Abstract
Background
Long-term treatment with antipsychotic medications in early episode schizophrenia spectrum disorders is common, but both short and long-term effects on the illness are unclear. There have been numerous suggestions that people with early episodes of schizophrenia appear to respond differently than those with multiple prior episodes. The number of episodes may moderate response to drug treatment.
Objectives
To assess the effects of antipsychotic medication treatment on people with early episode schizophrenia spectrum disorders.
Search methods
We searched the Cochrane Schizophrenia Group register (July 2007) as well as references of included studies. We contacted authors of studies for further data.
Selection criteria
Studies with a majority of first and second episode schizophrenia spectrum disorders comparing initial antipsychotic medication treatment with placebo, milieu, or psychosocial treatment.
Data collection and analysis
Working independently, we critically appraised records from 681 studies, of which five studies met inclusion criteria. We calculated risk ratios (RR) and their 95% confidence intervals (CI) where possible. For continuous data, we calculated mean difference (MD). We calculated numbers needed to treat/harm (NNT/NNH) where appropriate.
Main results
Five studies (combined total n=998) met inclusion criteria. Four studies (n=724) provided leaving the study early data and results suggested that individuals treated with a typical antipsychotic medication are less likely to leave the study early than those treated with placebo (Chlorpromazine: 3 RCTs n=353, RR 0.4 CI 0.3 to 0.5, NNT 3.2, Fluphenaxine: 1 RCT n=240, RR 0.5 CI 0.3 to 0.8, NNT 5; Thioridazine: 1 RCT n=236, RR 0.44 CI 0.3 to 0.7, NNT 4.3, Trifulperazine: 1 RCT n=94, RR 0.96 CI 0.3 to 3.6). Two studies contributed data to assessment of adverse effects and present a general pattern of more frequent side effects among individuals treated with typical antipsychotic medications compared to placebo. One trial suggested a higher rehospitalisation rate for those receiving chlorpromazine compared to placebo (n=80, RR 2.29 CI 1.3 to 4.0, NNH 2.9). However, a higher attrition in the placebo group is likely to have introduced a survivor bias into this comparison, as this difference becomes non-significant in a sensitivity analysis on intent-to-treat participants (n=127, RR 1.69 CI 0.9 to 3.0). One study contributes data to a comparison of trifluoperazine to psychotherapy on long-term health in favour of the trifluoperazine group (n=92, MD 5.8 CI 1.6 to 0.0); however, data from this study are also likely to contain biases due to selection and attrition. One other study contributes data to a comparison of typical antipsychotic medication to psychosocial treatment on six-week outcome measures of global psychopathology (n=89, MD 0.01 CI −0.6 to 0.6) and global improvement (n=89, MD −0.03 CI −0.5 to 0.4), indicating no between-group differences. On the whole, there is very little useable data in the few studies meeting inclusion criteria.
Authors’ conclusions
With only a few studies meeting inclusion criteria, and with limited useable data in these studies, it is not possible to arrive at definitive conclusions. The preliminary pattern of evidence suggests that people with early episode schizophrenia treated with typical antipsychotic medications are less likely to leave the study early, but more likely to experience medication-related side effects. Data are too sparse to assess the effects of antipsychotic medication on outcomes in early episode schizophrenia.
Medical Subject Headings (MeSH): Antipsychotic Agents [adverse effects; *therapeutic use], Chlorpromazine [therapeutic use], Fluphenazine [therapeutic use], Patient Dropouts, Randomized Controlled Trials as Topic, Schizophrenia [*drug therapy], Thioridazine [therapeutic use], Trifluoperazine [therapeutic use]
MeSH check words: Humans
BACKGROUND
In early-episode schizophrenia spectrum psychosis, clinical practice guidelines recommend intervention with conventional or atypical antipsychotic medication for at least one year (APA 2004;CPA 1998; Frances 1996; Gaebel 2005; National 2002). At the heart of this recommendation is an assumption that early antipsychotic treatment is beneficial. The overall risk-benefit balance is thought, in the short term (Kane 1993; Lehman 1998) as well as long term (Dixon 1995; Kane 1993; Lehman 1998; Wyatt 1991) to be favourable and outweighs risks of drug-induced adverse effects (Popp 1998). This is thought to be particularly true in view of the more benign adverse effect profiles of the atypical or second-generation medications.
The conclusion of a long-term benefit from immediate antipsychotic treatment in early episodes (Wyatt 1991) has several far-reaching implications, including:
emphasising the importance of early antipsychotic treatment in psychosis (DeQuardo 1998; Falloon 1998; Lewander 1996;Linszen 1998);
discouraging drug-free research on the ethical grounds of withholding a proven treatment (Kirch 1992);
contributing to the psychosis may be biologically toxic hypothesis (Norman 2001); and
stimulating interest in primary prevention through antipsychotic treatment of high-risk adolescents (Cornblatt 2001;DeGrazia 2001; McGlashan 2001; McGorry 2001; Warner 2001;Yung 1998).
An influential review on this important early treatment question incorporated many uncontrolled studies and used an unspecified analytic method (Wyatt 1991). A recent meta-analysis failed to find a long-term advantage from initial antipsychotic treatment in early episodes (Bola 2006), yet included only published studies. The Cochrane review on chlorpromazine for schizophrenia (Thornley 2006) acknowledged that there may be differences in treatment response for people in their first episode of illness, such as the lower effect observed in the first-episode, multi-site, double-blind NIMH study (Cole 1966; Schooler 1967). Thornley 2006, however, only assesses one medication. The few available early episode studies of chlorpromazine do not permit a sensitivity analysis comparing response across episodes. In evaluating relapse rates in people who have been withdrawn from medication, Gaebel 2002 found different rates of relapse across treatments when comparing people in their first episodes with those later in their illnesses (Pietzcker 1993a). This suggests that episode may moderate treatment response. A related Cochrane review, Rummel 2003, compares second generation antipsychotic medications with conventional (first-generation medications) in people in their first episode of illness. Rummel 2003 identified few relevant studies and, although outcomes such as leaving the study early did favour the newer drugs, other findings on global and mental state were not convincing.
In this review we examine the evidence on the effects of antipsychotic medications in early episode schizophrenia spectrum, which has a broader definition than simply first episode. There is a lack of evidence for any differential responsiveness to treatment when comparing people in their first episode with those in their second (Bola 1998). We therefore decided to include people in their first or second episode of psychotic illness in this review and by doing so hope to thoroughly examine the literature in relation to a pragmatic definition of early episode schizophrenia spectrum disorders.
OBJECTIVES
To assess the effects of antipsychotic medication treatment on people with early episode schizophrenia spectrum disorders.
METHODS
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. (RCTs) If a trial was described as ‘double blind’ but implied randomisation, we included such trials in a sensitivity analysis. If their inclusion did not result in a substantive difference, they remained in the analyses. If their inclusion resulted in statistically significant differences, we did not add the data from these lower quality studies to the results of the better trials, but presented such data within a subcategory. We excluded quasi-randomised studies, such as those allocating by alternate days of the week.
Types of participants
We included people with first and second episode schizophrenia spectrum disorders. Studies needed to have more than 50% of participants with these disorders. There is no clear evidence that the different diagnostic categories included in the schizophrenia spectrum (e.g. brief reactive psychosis, schizophreniform disorder, schizophrenia, schizoaffective disorder, delusional disorder, etc.) are caused by fundamentally different processes or require different treatment approaches (Carpenter 1994).
Types of interventions
1. Antipsychotic medications
Conventional or first-generation medications or atypical, second-generation medications, any dose range.
2. Placebo
3. No treatment, milieu
4. Psychosocial interventions
Types of outcome measures
We divided outcomes into very short-term (up to twelve weeks), short-term (less than six months), medium-term (7-12 months) and long-term (more than one year).
Primary outcomes
1. Global state
1.1 Relapse
2. Service outcomes
2.1 Hospitalisation
3. Mental state
3.1 No clinically important change in general mental state
4. Adverse effects
4.1 Clinically important general adverse effects
Secondary outcomes
1. Death - suicide or natural causes
2. Leaving the study early
3. Global state
3.1 Time to relapse
3.2 No clinically important change in global state
3.3 Not any change in global state
3.4 Average endpoint global state score
3.5 Average change in global state scores
4. Service outcomes
4.1 Time to hospitalisation
4.2 Days in hospital
4.3 Change in hospital status
5. Mental state
5.1 Not any change in general mental state
5.2 Average endpoint general mental state score
5.3 Average change in general mental state scores
5.4 No clinically important change in specific symptoms
5.5 Not any change in specific symptoms
5.6 Average endpoint specific symptom score
5.7 Average change in specific symptom scores
6. Leaving the study early
6.1 For specific reasons
6.2 For general reasons
7. General functioning
7.1 No clinically important change in general functioning
7.2 Not any change in general functioning
7.3 Average endpoint general functioning score
7.4 Average change in general functioning scores
7.5 No clinically important change in specific aspects of functioning, such as social or life skills
7.6 Not any change in specific aspects of functioning, such social or life skills
7.7 Average endpoint specific aspects of functioning, such as social or life skills
7.8 Average change in specific aspects of functioning, such as social or life skills
8. Behaviour
8.1 No clinically important change in general behaviour
8.2 Not any change in general behaviour
8.3 Average endpoint general behaviour score
8.4 Average change in general behaviour scores
8.5 No clinically important change in specific aspects of behaviour
8.6 Not any change in specific aspects of behaviour
8.7 Average endpoint specific aspects of behaviour
8.8 Average change in specific aspects of behaviour
9. Adverse effects
9.1 Any general adverse effects
9.2 Average endpoint general adverse effect score
9.3 Average change in general adverse effect scores
9.4 No clinically important change in specific adverse effects
9.5 Not any change in specific adverse effects
9.6 Average endpoint specific adverse effects
9.7 Average change in specific adverse effects
10. Engagement with services
10.1 No clinically important engagement
10.2 Not any engagement
10.3 Average endpoint engagement score
10.4 Average change in engagement scores
11. Satisfaction with treatment
11.1 Recipient of care not satisfied with treatment
11.2 Recipient of care average satisfaction score
11.3 Recipient of care average change in satisfaction scores
11.4 Carer not satisfied with treatment
11.5 Carer average satisfaction score
11.6 Carer average change in satisfaction scores
12. Quality of life
12.1 No clinically important change in quality of life
12.2 Not any change in quality of life
12.3 Average endpoint quality of life score
12.4 Average change in quality of life scores
12.5 No clinically important change in specific aspects of quality of life
12.6 Not any change in specific aspects of quality of life
12.7 Average endpoint specific aspects of quality of life
12.8 Average change in specific aspects of quality of life
13. Economic outcomes
13.1 Direct costs
13.2 Indirect costs
Search methods for identification of studies
1. Electronic searching
We searched the Cochrane Schizophrenia Group register with the phrases: [*early* OR *prodrom* OR *first?episo* OR *second?episo* OR *primary?episo* OR *secondary?episo* in title, abstract and index terms of REFERENCE] or [Antip* or drug*or tranquil* in interventions of STUDY]
The Schizophrenia Group’s trials register is based on regular searches of BIOSIS Inside, CENTRAL, CINAHL, EMBASE, MEDLINE and PsycINFO; the hand searching of relevant journals and conference proceedings, and searches of several key grey literature sources. A full description is given in the Group’s module.
1. Reference searching
We inspected references of all identified studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
JB and DK independently inspected citations from the searches and identify relevant abstracts. SH independently re-inspected a random 20% sample to ensure reliability. Where disputes arose, we acquired the full report for more detailed scrutiny. JB and DK obtained and inspected full reports of the abstracts meeting the review criteria. Again, SH re-inspected a random 20% of reports in order to ensure reliable selection. When it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
Reviewers (JB, DK) extracted data from all included studies. In addition, to ensure reliability, HS independently extracted data from a random sample of these studies, comprising 10% of the total. Again, we discussed any disagreements, documented decisions and, if necessary, contacted authors of studies for clarification. We extracted data presented only in graphs and figures whenever possible, but included only if two reviewers independently had the same result. We attempted to contact authors through an open-ended request in order to obtain missing information or for clarification whenever necessary. If studies were multi-centre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale-derived data
We included continuous data from rating scales only if a. the psychometric properties of the measuring instrument have been described in a peer-reviewed journal (Marshall 2000); and b. the measuring instrument has not been written or modified by one of the trialists for that particular trial. Ideally the measuring instrument should either be i. a self-report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, and we noted in the Description of studies if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between person variability from the analysis. On the other hand calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided to primarily use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis and we used mean differences (MD) rather than standardised mean differences throughout (Higgins 2009, Chapter 9.4.5.2).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non-parametric data, we aimed to apply the following standards to all data before inclusion: a) standard deviations and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the standard deviation, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996); c) if a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(SS min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. Skewed data from studies of less than 200 participants were entered into additional tables rather than into an analysis. Skewed data pose less of a problem when looking at means, if the sample size is large we entered this data into syntheses.
2.5 Common measure
To facilitate comparison between trials, where relevant we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This was done by identifying cut-off points on rating scales and dividing participants accordingly into ‘clinically improved’ or ‘not clinically improved’. It is generally assumed that if there is a 50% reduction in a scale-derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut-off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for typical antipsychotic training. Where keeping to this made it impossible to avoid outcome titles with clumsy double-negatives (e.g. ‘Not improved’) we reported data where the left of the line indicated an unfavourable outcome. This was noted in the relevant graphs.
2.8 Summary of findings table
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADE Profiler (GRADE Profiler) to import data from Review Manager 5 (Review Manager (RevMan)) to create ‘Summary of findings’ tables. These tables provide outcome-specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient-care and decision making. We selected the following main outcomes for inclusion in the summary of findings table:
1. Leaving the study early
2. Clinical response
Clinically significant response in global state - as defined by each of the studies
3. Service utilisation outcomes
Hospital admission, readmission
4. Adverse effects
Any important adverse event
Assessment of risk of bias in included studies
Again JB and DK worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting. If the raters disagreed, the final rating was made by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, authors of the studies were contacted in order to obtain further information. Non-concurrence in quality assessment was reported, but if disputes arose as to which category a trial should be allocated, again, resolution was made by discussion. The level of risk of bias was noted in both the text of the review and in the Summary of findings tables.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). For statistically significant results we had planned to calculate the number needed to treat to provide benefit/to induce harm statistic (NNTB/H), and its 95% confidence interval (CI) using Visual Rx (http://www.nntonline.net/) taking account of the event rate in the control group. This, however, has been superseded by Summary of findings tables and calculations therein.
2. Continuous data
For continuous outcomes we estimated the mean difference (MD) between groups. We prefer not to calculate effect size measures (standardised mean difference SMD). However, if scales of very considerable similarity are used, we would have presumed there was a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ ‘cluster randomisation’ (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra-class correlation in clustered studies, leading to a ‘unit of analysis’ error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999). Where clustering is not accounted for in primary studies, we presented such data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra-class correlation coefficients for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we presented these data as if from a non-cluster randomised study, but adjusted for the clustering effect. We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a ‘design effect’. This is calculated using the mean number of participants per cluster (m) and the intra-class correlation coefficient (ICC) (Design effect= 1+(m-1)*ICC) (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account intra-class correlation coefficients and relevant data documented in the report, synthesis with other studies was possible using the generic inverse variance technique.
2. Cross-over trials
A major concern of cross-over trials is the carry-over effect. It occurs if an effect (e.g. pharmacological, physiological, or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase, the participants can differ systematically from their initial state despite a wash-out phase. For the same reason cross-over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data of the first phase of cross-over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added and combined these data within the two-by-two table. If data were continuous we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook. Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow-up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome is between 0% and 50% and where these data are not clearly described, we will present data on a ‘once-randomised-always-analyse’ basis (an intention-to-treat analysis). Those leaving the study early are all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes we will use the rate of those who stayed in the study - in that particular arm of the trial - for those who did not. We will undertake a sensitivity analysis testing how prone the primary outcomes are to change when ‘completer’ data only are compared to the intention-to-treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome is between 0% and 50%, we reported completer-only data.
3.2 Standard deviations
If standard deviations were not reported, we tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error and confidence intervals available for group means, and either P value or T value available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook (Higgins 2009): when only the standard error (SE) is reported, standard deviations (SDs) were calculated by the formula SD=SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook (Higgins 2009) present detailed formula for estimating SDs from P values, T or F values, confidence intervals, ranges or other statistics. If these formula did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data have been used in the trial, if less than 50% of the data have been assumed, we reproduced these data and indicated that they are the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arise, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arise, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies was investigated by considering the I2 method alongside the Chi2 ‘p’ value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chanceHiggins 2003. The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. ‘p’ value from Chi2 test, or a confidence interval for I2). I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, was interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 - Higgins 2009. When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions Higgins 2009). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small-study effects. We will not use funnel plots for outcomes where there are ten or fewer studies, or where all studies are of similar sizes. In other cases, where funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed or random-effects models. The random-effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random-effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect these studies can either inflate or deflate the effect size. We chose the fixed effects model for all analyses. The reader is, however, able to choose to inspect the data using the random model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses - only primary outcomes
1.1 Gender
If sufficient data were available we used subgroup analyses to determine if initial antipsychotic treatment had different effects on the primary outcomes for men or women.
2. Investigation of heterogeneity
If inconsistency is high, this was reported. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed studies outside of the company of the rest to see if heterogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, we have not pooled data and have discussed issues. We know of no supporting research for this 10% cut off but are investigating use of prediction intervals as an alternative to this unsatisfactory state. When unanticipated clinical or methodological heterogeneity were obvious we simply stated hypotheses regarding these for future reviews or versions of this review. We do not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they are described in some way as to imply randomisation. For the primary outcomes we included these studies and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we employed all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow-up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with complete data only. We undertook a sensitivity analysis testing how prone results were to change when we compared ‘completer’ data only to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available): allocation concealment, blinding and outcome reporting for the meta-analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then data from these trials were included in the analysis
4. Imputed values
We also undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster randomised trials.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
For substantive descriptions of studies please see: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We inspected 670 records provided by the Cochrane Schizophrenia Group search (July 2007) and an additional 11 records known to us or suggested by reviewers. Only five studies with a combined sample of n=998, all using typical antipsychotic medications compared to placebo or psychosocial treatment, met inclusion criteria. Data reporting is generally poor, with most studies providing no useable outcome data.
Included studies
We found five studies for inclusion (Cole 1964; May 1976; Mosher 1995; Rappaport 1978; Simon 1965) that randomised a total of 998 participants.
Cole 1964 (n=463) conducted a multi-site double-blind placebo versus chlorpromazine acute treatment trial of six weeks for people (mostly) diagnosed with first-episode acute schizophrenia sponsored by the National Institute of Mental Health in the United States. The acute trial was followed up a year later (Schooler 1967).May 1976 (n=228) conducted an acute treatment comparison (of unspecified duration) of five treatments (psychotherapy, trifluoperazine, psychotherapy plus trifluoperazine, ECT, and milieu therapy) for people with first episode of schizophrenia deemed in the middle third of the prognostic spectrum (i.e., not remitting within an average 18-day waiting period, and deemed to not be at high risk for long-term schizophrenia). The acute treatment comparison study was followed up in successive studies up to 10 years.Mosher 1995 (n=100) conducted a six-week randomised comparison of hospital treatment with a typical antipsychotic to milieu treatment in a supervised community residence for people with first-episode schizophrenia type psychosis.
Rappaport 1978 (n=127) conducted an randomised comparison of chlorpromazine versus placebo in the hospital for men diagnosed with first episode of schizophrenia. The length of the initial treatment period is unclear, and there was a post-discharge follow-up three years later.
Simon 1965 (n=80) conducted a 30-day acute treatment comparison of four treatments (chlorpromazine, reserpine, clinical judgement, and hospital routine) in a hospital setting for males diagnosed with schizophrenia that had no prior treatment.
1. Length of studies
Five of the studies were, in the acute treatment phase, “very short-term” with durations of 30 days to eight weeks. Two others (May 1976; Rappaport 1978) were of unclear duration. Follow-up periods ranged widely, from none (Simon 1965) to one year (Cole 1964); one to three years (Rappaport 1978); two years (Mosher 1995); and in successive studies up to 10 years (May 1976).
2. Participants
The majority of participants were adults with a first or second episode of schizophrenia-type psychosis, or (in some cases) experiencing their first hospitalisation for psychosis (e.g. Cole 1964).
3. Setting
Five of the studies were entirely based in the hospital, with one study (Mosher 1995) comparing hospital treatment with treatment in a supervised community facility. All studies were conducted in the USA.
4. Study Size
The numbers of participants were 463 (Cole 1964), 228 (May 1976), 100 (Mosher 1995), 127 (Rappaport 1978), and 80 (Simon 1965).
5. Interventions
5.1 Antipsychotics
5.1.1 Chlorpromazine
In Cole 1964, chlorpromazine dosage ranged 200 to 1600 mg/day or 50 to 400 mg/day (IM); in Rappaport 1978, the dosage was 300 to 900 mg/day; in Simon 1965 the dosage was from 200 mg/day to no maximum dose. May 1976 did not use chlorpromazine, and in Mosher 1995 the typical antipsychotics used were unspecified.
5.1.2 Fluphenazine
Used in Cole 1964, with dosages of 2 to 16 mg/day or 1 to 8 mg/ day (IM).
5.1.3 Thioridazine
Used in Cole 1964, with dosages of 200 to 1600 mg/day or 50 to 400 mg/day (IM).
5.1.4 Trifluoperazine
Used in May 1976 at dosages of 10 to 120mg/day.
5.1.5 Resperine
Used in Simon 1965, dosages from 2 mg/day up to no maximum dosage.
5.2 Other therapies
5.2.1 Individual psychotherapy
Used in May 1976 - Psychotherapy for a minimum of two hours per week.
5.2.2 Individual psychotherapy plus trifluoperazine
Used in May 1976. Psychotherapy plus 10 to 40mg/day of trifuloperazine.
5.2.3 Electroconvulsive treatment (ECT)
Used in May 1976.
5.2.4 Milieu therapy
Used in May 1976. In Simon 1965 this is described as “hospital routine” treatment.
5.2.5 Hospital treatment with antipsychotic medications
Used in Mosher 1995, the type of typical antipsychotic medication and the dosages used were unspecified.
5.2.6 Non-hospital milieu treatment
Used in Mosher 1995, one group received a non-hospital milieu treatment combined with a time-limited antipsychotic postponement period of up to six weeks.
5.3 Placebo
Used in Cole 1964 and Rappaport 1978.
6. Outcomes
Data reporting in the studies was generally very poor. The studies compared a total of 13 different treatments, yet we could only analyse data for five comparisons.
6.1 Outcome scales
Scale data reporting, again was poor. The studies used 14 different scales to collect scale data but we could only use data from three scales. These are described below; reasons for excluding data from the other scales are given in the outcome sections of the Characteristics of included studies table.
6.1.1 Global outcomes
6.1.1.1 Global Rating Scale (Cole 1964; Mosher 1995 )
A seven-point ordinal global rating of mental illness developed byCole 1964 and also used by Mosher 1995
6.1.1.2 Global Improvement Scale (Cole 1964; Mosher 1995)
A seven-point ordinal rating of improvement in mental illness developed by Cole 1964 and also used by Mosher 1995.
6.1.1.3 Menninger Health-Sickness Rating Scale (MHSRS;Luborsky 1962)
One hundred point scale; higher score is better.
6.2 Missing outcomes
None of the included studies attempted to quantify death, service use, satisfaction, or quality of life. There is no evidence of any direct economic evaluation of treatments for early episode schizophrenia.
Excluded studies
1. Excluded studies
We excluded 675 of 681 studies. The primary reason for excluding studies was the lack of a non-medication treated group. Many studies compared types of medications, including some that compared conventional and atypical antipsychotic medications. It might be reasonable to address these comparisons in a subsequent version of this review. A second main reason for excluding studies is that they were medication-withdrawal, follow-up or other types of non-acute studies that address questions other than the effectiveness of initial treatment for early episode schizophrenia psychoses.
2. Awaiting Assessment
We are still seeking unpublished data for one study that appears to meet inclusion criteria for this review (Johnstone 1988).
3. Ongoing studies
One study in Melbourne (Francey 2010) is currently recruiting participants into an RCT of psychosocial treatment (Cognitive Behavioural Therapy plus Family Psycho-education) in both groups, and either placebo or low-dose antipsychotic medication for people with an acute first episode of psychosis.
Risk of bias in included studies
We used the tool for assessment of bias described in the Cochrane Handbook (Higgins 2009). The quality of randomisation in the studies is generally unclear. Several studies report higher rates of attrition in the non-medicated groups, with the potential for survivor bias. One study (May 1976) intentionally selected a “middle-third” of first-episode patients, but did not report operational selection criteria.
For overall view of risk of bias, see Figure 1 and Figure 2.
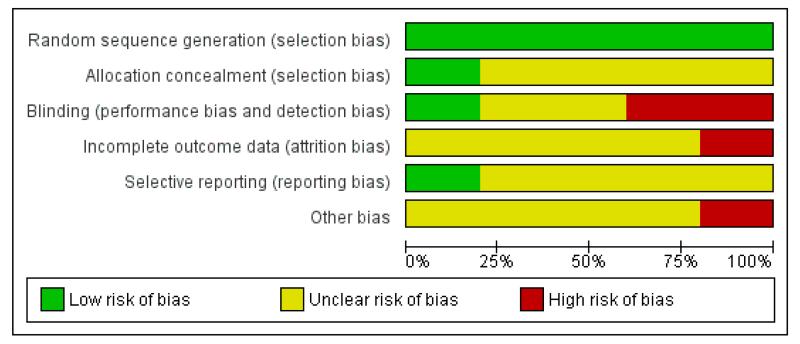
Figure 1.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
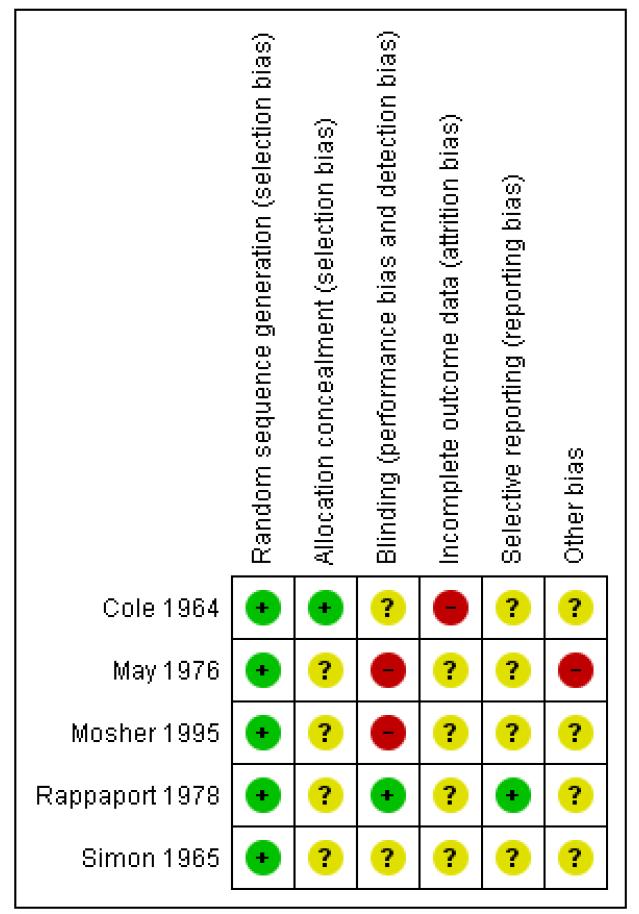
Figure 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
Each included study indicated that allocation to treatment was made by random assignment.
Blinding
Two studies reported double blinding (Cole 1964), two reported single blinding (May 1976; Rappaport 1978), one was an open study with treatments at different sites (Mosher 1995), and there are no details on blinding reported from one study (Simon 1965).
Incomplete outcome data
May 1976 did not report attrition, but successive follow-up studies have diminishing sample sizes. Mosher 1995 reports six-week outcomes for subjects completing different minimum durations of treatment (seven days in the hospital versus 28 days in the community milieu treatment). Rappaport 1978 reported differential attrition by treatment group and suggested it as a possible bias regarding treatment differences. One study (Simon 1965) did not provide attrition information.
Selective reporting
There are few details on selective reporting, aside from the differential attrition acknowledged in Rappaport 1978.
Other potential sources of bias
The May 1976 study selected first-episode subjects judged to be in the “middle third” of the prognostic spectrum, but did not provide operational criteria for this selection that could be used in a replication. Generalisability of results from this study is therefore limited.
Effects of interventions
We found only five very short-term trials that used a total of 15 different treatments. Several studies had follow-ups of varying lengths. Data were not available for all outcomes, as reporting was generally poor.
1. Comparison 1: Chlorpromazine versus placebo
1.1 Leaving the study early
Three studies (Cole 1964; Rappaport 1978; Simon 1965) provided data indicating those in the placebo groups were significantly more likely to leave the study early (3 RCTs, n=353, RR 0.40 CI 0.29 to 0.54, NNT 3.2 CI 2.5 to 4.55) (Analysis 1.1).
1.2 Global state: not improved after eight years
One study (Simon 1965) (n=40) contributed data to an assessment of improvement versus non-improvement that does not find a significant between group difference in rates of improvement (1 RCT, n=40, RR 0.76 CI 0.53 to 1.11, NNT 5 CI 2.17 to 16.67) (Analysis 1.2).
1.3 Rehospitalisation within three years - completer
One study (Rappaport 1978) (n=80) indicated a higher rehospitalisation rate for chlorpromazine treated completing patients compared to placebo (1 RCT, n=80, RR 2.29 CI 1.31 to 4.03, NNT 2.9 CI 1.82 to 7.14) (Analysis 1.3).
1.4 Rehospitalisation within three years - intent to treat
Due to higher attrition in the placebo group in Rappaport 1978, we conducted a sensitivity analysis on an intent-to-treat basis, which remains statistically significant (1 RCT, n=127, RR 3.05 CI 1.64 to 5.67, NNT 3.33 CI 2.17 to 6.67), indicating that placebo treated subjects had lower rehospitalisation rates (Analysis 1.4).
1.5 Adverse effects: various outcomes
Only one study (Cole 1964) (n=162) contributed data to an assessment of side effects and presents a pattern of more frequent side effects among individuals treated with chlorpromazine compared to placebo. Five of 13 adverse effect measures were statistically significant, each in the direction indicating more adverse effects associated with chlorpromazine treatment compared to placebo (Summary of findings table 1; Analysis 1.5).
1.5.1 Drowsiness
Individuals treated with chlorpromazine were more likely to experience drowsiness (1 RCT, n=162, RR 5.65 CI 2.72 to 11.73, NNT2.27 CI 1.79 to 3.13).
1.5.2 Restlessness
Data were equivocal for restlessness (1 RCT, n=162, RR 1.19 CI 0.83 to 1.71).
1.5.3 Constipation
Individuals treated with chlorpromazine were more likely to experience constipation (1 RCT, n=162, RR 2.71, CI 1.37 to 5.35, NNT4.76 CI 3.03 to 12.5).
1.5.3 Nausea or upper gastrointestinal distress
Individuals treated with chlorpromazine were more likely to experience nausea or upper gastrointestinal distress (1 RCT, n=162, RR 6.17 CI 1.92 to 19.79, NNT 4.76, CI 3.23 to 9.09).
1.5.5 Dryness of mouth or throat
Individuals treated with chlorpromazine were more likely to experience dryness of mouth or throat (1 RCT, n=162, RR 4.63 CI 1.67 to 12.82, NNT 5.0 CI 3.3 to 11.11).
1.5.6 Dizziness, faintness, or weakness
Individuals treated with chlorpromazine were more likely to experience dizziness, faintness or weakness (1 RCT, n=162, RR 4.41 CI 1.59 to 12.29, NNT 5.56 CI 3.45 to 12.5).
2. Comparison 2: Fluphenazine versus placebo
Only one study (Cole 1964) compared fluphenazine with placebo
2.1 Leaving the study early
Those treated with placebo were more likely to leave early (1 RCT, n=240, RR 0.51 CI 0.34 to 0.77, NNT 5 CI 3.23 to 11.11) (Analysis 2.1).
2.2 Adverse effects: various outcomes
Data indicated a pattern of fewer side effects in the placebo group (n=74). We present six of 13 adverse effect measures that were statistically significant, each in the direction indicating more adverse effects associated with fluphenazine treatment compared to placebo (Analysis 2.2). The other results were equivocal with no significant differences between treatment groups.
2.2.1 Drowsiness
Individuals treated with fluphenazine were more likely to experience drowsiness (1 RCT, n=165, RR 4.07 CI 1.12 to 4.54, NNT 3.45 CI 2.44 to 5.88).
2.2.3 Constipation
Individuals treated with fluphenazine were more likely to experience constipation (1 RCT, n=165, RR 2.26 CI 1.12 to 4.54, NNT 6.67 CI 3.7 to 33.3).
2.2.5 Dryness of mouth or throat
Individuals treated with fluphenazine were more likely to experience dryness of mouth or throat (1 RCT, n=165, RR 3.46 CI 1.22 to 9.83, NNT 7.69 CI 4.35 to 25.0).
2.2.7 Muscle rigidity
Individuals treated with fluphenazine were more likely to experience muscle rigidity (1 RCT, n=165, RR 2.98 CI 1.28 to 6.97, NNT 6.25 CI 3.7 to 20.0).
2.2.12 Loss of associated movements
Individuals treated with fluphenazine were more likely to experience loss of associated movements (1 RCT, n=165, RR 7.32 CI 1.75 to 30.53, NNT5.88 CI 3.85 to 12.5).
2.2.13 Akathesis - restlessness of feet
Individuals treated with fluphenazine were more likely to experience akathesis (1 RCT, n=165, RR 3.52 CI 1.04 to 11.90, NNT10.0 CI 5.26 to 50.0).
3. Comparison 3: Thioridazine versus placebo
Again, Cole 1964 was the only study to provide data for this comparison
3.1 Leaving the study early
Data suggested that those treated with placebo were more likely to leave early (1 RCT, n=240, RR 0.44 CI 0.28 to 0.69, NNT 4.3 CI 2.94 to 8.33) (Analysis 3.1).
3.2 Adverse effects: various outcomes
One study (Cole 1964) provided data suggesting more frequent side effects among those treated with thioridazine (n=165). Five of 13 adverse effect measures were statistically significant, each in the direction indicating more adverse effects associated with thioridazine treatment compared to placebo (Analysis 3.2). Only the significant results are presented below.
3.2.1 Drowsiness
Individuals treated with thioridazine were more likely to experience drowsiness (1 RCT, n=165, RR 5.46 CI 2.62 to 11.36, NNT 2.38 1.85 to 3.33).
3.2.4 Nausea or upper gastrointestinal distress
Individuals treated with thioridazine were more likely to experience nausea of upper gastrointestinal distress (1 RCT, n=165, RR 8.13 CI 2.58 to 25.59, NNT3.45 CI 2.5 to 5.56).
3.4.5 Dryness of mouth or throat
Individuals treated with thioridazine were more likely to experience dryness of mouth or throat (1 RCT, n=165, RR 5.69 CI 2.09 to 15.5, NNT4.0 CI 2.78 to 6.67).
3.4.6 Dizziness, faintness, or weakness
Individuals treated with thioridazine were more likely to experience dizziness, faintness, or weakness (1 RCT, n=165, RR 4.47 CI 1.61 to 12.41, NNT5.26 CI 3.45 to 11.11).
3.4.8 Nasal congestion
Individuals treated with thioridazine were more likely to experience nasal congestion (1 RCT, n=165, RR 3.25 CI 1.14 to 9.31, NNT 8.33 CI 4.55 to 33.3).
4. Comparison 4: Trifluoperazine versus psychotherapy
May 1976 was the only study to provide useable data for this comparison.
4.1 Leaving the study early
The data indicate no difference in the rates of leaving the study early (1 RCT, n=94, RR 0.96 CI 0.25 to 3.61) (Analysis 4.1).
4.2 Global State: overall health score - mean endpoint score Meninger Health Sickness Scale
Significantly higher endpoint scores on the Meninger Health sickness scale were found among those treated with trifluoperazine ( RCT, n=92, MD 5.8 CI 1.61 to 9.99) (Analysis 4.2).
4.3 Adverse effects
More frequent side effects were found among those treated with trifluoperazine compared to psychotherapy (1 RCT, n=162, RR 5.65 CI 2.72 to 11.73, NNT 2.3 CI 1.79 to 3.13) (Analysis 4.3).
5. Comparison 5: Typical antipsychotic versus psychosocial treatment (milieu therapy)
5.1 Global state - global psychopathology scale
One study (Mosher 1995) contributed data to an assessment of global psychopathology suggesting no between group differences at six weeks (1 RCT, n=89, MD 0.01 CI −0.55 to 0.57) (Analysis 5.1).
5.2 Global state - global improvement scale
Mosher 1995 contributed data to an assessment of global improvement in psychopathology suggesting no between group differences at six weeks (1 RCT, n=89, MD −0.03 CI −0.49 to 0.43) (Analysis 5.2).
DISCUSSION
The searches
The Cochrane Schizophrenia Group provided search results that included records from 670 studies. An additional 11 studies were either known to us or suggested by reviewers. Although antipsychotic treatment of acute early episode schizophrenia psychoses is uniformly recommended around the world in published clinical practice guidelines (Gaebel 2005b), we found only five studies meeting inclusion criteria for this review. It is possible that we have failed to identify all relevant studies. We have as yet been unsuccessful in gaining access to unpublished data from one study (Johnstone 1988), thus we have not included these data in the review.
Summary of main results
We found only five very short-term trials that used a total of 15 different treatments. Data reporting was generally very poor. Data were not available for all outcomes, and we could only analyse four outcomes, global state, rehospitalisation, adverse effects and leaving the study early.
With only a few studies meeting inclusion criteria and with limited useable data in these studies, it is not possible to arrive at definitive conclusions. The data suggest that early episode patients treated with typical antipsychotic medications are less likely to leave the study early and more likely to experience medication-related side effects. Data are too sparse to assess the effects of antipsychotic medication on outcomes in early episode schizophrenia.
1. Global outcomes
1.1 Global state
One study Mosher 1995 contributed data to a comparison of typical antipsychotic medication to psychosocial treatment on six-week outcome measures of global psychopathology (1 RCT, n= 89, MD 0.01 CI −0.6, 0.6) and global improvement (1 RCT, n= 89, MD −0.03 CI −0.5, 0.4), indicating no between-group differences (Analysis 5.1). This same study did not find between-group differences on the six-week measurement of improvement in psychopathology (1 RCT, n=89, MD −0.03 CI −0.49 to 0.43) (Analysis 5.2). One study (May 1976) contributed data to a two-year post-discharge comparison of global state using the Menninger Health-Sickness Scale Luborsky 1962 finding that trifluoperazine-treated individuals had higher mean scores than psychotherapy treated individuals (1 RCT, n=92, MD 5.8 CI 1.61 to 9.99) (Analysis 4.2). However, data from this study contain both selection and attrition biases.
One study (Simon 1965) contributed data to an eight-year post-discharge comparison of chlorpromazine versus placebo on a dichotomised rating scale (improved or not improved) that did not find a significant between group difference (1 RCT, n=40, RR 0.76 CI 0.53 to 1.11, NNT 5 CI 2.17 to 16.67) (Analysis 1.2).
1.2. Rehospitalisation
One study (Rappaport 1978) (n=80) suggested a higher rehospitalisation rate after two years for chlorpromazine treated completing participants compared to placebo (1 RCT, n=80, RR 2.29 CI 1.31 to 4.03, NNT 2.9 CI: 1.82 to 7.14) (Analysis 1.3). Due to higher attrition in the placebo group in Rappaport 1978, we conducted a sensitivity analysis on an intent-to-treat basis, which remains statistically significant (1 RCT, n=127, RR 3.05 CI 1.64 to 5.67, NNT 3.33 CI 2.17 to 6.67) (Analysis 1.4). This is similar to the finding of lower rehospitalisation in the placebo treated group at the one-year follow-up to Cole 1964 reported in Schooler 1967. However the Schooler 1967 paper does not quantify the differences in rehospitalisation and the original data appear to have been lost.
1.3. Adverse effects
Two studies (Cole 1964; May 1976; n=506) contributed data to an assessment of adverse effects and present a general pattern of more frequent adverse effects among individuals treated with typical antipsychotic medications compared to placebo.
2.4. Leaving the study early
Four studies (Cole 1964; May 1976; Rappaport 1978; Simon 1965; n=724) contributed data to an assessment of the likelihood of leaving the study early, suggesting that individuals treated with a typical antipsychotic medication are less likely to leave the study early than those treated with placebo (Analysis 1.1; Analysis 2.1; Analysis 3.1 Analysis 4.1).
The preliminary pattern from the limited quantity of available evidence suggests that early episode participants treated with typical antipsychotic medications are less likely to leave the study early and more likely to experience medication-related adverse effects. Data are too sparse to assess the effects of antipsychotic medication on outcomes in early episode schizophrenia.
Overall completeness and applicability of evidence
A majority of participants (n=998) in the five included studies had an early (first or second) episode of schizophrenia-type psychosis or a first hospitalisation for psychosis. The acute treatment phase in each study was very short-term (30 days to eight weeks) and follow-up periods ranged from no follow-up to 10 years. Available data were severely limited by the limited number of studies and by poor data reporting.
Quality of the evidence
We included five trials (n=998). The methodological quality of these studies was judged to be poor to fair and data reporting was generally poor.
Potential biases in the review process
We endeavoured to avoid publication bias; however, it is possible that all relevant studies have not yet been discovered. This review found five studies, each with methodological problems, and most with inadequate data reporting. Selection bias was apparent in one study (May 1976) and attrition was significant in at least two studies (May 1976; Rappaport 1978). This review found very few studies, and available evidence does not support a conclusion that antipsychotic treatment in an acute early episode of schizophrenia is effective. This does not mean that antipsychotic treatment is not effective, only that evidence is not available to adequately evaluate its effectiveness. This is of particular concern given the widespread use of antipsychotic medications around the world in the acute treatment of early episode schizophrenia-type psychoses (Gaebel 2005b).
Agreements and disagreements with other studies or reviews
Many reviews examine the effectiveness of first-generation antipsychotic medications (FGAs; e.g., chlorpromazine, Adams 2007; fluphenazine, Matar 2007, 2007; haloperidol, Irving 2006a; perphenazine, Hartung 2005; trifluoperazine, Marques 2004) or second-generation antipsychotics (SGAs; e.g., amisulpride, Silveira da Mota Neto 2002; aripiprazole, El-Sayeh 2006; olanzapine,Duggan 2005; risperidone, Rattehalli 2010) for schizophrenia. One review compares the two FGAs haloperidol and chlorpromazine (Leucht 2008). Two reviews compare the SGAs ziprasi-done (Komossa 2009) or zotepine (Komossa 2010) to other SGAs. There are reviews of ayurvedic medicine (Agarwal 2007), Chinese herbal medicine (Rathbone 2005), and Omega-3 fatty acid supplementation (Irving 2006b) for schizophrenia.
In each of these reviews, individuals at different stages of illness are grouped together, allowing an overall estimate of effectiveness (data permitting) that is not specific to stage of illness. To the best of our knowledge, the present review is the only effort to estimate the effectiveness of antipsychotic medications in early episode schizophrenia-spectrum disorder, in which a majority of treated individuals are experiencing a first or second acute episode.
AUTHORS’ CONCLUSIONS
Implications for practice
Clinical practice guidelines for treating early episodes of schizophrenia psychoses uniformly advise treatment with antipsychotic medications for six to 24 months (Gaebel 2005b). Evidence supporting this guideline is very limited. A more cautious approach to medication use in early episodes might be advisable while additional research is conducted.
Implications for research
1. General
Trials in this review preceded the international review of schizophrenia practice guidelines (Gaebel 2005b) uniformly recommending treatment with an antipsychotic medication in early episodes. Clear reporting of outcomes would certainly have resulted in this review being more informative.
2. Specific
The effectiveness of antipsychotic medications in early episode schizophrenia is under-researched and current evidence is inadequate to support international practice guideline recommendations. Even though antipsychotic medications have been used for decades, there are only a small number of randomised, placebo- controlled trials measuring the efficacy of these medications for people with an early episode of schizophrenia. The use of antipsychotic medications for millions of people with an early episode appears based on the evidence for those with multiple previous episodes (e.g. Thornley 2006). It is possible that early episode schizophrenia includes a higher proportion of people with a relatively better prognosis and potentially different response to treatment. Undertaking placebo-controlled trials for people with schizophrenia is problematic and many would disagree as to whether such a study was ethical (Fleischhacker 2003). There is however, some evidence that carefully conducted short-term placebo controlled trials can be conducted safely and without long-term harm to those later found to need medications (Bola 2006;Johnstone 1999). We feel that one or more large, well-planned, conducted and reported randomised, placebo-controlled trials is indicated. Preliminary evidence also suggests a possible benefit from an active therapeutic milieu or other psychosocial intervention (Bola 2006) that might be considered in a three- or four-arm study. Concrete and simple outcomes are of interest such as clearly reporting improvement, ’hospital admission’ ’days in hospital’ or even ’healthy days’. In addition, future trials need to report not only those clinically useful data but also information relating to cost effectiveness, employment, family burden, and satisfaction with care which are currently lacking. Any data on adverse effects, including those of medium- or long-term, would be most welcome. Most of these outcomes do not necessitate the use of scales as outcome measures.
PLAIN LANGUAGE SUMMARY.
Antipsychotic medication for early episode schizophrenia
There are only a few good quality studies comparing the acute treatment of early episode schizophrenia with an antipsychotic medication compared to placebo or psychosocial treatment. It appears that initial medication treatment reduces the study attrition rates while also increasing the risk for medication-induced side effects. Data are too limited to assess the effects of initial antipsychotic medication treatment on outcomes for individuals with an early episode of schizophrenia.
ACKNOWLEDGEMENTS
We would like to thank the School of Social Work, University of Southern California and the Cochrane Schizophrenia Group for providing staff time and resources to facilitate this review. We also acknowledge our use of the Cochrane Schizophrenia Group’s methods section template, which we have adapted to our requirements.
SOURCES OF SUPPORT
Internal sources
School of Social Work, University of Southern California, USA.
External sources
No sources of support supplied
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised (individually numbered containers of medicines). Blindness: double-blind. Duration: 6 weeks. Setting: multi-centre. |
|
| Participants | Diagnosis: DSM schizophrenia (50% first episode). N=463. Age: 16-45 years, mean ~ 28 years. Sex: male and female (proportions not given). History: acute, 60% first hospitalisation, no significant hospitalisation 12 months prior to current admission |
|
| Interventions | 1. Chlorpromazine: dose range 200-1200 mg/day. N=112. 2. Fluphenazine: dose range 2-16 mg/day. N=115. 3. Thioridazine: dose range 200-1600 mg/day. n=111. 4. Placebo. 2-16 doses. N=125. Plus antiparkinsonian medication as needed for extrapyramidal side effects |
|
| Outcomes | Leaving the study early. Adverse effects. Unable to use. Global state: Global rating of severity of illness, improved/not improved -no usable data. Inpatient Multidimensional Psychiatric Scale (IMPS) - no usable data. Ward Behaviour Rating Scale (WBRS) - no usable data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised - no further details. |
| Allocation concealment (selection bias) | Low risk | Individually numbered containers of medicines. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Double blind, untested. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Study attrition reported (not addressed in analysis). |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | No details. |
| Methods | Allocation: random, no further details. Blinding: single. Duration: until discharge or 6-12 months. Post-discharge follow up to 5 years. |
|
| Participants | Diagnosis: schizophrenia (clinical consensus); selected ‘middle third of prognostic spectrum’ (selection criteria unspecified). N=228. Age: range 16-45 years. Sex: male and female. History: first admission, ‘middle prognostic range’, not remitted with average 14 day observation period. Excluded: people who were assessed as unlikely to be discharged within 2 years, and those whose illness went into remission during 14 day average assessment period |
|
| Interventions | 1. Individual psychotherapy. N=46. 2. Ataraxic drugs (trifluoperazine). N=48. 3. Individual psychotherapy and ataraxic drugs. N=44. 4. ECT. N=47. 5. Milieu therapy and ataraxic drugs. N=43. |
|
| Outcomes | Leaving the study early Menninger Health-Sickness Rating Scale (HSRS). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Open study. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Study attrition not reported. Successive follow-up studies have diminishing sample size |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | High risk | Criteria used to select the “middle third of prognostic spectrum” not specified |
| Methods | Allocation: random. Blinding: single (evaluators presumed to be blind, however groups were treated at different facilities). Duration: 6 weeks, with follow-up to two years. |
|
| Participants | Diagnosis: DSM-II schizophrenia, “in need of hospitalisation” N=100. History: No more than one prior hospitalisation (51% first-episode). Sex: 80 M, 20 F. Age: range 18-30 yrs. |
|
| Interventions | 1. Hospital treatment with antipsychotic medications (100% received antipsychotic medications, 98% continuously), n=55 2. Non-hospital milieu treatment + postponement of antipsychotic medications for a maximum of 6 weeks (67% received no antipsychotics, 31% > 7 days of antipsychotic treatment, 12% continuous antipsychotic treatment), n=45 |
|
| Outcomes | Global Rating: Severity of Mental Illness (7-point scale). Global Rating of Improvement (7-point scale). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as “randomly assigned”. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Treated at different sites. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Data reported for patients receiving minimum duration of treatments (7+ days of hospital treatment or 28+ days of therapeutic milieu) |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | No details. |
| Methods | Allocation: randomly assigned (no further description). Blinding: single, staff ‘remained blind as to whether the patient was receiving medication or placebo’. Duration: unclear; mean hospitalisation=43 days, follow-up at 1-36 months after discharge |
|
| Participants | Diagnosis: schizophrenia (criteria not specified). N=127. Sex: all male. Age: range 16-40 yrs. History: ‘acute’ illness. |
|
| Interventions | 1. Chlorpromazine: dose variable 300-900 mg/day. N=53. 2. Placebo. N=74. |
|
| Outcomes | Leaving study early. Rehospitalisation. Unable to use. Clinical Change Index and Global Assessment Scores (data skewed) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (no further description). |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Reported that “staff remain blind to whether the patient was receiving medication or placebo” |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No details. |
| Selective reporting (reporting bias) | Low risk | Differential attrition reported by authors. |
| Other bias | Unclear risk | No further details. |
| Methods | Allocation: random. Blinding: unclear. Duration: 30 days. Setting: hospital. |
|
| Participants | Diagnosis: DSM-I schizophrenia (no further details), no prior treatment for schizophrenia, an average of 32.7 days treatment prior to evaluation for this study. N=80. Age: average ~ 31 years. Sex: all male. |
|
| Interventions | 1. Chlorpromazine: dose minimum 200 mg/day, maximum 1200 mg/day, average 400mg/day. n=20 2. Hospital routine care (occupational and manual arts therapy, special services activities). N=20 3. Reserpine: dose minimum 2 mg/day, maximum 16 mg/da, average 6 mg/day. N=20 4. Clinical judgement. N=20. |
|
| Outcomes | Leaving the study early. Not improved (Psychiatric improvement rating scale). Unable to use. Behaviour rating scale - no usable data. Minnesota Multiphasic Personality Iinventory (MMPI) - no usable data |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised - no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No details. |
| Selective reporting (reporting bias) | Unclear risk | No details. |
| Other bias | Unclear risk | No details. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACE 2003 | Allocation: randomised Participants: people with first episode schizophrenia Interventions: CBT + medications vs befriending + medications (no un-medicated group) |
| Adson 2003 | Allocation: randomised Participants: people with schizophrenia (unknown proportion of first and second episode participants) |
| Aguilar 1994 | Allocation: randomised Participants: people with first episode psychosis Interventions: haloperidol + biperiden vs. haloperidol + placebo (no un-medicated group) |
| Ahmed 1997 | Allocation: unknown method of assignment to treatment Participants: people with first episode psychosis Interventions: haloperidol vs. risperidone (no un-medicated group) |
| Alaghband-rad 2006a | Allocation: randomised Participants: people with first episode psychosis Interventions: treatment as usual + standard telephone follow-up vs. treatment as usual + home visit groups (both groups received standard or low dose medications) |
| Allison 2001 | Allocation: randomised Participants: people with psychosis Interventions: Clozapine, Haloperidol, Olanzapine, Risperidone or Placebos (unknown proportion of first and second episodes) |
| Altamura 1985 | Allocation: randomised Participants: people with schizophrenia (n=7) Interventions: fluphenazine (unknown proportion of early episodes; no un-medicated group) |
| Altamura 1999b | Allocation: random assignment to adjunctive antidepressant medication Participants: people (n=76) with diagnosis of schizophrenia or schizoaffective disorder and with a concomitant major depressive disorder Interventions: atypical antipsychotic drugs (AAD) vs. haloperidol decanoate (HL-D) (not an acute schizophrenia treatment study; unknown proportion of early episodes; no un-medicated group) |
| Alvarez 2005 | Allocation: randomised Participants: people with first episode schizophrenia Intervention: an early behavioural intervention (n=35) vs. routine care (n=27). All had been received antipsychotic treatments (risperidone n=23), olanzapine (n=18) and haloperidol (n=21) before the randomisation (no un-medicated group) |
| Amminger 2006 | Allocation: randomised Participants: people assessed at ultra high risk for psychosis (UHR), a prodromal phase intervention study Intervention: omega-3 fatty acids + standard care vs. placebo + standard care (not acute schizophrenia treatment study) |
| An 2006b | Allocation: randomised Participants: people with first episode schizophrenia Intervention: olanzapine vs. quetiapine (no un-medicated group) |
| Anonymous 1972 | Allocation: randomised Participants: people with chronic schizophrenia (n=20) Interventions: Piperacetazine vs. Thioridazine (not treatment for people in acute schizophrenia; no un-medicated group) |
| Apicella 2001 | Allocation: unknown method of assignment to treatment Participants: people with schizophreniform disorder, between the ages of 16 and 40 years of age and who have been recently diagnoses (within the last five years) with schizophrenia, schizophreniform disorder or schizoaffective disorder Interventions: haloperidol vs. olanzapine (unknown method of assignment to treatment; unknown proportion of first and second episodes; no un-medicated group) |
| Apiquian 2003 | Allocation: unknown method of assignment to treatment Participants: people with first episode psychosis Interventions: haloperidol (the minimum dose) vs. olanzapine vs. risperidone (unknown method of assignment to treatment; no un-medicated group) |
| Appelberg 2004a | Allocation: randomised Participants: people in the clinically stable status of psychosis Interventions: conventional neuroleptic(s), (with a mean dose of 312 chlorpromazine equivalents) vs. olanzapine (unknown proportion of first and second episodes; no un-medicated group) |
| Archie 2006 | Allocation: randomised Participants: people with first episode psychosis (n=547) Interventions: Integrated care (based on the Assertive Community Treatment model and delivered by a multidisciplinary team and people received social skill training or general psychoeducation as required) vs. standard care (the usual mental health services). Both integrated and standard care could include standard antipsychotic medication (no un-medicated group) |
| Ascher-Svanum 2006a | Allocation: randomised Participants: people with schizophrenia (n=664) Interventions: olanzapine vs. risperidone vs. typical antipsychotics (unknown proportion of first and second episodes; no un-medicated group) |
| Auby 2002 | Allocation: randomised Participants: people with stable schizophrenia or schizoaffective disorder (mean baseline PANSS 43-64) Interventions: aripiprazole 30 mg/day (n=12) vs. 45 mg/day (n=7) vs. 60 mg/day (n=7) vs. 75 mg/day (n=7) vs. 90 mg/day (n=7) Outcomes: positive and negative symptoms, akathisia and tachycardia, adverse and side effects (not treatment for acute schizophrenia; no un-medicated group) |
| Awad 2006 | Allocation: randomised Participants: people with first-episode schizophrenia (ICD-10) Interventions: olanzapine vs. haloperidol Outcome: psychosocial functioning and QOL (quality of life) (no un-medicated group) |
| Bai 2005d | Allocation: randomised Participants: people with schizophrenia Interventions: quetapine and chlorpromazine (unclear proportion of first and second episodes; no un-medicated group) |
| Bandelow 1992 | Allocation: randomised Participants: people with schizophrenia (ICD-9). Interventions: 3 groups: continuous medication, intermittent medication with crisis intervention, intermittent medication with early intervention (unclear proportion of first and second episodes; not an acute treatment study, but a follow-up maintenance treatment study) |
| Barrowclough 2001b | Allocation: randomised Participants: people with recent onset of schizophrenia (within 2 years) Interventions: CBT + usual treatment vs usual treatment only (no un-medicated group; usual treatment is unspecified and group assignment is unspecified; this is not an acute treatment study) |
| Beasley 1996a | Allocation: randomised Participants: people with psychosis Interventions: Olanzapine vs Risperidone or Olanzapine vs. placebo (no un-medicated group; unknown proportion of early episodes) |
| Beasley 1997 | Allocation: randomised Participants: people with schizophrenia Interventions: olanzapine and haloperidol (Unclear proportion of first and second episodes; no un-medicated group) |
| Bechdolf 2004a | Allocation: randomised Participants: people in the pre-psychotic prodromal period Interventions: CBT vs. antipsychotic amisulpride + clinical management vs. clinical management only (not treatment for acute episode schizophrenia) |
| Bechdolf 2004c | Allocation: randomised Participants: people in the pre-psychotic prodromal period Interventions: CBT vs. antipsychotic amisulpride + clinical management vs. clinical management only (not treatment for acute episode schizophrenia) |
| Bechdolf 2005a | Allocation: randomised Participants: people in pre-psychotic prodromal period Interventions: CBT vs. antipsychotic amisulpride + clinical management vs. clinical management only (not treatment for acute episode schizophrenia) |
| Bechdolf 2006 | Allocation: randomised Participants: people with “subthreshhold psychosis” (5.5% in CBT gruop vs 18.3% in supportive therapy) Interventions: cognitive behavioral therapy vs supportive therapy (Not psychotic; No contrast of medication vs not medication treatment) |
| Bendall 2004 | Allocation: randomised Participants: people with first episode psychosis. Interventions: Befriending vs CBT (No contrast of medication vs not medication treatment) |
| Bentall 2000 | Allocation: randomised Participants: people with first and second episode schizophrenia Interventions: Usual treatment, usual treatment + CBT, or medication + supportive counseling (No un-medicated group) |
| Berger 2004a | Allocation: randomised Participants: people with episode psychosis Interventions:Ethyl-eicosapentaenoic Acid (E-EPA) vs. placebo (i.e. as a supplement to antipsychotic treatment) (No un-medicated group) |
| Berger 2004b | Allocation: randomised Participants: people with episode psychosis Interventions: Ethyl-eicosapentaenoic Acid (E-EPA) vs placebo (i.e. as a supplement to antipsychotic treatment) (no un-medicated group) |
| Berger 2005 | Allocation: randomised Participants: people with first episode psychosis Interventions: antipsychotic medications + Ethyl-eicosapentaenoic Acid vs antipsychotic medications + placebo (No un-medicated group) |
| Berger 2006 | Allocation: randomised Participants: people assessed as having high risk for psychosis Interventions:lithium vs placebo (Persons not psychotic; No antipsychotic medication use) |
| Bertelsen 2004 | Allocation: randomised Participants: people with first episode psychosis Interventions: Integrated treatment (standard treatment + ACT) vs standard treatment (No un-medicated group) |
| Bertelsen 2005 | Allocation: randomised Participants: people with first episode psychosis Interventions: Integrated treatment (standard treatment + ACT) vs standard treatment (No un-medicated group) |
| Bertelsen 2006 | Allocation: randomised Participants: people with first episode psychosis Interventions: Integrated treatment (ACT + family involvement + social skills training) vs standard treatment (No un-medicated group) |
| Binder 2006 | Allocation: randomised Participants: people with recent onset schizophrenia (3 or less years from onset) Interventions: risperidol vs. oral olanzapine vs. oral quetiapine (Unclear proportion of first and second episodes; No un-medicated group) |
| Birchwood 2000a | Allocation: unclear Participants: people with multiple episodes of severe relapses Interventions: early intervention vs psychoeducation (Not early episodes; no clear specification of medicated vs un-medicated groups) |
| Birchwood 2000b | Allocation: randomised Participants: people with “relapsing psychosis”. Interventions: medication vs placebo (Not first and second episodes) |
| Birchwood 2000c | Allocation: randomised Participants: people with schizophrenia (n=60) Interventions: targeted medication for 4 weeks (placebo) vs. active medication (Unclear proportion of first and second episodes; no un-medicated group; not an acute treatment study) |
| Blaha 1980 | Allocation: Unclear Participants: People with schizophrenia (n=32) Interventions: Haloperidol at differing dosages (Unclear proportion of early episodes; No un-medicated group) |
| Bola 2003 | Allocation: Combination of 2 cohorts, one cohort assigned to treatment with a quasi-random procedure (consecutive space available), and the second cohort randomly assigned Participants: People with first and second episode schizophrenia (n=179) Interventions: Immediate antipsychotic medication in the hospital vs. psychosocial therapeutic milieu with up to 6 week postponement of antipsychotic treatment (Combines randomly assigned and quasi-randomly assigned cohorts) |
| Borison 1991b | Allocation: randomised Participants: people with chronic schizophrenia. Interventions: Risperidone vs Haloperidol vs placebo (Not first and second episodes) |
| Brecher 1998 | Allocation: randomised Participants: people with schizophrenia, schizophrenic disorder or psychotic disorder Interventions: Risperidone vs Olanzapine (Unclear proportion of first and second episodes; No un-medicated group) |
| Bredkjar 1999 | Allocation: randomised Participants: people with first episode psychosis Interventions: integrated care vs standard care (No un-medicated group) |
| Bredkjar 2000 | Allocation: randomised Participants: people with first episode psychosis Interventions: integrated care vs. standard care (No un-medicated group) |
| Breier 2002b | Allocation: randomised Participants: people at high-risk for psychosis, symptomatic, prodromal states Interventions: 1 year medication (PBO or Olanzapine) followed by 1 year of no medication (persons not psychotic) |
| Brewer 2002 | Allocation: randomised Participants: neuroleptics-naïve people with first episode psychosis Interventions: Haloperidol vs. Risperidone (No un-medicated group) |
| Brooker 1992 | Allocation: quasi-experimental design Participants: people with recent diagnosis of schizophrenia. Interventions: psychosocial interventions delivered by community psychiatric nurses plus usual treatment vs usual treatment (unclear proportion of first and second episodes; No contrast of medicated vs un-medicated group) |
| Burns 2002b | Allocation: no treatment assignment Participants: people with first episode psychosis Interventions: Not an intervention study (looks for neuroimaging correlates of social functioning) (not an intervention study; no contrast of medicated vs. un-medicated group; unclear proportion of early episodes) |
| Burrell 1960 | Allocation: randomised Participants: people with acute, multi-episode schizophrenia and bipolar disorder clinically assessed as “tense” Interventions: Chlorpromazine vs Hydroxyzine vs Placebos (mix of schizophrenia and bipolar cases; not early episodes) |
| Caffey 1968 | Allocation: randomised Participants: people with “all types of acute emotional disturbances” Interventions: brief hospitalization, crisis therapy, and family involvement (mix of diagnoses; Unclear proportion of first and second episodes; Unclear contrast of medication use vs. non-medication use) |
| Cao 2000 | Allocation: not an intervention study Participants: people with first episode schizophrenia (<=2 years) (coded for types of traditional Chinese medicine syndromes) Interventions: Risperidone (not an intervention study; No un-medicated group) |
| Carpenter 1977 | Allocation: historical two-group comparison (NIH acute treatment vs. IPSS Washington, DC cohort), one and two year follow-ups Participants: people with acute schizophrenia, adequate prior work and social functioning, >50% not first-episode, n=122 Interventions: (after a 3-week medication washout period) 1. TAU (hospitalization and antipsychotic medications) n=73; and 2. Milieu treatment (therapeutic community) with minimal antipsychotic medications, n=49 (Historical comparison group study (subjects not randomly allocated to treatment), unclear proportion of first and second episodes) |
| Carpenter 1982 | Allocation: randomised Participants: people with schizophrenia Interventions: targeted and time limited drug use vs continuous drug use (Not an acute treatment study; Unclear proportion of first and second episodes) |
| Carpenter 1983b | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder Interventions: targeted drug use vs continuous drug use (not an acute treatment study; Unclear proportion of first and second episodes) |
| Carpenter 1999a | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder (DSM-III-R) Interventions: Diazepam vs. fluphenazine vs. placebo (Not an acute treatment study; Unclear proportion of first and second episodes) |
| Carson 2000 | Allocation: randomised Participants: people with acute relapse of schizophrenia or schizoaffective disorder Interventions: aripiprazole, haloperidol and placebo (Not first and second episodes) |
| Carson 2000b | Allocation: randomised Participants: people with chronic schizophrenia. Interventions: aripiprazole and placebo (Not first and second episodes) |
| Casey 2002 | Allocation: randomised Participants: people with chronic and stable schizophrenia or schizoaffective disorder Interventions: aripiprazole (Not first and second episodes; No un-medicated group) |
| Castilla 2002 | Allocation: randomised Participants: children with onset of psychotic symptoms, hallucinations and delusions within 7 days Interventions: Olanzapine and Haloperidol (Not first and second episodes; not adults; No un-medicated group) |
| Cavozzoni 2002a | Allocation: randomised Participants: people with schizophrenia during the acute phase (<= 8 weeks) Interventions: haloperidol, risperidone or Clozapine and placebo (Unclear proportion of early episodes) |
| Centorrino 2003 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder Interventions: haloperidol and Olanzapine (Unclear proportion of first and second episodes; no un-medicated group; not an acute treatment study) |
| Chaudhry 2004 | Allocation: randomised Participants: people with first-episode schizophrenia Interventions: Randomised trial of the addition of Lamotrigine and Minocycline to standard medication treatment (inadequate detail to determine types of medications used) (No un-medicated group) |
| Chen 2000a | Allocation: randomised Participants: people with first-episode schizophrenia Interventions: Risperidone (fixed vs. curative effect dosage groups) (No un-medicated group) |
| Chen 2000c | Allocation: unclear method of assignment to treatment Participants: males with first-episodes schizophrenia Interventions: Risperidone (unclear method of assignment to treatment; No un-medicated group) |
| Chen 2004a | Allocation: unclear method of assignment to treatment Participants: people with first-episodes schizophrenia Interventions: Risperidone (unclear method of assignment to treatment; controls were not people with schizophrenia; no un-medicated group) |
| Chen 2004c | Allocation: unclear method of assignment to treatment Participants: people in a difficult situation and people with stress-induced schizophrenia Interventions: Neither group receives medication (unclear method of assignment to treatment; Unclear proportion of first and second episodes; No contrast of medicated vs un-medicated group) |
| Chen 2006d | Allocation: randomised Participants: people with first episode schizophrenia Interventions: Chlorpromazine and Clozapine (No un-medicated group) |
| Cheng 2006b | Allocation: unclear method of assignment to treatment Participants: children with schizophrenia. Interventions: Perphenazine and Risperidone (unclear method of assignment to treatment; Unclear proportion of first and second episodes; No un-medicated group) |
| Chiu 2006b | Allocation: randomised Participants: people with atypical schizophrenic Interventions: Olanzapine and Risperidone (Unclear proportion of first and second episode; No un-medicated group) |
| Chouinard 1992 | Allocation: randomised Participants: people with chronic schizophrenia Interventions: Risperidone, Haloperidol or placebo (Not first and second episodes) |
| Ciompi 1993 | Allocation: case-control Participants: People with DSM-IIIR Schizophrenia or Schizophreniform disorder, onset within one-year, ages 17-35, ≥2 of 6 cardinal symptoms of schizophrenia (hallucinations, delusions, thought disorders, catatonia, schizophrenic disorders of affect, severely deviant social behavior), n=44 Interventions: TAU Hospitalization and antipsychotic medications, n=22; therapeutic milieu with time-limited postponement (up to 4 weeks) of antipsychotic medications, n=22 (Not randomly assigned to treatment) |
| Claus 1992 | Allocation: randomised Participants: people with chronic schizophrenia. Interventions: Risperidone, Haloperidol (Not first and second episodes; No un-medicated group) |
| Conley 1999 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia. Interventions: Risperidone, Clozapine, Olanzapine and typical antipsychotic (unclear method of assignment to treatment; No un-medicated group) |
| Craig 2004b | Allocation: randomised Participants: people with first or second episode schizophrenia Interventions: assertive outreach with evidence based biopsychosocial interventions (CBT, medication, family support) vs. standard care (control group) delivered by community mental health teams (not an acute treatment study; no un-medicated group) |
| Crespo-Facorro 2006a | Allocation: randomised Participants: people with first episode schizophrenia Interventions: Olanzapine vs Risperidone vs Haloperidol (No un-medicated group) |
| Csernansky 2003 | Allocation: randomised Participants: people in acute relapse and requiring hospitalization Interventions: Aripiprazole vs. placebo (Not first or second episodes) |
| Cullberg 2002 | Allocation: Quasi-random (assigned to treatment available in catchment area) plus one historical (past) comparison group Participants: people with first-episode acute schizophrenia, n=388 Interventions: Milieu treatment with time-limited (up to 3 week) postponement of antipsychotic medications, n=253; hospital treatment with time-limited (duration unspecified) antipsychotic medication postponement, n=71; hospital treatment with antipsychotic medications (at a previous time), n=64 (Non-random assignment to treatments; both contemporary treatments postponed use of antipsychotic medications (i.e., no initial antipsychotic use vs non-use comparison); unable to assure equality of selection in the historical group) |
| Dahl 2000 | Allocation: not randomised (consecutive) Participants: people with first episode psychosis Interventions: a special program including education, medical/social detection network, early detection team of clinicians (not randomised; not a treatment comparison study; no un-medicated group) |
| Daniel 2000b | Allocation: randomised Participants: people in acute schizophrenic relapse and hospitalized Interventions: aripiprazole vs. haloperidol vs. placebo (Not first and second episodes) |
| David 1999a | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia Interventions: Olanzapine vs Risperidone (unclear method of assignment to treatment; Unclear proportion of first and second episodes; No un-medicated group) |
| David 1999b | Allocation: unclear method of assignment to treatment Participants: people in early phase schizophrenia or schizophrenic disorder Interventions: Olanzapine vs. Risperidone vs. Haloperidol (unclear method of assignment to treatment; Unclear proportion of first and second episodes; No un-medicated group) |
| David 2000a | Allocation: randomised Participants: people with schizophrenia, schizophriform disorder, or schizoaffective disorder Interventions: Olanzapine vs. Risperidone vs. Haloperidol (Unclear proportion of first and second episodes; No un-medicated group) |
| Davidson 2003 | Allocation: randomised Participants: people with first episode schizophrenia Interventions: low-dose Risperidone vs. low-dose Haloperidol (No un-medicated group) |
| Davidson 2004 | Allocation: randomised Participants: people with early psychosis Interventions: Risperidone vs. Haloperidol (No un-medicated group) |
| Davis 1977 | Allocation: randomised Participants: people with schizophrenia and affective disorders (n=19) Interventions: naloxone vs placebo (Unclear proportion of first and second episodes; mixture of people with schizophrenia and affective disorder) |
| De Smedt 1999 | Allocation: randomised Participants: people with first episode psychosis (DSM-IV diagnosis of schizophreniform, schizophrenia, or schizoaffective disorder) Interventions: Risperidone vs. Haloperidol (No un-medicated group) |
| Deng 2006b | Allocation: randomised Participants: people with first episode psychosis Interventions: early treatment vs. routine treatment (No un-medicated group) |
| Ding 2001 | Allocation: case-control group selection Participants: people with first episode psychosis and normals Interventions: Clozapine, unspecified additional antipsychotic, no treatment (not randomly assigned to treatment; not a treatment comparison study) |
| Dollfus 2006 | Allocation: randomised Participants: people with a post-psychotic depression (DSMIV) Interventions: Olanzapine vs. Risperidone (Not first and second episodes; No un-medicated group) |
| Dossenbach 1997 | Allocation: randomised Participants: people with schizophrenia Interventions: Olanzapine vs. Fluphenazine (Unknown proportion of first and second episodes; No un-medicated group) |
| Dubitsky 2002a | Allocation: randomised Participants: people with stable schizophrenia or schizoaffective disorders Interventions: aripiprazole vs. olanzapine (Not first and second episode schizophrenia; no un-medicated group; not an acute treatment study) |
| Dursun 2002 | Allocation: randomised Participants: people with first episode psychosis Interventions: lamotrigine, minocycline and placebo added to treatment as usual (No un-medicated group) |
| Eack 2007 | Allocation: randomised Participants: people with schizophrenia. Interventions: Cognitive Enhancement Therapy (CET) vs. Enriched Supportive Therapy (Unclear proportion of first and second episodes; Both groups received medications (no contrast of medicated to un-medicated subjects)) |
| Edwards 1999 | Allocation: randomised Participants: people with first episode psychosis Interventions: Clozapine vs. Clozapine plus CBT vs. thioridazine vs. thioridazine plus CBT (No un-medicated group) |
| Edwards 2003 | Allocation: randomised Participants: people with first episode psychosis not meeting remission criteria within 12 weeks Interventions: Clozapine vs. Clozapine plus CBT vs. thioridazine vs. thioridazine plus CBT (No un-medicated group) |
| Edwards 2004 | Allocation: randomised Participants: young people with first-episode psychosis; Interventions: Cannabis + Psychosis (CAP) therapy versus psycho-education (PE) (Not a study of treatment of schizophrenia but of interventions to reduce cannabis use among people with schizophrenia) |
| Edwards 2006 | Allocation: randomised; Participants: people with first-episode psychosis; Interventions: Cannabis + Psychosis (CAP) therapy versus psycho-education (PE) (Not a study of treatment of schizophrenia but of interventions to reduce cannabis use among people with schizophrenia) |
| Eguiluz 1998 | Allocation: randomised Participants: people with first-episode psychosis (n=79) Interventions: Psychoeducation plusmedications compared to standard treatment (no un-medicated group, unclear proportion of early episodes, not an acute treatment study) |
| Eli Lilly 2006d | Allocation: randomised Participants: people experiencing exacerbation of psychotic symptoms within the previous 2 weeks Interventions: Risperidone vs. Olanzapine (No un-medicated group, unclear proportion of first and second episodes) |
| Emsley 1999 | Allocation: randomised Participants: people with first episode psychosis (n=183) Interventions: Risperidone vs. Haloperidol (No un-medicated group) |
| Emsley 2004b | Allocation: randomised Participants: people with recent onset schizophrenia Interventions: Risperidone (n=278) vs. Haloperidol (n=277) (No un-medicated group, unclear proportion of first and second episodes) |
| Emsley 2006b | Allocation: randomised Participants: people with first episode psychosis (n=522) Interventions: Risperidone vs. Haloperidol (No un-medicated group) |
| Emsley 2007 | Allocation: randomised Participants: people with first episode psychosis Interventions: Risperidone vs. Haloperidol (No un-medicated group) |
| Engelhardt 1994 | Allocation: Randomised Participants: people with schizophrenia with at least one year of illness (Studies 1 and 2); children with schizophrenia (Study 3) Inverventions: Butaperazine and fluphenazine (Study 2) (Unclear proportion of first and second episodes; No un-medicated group) |
| Faber 2005 | Allocation: randomised Participants: people with first episode psychosis (n=54) Interventions: Risperidone vs. Olanzapine. One group discontinued their medication after 6 months of stable remission, the other group continued medication and served as the control group (Not an acute treatment study (medication withdrawal post-stabilization)) |
| Fabre 1995 | Allocation: randomised Participants: 12 males with chronic and sub-chronic schizophrenia Interventions: Quetiapine vs. placebo (Not first and second episodes) |
| Fan 2006 | Allocation: randomised Participants: people with first episode psychosis Interventions: Risperidone vs. Chlorpromazine (No un-medicated group) |
| Fang 2003 | Allocation: randomised Participants: people with first episode psychosis (n=126) Interventions: Risperidone plus psychosocial treatment vs. Risperidone (No un-medicated group) |
| Ferenc 2000 | Allocation: unclear method of allocation to treatment Participants: people with schizophrenia. Interventions: Olanzapine vs. Fluphenazine (unclear method of allocation to treatment; Unclear proportion of first and second episodes; No un-medicated group) |
| Ferrari 1997 | Allocation: randomised Participants: young people with chronic schizophrenia Interventions: Risperidone vs. conventional neuroleptics (Not first and second episodes; No un-medicated group) |
| Filatre 1998 | Allocation: randomised Participants: people with first episode psychosis Interventions: antipsychotic medications vs. antidepressant medications (No un-medicated group) |
| Fleischhacker 2005 | Allocation: randomised Participants: people with first episode schizophrenia (n=500) Interventions: second-generation antipsychotic medications (amisulpride, quetiapine, olanzapine and ziprasidone) vs. low dose of haloperidol (No un-medicated group) |
| Fowler 2004 | Allocation: randomised Participants: young people with duration less than five years and relative remission of psychotic symptoms (less than moderate severity on the PANSS) Interventions: SRCBT (Social Recovery oriented CBT) vs. standardized treatment as usual. (standard use of medication in both group) (Not an acutetreatment study; Unclear proportion of first and second episodes; no un-medicated group) |
| Gaebel 1993 | Allocation: unclear Participants: People with stabilized schizophrenia with an average duration of 7.2 years since onset and an average of 3.0 prior hospitalizaitons Interventions: Maintenance treatment vs. early intervention vs. crisis intervention (Not an acute treatment study, predominantly multi-episodes) |
| Gaebel 1995 | Allocation: randomised Participants: people with schizophrenia (n=364) Interventions: maintenance does vs. early intervention vs. crisis intervention (Unclear proportion of first and second episodes; No un-medicated group; a study of maintenance treatments not acute treatment) |
| Gaebel 2001 | Allocation: randomised Participants: people with schizophrenia (n=115 first-episodes; n=248 multi-episodes) Interventions: maintenance does vs. early intervention vs. crisis intervention (the proportion of first episodes is less than 50% (115/363=32%); No un-medicated group, a study of maintenance treatments not acute treatment) |
| Gaebel 2002a | Allocation: randomised Participants: people with first-episode schizophrenia Interventions: Risperidone vs Haloperidol (8 weeks acute, n=360; 1 year maintenance, n=280; 1 year randomised open withdrawal plus early intervention with either neuroleptic or lorazepam, n=136) (No un-medicated group during acute treatment) |
| Gaebel 2004 | Allocation: randomised Participants: people with first episode schizophrenia (n=142) Interventions: Risperidone vs. low-dose haloperidol (No un-medicated group) |
| Gaebel 2005 | Allocation: randomised Participants: people with first episode schizophrenia (n=159) Interventions: Risperidone vs. low-dose haloperidol (No un-medicated group) |
| Gaebel 2006 | Allocation: randomised Participants: people with first-episode schizophrenia Interventions: Risperidone vs Haloperidol (8 weeks acute, n=302; 1 year maintenance, n=176; 1 year randomised open withdrawal plus early intervention with either neuroleptic or lorazepam, n=57) (No un-medicated group during acute treatment phase) |
| Gafoor 2005a | Allocation: randomised Participants: people with first episode schizophreniform psychosis (n=60) Interventions: Risperidone vs. Quetiapine Outcomes: depressive and anxiety symptoms (No un-medicated group, treatment for depression within schizophrenia) |
| Gafoor 2006 | Allocation: randomised Participants: people with first episode schizophreniform psychosis (n=72) Interventions: Risperidone vs. Quetiapine (No un-medicated group) |
| Gallo 2006 | Allocation: Randomised (n=180) Participants: Persons with first-episode schizophrenia Interventions: trimethoprim sulfamethoxazole plus anpipsychotics compared to antipsychotic treatment only (no un-medicated group) |
| Gan 1999 | Allocation: randomised Participants: people with first episode schizophrenia (n=60, BPRS>=40, CCMD-2-R) Interventions: Clozapine vs Risperidone (No un-medicated group) |
| Gan 2000 | Allocation: unclear method of allocation to treatment Participants: people with first episode schizophrenia (n=46) Interventions: Risperidone (unclear method of allocation to treatment; No un-medicated group) |
| Garcia 2006 | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia Interventions: atypical vs typical antipsychotics (no definition of medicines) (unclear method of assignment to treatment; Unclear proportion of first and second episodes; No un-medicated group) |
| Garety 2000a | Allocation: randomised Participants: people with early schizophrenia (first or second episode) Interventions: Cognitive Behavioral Therapy (No contrast of medicated vs. un-medicated group) |
| Garety 2006 | Allocation: randomised; Participants: people with first or second episode schizophrenia (n=144) Interventions: care by the early onset team (a mix of medication, cognitive behavioral therapy, vocational input and family interventions, which provided based on individual need) vs. standard care (No un-medicated group) |
| Garver 2005 | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia Interventions: Risperidone vs. Ziprasidone vs. Haloperidol (Unclear proportion of first or second episodes; unclear method of assignment to treatment) |
| Gary 1990 | Allocation: randomised Participants: people with schizophrenia Interventions: experimental group (n=11) vs. control group (n=12): both group received medication and experimental group was given instructions regarding self-assessment of extrapyramidal side effects (unclear proportion of first and second episodes; No un-medicated group) |
| Gattaz 1989 | Allocation: randomised Participants: people with schizophrenia (n=30; 8 first episodes) Interventions: haloperidol plus bromocriptine (n= 15) vs. haloperidol plus placebo (n= 15) (proportion of first episodes is less than 50% (8/30=27%); No un-medicated group) |
| Genduso 1996 | Allocation: randomised Participants: people with schizophrenia, schizophreniform disorder, or schizoaffective disorder (n=1996) Interventions: Olanzapine (n=1,336) vs. Haloperidol (n=660) (Unclear proportion of first and second episodes; No un-medicated group) |
| Gharabawi 2006d | Allocation: unclear method of assignment to treatment Participants: people with first-episode psychosis Interventions: haloperidol vs. Risperidone (unclear method of assignment to treatment; No un-medicated group) |
| Gillin 1978 | Allocation: unclear method of assignment to treatment Participants: People with schizophrenia Interventions: pretreatment with pimozide (unclear method of assignment to treatment; Unknown proportion of first and second episodes; No un-medicated group) |
| Glenthoj 2000 | Allocation: randomised Participants: people with first-episode and drug-naïve schizophrenia Interventions: Risperidone vs. zuclopenthixol (No un-medicated group) |
| Glenthoj 2005 | Allocation: randomised Participants: people with first-episode and drug-naïve schizophrenia (n=19) Interventions: low doses of the typical drug zuclopenthixol vs. the atypical compound risperidone (No un-medicated group) |
| Godemann 1999 | Allocation: randomised Participants: people with psychosis Interventions: long-term vs. interval medication (Unknown proportion of first and second episodes; No un-medicated group) |
| Good 2004 | Allocation: randomised Participants: people with schizophrenia-like illnesses and neuroleptics-naive Interventions: haloperidol vs. risperidone (No un-medicated group) |
| Grasso 1974 | Not related to medication treatment |
| Grawe 1998 | Allocation: randomised Participants: people with recent onset schizophrenia Interventions: optimal multimodal treatment (neuroleptics, family psycho-education, family communicational problem-solving and stress management training, individualized psychotherapy) VS. treatment-asusual (No un-medicated group) |
| Grawe 2006 | Allocation: randomised Participants: people with less than 2-year duration of schizophrenia Interventions: integrated (pharmacotherapy, case management, cognitive-behavioural family treatment) vs. standard treatment (optimal pharmacotherapy and case management) (No un-medicated group) |
| Green 2001b | Allocation: randomised; Participants: people with first episode schizophrenia, schizoaffective disorder or schizophreniform disorder Interventions: olanzapine vs. haloperidol (No un-medicated group) |
| Gumley 2001b | Allocation: randomised Participants: people with schizophrenia-spectrum disorder Interventions: two methods of early signs monitoring : standardized vs. individualized early signs monitoring systems (Unknown proportion of first and second episodes; Unknown medication) |
| Gumley 2003a | Allocation: randomised Participants: people with schizophrenia or a related disorder and receiving antipsychotic medication and considered relapse prone Interventions: treatment as usual (n=72) vs. treatment as usual +CBT(n=72) (not an acute treatment study; No un-medicated group) |
| Gumley 2003b | Allocation: randomised Participants: people with a diagnosis of schizophrenia spectrum disorder and admitted to an acute psychiatric ward with a first or subsequent episode of psychosis Interventions: CBT plus antipsychotic medications vs. medications alone (No un-medicated group, unclear proportion of first and second episodes) |
| Gumley 2006 | Allocation: randomised Participants: people with schizophrenia or a related disorder and receiving antipsychotic medication, and considered relapse-prone Interventions: treatment as usual (n=72) vs. CBT (n=72) (not treatment for acute schizophrenia, no un-medicated group, unclear proportion of early episodes) |
| Guo 1995 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia Interventions: Clozapine vs. Risperidone (unclear method of assignment to treatment; No un-medicated group) |
| Guo 2001a | Allocation: unclear method of assignment to treatment Participants: People with schizophrenia Interventions: Risperidone (unclear method of assignment to treatment; the proportion of first episodes does not exceed 50%; No un-medicated group) |
| Guo 2004 | Allocation: randomised Participants: people with schizophrenia Interventions: treatment group (modified-ECT) plus medications vs. control group (without modified-ECT) plus medications (risperide and clozapine) (unclear proportion of first and second episodes; not a study of medication effectiveness) |
| Haddock 1999 | Allocation: randomised Participants: people with acute schizophrenia within 5 years of first onset Interventions: short-term individual CBT vs. supportive counseling/Psychoeducation + standard inpatient hospital care and medication (no un-medicated group) |
| Haddock 2000a | Allocation: randomised Participants: people dually diagnosed with recent onset schizophrenia and substance abuse Interventions: combination of cognitive behavior therapy for individuals and cognitive behavioral interventions for family and carergivers, compared to usual treatment (no contrast of medicated versus un-medicated groups) |
| Haddock 2000b | Allocation: unclear method of assignment to treatment Participants: people with an ICD10 diagnosis of schizophrenia, schizo-affective disorder or delusional disorder and have less than five years since onset and with alcohol or drug abuse Intervention: a family support and cognitive behavioural treatment service (unclear method of assignment to treatment; no contrast of medicated versus un-medicated groups) |
| Haddock 2006 | Allocation: randomised Participants: people with first or second admission (within 2 years of a first admission) Interventions: cognitive behavioral therapy (CBT) + treatment as usual, vs. supportive counseling + treatment as usual, vs. treatment as usual (no un-medicated group (not a medication effectiveness study)) |
| Haldun 2002 | Allocation: randomised Participants: people with a history of schizophrenia less than 10 years Interventions: optimal clinical management vs. routine case management (not acute schizophrenia treatment comparison; No un-medicated group) |
| Hawkins 2004a | Allocation: randomised Participants: people in the prodromal phase of schizophrenia Interventions: placebo (n= 29) vs. Olanzapine (n=31) (not acute schizophrenia treatment comparison) |
| Hawkins 2004b | Allocation: randomised Participants: people in the prodromal phase of schizophrenia Interventions: placebo vs. Olanzapine (not treatment for acute schizophrenia) |
| Herrmann 1991 | Allocation: randomised Participant: young healthy males (n=15) Interventions: Maroxepin vs. Chlorpromazine vs. Imipramine vs. Methanesulfonate salt vs. Savoxepine vs. Placebos (not treatment for people with first and second episode schizophrenia) |
| Herz 1982 | Allocation: consecutive Participants: People with schizophrenia Interventions: intermittent vs. continuious antipsychotic medication (not randomised; not an acute schizophrenia treatment comparison; no un-medicated group) |
| Herz 1989a | Allocation: randomised Participants: people with schizophrenia Interventions: Stage 1: drug washout for 8 weeks; Stage 2: active medication vs. placebo (no definition of the medications used) (not acute schizophrenia treatment comparison; unclear proportion of first and second episodes) |
| Herz 1998 | Allocation: randomised Participants: people with schizophrenia maintained on antipsychotic medication Interventions: early intervention treatment vs. treatment as usual (not treatment for acute schizophrenia; no un-medicated group; unclear proportion of early episodes) |
| Heydebrand 2004 | Allocation: randomised Participants: people with first episode schizophrenia Interventions: haloperidol and risperidone (no un-medicated group) |
| Himei 2005 | Allocation: randomised Participants: people with first episode schizophrenia (n=14) or not receiving drug treatment within the previous 6 months (n=6) or receiving therapy with haloperidol only for more than 5 years (treated group, n=100) Interventions: Risperidone (group A: increasing the dose; group B: decreasing the dose; group C: abruptly to a new regimen) (proportion of early episodes less than 50%; no unmedicatd group) |
| Hirsch 1986 | Allocation: Randomised (n=45) Participants: people with schizophrenia Interventions: Depot Preparations/fluphenazine and placebo (Not an acute treatment study; unclear proportion of early episodes) |
| Hodgekins 2006a | Allocation: Randomised Participants: People with early psychosis Interventions: Usual treatment plus cognitive treatment versus usual treatment (Combined consecutive referral allocation with random assignment; no un-medicated group) |
| Hoffman 2006 | Allocation: randomised Participants: people in prodromal status of psychosis Interventions: Olanzapine vs. Placebo (not treatment for acute schizophrenia) |
| Hogarty 1991 | Allocation: randomised Participants: people with schizophrenia (n=103) Interventions: family psychoeducation/management (FT) vs. individual social skills training (SST) vs. the combination of FT and SST vs. medication controls (unclear proportion of first and second episodes; no un-medicated group; not an acute treatment study) |
| Honer 2005b | Allocation: randomised Participants: people with first episode psychosis (n=533) Interventions: Haloperidol vs. Risperidone (no un-medicated group) |
| Hornung 1995 | Allocation: randomised Participants: people with schizophrenia, having at least two acute psychotic episode within 5 years Interventions: psychoeducational medication training (PMT) vs. Cognitive psychotherapy (CP) vs. Key-person counselling (KC) vs. non-specific treatment in the control group (consisted of regular leisure-time group activities: games, excursions, visits to organized functions, etc.) (not early episodes; not acute treatment; no un-medicated group) |
| Hu 2003b | Allocation: randomised Participants: people with first episode schizophrenia (n=62) Interventions: Chlorpromazine vs. Risperidone vs. Quetiapine (no un-medicated group) |
| Huang 2004c | Allocation: randomised Participants: senile people with first episode schizophrenia Interventions: trilafon+ nimodipine vs. trilafon (only include senile people; no un-medicated group) |
| Huang 2006d | Allocation: randomised Participants: adolescents with first episode schizophrenia Interventions: Olanzapine vs. Risperidone (no un-medicated group) |
| Ishigooka 2001 | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia Interventions: Olanzapine vs. Haloperidol (unclear method of assignment to treatment; unclear proportion of first and second episodes; no un-medicated group) |
| Ivarson 1998 | Allocation: randomised Participants: people with recent onset of schizophrenic disorders Interventions: integrated treatment (medication + psychosocial interventions) vs. standard treatment (no un-medicated group) |
| Jackson 2001a | Allocation: nonrandomised Participants: people with first episode psychosis (n=80) Interventions: Cognitively oriented psychotherapy for early psychosis (COPE). There are three group of comparison: those who were offered and accepted COPE; (2) those who were offered COPE but refused it, and continued to receive other services; and (3) those who were offered neither COPE nor any other continuing treatment (control subjects) (treatment assignment by choice; unclear use of medications) |
| Jackson 2001b | Allocation: randomised Participants: people with first episode schizophrenia Interventions: recovery intervention (cognitive therapy) vs. treatment-as-usual (Not an acute treatment study; no un-medicated group) |
| Jackson 2004a | Allocation: randomised Participants: people with first episode schizophrenia (n=66) Interventions: cognitive therapy vs. treatment-as-usual (no un-medicated group; not an acute treatment study) |
| Jackson 2004b | Allocation: randomised Participants: people with first episode schizophrenia (n=79) Interventions: Cognitively oriented psychotherapy for early psychosis (COPE) vs. no COPE (not an acute treatment study; no un-medicated group) |
| Jackson 2005 | Allocation: randomised Participants: people in the early phase of schizophrenia (n=62) Interventions: Active Cognitive Therapy for Early Psychosis (ACE) plus medications vs. Befriending plus medications (no un-medicated group) |
| Jackson 2006 | Allocation: randomised Participants: people with first episode psychosis (n=66) Interventions: cognitive therapy vs. treatment as usual (Not an acute treatment study; no contrast of medicated versus un-medicated group) |
| Janicak 1998 | Allocation: randomised Participants: People with acute mania (n=33) Interventions: Verapamil versus placebo (Not people with schizophrenia) |
| Jarboe 2001 | Allocation: unclear method of allocation to treatment Participants: people with first episode schizophrenia or schizoaffective disorder Interventions: Haloperidol vs. Olanzapine (unclear method of allocation to treatment; no un-medicated group) |
| Jasovic 1995 | Allocation: randomised Participants: people with schizophrenia and depression Intervention: active drug (moclobemide) vs. placebo (moclobemide free). Both groups also receive antipsychotic medications (dually diagnosed persons (schizophrenia and depression); unclear proportion of first and second episodes; no un-medicated group) |
| Jasovic 1998 | Allocation: randomised Participants: people with schizophrenia and depression Intervention: Mianserin, Moclobemide, or placebo, as an adjunctive therapy with classical neuroleptic medication (dually diagnosed persons (schizophrenia and depression); unclear proportion of first and second episodes; no un-medicated group) |
| Jenner 2004b | Allocation: randomised Participants: people with treatment refractory schizophrenia (n=76) Interventions: Hallucination-focused Integrative Treatment (HIT) vs. routine treatment (Not an acute treatment study; Unclear proportion of first and second episodes; No un-medicated group) |
| Ji 2006 | Allocation: randomised Participants: people with first episode schizophrenia (n=82) Interventions: antipsychotic medication + general nursing + system health education intervention vs. antipsychotic medication + general nursing (no un-medicated group) |
| Jiang 2006 | Allocation: randomised Participants: people with first episode schizophrenia (n=120) Interventions: antipsychotic medications + CBT vs. antipsychotic medications (no un-medicated group) |
| Jiang Xinyan 2004 | Allocation: randomised Participants: Older adults (over 60 years of age) with first episode schizophrenia (n=62) Interventions: Olanzapine vs. Risperidone (only older adults with schizophrenia; no un-medicated group) |
| Johnson 2004b | Allocation: unclear method of allocation to treatment Participants: people in early psychosis Intervention: unclear (Not enough information) |
| Johnston-Cronk 1993 | Allocation: randomised Participants: people with schizophrenia Interventions: antipsychotic medication plus placebo supplement vs antipsychotic medication and active medication supplement (not an acute treatment study; unclear proportion of early episodes; no un-medicated group) |
| Johnstone 1998b | Allocation: randomised Participants: people with schizophrenia (n=814) Interventions: olanzapine (OLZ) vs. haloperidol (HAL) (Unclear proportion of first and second episodes; No un-medicated group) |
| Jolley 1989 | Allocation: randomised Participants: people with chronic schizophrenia (n=54) Interventions: intermittent treatment group (n=27, placebo injection) vs. control group (n=27, Fluphenazine injections) (maintenance treatment study (not acute schizophrenia); unclear proportion of early episodes) |
| Jolley 2003 | Allocation: randomised Participants: people with first or second episode schizophrenia spectrum disorder and diagnosed within five years (n=21) Interventions: cognitive therapy + treatment as usual vs. treatment as usual (no un-medicated group) |
| Jones 1998 | Allocation: randomised Participants: people with schizophrenia (n=65) Interventions: Haloperidol vs. Olanzapine vs. Risperidone (not treatment for acute schizophrenia; No un-medicated group) |
| Jones 2005b | Allocation: randomised Participants: young people with early psychosis and severe mood disorder (n=100) Interventions: Social Recovery oriented CBT (SRCBT) vs. standard case management (Not a medication treatment study) |
| Jones 2006 | Allocation: randomised Participants: people with schizophrenia and related disorders (n=227) Interventions: first generation antipsychotics vs. second generation antipsychotics (No un-medicated group, Less than 50% first and second episodes, not an acute treatment study) |
| Kahn 2003 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorders Interventions: Haloperidol; Olanzapine (Unclear proportion of first and second episodes; No un-medicated group) |
| Kahn 2006 | Allocation: randomised Participants: people with first episode schizophrenia, schizoaffective disorders or schizophreniform disorders (n=500) Interventions: Amisulpride or Olanzapine or Quetiapine or Ziprasidone vs. low-dose Haloperidol (No un-medicated group) |
| Kane 1982a | Allocation: randomised Participants: people with remitted, first-episode schizophrenia (n=28) Interventions: Fluphenazine vs. Placebo (not treatment for acute schizophrenia) |
| Kane 2001b | Allocation: randomised Participants: people with schizophrenia (n=370) Interventions: 25mg, or 50mg or 75mg Risperidone microspheres vs. Placebo (Unclear proportion of first and second episodes) |
| Kapur 2000b | Allocation: randomised Participants: people with first-episode schizophrenia (n=22) Interventions: 1.0 mg/day haloperidol vs. 2.5 mg/day haloperidol (No un-medicated group) |
| Kavanagh 2004 | Allocation: randomised Participants: people with early psychosis and current misuse of non-opioid drugs (n=25) Interventions: Start Over and Survive (SOS) + standard care vs. standard care (No un-medicated group) |
| Keefe 2005 | Allocation: randomised Participants: people with first episode psychosis Interventions: Olanzapine vs. Quetiapine vs. Risperidone (no un-medicated group) |
| Keefe 2006b | Allocation: randomised Participants: people with first episode psychosis Interventions: Olanzapine vs. low dose haloperidol (no un-medicated group) |
| Kenny 1992 | Allocation: Randomised Participants: people with treatment resistant schizophrenia Interventions: Clozapine (n=24) vs. standard neuroleptics (n=13) (unclear proportion of first and second episodes; no un-medicated group) |
| Keri 2006 | Allocation: Not a treatment comparison study (one group study) Participants: People meeting ACE criteria for ultra-high risk of psychsis Interventions: Risperidone plus psychoeducation an supportive psychotherapy (Not a treatment comparison study (one group study), not acute schizophrenia, no un-medicated group) |
| Kern 2001 | Allocation: randomised Participants: people with clinically stable schizophrenia or schizoaffective disorder Interventions: Aripiprazole versus. Olanzapine (unclear proportion of first and second episodes; no un-medicated group) |
| Keshavan 1998 | Allocation: Not a treatment comparison study; studies brain morphology over time in first-episodes treated with conventional antipsychotics or risperidone Participants: people with first episode schizophrenia Interventions: Haloperidol (n=19) or Risperidone (n=16) (not a treatment comparison study, no un-medicated group) |
| Keshavan 2003 | Allocation: unclear method of assignment to treatment Participants: people with recent onset of psychosis (n=60) Interventions: Psycho Education and Collaboration Enhancement (PEACE) (unclear method of assignment to treatment; not a medication treatment comparison study) |
| Killackey 2006 | Allocation: randomised Participants: young people with early psychosis (n=40) Interventions: treatment as usual + Individual Placement and Support Model versus treatment as usual (not a medication treatment comparison study) |
| Kingdon 2000 | Allocation: randomised Participants: people with first or second episode (unclear number) Interventions: CBT + treatment as usual versus treatment as usual (drug only) (no un-medicated group) |
| Kistrup 1991 | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia and a duration of illness of 2 or more years Interventions: cis(z)- flupenthixol decanoate (n=24) versus Perphenazine decanoate (n=24) (unclear method of assignment to treatment; not treatment for recent onset schizophrenia but maintenance treatment, no un-medicated group) |
| Klier 2005 | Allocation: randomised Participants: adolescents with “At-Risk-Mental-State” Interventions: fish oil (EPA/DHA) (Omega-3 fatty acids) + standard care versus standard care (not treatment for recent onset of schizophrenia; no un-medicated group) |
| Knapp 2004 | Allocation: randomised Participants: people with first or second episode schizophrenia Interventions: Early psychosis service versus. Standard service (unclear definition of these two services) (inadequate information on the types of treatments provided) |
| Kolivakis 2001 | Allocation: randomised Participants: people with schizophreniform disorder and early paranoid schizophrenia (n=20) Interventions: Risperidone versus haloperidol, with or without anticonvulsant medications (no un-medicated group) |
| Kopala 2003 | Allocation: randomised Participants: people with recent onset schizophrenia Interventions: Haloperidol (n=277) versus Risperidone (n=278) (no un-medicated group) |
| Kuipers 2004 | Allocation: randomised Participants: people with a diagnosis of any functional psychosis Interventions: Croydon Outreach and Assertive Support Team or COAST (optimum atypical medication, and psychological interventions, e.g. individual CBT and family intervention, and a range of vocational and welfare support) vs. treatment as usual (n=27) (unclear proportion of first and second episodes; no un-medicated group, not an acute treatment study) |
| Kujawa 2002 | Allocation: randomised Participants: people with acute relapse of chronic schizophrenia Interventions: aripiprazole 30 mg (n=861) or haloperidol 10 mg (n=433) (not treatment for recent onset of schizophrenia; no un-medicated group) |
| Lambert 1995 | Allocation: unknown method of assignment to treatment Participants: people with schizophrenia (n=144) but only 28 first episodes Interventions: Remoxipride versus. Thioridazine (unknown method of assignment to treatment; no un-medicated group, majority are not early episodes) |
| Lambert 2006 | Allocation: randomised Participants: people with schizophrenia, schizophreniform disorder, or schizoaffective disorder (n=263) Interventions: Olanzapine versus haloperidol (unclear proportion of first and second episodes; no un-medicated group) |
| Lane 2001 | Allocation: randomised Participants: people with first episode schizophrenia (n=24) Interventions: risperidone 3mg/day versus. risperidone 6mg/day (no un-medicated group) |
| Lauriello 2005 | Allocation: randomised Participants: people with schizophrenia (n=34) Interventions: Haloperidol versus Quetiapine (unclear proportion of first and second episodes; no un-medicated group) |
| Lavalaye 1999 | Allocation: randomised Participants: young people with first episode schizophrenia (n=36) Interventions: Olanzapine versus. Risperidone (no un-medicated group, not a treatment outcome comparison study (but a study of dopamine occupancy)) |
| Leavey 2004 | Not an acute early-episode medication treatment comparison study, but a study of the response and satisfaction to adjunctive psychosocial treatment among relatives of persons with schizophrenia |
| Leblanc 2006 | Allocation: randomised Participants: people with schizophrenia or related psychosis and in a stable status (no score >=5 at PANSS positive symptom subscale) Interventions: Modafinil versus placebo (not treatment for acute schizophrenia) |
| Leclerc 2006 | Allocation: randomised Participants: people with first episode psychosis (n=19) Interventions: group CBT versus control group (not a medication treatment study, not acute treatment study, unclear use of medications) |
| Lecomte 2006 | Allocation: randomised Participants: people with first episode psychosis (n=129) Interventions: group CBT versus. group skills training focusing on symptom management versus a wait-list control group (not an acute treatment study, not a medications treatment comparison study, unclear use of medications) |
| Lecrubier 2003 | Allocation: randomised Participants: people with acute or sub-acute episode of schizophrenia (with a paranoid, disorganized or undifferentiated subtype), excluding first-episode Interventions: BP4897 (n=52) versus placebos (n=25) (not treatment for early episode schizophrenia (recent onsets excluded)) |
| Lehtinen 1990 | Allocation: Quasi-random (assigned to treatment available in the catchment area) Participants: People with first-episode functional non-affective psychosis, n=135, 80M, 55W Interventions: ‘Finnish need-specific treatment’ plus usual use of antipsychotic medications, n=51, vs ‘Finnish need-specific treatment’ plus ‘minimal neuroleptic regimen’, n=84 (Not randomly allocated to treatments) |
| Lehtinen 2000 | Allocation: Subjects were consecutively recruited in three experimental centers and other three control centers separately, but not randomly assigned to treatement Participants: people with first-episode functional non-affective psychosis (n=106) Interventions: integrated treatment versus standard treatment (in the former, a minimal neuroleptic regime was applied while in the latter neuroleptics were used according to the usual practice) (No random assignment) |
| Lei 2006 | Allocation: Randomised Participants: Relatives of children with first-episode schizophrenia (n=60) Interventions: Health education and psychotherapy (focused on relatives of people with schizophrenia) |
| Lemmer 2001 | Allocation: unclear method of assignment to treatment Participants: people with acute paranoid halluzinatory schizophrenia (n=46) Interventions: Zotepine versus Haloperidol (unclear method of assignment to treatment ; unclear proportion of early episodes; no un-medicated group) |
| Lencz 2006 | Allocation: randomised Participants: people with first episode schizophrenia (n=61) Interventions: Risperidone versus olanzapine (no un-medicated group) |
| Lenior 2001 | Allocation: randomised Participants: people with early-onset schizophrenia (n=72) Interventions: standard intervention versus family intervention + standard intervention (no un-medicated group) |
| Lenior 2002 | Allocation: randomised Participants: young people with early onset schizophrenia and related disorders Interventions: standard intervention versus family intervention + standard intervention (no un-medicated group) |
| Lester 2004a | Allocation: unknown method of assignment to treatment Participants: General practitioners (GPs) Interventions: video-based educational programme for GPs about first episode psychosis (FEP) (Not an early episode acute treatment medication comparison study) |
| Lester 2004b | Allocation: unknown method of assignment to treatment Participants: People with a developing first episode psychosis and their caregivers and family members Interventions: Primary care training programme for General Practitioners (GPs) regarding the early recognition of psychosis and adherence to guidelines (Not an early episode acute treatment medication comparison study; no people with acute schizophrenia) |
| Lewis 2000a | Allocation: randomised Participants: people with early schizophrenia with 82% first episode (total n=360) Interventions: routine care + CBT versus routine care + supportive counseling versus routine care only (no un-medicated group) |
| Lewis 2000d | Allocation: randomised Participants: people with recent onset schizophrenia and substance use Intervention: psychosocial intervention versus routine treatments (unclear use of medications) |
| Lewis 2000f | Allocation: randomised Participants: people with first or second episode schizophrenia Interventions: CBT (unclear use of medications) |
| Lewis 2001e | Allocation: randomised Participants: people with psychosis Interventions: monitoring only + routine care versus CBT + routine care (unclear proportion of first and second episodes) |
| Lewis 2002a | Allocation: randomised Participants: people with first or second episode psychosis (n=315) Interventions: CBT + routine care versus 1st control group (supportive counseling + routine care) versus 2nd control group (routine care only) (no un-medicated group) |
| Lewis 2006b | Allocation: randomised Participants: people with schizophrenia Interventions: one of the second-generation antipsychotics (risperidone, olanzapine, quetiapine, amisulpride) versus. Clozapine (n=136) (unclear proportion of first and second episodes; no un-medicated group) |
| Lewis 2006c | Allocation: randomised (2 RCTs in this study) Participants: people with schizophrenia Interventions: first RCT: atypical drugs (risperidone, olanzapine, quetiapine and amisulpride) versus conventional drugs (n=227); second RCT: new (non-clozapine) atypical drugs versus. Clozapine (n=136) (unclear proportion of first and second episodes; no un-medicated group) |
| Li 2003f | Allocation: only one treatment group Participants: people with first episode schizophrenia (n=36) Interventions: Quetiapine (no contrast of medicated versus un-medicated group) |
| Li 2004a | Allocation: not randomised Participants: people with first episode schizophrenia Interventions: psychological and social intervention + treated with medication (n=50) versus treatment with medication only (n=50) (no random assignment; no un-medicated group) |
| Li 2004f | Allocation: randomised Participants: people with first episode schizophrenia (n=86) Interventions: family mental intervention + medicine treatment versus medicine treatment only (no un-medicated group) |
| Li 2004h | Allocation: randomised Participants: people with early schizophrenia (n=80) Interventions: Clozapine + nursing care + self care versus Clozapine only (no un-medicated group) |
| Li 2005d | Allocation: randomised Participants: people with first episode schizophrenia (n=46) Interventions: Quetiapine versus Risperidone (no un-medicated group) |
| Liang 2003a | Allocation: only one treatment group Participants: children with age <14 years and with first episode schizophrenia Intervention: Risperidone (not treatment for adults; no contrast of medicated versus un-medicated) |
| Liao Chunping 2004 | Allocation: randomised Participants: people with first episode schizophrenia (n=60) Interventions: Risperidone (n= 30) and Clozapine (n= 30) (no un-medicated group) |
| Liberman 1988 | Allocation: randomised Participants: people with schizophrenia Interventions: low-dose neuroleptic therapy + highly structured skills training versus. low-dose neuroleptic therapy + unstructured, goup discussion (unclear proportion of first and second episodes; no un-medicated group) |
| Lieberman 2001b | Allocation: randomised Participants: people with first episode schizophrenia and drug naïve (n=164) Interventions: Clozapine versus chlorpromazine (no un-medicated group) |
| Lieberman 2003a | Allocation: randomised Participants: people with schizophrenia and schizoaffective disorders Interventions: Haloperidol versus Olanzapine (unclear proportion of first and second episodes; no un-medicated group) |
| Lieberman 2003c | Allocation: randomised Participants: people with first episode schizophrenia and drug naïve (n=160) Interventions: Clozapine versus chlorpromazine (no un-medicated group) |
| Lieberman 2005b | Allocation: randomised Participants: people with first episode schizophrenia (n=263) Interventions: haloperidol versus olanzapine (no un-medicated group) |
| Lieberman 2005c | Allocation: randomised Participants: people with first episode schizophrenia (n=263) Interventions: haloperidol versus olanzapine (no un-medicated group) |
| Lin 2006b | Allocation: randomised Participants: people with first episode schizophrenia (n=84) Interventions: Aripiprazole (n=42) versus Chlorpromazine(n=42) (no un-medicated group) |
| Lin 2006c | Allocation: consecutively according to admission time Participants: females with first episode schizophrenia (n=60) Interventions: Aripiprazole versus Chlorpromazine (no random assignment; no un-medicated group) |
| Linszen 1994 | Allocation: randomised Participants: people with recent onset schizophrenia or related disorders post-hospitaliztion Interventions: individually oriented early (psychosocial) intervention program + family intervention and medications versus individually oriented early (psychosocial) intervention program and medications (not an acute treatment comparison study, no un-medicated group) |
| Linszen 2004a | Allocation: randomised Participants: young people with first episode schizophrenia (n=200) Interventions: outpatient intervention program versus standard outpatient facilities (not an acute treatment comparison study, no un-medicated group) |
| Linszen 2006 | Allocation: randomised Participants: young people with first episode psychosis (n=183) Interventions: early and sustained intervention (not an acute treatment comparison, no un-medicated group) |
| Lis 2003 | Allocation: randomised Participants: people with schizophrenia (n=34, a majority with first episodes) Interventions: Haloperidol versus Sertindole (no un-medicated group) |
| Liu 2006c | Allocation: randomised Participants: people with first episode schizophrenia (n=60) Interventions: Aripiprazole versus Clozapine (no un-medicated group) |
| Liu Lin 2004b | Allocation: randomised Participants: people with first episode schizophrenia (n=112) Interventions: chlorpromazine therapy group ( n= 56) + health education versus chlorpromazine therapy group only ( n= 56) (no un-medicated group) |
| Loza 1999 | Allocation: randomised Participants: people with acute schizophrenia Interventions: Olanzapine (n=27) versus Chlorpromazine (n=14) (no un-medicated group; unspecified proportion of first-episodes) |
| Loza 2001 | Allocation: randomised Participants: people with first-episode paranoid schizophrenia (n=32) Interventions: typical antipsychotics (zuclopenthixol, perphenazine, haloperidol, perazine) versus atypical antipsychotics (risperidone, olanzapine, quetiapine) (no un-medicated group) |
| Loza 2002 | Allocation: randomised Participants: people with first-episode paranoidschizophrenia (n=39) Interventions: Clozapine versus Olanzapine versus Risperidone (no un-medicated group) |
| Lu 2002b | Allocation: not random (case-control ) Participants: people with first episode schizophrenia (n=19) and healthy controls (n=22) Interventions: Clozapine (no random assignment; no contrast of medicated versus un-medicated groups with acute psychosis; not a treatment comparison study) |
| Ma 2000a | Allocation: randomised Participants: people with first episode schizophrenia (n=56) Interventions: Chlorpromazine and Clozapine (no un-medicated group; not a treatment comparison study) |
| Ma 2002 | Allocation: randomised Participants: people with first episode schizophrenia (n=38) and healthy controls (n=20) Interventions: Chlorpromazine and Clozapine (no contrast of medicated versus un-medicated group; not a treatment comparison study) |
| Ma 2004b | Allocation: randomised Participants: people with first episode schizophrenia (n=118) Interventions: Risperidone (n=59) + nursing intervention versus Risperidone (n=59) (no un-medicated group) |
| Ma Xiaozhi 2004 | Allocation: randomised Participants: people with first episode schizophrenia (n=106) Interventions: medications + individualized quantitative healthy education versus. medications + random healthy education (no un-medicated group; not an acute treatment study) |
| Mackeprang 2001 | Allocation: randomised Participants: people with drug-naïve first episode schizophrenia Interventions: Risperidone versus zuclopenthixol (no un-medicated group; not a treatment outcome study) |
| Malla 2000 | Allocation: unclear method of assignment to treatment Participants: people with first episode of psychosis Interventions: a community focused early intervention (antipsychotics and adjunct medications, youth education and support, cognitively oriented skills training, case management and group intervention, and family intervention) versus standard treatment (unclear method of assignment to treatment; no un-medicated group) |
| Malla 2001 | Allocation: not random (matched case-control study: matched on age, gender, length of illness and length of treatment) Participants: people with first episode schizophrenia (n=38) Interventions: Risperidone versus typical antipsychotics (not randomly assigned to treatment; no un-medicated group) |
| Mandelson 2000 | Allocation: randomised Participants: people with first or second episode schizophrenia Interventions: CBT + medications versus psychoeducational and supportive counseling + medication versus Treatment as usual (no un-medicated group; unclear proportion of first-episodes) |
| Marder 1991 | Allocation: randomised Participants: people with stabilized schizophrenia (n=50) Interventions: in the beginning, all subjects randomly received either behavioural skills training or supportive group therapy; then in the prodromal period, subjects were randomly treated with Fluphenazine or placebo (not an acute treatment study; unclear proportion of first-episodes) |
| Marder 1994 | Allocation: randomised Participants: people with schizophrenia Interventions: 2mg or 6mg or 10mg or 16mg risperidone versus 20mg haloperidol versus placebo (unclear proportion of first and second episodes) |
| Marder 1996 | Allocation: Randomised Participants: males with schizophrenia undergoing treatment at West Los Angeles Veterans Affair Medical Center (n=80) Interventions: behaviorally oriented social skills training or supportive group therapy (not an acute treatment comparison study, no un-medicated group, and unclear proportion of first-epsiodes) |
| Marques 2001b | Allocation: randomised Participants: women with acute schizophrenia (n=40) Interventions: haloperidol + conjugated estrogens versus haloperidol + placebo (no un-medicated group; unclear proportion of first-episodes) |
| Marquez 2004a | Allocation: randomised Participants: people with first episode, early phase and stabilized chronic schizophrenia Interventions: Olanzapine versus Haloperidol (unclear proportion of first and second episodes; no un-medicated group) |
| Martényi 2000 | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia Interventions: Olanzapine versus. Fluphenazine (unclear method of assignment to treatment; unclear proportion of first episodes; no un-medicated group) |
| McConchie 2004 | Allocation: randomised Participants: people with first episode psychosis Interventions: essential fatty acid (EAC) versus placebo (no antipsychotics were included) |
| McEvoy 2003 | Allocation: randomised Participants: people with first episode of schizophrenia and schizoaffective disorders (n=262) Interventions: Olanzapine versus haloperidol (no un-medicated group) |
| McEvoy 2006b | Allocation: randomised Participants: people with first episode of schizophrenia and schizophreniform or schizoaffective disorders (n=400) Interventions: Olanzapine (n=133) versus Quetiapine (n=134) versus Risperidone (n=133) (no un-medicated group) |
| McEvoy 2006d | Allocation: randomised Participants: people with first episode of schizophrenia and schizophreniform or schizoaffective disorders (n=400) Interventions: Olanzapine versus Quetiapine versus Risperidone (no un-medicated group) |
| McEvoy 2006f | Allocation: randomised Participants: people with first episode psychosis (n=251) Interventions: olanzapine versus haloperidol (no un-medicated group) |
| McGlashan 1999 | Allocation: randomised Participants: people in the prodromal period of psychosis Interventions: Olanzapine versus placebo (not treatment for acute schizophrenia) |
| McGlashan 2006 | Allocation: randomised Participants: people in the pre-onset phase of the prodromal to schizophrenia (n=60) Interventions: Olanzapine (n=31) versus placebo (n=29) (not treatment for acute schizophrenia) |
| McGorry 1997a | Allocation: Randomised Participants: Young people aged 16 to 30 years, experiencing a first (non-affective) psychotic episode, nonresponders (slow) Inteverntions: 2 mg or 4 mg of risperidone or 2 mg of risperidone + Lithium therapy (No un-medicated group) |
| McGorry 1997b | Allocation: not random (matched cohorts) Participants: people with first episode schizophrenia Interventions: standard inpatient care versus. intensive community based early intervention (not randomised; no un-medicated group) |
| McGorry 2002b | Allocation: randomised Participants: people at incipient risk of progression to first episode schizophrenia (n=59) Interventions: needs based intervention (no antipsychotics but could receive antidepressants if necessary) versus low dose risperidone + cognitive behavioral therapy (not treatment for acute schizophrenia) |
| McGorry 2002c | Allocation: not random Participants: people with first episode schizophrenia (n=95) Interventions: in Phase I, all subjects received 2mg Risperidone for 4 weeks; in Phase II, fast responders continue 2mg Risperidone while slow responders were randomised to the following 3 groups: 2mg Risperidone; 4mg Risperidone; lithium + 2gm risperidone (not randomly assigned to treatment; no un-medicated group) |
| McQuade 2003 | Allocation: randomised Participants: people in acute relapse of schizophrenia and requiring hospitalization (n=317) Interventions: Aripiprazole (n=156) versus Olanzapine (n=161) (Unclear proportion of first episodes; no un-medicated group) |
| Melle 2006 | Not a study of the treatment of early onset schizophrenia but of the early detection of susicde attempts among peole with first episode schizophrenia in areas with and without early detection programs |
| Melnyk 1966 | Allocation: randomised Participants: people with schizophrenia (n=40) after stabilization Interventions: Chlorpromazine or Thioridazine versus Placebos (unclear proportion of first and second episodes; study of medication withdrawal study not of acute treatment) |
| Merlo 2000 | Allocation: randomised Participants: people with first episode psychosis (n=52) Interventions: Risperidone (2 mg or 4 mg) (no un-medicated group) |
| Merlo 2002b | Allocation: randomised Participants: people with acute psychosis and drug naïve (n=49) Interventions: 2mg Risperidone versus 4mg Risperidone (no un-medicated group) |
| Merson 1992 | Allocation: randomised Participants: people with psychosis (n=100) Interventions: multidiscipline community based intervention (n=48) versus. conventional hospital based psychiatric intervention (n=52) (unclear proportion of first episodes; no un-medicated group) |
| Michael 2005 | Allocation: randomised Participants: people with affective or nonaffective functional psychosis Interventions: SRCBT (Social Recovery Cognitive Behaviour Therapy) versus. standard case management (not an acute treatment study; unclear proportion of first episodes; no un-medicated group) |
| Miller 2004 | Not a study of the treatment for acute schizophrenia but a validation study of Structured Interview for Prodromal Syndromes (the SIPS), which is used to identify people in prodromal phase to schizophrenia |
| Min 2001 | Allocation: randomised Participants: people with first episode schizophrenia (n=81) Interventions: systematic early intervention + risperidone versus risperidone alone (no un-medicated group) |
| Montero 2005 | Allocation: randomised Participants: people with schizophrenia Interventions: Behavioral Family Intervention Group (n=46) versus Relatives Group (n=41) (unclear proportion of first episodes; no contrast of medicated versus. un-medicated group) |
| Morken 2005 | Allocation: randomised Participants: people with recent onset of schizophrenia (within 2 years) (n=50) Interventions: standard treatment + a multidiscipline team with a low case-load (patient-staff ratio about 1:10) versus. standard treatment (no un-medicated group; not an acute treatment study) |
| Morrison 2004 | Allocation: randomised Participants: people at high risk to develop a first episode psychosis (n=58) Interventions: cognitive therapy versus treatment as usual (not treatment for acute schizophrenia) |
| Morrison 2006b | Allocation: randomised Participants: young people at high risk of developing psychosis Interventions: cognitive therapy + monitoring versus monitoring only (not treatment for acute schizophrenia) |
| Mortimer 2003c | Allocation: randomised Participants: people with recent onset schizophrenia, schizoaffective and schizophreniform disorder Interventions: haloperidol, olanzapine, quetiapine, amisulpride, and ziprasidone (no un-medicated group) |
| Mosher 1978 | Allocation: Not randomised Participants: people with DSM-II Schizophrenia, nor more than one prior hospitalization, ages 16-35, unmarried, n=79 Interventions: TAU Hospitalization and antipsychotic medications, n=42; and therapeutic milieu with time-limited postponement (up to 6 weeks) of antipsychotic medications, n=37 (Not randomly assigned to treatment) |
| Mottaghipour 2000 | Allocation: not a treatment comparison study (no assignment to comparative treatments) Participants: families with early onset psychosis (n=34) and families with chronic psychosis (n=39) Interventions: family education (no assignment to comparative treatments; not an acute treatment comparison study) |
| Mottaghipour 2006 | Allocation: randomised Participants: families with first episode psychosis (n=22) Interventions: two models of family education: home based family education versus. family groups at hospital (not an acute treatment comparison study) |
| Mozes 2006 | Allocation: randomised Participants: children with childhood onset schizophrenia (n=25) Interventions: Olanzapine versus risperidone (no un-medicated group) |
| Mueller 2005b | Allocation: randomised Participants: people with schizophrenia or major depression Interventions: risperidone + celecoxib vs. riperidone + placebo (no un-medicated group, unclear proportion of first and second episodes) |
| Muller 2004 | Allocation: randomised Participants: people with recent onset of schizophrenia (n=50) Interventions: Celecoxibplus + Amisulpride versus Amisulpride alone (no un-medicated group) |
| Murasaki 1999a | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia (n=53) (13 first episodes and 9 second episodes) Interventions: Unclear (needs translation) (unclear method of assignment to treatment; less than 50% first and second episodes (22/54=41%)) |
| Newton 2005 | Allocation: unclear (wait list control) Participants: young people with recent onset of auditory hallucinations (n=22) Interventions: group CBT plus medications vs. medications alone (no un-medicated group, not an acute treatment comparison study; unclear proportion of early episodes) |
| Newton 2006 | Allocation: randomised (unclear number of subjects) Participants: young people with recent onset schizophrenia Interventions: Cognitive Remediation Therapy plus standard care versus standard care alone (unclear use of medications; no outcome data reported) |
| Nienhuis 2006 | Allocation: randomised Participants: people with a first onset of non-affective psychosis (n=131) Interventions: after a stable remission phase of 6 months, individuals were randomly assigned to either maintenance treatment group or targeted treatment group (not an acute treatment comparison (medication withdrawal post-stabilization)) |
| Nordentoft 2002 | Allocation: randomised Participants: people with first episode psychosis (n=547) Interventions: integrated treatment (assertive community treatment, psychoeducational, multi-family groups, social skills training and antipsychotic, medication) versus treatment as usual (no un-medicated group) |
| Nuechterlein 1992 | Allocation: randomised Participants: people indicating stable remission of psychosis after 1 year of maintenance psychotics Interventions: fluphenazine versus placebo (not an acute treatment comparison study) |
| Nuechterlein 2005 | Allocation: randomised Participants: people with recent onset of schizophrenia (n=51) Interventions: Individual Placement and Support (IPS) + a Workplace Fundamental Module (WFM) versus traditional vocational rehabilitation (not an acute treatment comparision study; unclear use of medications) |
| Nugter 1997 | Allocation: randomised Participants: people with recent onset of psychosis and their parents Interventions: individual out-patient treatment versus. a combination of individual out-patient and family treatment (not an acute treatment comparions study, no un-medicated group) |
| O’Donnell 2003b | Allocation: randomised Participants: people with early psychosis Interventions: Vitamin B (Folic acid and Pyridoxine and Hydroxycobalamin) versus placebo (no antipsychotics were used (no contrast of antipsychotic treated versus un-medicated subjects)) |
| O’Regan 2005 | Allocation: randomised Participants: young people with early psychosis (n=40) Interventions: B-complex Vitamin B + antipsychotics versus placebo + antipsychotics (no un-medicated group) |
| O’Sullivan 2001 | Allocation: randomised Participants: people with acute psychosis (n=92) Interventions: Olanzapine (n=46) versus. Ziprasidone (n=46) (unclear proportion of first and second episodes; no un-medicated group) |
| Offord 1998 | Allocation: randomised Participants: people with schizophrenia (n=47) Interventions: M100907 versus placebo (unclear proportion of first and second episode) |
| Ohlenschlaeger 2002 | Allocation: randomised Participants: People with first episode Schizophrenia Interventions: Standard treatment, integrated OPUSteam ACT or inpatient rehabilitation (no un-medicated group) |
| Oosthuizen 2002a | Allocation: subjects were recruited from 2 trials (one is RCT and the other is an open trial) Participants: people with first-episode schizophrenia or schizophreniform disorder (n=80) Interventions: low-dose Risperidone versus low-dose haloperidol (no un-medicated group) |
| Oosthuizen 2004 | Allocation: randomised Participants: people with first episode schizophreniform disorder, schizophrenia or schizoaffective disorder Interventions: 2 mg/d haloperidol versus. 8 mg/d haloperidol (no un-medicated group) |
| Opjordsmoen 2000 | Allocation: not randomly assigned to treatment (consecutively) Participants: people with early psychosis (n=134) Interventions: Olanzapine, risperidone, perphenazine, clozapine (not randomly assigned to treatment; no un-medicated group) |
| Pagsberg 2004 | Allocation: randomised Participants: people with first-episode schizophrenia (n=20) Interventions: Zyclopenthixol (n= 8) versus risperidone (n= 12) (no un-medicated group) |
| Pai 1982 | Allocation: not randomised Participants: people with first episode of psychosis and no previous treatment Interventions: hospital group versus home group (not randomly assigned; no un-medicated group) |
| Painter 2001 | Allocation: unclear method of assignment to treatment Participants: people with schizophrenia (n=50) Interventions: a relapse prevention program versus standard outpatient treatment (unclear method of assignment to treatment; unclear proportion of first and second episodes; unclear use of medications, not a comparison of acute treatments) |
| Pan Miao 2004b | Allocation: randomised Participants: people with first episode schizophrenia (n=120) Interventions: Quetiapine (n=60) versus Risperidone (n=60) (no un-medicated group) |
| Papas 2005 | Allocation: randomised Participants: young people with first episode psychosis Interventions: B-complex Vitamin versus. placebo (unclear use of antipsychotics) |
| Parellada 2006 | Allocation: randomised Participants: people with first episode psychosis (n=50) Interventions: olanzapine (n= 26) or quetiapine (n= 24) (no un-medicated group) |
| Parent 1983 | Allocation: unclear method of assignment to treatment Participants: people with acute psychosis (n=40) Interventions: Flupenthixol versus haloperidol (unclear method of assignment to treatment; unclear proportion of first and second episodes; no un-medicated group) |
| Paulman 1980 | Not a treatment study for early onset of schizophrenia, but rather a comparison of two theoretical models used to explain schizophrenia |
| Perez 2003 | Allocation: randomised Participants: people with first episode psychosis (n=44) Interventions: Olanzapine, haloperidol or risperidone (no un-medicated group) |
| Perkins 2000 | CBT to improve medication adherence in first-episode psychosis (unclear assignment to treatment, unclear use of medications) |
| Perkins 2006 | Allocation: randomised Participants: people with first episode schizophrenia, schizophreniform, or schizoaffective disorder (n=254) Interventions: Olanzapine versus haloperidol (no un-medicated group) |
| Petersen 2005a | Allocation: randomised Participants: people with first episode of schizophrenia spectrum disorder (n=547) Interventions: integrated treatment (assertive community treatment + programmes for family involvement + social skills training) versus. treatment as usual (no un-medicated group) |
| Peuskens 1992 | Allocation: randomised Participants: people with chronic schizophrenia Interventions: Risperidone (1, 4, 8, 12, 16mg/day) versus haloperidol (10mg/day) (not treatment for recent onset schizophrenia; no un-medicated group) |
| Philips 1999 | Allocation: randomised Participants: young people describing state and trait risk factors of psychosis (n=64) Interventions: a combined medical and psychological (specific) approach versus supportive (non-specific) case management (not acute-phase schizophrenia subjects; unclear use of medications) |
| Pietzcker 1993 | Allocation: randomised Participants: people with schizophrenia and in stabilized phase (n=79 for the randomization) Interventions: prophylactic early intervention treatment versus. prophylactic maintenance treatment versus neuroleptics crisis intervention (unclear proportion of first and second episodes; not an acute treatment comparison study; no un-medicated group) |
| Potkin 2003b | Allocation: randomised Participants: people with acute relapse of schizophrenia (n=404) Interventions: Aripiprazole 20 mg/d (n=101) versus Aripiprazole 30 mg/d (n=101) versus Risperidone 6mg/d (n=99) versus placebo (n=103) (unclear proportion of first and second episode schizophrenia (acute treatment comparision with multi-episodes)) |
| Power 2002 | Allocation: randomised Participants: people with non-affective early psychosis Interventions: an assertive outreach multidisciplinary team versus local community mental health team (unclear use of medications; not an acute treatment comparison (follow-up after acute initial treatment)) |
| Power 2003 | Allocation: randomised Participants: young people with first episode psychosis (n=56) Interventions: LifeSPAN Therapy (a brief individual cognitively, oriented therapy) + standard clinical care (n=31) versus standard clinical care (n= 25) (no un-medicated group; not an acute medication treatment study) |
| Power 2006 | Allocation: randomised Participants: young people with first episode psychosis Interventions: Early Detection and Crisis Assessment team (LEOCAT) versus standard community mental health (unclear use of medications; not an acute medication treatment study) |
| Poyurovsky 2002b | Allocation: randomised Participants: people with first episode schizophrenia (n=30) Interventions: Olanzapine + Fluoxetine (n= 15) versus Olanzapine + placebo (n=15) (no un-medicated group) |
| Poyurovsky 2003b | Allocation: randomised Participants: people with first episode schizophrenia (n=26) Interventions: Olanzapine + reboxetine (n= 13) versus Olanzapine + placebo (n=13) (no un-medicated group) |
| Poyurovsky 2004 | Allocation: randomised Participants: people with first episode psychosis (n=13) Interventions: Olanzapine + famotidine (n=7) versus Olanzapine + placebo (n=6) (no un-medicated group) |
| Proffitt 2004 | Allocation: randomised Participants: people with first episode psychosis (n=80) Interventions: Ethyl-Eicosapentenoic Acid (essential fatty acid supplements) versus placebo (not an acute medication treatment study) |
| Qian 2002b | Allocation: only one treatment group Participants: people with first episode schizophrenia (n=88) Interventions: Risperidone (not randomly assigned; no un-medicated group) |
| Qiu 2005 | Allocation: randomised Participants: people with first episode schizophrenia (n=92) Interventions: Clozapine + family circumstance group versus control group (Clozapine + close circumstance in hospital) (no un-medicated group) |
| Qu 2005 | Allocation: randomised Participants: people with schizophrenia Interventions: chlorpromazine versus risperidal (unclear proportion of first and second episodes; no un-medicated group) |
| Rabinowitz 2004 | Allocation: Post-hoc analysis of clinical dosage of risperidone (not randomly assigned) Participants: people with early episode psychosis (n=276) Interventions: risperidone <= 4mg/d versus risperidone <=5 mg/d versus risperidone >5mg/d (not randomly assigned to treatment; no un-medicated group) |
| Rabinowitz 2006 | Allocation: randomised Participants: people with recent onset psychosis Interventions: Haloperidol (n=278) versus Risperidone (n=281) (no un-medicated group) |
| Rasmussen 1998 | Allocation: randomised Participants: people with first episode psychosis (n=500) Interventions: Haloperidol versus risperidone (no un-medicated group) |
| Reeder 2004 | Allocation: Unclear Participants: people with schizophrenia Interventions: individual cognitive remediation therapy (n=18) versus occupational therapy activities (n= 14) versus treatment as usual (n=19) (majority multiple-episodes; unclear allocation of treatment; not an acute treatment comparison study) |
| Reilly 2006 | Allocation: randomised Participants: people with early psychosis Interventions: CBT (unclear control group treatment) (unclear use of medications; unclear whether this is an acute or post-acute treatment comparison) |
| Ren 2005c | Allocation: randomised Participants: people with first episode schizophrenia (n=104) Interventions: antipsychotic drug treatment + CBT (n=54) versus antipsychotic drug treatment (n=50) (no un-medicated group) |
| Renshaw 2003 | Allocation: randomised Participants: people with first episode psychosis (n=263) Interventions: Olanzapine versus haloperidol (no un-medicated group) |
| Renton 2004 | Allocation: unclear method of assignment to treatment Participants: people with psychosis Interventions: cognitive therapy versus treatment as usual (unclear method of assignment to treatment ; unclear proportion of first and second episodes; no un-medicated group) |
| Reveley 2000a | Allocation: randomised Participants: people with early psychosis (n=26) Interventions: Risperidone versus haloperidol (no un-medicated group) |
| Rimon 2004 | Allocation: randomised Participants: people with acute schizophrenia or chronic schizophrenia with acute symptoms (n=46) Interventions: Olanzapine versus Perphenazine (unclear proportion of first and second episodes; no un-medicated group) |
| Robles 2006 | Allocation: randomised Participants: young people with first episode psychosis (n=50) Interventions: quetiapine (n=24) or olanzapine (n=26) (no un-medicated group) |
| Ropert 1973 | Allocation: unclear method of assignment to treatment Participants: people with acute onset of psychosis (n=17) Interventions: fluphenazine versus pipothiazine (unclear method of assignment to treatment ; unclear proportion of first and second episodes; no un-medicated group) |
| Rosebush 2000 | Allocation: randomised Participants: people with first episode schizophrenia Interventions: olanzapine versus haloperidol (no un-medicated group) |
| Rosen 2002 | Allocation: randomised Participants: people in late prodromal phase of psychosis (n=8) Interventions: medication versus placebo (not an acute schizophrenia treatment comparison study) |
| Ruhrmann 2006a | Allocation: randomised Participants: people in imminent prodromal state of psychosis (n=124) Interventions: Amisulpride + a needs focused intervention versus a needs focused intervention (not an acute schizophrenia treatment comparison study; no un-medicated group) |
| Ryu 2006 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia or people with chronic schizophrenia in acute exacerbation (n=71) Interventions: risperidone, olanzapine, quetiatpine, amisulpride, haloperidol and trifluoperazine (unclear method of assignment to treatment; unclear proportion of first and second episodes; no un-medicated group) |
| Sanger 1999 | Allocation: randomised Participants: people with first episode psychosis (with duration <=5 years and age<=45) Interventions: Olanzapine versus haloperidol (no un-medicated group) |
| Sarkar 1994 | Allocation: randomised Participants: people with first episode schizophrenia (n=30) Interventions: electroconvulsive therapy + haloperidol versus placebo electroconvulsive therapy plus haloperidol (no un-medicated group) |
| Schlogelhofer 2006 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia (n=30) Interventions: Clozapine, Olanzapine, Quetiapine, or Risperidone (unclear method of assignment to treatment; no un-medicated group) |
| Schooler 1989 | No unmedicatred group (in the acute treatment portion of the study), and not an acute treatment study (in the medication withdrawal phase) |
| Schooler 2005 | Allocation: randomised Participants: people with first episode psychosis (n=555) Interventions: Risperidone versus Haloperidol (no un-medicated group) |
| Schulz 1997 | Allocation: unclear method of assignment to treatment Participants: young people with early onset schizophrenia (n=40) Interventions: Clozapine versus standard neuroleptics medications (unclear method of assignment to treatment; no un-medicated group) |
| Schwannauer 2002 | Allocation: randomised Participants: people with first episode of bipolar disorder Interventions: psychosocial intervention versus waiting list control (not treatment for first and second episode schizophrenia; unclear use of medications) |
| Scottish 1992 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia (n=44) Interventions: Flupenthixol versus Pimozide (unclear method of assignment to treatment; no un-medicated group) |
| Sharifi 2006 | Allocation: randomised Participants: people with first episode psychosis Interventions: routine practice versus telephone follow-up aftercare versus home visits by a team of the general trained practitioners, nurses and social workers (unclear use of medications; not an acute treatment comparison study) |
| SharMa 2000a | Allocation: randomised Participants: people with first episode psychosis (n=35) Interventions: Haloperidol versus Risperidone (no un-medicated group) |
| Sharma 2003 | Allocation: randomised Participants: people with first episode schizophrenia and schizoaffective disorders (n=263) Interventions: Haloperidol versus Olanzapine (no un-medicated group) |
| Sheng 2005 | Allocation: randomised Participants: people with first episode schizophrenia (n=62) Interventions: Clozapine (n=31) versus risperidone (n=31) (no un-medicated group) |
| Shi Tianyuan 2004 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia (n=60) Interventions: Clozapine versus Risperidone (unclear method of assignment to treatment; no un-medicated group) |
| Silverstone 1984b | Allocation: randomised Participants: people with first episode schizophrenia or acute relapse of schizophrenia (n=56) Interventions: Haloperidol versus Zetidoline (unclear proportion of first and second episodes; no un-medicated group) |
| Simonsen 2000 | Allocation: randomised Participants: people with non-affective psychosis (n=281) Interventions: an early detection program versus treatment as usual (unclear proportion of first and second episodes; no un-medicated group) |
| Spencer 1992 | Allocation: randomised Participants: children with schizophrenia (n=12) Interventions: Haloperidol versus placebo (unclear proportion of first and second episodes) |
| Srihari 2006 | Allocation: randomised Participants: people with first episode psychosis Interventions: STEP program (antipsychotics, multi-family psycho-education, group CBT, case management and cognitive remediation) versus usual community care (no un-medicated group) |
| SSRG 1987 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia (n=49) Interventions: In the first year : Flupenthixol versus Pimozide; In the second year: active medications versus placebos (unclear method of assignment to treatment; no initial un-medicated group) |
| Stain 2006 | Allocation: randomised Participants: young people at risk of developing psychotic disorders Interventions: an early intervention (CBT + motivational interviewing) for rural and remote communities (not an acute treatment comparison study (prodromal phase)) |
| Stotsky 1977 | Allocation: randomised Participants: people with acute excitement and agitation (n=30) Interventions: Haloperidol versus Thiothixene (not an acute psychosis treatment comparison study; no un-medicated group) |
| Strakowski 1997 | Allocation: randomised Participants: people with first episode manic or schizophrenic psychosis (n=13) Interventions: amphetamine, placebos (not an acute treatment comparison of antipsychotic medications (amphetamine challenge study)) |
| Strakowski 2005 | Allocation: randomised Participants: people with first episode schizophrenia (n=195) Interventions: olanzapine versus haloperidol (no un-medicated group) |
| Stuart 2004 | Allocation: unclear method of assignment to treatment Participants: people with first episode psychosis Interventions: amisulpride (unclear method of assignment to treatment; no un-medicated group) |
| Su 2002b | Allocation: randomised Participants: people with first episode schizophrenia (n=94) Interventions: Chlorpromazine versus Risperidone (no un-medicated group) |
| Sun 2000a | Allocation: randomised Participants: people with first episode schizophrenia (n=117) Interventions: Clozapine versus Risperidone (no un-medicated group) |
| Sun 2006a | Allocation: randomised Participants: people with first episode schizophrenia (n=117) Interventions: Clozapine versus Risperidone (no un-medicated group) |
| Sun 2006e | Allocation: randomised Participants: people with first episode schizophrenia (n=71) Interventions: Chlorpromazine versus Quetiapine (no un-medicated group) |
| Suri 2001 | Allocation: randomised Participants: people with early schizophrenia Interventions: CBT plus medications versus medications alone (no un-medicated group) |
| Svestka 2003a | Allocation: randomised Participants: people with first episode schizophrenia and schizophreniform disorders (n=42 females) Interventions: Olanzapine versus Risperidone (no un-medicated group) |
| Tait 2002 | Allocation: randomised Participants: people with schizophrenia spectrum disorders (n=20) Interventions: cognitively oriented intervention (3 stages: initial engagement and formulation; early sings monitoring; targeted cognitive therapy if required) versus treatment as usual (unclear proportion of first and second episodes; unclear use of medications; not an acute treatment comparison study (relapse prevention)) |
| Tait 2005 | Allocation: randomised Participants: young people with first episode psychosis Interventions: educational intervention versus alternative educational session on cognitive behavior therapy for depression (control practices) (not an acute treatment comparison study (reduction of DUP)) |
| Tan 2005b | Allocation: randomised Participants: older adults with first episode schizophrenia (n=51) Interventions: Haloperidol versus Risperidone (no un-medicated group) |
| Tao 2005a | Allocation: randomised Participants: people with first episode schizophrenia (n=177) Interventions: medications plus CBT versus medications alone (no un-medicated group) |
| Tao Yuan Li 2004 | Allocation: randomised Participants: people with first episode schizophrenia (n=97) Interventions: antipsychotic medications plus psychological and social interventions versus antipsychotic medications alone (no un-medicated group) |
| Tarrier 2000 | Allocation: randomised Participants: people with recent onset schizophrenia and substance abuse (dual diagnosis) Interventions: psychological intervention (dual diagnosed persons; unclear use of medications) |
| Thompson 2005 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia (n=39) Interventions: Haloperidol (n=18) versus Olanzapine (n=21) (unclear method of assignment to treatment; no un-medicated group) |
| Tian 2005 | Allocation: randomised Participants: children with first episode schizophrenia (n=60) Interventions: family nursing intervention plus Risperidone versus routine treatment plus Risperidone (no un-medicated group) |
| Toben 1998 | Allocation: randomised Participants: people with an acute episode of bipolar disorder (manic or mixed) Interventions: Olanzapine (n=70) versus Placebos (n=69) (unclear proportion of first and second episodes) |
| Tohen 1997a | Allocation: randomised Participants: people with first episode psychosis (n=82) Interventions: Haloperidol versus Olanzapine (no un-medicated group) |
| Tohen 2000b | Allocation: randomised Participants: people with bipolar I disorder and manic or mixed, with or without psychotic features Interventions: Olazanpine versus placebo (unclear proportion of first and second episodes) |
| Tollefson 1997 HGAJ | Allocation: randomised Participants: people with schizophrenia, schizophreniform, or schizoaffective disorder Interventions: Olanzapine or haloperidol (unclear proportion of first and second episodes; no un-medicated group) |
| Tollefson 1997b | Allocation: controlled longitudinal study Participants: people with chronic schizophrenia, schizophreniform disorder, or schizoaffective disorder Interventions: olanzapine (n=707) or haloperidol (n=197) (not randomly assigned to treatment; not an acute treatment comparison study (tardive dyskinesia in long-term treatment); not first and second episodes; no un-medicated group) |
| Tollefson 1997c | Allocation: randomised Participants: people with schizophrenia or related diagnosis (n=1996 Interventions: Olanzapine or haloperidol (unclear proportion of first and second episodes; no un-medicated group) |
| Tong 2003 | Allocation: only one treatment group Participants: people with first episode schizophrenia (n=30) and recurrent schizophrenia (n=36) Interventions: Risperidone (not randomly assigned to treatment; the proportion of first episodes does not exceed 50%; no un-medicated group) |
| Tran 1997a | Allocation: randomised Participants: people with schizophrenia, schizophreniform or schizoaffective disorders Interventions: Olanzapine versus Risperidone (unclear proportion of first and second episodes; no un-medicated group) |
| Ueland 2004 | Allocation: randomised Participants: adolescents with early onset psychosis Interventions: cognitive remediation (four modules: four modules: cognitive differentiation, attention, memory and social perception) (n=14) versus control group (n=12) (not an acute treatment comparison study; unclear use of medications) |
| Vaglum 2002 | Allocation: unclear method of assignment to treatment Participants: people with first episode schizophrenia Interventions: an early detection program (unclear method of assignment to treatment; unclear use of medications; not an acute treatment comparison study) |
| Van Bruggen 1999 | Allocation: randomised Participants: young people with a relatively short duration of untreated first or second psychosis Interventions: Olanzapine versus Risperidone (no un-medicated group) |
| Van Bruggen 2003 | Allocation: randomised Participants: young people with recent onset schizophrenia (n=44) Interventions: Olanzapine versus Risperidone (no un-medicated group) |
| Van Meijel 2006a | Allocation: randomised Participants: people with stable schizophrenia or related psychosis Interventions: experimental group (Relapse Prevention plans) or control condition (care as usual) (not an acute treatment comparison study; not first and second episodes; unclear use of medications) |
| Van Meijel 2006b | Allocation: randomised Participants: people with first episode non-affective psychosis (n=144) Interventions: adapted interventions (cognitive behavioural therapy plus medication management plus vocational support plus family interventions) versus standard generic community services (no un-medicated group) |
| Van Nimwegen 2006a | Allocation: randomised Participants: people with first episode psychosis (n=131) Interventions: Olanzapine versus Risperidone (no un-medicated group) |
| Van Nimwegen 2006b | Allocation: randomised Participants: young people with recent onset schizophrenia or related disorders (n=131) Interventions: Olanzapine versus Risperidone (no un-medicated group) |
| Van Nimwegen 2006c | Allocation: randomised Participants: adolescents with first episode psychosis (n=78) Interventions: Olanzapine versus Risperidone (no un-medicated group) |
| Verhaegh 2006 | Allocation: not randomly assigned to treatment Participants: young people with first episode psychosis Interventions: assertive community treatment versus care as usual (unclear use of medications; not an acute treatment comparison study; not randomly assigned to treatment) |
| Vollenweider 2003 | Allocation: matched case-control study Participants: males with first episode schizophrenia (n=15) and controls Interventions: scanned brain activity (not randomly assigned to treatment; not an acute treatment comparison study) |
| Volterra 1996 | Allocation: randomised Participants: people with recent onset schizophrenia (n=40) Interventions: a group or individual one-year treatment with insight-oriented therapy plus haloperidol (n= 22) versus drug therapy alone (n=18) (unclear proportion of first and second episodes; no un-medicated group) |
| Wang 2000a | Allocation: randomised Participants: people with first episode psychosis (n=100) Interventions: Clozapine versus Risperidone (no un-medicated group) |
| Wang 2003a | Allocation: randomised Participants: people with first episode schizophrenia (n=251) Interventions: CBT + regular antipsychotics (Risperidone and Clozapine) versus antipsychotics alone (Risperidone and Clozapine) (no un-medicated group) |
| Wang 2003i | Allocation: randomised Participants: people with first episode schizophrenia (n=200) Interventions: Risperidone plus Valproic Acid versus Risperidone alone (no un-medicated group) |
| Wang 2004d | Allocation: randomised Participants: people with first episode schizophrenia (n=80) Interventions: Hyberzine plus Quetiapine versus Quetiapine alone (control group) (no un-medicated group) |
| Wang 2004k | Allocation: randomised Participants: people with first-episode schizophrenia (n=64) Interventions: Olazepine or risperdal (No un-medicated group) |
| Wang 2005c | Allocation: randomised Participants: people with first-episode schizophrenia (n=72) Interventions: Aripiprazole or chlorpromazine (No un-medicated group) |
| Wang 2005d | Allocation: randomised Participants: people with first episode schizophrenia (n=96) Interventions: Risperidone or perphenazine (No un-medicated group) |
| Wang 2005e | Allocation: randomised Participants: people with first episode schizophrenia (n= 67) Interventions: Quetiapine or risperidone (No un-medicated group) |
| Wang 2005g | Allocation: randomised Participants: people with first episode schizophrenia (n=64) Interventions: Risperidone and clozapine (No un-medicated group) |
| Wang 2005h | Allocation: randomised Participants: people with first episode schizophrenia (n= 55) Interventions: Risperidone and chlorpromazine (No un-medicated group) |
| Wang 2005j | Allocation: randomised Participants: people with first episode schizophrenia (n=60) Interventions: Aripiprazloe and clozapine (No un-medicated group) |
| Wang 2005m | Allocation: randomised Participants: people with first episode schizophrenia (n=100) Interventions: Chlorpromazine and risperidone (No un-medicated group) |
| Wang 2006b | Allocation: randomised Participants: people with first episode schizophrenia (n=86) Interventions: Chlorpromazine, clozapine, and risperidone (No un-medicated group) |
| Wang 2006c | Allocation: randomised Participants: people with first episode schizophrenia (n=117) Interventions: Risperidone, clozapine, and chlorpromazine (No un-medicated group) |
| Wang 2006e | Allocation: randomised Participants: people with first episode schizophrenia (n=64) Interventions: Aripiprazole and clozapine (No un-medicated group) |
| Wang 2006i | Allocation: randomised Participants: people with first episode schizophrenia (n=60) Interventions: Quetiapine and clozapine (No un-medicated group) |
| Wang 2006k | Allocation: randomised Participants: people with first episode schizophrenia (n=61) Interventions: Clozapine and risperidone (No un-medicated group) |
| Warrington 2006 | Allocation: randomised Participants: Unknown Interventions: 2 mg vs. 20 mg of ziprasidone (No un-medicated group) |
| Wei 2006a | Allocation: randomised Participants: people with first-episode schizophrenia (n=58) Interventions: Quetiapine and risperidone (No un-medicated group) |
| Wei 2006b | Allocation: randomised Participants: people with first episode schizophrenia (n=101 females) Interventions: Aripiprazole and quetiapine (No un-medicated group) |
| Wei 2006c | Allocation: randomised Participants: people with first episode schizophrenia (n=101 females) Interventions: Aripiprazole and quetiapine (No un-medicated group) |
| WHO 1979 | Allocation: Multi-site study (no allocation to treatment) |
| Williams 2005b | Allocation: randomised Participants: Persons aged 14-35 with early psychosis Interventions: Systematic psychosocial interventions + treatment as usual VS. treatment as usual alone (No un-medicated group; not an acute treatment comparison study) |
| Wilson 1982b | Allocation: Randomised Participants: People with schizophrenia (n=39) Interventions: Flutroline (4 dosage groups: 1, 5, 10, and 20 mg) (unclear proportion of first and second episodes; no un-medicated group) |
| Wirshing 1992b | Allocation: Randomised Participants: People with schizophrenia (n=81) Interventions: Oral neuroleptic supplementation vs. Placebo supplementation to active medication in both groups (Unclear proportion of first and second episodes; not an acute treatment study (dosage reduction study); no un-medicated group) |
| Woggon 1978 | Allocation: Randomised Participants: people with schizophrenia (n=40) Interventions: Bromperidol vs. perphenazine (no un-medicated group; unclear proportion of first and second episodes) |
| Woods 2002a | Allocation: Randomised Participants: People with schizophrenia, diagnosed as prodromal Interventions: Olanzapine vs. placebo (Pre-acute treatment comparison study) |
| Woods 2002b | Allocation: Unknown Participants: people with schizophrenia patients (n=25) Interventions: Glycine (not sure of whether there is a comparison or control group) (unclear method of treatment assignment; pre-acute treatment comparison study) |
| Woods 2003 | Allocation: Randomised Participants: people with schizophrenia (n=60) Interventions: Olanzapine vs. placebo (Pre-acute treatment comparison study) |
| Woods 2004 | Allocation: Non random Participants: people with first-episode schizophrenia with zero duration of untreated psychosis (vs. two historical first episode samples treated after usual DUP) Interventions: Olanzapine (Not randomly assigned to treatment; no un-medicated group; not an acute treatment study) |
| Wu 2001a | Allocation: Unknown Participants: people with first episode schizophrenia (n=97) Interventions: Three groups: clozapine and 2 risperdione groups (middle dosage and very low dosage) (unknown method of assignment to treatment; no un-medicated group) |
| Wu 2002c | Allocation: Unknown Participants: people with schizophrenia (negative vs. positive subtypes) Interventions: Clozapine (unknown method of assignment to treatment; no un-medicated group) |
| Wu 2006 | Allocation: Unknown Participants: People with first-episode schizophrenia Interventions: Aripiprazole vs. haldol (unknown method of assignment to treatment; no un-medicated group) |
| Wu 2006a | Allocation: Randomised Participants: people with first episode schizophrenia (n=112) Interventions: Clozapine, olanzapine, risperidone, and sulpiride (no un-medicated group) |
| Wunderink 2003 | Allocation: Randomised Participants: People with first episode schizophrenia Interventions: short (6months) vs. sustained (2 years) antipsychotic drug treatment (not an acute treatment study, no un-medicated group) |
| Wunderink 2006 | Allocation: Randomised Participants: people with remitted first episode schizophrenia (n=131) Interventions: Discontinuation strategy vs. maintenance treatment (not an acute treatment study) |
| Xie 1998 | Allocation: Randomised Participants: people with first episode schizophrenia (n=122) Interventions: Clozapine vs. chlorpromazine (no un-medicated group) |
| Xu 2003d | Allocation: Randomised Participants: people with first episode schizophrenia (n=287) Interventions: Recovery psychotherapy vs. control (not an acute treatment study; not amedication treatment comparison study) |
| Xu 2005b | Allocation: Randomised Participants: people with first episode schizophrenia (n=110) Interventions: Insight education + risperidone vs. risperidone (no un-medicated group) |
| Yang 1999c | Allocation: Unknown Participants: people with first episode schizophrenia (n=78) Interventions: Chlorpromazine vs. clozapine (unknown method of assignment to treatment; no un-medicated group) |
| Yang 2000b | Allocation: Randomised Participants: people with first episode schizophrenia (n=164) Interventions: chlorpromazine or clozapine Outcomes: Brief Psychiatric Rating Scale (BPRS), Scale for Assessment of Negative Symptoms (SANS; Chinese version), Global Assessment of Functioning Scale (GAF) (No un-medicated group) |
| Yang 2001 | Allocation: Unknown Participants: people with first episode schizophrenia (n=124) Interventions: Chlorpromazine or clozapine (unknown method of assignment to treatment; no un-medicated group) |
| Yang 2003a | Allocation: Randomised Participants: people with first episode schizophrenia (n=70) Interventions: Olanzapine or risperidone (no un-medicated group) |
| Yang 2004b | Allocation: Randomised Participants: people with first episode schizophrenia (n=160) Interventions: Chlorpromazine or clozapine (no un-medicated group) |
| Yang 2005c | Allocation: Randomised Participants: people with first episode schizophrenia (n=60) Interventions: quetiapine or risperidone (no un-medicated group) |
| Yang 2006b | Allocation: Randomised Participants: people with first episode schizophrenia (n=100) Interventions: Aripirazole or haloperidol (no un-medicated group) |
| Yang 2006g | Allocation: Randomised Participants: people with first episode schizophrenia (n=75) Interventions: Ximin or Zyprexa (no un-medicated group) |
| Yang Bin 2004 | Allocation: Randomised Participants: people with first episode schizophrenia (n=95) Interventions: Clozapine, risperidone, and haloperidol (no un-medicated group) |
| Yanos 2004 | Allocation: Nonrandomised Participants: homeless participants with severe mental illness (38.8% with schizophrenia or related) Interventions: Referral to Pathways to Housing (not randomly assigned to treatment; unclear proportion of first and second episodes; not an acute treatment study; no medicated group) |
| Ye 2005a | Allocation: Randomised Participants: people with first episode schizophrenia (n=34) Interventions: Aripiprazole or risperidone (no un-medicated group) |
| Ye 2005b | Allocation: Randomised Participants: people with first episode schizophrenia (n=54) Interventions: Clozapine or risperidone (no un-medicated group) |
| Yu 2001b | Allocation: Randomised Participants: people with first episode schizophrenia (n=62) Interventions: Risperidone or chlorpromazine (no un-medicated group) |
| Yu E Li 2004 | Allocation: Randomised Participants: people with first-episode schizophrenia (n=66) Interventions: Clozapine vs. clozapine + psychological/social intervention (no un-medicated group) |
| Zeng 2003 | Allocation: Randomised Participants: people with first episode schizophrenia (n=136) Interventions: Clozapine vs. clozapine plus psychological education (no un-medicated group) |
| Zeng 2006 | Allocation: Randomised Participants: people with first episode schizophrenia (n=116) Interventions: anti-psychotics vs. anti-psychotics plus comprehensive intervention (no un-medicated group) |
| Zhang 1994a | Allocation: Randomised Participants: males with first episode schizophrenia (n=78) Interventions: Family intervention vs. control group (both medicated) (no un-medicated group) |
| Zhang 1998c | Allocation: Randomised, Cross-sectional 4-group design Participants: Children with autism and first-episode schizophrenia Interventions: This is not an intervention study (not an acute treatment study (4 group comparison of lymphocyte levels)) |
| Zhang 1998d | Allocation: Unknown Participants: relatives of people with schizophrenia (682 experimental; 366 control) Interventions: Group psychotherapy + conventional services vs. conventional services for the family members of persons with schizophrenia (not an acute treatment study of people with schizophrenia-type psychoses) |
| Zhang 2000f | Allocation: Nonrandomised Participants: women with first episode psychosis (119 pregnancy/parturition; 55 non-pregnancy/parturition) Interventions: None (not an acute treatment comparison study; no un-medicated group) |
| Zhang 2002j | Allocation: Nonrandomised Participants: people with first episode schizophrenia or schizophrenic form psychosis (n=24) Interventions: Clozapine (not randomly assigned to treatments (a single treatment group study); no un-medicated group) |
| Zhang 2003l | Allocation: Randomised Participants: people with first episode schizophrenia (n=250) Interventions: Celexib plus risperidone vs. risperidone (no un-medicated group) |
| Zhang 2004a | Allocation: Randomised Participants: people with first episode schizophrenia (n=126) Interventions: Varying doses of risperidone (2, 3, 4, or 5 mg) (no un-medicated group) |
| Zhang 2005k | Allocation: Randomised Participants: people with first episode schizophrenia (n=111) Interventions: Parents health education vs. routine services (not an acute treatment study; no contrast of medicated vs. un-medicated subject groups) |
| Zhang 2005l | Allocation: Randomised Participants: people with first episode schizophrenia (n=200) Interventions: Artemisinin (an anti-malarial medication) vs. placebo (adjunctive treatment comparison) (no contrast of a treatment group receiving antipsychotic medication treatment and another group not receiving antipsychotics) |
| Zhang Fuying 2005 | Allocation: Randomised Participants: people with first episode schizophrenia (n=93) Interventions: Nurse home visits vs. none (not an acute treatment study; no contrast of medicated vs. un-medicated subject groups) |
| Zhao 2006 | Allocation: Randomised Participants: people with first-episode schizophrenia (n=68) Interventions: Aripiprazole vs. quetiapine (no un-medicated group) |
| Zheng 2003c | Allocation: Randomised Participants: people with first episode schizophrenia (n=68) Interventions: Clozapine vs. risperidone (no un-medicated group) |
| Zhi 2006 | Allocation: Randomised Participants: females with first episode schizophrenia (n=124) Interventions: risperidone vs. self-efficacy plus risperidone (no un-medicated group) |
| Zhou 2005c | Allocation: Randomised Participants: people with first episode schizophrenia (n=118) Interventions: Risperidone vs. clozapine (no un-medicated group) |
| Zhu 2001a | Allocation: Nonrandomised Participants: people with first episode schizophrenia or schizophreniform psychosis (n=28) Interventions: Clozapine (varying dosages) (not randomly assigned to treatment; not a treatment comparison study (one-group design); no un-medicated group) |
| Zhu 2001b | Allocation: Randomised Participants: people with first episode schizophrenia (n=23) Interventions: Risperidone: full vs. half dosage (no un-medicated group) |
| Zhu 2002g | Allocation: Randomised Participants: people with first episode schizophrenia (n=90) Interventions: He-Ne laser intravascular irradiation vs. none (both groups received risperidone) (no un-medicated group) |
| Zhu 2002i | Allocation: Randomised Participants: 68 people with first episode schizophrenia (n=68) Interventions: Haloperidol, clozapine, and risperidone (no un-medicated group) |
| Zipursky 2004 | Allocation: Randomised Participants: people with first episode psychosis (n=25; 80.4% diagnosed with schizophrenia) Interventions: Home intervention for psychosis (HIP) vs. specialized first-episode psychosis clinic (FEPC) (no un-medicated group) |
| Zipursky 2005a | Allocation: Randomised Participants: People with first episode schizophrenia (n=239) Interventions: Olanzapine or haloperidol (no un-medicated group) |
| Zipursky 2005b | Allocation: Randomised Participants: people with first episode schizophrenia, schizophreniform disorder, or schizoaffective disorder (n=263) Interventions: Olanzapine or haloperidol (no un-medicated group) |
| Zuo 2000 | Allocation: Unknown Participants: people with first episode schizophrenia (n=35) Interventions: Risperidone (dosage ranging from 2 to 8 mg a day) (no un-medicated group) |
| Zuo 2002 | Allocation: Unknown Participants: People with first episode schizophrenia Interventions: Clozapine or risperidone (no un-medicated group) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised. |
| Participants | People with definite or possible psychosis. Stage/state: admitted under care of participating clinicians Age: 16-69 years of age. N=120. |
| Interventions | 1. Lithium and pimizode. 2. Lithium. 3. Pimizode. 4. Placebo. |
| Outcomes | No useable data published, seeking unpublished data from authors |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | |
| Methods | Randomised. |
| Participants | People with first-episode psychosis. |
| Interventions | 1. Cognitive Behavioural Treatment plus Family Treatment and placebo 2. Cognitive Behavioural Treatment plus Family Treatment and low dose antipsychotic medication |
| Outcomes | Social functioning: SOFAS. Clinical symptoms: remission and recovery. |
| Starting date | July 2009. |
| Contact information | Patrick McGorry, pmcgorry@unimelb.edu.au |
| Notes |
DATA AND ANALYSES
Comparison 1.
CHLORPROMAZINE vs PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 3 | 353 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.29, 0.54] |
| 2 Global state: not improved after 8 years (Psychiatric rating scale, not improved=1,2; improved=4,5) | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.76 [0.53, 1.11] |
| 3 Rehospitalisation within 3 years - completer | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 2.29 [1.31, 4.03] |
| 4 Rehospitalisation within 3 years - intent to treat | 1 | 127 | Risk Ratio (M-H, Fixed, 95% CI) | 3.05 [1.64, 5.67] |
| 5 Adverse effects: various outcomes | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 5.1 drowsiness | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 5.65 [2.72, 11.73] |
| 5.2 restlessness | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 1.19 [0.83, 1.71] |
| 5.3 constipation | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 2.71 [1.37, 5.35] |
| 5.4 nausea or upper gastrointestinal distress | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 6.17 [1.92, 19.79] |
| 5.5 dryness of mouth or throat | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 4.63 [1.67, 12.82] |
| 5.6 dizziness, faintness or weakness | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 4.41 [1.59, 12.29] |
| 5.7 muscle rigidity | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 1.54 [0.60, 3.97] |
| 5.8 nasal congestion | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 2.10 [0.69, 6.43] |
| 5.9 facial rigidity | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 2.31 [0.77, 6.96] |
| 5.10 tremor of hands, arms or face | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 [0.29, 3.77] |
| 5.11 headache | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 0.84 [0.33, 2.13] |
| 5.12 loss of associated movements | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 1.26 [0.22, 7.35] |
| 5.13 akathesis-restlessness of feet | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 1.40 [0.35, 5.67] |
Comparison 2.
FLUPHENAZINE vs PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | 240 | Risk Ratio (M-H, Fixed, 95% CI) | 0.51 [0.34, 0.77] |
| 2 Adverse effects: various outcomes | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 drowsiness | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 4.07 [1.92, 8.62] |
| 2.2 restlessness | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.60, 1.34] |
| 2.3 constipation | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 2.26 [1.12, 4.54] |
| 2.4 nausea or upper gastrointestinal distress | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [0.33, 5.49] |
| 2.5 dryness of mouth or throat | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 3.46 [1.22, 9.83] |
| 2.6 dizziness, faintness, weakness | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 2.24 [0.74, 6.73] |
| 2.7 muscle rigidity | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 2.98 [1.28, 6.97] |
| 2.8 nasal congestion | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 2.24 [0.74, 6.73] |
| 2.9 facial rigidity | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 2.64 [0.90, 7.77] |
| 2.10 tremor of hands, arms, face | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 2.24 [0.74, 6.73] |
| 2.11 headache | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 1.12 [0.47, 2.64] |
| 2.12 loss of associated movements | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 7.32 [1.75, 30.53] |
| 2.13 akathesis-restlessness of feet | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 3.52 [1.04, 11.90] |
Comparison 3.
THIORIDAZINE vs PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | 236 | Risk Ratio (M-H, Fixed, 95% CI) | 0.44 [0.28, 0.69] |
| 2 Adverse effects: various outcomes | 1 | Risk Ratio (M-H, Fixed, 95% CI) | Subtotals only | |
| 2.1 drowsiness | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 5.46 [2.62, 11.36] |
| 2.2 restlessness | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.69, 1.48] |
| 2.3 constipation | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 1.72 [0.83, 3.57] |
| 2.4 nausea or upper gastrointestinal distress | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 8.13 [2.58, 25.59] |
| 2.5 dryness of mouth or throat | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 5.69 [2.09, 15.50] |
| 2.6 dizziness, faintness, weakness | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 4.47 [1.61, 12.41] |
| 2.7 muscle rigidity | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 0.54 [0.16, 1.85] |
| 2.8 nasal congestion | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 3.25 [1.14, 9.31] |
| 2.9 facial rigidity | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 1.63 [0.51, 5.19] |
| 2.10 tremor of hands, arms, face | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 2.44 [0.82, 7.25] |
| 2.11 headache | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 0.81 [0.32, 2.06] |
| 2.12 loss of associated movements | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 0.16 [0.01, 3.34] |
| 2.13 akathesis-restlessness of feet | 1 | 165 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [0.33, 5.49] |
Comparison 4.
TRIFLUOPERAZINE vs PSYCHOTHERAPY
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | 94 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.25, 3.61] |
| 2 Global state: Overall Health Score - Meninger Health Sickness Scale (higher score=better) 2-years post-discharge | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [1.61, 9.99] |
| 3 Adverse effects: number of adverse events | 1 | 162 | Risk Ratio (M-H, Fixed, 95% CI) | 5.65 [2.72, 11.73] |
Comparison 5.
TYPICAL ANTIPSYCHOTIC vs PSYCHOSOCIAL TREATMENT
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1.Global Psychopathology Scale | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [−0.55, 0.57] |
| 2 Global state: 2. Global Improvement Scale | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | −0.03 [−0.49, 0.43] |
Analysis 1.1. Comparison 1 CHLORPROMAZINE vs PLACEBO, Outcome 1 Leaving the study early
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 1 CHLORPROMAZINE vs PLACEBO
Outcome: 1 Leaving the study early
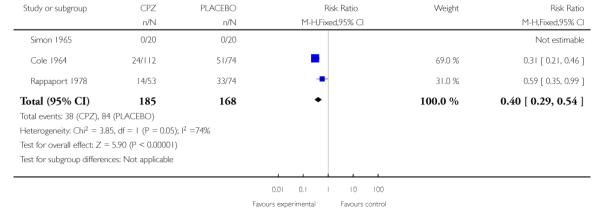 |
Analysis 1.2. Comparison 1 CHLORPROMAZINE vs PLACEBO, Outcome 2 Global state: not improved after 8 years (Psychiatric rating scale, not improved=1,2; improved=4,5)
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 1 CHLORPROMAZINE vs PLACEBO
Outcome: 2 Global state: not improved after 8 years (Psychiatric rating scale, not improved=1,2; improved=4,5)
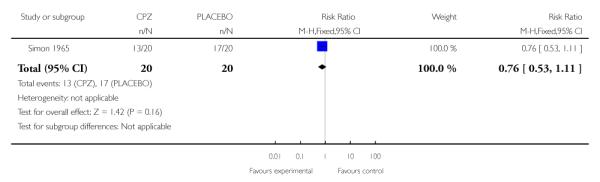 |
Analysis 1.3. Comparison 1 CHLORPROMAZINE vs PLACEBO, Outcome 3 Rehospitalisation within 3 years - completer
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 1 CHLORPROMAZINE vs PLACEBO
Outcome: 3 Rehospitalisation within 3 years - completer
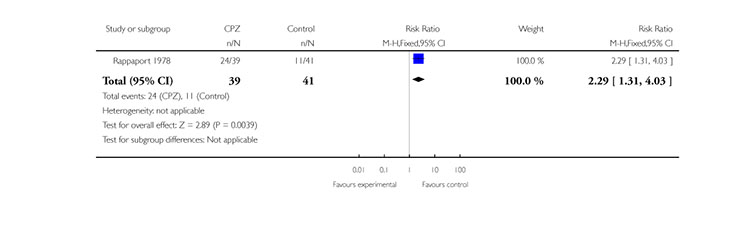 |
Analysis 1.4. Comparison 1 CHLORPROMAZINE vs PLACEBO, Outcome 4 Rehospitalisation within 3 years - intent to treat
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 1 CHLORPROMAZINE vs PLACEBO
Outcome: 4 Rehospitalisation within 3 years - intent to treat
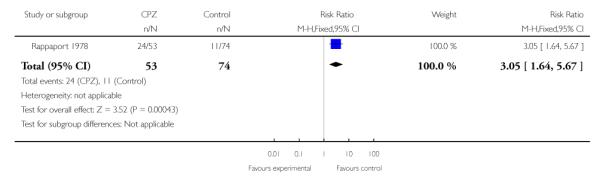 |
Analysis 1.5. Comparison 1 CHLORPROMAZINE vs PLACEBO, Outcome 5 Adverse effects: various outcomes
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 1 CHLORPROMAZINE vs PLACEBO
Outcome: 5 Adverse effects: various outcomes
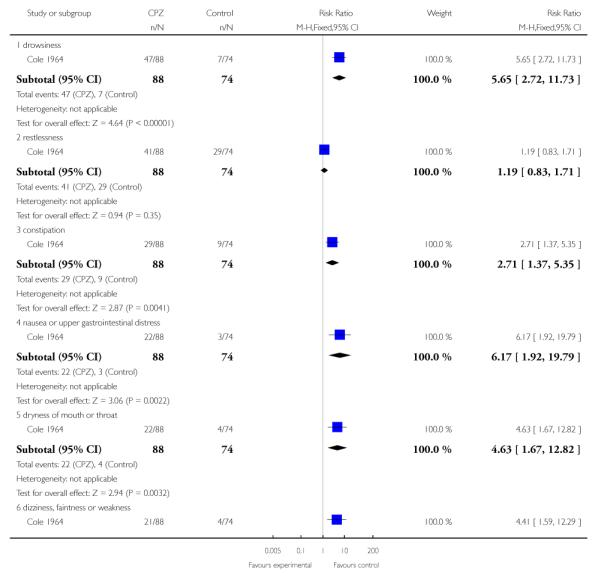 |
 |
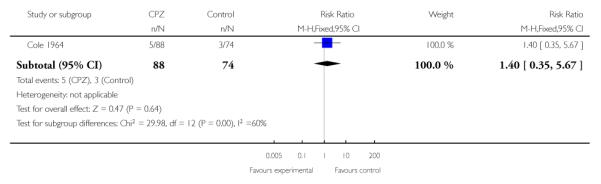 |
Analysis 2.1. Comparison 2 FLUPHENAZINE vs PLACEBO, Outcome 1 Leaving the study early
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 2 FLUPHENAZINE vs PLACEBO
Outcome: 1 Leaving the study early
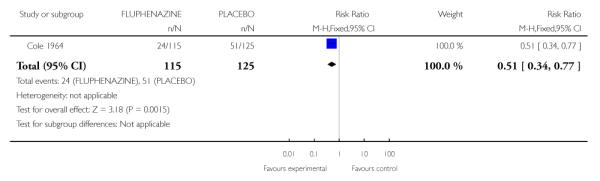 |
Analysis 2.2. Comparison 2 FLUPHENAZINE vs PLACEBO, Outcome 2 Adverse effects: various outcomes
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 2 FLUPHENAZINE vs PLACEBO
Outcome: 2 Adverse effects: various outcomes
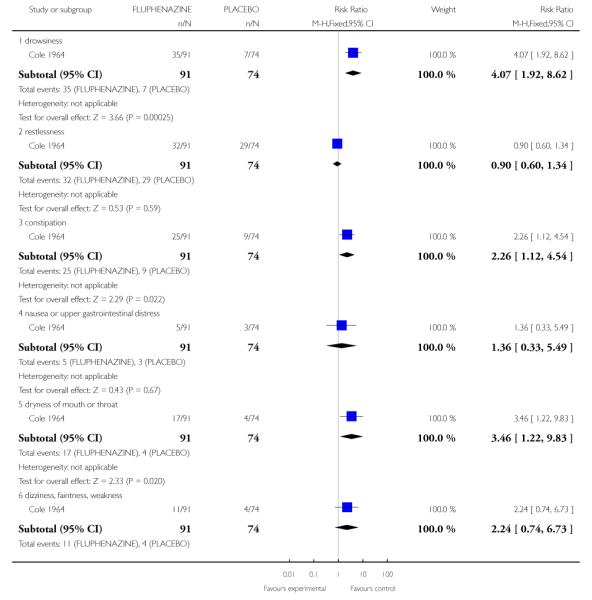 |
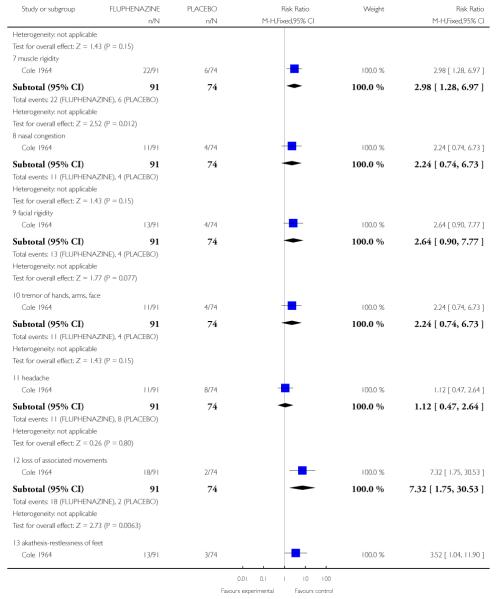 |
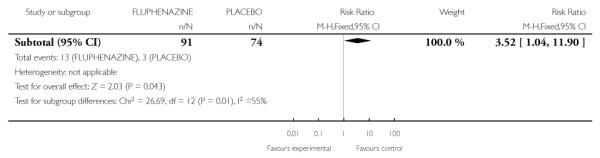 |
Analysis 3.1. Comparison 3 THIORIDAZINE vs PLACEBO, Outcome 1 Leaving the study early
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 3 THIORIDAZINE vs PLACEBO
Outcome: 1 Leaving the study early
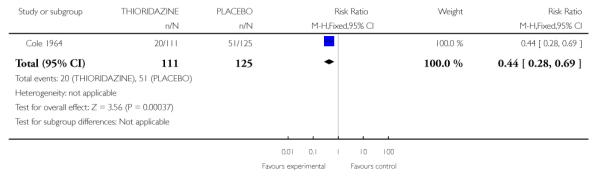 |
Analysis 3.2. Comparison 3 THIORIDAZINE vs PLACEBO, Outcome 2 Adverse effects: various outcomes
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 3 THIORIDAZINE vs PLACEBO
Outcome: 2 Adverse effects: various outcomes
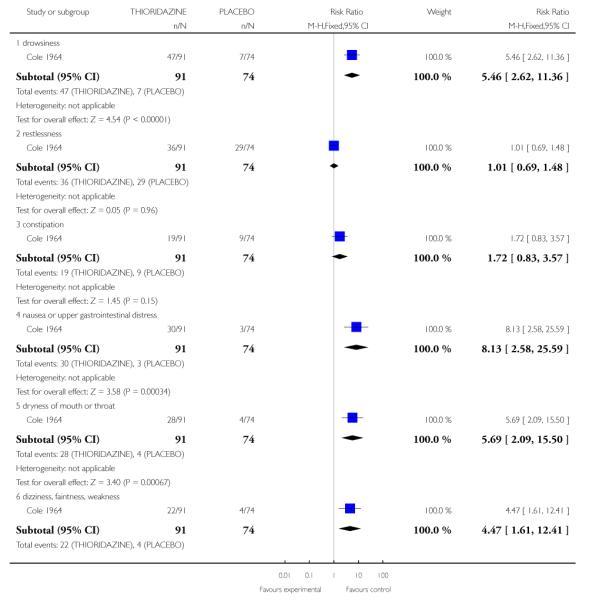 |
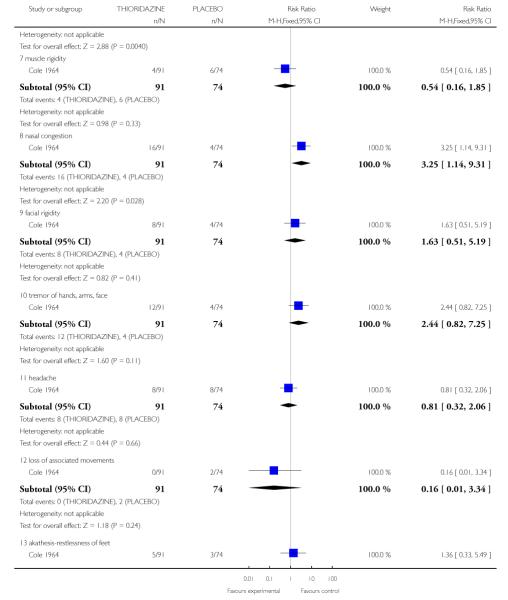 |
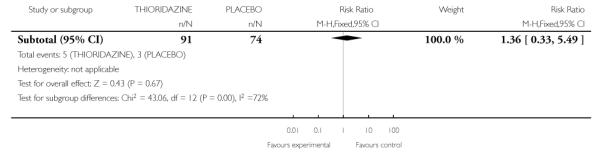 |
Analysis 4.1. Comparison 4 TRIFLUOPERAZINE vs PSYCHOTHERAPY, Outcome 1 Leaving the study early
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 4 TRIFLUOPERAZINE vs PSYCHOTHERAPY
Outcome: 1 Leaving the study early
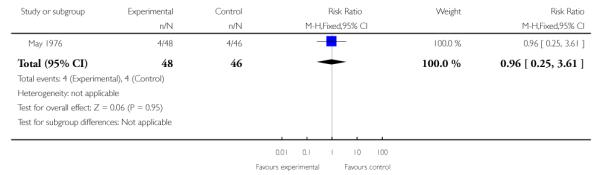 |
Analysis 4.2. Comparison 4 TRIFLUOPERAZINE vs PSYCHOTHERAPY, Outcome 2 Global state: Overall Health Score - Meninger Health Sickness Scale (higher score=better) 2-years post-discharge
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 4 TRIFLUOPERAZINE vs PSYCHOTHERAPY
Outcome: 2 Global state: Overall Health Score - Meninger Health Sickness Scale (higher score=better) 2-years post-discharge
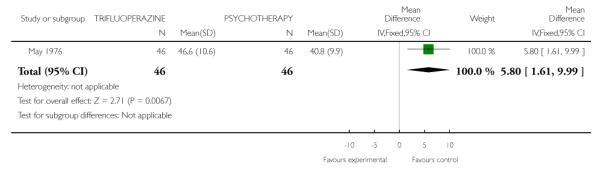 |
Analysis 4.3. Comparison 4 TRIFLUOPERAZINE vs PSYCHOTHERAPY, Outcome 3 Adverse effects: number of adverse events
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 4 TRIFLUOPERAZINE vs PSYCHOTHERAPY
Outcome: 3 Adverse effects: number of adverse events
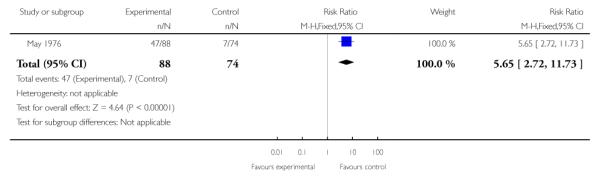 |
Analysis 5.1. Comparison 5 TYPICAL ANTIPSYCHOTIC vs PSYCHOSOCIAL TREATMENT, Outcome 1 Global state: 1.Global Psychopathology Scale
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 5 TYPICAL ANTIPSYCHOTIC vs PSYCHOSOCIAL TREATMENT
Outcome: 1 Global state: 1.Global Psychopathology Scale
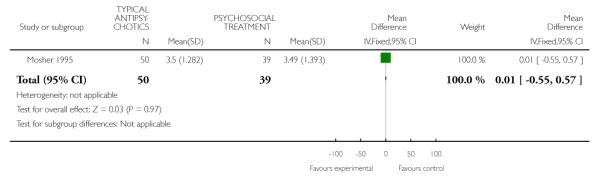 |
Analysis 5.2. Comparison 5 TYPICAL ANTIPSYCHOTIC vs PSYCHOSOCIAL TREATMENT, Outcome 2 Global state: 2. Global Improvement Scale
Review: Antipsychotic medication for early episode schizophrenia
Comparison: 5 TYPICAL ANTIPSYCHOTIC vs PSYCHOSOCIAL TREATMENT
Outcome: 2 Global state: 2. Global Improvement Scale
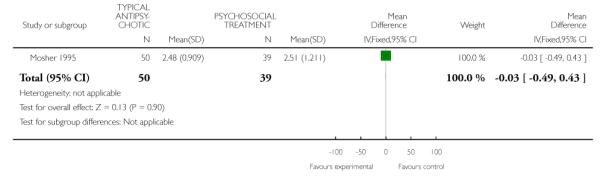 |
HISTORY
Protocol first published: Issue 1, 2007
Review first published: Issue 6, 2011
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
In reviewing studies for inclusion in this review, we realised that additional focusing of our review question was needed. The initial intent of this review was to assess the evidence supporting the clinical practice guideline to treat early episodes of schizophrenia psychosis in the acute phase with antipsychotic medications. We have therefore excluded (and left for a subsequent edition of this review) studies addressing the question about medication maintenance in the post-acute phase. For example, the Schooler 1989a study treated all first-episode patients in the acute phase with antipsychotic medications and, after stabilisation, randomised to two medication dosages and placebo that was followed up for two years. This, and similar studies, can address the effectiveness of maintenance medications in the post-acute phase of early episodes, but not the effectiveness of antipsychotics in the acute episode, since in that phase there was no un-medicated group. In addition, we have preliminarily excluded RCTS that compare two or more antipsychotic medications in the acute treatment of individuals with early episode schizophrenia. Subsequent editions of this review might include the medication to medication comparisons to address questions of differential medication effectiveness. Pseudo-random studies have not yet been included in this version of the review, but might be incorporated in a subsequent version of the review, along with a sensitivity analysis to assess the influence of their inclusion. We have also updated the protocol method’s section with the Cochrane schizophrenia group’s current guidelines.
WHAT’S NEW
Last assessed as up-to-date: 30 January 2009.
| Date | Event | Description |
|---|---|---|
| 5 October 2011 | Amended | Format updated, search undertaken, data extracted and conclusions revised |
Footnotes
DECLARATIONS OF INTEREST None known.
References to studies included in this review
* Indicates the major publication for the study
- Cole 1964 {published data only} .Cole JO, Goldberg SC, Klerman GL. Phenothiazine treatment in acute schizophrenia. Archives of General Psychiatry. 1964;10:246–61. [MEDLINE: 71204770] [PubMed] [Google Scholar]; Gibbons RD, Lewine RRJ, Davis JM, Schooler NR, Cole JO. An empirical test of a kraepelinian vs. a bleulerian view of negative symptoms. Schizophrenia Bulletin. 1985;11(3):390–5. doi: 10.1093/schbul/11.3.390. [MEDLINE: 71204770] [DOI] [PubMed] [Google Scholar]; Goldberg SC, Klerman GL, Cole JO. Changes in schizophrenic psychopathology and ward behaviour as a function of phenothiazine treatment. British Journal of Psychiatry. 1965;111:120–33. doi: 10.1192/bjp.111.471.120. [MEDLINE: 78162829] [DOI] [PubMed] [Google Scholar]; Goldberg SC, Mattsson N, Cole JO, Klerman GL. Prediction of improvement in schizophrenia under four phenothiazines. Archives of General Psychiatry. 1967;16:107–17. doi: 10.1001/archpsyc.1967.01730190109015. [MEDLINE: 6066689] [DOI] [PubMed] [Google Scholar]; Goldberg SC, Mattsson NB. Schizophrenic subtypes defined by response to drugs and placebo. Diseases of the Nervous System. 1968;29(5):S153–8. [MEDLINE: 4876985] [PubMed] [Google Scholar]; Klerman GL, Goldberg SG, Davis D. Relationship betweenthe hospital milieu and the response to phenothiazines in the treatment of schizophrenics. Acta Psychiatrica Belgica. 1970;70(6):716–29. [MEDLINE: 71204770] [PubMed] [Google Scholar]; *; Schooler NR, Goldberg SC, Boothe H, Cole JO. One year after discharge: Community adjustment of schizophrenic patients. American Journal of Psychiatry. 1967;123(8):986–95. doi: 10.1176/ajp.123.8.986. [DOI] [PubMed] [Google Scholar]
- May 1976 {published data only} .May PR, Tuma AH, Dixon WJ. Schizophrenia - a follow-up study of results of treatment. I. Design and other problems. Archives of General Psychiatry. 1976;33(4):474–8. doi: 10.1001/archpsyc.1976.01770040042008. [MEDLINE: 938184] [DOI] [PubMed] [Google Scholar]; May PR, Tuma AH, Dixon WJ, Yale C, Thiele DA, Kraude WH. Schizophrenia. A follow-up study of the results of five forms of treatment. Archives of General Psychiatry. 1981;38(7):776–84. doi: 10.1001/archpsyc.1981.01780320056006. [MEDLINE: 6113821] [DOI] [PubMed] [Google Scholar]; May PR, Tuma AH, Yale C, Potepan P, Dixon WJ. Schizophrenia - a follow-up study of results of treatment. Archives of General Psychiatry. 1976;33(4):481–6. doi: 10.1001/archpsyc.1976.01770040047009. [MEDLINE: 938185] [DOI] [PubMed] [Google Scholar]; May PRA. Treatment of Schizophrenia: A Comparative Study of Five Treatment Methods. Science House; New York, USA: 1968. Design and procedures of the schizophrenia research project; pp. 56–105. [MEDLINE: 9089819] [Google Scholar]; May PRA. Psychotherapy and ataraxic drugs in schizophrenia. Proceedings of the 131st Annual Meeting of the American Psychiatric Association; Atlanta, Georgia, USA. 1978 May 8-12; 1978. [MEDLINE: 9089819] [Google Scholar]; May PRA. Schizophrenia follow up: a controlled treatment study. Unknown source. 1974;10:55. [Google Scholar]; May PRA, Tuma AH. Treatment of schizophrenia: an experimental study of five treatments. British Journal of Psychiatry. 1965;111:503–10. [MEDLINE: 938185] [Google Scholar]; Tuma AH, May PR, Yale C, Forsythe AB. Therapist characteristics and the outcome of treatment in schizophrenia. Archives of General Psychiatry. 1978;35(1):81–5. doi: 10.1001/archpsyc.1978.01770250083008. [MEDLINE: 619842] [DOI] [PubMed] [Google Scholar]; Tuma AH, May PR, Yale C, Forsythe AB. Therapist experience, general clinical ability, and treatment outcome in schizophrenia. Journal of Consulting and Clinical Psychology. 1978;46(5):1120–6. doi: 10.1037//0022-006x.46.5.1120. [PSYCINFO: 62–06485] [DOI] [PubMed] [Google Scholar]; Wyatt RJ. Early intervention in schizophrenia improves the long-term course of the illness. Schizophrenia Research. 1995;Vol. 15(issue 1,2):170. [MEDLINE: 9089819] [Google Scholar]; *; Wyatt RJ, Green MF, Tuma AH. Long-term morbidity associated with delayed treatment of first admission schizophrenic patients: a re-analysis of the Camarillo State Hospital data. Psychological Medicine. 1997;27:261–8. doi: 10.1017/s0033291796004345. [MEDLINE: 9089819] [DOI] [PubMed] [Google Scholar]
- Mosher 1995 {published data only} .Mosher LR, Vallone R. Final progress report. NIMH; 1992. Soteria project. [Google Scholar]; Mosher LR, Vallone R, Menn A. The treatment of acute psychosis without neuroleptics: Six week psychopathology outcome data from the Soteria project. International Journal of Social Psychiatry. 1995;41(3):157–73. doi: 10.1177/002076409504100301. [DOI] [PubMed] [Google Scholar]
- Rappaport 1978 {published data only} .Rappaport M, Hopkins HK, Hall K, Belleza T. Silverman J. Are there schizophrenics for whom drugs may be unnecessary or contraindicated? International Pharmacopsychiatry. 1978;13(2):100–11. doi: 10.1159/000468327. [DOI] [PubMed] [Google Scholar]
- Simon 1965 {published data only} .Simon W, Wirt AL, Wirt RD, Halloran AV. Long-term follow-up study of schizophrenic patients. Archives of General Psychiatry. 1965;12:510–15. doi: 10.1001/archpsyc.1965.01720350078010. [DOI] [PubMed] [Google Scholar]; *; Wirt RD, Simon W. Differential Treatment and Prognosis in Schizophrenia. Charles C. Thomas; Springfield, Illinois: 1959. [Google Scholar]
References to studies excluded from this review
- ACE 2003 {published data only} .Jackson H, Mcgorry P, Killackey E, Bendall S, Allott K, Johnston T, Gleeson J, Harrigan S. The ace project: a randomised controlled trial of CBT versus befriending: acute phase results. Schizophrenia Research. 2004;70(1):56–7. doi: 10.1017/S0033291707002061. [ISI: 000224551100150] [DOI] [PubMed] [Google Scholar]; Killackey E, Jackson H, Bendall S, Allott K, Harrigan S, Gleeson J, Johnson T. An exploration of the relationship between dose and type of therapy and outcome in the ace trial of CBT for first episode psychosis. Schizophrenia Research. 2004;70(1):57. [ISI: 000224551100151] [Google Scholar]; Killackey E, Jackson H, McGorry P, Bendall S, Allott K, Johnson T, Gleeson J, Harrigan S. Controlled trial of CBT versus befriending for acute first episode psychosis: acute phase results. Schizophrenia Bulletin. 2005;31:526–527. [: 114097] [Google Scholar]
- Adson 2003 {published data only} .Aquino P, Adams CE, Crow T, Wood I. Personal communication. 2006. ; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.
- Aguilar 1994 {published data only} .*; Aguilar EJ, Keshavan MS, Martinez Quiles MD, Hernandez J, Gomez Beneyto M, Schooler NR. Predictors of acute dystonia in first-episode psychotic patients. American Journal of Psychiatry. 1994;151(12):1819–21. doi: 10.1176/ajp.151.12.1819. [MEDLINE: 7977894] [DOI] [PubMed] [Google Scholar]
- Ahmed 1997 {published data only} .*; Ahmed S, Schooler N, Montrose D, Haas G, Sweeney J, Keshavan MS. Efficacy of risperidone in first-episode schizophrenia. Schizophrenia Research. 1997;24(1,2):193. [MEDLINE: 12409165] [Google Scholar]
- Alaghband-rad 2006a {published data only} .Alaghband-Rad J, Shahrivar Z, Mahmoodi J, Salesian N. First episode psychoses among Iranian adolescents. Schizophrenia Research. 2006;86(Suppl 1):S65. [Google Scholar]; *; Alaghband-Rad J, Sharifi V, Amini H, Shahrivar Z, Mottaghipour Y, Mahmoodi J, Seddigh A, Salesian N, Ali-Malayeri A, Tabatabaee M. Management of first episode psychoses in Iran: unique features and challenges. Schizophrenia Research. 2006;86(Suppl 1):S42. [Google Scholar]
- Allison 2001 {published data only} .Allison D, Cavazzoni P, Beasley C, Holcombe J, Buse J. Analysis of random glucose concentration data from patients with schizophrenia treated with typical and atypical agents during double-blind, randomised, controlled clinical trials. Biological Psychiatry. 2001;2(Suppl 1):P010–25. [Google Scholar]; Allison DB, Cavazzoni P, Beasley CM, Jr, Berg PH, Mukhopadhyay N, Mallinckrodt C, Baker RW, Holcombe J, Taylor CC, Breier A, Buse JB. Random blood glucose levels in patients with schizophrenia treated with typical and atypical antipsychotic agents: an analysis of data from double-blind, randomised, controlled clinical trial. European Neuropsychopharmacology. 2001;11(3):280. [Google Scholar]
- Altamura 1985 {published data only} .*; Altamura AC, Curry SH, Montgomery S, Wiles DH. Early unwanted effects of fluphenazine esters related to plasma fluphenazine concentrations in schizophrenic patients. Psychopharmacology. 1985;87(1):30–3. doi: 10.1007/BF00431773. [MEDLINE: 3933035] [DOI] [PubMed] [Google Scholar]
- Altamura 1999b {published data only} .*; Altamura AC, Cao A, La Croce ML, Serri L, Soddu A, Laddomada A, Percudani M. Are atypical antipsychotics less depressogenic than typical compounds? Journal of the European College of Neuropsychopharmacology. 1999;9:S168. [Google Scholar]
- Alvarez 2005 {published data only} .Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, Perez-Iglesias R, Martinez-Garcia O, Perez-Pardal T, Ramirez-Bonilla ML, Crespo-Facorro B. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: a randomised controlled trial. Journal of Clinical Psychiatry. 2006;67(8):1253–60. doi: 10.4088/jcp.v67n0812. [EMBASE: 2006468531; MEDLINE: 16965204] [DOI] [PubMed] [Google Scholar]; *; Alvarez M, Gonzalez-Blanch C, Perez-Iglesias R, Perez-Pardal T, Martinez-Garcia O, Crespo-Facorro B, Vazquez-Barquero JL. Early intervention in antipsychotic - induced weight gain in first episode psychosis. Schizophrenia Bulletin. 2005;31:518. [Google Scholar]
- Amminger 2006 {published data only} .Amminger GP, Schafer MR. Indicated prevention with omega-3 fatty acids in adolescents at ultra-high risk for psychosis - rationale, methods, and 3-months outcome. Schizophrenia Research. 2006;86(Suppl 1):S97–8. [Google Scholar]; Amminger GP, Schafer MR. Is it feasible to conduct a RCT in ultra-high risk individuals at a child and adolescent psychiatric service? Schizophrenia Research. 2006;86(Suppl 1):S98. [Google Scholar]
- An 2006b {published data only} .*; An B-F, Zhang M-L, Qi S-G. Comparative study between the effect olanzapine and quetiapine in first-episode schizophrenia. Journal of Clinical Psychiatry. 2006;16(2):84–5. [Google Scholar]
- Anonymous 1972 {published data only} .*; Anon Early clinical drug evaluation units reports. Psychopharmacology Bulletin. 1972;8(1):60–72. [MEDLINE: 72188780] [PubMed] [Google Scholar]
- Apicella 2001 {published data only} .*; Apicella A. [accessed 01 February 2001];National alliance for research on schizophrenia and depression. http://www.mhsource.com/narsad/bd/studyops.html accessed February 2001)
- Apiquian 2003 {published data only} .*; Apiquian R, Fresan A, Herrera K, Ulloa RE, Loyzaga C, De LaFuente-Sandoval C, Gutierrez D, Nicolini H. Minimum effective doses of haloperidol for the treatment of first psychotic episode: a comparative study with risperidone and olanzapine. International Journal of Neuropsychopharmacology. 2003;6(4):403–8. doi: 10.1017/S1461145703003742. [EMBASE: 2004054013] [DOI] [PubMed] [Google Scholar]
- Appelberg 2004a {published data only} .*; Appelberg B, Tuisku K, Joffe G. Is it worthwhile changing clinically stable schizophrenic out-patients with mild to moderate residual symptoms and/or side effects from conventional to atypical antipsychotics? Schizophrenia Research. 2004;67(1):140–1. doi: 10.1016/j.eurpsy.2004.06.035. [ISI: 000188788100335] [DOI] [PubMed] [Google Scholar]
- Archie 2006 {published data only} .*; Archie S. Integrated care improves one year outcomes in first episode psychosis. Evidence-Based Mental Health. 2006;9(2):46. doi: 10.1136/ebmh.9.2.46. [CINAHL: 2009195566; MEDLINE: 16638895] [DOI] [PubMed] [Google Scholar]
- Ascher-Svanum 2006a {published data only} .*; Ascher-Svanum H, Nyhuis A, Faries DE, Kinon BJ. Early response to antipsychotics as predictor of later response in the naturalistic treatment of schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Auby 2002 {published data only} .Auby P, Saha A, Ali M, Ingenito G, Wilber R, Bramer S. Safety and tolerability of aripiprazole at doses higher than 30 mg. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S288. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Saha A, Ali MW, Ingenito GG, Wilber R, Luo X, Bramer S. Safety and tolerability of aripiprazole at doses higher than 30 mg. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S185. [Google Scholar]; Saha AR, Ali MW, Ingenito GG, Wilber R, Luo X, Bramer S. Safety and tolerability of aripiprazole at doses higher than 30mg. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Awad 2006 {published data only} .*; Awad G. First episode psychosis: olanzapine and haloperidol provide similar improvements in quality of life and social functioning. Evidence-Based Mental Health. 2006;9(2):47. doi: 10.1136/ebmh.9.2.47. [CINAHL: 2009195567; MEDLINE: 16638896] [DOI] [PubMed] [Google Scholar]
- Bai 2005d {published data only} .*; Bai Y, Jiang K, Wang L. A random chlorpromazine-controlled study of the effects of quetiapine on cognition in schizophrenia. Shanghai Archives of Psychiatry. 2005;17(3):151–4. [CAJ: MEDI0509] [Google Scholar]
- Bandelow 1992 {published data only} .*; Bandelow B, Muller P, Frick U, Gaebel W, Linden M, Muller Spahn F, Pietzcker A, Tegeler J. Depressive syndromes in schizophrenic patients under neuroleptic therapy. ANI Study Group Berlin, Dusseldorf, Gottingen, Munich, Federal Republic of Germany. European Archives of Psychiatry and Clinical Neuroscience. 1992;241(5):291–5. doi: 10.1007/BF02195978. [MEDLINE: 1351405] [DOI] [PubMed] [Google Scholar]
- Barrowclough 2001b {published data only} .*; Barrowclough C. An evaluation of the effectiveness of group cognitive therapy for people with recent onset schizophrenia. National Research Register. 2001;Vol. 1 [Google Scholar]
- Beasley 1996a {published data only} .Baker RW, Ames D. Umbricht DSG, Chengappa KNR, Schooler NR. Obsessive-compulsive symptoms in schizophrenia - a comparison of olanzapine and placebo. Psychopharmacology Bulletin. 1996;32(1):89–93. [EMBASE: 1996176660; MEDLINE: 96267995] [PubMed] [Google Scholar]; Baker RW, Julier B, Stauffer V. Manic-like symptoms in schizophrenic patients treated with olanzapine, haloperidol and placebo. Biological Psychiatry. 2001;2(Suppl 1):P027–34. [Google Scholar]; Beasley C, Tran P, Beuzen JN, Tamura R, Dellva MA, Bailey J, Krueger J, Tollefson G. Olanzapine versus haloperidol: long-term results of the multi-center international trial. Proceedings of the 20th Collegium Internationale Neuro-Psychopharmacologicum Congress; Melbourne, Australia. 1996 Jun 23-27.1996. [Google Scholar]; Beasley C, Tran P, Satterlee W, Tollefson G, Lu Y, Kuntz A, Bradley P, Paul S. Olanzapine versus placebo, results of the United-States double-blind olanzapine trial. Proceedings of the 20th Collegium Internationale Neuro-Psychopharmacologicum Congress; Melbourne, Australia. 1996 Jun 23-27.1996. [Google Scholar]; Beasley C, Jr, Tollefson GD, Beuzen JN, Dellva MA, Sanger TM, Paul S. Acute and long-term results of the North American double-blind olanzapine trial. Proceedings of the 4th International Conference on Schizophrenia - 1996: Breaking down the Barriers; Vancouver, Canada. 1996 Oct 6-9.1996. [Google Scholar]; Beasley CM, Dellva MA, Tamura RN, Morgenstern H, Glazer WM, Ferguson K, Tollefson GD. Double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. British Journal of Psychiatry. 1999;174:23–30. doi: 10.1192/bjp.174.1.23. [MEDLINE: 99227593] [DOI] [PubMed] [Google Scholar]; Beasley CM, Sayler ME, Keisler GM, Potvin JH, Sanger TM, Tollefson GD. The influence of pharamacotherapy on self-directed and externally-directed aggression in schizophrenia. Schizophrenia Research. 1998;29(1-2):28. [Google Scholar]; Beasley CM, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropyschopharmacology. 1996;14(2):111–23. doi: 10.1016/0893-133X(95)00069-P. [MEDLINE: 8822534] [DOI] [PubMed] [Google Scholar]; Beasley CM, Jr, Sanger T, Satterlee W, Tollefson G, Tran P, Hamilton S. Olanzapine versus placebo - results of a double-blind, fixed dose olanzapine trial. Psychopharmacology. 1996;124(1-2):159–67. doi: 10.1007/BF02245617. [EMBASE: 1996106370] [DOI] [PubMed] [Google Scholar]; Crawford AM, Beasley CM, Tollefson GD. Olanzapine -impact of an atypical antipsychotic candidate on prolactin release; Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, New York, USA. 1996 May 4-9.1996. [Google Scholar]; Crawford AM, Beasley CM, Tollefson GD. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Journal of the European College of Neuropsychopharmacology. 1997;9:P.2.016. doi: 10.1016/S0920-9964(97)00036-4. [MEDLINE: 11199942; PsycINFO: 2001–14228–003] [DOI] [PubMed] [Google Scholar]; Crawford AM, Beasley CM, Tollefson GD. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Schizophrenia Research. 1997;26(1):41–54. doi: 10.1016/S0920-9964(97)00036-4. [MEDLINE: 9376336] [DOI] [PubMed] [Google Scholar]; Crawford AMK, Beasley CM, Tollefson GD. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentration. Proceedings of the 10th European College of Neuropsychopharmacology Congress; Vienna, Austria. 1997 Sep 13-17; 1997. [DOI] [PubMed] [Google Scholar]; Czekalla J, Beasley CM, Jr, Dellva MA, Berg PH, Grundy S. Analysis of the qtc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry. 2001;62(3):191–8. doi: 10.4088/jcp.v62n0310. [MEDLINE: 21200321] [DOI] [PubMed] [Google Scholar]; Czekalla J, Beasley CM, Jr, Dellva MA, Berg PH, Grundy S. Analysis of the qtc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry. 2001;62(3):191–8. doi: 10.4088/jcp.v62n0310. [MEDLINE: 21200321] [DOI] [PubMed] [Google Scholar]; Dellva MA, Tran P, Tollefson GD, Wentley AL, Beasley CM., Jr Standard olanzapine versus placebo and ineffective-dose olanzapine in the maintenance treatment of schizophrenia. Psychiatric Services. 1997;48(12):1571–7. doi: 10.1176/ps.48.12.1571. [MEDLINE:9406266] [DOI] [PubMed] [Google Scholar]; Edgell ET, Hamilton SH, Revicki DA, Genduso LA, Tollefson GD. Costs of olanzapine treatment compared with haloperidol for schizophrenia: results from a randomised clinical trial. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-6.1998. [Google Scholar]; Eli Lilly. Company. Beasley 1996a - olz vs placebo vs hpl (N America) 2001. Unpublished Report.; Hamilton SH, Revicki DA, Genduso LA, Beasley CM., Jr Olanzapine versus placebo and haloperidol: quality of life and efficacy results of the North American double-blind trial. Neuropyschopharmacology. 1998;18(1):41–9. doi: 10.1016/S0893-133X(97)00111-5. [MEDLINE: 9408917] [DOI] [PubMed] [Google Scholar]; Jones B, Crawford AMK, Beasley CM, Jr, Tollefson GD. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentration. Schizophrenia Research. 1998;29(1, 2):204. doi: 10.1016/S0920-9964(97)00036-4. [MEDLINE: 9376336] [DOI] [PubMed] [Google Scholar]; Martin C, Genduso L, Revicki D, Hamilton S, Tran P, Beasley C. Quality of life outcomes of olanzapine, a new atypical antipsychotic agent. Schizophrenia Research. 1996;18(2, 3):130. [MEDLINE: 21200321] [Google Scholar]; Perry PJ, Lund BC, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response: acute phase results of the North American olanzapine trial. Journal of Clinical Psychopharmacology. 2001;21(1):14–20. doi: 10.1097/00004714-200102000-00004. [MEDLINE: 11199942; PsycINFO: 2001–14228–003] [DOI] [PubMed] [Google Scholar]; Revicki D, Genduso L. Effect of olanzapine on deficit syndrome symptoms in chronic schizophrenia. Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30 - Oct 4.1995. [Google Scholar]; Sanger T, Tollefson GD. A controlled study on the course of primary and secondary negative symptoms. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]; Sanger T, Tollefson GD, Lieberman JA, Tohen M. Olanzapine versus haloperidol in the treatment of first-episode psychosis. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]; Satterlee W, Beasley C, Sanger T, Tollefson G. Additional clinical experience with olanzapine, an “atypical” antipsychotic. Schizophrenia Research. 1995;15(1, 2):163. [EMBASE: 1996106370] [Google Scholar]; Satterlee WG, Beasley CM, Tollefson GD, Moore N, Tran PV. Preclinical and clinical observations of olanzapine (a new antipsychotic) Psychopharmacology Bulletin. 1994;30(4):638. [MEDLINE: 9408917] [Google Scholar]; Street J, Dellva M, Tamura R, Sanger T, Tollefson G. A comparison of extrapyramidal syndromes between olanzapine and placebo in schizophrenia. Proceedings of the 9th European College of Neuropsychopharmacology Congress; Amsterdam, Netherlands. 1996 Sep 21-25.1996. [Google Scholar]; Street JS, Dellva MA, Tamura RN, Sanger T, Tollefson GD. Comparison of extrapyramidal syndromes between olanzapine and placebo in schizophrenia. 20th Collegium Internationale Neuro-Psychopharmacologicum Congress; Melbourne, Australia. 1996 Jun 23-27; 1996. [MEDLINE: 9408917] [Google Scholar]; Street JS, Dellva MA, Tamura RN, Sanger T, Tollefson GD. Comparison of extrapyramidal syndromes between olanzapine and placebo in schizophrenia. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, USA. 1996 May 4-9.1996. [Google Scholar]; Tamura RN, Tollefson GD, Dellva MA, Beasley CM, Glazer WM, Morgenstern H. What is the differential risk of tardive dyskinesia with the novel antipsychotic olanzapine? Schizophrenia Research. 1998;29(1-2):176. [MEDLINE: 9376336] [Google Scholar]; Tollefson G, Beasley C, Tran P, Sanger T. Olanzapine: an exciting atypical antipsychotic; the clinical experience. Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30 - Oct 4.1995. [Google Scholar]; Tollefson GD. The value of atypical antipsychotic medications. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]; Tollefson GD. Update on new atypical antipsychotics. Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30-Oct 4.1995. [Google Scholar]; Tollefson GD, Beasley CM, Tamura RN, Tran PV, Potvin JH. Blind, controlled, long-term study of the comparative incidence of treatment emergent tardive dyskinesia with olanzapine or haloperidol. American Journal of Psychiatry. 1997;154(9):1248–54. doi: 10.1176/ajp.154.9.1248. [DOI] [PubMed] [Google Scholar]; Tollefson GD, Sanger TM. Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. American Journal of Psychiatry. 1997;154(4):466–74. doi: 10.1176/ajp.154.4.466. [MEDLINE: 97245664] [DOI] [PubMed] [Google Scholar]; Tollefson GD, Sanger TM, Beasley CM. The course of primary and secondary negative symptoms in a controlled trial with olanzapine. 20th Collegium Internationale Neuro-Psychopharmacologicum Congress; Melbourne, Australia. 1996 Jun 23-27; 1996. [MEDLINE: 97245664] [Google Scholar]; Tollefson GD, Sanger TM, Beasley CM. The course of primary and secondary negative symptoms in a controlled trial with olanzapine. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, USA. 1996 May 4-9.1996. [Google Scholar]; Tollefson GD, Sanger TM, Beasley CM. The course of primary and secondary negative symptoms in a controlled trial with olanzapine. Schizophrenia Research. 1997;24(1, 2):192. [MEDLINE: 97245664] [Google Scholar]; Tollefson GD, Sanger TM, Beasley CM. The course of primary and secondary negative symptoms in a placebo-and comparator-controlled trial of the typical antipsychotic olanzapine. Proceedings of the 9th European College of Neuropsychopharmacology Congress; Amsterdam, Netherlands. 1996 Sep 21-25.1996. [Google Scholar]; Tollefson GD, Sanger TM, Beasley CM, Tran PV. A double blind, controlled comparison of the novel antipsychotic olanzapine versus haloperidol or placebo on anxious and depressive symptoms accompanying schizophrenia. Biological Psychiatry. 1998;43(11):803–10. doi: 10.1016/s0006-3223(98)00093-6. [MEDLINE: 98274603] [DOI] [PubMed] [Google Scholar]; Tran P, Beasley C, Tollefson G, Beuzen J, Dellva M, Sanger T, Paul S. Acute and long-term results of the North American double-blind olanzapine trial. Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30-Oct 4.1995. [Google Scholar]; Tran PV, Beasley CM, Tollefosn GD, Sanger T, Satterlee WG. Clinical efficacy and safety of olanzapine, a new atypical antipsychotic agent. Neuropyschopharmacology. 1994;10(3S):267S. [MEDLINE: 11199942; PsycINFO: 2001–14228–003] [Google Scholar]; Tran PV, Beasley CM, Tollefson GD, Beuzen JN, Holman SL, Sanger TM, Satterlee WG. Olanzapine: a promising “atypical” antipsychotic agent. Schizophrenia Research. 1995;15(1, 2):169. [MEDLINE: 21200321] [Google Scholar]; Tran PV, Dellva MA, Tollefson GD, Beasley CM, Jr, Potvin JH, Kiesler GM. Extrapyramidal symptoms and tolerability of olanzapine versus haloperidol in the acute treatment of schizophrenia. Journal of Clinical Psychiatry. 1997;58(5):205–11. doi: 10.4088/jcp.v58n0505. [MEDLINE: 9184614] [DOI] [PubMed] [Google Scholar]; Williamson DJ, Tran PV, Beasley CM, Satterlee WG, Sanger T, Paul S, Tollefson GD. Additional clinical experience with olanzapine, an atypical antipsychotic. Journal of Psychopharmacology. 1995;9(3):A47. [MEDLINE: 11199942; PsycINFO: 2001–14228–003] [Google Scholar]; Williamson DJ, Tran PV, Beasley CM, Tollefson GD, Sanger T, Satterlee WG. Clinical efficacy and safety of olanzapine, a new atypical antipsychotic agent. Journal of Psychopharmacology. 1995;9(3):A47. [MEDLINE: 11199942; PsycINFO: 2001–14228–003] [Google Scholar]; Wood AJ, Beasley CM, Tollefson GD, Tran PV. Efficacy of olanzapine in the positive and negative symptoms of schizophrenia. Proceedings of the 7th European College of Neuropsychopharmacology Congress; Jerusalem, Israel. 1994 Oct 16-21.1994. [Google Scholar]; Wright P, Tollefson GD, Beasley CM, Tamura RN, Tran PV, Potvin J. A blinded, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Schizophrenia Research. 1998;29(1-2):206. doi: 10.1176/ajp.154.9.1248. [DOI] [PubMed] [Google Scholar]
- Beasley 1997 {published data only} .Baker RW, Julier B, Stauffer V. Manic-like symptoms in schizophrenic patients treated with olanzapine, haloperidol and placebo. Proceedings of the 7th World Congress of Biological Psychiatry; Berlin, Germany. 2001 Jul 1-6.2001. [Google Scholar]; Beasley CM, Dellva MA, Tamura RN, Morgenstern H, Glazer WM, Ferguson K, Tollefson GD. A randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. British Journal of Psychiatry. 1999;174:23–30. doi: 10.1192/bjp.174.1.23. [MEDLINE: 99227593] [DOI] [PubMed] [Google Scholar]; Beasley CM, Hamilton SH, Crawford AM, Dellva MA, Tollefson GD, Tran PV, Blin O, Beuzen JN. Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. European Neuropsychopharmacology. 1997;7(2):125–37. doi: 10.1016/s0924-977x(96)00392-6. [MEDLINE: 9169300] [DOI] [PubMed] [Google Scholar]; Beasley CM, Sayler ME, Keisler GM, Potvin JH, Sanger TM, Tollefson GD. The influence of pharamacotherapy on self-directed and externally-directed aggression in schizophrenia. Schizophrenia Research. 1998;29(1-2):28. [Google Scholar]; Dellva MA, Tran P, Tollefson GD, Wentley AL, Beasley CM., Jr Standard olanzapine versus placebo and ineffective-dose olanzapine in the maintenance treatment of schizophrenia. Psychiatric Services. 1997;48(12):1571–7. doi: 10.1176/ps.48.12.1571. [MEDLINE: 9406266] [DOI] [PubMed] [Google Scholar]; Edgell ET, Hamilton SH, Revicki DA, Genduso LA, Tollefson GD. Costs of olanzapine treatment compared with haloperidol for schizophrenia: results from a randomised clinical trial. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-6.1998. [Google Scholar]; Nemeroff Quality of life and new antipsychotics. Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996 Aug 23-28.1996. [Google Scholar]; Revicki D, Genduso L. Olanzapine versus haloperidol therapy for chronic schizophrenia: impact on deficit syndrome. Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30-Oct 4.1995. [Google Scholar]; Tamura RN, Tollefson GD, Dellva MA, Beasley CM, Glazer WM, Morgenstern H. What is the differential risk of tardive dyskinesia with the novel antipsychotic olanzapine? Schizophrenia Research. 1998;29(1-2):176. [MEDLINE: 99227593] [Google Scholar]; Tollefson GD, Beasley CM, Tamura RN, Tran PV, Potvin JH. Blind, controlled, long-term study of the comparative incidence of treatment emergent tardive dyskinesia with olanzapine or haloperidol. American Journal of Psychiatry. 1997;154(9):1248–54. doi: 10.1176/ajp.154.9.1248. [DOI] [PubMed] [Google Scholar]; Tran P, Beasley C, Tollefson G, Crawford A, Dellva M, Gusman S, Wood A. Acute and long-term results of the dose ranging double-blind olanzapine trial. Proceedings of the 20th Collegium Internationale Neuro-Psychopharmacologicum Congress; Melbourne, Australia. 1996 Jun 23-27.1996. [Google Scholar]; Tran PV, Beasley CM, Tollefson GD, Beuzen JN, Holman SL, Sanger TM, Satterlee WG. Olanzapine: a promising “atypical” antipsychotic agent. Schizophrenia Research. 1995;15(1, 2):169. [MEDLINE: 9169300] [Google Scholar]; Tran PV, Dellva MA, Tollefson GD, Beasley CM, Jr, Potvin JH, Kiesler GM. Extrapyramidal symptoms and tolerability of olanzapine versus haloperidol in the acute treatment of schizophrenia. Journal of Clinical Psychiatry. 1997;58(5):205–11. doi: 10.4088/jcp.v58n0505. [MEDLINE: 9184614] [DOI] [PubMed] [Google Scholar]; Wright P, Tollefson GD, Beasley CM, Tamura RN, Tran PV, Potvin JII. A blinded, controlled, long-term study ofthe comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Schizophrenia Research. 1998;29(1-2):206. [MEDLINE: 9184614] [Google Scholar]
- Bechdolf 2004a {published data only} .Bechdolf A, Buhler B, Berning J, Wagner M, Stamm E, Streit M. Cognitive behavioural therapy in the early initial prodromal state of psychosis: first results. Schizophrenia Research. 2004;67(1):202. [ISI: 000188788100483] [Google Scholar]; *; Bechdolf A, Klosterkotter J. Cognitive-behavioural treatment (CBT) in the early initial prodromal state of psychosis: concept and practical approach. Schizophrenia Research. 2004;70(1):52. [ISI: 000224551100137] [Google Scholar]
- Bechdolf 2004c {published data only} .*; Bechdolf A, Veith V, Berning J, Stamm E, Decker P, Janssen B, Bottlender R, Wagner M, Klosterkotter J. Cognitive behavioral therapy (cbt) in the early initial prodromal state of psychosis: first results of a randomised trial. Schizophrenia Research. 2004;70(1):62–3. [ISI: 000224551100168] [Google Scholar]
- Bechdolf 2005a {published data only} .*; Bechdolf A, Ruhrmann S, Wagner M, Kuhn KU, Janssen B, Bottlender R, Wieneke A, Schulze-Lutter F, Maier W, Klosterkotter J. Interventions in the initial prodromal states of psychosis in Germany: concept and recruitment. British Journal of Psychiatry Supplementum. 2005;187(Suppl 48):S45–8. doi: 10.1192/bjp.187.48.s45. [MEDLINE: 16055807] [DOI] [PubMed] [Google Scholar]
- Bechdolf 2006 {published data only} .*; Bechdolf A, Wagner M, Veith V, Ruhrmann R, Janssen B, Bottlender R, Moeller HJ, Gaebel W, Maier W, Klosterkotter J. Controlled multicenter trial of cognitive behaviour therapy in the early initial prodromal state of psychosis. Schizophrenia Research. 2006;86(Suppl 1):S8. [Google Scholar]
- Bendall 2004 {published data only} .*; Bendall S. A comparison of the nonspecific effects of a control treatment with cognitive behavioural therapy in first episode psychosis. Schizophrenia Research. 2004;70(1):57. [ISI: 000224551100152] [Google Scholar]
- Bentall 2000 {published data only} .Bentall R. A multi-centre randomised controlled trial of cognitive behaviour therapy in early schizophrenia; the SOCRATES trial. National Research Register. 2000. ; *; Bentall R. A multi-centre randomised controlled trial of cognitive behaviour therapy in early schizophrenia; the SOCRATES trial. National Research Register. 2001;Vol. 1 [Google Scholar]
- Berger 2004a {published data only} .*; Berger GB, Proffitt TM, Mcconchie MA, Wood SJ, Yuen HP, Smith D, Horrobin D, Mcgorry PD. Ethyleicosapentaenoic acid (e-epa) supplementation in early psychosis. Schizophrenia Research. 2004;67(1):7–8. [ISI: 000188788100014] [Google Scholar]
- Berger 2004b {published data only} .Berger G, Wood S, Proffitt T, Mcconchie M, Khan A, O’Donnell C, Yuen H, Smith D, Horrobin D, Mcgorry P. Ethyl-eicosapentaenoic acid (e-epa) supplementation in early psychosis. A double-blind randomised add on standard therapy study in 80 drug- native or early treated first episode psychosis patients. Schizophrenia Research. 2004;70(1):41. [ISI: 000224551100105] [Google Scholar]
- Berger 2005 {published data only} .*; Berger GE, Proffitt TM, McConchie MA, Wood SJ, Yuen HP, McGorry PD. Ethyl - eicosapentaenoic acid (e - epa) supplementation in early psychosis. A double - blind, randomised, placebo - controlled trial (RCT) comparing 2g e - epa versus placebo add - on therapy in 80 drug - naive or early treated first - episode psychos. Schizophrenia Bulletin. 2005;31:475. [Google Scholar]
- Berger 2006 {published data only} .*; Berger G. Lithium in patients at ultra high risk of developing a first psychotic episode. Stanley Foundation Research Programs. 2006.
- Bertelsen 2004 {published data only} .*; Bertelsen MB. Rct of integrated treatment versus standard treatment of patients with first-episode of schizophrenia -5 years follow up. Schizophrenia Research. 2004;70(1):32. [ISI: 000224551100081] [Google Scholar]
- Bertelsen 2005 {published data only} .*; Bertelsen M. Randomized controlled trial of two-years integrated treatment versus standard treatment of patients with first - episode of schizophrenia or psychosis, five years follow-up. The opus trial. Schizophrenia Bulletin. 2005;31:519–20. [Google Scholar]
- Bertelsen 2006 {published data only} .*; Bertelsen M, Thorup A, Petersen L, Jeppesen P, Oehlenschlager J, Joergensen P, Le Quach P, Krarup G, Mortensen P, Nordentoft M. The OPUS trial: results from the five-year follow-up. Schizophrenia Research. 2006;86(Suppl 1):S43. [Google Scholar]
- Binder 2006 {published data only} .*; Binder C, Chue P, Malla A. Does long acting risperidone have a place in the treatment of early episode psychosis?. Proceedings of the Collegium Internationale Neuro-Psychopharmacologium 25th Biennial Congress; Chicago, Illinois. 2006 July 9-13.2006. [Google Scholar]
- Birchwood 2000a {published data only} .Birchwood M. Early detection and prevention of psychotic relapse. National Research Register. 2000. ; Birchwood M. Early detection and prevention of psychotic relapse. National Research Register. 2001;Vol. 1 [Google Scholar]
- Birchwood 2000b {published data only} .Birchwood M. Randomised controlled trial of early intervention in psychotic relapse. National Research Register. 2000 [Google Scholar]; Birchwood M. Randomised controlled trial of early intervention in psychotic relapse. National Research Register. 2002;Vol. 1 [Google Scholar]
- Birchwood 2000c {published data only} .Birchwood M. Early signs project. National Research Register. 2000. ; Birchwood M. Early signs project. National Research Register. 2001;Vol. 1 [Google Scholar]
- Blaha 1980 {published data only} .*; Blaha L. A strategy to reduce the frequency of early dyskinesias under high-dosed haloperidol treatment [Therapiekonzept zur reduktion der fruehdyskinesie– frequenz bei hochdosierter haloperidolbehandlung] Arzneimittel Forschung. 1980;30(8):1208. 00MEDLINE: 222524640. [Google Scholar]
- Bola 2003 {published data only} .*; Bola JR, Mosher LR. Treatment of acute psychosis without neuroleptics: two-year outcomes from the Soteria project. Journal of Nervous and Mental Disease. 2003;191(4):219–29. doi: 10.1097/01.NMD.0000061148.84257.F9. 00EMBASE: 20031773940. [DOI] [PubMed] [Google Scholar]
- Borison 1991b {published data only} .Borison R. Risperidone versus haloperidol in acute exacerbations of chronic schizophrenia. Proceedings of the 1st International Risperidone Investigators’ Meeting; Paris, France. 1992 Mar 9-10.1992. [Google Scholar]; Borison R. Risperidone versus haloperidol versus placebo in the treatment of schizophrenia. Clinical Report. 1991 [Google Scholar]; Borison R, Pathiraja A, Diamond B, Meibach R. Risperidone and schizophrenia. Biological Psychiatry. 1991;29:417. [Google Scholar]; Borison RL, Diamond BI, Augusta GA. Serotonin modulation of dopaminergic-medicated extrapyramidal side effects. Neurology. 1991;41(Suppl 1):396. [Google Scholar]; Borison RL, Diamond BI, Pathiraja A, Meibach RC. Clinical profile of risperidone in chronic schizophrenia. Proceedings of the 17th Collegium Internationale Neuro-Psychopharmacologicum Congress; Kyoto, Japan. 1990 Sep 10-14.1991. [Google Scholar]; Borison RL, Pathiraja AP, Diamond BI, Meibach RC. Risperidone - clinical safety and efficacy in schizophrenia. Psychopharmacology Bulletin. 1992;28(2):213–8. 00EMBASE: 1992238962; MEDLINE: 923833710. [PubMed] [Google Scholar]; Borison RL, Pathiraja AP, Diamond BI, Meibach RC. Risperidone in the treatment of acute exacerbation of chronic schizophrenia. Schizophrenia Research. 1991;4(3):314–5. [Google Scholar]; Borison Rl, Pathiraja AP, Diamond BIl, Meibach RC. Antidopaminergic and antiserotonergic actions of risperidone in schizophrenia. Biological Psychiatry. 1991;29(Suppl):114A. [Google Scholar]; Marder SR. Risperidone: efficacy. Journal of Clinical Psychiatry. 1994;12:49–52. 00EMBASE: 1992238962; MEDLINE: 923833710. [Google Scholar]; Meibach R. Risperidone versus haloperidol in acute exacerbations of chronic schizophrenia. Proceedings of the 1st International Risperidone Investigators’ Meeting; Paris, France. 1992 Mar 9-10.1992. [Google Scholar]
- Brecher 1998 {published data only} .Berry S, Martinez R, Myers JE, Mahmoud R. Serum prolactin in schizophrenia. Proceedings of the 7th World Congress of Biological Psychiatry; Berlin, Germany. 2001 Jul 1-6.2001. [Google Scholar]; Berry S, Martinez RA, Myers JE, Mahmoud R. Serum prolactin in schizophrenia. European Neuropsychopharmacology. 2001;11(3):257. [Google Scholar]; Berry SA, Gudelsky GA, Mahmoud RA. Serum prolactin levels in schizophrenia. Biological Psychiatry. 2001;49(8):22S. [Google Scholar]; Berry SA, Martinez RA, Gudelsky GA, Mahmoud R, Myers J. Serum prolactin levels in schizophrenia. Schizophrenia Research. 2001;49(1, 2):280–1. [Google Scholar]; Berry SA, Martinez RA, Gudelsky GA, Myers JE, Mahmoud RA. Serum prolactin in schizophrenia. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]; Berry SA, Martinez RA, Gudelsky GA, Myers JE, Mahmoud RA. Serum prolactin levels in schizophrenia. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10.2001. [Google Scholar]; Brecher M. Risperidone versus olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31-Nov 4.1998. [Google Scholar]; Brecher M, The Risperidone Olanzapine Study Group Risperidone versus olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16.1998. [Google Scholar]; Brecher M, The Risperidone-Olanzapine Study Group Risperidone versus olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder. Schizophrenia Research. 1999;36(1-3):271. [Google Scholar]; Conley RR, Brecher M, The Risperidone, Olanzapine Study Group Risperidone versus olanzapine in patients with schizophrenia or schizoaffective disorders. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31-Nov 4.1998. [Google Scholar]; Conley RR, Brecher MB, Olanzapine-Risperidone Study Group Risperidone versus olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]; Conley RR, Mahmoud R. A randomised double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 2001;Vol. 158(issue 5):765–74. doi: 10.1176/appi.ajp.158.5.765. 00MEDLINE: 212290380. [DOI] [PubMed] [Google Scholar]; Conley RR, Mahmoud R. Efficacy of risperidone vs. olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S151. [Google Scholar]; Conley RR, Mahmoud R. Efficacy of risperidone vs. olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder. Journal of the European College of Neuropsychopharmacology. 2000;10(Suppl 3):S343. [Google Scholar]; Conley RR, Mahmoud R. Risperidone and olanzapinein people with schizophrenia or schizoaffective disorder: a randomised double-blind study. Poster supplied by Company. 2001 doi: 10.1176/appi.ajp.158.5.765. [DOI] [PubMed] [Google Scholar]; Conley RR, Mahmoud R. Risperidone vs. olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder: safety comparisons. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S151. [Google Scholar]; Conley RR, Mahmoud R. Risperidone vs. olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder: safety comparisons. Journal of the European College of Neuropsychopharmacology. 2000;10(Suppl 3):S342. [Google Scholar]; Conley RR, Mahmoud R. Risperidone vs olanzapine in patients with schizophrenia & schizoaffective disorder. Proceedings of the 40th Annual Meeting of the New Clinical Drug Evaluation Unit; Boca Raton, Florida, USA. 2000 May 30 - Jun 2.2000. [Google Scholar]; Conley RR, Mahmoud R, Risperidone Study Group Risperidone versus olanzapine in patients with schizophrenia and schizoaffective disorder. Proceedings of the 10th Biennial Winter Workshop on Schizophrenia; Davos, Switzerland. 2000 Feb 5-11.2000. [Google Scholar]; Conley RR, Mahmoud R. Risperidone versus olanzapine in patients with schizophrenia and schizoaffective psychosis [Risperidon versus olanzapin bei patienten mit schizophrenie und schizoaffektiven psychosen] Nervenheilkunde. 2000;19(5):110–2. 00EMBASE: 20002472440. [Google Scholar]; Conley RR, Mahmoud R, Risperidone Study Group Risperidone vs. olanzapine in patients with schizophrenia and schizoaffective disorder. Biological Psychiatry. 2000;47:32S. [Google Scholar]; Conley RR, Mahmoud RA. Risperidone versus olanzapine in patients with schizophrenia and schizoaffective disorder. 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]; Conley RR, Mahmoud RA. Risperidone versus olanzapine in patients with schizophrenia and schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; Conley RR, Mahmoud RA. Risperidone vs olanzapine in patients with schizophrenia and schizo-affective disorder. International Drug Therapy Newsletter. 2000;35(10):77–8. [Google Scholar]; Harvey K, Burns T, Sedgwick P, Higgitt A, Creed F, Fahy T. Relatives of patients with severe psychotic disorders: factors that influence contact frequency. Report from the UK700 trial. British Journal of Psychiatry. 2001;178:248–54. doi: 10.1192/bjp.178.3.248. 00MEDLINE: 211529660. [DOI] [PubMed] [Google Scholar]; Harvey P, Meltzer H, Green M. Risperidone cognitive effects in schizophrenia and schizoaffective patients. International Drug Therapy Newsletter. 2001;36(8):59. [Google Scholar]; Harvey P, Meltzer HY, Green MP. Cognitive effects of risperidone versus olanzapine in patients with schizophrenia or schizoaffective disorder. Biological Psychiatry. 2001;2(Suppl 1):P021–48. [Google Scholar]; Harvey P, Melzer H, Green M. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Biological Psychiatry. 2001;49(8):123S. [Google Scholar]; Harvey PD. Cognitive effects of risperidone and olanzapine in patients with schizophrenia. Proceedings of the 52nd Institute on Psychiatric Services; Philadelphia, Pennsylvania, USA. 2000 Oct 25-29.2000. [Google Scholar]; Harvey PD. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]; Harvey PD, Gharabawi G. Risperidone and cognition in schizophrenic elderly. Proceedings of the 12th World Congress of Psychiatry; Yokohama, Japan. 2002 Aug 24-29.2002. [Google Scholar]; Harvey PD, Green MF, McGurk SR, Meltzer HY. Changes in cognitive functioning with risperidone and olanzapine treatment: a large-scale, double-blind, randomised study. Psychopharmacology. 2003;169(3-4):404–11. doi: 10.1007/s00213-002-1342-5. 00MEDLINE: 125903560. [DOI] [PubMed] [Google Scholar]; Harvey PD, Mahmoud R, Meltzer HY, Green MP. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. European Neuropsychopharmacology. 2001;11(3):257. [Google Scholar]; Harvey PD, Meltzer HY, Green M. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]; Harvey PD, Meltzer HY, Green MF. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Proceedings of the 40th Annual Meeting of the New Clinical Drug Evaluation Unit; Boca Raton, Florida, USA. 2000 May 30-Jun 2.2000. [Google Scholar]; Lasser RA, Mao L, Gharabawi G. Smokers and nonsmokers equally affected by olanzapine-induced weight gain: metabolic implications. Schizophrenia Research. 2004;66(2-3):163–7. doi: 10.1016/S0920-9964(03)00153-1. 00EMBASE: 2004015909; MEDLINE: 150612490. [DOI] [PubMed] [Google Scholar]; Mahmoud R, Harvey PD, Meltzer HY, Green MF. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Schizophrenia Research. 2001;49(1, 2):236–7. [Google Scholar]; Mahmoud RA, Engelhart LM, Janagap C, Awad G. Assessment of symptoms affecting quality of life and patient satisfaction with antipsychotic drugs: new insights for a trial of risperidone/olanzapine. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]; Martinez RA, Berry SA, Gudelsky GA, Myers JE, Mahmoud RA. Serum prolactin levels in schizophrenia. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; Myers J, Mahmoud R, Berry S, Conley R. Risperidoneversus olanzapine for the treatment of mood symptoms in patients with schizophrenia and schizoaffective disorder. Bipolar Disorders. 2001;3(Suppl 1):49. [Google Scholar]; Myers JE, Mahmoud R, Berry S, Conley RR. Risperidone versus olanzapine for the treatment of mood symptoms in patients with schizophrenia and schizoaffective disorder. European Neuropsychopharmacology. 2001;11(3):254. [Google Scholar]; Myers JE, Mahmoud RA, Berry SA, Conley RR. Risperidone versus olanzapine for the treatment of mood symptoms in patients with schizophrenia and schizoaffective disorder. 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23; 2002. 00: NR267 Tuesday, May 8, 12:00 p.m.–2:00 p.m0. [Google Scholar]; Myers JE, Mahmoud RA, Berry SA, Conley RR. Risperidone versus olanzapine for the treatment of mood symptoms in patients with schizophrenia and schizoaffective disorder. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10.2001. [Google Scholar]; Myers JE, Mahmoud RA, Keith SJ, Csernansky JG. Long-term benefit of risperidone versus haloperidol for affective symptoms in patients with schizophrenia and schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; Robinson G, Wheeler A, Byrd J, Visser S. Longer-term effects of switching from typical to atypical antipsychotics in patients with stable schizophrenia. Journal of the European College of Neuropsychopharmacology. 2000;10(Suppl 3):S291. [Google Scholar]
- Bredkjar 1999 {published data only} .Bredkjar S, Koster A. Continuity of care in first episode psychosis. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]
- Bredkjar 2000 {published data only} .Bredkjar SR, Koster A. Continuity of care of severely disturbed first episode psychosis. Schizophrenia Research. 2000;41(1):233. 00ISI: 0002245511001690. [Google Scholar]
- Breier 2002b {published data only} .*; Breier AF, Zipursky RB, Perkins DO, Addington JM, Tohen MF, David SR, McGlashan TH. A trial of olanzapine versus PBO in the prodrome: protocol and baseline sample. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Brewer 2002 {published data only} .*; Brewer WJ, McGorry PD, O’Keefe G, Olver J, Egan G, Velakoulis D, Pipingas A, Maruff P, Scott A, Yucel M, Stuart G, Pantelis C. Functional neuroimaging follow-up of stroop performance in neuroleptic-naive first-episode psychosis. Schizophrenia Research. 2002;53(3 Suppl 1):109. 00MEDLINE: 124091650. [Google Scholar]
- Brooker 1992 {published data only} .Brooker C, Falloon I, Butterworth A, Goldberg D, Graham-Hole V, Hillier V. The outcome of training community psychiatric nurses to deliver psychosocial intervention. British Journal of Psychiatry. 1994;164(August):222–30. doi: 10.1192/bjp.165.2.222. 00MEDLINE: 16173670. [DOI] [PubMed] [Google Scholar]; Brooker C, Tarrier N, Barrowclough C, Butterworth A, Goldberg D. Training community psychiatric nurses for psychosocial intervention. Report of a pilot study. British Journal of Psychiatry. 1992;160:836–44. doi: 10.1192/bjp.160.6.836. 00MEDLINE: 16173670. [DOI] [PubMed] [Google Scholar]
- Burns 2002b {published data only} .*; Burns T. The West London longitudinal first episode study. Vol. 3. National Research Register; 2002. Cognitive and neuroimaging abnormalities in first-episode psychosis. [Google Scholar]
- Burrell 1960 {published data only} .*; Burrell R, Clancey I, Rejskind M, Weckowicz TE. Hydroxyzine hydrochloride and chlorpromazine. A clinical trial with a group of “tense” psychiatric patients. Canadian Psychiatric Association Journal. 1960;5:124–9. doi: 10.1177/070674376000500212. 00PsycINFO: 1966–13288–0010. [DOI] [PubMed] [Google Scholar]
- Caffey 1968 {published data only} .Caffey EM, Jones RD. Brief hospital treatment of schizophrenia: early results of a multiple hospital study. Hospital and Community Psychiatry. 1968;19:282–7. 00MEDLINE: 201750780. [PubMed] [Google Scholar]; Caffey EM, Jr, Galbrecht CR, Klett CJ. Brief hospitalization and aftercare in the treatment of schizophrenia. Archives of General Psychiatry. 1971;24(1):81–6. doi: 10.1001/archpsyc.1971.01750070083012. 00MEDLINE: 43214070. [DOI] [PubMed] [Google Scholar]
- Cao 2000 {published data only} .*; Cao X, Wang W. Comparison between therapeutic effect of risperidone in treating different traditional Chinese medicine syndrome types of first-episode schizophrenia. Chinese Journal of Integrated Traditional and Western Medicine. 2000;20(6):421–3. 00CAJ: MEDI00120. [PubMed] [Google Scholar]
- Carpenter 1977 {published data only} .Carpenter WT, McGlashan TH, Strauss JS. The treatment of acute schizophrenia without drugs: An investigation of some current assumptions. American Journal of Psychiatry. 1977;134(1):14–20. doi: 10.1176/ajp.134.1.14. [DOI] [PubMed] [Google Scholar]
- Carpenter 1982 {published data only} .*; Carpenter WT, Stephens JH, Rey AC. Early intervention vs. continuous pharmacotherapy of schizophrenia. Psychopharmacology Bulletin. 1982;18(1):21–3. 00EMBASE: 19821460410. [Google Scholar]
- Carpenter 1983b {published data only} .*; Carpenter WT, Heinrichs DW. Early intervention, time limited, targeted pharmacotherapy of schizophrenia. Schizophrenia Bulletin. 1983;9(4):533–42. doi: 10.1093/schbul/9.4.533. 00MEDLINE: 6140752; PsycINFO: 71–212290. [DOI] [PubMed] [Google Scholar]
- Carpenter 1999a {published data only} .*; Carpenter WT, Jr, Buchanan RW, Kirkpatrick B, Breier AF. Diazepam treatment of early signs of exacerbation in schizophrenia. American Journal of Psychiatry. 1999;156(2):299–303. doi: 10.1176/ajp.156.2.299. 00MEDLINE: 991426730. [DOI] [PubMed] [Google Scholar]
- Carson 2000 {published data only} .Anutosh S, Ali MW, Ingenito G, Carson WH. Controlled study of aripiprazole and haloperidol in schizophrenia. European Psychiatry. 2002;17(Suppl 1):103s. [Google Scholar]; Aquino P, Adams CE, Crow T, Wood I. Personal communication. 2006. ; Carson WH, Ali M, Dunbar G, Ingenito G, Saha AR. A double-blind, placebo-controlled trial of aripiprazole and haloperidol. Schizophrenia Research. 2001;49(1, 2):221–2. [Google Scholar]; Carson WH, Ali M, Saha AR, Dunbar GC, Ingenito G. A double-blind, placebo-controlled trial of aripiprazole and haloperidol in patients with schizophrenia or schizoaffective disorder. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]; Carson WH, Kane JM, Ali M, Dunbar GC, Ingenito G. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. European Neuropsychopharmacology. 2000;10(Suppl 3):S309. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Kane J, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. Schizophrenia Research. 2000;41(1):39. [Google Scholar]; Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, Ali MW. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. Journal of Clinical Psychiatry. 2002;63(9):763–71. doi: 10.4088/jcp.v63n0903. [DOI] [PubMed] [Google Scholar]; Kane JM, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23; 2002. 00: NR345 Tuesday, May 16, 3:00 p.m.–5:00 p.m0. [Google Scholar]; Kane JM, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S124. [Google Scholar]; Kane JM, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]
- Carson 2000b {published data only} .Carson W, McQuade R, Saha A, Torbeyns A, Stock E. Aripiprazole versus placebo for relapse prevention in patients with chronic schizophrenia. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S288. [Google Scholar]; Carson W, Pigott T, Saha A, Ali M, McQuade RD, Torbeyns AF, Stock E. Aripiprazole vs placebo in the treatment of stable, chronic schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; Carson WH, Pigott TA, Saha AR, Ali MW, McQuade RD, Torbeyns AF, Stock EG. Aripiprazole vs placebo in the treatment of chronic schizophrenia. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S187. [Google Scholar]; Casey D, Saha A, Marcus R, Carson WH, McQuade RD, Torbeyns AF, Stock E. Aripiprazole versus placebo for relapse prevention in patients with chronic schizophrenia. Schizophrenia Research. 2003;60(1):276. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Marder SR, Kaplita S, Saha AR, Carson WH, Jr, Torbeyns A, Stock EG. Glycemic control and plasma lipids in long-term aripiprazole treatment. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: A placebo-controlled 26-week study. Journal of Clinical Psychiatry. 2003;64(9):1048–56. doi: 10.4088/jcp.v64n0910. 00EMBASE: 20033832620. [DOI] [PubMed] [Google Scholar]; Pigott TA, Saha AR, Ali MW, McQuade RD, Torbeyns AF, William HC, Jr, Stock EG. Aripiprazole versus placebo in the treatment of chronic schizophrenia. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; Stock E, Marder SR, Jody D, Kaplita S, Saha A, Carson W, Torbeyns A. Plasma lipids levels and glycemic control in long-term treatment with aripiprazole. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S327. [Google Scholar]; Torbeyns A, Marder SR, Carson W, Jody D, Kaplita S, Saba A, Stock E. Glycemic control and plasma lipids in long-term treatment with aripiprazole. Schizophrenia Research. 2004;67(1):192–3. 00ISI: 0001887881004610. [Google Scholar]
- Casey 2002 {published data only} .Casey D, Saha AR, Ali MW, Jody DN, Kujawa MJ, Stock EG, Ingenito GG. Switching to aripiprazole monotherapy. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S187. [Google Scholar]; Casey DE, Carson WH, Saha AR, Liebeskind A, Ali MW, Jody D, Ingenito GG. Switching patients to aripiprazole from other antipsychotic agents: A multicenter randomised study. Psychopharmacology. 2003;166(4):391–9. doi: 10.1007/s00213-002-1344-3. 00EMBASE: 20031674180. [DOI] [PubMed] [Google Scholar]; Casey DE, Saha AR, Ali MW, Jody D, Kujawa MJ, Stock EG, Ingenito GG. Switching to aripiprazole monotherapy. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00EMBASE: 20031674180.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00EMBASE: 20031674180.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00EMBASE: 20031674180.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00EMBASE: 20031674180.; Kujawa M, Saha AR, Ali MW, Jody DN, McQuade RD, Ingenito GG. Switching to aripiprazole monotherapy. Schizophrenia Research. 2003;60:290. [Google Scholar]; Medori R, Gharbia N, Saha A, Ali M, Stock E, Ingenito G. Switching to aripiprazole monotherapy. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S292. [Google Scholar]
- Castilla 2002 {published data only} .*; Castilla R. Early medication intervention in the treatment of psychosis in children. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S51. [Google Scholar]
- Cavozzoni 2002a {published data only} .*; Cavazzoni P, Berg PH, Millikan M, Carlson C, Beasley CM. An integrated analysis of treatment-emergent extrapyramidal syndrome in schizophrenic patients during olanzapine clinical trials versus placebo, haloperidol, risperidone or clozapine. Schizophrenia Research. 2002;53(3 Suppl 1):171. doi: 10.4088/jcp.v64n0807. 00MEDLINE: 124091650. [DOI] [PubMed] [Google Scholar]
- Centorrino 2003 {published data only} .*; Centorrino F, Hamer RM, Tohen M, Lieberman JA. Drug attitudes and treatment adherence in a clinical trial comparing haloperidol and olanzapine in first episode schizophrenia. Schizophrenia Research. 2003;60(1):277. 00MEDLINE: 124091650. [Google Scholar]
- Chaudhry 2004 {published data only} .*; Chaudhry I. The addition of lamotrigine or minocycline to treat 80 first-episode subjects with schizophrenia. Stanley Foundation Research Programs. 2004.
- Chen 2000a {published data only} .*; Chen S, Sun M. A controlled study of fixed dose of risperidone in treatment of the first episode schizophrenia. Sichuan Mental Health. 2000;13(4):238–9. 00CAJ: MEDI01010. [Google Scholar]
- Chen 2000c {published data only} .*; Chen F, Cai C, Wan C. Risperidone in the treatment of first-episode schizophrenia. Journal of Wannan Medical College. 2000;19(3):192–3. 00CAJ: MEDI0011S10. [Google Scholar]
- Chen 2004a {published data only} .*; Chen DC, Ji ZF, Yao SW. Effect of clozapine on paroxetine neuroendocrine challenge test in first-episode schizophrenics. Chinese Journal of Psychiatry. 2004;37(1):30–2. 00CAJ: MEDI04040. [Google Scholar]
- Chen 2004c {published data only} .*; Chen Q, Wang W, Cao D. An analysis of prodromal symptoms in schizophrenia. Sichuan Mental Health. 2004;17(3):147–8. [Google Scholar]
- Chen 2006d {published data only} .*; Chen W, Li L. Study on influence of classical and non-classical antipsychotics on EEG of schizophrenia in first episode. Journal of Modern Electrophysiology. 2006;13(2):86–7. [Google Scholar]
- Cheng 2006b {published data only} .Cheng P, Wang T-L, Tang P. The contrast investigation between risperidone and perphenazlne in the treatment of first episode of schizophrenia in children and adolescents. Nervous Diseases and Mental Hygiene. 2006;6(2):120–1. [Google Scholar]; *; Crespo-Facorro B, Perez-Iglesias R, Ramirez-Bonilla M, Martinez-Garcia O, Llorca J, Vazquez-Barquero JL. A practical clinical trial comparing haloperidol, risperidone, and olanzapine for the acute treatment of first-episode nonaffective psychosis. Journal of Clinical Psychiatry. 2006;67(10):1511–21. doi: 10.4088/jcp.v67n1004. 00EMBASE: 2006573618; MEDLINE: 171072410. [DOI] [PubMed] [Google Scholar]
- Chiu 2006b {published data only} .*; Chiu C-C, Chen K-P, Liu H-C, Lu M-L. The early effect of olanzapine and risperidone on insulin secretion in atypical-naive schizophrenic patients. Journal of Clinical Psychopharmacology. 2006;26(5):504–7. doi: 10.1097/01.jcp.0000237947.80764.d9. 00MEDLINE: 169741930. [DOI] [PubMed] [Google Scholar]
- Chouinard 1992 {published data only} .Anderson C, Clark WR, True J, Ereshefsky L. Risperidone, a novel antipsychotic and weight change. Pharmacotherapy. 1993;13:292. 00MEDLINE: 923618500. [Google Scholar]; Anderson C, True J, Ereshefsky L, Miller A. Risperidone clinical efficacy: role of the metabolite 9-hydroxyrisperidone. Psychopharmacology Bulletin. 1994;30(4):88. 00MEDLINE: 923618500. [Google Scholar]; Anderson CB, True JE, Ereshefsky L, Miller AL, Peters BL, Velligan DI. Risperidone dose, plasma levels and response. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 1993 May 22-27.1993. [Google Scholar]; Chouinard G. Effects of risperidone in tardive dyskinesia: an analysis of the Canadian multicenter risperidone study. Journal of Clinical Psychopharmacology. 1995;15(Suppl 1):S36–S44. doi: 10.1097/00004714-199502001-00007. 00MEDLINE: 75372860. [DOI] [PubMed] [Google Scholar]; Chouinard G, Albright PS. Economic and health state utility determinations for schizophrenic patients treated with risperidone or haloperidol. Journal of Clinical Psychopharmacology. 1997;17(4):298–307. doi: 10.1097/00004714-199708000-00010. 00MEDLINE: 92410100. [DOI] [PubMed] [Google Scholar]; Chouinard G, Arnott W. An antidyskinetic effect of risperidone. Proceedings of the 9th World Congress of Psychiatry; Rio de Janeiro, Brazil. 1993 Jun 6-12.1993. [Google Scholar]; Chouinard G, Arnott W. Antidyskinetic effect of risperidone in chronic schizophrenic patients. Clinical Neuropharmacology. 1992;15(Suppl 1 Pt B):266. 00MEDLINE: 90898190. [Google Scholar]; Chouinard G, Arnott W. The effect of risperidone on extrapyramidal symptoms in chronic schizophrenic patients. Biological Psychiatry. 1992;31:158. 00MEDLINE: 92410100. [Google Scholar]; Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, Labelle A, Beauclair L, Arnott W. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. Journal of Clinical Psychopharmacology. 1993;13(1):25–40. 00MEDLINE: 932531200. [PubMed] [Google Scholar]; Chouinard G, Vainer JL, Beauclair L. Dose regimens of neuroleptics in negative symptoms. Proceedings of the 19th Collegium Internationale Neuro-Psychopharmacologicum Congress; Washington DC, USA. 1994 Jun 27-Jul 1.1994. [Google Scholar]; Davis JM, Chen N. Evidence of efficacy of risperidone in schizophrenia. Schizophrenia Research. 2001;49(1-2):224–5. 00MEDLINE: 97313564; PsycINFO: 1997–06350–0090. [Google Scholar]; De Coster R, Bowden C, Byloos M, Voina S, Coussement W, Meibach R, Arnott W, Heylen S. Endocrine effects of the new antipsychotic risperidone. Proceedings of the 9th International Congress of Endocrinology; Nice, France. 1992 Aug 30-Sep 5; 1992. 00MEDLINE: 97313564; PsycINFO: 1997–06350–0090. [Google Scholar]; Heylen SL, Gelders YG. Risperidone, a new antipsychotic with serotonin 5-HT2 and dopamine D2 antagonistic properties. Clinical Neuropharmacology. 1992;15(Suppl 1):180A–1A. doi: 10.1097/00002826-199201001-00095. 00MEDLINE: 923615480. [DOI] [PubMed] [Google Scholar]; Lindenmayer JP, The Risperidone Study Group Incidence of EPS with risperidone compared with haloperidol and placebo in patients with chronic schizophrenia. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA; 1993 May 22-27.1993. [Google Scholar]; Marder SR. Risperidone: clinical development: North American results. Clinical Neuropharmacology. 1992;15(Suppl 1 Pt A):92A–93A. doi: 10.1097/00002826-199201001-00049. 00MEDLINE: 923618500. [DOI] [PubMed] [Google Scholar]; Marder SR. Risperidone: efficacy. Journal of Clinical Psychiatry. 1994;12:49–52. 00MEDLINE: 923618500. [Google Scholar]; Meibach RC, The Risperidone Study Group A fixed dose, parallel group study of risperidone vs. haloperidol vs. placebo. Schizophrenia Research. 1993;9(2, 3):245. 00MEDLINE: 943509250. [Google Scholar]; Moller HJ, Muller H, Borison RL, Schooler NR, Chouinard G. A path-analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients. A re-evaluation of the North American risperidone study. European Archives of Psychiatry and Clinical Neuroscience. 1995;245(1):45–9. doi: 10.1007/BF02191543. 00MEDLINE: 953065060. [DOI] [PubMed] [Google Scholar]; Schooler NR. Negative symptoms, risperidone and dose. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 1993 May 22-27.1993. [Google Scholar]; Schooler NR. Negative symptoms in schizophrenia: assessment of the effect of risperidone. Journal of Clinical Psychiatry. 1994;55(Suppl 5):22–8. 00MEDLINE: 943509250. [PubMed] [Google Scholar]; Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. Journal of Clinical Psychopharmacology. 1997;17(3):194–201. doi: 10.1097/00004714-199706000-00010. 00MEDLINE: 97313564; PsycINFO: 1997–06350–0090. [DOI] [PubMed] [Google Scholar]
- Ciompi 1993 {published data only} .Ciompi L, Dauwalder HP, Maier C, Aebi E, Trutsch K, Kupper Z. The pilot project “Soteria Berne”: Clinical experiences and results. British Journal of Psychiatry. 1992;161(Suppl. 18):145–53. [PubMed] [Google Scholar]; *; Ciompi L, Kupper Z, Aebi E, Duwalder HP, Hubschmidt T, Trutsch K, Rutishauser C. The pilot project “Soteria Berne” for the treatment of acute schizophrenics: II. Results of a comparative prospective study over 2 years [Das Pilot–Projekt “Soteria Berne” zur Behandlung akut Schizophener: II. Ergebnisse der vergleichenden prospektiven Verlaufsstudie uber 2 Jahre.] Der Nervenarzt. 1993;64:440–50. [PubMed] [Google Scholar]
- Claus 1992 {published data only} .Claus A, Bollen J, De Cuyper H, Eneman M, Malfroid M, Peuskens J, Heylen S. Risperidone versus haloperidol in the treatment of chronic schizophrenic inpatients: a multicentre double-blind comparative study. Acta Psychiatrica Scandinavica. 1992;85(4):295–305. doi: 10.1111/j.1600-0447.1992.tb01473.x. 00MEDLINE: 13758010. [DOI] [PubMed] [Google Scholar]; Marder SR. Risperidone: efficacy. Journal of Clinical Psychiatry. 1994;12:49–52. 00MEDLINE: 13758020. [Google Scholar]; Wilms G, Van Ongeval C, Baert AL, Claus A, Bollen J, De Cuyper H, Eneman M, Malfroid M, Peuskens J, Heylen S. Ventricular enlargement, clinical correlates and treatment outcome in chronic schizophrenic inpatients. Acta Psychiatrica Scandinavica. 1992;85(4):306–12. doi: 10.1111/j.1600-0447.1992.tb01474.x. 00MEDLINE: 13758020. [DOI] [PubMed] [Google Scholar]
- Conley 1999 {published data only} .*; Conley J, Goldman RS, Bilder RM, Bates J, Reiter G, Pappadopulos E, Robinson D, Alvir JMA, Liebrman J, Schooler N. A comparison of the neurocognitive effects of treatment with typical and atypical neuroleptics in first-episode schizophrenia. Schizophrenia Research. 1999;36(1-3):128. 00ISI: 0001887881000820. [Google Scholar]
- Craig 2004b {published data only} .Craig T. Brixton early psychosis project. National Research Register. 2001;Vol. 3 [Google Scholar]; Craig T, Garety P, Power P, Rahaman N, Colbert S, Fornells-Ambrojo M. Lambeth early onset service: a randomisedcontrolled trial. Schizophrenia Research. 2004;70(1):145–6. 00ISI: 0002245511004330. [Google Scholar]; Craig TKJ. Brixton early psychosis project. National Research Register. 2000 [Google Scholar]; *; Craig TKJ, Garety P, Power P, Rahaman N, Colbert S, Fornells-Ambrojo M, Dunn G. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. British Medical Journal. 2004;329(7474):1067–70. doi: 10.1136/bmj.38246.594873.7C. 00CINAHL: 2005085280; MEDLINE: 154859340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro 2006a {published data only} .Crespo-Facorro B, Perez-Iglesias R, Ramirez-Bonilla M, Martinez-Garcia O, Llorca J, Vazquez-Barquero JL. A practical clinical trial comparing haloperidol, risperidone, and olanzapine for the acute treatment of first-episode nonaffective psychosis. Journal of Clinical Psychiatry. 2006;67(10):1511–21. doi: 10.4088/jcp.v67n1004. 00EMBASE: 2006573618; MEDLINE: 171072410. [DOI] [PubMed] [Google Scholar]; Crespo-Facorro B, Perez-Iglesias R, Ramirez ML, Pelayo-Teran JM, Martinez O, Vazquez-Barquero JL. A practical clinical trial comparing haloperidol, risperidone and olanzapine for the acute treatment of first episode of non-affective psychosis. Schizophrenia Research. 2006;86(Suppl 1):S39. [Google Scholar]
- Csernansky 2003 {published data only} .Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00ISI: 0001887881004610.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00ISI: 0001887881004610.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00ISI: 0001887881004610.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00ISI: 0001887881004610.
- Cullberg 2002 {published data only} .Cullberg J, Levander S, Holmquist R, Mattsson M, Weiselgren IM. One-year outcome in first episode psychosis patients in the Swedish Parachute project. Acta Psychiatrica Scandinavica. 2002;106(4):276–85. doi: 10.1034/j.1600-0447.2002.02376.x. [DOI] [PubMed] [Google Scholar]; Cullberg J, Mattsson M, Levander S, Holmqvist R, Tomsmark L, Elingfors C, Wieselgren IM. Treatment costs and clinical outcome for first episode schizophrenia patients: a 3-year follow-up of the Swedish ’Parachute Project’ and two Comparison Groups. Acta Psychiatrica Scandinavica. 2006;114(4):274–81. doi: 10.1111/j.1600-0447.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- Dahl 2000 {published data only} .Dahl A, Haahr UH, Simonsen E. Improving compliance in treatment of first episode non-affective psychotic patients. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]; Larsen TK. First episode schizophrenia: reducing delays in treatment (oral presentation). Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]
- Daniel 2000b {published data only} .Daniel DG, Saha AR, Ingenito G, Carson WH, Dunbar G. Aripiprazole, a novel antipsychotic: overview of a phase II study result. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S157. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Saha AR, Petrie JL, Ali MW. Safety and efficacy profile of aripiprazole, a novel antipsychotic. Schizophrenia Research. 1999;36(1-3):295. [Google Scholar]
- David 1999a {published data only} .David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. Journal of the European College of Neuropsychopharmacology. 1999;9:S292. [Google Scholar]
- David 1999b {published data only} .David SR, Rubin DB, Wu Y. Modeling schizophrenic behaviour and testing drug efficacy using general mixture components on finger tapping data from a twelve month prospective double-blind clinical trial. Schizophrenia Research. 1999;36(1-3):163–4. 00ISI: 0001887881004480. [Google Scholar]
- David 2000a {published data only} .David SR, Taylor CC, Kinon BJ, Breier A. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clinical Therapeutics. 2000;22(9):1085–96. doi: 10.1016/S0149-2918(00)80086-7. [DOI] [PubMed] [Google Scholar]
- Davidson 2003 {published data only} .Davidson M, Schooler N, Rabinowitz J. Treatment of cognitive impairment in recent onset psychosis; a comparison of risperidone and haloperidol. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S334. [Google Scholar]; Davidson M, Schooler N, Rabinowitz J. Treatment of cognitive impairment in recent onset psychosis; a comparison of risperidone and haloperidol. Proceedings of the 16th European College of Neuropsychopharmacology Congress; Prague, Czech Republic. 2003 Sep 20-24.2003. [Google Scholar]
- Davidson 2004 {published data only} .*; Davidson M. Reducing the risk of early transition to psychosis: using long-acting atypical antipsychotics in young patients. Proceedings of the Thematic Conference of the World Psychiatric Association on “Treatments in Psychiatry: An Update”; Florence, Italy. 2004 Nov 10-13.2004. [Google Scholar]
- Davis 1977 {published data only} .*; Davis GC, Bunney WE, DeFraites EG, Kleinman JE, Van Kammen DP, Post RM, Wyatt RJ. Intravenous naloxone administration in schizophrenia and affective illness. Science. 1977;197(4298):74–7. doi: 10.1126/science.325650. 00MEDLINE: 3256500. [DOI] [PubMed] [Google Scholar]
- De Smedt 1999 {published data only} .*; De Smedt G. Risperidone vs. haloperidol in first episode psychosis. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]
- Deng 2006b {published data only} .*; Deng Q-Y, Huang Z-L, Xie Z-Y. Research the effect of early intervention of first episode schizophrenia. Medical Journal of Chinese Civil Administration. 2006;18(9):794–6. [Google Scholar]
- Ding 2001 {published data only} .*; Ding YQ, Zhang Y. Effect of clozapine on leukocyte in patients with first-episode schizophrenia. Jiujiang Medical Journal. 2001;16(1):6–7. 00CAJ: MEDI01060. [Google Scholar]
- Dollfus 2006 {published data only} .*; Dollfus S. The treatment of post-psychotic depression. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S165. [Google Scholar]
- Dossenbach 1997 {published data only} .Dossenbach M, Friedel P, Jakovljevic M, Hotujac L, Folnegovic V, Uglesic B, Dodig G. Olanzapine versus fluphenazine - six weeks’ treatment of acute schizophrenia. Proceedings of the 10th European College of Neuropsychopharmacology Congress; Vienna, Austria. 1997 Sep 13-17.1997. [Google Scholar]; Dossenbach M, Jakovljevic M, Folnegovic F, Uglesic B, Dodig G, Friedel P, Hotujac L. Olanzapine versus fluphenazine - 6 weeks treatment of anxiety symptoms during acute schizophrenia. Schizophrenia Research. 1998;29(1, 2):203. 00MEDLINE: 212003210. [Google Scholar]; Martenyi F, Dossenbach M, Jakovljevic M, Metcalfe S. Predictive value of early anti anxiety effect on the acute antipsychotic outcome: a comparison of fluphenazine and olanzapine. Schizophrenia Research. 2000;41(1):191. [Google Scholar]; Martin C, Genduso L, Revicki D, Hamilton S, Tran P, Beasley C. Quality of life outcomes of olanzapine, a new atypical antipsychotic agent. Schizophrenia Research. 1996;18(2, 3):130. [Google Scholar]; Mimica N, Dossenbach M, Friedel P, Folnegovic-Smalc V, Makaric G, Jakovlijevic M, Uglesic B. Olanzapine compared to fluphenazine in the treatment of schizophrenia. Schizophrenia Research. 1998;29(1, 2):150. 00MEDLINE: 212003210. [Google Scholar]; Schausberger B, Dossenbach M, Hotujac L, Folnegovic-Smalc V, Uglesic B, Jakovljevic M. Impact of olanzapine versus fluphenazine on patient’s drug acceptance during acute treatment of schizophrenia. Journal of the European College of Neuropsychopharmacology. 1999;9:S292. [Google Scholar]; Schausberger B, Folnegovic-Smalc V, Hotujac L, Uglesic B, Jakovljevic M, Dossenbach M. Impact of olanzapine vs. fluphenazine on patient’s drug acceptance during acute treatment of schizophrenia. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. pp. PO–16–17. [Google Scholar]; Tran PV, Tollefson GD, Crawford AM, Dossenbach M, Friedel P, Folnegovic V, Jaklovljevic M. Olanzapine versus fluphenazine in schizophrenia. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30-Jun 4.1998. [Google Scholar]
- Dubitsky 2002a {published data only} .*; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm. 00EMBASE: 20031674180.
- Dursun 2002 {published data only} .*; Dursun S. A randomised, rater-blind trial of lamotrigine, minocycline and placebo added to treatment as usual (TAU) in first episode psychosis. National Research Register. 2002;Vol. 4 [Google Scholar]
- Eack 2007 {published data only} .*; Eack SM, Hogarty GE, Greenwald DP, Hogarty SS, Keshavan MS. Cognitive enhancement therapy improves emotional intelligence in early course schizophrenia: preliminary effects. Schizophrenia Research. 2007;89(1-3):308–11. doi: 10.1016/j.schres.2006.08.018. 00MEDLINE: 170552270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards 1999 {published data only} .*; Edwards J, Maude D, McGorry P, Cocks J, Burnett P, Davern M, Bennett C, Harrigan S, Herman T, Wade D, Bell R. Treatment of enduring positive symptoms in first-episode psychosis: a randomised controlled trial of CBT and clozapine. Schizophrenia Research. 1999;1, 2 & 3:278. 00ISI: 0001887881000820. [Google Scholar]
- Edwards 2003 {published data only} .Edwards J. Early psychosis service developments: becoming real. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. p. 56. [Google Scholar]; *; Edwards J, Wong L, Burnett P, Harrigan SM, McGorry PD, Wade D, Murphy B, Drew L, Albiston D. Enduring positive symptoms in first episode psychosis: a randomised controlled trial of clozapine and CBT. Schizophrenia Research. 2003;60(1):321. doi: 10.1155/2011/394896. 00MEDLINE: 124091650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards 2004 {published data only} .Edwards J, Elkins KS, Hinton MF, Harrigan SM, Donovan KD, Athanasopoulos O. Randomized controlled trial of a cannabis-focused intervention versus psychoeducation for young people continuing to use cannabis in the 12 months following entry to treatment for first-episode psychosis. Schizophrenia Research. 2004;70(1):61. 00ISI: 0002245511001640. [Google Scholar]
- Edwards 2006 {published data only} .*; Edwards J, Elkins K, Hinton M, Harrigan SM, Donovan K, Athanasopoulos O, McGorry PD. Randomized controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatrica Scandinavica. 2006;114(2):109–17. doi: 10.1111/j.1600-0447.2006.00783.x. 00MEDLINE: 168365980. [DOI] [PubMed] [Google Scholar]
- Eguiluz 1998 {published data only} .*; Eguiluz I, Gonzalez-Torres MA, Munoz P, Guadilla M, Gonzalez G. Evaluation of the efficacy of psychoeducative groups in schizophrenic patients [Evaluacion de la eficacia de los grupos psicoeducativos en pacientes esquizofrenicos] Actas Luso Espanolas de Neurologia Psiquiatria y Ciencias Afines. 1998;26(1):29–34. [PubMed] [Google Scholar]
- Eli Lilly 2006d {published data only} .Eli Lilly, Company . Efficacy study of early onset of antipsychotic drug action in schizophrenia. Eli Lilly and Company Clinical Trial Registry; 2006. [Google Scholar]
- Emsley 1999 {published data only} .Emsley R, McCreadie R, Livingston M, De Smedt G, Lemmens P. Risperidone in the treatment of first-episode patients with schizophreniform disorder: a double-blind multicenter study. Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30-Oct 4.1995. [Google Scholar]; Emsley R, McCreadie R, Livingston M, De Smedt G, Lemmens P. Risperidone in the treatment of first-episode schizophrenic patients with schizophreniform disorder: a double blind study. Proceedings of the International Academy for Biomedical and Drug Research on Critical Issues in the Treatment of Schizophrenia; Palazzo dei Congressi, Florence, Italy. 1995 Mar 10-12.1995. [Google Scholar]; Emsley RA, McCreadie R, Lemmens P. Risperidone in the treatment of first episode psychotic patients: a double-blind multicenter comparison with haloperidol. Proceedings of the 148th Annual Meeting of the American Psychiatric Association; Miami, Florida, USA. 1995 May 20-25.1995. [Google Scholar]; Emsley RA, McCreadie R, Livingston M, De Smedt G, Lemmens P. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Proceedings of the Workshop on Critical Issues in the Treatment of Schizophrenia; Florence, Italy. 1995 Mar 10-12.1995. [Google Scholar]; *; Emsley RA, Risperidone Working Group Risperidone in the treatment of first episode psychotic patients: a double blind multicenter study. Schizophrenia Bulletin. 1999;25(4):721–9. doi: 10.1093/oxfordjournals.schbul.a033413. 00MEDLINE: 201292040. [DOI] [PubMed] [Google Scholar]
- Emsley 2004b {published data only} .Emsley R, Davidson M, Rabinowitz J. Risk for akathisia in patients with recent onset schizophrenia treated with risperidone and haloperidol and its association with suicidality. Schizophrenia Research. 2004;67(1):183. 00ISI: 0001887881004380. [Google Scholar]
- Emsley 2006b {published data only} .Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. American Journal of Psychiatry. 2006;163(4):743–5. doi: 10.1176/ajp.2006.163.4.743. 00CINAHL: 2009247684; EMBASE: 2006475079; MEDLINE: 165854550. [DOI] [PubMed] [Google Scholar]
- Emsley 2007 {published data only} .Emsley R, Rabinowitz J, Medori R. Remission in early psychosis: rates, predictors, and clinical and functional outcome correlates. Schizophrenia Research. 2007;89(1-3):129–39. doi: 10.1016/j.schres.2006.09.013. 00EMBASE: 2006601347; MEDLINE: 170951940. [DOI] [PubMed] [Google Scholar]
- Engelhardt 1994 {published data only} .Engelhardt DM. Early evaluation of psychopharmacological agents. Unknown Source. 1994:16–7. [Google Scholar]
- Faber 2005 {published data only} .Faber G, Smid HG, Van Den Bosch RJ. Effects of discontinuation of atypical antipsychotics on neurocognition in first onset psychosis. Schizophrenia Bulletin. 2005;31:482. doi: 10.1016/j.eurpsy.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Fabre 1995 {published data only} .Fabre L, Slotnick V, Jones V, Murray G, Malick J. ICI 204, 636 a novel atypical antipsychotic: early indication for safety and efficacy in man. Proceedings of the 17th Congress of Collegium Internationale Neuro-Psychophramacologicum; Kyoto, Japan. 1990 Sep 10-14.1990. [Google Scholar]; *; Fabre LF, Arvanitis L, Pultz J, Jones VM, Malick JB, Slotnick VB. ICI 204,636, a Novel, atypical antipsychotic - early indication of safety and efficacy in patients with chronic and subchronic schizophrenia. Clinical Therapeutics. 1995;17(3):366–78. doi: 10.1016/0149-2918(95)80102-2. 00MEDLEY: via DataStar 0036382 9606; MEDLINE: 75858410. [DOI] [PubMed] [Google Scholar]
- Fan 2006 {published data only} .Fan J, Yang F, Lu Z. Effect of risperidone and chlorpromazine on cognition in first-episode schizophrenia. Chinese Journal of Health Psychology. 2006;14(4):422–4. [Google Scholar]
- Fang 2003 {published data only} .Fang R, Sun F, Zhang Y. Effect of comprehensive intervention on quality of life and prognosis of inpatients with schizophrenia. Chinese Mental Health Journal. 2003;17(10):687–9. 00CAJ: MEDI03110. [Google Scholar]
- Ferenc 2000 {published data only} .Ferenc M, Dossenbach M, Jakovljevic M, Metcalfe S. Predictive value of early anti anxiety effect on the acute antipsychotic outcome: a comparison of fluphenazine and olanzapine. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]; *; Ferenc M, Dossenbach M, Jakovljevic M, Metcalfe S. Predictive value of early anti-anxiety effect on the acute-antipsychotic outcome: a comparison of fluphenazine and olanzapine. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Ferrari 1997 {published data only} .Ferrari MCL, Elkis H. A six month trial of risperidone versus conventional neuroleptics in young patients with early onset schizophrenia. Schizophrenia Research. 1997;Vol. 24(issue 1, 2):194. 00MEDLINE: 170552270. [Google Scholar]
- Filatre 1998 {published data only} .Fillatre M, Fahs HR, Reinhardt H, Degiovanni A, Marcelli D. First acute psychotic disorder of the young [Premier episode psychotique de l’adolescent et de l’adulte jeune] Annales de Psychiatrie. 1998;13(4):256–61. 00EMBASE: 19990433620. [Google Scholar]
- Fleischhacker 2005 {published data only} .Fleischhacker WW, for the EUFEST study group The European first episode schizophrenia trial (eufest). Proceedings of the Thematic Conference of the World Psychiatric Association on “Treatments in Psychiatry: An Update”; Florence, Italy. 2004 Nov 10-13.2004. [Google Scholar]; *; Fleischhacker WW, Keet IPM, Kahn RS. The European first episode schizophrenia trial (EUFEST): rationale and design of the trial. Schizophrenia Research. 2005;78(2-3):147–56. doi: 10.1016/j.schres.2005.06.004. 00MEDLINE: 160553080. [DOI] [PubMed] [Google Scholar]
- Fowler 2004 {published data only} .Fowler D. Improving social recovery in early affective and non-affective psychosis: a randomised controlled trial of social recovery oriented cognitive behavioural therapy (SRCBT) National Research Register. 2004;Vol. 4 [Google Scholar]; *; Fowler D, Jones P. Improving social recovery in early psychosis (ISREP) study. Unpublished Report. 2007.
- Gaebel 1993 {published data only} .Gaebel W, Frick U, Kopcke W, Linden M, Muller P, Muller Spahn F, Pietzcker A, Tegeler J. Early neuroleptic intervention in schizophrenia: are prodromal symptoms valid predictors of relapse? British Journal of Psychiatry Supplementum. 1993;163(Suppl 21):8–12. 00MEDLINE: 81058140. [PubMed] [Google Scholar]
- Gaebel 1995 {published data only} .Gaebel W. Predictors of response to different neuroleptic long-term treatment strategies in schizophrenia. Schizophrenia Research. 1995;15(1, 2):150. [Google Scholar]
- Gaebel 2001 {published data only} .Gaebel W, Janner M, Frommann N, Pietzcker A, Kopcke W, Linden M, Muller P, Muller-Spahn F, Tegeler J. First vs multiple episode schizophrenia: two-year outcome of intermittent and maintenance medication strategies. Schizophrenia Research. 2002;53(1-2):145–59. doi: 10.1016/s0920-9964(01)00182-7. 00MEDLINE: 117288460. [DOI] [PubMed] [Google Scholar]; *; Gaebel W, Janner M, Pietzcker A, Kopcke W, Muller P, Muller-Spahn,, Tegeler J. Long-term treatment in first and multiple episode schizophrenia. Two year outcome of intermittent and maintenance medication strategies. Schizophrenia Research. 2001;49(1, 2):228. doi: 10.1016/s0920-9964(01)00182-7. 00MEDLINE: 124091650. [DOI] [PubMed] [Google Scholar]
- Gaebel 2002a {published data only} .*; Gaebel W, Hans-Juergen M. Treatment strategies in first episode schizophrenia. Proceedings of the 12th World Congress of Psychiatry; Yokohama, Japan. 2002 Aug 24-29.2002. [Google Scholar]
- Gaebel 2004 {published data only} .*; Gaebel W, Moller HJ, Buchkremer G, Ohmann C, Riesbeck M, Wolwer WVWM, Bottlender R, Klingberg S. Pharmacological long-term treatment strategies in first episode schizophrenia--study design and preliminary results of an ongoing RCT within the German research network on schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2004;254(2):129–40. doi: 10.1007/s00406-004-0509-y. 00EMBASE: 2004421362; MEDLINE: 151463420. [DOI] [PubMed] [Google Scholar]
- Gaebel 2005 {published data only} .*; Gaebel W, Riesbeck M, von Wilmsdorff M, Zielasek J. Pharmacological long - term treatment strategies in first episode schizophrenia: preliminary results of an ongoing randomised clinical trial within the German research network on schizophrenia. Schizophrenia Bulletin. 2005;31:483. doi: 10.1007/s00406-004-0509-y. [DOI] [PubMed] [Google Scholar]
- Gaebel 2006 {published data only} .*; Gaebel W, Moeller HJ. Measures to prevent relapse in long-term treatment: results from the German research network on schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Gafoor 2005a {published data only} .Gafoor R, Kerwin R, Power P, Craig T, McGuire P. Equiefficacy of quetiapine versus risperidone in the treatment of depression in patients in their first episode of a schizophreniform psychotic illness: interim results of an 8 week randomised single blind trial. Schizophrenia Bulletin. 2005;31:483–84. [Google Scholar]; *; Gafoor R, Power P, Craig T, Kerwin R, McGuire P. A comparative study of quetiapine and risperidone in patients with first-episode psychosis. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):S509. [Google Scholar]
- Gafoor 2006 {published data only} .*; Gafoor RA, Craig T, Power P, Kerwin R, McGuire P. week randomised blinded trial of quetiapine versus risperidone: relative antipsychotic efficacies and side effects. Schizophrenia Research. 2006;86(Suppl 1):S38. [Google Scholar]
- Gallo 2006 {published data only} .*; Gallo C. A 6-week, randomised, double-blind study of adjunctive trimethoprim-sulfamethoxazole in 180 first-episode schizophrenic patients seropositive for toxoplasma gondii. Stanley Foundation Research Programs. 2006 [Google Scholar]
- Gan 1999 {published data only} .*; Gan JL. A control study of risperidone and clozapine in the treatment of first episode schizophrenia. Medical Journal of Chinese Civil Administration. 1999;11(1):4–7. 61. 00CAJ: MEDI99040. [Google Scholar]
- Gan 2000 {published data only} .*; Gan J, Hu X, Zhang W. Extrapyramidal side effects of risperidone. Journal of Clinical Psychological Medicine. 2000;10(5):280–2. 00CAJ: MEDI0012S10. [Google Scholar]
- Garcia 2006 {published data only} .*; Garcia MC, Vidal M, Ramos R. Sexual side effects of antipsychotics and treatment adherence. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S378. [Google Scholar]
- Garety 2000a {published data only} .Garety P. Pilot RCT of cognitive behavioural therapy for early psychosis. National Research Register. 2000. ; Garety P. Pilot RCT of cognitive behavioural therapy for early psychosis. National Research Register. 2001;Vol. 1 [Google Scholar]
- Garety 2006 {published data only} .Garety P. Randomised controlled trial of early psychosis in Lambeth. National Research Register. 2000. ; Garety P. Randomised controlled trial of early psychosis in Lambeth. National Research Register. 2001;Vol. 1 [Google Scholar]; Garety PA, Craig TKJ, Dunn G, Fornells-Ambrojo M, Colbert S, Rahaman N, Reed J, Power P. Specialised care for early psychosis: symptoms, social functioning and patient satisfaction: randomised controlled trial. British Journal of Psychiatry. 2006;188:37–45. doi: 10.1192/bjp.bp.104.007286. 00MEDLINE: 163880680. [DOI] [PubMed] [Google Scholar]
- Garver 2005 {published data only} .Garver DL, Holcomb JA, Christensen JD. Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biological Psychiatry. 2005;58(1):62–6. doi: 10.1016/j.biopsych.2005.02.008. 00EMBASE: 20052950220. [DOI] [PubMed] [Google Scholar]
- Gary 1990 {published data only} .Gary M-A. Self-assessment of neuroleptic-induced early-onset extrapyramidal symptoms among persons with chronic mental illness. Texas Tech University; Texas, USA: 1990. [Google Scholar]
- Gattaz 1989 {published data only} .Gattaz WF, Rost W, Hubner CK, Bauer K. Acute and subchronic effects of low-dose bromocriptine in haloperidol-treated schizophrenics. Biological Psychiatry. 1989;25(3):247–55. doi: 10.1016/0006-3223(89)90172-8. 00MEDLINE: 26439960. [DOI] [PubMed] [Google Scholar]
- Genduso 1996 {published data only} .Hamilton SH, Genduso LA, Haley JC, Revicki DA. Medical resource use and socioeconomics in the treatment of schizophrenia: olanzapine compared with haloperidol. Proceedings of the 10th European College of Neuropsychopharmacology Congress; Vienna, Austria. 1997 Sep 13-17; 1997. 00CENTRAL: CN–005179380. [Google Scholar]; Martin C. Impact of olanzapine on quality of life in schizophrenia. Proceedings of the 8th Congress of the Association of European Psychiatrists; London, UK. 1996 Jul 7-12.1996. [Google Scholar]; Martin C, Genduso L, Revicki D, Hamilton S, Tran P, Beasley C. Impact of olanzapine on patient quality of life in schizophrenia. Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996 Aug 23-28.1996. [Google Scholar]; Revicki D, Genduso LA, Hamilton SL, Martin C, Reblando J, Tran PV. Quality of life outcomes for olanzapine and haloperidol treatment for schizophrenia and related psychotic disorders. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, USA. 1996 May 4-9.1996. [Google Scholar]; Satterlee WG, Beasley CM, Tran PV, Tamura RN, Krueger JA, Tollefson GD. Olanzapine versus haloperidol - results of a large multi-center international trial. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, USA. 1996 May 4-9.1996. [Google Scholar]; Satterlee WG, Beasley CM, Tran PV, Tamura RN, Krueger JA, Tollefson GD. Olanzapine vs haloperidol in a large international trial. Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996 Aug 23-28.1996. [Google Scholar]; Tollefson GD, Beasley CM, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. American Journal of Psychiatry. 1997;154(4):457–65. doi: 10.1176/ajp.154.4.457. 00MEDLINE: 90903310. [DOI] [PubMed] [Google Scholar]; Tollefson GD, Lu Y. Comorbid mood disturbance in schizophrenia. 9th European College of Neuropsychopharmacology Congress; Amsterdam, Netherlands. 1996 Sep 21-25; 1996. 00MEDLINE: 90903310. [Google Scholar]; Tollefson GD, Lu Y. Comorbid mood disturbance in schizophrenia. Proceedings of the 20th Collegium Internationale Neuro-Psychopharmacologicum Congress; Melbourne, Australia. 1996 Jun 23-27.1996. [Google Scholar]; Tollefson GD, Lu Y, Sanger TM, Beasley CM, Tran PV. Olanzapine in the treatment of schizoaffective disorder. Schizophrenia Research. 1997;24(1, 2):192. 00MEDLINE: 90903310. [Google Scholar]; Tollefson GD, Sanger TM, Leiberman JA. Olanzapine versus haloperidol in the treatment of first episode psychosis. Schizophrenia Research. 1997;24(1, 2):193. doi: 10.1176/ajp.156.1.79. 00MEDLINE: 90903310. [DOI] [PubMed] [Google Scholar]; Tran P, Beasley C, Street J, Tamura R, Dellva MA, Graffeo K, Krueger J, Tollefson G. Olanzapine versus haloperidol: acute results of the multi-center international trial. Proceedings of the 20th Collegium Internationale Neuro-Psychopharmacologicum Congress; Melbourne, Australia. 1996 Jun 23-27.1996. [Google Scholar]; Tran P, Sanger TM, Satterlee W, Beasley CJr, Tamura RN, Tollefson GD. Olanzapine vs haloperidol - results of a large multi-centre international trial. Proceedings of the 4th International Conference on Schizophrenia - 1996: Breaking down the Barriers; Vancouver, Canada. 1996 Oct 6-9.1996. [Google Scholar]; Tunis SL. The impact of schizophrenic patient functionality on service utilization and cost. Based on a presentation by Sandra L. Tunis, PhD. American Journal of Managed Care. 1999;5(10 Suppl):S583–90. 00CENTRAL: CN–003807750. [PubMed] [Google Scholar]; Williamson D, Beasley C, Tran P, Tamura N, Sanger T, Tollefson G. Olanzapine versus haloperidol: results of the multi-center international trial. Proceedings of the 8th Congress of the Association of European Psychiatrists; London, UK. 1996 Jul 7-12.1996. [Google Scholar]
- Gharabawi 2006d {published data only} .*; Gharabawi GM, Bossie CA, Zhu Y. New-onset tardive dyskinesia in patients with first-episode psychosis receiving risperidone or haloperidol...Schooler N, Rabinowitz J, Davidson M, Emsley R, Harvey PD, Kopala L, McGorry PD, Van Hove I, Eerdekens M, Swyzen W, De Smedt G, Early Psychosis Global Working Group: Risperidone and haloperidol in first-episode psychosis: a long-termrandomised trial. Am J Psychiatry. 2005;162:947–953. [Google Scholar]; American Journal of Psychiatry. 2006;163(5):938–9. doi: 10.1176/ajp.2006.163.5.938a. 00CINAHL: 2009175503; MEDLINE: 166483430. [DOI] [PubMed] [Google Scholar]
- Gillin 1978 {published data only} .*; Gillin JC, Van Kammen DP, Bunney WE., Jr Pimozide attentuates d-amphetamine-induced sleep changes in man. Life Sciences. 1978;22(20):1805–10. doi: 10.1016/0024-3205(78)90596-9. 00MEDLINE: 2092740. [DOI] [PubMed] [Google Scholar]
- Glenthoj 2000 {published data only} .Fagerlund B, Mackeprang T, Gade A, Glenthoj BY. Effects of risperidone and zuclopenthixol on cognitive deficits in drug-naive first episode schizophrenic patients. Schizophrenia Research. 2003;60(1):133–4. 00MEDLINE: 991073190. [Google Scholar]; Glenthoj BY, Mackeprang T, Fagerlund B, Hemmingsen R. Effects of antipsychotics on information-processing and extrastriatal dopamine d2/d3 receptors in first-episode drug-naive schizophrenic patients. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]; Glenthoj BY, Mackeprang T, Fagerlund B, Hemmingsen RP. Effects of antipsychotic treatment on prepulse inhibition of the startle response (ppi) and cognition in first episode drug-naive schizophrenic patients. Schizophrenia Research. 2001;49(1, 2):133. 00MEDLINE: 991073190. [Google Scholar]
- Glenthoj 2005 {published data only} .*; Glenthoj B, Glenthoj A, Jagersma E, Pagsberg AK, Hemmingsen RP, Svarer C, Videbaek C, Baare W. Effects of typical and atypical antipsychotic medication on caudate nucleus volume in first-episode schizophrenic patients: relation to dopamine d2/3 receptor occupancy psychopathology and extrapyramidal side-effects. Proceedings in the XIII World Congress of Psychiatry; Cairo, Egypt. 2005 10-15th Sept.2005. [Google Scholar]
- Godemann 1999 {published data only} .*; Godemann F, Linden M, Seidel K. Interval long-term neuroleptic medication an alternative for patients who refuse continuous medication?. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. p. 151. [Google Scholar]
- Good 2004 {published data only} .*; Good KP, Rabinowitz J, Whitehorn D, Harvey PD, DeSmedt G, Kopala LC. The relationship of neuropsychological test performance with the PANSS in antipsychotic naive, first-episode psychosis patients. Schizophrenia Research. 2004;68(1):11–9. doi: 10.1016/j.schres.2003.07.001. 00MEDLINE: 150373350. [DOI] [PubMed] [Google Scholar]
- Grasso 1974 {published data only} .*; Grasso AM, Nicoletti F. Psycho-neurophysiological observations in schizophrenics: analysis of gastrocnemius motor and H and T responses after “click”. Acta Neurologica. 1974;29(3):299–306. 00MEDLINE: 4418354; PsycINFO : 53–075600. [PubMed] [Google Scholar]
- Grawe 1998 {published data only} .*; Grawe RW, Widen JH. Result of two years optimal out-patient treatment of first episode schizophrenia: a controlled study. Nordisk Psykiatrisk Tidsskrift. 1998;52(41):76. 00EMBASE: 19981242070. [Google Scholar]
- Grawe 2006 {published data only} .*; Grawe RW, Falloon IRH, Widen JH, Skogvoll E. Two years of continued early treatment for recent-onset schizophrenia: a randomised controlled study. Acta Psychiatrica Scandinavica. 2006;114(5):328–36. doi: 10.1111/j.1600-0447.2006.00799.x. 00EMBASE: 2006491992; MEDLINE: 170227920. [DOI] [PubMed] [Google Scholar]
- Green 2001b {published data only} .Green A, Tohen M, Strakowski SM, Lieberman JA, Glick D, Clarke SW, HGDH Study Group Comorbid substance use disorder and first episode schizophrenia: acute effects of olanzapine versus haloperidol. Schizophrenia Research. 2001;49(1, 2):230. 00ISI: 0001887881003570. [Google Scholar]; Green AI, Lieberman JA, Hamer RM, Glick ID, Gur RE, Kahn RS, McEvoy JP, Perkins DO, Rothschild AJ, Sharma T, Tohen MF, Woolson S, Zipursky RB. Olanzapine and haloperidol in first episode psychosis: two-year data. Schizophrenia Research. 2006;86(1-3):234–43. doi: 10.1016/j.schres.2006.06.021. 00EMBASE: 2006398134; MEDLINE: 168873340. [DOI] [PubMed] [Google Scholar]; *; Green AI, Tohen MF, Hamer RM, Strakowski SM, Lieberman JA, Glick ICWS, HGDH Research Group First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophrenia Research. 2004;66(2-3):125–35. doi: 10.1016/j.schres.2003.08.001. 00MEDLINE: 150612440. [DOI] [PubMed] [Google Scholar]
- Gumley 2001b {published data only} .*; Gumley A. A randomised controlled trial of two methods of early signs monitoring to detect relapse in schizophrenia. National Research Register. 2001;Vol. 3 [Google Scholar]; Gumley A. A randomised controlled trial of two methods of early signs monitoring to detect relapse in schizophrenia. National Research Register. 2002;Vol. 1 [Google Scholar]
- Gumley 2003a {published data only} .Gumley A, O’Grady M, McNay L, Reilly J, Power K, Norrie J. Early intervention for relapse in schizophrenia: results of a 12-month randomised controlled trial of cognitive behavioural therapy. Psychological Medicine. 2003;33(3):419–31. doi: 10.1017/s0033291703007323. 00EMBASE: 20031605760. [DOI] [PubMed] [Google Scholar]
- Gumley 2003b {published data only} .Gumley A. A pilot study of cognitive therapy during the acute phase of psychosis: impact on adjustment to and recovery from psychosis. National Research Register. 2003;Vol. 3 [Google Scholar]
- Gumley 2006 {published data only} .Gumley A, Karatzias A, Power K, Reilly J, McNay L, O’Grady M. Early intervention for relapse in schizophrenia: impact of cognitive behavioural therapy on negative beliefs about psychosis and self-esteem. British Journal of Clinical Psychology. 2006;45(Pt 2):247–60. doi: 10.1348/014466505X49925. 00CINAHL: 2009206992; MEDLINE: 167199820. [DOI] [PubMed] [Google Scholar]
- Guo 1995 {published data only} .Guo S, Lu L, Cheng W. Effect of clozapine and risperidone on interleukin-2 on schizophrenia. Shanghai Archives of Psychiatry. 2003;15(1):35–8. 00CAJ: MEDI03110. [Google Scholar]
- Guo 2001a {published data only} .Guo Z, Shi J, Dai Z. A controlled trial comparing the efficacy of risperidone in the treatment of the recurrent schizophrenia. Chinese Journal of Behavioral Medical Science. 2001;10(6):575–7. 00CAJ: MEDI02010. [Google Scholar]
- Guo 2004 {published data only} .Guo SQ, Chen W, Lü LX, Zhang SL, Cheng J, Li Q. Effect of clozapine and risperide on serum cytokine in first episode paranoid schizophrenia patient. Chinese Journal of Immunology. 2004;2:117–9. 120. 00CAJ: MEDI0403S10. [Google Scholar]
- Haddock 1999 {published data only} .Haddock G, Tarrier N, Morrison AP, Hopkins R, Drake R, Lewis S. A pilot study evaluating the effectiveness of individual inpatient cognitive-behavioural therapy in early psychosis. Social Psychiatry and Psychiatric Epidemiology. 1999;34(5):254–8. doi: 10.1007/s001270050141. 00MEDLINE: 103961670. [DOI] [PubMed] [Google Scholar]
- Haddock 2000a {published data only} .Haddock G. Dual diagnosis project: evaluation of family support and cognitive behaviour therapy for recent onset schizophrenia sufferers with substance misuse problems. National Research Register. 2000. ; Haddock G. Dual diagnosis project: evaluation of family support and cognitive behaviour therapy for recent onset schizophrenia sufferers with substance misuse problems. National Research Register. 2001;Vol. 1 [Google Scholar]
- Haddock 2000b {published data only} .Haddock G. Evaluation of a family support and cognitive-behavioural treatment service for recent onset schizophrenia sufferers with substance misuse problems. National Research Register. 2000.
- Haddock 2006 {published data only} .Haddock G, Lewis S, Bentall R, Dunn G, Drake R, Tarrier N. Influence of age on outcome of psychological treatments in first-episode psychosis. British Journal of Psychiatry. 2006;188:250–4. doi: 10.1192/bjp.188.3.250. 00MEDLINE: 165079670. [DOI] [PubMed] [Google Scholar]
- Haldun 2002 {published data only} .Haldun S, Mehmet S, Perihan G, Ilkten C, Besti U. Optimal treatment of schizophrenia. Proceedings of the 12th World Congress of Psychiatry; Yokohama, Japan. 2002 Aug 24-29.2002. [Google Scholar]
- Hawkins 2004a {published data only} .Hawkins KA, Addington J, Keefe RS, Christensen B, Woods SW, Miller TJ, Trzaskoma QN, Breier A, Zipursky RB, Perkins DO. Effect of olanzapine versus placebo on the neuropsychological status of prodromal subjects. Schizophrenia Research. 2004;67(1):205. doi: 10.1016/j.schres.2003.08.007. 00ISI: 0001887881004920. [DOI] [PubMed] [Google Scholar]
- Hawkins 2004b {published data only} .Hawkins KA, Addington J, Keefe R, Christensen B, Woods S, Zipursky R, Perkins D, Mcglashan T. Neuropsychological functioning in the first episode prodrome and early psychosis. Schizophrenia Research. 2004;70(1):101. doi: 10.1016/j.schres.2003.08.007. 00ISI: 0002245511002870. [DOI] [PubMed] [Google Scholar]
- Herrmann 1991 {published data only} .Herrmann WM, Scharer E, Delini Stula A. Predictive value of pharmaco electroencephalography in early human pharmacological evaluations of psychoactive drugs -first example - Savoxepine. Pharmacopsychiatry. 1991;24(6):196–205. doi: 10.1055/s-2007-1014469. 00MEDLINE: 92253604; PSYCINFO: 79–323490. [DOI] [PubMed] [Google Scholar]; Herrmann WM, Scharer E, Wendt G, Delini Stula A. Pharmaco EEG profile of maroxepine - third example to discuss the predictive value of pharmaco electroencephalography in early human pharmacological evaluations of psychoactive drugs. Pharmacopsychiatry. 1991;24(6):214–24. doi: 10.1055/s-2007-1014471. 00MEDLINE: 92253606; PSYCINFO: 79–323500. [DOI] [PubMed] [Google Scholar]
- Herz 1982 {published data only} .Herz MI, Szymanski HV, Simon JC. Intermittent medication for stable schizophrenic outpatients: an alternative to maintenance medication. American Journal of Psychiatry. 1982;139:918–22. doi: 10.1176/ajp.139.7.918. 00MEDLINE: 822281600. [DOI] [PubMed] [Google Scholar]
- Herz 1989a {published data only} .Herz MI, Glazer W, Mirza M, Mostert M, Hafez H. Treating prodromal episodes to prevent relapse in schizophrenia. British Journal of Psychiatry Supplementum. 1989;155(Suppl 5):123–7. 00MEDLINE: 25749840. [PubMed] [Google Scholar]; Herz MI, Glazer WM, Mostert MA, Sheard MA, Szymanski HV, Hafez H, Mirza M, Vana J. Intermittent vs maintenance medication in schizophrenia. Two-year results. Archives of General Psychiatry. 1991;48(4):333–9. doi: 10.1001/archpsyc.1991.01810280049007. 00MEDLINE: 16725880. [DOI] [PubMed] [Google Scholar]
- Herz 1998 {published data only} .Herz MI, Lamberti JS. Prodromal symptoms and early intervention in schizophrenia. Neurology Psychiatry and Brain Research. 1998;6(1):37–44. 00EMBASE: 19983338060. [Google Scholar]; Herz MI, Lamberti JS, Mintz J, Scott R, O’Dell SP, McCartan L, Nix G. A program for relapse prevention in schizophrenia: a controlled study. Archives of General Psychiatry. 2000;57(3):277–83. doi: 10.1001/archpsyc.57.3.277. 00MEDLINE: 201750780. [DOI] [PubMed] [Google Scholar]
- Heydebrand 2004 {published data only} .Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, Csernansky JG. Correlates of cognitive deficits in first episode schizophrenia. Schizophrenia Research. 2004;68(1):1–9. doi: 10.1016/S0920-9964(03)00097-5. 00EMBASE: 20041306720. [DOI] [PubMed] [Google Scholar]
- Himei 2005 {published data only} .Himei A, Okamura T. Evaluation of the clinical efficacy of risperidone for untreated and treated cases of schizophrenia from various aspects. Psychiatry and Clinical Neurosciences. 2005;59(5):556–62. doi: 10.1111/j.1440-1819.2005.01414.x. 00MEDLINE: 161942580. [DOI] [PubMed] [Google Scholar]
- Hirsch 1986 {published data only} .Hirsch SR, Jolley AG, Manchanda R, McRink A. Intermittent medication as an alternative to maintenance medication in the treatment of schizophrenia: a preliminary report [Fruehzeitige medikamentoese intervention als alternative zur depot–dauer–medikation in der schizophreniebehandlung ein vorlaeufiger bericht] In: Boeker W, Brenner HD, editors. Bewaltigung der Schizophrenie. Verlag Hans Huber; Bern, Switzerland: 1986. pp. 62–71. 00PSYNDEX: 00205910. [Google Scholar]
- Hodgekins 2006a {published data only} .Hodgekins J, Fowler D, Bradford S, Macmillan I, Jones P. Psychological and neuropsychological predictors of time use and social recovery in early psychosis. Schizophrenia Research. 2006;86(Suppl 1):S28. [Google Scholar]
- Hoffman 2006 {published data only} .Hoffman RE, Woods S, Preda A, Tohen M, Breier A, Glist J, Addington J, Perkins DO, Hawkins K, McGlashan TH. Excessive top-down perceptual processing and reduced real-world investment exhibited by prodromal patients predict subsequent conversion to schizophrenia. Schizophrenia Research. 2006;86(Suppl 1):S46. [Google Scholar]
- Hogarty 1991 {published data only} .Hogarty GE, Anderson CM, Reiss DJ, Kornblith SJ, Greenwald DP, Ulrich RF, Carter M. Environmental-Personal Indicators in the Course of Schizophrenia (EPICS) Research Group. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. II. Two-year effects of a controlled study on relapse and adjustment. Archives of General Psychiatry. 1991;Vol. 48(issue 4):340–7. doi: 10.1001/archpsyc.1991.01810280056008. 00MEDLINE: 911819460. [DOI] [PubMed] [Google Scholar]
- Honer 2005b {published data only} .Honer WG, Kopala LC, Rabinowit J. Extrapyramidal symptoms and signs in first-episode, antipsychotic exposed and non-exposed patients with schizophrenia or related psychotic illness. Journal of Psychopharmacology. 2005;19(3):277–85. doi: 10.1177/0269881105051539. 00PSYCINFO: 2005–05193–0100. [DOI] [PubMed] [Google Scholar]
- Hornung 1995 {published data only} .Buchkremer G, Klingberg S, Holle R, Monking HS, Hornung WP. Psychoeducational psychotherapy for schizophrenic patients and their key relatives or care-givers: results of a 2-year follow-up. Acta Psychiatrica Scandinavica. 1997;96(6):483–91. doi: 10.1111/j.1600-0447.1997.tb09951.x. 00MEDLINE: 94213460. [DOI] [PubMed] [Google Scholar]; Feldmann R, Buchkremer G, Hornung WP. Prognostic and therapeutic relevance of cognitive characteristics for the long-term course of schizophrenic illness following psychoeducational psychotherapeutic treatment [Prognostische und therapeutische relevanz kognitiver ressourcen fur den langzeitverlauf schizophrener erkrankung nach psychoedukativ–psychotherapeutischer behandlung] Fortschritte der Neurologie-Psychiatrie. 2000;68(2):54–60. doi: 10.1055/s-2000-11649. 00MEDLINE: 107196580. [DOI] [PubMed] [Google Scholar]; Hornung WP, Buchkremer G, Redbrake M, Klingberg S. Patient modified treatment. What are the effects of neuroleptic drugs on people with schizophrenia? [Patientmodifizierte medikation: Wie gehen schizophrene patienten mit ihren neuroleptika um?] Nervenarzt. 1993;64:434–9. 00MEDLINE: 104331310. [PubMed] [Google Scholar]; Hornung WP, Feldmann R, Klingberg S, Buchkremer G, Reker T. Long term effects of a psychoeducational psychotherapeutic intervention for schizophrenic outpatients and their key persons: results of a five year follow-up [Langzeitwirkungen einer psychoedukativen– psychotherapeutischen intervention bei schizophrenen ambulanten patienten und ihren bezugspersonen – ergebnisse einer fuenf–jahres–katamnese] European Archives of Psychiatry and Clinical Neuroscience. 1999;249(3):162–7. doi: 10.1007/s004060050082. 00MEDLINE: 104331310. [DOI] [PubMed] [Google Scholar]; Hornung WP, Feldmann R, Schonauer K, Schafer A, Monking HS, Klingberg S, Buchkremer G. Psychoeducational psychotherapeutic treatment of schizophrenic patients and their caregivers. II. Supplementary findings at a 2-year follow-up [Psychoedukativ–psychotherapeutische behandlung von schizophrenen patienten und ihren bezugspersonen. II. Erganzende befunde der 2–jahres– katamnese] Nervenarzt. 1999;70(5):444–9. doi: 10.1007/s001150050460. 00MEDLINE: 104078400. [DOI] [PubMed] [Google Scholar]; Hornung WP, Holle R, Schulze Monking H, Klingberg S, Buchkremer G. Psychoeducational-psychotherapeutic treatment of schizophrenic patients and their caregivers. Results of a 1-year catamnestic study [Psychoedukativ– psychotherapeutische behandlung von schizophrenen patienten und ihren bezugspersonen. Ergebnisse einer 1–jahres–katamnese] Nervenarzt. 1995;66(11):828–34. 00MEDLINE: 96091921; PSYNDEX: 00977640. [PubMed] [Google Scholar]; Hornung WP, Kieserg A, Feldmann R, Buchkremer G. Psychoeducational training for schizophrenic patients: background, procedure and empirical findings. Patient Education and Counseling. 1996;29(3):257–68. doi: 10.1016/s0738-3991(96)00918-4. 00CINAHL: 19970186140. [DOI] [PubMed] [Google Scholar]; Hornung WP, Klingberg S, Feldmann R, Schonauer K, Schulze Monking H. Collaboration with drug treatment by schizophrenic patients with and without psychoeducational training: Results of a 1-year follow-up. Acta Psychiatrica Scandinavica. 1998;97(3):213–9. doi: 10.1111/j.1600-0447.1998.tb09990.x. 00EMBASE: 1998058470; MEDLINE: 982023110. [DOI] [PubMed] [Google Scholar]; Hornung WP, Schonauer K, Feldmann R, Monking HS. Medication related attitudes of chronic schizophrenic patients. A follow up study after psycho educational intervention [Medikationsbezogene einstellungen chronisch schizophrener patienten. Eine follow up untersuchung 24 monate nach psychoedukativer intervention] Psychiatrische Praxis. 1998;25(1):25–8. 00MEDLINE: 981918980. [PubMed] [Google Scholar]; Klingberg S, Buchkremer G, Holle R, Schulze-Monking H, Hornung WP. Differential therapy effects of psychoeducational psychotherapy for schizophrenic patients: results of a 2-year follow-up. European Archives of Psychiatry and Clinical Neuroscience. 1999;249(2):66–72. doi: 10.1007/s004060050068. 00MEDLINE: 992958260. [DOI] [PubMed] [Google Scholar]
- Hu 2003b {published data only} .Hu JM, Li Yi, Li Tao, Wang HM, Liu XH, Huo KJ. The effects of antipsychotics on serum prolactin in the first-episode schizophrenia patients. West China Journal of Pharmaceutical Sciences. 2003;18(6):467–9. 00CAJ: MEDI04020. [Google Scholar]
- Huang 2004c {published data only} .Huang J-L, Fu Z-C, Sun Q-Q. Trilafon in combination with nimodipine in the treatment of 30 senile patients with first-episode schizophrenia. Herald of Medicine. 2004;23(3):152–3. 00CAJ: MEDI05110. [Google Scholar]
- Huang 2006d {published data only} .Huang Y-P, Zhu J-Z, Jiang X-Y. A comparative study of olanzapine and risperidone in the treatment of adolescentswith first-episode schizophrenia. Shandong Jingshen Yixue. 2006;19(2):105–7. [Google Scholar]
- Ishigooka 2001 {published data only} .Inada T, Beasley CM, JR, Tanaka Y, Walker DJ. Extrapyramidal symptom profiles assessed with the drug-induced extrapyramidal symptom scale: comparison with western scales in the clinical double-blind studies of schizophrenic patients treated with either olanzapine or haloperidol. International Clinical Psychopharmacology. 2003;18(1):39–48. doi: 10.1097/00004850-200301000-00007. [DOI] [PubMed] [Google Scholar]; Inada T, Miura S. Favorable EPS profiles of olanzapine measured by the DIEPSS: predictive validity of this scale to differentiate the EPS profiles of atypical antipsychotic drugs from conventional antipsychotic drugs in the clinical trial. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S147. [Google Scholar]; Inada T, Yagi G, Miura S. Extrapyramidal symptom profiles in Japanese patients with schizophrenia treated with olanzapine or haloperidol. Schizophrenia Research. 2002;57(2-3):227–38. doi: 10.1016/s0920-9964(01)00314-0. 00EMBASE: 20023865020. [DOI] [PubMed] [Google Scholar]; Ishigooka J, Hirotsu C, Kurihara M, Inada T, Miura S, Olanzapine 301E study group Olanzapine versus haloperidol in the treatment of patients with chronic schizophrenia: results of the Japan multi-center double-blind olanzapine trial. Psychiatry and Clinical Neurosciences. 2001;55(4):403–14. doi: 10.1046/j.1440-1819.2001.00882.x. 00PSYCINFO: 2004–16292–0050. [DOI] [PubMed] [Google Scholar]
- Ivarson 1998 {published data only} .Ivarson B, Karrang L, Malm U. Implementing the integrated care model in a Swedish setting. Nordisk Psykiatrisk Tidsskrift. 1998;52(41):164. 00EMBASE: 19981243750. [Google Scholar]
- Jackson 2001a {published data only} .Jackson H, McGorry P, Henry L, Edwards J, Hulbert C, Harrigan S, Dudgeon P, Francey S, Maude D, Cocks J, Power P. Cognitively oriented psychotherapy for early psychosis (COPE): a 1-year follow-up. British Journal of Clinical Psychology. 2001;40(1):57–70. doi: 10.1348/014466501163481. [DOI] [PubMed] [Google Scholar]
- Jackson 2001b {published data only} .Jackson C. Promoting personal recovery from psychosis: a randomised controlled trial in first episode schizophrenia. National Research Register. 2001;Vol. 1 [Google Scholar]; Jackson C. Promoting personal recovery from psychosis: a randomised control trial in first episode schizophrenia. National Research Register. 2001 [Google Scholar]
- Jackson 2004a {published data only} .Jackson C, Smith J, Birchwood M, Trower P, Reid I, Townend M, Hall M, Newton L, Barton K, Jones J. Preventing the traumatic sequelae of first episode psychosis: a randomised controlled trial. Schizophrenia Research. 2004;70(1):45–6. doi: 10.1016/j.brat.2009.02.009. 00ISI: 0002245511001190. [DOI] [PubMed] [Google Scholar]
- Jackson 2004b {published data only} .Jackson H, Mcgorry P, Edwards J, Hulbert C, Henry L, Harrigan S, Dudgeon P, Francey S, Cocks J, Killackey E. A randomised controlled trial of cognitively-orientated psychotherapy for early psychosis (cope) with 5-year follow-up relapse data. Schizophrenia Research. 2004;70(1):61. 00ISI: 0002245511001630. [Google Scholar]
- Jackson 2005 {published data only} .Jackson HJ, Killackey E, Bendall S, Allott K, Dudgeon P, Harrigan S, Gleeson J, McGorry PD. A randomised controlled trial of CBT for early psychosis with 1 year follow-up. Australian and New Zealand Journal of Psychiatry. 2005;39(Suppl 2):A42. [Google Scholar]
- Jackson 2006 {published data only} .Jackson C, Trower P, Reid I, Smith J, Birchwood M, Hall M, Townend M, Barton K, Jones J, Ross K, Russell R, Newton E. Improving psychological adjustment following a first episode of psychosis: a randomised controlled trial of cognitive therapy to reduce post-psychotic trauma symptoms. Schizophrenia Research. 2006;86(Suppl 1):S39. doi: 10.1016/j.brat.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Janicak 1998 {published data only} .Janicak PG, Sharma RP, Pandey G, Davis JM. Verapamil for the treatment of acute mania: a double-blind, placebo-controlled trial. American Journal of Psychiatry. 1998;155(7):972–3. doi: 10.1176/ajp.155.7.972. 00MEDLINE: 983240530. [DOI] [PubMed] [Google Scholar]
- Jarboe 2001 {published data only} .Jarboe KS, Lewine RR. Haloperidol versus olanzapine induced weight gain and clinical relevance, a double-blind and open label comparison. Schizophrenia Research. 2001;49(1, 2):232. 00ISI: 0002245511002170. [Google Scholar]
- Jasovic 1995 {published data only} .Jasovic-Gasic M, Nikolic-Balkoski G, Totic S, Acimovic B, Kuzmanovic V. Moclobemide in the treatment of depressive syndrome in schizophrenia. Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30-Oct 4.1995. [Google Scholar]
- Jasovic 1998 {published data only} .Jasovic-Gasic M, Crnobaric C, Milovanovic S, Miljevic C. Mianserin versus moclobemide in the treatment of depressive syndrome in schizophrenia. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16.1998. [Google Scholar]
- Jenner 2004b {published data only} .Jenner J, Van De Willige G. Results of integrated treatment (hit) in early psychoses: a pilot study. Schizophrenia Research. 2004;70(1):16. 00ISI: 0002245511000380. [Google Scholar]
- Ji 2006 {published data only} .Ji H-Y, Zhang Y-C, Sun X-Z. Effect of health education intervention on the recovery of first onset schizophrenia. Chinese Journal of Rehabilitation Theory and Practice. 2006;12(9):823–4. [Google Scholar]
- Jiang 2006 {published data only} .Jiang Y-B, Sui A-M, Lin Y-M. The short-term effect of cognitive behavior therapy on first episode schizophrenia. Chinese Journal of Rehabilitation. 2006;21(1):63–4. 00CAJ: MEDI0603S10. [Google Scholar]
- Jiang Xinyan 2004 {published data only} .Jiang X, Zhu J, Zhang F, Wu X. Clinical observation of risperidone on first episode late-life schizophrenic patients. Shandong Archives of Psychiatry. 2004;17(4):200–1. [Google Scholar]
- Johnson 2004b {published data only} .Johnson S. Establishing a framework for long-term research in early psychosis: use for research of routine outcome measures. National Research Register. 2004;Vol. 2 [Google Scholar]
- Johnston-Cronk 1993 {published data only} .Johnston-Cronk K, Marder SR, Wirshing WC, Mintz J, McKenzie J, Van Putten T, Lebell M, Liberman RP. Prediction of schizophrenic relapse using prodromal symptoms. Schizophrenia Research. 1993;Vol. 9:259. 00MEDLINE: 124091650. [Google Scholar]
- Johnstone 1998b {published data only} .Johnstone BM, Obenchain RL, Edgell ET, Croghan TW, Tunis SL, Kniesner TJ. Intent to treat analysis of repeated measures data from a randomised clinical trial comparing the cost and effectiveness of treatment for schizophrenia with olanzapine or haloperidol. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31-Nov 4.1998. [Google Scholar]; Tunis SL. The impact of schizophrenic patient functionality on service utilization and cost. Based on a presentation by Sandra L. Tunis, PhD. American Journal of Managed Care. 1999;5(10 Suppl):S583–90. 00CENTRAL: CN–00380775; MEDLINE: 994166840. [PubMed] [Google Scholar]
- Jolley 1989 {published data only} .Jolley AG, Hirsch SR. Therapie der Schizophrenie. Kohlhammer; UK: 1990. Intermittent and low-dosage neuroleptic treatment: preventive strategies in schizophrenia [Intermittierende und niedrigdosierte neuroleptika– therapie: Prophylaktische strategien bei der schizophrenie] pp. 53–63. 00PSYNDEX: 00603660. [Google Scholar]; Jolley AG, Hirsch SR, McRink A, Manchanda R. Trial of brief intermittent neuroleptic prophylaxis for selected schizophrenic outpatients: clinical outcome at one year. BMJ. 1989;298(6679):985–90. doi: 10.1136/bmj.298.6679.985. 00MEDLINE: 892744550. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jolley AG, Hirsch SR, Morrison E, McRink A, Wilson L. Trial of brief intermittent neuroleptic prophylaxis for selected schizophrenic outpatients: clinical and social outcome at two years. BMJ. 1990;301(6756):837–42. doi: 10.1136/bmj.301.6756.837. 00MEDLINE: 892744550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley 2003 {published data only} .Jolley S, Garety P, Craig T, Dunn G, White J, Aitken M. Cognitive therapy in early psychosis: a pilot randomised controlled trial. Behavioural and Cognitive Psychotherapy. 2003;31(4):473–8. 00AMED: 0058120; CENTRAL: CN–00474925.0. [Google Scholar]
- Jones 1998 {published data only} .David SR, Purdon S, Jones BD, Stip E, Labelle A, Breier AF, Tollefson GD, Kutcher SP, Maclaren C, Hadrava V, Thompson PM, Leblanc Olanzapine versus risperidone versus haloperidol in early illness schizophrenia. Education improves outcomes in MDD. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]; Jones B. Olanzapine versus risperidone and haloperidol in the treatment of schizophrenia. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30 - Jun 4.1998. [Google Scholar]; Jones B. Treatment of cognitive deficits with antipsychotic drugs. Neurobiology of Aging. 1998;14(4S):S152–3. [Google Scholar]; Jones B, Tollefson G. Olanzapine versus risperidone and haloperidol in the treatment of schizophrenia. Schizophrenia Research. 1998;29:150–1. 00MEDLINE: 107119110. [Google Scholar]; Purdon SE, Canadian Cognition and Outcome Study Group Neuropsychological change in early phase schizophrenia over twelve months of treatment with olanzapine, risperidone, or haloperidol. Schizophrenia Research. 1998;29(1, 2):132–3. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]; Purdon SE, Jones B, Labelle A, Addington D, Tollefson G, Study Group A multicentre comparison of olanzapine, risperidone, and haloperidol on working memory, new learning, and delayed recall of verbal and nonverbal materials in early-phase schizophrenia over a 12-month prospective double-blind clinical trial. Schizophrenia Research. 1999;36(1-3):150. [Google Scholar]; Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Archives of General Psychiatry. 2000;57(3):249–58. doi: 10.1001/archpsyc.57.3.249. 00MEDLINE: 107119110. [DOI] [PubMed] [Google Scholar]; Purdon SE, Jones BDW, Stip E, Labelle A, Addington D, Breier A, Tollefson GD, The Canadian Collaborative Group for Research on Cognition in Schizophrenia Olanzapine versus haloperidol versus risperidone in early illness schizophrenia. Unpublished Report. 2001. [DOI] [PubMed]; Purdon SE, Woodward N, Lindborg SR, Stip E. Procedural learning in schizophrenia after 6 months of double-blind treatment with olanzapine, risperidone, and haloperidol. Psychopharmacology. 2003;169(3-4):390–7. doi: 10.1007/s00213-003-1505-z. 00MEDLINE: 128273470. [DOI] [PubMed] [Google Scholar]; Woodward ND, Purdon SE, David SR, Stip E. Procedural learning over six months double blind treatment with haloperidol, risperidone or olanzapine. Schizophrenia Research. 2001;49(1, 2):125. [Google Scholar]
- Jones 2005b {published data only} .Jones PB. Improving social recovery in early affective and non-affective psychosis: a randomised controlled trial of social recovery orientated cognitive behaviour (SRCBT) National Research Register. 2005;Vol. 3 [Google Scholar]
- Jones 2006 {published data only} .Jones PB, Barnes TRE, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1) Archives of General Psychiatry. 2006;63(10):1079–87. doi: 10.1001/archpsyc.63.10.1079. 00EMBASE: 2006486244; MEDLINE: 170158100. [DOI] [PubMed] [Google Scholar]
- Kahn 2003 {published data only} .Kahn RS, Lieberman JA, Charles C, Sharma T, Zipursky RB, Gur R, Tohen M, Green AI, McEvoy JP, Perkins DO, Hamer RM, Nemeroff CB, Rothschild AJ, Kuldau J, Strakowski SM, Tollefson GD. Antipsychotic treatment effects on progression of brain pathomorphology in first episode schizophrenia. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S336. [Google Scholar]; Kahn RS, Lieberman JA, Charles C, Sharma T, Zipursky RB, Gur R, Tohen M, Green AI, McEvoy JP, Perkins DO, Hamer RM, Nemeroff CB, Rothschild AJ, Kuldau J, Strakowski SM, Tollefson GD. Antipsychotic treatment effects on progression of brain pathomorphology in first episode schizophrenia. Proceedings of the 16th European College of Neuropsychopharmacology Congress; Prague, Czech Republic. 2003 Sep 20-24.2003. [Google Scholar]
- Kahn 2006 {published data only} .Kahn RS, Fleischhacker WW, The EUFEST study Schizophrenia Research. 2006;86(Suppl 1):S3. [Google Scholar]
- Kane 1982a {published data only} .Kane JM, Rifkin A, Quitkin F, Nayak D, Ramos Lorenzi J. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Archives of General Psychiatry. 1982;39(1):70–3. doi: 10.1001/archpsyc.1982.04290010048009. 00MEDLINE: 62758110. [DOI] [PubMed] [Google Scholar]
- Kane 2001b {published data only} .Anonymous Injectible, long-acting risperidone effective. Journal of Pharmacy Technology. 2001;17(4):157. 00EMBASE: 20013200400. [Google Scholar]; Kane J, Eerdekens M, Keith S, Lesem M, Karcher K, Lindenmayer JP. Efficacy and safety of Risperdal Consta, a long-acting injection risperidone formulation RIS-USA-121. Promational slides on file from Jannssen-Cilag UK Ltd; 2002. [Google Scholar]; Kane J, Eerdekens M, Keith S, Lesem M, Karcher K, Lindenmayer JP. Long-acting risperidone microspheres for treatment of patients with schizophrenia. European Neuropsychopharmacology. 2001;11(3):291. [Google Scholar]; Kane J, Eerdekens M, Keith S, Lesem M, Karcher K, Lindenmayer J-P. Efficacy and safety of a novel long-acting risperidone formulation. European Psychiatry. 2002;17(Suppl 1):S193. [Google Scholar]; Kane J, Eerdekens M, Keith S, Lesem M, Karcher K, Lindenmayer J-P. Efficacy and safety of a novel long-acting risperidone microspheres formulation. 11th Biennial Winter Workshop on Schizophrenia; Davos, Switzerland. 2002 Feb 24 - Mar 1; 2002. 00EMBASE: 2001320040; MEDLINE: 0. [Google Scholar]; Kane J, Eerdekens M, Keith SJ, Lesem M, Karcher K, Lindenmayer JP. Efficacy and safety of risperdal constatm, the long-acting injection risperidone formulation. International Journal of Neuropsychopharmacology. 2002;Vol. 5(issue Suppl 1):S188. [Google Scholar]; Kane JM. [accessed 16th Feb 2001];Preventing morbidity in first-episode schizophrenia. http://www.clinicaltrials.gov/; Kane JM, Eerdekens M, Keith SJ, Lesem M, Karcher K, Lindenmayer JP. Efficacy and safety of a novel long-acting risperidone microspheres formulation. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):174. 00EMBASE: 20013200400. [Google Scholar]; Kane JM, Eerdekens M, Keith SJ, Lesem M, Karcher K, Lindenmayer JP. Long-acting injectable risperidone: Efficacy and safety. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S325. [Google Scholar]; Kane JM, Eerdekens M, Lindenmayer JP, Keith SJ, Lesem M, Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. American Journal of Psychiatry. 2003;160:1125–32. doi: 10.1176/appi.ajp.160.6.1125. 00MEDLINE: 127772710. [DOI] [PubMed] [Google Scholar]
- Kapur 2000b {published data only} .Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double blind PET study of first episode schizophrenia. American Journal of Psychiatry. 2000;157(4):514–20. doi: 10.1176/appi.ajp.157.4.514. 00MEDLINE: 202040610. [DOI] [PubMed] [Google Scholar]
- Kavanagh 2004 {published data only} .Kavanagh DJ, Young R, White A, Saunders JB, Wallis J, Shockley N, Jenner L, Clair A. A brief motivational intervention for substance misuse in recent-onset psychosis. Drug and Alcohol Review. 2004;23(2):151–5. doi: 10.1080/09595230410001704127. 00MEDLINE: 153700200. [DOI] [PubMed] [Google Scholar]
- Keefe 2005 {published data only} .Keefe R, Gu HB, Sweeney J, Perkins D, McEvoy J, Hamer R, Lieberman J. A comparison of the effects of olanzapine, quetiapine, and risperidone on neurocognitive function in first-episode psychosis. Neuropsychopharmacology. 2005;30(Suppl 1):S192. 00ISIP: 0002334421005010. [Google Scholar]; *; Keefe RSE, Gu H, Sweeney JA, Perkins DO, McEvoy JP, Hamer RM, Lieberman JA. The effects of olanzapine, quetiapine and risperidone on neurocognitive function in first-episode psychosis: a double-blind 52-week comparison. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Keefe 2006b {published data only} .Keefe R, Seidman LJ, Christensen B, Hamer RM, Yurgelun-Todd D, Lewine R, Sitskoorn M, Sharma T, Tohen M, Lieberman JA. Neurocognitive effects of olanzapine and low-dose haloperidol: a two-year treatment study in first episode psychosis. Schizophrenia Research. 2003;60(1):289–90. 00MEDLINE: 822281600. [Google Scholar]; Keefe RS, Seiden LJ, Christensen B, Yurgelun-Todd DA, Lewine RR, Sitskoorn M, Sharma T, Clark WS, Sanger TM, Tohen M, Lieberman JA. Treatment of neurocognitive deficits with olanzapine or low-dose haloperidol in first episode psychosis. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]; Keefe RS, Seidman LJ, Christensen BK, Yurgelun-Todd DA, Lewine RR, Lieberman MM, Sitskoorn J. Treatment of neurocognitive deficits with olanzapine or low-dose haloperidol in first episode psychosis. Schizophrenia Research. 2001;49(1, 2):234. 00MEDLINE: 170552270. [Google Scholar]; Keefe RS, Seidman LJ, Hamer RM, Todd DY, Christensen B, Sitskoorn MM, Lieberman JA. Neurocognition aftertwo years olanzapine or low-dose haldol in FE psychosis. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; Keefe RSE. Treatment of neurocognitive deficits with olanzapine or haloperidol in first episode psychosis. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]; Keefe RSE, Seidman LJ, Christensen B, Hamer RM, Yurgelun-Todd D. Neurocognitive effects of olanzapine and low-dose haloperidol: a two- year treatment study in first episode psychosis. Schizophrenia Research. 2004;67(1):206. 00ISI: 0001887881004940. [Google Scholar]; Keefe RSE, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RRJ, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomised, double-blind trial of olanzapine versus low doses of haloperidol. American Journal of Psychiatry. 2004;161(6):985–95. doi: 10.1176/appi.ajp.161.6.985. 00EMBASE: 20042456620. [DOI] [PubMed] [Google Scholar]; *; Keefe RSE, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Rock SL, Woolson S, Tohen M, Tollefson GD, Sanger TM, Lieberman JA. Long-term neurocognitive effects of olanzapine or low-dose haloperidol in first-episode psychosis. Biological Psychiatry. 2006;59(2):97–105. doi: 10.1016/j.biopsych.2005.06.022. 00MEDLINE: 161402820. [DOI] [PubMed] [Google Scholar]
- Kenny 1992 {published data only} .Kenny JT, Meltzer HY. Effect of atypical and typical antipsychotic drugs on neuropsychological functions in early- stage schizophrenic patients. Proceedings of the 7th Biennial Winter Workshop on Schizophrenia; Les Diablerets, Switzerland. 1994 Jan 23-28.1992. [Google Scholar]
- Keri 2006 {published data only} .Keri S, Kelemen O, Janka Z. Therapy of mental states at high risk for psychosis: preliminary results from Hungary [A psychosis szempontjából nagy kockázatú mentális állapotok és kezelésük: els hazai eredmények] Orvosi Hetilap. 2006;147(5):201–4. 00MEDLINE: 165092200. [PubMed] [Google Scholar]
- Kern 2001 {published data only} .Carson W, Cornblatt B, Saha A, Ali M, Kern R, Green M. Neurocognitive benefits of aripiprazole versus olanzapine in stable psychosis. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S291. [Google Scholar]; Cornblatt B, Kern RS, Carson WH, Ali MW, Luo X, Green M. Neurocognitive effects of aripiprazole versus olanzapine in stable psychosis. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S185. [Google Scholar]; Cornblatt B, Kern RS, Carson WH, Stock E, Ali M, Ingenito G, Green MF. Neurocognitive effects of aripiprazole versus olanzapine in patients with stabile psychosis. Journal of Psychopharmacology. 2002;Vol. 16(issue Suppl 3):A15. [Google Scholar]; Cornblatt B, Kern RS, Carson WH, Stock E, Ali M, Ingenito G, Green MF. Neurocognitive effects of aripiprazole versus olanzapine in patients with stable psychosis. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):27. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Kern RS, Cornblatt B, Carson WH, Dunbar G, Ali M, Ingenito G, Green MF. An open-label comparison of the neurocognitive effects of aripiprazole versus olanzapine in patients with stable psychosis. Schizophrenia Research. 2001;49(1, 2):234. [Google Scholar]; Kern RS, Cornblatt B, Carson WH, Stock E, Saha AR, Ali MW, Ingenito G, Green MF. Neurocognitive effects: aripiprazole vs olanzapine in stable psychosis. European Psychiatry. 2002;17(Suppl 1):104s. [Google Scholar]
- Keshavan 1998 {published data only} .Keshavan MS, Schooler NR, Sweeney JA, Haas GL, Pettegrew JW. Research and treatment strategies in first episode psychoses: the Pittsburgh experience. British Journal of Psychiatry. 1998;172(Suppl 33):60–5. 00EMBASE: 19981872010. [PubMed] [Google Scholar]
- Keshavan 2003 {published data only} .Keshavan M. A 6-month controlled study of psychoeducation and collaboration enhancement (PEACE) in early psychosis to improve treatment compliance. Stanley Foundation Research Programs. 2003.
- Killackey 2006 {published data only} .Killackey E, Jackson H, McGorry P. Vocational intervention in a specialist early psychosis service: first results of a pilot randomised controlled trial. Schizophrenia Research. 2006;86(Suppl 1):S28–9. [Google Scholar]
- Kingdon 2000 {published data only} .Kingdon D. SOCRATES - a multicentre, randomised controlled trial of cognitive behavioural therapy in early schizophrenia. National Research Register. 2000 [Google Scholar]
- Kistrup 1991 {published data only} .Kistrup K, Gerlach J, Aaes Jorgensen T, Larsen NE. Perphenazine decanoate and cis(z)-flupentixol decanoate in maintenance treatment of schizophrenic outpatients. Serum levels at the minimum effective dose. Psychopharmacology. 1991;105(1):42–8. doi: 10.1007/BF02316862. 00MEDLINE: 920806170. [DOI] [PubMed] [Google Scholar]
- Klier 2005 {published data only} .Klier C, Hollmann M, Schlögelhofer M, Mossaheb N, Friedrich M, Amminger PG. Indicated prevention with omega-3 fatty acids (epa/dha) in adolescents with “at-risk-mental-state“ for psychosis. Proceedings of the XIII World Congress of Psychiatry; Cairo, Egypt. 2005 10-15th Sept.2005. [Google Scholar]
- Knapp 2004 {published data only} .Knapp M. Brixton early psychosis study project #432. National Research Register. 2004;Vol. 1 [Google Scholar]
- Kolivakis 2001 {published data only} .Kolivakis TT, Margolese HC, Beauclair L, Chouinard G. Adjunctive anticonvulsant use in first-episode schizophrenia. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10.2001. [Google Scholar]; Kolivakis TT, Margolese HC, Beauclair L, Chouinard G. Adjunctive anticonvulsant use in first-episode schizophrenia. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Kopala 2003 {published data only} .Kopala L, Rabinowitz J, Davidson M. EPS in recent onset schizophrenia: a comparison of risperidone and haloperidol. Proceedings of the 16th European College of Neuropsychopharmacology Congress; Prague, Czech Republic. 2003 Sep 20-24.2003. [Google Scholar]; Kopala L, Rabinowitz J, Davidson M. Extra-pyramidal signs and symptoms (eps) in recent onset schizophrenia: a comparison of risperidone and haloperidol. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S338. [Google Scholar]; Kopala L, Rabinowitz J, Emsley R, Mcgorry P. Extra-pyramidal signs and symptoms (eps) in recent onset schizophrenia: a comparison of risperidone and haloperidol. Schizophrenia Research. 2004;67(1):187. 00ISI: 0001887881004480. [Google Scholar]
- Kuipers 2004 {published data only} .Kuipers E, Holloway F, Rabe-Hesketh S, Tennakoon L. An RCT of early intervention in psychosis: Croydon outreach and assertive support team (COAST) Social Psychiatry and Psychiatric Epidemiology. 2004;39(5):358–63. doi: 10.1007/s00127-004-0754-4. 00MEDLINE: 15133591; : CN–00468266.0. [DOI] [PubMed] [Google Scholar]
- Kujawa 2002 {published data only} .Archibald DG, Manos G, Stock E, Jody D, Tourkodimitris S, Marcus R, Iwamoto T, Yamamoto Y. Effects of long-term aripiprazole therapy on the negative symptoms of schizophrenia. Schizophrenia Research. 2004;67(1):155. 00ISI: 0001887881003690. [Google Scholar]; Archibald DG, Manos G, Tourkodimitris S, Iwamoto T, Carson WH, Stock E, Marcus R. Reduction in negative symptoms of schizophrenia during long-term therapy with aripiprazole. Schizophrenia Research. 2003;60(1):271. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, Archibald D, Ingenito G, Marcus R, Pigott T. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. International Journal of Neuropsychopharmacology. 2003;6(4):325–37. doi: 10.1017/S1461145703003651. 00EMBASE: 2004054004; MEDLINE: 146094390. [DOI] [PubMed] [Google Scholar]; Kostic D, Manos G, Stock E, Jody D, Archibald D, Tourkodimitris S, Marcus R. Long-term effects of aripirazole on the negative symptoms of schizophrenia. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S328. [Google Scholar]; Kujawa M, Saha AR, Ingenito GG, Ali MW, Luo X, Archibald DG, Carson WH. Aripiprazole for long-term maintenance treatment of schizophrenia. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S186. [Google Scholar]; Kujawa MJ, Saha AR, Ingenito GG, Ali MW, Luo X, Archibald DG, William HC., Jr Aripiprazole for long-term maintenance treatment in schizophrenia. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; Manos G, Stock EG, Jody D, Archibald DG, Tourkodimitris S, Marcus RN. Long-term effects of aripiprazole on the negative symptoms of schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; McQuade RD, Kujawa M, Saha AR, Ingenito GG, Ali MW, Luo X, Archibald DG, Carson WH. Aripiprazole for long-term maintenance treatment of schizophrenia. Schizophrenia Research. 2003;60(1):295. [Google Scholar]; Saha AR, Carson WH, Mcquade RD, Stock EG, Inada I, Ali MW. Long-term aripiprazole therapy in schizophrenia. Proceedings of the 12th World Congress of Psychiatry; Yokohama, Japan. 2002 Aug 24-29.2002. [Google Scholar]; Stock E, Archibald DG, Tourkodimitris S, Kujawa M, Marcus R, Carson W, Iwamoto T. Long-term effects of aripiprazole and haloperidol on affective symptoms of schizophrenia. Schizophrenia Research. 2004;67(1):158–9. 00ISI: 0001887881003780. [Google Scholar]
- Lambert 1995 {published data only} .Lambert T, Keks N, McGrath J, Catts S, Hustig H, Vaddadi K, Burrows G, Varghese F, George T, Kerr K, Johnson G, Burnett P, Zorbas A, Hill Remoxipride versus thioridazine in the treatment of first episodes of schizophrenia in drug-naive patients - A case for specific, low potency D2 antagonists. Human Psychopharmacology. 1995;10(6):455–60. 00EMBASE: 19960268970. [Google Scholar]
- Lambert 2006 {published data only} .Lambert MT. Olanzapine is associated with more rapid weight gain than haloperidol in people with first episode psychosis. Evidence-Based Mental Health. 2006;9(3):72. doi: 10.1136/ebmh.9.3.72. 00CINAHL: 2009277049; MEDLINE: 168681910. [DOI] [PubMed] [Google Scholar]
- Lane 2001 {published data only} .Lane HY, Chang WH, Chiu CC, Huang MC, Lee SH, Chen JY. A pilot double-blind, dose-comparison study of risperidone in drug-naive, first-episode schizophrenia. Journal of Clinical Psychiatry. 2001;62(12):994–5. doi: 10.4088/jcp.v62n1214c. 00MEDLINE: 216392450. [DOI] [PubMed] [Google Scholar]
- Lauriello 2005 {published data only} .Lauriello J, Rowland L, Hammond LR, Brooks W, Bustillo J. Longitudinal assessment of brain chemistry early in schizophrenia: a randomised controlled trial of quetiapine and haloperidol. Schizophrenia Bulletin. 2005;31:444. [Google Scholar]
- Lavalaye 1999 {published data only} .Lavalaye J, Linszen DH, Booij J, Reneman L, Gersons BP, Van-Royen EA. Dopamine D2 receptor occupancy by olanzapine or risperidone in young patients with schizophrenia. Psychiatry Research. 1999;92(1):33–44. doi: 10.1016/s0925-4927(99)00032-3. 00MEDLINE: 106881580. [DOI] [PubMed] [Google Scholar]
- Leavey 2004 {published data only} .Leavey G. A controlled evaluation of an individualised service to relatives of patients with a first episode of psychosis illness. National Research Register. 2000. ; Leavey G. A controlled evaluation of an individualised service to relatives of patients with a first episode of psychosis illness. National Research Register. 2001;Vol. 1 [Google Scholar]; Leavey G. A controlled evaluation of an individualised service to relatives of patients with a first onset of psychotic illness. National Research Register. 2000. ; *; Leavey G, Gulamhussein S, Papadopoulos C, Johnson-Sabine E, Blizard BKM. A randomised controlled trial of a brief intervention for families of patients with a first episode of psychosis. Psychological Medicine. 2004;34(3):423–31. doi: 10.1017/s0033291703001594. 00CENTRAL: CN–00482786; MEDLINE: 152598270. [DOI] [PubMed] [Google Scholar]
- Leblanc 2006 {published data only} .Leblanc J, Letourneau K, Demers MF, Bouchard RH, Roy MA. The impact of modafinil as an adjunct to a second generation antipsychotic on cognitive functioning in patients with first psychotic episode. Schizophrenia Research. 2006;86(Suppl 1):S131. [Google Scholar]
- Leclerc 2006 {published data only} .Leclerc C, Gauvin D, Lecomte T. CBT group to maintain and improve relationships and intimacy abilities of young adults with a first psychotic episode - a pilot study. Schizophrenia Research. 2006;86(Suppl 1):S138–9. [Google Scholar]
- Lecomte 2006 {published data only} .Lecomte T, Leclerc C, Wykes T, Wallace CJ. Group CBT versus group symptom management for treating psychotic symptoms of young individuals presenting a first episode of schizophrenia: preliminary results. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]; *; Lecomte T, Leclerc C, Wykes T, Wallace CJ, Spidel A, Corbiere M. Group CBT vs skills training for first episodes of psychosis - results of a RCT. Schizophrenia Research. 2006;86(Suppl 1):S45–6. [Google Scholar]
- Lecrubier 2003 {published data only} .Lecrubier Y. A partial d3 receptor agonist in schizophrenia. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S167. [Google Scholar]
- Lehtinen 1990 {published data only} .Lehtinen V, Aaltonen J, Koffert T, Rakkolainen V, Syvalahti E. Two-year outcome in first-episode psychosis treated according to an integrated model. Is immediate neuroleptisation always needed? European Psychiatry. 2000;15(5):312–20. doi: 10.1016/s0924-9338(00)00400-4. [DOI] [PubMed] [Google Scholar]
- Lehtinen 2000 {published data only} .Lehtinen V, Aaltonen J, Koffert T, Rakkolainen V, Syvalahti E. Two-year outcome in first-episode psychosis treated according to an integrated model. Is immediate neuroleptisation always needed? European Psychiatry. 2000;15(5):312–20. doi: 10.1016/s0924-9338(00)00400-4. 00MEDLINE: 151634440. [DOI] [PubMed] [Google Scholar]
- Lei 2006 {published data only} .Lei Z, Huang H. Analyses on mental health status and psycho-intervention of accompanying relatives of first-episode childhood schizophrenics. Journal of Clinical Psychosomatic Diseases. 2006;12(6):447–8. [Google Scholar]
- Lemmer 2001 {published data only} .Lemmer W, Lehmann E. The efficacy and tolerance of zotepine and haloperidol in acute schizophrenia and the differential therapy of kava-kava. Biological Psychiatry. 2001;2(Suppl 1):P021–39. [Google Scholar]
- Lencz 2006 {published data only} .Lencz T, Robinson DG, Xu K, Ekholm J, Sevy S, Gunduz-Bruce H, Woerner MG, Kane JM, Goldman D, Malhotra AK. DRD2 promotor region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. American Journal of Psychiatry. 2006;163(3):529–31. doi: 10.1176/appi.ajp.163.3.529. 00CINAHL: 2009215507; MEDLINE: 165138770. [DOI] [PubMed] [Google Scholar]
- Lenior 2001 {published data only} .Lenior ME, Dingemans PM, Linszen DH, De Haan L, Schene AH. Social functioning and the course of early-onset schizophrenia: five-year follow-up of a psychosocial intervention. British Journal of Psychiatry. 2001;179:53–8. doi: 10.1192/bjp.179.1.53. 00MEDLINE: 213287610. [DOI] [PubMed] [Google Scholar]; Lenior ME, Linszen DH, Dingemans PM, Schene AH. Social functioning and the course of recent-onset schizophrenia a 5-year follow-up study. Schizophrenia Research. 1999;36(1-3):328–9. 00ISI: 0001887881000820. [Google Scholar]; Lenior ME, Linszen DH, Dingemans PM, Schene H. Family intervention and the course of parental expressed emotion: a longitudinal study. Schizophrenia Research. 2001;49(1, 2):263. doi: 10.1016/s0920-9964(01)00305-x. 00MEDLINE: 213287610. [DOI] [PubMed] [Google Scholar]
- Lenior 2002 {published data only} .Lenior M, Dingemans P, Schene A, Linszen D. Predictors and 5-year outcome in early onset schizophrenia. A path analysis. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]
- Lester 2004a {published data only} .Lester H, Birchwood M, Tait L, Shanks A. Redirect: evaluating the effectiveness of an educational interventionon first episode psychosis in primary care. Schizophrenia Research. 2004;70(1):39. 00ISI: 0002245511001000. [Google Scholar]; *; Lester HE, Birchwood M, Tait L, Freemantle N. Redirect: evaluating the effectiveness of an educational intervention about first episode psychosis in primary care. Schizophrenia Research. 2006;86(Suppl 1):S39–40. [Google Scholar]
- Lester 2004b {published data only} .*; Lester H. Birmingham early detection in untreated psychosis trial (REDIRECT) National Research Register. 2004;Vol. 1 doi: 10.1186/1472-6963-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis 2000a {published data only} .Lewis S, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D, Siddle R, Everitt J, Benn A, Leadley K, Glazebrook K, Drake R, Haley C, Akhtar S, Faragher B. A randomised, controlled trial of cognitive-behaviour therapy in acute, early schizophrenia: the SoCRATES trial; Summary. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]; Lewis SW, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D. A multicentre, randomised controlled trial of cognitive-behaviour therapy in first-and second-episode schizophrenia: the Socrates trial. Schizophrenia Research. 1999;36(1-3):329. [Google Scholar]; Lewis SW, Tarrier N, Haddock R, Bentall R, Kinderman P, Kingdon D, Siddle R, Leadley K, Grazebrook K, Everitt J, Benn A, Faragher B. The Socrates trial: a multicentre, randomised, controlled trial of cognitive behaviour therapy in early schizophrenia. Schizophrenia Research. 2000;41(1):9. 00MEDLINE: 170552270. [Google Scholar]
- Lewis 2000d {published data only} .Lewis S. Evaluation of family support and cognitive behavioural treatment service for recent onset schizophrenia sufferers with substance misuse problems. National Research Register. 2000. ; Lewis S. Evaluation of family support and cognitive behavioural treatment service for recent onset schizophrenia sufferers with substance misuse problems. National Research Register. 2001;Vol. 1 [Google Scholar]
- Lewis 2000f {published data only} .Lewis S. The SOCRATES study: cognitive therapy in early schizophrenia. National Research Register. 2000 [Google Scholar]; Lewis S. The SOCRATES study: cognitive therapy in early schizophrenia. National Research Register. 2004;Vol. 3 [Google Scholar]
- Lewis 2001e {published data only} .Lewis L, Unkefer EP, O’Neal SK, Crith CJ, Fultz J. Cognitive rehabilitation with patients having persistent, severe psychiatric disabilities. Psychiatric Rehabilitation Journal. 2003;26(4):325–31. doi: 10.2975/26.2003.325.331. 00EMBASE: 20032063550. [DOI] [PubMed] [Google Scholar]; Lewis S. EDIE - Early detection and intervention for psychosis. National Research Register. 2001;Vol. 3 [Google Scholar]; Lewis S. EDIE - Early detection and intervention for psychosis (in primary care) National Research Register. 2002;Vol. 1 [Google Scholar]
- Lewis 2002a {published data only} .Lewis S, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D, Siddle R, Drake R, Everitt J, Leadley K, Benn A, Grazebrook K, Haley C, Akhtar S, Davies L, Palmer S, Faragher B, Dunn G. Randomised controlled trial of cognitive-behavioural therapy in early schizophrenia: acute-phase outcomes. British Journal of Psychiatry Supplementum. 2002;181(Suppl 43):S91–7. doi: 10.1192/bjp.181.43.s91. 00MEDLINE: 12271807; PASCAL: 2002–0516453 INIST0100 INRA0200 BRGM0204 200210310. [DOI] [PubMed] [Google Scholar]; Lewis SW. SOCRATES: study of cognitive realignment therapy in early schizophrenia. Current Controlled Trials. 2001 00ISRCTN: 658185270. [Google Scholar]; Lewis SW, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D, Drake R, Dunn G. Cognitive therapy improves 18-month outcomes but not time to relapse in first episode schizophrenia. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):14. [Google Scholar]; Lewis SW, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D, Drake RJ. A randomised controlled trial of cognitive behaviour therapy in early schizophrenia. Schizophrenia Research. 2001;49(1, 2):263–4. 00MEDLINE: 170552270. [Google Scholar]; Lewis SW, Tarrier N, Hadock G, Bentall R, Kinderman P, Kingdon D. Cognitive therapy improves outcomes in first episode psychosis. Proceedings of the 12th World Congress of Psychiatry; Yokohama, Japan. 2002 Aug 24-29.2002. [Google Scholar]; Tarrier N, Lewis S, Haddock G, Bentall R, Drake R, Kinderman P, Kingdon D, Siddle R, Everitt J, Leadley K, Benn A, Grazebrook K, Haley C, Akhtar S, Davies L, Palmer S, Dunn G. Cognitive-behavioural therapy in first-episode and early schizophrenia: 18-month follow-up of a randomised controlled trial. British Journal of Psychiatry. 2004;184:231–9. doi: 10.1192/bjp.184.3.231. 00AMED: 0061364; EMBASE: 20041218900. [DOI] [PubMed] [Google Scholar]
- Lewis 2006b {published data only} .Lewis SW, Barnes TRE, Davies L, Murray RM, Dunn G, Hayhurst KP, Markwick A, Lloyd H, Jones PB. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophrenia Bulletin. 2006;32(4):715–23. doi: 10.1093/schbul/sbj067. 00EMBASE: 2006459790; MEDLINE: 165407020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis 2006c {published data only} .Lewis S, Davies L, Jones P, Barnes T, Murray R, Kerwin R, Taylor D, Hayhurst K, Markwick A, Lloyd H, Dunn G. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Draft final report for NCCHTA. 2003 doi: 10.3310/hta10170. [DOI] [PubMed] [Google Scholar]; *; Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, Taylor D, Hayhurst KP, Markwick A, Lloyd H, Dunn G. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment. 2006;10(17):1–182. doi: 10.3310/hta10170. 00CINAHL: 2009206784; EMBASE: 2006300640; MEDLINE: 167070740. [DOI] [PubMed] [Google Scholar]
- Li 2003f {published data only} .Li Z, Liu Y, Wang M. Therapeutic effect of quetiapine in the treatment of first episode schizophrenia. Health Psychology Journal. 2003;11(6):424–6. 00CAJ: MEDI04010. [Google Scholar]
- Li 2004a {published data only} .Li YY, Wu TC, Li M. Efficacy of early intervention on first-episode schizophrenic patients. Journal of Clinical Psychological Medicine. 2004;14(1):24–6. 00CAJ: MEDI04040. [Google Scholar]
- Li 2004f {published data only} .Li Y, Jia J-D, Zhang M-S. Influence of family mental intervention on social function, family environment and relapse rate in first-episode schizophrenics. Chinese Journal of Clinical Rehabilitation. 2004;8(21):4184–5. [Google Scholar]
- Li 2004h {published data only} .Li X-H, Wan J. Effects of the nursing care of mutual participation model on the rehabilitation of inpatients with early schizophrenia. Chinese Journal of Clinical Rehabilitation. 2004;8(24):4958–9. 00CAJ: MEDI0410; CENTRAL: CN–005166940. [Google Scholar]
- Li 2005d {published data only} .Li G, Chen Q-G, Zhang Q-H. A comparison analysis on the efficacy of quetiapine and risperidone in cognitive function of schizophrenia. Chinese Journal of Behavioral Medical Science. 2005;14(11):1007–8. 00CAJ: MEDI0512S10. [Google Scholar]
- Liang 2003a {published data only} .Liang L. A clinical trial of risperidone in treatment of childhood patients with first-episode schizophrenia. Journal of Clinical Psychological Medicine. 2003;7467:1. [Google Scholar]
- Liao Chunping 2004 {published data only} .Liao C, Yang H, Gao H. Effects of clozapine and risperidone on blood routine examinations of patients with schizophrenia. Journal of Clinical Psychosomatic Diseases. 2004;10(3):156–7. 00MEDLINE: 169741930. [Google Scholar]
- Liberman 1988 {published data only} .Liberman PR, Eclam AT, Marder RS, Wirshing W, Johnston-Cronk K. Symptom management training for schizophrenics. Proceedings of the 141st Annual Meeting of the American Psychiatric Association; Montreal, Quebec, Canada. 1988 May 7-12.1988. [Google Scholar]
- Lieberman 2001b {published data only} .Lieberman JA, Phillips M, Kong L, Gu H, Koch G. Efficacy and safety of clozapine versus chlorpromazine in first episode psychosis: results of a 52-week randomised double-blind trial. Schizophrenia Research. 2001;49(1, 2):236. [Google Scholar]; *; Lieberman JA, Phillips M, Kong L, Gu H, Koch G. Efficacy and safety of clozapine versus chlorpromazine in first-episode psychosis: results of a 52 week randomised double blind trial. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]
- Lieberman 2003a {published data only} .Lieberman J, Charles HC, Sharma T, Zipursky R, Kahn R, Gur R, Tohen M, Green AI, McEvoy J, Perkins D. Antipsychotic treatment effects on progression of brain pathomorphology in first episode schizophrenia. Schizophrenia Research. 2003;60(1):293. [Google Scholar]; Lieberman JA, Charles C, Sharma T, Zipursky RB, Hamer RM, Tollefson GD. Effect of olanzapine versus haloperidol on brain pathomorphology in first-episode psychosis. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; Perkins DO, Lieberman J, Gu H, Tohen M, McEvoy J, Green A, Zipursky R, Strakowski S, Sharma T, Kahn R, Gur R, Tollefson G, HGDH Research Group Predictors of antipsychotic treatment response in patients with first-episode schizophrenia, schizoaffective and schizophreniform disorders. British Journal of Psychiatry. 2004;185:18–24. doi: 10.1192/bjp.185.1.18. 00EMBASE: 2004310667; MEDLINE: 152315510. [DOI] [PubMed] [Google Scholar]
- Lieberman 2003c {published data only} .Lieberman JA, Phillips M, Gu H, Bilder R, Zhang P, Ji Z, Koch G. Effects of atypical and conventional antipsychotic drugs on cognitive performance in treatment naive first-episode schizophrenia: a 3 year randomised trial of clozapine versus chlorpromazine. Schizophrenia Bulletin. 2005;31:493. [Google Scholar]; *; Lieberman JA, Phillips M, Gu H, Stroup S, Zhang P, Kong L, Ji Z, Koch G, Hamer RM. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomised trial of clozapine vs chlorpromazine. Neuropyschopharmacology. 2003;28:995–1003. doi: 10.1038/sj.npp.1300157. 00ISIP: 0002334421005010. [DOI] [PubMed] [Google Scholar]
- Lieberman 2005b {published data only} .Lieberman J, McEvoy JP, Perkins D, Hamer RH. Comparison of atypicals in first-episode psychosis: a randomised, 52-week comparison of olanzapine, quetiapine, and risperidone. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):S525. [Google Scholar]; Lieberman JA, Tollefson G, Tohen M, Green AI, Gur RE, Kahn R, McEvoy J, Perkins D, Sharma T, Zipursky R, Wei H, Hamer RM, HGDH Study Group Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomised, double-blind trial of olanzapine versus haloperidol. American Journal of Psychiatry. 2003;160(8):1396–404. doi: 10.1176/appi.ajp.160.8.1396. 00EMBASE: 2005343018; MEDLINE: 129003000. [DOI] [PubMed] [Google Scholar]
- Lieberman 2005c {published data only} .Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RSE, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Archives of General Psychiatry. 2005;62(4):361–70. doi: 10.1001/archpsyc.62.4.361. 00EMBASE: 2005157308; MEDLINE: 158094030. [DOI] [PubMed] [Google Scholar]
- Lin 2006b {published data only} .Lin H-L, Shi W-H, Wang J-H. Effects of aripiprazole and chlorpromazine on the cognitive function in first-episode schizophrenia patients. Chinese Journal of Behavioral Medical Science. 2006;15(5):440–2. [Google Scholar]
- Lin 2006c {published data only} .Lin C. A controlIed study of aripiprazole and risperidone in treatment of first-episode schizophrenia. Chinese Journal of Health Psychology. 2006;14(6):660–1. [Google Scholar]
- Linszen 1994 {published data only} .Linszen D, Dingemans P, Lenior M. Early intervention and a five year follow up in young adults with a short duration of untreated psychosis: ethical implications. Schizophrenia Research. 2001;51(1):55–61. doi: 10.1016/s0920-9964(01)00239-0. 00MEDLINE: 213726260. [DOI] [PubMed] [Google Scholar]; Linszen D, Dingemans P, Lenior M, Scholte W. Early individual and family intervention in schizophrenia. Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996 Aug 23-28.1996. [Google Scholar]; Linszen D, Dingemans P, Van der Does JW, Nugter A, Scholte P, Lenior R, Goldstein MJ. Treatment, expressed emotion and relapse in recent onset schizophrenic disorders. Psychological Medicine. 1996;26(2):333–42. doi: 10.1017/s0033291700034723. 00MEDLINE: 86852890. [DOI] [PubMed] [Google Scholar]; Linszen D, Lenior M, De Haan L, Dingemans P, Gersons B. Early intervention, untreated psychosis and the course of early schizophrenia. British Journal of Psychiatry Supplementum. 1998;172(33):84–9. 00MEDLINE: 984366230. [PubMed] [Google Scholar]; Linszen DH, Dingemans PM. Early intervention in recent onset schizophrenia preliminary results. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]; Linszen DH, Dingemans PM, Lenior ME, Nugter MA, Scholte WF, Van der Does AJ. Relapse criteria in schizophrenic disorders: different perspectives. Psychiatry Research. 1994;54(3):273–81. doi: 10.1016/0165-1781(94)90021-3. 00MEDLINE: 953125870. [DOI] [PubMed] [Google Scholar]; Linszen H, Lenior RM, Dingemans PM. Early intervention in first episode psychoses and the critical period. Schizophrenia Research. 2001;49(1, 2):264. 00MEDLINE: 170552270. [Google Scholar]; Nugter A, Dingemans P, Van der Does JW, Linszen D, Gersons B. Family treatment, expressed emotion and relapse in recent onset schizophrenia. Psychiatry Research. 1997;72(1):23–31. doi: 10.1016/s0165-1781(97)00086-3. 00MEDLINE: 9355816; PSYCINFO: 1997–41313–0040. [DOI] [PubMed] [Google Scholar]
- Linszen 2004a {published data only} .Linszen D. Early and critical period intervention in first episode schizophrenia: relapse, chronicity, early stabilisation, predictors over 4 years and new research. Schizophrenia Research. 2004;70(1):66. 00ISI: 0002245511001770. [Google Scholar]; Linszen D, Wouters L, Dingemans P, De Haan L, Nieman D. Early and 3-year sustained intervention in first episode schizophrenia: relapse, stabilization and its predictors. Schizophrenia Research. 2004;67(1):18. 00ISI: 0001887881000380. [Google Scholar]; Linszen DH, De Haan L, Dingemans P, Van Bruggen M, Hofstra N, Van Engelsdorp H, Smitten M. Treatment reluctance in first episode schizophrenia: lack of insight, non-compliance and cannabis abuse predict bad outcome after eighteen months intervention. Schizophrenia Research. 2003;60(1):325. 00MEDLINE: 124091650. [Google Scholar]
- Linszen 2006 {published data only} .Linszen D, De Haan L, Dingemans P, Lenior R, Van Amelsvoort T, Wouters L. The Amsterdam critical period intervention in the early phase of schizophrenia-like psychoses. Schizophrenia Research. 2006;86(Suppl 1):S61. [Google Scholar]
- Lis 2003 {published data only} .Lis S, Krieger S, Gallhofer B, Torre P, Mittoux A, Menard F. Sertindole is superior to haloperidol in cognitive performance in patients with schizophrenia: a comparative study. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S323. [Google Scholar]; Lis S, Krieger S, Gallhofer B, Torre P, Mittoux A, Menard F. Sertindole is superior to haloperidol in cognitive performance in patients with schizophrenia: a comparative study. Proceedings of the 16th European College of Neuropsychopharmacology Congress; Prague, Czech Republic. 2003 Sep 20-24.2003. [Google Scholar]
- Liu 2006c {published data only} .Liu S, Ren C, Song X. Comparative study between aripiprazole and clozapine in the treatment of first-episode schizophrenia. Chinese Journal of Health Psychology. 2006;14(6):658–9. [Google Scholar]
- Liu Lin 2004b {published data only} .Liu L, Yue S, Li J. Effect of health education on insight recovering drug therapy compliance and recurrence in first-episode schizophrenic patients. Chinese Journal of Nursing. 2004;39(5):330–2. 00CAJ: MEDI0512S10. [Google Scholar]
- Loza 1999 {published data only} .Loza N, El-Dosoky AM, Okasha TA, Khalil AH, Hasan NM, Dossenbach M, Kratky P, Okasha A. Olanzapine compared to chlorpromazine in acute schizophrenia. Journal of the European College of Neuropsychopharmacology. 1999;Vol. 9(issue Suppl 5):S291. [Google Scholar]
- Loza 2001 {published data only} .Loza B, Kucharska-Pietura K, Debowska G. Atypical versus typical antipsychotic treatment prognosis in first-episode paranoid schizophrenia based on WCST and dichotic listening scores. European Neuropsychopharmacology. 2001;Vol. 11(issue 3):285. [Google Scholar]
- Loza 2002 {published data only} .Loza B. Atypical antipsychotic treatment prognosis in first-episode paranoid schizophrenia based on syllabic and language-related dichotic listening scores. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S298. [Google Scholar]
- Lu 2002b {published data only} .Lü L, Guo S, Ji M. A control study of the effect of one single dosage of clozapine on EEG in schizophrenia. Journal of Clinical Psychological Medicine. 2002;12(3):131–2. 00CAJ: MEDI02080. [Google Scholar]
- Ma 2000a {published data only} .Ma W, Yang L, Liu G. The effects of chlorpromazine and clozapine on the serum prolactin and growth hormone in schizophrenia. Journal of Clinical Psychological Medicine. 2000;10(6):323–5. 00CAJ: MEDI01020. [Google Scholar]
- Ma 2002 {published data only} .Ma W, Yang L, Zhang H. Changes of plasma cytokine levels before and after treatment in first-episode schizophrenics. Chinese Mental Health Journal. 2002;16(5):345–7. 00CAJ: MEDI02070. [Google Scholar]
- Ma 2004b {published data only} .*; Ma Z-F, Liu L, Fu F-Z, Guo J, Li X-R. The effect of nursing intervention on quality of life in patients with first-episode schizophrenia. Journal of Nursing Administration. 2004;4(4):3–5. 00EMBASE: 19980213970. [Google Scholar]
- Ma Xiaozhi 2004 {published data only} .Ma X, Guo P. Effects of individualized quantitative healthy education on improving medication compliance of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2004;10(4):242–4. 00MEDLINE: 169741930. [Google Scholar]
- Mackeprang 2001 {published data only} .Mackeprang T, Fagerlund B, Videbaek C, Hemmingsen RP, Glenthoj BY. Extrastriatal dopamine(d2/d3)-receptor occupancy and cognition in first episode schizophrenic patients. Schizophrenia Research. 2001;Vol. 49(issue Suppl 1, 2):194. [Google Scholar]
- Malla 2000 {published data only} .Malla A, Norman R, McLean T, Manchanda R, Townsend L, Cortese L, Harricharan R, Takhar J. Development of a community focussed early intervention program for psychosis: combining service, research and public education. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31 - Apr 2.2000. [Google Scholar]
- Malla 2001 {published data only} .Malla AK, Norman RM, Scholten DJ, Zirul S, Kotteda V. A comparison of long-term outcome in first-episode schizophrenia following treatment with risperidone or a typical antipsychotic. Journal of Clinical Psychiatry. 2001;62(3):179–84. doi: 10.4088/jcp.v62n0308. 00MEDLINE: 113057040. [DOI] [PubMed] [Google Scholar]
- Mandelson 2000 {published data only} .Mandelson M. A multi-centre, randomised, controlled trial of cognitive behavioural therapy in early schizophrenia (SOCRATES) National Research Register. 2000.
- Marder 1991 {published data only} .Marder SR, Mintz J, Van Putten T, Lebell M, Wirshing WC, Cronk KJ. Early prediction of relapse in schizophrenia: an application of receiver operating characteristic (ROC) methods. Psychopharmacology Bulletin. 1991;27(1):79–82. 00MEDLINE: 913198940. [PubMed] [Google Scholar]
- Marder 1994 {published data only} .Anderson C, Clark WR, True J, Ereshefsky L. Risperidone, a novel antipsychotic and weight change. Pharmacotherapy. 1993;Vol. 13:292. 00MEDLINE: 923618500. [Google Scholar]; Anderson C, True J, Ereshefsky L, Miller A. Risperidone clinical efficacy: role of the metabolite 9-hydroxyrisperidone. Psychopharmacology Bulletin. 1994;Vol. 30(issue 4):88. 00MEDLINE: 97313564; PSYCINFO: 1997–06350–0090. [Google Scholar]; Anderson CB, True JE, Ereshefsky L, Miller AL, Peters BL, Velligan DI. Risperidone dose, plasma levels and response. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 1993 May 22-27.1993. [Google Scholar]; Blaeser-Kiel G. Successful therapy with risperidone in schizophrenic negative syndrome [Schizophrenes negativsyndrom. Risperidon erfolgreich] TW Neurologie Psychiatrie. 1994;8(11):614–5. 00EMBASE: 19943424040. [Google Scholar]; Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. Journal of Clinical Psychopharmacology. 1995;15(4):243–9. doi: 10.1097/00004714-199508000-00002. 00MEDLINE: 75937060. [DOI] [PubMed] [Google Scholar]; Davis JM, Chen N. Evidence of efficacy of risperidone in schizophrenia. Schizophrenia Research. 2001;Vol. 49(issue 1–2):224–5. 00MEDLINE: 97313564; PSYCINFO: 1997–06350–0090. [Google Scholar]; De Coster R, Bowden C, Byloos M, Voina S, Coussement W, Meibach R, Arnott W, Heylen S. Endocrine effects of the new antipsychotic risperidone. Proceedings of the 9th International Congress of Endocrinology; Nice, France. 1992 Aug 30 -Sep 5.1992. [Google Scholar]; Lindenmayer J, Grochowski S, Hyman RB. Five factor model of schizophrenia - replication across samples. Schizophrenia Research. 1995;14(3):229–34. doi: 10.1016/0920-9964(94)00041-6. 00EMBASE: 1995049102; MEDLINE: 952839550. [DOI] [PubMed] [Google Scholar]; Lindenmayer JP, The Risperidone Study Group Incidence of EPS with risperidone compared with haloperidol and placebo in patients with chronic schizophrenia. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 1993 May 22-27.1993. [Google Scholar]; Marder SR. Risperidone: Clinical development: North American results. Clinical Neuropharmacology. 1992;15(Suppl 1 Pt A):92A–3A. doi: 10.1097/00002826-199201001-00049. [DOI] [PubMed] [Google Scholar]; Marder SR. Risperidone: Efficacy. Journal of Clinical Psychiatry. 1994;12:49–52. 00EMBASE: 19943424040. [Google Scholar]; Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. American Journal of Psychiatry. 1994;151(6):825–35. doi: 10.1176/ajp.151.6.825. 00EMBASE: 19942021810. [DOI] [PubMed] [Google Scholar]; Marder SR, Wirshing WC, Van Putten T, Mintz J, McKenzie J, Johnston Cronk K, Lebell M, Liberman RP. Fluphenazine vs placebo supplementation for prodromal signs of relapse in schizophrenia. Archives of General Psychiatry. 1994;51(4):280–7. doi: 10.1001/archpsyc.1994.03950040024003. 00MEDLINE: 942135750. [DOI] [PubMed] [Google Scholar]; McEvoy JP. Efficacy of risperidone on positive features of schizophrenia. Journal of Clinical Psychiatry. 1994;55(5 Suppl):18–21. 00EMBASE: 19941820020. [PubMed] [Google Scholar]; Meibach RC, The Risperidone Study Group A fixed dose, parallel group study of risperidone vs. haloperidol vs. placebo. Schizophrenia Research. 1993;9(2, 3):245. 00MEDLINE: 923618500. [Google Scholar]; Schooler NR. Negative symptoms in schizophrenia: Assessment of the effect of risperidone. Journal of Clinical Psychiatry. 1994;55(Suppl 5):22–8. 00MEDLINE: 943509250. [PubMed] [Google Scholar]; Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. Journal of Clinical Psychopharmacology. 1997;17(3):194–201. doi: 10.1097/00004714-199706000-00010. 00MEDLINE:97313564; PSYCINFO: 1997–06350–0090. [DOI] [PubMed] [Google Scholar]
- Marder 1996 {published data only} .Marder SR, Wirshing WC, Mintz J, McKenzie J, Johnston K, Eckman TA, Lebell M, Zimmerman K, Liberman RP. Two-year outcome of social skills training and group psychotherapy for outpatients with schizophrenia. American Journal of Psychiatry. 1996;153(12):1585–92. doi: 10.1176/ajp.153.12.1585. 00MEDLINE: 970979140. [DOI] [PubMed] [Google Scholar]
- Marques 2001b {published data only} .Marques AO, Elkis H, Louza MR, Yacubian J, Diegoli MS, Gattaz WF. A double blind placebo controlled study of conjugated estrogens added to haloperidol in patients with schizophrenia. Schizophrenia Research. 2001;49(1, 2):254. 00EMBASE: 20041306720. [Google Scholar]
- Marquez 2004a {published data only} .Marquez E, Keefe RSE, Purdon SE, Rock S, Alaka K, Ahmed S, Mohs RC. Is cognitive improvement with antipsychotic treatment pseudospecific?. Proceedings of the 157th Annual Meeting of the American Psychiatric Association; New York, USA. 2004 May 1-6.2004. [Google Scholar]
- Martényi 2000 {published data only} .Martényi F, Dossenbach M, Jakovljevic M, Metcalfe S. Predictive value of early antianxiety effect on the acute-antipsychotic outcome: a comparison of fluphenazine and olanzapine. Journal of the European College of Neuropsychopharmacology. 2000;10(Suppl 3):S305. [Google Scholar]
- McConchie 2004 {published data only} .Mcconchie MA, Berger GE, Proffitt TM, Yuen HP, Wood S, Smith D, Horrobin D, Mcgorry PD. Effect of diagnostic heterogeneity on response to ethyl- eicosapentaenoic acid (epa) in first-episode psychosis. Schizophrenia Research. 2004;67(1):147–8. 00ISI: 0001887881003520. [Google Scholar]
- McEvoy 2003 {published data only} .McEvoy J, Lieberman JA, Perkins D, Hamer RM, Sharma T, Zipursky R, Kahn R, Gur R, Centorrino F, Glick I. Long-term efficacy and safety of atypical and conventional antipsychotic drugs in first episode schizophrenia. Schizophrenia Research. 2003;60(1):313. [Google Scholar]; McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Sharma T, Zipursky RB. Long-term olanzapine treatment versus haloperidol in first-episode psychosis. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]
- McEvoy 2006b {published data only} .McEvoy JP. Efficacy and tolerability of olanzapine, quetiapine and risperidone in the treatment of first-episode psychosis a randomised double-blind 52-week comparison. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- McEvoy 2006d {published data only} .McEvoy JP, Perkins DO, Gu H, Hamer RM, Lieberman JA. Clinical effectiveness and predictors of treatment non-adherence: comparison of olanzapine, quetiapine, and risperidone in first-episode psychosis. Schizophrenia Research. 2006;86(Suppl 1):S130. doi: 10.1016/j.schres.2006.06.021. [DOI] [PubMed] [Google Scholar]; McEvoy JP, Perkins DO, Gu H, Hamer RM, Lieberman JA. Olanzapine, quetiapine, and risperidone in the treatment of first-episode psychosis: effectiveness and factors influencing adherence to treatment. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S425. [Google Scholar]
- McEvoy 2006f {published data only} .McEvoy JP, Johnson J, Perkins D, Lieberman JA, Hamer RM, Keefe RSE, Tohen M, Glick ID, Sharma T. Insight in first-episode psychosis. Psychological Medicine. 2006;36(10):1385–93. doi: 10.1017/S0033291706007793. 00EMBASE: 2006439216; MEDLINE: 16740175; : 2006185350. [DOI] [PubMed] [Google Scholar]
- McGlashan 1999 {published data only} .McGlashan TH. Treatment intervention in the New Haven prime clinic prodromal sample. Current Opinion in Psychiatry. 1999;Vol. 12(issue Suppl 1):S62. [Google Scholar]
- McGlashan 2006 {published data only} .Mcglashan T, Zipursky R, Perkins D, Addington J, Miller T, Woods S, Hawkins K, Hoffman R, Tohen M, Breier A. Pharmacotherapy in the prodromal phase of first psychosis: results and implications. Schizophrenia Research. 2004;70(1):6. 00ISI: 0002245511000130. [Google Scholar]; McGlashan TH. Intervention in the prodrome to first psychosis. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]; McGlashan TH, Miller TJ, Woods SW. Psychosis treatment prior to psychosis onset: ethical issues. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):15. doi: 10.1016/s0920-9964(01)00238-9. [DOI] [PubMed] [Google Scholar]; McGlashan TH, Miller TJ, Woods SW, Rosen J, Davidson L, Preda A, Markovich P. Ethical issues in the pre-onset treatment of schizophrenia. 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; McGlashan TH, Miller TJ, Woods SW, Rosen J, Davidson L, Preda A, Markovich P. Ethical issues in the pre-onset treatment of schizophrenia. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10.2001. [Google Scholar]; McGlashan TH, Miller TJ, Zipursky RB, Woods SW, Perkins DO, Hawkins KA, Addington JM. Intervention in the schizophrenic prodrome: the prevention through risk identification, management, and education initiative. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; McGlashan TH, Vaglum P, Friis S, Johannessen JO, Simonsen E, Larsen TK, Melle I, Haahr U, Opjordsmoen S, Zipursky R, Perkins D, Addington J, Miller T, Woods S, Hoffman R, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Early detection and intervention in first episode psychosis: empirical update of the tips and prime projects. Schizophrenia Bulletin. 2005;31:496. [Google Scholar]; McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein L, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. American Journal of Psychiatry. 2006;163(5):790–9. doi: 10.1176/ajp.2006.163.5.790. 00CINAHL: 2009175479; MEDLINE: 166483180. [DOI] [PubMed] [Google Scholar]; McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller TJ, Woods SW, Hawkins KA, Hoffman R, Lindborg S, Tohen M, Breier A. The PRIME North America randomised double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophrenia Research. 2003;61(1):7–18. doi: 10.1016/s0920-9964(02)00439-5. 00MEDLINE: 126487310. [DOI] [PubMed] [Google Scholar]; Mcglashan TH, Zipursky RB, Perkins DO, Addington J. Olanzapine for treatment of the schizophrenia prodrome: 2-year results of a randomised placebo-controlled study. Schizophrenia Research. 2004;67(1):164. 00ISI: 0001887881003900. [Google Scholar]; Mcglashan TH, Zipursky RB, Perkins DO, Addington J, Woods SW, Miller TJ, Lindborg S. Olanzapine vs. placebo for prodromal schizophrenia. Schizophrenia Research. 2004;67(1):6. 00ISI: 0001887881000100. [Google Scholar]; McGlashan TH, Zipursky RB, Perkins DO, Addington J, Woods SW, Miller TJ, Lindborg S, Marquez E, Hawkins K, Hoffman RE. Olanzapine versus placebo treatment of the schizophrenia prodrome: One year results. Schizophrenia Research. 2003;60(1):295. [Google Scholar]; McGlashan TH, Zipursky RB, Perkins DO, Addington JM, Woods SW, Lindborg S, Breier AF. Olanzapine versus PBO for the schizophrenic prodrome: one-year results. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; McGlashlan TH, Zipursky RB, Perkins DO, Addington J, Miller TH, Woods SW, Marquez E, David SM, Tohen M, Breier A. A prodromal trial of olanzapine versus placebo baseline results. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]; Miller TJ, Zipursky RB, Perkins D, Addington J, Woods SW, Hawkins KAHR, Preda A, Epstein I, Addington D, Lindborg S, Marquez ETM, Breier A, McGlashan TH. The PRIME North America randomised double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. II. Baseline characteristics of the ”prodromal“ sample. Schizophrenia Research. 2003;61(1):19–30. doi: 10.1016/s0920-9964(02)00440-1. 00MEDLINE: 126487320. [DOI] [PubMed] [Google Scholar]; *; Woods SW, Breier A, Zipursky RB, Perkins DO, Addington J, Miller TJ, Hawkins KA, Marquez E, Lindborg SR, Tohen M, McGlashan TH. Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biological Psychiatry. 2003;Vol. 54(issue 4):453–64. doi: 10.1016/s0006-3223(03)00321-4. 00MEDLINE: 129152900. [DOI] [PubMed] [Google Scholar]
- McGorry 1997a {published data only} .McGorry PD, Cocks J, Longley T, Webster K, Ellkins K, Hallgren M, Plowright D. Very low dose neuroleptic-treatment of first episode psychosis - is it feasible in routine clinical care? Schizophrenia Research. 1997;Vol. 24(issue 1, 2):208. 00MEDLINE: 150373350. [Google Scholar]
- McGorry 1997b {published data only} .McGorry PD, Edwards J, Mihalopoulos C, Jackson H. Is early intervention effective and cost effective? Schizophrenia Research. 1997;Vol. 24(issue 1, 2):208. 00MEDLINE: 150373350. [Google Scholar]
- McGorry 2002b {published data only} .Gleeson J, Wade D, Mcgorry P, Albiston D, Castle D, Gilbert M, Young D. Episode ii: prevention of relapse following early psychosis. Schizophrenia Research. 2004;70(1):61–2. 00ISI: 0002245511001650. [Google Scholar]; McGorry P. Can the onset of schizophrenia be delayed or prevented? International Journal of Neuropsychopharmacology. 2002;Vol. 5(issue Suppl 1):S26. [Google Scholar]; McGorry P, Adlard S, Yung A, McDonald A, Phillips L, Hearn N. Detection and intervention in pre-psychotic schizophrenia. Current Opinion in Psychiatry. 1999;Vol. 12(issue Suppl 1):S62. [Google Scholar]; McGorry PD, Hearn N, Germano D, Bravin J, Phillips LJ, Yung AR, Blair A, Francey S, Patton G. Prepsychotic intervention in schizophrenia: a stitch in time?. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]; McGorry PD, Yung AR, Phillips L, Adlard S, Hallgren M, Patton G, Hearn N. Pre-psychotic intervention in schizophrenia: a stitch in time? Schizophrenia Research. 1998;Vol. 29(issue 1, 2):160. [Google Scholar]; *; McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, McDonald T, Blair A, Adlard S, Jackson H. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Archives of General Psychiatry. 2002;59(10):921–8. doi: 10.1001/archpsyc.59.10.921. 00MEDLINE: 22252464; : 123658790. [DOI] [PubMed] [Google Scholar]
- McGorry 2002c {published data only} .McGorry PD, Cocks J, Harrigan S, Elkins K, Lambert T, Owen S. Very low-dose risperidone treatment in first-episode psychosis: how effective is it?. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]
- McQuade 2003 {published data only} .Abou Gharbia N, McQuade R, Jody D, Kujawa M, Carson W, Iwamoto T, Archibald D, Stock E. Comparative study of the long-term effects of aripiprazole and olanzapine treatment on body weight. Proceedings of the Thematic Conference of the World Psychiatric Association on ”Treatments in Psychiatry: An Update“; Florence, Italy. 2004 Nov 10-13.2004. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 2. 2002:50–110. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Jody D, Mcquade Rd, Kujawa M, Carson W, Iwamoto T, Archibald D, Stock E. Long-term weight effects of aripiprazole versus olanzapine. Schizophrenia Research. 2004;67(1):187. 00ISI: 0001887881004470. [Google Scholar]; McQuade RD, Jody D, Kujawa MJ, Jr WHC, Iwamoto T, Archibald DG, Stock EG. Long-term weight effects of aripiprazole versus olanzapine. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; McQuade RD, Stock E, Marcus R, Jody D, Gharbia NA, Vanveggel S, Carson WH. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomised, double-blind study. Journal of Clinical Psychiatry. 2004;65(Suppl 18):47–56. 00MEDLINE: 156003840. [PubMed] [Google Scholar]
- Melle 2006 {published data only} .Melle I, Johannesen JO, Friis S, Haahr U, Joa I, Larsen TK, Opjordsmoen S, Rund BR, Simonsen E, Vaglum P, McGlashan T. Early detection of the first episode of schizophrenia and suicidal behavior. American Journal of Psychiatry. 2006;163(5):800–4. doi: 10.1176/ajp.2006.163.5.800. 00MEDLINE: 166483190. [DOI] [PubMed] [Google Scholar]
- Melnyk 1966 {published data only} .Melnyk WT, Worthington AG, Laverty SG. Abrupt withdrawal of chlorpromazine and thioridazine from schizophrenic in-patients. Canadian Psychiatric Association Journal. 1966;11(5):410–3. 00PSYCINFO: 1966–13288–0010. [Google Scholar]
- Merlo 2000 {published data only} .Merlo MCG, Hofer H, Marder SR. Effects on clinical psychopathology and fine motor functions of 2 versus 4 mg risperidone in first episode psychotic patients. Schizophrenia Research. 2000;41(1):26. 00ISI: 0001887881002360. [Google Scholar]
- Merlo 2002b {published data only} .Merlo MCG, Hofer H, Fabry MG, Marder SR. Improvement of cognitive functions in acute first-episode psychosis treated with risperidone. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):27. 00EMBASE: 20041306720. [Google Scholar]; Merlo MCG, Hofer H, Gekle W, Berger G, Ventura J, Panhuber I, Latour G, Marder SR. Risperidone, 2 mg/ day vs. 4 mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioning. Journal of Clinical Psychiatry. 2002;63(10):885–91. doi: 10.4088/jcp.v63n1006. 00EMBASE: 20023835270. [DOI] [PubMed] [Google Scholar]
- Merson 1992 {published data only} .Merson S, Tyrer P, Carlen D, Johnson T. The cost of treatment of psychiatric emergencies: a comparison of hospital and community services. Psychological Medicine. 1996;26(4):727–34. doi: 10.1017/s0033291700037740. 00MEDLINE: 88177070. [DOI] [PubMed] [Google Scholar]; Merson S, Tyrer P, Onyett S, Lack S, Birkett P, Lynch S, Johnson T. Early intervention in psychiatric emergencies: a controlled clinical trial. Lancet. 1992;339(8805):1311–4. doi: 10.1016/0140-6736(92)91959-c. 00MEDLINE: 922611940. [DOI] [PubMed] [Google Scholar]; Tyrer P, Merson S, Onyett S, Johnson T. The effect of personality disorder on clinical outcome, social networks and adjustment: a controlled clinical trial of psychiatric emergencies. Psychological Medicine. 1994;24(3):731–40. doi: 10.1017/s0033291700027884. 00MEDLINE: 950838280. [DOI] [PubMed] [Google Scholar]
- Michael 2005 {published data only} .Michael A. Improving social recovery in early affective and non-affective psychosis: a randomised controlled trial of Social Recovery oriented Cognitive Behaviour Therapy (ISREP) National Research Register. 2005;Vol. 4 [Google Scholar]
- Miller 2004 {published data only} .Miller TJ, Rosen JI, D’andrea J, Woods SW, Mcglashan TH. Outcome of prodromal syndromes: Sips predictive validity. Schizophrenia Research. 2004;70(1):44. 00ISI: 0002245511001130. [Google Scholar]
- Min 2001 {published data only} .Min Q, Li M, Wu T. Early rehabilitation of schizophrenics. Chinese Mental Health Journal. 2001;15(5):352–4. 00CAJ: MEDI01110. [Google Scholar]
- Montero 2005 {published data only} .Montero I, Hernandez I, Asencio A, Bellver F, LaCruz M, Masanet MJ. Do all people with schizophrenia receive the same benefit from different family intervention programs? Psychiatry Research. 2005;133(2-3):187–95. doi: 10.1016/j.psychres.2002.12.001. 00CENTRAL: CN–00512983; PUBMED: 157409940. [DOI] [PubMed] [Google Scholar]
- Morken 2005 {published data only} .Morken G, Grawe RW, Widen JH. A randomised controlled trial in recent-onset schizophrenia. Effects on compliance of two years of continued intervention. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):S521. [Google Scholar]
- Morrison 2004d {published data only} .Morrison A. Findings from a randomised controlled trial and clinical service delivering cognitive therapy to people at ultra-high risk of developing psychosis. Schizophrenia Research. 2004;70(1):43–4. 00ISI: 0002245511001120. [Google Scholar]; Morrison A. Follow-up of prodromal symptoms. National Research Register. 2004;Vol. 3 [Google Scholar]; Morrison A, Bentall R, French P, Kilcommons A, Lewis SW. Very early intervention in prodromal psychosis: a randomised trial. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):42. 00EMBASE: 20041306720. [Google Scholar]; Morrison A, French P, Watford L, Lewis S, Kilcommons A, Green J, Lomax S, Bentall R. Randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultra-high risk. Schizophrenia Research. 2004;70(1):63. doi: 10.1192/bjp.185.4.291. 00ISI: 0002245511001690. [DOI] [PubMed] [Google Scholar]; Morrison AP. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: results of arandomised controlled trial. Schizophrenia Research. 2006;86(Suppl 1):S59. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]; Morrison AP. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: results of a randomised controlled trial. Schizophrenia Research. 2006;86(Suppl 1):S6. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]; Morrison AP, Bentall RP, French P, Kilcommons A, Green J, Walford L, Lewis SW. Cognitive therapy in ultra high risk individuals for psychosis: randomised controlled trial. Schizophrenia Research. 2003;60(1):326. 00EMBASE: 20041306720. [Google Scholar]; Morrison AP, Bentall RP, French P, Walford L, Kilcommons A, Knight A, Kreutz M, Lewis SW. Randomised controlled trial of early detection and cognitive therapy for preventing transition to psychosis in high-risk individuals. Study design and interim analysis of transition rate and psychological risk factors. British Journal of Psychiatry Supplementum. 2002;181(43):s78–84. doi: 10.1192/bjp.181.43.s78. 00MEDLINE: 12271805; PASCAL: 2002–0516356 INIST0100 INRA0200 BRGM0204 200210310. [DOI] [PubMed] [Google Scholar]; Morrison AP, Bentall RP, French P, Walford L, Kilcommons A, Lewis S. Early detection and intervention for psychosis in primary care. Proceedings of the 12th World Congress of Psychiatry; Yokohama, Japan. 2002 Aug 24-29.2002. [Google Scholar]; Morrison AP, French P, Walford L, Lewis S, Kilcommons A, Green J, Lomax S, Bentall R. A randomised controlled trial of cognitive therapy for the prevention of psychosis in people at ultra-high risk. Schizophrenia Research. 2004;67(1):7. doi: 10.1192/bjp.185.4.291. 00ISI: 0001887881000120. [DOI] [PubMed] [Google Scholar]; Morrison AP, French P, Walford L, Lewis SW, Kilcommons A, Green J, Parker S, Bentall RP. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. British Journal of Psychiatry. 2004;185:291–7. doi: 10.1192/bjp.185.4.291. 00EMBASE: 19981872010. [DOI] [PubMed] [Google Scholar]; *; Morrison T. Early detection and intervention for psychosis in primary care. National Research Register. 2001;Vol. 1 [Google Scholar]; Morrison T, Bentall R, French P, Kilcommons A, Green J, Lewis S. Early detection and intervention for psychosis in primary care. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]
- Morrison 2006b {published data only} .Morrison T. Early detection and psychological intervention for individuals at high risk of psychosis. EDIE-2. Unpublished Report. 2006.
- Mortimer 2003c {published data only} .Mortimer A. The European first episode schizophrenia trial: comparison of outcome in first episode schizophrenia with different low dose antipsychotic regimens (EUFEST) National Research Register. 2003;Vol. 1 [Google Scholar]
- Mosher 1978 {published data only} .*; Matthews SM, Roper MT, Mosher LR, Menn AZ. A non-neuroleptic treatment for schizophrenia: analysis of the two-year post-discharge risk of relapse. Schizophrenia Bulletin. 1979;5(2):322–33. doi: 10.1093/schbul/5.2.322. [DOI] [PubMed] [Google Scholar]; Mosher LR, Menn AZ. Community residential treatment for schizophrenia: two-year follow-up. Hospital and Community Psychiatry. 1978;29(11):715–23. doi: 10.1176/ps.29.11.715. [DOI] [PubMed] [Google Scholar]
- Mottaghipour 2000 {published data only} .Mottaghipour Y, Woodland L, Sara G. Efficacy and effectiveness in early psychosis family education group program. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31- Apr 2.2000. [Google Scholar]
- Mottaghipour 2006 {published data only} .Mottaghipour Y, Shari V, Salesian N, Seddigh A, Alaghbandrad J, Shahrivar Z, Mahmoudi J. Development and evaluation of culturally appropriate services for families of patients with first-episode psychosis in Iran. Schizophrenia Research. 2006;86(Suppl 1):S149–50. [Google Scholar]
- Mozes 2006 {published data only} .Mozes T, Ebert T, Michal S-E, Spivak B, Weizman A. An open-label randomised comparison of olanzapine versus risperidone in the treatment of childhood-onset schizophrenia. Journal of Child and Adolescent Psychopharmacology. 2006;16(4):393–403. doi: 10.1089/cap.2006.16.393. 00EMBASE: 2006538413; MEDLINE: 16958565; PSYCINFO: 2006–12802–0030. [DOI] [PubMed] [Google Scholar]
- Mueller 2005b {published data only} .Mueller N, Riedel M, Dehning S, Spellmann I, Mueller-Arends A, Cerovecki A, Goldstein-Mueller B, Moeller H-J, Schwarz M. Cox-2 inhibition in schizophrenia and major depression - the advent of immunotherapy in psychiatry?. XIII World Congress of Psychiatry; Cairo, Egypt. 2005 10-15th Sept.2005. [Google Scholar]
- Muller 2004 {published data only} .Muller N. A randomised, controlled trial of celecoxib adjunctive to amisulpride in recent-onset schizophrenia. Stanley Foundation Research Programs. 2004.
- Murasaki 1999a {published data only} .Murasaki M, Yamauchi T, Yagi G, Nakajima T, Nakane Y, Kudo Y. Early phase II study of quetiapine fumarate on schizophrenia. Nihon Shinkei Seishin Yakurigaku Zasshi. 1999;19(2):53–66. 00ISIP: 0002334421005010. [PubMed] [Google Scholar]
- Newton 2005 {published data only} .Newton E, Landau S, Smith P, Monks P, Shergill S, Wykes T. Early psychological intervention for auditory hallucinations: an exploratory study of young people’s voices groups. Journal of Nervous and Mental Disease. 2005;193(1):58–61. doi: 10.1097/01.nmd.0000149220.91667.fa. 00MEDLINE: 20050889800. [DOI] [PubMed] [Google Scholar]
- Newton 2006 {published data only} .Newton E, Landau S. Cognitive remediation therapy (CRT): a new psychological treatment for young patients with schizophrenia. Schizophrenia Research. 2006;86(Suppl 1):S143–4. doi: 10.1016/j.schres.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Nienhuis 2006 {published data only} .Nienhuis F, Wunderink A, Wiersma D. Feasibility of carrying out maintenance or targeted medication treatment in first onset schizophrenia: the mesifos RCT. Schizophrenia Bulletin. 2005;31:498. [Google Scholar]; Nienhuis F, Wunderink A, Wiersma D, Van Den Bosch RJ, Bruggeman R, Faber G, Van Der Linde J, Noorthoorn E, Slooff CJ, Vlaminck P. Mesifos: medication strategies in first onset schizophrenia effects of short versus sustained antipsychotic treatment on quality of life after first episode psychosis: a randomised trial. Schizophrenia Research. 2003;60(1):296–7. [Google Scholar]; *; Nienhuis FJ, Wunderink A, Wiersma D. Feasibility of carrying out targeted and maintenance treatment in first episode psychosis. Schizophrenia Research. 2006;86(Suppl 1):S50–1. [Google Scholar]
- Nordentoft 2002 {published data only} .Jeppesen P, Abel MB, Krarup G, Jorgensen P, Nordentoft M. Family burden and expressed emotion in first episode psychosis. The OPUS-trial. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]; Jeppesen P, Hemmingsen R, Jírgensen P, Reisby N, Abel MB, Nordentoft M. Opus project: impact of mental disorder on caregivers. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]; Jeppesen P, Hemmingsen R, Reisby N, Jørgensen P, Nordentoft M, Abel M-B. The impact of mental disorder on caregivers. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]; Jeppesen P, Nordentoft M, Abel M, Hemmingsen RP, Joergensen, Kassow P. Opus-project: a RCT of integrated psychiatric treatment for recent onset psychotic patients. Schizophrenia Research. 2001;49(1, 2):262. [Google Scholar]; Jeppesen P, Nordentoft M, Jorgensen P, Abel MB, Reisby N, Hemmingsen R, Kassow P, Guliaelev G. Opus-project: better compliance? A randomised-controlled trial of integrated care of first-episode psychotic patients conference abstract. Schizophrenia Research. 1999;36(1-3):327. 00ISI: 0001887881000820. [Google Scholar]; Jeppesen P, Petersen L, Thorup A, Abel M-B, Oehlenschlaeger J, Christensen TO, Krarup G, Hemmingsen R, Jorgensen P, Nordentoft M. Integrated treatment of first-episode psychosis: effect of treatment on family burden: OPUS trial. British Journal of Psychiatry. 2005;48(Suppl):s85–90. doi: 10.1192/bjp.187.48.s85. [DOI] [PubMed] [Google Scholar]; Jørgensen P, Jeppesen P, Abel MB, Kassow P, Krarup G, Hemmingsen R, Nordentoft M. Early intervention in schizophrenia. Nordic Journal of Psychiatry. 2002;Vol. 56(issue 2):8. 00ISIP: 0002334421005010. [Google Scholar]; Jørgensen P, Nordentoft M, Abel MB, Gouliaev G, Jeppesen P, Kassow P. Early detection and assertive community treatment of young psychotics: the Opus study rationale and design of the trial. Social Psychiatry and Psychiatric Epidemiology. 2000;35(7):283–7. doi: 10.1007/s001270050240. 00MEDLINE: 110165220. [DOI] [PubMed] [Google Scholar]; Nordentoft M, Jeppesen P, Abel M, Kassow P, Petersen L, Thorup A, Krarup G, Hemmingsen R, Jorgensen P. OPUS study: suicidal behaviour, suicidal ideation and hopelessness among patients with first-episode psychosis. One-year follow-up of a randomised controlled trial. British Journal of Psychiatry Supplementum. 2002;181(Suppl 43):S98–106. doi: 10.1192/bjp.181.43.s98. 00MEDLINE: 12271808; PASCAL: 2002–0516752 INIST0100 INRA0200 BRGM0204 200210310. [DOI] [PubMed] [Google Scholar]; Nordentoft M, Jeppesen P, Abel M, Petersen L, Thorup A, Christensen T, Ohlenschlæger J, Jorgensen P. Controlled trial of integrated psychiatric treatment in first-episode psychosis - clinical outcome improved. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]; Nordentoft M, Jeppesen P, Abel MB, Hemmingsen R, Reisby N. Can duration of untreated psychosis be shortened and does optimal treatment program improve outcome? A randomised controlled study. Nordisk Psykiatrisk Tidsskrift. 1998;52(41):76. 00EMBASE: 19981242080. [Google Scholar]; Nordentoft M, Jeppesen P, Jorgensen P, Abel MB, Kassow P, Reisby N, Hemmingsen R. OPUS - project: a randomised controlled trial of first episode psychotic patients better compliance. Proceedings of the 2nd International Conference on Early Psychosis; New York, New York, USA. 2000 Mar 31-Apr 2; 2000. 00MEDLINE: 12271808; PASCAL: 2002–0516752 INIST0100 INRA0200 BRGM0204 200210310. [Google Scholar]; Nordentoft M, Jeppesen P, Kassow P, Abel M, Petersen L, Thorup A, Cristensen T, Øhlenschlæger J, Jørgensen P. Opus-project: a randomised controlled trial of integrated psychiatric treatment in first-episode psychosis-clinical outcome improved. Schizophrenia Research. 2002;53(3 Suppl 1):51. 00MEDLINE: 110165220. [Google Scholar]; Nordentoft M, Jeppesen P, Petersen L, Thorup A, Abel M, Ohlenschlaeger JK, Jorgensen P, Christensen T. Controlled trial of integrated psychiatric treatment in first episode psychosis. Schizophrenia Research. 2003;60(1):297. [Google Scholar]; Nordentoft M, Jeppesen P, Petersen L, Thorup A, Jorgensen P. Duration of untreated psychosis predicts psychotic symptoms but not negative symptoms. Schizophrenia Bulletin. 2005;31:234. [Google Scholar]; Nordentoft M, Jeppesen P, Petersen L, Thorup a, Krarup G, Abel M, Oehlenschlaeger J, Christensen T, Jorgensen P. The Danish opus-trial: a randomised controlled trial of integrated treatment among 547 first-episode psychotic patients. One and two years follow-up. Schizophrenia Research. 2004;67(1):35–6. 00ISI: 0001887881000810. [Google Scholar]; Nordentoft M, Jeppesen P, Ventegodt AT, Joergensen P, Abel M, Petersen L, Hemmingsen RP. Opus-project: a randomised controlled trial of first episode psychotic patients: patient satisfaction, depression and suicidal behaviour. Schizophrenia Research. 2001;49(1, 2):265. 00MEDLINE: 170552270. [Google Scholar]; Nordentoft M, Jorgensen P, Jeppesen P, Kassow P, Abel MB, Resiby N, Hemmingsen R, Guliaelev G. Opus-project: differences in clinical and social outcome of a randomised controlled trial of integrated care of first-episode psychotic patients. Schizophrenia Research. 1999;36(1-3):330. 00ISI: 0001887881000820. [Google Scholar]; Nordentoft M, Reisby N, Jeppesen P, Abel M-B, Kassow P, Jírgensen P. Opus-project: differences in clinical and social outcome of a randomized controlled trial of integrated care of first-episode psychotic patients. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]; Rosenbaum B, Valbak K, Harder S, Knudsen P, Koster A, Lajer M, Lindhardt A, Winther G, Petersen L, Jorgensen P, Nordentoft M, Andreasen AH. The Danish National Schizophrenia Project: prospective, comparative longitudinal treatment study of first-episode psychosis. British Journal of Psychiatry. 2005;186:394–9. doi: 10.1192/bjp.186.5.394. 00MEDLINE: 158637430. [DOI] [PubMed] [Google Scholar]; Thorup A. The influence of social network, dup, age, gender and treatment on negative symptoms in first-episode psychosis. Schizophrenia Research. 2004;70(1):89–90. 00ISI: 0002245511002490. [Google Scholar]; Thorup A, Nordentoft M, Petersen L, Oehlensschlaeger J, Abel M, Jeppesen P, Hemmingsen R. The Danish OPUS-project: psychopathology and gender differences in first episode psychotic patients. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]; Thorup A, Petersen L, Jeppesen P, Christensen T, Nordentoft M. The opus trial: gender differences in a sample of 547 first - episode psychotic patients. Schizophrenia Bulletin. 2005;31:505. [Google Scholar]; *; Ventegodt AT, Jeppesen P, Petersen L, Abel M, Nordentoft M, Kassow P, Hemmingsen RP. Controlled trial of first episode psychotic patients: gender differences, social network and negative symptoms. Schizophrenia Research. 2001;49(1,2):267. 00MEDLINE: 170552270. [Google Scholar]
- Nuechterlein 1992 {published data only} .Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental processes in schizophrenic disorders: longitudinal studies of vulnerability and stress. Schizophrenia Bulletin. 1992;18(3):387–425. doi: 10.1093/schbul/18.3.387. 00MEDLINE: 14113290. [DOI] [PubMed] [Google Scholar]
- Nuechterlein 2005 {published data only} .Nuechterlein KH, Subotnik KL, Ventura J, Gitlin MJ, Green MF, Wallace CJ, Becker DR, Liberman RP, Drake RE, Mintz J. Advances in improving and predicting work outcome in recent - onset schizophrenia. Schizophrenia Bulletin. 2005;31:530. [Google Scholar]
- Nugter 1997 {published data only} .Nugter MA, Dingemans PMAJ, Linszen DH, Van der Does AJW, Gersons BPR. Parental communication deviance: its stability and the effect of family treatment in recent-onset schizophrenia. Acta Psychiatrica Scandinavica. 1997;95(3):199–204. doi: 10.1111/j.1600-0447.1997.tb09620.x. 00MEDLINE: 91118520. [DOI] [PubMed] [Google Scholar]
- O’Donnell 2003b {published data only} .O’Donnell C. VIP (Vitamins In Psychosis) study. A randomised double blind placebo controlled trial of the effects of vitamin B12, B6 and folic acid augmentation on cognition and symptoms in early psychosis. Stanley Foundation Research Programs. 2003.
- O’Regan 2005 {published data only} .O’Regan MK, O’Donnell C, Papas A, Dell’Olio M, Purcell R, McGorry PD. medication compliance needed to achieve therapeutic benefit in first episode psychosis (FEP) Australian and New Zealand Journal of Psychiatry. 2005;39(Suppl 2):A74. 00MEDLINE: 22252464; : 123658790. [Google Scholar]
- O’Sullivan 2001 {published data only} .O’Sullivan R, Fryburg D, Siu C, Simpson G. Insulin resistance in olanzapine and ziprasidone treated subjects: interim results of a double-blind controlled six-week trial. Schizophrenia Research. 2001;49(1, 2):241. [Google Scholar]
- Offord 1998 {published data only} .Offord SJ. M100907, a highly selective 5-HT2A antagonist for treatment of schizophrenia: early indication of safety and clinical activity in schizophrenic patients. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16.1998. [Google Scholar]
- Ohlenschlaeger 2002 {published data only} .Ohlenschlaeger J, Thorup A, Petersen L, Jeppesen P, Abel M, Nordentoft M. Coercion in first episode psychosis. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]
- Oosthuizen 2002a {published data only} .Oosthuizen P, Emsley RA, Roberts MC, Turner J, Keyter L, Keyter NTM. Depressive symptoms at baseline predict fewer negative symptoms at follow-up in patients with first-episode schizophrenia. Schizophrenia Research. 2002;58(2-3):247–52. doi: 10.1016/s0920-9964(01)00375-9. 00MEDLINE: 124091650. [DOI] [PubMed] [Google Scholar]
- Oosthuizen 2004 {published data only} .Oosthuizen P, Emsley R, Turner HJ, Keyter N. A randomised, controlled comparison of the efficacy and tolerability of low and high doses of haloperidol in the treatment of first-episode psychosis. International Journal of Neuropsychopharmacology. 2004;7(2):125–31. doi: 10.1017/S1461145704004262. 00EMBASE: 2004272629; MEDLINE: 150031470. [DOI] [PubMed] [Google Scholar]
- Opjordsmoen 2000 {published data only} .Opjordsmoen S, Brunsvik S, Melle I, Dahl A, Friis S, Haahr U, Hustoft K, Johannessen JO, Larsen TK, McGlashan TH, Simonsen E, Vaglum P. A comparison between novel and traditional antipsychotics as first-line medication in early psychosis. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2; 2000. 00MEDLINE: 991073190. [Google Scholar]
- Pagsberg 2004 {published data only} .Pagsberg AK, Jagersma E, Baare WFC, Mackeprang T, Glenthoej BY. Change in caudate nucleus volume after three-month treatment in drug-naive first-episode schizophrenia patients. Schizophrenia Research. 2004;67(1):99. 00ISI: 0001887881002360. [Google Scholar]
- Pai 1982 {published data only} .Pai S, Kapur RL. Evaluation of home care treatment for schizophrenic patients. Acta Psychiatrica Scandinavica. 1983;67(2):80–8. doi: 10.1111/j.1600-0447.1983.tb06726.x. 00MEDLINE: 832004430. [DOI] [PubMed] [Google Scholar]; Pai S, Kapur RL. Impact of treatment intervention on the relationship between dimensions of clinical psychopathology, social dysfunction and burden on the family of psychiatric patients. Psychological Medicine. 1982;12(3):651–8. doi: 10.1017/s0033291700055756. 00MEDLINE: 830397850. [DOI] [PubMed] [Google Scholar]; Pai S. Nagarajaiah. Treatment of schizophrenic patients in their homes through a visiting nurse - some issues in the nurse’s training. International Journal of Nursing Studies. 1982;19(3):167–72. doi: 10.1016/0020-7489(82)90034-7. 00MEDLINE: 83081824; PSYCINFO: 71–075150. [DOI] [PubMed] [Google Scholar]; Pai S, Roberts EJ. Follow-up study of schizophrenic patients initially treated with home care. British Journal of Psychiatry. 1983;143:447–50. doi: 10.1192/bjp.143.5.447. 00MEDLINE: 66402120. [DOI] [PubMed] [Google Scholar]
- Painter 2001 {published data only} .Painter M. The use of relapse prevention plans based on early warning signs and coping strategy enhancement as a means of reducing relapse in schizophrenia. National Research Register. 2001;Vol. 3 [Google Scholar]
- Pan Miao 2004b {published data only} .Pan M, Wang H, Zhang S. A controlled study of domestic quetiapine and risperidone in the treatment of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2004;10(4):244–6. 00MEDLINE: 169741930. [Google Scholar]
- Papas 2005 {published data only} .Papas A, O’Donnell C, O’Regan MK, Proffitt TM, Maruff P, Berger G, Stephens T, McGorry PD. Reduction in homocysteine improves cognitive functioning in first episode psychosis. Australian and New Zealand Journal of Psychiatry. 2005;39(Suppl 2):A73. 00MEDLINE: 222524640. [Google Scholar]
- Parellada 2006 {published data only} .Parellada MJ, Moreno D, Ruiz-Sancho A, Medina O, Arango C. Open-label randomised trial comparing the efficacy and tolerability of quetiapine and olanzapine in first episode psychosis in adolescents. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S377. [Google Scholar]
- Parent 1983 {published data only} .Parent M, Toussaint C. Flupenthixol versus haloperidol in acute psychosis. Pharmatherapeutica. 1983;3(5):354–64. 00MEDLINE: 68443720. [PubMed] [Google Scholar]
- Paulman 1980 {published data only} .Paulman R, Meyers Abell J. Chapman’s versus Broen and Storms’ theory of schizophrenic thought disorder: an empirical comparison. Journal of Clinical Psychology. 1980;36:844–52. doi: 10.1002/1097-4679(198010)36:4<844::aid-jclp2270360402>3.0.co;2-m. 00MEDLINE: 74407340. [DOI] [PubMed] [Google Scholar]
- Perez 2003 {published data only} .Perez R, Gonzalez-Blanch C, Sierra-Biddle D, Martinez I, Vazquez-Barquero JL, Crespo-Facorro B. Efficacy and safety of olanzapine, risperidone and haloperidol in acute treatment of patients with first episode psychosis. Schizophrenia Research. 2003;60(1):298–9. [Google Scholar]
- Perkins 2000 {published data only} .Perkins D. Modified cognitive behavior therapy for first-episode schizophrenia. Stanley Foundation Research Programs. 2000.
- Perkins 2006 {published data only} .Perkins DO, Johnson JL, Hamer RM, Zipursky RB, Keefe RS, Centorrhino F, Green AI, Glick IB, Kahn RS, Sharma T, Tohen M, McEvoy JP, Weiden PJ, Lieberman JA, HGDH Research Group Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophrenia Research. 2006;83(1):53–63. doi: 10.1016/j.schres.2005.10.016. 00MEDLINE: 165299100. [DOI] [PubMed] [Google Scholar]
- Petersen 2005a {published data only} .Petersen L, Jeppesen P, Thorup A, Abel MB, Ohlenschlaeger J, Christensen TO, Krarup G, Jorgensen P, Nordentoft M. Multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. BMJ. 2005;331(7517):602–8. doi: 10.1136/bmj.38565.415000.E01. 00CINAHL: 2009245707; MEDLINE: 16141449] [DOI] [PMC free article] [PubMed] [Google Scholar]; Petersen L, Jeppesen P, Thorup A, Ohlenschlaeger J, Christensen T, Krarup G, Jorgensen P, Nordentoft M. Substance abuse in first-episode schizophrenia-spectrum disorders. Schizophrenia Research. 2006;86(Suppl 1):S44. [Google Scholar]; Petersen L, Jeppesen P, Ventegodt AT, Abel M, Nordentoft M, Kassow P, Christensen T. Controlled trial of first episode psychotic patients: predictors of outcome. Schizophrenia Research. 2001;49(1, 2):266. 00MEDLINE: 170552270. [Google Scholar]; Petersen L, Nordentoft M, Jeppesen P, Ohlenschaeger J, Thorup A, Christensen TO, Krarup G, Dahlstrom J, Haastrup B, Jorgensen P. Improving 1-year outcome in first-episode psychosis: OPUS trial. British Journal of Psychiatry. 2005;48(Suppl):s98–103. doi: 10.1192/bjp.187.48.s98. 00MEDLINE: 160558170. [DOI] [PubMed] [Google Scholar]; Petersen L, Nordentoft M, Thorup A, Oehlenschlaeger J, Jeppesen P, Christensen T, Krarup G, Joergensen P. Multi-centre trial of integrated versus standard treatment for 547 first - episode psychotic patients. Schizophrenia Bulletin. 2005;31:531. [Google Scholar]; *; Petersen L, Thorup a, Jeppesen P, Ohlenschaeger J, Krarup G, Christensen T, Jorgensen P, Nordentoft M. Predictors of poor outcome. The opus-trial. Schizophrenia Research. 2004;70(1):32. 00ISI: 0002245511000820. [Google Scholar]
- Peuskens 1992 {published data only} .Bechelli LP, Moreno R, Versiani M, Caetano D, Mari J, Acioli A. Risperidone x haloperidol: Brazilian results of an international trial. Proceedings of the 9th World Congress of Psychiatry; Rio de Janeiro, Brazil. 1993 Jun 6-12.1993. [Google Scholar]; Crocket G, Mortimer AM, Livingston MG, Cookson JC, Jauhar P, Luthra JS, Batchelor DH, Hammond GL. Risperidone versus haloperidol in the treatment of chronic schizophrenic patients: UK centres in an international dose finding study. Proceedings of the 18th Collegium Internationale Neuro-Psychopharmacologicum Congress; Nice, France. 1992 Jun 28-Jul 2.1992. p. 197. [Google Scholar]; Duarte A, Borda JT. Risperidone vs haloperidol in schizophrenic patients. Argentine results. Proceedings of the 9th World Congress of Psychiatry; Rio de Janeiro, Brazil. 1993 Jun 6-12.1993. [Google Scholar]; Heylen SL, Gelders YG. Risperidone, a new antipsychotic with serotonin 5-HT2 and dopamine D2 antagonistic properties. Clinical Neuropharmacology. 1992;15(Suppl 1):180A–1A. doi: 10.1097/00002826-199201001-00095. 00MEDLINE: 923615480. [DOI] [PubMed] [Google Scholar]; Lindstrom E, Eriksson B, Hellgren A, Von Knorring L, Eberhard G. Efficacy and safety of risperidone in the long-term treatment of patients with schizophrenia. Clinical Therapeutics. 1995;17(3):402–12. doi: 10.1016/0149-2918(95)80105-7. 00MEDLINE: 75858440. [DOI] [PubMed] [Google Scholar]; Lindstrom E, Von Knorring L. Changes in single symptoms and separate factors of the schizophrenic syndrome after treatment with risperidone or haloperidol. Pharmacopsychiatry. 1994;27(3):108–13. doi: 10.1055/s-2007-1014288. 00MEDLINE:75215340. [DOI] [PubMed] [Google Scholar]; Marder SR. Risperidone: efficacy. Journal of Clinical Psychiatry. 1994;12:49–52. 00MEDLINE: 94770070. [Google Scholar]; Moller HJ, Bauml J, Ferrero F, Fuger J, Geretsegger C, Kasper S, Kissling W, Schubert H. Risperidone in the treatment of schizophrenia: results of a study of patients from Germany, Austria, and Switzerland. European Archives of Psychiatry and Clinical Neuroscience. 1997;247(6):291–6. doi: 10.1007/BF02922257. 00MEDLINE: 94770070. [DOI] [PubMed] [Google Scholar]; Mueller-Spahn F. The International Risperidone Research Group. Risperidone in the treatment of chronic schizophrenic patients: an international double-blind parallel-group study versus haloperidol. Clinical Neuropharmacology. 1992;15(Suppl 1 Pt A):90A–1A. doi: 10.1097/00002826-199201001-00048. 00MEDLINE: 923618490. [DOI] [PubMed] [Google Scholar]; Peuskens J. Risperidone in the treatment of chronic schizophrenic patients: an international double blind parallel group comparative study versus haloperidol. Clinical Report. 1992 doi: 10.1097/00002826-199201001-00048. [DOI] [PubMed] [Google Scholar]; Peuskens J, Risperidone Study Group Risperidone in the treatment of patients with chronic schizophrenia: a multinational, multi-centre, double-blind, parallel-group study versus haloperidol. British Journal of Psychiatry. 1995;166:712–26. doi: 10.1192/bjp.166.6.712. [DOI] [PubMed] [Google Scholar]; Rabinowitz J, Davidson M. Risperidone versus haloperidol in long-term hospitalized chronic patients in a double blind randomised trial: a post hoc analysis. Schizophrenia Research. 2001;50(1-2):89–93. doi: 10.1016/s0920-9964(00)00163-8. 00MEDLINE: 212733940. [DOI] [PubMed] [Google Scholar]; Rabinowitz J, Davidson M, Hornik T. Onset of therapeutic effect of risperidone versus haloperidol in a double blind randomised trial. Biological Psychiatry. 2001;2(Suppl 1):P021–29. doi: 10.4088/jcp.v62n0506. [DOI] [PubMed] [Google Scholar]; Rabinowitz J, Hornik T, Davidson M. Rapid onset of therapeutic effect of risperidone versus haloperidol in a double-blind randomised trial. Journal of Clinical Psychiatry. 2001;62(5):343–6. doi: 10.4088/jcp.v62n0506. 00MEDLINE: 213045620. [DOI] [PubMed] [Google Scholar]; Von Knorring L, Lindstrom E. The Swedish version of the positive and negative syndrome scale (PANSS) for schizophrenia. Construct validity and interrater reliability. Acta Psychiatrica Scandinavica. 1992;86(6):463–8. doi: 10.1111/j.1600-0447.1992.tb03298.x. 00MEDLINE: 931111420. [DOI] [PubMed] [Google Scholar]
- Philips 1999 {published data only} .Philipps LJ, McGorry P, Yung A, Francey D, Germano F, Bravin J, MacDonald A. The development of preventive interventions for early psychosis: early findings and directions for the future. Biological Psychiatry. 2001;2(Suppl 1):S066–02. [Google Scholar]; *; Phillips LJ, McGorry PD, Yung AR, Francey S, Cosgrave L, Germano D, Bravin J, MacDonald A, Hallgren M, Hearn N, Adlard S, Patton G. The development of preventive interventions for early psychosis: early findings and directions for the future conference abstract. Schizophrenia Research. 1999;36(1-3):331–2. 00MEDLINE: 150373350. [Google Scholar]
- Pietzcker 1993 {published data only} .Bandelow B, Muller P, Frick U, Gaebel W, Linden M, Muller Spahn F, Pietzcker A, Tegeler J. Depressive syndromes in schizophrenic patients under neuroleptic therapy. ANI study group Berlin, Dusseldorf, Gottingen, Munich, Federal Republic of Germany. European Archives of Psychiatry and Clinical Neuroscience. 1992;241(5):291–5. doi: 10.1007/BF02195978. 00MEDLINE: 13514050. [DOI] [PubMed] [Google Scholar]; Pietzcker A. A German multicentre study on the long-term treatment of schizophrenic outpatients. Pharmacopsychiatry. 1985;18(6):333–8. doi: 10.1055/s-2007-1017392. 00MEDLINE: 861212000. [DOI] [PubMed] [Google Scholar]; Pietzcker A, Gaebel W, Kopcke W, Linden M, Muller P, Muller-Spahn, Schussler G, Tegeler J. A German multicenter study on the neuroleptic long-term therapy of schizophrenic patients. Preliminary report. Pharmacopsychiatry. 1986;19(4):161–6. [Google Scholar]; *; Pietzcker A, Gaebel W, Kopcke W, Linden M, Muller P, MullerSpahn F, Tegeler J. Intermittent versus maintenance neuroleptic long-term treatment in schizophrenia - 2-year results of a German multicenter study. Journal of Psychiatric Research. 1993;27(4):321–39. [Google Scholar]
- Potkin 2003b {published data only} .Carson WH, Saha AR, Ali M, Dunbar GC, Ingenito G. Aripiprazole and risperidone versus placebo in schizophrenia and schizoaffective disorder. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10.2001. [Google Scholar]; Carson WHJ, Saha AR, Ali M, Dunbar GC, Ingenito G. Aripiprazole and risperidone versus placebo in schizophrenia and schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 1. 2002:1–50. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 3. 2002:111–75. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Dubitsky GM, Harris R, Laughren T, Hardeman S. Abilify (aripiprazole) tablets; medical review part 4. 2002:176–232. www.fda.gov/cder/foi/nda/2002/21-436·Abilify.htm.; Potkin SG, Kujawa M, Carson WH, Saha AR, Ali M, Ingenito G. Aripiprazole and risperidone versus placebo in schizophrenia and schizoaffective disorder. Schizophrenia Research. 2003;60(1):300. doi: 10.1001/archpsyc.60.7.681. 00EMBASE: 20032755420. [DOI] [PubMed] [Google Scholar]; Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, Stringfellow J, Ingenito G, Marder SR. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Archives of General Psychiatry. 2003;60(7):681–90. doi: 10.1001/archpsyc.60.7.681. 00EMBASE: 20032755420. [DOI] [PubMed] [Google Scholar]; Saha A, Carson W, Ali M, Dunbar G, Ingenito G. Efficacy and safety of aripiprazole and risperidone vs. placebo in patients with schizophrenia and schizoaffective disorder. Biological Psychiatry. 2001;2(Suppl 1):P021–24. [Google Scholar]; Yeung P, Kujawa M, Carson WH, Saha A, Alid M, Ingenito G. Aripiprazole and risperidone versus placebo in schizophrenia and schizoaffective disorder. Schizophrenia Research. 2002;53(3 Suppl 1):185–6. [Google Scholar]; Yeung P, McQuade RD, Carson WH, Saha A, Ali MW, Ingenito G. Aripiprazole and risperidone versus placebo in schizophrenia. European Psychiatry. 2002;17(Suppl 1):102s. [Google Scholar]; *; Yeung PP, Carson WH, Saha A, McQuade RD, Ali M, Stringfellow JC, Ingenito G. Efficacy of aripiprazole, a novel antipsychotic, in schizophrenia and schizoaffective disorder: results of a placebo-controlled trial with risperidone. European Neuropsychopharmacology. 2001;11(3):259. [Google Scholar]
- Power 2002 {published data only} .Power P, Craig T, Garety P, Rahaman N, Colbert S, Fornells-Ambrojo M. Lambeth early onset (LEO) trial: a randomised controlled trial of assertive community follow-up in early psychosis: initial 6 month data. Unknown Source. 1994 [Google Scholar]; *; Power P, Craig T, Garety P, Rahaman N, Fornells-Ambrojo M, Colbert S. A randomised controlled trial of assertive community follow-up in early psychosis: preliminary results. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):42. 00EMBASE: 20041306720. [Google Scholar]
- Power 2003 {published data only} .Power P, Bell R, Mills R, Herrmann-Doig T, Davern M, Henry L, McGorry P, Khademy-Deljo A. A randomised controlled trial of suicide prevention therapy for young people with first episode psychosis. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]; Power P, Bell R, Mills R, Herrmann-Doig T, Davern M, Yuen H, McGorry P. A randomised controlled trial of a suicide preventative cognitive oriented psychotherapy for suicidal young people with first episode psychosis. Schizophrenia Research. 1999;36(1-3):332. 00ISI: 000188788100082] [Google Scholar]; *; Power PJ, Bell RJ, Mills R, Herrman-Doig T, Davern M, Henry L, Yuen HP, Khademy-Deljo A, McGorry PD. Suicide prevention in first episode psychosis: the development of a randomised controlled trial of cognitive therapy for acutely suicidal patients with early psychosis. Australian and New Zealand Journal of Psychiatry. 2003;37(4):414–20. doi: 10.1046/j.1440-1614.2003.01209.x. 00MEDLINE: 128733250. [DOI] [PubMed] [Google Scholar]
- Power 2006 {published data only} .Power P, Craig T, Mcguire P, Lacoponi E, Garety P, Russell M. A randomised controlled trial of an early detection team in first episode psychosis: the leo cat trial. Schizophrenia Research. 2004;67(1):36. 00ISI: 0001887881000820. [Google Scholar]; Power P, Lacoponi E, Russell M, Fisher H, Mcguire P, Garety P, Valmaggio L, Craig T. A randomised controlled trial of an early detection team in first- episode psychosis: provisional findings of the leo cat study. Schizophrenia Research. 2004;70(1):131. 00ISI: 0002245511003860. [Google Scholar]; *; Power P, Reynolds N, Fisher H, Iacoponi E, Garety P, McGuire P, Russell M, Morris E, Valmaggia L, Craig T. The impact of an early detection team (LEO CAT) and GP education in first episode psychosis. Schizophrenia Research. 2006;86(Suppl 1):S41. [Google Scholar]
- Poyurovsky 2002b {published data only} .Poyurovsky M, Isaaks I, Pashnian A, Fuchs C, Schneidman M, Faragian S, Weizman R, Weizman A. Reboxetine but not fluoxetine attenuates olanzapine-induced weight gain in first-episode schizophrenia patients. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S312. [Google Scholar]; Poyurovsky M, Pashinian A, Gil-Ad I, Maayan R, Schneidman M, Fuchs C, Weizman A. Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. American Journal of Psychiatry. 2002;159(6):1058–60. doi: 10.1176/appi.ajp.159.6.1058. 00CENTRAL: CN–00380340; MEDLINE: 220374580. [DOI] [PubMed] [Google Scholar]; *; Poyurovsky M, Pashnian A, Fuchs C, Gilad I, Maayan R, Weizman A. Olanzapine-induced weight gain in patients with first-episode schizophrenia. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10.2001. [Google Scholar]
- Poyurovsky 2003b {published data only} .Poyurovsky M, Isaacs I, Fuchs C, Schneidman M, Faragian S, Weizman RWA. Attenuation of olanzapine-induced weight gain with reboxetine in patients with schizophrenia: a double-blind, placebo-controlled study. American Journal of Psychiatry. 2003;160(2):297–302. doi: 10.1176/appi.ajp.160.2.297. 00MEDLINE: 125625760. [DOI] [PubMed] [Google Scholar]; Poyurovsky M, Isaaks I, Fuchs C, Schneidman M, Faragian S, Weizman R, Weizman A. Reboxetine and attenuation of olanzapine-induced weight gain in first-episode schizophrenia patients. A double-blind placebo-controlled study. International Journal of Neuropsychopharmacology. 2002;Vol. 5(issue Suppl 1):S171. [Google Scholar]; *; Poyurovsky M, Isaaks I, Pashnian A, Fuchs C, Schneidman M, Faragian S, Weizman R, Weizman A. Reboxetine but not fluoxetine attenuates olanzapine-induced weight gain in first-episode schizophrenia patients. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S312. [Google Scholar]
- Poyurovsky 2004 {published data only} .Poyurovsky M, Tal V, Maayan R, Gil-Ad I, Fuchs C, Weizman A. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a double-blind placebo-controlled pilot study. European Neuropsychopharmacology. 2004;14(4):332–6. doi: 10.1016/j.euroneuro.2003.10.004. 00MEDLINE: 151634440. [DOI] [PubMed] [Google Scholar]
- Proffitt 2004 {published data only} .Proffitt T, Wood S, Yuen H, Mcconchie M, Brewer W, Horrobin D, Mcgorry P, Berger G. Ethyl-eicosapentaenoic acid (e-epa) supplementation in first-episode psychosis: effects on cognition mediated by lipid metabolic status. Schizophrenia Research. 2004;70(1):40. 00ISI: 0002245511001030. [Google Scholar]
- Qian 2002b {published data only} .Qian H, Zhang S, Cao R, Chen S, Song H. Risperidone in the treatment of first-episode schizophrenia. Chinese New Drugs Journal. 2002;11(2):158–60. 00CAJ: MEDI02070. [Google Scholar]
- Qiu 2005 {published data only} .Qiu C-Q, Zeng Z-X. Follow-up study of effects of family circumstance in schizophrenic patients. Journal of Clinical Psychological Medicine. 2005;15(4):209–11. 00CAJ: MEDI05090. [Google Scholar]
- Qu 2005 {published data only} .Qu Z-W, Chen M-D, Gu J-Q. Initial searching for the early influence and interfere about the regulating function of the blood-glucose affected by chlorpromazine and risperidal. Journal of the Science of Modern China. 2005;2(12):1061–3. [Google Scholar]
- Rabinowitz 2004 {published data only} .Rabinowitz J, Davidson M, Kopala L. A method for examining efficacy by dosage in flexible dose clinical trials. Schizophrenia Research. 2004;67(1):150. 00ISI: 0001887881003570. [Google Scholar]
- Rabinowitz 2006 {published data only} .Rabinowitz J, De Smedt G, Davidson M. Premorbid functioning and outcomes in recent onset schizophrenia. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S338. [Google Scholar]; *; Rabinowitz J, Harvey PD, Eerdekens M, Davidson M. Premorbid functioning and treatment response in recent-onset schizophrenia. British Journal of Psychiatry. 2006;189:31–5. doi: 10.1192/bjp.bp.105.013276. [DOI] [PubMed] [Google Scholar]
- Rasmussen 1998 {published data only} .Rasmussen M, FutuRis Study Group Long-term outcome with risperidone or haloperidol in first episode psychosis. Biological Psychiatry. 1999;Vol. 45:35S. [Google Scholar]; Rasmussen M, RIS-INT-35 Study Group The impact on long-term outcome of early intervention with risperidone or haloperidol in first episode psychosis: characteristics at baseline. Proceedings of the 37th Annual Meeting of the American College of Neuropsychopharmacology; Las Croabas, Puerto Rico. 1998 Dec 14-18.1998. [Google Scholar]; Rasmussen M. Risperiderone International Study Group. The impact on long-term outcome of early intervention with risperidone or haloperidol in first episode psychosis: characteristics at baseline. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16.1998. [Google Scholar]; Rasmussen M, The RIS-INT-35 Study Group The impact on long-term outcome of early intervention with risperidone or haloperidol in first episode psychosis:characteristics at baseline. Schizophrenia Research. 1999;36(1-3):293. [Google Scholar]; Schooler NR, Risperidone International Study Group The FutuRIS study - a prospective long-term evaluation of risperidone versus haloperidol in early psychosis patients. Proceedings of the 6th World Congress of Biological Psychiatry; Nice, France. 1997 Jun 22-27.1997. [Google Scholar]
- Reeder 2004 {published data only} .Reeder C, Newton E, Frangou S, Wykes T. Which executive skills should we target to affect social functioning and symptom change? A study of a cognitive remediation therapy program. Schizophrenia Bulletin. 2004;30(1):87–100. doi: 10.1093/oxfordjournals.schbul.a007070. 00CENTRAL: CN–004821790. [DOI] [PubMed] [Google Scholar]
- Reilly 2006 {published data only} .Reilly T, Wymbs P, Painter M, Fowler D. From psychological understanding to psychological therapy for social anxiety and paranoia in early psychosis: addressing psychological issues while improving social recovery. Schizophrenia Research. 2006;86(Suppl 1):S139. [Google Scholar]
- Ren 2005c {published data only} .Ren X, Chu P, Yan J. The study of cognitive behavior intervention to long period efficacy of first-episode schizophrenic patients. Practice and Research. 2005;2(4):6–8. [Google Scholar]
- Renshaw 2003 {published data only} .Renshaw PF, Todd DY, Wei H, Charles C, Tollefson GD, Lieberman JA. Olanzapine-induced reduction in frontal lobe lactate in first episode psychosis. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; *; Renshaw PF, Yurgelun-Todd DA, Wei H, Charles HC, Tohen M, Lieberman JA. Olanzapine induced reductions in frontal lobe lactate levels correlate with treatment response in first episode psychosis. Schizophrenia Research. 2003;60(1):301. [Google Scholar]
- Renton 2004 {published data only} .Renton J, Morrison AP. Effectiveness of cognitive therapy for psychosis and implications for early intervention. Schizophrenia Research. 2004;70(1):142. 00ISI: 0002245511004210. [Google Scholar]
- Reveley 2000a {published data only} .Reveley M. Ris-int-35 a double blind evaluation of risperidone versus haloperidol on the long-term morbidity of early psychotic patients. National Research Register. 2000. ; Reveley M. RIS-int-35 a double blind evaluation of risperidone versus haloperidol on the long-term morbidity of early psychotic patients. National Research Register. 2001;Vol. 1 [Google Scholar]
- Rimon 2004 {published data only} .Rimon RH. Olanzapine versus perphenazine in the treatment of schizophrenia: a double-blind study. Schizophrenia Research. 2004;67(1):164–5. 00ISI: 0001887881003920. [Google Scholar]
- Robles 2006 {published data only} .Robles O, Zabala A, Parellada MJ, Ruiz A, Moreno MD, Burdalo MT, Arango C. Cognitive efficacy of quetiapine and olanzapine in early onset first episode psychosis. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S381. [Google Scholar]
- Ropert 1973 {published data only} .Ropert R, Levy L, Ropert M. Problems posed by trial use of prolonged action neuroleptics in acute psychiatric syndromes [Problemes poses par les essais d’emploi des neuroleptiques a action prolongee (N A P ) dans les syndromes psychiatriques aigus] Annales Medico-Psychologiques. 1973;1(2):259–67. 00PSYCINFO: 51–016130. [PubMed] [Google Scholar]
- Rosebush 2000 {published data only} .Rosebush P, Mazurek M. Olanzapine versus haloperidol in randomised trials of first-episode patients with schizophrenia. Stanley Foundation Research Programs. 2000.
- Rosen 2002 {published data only} .Rosen JL, Woods SW, Miller TJ, McGlashan TH. Prospective observations of emerging psychosis. Journal of Nervous and Mental Disease. 2002;190(3):133–41. doi: 10.1097/00005053-200203000-00001. 00MEDLINE: 119236470. [DOI] [PubMed] [Google Scholar]
- Ruhrmann 2006a {published data only} .Ruhrmann S, Hoppmann B, Theysohn S, Picker H, Kuhn K-U, Schultze-Lutter F, Wagner M, Bechdolf A, Moller HJ, Gaebel W, Maier W, Klosterkotter J. Acute symptomatic treatment effects in persons clinically at risk for psychosis. Schizophrenia Research. 2006;86(Suppl 1):S8. [Google Scholar]; Ruhrmann S, Hoppmann B, Theysohn S, Picker H, Kuhn K-U, Schultze-Lutter F, Wagner M, Bechdolf A, Moller H-J, Gaebel W, Maier W, Klosterkotter J. Intervention in the late initial prodromal state (LIPS) of psychosis. Schizophrenia Research. 2006;86(Suppl 1):S96. [Google Scholar]
- Ryu 2006 {published data only} .Ryu SH, Jang WS, Cho EY, Kim SK, Lee DS, Hong KS. Association of leptin gene polymorphism with antipsychotic drug-induced weight gain. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S419. [Google Scholar]
- Sanger 1999 {published data only} .Lieberman J, Sanger T, Tohen M, First Episode Collaborative Study Group Olanzapine and haloperidol treatment of first episode schizophrenia and schizoaffective disorder: 12 week outcome of a two year randomised double blind trial. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]; Lieberman J, Tohen M, McEvoy J, Sanger T, Keefe R, Charles C, Clark S, Brier A, Tollefson G, the HGDH Study Group Olanzapine versus haldoperidol in the treatment of first episode psychosis. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]; Sanger T. Olanzapine versus haloperidol treatment in first episode psychosis. Proceedings of the 2nd International Conference on Early Psychosis; New York, New York, USA. 2000 Mar 31 - Apr 2.2000. [Google Scholar]; Sanger T. Treatment of neurocognitive deficits with olanzapine or haloperidol in first episode psychosis. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]; Sanger T, Lieberman J, Tohen M, Grundy S, Beasley C, Jr, Tollefson G. Olanzapine versus haloperidol treatment in first episode psychosis. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31-Nov 4.1998. [Google Scholar]; Sanger T, Lieberman JA, Tohen M, Tollefson GD. Olanzapine versus haloperidol in the treatment of first episode psychosis. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16.1998. [Google Scholar]; Sanger T, Lieberman JA, Tohen M, Tollefson GD. Olanzapine versus haloperidol in the treatment of psychosis. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30-Jun 4.1998. [Google Scholar]; Sanger T, Tollefson GD, Lieberman JA, Tohen M. Olanzapine versus haloperidol in the treatment of first-episode psychosis. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]; Sanger TM, Lieberman JA, Tohen M, Grundy S, Beasley C., Jr Tollefson GD. Olanzapine versus haloperidol treatment in first-episode psychosis. American Journal of Psychiatry. 1999;156(1):79–87. doi: 10.1176/ajp.156.1.79. 00MEDLINE: 99107319] [DOI] [PubMed] [Google Scholar]; *; Sanger TM, Lieberman JA, Tohen M, Tollefson GD. Olanzapine versus haloperidol in the treatment of first episode psychosis. Schizophrenia Research. 1998;29(1, 2):151. doi: 10.1176/ajp.156.1.79. [DOI] [PubMed] [Google Scholar]
- Sarkar 1994 {published data only} .Sarkar P, Andrade C, Kapur B, Das P, Sivaramakrishna Y, Harihar C, Pandey A, Anand A, Dharmendra MS. An exploratory evaluation of ECT in haloperidol-treated DSMIIIR schizophreniform disorder. Convulsive Therapy. 1994;10(4):271–8. 00MEDLINE: 951532520. [PubMed] [Google Scholar]
- Schlogelhofer 2006 {published data only} .Schlogelhofer M, Amminger GP, Werneck-Rohrer S, Aschauer HN, Edwards J. Emotion recognition deficit in first-episode schizophrenic patients and atypical antipsychotics. Proceedings of the 19th European College of Neuropsychopharmacology Congress; Paris, France. 2006 Sep 16-20.2006. [Google Scholar]
- Schooler 1989 {published data only} .Pandalai SP. The relationship of neurological, psychopathology, family, psychosocial, demographic, and history factors to relapse in the maintenance treatment of schizophrenia. Univ. of Pittsburgh; Pittsburgh, USA: 1998. [Google Scholar]; Schooler NR, Keith SJ, Severe JB, Matthews S. Acute treatment response and short-term outcome in schizophrenia - 1st results of the NIMH treatment strategies in schizophrenia study. Psychopharmacology Bulletin. 1989;25(3):331–5. [PubMed] [Google Scholar]
- Schooler 2005 {published data only} .Pandalai SP. The relationship of neurological, psychopathology, family, psychosocial, demographic, and history factors to relapse in the maintenance treatment of schizophrenia. Univ. of Pittsburgh; Pittsburgh, USA: 1998. [Google Scholar]; Schooler N, Davidson M, Kopala L. Reduced relapse rates in recent onset schizophrenia patients treated with risperidone vs. haloperidol. Journal of the European College of Neuropsychopharmacology. 2003;Vol. 13(issue 4):S337. [Google Scholar]; Schooler N, Rabinowitz J, Davidson M, Emsley R, Harvey PD, Kopala L, McGorry PD, Hove IV, Eerdekens M, Swyzen W, Smedt GD. Risperidone and haloperidol in first-episode psychosis: a long-term randomised trial. American Journal of Psychiatry. 2005;162(5):947–53. doi: 10.1176/appi.ajp.162.5.947. 00CINAHL: 2005116142; EMBASE: 2005219788; MEDLINE: 158637970. [DOI] [PubMed] [Google Scholar]; *; Schooler NR, Emsley R, Kopala L, Martinez R, McGorry P. Characteristics of first episode clinical trial subjects. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31 - Apr 2.2000. [Google Scholar]
- Schulz 1997 {published data only} .Schulz E, Fleischhaker C, Clement HW, Remschmidt H. Blood biogenic amines during clozapine treatment of early onset schizophrenia. Journal of Neural Transmission. 1997;104(10):1077–89. doi: 10.1007/BF01273320. 00EMBASE: 19980213970. [DOI] [PubMed] [Google Scholar]
- Schwannauer 2002 {published data only} .Schwannauer M, Brodie C, Power M. Early recognition and intervention for bipolar disorders - an investigation into cognitive, emotional and psychosocial factors influencing vulnerability and recovery in early onset bipolar disorders. Proceeding of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]
- Scottish 1992 {published data only} .Scottish Schizophrenia Research Group The Scottish first episode schizophrenia study. VIII. Five-year follow-up: clinical and psychosocial findings. The Scottish Schizophrenia Research Group. British Journal of Psychiatry. 1992;161:496–500. 00MEDLINE: 930068190. [PubMed] [Google Scholar]
- Sharifi 2006 {published data only} .Sharifi V, Alaghband-rad J, Amini H, Mottaghipour Y, Jalali M, Seddigh A, Salesian N, Shahrivar Z. Towards models for aftercare of patients with a first episode of psychosis in Iran as a developing country. Schizophrenia Research. 2006;86(Suppl 1):S164–5. [Google Scholar]
- Sharma 2000a {published data only} .Sharma T. A double-blind evaluation of risperidone versus haloperidol on the long-term morbidity of early psychotic patients. National Research Register. 2000. ; Sharma T. A double-blind evaluation of risperidone versus haloperidol on the long-term morbidity of early psychotic patients. National Research Register. 2001;Vol. 1 [Google Scholar]
- Sharma 2003 {published data only} .Sharma T. A double-blind evaluation of risperidone versus haloperidol on the long-term morbidity of early psychotic patients. National Research Register. 2000. ; Sharma T. A double-blind evaluation of risperidone versus haloperidol on the long-term morbidity of early psychotic patients. National Research Register. 2001;Vol. 1 [Google Scholar]; *; Sharma T, Lieberman JA, McEvoy JP, Perkins DO, Hamer RM, Zipursky RB, Kahn RS, Gur RE, Centorrino F, Glick I, Green AI, Nemeroff CB, Rothschild AJ, Strakowski SM, Tohen M, Tollefson GD. Long-term efficacy and safety of atypical and conventional antipsychotic drugs in first episode schizophrenia. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S134. [Google Scholar]
- Sheng 2005 {published data only} .Sheng Y. Effects of clozapine and risperidone on serum interleukin-2 and interleukin-6 of patients with first episode schizophrenia. Qilu Journal of Medicall Aboratory Sciences. 2005;16(5):14–5. 00CAJ: MEDI05120. [Google Scholar]
- Shi Tianyuan 2004 {published data only} .Shi T, Zhang R, Guo X. Influences of risperidone and clozapine on plasma levels of cytokine in first-episode schizophrenics. Chinese Journal of Nervous and Mental Diseases. 2004;30(5):339–41. 00CAJ: MEDI00120. [Google Scholar]
- Silverstone 1984b {published data only} .Silverstone T, Levine S, Freeman HL, Dubini A. Zetidoline, a new antipsychotic. First controlled trial in acute schizophrenia. British Journal of Psychiatry. 1984;145:294–9. doi: 10.1192/bjp.145.3.294. 00MEDLINE: 850010460. [DOI] [PubMed] [Google Scholar]
- Simonsen 2000 {published data only} .Simonsen E. A randomised comparative trial of an early detection program and treatment as usual in the duration of untreated psychosis. Stanley Foundation Research Programs. 2000.
- Spencer 1992 {published data only} .Spencer EK, Alpert M, Pouget ER. Scales for the assessment of neuroleptic response in schizophrenic children: specific measures derived from the CPRS. Psychopharmacology Bulletin. 1994;30(2):199–202. 00MEDLINE: 951328180. [PubMed] [Google Scholar]; *; Spencer EK, Kafantaris V, Padron-Gayol MV, Rosenberg CR, Campbell M. Haloperidol in schizophrenic children: early findings from a study in progress. Psychopharmacology Bulletin. 1992;28(2):183–6. 00MEDLINE: 1513922; PSYCINFO: 80–026730. [PubMed] [Google Scholar]
- Srihari 2006 {published data only} .Srihari VH, Woods SW, Walsh B, Saksa JR, Pollard J, Hyman L, Walsh K, Cartier S. Specialized treatment early in psychosis (STEP): a pragmatic randomised controlled trial in the US public sector. Schizophrenia Research. 2006;86(Suppl 1):S165. [Google Scholar]
- SSRG 1987 {published data only} .McCreadie RG, Wiles D, Grant S, Crockett GT, Mahmood Z, Livingston MG, Watt JA, Greene JG, Kershaw PW, Todd NA, Scott AM, Loudon J, Dyer JAT, Philip AE, Batchelor D. The Scottish first episode schizophrenia study. VII. Two-year follow-up. Scottish Schizophrenia Research Group. Acta Psychiatrica Scandinavica. 1989;80(6):597–602. doi: 10.1111/j.1600-0447.1989.tb03032.x. 00MEDLINE: 901440400. [DOI] [PubMed] [Google Scholar]; Scottish Schizophrenia Research Group The Scottish first episode schizophrenia study. II. Treatment: pimozide versus flupenthixol. British Journal of Psychiatry. 1987;150:334–8. doi: 10.1192/bjp.150.3.334. 00MEDLINE: 901440400. [DOI] [PubMed] [Google Scholar]; Scottish Schizophrenia Research Group The Scottish first episode schizophrenia study v. one-year follow-up. The Scottish Schizophrenia Research Group. British Journal of Psychiatry. 1988;152:470–6. doi: 10.1192/bjp.152.4.470. 00MEDLINE: 890017380. [DOI] [PubMed] [Google Scholar]
- Stain 2006 {published data only} .Stain HJ, Startup M, Carr V, Baker A, Schall U. The depth project: a multisite RCT for youths at risk for psychosis. Schizophrenia Research. 2006;86(Suppl 1):S51–2. [Google Scholar]
- Stotsky 1977 {published data only} .Stotsky BA. Relative efficacy of parenteral haloperidol and thiothixene for the emergency treatment of acutely excited and agitated patients. Diseases of the Nervous System. 1977;38(12):967–73. 00MEDLINE: 780637820. [PubMed] [Google Scholar]
- Strakowski 1997 {published data only} .Strakowski SM, Sax KW, Setters MJ, Stanton SP, Keck PE., Jr Lack of enhanced response to repeated d-amphetamine challenge in first-episode psychosis: Implications for a sensitization model of psychosis in humans. Biological Psychiatry. 1997;42(9):749–55. doi: 10.1016/s0006-3223(97)00052-8. 00MEDLINE: 9347122; PSYCINFO: 1997–43428–0010. [DOI] [PubMed] [Google Scholar]
- Strakowski 2005 {published data only} .Strakowski SM, Johnson JL, Delbello MP, Hamer RM, Green AI, Tohen M, Lieberman JA, Glick I, Patel JK. Quality of life during treatment with haloperidol or olanzapine in the year following a first psychotic episode. Schizophrenia Research. 2005;78(2-3):161–9. doi: 10.1016/j.schres.2005.04.017. 00MEDLINE: 159504360. [DOI] [PubMed] [Google Scholar]
- Stuart 2004 {published data only} .Stuart A, Wade D, Murphy B, Macneil C, Wong L, Mcgorry P. Enduring negative symptoms in first-episode psychosis. Schizophrenia Research. 2004;70(1):146. 00ISI: 0002245511004360. [Google Scholar]
- Su 2002b {published data only} .Su YQ. Effects of low dosage risperdal to treat the first attack of schizophrenia. Youjiang Medical Journal. 2002;30(5):380–1. 00CAJ: MEDI02120. [Google Scholar]
- Sun 2000a {published data only} .Sun T. A controlled study comparing risperidone and clozapine in the treatment of schizophrenia. Heath Psychology Journal. 2000;8(3):290–2. 00CAJ: MEDI0008S20. [Google Scholar]
- Sun 2006a {published data only} .Sun F, Dong R, Wang Y. A randomised control study aripiprazole in the treatment of first-episode schizophrenia. Chinese Journal of Health Psychology. 2006;14(6):642–3. [Google Scholar]
- Sun 2006e {published data only} .Sun X-D, Zhou S-B, Li Z-M, Han Z-F. Effect of cognitive function in first-episode schizophrenia treated with quetiapine. Journal of Clinical Psychiatry. 2006;16(2):94–5. [Google Scholar]
- Suri 2001 {published data only} .Suri AK. Cognitive behavioural therapy in early schizophrenia. National Research Register. 2001;Vol. 1 [Google Scholar]
- Svestka 2003a {published data only} .Svestka J, Synek O, Zourkova A. Olanzapine versus risperidone in first-episode schizophrenic and schizoform disorders: a double-blind comparison. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S291. [Google Scholar]
- Tait 2002 {published data only} .Tait A, McNay L, Gumley A, O’Grady M. The development and implementation of an individualised early signs monitoring system in the prediction of relapse in schizophrenia. Journal of Mental Health. 2002;11(2):141–53. 00CAJ: MEDI05110. [Google Scholar]
- Tait 2005 {published data only} .Tait L, Lester H, Birchwood M, Freemantle N, Wilson S. Design of the Birmingham early detection in untreated psychosis trial (REDIRECT): cluster randomised controlled trial of general practitioner education in detection of first episode psychosis [ISRCTN87898421] BMC Health Services Research. 2005;5(1):19. doi: 10.1186/1472-6963-5-19. 00MEDLINE: 157553210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan 2005b {published data only} .Tan X-G, Li J-M, LV J-Q, Liu Y-Z, Cui F-Z. Clinical study on the efficacy of oral risperidone solution on aged- schizophrenic patients. Journal of Clinical Psychological Medicine. 2005;15(3):146–7. 00CAJ: MEDI05080. [Google Scholar]
- Tao 2005a {published data only} .Tao J-Q, Liang J, Zeng Q, Kong H, Ye M, Su D, Shi J. Controlled trial of cognitive behavioral therapy for first-episode schizophrenia. Journal of Clinical Psychological Medicine. 2005;15(3):135–7. 00CAJ: MEDI05080. [Google Scholar]
- Tao Yuan Li 2004 {published data only} .Tao YL, Yong YL, Tian CW, Wei YY, Yu HC, Yuan A, Chang YL, Hong LZ, Cai XJ. Effect of early intervention on quality of life in patients with first episode schizophrenia. Chinese Journal of Clinical Rehabilitation. 2004;8(18):3464–5. 00CAJ: MEDI05070. [Google Scholar]
- Tarrier 2000d {published data only} .Tarrier N. Evaluation of the family support and cognitive service for recent onset schizophrenia sufferers with substance misuse. National Research Register. 2000. ; Tarrier N. Evaluation of the family support and cognitive service for recent onset schizophrenia sufferers with substance misuse. National Research Register. 2001;Vol. 1 [Google Scholar]
- Thompson 2005 {published data only} .Thompson P. Significant slowing of grey matter loss rates with olanzapine versus haloperidol. Unpublished Report. 2005.
- Tian 2005 {published data only} .Tian S, Li S, Xie Z. Influence of family nursing intervention on therapeutic effect of childhood patients with schizophrenia of first episode. Chinese Nursing Research. 2005;19(8B):1551–2. 00CAJ: MEDI0510; CINAHL: 20090536640. [Google Scholar]
- Toben 1998 {published data only} .Toben M, Zarate C, Jr, Sanger IM. A placebo-controlled trial of olanzapine in psychotic or non-psychotic acute mania. Schizophrenia Research. 1998;Vol. 29(issue 1,2):204. 00EMBASE: 2006459790; MEDLINE: 165407020. [Google Scholar]
- Tohen 1997a {published data only} .Tohen M, Sanger T, Tollefson GD. Gender differences in the response of olanzapine versus haloperidol in the treatment of first-episode psychosis. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]
- Tohen 2000b {published data only} .Tohen M, Jacobs T, Toma V, Francis J, Zhang F, Gannon KS, Sanger TM, Breier A. Is olanzapine a mood stabilizer? Schizophrenia Research. 2000;41(1):193–4. 00ISI: 0002245511001650. [Google Scholar]
- Tollefson 1997 HGAJ {published data only} .Alan B, Hamilton SH. Comparative efficacy of olanzapine and haloperidol for patients with treatment resistant schizophrenia. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11; 1999. [DOI] [PubMed] [Google Scholar]; Allison D, Cavazzoni P, Beasley C, Holcombe J, Buse J. Analysis of random glucose concentration data from patients with schizophrenia treated with typical and atypical agents during double-blind, randomised, controlled clinicaltrials. Proceedings of the 7th World Congress of Biological Psychiatry; Berlin, Germany. 2001 Jul 1-6.2001. [Google Scholar]; Andersen S, Cohen L, Goldstein J, Tohen M, Tollefson G. Sex and neuroendocrine differences in response to treatment with olanzapine: a preliminary analysis. Schizophrenia Research. 1998;29(1-2):186–7. [Google Scholar]; Andersen SW, Tollefson GD, Sanger TM. Depressive signs and symptoms in schizophrenia: a prospective blinded trial of olanzapine and haloperidol. Schizophrenia Research. 1999;36(1-3):269. doi: 10.1001/archpsyc.55.3.250. [DOI] [PubMed] [Google Scholar]; Basson B, Kinon BJ, Gilmore JA, Taylor CC, Tollefson GD, Czekalla J. Factors influencing weight change in patients with schizophrenia treated with olanzapine verus haloperidol or risperidone. Journal of Psychopharmacology. 2000;14(3 Suppl):A60. 00EMBASE: 19993586740. [Google Scholar]; Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. Journal of Clinical Psychiatry. 2001;62(4):231–8. doi: 10.4088/jcp.v62n0404. 00MEDLINE: 113798360. [DOI] [PubMed] [Google Scholar]; Beasley CM. Safety of olanzapine. Journal of Clinical Psychiatry. 1997;15(2):19–21. 00EMBASE: 2003491563; MEDLINE: 14618553; PSYCINFO: 2003–10341–0080. [PubMed] [Google Scholar]; Beasley CM, Sayler ME, Keisler GM, Potvin JH, Sanger TM, Tollefson GD. The influence of pharamacotherapy on self-directed and externally-directed aggression in schizophrenia. Schizophrenia Research. 1998;29(1-2):28. [Google Scholar]; Beasley CM, Tollefson GD, Dellva MA, Tamura R, Glazer WM, Morgenstern H. The differential risk of tardive dyskinesia with olanzapine. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30 - Jun 4.1998. [Google Scholar]; Breier AF, Hamilton SH. Comparative efficacy of olanzapine and haloperidol for patients with treatment-resistant schizophrenia. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20; 1999. [DOI] [PubMed] [Google Scholar]; Conley RR, Brecher M. The Risperidone/Olanzapine Study Group. Risperidone versus olanzapine in patients with schizophrenia or schizoaffective disorders. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31-Nov 4.1998. [Google Scholar]; Crawford AMK, Gomez JC, Beasley CM, Jr, Tollefson GD. Olanzapine versus haloperidol: analysis of schizophrenic patients from the multi-center international trial. Journal of the European College of Neuropsychopharmacology. 1997;7:P.2.015. doi: 10.1016/s0924-977x(96)00392-6. [DOI] [PubMed] [Google Scholar]; Czekalla J, Beasley CM, Jr, Dellva MA, Berg PH, Grundy S. Analysis of the qtc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry. 2001;62(3):191–8. doi: 10.4088/jcp.v62n0310. 00MEDLINE: 212003210. [DOI] [PubMed] [Google Scholar]; David SR, Taylor CC, Kinon BJ, Breier A. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clinical Therapeutics. 2000;22(9):1085–96. doi: 10.1016/S0149-2918(00)80086-7. [DOI] [PubMed] [Google Scholar]; Eli Lilly, Company Tollefson 1997 - olz vs hpl. Unpublished Report. 2001. ; Gilmore J, Kinon BJ, Basson BR, Tollefson GD. Effect of long-term olanzapine treatment on weight change in schizophrenia. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S155. [Google Scholar]; Gilmore J, Kinon BJ, Basson BR, Tollefson GD. Effect of long-term olanzapine treatment on weight change in schizophrenia. Journal of the European College of Neuropsychopharmacology. 2000;10(Suppl 3):S305. [Google Scholar]; Gilmore JA, Kinon BJ, Zhao Z, Barber B. Improvement in quality of life and depressive symptoms in schizophrenic patients is associated with robust acute treatment response of olanzapine versus haloperidol. Schizophrenia Research. 2002;53(3 Suppl 1):177. [Google Scholar]; Gilmore JA, Kinon BJ, Zhao Z, Barber B. Rigorous criteria for treatment response differentiated efficacy of olanzapine versus haloperidol in patients with schizophrenia. Schizophrenia Research. 2002;53(3 Suppl 1):177. [Google Scholar]; Glick ID, Berg PH. Time to study discontinuation, relapse, and compliance with atypical or conventional antipsychotics in schizophrenia and related disorders. International Clinical Psychopharmacology. 2002;17(2):65–8. doi: 10.1097/00004850-200203000-00004. 00MEDLINE: 212003210. [DOI] [PubMed] [Google Scholar]; Goldstein JM, Cohen LS, Horton NJ, Lee H, Andersen S, Tohen M, Crawford A, Tollefson G. Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Research. 2002;110(1):27–37. doi: 10.1016/s0165-1781(02)00028-8. 00CENTRAL: CN–00389114; MEDLINE: 220041660. [DOI] [PubMed] [Google Scholar]; Gregor K, Hamilton S, Edgell E. Functional outcomes in schizophrenia: a European comparison of olanzapine and haloperidol. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]; Gregor KJ, Allicar MP, Lilliu H, Olivier V, Le Pen C, Gavart S. An economic comparison of olanzapine versus haloperidol in the treatment of schizophrenia in France. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S161. [Google Scholar]; Gregor KJ, Hamilton SH, Edgell ET. Functional outcomes in schizophrenia: a European comparison of olanzapine and haloperidol. Journal of the European College of Neuropsychopharmacology. 1999;9(Suppl 1):S11. [Google Scholar]; Gregor KJ, Hamilton SH, Edgell ET. Functional outcomes in schizophrenia: a European comparison of olanzapine and haloperidol. Journal of the European College of Neuropsychopharmacology. 1999;9:S289. [Google Scholar]; Gregor KJ, Hamilton SH, Edgell ET. Functional outcomes in schizophrenia: a European comparison of olanzapine and haloperidol. Schizophrenia Research. 2000;41(1):189. doi: 10.1097/00004850-200015050-00001. [DOI] [PubMed] [Google Scholar]; 11TH: World Congress of Psychiatry [CD–ROM]: Conifer, Excerpta Medica Medical Communications BV; 1999. PO–04–22; MEDLINE: 0. [Google Scholar]; Hamilton S, Breier A, David S. Comparative efficacy of olanzapine and haloperidol for patients with treatment resistant schizophrenia. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct31 - Nov 4.1998. [Google Scholar]; Inada T, Beasley CM, JR, Tanaka Y, Walker DJ. Extrapyramidal symptom profiles assessed with the drug-induced extrapyramidal symptom scale: comparison with western scales in the clinical double-blind studies of schizophrenic patients treated with either olanzapine or haloperidol. International Clinical Psychopharmacology. 2003;18(1):39–48. doi: 10.1097/00004850-200301000-00007. [DOI] [PubMed] [Google Scholar]; Jaton LA, Kinon BJ, Rotelli MD, Kaiser C, Kollack-Walker S. Differential rate of weight gain present among patients treated with olanzapine. Schizophrenia Research. 2003;60(1):357. 00EMBASE: 2003491563; MEDLINE: 14618553; PSYCINFO: 2003–10341–0080. [Google Scholar]; Javor K, Kinon BJ, Gilmore J. Continued improvement in quality of life despite weight change during olanzapine treatment. Schizophrenia Research. 2001;49(1, 2):233. [Google Scholar]; Kennedy JS, Jeste D, Kaiser CJ, Golsham S, Maguire GA, Tollefson G, Sanger T, Bymaster FP, Kinon BJ, Dossenbach M, Gilmore JA, Breier A. Olanzapine vs haloperidol in geriatric schizophrenia: analysis of data from a double-blind controlled trial. International Journal of Geriatric Psychiatry. 2003;18:1013–20. doi: 10.1002/gps.1007. 00EMBASE: 2003491563; MEDLINE: 14618553; PSYCINFO: 2003–10341–0080. [DOI] [PubMed] [Google Scholar]; Kinon B, Basson B, Tollefson GD. Gender-specific prolactin olanzapine versus haloperidol in schizophrenia. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30-Jun 4.1998. [Google Scholar]; Kinon BJ, Basson B, Hill AL, Berg PH. Effective resolution of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia with olanzapine. Journal of the European College of Neuropsychopharmacology. 2000;10(Suppl 3):S305. [Google Scholar]; Kinon BJ, Basson B, Szymanski K, Tollefson GD. Predictors of weight gain during olanzapine treatment. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31-Nov 4.1998. [Google Scholar]; Kinon BJ, Basson BR, Gilmore JA, Tollefson GD. Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. Journal of Clinical Psychiatry. 2001;62(2):92–100. [PubMed] [Google Scholar]; Kinon BJ, Basson MS, Tollefson GD. Gender-specific prolactin response to treatment with olanzapine versus haloperidol in schizophrenia. Proceedings of the 9th Biennial Winter Workshop on Schizophrenia; Davos, Switzerland. 1998 Feb 7-13.1998. [Google Scholar]; Kinon BJ, Gilmore JA, Gottschalk LA. Continued improvement in quality of life despite weight change during olanzapine treatment. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]; Kinon BJ, Milton DR, Gilmore JA. Continued improvement in quality of life despite weight change during olanzapine treatment. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S154. [Google Scholar]; Kinon BJ, Milton DR, Hill AL. Effective resolution of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia with olanzapine. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S154. [PubMed] [Google Scholar]; Kinon BJ, Milton DR, Hill AL, Williamson DJ. Effective resolution of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia with olanzapine. Journal of Psychopharmacology. 2000;14(3 Suppl):A60. [Google Scholar]; Kinon BJ, Roychowdhury SM, Milton DR, Hill AL. Effective resolution with olanzapine of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia. Journal of Clinical Psychiatry. 2001;62(Suppl 2):17–21. 00PSYCINFO: 2001–14523–0040. [PubMed] [Google Scholar]; Le Pen C, Lilliu H, Allicar MP, Olivier V, Gregor KJ. An economic comparison of olanzapine versus haloperidol in the treatment of schizophrenia in France [Comparaison economique de l’olanzapine versus haloperidol dans le traitement de la schizophrenie en France] Encephale. 1999;25(4):281–6. 00EMBASE: 19993586740. [PubMed] [Google Scholar]; Tollefson G, Beasley C, Tran P, Dellva MA, Krueger J, Tamura R. Olanzapine versus haloperidol: acute results of the multicenter international trial. Psychopharmacology Bulletin. 1996;32(3):401. [PSYCINFO: 2001–14523–0040. [Google Scholar]; Tollefson G, Lu Y. Comorbid mood disturbance in schizophrenia. Schizophrenia Research. 1997;24:192. 00MEDLINE: 127772710. [Google Scholar]; Tollefson GD. Olanzapine: a novel antipsychotic with a broad spectrum profile. Neuropyschopharmacology. 1994;10(Suppl 3 Pt 1):805S. [Google Scholar]; Tollefson GD. Treatment consideration for comorbid mood disorders in schizophrenic patients. Proceedings of the 6th World Congress of Biological Psychiatry; Nice, France. 1997 Jun 22-27.1997. [Google Scholar]; Tollefson GD, Lu Y. Comorbid mood disturbance in schizophrenia. Biological Psychiatry. 1997;41:101S. 00MEDLINE: 127772710. [Google Scholar]; Tollefson GD, Sanger TD, Lieberman JA. Olanzapine versus haloperidol in the treatment of first episode psychosis. Biological Psychiatry. 1997;41:73S. doi: 10.1176/ajp.156.1.79. 00PSYCINFO: 2001–14523–0040. [DOI] [PubMed] [Google Scholar]; Tollefson GD, Sanger TM. A blinded trial on the course and relationship of depressive symptoms in schizophrenia. Schizophrenia Research. 1998;29(1, 2):205. 00PSYCINFO: 2001–14523–0040. [Google Scholar]; Tollefson GD, Tran PV, Hamilton S, Kuntz A. Olanzapine versus risperidone in the treatment of psychosis. Preliminary report. Biological Psychiatry. 1997;41:20S. [Google Scholar]; Tran P, Lu Y, Sanger T, Beasley C, Tollefson G. Olanzapine in the treatment of schizoaffective disorder. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16; 1998. 00PSYCINFO: 2001–14523–0040. [Google Scholar]; Tran PV, Dellva MA, Beasley CM, Jr, Satterlee WG, Cousins LM, Tollefson GD. Clinical experience with long-term continuation treatment with olanzapine. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, USA. 1996 May 4-9.1996. [Google Scholar]; Tunis SL, Croghan TW, Heilman DK. Validity of SF36 for severely mentally ill patients. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30-Jun 4.1998. [Google Scholar]; Wright P, Tollefson GD, Beasley CM, Tamura RN, Tran PV, Potvin JII. A blinded, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Schizophrenia Research. 1998;29(1-2):206. doi: 10.1176/ajp.154.9.1248. 00PSYCINFO: 2001–14523–0040. [DOI] [PubMed] [Google Scholar]; Zhang F, Tohen M, Gannon KS, Breier A. Olanzapine versus haloperidol: assessment of cognitive function in patients with schizoaffective disorder, bipolar type. Journal of the European College of Neuropsychopharmacology. 1999;9:S247. 00PSYCINFO: 2001–14523–0040. [Google Scholar]; Zhang F, Tohen M, Sanger T, Gannon KS, Tollefson GD, Breier A. Olanzapine versus haloperidol in schizoaffective disorder, bipolar type: a repeated measures analysis. Journal of the European College of Neuropsychopharmacology. 1999;9:S246. 00PSYCINFO: 2001–14523–0040. [Google Scholar]
- Tollefson 1997b {published data only} .Tollefson GD, Beasley CM, Tamura RN, Tran PV, Potvin JH. Blind, controlled, long-term study of the comparative incidence of treatment emergent tardive dyskinesia with olanzapine or haloperidol. American Journal of Psychiatry. 1997;154(9):1248–54. doi: 10.1176/ajp.154.9.1248. 00ASSIA: 981810. [DOI] [PubMed] [Google Scholar]
- Tollefson 1997c {published data only} .Tollefson GD, Lu Y. A blinded trial on the course and relationship of depressive symptoms in schizophrenia. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]
- Tong 2003 {published data only} .Tong L. A controlled trial comparing the efficacy of risperidone in the treatment of first-onset and recurrent schizophrenia. Chinese Journal of the Practical Chinese With Modern Medicine. 2003;3(14):2071. 00CAJ: MEDI04010. [Google Scholar]
- Tran 1997a {published data only} .Ahmed S, Zhang F, Lindborg S, Tohen M, Breier A. A comparison of olanzapine versus risperidone on improvement in negative symptoms and emotional discomfort in patients with schizophrenia. Schizophrenia Research. 2003;60(1):270. [Google Scholar]; Ahmed S, Zhang F, Walker D, Beglinger L, Earley WR, Tran PV. Olanzapine versus risperidone for treatment of negative symptoms in schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; Allison D, Cavazzoni P, Beasley C, Holcombe J, Buse J. Analysis of random glucose concentration data from patients with schizophrenia treated with typical and atypical agents during double-blind, randomised, controlled clinical trials. Biological Psychiatry. 2001;2(Suppl 1):P010–25. [Google Scholar]; Basson B, Kennedy J, Tollefson G, Tran P, Beasley C, Bymaster F. The comparative anti muscarinic like adverse event profiles of olanzapine and risperidone treatment in patients with schizophrenia spectrum psychosis. Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]; Basson B, Kinon BJ, Gilmore JA, Taylor CC, Tollefson GD, Czekalla J. Factors influencing weight change in patients with schizophrenia treated with olanzapine verus haloperidol or risperidone. Journal of Psychopharmacology. 2000;14(3 Suppl):A60. [Google Scholar]; Basson BR, Kennedy JS, Tran PV, Beasley CM, Bymaster FP, Tollefson GD. The comparative anti-muscarinic like side effect profiles of olanzapine and risperidone treatment in patients with schizophrenia spectrum psychosis. Schizophrenia Research. 1999;36(1-3):270. [Google Scholar]; Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. Journal of Clinical Psychiatry. 2001;62(4):231–8. doi: 10.4088/jcp.v62n0404. 00MEDLINE: 113798360. [DOI] [PubMed] [Google Scholar]; Cavazzoni P, Berg PH, Millikan M, Carlson C, Beasley CM. An integrated analysis of treatment-emergent extrapyramidal syndrome in schizophrenic patients during olanzapine clinical trials versus placebo, haloperidol, risperidone or clozapine. Schizophrenia Research. 2002;53(3 Suppl 1):171. doi: 10.4088/jcp.v64n0807. [DOI] [PubMed] [Google Scholar]; Czekalla J, Beasley CM, Jr, Dellva MA, Berg PH, Grundy S. Analysis of the qtc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry. 2001;62(3):191–8. doi: 10.4088/jcp.v62n0310. 00MEDLINE: 212003210. [DOI] [PubMed] [Google Scholar]; David S, Crawford AM, Breier A. Prolactin levels in olanzapine versus typical and atypical antipsychotics. European Neuropsychopharmacology. 1998;8(Suppl 2):s229. [MEDLINE: 99206622] [Google Scholar]; David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. Journal of the European College of Neuropsychopharmacology. 1999;9:S292. [Google Scholar]; David SR, Taylor CC, Kinon BJ, Breier A. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clinical Therapeutics. 2000;22(9):1085–96. doi: 10.1016/S0149-2918(00)80086-7. [DOI] [PubMed] [Google Scholar]; Edgell ET, Andersen SW, Grainger D, Wang J. Resource use and quality of life of olanzapine compared with risperidone: results from an international randomised clinical trial. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16.1998. [Google Scholar]; Edgell ET, Andersen SW, Johnstone BM, Dulisse B, Revicki D, Breier A. Olanzapine versus risperidone: a prospective comparison of clinical and economic outcomes in schizophrenia. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S92. doi: 10.2165/00019053-200018060-00004. [DOI] [PubMed] [Google Scholar]; Edgell ET, Andersen SW, Johnstone BM, Dulisse B, Revicki D, Breier A. Olanzapine versus risperidone: a prospective comparison of clinical and economic outcomes in schizophrenia. Pharmacoeconomics. 2000;18(6):567–79. doi: 10.2165/00019053-200018060-00004. 00EMBASE: 2001017965; MEDLINE: 112273950. [DOI] [PubMed] [Google Scholar]; Edgell ET, Andersen SW, Johnstone BM, Dulisse B, Revicki D, Breier A, Gavart S. Olanzapine versus risperidone: a prospective comparison of clinical and economic outcomes in schizophrenia. European Psychiatry. 2000;15(Suppl 2):408s. doi: 10.2165/00019053-200018060-00004. [DOI] [PubMed] [Google Scholar]; Edgell ET, Grainger DL, Andersen SW, Wang J. Resource use and quality of life associated with olanzapine compared with risperidone. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30-Jun 4.1998. [Google Scholar]; Edgell ET, Hamilton SH, Revicki DA, Genduso LA, Tollefson GD. Costs of olanzapine treatment compared with haloperidol for schizophrenia: results from a randomised clinical trial. Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-6.1998. [Google Scholar]; Eli Lilly, Company Tran 1997 - olz vs risp. Unpublished Report. 2001 [Google Scholar]; Feldman PD, Kaiser CJ, Kennedy JS, Sutton VK, Tran PV, Tollefson GD, Zhang F, Breier A. Comparison of risperidone and olanzapine in the control of negative symptoms of chronic schizophrenia and related psychotic disorders in patients aged 50 to 65 years. Journal of Clinical Psychiatry. 2003;64(9):998–1004. doi: 10.4088/jcp.v64n0904. 00EMBASE: 2003383256; MEDLINE: 146289740. [DOI] [PubMed] [Google Scholar]; Glick ID, Berg PH. Time to study discontinuation, relapse, and compliance with atypical or conventional antipsychotics in schizophrenia and related disorders. International Clinical Psychopharmacology. 2002;17(2):65–8. doi: 10.1097/00004850-200203000-00004. 00MEDLINE: 212003210. [DOI] [PubMed] [Google Scholar]; Grainger D, Edgell ET, Andersen SW, Wang J. Resource use and QOL of olanzapine compared with risperidone: results from an international randomised clinical trial. European Neuropsychopharmacology. 1998;8(Suppl 2):S225–6. [Google Scholar]; Grainger D, Edgell ET, Andersen SW, Wang J. Resource use and quality of life of olanzapine compared with risperidone: results from an international randomised clinical trial. Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31-Nov 4.1998. [Google Scholar]; Kennedy J, Basson B, Tran P, Beasley C, Bymaster F, Breier A. The comparative anti-muscarinic-like adverse event profiles of olanzapine and risperidone treatment in patients with schizophrenia spectrum psychosis. Proceedings of the 39th Annual Meeting of the New Clinical Drug Evaluation Unit; Boca Raton, Florida, USA. 1999 Jun 1-4.1999. [Google Scholar]; Kennedy JS, Basson BR, Tran PV, Beasley CM, Bymaster FP, Tollefson GD. The comparative anti-muscarinic-like adverse event profiles of olanzapine and risperidone treatment in patients with schizophrenia spectrum psychosis. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]; Kinon B, Basson B, Tollefson GD. Gender-specific prolactin olanzapine versus haloperidol in schizophrenia. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; Toronto, Ontario, Canada. 1998 May 30-Jun 4.1998. [Google Scholar]; Kollack-Walker S, Lipkovich I, Ahmed S. Treatment-emergent EPS symptoms during treatment with olanzapine or risperidone. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]; Owens DG. Olanzapine produced a higher clinical response rate than risperidone in schizophrenia. Evidence-Based Mental Health. 1998;1(2):55. [Google Scholar]; Pickar D. Clinical profiles of the new antipsychotic agents. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]; Sutton VK, Street JS, Kennedy JS, Feldman PD, Breier A. Superiority of olanzapine over risperidone in the control of negative symptoms of schizophrenia and related psychotic disorders in older patients. European Neuropsychopharmacology. 2001;11(3):276. [Google Scholar]; Tollefson GD, Andersen SW. Should we consider mood disturbance in schizophrenia as an important determinant of quality of life? Journal of Clinical Psychiatry. 1999;60(Suppl 5):23–9. 00MEDLINE: 992066220. [PubMed] [Google Scholar]; Tollefson GD, Andersen SW, Tran PV. The course of depressive symptoms in predicting relapse in schizophrenia: a double blind, randomised comparison of olanzapine and risperidone. Biological Psychiatry. 1999;46(3):365–73. doi: 10.1016/s0006-3223(99)00049-9. 00EMBASE: 1999252174; MEDLINE: 10435202] [DOI] [PubMed] [Google Scholar]; Tollefson GD, Tran PV, Hamilton S. Kuntz A. Olanzapine versus risperidone in the treatment of psychosis. Preliminary report. Biological Psychiatry. 1997;41:20S. [MEDLINE: 974617170. [Google Scholar]; Tollefson GD, Tran PV, Hamilton S, Kuntz A. Olanzapine versus risperidone in the treatment of psychosis. Preliminary report. Schizophrenia Research. 1997;24(1, 2):191. [Google Scholar]; Tran PV, Basson BR, Kennedy JS, Beasley CM, Jr, Bymaster FP, Tollefson GD. The comparative anti-muscarinic-like side effect profiles of olanzapine and risperidone treatment in patients with schizophrenia spectrum psychosis. Journal of the European College of Neuropsychopharmacology. 1999;9:S290. [Google Scholar]; Tran PV, Hamiliton SH, Kuntz AJ, Tollefson GD. Olanzapine versus risperidone in the treatment of psychosis disorders: a preliminary report. Proceedings of the 35th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 1996 Dec 9-13.1996. [Google Scholar]; Tran PV, Hamilton SH, Kuntz AJ, Potvin JH, Andersen SW, Beasley C, Tollefson GD. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. Journal of Clinical Psychopharmacology. 1997;17(5):407–18. doi: 10.1097/00004714-199710000-00010. 00MEDLINE: 974617170. [DOI] [PubMed] [Google Scholar]; Tran PV, Tollefson GD, Andersen SW, Kuntz AJ, Hamilton SH. Olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. Proceedings of the 10th European College of Neuropsychopharmacology Congress; Vienna, Austria. 1997 Sep 13-17; 1997. [DOI] [PubMed] [Google Scholar]; Tran PV, Tollefson GD, Anderson SW, Kuntz A, Hamilton SH. Olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. Schizophrenia Research. 1998;29(1, 2):205. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]; Tran PV, Tollefson GD, Hamilton S. Olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]; Tran PV, Tollefson GD, Hamilton S, Kuntz A. Olanzapine vs. risperidone in the treatment of psychosis. Proceedings of the 6th World Congress of Biological Psychiatry; Nice, France. 1997 Jun 22-27.1997. [Google Scholar]; Wirtz HS, Kinon BJ, Zhao Z, Barber BL. Acute response to olanzapine but not to risperidone predicts the likelihood of continued improvement over time in patients with schizophrenia. Schizophrenia Research. 2002;53(3 Suppl 1):181. [Google Scholar]; *; Zhao Z, Kinon BJ, Barber BL, Wirtz HS. Acute response to olanzapine predicts continued improvement in schizophrenia. Schizophrenia Research. 2003;60(1):308. [Google Scholar]
- Ueland 2004 {published data only} .Ueland T, Rund BR. A controlled randomised treatment study: the effects of a cognitive remediation program on adolescents with early onset psychosis. Acta Psychiatrica Scandinavica. 2004;109(1):70–4. doi: 10.1046/j.0001-690x.2003.00239.x. 00MEDLINE: 146749610. [DOI] [PubMed] [Google Scholar]
- Vaglum 2002 {published data only} .Vaglum P, Friis S, Melle I, Opjordsmoen S, Larsen TK, Simonsen E, McGlashan TH. Does duration of untreated psychosis bias schizophrenia study samples?. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Van Bruggen 1999 {published data only} .Van Bruggen JM, Linszen DH, Dingemans PM. An open study of olanzapine versus risperidone in the management of early-phase schizophrenia and related disorders conference abstract. Schizophrenia Research. 1999;36(1-3):316–7. 00ISI: 0001887881000820. [Google Scholar]
- Van Bruggen 2003 {published data only} .Van Bruggen J, Tijssen J, Dingemans P, Gersons B, Linszen D. Symptom response and side-effects of olanzapine and risperidone in young adults with recent onset schizophrenia. International Clinical Psychopharmacology. 2003;18(6):341–6. doi: 10.1097/00004850-200311000-00005. 00PSYCINFO: 2004–16292–0050. [DOI] [PubMed] [Google Scholar]
- Van Meijel 2006a {published data only} .Van Meijel B, Kruitwagen C, Van der Gaag M, Kahn RS, Grypdonck MHF. An intervention study to prevent relapse in patients with schizophrenia. Journal of Nursing Scholarship. 2006;38(1):42–9. doi: 10.1111/j.1547-5069.2006.00076.x. 00MEDLINE: 165793230. [DOI] [PubMed] [Google Scholar]
- Van Meijel 2006b {published data only} .Van Meijel B. Early intervention has no effect on symptoms in people with first episode, non-affective psychosis, although it may improve overall function and medication adherence. Evidence-Based Mental Health. 2006;9(3):69. doi: 10.1136/ebmh.9.3.69. [CINAHL: 2009277046; MEDLINE: 168681880. [DOI] [PubMed] [Google Scholar]
- Van Nimwegen 2006a {published data only} .Van Nimwegen L, De Haan L, Van Beveren N, Laan W, Van de Brink W, Linszen D. Subjective well-being and craving for cannabis in first psychosis, a randomised double blind comparison of olanzapine versus risperidone. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; Davos, Switzerland. 2006 Feb 6-10.2006. [Google Scholar]
- Van Nimwegen 2006b {published data only} .Van Nimwegen L, De Haan L, Van Beveren N, Laan W, Van de Brink W, Linszen D. Obsessive compulsive symptoms in a randomised double blind. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; Davos, Switzerland. 2006 Feb 6-10.2006. [Google Scholar]
- Van Nimwegen 2006c {published data only} .Van Nimwegen L, De Haan L. Early withdrawal in a double-blind randomised clinical trial with olanzapine and risperidone performed in adolescents with first psychosis. Psychopathology. 2006;39(3):158. doi: 10.1159/000091802. [EMBASE: 160407; MEDLINE: 165316920. [DOI] [PubMed] [Google Scholar]
- Verhaegh 2006 {published data only} .Verhaegh MJM. Researching the effect of assertive community treatment (ACT) for first episode psychosis: the use of peer-interviewers in a quasi-experimental study. Schizophrenia Research. 2006;86(Suppl 1):S170. [Google Scholar]
- Vollenweider 2003 {published data only} .Vollenweider FX, Benz M, Etzensberger M, Ludewig K, Hell D. Brain activation patterns during prepulse inhibition (ppi) in first episode schizophrenia and healthy controls using h2 150-pet. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S327. [Google Scholar]
- Volterra 1996 {published data only} .Volterra V, De Ronchi D, Belelli G, Ruggeri M, Borsetti G, Cotani P. Effects of psychodynamic therapy in schizophrenic patients. Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996 Aug 23-28.1996. [Google Scholar]; Volterra V, De Ronchi D, Belelli G, Ruggeri M, Lunardi A. Effects of psychodynamic therapy in schizophrenic patients. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, USA. 1996 May 4-9.1996. [Google Scholar]
- Wang 2000a {published data only} .Wang L, Wang Z. Risperidone versus clozapine treatment in first-episode psychosis. Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31-Apr 2.2000. [Google Scholar]
- Wang 2003a {published data only} .Wang C, Li Y, Zhao Z. Controlled study on long-term effect of cognitive behavior intervention on first episode schizophrenia. Chinese Mental Health Journal. 2003;17(3):200–2. 00: MEDI03050. [Google Scholar]
- Wang 2003i {published data only} .Wang G. 60 week, randomised, double-blind, placebo-controlled trial of valproate added to risperidone in 200treatment-naive, first-episode patients with schizophrenia. Stanley Foundation Research Programs. 2003.
- Wang 2004d {published data only} .Wang Z-Y, Zhang L. Clinical efficacy of hyperzine on memory function of schizophrenia. Journal of Clinical Psychological Medicine. 2004;14(5):280–1. 00MEDLINE: 113057040. [Google Scholar]
- Wang 2004k {published data only} .Wang X, Wen Q, Jiang F. Comparison of efficacy safety of olanzapine and risperidone in the treatment of first episode schizophrenia. International Medicine and Health Guidance News. 2004;10(8):9–11. 00EMBASE:: 20040540130. [Google Scholar]
- Wang 2005c {published data only} .Wang G-P, Xie R, Pei G-X. A comparative study between aripiprazole and chlorpromazine in the treatment of schizophrenia. Shandong Archives of Psychiatry. 2005;18(4):250–1. 00CAJ: MEDI05100. [Google Scholar]
- Wang 2005d {published data only} .Wang Z-J, Du X-S, Wang J-L. Comparative study on treatment of aged schizophrenic patients with risperidone. Journal of Clinical Psychological Medicine. 2005;15(5):289–90. 00CAJ: MEDI05110. [Google Scholar]
- Wang 2005e {published data only} .Wang C-H, Li Y, Liu X. Cognitive function and p300 potentials in first-episode schizophrenia treated with quetiapine and risperidone. Chinese Mental Health Journal. 2005;19(5):333–6. 00CAJ: MEDI0512S1] [Google Scholar]; Wang C-H, Li Y, Wang L-H, Pan M, ZHao Z, Mu JL. Comparison of cognitive function and event related potentials in first episode schizophrenic treated with antipsychotic drugs. Journal of Clinical Psychological Medicine. 2005;15(3):168–70. 00CAJ: MEDI05080. [Google Scholar]
- Wang 2005g {published data only} .Wang C-H, Li Y, Ma J-D, Wang L-H, Pan M, Mu J-L. Comparison of cognitive function and p300 potentials in first-episode schizophrenia treated with risperidone and clozapine. Chinese Journal of Nervous and Mental Diseases. 2005;31(4):267–71. 00CAJ: MEDI0512S10. [Google Scholar]
- Wang 2005h {published data only} .Wang C-H, Li Y, Pan M. Comparison of cognitive function and p300 potentials in first-episode schizophrenia treated with risperidone and chlorpromazine. Chinese Journal of Behavioral Medical Science. 2005;14(5):405–7. 00CAJ: MEDI05070. [Google Scholar]
- Wang 2005j {published data only} .Wang R, Liu Y, Ning Z. A controlled study of aripiprazole vs clozapine in the treatment of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(4):301–2. 00CAJ: MEDI05110. [Google Scholar]
- Wang 2005m {published data only} .Wang X, Hu X, Yang J. Effect of chlorpromazine and risperidone on cognition function of the patients with first episode schizophrenia. Heath Psychology Journal. 2005;13(5):342–4. 00CAJ: MEDI05110. [Google Scholar]
- Wang 2006b {published data only} .Wang C, Li Y, Pan M. A clinical study of risperidone, clozapine and chlorpromazine in patients with first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2006;12(1):7–9. [Google Scholar]
- Wang 2006c {published data only} .Wang C, Li Y, Li Y. Comparative study on the life quality of first-episode schizophrenics treated with risperidone, clozapine and chlorpromazine. Journal of Clinical Psychosomatic Diseases. 2006;12(2):87–9. [Google Scholar]
- Wang 2006e {published data only} .Wang G-P, Xie R, Pei G-X, Zhang Y-L. Comparative study between aripiprazole and clozapine in the treatment of patients with schizophrenia. Journal of Clinical Psychiatry. 2006;16(1):44–5. [Google Scholar]
- Wang 2006i {published data only} .Wang J, Yan R. Effects of quetiapine vs. clozapine on the quality of life in first-episode schizophrenics. Journal of Clinical Psychosomatic Diseases. 2006;12(6):416–7. [Google Scholar]
- Wang 2006k {published data only} .Wang M, Lin Y, Liu K. Effects of clozapine and risperidone on the body weight and blood glucose in patients with schizophrenia. Chinese Journal of Health Psychology. 2006;14(3):315–6. [Google Scholar]
- Warrington 2006 {published data only} .Warrington LE, Loebel AD, Siu C, Kapur S. Early onset of antipsychotic action in the treatment of acutely agitated patients with schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Wei 2006a {published data only} .Wei Q-W, Fang Y-R, Shen X-L. A comparative study of social function in first-episode schizophrenic patients treated with quetiapine and risperidone. Shandong Jingshen Yixue. 2006;19(1):34–6. [Google Scholar]
- Wei 2006b {published data only} .Wei S-Z, Tang Q-S, Xu Z-N, Ruan X-J, Lu B. A randomised, double blind, double dummy parallel controlled study in the female first-episode schizophrenia treated with aripiprazole and quetiapine. Chinese Journal of Nervous and Mental Diseases. 2006;32(6):511–7. [Google Scholar]
- Wei 2006c {published data only} .Wei S-Z. A randomised, double blind, double dummy parallel controlled study in the female first-onset schizophrenia treated with aripiprazole and quetiapine. Nervous Diseases and Mental Hygiene. 2006;6(3):194–7. [Google Scholar]
- WHO 1979 {published data only} .World Health Organization . Schizophrenia: An International Follow-up Study. John Wiley & Sons; New York: 1979. [Google Scholar]
- Williams 2005b {published data only} .Williams S. Effectiveness trial of systematic psychosocial interventions in early psychosis. National Research Register. 2005;Vol. 4 [Google Scholar]
- Wilson 1982b {published data only} .Wilson WH, Guy W, Ban TA, Adelson LM, Perez-Payan H. A double-blind dose-determination study with flutroline - a new neuroleptic. Drug Development Research. 1982;2(4):357–62. 00MEDLINE: 153700200. [Google Scholar]
- Wirshing 1992b {published data only} .Wirshing WC, Marder SR, Johnston-Cronk K, Lebell M, Mackenzie J, Mintz J, Eckman T, Liberman RP. Management of risk of relapse in schizophrenia. Schizophrenia Research. 1992;Vol. 6(issue 2):107. 00EMBASE: 2006601347; MEDLINE: 170951940. [Google Scholar]
- Woggon 1978 {published data only} .Woggon B. Effects and side-effects of bromperidol in comparison with other antipsychotic drugs. Acta Psychiatrica Belgica. 1978;78(1):155–72. 00MEDLINE: 781628290. [PubMed] [Google Scholar]; Woggon B, Angst J. Double-blind comparison of bromperidol and perphenazine. International Pharmacopsychiatry. 1978;13(3):165–76. doi: 10.1159/000468337. 00MEDLINE: 3551820. [DOI] [PubMed] [Google Scholar]
- Woods 2002a {published data only} .Woods SW, McGlashan TH. Sample size planning for prodromal intervention trials. Schizophrenia Research. 2002;Vol. 53(issue 3 Suppl 1):40. 00MEDLINE: 124091650. [Google Scholar]
- Woods 2002b {published data only} .Woods S. A l2-week open label trial of glycine treatment of prodromal symptoms of schizophrenia in 25 patients. Glycine is an amino acid that stimulates the NMDA receptor. Some theories of schizophrenia posit that NMDA receptors may be hypoactive. Stanley Foundation Research Programs. 2002.
- Woods 2003 {published data only} .Woods S, Zipursky R, Perkins D, Addington J, Marquez E, Breier A, McGlashan TH. Olanzapine versus placebo for prodromal symptoms. Proceedings of the 3rd International Conference on Early Psychosis; Copenhagen, Denmark. 2002 Sep 25-28.2002. [Google Scholar]; Woods SW, Breier A, Zipursky RB, Perkins DO, Addington J, Miller TJ, Hawkins KA, Marquez E, Lindborg SR, Tohen M, McGlashan TH. Olanzapine versus placebo for prodromal symptoms. Schizophrenia Research. 2003;60(1):306–7. doi: 10.1016/s0920-9964(02)00440-1. [DOI] [PubMed] [Google Scholar]; *; Woods SW, Zipursky RB, Perkins DO, Addington JM, Miller TJ, Breier AF, McGlashan TH. Olanzapine versus placebo for prodromal symptoms. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Woods 2004 {published data only} .Woods SW, Miller TJ, Zipursky RB, Perkins DO, Addington J, Tohen M, Breier A, McGlashan T. Treatment responsiveness with olanzapine in first-episode psychosis after zero dup. Schizophrenia Research. 2004;70(1):80. [ISI: 0002245511002170. [Google Scholar]
- Wu 2001a {published data only} .Wu T, Li Y, Li M. Efficacy of very low dosage of risperidone in the treatment of first-episode schizophrenia. Journal of Clinical Psychological Medicine. 2001;11(3):152–4. 00CAJ: MEDI01090. [Google Scholar]
- Wu 2002c {published data only} .Wu DX, Yao SQ, Cheng ZH. The study of t-test significance probability mapping of auditory p·(300) between positive and negative subtype schizophrenics. Chinese Journal of Behavioral Medical Science. 2002;11(2):154–5. 00CAJ: MEDI02070. [Google Scholar]
- Wu 2006 {published data only} .Wu J-D, Li Y-D, Song Z-W. Effect of aripiprazole or haldol on intelligence and memory in the first-onset schizophrenia. Chinese Journal of Rehabilitation Theory and Practice. 2006;12(1):64–5. 00CAJ: MEDI0603S10. [Google Scholar]
- Wu 2006a {published data only} .Wu R-R, Zhao J-P, Liu Z-N, Zhai J-G, Guo X-F, Guo WB, Tang J-S. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology. 2006;186(4):572–8. doi: 10.1007/s00213-006-0384-5. 00EMBASE: 20062757960. [DOI] [PubMed] [Google Scholar]
- Wunderink 2003 {published data only} .Wunderink L, Nienhuis FJ, Wiersma D, van den Bosch RJ, Bruggeman R, Faber G, van der Linde J, Noorthoorn E, Slooff CJ, Vlaminck P. Medication strategies in first onset schizophrenia: a randomised trial of effectiveness of short versus sustained antipsychotic treatment and quality of life in first episode psychosis preliminary findings: Incidence, compliance and early course. Schizophrenia Research. 2003;60(1):307. [Google Scholar]
- Wunderink 2006 {published data only} .Wunderink A, Nienhuis FJ, Sytema S, Wiersma D. Guided discontinuation versus maintenance treatment in remitted first episode psychosis: relapse rates and functional outcome. Schizophrenia Research. 2006;86(Suppl 1):S51. doi: 10.4088/jcp.v68n0502. [DOI] [PubMed] [Google Scholar]
- Xie 1998 {published data only} .Xie Q, Li J, Li Z. Clozapine versus chlorpromazine in the treatment of schizophrenia. Sichuan Mental Health. 1998;11(1):18–20. 00CAJ: MEDI98020. [Google Scholar]
- Xu 2003d {published data only} .Xu L, Zhang F, Meng Q. Early recovery psychotherapy and first episode schizophrenics. Journal of Nursing Science. 2003;18(10):723–6. 00CAJ: MEDI04020. [Google Scholar]
- Xu 2005b {published data only} .Xu L, Chen J, Xu K. Prevention of first episode schizophrenia relapse by insight education. Journal of Nursing Science. 2005;20(9):3–5. 00CAJ: MEDI05060. [Google Scholar]
- Yang 1999c {published data only} .Yang F, Ji Z. Effect of pharmacotherapy on the cognitive functions in patients with first- episode schizophrenia. Herald of Medicine. 2002;21(10):624–8. 00CAJ: MEDI02120. [Google Scholar]; *; Yang F, Ji Z. Relationships of cognitive function, positive or negative symptoms, and response to antipsychotic in first-episode schizophrenic patients. Sichuan Mental Health. 1999;12(4):223–6. 00CAJ: MEDI0001] [Google Scholar]; Yang FD, Ji ZF. Effects of chlorpromazine and clozapine on the cognitive function in first-episode schizophrenia patients. Chinese New Drugs Journal. 2002;11(2):152–5. 00CAJ: MEDI02070. [Google Scholar]
- Yang 2000b {published data only} .Yang F, Ji Z. Relationship of neurocognitive functioning to clinical prognosis in first-episode schizophrenic patients. Shanghai Archives of Psychiatry. 2000;12(3):128–31. 00CAJ: MEDI0510] [Google Scholar]; Yang F, Ji Z, Zhang P. A long follow-up controlled study of schizophrenia-like psychosis and first-episode schizophrenia. Nervous Diseases and Mental Hygjene. 2002;2(2):76–80. 00CAJ: MEDI0308] [Google Scholar]; Yang F, Phillips M, Zhang P. Cognitive function in first episode schizophrenics. Chinese Mental Health Journal. 2000;14(6):383–5. [Google Scholar]
- Yang 2001 {published data only} .Yang F, Fei L, Ji Z. Comparison of neuropsychological characteristics between paranoid and non-paranoid schizophrenia. Nervous Diseases and Mental Hygiene. 2001;1(1):26–8. [Google Scholar]
- Yang 2003a {published data only} .Yang X, Mei Q. A comparison of olanzapine and risperidone in the treatment of first-episode schizophrenia. Shanghai Archives of Psychiatry. 2003;15(6):338–48. 00CAJ: MEDI04040. [Google Scholar]
- Yang 2004b {published data only} .Yang F, Zhang P. Blood glucose in first-episode schizophrenia with chlorpromazine and clozapine treatment. Shanghai Archives of Psychiatry. 2004;16(1):1–3. 00CAJ: MEDI03110. [Google Scholar]; Yang F, Zhang P, Fei L. Blood glucose in first-episode schizophrenia with chlorpromazine and clozapine treatment. Shanghai Archives of Psychiatry. 2004;16(1):1–3. [Google Scholar]
- Yang 2005c {published data only} .Yang Q-P, Huang Y-P, Yang B-X. The effect of quetiapine and risperidone on the EEG of first-episode schizophrenic patients. Shandong Archives of Psychiatry. 2005;18(3):159–60. 00CAJ: MEDI05090. [Google Scholar]
- Yang 2006b {published data only} .Yang J, Sun M, Meng Y. Clinical study of aripirazole in the treatment of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2006;12(2):90–2. [Google Scholar]
- Yang 2006g {published data only} .Yang Z, Pan F-Y, Yin J-B. A comparative study between the domestic and imported olanzapine in first-episode schizophrenia. Nervous Diseases and Mental Hygiene. 2006;6(5):345–6. [Google Scholar]
- Yang Bin 2004 {published data only} .Yang B, Wang YD, Zhang L. Effects of clozapine, risperidone and haloperidol on plasma leptin in first-episode schizophrenic patients. Journal of The Fourth Military Medical University. 2004;25(14):1323–5. [Google Scholar]
- Yanos 2004 {published data only} .Yanos PT, Barrow SM, Tsemberis S. Community integration in the early phase of housing among homeless persons diagnosed with severe mental illness: successes and challenges. Community Mental Health Journal. 2004;40(2):133–50. doi: 10.1023/b:comh.0000022733.12858.cb. 00CENTRAL: CN–00489856; PUBMED: 152066380. [DOI] [PubMed] [Google Scholar]
- Ye 2005a {published data only} .Ye X-R, Xia X-L. A comparative study between aripiprazole and risperidone in treatment of first-onset schizophrenia. Medical Journal of National Defending Forces in Southwest China. 2005;15(6):616–8. 00CAJ: MEDI06020. [Google Scholar]
- Ye 2005b {published data only} .Ye S-C, Zhang W-Y, Zhang Y-M. Observation of developments of the level of serum prolactin of first-episode schizophrenia patients during the treatment. Medical Journal of Chinese Peoples Health. 2005;17(6):267–8. 00CAJ: MEDI0507S10. [Google Scholar]
- Yu 2001b {published data only} .Yu J, Chai M, Chen J. A comparison of cognitive function in schizophrenia treated with risperidone and chlorpromazine. Journal of Clinical Psychological Medicine. 2001;11(5):265–6. 00CAJ: MEDI01120. [Google Scholar]
- Yu E Li 2004 {published data only} .Yu EL, Xiu YL, Zhao XZ. One-year follow-up study of systemic early intervention to first episode schizophrene. Chinese Journal of Clinical Rehabilitation. 2004;8(36):8178–81. 00CAJ: MEDI05070. [Google Scholar]
- Zeng 2003 {published data only} .Zeng Z. An extended two-year follow up of psychological education on insight recovery and drug therapy compliance and recurrence in schizophrenic patients. Modern Rehabilitation. 2003;7(12):1774–5. 00CAJ: MEDI03090. [Google Scholar]
- Zeng 2006 {published data only} .Zeng Z-X, Liu Q-F, Li Z-C, Li Y-E. Effect of comprehensive intervention in the whole process in patients with first episode schizophrenia. Journal of Clinical Psychiatry. 2006;16(5):284–6. [Google Scholar]
- Zhang 1994a {published data only} .Zhang M, Wang M, Li J, Phillips MR. Randomised-control trial of family intervention for 78 first-episode male schizophrenic patients: an 18-month study in suzhou, jiangsu. British Journal of Psychiatry. 1994;165(Suppl 24):96–102. 00MEDLINE: 163194060. [PubMed] [Google Scholar]; Zhang MD. A systematic study of brain evoked potentials on schizophrenics and its clinical applications. Shanghai Medical Journal. 1994;17(6):319–22. 00CAJ: MEDI94220. [Google Scholar]
- Zhang 1998c {published data only} .Zhang X, Zhou D, Xiang Y. Atypical lymphocyte in the free naive autistic children and first episode schizophrenia. Chinese Journal of Psychiatry. 1998;31(1):26–9. 00EMBASE: 19983618280. [Google Scholar]
- Zhang 1998d {published data only} .Zhang M, Weng Z, Yan H. Two year experience of psychosocial education for relatives of schizophrenics. Chinese Journal of Psychiatry. 1998;31(2):90–3. 00CAJ: MEDI98080. [Google Scholar]
- Zhang 2000f {published data only} .Zhang X, Chen Y, Zhang X. Influence of pregnancy and parturition on patients with first episode psychosis. Chinese Journal of Psychiatry. 2000;33(3):182–4. 00CAJ: MEDI0012S30. [Google Scholar]
- Zhang 2002j {published data only} .Zhang W, Wang X, Tao J. The effect of clozapine on the insulin sensitivity. Journal of Clinical Psychological Medicine. 2002;12(4):196–8. 00CAJ: MEDI02100. [Google Scholar]
- Zhang 2003l {published data only} .Zhang XY. A 24 week double-blind, placebo-controlled trial of the anti-inflammatory drug celecoxib added to risperidone in 250 treatment-naive, first-episode patients with schizophrenia. Stanley Foundation Research Programs. 2003.
- Zhang 2004a {published data only} .Zhang H, Liu ZC, Wang GH. Efficacy and safety of different fixed dose of risperidone in treatment of first-episode schizophrenia. Chinese Journal of Psychiatry. 2004;37(1):26–9. 00CAJ: MEDI04040. [Google Scholar]
- Zhang 2005k {published data only} .Zhang C-J, Li C, Yang F-S, Yang R-L, Zhao Z. Effect of health education for parents in preventing the recurrence of schizophrenia in their children after first episode. Chinese Journal of Clinical Rehabilitation. 2005;9(28):19–20. 00CAJ: MEDI05090. [Google Scholar]
- Zhang 2005l {published data only} .Zhang XY. 20-week, randomised, double-blind, placebo-controlled study of artemisinin as an adjunct in the treatment of 200 first-episode patients with schizophrenia. Stanley Foundation Research Programs. 2005.
- Zhang Fuying 2005 {published data only} .Zhang F, Meng Q, Xu L. The function of the prognosis to first episode schizophrenics to home visiting by nurses. Chinese Journal of Nursing. 2005;40(1):5–8. [Google Scholar]
- Zhao 2006 {published data only} .Zhao L, Wang M, Wang B. A controlled study of aripiprazole versus quetiapine in the treatment of first-episode schizophrenia. Chinese Journal of Health Psychology. 2006;14(6):663–4. [Google Scholar]
- Zheng 2003c {published data only} .Zheng YJ, Wang GH, Cheng ZL. Effects of clozapine and risperidone on the glucose metabolism in first-episode schizophrenic patients. Chinese Journal of Psychiatry. 2003;36(4):207–10. 00CAJ: MEDI04010. [Google Scholar]
- Zhi 2006 {published data only} .Zhi X-Y, Yan F, Yang L-Q. Effect of self-efficacy on drug therapy compliance in female first-episode schizophrenic patients. Journal of the Xinxiang Medical College. 2006;23(5):509–11. [Google Scholar]
- Zhou 2005c {published data only} .Zhou B, Tang Y, Zhu L. Effects of risperidone vs clozapine on blood-lipid of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(4):315–6. 00CAJ: MEDI05110. [Google Scholar]
- Zhu 2001a {published data only} .Zhu F, Ji Z, Fie L. The pharmacokinetics of clozapine and its clinical utilization. Journal of Clinical Psychological Medicine. 2001;11(1):12–5. 00CAJ: MEDI01040. [Google Scholar]
- Zhu 2001b {published data only} .Zhu J, Xia Z, Pan X. An investigation on the optimal dose of risperidone and follow-up of the result in the maintenance treatment of first-episode schizophrenia. Sichuan Mental Health. 2001;14(3):151–2. [Google Scholar]
- Zhu 2002g {published data only} .Zhu YP, Zhou HJ. Clinical and experimental observation of he-ne laser intravascular irradiation for schizophrenia. Modern Rehabilitation. 2002;6(9):1271–2. 00CAJ: MEDI02070. [Google Scholar]
- Zhu 2002i {published data only} .Zhu H, Ma C, Hou J, Yin Q, Hu H, Guo Y. Study of serum il-6 and a-ifn in the pre-and post-treatment of 68 patients with first episode schizophrenia. Chinese Journal of Nervous and Mental Diseases. 2002;28(5):324–6. 00CAJ: MEDI03010. [Google Scholar]
- Zipursky 2004 {published data only} .Zipursky R. Home-based versus clinic-based care for first-episode psychosis: findings from the pilot phase of a randomised clinical trial. Schizophrenia Research. 2004;70(1):16. 00ISI: 0002245511000390. [Google Scholar]
- Zipursky 2005a {published data only} .Zipursky RB, Gu H, Charles C, Sharma T, Green AI, Gur RE, Kahn RS, Perkins D, Keefe R, Hamer RM, Tollefson GD, Tohen M, Lieberman JA. Clinical correlates of MRI brain volumes in first episode psychosis. Schizophrenia Bulletin. 2005;31:408. [Google Scholar]
- Zipursky 2005b {published data only} .Zipursky R, Gu H, Green AI, Centorrina F, Glick I, Perkins DO, McEvoy J, Sharma T, Gur R, Strakowski SM. Clinical correlates of weight gain in first episode psychosis patients treated with olanzapine. Schizophrenia Research. 2003;60(1):372. [Google Scholar]; Zipursky RB, Gu H, Green AI, Perkins DO, Tohen MF, McEvoy JP, Strakowski SM, Sharma T, Kahn RS, Gur RE, Tollefson GD, Lieberman JA. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. British Journal of Psychiatry. 2005;187:537–43. doi: 10.1192/bjp.187.6.537. 00MEDLINE: 163194060. [DOI] [PubMed] [Google Scholar]; *; Zipursky RB, Hongbin G, Green AI, Centorrina F, Glick ID, Lieberman JA. Clinical correlates of weight gain in first-episode patients on olanzapine. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]
- Zuo 2000 {published data only} .Zuo J. Risperidone in the treatment of first-episode schizophrenia. Tianjin Pharmacy. 2000;12(3):44–5. 00CAJ: MEDI0012S20. [Google Scholar]
- Zuo 2002 {published data only} .Zuo J, Xun Z, Wang M. A comparison of social ability of patients with schizophrenia treated with risperidone and those treated with clozapine. Tianjin Pharmacy. 2002;20(5):54–5. 00CAJ: MEDI02120. [Google Scholar]
References to studies awaiting assessment
- Johnstone 1988 {published data only (unpublished sought but not used)} .Johnstone EC, Crow TJ, Frith CD, Owens DGC. The Northwick Park ”functional“ psychosis study: diagnosis and treatment response. Lancet. 1988;8603:119–25. doi: 10.1016/s0140-6736(88)90682-4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Francey 2010 {unpublished data only} .Francey SM, Nelson B, Thompson A, Parker A, Kerr M, MacNeil C, Fraser R, Hughes F, Crisp K, Harrigan S, Wood SJ, Berk M, McGorry PD. Who needs antipsychotic medication in the earliest stages of psychosis? A reconsideration of benefits, risks, neurobiology and ethics in the era of early intervention. Schizophrenia Research. 2010;119:1–10. doi: 10.1016/j.schres.2010.02.1071. [DOI] [PubMed] [Google Scholar]
Additional references
- Adams 2007 .Adams CE, Awad G, Rathbone J, Thornley B. Chlorpromazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD000284.pub2. 00DOI: 10.1002/14651858.CD000284.pub20. [DOI] [PubMed] [Google Scholar]
- Agarwal 2007 .Agarwal V, Abhijnhan A, Raviraj P. Ayurvedic medicine for schizophrenia. Cochrane Database of Systematic Reviews. 2007;(Issue 4) doi: 10.1002/14651858.CD006867. 00DOI: 10.1002/14651858.CD0068670. [DOI] [PubMed] [Google Scholar]
- Altman 1996 .Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA 2004 .American Psychiatric Association Practice guidelines for the treatment of patients with schizophrenia, second edition. American Journal of Psychiatry Supplement. 2004;161(2):1–56. [PubMed] [Google Scholar]
- Bland 1997 .Bland JM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel 1999 .Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d’efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d’Etude des Indices D’efficacite] Therapie. 1999;54(4):405–11. 00PUBMED: 106671060. [PubMed] [Google Scholar]
- Bola 1998 .Bola JR. Evaluation of Treatment in Early Episode Acute Psychosis: A Secondary Analysis of the Soteria study [Dissertation] Univ. of California; Berkeley, CA: 1998. [Google Scholar]
- Bola 2006 .Bola JR. At Issue: Medication-free research in early episode schizophrenia: evidence of long-term harm? Schizophrenia Bulletin. 2006;32(2):288–96. doi: 10.1093/schbul/sbj019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter 1994 .Carpenter WT, Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine. 1994;330:681–90. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- Cole 1966 .Cole JO, Goldberg SC, Davis JM. Drugs in the treatment of psychosis: controlled studies. In: Solomon P, editor. Psychiatric Drugs. Grune and Stratton; New York: 1966. pp. 153–80. [Google Scholar]
- Cornblatt 2001 .Cornblatt BA, Lencz T, Kane JM. Treatment of the schizophrenia prodrome: is it presently ethical? Schizophrenia Research. 2001;51:31–8. doi: 10.1016/s0920-9964(01)00236-5. [DOI] [PubMed] [Google Scholar]
- CPA 1998 .Canadian Psychiatric Association Canadian clinical practice guidelines for the treatment of schizophrenia. Canadian Journal of Psychiatry. 1998;43(Suppl 2):19. [PubMed] [Google Scholar]
- Deeks 2000 .Deeks J. Issues in the selection for meta-analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; Cape town. 2000 Oct 25-28; Cape Town: The Cochrane Collaboration; 2000. [Google Scholar]
- DeGrazia 2001 .DeGrazia D. Ethical issues in early-intervention clinical trials involving minors at risk for schizophrenia. Schizophrenia Research. 2001;51:77–86. doi: 10.1016/s0920-9964(01)00243-2. [DOI] [PubMed] [Google Scholar]
- DeQuardo 1998 .DeQuardo JR. Pharmacologic treatment of first-episode schizophrenia: early intervention is key to outcome. Journal of Clinical Psychiatry. 1998;59(Suppl 19):9–17. [PubMed] [Google Scholar]
- Divine 1992 .Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians’ patient care behavior. Journal of General Internal Medicine. 1992;7(6):623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- Dixon 1995 .Dixon LB, Lehman AF, Levine J. Conventional antipsychotic medications for schizophrenia. Schizophrenia Bulletin. 1995;21(4):567–77. doi: 10.1093/schbul/21.4.567. [DOI] [PubMed] [Google Scholar]
- Donner 2002 .Donner A, Klar N. Issues in the meta-analysis of cluster randomised trials. Statistics in Medicine. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- Duggan 2005 .Duggan L, Fenton M, Rathbone J, Dardennes R, El-Dosoky A, Aidran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD001359.pub2. 00DOI: 10.1002/14651858.CD001359.pub20. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey-Smith G, Schneider M, Minder CSO. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;13:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayeh 2006 .El-Sayeh HGG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database of Systematic Reviews. 2006;(Issue 2) doi: 10.1002/14651858.CD004578.pub3. 00DOI: 10.1002/14651858.CD004578.pub30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 2002 .Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta-analyses involving crossover trials: methodological issues. International Journal of Epidemiology. 2002;31(1):140–9. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- Falloon 1998 .Falloon IRH, Cloverdale JH, Laidlaw TM, Merry S, Kydd RR, Morosini P. Early intervention for schizophrenic disorders: Implementing optimal treatment strategies in routine clinical services. British Journal of Psychiatry. 1998;172(33S):33–8. [PubMed] [Google Scholar]
- Fleischhacker 2003 .Fleischhacker WW, Czobor P, Hummer M, Kemmler G, Kohnen R, Volavka J. Placebo or active control trials of antipsychotic drugs? Archives of General Psychiatry. 2003;60(5):458–64. doi: 10.1001/archpsyc.60.5.458. [DOI] [PubMed] [Google Scholar]
- Frances 1996 .Frances A, Docherty JP, Kahn DA. The expert consensus guidelines series: treatment of schizophrenia. Journal of Clinical Psychiatry. 1996;57(Suppl 12B):5–58. [Google Scholar]
- Furukawa 2006 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology. 2006;59(7):7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Gaebel 2002 .Gaebel W, Janner M, Frommann N, Pietzckier A, Kopcke W, Linden M, Muller P, Muller-Spahn F, Tegeler J. First vs multiple episode schizophrenia: two-year outcome of intermittent and maintenance medication strategies. Schizophrenia Research. 2002;53:145–59. doi: 10.1016/s0920-9964(01)00182-7. [DOI] [PubMed] [Google Scholar]
- Gaebel 2005b .Gaebel W, Weinmann S, Sartorius N, Rutz W, McIntyre JS. Schizophrenia practice guidelines: international survey and comparison. British Journal of Psychiatry. 2005;187:248–55. doi: 10.1192/bjp.187.3.248. [DOI] [PubMed] [Google Scholar]
- GRADE Profiler .GRADE Working Group . GRADE Profiler. 3.2. GRADE Working Group; 2004. [Google Scholar]
- Gulliford 1999 .Gulliford MC. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology. 1999;149:876–83. doi: 10.1093/oxfordjournals.aje.a009904. [DOI] [PubMed] [Google Scholar]
- Hartung 2005 .Hargtung B, Wada M, Laux G, Leucht S. Perphenazine for schizophrenia. Cochrane Database of Systematic Reviews. 2005;(Issue 1) doi: 10.1002/14651858.CD003443.pub2. 00DOI: 10.1002/14651858.CD003443.pub20. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2009 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. The Cochrane Collaboration; [updated September 2009]. 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Irving 2006a .Irving CB, Adams CE, Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD003082.pub3. 00DOI: 10.1002/ 14651858.CD003082.pub20. [DOI] [PubMed] [Google Scholar]
- Irving 2006b .Irving CB, Mumby-Croft R, Joy LA. Polyunsaturate fatty acid supplementation for schizophrenia. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD001257.pub2. 00DOI: 10.1002/ 14651858.CD001257.pub20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone 1999 .Johnstone EC, Owens DG, Crow TJ, Davis JM. Does a four-week delay in the introduction of medication alter the course of functional psychosis? Journal of Psychopharmacology. 1999;13(3):238–44. doi: 10.1177/026988119901300305. [DOI] [PubMed] [Google Scholar]
- Kane 1993 .Kane JM, Marder SR. Pharmacologic treatment of schizophrenia. Schizophrenia Bulletin. 1993;19(2):287–302. doi: 10.1093/schbul/19.2.287. [DOI] [PubMed] [Google Scholar]
- Kay 1986 .Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. Multi-Health Systems; North Tonawanda, NY: 1986. [Google Scholar]
- Kirch 1992 .Kirch DG, Keith SJ, Matthews SM. Research on first episode psychosis: report on a National Institute of Mental Health workshop. Schizophrenia Bulletin. 1992;18(2):179–84. doi: 10.1093/schbul/18.2.179. [DOI] [PubMed] [Google Scholar]
- Komossa 2009 .Komossa K, Rummel-Kluge C, Hunger H, Schwarz S, Bhoopathi PS, Kissling W, Leucht S. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews. 2009;(Issue 4) doi: 10.1002/14651858.CD006627.pub2. 00DOI: 10.1002/14651858.CD006627.pub20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa 2010 .Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Kissling W, Leucht S. Zotepine versus other atypical antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews. 2010;(Issue 1) doi: 10.1002/14651858.CD006628.pub2. 00DOI: 10.1002/ 14651858.CD006628.pub30. [DOI] [PubMed] [Google Scholar]
- Lehman 1998 .Lehman AF, Steinwachs DM. The schizophrenia patient outcomes research team (PORT) treatment recommendations. Schizophrenia Bulletin. 1998;24(1):1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- Leucht 2005 .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry. 2005;187:366–71. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- Leucht 2005a .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophrenia Research. 2005;79(2-3):231–8. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Leucht 2007 .Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF? Schizophrenia Bulletin. 2007;33(1):183–91. doi: 10.1093/schbul/sbl025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht 2008 .Leucht C, Kitzmantel M, Kane J, Leucht S, Chua WLLC. Haloperidol versus chlorpromazine for schizophrenia. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD004278.pub2. 00DOI: 10.1002/14651858.CD004278.pub20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewander 1996 .Lewander T. Long-term studies of antipsychotic drugs in schizophrenia. European Psychiatry. 1996;11:127–34. doi: 10.1016/0924-9338(96)85177-7. [DOI] [PubMed] [Google Scholar]
- Linszen 1998 .Linszen D, Lenior M, DeHaan L, Dingemas P, Gersons B. Early intervention, untreated psychosis and the course of early schizophrenia. British Journal of Psychiatry. 1998;172:84–9. [PubMed] [Google Scholar]
- Luborsky 1962 .Luborsky R. Clinicians’ judgements of mental health: a proposed scale. Archives of General Psychiatry. 1962;7:407–17. doi: 10.1001/archpsyc.1962.01720060019002. [DOI] [PubMed] [Google Scholar]
- Marques 2004 .Marques LDO, Soared B, Silva de Lima M. Trifluoperazine for schizophrenia. Cochrane Database of Systematic Reviews. 2004;(Issue 1) doi: 10.1002/14651858.CD003545.pub2. 00DOI: 10.1002/14651858.CD003545.pub20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall 2000 .Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry. 2000;176:249–52. doi: 10.1192/bjp.176.3.249. [DOI] [PubMed] [Google Scholar]
- Matar 2007 .Matar HE, Almerie MQ. Oral fluphenazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD006352. 00DOI: 10.1002/14651858.CD0063520. [DOI] [PubMed] [Google Scholar]
- McGlashan 2001 .McGlashan TH. Psychosis treatment prior to psychosis onset: Ethical issues. Schizophrenia Research. 2001;51:47–54. doi: 10.1016/s0920-9964(01)00238-9. [DOI] [PubMed] [Google Scholar]
- McGorry 2001 .McGorry PD, Yung A, Phillips L. Ethics and early intervention in psychosis: Keeping up the pace and staying in step. Schizophrenia Research. 2001;51:17–29. doi: 10.1016/s0920-9964(01)00235-3. [DOI] [PubMed] [Google Scholar]
- National 2002 .National Institute for Clinical Excellence (NICE) Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care. National Institute for Clinical Excellence; London: 2002. [Google Scholar]
- Norman 2001 .Norman RMG, Malla AK. Duration of untreated psychosis: a critical examination of the concept and its importance. Psychological Medicine. 2001;31(3):381–400. doi: 10.1017/s0033291701003488. [DOI] [PubMed] [Google Scholar]
- Overall 1962 .Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pietzcker 1993a .Pietzcker A, Gaebel W, Kopcke W, Linden M, Muller P, Muller-Spahn F, Tegeler J. Intermittent versus maintenance neuroleptic long-term treatment in schizophrenia: 2-year results of a German multicenter study. Journal of Psychiatric Research. 1993;27(4):321–39. [Google Scholar]
- Popp 1998 .Popp SM, Trezza GR. Side effects of and reactions to psychotropic medications. In: Kleespies PM, editor. Emergencies in Mental Health Practice. Guilford; New York: 1998. pp. 279–311. [Google Scholar]
- Rathbone 2005 .Rathbone J, Zhang L, Zhang M, Xia J, Liu X, Yang Y. Chinese herbal medicine for schizophrenia. Cochrane Database of Systematic Reviews. 2005;(Issue 4) doi: 10.1002/14651858.CD003444.pub2. 00DOI: 10.1002/14651858.CD003444.pub20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattehalli 2010 .Ratehalli RD, Jayaram MB, Smith M. Risperidone versus placebo for schizophrenia. Cochrane Database of Systematic Reviews. 2010;(Issue 1) 00DOI: 10.1002/ 14651858.CD006918.pub20. [PubMed] [Google Scholar]
- Review Manager (RevMan) .The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Rummel 2003 .Rummel C, Harmann J, Kissling W, Leucht S. New generation antipsychotics for first-episode schizophrenia. Cochrane Database of Systematic Reviews. 2003;(Issue 3) doi: 10.1002/14651858.CD004410. 00DOI: 10.1002/14651858.CD0044100. [DOI] [PubMed] [Google Scholar]
- Schooler 1967 .Schooler NR, Goldberg SC, Boothe H, Cole JO. One year after discharge: community adjustment of schizophrenic patients. American Journal of Psychiatry. 1967;123(8):986–95. doi: 10.1176/ajp.123.8.986. [DOI] [PubMed] [Google Scholar]
- Schooler 1989a .Schooler NR, Keith SJ, Severe JB, Matthews S. Acute treatment response and short-term outcome in schizophrenia - 1st results of the NIMH treatment strategies in schizophrenia study. Psychopharmacology Bulletin. 1989;25(3):331–35. [PubMed] [Google Scholar]
- Schünemann 2008 .Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Chichester: 2008. pp. 359–83. [Google Scholar]
- Silveira da Mota Neto 2002 .Silveira da Mota Neto JI, Soares B, Silva de Lima M. Amisulpride for schizophrenia. Cochrane Database of Systematic Reviews. 2002;(Issue 2) doi: 10.1002/14651858.CD001357. 00DOI: 10.1002/ 14651858.CD0013570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley 2006 .Thornley B, Rathbone J, Adams CE, Awad G. Chlorpromazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews. 2006;(Issue 4) 00DOI: 10.1002/14651858.CD000284.pub20. [Google Scholar]
- Ukoumunne 1999 .Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area-wide and organisation-based intervention in health and health care: a systematic review. Health Technology Assessment. 1999;3(5):1–75. [PubMed] [Google Scholar]
- Warner 2001 .Warner R. The prevention of schizophrenia: what interventions are safe and effective? Schizophrenia Bulletin. 2001;27(4):551–62. doi: 10.1093/oxfordjournals.schbul.a006895. [DOI] [PubMed] [Google Scholar]
- Wyatt 1991 .Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophrenia Bulletin. 1991;17(2):325–51. doi: 10.1093/schbul/17.2.325. [DOI] [PubMed] [Google Scholar]
- Xia 2009 .Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin. 2009;33(7):254–7. [Google Scholar]
- Yung 1998 .Yung AR, Phillips LJ, McGorrey PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ. Prediction of psychosis: a step towards indicated prevention of schizophrenia. British Journal of Psychiatry. 1998;172:14–20. [PubMed] [Google Scholar]