Abstract
An assessment of the anatomical costs of extremely long proboscid mouthparts can contribute to the understanding of the evolution of form and function in the context of insect feeding behaviour. An integrative analysis of expenses relating to an exceptionally long proboscis in butterflies includes all organs involved in fluid feeding, such as the proboscis plus its musculature, sensilla, and food canal, as well as organs for proboscis movements and the suction pump for fluid uptake. In the present study, we report a morphometric comparison of derived long-tongued (proboscis approximately twice as long as the body) and short-tongued Riodinidae (proboscis half as long as the body), which reveals the non-linear scaling relationships of an extremely long proboscis. We found no elongation of the tip region, low numbers of proboscis sensilla, short sensilla styloconica, and no increase of galeal musculature in relation to galeal volume, but a larger food canal, as well as larger head musculature in relation to the head capsule. The results indicate the relatively low extra expense on the proboscis musculature and sensilla equipment but significant anatomical costs, such as reinforced haemolymph and suction pump musculature, as well as thick cuticular proboscis walls, which are functionally related to feeding performance in species possessing an extremely long proboscis.
Keywords: flower-visitor, haemolymph pump, insect, mouthparts, sensilla, suction pump
INTRODUCTION
Feeding in adult butterflies is of direct importance to individual fitness because the intake of carbohydrates and amino acids contributes to increased longevity, body weight maintenance, and lifelong fecundity in females (Murphy, Launer & Ehrlich, 1983; Hill & Pierce, 1989; Fischer, O’Brien & Boggs, 2004; Bauernfeind & Fischer, 2005). Additionally, a case-study on Euphydryas editha has shown that egg distribution is constrained by the proximity of nectar plants (Murphy, 1983). Optimal foraging theory predicts that insects are bound to maximize their rate of food intake (Pyke, Pulliam & Charnov, 1977). Therefore, the performance of the feeding apparatus should be subject to a high selective pressure and allow these insects to feed even tiny amounts of nectar from variously shaped flowers in an efficient way (Krenn, Plant & Szucsich, 2005; Borrell & Krenn, 2006; Krenn, 2010).
Nectar foraging in insects comprises searching for a flower, the discovery of nectar within the flower, and the uptake of nectar (Boggs, 1988). Searching for a suitable flower requires locomotion organs and adequate sensory organs (i.e. colour vision and olfactory receptors on the antennae and mouthparts). The feeding apparatus is primarily responsible for flower handling, which includes the discovery and uptake of nectar. The feeding apparatus of butterflies comprises the tubular proboscis, a basal haemolymph pump for uncoiling the proboscis, and a suction pump located in the butterfly’s head. The activity of the suction pump produces a pressure gradient to take up fluid through the food canal of the proboscis, which functions like a drinking straw (Krenn, 2010). Recent work on Danaus plexippus showed that the proboscis tip additionally functions like a nanosponge using capillary forces for initial fluid uptake (Monaenkova et al., 2012). The production of a pressure gradient and resulting fluid transport from the tip of the proboscis through the food canal into the oesophagus imposes physical constraints such that fluid flow rate decreases as proboscis length increases (Kingsolver & Daniel, 1979). Evolutionary scenarios have been proposed that relate proboscis length of some Lepidoptera and Diptera with host flower morphology (Nilsson et al., 1985; Nilsson, 1988; Johnson & Steiner, 1997; Alexandersson & Johnson, 2002; Anderson & Johnson, 2008, 2009; Pauw, Stofberg & Waterman, 2009). These well-studied model systems for insect–plant interactions demonstrate reciprocal adaptations and/or coevolutionary relationships between flower depth and insect proboscis length. However, with the exception of South African flies (Karolyi et al., 2012), these studies focused mainly on the pollinator mediated selection on flowers. Apart from proboscis length, they do not consider other morphological adaptations of the mouthparts (e.g. haemolymph pump, additional musculature, and sensilla) or suction pumps.
Despite the putative advantage for nectar uptake from deep floral tubes, which cannot be used by insects with short proboscides, only a few species of butterflies possess a proboscis that is longer than the body. In the present study, we search for reasons for this phenomenon and pose the hypothesis that the haemolymph and nectar pumping organs of the feeding apparatus might cause constraints for the evolution of a particularly long proboscis. Thus, the functional morphology of proboscides that greatly exceed the body length is of interest in comparison to the morphology of short feeding organs. Neotropical representatives of the riodinid butterflies are suitable for comparing the whole feeding apparatus because the proboscis of representatives of the genus Eurybia may reach 49.9 mm, equivalent to double the body length (Kunte, 2007; Bauder, Lieskonig & Krenn, 2011), whereas, in other species of Neotropical Riodinidae, the proboscis is rather short. Despite these differences in length, neither the micro-morphology of these mouthparts, nor the biomechanics of movements or of suction pumps have been studied so far. The purpose of the present study is to investigate exceptionally long proboscides and to estimate additional costs compared to short proboscides in Riodinidae. In this way, constraints on butterfly mouthpart length that might limit the adaptability to flower spur length should be discovered.
The coilable proboscis of Lepidoptera is composed of the two interlocked galeae of the maxillae. The basal parts of the maxillae connect the proboscis to the head and form a pair of pumping structures: the haemolymph pump. Uncoiling of the proboscis spiral results from the increase of an internal haemolymph pressure inside each galea as a result of the activity of the haemolymph pump. Coiling results from the elastic properties of the proboscis cuticle and the internal proboscis musculature (Bänziger, 1971; Krenn, 1990; Krenn, 2000; Wannenmacher & Wasserthal, 2003), which is arranged as a lateral and median series of muscles in the haemocoel of each galea (Eastham & Eassa, 1955; Krenn & Mühlberger, 2002; Krenn & Kristensen, 2004). During flower probing and nectar feeding the proboscis shows a distinct bend, located at 30–35% of the total proboscis length, which divides the proboscis into the proximal and distal regions (Eastham & Eassa, 1955; Bänziger, 1971; Krenn, 1990; Krenn & Mühlberger, 2002; Krenn et al., 2005). The tip region at the apical end of the proboscis is characterized by rows of slits for fluid uptake (Paulus & Krenn, 1996; Krenn & Mühlberger, 2002). The morphology and functional mechanism of the proboscis of Lepidoptera has been reviewed by Krenn et al. (2005) and Krenn (2010).
Most previous studies are concerned with proboscis morphology and adaptations to various food sources in Lepidoptera (Büttiker, Krenn & Putterill, 1996; Krenn & Penz, 1998; Krenn, Zulka & Gatschnegg, 2001; Knopp & Krenn, 2003; Molleman et al., 2005; Bauder et al., 2011; Zaspel, Weller & Branham, 2011). In butterflies, variations of proboscis length and sensilla morphology were found to be candidates for adaptations to various feeding strategies and food sources (Krenn et al., 2001). The sensory equipment of a proboscis of higher Lepidoptera includes mechanosensitive sensilla trichodea, chemoreceptive sensilla basiconica, and sensilla styloconica (Altner & Altner, 1986; Paulus & Krenn, 1996; Krenn, 1998). Sensilla styloconica are unique to the Eulepidoptera and evolved along with nectar feeding (Krenn & Kristensen, 2000). They are composed of a stylus and an apical sensory peg functioning as combined chemo-mechanoreceptors that are restricted exclusively to the tip region (Altner & Altner, 1986).
The present study aimed to determine whether there are morphological and/or biometrical adaptations of the feeding apparatus of long-tongued Neotropical riodinid butterflies in comparison to short-tongued, related species. The present study is the first to take into account that the haemolymph pump and the suction pump have to be considered in addition to the proboscis itself to understand the evolution of extremely long mouthparts. Based on morphometric analyses of all functional components of the feeding apparatus, the extra expenses and material costs of disproportionally long proboscides in butterflies can be estimated.
MATERIAL AND METHODS
Field work
The garden and surroundings of the Tropical Research Station La Gamba (Costa Rica, Puntarenas, 8°45′N, 83°10′W; 81 m above sea level) house a rich fauna of butterflies including 45 species of Riodinidae, also known as metalmark butterflies (Krenn et al., 2010). All studied individuals were collected with a hand net in September and October 2010. The butterflies were identified with reference to DeVries (1997). In total, 50 metalmarks belonging to 16 different species were sampled (Table 1). Live butterflies were immobilized by cooling to approximately 15 °C to measure body length with a digital caliper. Images of the manually uncoiled proboscides were taken with a digital camera (Olympus-μ-Tough-6010) to measure proboscis length using the software IMAGEJ (NIH). Afterwards, specimens were fixed in a mixture of ethanol, acetic acid, and formaldehyde for 1–2 days; subsequently, they were transferred to 70% ethanol for storage.
Table 1. Numbers of individuals of 16 riodinid species (collected at the Tropical Station La Gamba, Costa Rica, September–October 2010) in alphabetical order, which were measured regarding body length and proboscis length; given as minimum and maximum values where more than one individual per species was found.
| Species | N | Body length [mm] | Proboscis length [mm] |
|---|---|---|---|
| Detritivora hermodora (C. Felder & R. Felder, 1861) | 1 | 9.0 | 5.9 |
| Emesis mandana (Cramer, 1780) | 1 | 15.0 | 9.7 |
| Eurybia elvina Stichel, 1910 | 3 | 18.2–19.6 | 33.5–38.5 |
| Eurybia lycisca Westwood, 1851 | 8 | 15.1–20.7 | 34.8–42.4 |
| Eurybia unxia Godman & Salvin, 1885 | 8 | 14.0–19.1 | 26.2–32.0 |
| Euselasia aurantia (A. Butler & H. Druce, 1872) | 8 | 10.0–11.5 | 1.9–3.7 |
| Juditha molpe (Hubner, [1808]) | 2 | 10.6–12.6 | 7.7–7.9 |
| Leucochimona lepida (Godman & Salvin, 1885) | 1 | 10.1 | 4.0 |
| Mesene phareus (Cramer, 1777) | 1 | 9.2 | 5.1 |
| Mesosemia asa Hewitson, 1869 | 5 | 10.7–11.9 | 4.4–6.1 |
| Mesosemia zonalis Godman & Salvin, 1885 | 1 | 12.5 | 5.2 |
| Napaea eucharila (H. Bates, 1867) | 1 | 14.4 | 6.6 |
| Nymphidium ascolia Hewitson, [1853] | 1 | 11.8 | 7.5 |
| Perophthalma lasus Westwood, 1851 | 2 | 7.1–9.7 | 3.3–4.2 |
| Sarota chrysus (Stoll, 1781) | 1 | 9.9 | 3.1 |
| Sarota gyas (Cramer, 1775) | 6 | 6.2–8.0 | 2.6–3.3 |
Choosing species for morphological comparisons
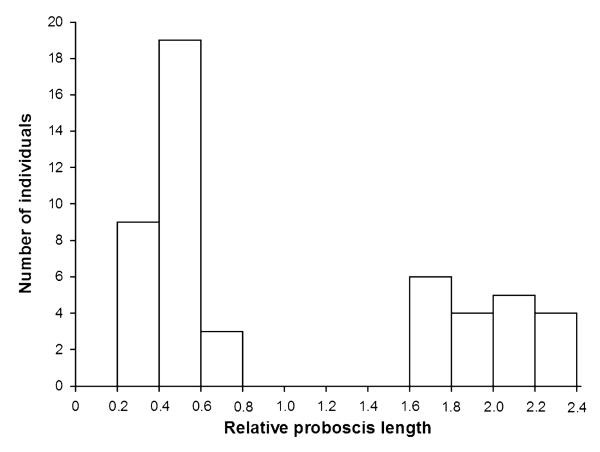
Relative proboscis length of all collected specimens was calculated by dividing proboscis length by body length. Representatives of 13 species have a relative short proboscis ranging between 0.2 and 0.7 times the body length. These species differ significantly from Eurybia elvina, Eurybia lycisca, and Eurybia unxia, which have a relative proboscis length ranging between 1.6 and 2.4 times the body length (Mann– Whitney U-test: Z = −5.939, P = 0.0001, N = 50; Fig. 1). Accordingly, all species possessing a proboscis that is shorter than the body are referred to as the short-tongued group, whereas the three species belonging to the genus Eurybia are termed the long-tongued group. For detailed anatomical analyses, all species of which we could collect at least three individuals were chosen in both groups (Table 1): Euselasia aurantia, Mesosemia asa, and Sarota gyas (short-tongued group), and E. elvina, E. lycisca, and E. unxia (long-tongued group). For each species, we analyzed two individuals, the third collected specimen served as a back-up in case of methodological problems. As far as possible, we chose males and females of each species to rule out sexual dimorphism of proboscis morphology, although we were not able to collect both sexes of E. lycisca, E. elvina, and E. aurantia. However, sexual dimorphisms relating to proboscis morphology are unlikely because previous work on nymphalids showed that these do not exist even though puddling behaviour is performed by males (Molleman et al., 2005).
Figure 1. Frequency distribution of the ratio of proboscis length and body length in 50 collected Riodindae belonging to 16 species.
Species with a proboscis that is shorter than the body (short-tongued group: relative proboscis length = 0.2–0.8) are distinct from Eurybia species (long-tongued group: relative proboscis length = 1.6–2.4) with a long proboscis (Mann-Whitney U-test: Z = −5.939, P = 0.0001, N = 50).
On each specimen, 15 biometrical characters were measured and categorized into characters of: (1) body size; (2) suction pump; (3) haemolymph pump; and (4) galea (Table 2).
Table 2. Biometrical characters of three long-tongued species (Eurybia elvina, Eurybia lycisca, Eurybia unxia) and three short-tongued species (Euselasia aurantia, Mesosemia asa, Sarota gyas) were measured.
| Biometrical parameters | Scale unit | Method of measurement |
|---|---|---|
| Body size | ||
| Body length | mm | Digital caliper |
| Head capsule volume | mm3 | μ-CT, 3D-reconstruction |
| Suction pump | ||
| Volume of compressors | mm3 | μ-CT, 3D-reconstruction |
| Volume of dilators | mm3 | μ-CT, 3D-reconstruction |
| Haemolymph pump | ||
| Number of external stipes muscles | μ-CT, 3D-reconstruction | |
| Volume of external stipes muscles | mm3 | μ-CT, 3D-reconstruction |
| Volume of internal stipes muscles | mm3 | μ-CT, 3D-reconstruction |
| Galea | ||
| Cuticula volume | mm3 | Serial semithin sectioning, 3D-reconstruction |
| Food canal volume | mm3 | Serial semithin sectioning, approximated to truncated cone |
| Galeal volume | mm3 | Serial semithin sectioning, 3D-reconstruction |
| Intragaleal muscle volume | mm3 | Serial semithin sectioning, 3D-reconstruction |
| Number of sensilla styloconica | Light microscopy | |
| Number of sensilla trichodea | Light microscopy | |
| Proboscis length | mm | Photographic images, IMAGEJ |
| Tip length | mm | Light microscopy |
Two individuals per species were studied. CT, computed tomography.
Because long-tongued Eurybia species are larger than the three short-tongued species (Table 3), it was necessary to correct for differences in body size by comparing relative values (e.g. galeal cuticle volume in relation to the total galeal volume) for the comparisons between these groups. Statistical comparisons of all feeding apparatus characters in relation to head capsule volume, galeal length or galeal volume were made using SPSS, version 15.0 (SPSS Inc.). The Mann–Whitney U-test for non-parametric data was used, and the significance level was set at 0.05. Graphical figures were created with SIGMAPLOT, version 11.0 (Systat Software Inc.), CORELDRAW X5 (Corel Corp.), and PHOTOSHOP CS 4 (Adobe Systems).
Table 3. Morphometry of the feeding apparatus of the long-tongued species (Eurybia elvina, Eurybia lycisca, Eurybia unxia) and the short-tongued species (Euselasia aurantia, Mesosemia asa, Sarota gyas).
| Long-tongued (N = 6) |
Short-tongued (N = 6) |
Mann–Whitney U-test |
||||||
|---|---|---|---|---|---|---|---|---|
| Median | Minimum | Maximum | Median | Minimum | Maximum | Z | P | |
| Body size | ||||||||
| Body length (mm) | 18.9 | 16.8 | 20.7 | 11.3 | 6.2 | 11.9 | ||
| Head capsule volume (mm3) | 1.6 | 1.2 | 2.0 | 0.8 | 0.2 | 0.9 | ||
| Suction pump | ||||||||
| Dilators (mm3) | 0.056 | 0.045 | 0.077 | 0.020 | 0.003 | 0.026 | ||
| Dilators (% of head capsule) | 3.6 | 3.4 | 3.8 | 2.2 | 1.3 | 3.6 | −2.242 | 0.026 |
| Compressors (mm3) | 0.025 | 0.018 | 0.030 | 0.011 | 0.003 | 0.021 | ||
| Compressors (% of head capsule) | 1.4 | 1.2 | 2.0 | 1.6 | 1.0 | 2.6 | −0.641 | 0.589 |
| Haemolymph pump | ||||||||
| External stipes muscles (mm3) | 0.115 | 0.091 | 0.151 | 0.007 | 0.003 | 0.014 | ||
| External stipes muscles (% of head capsule) | 7.4 | 6.4 | 7.6 | 1.4 | 0.7 | 1.7 | −2.882 | 0.002 |
| Internal stipes muscles (mm3) | 0.015 | 0.010 | 0.020 | 0.001 | 0.001 | 0.002 | ||
| Internal stipes muscles (% of head capsule) | 0.9 | 0.7 | 1.0 | 0.2 | 0.1 | 0.3 | −2.882 | 0.002 |
| Galea | ||||||||
| Galeal length (mm) | 35.1 | 29.1 | 40.2 | 3.4 | 2.6 | 5.7 | ||
| Tip length (mm) | 1.4 | 1.0 | 1.7 | 1.5 | 0.6 | 2.1 | ||
| Tip length (% of galeal length) | 3.8 | 3.0 | 5.5 | 33.0 | 21.3 | 47.3 | −2.887 | 0.002 |
| Galea (mm3) | 0.189 | 0.136 | 0.233 | 0.022 | 0.020 | 0.043 | ||
| Cuticula (mm3) | 0.110 | 0.072 | 0.133 | 0.007 | 0.006 | 0.012 | ||
| Cuticula (% of galeal volume) | 57.3 | 52.9 | 61.7 | 31.3 | 24.7 | 36.4 | −2.882 | 0.002 |
| Food canal (mm3) | 0.050 | 0.033 | 0.060 | 0.004 | 0.001 | 0.005 | ||
| Food canal (% of galeal volume) | 24.7 | 24.2 | 30.3 | 14.6 | 1.5 | 23.4 | −2.882 | 0.002 |
| Internal galeal muscles (mm3) | 0.010 | 0.007 | 0.014 | 0.002 | 0.001 | 0.002 | ||
| Internal galeal muscles (% of galeal volume) | 5.7 | 3.9 | 6.3 | 6.2 | 4.3 | 10.9 | −0.801 | 0.485 |
| Sensilla styloconica number | 30.5 | 20.0 | 38.0 | 64.5 | 23.0 | 83.0 | ||
| Sensilla styloconica (per mm tip length) | 22.2 | 11.8 | 38.0 | 40.7 | 38.3 | 48.0 | −2.882 | 0.002 |
| Sensilla trichodea number | 294.0 | 192.0 | 373.0 | 71.0 | 55.0 | 91.0 | ||
| Sensilla trichodea (per mm galeal length) | 8.8 | 5.3 | 9.8 | 21.0 | 11.6 | 27.1 | −2.882 | 0.004 |
Volumetric parameters were compared using the Mann–Whitney U-test (N = 6 individuals per group).
Number of proboscis sensilla
The number and distribution of sensilla trichodea and styloconica along the proboscis were assessed by light microscopy. Because reliable counts of the very small sensilla basiconica can only be acquired from scanning electron microscopy samples, we chose to exclude these sensilla from our analysis. Proboscides were prepared by separating the two galeae, embedding in glycerine, and mounting on microscopic slides. Drawings of the galea and its sensilla equipment were made for a total of 12 specimens belonging to six species with the aid of a drawing tube attached to the microscope. Accordingly, counts of sensilla occurring along a single galea were made. Proboscis tip length was measured from the apical apex of the proboscis to the most proximal nectar intake slits and/or sensilla styloconica. Additionally, digital micrographs of the proboscis tip and its sensilla were made with an Olympus E-330 camera on an Olympus CX-41 microscope. Each proboscis tip was photographed in several focus planes. These images were imported into the software HELICONFOCUS, version 5.2 (HeliconSoft Ltd) and compiled into one completely focused image. After this treatment, the proboscides were again stored in 70% ethanol for further analyses.
Serial semithin sections
The proboscides were cut at the middle of their length and near the tip to ensure infiltration of Agar low viscosity resin (Agar Scientific) under vacuum after dehydration in an ascending ethanol series. Serial sections were cut on a microtome (Leica EM UC6) with a diamond knife (Histo Jumbo Diatome) at a thickness of 1 μm. Serial cross-sections of 200 sections per region for long-tongued species or 100 sections per region for short-tongued species were taken from the proximal region, the knee bend, three sub-regions of the distal region (at 25%, 50%, and 75% of its length), and the proboscis tip. Sections were stained with a mixture of 1% azure II and 1% methylene blue in an aqueous 1% borax solution (Romeis, 1989) at 60–70 °C.
Sections were digitally photographed with an Olympus E-330 camera on an Olympus CX-41 microscope. Microphotographs were enhanced in contrast and converted to grayscale using ADOBE PHOTOSHOP CS4 extended, version 11.0.2. After importing into the 3D reconstruction software AMIRA, version 5.3.3 (Zuse Institute), image stacks were aligned with the AlignSlices tool. Segmentation of the whole galea, its muscles and cuticle was performed manually using the brush tool. The MaterialStatistics tool was used to make volumetric calculations of the galea, as well as its muscles and cuticle, in each proboscis region (proximal: 0–30%, knee bend: 30–35%, distal: 35% to the beginning of the proboscis tip, and tip region, which is characterized by intake slits for nectar and the presence of sensilla styloconica). This procedure gave the volume of the galea, its muscles and cuticle for each proboscis region with spans of 200 μm in long-tongued species or spans of 100 μm in short-tongued riodinids. By multiplying the volume of the galea/muscles/cuticle per 1 μm proboscis length by the length of the respective proboscis region, and adding the volumes of each region, we can estimate the volume of a whole galea, its muscles and cuticle. Furthermore, we estimated the volume of the food canal by measuring the area of the food canal of the most proximal cross-section of the proboscis and of the most distal cross-section near the proboscis tip with IMAGEJ. The volume of the food canal was calculated by approximating it as a truncated cone.
Micro-computed tomography (CT)
Butterfly heads were prepared for X-ray micro-tomography by dehydration in an ascending ethanol series. Afterwards, the specimens were stained overnight in 1% elemental iodine dissolved in 100% ethanol. The samples were mounted in pipet tips filled with 100% ethanol (Metscher, 2009) and imaged using the Xradia MicroXCT-200 system with source settings of 40 kV and 200 μA and secondary optical magnification of either × 2 or × 4 depending on specimen size. Volume images were reconstructed with voxel sizes ranging from 3.6 to 7.3 μm. 3D reconstructions of the suction pump and the haemolymph pump were performed using AMIRA, version 5.3.3. Segmentation was done manually using the brush tool; volumetric calculations were made with the MaterialStatistics tool. The head capsule volume was reconstructed with compound eyes excluded because of a sexual dimorphism in eye size of some metalmark species (DeVries, 1997). The nomenclature of the suction pump musculature is given sensu Eberhard & Krenn (2005) and the nomenclature of the haemolymph pump musculature is given sensu Krenn (2010).
RESULTS
Body size and proboscis length
In the studied individuals of Riodinidae body length measured from 6.2 to 20.7 mm and proboscis length ranged between 1.9 and 42.4 mm (N = 50; Table 1). The comparison of the body length and proboscis length revealed two non-overlapping groups; one having a proboscis longer than the body and a second group possessing a proboscis that measures approximately half of the body length (Fig. 1).
With only a moderately larger body size, the long-tongued species of Riodinidae exhibit a ten-fold longer proboscis than the short-tongued group. The long-tongued and short-tongued species differ significantly in the proportions of the proboscis regions (Table 3). In relation to proboscis length, the representatives of the Eurybia species have a significantly shorter tip region than short-tongued species (Mann–Whitney U-test: Z = −2.887, P = 0.002, N = 12), because the absolute tip lengths are similar in all species (Table 3).
Sensilla
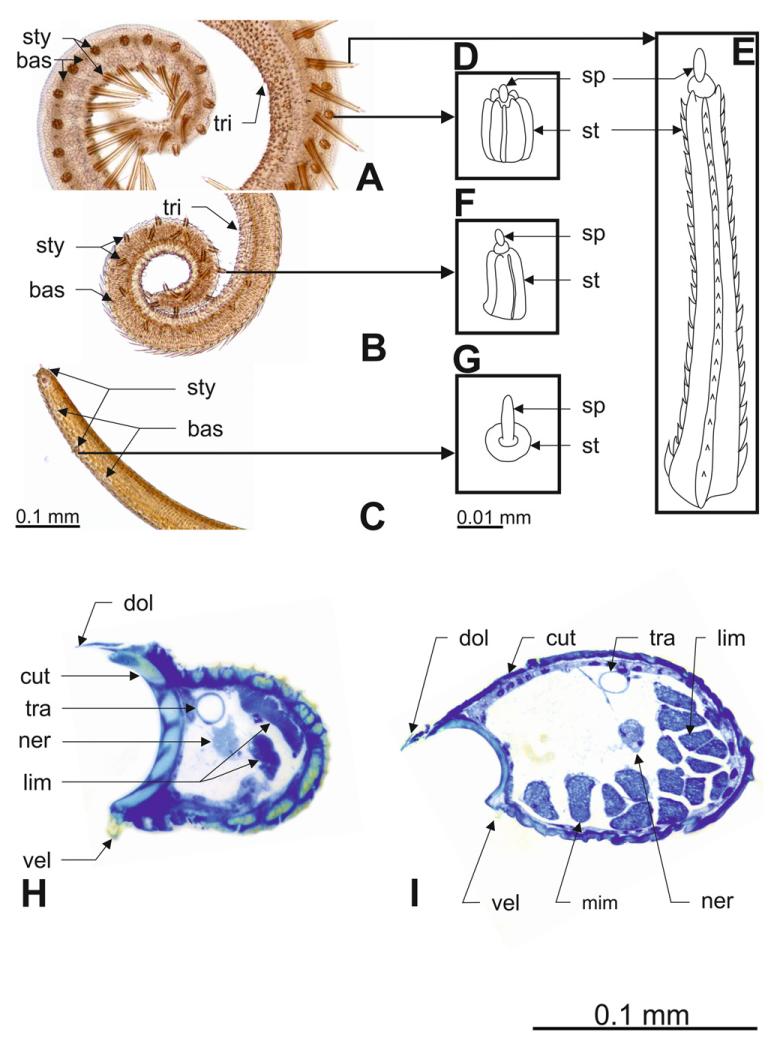
The external surface of the proboscis is covered with various types of sensilla. Bristle-shaped sensilla trichodea are the most numerous (Table 3). Their numbers ranged from 192 to 373 in long-tongued metalmarks, whereas short-tongued metalmarks have lower absolute numbers of sensilla trichodea, ranging between 55 and 91. However, the long-tongued genus Eurybia is characterized by significantly lower sensilla trichodea numbers per mm proboscis length (Mann–Whitney U-test: Z = −2.882, P = 0.004, N = 12). All in all, short-tongued species have fewer sensilla trichodea than Eurybia species, although they are arranged more densely (Fig. 2A, B, C; Table 3).
Figure 2. Proboscis morphology of long-tongued and short-tongued Riodinidae.
A–C, proboscis tip equipped with various sensilla types (bas, sensilla basiconica; sty, sensilla styloconica; tri, sensilla trichodea): A, Euselasia aurantia (short-tongued). B, Sarota gyas (short-tongued). C, Eurybia lycisca (long-tongued). D, E, F, G, morphology of sensilla styloconica in these three species (sp, sensory peg; st, stylus). H, I, cross-sections of the distal proboscis of Eurybia elvina (long-tongued, H) and Euselasia aurantia (short-tongued, I) (cut, cuticula; dol, dorsal linkage; lim, lateral intrinsic muscles; mim, median intrinsic muscles; ner, nerve; tra, trachea; vel, ventral linkage).
Numbers of sensilla styloconica ranged from 20 to 38 in long-tongued species and from 23 to 83 in short-tongued species. Consequently, long-tongued species have a significantly lower number of sensilla styloconica per mm tip length than short-tongued species (Mann–Whitney U-test: Z = −2.882, P = 0.002, N = 12; Table 3). In the long-tongued Eurybia species, sensilla styloconcia occur in relatively low numbers in the distal part of the distal region, as well as in the tip region. Four sensilla styloconica form a characteristic cluster at the proboscis tip (Fig. 2C). The stylus is very short and resembles a collared socket. The sensory peg of these sensilla exceeds the length of the stylus (Fig. 2C, G).
In the short-tongued species E. aurantia (Fig. 2A, D, E) and M. asa, two different kinds of sensilla styloconica occur on the proboscis tip: (1) short sensilla styloconica (Fig. 2D), distributed in a row on the dorsal side of the galea, and (2) long sensilla styloconica (Fig. 2E). The long sensilla styloconica are arranged in a row on the ventral side near the tip, changing their position from ventral to lateral/dorsal at the proximal end of the tip region where they form more or less one row of alternating short and long sensilla styloconica. In both sensilla styloconica types, the stylus is longer than the sensory peg but, in the long sensilla styloconica, this difference is extremely pronounced. The short sensilla styloconica are characterized by a cylindriform stylus with seven smooth ridges and a central sensory peg. The stylus of the long sensilla styloconica has an asymmetrical cross-section and consists of seven ridges, with at least three of them serrated, and bears a central sensory peg.
The sensilla styloconica of the short-tongued species S. gyas (Fig. 2B, F) are cylindriform, their stylus is longer than the sensory peg, has five smooth ridges, and the sensory peg is in a central position. These sensilla are distributed in two rows: one on the dorsal side and one on the lateral side of the galea. The row on the dorsal side terminates in the middle of the tip region; the row on the lateral side stretches across the whole proboscis tip.
Muscles and cuticle of the galea
The long-tongued Eurybia species (Fig. 2H), as well as two of the short-tongued species, M. asa and S. gyas, are characterized by the occurrence of lateral intrinsic muscles throughout the whole galea and the presence of median intrinsic muscles only in the knee bend. In the short-tongued species E. aurantia (Fig. 2I), however, median intrinsic muscles occur in the knee bend as well as in the distal region.
Comparisons of the percentage of the intragaleal muscle volume per galeal volume and of the cuticular volume per galeal volume reveal that long-tongued species have a significantly thicker cuticle than short-tongued species (Mann–Whitney U-test: Z = −2.882, P = 0.002, N = 12), whereas the muscle volume is similar in all species (Table 3). Furthermore, long-tongued Eurybia species have a significantly larger food canal volume in relation to the galeal volume than the short-tongued species (Mann–Whitney U-test: Z = −2.882, P = 0.002, N = 12; Table 3).
Muscles of the haemolymph pump
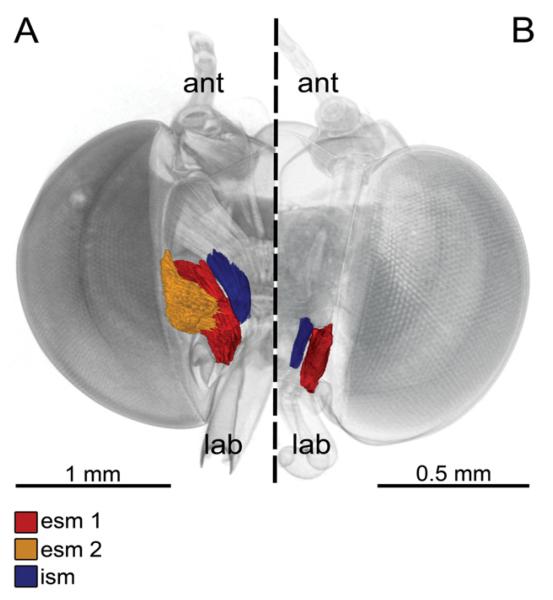
The haemolymph pump morphology correlates with proboscis length. In long-tongued Eurybia species, there are three stipes muscles (Fig. 3A), which are significantly larger in relation to the head capsule volume than in short-tongued species, which possess only two stipes muscles (Fig. 3B, Table 3).
Figure 3. 3D reconstruction based on micro-computed tomography images of the stipes pump of long-tongued (A) and short-tongued (B) Riodindae, frontal view, proboscis removed.
A, Eurybia lycisca (long-tongued): right half of the head with two external and one internal stipes muscles. External stipes muscle 1 (esm 1) originates on the gena and extends to a broad apodeme of the flat stipes part. External stipes muscle 2 (esm 2) originates on the tentorium and extends to the flat part of the stipes. The internal stipes muscle (ism) originates on the tentorium and extends to the stipes near the galeal base (ant, antenna; lab, labial palpus). B, Sarota gyas (short-tongued): left half of the head with one external and one internal stipes muscle. Esm 2 is missing in all short-tongued species.
The external stipes muscles attach to an apodeme on the flat part of the stipes. In long-tongued Eurybia species (Fig. 3A), two external stipes muscles can be found: the external stipes muscle 1 is paired and originates on the gena at the lateral frontoclypeus, whereas the paired external stipes muscle 2 takes its origin on the anterior tentorium. In short-tongued species (Fig. 3B), there is only one external stipes muscle present (external stipes muscle 1), and the external stipes muscle 2 is missing. In all species, the internal stipes muscle originates on the posterior tentorium and extends to the posterior stipes part near the base of the galea.
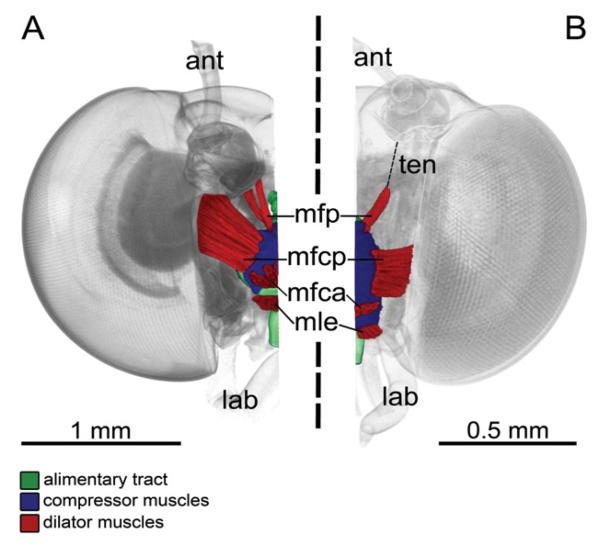
Muscles of the suction pump
The suction pump for nectar uptake is the largest muscular structure in the head and lies in the anterior half of the head (Fig. 4). The dorsal roof of the suction pump is covered with layers of transversal compressor muscles. Three dilator muscles attach on different sites of the suction pump: (1) The unpaired musculus frontoclypeo-cibarialis anterior originates at the median frontoclypeus and extends to the anterior part of the dorsal wall of the suction pump. (2) The musculus frontoclypeo-cibarialis posterior is paired and originates on the lateral frontoclypeus and extends to the lateral dorsal wall of the suction pump. In Eurybia species, the origin of this muscle lies at the lateral/posterior frontoclypeus at the base of the antennae (Fig. 4A), whereas in the short-tongued species the origin is located at the lateral frontoclypeus (Fig. 4B). (3) The paired musculus frontoclypeo-pharyngealis originates on the lateral frontoclypeus near the base of the antennae and extends to the posterior part of the dorsal wall of the suction pump. This muscle is separated into two parts in the long-tongued species of genus Eurybia (Fig. 4A) but not in the short-tongued species (Fig. 4B). Another dilator muscle, the musculus labro-epipharyngealis attaches frontally at the median part of the labrum and extends to the transition of the suction pump lumen into the proboscis food canal.
Figure 4. 3D reconstruction based on micro-CT images of the suction pump musculature of long-tongued (A) and short-tongued (B) Riodinidae, frontal view, proboscis removed.
A, Eurybia lycisca (long-tongued): right half of the head with alimentary tract, compressor muscles and dilator muscles [musculus frontoclypeo-pharyngealis (mfp), musculus frontoclypeo-cibarialis posterior (mfcp), musculus frontoclypeo-cibarialis anterior (mfca), musculus labro-epipharyngealis (mle)] of the suction pump. Compressors contract and reduce the suctorial cavity to swallow nectar into the oesophagus. Dilator muscles expand the suctorial cavity to produce a pressure gradient to take up nectar through the food canal of the straw-like proboscis (ant, antenna; lab, labial palpus). B, Sarota gyas (short-tongued): left half of the head, with alimentary tract, compressor muscles and dilator muscles of the suction pump. The dilator mfp is connected to its attachment site via a tendon (ten).
Comparison of the proportions of the dilator and compressor muscles in relation to the head capsule volume reveals significant differences concerning only the dilator muscles. The long-tongued Eurybia species have larger dilators than the short-tongued species (Table 3).
DISCUSSION
Insects present many examples of organs with disproportional size that evolved in context with sexual selection, such as horns in beetles (Emlen & Nijhout, 2000; Emlen, 2001). Similar extremely long organs that have been shaped by natural selection have received less attention, although there are examples of amazing lengths in proboscides of flower-visiting insects (Borrell & Krenn, 2006). Because great size variations (an up to ten-fold difference in proboscis length) exist among related riodinid species, these mouthparts should be promising objects to study organ evolution and its limits from a quantitative point of view.
Because the Eurybiini nest within the subfamily Riodininae (Brower, 2008), it is likely that the extremely long proboscis of the representatives of the genus Eurybia is a derived feature that must have evolved in context with nectar feeding (Hall & Willmott, 2000).
The configuration of the feeding apparatus differs between the investigated long-tongued and short-tongued metalmarks regarding proboscis features and the size and composition of the haemolymph pump and suction pump. The proboscis of the representatives of Eurybia is unique in its extraordinary length, which may reach 49.9 mm (Kunte, 2007), the standing record in butterflies. The extremely long proboscis of Eurybia species comes from the elongation of the proximal and distal regions of the proboscis. The comparison of the proboscis proportions in long-tongued and short-tongued metalmarks revealed that the length of the tip region is the same in all species, even when the whole proboscis is ten times longer. The proboscis morphology of long-tongued Eurybia species is remarkable because of its low density of sensilla on the galeae, a large food canal and thick cuticular walls, as well as a short tip region equipped with relatively few and very short sensilla styloconica. The morphology of the associated inner structures indicates that the very long proboscis in metalmarks comes hand in hand with the presence of two, large external stipital muscles for uncoiling the very long and remarkably thin proboscis (Bauder et al., 2011), as well as with reinforced dilator musculature of the suction pump for creating the pressure drop required to suck up fluid through a proboscis twice the length of the body. The necessity of reinforcing the fluid pumps in the head and of developing a thick proboscis wall, as well as a large food canal in a thin proboscis, could therefore limit proboscis elongation in flower-visiting insects. The short proboscides of the metalmarks are characterized by a relatively high number of sensory sensilla, a small food canal, and thin cuticular walls, as well as a relatively long tip region covered with densely arranged and long sensilla styloconica. The morphology and arrangement of the sensilla styloconica predicts that these butterflies likely use other food sources than floral nectar since these features are similar to non-flower-visiting nymphalid butterflies (Krenn et al., 2001; Molleman et al., 2005).
Functional explanations for the sensilla densities and various morphologies in metalmarks come from other studies that analyzed flower-visiting behaviour and food preferences of Lepidoptera.
Sensilla trichodea function as mechanoreceptors and are assumed to provide information on the insertion depth of the proboscis (Krenn, 1990; Krenn, 1998). The sensilla styloconica of most butterflies are characterized by a long stylus that allows for the terminal sensory peg to function at a considerable distance from the surface of the galea in adult feeding (Paulus & Krenn, 1996; Krenn, 2010). These sensilla were found to be sensitive to solutions of sucrose and sodium chloride and to gather sensory information via chemical stimuli during feeding (Frings & Frings, 1956; Altner & Altner, 1986; Petr & Stewart, 2004). In addition, they are also assumed to provide tactile stimuli for detecting the opening of corolla tubes (Krenn, 1998). The morphology of these sensilla is highly variable in Lepidoptera and is known to be closely correlated with the feeding habits and preferred food sources of adult Lepidoptera (Paulus & Krenn, 1996; Krenn et al., 2001; Knopp & Krenn, 2003; Petr & Stewart, 2004; Zaspel et al., 2011).
Investigations on the flower-visiting behaviour of sphingid moths revealed that tactile information gathered by the proboscis on the corolla surface is needed to forage efficiently. Hawk moths decrease the time spent on individual flowers at the same time as increasing foraging efficiency (Goyret & Raguso, 2006). However, this may not apply for butterflies to such an extent. Because Eurybia butterflies do not hover in front of a flower like hawk moths but, instead, sit on the inflorescences and search for corolla entrances at a more leisurely pace, they do not expend high energetic costs (Bauder et al., 2011). The present study suggests that there is no need for extra-investments concerning the sensory equipment of a very long proboscis. Alternatively, the prolonged handling times at flowers of long-tongued butterflies (Kunte, 2007; Bauder et al., 2011) could arise partly from a poor supply of tactile information as a result of the low number of tip sensilla and the consequently longer search times for the corolla opening.
The absence of prominent sensilla styloconica, which was detected for Eurybia species in the present study, has been found so far in only a few Eulepidoptera belonging to Danainae, Papilionidae, and Sphingidae (Paulus & Krenn, 1996; Petr & Stewart, 2004). This character state may be an adaptation to nectar feeding from deep floral tubes filled with nectar and may be viewed as an independent autapomorphy for each group (Petr & Stewart, 2004). A tip region equipped with short sensilla styloconica simply fits better even in narrow spurs and can be interpreted as a way of mitigating the material costs of the long proboscis but might restrict the food source to long-spurred flowers.
The sensilla styloconica morphology of the short-tongued metalmark species differs markedly from the long-tongued species and is similar to other, non-flower-visiting lycaenid and riodinid species. Because of this, it is likely that these species use food sources other than nectar, although DeVries (1997) reported that Mesosemia asa feeds on tiny flowers. Most non-flower-visiting butterflies possess conspicuously shaped long sensilla styloconica, which allow for feeding on evenly distributed fluids instead of fluid inside a floral tube (Paulus & Krenn, 1996; Petr & Stewart, 2004). The long styli of densely arranged sensilla styloconica form a brush-like structure to accumulate evenly distributed fluids near the food canal by capillary action in nymphalids (Krenn et al., 2001).
The two differently sized types of sensilla styloconica occuring on the proboscis tip of E. aurantia and M. asa were also found in other lycaenid and riodinid species (Paulus & Krenn, 1996; Petr & Stewart, 2004). They use mainly food sources other than nectar (e.g. tree sap, honey dew from aphids) or exudates from insect galls and wet sand (Scott, 1974; Garling, 1984; Ebert & Rennwald, 1991; Stoffolano, 1995; Anthes, Fartmann & Hermann, 2008). Similar feeding preferences are also known from some metalmark species that feed on carrion and take up nutrients from puddles (Hall & Willmott, 2000).
The internal anatomy of the proboscis in the investigated species allows conclusions to be drawn about proboscis stiffness and recoiling. The muscles inside the galeae account for recoiling the proboscis into a tight spiral (Krenn, 2000). The longitudinal arrangement of these muscles differs between the investigated species: the short-tongued E. aurantia possesses both lateral and median muscles in the knee bend and in the distal region, whereas all the other species investigated, regardless of proboscis length, have median intrinsic muscles only in the knee bend. Whether these groups of muscles carry out different functions is not yet known, although most butterflies possess both throughout the proboscis (Krenn & Mühlberger, 2002). Our study shows that the volume of intrinsic muscles per galeal volume is reduced in long-tongued Eurybia species. The proboscis of Eurybia species, which is ten times longer than those measured in the short-tongued group, contains approximately the same percentage of muscle volume per galeal volume. Although absolutely larger proboscides require absolutely more muscles, we conclude that galeal musculature constitutes no significant limit to proboscis length adaptation because galeal muscle volume is very small in comparison to the muscle volumes of the haemolymph and suction pump (Table 3). Therefore, no anatomical extra-investments in the galeal musculature appear to be necessary to move a very long proboscis. On the other hand, material costs of proboscis elongation affect the cuticular walls of the proboscis: the cuticle of long-tongued species is approximately two times thicker than the cuticle of short-tongued species. Krenn et al. (2001) suggested that long proboscides, which are suited for taking up nectar from narrow flower tubes, require a stiffer proboscis to retain their maneuverability.
The haemolymph pump of Eurybia species features three muscles, whereas, in short-tongued species, one of the external stipes muscles is absent. During proboscis uncoiling, compression of the stipital tube is accomplished by the external stipes muscles pumping haemolymph from the head into the galeae (Krenn, 1990). The internal stipes muscle opens the stipital valve during coiling to allow back-flow of the haemolymph into the head (Bänziger, 1971; Krenn, 1990) and pulls back the galea base to lower the coiled proboscis (Wannenmacher & Wasserthal, 2003). Korzeev & Stekolnikov (2011) compared the musculature of the head between representatives of the superfamilies Cossoidea, Zygaenoidea, and Sesioidea and found that the additional external stipes muscle first appeared in Zygaenidae, most probably from a splitting of the external stipes muscle 1. If the presence of three stipital muscles is a character configuration associated with proboscis length, we would expect representatives that possess a short proboscis to have a reduced stipital muscle set, whereas species with functional and long proboscides should have a complete set. So far, an additional external stipes muscle has been demonstrated in several members of Ditrysia, including many Papilionoidea (Schmitt, 1938; Eastham & Eassa, 1955; MacFarlane & Eaton, 1973; Wannenmacher & Wasserthal, 2003). Additional external stipes muscles are assumed to improve the mechanism of proboscis uncoiling because it was detected in species possessing a long and movable proboscis and is absent in species belonging to Cossidae, Limacodidae, and Sesiidae with atrophic mouthparts (Korzeev & Stekolnikov, 2011). Comparative anatomy suggests that there are also butterfly species with a functional proboscis, such as members of Papilionidae, Nymphalidae, and Pieridae, which possess only one external stipes muscle, (Schmitt, 1938), as is the case in E. aurantia, M. asa, and S. gyas (Riodinidae). Consequently, the presence of a second external stipes muscle in the investigated long-tongued species cannot be interpreted as an exclusive adaptation necessary for uncoiling an extremely long proboscis. The larger size of the stipes muscles in relation to the head capsule of long-tongued Eurybia species is likely to improve proboscis uncoiling. Further studies should focus on the configuration of the haemolymph pump in other extremely long-tongued Lepidoptera, such as hawk moths and skippers, aiming to find a correlation between stipes musculature and proboscis length. Accordingly, we could determine whether the presence of three stipes muscles is a precondition for evolving a long proboscis as an adaptation to long nectar spurs.
The morphology of the suction pump is similar in all studied riodinid species and corresponds with other butterflies and moths (Eastham & Eassa, 1955; Srivastava & Bogawat, 1969; MacFarlane & Eaton, 1973; Miles & Booker, 1998; Eberhard & Krenn, 2005; Davis & Hildebrand, 2006). Differences between the studied species concern the number of dilator muscle bundles, as well as their volume. These muscles serve to raise the flexible roof of the suction pump when the opening to the food canal of the proboscis is opened. Fluid is transported into the chamber, and then either sphincter muscles or an oral valve close the opening into the proboscis, at the same time as the compressor muscles depress the lumen of the suction pump. In this way, fluid can be transported into the oesophagus (Schmitt, 1938; Eastham & Eassa, 1955; Srivastava & Bogawat, 1969; MacFarlane & Eaton, 1973; Miles & Booker, 1998; Eberhard & Krenn, 2005; Davis & Hildebrand, 2006). Because the proportion of dilator muscles in relation to the head capsule is larger in long-tongued riodinids and because the dilator muscle is divided into four bundles in Eurybia species in contrast to two bundles in the short-tongued riodinids, we hypothesize that a reinforcement of the suction pump evolved in context with a very long proboscis in Eurybia. This is consistent with the results showing that the suction pump of a fruit-piercing moth contain more muscle bundles compared to nectar feeding Lepidoptera and that this is an adaptation to drinking thick and viscous fruit sap (Srivastava & Bogawat, 1969).
Kunte (2007) noted that there is a trade-off between proboscis length and food handling time. He concluded that long-tongued butterflies harvest less nectar per time from the same flower than butterflies with normal sized proboscides. Because of this, he regarded the reduced foraging efficiency as a functional constraint for evolving disproportionately long proboscides. His presumption for this study was that, because of an allometric relationship between body size and cibarial muscle mass, a disproportionately long proboscis has a reduced nectar uptake rate resulting from a higher nectar flow resistance and therefore a greater handling time. Both Kingsolver & Daniel (1979), Pivnick & McNeil (1985) and Daniel, Kingsolver & Meyhöfer (1989) constructed mathematical models describing the mechanics and energetics of nectar feeding in butterflies, based on the Hagen-Poiseuille equation that describes the volume flow per time through a tube: the rate of volume intake is given by the pressure drop divided by the resistance to flow. This volumetric flow rate is inversely correlated with the length of the proboscis and directly correlated with the radius of the food canal, meaning that an increase in proboscis length leads to a decrease in the rate of intake and an increase of the food canal size leads to an increase of intake rate. In the present study, we show that, at least in extremely long-tongued Eurybia species, there are enlargements of the suction pump muscles, which probably create a higher pressure drop, and of the relative food canal size, which is larger than the food canal of short-tongued species. Especially an increase in the food canal radius is known to dramatically increase the rate of energy intake at all nectar concentrations, because nectar flow depends on the fourth power of the radius (Kingsolver & Daniel, 1979). Therefore, the enlargement of the food canal is a very efficient way for butterflies to compensate for lower flow velocities resulting from proboscis elongation but comes at the cost of cuticular investments. Also, a ten-fold increase in the cross-sectional area of the pump dilators leads to an approximately two-fold increase in energy intake rate at all nectar concentrations (Daniel et al., 1989). Interestingly, Daniel et al. (1989) stated that: (1) to achieve the highest intake rates, the geometry of the entire feeding apparatus must be tuned, and (2) that isometric size increase of the feeding apparatus increases the energy intake rate at all nectar concentrations. In Eurybia species, we demonstrate a combination of these adaptations: (1) fine-tuning of dilator muscles and food canal size, and (2) scaling to a larger body size.
The present study has shown that anatomical investments in the suction pump dilators, the stipital muscles, and the proboscis walls can be considered to maintain feeding performance in long-tongued riodinid butterflies. At the same time, these parts of the feeding apparatus can also limit further proboscis elongation because the space in a butterfly’s head is finite. The present study provides novel information about the functional tuning of the feeding apparatus that may constrain organ evolution in an evolutionary arms race between plants and visiting nectar feeding insects.
ACKNOWLEDGEMENTS
We are grateful to the Tropical Research Station La Gamba, Costa Rica, for making available their laboratory facilities and the Department of Theoretical Biology, Vienna, for providing their Micro-CT facilities. We want to thank Mag. Philipp Oberrisser for his enthusiastic support in the field catching butterflies. We also thank Dr Norbert Milasowszky for his valuable comments and discussions. Furthermore, we are grateful to Eddy Gómez Ramírez, Centro de Investigacíon en Ciencias del Mar y Limnología, Universidad de Costa Rica, for providing us with fixatives. The Costa Rican Ministerio del Ambients y Energia kindly granted research permits. We thank three anonymous reviewers for their helpful suggestions. The study was funded by FWF (P 22248 B17).
REFERENCES
- Alexandersson R, Johnson SD. Pollinator–mediated selection on flower-tube length in a hawkmoth-pollinated Gladiolus (Iridaceae) Proceedings of the Royal Society of London Series B, Biological Sciences. 2002;269:631–636. doi: 10.1098/rspb.2001.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altner H, Altner I. Sensilla with both terminal pore and wall pores on the proboscis of the moth Rhogogastria bubo Walker (Lepidoptera: Arctiidae) Zoologischer Anzeiger – A Journal of Comparative Zoology. 1986;216:129–150. [Google Scholar]
- Anderson B, Johnson SD. The geographical mosaic of coevolution in a plant–pollinator mutualism. Evolution. 2008;62:220–225. doi: 10.1111/j.1558-5646.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Johnson SD. Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants. New Phytologist. 2009;182:533–540. doi: 10.1111/j.1469-8137.2009.02764.x. [DOI] [PubMed] [Google Scholar]
- Anthes N, Fartmann T, Hermann G. The Duke of Burgundy butterfly and its dukedom: larval niche variation in Hamearis lucina across Central Europe. Journal of Insect Conservation. 2008;12:3–14. [Google Scholar]
- Bänziger H. Extension and coiling of the lepidopterous proboscis – a new interpretation of the blood-pressure theory. Mitteilungen der schweizerischen entomologischen Gesellschaft. 1971;43:225–239. [Google Scholar]
- Bauder JAS, Lieskonig NR, Krenn HW. The extremely long-tongued Neotropical butterfly Eurybia lycisca (Riodinidae): proboscis morphology and flower handling. Arthropod Structure & Development. 2011;40:122–127. doi: 10.1016/j.asd.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind SS, Fischer K. Effects of adult-derived carbohydrates, amino acids and micronutrients on female reproduction in a fruit-feeding butterfly. Journal of Insect Physiology. 2005;51:545–554. doi: 10.1016/j.jinsphys.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Boggs CL. Rates of nectar feeding in butterflies: effects of sex, size, age and nectar concentration. Functional Ecology. 1988;2:289–295. [Google Scholar]
- Borrell BJ, Krenn HW. Nectar feeding in long-proboscid insects. In: Herrel A, Speck T, Rowe NP, editors. Ecology and biomechanics – a mechanical approach to the ecology of animals and plants. CRC Group Taylor & Francis Group; Boca Raton: 2006. pp. 185–212. [Google Scholar]
- Brower AVZ. Riodinidae Grote 1895. Metalmarks. (Version 1.08) 2008 Available at: http://tolweb.org/Riodinidae/12174.
- Büttiker W, Krenn HW, Putterill J. The proboscis of eye-frequenting and piercing Lepidoptera (Insecta) Zoomorphology. 1996;116:77–83. [Google Scholar]
- Daniel TL, Kingsolver JG, Meyhöfer E. Mechanical determinants of nectar-feeding energetics in butterflies: muscle mechanics, feeding geometry, and functional equivalence. Oecologia. 1989;79:66–75. doi: 10.1007/BF00378241. [DOI] [PubMed] [Google Scholar]
- Davis NT, Hildebrand JG. Neuroanatomy of the sucking pump of the moth, Manduca sexta (Sphingidae, Lepidoptera) Arthropod Structure & Development. 2006;35:15–33. doi: 10.1016/j.asd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- DeVries PJ. The butterflies of Costa Rica and their natural history volume II: riodinidae. Princeton University Press; Chichester: 1997. [Google Scholar]
- Eastham LES, Eassa YEE. The feeding mechanism of the butterfly Pieris brassicae L. Philosophical Transactions of the Royal Society of London Series B. Biological Sciences. 1955;239:1–43. [Google Scholar]
- Eberhard SH, Krenn HW. Anatomy of the oral valve in nymphalid butterflies and a functional model for fluid uptake in Lepidoptera. Zoologischer Anzeiger – A Journal of Comparative Zoology. 2005;243:305–312. [Google Scholar]
- Ebert G, Rennwald E. Die Schmetterlinge Baden-Württembergs Band 2: Tagfalter II. Eugen Ulmer; Stuttgart: 1991. [Google Scholar]
- Emlen DJ. Costs and the diversification of exaggerated animal structures. Science. 2001;291:1534–1536. doi: 10.1126/science.1056607. [DOI] [PubMed] [Google Scholar]
- Emlen DJ, Nijhout HF. The development and evolution of exaggerated morphologies in insects. Annual Reviews of Entomology. 2000;45:661–708. doi: 10.1146/annurev.ento.45.1.661. [DOI] [PubMed] [Google Scholar]
- Fischer K, O’Brien DM, Boggs CL. Allocation of larval and adult resources to reproduction in a fruit-feeding butterfly. Functional Ecology. 2004;18:656–663. [Google Scholar]
- Frings H, Frings M. The loci of contact chemoreceptors involved in feeding reactions in certain Lepidoptera. Biological Bulletin. 1956;110:291–299. [Google Scholar]
- Garling B. Hamearis lucina L., der Braune Würfelfalter: Lebensraum, Flugzeiten und Entwicklungsdaten (Lep.: Riodinidae) Entomologische Zeitschrift. 1984;94:321–336. [Google Scholar]
- Goyret J, Raguso RA. The role of mechanosensory input in flower handling efficiency and learning by Manduca sexta. The Journal of Experimental Biology. 2006;209:1585–1593. doi: 10.1242/jeb.02169. [DOI] [PubMed] [Google Scholar]
- Hall JPW, Willmott KR. Patterns of feeding behaviour in adult male riodinid butterflies and their relationship to morphology and ecology. Biological Journal of the Linnean Society. 2000;69:1–23. [Google Scholar]
- Hill CJ, Pierce NE. The effect of adult diet on the biology of butterflies. 1. The common imperial blue, Jalmenus evagoras. Oecologia. 1989;81:249–257. doi: 10.1007/BF00379812. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Karolyi F, Szucsich NU, Colville JF, Krenn HW. Adaptations for nectar-feeding in the mouthparts of long-proboscid flies (Nemestrinidae: Prosoeca) Biological Journal of the Linnean Society. 2012;107:414–424. doi: 10.1111/j.1095-8312.2012.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver JG, Daniel TL. On the mechanics and energetics of nectar feeding in butterflies. Journal of Theoretical Biology. 1979;76:167–179. doi: 10.1016/0022-5193(79)90368-0. [DOI] [PubMed] [Google Scholar]
- Knopp MCN, Krenn HW. Efficiency of fruit juice feeding in Morpho peleides (Nymphalidae, Lepidoptera) Journal of Insect Behavior. 2003;16:67–77. [Google Scholar]
- Korzeev A, Stekolnikov A. Musculature of the head and cervical region in several palaearctic species from the superfamilies Cossoidea, Zygaenoidea, and Sesioidae (Lepidoptera, Cossiformes) Entomological Review. 2011;91:547–565. [Google Scholar]
- Krenn HW. Functional morphology and movements of the proboscis of Lepidoptera (Insecta) Zoomorphology. 1990;110:105–114. [Google Scholar]
- Krenn HW. Proboscis sensilla in Vanessa cardui (Nymphalidae, Lepidoptera): functional morphology and significance in flower-probing. Zoomorphology. 1998;118:23–30. [Google Scholar]
- Krenn HW. Proboscis musculature in the butterfly Vanessa cardui (Nymphalidae, Lepidoptera): settling the proboscis recoiling controversy. Acta Zoologica (Stockholm) 2000;81:259–266. [Google Scholar]
- Krenn HW. Feeding mechanisms of adult lepidoptera: structure, function, and evolution of the mouthparts. Annual Review of Entomology. 2010;55:307–327. doi: 10.1146/annurev-ento-112408-085338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn HW, Kristensen NP. Early evolution of the proboscis of Lepidoptera (Insecta): external morphology of the galea in basal glossatan moths lineages, with remarks on the origin of the pilifers. Zoologischer Anzeiger – A Journal of Comparative Zoology. 2000;239:179–196. [Google Scholar]
- Krenn HW, Kristensen NP. Evolution of proboscis musculature in Lepidoptera. European Journal of Entomology. 2004;101:565–575. [Google Scholar]
- Krenn HW, Mühlberger N. Groundplan anatomy of the proboscis of butterflies (Papilionoidea, Lepidoptera) Zoologischer Anzeiger – A Journal of Comparative Zoology. 2002;241:369–380. [Google Scholar]
- Krenn HW, Penz CM. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): a search for anatomical adaptations to pollen-feeding behavior. International Journal of Insect Morphology and Embryology. 1998;27:301–309. [Google Scholar]
- Krenn HW, Plant JD, Szucsich NU. Mouthparts of flower-visiting insects. Arthropod Structure & Development. 2005;34:1–40. [Google Scholar]
- Krenn HW, Wiemers M, Maurer L, Pemmer V, Huber W, Weissenhofer A. Butterflies of the Golfo Dulce Region, Costa Rica. Verein zur Förderung der Tropenstation La Gamba; Vienna: 2010. [Google Scholar]
- Krenn HW, Zulka KP, Gatschnegg T. Proboscis morphology and food preferences in nymphalid butterflies (Lepidoptera: Nymphalidae) Journal of Zoology. 2001;254:17–26. [Google Scholar]
- Kunte K. Allometry and functional constraints on proboscis lengths in butterflies. Functional Ecology. 2007;21:982–987. [Google Scholar]
- MacFarlane J, Eaton JL. Skeleton and musculature of the head and thorax of Trichoplusia ni (Hubner), (Lepidoptera: Noctuidae) Journal of Morphology. 1973;139:185–210. doi: 10.1002/jmor.1051390204. [DOI] [PubMed] [Google Scholar]
- Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology. 2009;9:11. doi: 10.1186/1472-6793-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles CL, Booker R. The role of the frontal ganglion in the feeding and eclosion behavior of the moth Manduca sexta. Journal of Experimental Biology. 1998;201:1785–1798. doi: 10.1242/jeb.201.11.1785. [DOI] [PubMed] [Google Scholar]
- Molleman F, Krenn HW, Van Alphen ME, Brakefield PM, DeVries PJ, Zwaan BJ. Food intake of fruit-feeding butterflies: evidence for adaptive variation in proboscis morphology. Biological Journal of the Linnean Society. 2005;86:333–343. [Google Scholar]
- Monaenkova D, Lehnert MS, Andrukh T, Beard CE, Rubin B, Tokarev A, Lee W-K, Adler PH, Kornev KG. Butterfly proboscis: combining a drinking straw with a nanosponge facilitated diversification of feeding habits. Journal of the Royal Society Interface. 2012;9:720–726. doi: 10.1098/rsif.2011.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD. Nectar sources as constraints on the distribution of egg masses by the checkerspot butterfly, Euphydryas chalcedona (Lepidoptera: Nymphalidae) Environmental Entomology. 1983;12:463–466. [Google Scholar]
- Murphy DD, Launer AE, Ehrlich PR. The role of adult feeding in egg production and population dynamics of the checkerspot butterfly Euphydryas editha. Oecologia. 1983;56:257–263. doi: 10.1007/BF00379699. [DOI] [PubMed] [Google Scholar]
- Nilsson LA. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. [Google Scholar]
- Nilsson LA, Jonsson L, Rason L, Randrianjohany E. Monophily and pollination mechanisms in Angraecum arachnites Schltr. (Orchidaceae) in a guild of long-tongued hawk-moths (Sphingidae) in Madagascar. Biological Journal of the Linnean Society. 1985;26:1–19. [Google Scholar]
- Paulus HF, Krenn HW. Vergleichende Morphologie des Schmetterlingsrüssels und seiner Sensillen – Ein Beitrag zur phylogenetischen Systematik der Papilionoidea (Insecta, Lepidoptera) Journal of Zoological Systematics and Evolutionary Research. 1996;34:203–216. [Google Scholar]
- Pauw A, Stofberg J, Waterman RJ. Flies and flowers in Darwin’s race. Evolution. 2009;63:268–279. doi: 10.1111/j.1558-5646.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- Petr D, Stewart KW. Comparative morphology of sensilla styloconica on the proboscis of North American nymphalidae and other selected taxa (Lepidoptera): systematic and ecological considerations. Transactions of the American Entomological Society. 2004;130:293–409. [Google Scholar]
- Pivnick KA, McNeil JN. Effects of nectar concentration on butterfly feeding: measured feeding rates for Thymelicus lineola (Lepidoptera: Hesperiidae) and a general feeding model for adult Lepidoptera. Oecologia. 1985;66:226–237. doi: 10.1007/BF00379859. [DOI] [PubMed] [Google Scholar]
- Pyke GH, Pulliam HR, Charnov EL. Optimal foraging: a selective review of theory and tests. Quarterly Review of Biology. 1977;52:137–154. [Google Scholar]
- Romeis B. Mikroskopische Technik. Urban Schwarzenberg; Wien: 1989. [Google Scholar]
- Schmitt JB. The feeding mechanism of adult Lepidoptera. Smithsonian Miscellaneous Collections. 1938;97:1–28. [Google Scholar]
- Scott JA. The interaction of behavior, population biology, and environment in Hypaurotis crysalus (Lepidoptera) American Midland Naturalist. 1974;91:383–394. [Google Scholar]
- Srivastava RP, Bogawat JK. Feeding mechanism of a fruit-sucking moth Othreis materna (Lepidoptera: Noctuidae) Journal of Natural History. 1969;3:165–181. [Google Scholar]
- Stoffolano JGJ. Regulation of a carbohydrate meal in Adult Diptera, Lepidoptera and Hymenoptera. In: Chapman RF, De Boer G, editors. Regulatory mechanisms in insect feeding. Chapman & Hall; New York, NY: 1995. pp. 210–238. [Google Scholar]
- Wannenmacher G, Wasserthal LT. Contribution of the maxillary muscles to proboscis movement in hawkmoths (Lepidoptera: Sphingidae) – an electrophysiological study. Journal of Insect Physiology. 2003;49:765–776. doi: 10.1016/s0022-1910(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Zaspel JM, Weller SJ, Branham MA. A comparative survey of proboscis morphology and associated structures in fruit-piercing, tear-feeding, and blood-feeding moths in Calpinae (Lepidoptera: Erebidae) Zoomorphology. 2011;130:203–225. [Google Scholar]