Abstract
The development of effective vaccines against difficult disease targets will require the identification of new subunit vaccination strategies that can induce and maintain effective immune responses in humans. Here we report on a Phase Ia clinical trial using the AMA1 antigen from the blood-stage Plasmodium falciparum malaria parasite delivered either as recombinant protein formulated with Alhydrogel adjuvant with and without CPG 7909, or using recombinant vectored vaccines – chimpanzee adenovirus ChAd63 and the orthopoxvirus MVA. A variety of promising ‘mixed-modality’ regimens were tested. All volunteers were primed with ChAd63, and then subsequently boosted with MVA and/or protein-in-adjuvant using either an 8 or 16 week prime-boost interval. We report on the safety of these regimens, as well as the T cell, B cell and serum antibody responses. Notably, IgG antibody responses primed by ChAd63 were comparably boosted by AMA1 protein vaccine, irrespective of whether CPG 7909 was included in the Alhydrogel adjuvant. The ability to improve the potency of a relatively weak aluminium-based adjuvant in humans, by previously priming with an adenoviral vaccine vector encoding the same antigen, thus offers a novel vaccination strategy for difficult or neglected disease targets when access to more potent adjuvants is not possible.
INTRODUCTION
Plasmodium falciparum, the primary causative agent of human malaria, remains the most significant parasitic infection worldwide, responsible for over 200 million infections and upwards of 600,000 deaths in 2012 [1, 2]. Development of a highly effective vaccine against P. falciparum thus remains an urgent global health priority with the potential to reduce both mortality and transmission [3]. However, in light of disappointing Phase III efficacy results reported for the leading pre-erythrocytic vaccine RTS,S in the target infant age group [4], there remains a strong rationale to pursue development of alternative vaccine strategies, including those targeting the asexual blood-stage and the sexual transmission-stages of the parasite’s lifecycle. Like the liver-invasive sporozoites targeted by RTS,S, these subsequent stages of the lifecycle are susceptible to antibodies – either acting within the blood to prevent red blood cell (RBC) invasion, or within the mosquito to interfere with sexual development [5, 6]. Development of subunit vaccination strategies that can induce and maintain effective cellular and humoral responses in humans will thus be a primary requisite for success, given the apparent need for extremely high antibody concentrations, against leading target antigens, in order to neutralize malaria parasites [7, 8].
Most blood-stage and transmission-blocking malaria vaccine candidates trialled to-date have been recombinant protein-in-adjuvant formulations. In many cases, these have been designed to induce high-titer functional antibodies that exert growth inhibitory activity (GIA) against merozoite antigens involved in the RBC invasion process [6, 9], or which inhibit sexual development of the parasite within the mosquito [10], respectively. However, in a number of cases experimental protein vaccine adjuvants have shown unacceptable safety or reactogenicity profiles in clinical trials [11-13], moreover, to-date, only a small number of adjuvants have been licensed for human use – including aluminium-based salts (aluminium phosphate and aluminium hydroxide); virosomes; the oil-in-water emulsion MF59 (Novartis), and the adjuvant-system platform AS03 and AS04 developed by GlaxoSmithKline (GSK) [14, 15]. Similarly, the lack of access to many promising adjuvants developed by some companies has had an adverse effect on vaccine development for difficult diseases, such as malaria, in which there is relatively limited commercial interest and very strong immune responses are required for protection [16].
In recent years we have evaluated an alternative approach to subunit vaccination aimed at inducing both cellular and humoral immune responses. In this case a heterologous prime-boost regimen is used with recombinant adeno- and pox-viral vectors [17, 18]. In order to translate this approach into human clinical trials, we utilized a chimpanzee adenovirus serotype 63 (ChAd63) vector to prime immune responses, followed by a boost 8 weeks later with the orthopoxvirus vector modified vaccinia virus Ankara (MVA) [19, 20]. In a series of Phase Ia clinical trials, we showed that vectors recombinant for the blood-stage P. falciparum antigens merozoite surface protein 1 (MSP1) or apical membrane antigen 1 (AMA1) can induce Th1-isotype-skewed serum IgG antibody responses and strong T cell and memory B cell (mBC) responses [21-23] [Biswas et al., submitted]. However, these vaccine candidates failed to demonstrate clinical efficacy, with no impact on blood-stage parasite multiplication rates (PMR) observed in vaccinated UK adults following controlled human malaria infection (CHMI) administered by mosquito bite [24]. The vectors targeting both blood-stage antigens induced on average 20-50 μg/mL of antigen-specific serum IgG [21, 22], and it is likely these antibody concentrations were too low to mediate functional GIA in vivo [7].
More recent preclinical studies have focused on combining viral vectored and protein-in-adjuvant vaccines in ‘mixed-modality’ regimens, with the aim of maximizing the induction of both cellular and humoral immune responses [18]. In most cases, a recombinant adenoviral vaccine is used to prime the immune response, followed by boosting with MVA and/or protein. Studies of these regimens in mice or rhesus macaques with the MSP1 or AMA1 antigens have shown that these mixed-modality approaches can achieve antibody and T cell responses which equal, or in some cases surpass, the best immune responses achieved by either vaccine technology alone [25, 26]. Priming with viral vectors also led to enhanced IgG antibody avidity and Th1-type IgG isotype skew in mice [25, 27]. Preclinical data from the fields of HIV-1 [28-32] and liver-stage malaria vaccine development [33, 34] also show encouraging results from the combination of adenoviral-vectored and protein-based vaccines. Whilst undertaking the above mouse studies, we also reported that priming with a recombinant adenovirus followed by a booster vaccination with protein-in-adjuvant, greatly reduced the hierarchy of humoral immunogenicity that is typically observed when comparing protein vaccine adjuvants. In this case, relatively weak protein adjuvants such as aluminium-based salts, boosted antibody responses comparably to far more immunogenic formulations, in contrast to results observed when the protein-in-adjuvant vaccines were used alone [27]. This adenovirus-protein regimen also induced a more cytophilic antibody response, dominated by IgG2a, and improved the efficacy of a weakly immunogenic MSP1 protein vaccine delivered in AdjuPhos (aluminium phosphate) in a P. yoelii rodent malaria blood-stage challenge model [27]. In rhesus macaques, an AMA1 vaccine formulated in Alhydrogel boosted IgG responses comparably to the same vaccine in CoVaccineHT™ adjuvant following ChAd63-MVA priming [26]. Overall these data suggested that the differential immunogenicity of protein-in-adjuvant vaccines may be overcome by prior immunization with recombinant adenovirus. They also indicated that a mixed-modality approach may circumvent the need to use more reactogenic adjuvants to achieve maximal antibody responses.
Here we made use of an opportunity to test the mixed-modality concept in a Phase Ia clinical trial, given adenoviral, poxviral and protein AMA1 vaccines were available, and the protein could be administered in Alhydrogel with or without a second potent immuno-stimulator adjuvant (CPG 7909). We thus present the safety and immunogenicity data of this vaccine delivery approach in humans.
RESULTS
VAC044 Study Recruitment and Vaccinations
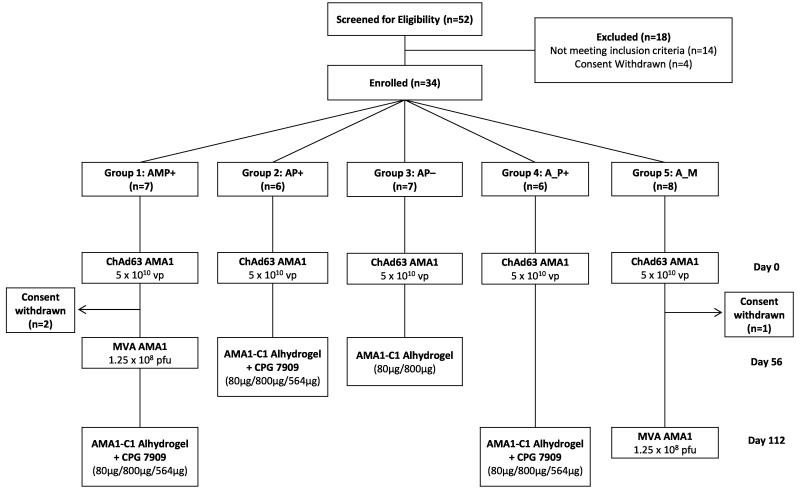
The VAC044 study was designed to assess a variety of promising mixed-modality regimens previously tested in mice and rhesus macaques. These included the use of 16 week (as opposed to 8 week) prime-boost regimens [25, 26], and using protein vaccine formulated in an aluminium-based adjuvant to boost antibody responses primed by an adenoviral vaccine [27]. Recruitment took place between May 2011 and July 2012. Thirty-four healthy malaria-naïve adult volunteers (14 female and 20 male) were enrolled (Fig. 1). The mean age of volunteers was 26.5 years (range 19 – 48). Vaccinations began in June 2011 and all follow-up visits were completed by March 2013. All vaccinees received their immunizations as scheduled with the exception of two volunteers in Group 1 and one volunteer in Group 5 who withdrew from the study for personal reasons following priming with ChAd63 AMA1. Their data was included for the safety analysis, but excluded from immunological analysis. All doses of vaccines were the same as those tested in previous Phase I/IIa studies [22, 24, 35, 36].
Figure 1. VAC044 flow chart of study design and volunteer recruitment.
18 volunteers were excluded following screening for the following reasons: prior malaria exposure (2 volunteers); excessive alcohol consumption (1 volunteer); proteinuria (1 volunteer); positive anti-nuclear antibody at screening (2 volunteers); nickel allergy (2 volunteers); anaemia (1 volunteer); psychiatric history (4 volunteers); haematuria (1 volunteer); consent withdrawn prior to enrolment (4 volunteers). All immunizations were administered intramuscularly with sequential vaccines administered into the deltoid of alternating arms. Two volunteers in Group 1 withdrew from the study 56 days post-ChAd63 AMA1 for personal reasons. One volunteer in Group 5 withdrew from the study 57 days post-ChAd63 AMA1 for personal reasons and was replaced with a new volunteer, thus n=8 recruited into this Group. Throughout the paper the immunization regimens are referred to as defined in the Group boxes, e.g AMP+ = ChAd63 prime, MVA boost, AMA1-C1 protein-in-Alhydrogel + CP7909 boost with 8 week intervals; A_P+ = ChAd63 prime, AMA1-C1 protein-in-Alhydrogel + CP7909 boost with a 16 week interval. Where the “AM” regimen is referred to, this relates to ChAd63 prime, MVA boost with an 8 week interval from Group 1 (before the protein vaccine boost).
Vaccine Safety and Reactogenicity
No unexpected or serious adverse events (AEs) occurred and no volunteers were withdrawn due to AEs. ChAd63 AMA1 administered at the dose of 5 × 1010 vp (n=34) on day 0 demonstrated a favourable safety profile, similar to that seen in the previous Phase I/IIa studies [22, 24], with the majority of AEs mild in severity (82%), all resolving completely (Fig. S1). One volunteer (3%) experienced severe feverishness, myalgia and malaise post-vaccination which resolved within 72 hours.
MVA AMA1 administered at the dose of 1.25 × 108 pfu on day 56 (Group 1) or day 112 (Group 5) was well tolerated (n=12) with the majority of AEs experienced mild in severity (83%) (Fig. S2). As seen with MVA AMA1 and other MVA vectored vaccines [21, 22, 24], the majority of volunteers experienced injection site pain (92%), which was moderate or severe in intensity in 58% of volunteers. Five volunteers (42%) experienced at least one systemic AE that was moderate or severe in intensity. All resolved within 96 hours with the exception of a moderate exacerbation of previously quiescent childhood asthma for which the volunteer was still receiving treatment at the end of the study. No difference was seen in the local or systemic reactogenicity profile of MVA AMA1 when administered 8 or 16 weeks after ChAd63 AMA1 (i.e. between Groups 1 and 5).
As seen in a previous study of malaria-naïve volunteers [36], the addition of CPG 7909 to AMA1-C1/Alhydrogel increased reactogenicity, especially systemic AEs, however both vaccines were overall well tolerated (Fig. S3). Priming with ChAd63 AMA1 (with or without MVA AMA1 booster) did not appear to significantly change the reactogenicity of AMA1-C1/Alhydrogel ± CPG 7909, i.e. when comparing between Groups 1 and 2, or when comparing safety data with those from other studies including malaria-naïve volunteers [35, 36]. No difference was seen in the local or systemic reactogenicity profile of AMA1-C1/Alhydrogel + CPG 7909 administered 8 or 16 weeks after ChAd63 AMA1 (i.e. comparing between Groups 2 and 4).
T cell Responses and Phenotype
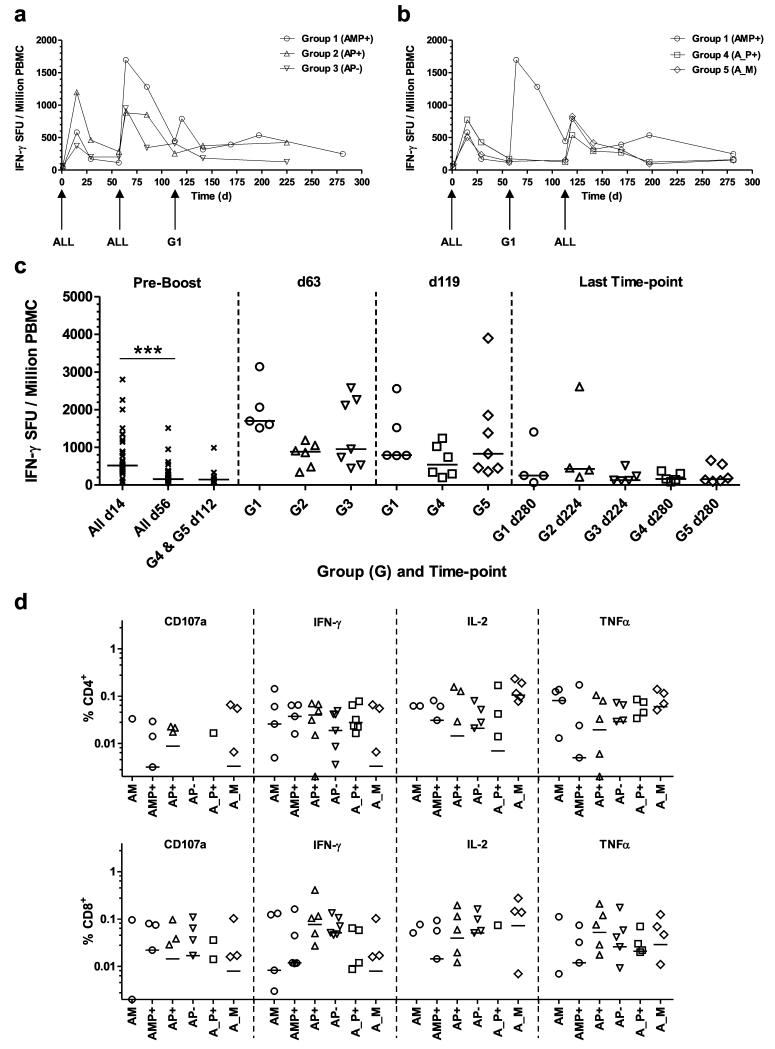
T cell responses provide important help to B cell responses induced by subunit vaccines and in many instances will contribute to protective efficacy against difficult pathogens. T cell responses were thus monitored over time in all groups by ex-vivo IFN-γ ELISPOT. Median responses in each group are shown (Figs. 2a,b) as well as individual responses at key time-points (Fig. 2c). All volunteers in the study were primed with the same dose of ChAd63 AMA1, resulting in a median response at day 14 of 518 SFU / million PBMC (range 74 – 2796) (Fig. 2c), in good agreement with previous studies of the same vaccine [24]. These responses contracted prior to boosting on days 56 and/or 112. Following boosting on day 56 (d56), significantly increased responses were observed across all three vaccinated groups by d63 when the data from the three groups were combined (P=0.0004, n=18, Wilcoxon matched pairs signed-rank test). MVA AMA1 (Group 1) tended to show the strongest responses post-boost on d63 (median of 1698 SFU/million PBMC) but this did not reach significance (P=0.06, Kruskal-Wallis test), whilst the AMA1-C1/Alhydrogel vaccines boosted comparably irrespective of whether CPG 7909 was included in the formulation (medians of 883 and 952 SFU/million PBMC, respectively) (Figs. 2a,c). Following boosting on d112, significantly increased responses were again observed across all three vaccinated groups by d119 when the data from the three groups were combined (P=0.0002, n=18, Wilcoxon matched pairs signed-rank test). A modest increase was observed in the AMP+ group with a median response of 790 SFU/million PBMC (lower than that observed after the first MVA AMA1 boost at week 8). Responses in the A_P+ and A_M groups showed medians of 538 and 828 SFU/million PBMC respectively, indicating that T cell immunogenicity tended to be lower following a vaccine boost at 16 weeks in comparison to 8 weeks (Figs. 2b,c). Following contraction of all responses into the memory phase, there was no significant difference between any of the regimens, irrespective of number of immunizations, prime-boost interval or adjuvant formulation, by the last time-point 24 weeks post-final immunization (d224 or d280) (P=0.45, Kruskal-Wallis test) (Fig. 2c).
Figure 2. T cell responses of mixed-modality AMA1 immunization regimens.
T cell responses were assessed in each group by ex-vivo IFN-γ ELISPOT using fresh PBMC. All volunteers received the same prime with ChAd63 AMA1 on d0. Median responses are shown over time for the 3D7 AMA1 allele in (a) Groups 1, 2 and 3 which all received a booster immunization on day 56, and (b) Groups 1, 4 and 5 which all received a booster immunization on d112, (note Group 1 received a booster immunization at both of these time-points). (c) Median and individual IFN-γ ELISPOT responses are shown for key time-points: all volunteers combined before boosting at days 14 and 56 (n=31) and day 112 (n=13); one week after the booster immunizations (d63 and d119); and the final time-point of follow-up 24 weeks after the last immunization (d224 or d280). *** P<0.0001, Wilcoxon matched-pairs signed rank test. (d) Frozen PBMC from 4 weeks post-booster immunization = d84 (AM, AP+, AP−) and d140 (AMP+, A_P+ and A_M), were re-stimulated with a pool of 3D7 AMA1-specific peptides and assayed by ICS for all volunteers (except for one in Group 5 (A_M) for which cells were not available). Individual and group median responses are shown for the % CD4+ (top) and CD8+ (bottom) T cells positive for CD107a, IFN-γ, IL-2 or TNFα. Any values <0.002% are not shown.
The phenotype of the AMA1-specific T cell responses was also assessed by flow cytometry and intracellular cytokine staining (ICS) for IFN-γ, TNFα, IL-2 and CD107a (Figs. 2d and S4). Frozen PBMC taken 4 weeks after each booster immunization (day 84 for the AM, AP+ and AP− regimens, and day 140 for the AMP+, A_P+ and A_M regimens) were thawed and re-stimulated with a pool of peptides corresponding to the 3D7 allele of AMA1. In agreement with the ex-vivo IFN-γ ELISPOT data for these specific time-points (Figs. 2a,b), responses were largely comparable across all the groups. A mixed CD4+ and CD8+ T cell response was observed following all regimens, suggesting that both the MVA and protein vaccines are capable of boosting these T cell responses primed by ChAd63.
Antibody Responses Assessed by ELISA
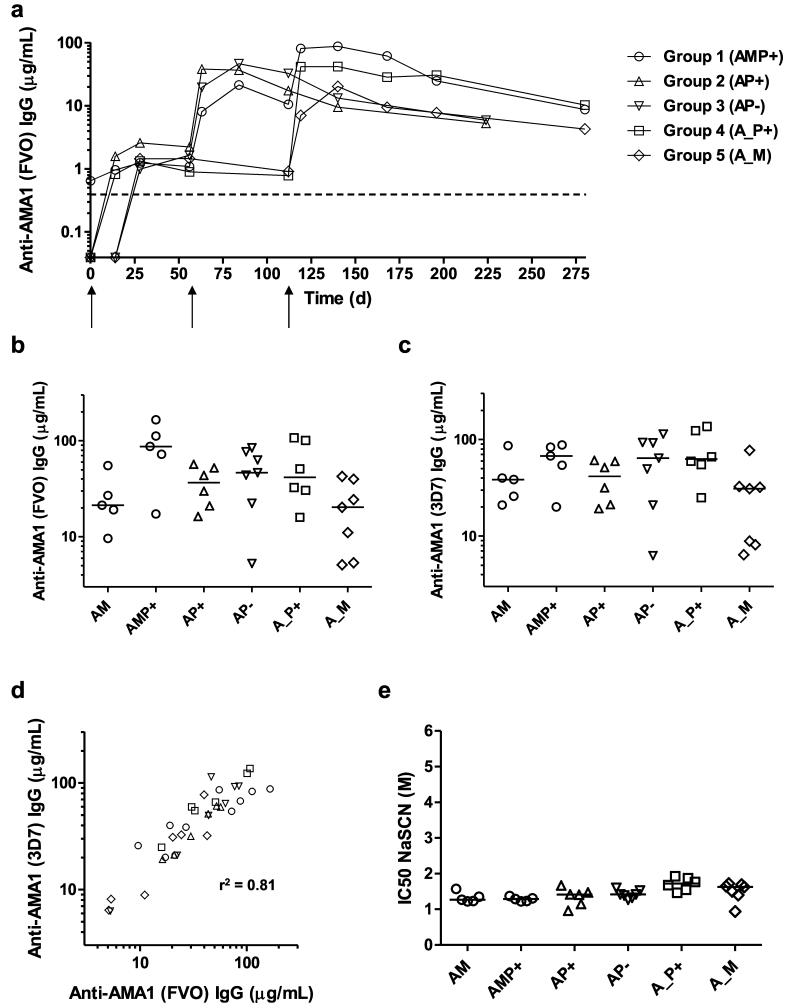
Serum IgG antibody responses against AMA1 were monitored over time in all groups by total IgG ELISA against recombinant AMA1 antigen. IgG ELISA antibody units were converted to antigen-specific μg/mL serum antibody concentrations by calibration-free concentration analysis (see Methods and Fig. S5). Median responses in each group are shown over time against FVO AMA1 (Fig. 3a) as well as individual responses at the peak time-points for both FVO and 3D7 AMA1 (Figs. 3b,c). All 31 volunteers in the study were primed with the same dose of ChAd63 AMA1 on d0 and showed a positive ELISA response at d56 (median 1.6, range 0.5 – 4.0 μg/mL anti-FVO AMA1 IgG) (Fig. 3a). Following boosting on d56, significantly increased responses were observed across all three vaccinated groups by d84 when the data from the three groups were combined (P=0.0002, n=18, Wilcoxon matched pairs signed-rank test). MVA AMA1 tended to show the lowest responses post-boost on d84 (median of 21.3 μg/mL anti-FVO AMA1 IgG) but this did not reach significance (P=0.29, Kruskal-Wallis test), whilst the AMA1-C1/Alhydrogel vaccines boosted comparably irrespective of whether CPG 7909 was included in the formulation (medians of 36.4 and 46.3 μg/mL anti-FVO AMA1 IgG, respectively) (Figs. 3a,b). Following boosting on d112, significantly increased responses were again observed across all three vaccinated groups by d140 when the data from the three groups were combined (P=0.0003, n=18, Wilcoxon matched pairs signed-rank test). The highest responses were observed in the AMP+ group with a median response of 87.2 μg/mL anti-FVO AMA1 IgG (four times higher than observed after the first MVA AMA1 boost at week 8). Responses in the A_P+ and A_M groups showed medians of 41.7 and 20.2 μg/mL anti-FVO AMA1 IgG, respectively. Similar to the observations with T cells, there was no difference in antibody immunogenicity following either a MVA AMA1 or AMA1-C1/Alyhdrogel + CPG 7909 vaccine boost at 16 weeks (Groups 5 and 4) in comparison to 8 weeks (Groups 1 and 2, respectively) (Figs. 3a,b).
Figure 3. Humoral responses of mixed-modality AMA1 immunization regimens.
Serum IgG antibody responses were assessed in each group by anti-AMA1 ELISA. All volunteers received the same prime with ChAd63 AMA1 on d0. (a) Median responses are shown over time for all groups for the FVO AMA1 allele. The dashed line indicates the limit of detection in the assay. (b) Median and individual ELISA responses against FVO AMA1 are shown four weeks after all booster vaccinations: day 84 following AM, AP+ and AP− immunization, and day 140 following AMP+, A_P+ and A_M immunization. (c) The same as in b except the ELISA was performed for 3D7 AMA1. (d) Concordance between the anti-AMA1 total IgG ELISA readouts between the two allelic variants of AMA1 (responses as shown in b and c). Linear regression r2 value is shown; slope = 0.96 (95% C.I. = 0.79 – 1.13); Y-intercept = 8.9 μg/mL (95% C.I. = 0.23 – 17.6) (n=36). (e) Avidity of serum IgG responses was assessed by NaSCN-displacement 3D7 AMA1 ELISA and is reported as the molar (M) concentration of NaSCN required to reduce the starting OD in the ELISA by 50% (IC50). Median and individual responses are shown. Regimens and time-points as in b and c.
Responses against the 3D7 allele of AMA1 showed overall similar results at the peak time-points (Fig. 3c), but anti-3D7 AMA1 serum IgG concentrations tended to be slightly higher for most regimens: medians of 38.4 μg/mL (AM); 67.4 μg/mL (AMP+); 41.4 μg/mL (AP+); 64.0 (AP−); 62.9 μg/mL (A_P+); and 30.9 μg/mL (A_M); also evidenced by an analysis of concordance between the responses against both alleles at the peak of the response (Fig. 3d). By the final time-point 24 weeks post-final immunization (d224 or d280), there was no significant difference between any of the regimens, irrespective of number of immunizations, prime-boost interval or adjuvant formulation (P=0.07, Kruskal-Wallis test) (Fig. 3a).
As part of the same analysis, we also tested 9 serum samples that were remaining and available to us from a previous Phase Ia vaccine trial of the AMA1-C1/Alhydrogel + CPG 7909 vaccine undertaken in healthy US adults [37]. These volunteers received two protein-in-adjuvant vaccine immunizations, at the same doses as used here and given 8 weeks apart, thus providing some comparison to the mixed-modality regimens. The responses induced by this protein-only regimen (PP+), as measured in this assay, were median 131, range 40-191 μg/mL (anti-3D7 AMA1) and median 82, range 25-151 μg/mL (anti-FVO AMA1), and thus higher on average than the other regimens tested here.
Qualitative Assessment of the anti-3D7 AMA1 Antibody Response
We further assessed qualitative aspects of the anti-3D7 AMA1 serum antibody response following immunization with these various mixed-modality regimens. Initially we assessed the avidity of the anti-AMA1 IgG antibodies using a NaSCN-displacement ELISA, whereby this is expressed as the concentration of the NaSCN chaotropic agent required to reduce the starting OD in the ELISA by 50%. Results across all of the regimens were largely similar at the peak of the antibody responses (4 weeks post-immunization), irrespective of adjuvant or prime-boost interval (Fig. 3e). Median responses ranged from 1.3 – 1.7 M, and the results for the ChAd63-MVA AMA1 (AM) regimen here were in very close agreement with data from previous Phase I/IIa trials of the same vaccines (Biswas et al., submitted).
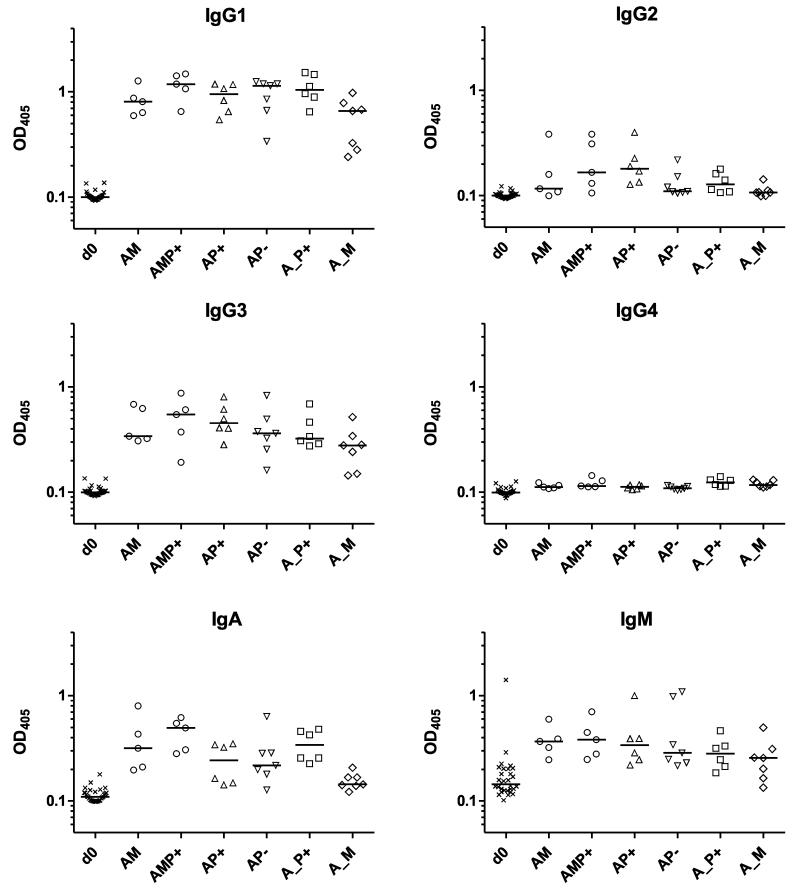
In terms of the serum antibody isotype profiles, again the results were largely comparable across all of the tested regimens (Fig. 4). Anti-3D7 AMA1 IgG responses at the peak of the responses were comprised of a mixed IgG1 and IgG3 response, with low levels of IgG2 in a subset of volunteers and no detectable IgG4. Serum IgA and IgM against AMA1 was also detected for all regimens, although responses were noticeably weaker for the A_M regimen – consistent with this group showing the weakest serum IgG antibody concentrations overall (Figs. 3b,c). Again, results for the AM regimen here were very consistent with those from previous clinical trials of the same vaccines (Biswas et al., submitted).
Figure 4. Antibody isotype profiles of mixed-modality AMA1 immunization regimens.
Isotype profiles of serum antibody responses were assessed by 3D7 AMA1 ELISA. Responses are shown at baseline (d0) for all volunteers and then four weeks after all booster vaccinations: day 84 following AM, AP+, and AP− immunization, and day 140 following AMP+, A_P+ and A_M immunization. In all panels, individual and median responses are shown.
B cell Responses Assessed by ELISPOT
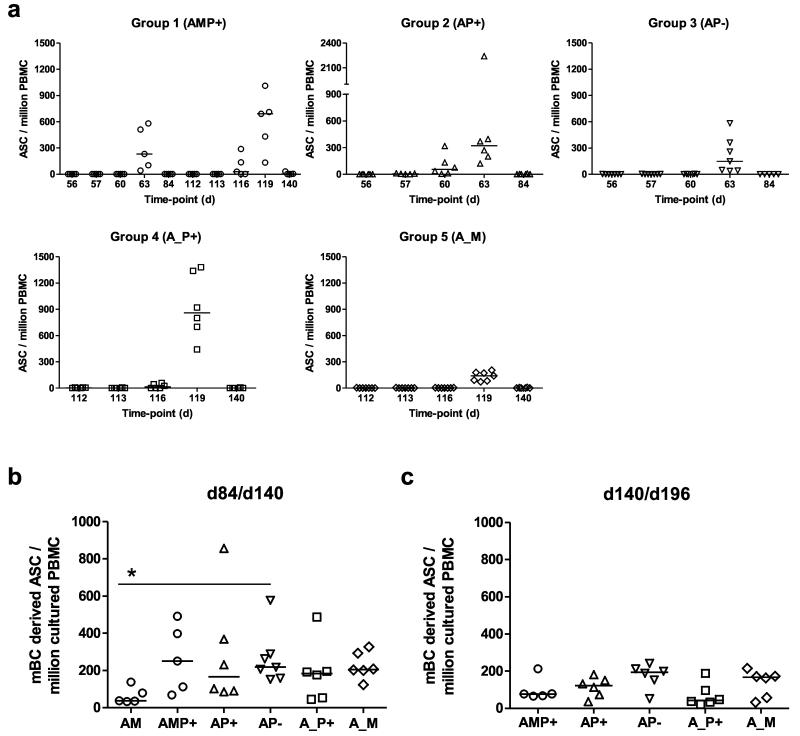
B cell responses were also monitored over time in all groups. Initially AMA1-specific antibody-secreting cell (ASC) responses were assessed at key time-points around the time of immunization by ex-vivo ELISPOT (including day of vaccination, and then 1, 4, 7 and 28 days thereafter) (Fig. 5a). In all volunteers, AMA1-specific ASC responses peaked 7 days post-vaccination, but some lower-level responses were also detected 4 days post-vaccination in volunteers receiving AMA1-C1 protein vaccine with CPG 7909. Group 4 (AP+) showed significantly higher responses than Group 3 (AP−) and Group 5 (A_M) (Fig. S6a). These responses also varied as a % of total IgG-secreting ASC, with the lowest median observed in Group 3 (AP−) at 10.7%, and the highest following the AMP+ immunization regimen in Group 1 at 68.0% (Fig. S6b).
Figure 5. B cell responses of mixed-modality AMA1 immunization regimens.
(a) AMA1-specific antibody-secreting cell (ASC) responses were assessed in each group by ex-vivo ELISPOT using 3D7 + FVO AMA1 protein and fresh PBMC from selected time-points post-booster vaccinations (including day of vaccination, and then 1, 4, 7 and 28 days thereafter). Individual and median responses are shown for each group, (note Group 1 received a booster immunization on both d56 and d112). Responses are reported as AMA1-specific ASC / million PBMC used in the assay. Inter-group comparisons, and AMA1-specific ASC reported as % total IgG ASC are shown in Fig. S6. (b) AMA1-specific memory B cell (mBC) responses were assessed in each group by ELISPOT assay using 3D7 + FVO AMA1 protein. Frozen PBMC were thawed and underwent a 6 day polyclonal restimulation during which ASC are derived from mBC, before testing in the assay. Responses are shown over time in Fig. S7. Here individual and median responses are reported four weeks after all booster vaccinations: day 84 following AM, AP+, and AP− immunization, and day 140 following AMP+, A_P+ and A_M immunization. (c) As for b, except the late time-point 12 weeks post-final immunization is reported (d140/d196). * P<0.05, Kruskal-Wallis test with Dunn’s correction for multiple comparisons.
Memory B cell (mBC) responses were also monitored over-time using an established cultured ELISPOT protocol, whereby mBC within PBMC undergo a 6 day polyclonal stimulation to form ASC which are then measured using the same assay protocol as for the ex-vivo assay. Responses for all volunteers were monitored over-time and are reported as number of mBC-derived AMA1-specific ASC per million cultured PBMC (Fig. S7a), and as a % of total IgG-secreting ASC (Fig. S7b). On average responses in all groups reached a peak 7-28 days post-vaccination. At this time-point, the AM regimen trended to induce the weakest responses, but only one significant difference was noted after correcting for multiple comparisons (Fig. 5b). At the late time-point (d140/196 = 12 weeks after the final immunization), responses were maintained with no significant differences between any of the five regimens (Fig. 5c). Over the entire time-course of the study, there were also no notable effects on mBC responses against a bystander antigen – responses against diphtheria toxoid remained stable over time in all groups (Fig. S7c).
Antibody Functional Activity – Assessment of in vitro GIA
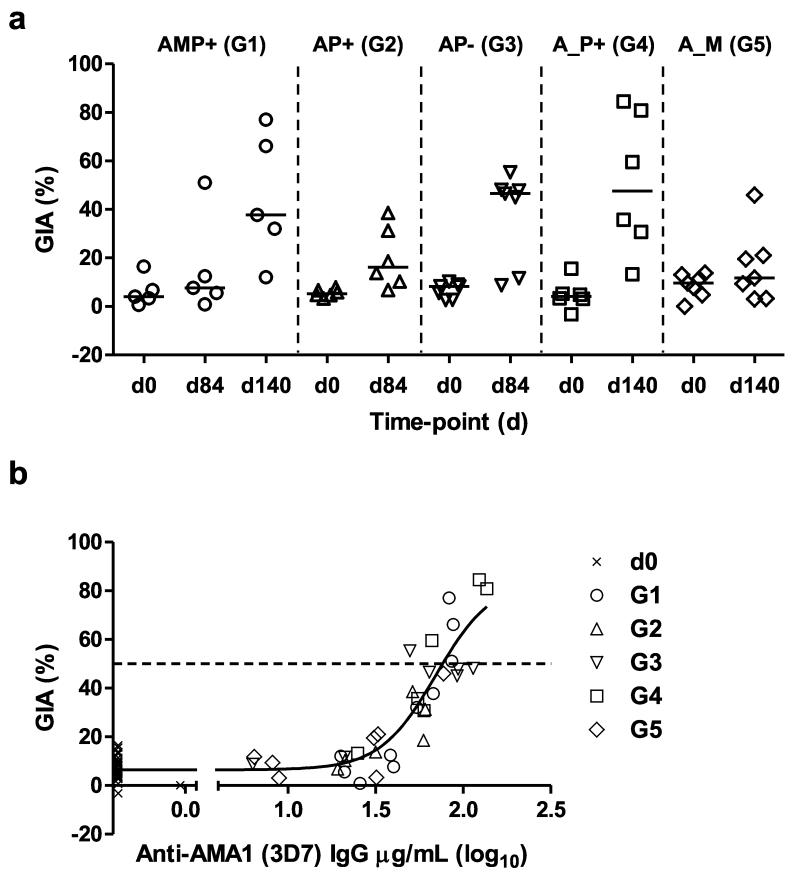
Finally we assessed the functionality of the anti-AMA1 IgG using the in vitro assay of GIA against 3D7 clone P. falciparum parasites. IgG was purified from serum at the peak of the antibody responses (four weeks post-immunization). The highest levels of GIA were observed in the groups receiving the AMP+, AP− or A_P+ regimens (Fig. 6a). Overall there was a strong sigmoidal relationship between % GIA and the anti-3D7 AMA1 serum IgG concentration across all groups, suggesting all five regimens induced a similar quality of functional anti-AMA1 antibody response (Fig. 6b).
Figure 6. Assessment of functional GIA induced by mixed-modality AMA1 immunization regimens.
(a) In vitro GIA of purified IgG was assessed at 10mg/mL against 3D7 clone P. falciparum parasites. Individual data and medians are shown for each group at d0 (baseline), and then four weeks following all booster vaccinations (d84 or d140). Responses >20% are typically regarded as positive. (b) Relationship between GIA and anti-3D7 AMA1 serum IgG concentrations measured by ELISA. Non-linear regression curve is also shown (n=67). The EC50 (level of anti-3D7 AMA1 response in this ELISA assay that gives 50% GIA, indicated by the dotted line) was 69.7 μg/mL, (95% C.I. = 50.2 – 97.0).
DISCUSSION
In the absence of safe, effective, scalable or deployable approaches to whole organism vaccination, subunit vaccines have formed the mainstay approach for the development of novel vaccines against difficult disease targets. Such strategies necessitate the identification of optimal target antigen(s) or immunogens, however, in parallel it has also been essential to develop effective delivery platforms capable of inducing appropriate and often strong immune responses in humans. To-date, leading approaches have included the use of replication-deficient recombinant viruses (in particular adenoviral and poxviral vectors) – suited for the induction of cellular immunity and moderate levels of antibodies [18-20, 38], as well recombinant protein-in-adjuvant formulations – suited for the induction of strong antibody responses. The latter endeavour requires access to a safe and immunogenic human-compatible adjuvant [39], which can be problematic for vaccine developers working on difficult or neglected disease targets [16]. Moreover, in the field of HIV-1 vaccines, a canarypox (ALVAC) viral prime – protein boost regimen was reported to show low-level efficacy in the RV144 trial [40], and future endeavours will likely explore inclusion of adenoviral vaccines as improved priming vectors [18, 41]. Here we report on a Phase Ia clinical trial of a ‘mixed-modality’ approach combining adenoviral, poxviral and protein subunit vaccines targeting the blood-stage malaria antigen P. falciparum AMA1.
All volunteers were primed with the ChAd63 AMA1 vector, and then subsequently boosted with MVA AMA1 or AMA1-C1 protein vaccine in adjuvant using either an 8 or 16 week prime-boost interval. Similar to previous reports of these two viral vectors [22, 24], the ChAd63 and MVA AMA1 vaccines showed a favourable safety profile irrespective of prime-boost time interval. A boost with AMA1-C1 protein in Alhydrogel also showed a very favourable safety profile when given 8 weeks after ChAd63 AMA1. Inclusion of the CPG 7909 with the protein-in-Alhydrogel vaccine led to a small increase in the number of moderate systemic AEs, more similar to ChAd63 AMA1, and this profile was consistent irrespective of prime-boost interval, or whether given 8 weeks after ChAd63 or MVA.
Following ChAd63 priming, T cell responses (detected by ex-vivo IFN-γ ELISPOT) were measurable in all volunteers and contracted by the time of boosting at 8 or 16 weeks. In agreement with data from mice [25] and rhesus macaques [26], the highest T cell responses were observed 7 days after the MVA AMA1 boost following an 8 week prime-boost interval (AM regimen), whilst the AMA1-C1 protein vaccine also boosted but to lower levels on average, irrespective of whether CPG 7909 was included in the Alhydrogel adjuvant formulation. Notably, boosting at 16 weeks with either protein or MVA showed a more modest level of T cell boosting, suggesting an 8 week prime-boost interval is preferable. Nevertheless, irrespective of regimen, all responses contracted and there was no significant difference between the five regimens by 24 weeks post-final immunization. Basic phenotyping of the T cell responses 4 weeks post-boost showed a mixed CD4+ / CD8+ T cell response with no clear differences between regimens, suggesting all delivery modalities (including protein in the relatively weak adjuvant Alhydrogel) could boost the T cell responses primed by ChAd63. It is possible further analyses could reveal more subtle differences in T cell phenotypes induced by MVA as opposed to protein-in-adjuvant vaccine boosting. Interestingly, a previous study using the same protein vaccine and ELISPOT assay reported an average of 198 AMA1 (3D7)-specific SFU/million PBMC 14 days after a single immunization with AMA1-C1 Alhydrogel + CPG 7909, and 282 SFU/million PBMC 14 days after a second immunization using an 8 week prime-boost interval (i.e. the PP+ regimen), and these T cells were predominantly CD4+ [35]. These data thus suggest that boosting with the protein vaccine after priming with ChAd63 (as opposed to priming with protein) leads to stronger cellular immune responses, including CD8+ T cells.
In the case of serum antibody responses, boosting with the protein-in-adjuvant vaccine tended to lead to the highest anti-AMA1 IgG concentrations, again in agreement with preclinical data [25-27] and suggesting the protein-in-adjuvant boost is more effective than MVA in humans. There was no discernable benefit of boosting at 16 weeks as opposed to 8 weeks, nor did an intervening MVA administration prior to the protein vaccination (the AMP+ regimen) significantly improve overall responses in comparison to the AP−, AP+ or A_P+ regimens. These results are highly comparable to previous data with very similar AMA1 vaccines tested in rhesus macaques, whereby the highest titers of antibody were observed following AMP+ or A_P+ immunization when using Alhydrogel or the more potent CoVaccineHT™ adjuvant [26]. Encouragingly, the AP− regimen tested here performed comparably to the AP+ regimen, with the AMA1-C1 Alhydrogel boost inducing on average 46 and 64 μg/mL anti-FVO and −3D7 AMA1 IgG respectively, (about 2-fold higher than the concentrations achieved by the AM regimen). These data confirm previous observations in mice that suggested adenoviral vaccine priming led to comparable boosting of antibody responses by protein-in-adjuvant vaccines, irrespective of the adjuvant or its relative potency when tested in a protein-only regimen [27]. It will also remain of interest in future studies to explore the merits of multiple protein-in-Alhydrogel booster immunizations to see whether peak antibody concentrations can be further improved by such a strategy.
Previous data in mice using MSP1 vaccines had suggested inclusion of both viral vectors in the immunization regimen may improve IgG avidity [25]. Here with AMA1 vaccines in humans, avidity was largely comparable across all regimens tested, irrespective of whether they included a boost with MVA. Further studies will be required to assess for more subtle differences in the quality of the IgG response, in terms of the possible differences at the level of the antibody repertoire, epitope fine specificity and degree of somatic hypermutation related to the induction of germinal centers and CD4+ T follicular helper cell responses. Nevertheless, there remained a strong relationship between serum anti-AMA1 IgG concentration and functional GIA in vitro across all regimens, suggesting no major differences in the quality of vaccine-induced IgG. The GIA EC50 defined here against 3D7 clone parasites (69.7 μg/mL anti-3D7 AMA1 IgG) was also in agreement with previous reports for the AMA1-C1 vaccine tested in malaria-naïve adults [7, 36, 42, 43]. Similar to avidity, the antibody isotype profiles were largely comparable across all regimens, with IgG1 and IgG3 the main isotypes induced against AMA1. In contrast the same protein in Alhydrogel administered to US adults induced predominantly IgG1 alone [44]. Induction of both Th1-type human IgG isotypes (IgG1 and IgG3) is consistent with adenoviral priming, as observed in mice where responses are skewed from predominantly IgG1 to both IgG1 and IgG2a when using viral vectors in conjunction with adjuvants such as Alhydrogel [27].
Previous studies with the AMA1-C1 vaccine reported significant improvements in B cell memory following addition of CPG 7909 to the Alhydrogel adjuvant in US adults [45], but surprisingly not in Malian adults [46]. In our study, all regimens tested produced largely comparable AMA1-specific mBC responses, both at the peak of the response and 12 weeks post-final immunization. These responses were largely comparable to those previously reported for the AM regimen and also the PP+ regimen in UK and US adults respectively [23, 45], again confirming that the protein vaccine in Alhydrogel (AP− regimen) is capable of boosting such responses once primed by ChAd63. Similar to other studies [23, 47], AMA1-specific ASC responses were also consistently detectable 7 days post-booster immunization irrespective of whether MVA or protein was used. Notably, inclusion of CPG 7909 tended to lead to earlier detection of peripheral AMA1-specific ASC responses (on day 4 post-boost) albeit at much lower levels as compared to day 7.
This study aimed to assess whether any of these ‘mixed-modality’ vaccine delivery regimens were largely superior in terms of antibody induction. A comparison (using the same ELISA assay) to historical sera that were available from US adults immunized with the PP+ regimen [37], suggests that protein vaccine formulated in a potent adjuvant remains the most immunogenic delivery platform. Indeed, antibody responses reported for this regimen here were median 131 μg/mL (anti-3D7 AMA1) and 82 μg/mL (anti-FVO AMA1), and largely comparable to other reports for the same vaccine tested with similar regimens elsewhere [35, 36]. Nevertheless, the same AMA1-C1 vaccine delivered in Alhydrogel alone in US adults induced on average only 5-20 μg/mL anti-AMA1 IgG [36, 48] and 16% GIA against 3D7 parasites [36], and thus it remains encouraging that the AP− regimen reported here improved on those reported data. Other AMA1 protein vaccines formulated with strong adjuvants (such as Montanide ISA 720, AS01B and AS02A), have also induced high serum IgG concentrations and consequently high level of in vitro GIA [42, 49], and one candidate has shown evidence of strain-specific efficacy in a Phase IIb field trial in Malian children [50]; whilst another novel candidate in preclinical development has shown an improved quality of vaccine-induced anti-AMA1 IgG response [51]. It thus remains important in future clinical studies to assess whether anti-AMA1 IgG and/or in vitro GIA associate with protective outcome [35, 52, 53], in order to guide the onward clinical development of blood-stage malaria vaccine candidates.
Access to potent human-compatible adjuvants is not always easy for vaccine developers working on neglected diseases, and similarly, experimental unlicensed adjuvants, although potent, may show unacceptable side-effects or reactogenicity during clinical development. CPG 7909 has also shown reduced potency in African adults (as compared to US adults) when formulated with AMA1-C1 in Alhydrogel [36, 54]. Moreover, even though exceptionally high antibody concentrations are likely required to neutralize merozoite invasion of RBCs (a level not achieved by the AMA1-C1/Alhydrogel + CPG 7909 vaccine in UK adults [35]), other disease targets may be inherently more susceptible – for example, anti-capsular antibody concentrations of >0.35 μg/mL are regarded as sufficient for protection against invasive pneumococcal disease [55]. Consequently, the ability to improve the potency of an aluminium-based adjuvant by priming with an adenoviral vaccine vector encoding the same antigen may find utility against other malaria antigens or disease targets; especially where the use of aluminium-based salts alone has proved insufficiently immunogenic, or where access to more potent adjuvants is not possible or their use is undesirable in the target population.
METHODS
Participants
The VAC044 study was conducted at the Oxford Vaccine Centre (OVC), part of the Centre for Clinical Vaccinology and Tropical Medicine (CCVTM), University of Oxford, UK. Healthy, malaria-naïve males and non-pregnant females aged 18-50 years were invited to participate in the study. All volunteers gave written informed consent prior to participation, and the study was conducted according to the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice (GCP). There was no selection of volunteers on the basis of pre-existing neutralizing antibodies (NAb) to the ChAd63 vector prior to enrolment (see Supplementary Material for the full list of inclusion and exclusion criteria).
Ethical & Regulatory Approval
All necessary approvals for VAC044 were granted by Oxfordshire Research Ethics Committee A (Ref: 11/H0604/2) and the UK Medicines and Healthcare products Regulatory Agency (Ref: 21584/0280/001-0001). Vaccine use was authorized by the Genetically Modified Organisms Safety Committee (GMSC) of the Oxford University Hospitals NHS Trust (Reference number GM462.10.59). The trial was registered with ClinicalTrials.gov (Ref: NCT01351948). The Local Safety Committee provided safety oversight and GCP compliance was independently monitored by an external organization (Appledown Clinical Research Ltd., Great Missenden, UK).
Vaccines
Generation, manufacture and QC testing of the recombinant ChAd63 and MVA vectors encoding AMA1 has been previously described [22, 26, 56]. Briefly, the AMA1 transgene insert contains from N- to C-terminus: the leader sequence from human tissue plasminogen activator (tPA) followed in-frame by sequence encoding the ectodomain of P. falciparum (clone 3D7) AMA1 linked to the ectodomain plus C-terminal transmembrane region of P. falciparum (strain FVO) AMA1. In the case of the recombinant protein-in-adjuvant vaccines, details of the manufacture and formulation of both AMA1-C1/Alhydrogel and CPG 7909 in saline, and the mixing procedure used in the clinic, have been described in detail elsewhere [37, 57]. Briefly, the AMA1-C1 vaccine contains an equal mixture of two 533 amino acid recombinant malaria proteins based on the AMA1 sequences of the FVO strain and 3D7 clone of P. falciparum. The recombinant proteins consist of the correctly folded ectodomain portions of the antigens, with the addition of a six-histidine C-terminal tag to enable protein purification, and are expressed separately in Pichia pastoris. CPG 7909 was provided by Coley Pharmaceuticals, now a Pfizer Company.
Study Design
This was a Phase Ia open-label, non-randomized observational and descriptive vaccine trial to assess the safety and immunogenicity of ChAd63 AMA, MVA AMA1 and AMA1-C1/Alhydrogel ± CPG 7909 administered in various regimens. Allocation to study groups (aiming for n=6-7 per group) (Fig. 1) occurred at screening based on volunteer preference as previously described [21]. All vaccinations were administered intramuscularly (IM) into the deltoid, with sequential vaccines administered into the deltoid of alternating arms. Details of dosing, clinical follow-up and safety monitoring are given in Supplementary Methods. Throughout the paper, study day refers to the nominal time-point for a group and not the actual day of sampling.
Peripheral Blood Mononuclear Cell (PBMC) and Serum Preparation
Blood samples were collected into lithium heparin-treated vacutainer blood collection systems (Becton Dickinson, UK). PBMC were isolated and used within 6 hours in fresh assays as previously described [21]. Excess cells were frozen in foetal calf serum (FCS) containing 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen. For serum preparation, untreated blood samples were stored at 4°C and then the clotted blood was centrifuged for 5 min (1000 xg). Serum was stored at −80°C. Historical serum samples from 9 healthy US volunteers previously immunized with AMA1-C1/Alhydrogel + CPG 7909 were the only ones available and were provided to us from a previous trial [37]. These volunteers were immunized twice, 8 weeks apart, with the same doses of vaccine as used in this study, and this regimen is referred to as “PP+”.
Ex-vivo interferon-γ (IFN-γ) ELISPOT
The kinetics and magnitude of the T cell response to AMA1 were assessed over time by ex-vivo IFN-γ ELISPOT following an 18-20 hour re-stimulation of PBMC with overlapping peptides spanning the entire AMA1 sequence present in the vaccines. Peptides were purchased from NEO Peptide (Cambridge, MA, USA) and the sequences have been previously described [22, 24]. 20mer peptides overlapping by 10 amino acids (aa) were generated for the whole of the bi-allelic AMA1 vaccine insert present in the ChAd63 and MVA vaccines which also matched the two AMA1 alleles in the AMA1-C1 protein vaccine [26, 56]. Peptides were reconstituted in 100% DMSO at 50-200 mg/mL and combined into various pools for ELISPOT and flow cytometry assays as previously described [24]. In brief, for the ELISPOT, peptides were divided into pools containing up to 10 peptides per pool according to whether they were 3D7-specific, FVO-specific, common peptides, or FVO C-terminus peptides. Fresh PBMC were used in all ELISPOT assays using a previously described protocol [21]. Spots were counted using an ELISPOT counter (Autoimmun Diagnostika (AID), Germany). Results are expressed as IFN-γ spot-forming units (SFU) per million PBMC. Background responses in un-stimulated control wells were almost always less than 20 spots per 250,000 cells, and were subtracted from those measured in peptide-stimulated wells. Responses are reported as the 3D7 allele-specific AMA1 response (summed response for 3D7-specific peptides + common peptides + C-terminal tail peptides).
Multiparameter Flow Cytometry
Cytokine secretion by PBMC was assayed by ICS followed by flow cytometry using a previously reported protocol [22] with some minor changes. Briefly, frozen PBMC were re-stimulated for 18 h in the presence of anti-human CD49d and CD28 (BD Biosciences) and CD107a. Re-stimulation for the final 16 h was carried out in the presence of Brefeldin A (Sigma) and Monensin (Golgi Stop, BD Biosciences). Each sample was re-stimulated with either: 1μg/mL Staphylococcal enterotoxin B (SEB, positive control samples); a single pool of 3D7 AMA1-specific peptides (n=56) at final concentration 2μg/mL each peptide (0.11% total DMSO concentration); and 0.11% DMSO final concentration (un-stimulated peptide control sample). Cells were stained the next day using a Live/Dead marker, as well as for CD4, CD14, CD19, CD8α, CD3, IFN-γ, TNFα, and IL-2. The staining antibodies differed from those previously reported only for CD3 (Alexa 700; clone: UCHTI) and CD19 (eFluor450; clone: HIB19) (eBioscience). Samples were analyzed using a LSRII Flow Cytometer (BD Biosciences) and FlowJo v9.7.5 (Tree Star Inc, USA). Dead cells, monocytes (CD14+), and B cells (CD19+) were excluded from the analysis (Fig. S4). Background responses in un-stimulated no peptide control cells were subtracted from the antigen-specific peptide responses.
Total IgG ELISA
Recombinant 3D7 AMA1 protein was produced in HEK293E cells. Briefly, suspension HEK293E cells were transiently transfected with an expression plasmid encoding: the ectodomain of 3D7 AMA1 (amino acids 25-546, with sites of potential N-linked glycosylation removed as previously described [26]), with the human tissue plasminogen activator (tPA) signal peptide fused to the N-terminus [17], and the AviTag biotin acceptor peptide (amino acids GLNDIFEAQKIEWHE) followed by a hexa-histidine (His6) tag fused to the C-terminus. When cell viability fell below 95%, culture supernatants were harvested and the recombinant protein was purified in a single step by immobilized metal ion (Ni++) chromatography and quantified by nanodrop. The recombinant FVO AMA1 protein used for ELISAs was a gift from Dr Mike Blackman (NIMR, London, UK) [56]. Total IgG ELISAs were performed with these proteins using standardized methodology, as previously described [22]. The limit of detection in the ELISA assays for both alleles of AMA1 was 10AU, and we assigned the AU value of 1.0 for any test samples with less than 10AU.
Calibration-Free Concentration Analysis (CFCA)
AMA1 IgG antibody OD-based ELISA units were converted to antigen-specific μg/mL as follows. CFCA was performed with a method similar to that previously described [58], using a Biacore T200 instrument, a Biotin CAP chip, and T200 control and evaluation software (all from GE Lifesciences, Amersham, UK). Recombinant 3D7 and FVO AMA1 proteins were produced by generating plasmids encoding each allele’s full-length AMA1 ectodomain sequence (identical to those included in both the AMA1-C1 protein [57] and viral vector vaccines, and reported elsewhere [26, 56]). Sequences were codon-optimized for human expression and possessed an N-terminal human tPA signal peptide and C-terminal AviTag (as above) and StrepII tag. Protein was produced as described above by transient transfection of HEK293E cells; both antigens were enzymatically mono-biotinylated by co-transfection of the cultures with a plasmid encoding BirA [59], then dialysed extensively against PBS prior to CFCA. The CFCA was performed using day 84 serum samples from three individuals with a range of ELISA-measured AMA1-specific IgG antibody responses. Two replicate dilutions of each individual’s sera (1:250 in running buffer [58]) were prepared and assayed independently on different days. Mass-transport limited binding conditions were obtained by capturing a minimum of 800 response units (RU) of AMA1 antigen on the active flow cell. The chip was regenerated with the manufacturer’s supplied regeneration and CAP reagents and fresh antigen prior to each application of antibody; variation in the level of antigen capture between cycles was typically <2%. Antigen-specific antibody binding was measured by double reference subtraction, firstly of binding to a flow cell coated only with the biotin capture reagent, and secondly of the binding of the same individual’s day 0 serum sample from that of the day 84 sample (Figs. S5a-c). Initial rates of antigen-specific binding at 5 μL/min and 100 μL/min were measured and compared to permit measurement of concentration and the level of mass-transport limitation. The binding model used a molecular weight of 150 kDa for IgG and a diffusion coefficient of IgG under the test conditions (37°C, running buffer) of 5.5×10−11 m2/s [58]. Initial binding rates were in the range 0.23 to 1.24 RU/s at 5 μL/min flow, and calculated quality control ratios exceeded the manufacturer’s recommended value of >0.13 (reflecting adequate mass transport limitation for concentration estimation). The CFCA-measured antigen-specific antibody concentrations for each individual against 3D7 and FVO sequence AMA1 were combined with the known total IgG ELISA AU measurements for the same samples to derive an AU-to-μg/mL conversion factor. For each AMA1 allele, the mean of the conversion factors measured for the three subjects was calculated and applied to express other subjects’ ELISA results in terms of μg/mL units. Conversion factors calculated using CFCA results (Fig. S5d) were 0.014 μg/mL per anti-3D7 AMA1 IgG antibody unit, and 0.039 μg/mL per anti-FVO AMA1 IgG antibody unit.
It should be noted that a proportion of the binding detected by the CFCA assay is likely to be due to AMA1-specific IgA and IgM. Given that the levels of AMA1-specific IgA and IgM are on the margins of detectability by ELISA whereas AMA1-specific IgG is detectable in serum diluted many thousand-fold, the substantial majority of the CFCA binding detected is likely to be attributable to AMA1-specific IgG. We therefore used the CFCA measurement of antigen-specific antibody concentration as a conversion factor for the IgG ELISA. To add support to this approach, we also tested 8 serum samples from a previous Phase Ia trial (VAC036) of ChAd63-MVA AMA1. In the previous trial the IgG antibody responses in these 8 sera were converted to AMA1-specific μg/mL IgG concentrations using a conversion factor assigned to an IgG reference standard prepared by affinity-purification on AMA1 antigen (as reported in our previous study) [22]. Here, we now re-measured these 8 samples by ELISA and converted them to AMA1-specific IgG antibody concentrations using the conversion factor from the CFCA. There was a strong concordance between the results (Figs. S5e,f).
IgG Antibody Avidity
IgG antibody avidity was assessed by sodium thiocyanate (NaSCN)-displacement ELISA as described in detail elsewhere (Biswas et al., submitted). In brief, the assays were performed using 3D7 AMA1 protein exactly as for total IgG except sera were individually diluted to a level calculated to give an OD405 = 1.0, plated and then exposed to an ascending concentration of the chaotropic agent NaSCN down the plate (0 to 7M NaSCN). Plates were incubated for 15 min before washing and development as for total IgG. The concentration of NaSCN required to reduce the OD405 to 50% of that without NaSCN was used as a measure of avidity.
Antibody Isotype ELISA
Antibody isotype ELISAs were performed as described in detail elsewhere (Biswas et al., submitted). In brief, the assays were performed using 3D7 AMA1 protein exactly as for total IgG except sera were individually diluted 1:100 and added to duplicate wells of six 96 well plates (one for each isotype analysis). After incubating for 2 h the plates were washed and the secondary antibodies added for human IgG1, IgG2, IgG3, IgG4, IgA and IgM. After incubation and washing, plates were developed as per the total IgG ELISA. Blank wells, internal development controls and a series of positive monoclonal antibody controls were included on each plate.
B cell ELISPOT Assays
B cell ELISPOT assays were performed as described in detail elsewhere [23]. In brief, to measure mBC responses, frozen PBMC were thawed before culturing with a polyclonal B cell stimulation mix containing Staphylococcus aureus Cowan strain Pansorbin cell ‘SAC’ (Calbiochem), the human TLR agonist CpG ODN-2006 (Invivogen) and pokeweed mitogen ‘PWM’ (Sigma) for six days, allowing mBC to differentiate into ASC. On day five of the experiment, ELISPOT plates were coated with recombinant AMA1 protein (a 50:50 mixture of the 3D7 and FVO alleles) to measure the antigen-specific response and polyvalent goat-anti human IgG (Caltag) to measure the total IgG response. A separate plate was coated with a non-malaria vaccine antigen (diphtheria toxoid (DT)), and PBS coated wells were used as a negative control. On day six, cultured cells were transferred to the ELISPOT plate and incubated for 18-20 h before developing with an anti-human IgG (γ-chain) antibody conjugated to alkaline phosphatase (Calbiochem) followed by a substrate buffer. Plates were counted using an AID ELISPOT plate reader. Ex-vivo ASC ELISPOT assays were performed exactly as above but using fresh PBMC directly prepared and added to the ELISPOT plate with no preceding 6 day culture.
In vitro Assay of Growth Inhibitory Activity (GIA)
The ability of induced anti-AMA1 antibodies to inhibit growth of P. falciparum 3D7 clone parasites in vitro were assessed by a standardized GIA assay using purified IgG as previously described [7]. Briefly, each test IgG (10mg/mL in a final test well) was incubated with synchronized P. falciparum parasites for a single growth cycle and relative parasitemia levels were quantified by biochemical determination of parasite lactate dehydrogenase.
Statistical Analysis
Data were analyzed using GraphPad Prism version 5.04 for Windows (GraphPad Software Inc., California, USA). Median responses for each group are described. Significance testing of differences between groups used the Kruskal-Wallis test with Dunn’s correction for multiple comparisons, or the Wilcoxon matched-pairs signed rank test was used when data were paired (e.g. between time-points). To analyse concordance, linear regression was performed without constraints but with automatic removal of outliers. A value of P < 0.05 was considered significant.
Supplementary Material
Figure S1: Local and systemic AEs deemed definitely, probably or possibly related to ChAd63 AMA1.
Only the highest intensity of each AE per subject is listed. Data are combined for all AEs for all volunteers receiving the same vaccine at the stated dose (n=34 including all enrolled volunteers). There were no immunization-related serious AEs. (a) Local AEs post-ChAd63 AMA1. ‘Other’ AE was shoulder pain. (b) Systemic AEs post-ChAd63 AMA1. ‘Other’ AEs included mild dizziness, light-headedness, coryzal symptoms, shoulder pain, anaemia and leukopenia; moderate anorexia, diarrhoea and light-headedness.
Figure S2: Local and systemic AEs deemed definitely, probably or possibly related to MVA AMA1.
Only the highest intensity of each AE per subject is listed. Data are combined for all AEs for all volunteers receiving the same vaccine at the stated dose. There were no immunization-related serious AEs. (a) Local AEs post-MVA AMA1. ‘Other’ AE was bruising at injection site. (b) Systemic AEs post-MVA AMA1. ‘Other’ AEs included mild dizziness, tender axilla, elevation of alanine transaminase; and moderate exacerbation of childhood asthma.
Figure S3: Local and systemic AEs deemed definitely, probably or possibly related to AMA1-C1/Alhydrogel ± CPG 7909.
Only the highest intensity of each AE per subject is listed. Data are combined for all AEs for all volunteers receiving the same vaccine at the stated dose. There were no immunization-related serious AEs. (a) Local AEs post-AMA1-C1/Alhydrogel. ‘Other’ AE was bruise at vaccination site. (b) Local AEs post-AMA1-C1/Alhydrogel + CPG 7909. (c) Systemic AEs post-AMA1-C1/Alhydrogel. ‘Other’ AE was diarrhoea. (d) Systemic AEs post-AMA1-C1/Alhydrogel + CPG 7909. ‘Other’ AEs included mild pharyngitis, anxiety, vasovagal syncope, coryzal symptoms and salivary gland infection; moderate leg cramps and sweating; and severe lower back pain.
Figure S4: Gating strategy for the analysis of antigen-specific T cell responses.
Representative flow cytometry plots are shown for the analysis of AMA1-specific T cells shown in Fig. 2d. (a) Initial gating used (from top left to bottom right) forward scatter area (FSC-A) versus side scatter area (SSC-A) to gate lymphocytes; then following this forward scatter height (FSC-H) versus FSC-A to remove doublet events and select singlet cells; then live cells were gated followed by CD14− CD19− CD3+ cells; then CD4 versus CD8 was used to select the total CD4+ CD8− cell population and the CD8+ CD4− population. (b) Subsequently the cytokines (IFN-γ, IL-2 and TNFα) and CD107a were analyzed against SSC-A as shown for CD4+ cells (left) and CD8+ cells (right). Representative plots for un-stimulated (UNS), AMA1 peptide stimulated (AMA1) and SEB stimulated samples are shown with IFN-γ (top row), IL-2 (second row), TNFα (third row) and CD107a (bottom row). Percentages refer to the % of CD8− CD4+ or CD4− CD8+ cells that express the specific cytokine or marker. Background responses in UNS control cells were subtracted from the AMA1 response during the analysis.
Figure S5: Calibration-free concentration analysis (CFCA).
CFCA was performed using a Biacore T200 instrument in order to measure absolute (μg/mL) concentrations of 3D7- and FVO-allele AMA1-specific antibody in day 84 serum samples from subjects 1019, 1050 and 1051. Throughout, binding at low sample flow rate (5 μL/min) is indicated by black lines while binding at high flow (100 μL/min) is indicated by red lines. (a-c) depict processing of the data used to make a single measurement of anti-3D7 AMA1 antibody concentration in subject 1019. (a) Binding of d84 serum (solid lines) and d0 pre-immune serum (dashed lines) to the 3D7 AMA1 coated active flow cell (Fc2) at the two flow rates, as above. (b) Binding of d84 and d0 serum at the two flow rates, after subtraction of binding to the non-3D7 AMA1 coated reference flow cell (i.e. graph shows Fc2-1). (c) Final double-subtracted 3D7 AMA1-specific binding at the two flow rates, after subtraction of pre-immune d0 serum binding from reference-subtracted d84 sample binding (i.e. graph shows difference between solid and dashed lines in b). The slopes of these lines, as estimated by the CFCA function in the T200 evaluation software, were used to calculate antigen-specific antibody concentration in the d84 sample. (d) Tabulates CFCA and relevant ELISA results for the same samples, indicating calculated IgG AU-to-μg/mL conversion factor. SE: standard error of fit. (e) Concordance of 3D7 AMA1-specific serum IgG concentration in 8 samples determined here by i) ELISA and then CFCA conversion factor, versus ii) ELISA followed by a conversion factor developed using an AMA1 antigen-affinity-purified IgG ELISA reference standard. The latter data were generated by an independent laboratory (the GIA Reference Centre at NIH, USA) using sera from a previous Phase Ia trial called VAC036 and have been reported elsewhere [22]. Linear regression r2 value is shown; slope = 0.96 (95% C.I. = 0.62 – 1.31); y-intercept = 3.8 μg/mL (95% C.I. = −14.7 – 22.3) (n=8). (f) Exactly as in e except for FVO AMA1 and (n=7). Linear regression r2 value is shown; slope = 0.63 (95% C.I. = 0.07 – 1.18); y-intercept = 12.2 μg/mL (95% C.I. = −17.6 – 42.0).
Figure S6: Ex-vivo ASC responses following mixed-modality AMA1 immunization regimens.
(a) AMA1-specific ASC responses were assessed in each group by ex-vivo ELISPOT as reported in Fig. 5. Individual and median responses are shown for each group at the indicated time-points all 7 days after a booster vaccination (d63 or d119). Responses are reported as AMA1-specific ASC / million PBMC used in the assay. (b) The same individuals and time-points as shown in a, but AMA1-specific ASC responses are reported as % total IgG ASC measured in the assay. * P<0.05, ** P<0.01, Kruskal-Wallis test with Dunn’s correction for multiple comparisons.
Figure S7. mBC responses over time.
AMA1-specific mBC responses were measured as in Fig. 5 for all volunteers. Box and whisker plots show the median, range and interquartile range of AMA1-specific mBC derived ASC responses measured over time using the mBC ELISPOT assay. Responses are defined as (a) mBC derived ASC per million cultured PBMC specific for AMA1; or (b) as mBC derived ASC specific for AMA1 as a % of total IgG+ ASC measured in the assay. (c) Median diphtheria toxoid-specific responses are also shown for each group over time.
ACKNOWLEDGEMENTS
We thank M. Smith, R. Lopez-Ramon, N. Anagnostou, R. Antrobus and J. Meyer for clinical assistance; N. Lella & S. French for logistical support; J. Furze and D. Worth for laboratory assistance; the Jenner Institute Flow Cytometry Core Facility for technical assistance; S. Moretz, A. Diouf and G. Tullo for technical support performing the GIA assays; Yves Durocher (CNRC-NRC, Canada) for provision of HEK293E cells; and all the study volunteers. The manufacture and QC control of AMA1-C1/Alhydrogel vaccine was supported by the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, USA.
FUNDING STATEMENT
This work was supported by the EMVDA (European Malaria Vaccine Development Association), a European Commission (EC) FP6-funded consortium [LSHP-CT-2007-037506]; the UK National Institute of Health Research through the Oxford Biomedical Research Centre (NIHR-BRC) [A91301 Adult Vaccine]; the Wellcome Trust [084113/Z/07/Z]; and EVIMalaR, an EC FP7-funded programme [Grant agreement No. 242095]. The GIA work was supported by the PATH Malaria Vaccine Initiative and the Intramural Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases. AVSH and SJD are Jenner Investigators; SHH holds a Wellcome Trust Research Training Fellowship [097940/Z/11/Z]; SB is a NDM Leadership Fellow and Junior Research Fellow of St Catherine’s College, Oxford University; and SJD is a UK MRC Career Development Fellow [G1000527] and Lister Institute Prize Research Fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
COMPETING INTEREST STATEMENT
ADD, JJI, SCdC, AVSH and SJD are named inventors on patent applications covering malaria vaccines and immunization regimens. AN is an employee of and / or shareholder in Okairòs, which is developing vectored vaccines for malaria and other diseases.
REFERENCES
- 1.World Health Organization World Malaria Report 2012. World Malaria Report. 2012;2012:1–249. [Google Scholar]
- 2.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 3.Birkett AJ, Moorthy VS, Loucq C, Chitnis CE, Kaslow DC. Malaria vaccine R&D in the Decade of Vaccines: Breakthroughs, challenges and opportunities. Vaccine. 2013;31(Suppl 2):B233–243. doi: 10.1016/j.vaccine.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill AV. Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci. 2011;366:2806–2814. doi: 10.1098/rstb.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman AL, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol. 2010;104:189–211. doi: 10.1179/136485910X12647085215534. [DOI] [PubMed] [Google Scholar]
- 7.Miura K, Zhou H, Diouf A, Moretz SE, Fay MP, Miller LH, et al. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin Vaccine Immunol. 2009;16:963–968. doi: 10.1128/CVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheru L, Wu Y, Diouf A, Moretz SE, Muratova OV, Song G, et al. The IC(50) of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine. 2010;28:4423–4429. doi: 10.1016/j.vaccine.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis RD, Sagara I, Doumbo O, Wu Y. Blood stage vaccines for Plasmodium falciparum: Current status and the way forward. Hum Vaccin. 2010;6 doi: 10.4161/hv.6.8.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Targett GA, Greenwood BM. Malaria vaccines and their potential role in the elimination of malaria. Malar J. 2008;7(Suppl 1):S10. doi: 10.1186/1475-2875-7-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roestenberg M, Remarque E, de Jonge E, Hermsen R, Blythman H, Leroy O, et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS ONE. 2008;3:e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert C. Boosting our best shot. Nat Med. 2009;15:984–988. doi: 10.1038/nm0909-984. [DOI] [PubMed] [Google Scholar]
- 14.Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines. 2003;2:197–203. doi: 10.1586/14760584.2.2.197. [DOI] [PubMed] [Google Scholar]
- 15.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24:310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coler RN, Carter D, Friede M, Reed SG. Adjuvants for malaria vaccines. Parasite Immunol. 2009;31:520–528. doi: 10.1111/j.1365-3024.2009.01142.x. [DOI] [PubMed] [Google Scholar]
- 17.Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC, et al. Effective induction of high-titer antibodies by viral vector vaccines. Nat Med. 2008;14:819–821. doi: 10.1038/nm.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Cassan SC, Draper SJ. Recent advances in antibody-inducing poxviral and adenoviral vectored vaccine delivery platforms for difficult disease targets. Expert Rev Vaccines. 2013;12:365–378. doi: 10.1586/erv.13.11. [DOI] [PubMed] [Google Scholar]
- 19.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 20.Capone S, D’Alise AM, Ammendola V, Colloca S, Cortese R, Nicosia A, et al. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines. 2013;12:379–393. doi: 10.1586/erv.13.15. [DOI] [PubMed] [Google Scholar]
- 21.Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, et al. Phase Ia Clinical Evaluation of the Plasmodium falciparum Blood-stage Antigen MSP1 in ChAd63 and MVA Vaccine Vectors. Mol Ther. 2011;19:2269–2276. doi: 10.1038/mt.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehy SH, Duncan CJ, Elias SC, Biswas S, Collins KA, O’Hara GA, et al. Phase Ia Clinical Evaluation of the Safety and Immunogenicity of the Plasmodium falciparum Blood-Stage Antigen AMA1 in ChAd63 and MVA Vaccine Vectors. PLoS One. 2012;7:e31208. doi: 10.1371/journal.pone.0031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias SC, Choudhary P, de Cassan SC, Biswas S, Collins KA, Halstead FD, et al. Analysis of human B-cell responses following ChAd63-MVA MSP1 and AMA1 immunization and controlled malaria infection. Immunology. 2014;141:628–644. doi: 10.1111/imm.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, et al. ChAd63-MVA-vectored Blood-stage Malaria Vaccines Targeting MSP1 and AMA1: Assessment of Efficacy Against Mosquito Bite Challenge in Humans. Mol Ther. 2012;20:2355–2368. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas AD, de Cassan SC, Dicks MD, Gilbert SC, Hill AV, Draper SJ. Tailoring subunit vaccine immunogenicity: Maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine. 2010;28:7167–7178. doi: 10.1016/j.vaccine.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, Naddeo M, et al. Enhancing blood-stage malaria subunit vaccine immunogenicity in rhesus macaques by combining adenovirus, poxvirus, and protein-in-adjuvant vaccines. J Immunol. 2010;185:7583–7595. doi: 10.4049/jimmunol.1001760. [DOI] [PubMed] [Google Scholar]
- 27.de Cassan SC, Forbes EK, Douglas AD, Milicic A, Singh B, Gupta P, et al. The requirement for potent adjuvants to enhance the immunogenicity and protective efficacy of protein vaccines can be overcome by prior immunization with a recombinant adenovirus. J Immunol. 2011;187:2602–2616. doi: 10.4049/jimmunol.1101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Roman VR, Florese RH, Peng B, Montefiori DC, Kalyanaraman VS, Venzon D, et al. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr. 2006;43:270–277. doi: 10.1097/01.qai.0000230318.40170.60. [DOI] [PubMed] [Google Scholar]
- 29.Vinner L, Therrien D, Wee E, Laursen I, Hanke T, Corbet SL, et al. Immune response in rhesus macaques after mixed modality immunisations with DNA, recombinant adenovirus and recombinant gp120 from human immunodeficiency virus type 1. Apmis. 2006;114:690–699. doi: 10.1111/j.1600-0463.2006.apm_395.x. [DOI] [PubMed] [Google Scholar]
- 30.Shu Y, Winfrey S, Yang ZY, Xu L, Rao SS, Srivastava I, et al. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25:1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zolla-Pazner S, Lubeck M, Xu S, Burda S, Natuk RJ, Sinangil F, et al. Induction of neutralizing antibodies to T-cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime-boost vaccine regimen in chimpanzees. J Virol. 1998;72:1052–1059. doi: 10.1128/jvi.72.2.1052-1059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson LJ, Malkevitch N, Pinczewski J, Venzon D, Lou Y, Peng B, et al. Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120. J Virol. 2003;77:8607–8620. doi: 10.1128/JVI.77.16.8607-8620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, Shott J, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect Immun. 2007;75:2283–2290. doi: 10.1128/IAI.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchings CL, Birkett AJ, Moore AC, Hill AV. Combination of protein and viral vaccines induces potent cellular and humoral immune responses and enhanced protection from murine malaria challenge. Infect Immun. 2007;75:5819–5826. doi: 10.1128/IAI.00828-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan CJ, Sheehy SH, Ewer KJ, Douglas AD, Collins KA, Halstead FD, et al. Impact on Malaria Parasite Multiplication Rates in Infected Volunteers of the Protein-in-Adjuvant Vaccine AMA1-C1/Alhydrogel+CPG 7909. PLoS One. 2011;6:e22271. doi: 10.1371/journal.pone.0022271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE. 2008;3:e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis RD, Mullen GE, Pierce M, Martin LB, Miura K, Fay MP, et al. A Phase 1 study of the blood-stage malaria vaccine candidate AMA1-C1/Alhydrogel with CPG 7909, using two different formulations and dosing intervals. Vaccine. 2009;27:4104–4109. doi: 10.1016/j.vaccine.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Draper SJ, Cottingham MG, Gilbert SC. Utilizing poxviral vectored vaccines for antibody induction-Progress and prospects. Vaccine. 2013;31:4223–4230. doi: 10.1016/j.vaccine.2013.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 41.Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, Ebrahimsa U, et al. Vaccine-elicited Human T Cells Recognizing Conserved Protein Regions Inhibit HIV-1. Mol Ther. 2014;22:464–475. doi: 10.1038/mt.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierce MA, Ellis RD, Martin LB, Malkin E, Tierney E, Miura K, et al. Phase 1 safety and immunogenicity trial of the Plasmodium falciparum blood-stage malaria vaccine AMA1-C1/ISA 720 in Australian adults. Vaccine. 2010;28:2236–2242. doi: 10.1016/j.vaccine.2009.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miura K, Zhou H, Moretz SE, Diouf A, Thera MA, Dolo A, et al. Comparison of biological activity of human anti-apical membrane antigen-1 antibodies induced by natural infection and vaccination. J Immunol. 2008;181:8776–8783. doi: 10.4049/jimmunol.181.12.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73:3677–3685. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crompton PD, Mircetic M, Weiss G, Baughman A, Huang CY, Topham DJ, et al. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–3326. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traore B, Kone Y, Doumbo S, Doumtabe D, Traore A, Crompton PD, et al. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine. 2009;27:7299–7303. doi: 10.1016/j.vaccine.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miura K, Zhou H, Diouf A, Tullo G, Moretz SE, Aebig JA, et al. Immunological responses against Plasmodium falciparum Apical Membrane Antigen 1 vaccines vary depending on the population immunized. Vaccine. 2011;29:2255–2261. doi: 10.1016/j.vaccine.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE. 2009;4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan P, Ekanem E, Diouf A, Tonkin ML, Miura K, Boulanger MJ, et al. Immunization with a functional protein complex required for erythrocyte invasion protects against lethal malaria. Proc Natl Acad Sci U S A. 2014;111:10311–10316. doi: 10.1073/pnas.1409928111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheehy SH, Douglas AD, Draper SJ. Challenges of assessing the clinical efficacy of asexual blood-stage Plasmodium falciparum malaria vaccines. Hum Vaccin Immunother. 2013;9:1831–1840. doi: 10.4161/hv.25383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duncan CJ, Hill AV, Ellis RD. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum Vaccin Immunother. 2012;8:706–714. doi: 10.4161/hv.19712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sagara I, Ellis RD, Dicko A, Niambele MB, Kamate B, Guindo O, et al. A randomized and controlled Phase 1 study of the safety and immunogenicity of the AMA1-C1/Alhydrogel + CPG 7909 vaccine for Plasmodium falciparum malaria in semi-immune Malian adults. Vaccine. 2009;27:7292–7298. doi: 10.1016/j.vaccine.2009.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paradiso P. Essential criteria for evaluation of pneumococcal conjugate vaccine candidates. Vaccine. 2009;27(Suppl 3):C15–18. doi: 10.1016/j.vaccine.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Biswas S, Dicks MD, Long CA, Remarque EJ, Siani L, Colloca S, et al. Transgene Optimization, Immunogenicity and In Vitro Efficacy of Viral Vectored Vaccines Expressing Two Alleles of Plasmodium falciparum AMA1. PLoS One. 2011;6:e20977. doi: 10.1371/journal.pone.0020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70:6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams AR, Douglas AD, Miura K, Illingworth JJ, Choudhary P, Murungi LM, et al. Enhancing Blockade of Plasmodium falciparum Erythrocyte Invasion: Assessing Combinations of Antibodies against PfRH5 and Other Merozoite Antigens. PLoS Pathog. 2012;8:e1002991. doi: 10.1371/journal.ppat.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bushell KM, Sollner C, Schuster-Boeckler B, Bateman A, Wright GJ. Large-scale screening for novel low-affinity extracellular protein interactions. Genome Res. 2008;18:622–630. doi: 10.1101/gr.7187808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Local and systemic AEs deemed definitely, probably or possibly related to ChAd63 AMA1.
Only the highest intensity of each AE per subject is listed. Data are combined for all AEs for all volunteers receiving the same vaccine at the stated dose (n=34 including all enrolled volunteers). There were no immunization-related serious AEs. (a) Local AEs post-ChAd63 AMA1. ‘Other’ AE was shoulder pain. (b) Systemic AEs post-ChAd63 AMA1. ‘Other’ AEs included mild dizziness, light-headedness, coryzal symptoms, shoulder pain, anaemia and leukopenia; moderate anorexia, diarrhoea and light-headedness.
Figure S2: Local and systemic AEs deemed definitely, probably or possibly related to MVA AMA1.
Only the highest intensity of each AE per subject is listed. Data are combined for all AEs for all volunteers receiving the same vaccine at the stated dose. There were no immunization-related serious AEs. (a) Local AEs post-MVA AMA1. ‘Other’ AE was bruising at injection site. (b) Systemic AEs post-MVA AMA1. ‘Other’ AEs included mild dizziness, tender axilla, elevation of alanine transaminase; and moderate exacerbation of childhood asthma.
Figure S3: Local and systemic AEs deemed definitely, probably or possibly related to AMA1-C1/Alhydrogel ± CPG 7909.
Only the highest intensity of each AE per subject is listed. Data are combined for all AEs for all volunteers receiving the same vaccine at the stated dose. There were no immunization-related serious AEs. (a) Local AEs post-AMA1-C1/Alhydrogel. ‘Other’ AE was bruise at vaccination site. (b) Local AEs post-AMA1-C1/Alhydrogel + CPG 7909. (c) Systemic AEs post-AMA1-C1/Alhydrogel. ‘Other’ AE was diarrhoea. (d) Systemic AEs post-AMA1-C1/Alhydrogel + CPG 7909. ‘Other’ AEs included mild pharyngitis, anxiety, vasovagal syncope, coryzal symptoms and salivary gland infection; moderate leg cramps and sweating; and severe lower back pain.
Figure S4: Gating strategy for the analysis of antigen-specific T cell responses.
Representative flow cytometry plots are shown for the analysis of AMA1-specific T cells shown in Fig. 2d. (a) Initial gating used (from top left to bottom right) forward scatter area (FSC-A) versus side scatter area (SSC-A) to gate lymphocytes; then following this forward scatter height (FSC-H) versus FSC-A to remove doublet events and select singlet cells; then live cells were gated followed by CD14− CD19− CD3+ cells; then CD4 versus CD8 was used to select the total CD4+ CD8− cell population and the CD8+ CD4− population. (b) Subsequently the cytokines (IFN-γ, IL-2 and TNFα) and CD107a were analyzed against SSC-A as shown for CD4+ cells (left) and CD8+ cells (right). Representative plots for un-stimulated (UNS), AMA1 peptide stimulated (AMA1) and SEB stimulated samples are shown with IFN-γ (top row), IL-2 (second row), TNFα (third row) and CD107a (bottom row). Percentages refer to the % of CD8− CD4+ or CD4− CD8+ cells that express the specific cytokine or marker. Background responses in UNS control cells were subtracted from the AMA1 response during the analysis.
Figure S5: Calibration-free concentration analysis (CFCA).
CFCA was performed using a Biacore T200 instrument in order to measure absolute (μg/mL) concentrations of 3D7- and FVO-allele AMA1-specific antibody in day 84 serum samples from subjects 1019, 1050 and 1051. Throughout, binding at low sample flow rate (5 μL/min) is indicated by black lines while binding at high flow (100 μL/min) is indicated by red lines. (a-c) depict processing of the data used to make a single measurement of anti-3D7 AMA1 antibody concentration in subject 1019. (a) Binding of d84 serum (solid lines) and d0 pre-immune serum (dashed lines) to the 3D7 AMA1 coated active flow cell (Fc2) at the two flow rates, as above. (b) Binding of d84 and d0 serum at the two flow rates, after subtraction of binding to the non-3D7 AMA1 coated reference flow cell (i.e. graph shows Fc2-1). (c) Final double-subtracted 3D7 AMA1-specific binding at the two flow rates, after subtraction of pre-immune d0 serum binding from reference-subtracted d84 sample binding (i.e. graph shows difference between solid and dashed lines in b). The slopes of these lines, as estimated by the CFCA function in the T200 evaluation software, were used to calculate antigen-specific antibody concentration in the d84 sample. (d) Tabulates CFCA and relevant ELISA results for the same samples, indicating calculated IgG AU-to-μg/mL conversion factor. SE: standard error of fit. (e) Concordance of 3D7 AMA1-specific serum IgG concentration in 8 samples determined here by i) ELISA and then CFCA conversion factor, versus ii) ELISA followed by a conversion factor developed using an AMA1 antigen-affinity-purified IgG ELISA reference standard. The latter data were generated by an independent laboratory (the GIA Reference Centre at NIH, USA) using sera from a previous Phase Ia trial called VAC036 and have been reported elsewhere [22]. Linear regression r2 value is shown; slope = 0.96 (95% C.I. = 0.62 – 1.31); y-intercept = 3.8 μg/mL (95% C.I. = −14.7 – 22.3) (n=8). (f) Exactly as in e except for FVO AMA1 and (n=7). Linear regression r2 value is shown; slope = 0.63 (95% C.I. = 0.07 – 1.18); y-intercept = 12.2 μg/mL (95% C.I. = −17.6 – 42.0).
Figure S6: Ex-vivo ASC responses following mixed-modality AMA1 immunization regimens.
(a) AMA1-specific ASC responses were assessed in each group by ex-vivo ELISPOT as reported in Fig. 5. Individual and median responses are shown for each group at the indicated time-points all 7 days after a booster vaccination (d63 or d119). Responses are reported as AMA1-specific ASC / million PBMC used in the assay. (b) The same individuals and time-points as shown in a, but AMA1-specific ASC responses are reported as % total IgG ASC measured in the assay. * P<0.05, ** P<0.01, Kruskal-Wallis test with Dunn’s correction for multiple comparisons.
Figure S7. mBC responses over time.
AMA1-specific mBC responses were measured as in Fig. 5 for all volunteers. Box and whisker plots show the median, range and interquartile range of AMA1-specific mBC derived ASC responses measured over time using the mBC ELISPOT assay. Responses are defined as (a) mBC derived ASC per million cultured PBMC specific for AMA1; or (b) as mBC derived ASC specific for AMA1 as a % of total IgG+ ASC measured in the assay. (c) Median diphtheria toxoid-specific responses are also shown for each group over time.