 1360 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 2 No. exposed to allogeneic blood (Orthopaedic) | 32 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Washed | 10 | 800 | Risk Ratio (M-H, Random, 95% CI) | 0.48 [0.36, 0.64] |
| 2.2 Unwashed | 22 | 2357 | Risk Ratio (M-H, Random, 95% CI) | 0.47 [0.36, 0.63] |
| 3 No. exposed to allogeneic blood (Vascular) | 4 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Washed | 4 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.34, 1.15] |
Comparison 3.
Cell salvage - blood transfused (active versus control)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. exposed to allogeneic blood (Active vs Control) | 43 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Cell Salvage vs Control | 43 | 3666 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.52, 0.71] |
| 2 No. exposed to allogeneic blood (Transfusion Protocol) | 43 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Transfusion Protocol | 34 | 3030 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.51, 0.73] |
| 2.2 No Transfusion Protocol | 9 | 636 | Risk Ratio (M-H, Random, 95% CI) | 0.56 [0.35, 0.89] |
| 3 No. exposed to allogeneic blood (Type of Surgery) | 43 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Cardiac | 18 | 1257 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.68, 0.91] |
| 3.2 Orthopaedic | 21 | 2142 | Risk Ratio (M-H, Random, 95% CI) | 0.45 [0.34, 0.60] |
| 3.3 Vascular | 4 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.34, 1.15] |
| 4 No. exposed to allogeneic blood (Washed vs Unwashed) | 42 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Washed | 18 | 1322 | Risk Ratio (M-H, Random, 95% CI) | 0.57 [0.45, 0.72] |
| 4.2 Unwashed | 24 | 2224 | Risk Ratio (M-H, Random, 95% CI) | 0.67 [0.56, 0.81] |
| 5 No. exposed to allogeneic blood (Timing) | 43 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 Intra-operative cell salvage | 8 | 564 | Risk Ratio (M-H, Random, 95% CI) | 0.64 [0.50, 0.83] |
| 5.2 Post-operative cell salvage | 29 | 2852 | Risk Ratio (M-H, Random, 95% CI) | 0.59 [0.48, 0.73] |
| 5.3 Intra & post-operative cell salvage | 6 | 250 | Risk Ratio (M-H, Random, 95% CI) | 0.58 [0.35, 0.95] |
| 6 Units of allogeneic blood transfused (Active vs Control) | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Cell Salvage vs Control | 23 | 1608 | Mean Difference (IV, Random, 95% CI) | −0.81 [−1.08, −0.54] |
| 7 Units of allogeneic blood transfused (Transfusion Protocol) | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Transfusion Protocol | 19 | 1339 | Mean Difference (IV, Random, 95% CI) | −0.86 [−1.17, −0.55] |
| 7.2 No Transfusion Protocol | 4 | 269 | Mean Difference (IV, Random, 95% CI) | −0.68 [−1.63, 0.27] |
| 8 Units of allogeneic blood transfused (Type of Surgery) | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Cardiac | 13 | 995 | Mean Difference (IV, Random, 95% CI) | −0.93 [−1.27, −0.59] |
| 8.2 Orthopaedic | 7 | 427 | Mean Difference (IV, Random, 95% CI) | −0.82 [−1.36, −0.27] |
| 8.3 Vascular | 3 | 186 | Mean Difference (IV, Random, 95% CI) | 0.02 [−0.34, 0.38] |
Comparison 4.
Cell salvage - blood transfused (active versus active)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. exposed to allogeneic blood (Active vs Active) | 25 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Cell Salvage (Active vs Active) | 25 | 2402 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.53, 0.76] |
| 2 No. exposed to allogeneic blood (Transfusion Protocol) | 25 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Transfusion Protocol | 19 | 1768 | Risk Ratio (M-H, Random, 95% CI) | 0.64 [0.52, 0.77] |
| 2.2 No Transfusion Protocol | 6 | 634 | Risk Ratio (M-H, Random, 95% CI) | 0.59 [0.35, 1.00] |
| 3 No. exposed to allogeneic blood (Type of Surgery) | 25 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Cardiac | 14 | 1304 | Risk Ratio (M-H, Random, 95% CI) | 0.73 [0.61, 0.87] |
| 3.2 Orthopaedic | 11 | 1098 | Risk Ratio (M-H, Random, 95% CI) | 0.46 [0.33, 0.65] |
| 4 No. exposed to allogeneic blood (Washed vs Unwashed) | 26 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Washed | 9 | 897 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.48, 0.78] |
| 4.2 Unwashed | 17 | 1543 | Risk Ratio (M-H, Random, 95% CI) | 0.68 [0.52, 0.89] |
| 5 No. exposed to allogeneic blood (Timing of cell salvage) | 26 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 Intra-operative cell salvage | 4 | 281 | Risk Ratio (M-H, Random, 95% CI) | 0.54 [0.35, 0.84] |
| 5.2 Post-operative cell salvage | 18 | 1629 | Risk Ratio (M-H, Random, 95% CI) | 0.65 [0.50, 0.85] |
| 5.3 Intra & post-operative cell salvage | 4 | 530 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.59, 0.98] |
| 6 Units of allogeneic blood transfused (Active vs Active) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Cell Salvage (Active vs Active) | 8 | 663 | Mean Difference (IV, Random, 95% CI) | −0.66 [−1.02, −0.30] |
| 7 Units of allogeneic blood transfused (Transfusion Protocol) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Transfusion Protocol | 7 | 561 | Mean Difference (IV, Random, 95% CI) | −0.67 [−1.09, −0.25] |
| 7.2 No Transfusion Protocol | 1 | 102 | Mean Difference (IV, Random, 95% CI) | −0.69 [−1.20, −0.18] |
| 8 Units allogeneic blood transfused (Type of Surgery) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Cardiac | 6 | 501 | Mean Difference (IV, Random, 95% CI) | −0.39 [−0.67, −0.12] |
| 8.2 Orthopaedic | 2 | 162 | Mean Difference (IV, Random, 95% CI) | −1.10 [−1.91, −0.29] |
Comparison 5.
Cell salvage - blood loss
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total blood loss (All Studies) | 32 | 2311 | Mean Difference (IV, Random, 95% CI) | −39.02 [−85.10, 7.05] |
| 2 Total blood loss (Active vs Control) | 24 | 1570 | Mean Difference (IV, Random, 95% CI) | −38.98 [−99.91, 21.95] |
| 3 Total blood loss (Active vs Active) | 8 | 741 | Mean Difference (IV, Random, 95% CI) | −48.32 [−116.38, 19.74] |
Comparison 6.
Cell salvage - blood transfused (language and methodological quality)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Language of Publication (All Studies) | 66 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 English | 60 | 5711 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.54, 0.70] |
| 1.2 Non-English | 6 | 274 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.50, 0.79] |
| 2 Methodological Quality - Allocation concealment (All Studies) | 67 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Allocation concealment - Yes | 1 | 47 | Risk Ratio (M-H, Random, 95% CI) | 0.35 [0.04, 3.11] |
| 2.2 Allocation concealment - Unclear | 42 | 3812 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.53, 0.72] |
| 2.3 Allocation concealment - No | 24 | 2166 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.51, 0.75] |
Comparison 7.
Adverse events and other outcomes
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality - All Studies | 22 | 1788 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.49, 1.88] |
| 2 Mortality - Active vs Control | 16 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Active vs Control | 16 | 1132 | Risk Ratio (M-H, Random, 95% CI) | 1.41 [0.66, 3.05] |
| 3 Mortality - Active vs Active | 6 | 656 | Risk Ratio (M-H, Random, 95% CI) | 0.27 [0.07, 1.08] |
| 4 Re-operation for bleeding - All Studies | 19 | 1683 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.53, 1.53] |
| 5 Re-operation for bleeding - Active vs Control | 10 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 Active vs Control | 10 | 688 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.45, 2.24] |
| 6 Re-operation for bleeding - Active vs Active | 9 | 995 | Risk Ratio (M-H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 7 Any infection - All Studies | 23 | 2892 | Risk Ratio (M-H, Random, 95% CI) | 0.68 [0.46, 0.99] |
| 8 Any infection - Active vs Control | 16 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 8.1 Active vs Control | 16 | 1860 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.41, 0.96] |
| 9 Any Infection - Active vs Active | 7 | 1032 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.37, 2.07] |
| 10 Wound complication - All Studies | 16 | 1962 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.57, 1.55] |
| 11 Wound complication - Active vs Control | 12 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 11.1 Active vs Control | 12 | 1464 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.49, 1.57] |
| 12 Wound complication - Active vs Active | 4 | 498 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.43, 2.99] |
| 13 Any thrombosis - All Studies | 11 | 925 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.48, 2.66] |
| 14 Any thrombosis - Active vs Control | 10 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 14.1 Active vs Control | 10 | 807 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.48, 2.66] |
| 15 Stroke - All Studies | 7 | 696 | Risk Ratio (M-H, Random, 95% CI) | 0.64 [0.21, 1.98] |
| 16 Stroke - Active vs Control | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 16.1 Active vs Control | 5 | 439 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.20, 3.21] |
| 17 Stroke - Active vs Active | 2 | 257 | Risk Ratio (M-H, Random, 95% CI) | 0.43 [0.06, 2.90] |
| 18 Non-fatal myocardial infarction - All Studies | 11 | 951 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.43, 1.46] |
| 19 Non-fatal myocardial infarction - Active vs Control | 6 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 19.1 Active vs Control | 6 | 509 | Risk Ratio (M-H, Random, 95% CI) | 0.65 [0.32, 1.31] |
| 20 Non-fatal myocardial infarction - Active vs Active | 5 | 442 | Risk Ratio (M-H, Random, 95% CI) | 1.46 [0.43, 4.88] |
| 21 Deep vein thrombosis (DVT) | 7 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 21.1 Active vs Control | 7 | 645 | Risk Ratio (M-H, Random, 95% CI) | 0.83 [0.31, 2.23] |
| 22 Hospital length of stay (LOS) - Active vs Control | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Active vs Control | 10 | 772 | Mean Difference (IV, Random, 95% CI) | −1.38 [−2.79, 0.03] |
| 23 Hospital length of stay (LOS) - Active vs Active | 1 | 196 | Mean Difference (IV, Random, 95% CI) | 2.8 [−2.11, 7.71] |
| 23.1 Active vs Active | 1 | 196 | Mean Difference (IV, Random, 95% CI) | 2.8 [−2.11, 7.71] |
Analysis 1.1. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 1 No. exposed to allogeneic blood (All Studies)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 1 No. exposed to allogeneic blood (All Studies)
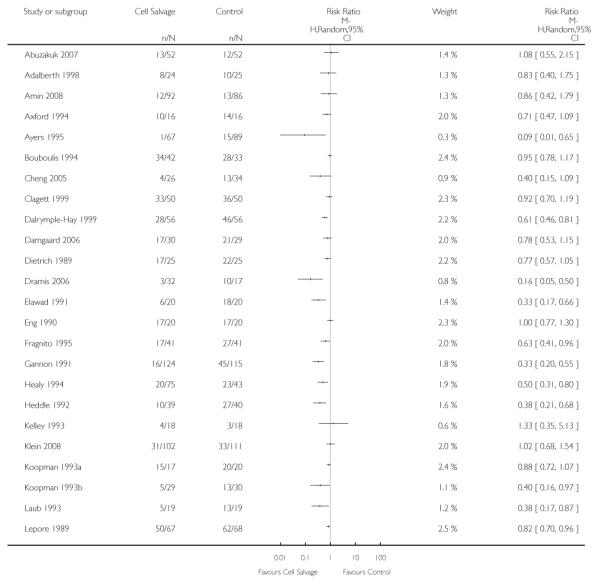 |
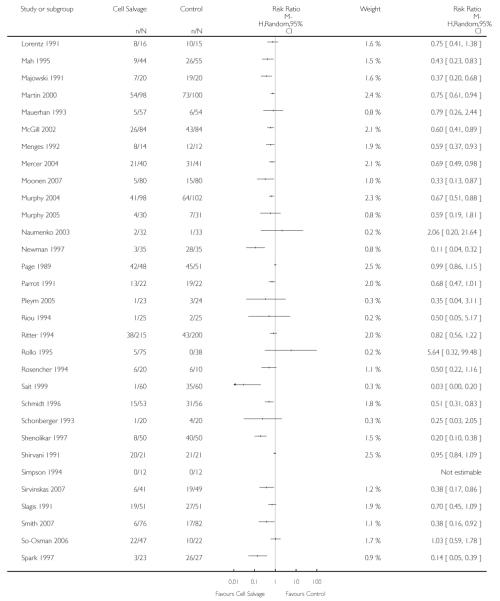 |
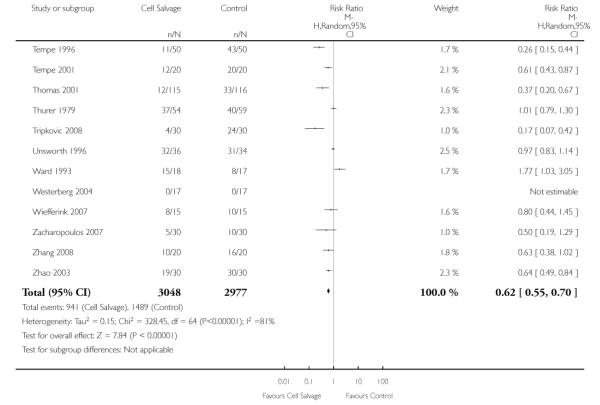 |
Analysis 1.2. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 2 No. exposed to allogeneic blood (Transfusion Protocol)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 2 No. exposed to allogeneic blood (Transfusion Protocol)
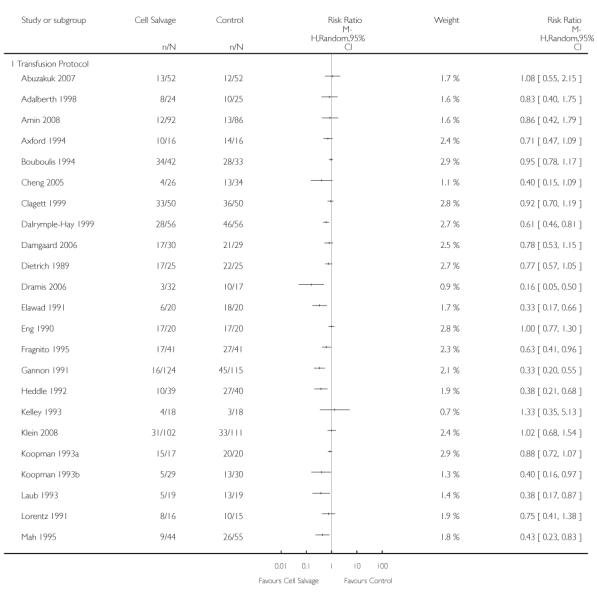 |
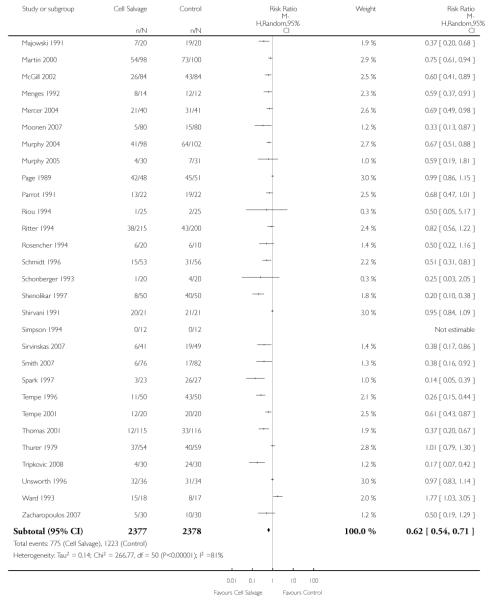 |
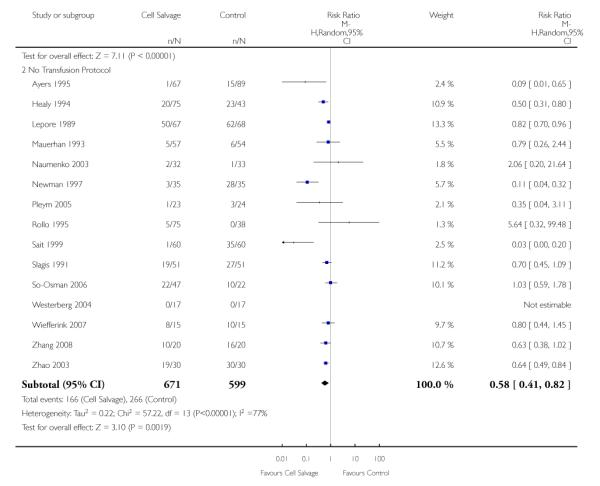 |
Analysis 1.3. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 3 No. exposed to allogeneic blood (Type of Surgery)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 3 No. exposed to allogeneic blood (Type of Surgery)
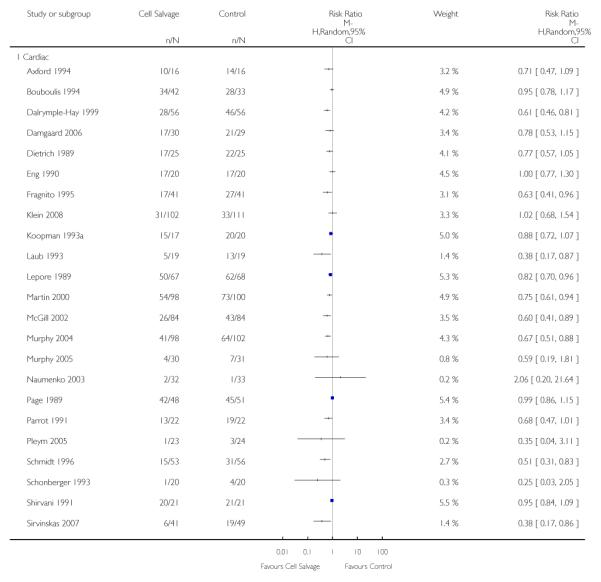 |
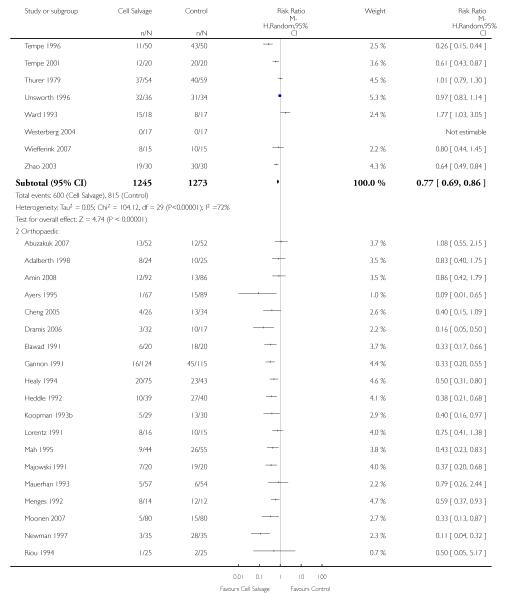 |
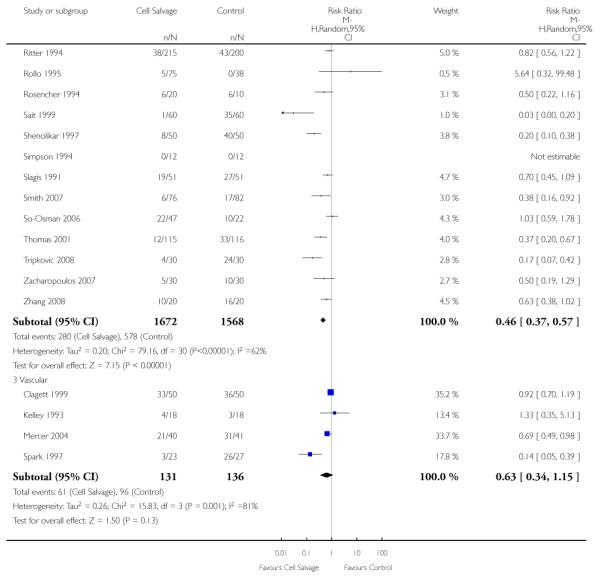 |
Analysis 1.4. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 4 No. exposed to allogeneic blood - (Washed vs Unwashed)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 4 No. exposed to allogeneic blood - (Washed vs Unwashed)
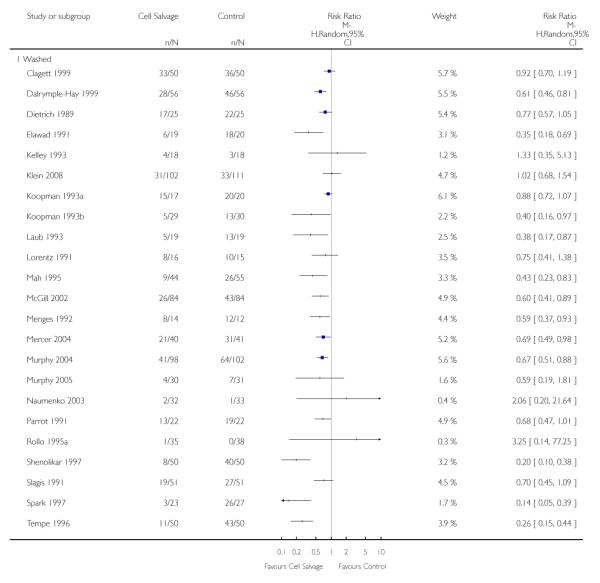 |
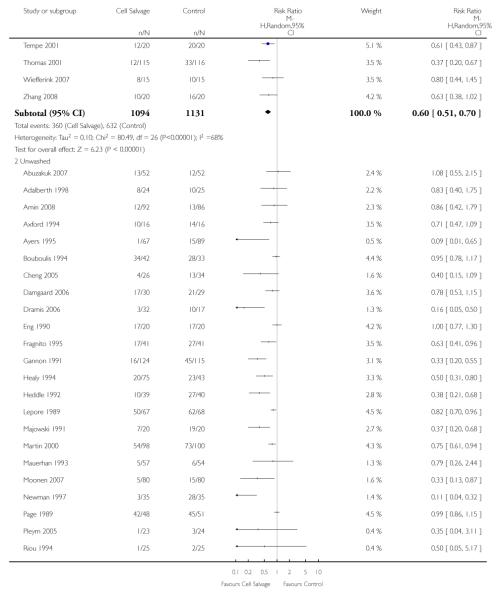 |
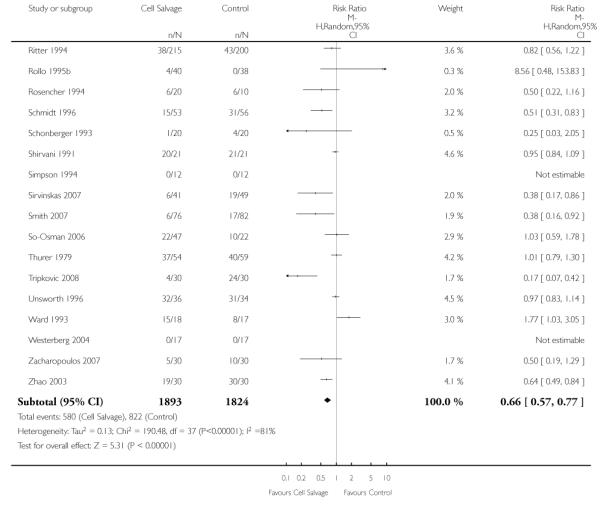 |
Analysis 1.5. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 5 No. exposed to allogeneic blood (Timing)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 5 No. exposed to allogeneic blood (Timing)
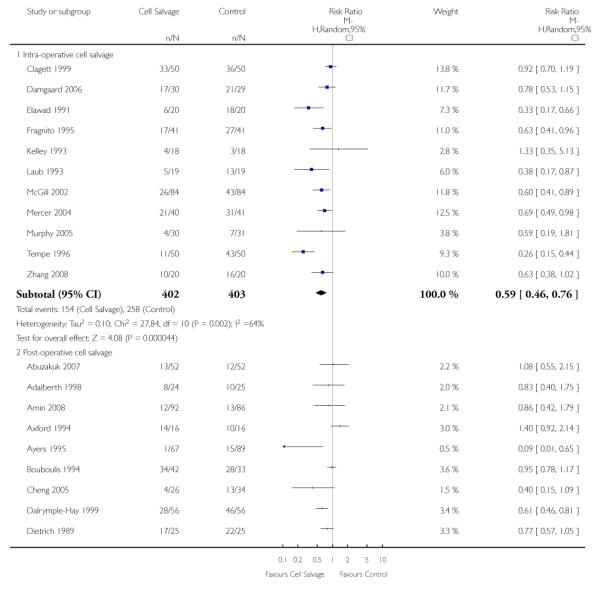 |
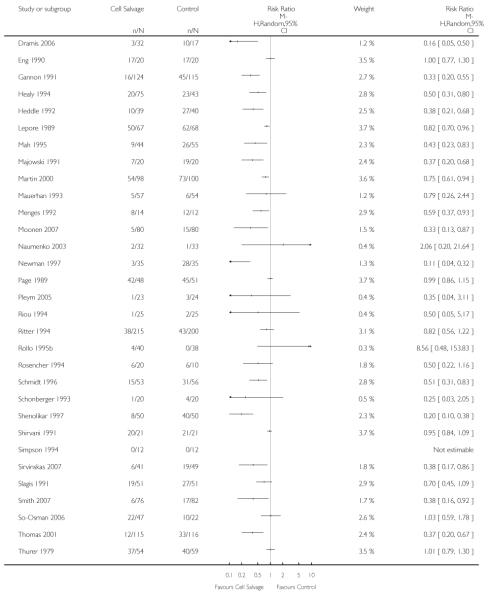 |
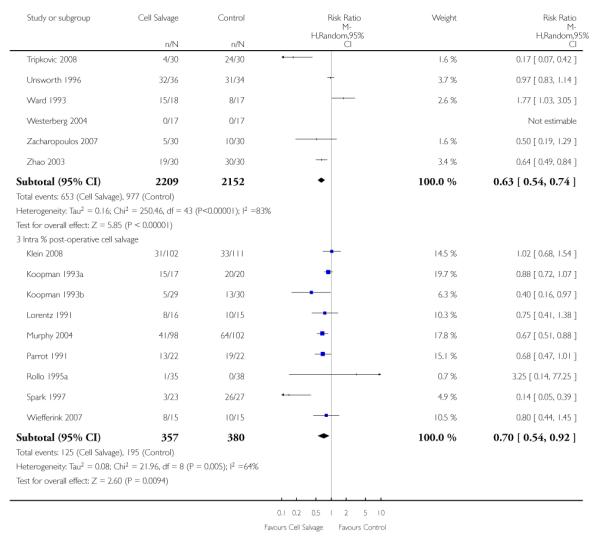 |
Analysis 1.6. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 6 Units of allogeneic blood transfused (All Studies)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 6 Units of allogeneic blood transfused (All Studies)
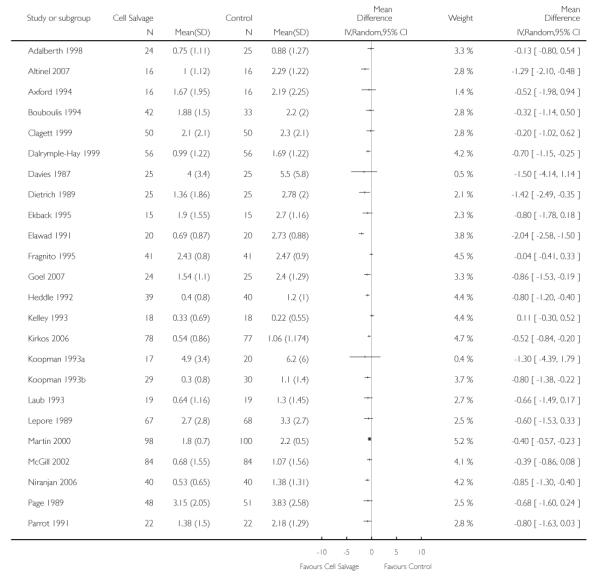 |
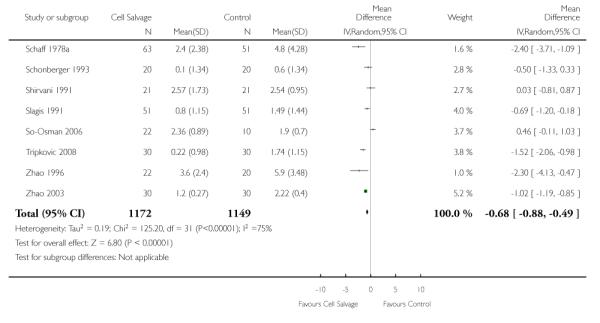 |
Analysis 1.7. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 7 Units of allogeneic blood transfused (Transfusion Protocol)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 7 Units of allogeneic blood transfused (Transfusion Protocol)
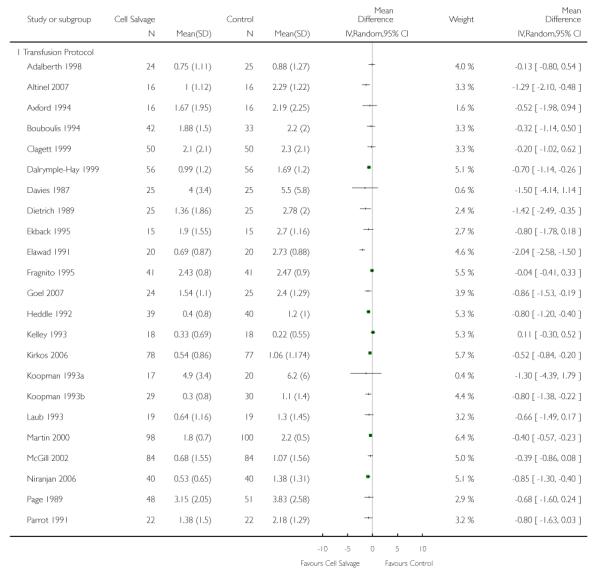 |
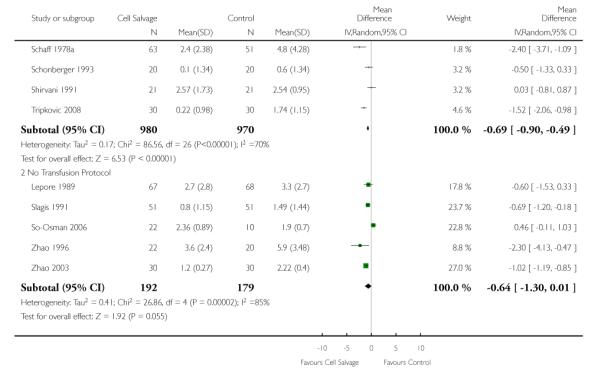 |
Analysis 1.8. Comparison 1 Cell salvage - blood transfused (all studies), Outcome 8 Units of allogeneic blood transfused (Type of Surgery)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 1 Cell salvage - blood transfused (all studies)
Outcome: 8 Units of allogeneic blood transfused (Type of Surgery)
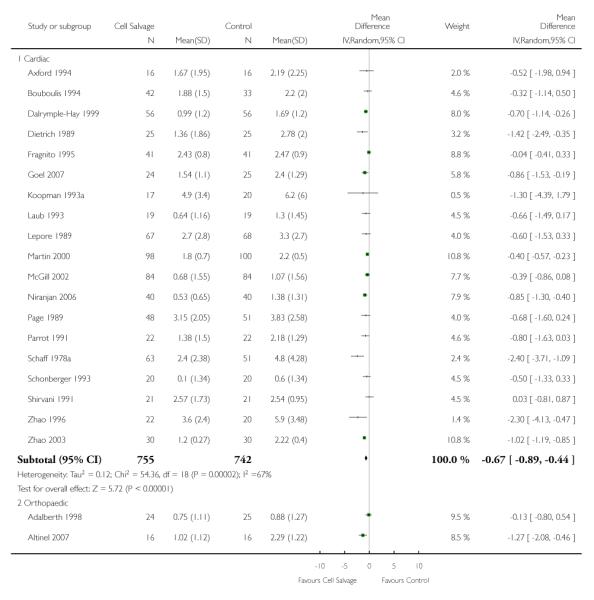 |
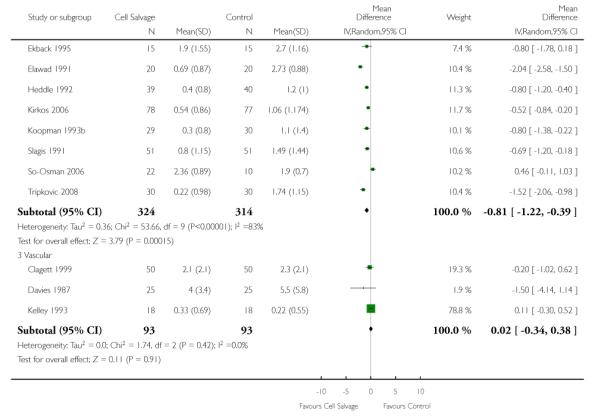 |
Analysis 2.1. Comparison 2 Cell salvage - blood transfused (washed versus unwashed), Outcome 1 No. exposed to allogeneic blood (Cardiac)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 2 Cell salvage - blood transfused (washed versus unwashed)
Outcome: 1 No. exposed to allogeneic blood (Cardiac)
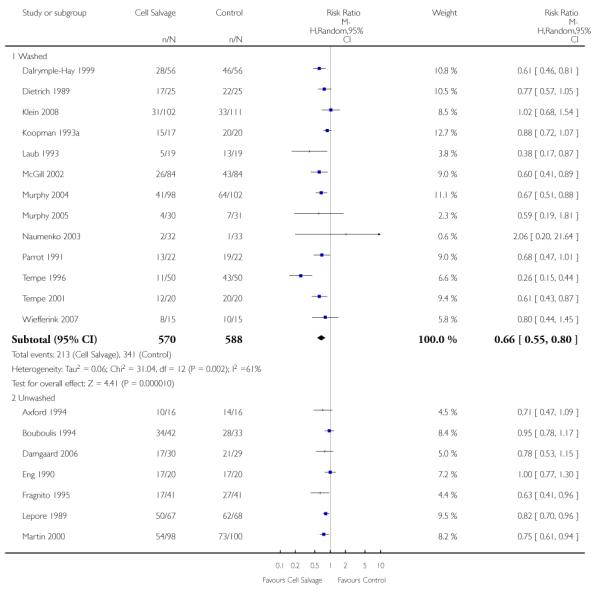 |
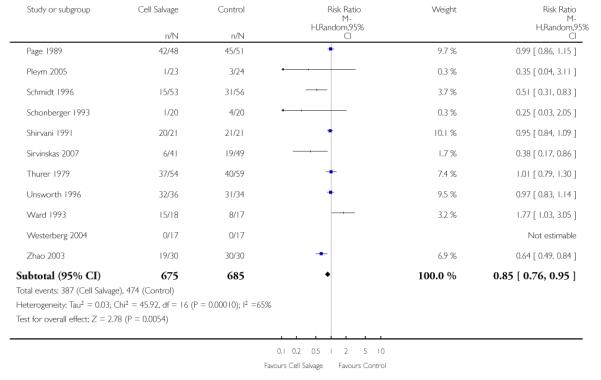 |
Analysis 2.2. Comparison 2 Cell salvage - blood transfused (washed versus unwashed), Outcome 2 No. exposed to allogeneic blood (Orthopaedic)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 2 Cell salvage - blood transfused (washed versus unwashed)
Outcome: 2 No. exposed to allogeneic blood (Orthopaedic)
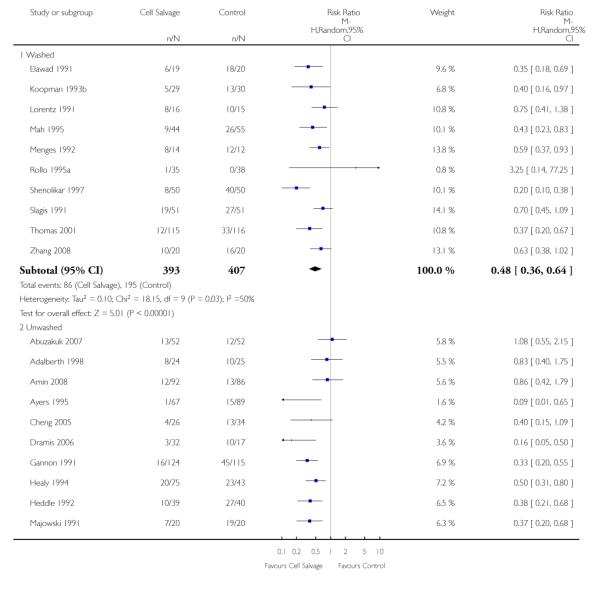 |
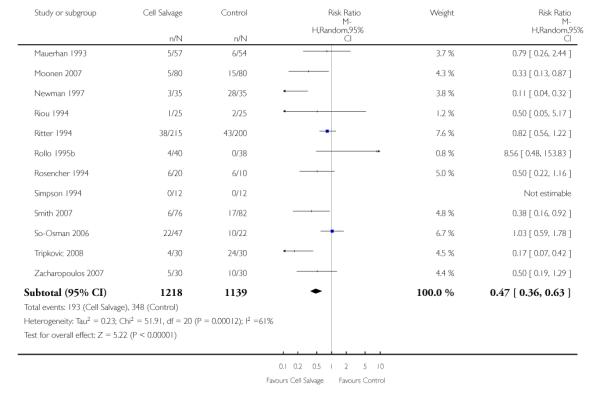 |
Analysis 2.3. Comparison 2 Cell salvage - blood transfused (washed versus unwashed), Outcome 3 No. exposed to allogeneic blood (Vascular)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 2 Cell salvage - blood transfused (washed versus unwashed)
Outcome: 3 No. exposed to allogeneic blood (Vascular)
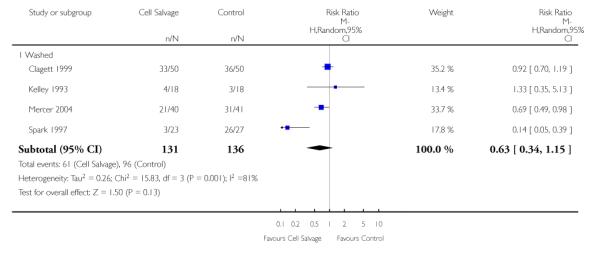 |
Analysis 3.1. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 1 No. exposed to allogeneic blood (Active vs Control)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 1 No. exposed to allogeneic blood (Active vs Control)
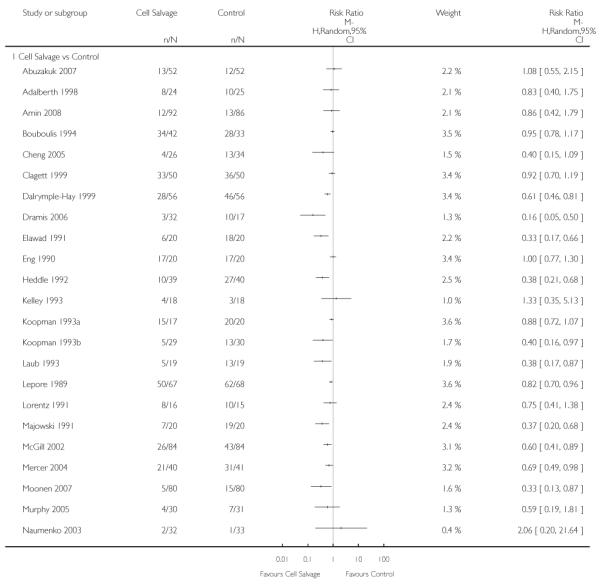 |
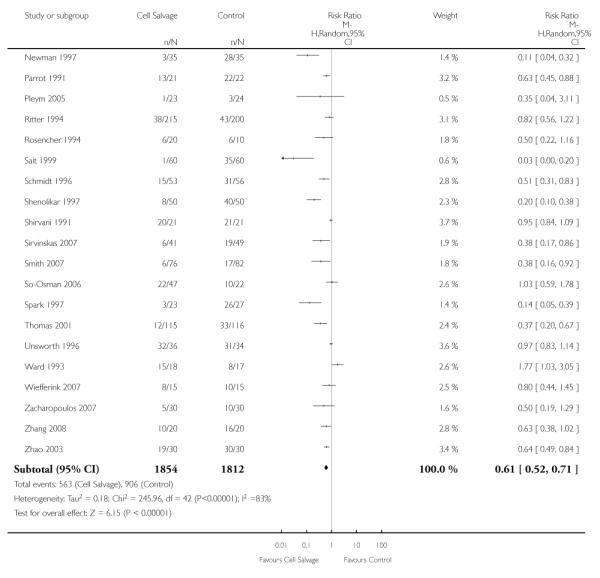 |
Analysis 3.2. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 2 No. exposed to allogeneic blood (Transfusion Protocol)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 2 No. exposed to allogeneic blood (Transfusion Protocol)
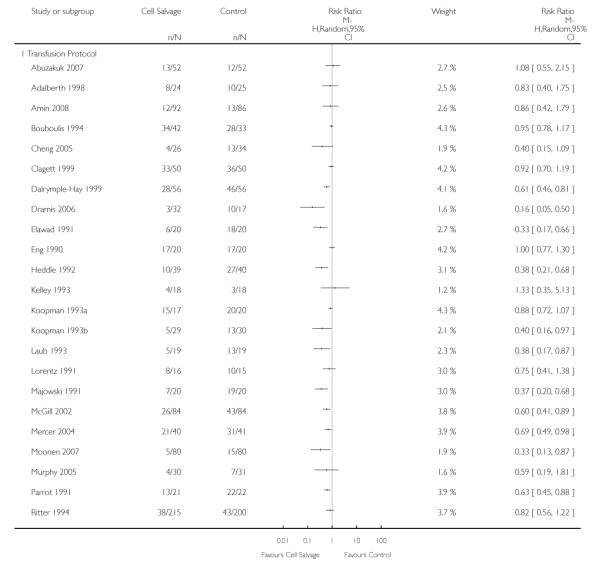 |
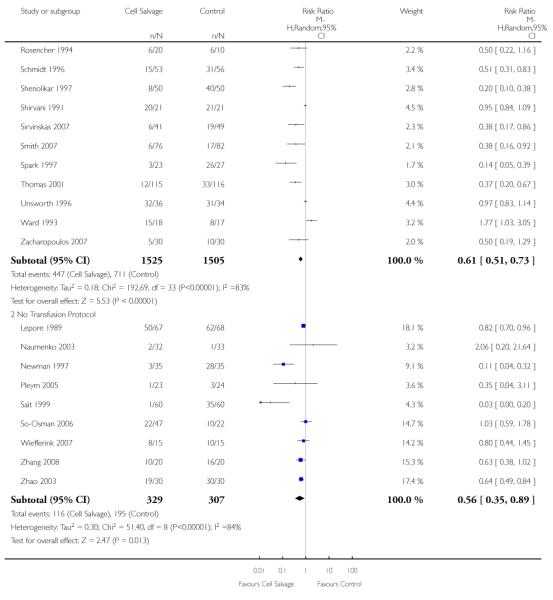 |
Analysis 3.3. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 3 No. exposed to allogeneic blood (Type of Surgery)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 3 No. exposed to allogeneic blood (Type of Surgery)
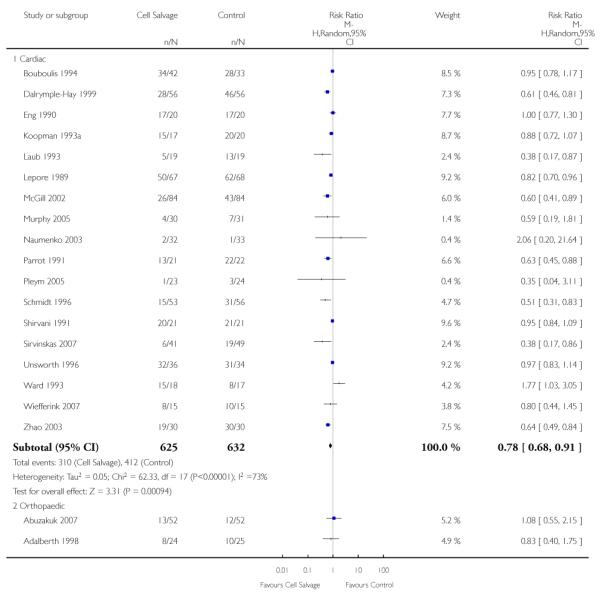 |
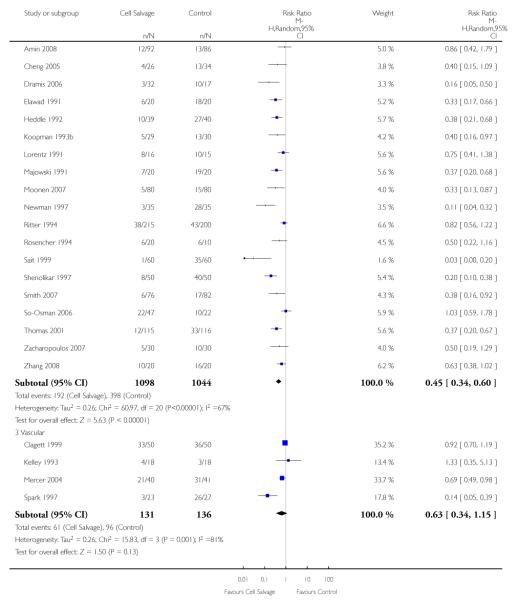 |
Analysis 3.4. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 4 No. exposed to allogeneic blood (Washed vs Unwashed)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 4 No. exposed to allogeneic blood (Washed vs Unwashed)
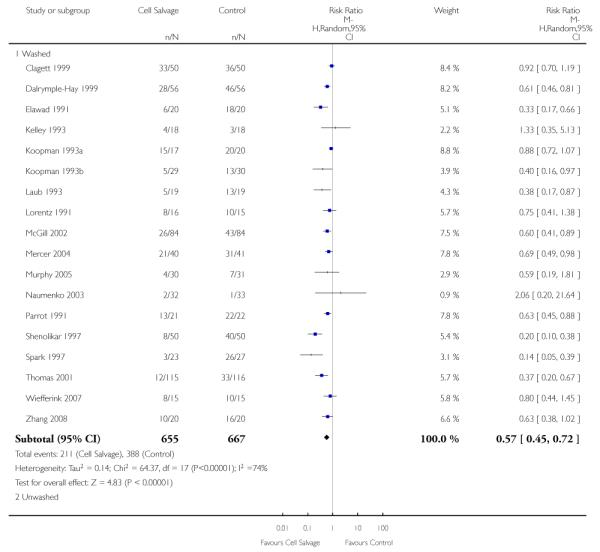 |
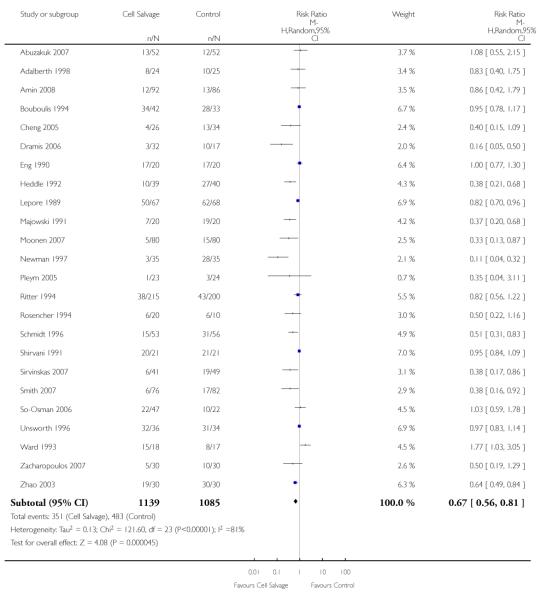 |
Analysis 3.5. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 5 No. exposed to allogeneic blood (Timing)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 5 No. exposed to allogeneic blood (Timing)
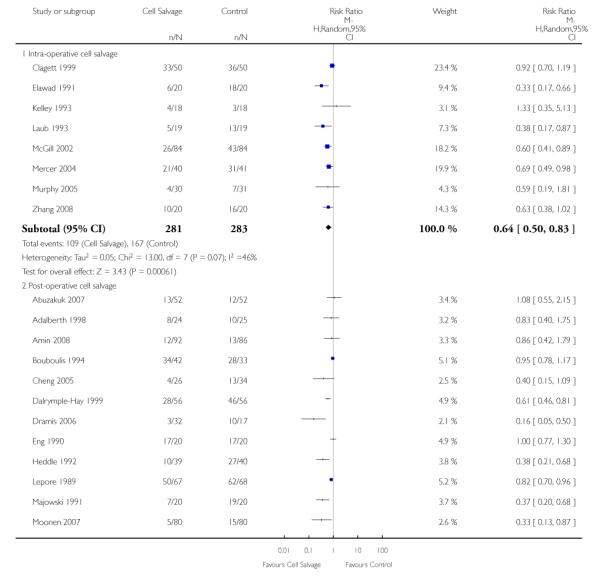 |
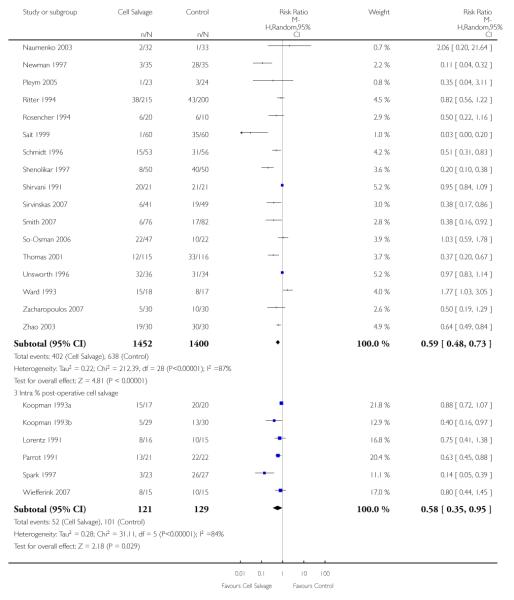 |
Analysis 3.6. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 6 Units of allogeneic blood transfused (Active vs Control)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 6 Units of allogeneic blood transfused (Active vs Control)
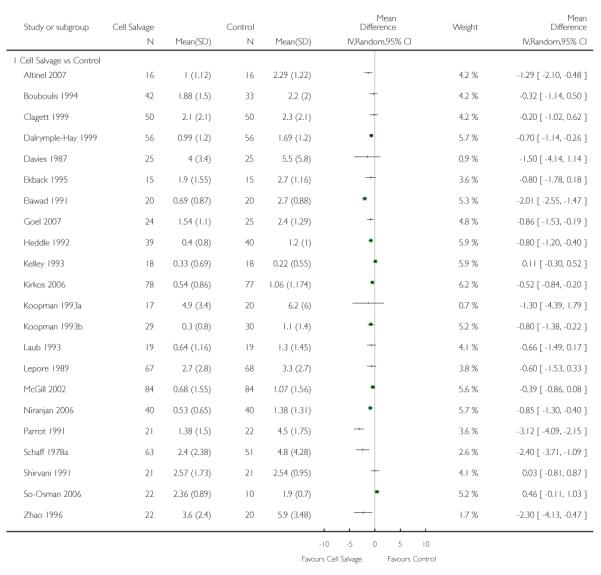 |
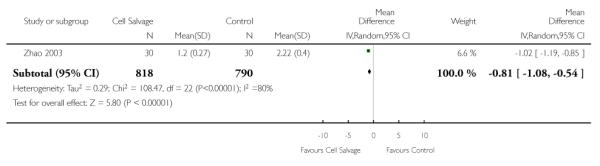 |
Analysis 3.7. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 7 Units of allogeneic blood transfused (Transfusion Protocol)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 7 Units of allogeneic blood transfused (Transfusion Protocol)
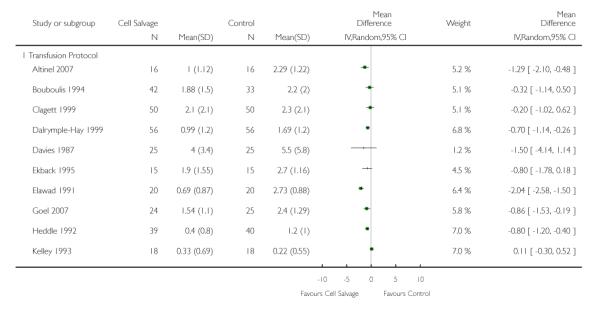 |
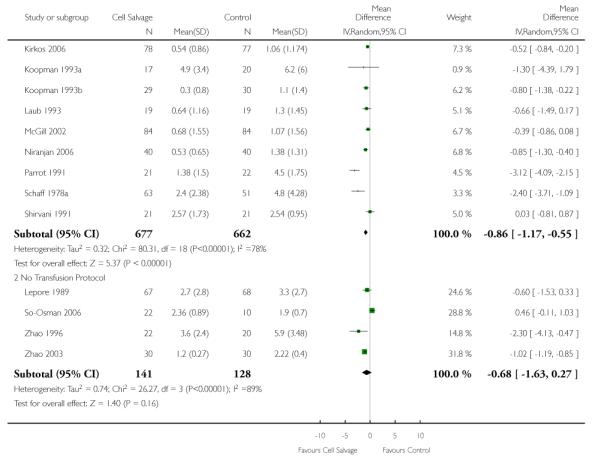 |
Analysis 3.8. Comparison 3 Cell salvage - blood transfused (active versus control), Outcome 8 Units of allogeneic blood transfused (Type of Surgery)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 3 Cell salvage - blood transfused (active versus control)
Outcome: 8 Units of allogeneic blood transfused (Type of Surgery)
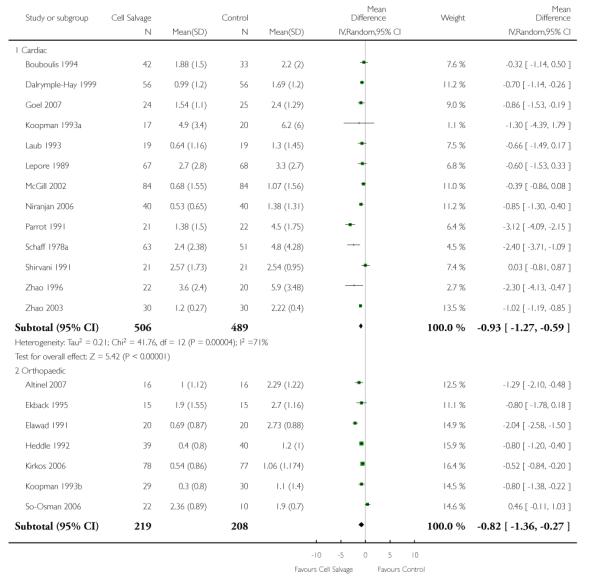 |
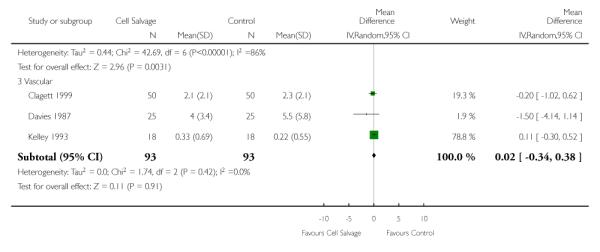 |
Analysis 4.1. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 1 No. exposed to allogeneic blood (Active vs Active)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 1 No. exposed to allogeneic blood (Active vs Active)
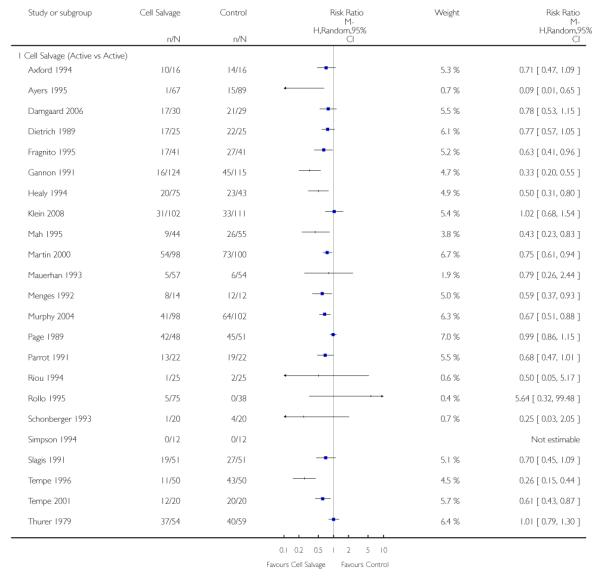 |
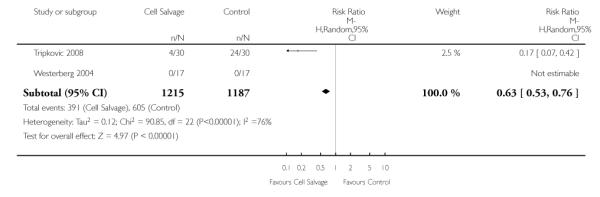 |
Analysis 4.2. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 2 No. exposed to allogeneic blood (Transfusion Protocol)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 2 No. exposed to allogeneic blood (Transfusion Protocol)
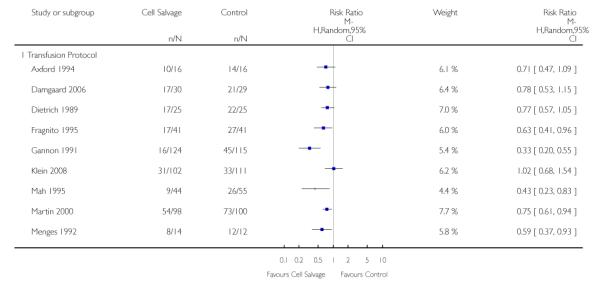 |
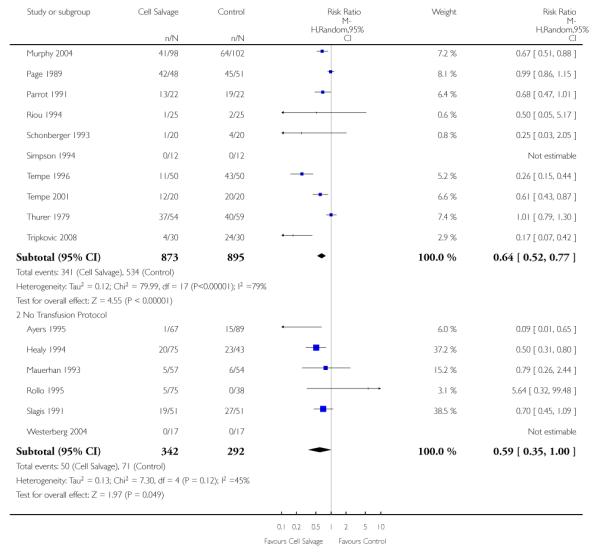 |
Analysis 4.3. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 3 No. exposed to allogeneic blood (Type of Surgery)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 3 No. exposed to allogeneic blood (Type of Surgery)
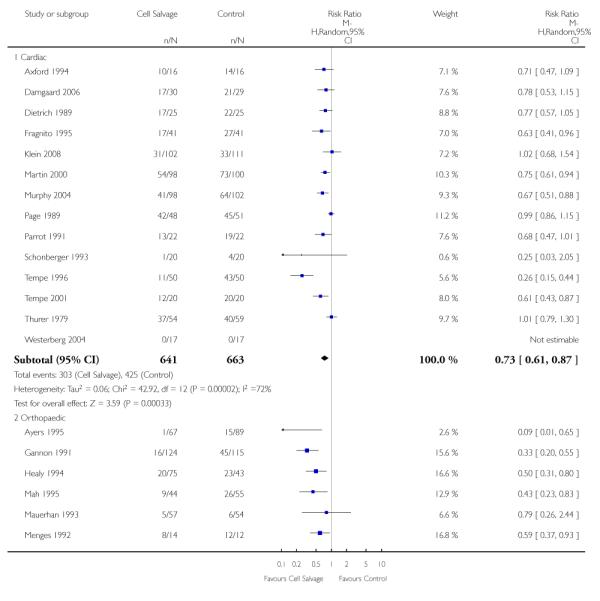 |
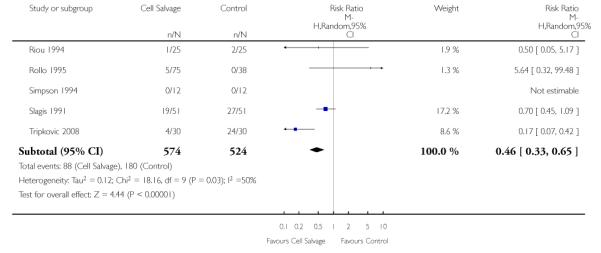 |
Analysis 4.4. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 4 No. exposed to allogeneic blood (Washed vs Unwashed)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 4 No. exposed to allogeneic blood (Washed vs Unwashed)
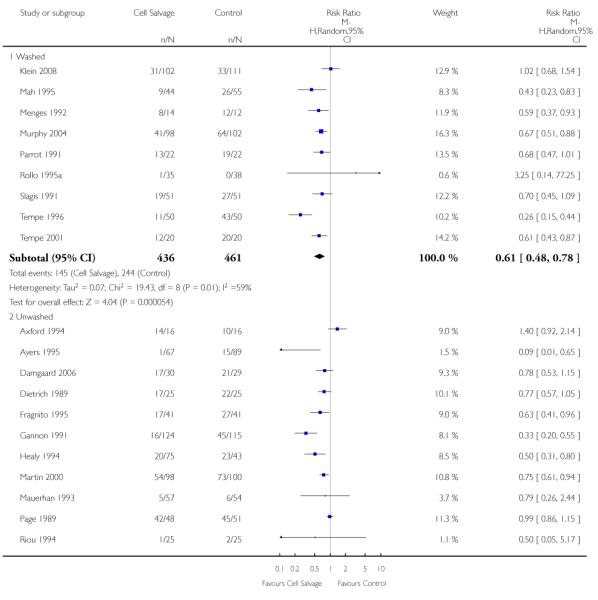 |
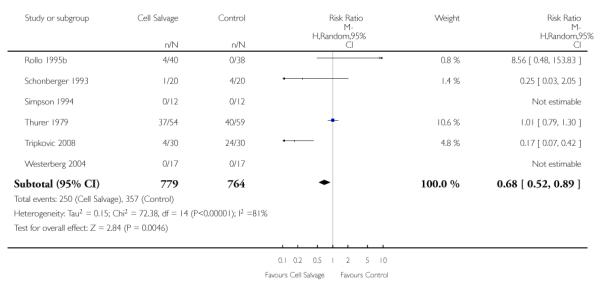 |
Analysis 4.5. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 5 No. exposed to allogeneic blood (Timing of cell salvage)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 5 No. exposed to allogeneic blood (Timing of cell salvage)
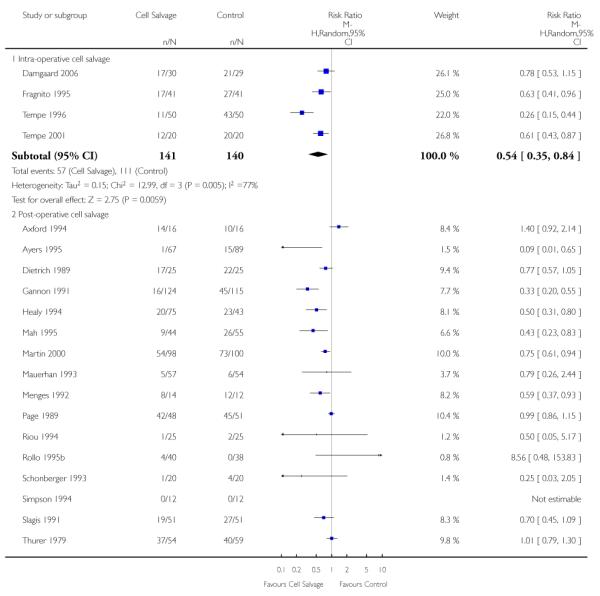 |
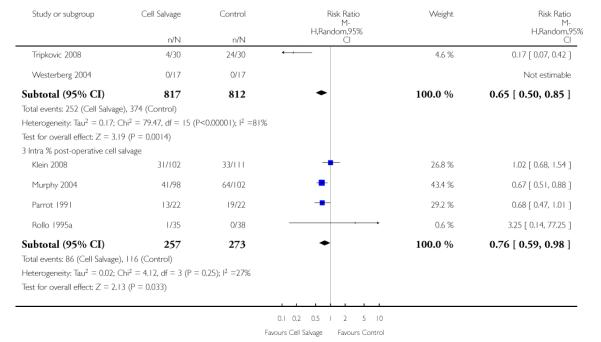 |
Analysis 4.6. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 6 Units of allogeneic blood transfused (Active vs Active)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 6 Units of allogeneic blood transfused (Active vs Active)
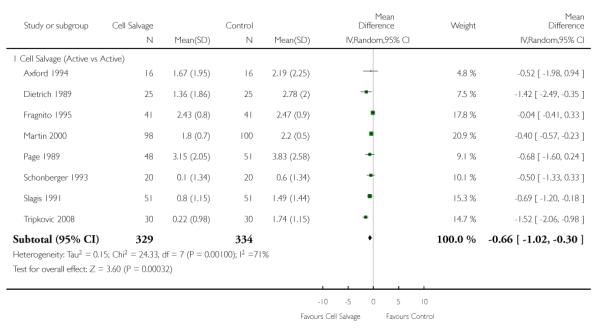 |
Analysis 4.7. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 7 Units of allogeneic blood transfused (Transfusion Protocol)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 7 Units of allogeneic blood transfused (Transfusion Protocol)
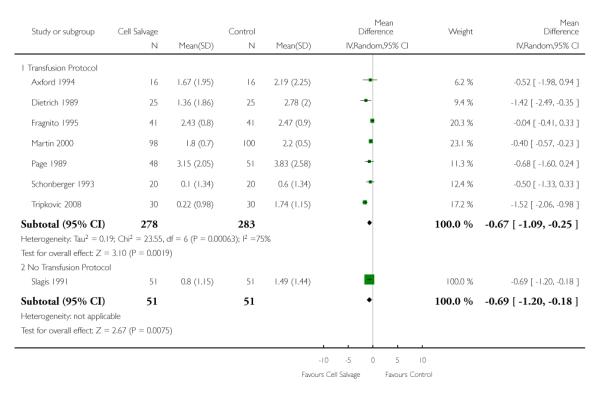 |
Analysis 4.8. Comparison 4 Cell salvage - blood transfused (active versus active), Outcome 8 Units allogeneic blood transfused (Type of Surgery)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 4 Cell salvage - blood transfused (active versus active)
Outcome: 8 Units allogeneic blood transfused (Type of Surgery)
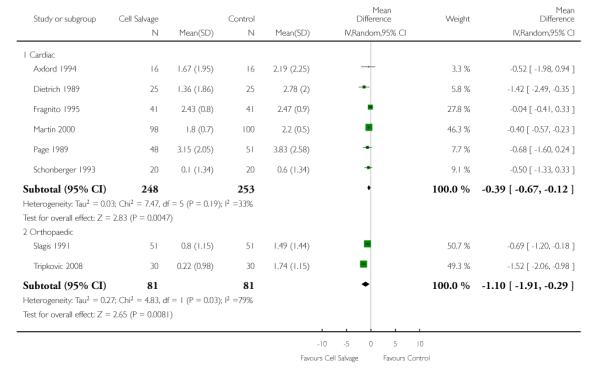 |
Analysis 5.1. Comparison 5 Cell salvage - blood loss, Outcome 1 Total blood loss (All Studies)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 5 Cell salvage - blood loss
Outcome: 1 Total blood loss (All Studies)
 |
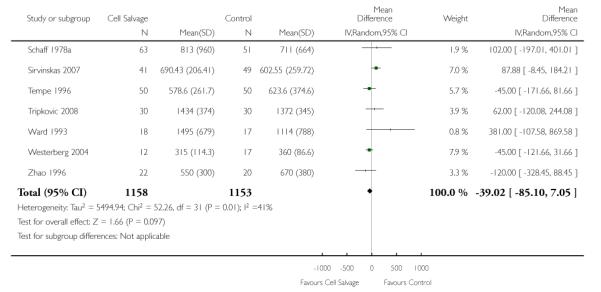 |
Analysis 5.2. Comparison 5 Cell salvage - blood loss, Outcome 2 Total blood loss (Active vs Control)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 5 Cell salvage - blood loss
Outcome: 2 Total blood loss (Active vs Control)
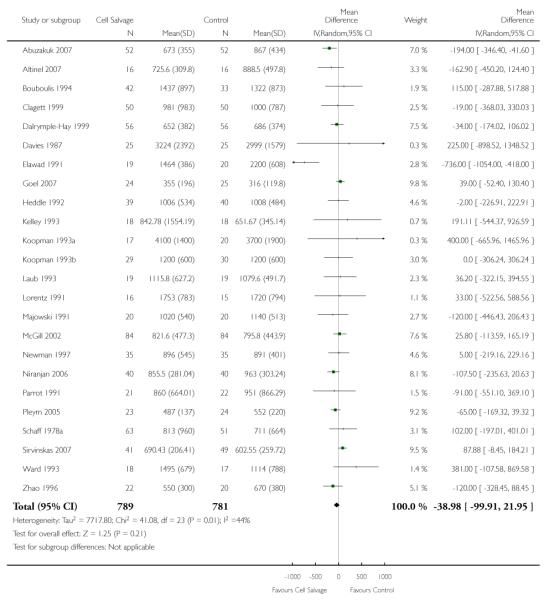 |
Analysis 5.3. Comparison 5 Cell salvage - blood loss, Outcome 3 Total blood loss (Active vs Active)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 5 Cell salvage - blood loss
Outcome: 3 Total blood loss (Active vs Active)
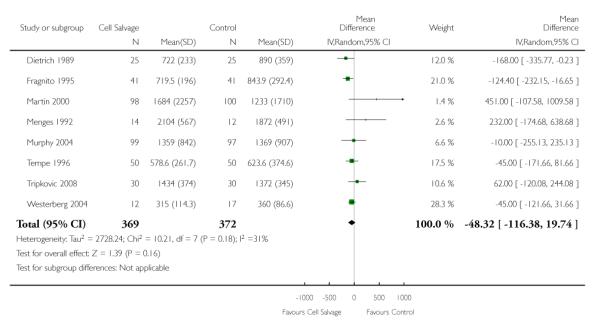 |
Analysis 6.1. Comparison 6 Cell salvage - blood transfused (language and methodological quality), Outcome 1 Language of Publication (All Studies)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 6 Cell salvage - blood transfused (language and methodological quality)
Outcome: 1 Language of Publication (All Studies)
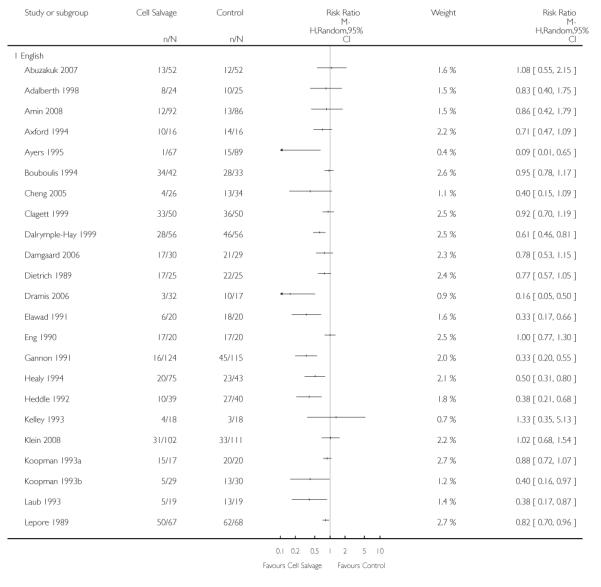 |
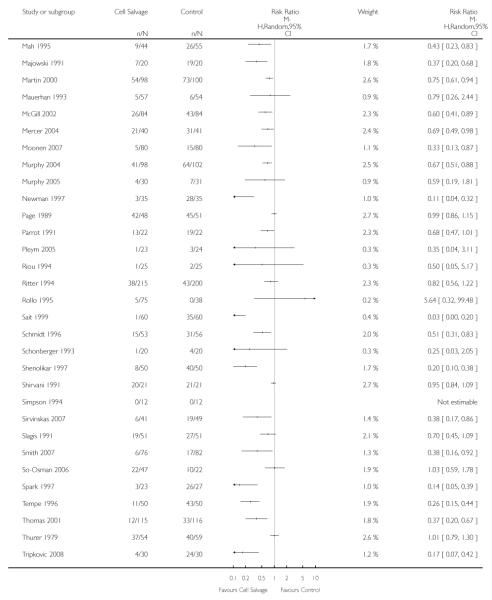 |
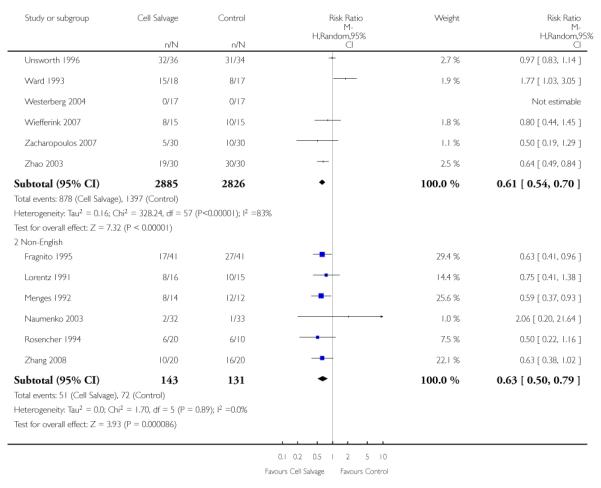 |
Analysis 6.2. Comparison 6 Cell salvage - blood transfused (language and methodological quality), Outcome 2 Methodological Quality - Allocation concealment (All Studies)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 6 Cell salvage - blood transfused (language and methodological quality)
Outcome: 2 Methodological Quality - Allocation concealment (All Studies)
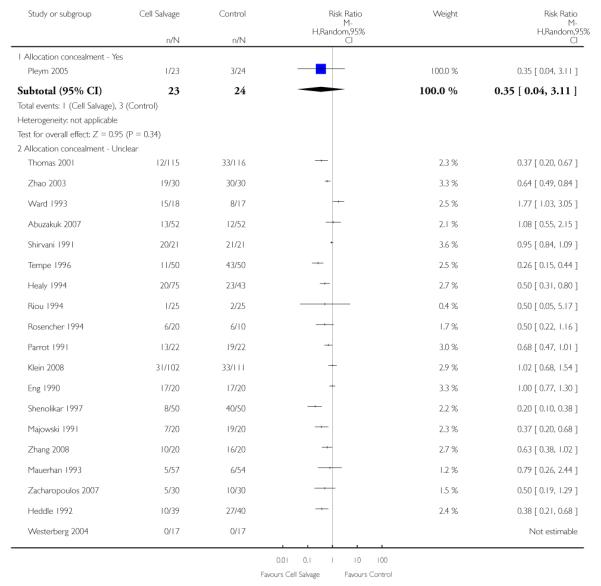 |
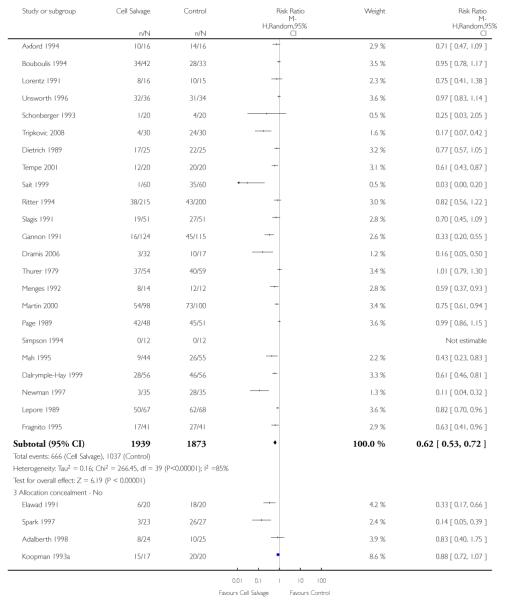 |
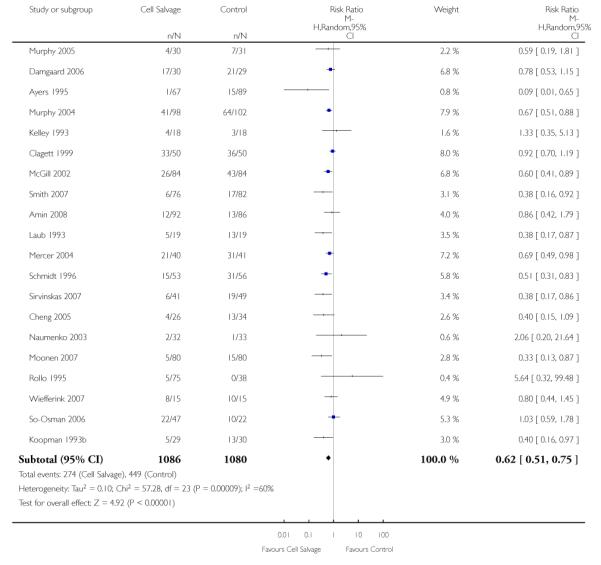 |
Analysis 7.1. Comparison 7 Adverse events and other outcomes, Outcome 1 Mortality - All Studies
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 1 Mortality - All Studies
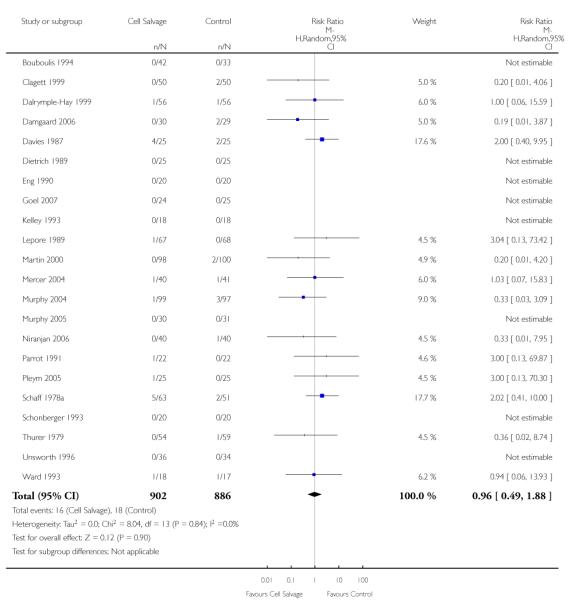 |
Analysis 7.2. Comparison 7 Adverse events and other outcomes, Outcome 2 Mortality - Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 2 Mortality - Active vs Control
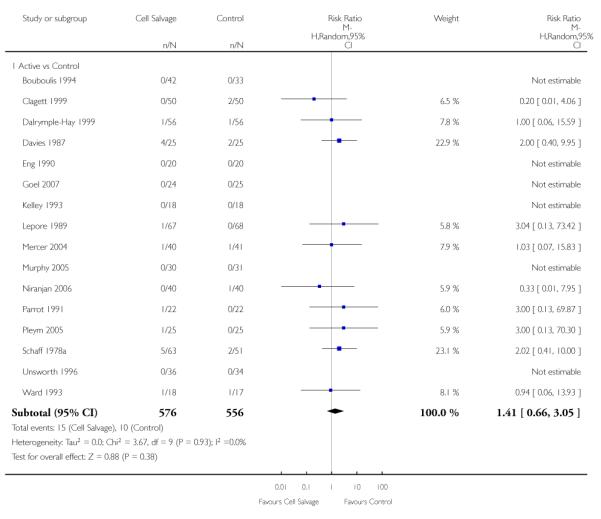 |
Analysis 7.3. Comparison 7 Adverse events and other outcomes, Outcome 3 Mortality - Active vs Active
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 3 Mortality - Active vs Active
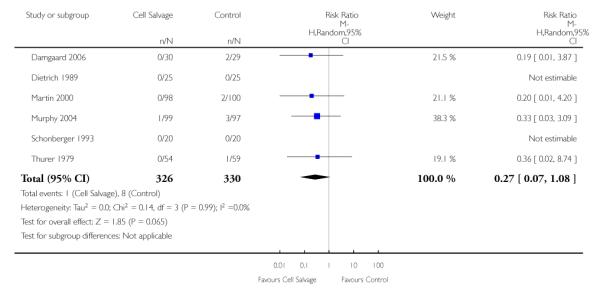 |
Analysis 7.4. Comparison 7 Adverse events and other outcomes, Outcome 4 Re-operation for bleeding - All Studies
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 4 Re-operation for bleeding - All Studies
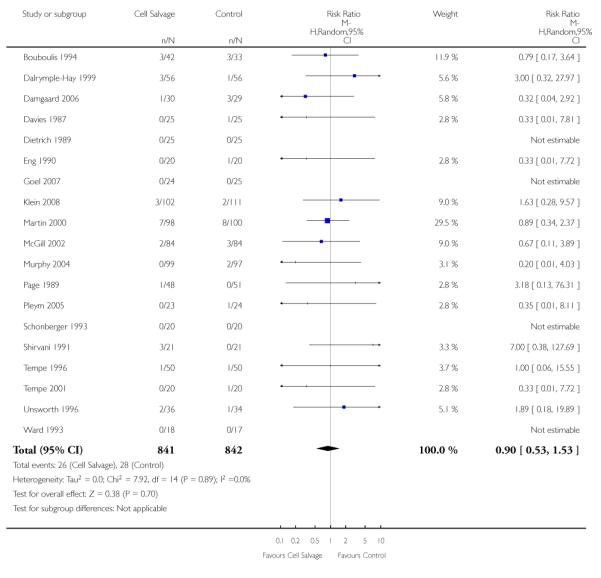 |
Analysis 7.5. Comparison 7 Adverse events and other outcomes, Outcome 5 Re-operation for bleeding -Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 5 Re-operation for bleeding - Active vs Control
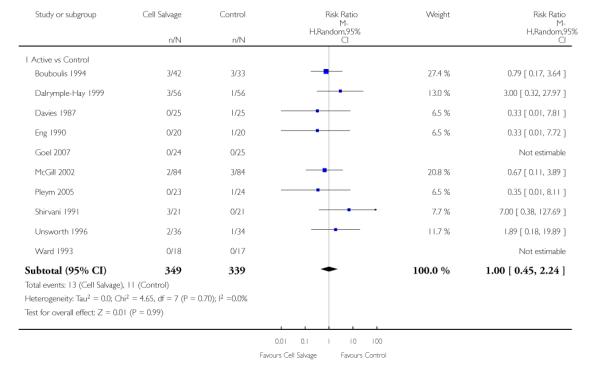 |
Analysis 7.6. Comparison 7 Adverse events and other outcomes, Outcome 6 Re-operation for bleeding -Active vs Active
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 6 Re-operation for bleeding - Active vs Active
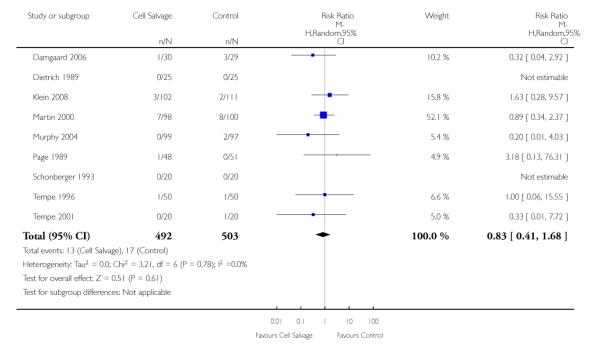 |
Analysis 7.7. Comparison 7 Adverse events and other outcomes, Outcome 7 Any infection - All Studies
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 7 Any infection - All Studies
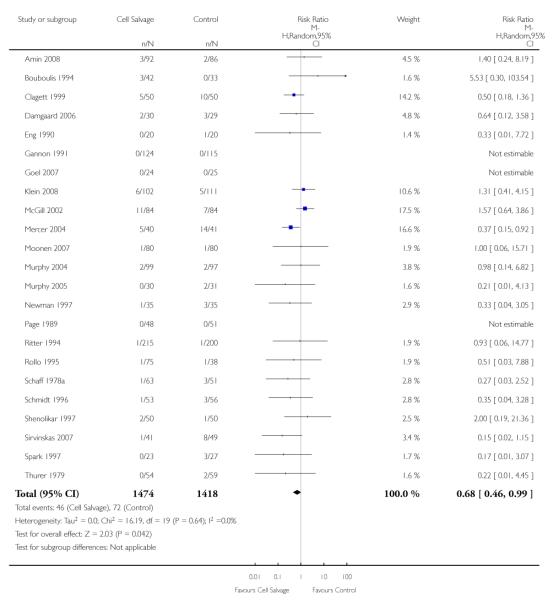 |
Analysis 7.8. Comparison 7 Adverse events and other outcomes, Outcome 8 Any infection - Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 8 Any infection - Active vs Control
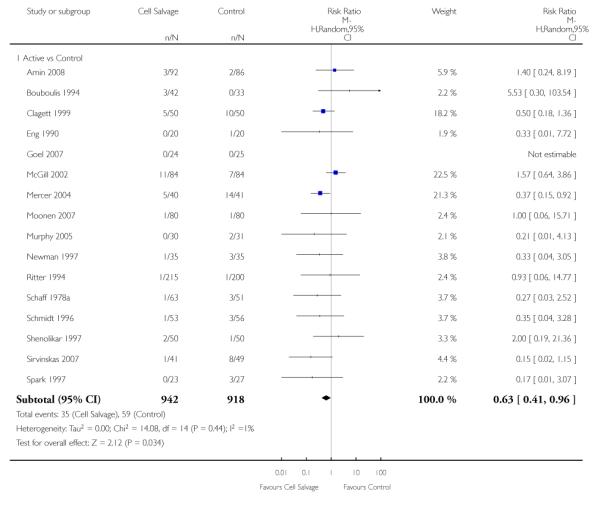 |
Analysis 7.9. Comparison 7 Adverse events and other outcomes, Outcome 9 Any Infection - Active vs Active
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 9 Any Infection - Active vs Active
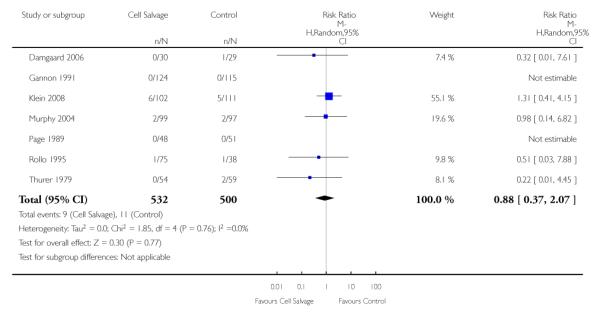 |
Analysis 7.10. Comparison 7 Adverse events and other outcomes, Outcome 10 Wound complication - All Studies
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 10 Wound complication - All Studies
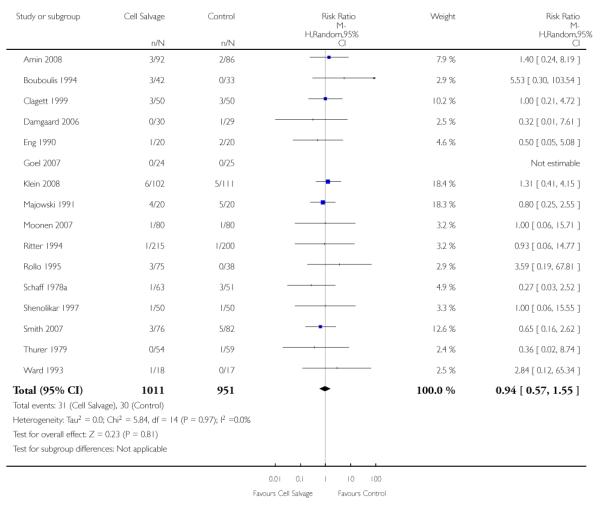 |
Analysis 7.11. Comparison 7 Adverse events and other outcomes, Outcome 11 Wound complication -Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 11 Wound complication - Active vs Control
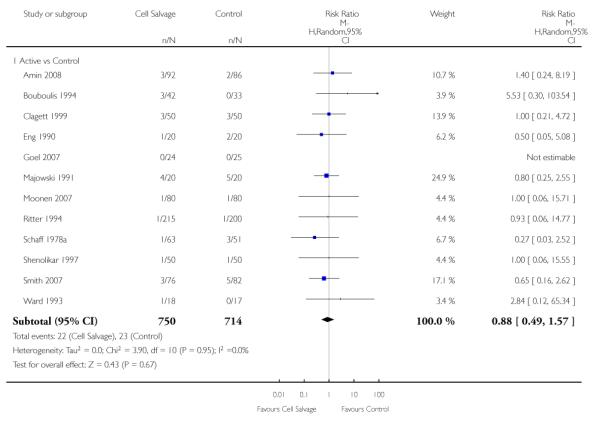 |
Analysis 7.12. Comparison 7 Adverse events and other outcomes, Outcome 12 Wound complication -Active vs Active
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 12 Wound complication - Active vs Active
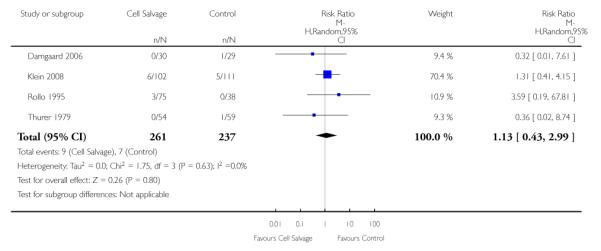 |
Analysis 7.13. Comparison 7 Adverse events and other outcomes, Outcome 13 Any thrombosis - All Studies
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 13 Any thrombosis - All Studies
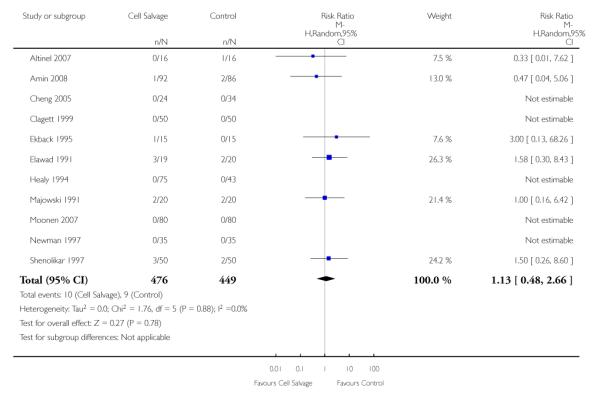 |
Analysis 7.14. Comparison 7 Adverse events and other outcomes, Outcome 14 Any thrombosis - Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 14 Any thrombosis - Active vs Control
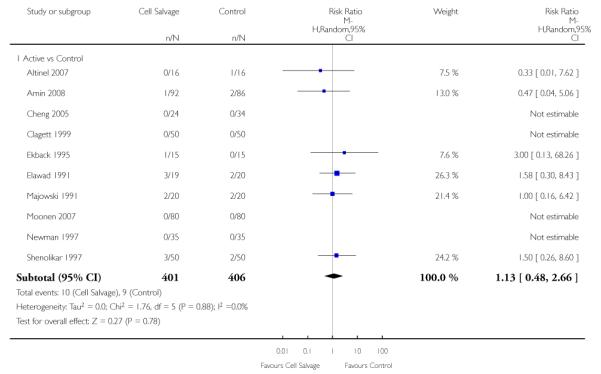 |
Analysis 7.15. Comparison 7 Adverse events and other outcomes, Outcome 15 Stroke - All Studies
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 15 Stroke - All Studies
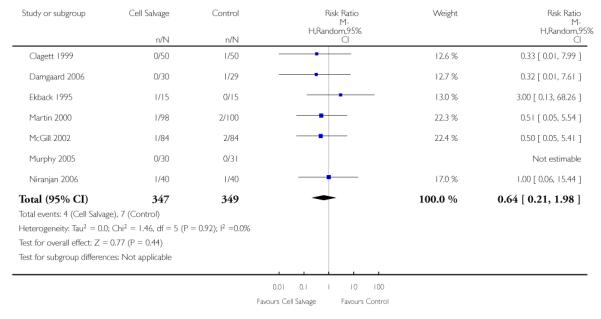 |
Analysis 7.16. Comparison 7 Adverse events and other outcomes, Outcome 16 Stroke - Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 16 Stroke - Active vs Control
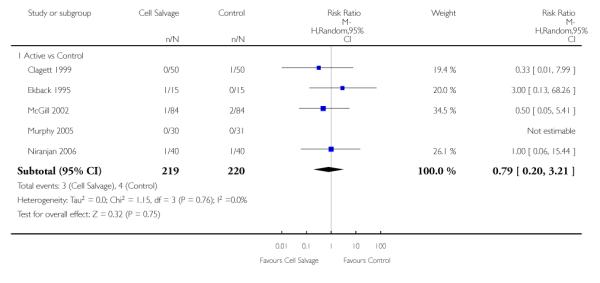 |
Analysis 7.17. Comparison 7 Adverse events and other outcomes, Outcome 17 Stroke - Active vs Active
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 17 Stroke - Active vs Active
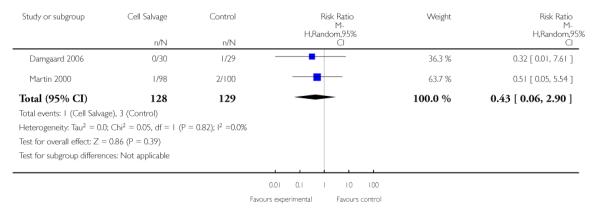 |
Analysis 7.18. Comparison 7 Adverse events and other outcomes, Outcome 18 Non-fatal myocardial infarction - All Studies
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 18 Non-fatal myocardial infarction - All Studies
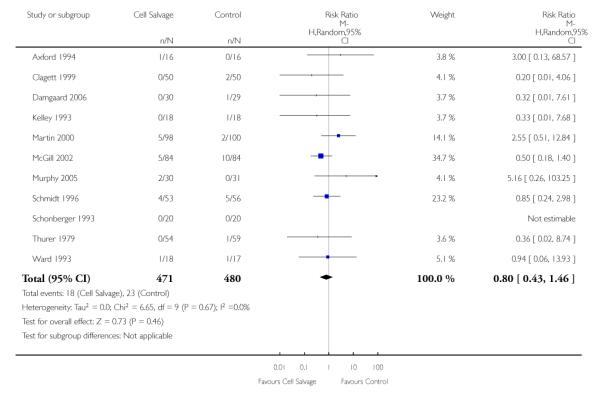 |
Analysis 7.19. Comparison 7 Adverse events and other outcomes, Outcome 19 Non-fatal myocardial infarction - Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 19 Non-fatal myocardial infarction - Active vs Control
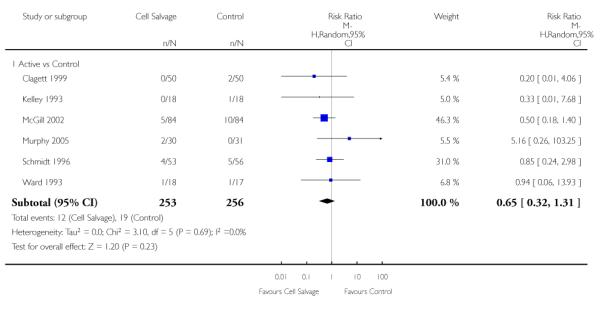 |
Analysis 7.20. Comparison 7 Adverse events and other outcomes, Outcome 20 Non-fatal myocardial infarction - Active vs Active
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 20 Non-fatal myocardial infarction - Active vs Active
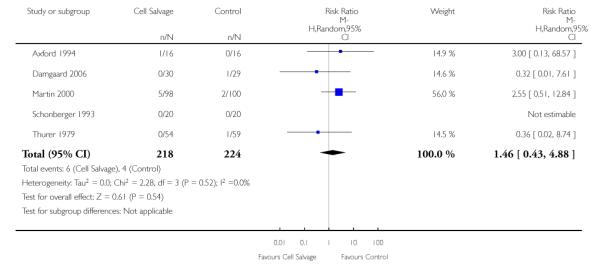 |
Analysis 7.21. Comparison 7 Adverse events and other outcomes, Outcome 21 Deep vein thrombosis (DVT)
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 21 Deep vein thrombosis (DVT)
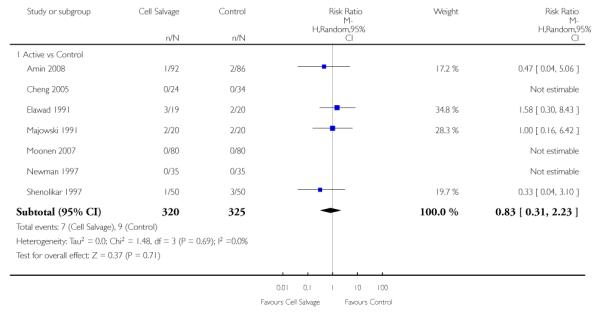 |
Analysis 7.22. Comparison 7 Adverse events and other outcomes, Outcome 22 Hospital length of stay (LOS) - Active vs Control
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 22 Hospital length of stay (LOS) - Active vs Control
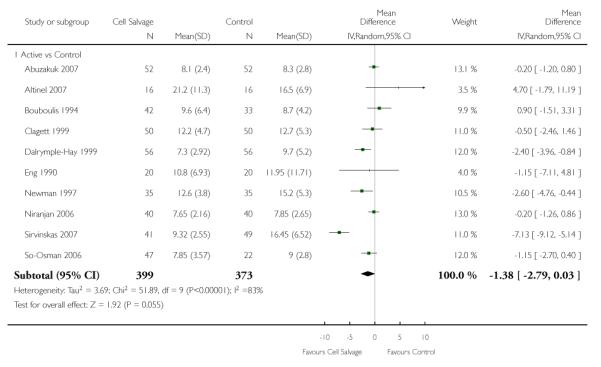 |
Analysis 7.23. Comparison 7 Adverse events and other outcomes, Outcome 23 Hospital length of stay (LOS) - Active vs Active
Review: Cell salvage for minimising perioperative allogeneic blood transfusion
Comparison: 7 Adverse events and other outcomes
Outcome: 23 Hospital length of stay (LOS) - Active vs Active
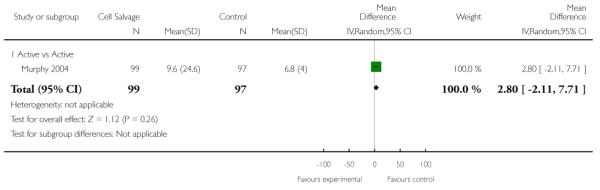 |
Appendix 1. Search strategy
MEDLINE search strategy
cell$ sav$.mp.
cell$ salvage.mp.
blood transfusion, autologous/
autotransfusion$.mp.
auto-transfusion$.mp.
blood salvage.mp.
autovac.mp.
solcotrans system.mp.
constavac.mp.
solcotrans.mp.
hemovac.mp.
BRAT.mp.
fresenius.mp.
consta vac.mp.
cell saver.mp.
dideco.mp.
electromedic.mp.
electromedics.mp.
gish biomedical.mp.
haemonetics.mp.
orth-evac.mp.
pleur-evac.mp.
sorenson.mp.
reinfusion system.mp.
sorin biomedical.mp.
or/1-25
exp blood transfusion/
exp hemorrhage/
exp anesthesia/
transfusion$.mp.
bleed$.mp.
blood loss$.mp.
hemorrhag$.mp.
haemorrhag$.mp.
or/27-34
26 and 35
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized controlled trials.sh.
random allocation.sh.
double blind method.sh.
single blind method.sh.
or/37-42
clinical trial.pt.
exp Clinical trials/
(clin$ adj25 trial$).ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
placebos.sh.
placebo$.ti,ab.
random$.ti,ab.
research design.sh.
or/44-51
comparative study.sh.
exp Evaluation studies/
follow up studies.sh.
prospective studies.sh.
(control$ or prospectiv$ or volunteer$).ti,ab.
or/53-57
43 or 52 or 58
36 and 59
animal/ not human/
60 not 61
HISTORY
Protocol first published: Issue 1, 2000
Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 10 February 2010 | New citation required and conclusions have changed | The review has been updated with the results of 24 additional trials |
| 1 June 2006 | New search has been performed | May 2006 The searches were updated in January 2004 as part of a Health Technology Assessment (HTA) project. Two new studies have been included (Naumenko 2003; Zhao 2003), with the results of the review amended accordingly |
WHAT’S NEW
Last assessed as up-to-date: 2 June 2009.
| Date | Event | Description |
|---|---|---|
| 19 December 2011 | Amended | The Plain Language Summary Title has been shortened to comply with new guidelines |
Footnotes
DECLARATIONS OF INTEREST None known.
References to studies included in this review
* Indicates the major publication for the study
- Abuzakuk 2007 {published data only} .Abuzakuk T, Senthil KV, Shenava Y, Bulstrode C, Skinner JA, et al. Autotransfusion drains in total knee replacement. Are they alternatives to homologous transfusion? International Orthopaedics. 2007;31(2):235–9. doi: 10.1007/s00264-006-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adalberth 1998 {published data only} .Adalberth G, Bystrom S, Kolstad K, Mallmin H, Milbrink J. Postoperative drainage of knee arthroplasty is not necessary - A randomised study of 90 patients. Acta Orthopaedica Scandinavica. 1998;69(5):475–8. doi: 10.3109/17453679808997781. [DOI] [PubMed] [Google Scholar]
- Altinel 2007 {published data only} .Altinel L, Kaya E, Kose KC, Fidan F, Ergan V, Fidan H. Effect of shed blood retransfusion on pulmonary perfusion after total knee arthroplasty: a prospective controlled study. International Orthopaedics. 2007;31(6):837–44. doi: 10.1007/s00264-006-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin 2008 {published data only} .Amin A, Watson A, Mangwani J, Nawabi D, Ahluwalia R, Loeffler M. A prospective randomised controlled trial of autologous retransfusion in total knee replacement. Journal of Bone & Joint Surgery - British Volume. 2008;90(4):451–4. doi: 10.1302/0301-620X.90B4.20044. [DOI] [PubMed] [Google Scholar]
- Axford 1994 {published data only} .Axford TC, Dearani JA, Ragno G, MacGregor H, Patel MA, Valeri CR, Khuri SF. Safety and therapeutic effectiveness of reinfused shed blood after open heart surgery. Annals of Thoracic Surgery. 1994;57(3):615–22. doi: 10.1016/0003-4975(94)90554-1. [DOI] [PubMed] [Google Scholar]
- Ayers 1995 {published data only} .Ayers DC, Murray DG, Duerr DM. Blood salvage after total hip arthroplasty. The Journal of Bone and Joint Surgery. American Volume. 1995;77(9):1347–51. doi: 10.2106/00004623-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Bouboulis 1994 {published data only} .Bouboulis N, Kardara M, Kesteven PJ, Jayakrishnan AG. Autotransfusion after coronary artery bypass surgery: is there any benefit? Journal of Cardiac Surgery. 1994;9(3):314–21. doi: 10.1111/j.1540-8191.1994.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Cheng 2005 {published data only} .Cheng SC, Hung TS, Tse PY. Investigation of the use of drained blood reinfusion after total knee arthroplasty: a prospective randomised controlled study. Journal of Orthopaedic Surgery. 2005;13(2):120–4. doi: 10.1177/230949900501300203. [DOI] [PubMed] [Google Scholar]
- Clagett 1999 {published data only} .Clagett GP, Valentine RJ, Jackson MR, Mathison C, Kakish HB, Bengtson TD. A randomised trial of intraoperative autotransfusion during aortic surgery. Journal of Vascular Surgery. 1999;29(1):22–31. doi: 10.1016/s0741-5214(99)70346-0. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Hay 1999 {published data only} .Dalrymple-Hay MJR, Pack L, Deakin CD, Shepherd S, Ohri SK, Haw MP, Livesey SA, Monro JL. Autotransfusion of washed shed mediastinal fluid decreases requirement for autologous blood transfusion following cardiac surgery: A prospective randomized trial. European Journal of Cardiothoracic Surgery. 1999;15:830–4. doi: 10.1016/s1010-7940(99)00112-8. [DOI] [PubMed] [Google Scholar]
- Damgaard 2006 {published data only} .Damgaard S, Steinbruchel DA. Autotransfusion with cell saver for off-pump coronary artery bypass surgery: a randomized trial. Scandinavian Cardiovascular Journal. 2006;40(3):194–8. doi: 10.1080/14017430600628292. [DOI] [PubMed] [Google Scholar]
- Davies 1987 {published data only} .Davies MJ, Cronin KC, Moran P, Mears L, Booth RJ. Autologous blood transfusion for major vascular surgery using the Sorenson Receptal Device. Anaesthesia and Intensive Care. 1987;15(3):282–8. doi: 10.1177/0310057X8701500307. [DOI] [PubMed] [Google Scholar]
- Dietrich 1989 {published data only} .Dietrich W, Barankay A, Dilthey G, Mitto HP, Richter JA. Reduction of blood utilization during myocardial revascularization. Journal of Thoracic and Cardiovascular Surgery. 1989;97(2):213–9. [PubMed] [Google Scholar]
- Dramis 2006 {published data only} .Dramis A, Plewes J. Autologous blood transfusion after primary unilateral total knee replacement surgery. Acta Orthopaedica Belgica. 2006;72(1):15–7. [PubMed] [Google Scholar]
- Ekback 1995 {published data only} .Ekback G, Schott U, Axelsson K, Carberg M. Perioperative autotransfusion and functional coagulation analysis in total hip replacement. Acta Anaesthesiologica Scandinavica. 1995;39:390–5. doi: 10.1111/j.1399-6576.1995.tb04083.x. [MEDLINE: 1919270] [DOI] [PubMed] [Google Scholar]
- Elawad 1991 {published data only} .Elawad AA, Ohlin AK, Berntorp E, Nilsson IM, Fredin H. Intraoperative autotransfusion in primary hip arthroplasty. A randomized comparison with homologous blood. Acta Orthopaedica Scandinavica. 1991;62(6):557–62. doi: 10.3109/17453679108994496. [DOI] [PubMed] [Google Scholar]
- Eng 1990 {published data only} .Eng J, Kay PH, Murday AJ, Shreiti I, Harrison DP, Norfolk DR, et al. Postoperative autologous transfusion in cardiac surgery. A prospective, randomised study. European Journal of Cardiothorac Surgery. 1990;4(11):595–600. doi: 10.1016/1010-7940(90)90018-u. [DOI] [PubMed] [Google Scholar]
- Fragnito 1995 {published data only} .Fragnito C, Beghi C, Cavozza C, Saccani S, Contini SA, Barboso G. Autotransfusion of the blood drained from mediastinum in the course of myocardial revascularization. Acta Bio-Medica de l Ateneo Parmense. 1995;66(5):195–201. [PubMed] [Google Scholar]
- Gannon 1991 {published data only} .Gannon DM, Lombardi AV, Jr, Mallory TH, Vaughn BK, Finney CR, Niemcryk S. An evaluation of the efficacy of postoperative blood salvage after total joint arthroplasty. A prospective randomized trial. Journal of Arthroplasty. 1991;6(2):109–14. doi: 10.1016/s0883-5403(11)80004-5. [DOI] [PubMed] [Google Scholar]
- Goel 2007 {published data only} .Goel P, Pannu H, Mohan D, Arora R. Efficacy of cell saver in reducing homologous blood transfusions during OPCAB surgery: a prospective randomized trial. Transfusion Medicine. 2007;17(4):285–9. doi: 10.1111/j.1365-3148.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- Healy 1994 {published data only} .Healy WL, Pfeifer BA, Kurtz SR, Johnson C, Johnson W, Johnston R, et al. Evaluation of autologous shed blood for autotransfusion after orthopaedic surgery. Clinical Orthopaedics and Related Research. 1994;299:53–9. [PubMed] [Google Scholar]
- Heddle 1992 {published data only} .Heddle NM, Brox WT, Klama LL, Dickson LL, Levine MN. A randomized trial on the efficacy of an autologous blood drainage and transfusion device in patients undergoing elective knee arthroplasty. Transfusion. 1992;32:742–6. doi: 10.1046/j.1537-2995.1992.32893032102.x. [DOI] [PubMed] [Google Scholar]
- Kelley 1993 {published data only} .Kelley-Patteson C, Ammar AD, Kelley H. Should the Cell Saver Autotransfusion Device be used routinely in all infrarenal abdominal aortic bypass operations? Journal of Vascular Surgery. 1993;18(2):261–5. [PubMed] [Google Scholar]
- Kirkos 2006 {published data only} .Kirkos JM, Krystallis CT, Konstantinidis PA, Papavasiliou KA, Kyrkos MJ, Ikonomidis LG. Postoperative reperfusion of drained blood in patients undergoing total knee arthroplasty: is it effective and cost-efficient? Acta Orthopaedica Belgica. 2006;72(1):18–23. [PubMed] [Google Scholar]
- Klein 2008 {published data only} .Klein AA, Nashef SA, Sharples L, Bottrill F, Dyer M, Armstrong J, Vuylsteke A. A randomized controlled trial of cell salvage in routine cardiac surgery. Anesthesia and Analgesia. 2008;107(5):1487–95. doi: 10.1213/ane.0b013e3181831e54. [DOI] [PubMed] [Google Scholar]
- Koopman 1993a {published data only} .Koopman-van Germert AWMM. Peri-operative autotransfusion by means of a blood cell separator. Processed autotransfusion and homologous red cell requirement in elective cardiac and orthopaedic surgery: a randomised prospective study. Den Haag: Cip-Data Koninklijke Bibliotheek. 1993:96–112. Vol. Chapter 5. [Google Scholar]
- Koopman 1993b {published data only} .Koopman-van Germert AWMM. Peri-operative autotransfusion by means of a blood cell separator. Processed autotransfusion and homologous red cell requirement in elective cardiac and orthopaedic surgery: a randomised prospective study. Den Haag: Cip-Data Koninklijke Bibliotheek. 1993:96–112. Vol. Chapter 5. [Google Scholar]
- Laub 1993 {published data only} .Laub GW, Dharan M, Riebman JB, Chen C, Moore R, Bailey BM, et al. The impact of intraoperative autotransfusion on cardiac surgery. A prospective randomized double-blind study. Chest. 1993;104(3):686–9. doi: 10.1378/chest.104.3.686. [DOI] [PubMed] [Google Scholar]
- Lepore 1989 {published data only} .Lepore V, Radegran K. Autotransfusion of mediastinal blood in cardiac surgery. Scandinavian Journal of Thoracic and Cardiovascular Surgery. 1989;23(1):47–9. doi: 10.3109/14017438909105967. [DOI] [PubMed] [Google Scholar]
- Lorentz 1991 {published data only} .Lorentz A, Osswald PM, Schilling M, Jani L. A comparison of autologous transfusion procedures in hip surgery. Anaesthesist. 1991;40(4):205–13. [PubMed] [Google Scholar]
- Mah 1995 {published data only} .Mah ET, Davis R, Seshadri P, Nyman TLM, Seshadri R. The role of autologous blood transfusion in joint replacement. Anaesthesia and Intensive Care. 1995;23:472–7. doi: 10.1177/0310057X9502300411. [DOI] [PubMed] [Google Scholar]
- Majowski 1991 {published data only} .Majkowski RS, Currie IC, Newman JH. Postoperative collection and reinfusion of autologous blood in total knee arthroplasty. Annals of the Royal College of Surgeons of England. 1991;73(6):381–4. [PMC free article] [PubMed] [Google Scholar]
- Martin 2000 {published data only} .Martin J, Robitaille D, Perrault LP, Pellerin M, Page P, Searle N, et al. Reinfusion of mediastinal blood after heart surgery. Journal of Thoracic and Cardiovascular Surgery. 2000;120(3):499–504. doi: 10.1067/mtc.2000.108691. [DOI] [PubMed] [Google Scholar]
- Mauerhan 1993 {published data only} .Mauerhan DR, Nussman D, Mokris JG, Beaver WB. Effect of postoperative reinfusion systems on hemoglobin levels in primary total hip and total knee arthroplasties. A prospective randomized study. Journal of Arthroplasty. 1993;8(5):523–7. [PubMed] [Google Scholar]
- McGill 2002 {published data only} .McGill N, O’Shaughnessy D, Pickering R, Herbertson M, Gill R. Mechanical methods of reducing blood transfusion in cardiac surgery: randomised controlled trial. [erratum appears in BMJ 2002 Jul 20;325(7356):142] BMJ. 2002;324(7349):1299. doi: 10.1136/bmj.324.7349.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges 1992 {published data only} .Menges T, Rupp D, van Lessen A, Hempelmann G. Effects on coagulation parameters of different methods of autologous blood transfusion. Anaesthesist. 1992;41:27–33. [PubMed] [Google Scholar]
- Mercer 2004 {published data only} .Mercer KG, Spark JI, Berridge DC, Kent PJ, Scott DJ. Randomized clinical trial of intraoperative autotransfusion in surgery for abdominal aortic aneurysm. The British Journal of Surgery. 2004;91(11):1443–8. doi: 10.1002/bjs.4793. [DOI] [PubMed] [Google Scholar]
- Moonen 2007 {published data only} .Moonen AF, Knoors NT, van Os JJ, Verburg AD, Pilot P. Retransfusion of filtered shed blood in primary total hip and knee arthroplasty: a prospective randomized clinical trial. Transfusion. 2007;47(3):379–84. doi: 10.1111/j.1537-2995.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- Murphy 2004 {published data only} .Murphy GJ, Allen SM, Unsworth-White J, Lewis CT, Dalrymple-Hay MJ. Safety and efficacy of perioperative cell salvage and autotransfusion after coronary artery bypass grafting: a randomized trial. Annals of Thoracic Surgery. 2004;77(5):1553–9. doi: 10.1016/j.athoracsur.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Murphy 2005 {published data only} .Murphy GJ, Rogers CS, Lansdowne WB, Channon I, Alwair H, Cohen A, et al. Safety, efficacy, and cost of intraoperative cell salvage and autotransfusion after off-pump coronary artery bypass surgery: a randomized trial. The Journal of Thoracic and Cardiovascular Surgery. 2005;130(1):20–8. doi: 10.1016/j.jtcvs.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Naumenko 2003 {published data only} .Naumenko SE, Pokrovskii MG, Belavin AS, Kim SF. Blood-saving effectiveness of preoperative reservation of autoblood for surgical treatment of ischaemic heart disease. Vestnik khirurgii imeni I. I. Grekova. 2003;162:59–64. [PubMed] [Google Scholar]
- Newman 1997 {published data only} .Newman JH, Bowers M, Murphy J. The clinical advantages of autologous transfusion. A randomized, controlled study after knee replacement. The Journal of Bone and Joint Surgery. British Volume. 1997;79(4):630–2. doi: 10.1302/0301-620x.79b4.7272. [DOI] [PubMed] [Google Scholar]
- Niranjan 2006 {published data only} .Niranjan G, Asimakopoulos G, Karagounis A, Cockerill G, Thompson M, Chandrasekaran V. Effects of cell saver autologous blood transfusion on blood loss and homologous blood transfusion requirements in patients undergoing cardiac surgery on- versus off-cardiopulmonary bypass: a randomised trial. European Journal of Cardio-thoracic Surgery. 2006;30(2):271–7. doi: 10.1016/j.ejcts.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Page 1989 {published data only} .Page R, Russell GN, Fox MA, Fabri BM, Lewis I, Williets T. Hard-shell cardiotomy reservoir for reinfusion of shed mediastinal blood. Annals of Thoracic Surgery. 1989;48(4):514–7. doi: 10.1016/s0003-4975(10)66852-x. [DOI] [PubMed] [Google Scholar]
- Parrot 1991 {published data only} .Parrot D, Lancon JP, Merle JP, Rerolle A, Bernard A, Obadia JF, Caillard B. Blood salvage in cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia. 1991;5(5):454–6. doi: 10.1016/1053-0770(91)90119-e. [DOI] [PubMed] [Google Scholar]
- Pleym 2005 {published data only} .Pleym H, Tjomsland O, Asberg A, Lydersen S, Wahba A, Bjella L, et al. Effects of autotransfusion of mediastinal shed blood on biochemical markers of myocardial damage in coronary surgery. Acta Anaesthesiologica Scandinavica. 2005;49(9):1248–54. doi: 10.1111/j.1399-6576.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- Riou 1994 {published data only} .Riou B, Arock M, Guerrero M, Ramos M, Thoreux P, Guillosson JJ, et al. Haematological effects of postoperative autotransfusion in spinal surgery. Acta Anaesthesiologica Scandinavica. 1994;38(4):336–41. doi: 10.1111/j.1399-6576.1994.tb03903.x. [DOI] [PubMed] [Google Scholar]
- Ritter 1994 {published data only} .Ritter MA, Keating EM, Faris PM. Closed wound drainage in total hip or total knee replacement. A prospective, randomized study. The Journal of Bone and Joint Surgery. American Volume. 1994;76(1):35–8. doi: 10.2106/00004623-199401000-00005. [DOI] [PubMed] [Google Scholar]
- Rollo 1995 {published data only} .Rollo VJ, Hozack WJ, Rothman RH, Chao W, Eng KO. Prospective randomized evaluation of blood salvage techniques for primary total hip arthroplasty. Journal of Arthroplasty. 1995;10(4):532–9. doi: 10.1016/s0883-5403(05)80157-3. [DOI] [PubMed] [Google Scholar]
- Rollo 1995a {published data only} .Rollo VJ, Hozack WJ, Rothman RH, Chao W, Eng KO. Prospective randomized evaluation of blood salvage techniques for primary total hip arthroplasty. Journal of Arthroplasty. 1995;10(4):532–9. doi: 10.1016/s0883-5403(05)80157-3. [DOI] [PubMed] [Google Scholar]
- Rollo 1995b {published data only} .Rollo VJ, Hozack WJ, Rothman RH, Chao W, Eng KO. Prospective randomized evaluation of blood salvage techniques for primary total hip arthroplasty. Journal of Arthroplasty. 1995;10(4):532–9. doi: 10.1016/s0883-5403(05)80157-3. [DOI] [PubMed] [Google Scholar]
- Rosencher 1994 {published data only} .Rosencher N, Vassilieff V, Tallet F, Toulon P, Leoni J, Tomeno B, Conseiller C. Comparison of Orth-Evac and Solcotrans Plus devices for the autotransfusion of blood drained after total knee joint arthroplasty. Annales Francaises d Anesthesie et de Reanimation. 1994;13(3):318–25. doi: 10.1016/s0750-7658(94)80040-5. [DOI] [PubMed] [Google Scholar]
- Sait 1999 {published data only} .Sait MS, Earnshaw P. Autotransfusion in total knee arthroplasty - Is it worth the effort? The Journal of Bone and Joint Surgery. British Volume. 1999;81(Suppl 2):244. [Google Scholar]
- Schaff 1978a {published data only} .Schaff HV, Hauer JM, Bell WR, Gardner TJ, Donahoo JS, Gott VL, Brawley RK. Autotransfusion of shed mediastinal blood after cardiac surgery. A prospective study. Journal of Thoracic and Cardiovascular Surgery. 1978;75(4):632–41. [PubMed] [Google Scholar]
- Schmidt 1996 {published data only} .Schmidt H, Folsgaard S, Mortensen PE, Jensen E. Impact of autotransfusion after coronary artery bypass grafting on oxygen transport. Acta Anaesthesiologica Scandinavica. 1997;41(8):995–1001. doi: 10.1111/j.1399-6576.1997.tb04826.x. [DOI] [PubMed] [Google Scholar]
- Schonberger 1993 {published data only} .Schonberger JP, Bredee J, Speekenbrink RG, Everts PA, Wildevuur CR. Autotransfusion of shed blood contributes additionally to blood saving in patients receiving aprotinin(2 million KIU) European Journal of Cardio-thoracic Surgery. 1993;7(9):474–7. doi: 10.1016/1010-7940(93)90276-h. [DOI] [PubMed] [Google Scholar]
- Shenolikar 1997 {published data only} .Shenolikar A, Wareham K, Newington D, Thomas D, Hughes J, Downes M. Cell salvage auto transfusion in total knee replacement surgery. Transfusion Medicine. 1997;7(4):277–80. doi: 10.1046/j.1365-3148.1997.d01-43.x. [DOI] [PubMed] [Google Scholar]
- Shirvani 1991 {published data only} .Shirvani R. An evaluation of clinical aspects of postoperative autotransfusion, either alone or in conjunction with pre-operative aspirin, in cardiac surgery. British Journal of Clinical Practice. 1991;45(2):105–8. [PubMed] [Google Scholar]
- Simpson 1994 {published data only} .Simpson MB, Murphy KP, Chambers HG, Bucknell AL. The effect of postoperative wound drainage reinfusion in reducing the need for blood transfusions in elective total joint arthroplasty: a prospective, randomized study. Orthopedics. 1994;17(2):133–7. doi: 10.3928/0147-7447-19940201-08. [DOI] [PubMed] [Google Scholar]
- Sirvinskas 2007 {published data only} .Sirvinskas E, Veikutiene A, Benetis R, Grybauskas P, Andrejaitiene J, Veikutis V, et al. Influence of early re-infusion of autologous shed mediastinal blood on clinical outcome after cardiac surgery. Perfusion. 2007;22(5):345–52. doi: 10.1177/0267659107088450. [DOI] [PubMed] [Google Scholar]
- Slagis 1991 {published data only} .Slagis SV, Benjamin JB, Volz RG, Giordano GF. Postoperative blood salvage in total hip and knee arthroplasty. A randomised controlled trial. The Journal of Bone and Joint Surgery. British Volume. 1991;73(4):591–4. doi: 10.1302/0301-620X.73B4.1906472. [DOI] [PubMed] [Google Scholar]
- Smith 2007 {published data only} .Smith LK, Williams DH, Langkamer VG. Post-operative blood salvage with autologous retransfusion in primary total hip replacement. The Journal of Bone and Joint Surgery. British Volume. 2007;89(8):1092–7. doi: 10.1302/0301-620X.89B8.18736. [DOI] [PubMed] [Google Scholar]
- So-Osman 2006 {published data only} .So-Osman C, Nelissen RG, Eikenboom HC, Brand A. Efficacy, safety and user-friendliness of two devices for postoperative autologous shed red blood cell re-infusion in elective orthopaedic surgery patients: A randomized pilot study. Transfusion Medicine. 2006;16(5):321–8. doi: 10.1111/j.1365-3148.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- Spark 1997 {published data only} .Spark JI, Chetter IC, Kester RC, Scott DJ. Allogeneic versus autologous blood during abdominal aortic aneurysm surgery. European Journal of Vascular and Endovascular Surgery. 1997;14(6):482–6. doi: 10.1016/s1078-5884(97)80128-1. [DOI] [PubMed] [Google Scholar]
- Tempe 1996 {published data only} .Tempe D, Bajwa R, Cooper A, Nag B, Tomar AS, Khanna SK, et al. Blood conservation in small adults undergoing valve surgery. Journal of Cardiothoracic and Vascular Anesthesia. 1996;10(4):502–6. doi: 10.1016/s1053-0770(05)80012-6. [DOI] [PubMed] [Google Scholar]
- Tempe 2001 {published data only} .Tempe DK, Banerjee A, Virmani S, Mehta N, Panwar S, Tomar AS, et al. Comparison of the effects of a cell saver and low dose aprotinin on blood loss and homologous blood usage in patients undergoing valve surgery. Journal of Cardiothoracic and Vascular Anesthesia. 2001;15(3):326–30. doi: 10.1053/jcan.2001.23282. [DOI] [PubMed] [Google Scholar]
- Thomas 2001 {published data only} .Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. British Journal of Anaesthesia. 2001;86(5):669–73. doi: 10.1093/bja/86.5.669. [DOI] [PubMed] [Google Scholar]
- Thurer 1979 {published data only} .Thurer RL, Lytle BW, Cosgrove DM, Loop FD. Autotransfusion following cardiac operations: a randomized, prospective study. Annals of Thoracic Surgery. 1979;27(6):500–7. doi: 10.1016/s0003-4975(10)63358-9. [DOI] [PubMed] [Google Scholar]
- Tripkovic 2008 {published data only} .Tripkovic B, Bukovic D, Sakic K, Sakic S, Bukovic N, Radakovic B. Quality of the blood sampled from surgical drainage after total hip arthroplasty. Collegium Antropologicum. 2008;32(1):153–60. [PubMed] [Google Scholar]
- Unsworth 1996 {published data only} .Unsworth-White MJ, Kallis P, Cowan D, Tooze JA, Bevan DH, Treasure T. A prospective randomised controlled trial of postoperative autotransfusion with and without a heparin-bonded circuit. European Journal of Cardio-thoracic Surgery. 1996;10(1):38–47. doi: 10.1016/s1010-7940(96)80264-8. [DOI] [PubMed] [Google Scholar]
- Ward 1993 {published data only} .Ward HB, Smith RR, Landis KP, Nemzek TG, Dalmasso AP, Swaim WR. Prospective, randomized trial of autotransfusion after routine cardiac operations. Annals of Thoracic Surgery. 1993;56(1):137–41. doi: 10.1016/0003-4975(93)90418-h. [DOI] [PubMed] [Google Scholar]
- Westerberg 2004 {published data only} .Westerberg M, Bengtsson A, Jeppsson A. Coronary surgery without cardiotomy suction and autotransfusion reduces the postoperative systemic inflammatory response. Annals of Thoracic Surgery. 2004;78(1):54–9. doi: 10.1016/j.athoracsur.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Wiefferink 2007 {published data only} .Wiefferink A, Weerwind PW, van Heerde W, Teerenstra S, Noyez L, de Pauw BE, Brouwer R. Autotransfusion management during and after cardiopulmonary bypass alters fibrin degradation and transfusion requirements. Journal of Extra-Corporeal Technology. 2007;39(2):66–70. [PMC free article] [PubMed] [Google Scholar]
- Zacharopoulos 2007 {published data only} .Zacharopoulos A, Apostolopoulos A, Kyriakidis A. The effectiveness of reinfusion after total knee replacement. A prospective randomised controlled study. International Orthopaedics. 2007;31(3):303–8. doi: 10.1007/s00264-006-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang 2008 {published data only} .Zhang XF, Dong JM, Gong ML, Shen SM, Zhou Y, Pan YF, Mao J. Effectiveness of preoperative autologous plateletpheresis combined with intraoperative autotransfusion on the blood coagulation in orthopaedic patients. [Chinese] Chung-Hua Wai Ko Tsa Chih [Chinese Journal of Surgery] 2008;46(2):118–21. [PubMed] [Google Scholar]
- Zhao 1996 {published data only} .Zhao K, Xiao M, Deng S. Autotransfusion of shed medistinal blood after open heart operation. Chinese Journal of Surgery. 1996;34(8):497–9. [PubMed] [Google Scholar]
- Zhao 2003 {published data only} .Zhao K, Xu J, Hu S, Wu Q, Wei Y, Liu Y. Autotransfusion of shed mediastinal blood after open heart surgery. Chinese Medical Journal. 2003;116:1179–82. [PubMed] [Google Scholar]
References to studies excluded from this review
- Adan 1988 {published data only} .Adan A, Brutel de la Riviere A, Haas F, van Zalk A, de Nooij E. Autotransfusion of drained mediastinal blood after cardiac surgery: a reappraisal. Thoracic and Cardiovascular Surgery. 1988;36(1):10–14. doi: 10.1055/s-2007-1020034. [DOI] [PubMed] [Google Scholar]
- Bartels 1996 {published data only} .Bartels C, Bechtel JV, Winkler C, Horsch S. Intraoperative autotransfusion in aortic surgery: comparison of whole blood autotransfusion versus cell separation. Journal of Vascular Surgery. 1996;24(1):102–8. doi: 10.1016/s0741-5214(96)70150-7. [DOI] [PubMed] [Google Scholar]
- Bell 1992 {published data only} .Bell K, Stott K, Sinclair CJ, Walker WS, Gillon J. A controlled trial of intra-operative autologous transfusion in cardiothoracic surgery measuring effect on transfusion requirements and clinical outcome. Transfusion Medicine. 1992;2(4):295–300. doi: 10.1111/j.1365-3148.1992.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Breakwell 2000 {published data only} .Breakwell LM, Getty CJM, Dobson P. The efficacy of autologous blood transfusion in bilateral total knee arthroplasty. Knee. 2000;7(3):145–7. doi: 10.1016/s0968-0160(00)00032-6. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Hay 2001 {published data only} .Dalrymple-Hay MJR, Dawkins S, Pack L, Deakin CD, Sheppard S, Ohri SK, et al. Autotransfusion decreases blood usage following cardiac surgery - A prospective randomized trial. Cardiovascular Surgery. 2001;9(2):184–7. doi: 10.1016/s0967-2109(00)00100-9. [DOI] [PubMed] [Google Scholar]
- Deramoudt 1991 {published data only} .Deramoudt V, Menestret P, Melledant Y, et al. Autologous transfusion in cardiac surgery [Transfusion autologue en chirurgie cardiaque (a propos de 74 patients)] Cahiers D Anesthesiologie. 1991;39(3):153–9. [PubMed] [Google Scholar]
- Elawad 1992 {published data only} .Elawad AA, Ohlin AK, Berntorp E, Nilsson IM, Fredin H. Autologous blood transfusion in revision hip arthroplasty. A prospective, controlled study of 30 patients. Acta Orthopaedica Scandinavica. 1992;63(4):373–6. doi: 10.3109/17453679209154747. [DOI] [PubMed] [Google Scholar]
- Farrer 1997 {published data only} .Farrer A, Spark JI, Scott DJ. Autologous blood transfusion: the benefits to the patient undergoing abdominal aortic aneurysm repair. Journal of Vascular Nursing. 1997;15(4):111–5. doi: 10.1016/s1062-0303(97)90028-5. [DOI] [PubMed] [Google Scholar]
- Jacobi 1997 {published data only} .Jacobi K, Walther A, Kuhn R, Dworak O, Neidhardt B, Rugheimer E. Advantages and limitations of intraoperative mechanical autotransfusion in radical prostatectomies. Anaesthetist. 1997;46(2):101–7. doi: 10.1007/s001010050378. [DOI] [PubMed] [Google Scholar]
- Kristensen 1992 {published data only} .Kristensen PW, Sorensen LS, Thyregod HC. Autotransfusion of drainage blood in arthroplasty. A prospective, controlled study of 31 operations. Acta Orthopaedica Scandinavica. 1992;63(4):377–80. doi: 10.3109/17453679209154748. [DOI] [PubMed] [Google Scholar]
- Mac 1993 {published data only} .Mac HL, Reynolds MA, Treston-Aurand J, Henke JA. Comparison of autoreinfusion and standard drainage systems in total joint arthroplasty patients. Orthopaedic Nursing. 1993;12(3):19–25. doi: 10.1097/00006416-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Mayer 1985 {published data only} .Mayer ED, Welsch M, Tanzeem A, Saggau W, Spath J, Hummels R, Schmitz W. Reduction of postoperative donor blood requirement by use of the cell separator. Scandinavian Journal of Thoracic Cardiovascular Surgery. 1985;19(2):165–71. doi: 10.3109/14017438509102713. [DOI] [PubMed] [Google Scholar]
- McShane 1987 {published data only} .McShane AJ, Power C, Jackson JF, Murphy DF, MacDonald A, Moriarty DC, Otridge BW. Autotransfusion: quality of blood prepared with a red cell processing device. British Journal of Anaesthesia. 1987;59(8):1035–9. doi: 10.1093/bja/59.8.1035. [DOI] [PubMed] [Google Scholar]
- Schaff 1978b {published data only} .Schaff HV, Hauer JM, Brawley RK. Autotransfusion in cardiac surgical patients after operation. Surgery. 1978;84(5):713–8. [PubMed] [Google Scholar]
- Schmidt 1997a {published data only} .Schmidt H, Folsgaard S, Mortensen PE, Jensen E. Impact of autotransfusion after coronary artery bypass grafting on oxygen transport. Acta Anaesthesiologica Scandinavica. 1997;41:995–1001. doi: 10.1111/j.1399-6576.1997.tb04826.x. [DOI] [PubMed] [Google Scholar]; *; Schmidt H, Lund JO, Nielsen SL. Autotransfused shed mediastinal blood has normal erythrocyte survival. Annals of Thoracic Surgery. 1996;62(1):105–8. doi: 10.1016/0003-4975(96)00219-6. [DOI] [PubMed] [Google Scholar]
- Schmidt 1997b {published data only} .Schmidt H, Folsgaard S, Mortensen PE, Jensen E. Cardiac enzymes and autotransfusion of shed mediastinal blood after myocardial revascularization. Annals of Thoracic Surgery. 1997;63:1288–92. doi: 10.1016/s0003-4975(97)00214-2. [DOI] [PubMed] [Google Scholar]
- Skoura 1997 {published data only} .Skoura H, Anastasiou E, Moshou A, Katsioulas A, Kostaki S. Value of autologous blood for intra-operative massive bleeding. European Journal of Anaesthesiology. 1997;14(1):103. [Google Scholar]
- Thompson 1990 {published data only} .Thompson JF, Webster JH, Chant AD. Prospective randomised evaluation of a new cell saving device in elective aortic reconstruction. European Journal of Vascular Surgery. 1990;4(5):507–12. doi: 10.1016/s0950-821x(05)80793-5. [DOI] [PubMed] [Google Scholar]
- Trubel 1995 {published data only} .Trubel W, Gunen E, Wuppinger G, Tschernko E, Gunen-Frank A, Staudacher M, et al. Recovery of intraoperatively shed blood in aortoiliac surgery: comparison of cell washing with simple filtration. The Thoracic and Cardiovascular Surgeon. 1995;43(3):165–70. doi: 10.1055/s-2007-1013792. [DOI] [PubMed] [Google Scholar]
- Vertrees 1996 {published data only} .Vertrees RA, Conti VR, Lick SD, Zwischenberger JB, McDaniel LB, Shulman G. Adverse effects of postoperative infusion of shed mediastinal blood. Annals of Thoracic Surgery. 1996;62(3):717–23. doi: 10.1016/s0003-4975(96)00390-6. [DOI] [PubMed] [Google Scholar]
Additional references
- Baker 2009 .Baker RA. Suction, salvage, sutures, and potions: blood management post-aprotinin. Seminars in Cardiothoracic and Vascular Anesthesia. 2009;13(2):122–6. doi: 10.1177/1089253209337159. [DOI] [PubMed] [Google Scholar]
- Brown 2000 .Brown P. BSE and transmission through blood. Lancet. 2000 Sep 16;356:955–6. doi: 10.1016/S0140-6736(00)02706-9. [DOI] [PubMed] [Google Scholar]
- Bryson 1998 .Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The International Study of Perioperative Transfusion. Anesthesia and Analgesia. 1998;86(1):9–15. doi: 10.1213/00000539-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Carson 1998 .Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion. 1998;38(6):522–9. doi: 10.1046/j.1537-2995.1998.38698326331.x. [DOI] [PubMed] [Google Scholar]
- Carson 2002 .Carson JL, Hill S, Carless P, Hebert P, Henry D. Transfusion triggers: a systematic review of the literature. Transfusion Medicine Reviews. 2002;16(3):187–99. doi: 10.1053/tmrv.2002.33461. [DOI] [PubMed] [Google Scholar]
- Cook 1991 .Cook SS, Epps J. Transfusion practice in central Virginia. Transfusion. 1991;31(4):355–60. doi: 10.1046/j.1537-2995.1991.31491213303.x. [DOI] [PubMed] [Google Scholar]
- Coyle 1999 .Coyle D, Lee KM, Fergusson DA, Laupacis A. Economic analysis of erythropoietin use in orthopaedic surgery. Transfusion Medicine. 1999;9(1):21–30. doi: 10.1046/j.1365-3148.1999.009001021.x. [DOI] [PubMed] [Google Scholar]
- Davies 2006 .Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technology Assessment. 2006;10(44):1–228. doi: 10.3310/hta10440. [DOI] [PubMed] [Google Scholar]
- Der Simonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dickersin 1996 .Dickersin K, Larson K. Cochrane Collaboration. Cochrane Collaboration; Oxford. United Kingdom. Oxford. United Kingdom.(UK): 1996. Establishing and maintaining an international register of RCTs. [Google Scholar]
- Faught 1998 .Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfusion Medicine Reviews. 1998;12(3):206–25. doi: 10.1016/s0887-7963(98)80061-8. [DOI] [PubMed] [Google Scholar]
- Fergusson 1999a .Fergusson D, van Walraven C, Coyle D, Laupacis A. Economic evaluations of technologies to minimize perioperative transfusion: a systematic review of published studies. International Study of Peri-operative Transfusion (ISPOT) Investigators. Transfusion Medicine Reviews. 1999;13(2):106–17. doi: 10.1016/s0887-7963(99)80005-4. [DOI] [PubMed] [Google Scholar]
- Fergusson 1999b .Fergusson D, Blair A, Henry D, Hisashige A, Huet C, Koopman-van Gemer A, et al. Technologies to minimize blood transfusion in cardiac and orthopedic surgery. Results of a practice variation survey in nine countries. International Study of Peri-operative Transfusion (ISPOT) Investigators. International Journal of Technology Assessment in Health Care. 1999;15(4):717–28. [PubMed] [Google Scholar]
- Fergusson 2008 .Fergusson DA, Hebert PC, Mazer CD, Fremes SE, MacAdams C, Murkin JM, et al. A comparison aprotinin and lysine analogues in high-risk cardiac surgery. New England Journal of Medicine. 2008;358(22):2319–31. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- Forgie 1998 .Forgie M, Wells P, Laupacis A, Fergusson D. Pre-operative autologous donation decreases allogeneic transfusion but increases exposure to all red cell transfusion - results of a meta analysis. Archives of Internal Medicine. 1998;Vol. 158:610–6. doi: 10.1001/archinte.158.6.610. [DOI] [PubMed] [Google Scholar]
- Glynn 2000 .Glynn SA, Kleinman SH, Schreiber GB. Trends in incidence and prevalence of major transfusion - Transmissible viral infections in US blood donors, 1991 to 1996. JAMA. 2000;284(2):229–35. doi: 10.1001/jama.284.2.229. [DOI] [PubMed] [Google Scholar]
- Hadjianastassiou 2002 .Hadjianastassiou VG, Virich G, Lennox IA. Use of the blood transfusion service in total knee replacement arthroplasty. The cost implications. Knee. 2002;9(2):145–8. doi: 10.1016/s0968-0160(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Hebert 1999 .Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators: Canadian Critical Care Trials Group. New England Journal of Medicine. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Henry 2007 .Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, McClelland B, Laupacis A, Fergusson D. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD001886.pub2. [DOI: 10.1002/14651858.CD001886.pub2] [DOI] [PubMed] [Google Scholar]
- Higgins 2009 .Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 The Cochrane Collaboration; 2009. [Google Scholar]
- Hill 2003 .Hill SR, Carless PA, McClelland B, Henry DA, Henderson KM, Carson J, et al. Transfusion thresholds and other strategies for guiding alogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews. 2003;(Issue 2) doi: 10.1002/14651858.CD002042. [DOI: 10.1002/14651858.CD002042] [DOI] [PubMed] [Google Scholar]
- Houston 2000 .Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Transmission of BSE by blood transfusion in sheep. Lancet. 2000 Sep 16;356:999–1000. doi: 10.1016/s0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- Huber 1997 .Huber TS, McGorray SP, Carlton LC, Irwin PB, Flug RR, Flynn TC, et al. Intraoperative autologous transfusion during elective infrarenal aortic reconstruction: A decision analysis model. Journal of Vascular Surgery. 1997;25:984–94. [PubMed] [Google Scholar]
- Huet 1999 .Huet C, Salmi LR, Fergusson D, Koopman-van Gemert AW, Rubens F, Laupacis A. A meta-analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesthesia and Analgesia. 1999;89(4):861–9. doi: 10.1097/00000539-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Kimball 1995 .Kimball AM, Berkley S, Ngugi E, Gayle H. International aspects of the AIDS/HIV epidemic. Annual Review of Public Health. 1995;16:253–82. doi: 10.1146/annurev.pu.16.050195.001345. [DOI] [PubMed] [Google Scholar]
- Lackritz 1998 .Lackritz EM. Prevention of HIV transmission by blood transfusion in the developing world: achievements and continuing challenges. AIDS. 1998;12(Suppl A):81–6. [PubMed] [Google Scholar]
- Laupacis 1997 .Laupacis A, Fergusson D. Drugs to minimize perioperative blood loss in cardiac surgery: meta-analyses using perioperative blood transfusion as the outcome. International Study of Peri-operative Transfusion (ISPOT) Investigators. Anesthesia and Analgesia. 1997;85(6):1258–67. doi: 10.1097/00000539-199712000-00014. [DOI] [PubMed] [Google Scholar]
- Lenfant 1992 .Lenfant C. Transfusion practice should be audited for both undertransfusion and overtransfusion. Transfusion. 1992;32(9):873–4. doi: 10.1046/j.1537-2995.1992.32993110764.x. [DOI] [PubMed] [Google Scholar]
- McFarland 1997 .McFarland W, Mvere D, Shandera W, Reingold A. Epidemiology and prevention of transfusion-associated human immunodeficiency virus transmission in sub-Saharan Africa. Vox Sanguinis. 1997;72(2):85–92. doi: 10.1046/j.1423-0410.1997.7220085.x. [DOI] [PubMed] [Google Scholar]
- Oliver 2002 .Oliver AM. Strategies for handling a potentially diminished blood supply. The potential shortage. Transfusion Medicine. 2002;Vol. 12:1–12. [Google Scholar]
- Ray 1999 .Ray MJ, Brown KF, Burrows CA, O’Brien MF. Economic evaluation of high-dose and low-dose aprotinin therapy during cardiopulmonary bypass. Annals of Thoracic Surgery. 1999;68(3):940–5. doi: 10.1016/s0003-4975(99)00682-7. [DOI] [PubMed] [Google Scholar]
- Regan 2002 .Regan F, Taylor C. Blood transfusion medicine. BMJ. 2002;325(7356):143–7. doi: 10.1136/bmj.325.7356.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz 1995 .Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Semmens 2000 .Semmens JB, Lawrence-Brown MM, Miles LE, Hellings MJ. Intraoperative blood salvage: the missing link in providing a safe and effective blood transfusion service.[comment] Medical Journal of Australia. 2000;173(5):266–8. doi: 10.5694/j.1326-5377.2000.tb125636.x. [DOI] [PubMed] [Google Scholar]
- Spahn 2000 .Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242–55. doi: 10.1097/00000542-200007000-00035. [DOI] [PubMed] [Google Scholar]
- Surgenor 1990 .Surgenor DM, Wallace EL, Hao SH, Chapman RH. Collection and transfusion of blood in the United States, 1982-1988. New England Journal of Medicine. 1990;322(23):1646–51. doi: 10.1056/NEJM199006073222306. [DOI] [PubMed] [Google Scholar]
- Wallace 1993 .Wallace EL, Surgenor DM, Hao HS, An J, Chapman RH, Churchill WH. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993;33(2):139–44. doi: 10.1046/j.1537-2995.1993.33293158046.x. [DOI] [PubMed] [Google Scholar]
- Whyte 1997 .Whyte GS, Savoia HF. The risk of transmitting HCV, HBV or HIV by blood transfusion in Victoria. Medical Journal of Australia. 1997;166(11):584–6. doi: 10.5694/j.1326-5377.1997.tb123269.x. [DOI] [PubMed] [Google Scholar]