Abstract
The cell adhesion molecule Neuroligin2 (NL2) is localized selectively at GABAergic synapses, where it interacts with the scaffolding protein gephyrin in the postsynaptic density. However, the role of this interaction for formation and plasticity of GABAergic synapses is unclear. Here we demonstrate that endogenous NL2 undergoes proline-directed phosphorylation at its unique S714-P consensus site, leading to the recruitment of the peptidyl-prolyl cis-trans isomerase Pin1. This signaling cascade negatively regulates NL2’s ability to interact with gephyrin at GABAergic postsynaptic sites. As a consequence, enhanced accumulation of NL2, gephyrin and GABAA receptors was detected at GABAergic synapses in the hippocampus of Pin1 knockout mice (Pin1−/−) associated with an increase in amplitude of spontaneous GABAA-mediated postsynaptic currents. Our results suggest that Pin1-dependent signaling represents a mechanism to modulate GABAergic transmission by regulating NL2/gephyrin interaction.
Introduction
Structural and functional changes of post-synaptic density (PSD) components contribute to regulate synapse formation and plasticity. These remodeling events can affect trafficking, lateral mobility and turnover of several classes of structural and signaling molecules. They often involve interactions among specific proteins regulated by post-translational modifications, such as phosphorylation. At GABAergic synapses, the impact of phosphorylation on the gating properties, surface mobility and trafficking of the gamma-aminobutyric acid A receptors (GABAARs) has been extensively studied1,2. Much less is known about the effects of phosphorylation of other post-synaptic proteins functionally linked to GABAARs.
An important class of molecules involved in synapse formation, maturation and stabilization comprizes the cell adhesion molecules of the neuroligin (NLs) family3. These post-synaptic proteins functionally coordinate pre- and post-synaptic rearrangements by binding, via their extracellular domain, the pre-synaptically localized neurexins (NRXs) and via specific intracellular motifs, synapse-specific scaffolding molecules4-6. NL2 isoform is the only known adhesion molecule constitutively present at GABAergic PSDs7, where it drives the recruitment of inhibitory neurotransmitter receptors as well as the scaffolding molecule gephyrin6. Gephyrin, initially identified as a constituent of purified glycine receptor preparations (GlyR)9-10, was soon recognized a key player in α2 and γ2 subunit-containing GABAARs clustering11-12 and to be a central component of the GABAergic (and glycinergic) PSD8. Based on its auto-oligomerization properties, gephyrin builds a bidimensional lattice underneath the synaptic membrane, which exposes a high number of binding sites to accumulate GlyR and GABAARs in front of the presynaptic releasing sites13-17.
NL2 interacts with gephyrin through a conserved stretch of amino acid residues highly conserved among all family members6. Site-directed mutagenesis within this binding module identified a specific tyrosine residue (Y770A) whose alanine substitution impairs NL2 ability to recruit recombinant and endogenous gephyrin to postsynaptic sites6. Notably, the corresponding tyrosine residue on NL1, the isoform enriched at excitatory synapses, was found to be phosphorylated in vivo, preventing NL1- gephyrin interaction while favoring PSD95 recruitment at excitatory synapses18. Altogether, these findings point to the existence of intracellular signaling mechanisms able to modulate neuroligin-scaffolding protein interactions by modifying specifically neuroligin properties, leading to alteration in excitatory and inhibitory synaptic transmission.
In the present study, we have investigated whether post-phosphorylation prolyl-isomerization may affect GABAergic transmission in a similar manner. This signaling cascade targets serine and threonine residues preceding a proline residue to promote conformational changes on its substrate19. This effect is achieved by a unique enzyme, peptidyl-prolyl isomerase Pin1, whose catalytic activity facilitates the cis-trans isomerization of the peptide bond20,21. Notably, Pin1 was found to interact with gephyrin and to alter its overall conformation, thus enhancing its ability to bind the GlyR22.
Here, we provide evidence that endogenous NL2 can be phosphorylated at its unique Pin1 consensus motif thus rendering it able to physically recruit the phospho-specific effector Pin1. We show that post-phosphorylation prolyl-isomerization can regulate NL2’s ability to complex with gephyrin. Specifically, Pin1-mediated propyl-isomerization of phosphorylated serine 714 negatively modulates NL2-gephyrin complex formation, down-regulating GABAergic synaptic transmission.
Results
Endogenous NL2 undergoes proline-directed phosphorylation
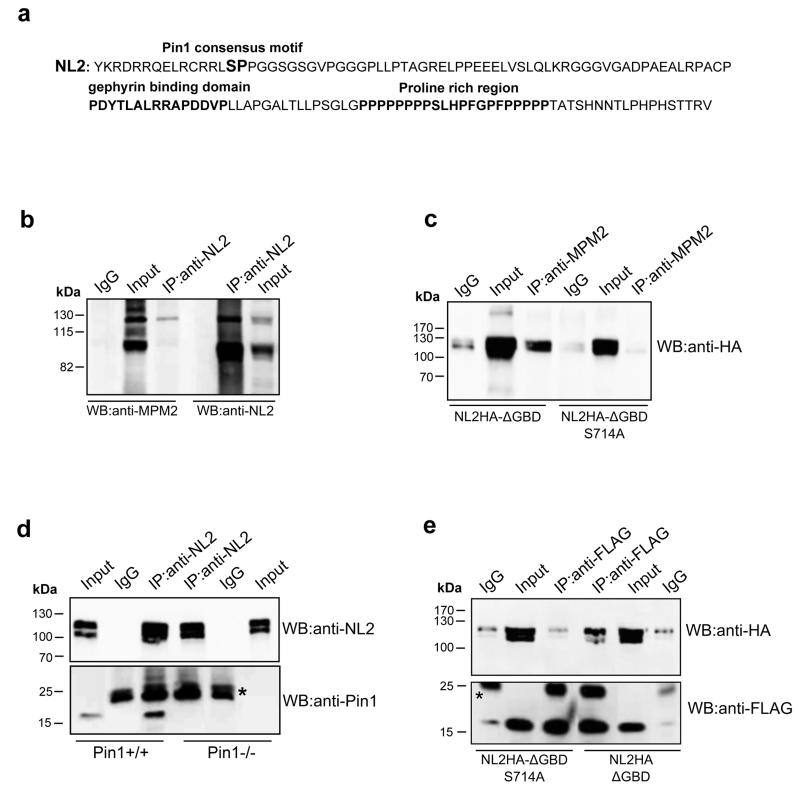
The cytoplasmic domain of NL2 possesses a unique consensus motif for proline-directed phosphorylation, S714-P, located 15 amino acids apart from the transmembrane domain (Fig. 1a). To assess whether this site can undergo phosphorylation in vivo we used the mitotic phosphoprotein monoclonal 2 (MPM-2) antibody that specifically recognizes phosphorylated S/T-P motifs23(Davis et al., 1983). Endogenous NL2 was therefore immunoprecipitated from mouse brain homogenates using an affinity-purified polyclonal antibody raised against its cytoplasmic domain or normal mouse IgG as negative control. Western blotting using the MPM-2 antibody revealed a band at around 120 kDa that corresponds to the upper band of the doublet recognized by the NL2 antibody in parallel immunoprecipitation experiments (Fig. 1b), suggesting that at least a fraction of NL2 can be phosphorylated at its unique Pin1 consensus motif. To demonstrate that phosphorylation at serine 714 is the event responsible for NL2 detection by the MPM2 antibody, we generated the phospho-defective point mutant NL2HAS714A. This mutation was introduced into a NL2HA hampered in gephyrin binding (NL2HAS714A-Δgephyrin binding domain, GBD) (see Supplementary Fig.1), to exclude the possibility that the MPM2 antibody would immune-react with phosphorylated Pin1 consensus motifs on endogenous gephyrin, which is, at the same time, a Pin1 target22 and an interacting partner of NL26. Under these conditions the MPM2 antibody efficiently immunoprecipitated only NL2HA-ΔGBD but not the corresponding point mutant, as indicated by the anti-HA immunoblot (Fig. 1c), thus demonstrating that S714 can be found phosphorylated on NL2.
Figure 1. NL2 is a proline-directed substrate.
(a) Amino acid sequence of the NL2 cytoplasmic domain. In bold is marked the unique Pin1 consensus motif (S714-P). The gephyrin-binding domain and the proline-rich region are highlighted in bold. (b) Representative immunoblotting of endogenous NL2 immunoprecipitated (IP) from mouse brain and probed with the anti-MPM2 that specifically recognizes phosphorylated S/T-P motifs and anti-NL2. Rabbit IgGs were used as negative control (IgG) (n=4). (c) Representative immunoblotting of overexpressed NL2HA lacking the gephyrin binding domain (NL2HA-ΔGBD) and the corresponding point mutant (NL2HA-ΔGBDSer714Ala) immunoprecipitated by the phospho-specific MPM2 antibody. Western blot analysis was carried out with anti-HA monoclonal antibody. Mouse IgGs were used as negative control (n=5). (d) Co-immunoprecipitation (CoIP) of endogenous NL2 and Pin1 from DSP cross-linked brain homogenates of Pin1+/+ or Pin1−/− mice. Western blots were performed with anti-NL2 polyclonal and anti-Pin1 monoclonal antibodies. Mouse IgGs were used as negative control. Asterisk indicate the IgG light chains (n=6). (e) FLAG epitopes from cross-linked samples of HEK293 cells co-expressing Pin1-FLAG and NL2HA-ΔGBD or NL2HA-ΔGBDS714 were immunoprecipitated by anti-FLAG antibody. Western blot was performed with anti-HA and anti-FLAG monoclonal antibodies. Mouse IgGs were used as negative control (n=4). Full images of western blots are in Supplementary Fig.5.
The essential feature of proline-directed phosphorylation as a signaling mechanism relies on the ability of phosphorylated S/T-P motifs to recruit the prolyl-isomerase Pin124,25. To test whether this unique phospho-epitope is able to recruit the effector molecule of the signaling cascade we performed co-immunoprecipitation experiments from Pin1+/+ and Pin1−/− brain lysates. This approach unveiled that Pin1 can be detected in NL2, but not in control, immunoprecipitates or in the absence of Pin1 expression (Fig. 1d). To exclude the possibility that Pin1 co-precipitated by NL2 is bound to endogenous gephyrin, these assays were performed upon co-expression of NL2HA-ΔGBD and Pin1-FLAG in HEK293 cells. Cell lysates were immunoprecipitated with the anti-FLAG antibody and bound protein complexes analyzed by Western blotting using anti-HA and anti-FLAG antibodies for NL2 and Pin1 detection, respectively. As shown in Figure 1e, while NL2HA-ΔGBD was still able to be immunoprecipitated from cells expressing Pin1-FLAG, S714 to alanine mutagenesis completely abolished such interaction, indicating that S714 represents a newly identified Pin1 target.
Pin1 modulates gephyrin-NL2 interaction
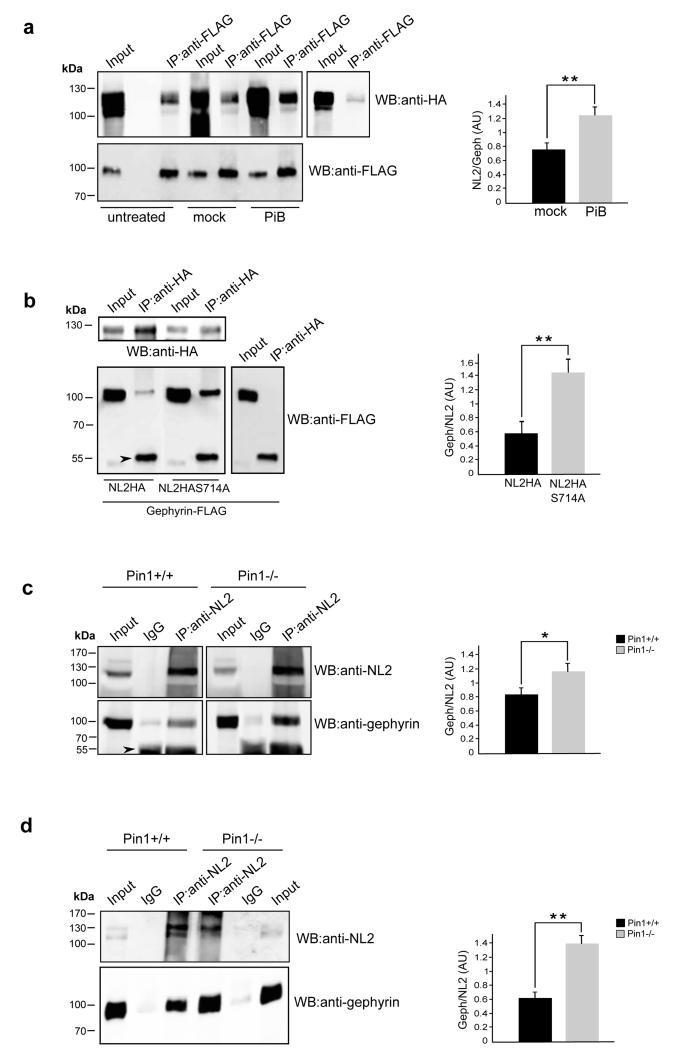
The observation that two fundamental components of the GABAergic PSD are both targets of proline-directed phosphorylation prompted us to investigate whether such signaling cascade would modulate their interaction. To this end, we initially co-expressed gephyrin-FLAG and NL2-HA in HEK 293 cells and examined the amount of NL2HA that complex with gephyrin-FLAG at 48 hours after treating the cells with the selective and reversible inhibitor of Pin1 isomerase activity PiB (IC50 of approximately 1.5μM)26. As shown in Figure 2a, even though the anti-FLAG antibody immunoprecipitated comparable amounts of gephyrin-FLAG, a significant increase (64%) in the amount of co-precipitated NL2HA was observed upon PiB treatment as compared to mock-treated cells (DMSO). Interestingly a marked increase (140%) was detected upon gephyrin-FLAG co-precipitation by NL2HA-S714A as compared to NL2HA, indicating that Pin1 exerts a negative control on NL2-gephyrin complex formation, at least in part, through NL2 prolyl-isomerization (Fig. 2b) .
Figure 2. Pin1 negatively modulates NL2/gephyrin interaction.
(a) Representative IP of FLAG epitopes from samples of HEK293 cells co-expressing gephyrin-FLAG and NL2HA and treated for 48h with PiB 2.5μM, DMSO (mock) or untreated. IP was also performed on NL2HA single transfected cells as a negative control. Nitrocellulose membranes were probed with anti-HA and anti-FLAG antibodies. The histogram on the right shows the relative amount of NL2 coprecipitated by gephyrin-FLAG in control and PiB treated cells obtained from densitometric analysis (n=5, mean values ± s.d., **P < 0.001, Student’s t-test). (b) Lysates of HEK cells transfected with gephyrin-FLAG in the presence of NL2HA or NL2HAS714A or with gephyrin alone (as a negative control) were immunoprecipitated with anti-HA agarose. Immunoprecipitates were analysed by Western blotting using anti-FLAG and anti-HA monoclonal antibodies. Arrowhead indicates the IgG heavy chains. The histogram on the right shows the relative amount of gephyrin-FLAG in complex with either NL2HA or NL2HAS714A coprecipitated by anti-HA agarose obtained from densitometric analysis (n=5, mean values ± s.d., **P < 0.001, Student’s t-test ). (c) CoIP of endogenous NL2/Pin1 complexes from DSP cross-linked brain homogenates of Pin1+/+ or Pin1−/− mice. Western blots were performed with anti-NL2 polyclonal and anti-Pin1 monoclonal antibodies. Rabbit IgGs were used as negative control. An increased amount of gephyrin co-precipitates in complex with NL2 in the absence of Pin1 expression. Arrowhead indicates the IgG heavy chains. The histogram on the right shows the relative amount (obtained from densitometric analysis) of endogenous gephyrin coprecipitated by endogenous NL2 from both mouse genotypes (n=8, mean values ± s.d., *P < 0.01, Student’s t-test ). (d) A similar experiment described in c was carries out on hippocampus isolated from of Pin1+/+ or Pin1−/− mice. The histogram on the right shows the relative amount (obtained from densitometric analysis) of endogenous gephyrin coprecipitated by endogenous NL2 from both mouse genotypes (n=4, mean values ± s.d., **P < 0.001, Student’s t-test ). AU arbitrary units. Full images of western blots are in Supplementary Fig.5.
This issue was then investigated using as a source of native NL2-gephyrin complexes mouse brain homogenates from both genotypes. For these experiments, endogenous NL2 was immunoprecipitated using a rabbit polyclonal anti-NL2 antibody and the co-precipitated gephyrin fraction was visualized by the monoclonal 3B11 antibody (Fig. 2c). In the absence of Pin1 expression the amount of gephyrin co-precipitated by NL2 was increased by 40% as compared to Pin1 expressing neurons. This approach was also applied on hippocampal tissues isolated from both mouse genotypes. Here, the enrichment of gephyrin co-precipitated by NL2 in the absence of Pin1 expression was even more dramatic as compared to the amount detected from whole brain (130% increase; Fig. 2d), suggesting a strong impact of such signaling pathway on GABAergic synapses of the hippocampus.
Characterization of gephyrin Pin1 sites S270-P and S319-P
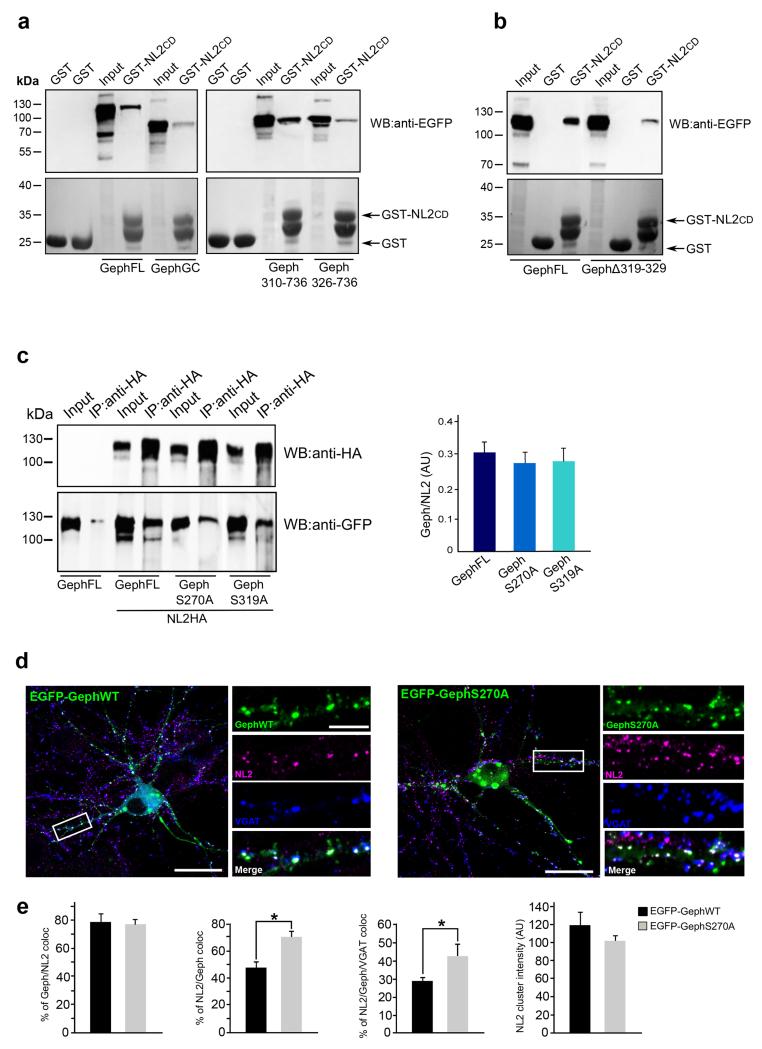
The scaffolding molecule gephyrin possesses ten putative Pin1 consensus motifs, the majority of them being concentrated in the central region (C-domain) 27. To determine whether specific Pin1 sites may contribute to enhance NL2/gephyrin complex formation we decided to focus on those located close to, or within, the NL2 binding site on gephyrin. A previous yeast two-hybrid screening identified a large portion of gephyrin encompassing the E-domain and part of the C-domain as the region involved in NL2 interaction6. We re-examine this issue by generating eGFP-tagged gephyrin truncated version to be tested in GST-NL2 cytoplasmic domain (CD) pull-down assays. HEK293 cells transfected with different eGFP-gephyrin variants were incubated with GST-NL2 CD loaded beads or with GST alone as negative controls. As shown in Figure 3a, while gephyrin 310-736 was recruited even better than the wild-type version, the mutants gephyrin 326-736 and gephyrin 1-310 (gephyrin-GC) displayed a reduced binding activity as compared to both gephyrin full length (FL) and the truncated version 310-736 (Fig. 3a). Since the two E-domain gephyrin versions, showing such a striking difference in the binding affinity, differ only for a short stretch of amino acids, we generated the deletion mutant removing, from the full-length protein, only the residues contained in this region but belonging to the E-domain itself (gephyrinΔ319-329) and assayed it for NL2 binding. Interestingly, the lack of this short sequence almost completely abolished the interaction of gephyrin with NL2 (Fig.3b), indicating that epitope(s) contained in the C-domain together with this minimal binding module are involved in gephyrin recruitment.
Figure 3. Impact of gephyrin S270A and S319A in NL2/gephyrin interaction.
(a) GST-NL2-CD pull-down from samples of HEK293 expressing EGFP-gephyrin full-length (FL), EGFP- gephyrin 310-736 (E-310), EGFP-gephyrin 326-736 (E-326) and EGFP-gephyrin GC. GST was used as negative control. Pulled down eGFP-gephyrin variants were detected using an anti-GFP monoclonal antibody. The bottom panels show the levels of GST and GST–NL2CD in the pull-down assays (Ponceau staining) (n=8). (b) EGFP-gephyrin Δ319 to 329 was tested in similar pull-down assays. Western blots in (a) and (b) were performed using anti-GFP antibody. Gephyrin requires amino acid sequence 319-329 for its efficient recruitment by NL2 (n=6). (c) Representative IP of HA epitopes from samples of HEK293 cells co-expressing NL2HA and EGFP-gephyrin wild type, EGFP-gephyrinS270A or EGFP-gephyrinS319A. Nitrocellulose membranes were probed with anti-HA and anti-GFP antibodies. EGFP-gephyrin single transfected cells incubated with HA-agarose were used as negative controls. The histogram on the right shows the relative amount of eGFP-gephyrinWT and point mutants coprecipitated by NL2HA (n=4, mean values ± s.d., P > 0.05). (d) Representative images of hippocampal neurons transfected with EGFP-gephyrin and EGFP-gephyrinS270A point mutant immunolabeled for endogenous NL2 (magenta) and VGAT (blue) at DIV10. Enlarged boxed areas are shown aside to the corresponding full view image. Post-synaptic clustering is demonstrated by apposition of gephyrin/NL2 clusters to VGAT positive terminals on the merge window. Scale bars: 20μm in full view images and 5μm in enlarged panels. (e) Distribution histograms of the % of gephyrin clusters colabeled with NL2 (79 ± 5% in EGFP-gephyrinWT vs 77 ± 4% in EGFP-gephyrinS270A), % of NL2 clusters colabeled with gephyrin (48 ± 5% in EGFP-gephyrinWT vs 71 ± 4% in EGFP-gephyrinS270A), % of NL2 synaptically localized (29 ± 2% in EGFP-gephyrinWT vs 43 ± 6% in EGFP-gephyrinS270A) and NL2 clusters intensity (119 ± 15 AU in EGFP-gephyrinWT vs 102AU ± 6 in EGFP-gephyrinS270A). The number of transfected hippocampal neurons investigated in each experiments (4 independent experiments) were as follow: n=15 for eGFP-gephyrinWT, n=10 for eGFP-gepyrinS270A (for each neurons at least 4 dendritic ROIs were measured, mean values ± s.d., *P < 0.01, Student’s t-test). AU: Arbitrary Units.
Based on these results two Pin1 consensus sites were further characterized, namely S319-P, located at the edge of the minimal binding module, and S270-P, positioned in its proximity, still contained, in the C-domain participating in NL2 binding. To this end we introduced point mutations in eGFP-gephyrin to create S319A and S270A mutants and tested them for their ability to interact with NL2HA. As judged by coimmunoprecipitation experiments, no significant differences were observed in binding capacity of the mutants as compared to gephyrin wild type (WT) (Fig. 3c). These constructs were also over-expressed in cultured hippocampal neurons to analyze and quantify their impact on endogenous NL2 distribution using immunofluorescence staining and confocal microscopy. As previously reported, neurons expressing the S270A mutants had an increased number, unchanged in size, of gephyrin clusters compared with eGFP-gephyrin WT28 (18.9 ± 1.7 per 20 μm dendritic segment vs 6.5 ± 0.6, P = 0.00015). The expression of the S319A construct produced a dramatic decrease in cluster density associated with a diffuse cytoplasmic staining. This latter effect seems to correlate with the intrinsic instability of the mutant protein that undergoes a high rate of degradation upon neuronal expression (data not shown), hampering its further characterization. Clusters formed by gephyrin S270A colocalized with NL2 at the same extent as the wild type protein (around 78%) (Fig. 3 d and e). The fraction of NL2 clusters co-localizing with S270A mutant as well as their synaptic localization were increased as compared to gephyrin WT but their intensity values (calculated by normalizing cluster fluorescence intensity to cluster area and expressed in Arbitrary Units: 119AU ± 15.2 vs 102 ± 6.3) were unchanged (Fig. 3e). These data indicate that the increase in NL2/gephyrin S270 interaction observed by immunoprecipitation is simply due to the augmented S270A cluster density and not to an enhance affinity of the mutant for NL2.
Pin1 selectively controls NL2 synaptic enrichment
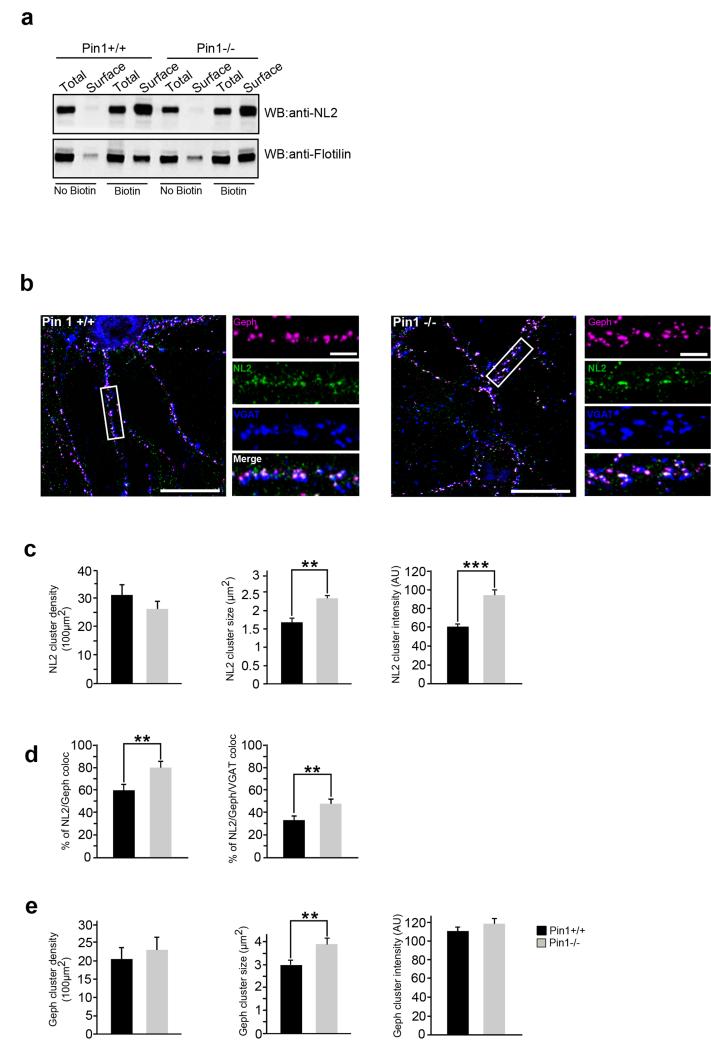
Pin1 has emerged as a negative regulator of gephyrin-NL2 interaction. Since these protein complexes are mainly localized at the plasma membrane, we tested whether Pin1 affects the amount of NL2 transported to, or maintained at, the neuronal plasma membrane. To this end, cultured hippocampal neurons derived from Pin1+/+ and Pin1−/− mice were subjected to surface biotinylation assay. Cell surface proteins were treated with the membrane-impermeant sulfo-NHS-biotin reagent, then isolated by binding to Streptavidin beads and probed with anti-NL2 antibody. To check for unspecific protein binding during surface biotinylation experiments, hippocampal neurons not labeled with biotin were processed with biotinylated samples. Western blot detecting the intracellular GPI–anchored protein Flotilin1 was included to ensure that similar amount of associated membrane proteins, biotinylated or not, where incubated with Streptavidin beads. No major differences on the total content of membrane localized NL2 was observed between Pin1+/+ and Pin1−/− (Fig. 4a).
Figure 4. Pin1 enhances NL2 synaptic content not its surface abundance.
(a) Surface NL2 derived from cultured hippocampal neurons of Pin1+/+ and Pin1−/− mice was isolated by biotinylation assay and detected by anti-NL2 antibody. No biotinylated neuronal cells were processed in parallel to evaluate unspecific NL2 binding. Western blot detecting GPI-anchored Flotilin was used as loading control (n=4). Full images of western blots are in Supplementary Fig.5. (b) Typical examples of hippocampal neurons from Pin1+/+ and Pin1−/− immunolabeled for endogenous gephyrin (magenta), NL2 (green) and VGAT (blue) at DIV10. Enlarged boxed areas are shown aside to the corresponding full view image. Post-synaptic clustering is demonstrated by apposition of gephyrin/NL2 clusters to VGAT positive terminals on the merge window. Scale bars: 20μm in full view images and 5μm in enlarged panels. (c) Distribution histograms of NL2 cluster density (normalized to 100μm2), the average cluster size and intensity in Pin1+/+ and Pin1−/− hippocampal neurons. (d) Distribution histograms of the percentage of NL2 colocalizing with gephryin and the percentage of double labeled NL2/gephyrin puncta overlapping with the pre-synaptic marker VGAT. (e) Distribution histograms of gephyrin cluster density (normalized to 100μm2), the average cluster size and intensity (calculated as described in c) in both mouse genotypes. The number of hippocampal neurons investigated in each experiments (3 independent experiments) were as follow: n=10 for Pin1+/+, n=12 for Pin1−/−. For each neurons at least 5 dendritic ROIs were measured, mean values ± s.d., **P < 0.001, ***P < 0.0001, Student’s t-test).
These results allow excluding the involvement of Pin1 in NL2 transport and/or turnover at the plasma membrane. Surface biotinylation represents an experimental approach that cannot provide an accurate analysis of protein distributions among different membrane domains. Since NL2 is enriched at GABAergic synapses, but is also distributed on extrasynaptic sites29, with this approach differences in NL2 partitioning between these two compartments might have been missed.
To this aim, immunocytochemical experiments were performed in dissociated Pin1+/+ and Pin1−/− hippocampal neurons co-labeled for NL2, gephyrin and VGAT, a specific marker of GABAergic innervations30 (Fig. 4b). In the absence of Pin1 expression a significant increase in NL2 cluster size (2.4 μm2 ± 0.2 vs 1.7 μm2 ± 0.2, P = 0.00044) and intensity (92 AU ± 4.0 vs 58 AU ± 2, p < 0.00048) was observed as compared to wild type neurons, while no major changes in NL2 cluster density were detected (Fig. 4c). The fraction of NL2 positive clusters co-localized with endogenous gephyrin puncta was also enhanced in Pin1−/− cells (80% ± 3.0% vs 60% ± 5%, P = 0.00013) and found enriched at postsynaptic sites, as demonstrated by the higher percentage of NL2/gephyrin co-stained puncta overlapping with the presynaptic marker VGAT (48% ± 4% vs 33% ± 4%, P = 0.0008; Fig. 4d). Gephyrin puncta appeared slightly, but significantly, increased in size while their density and intensity values were unchanged as compared to Pin1+/+ (Fig. 4e). These observations suggest that the absence of Pin1 promotes the formation and/or stabilization of NL2/gephyrin complexes at GABAergic post-synaptic sites.
NL2/gephyrin complex modulates synaptic abundance of GABAARs
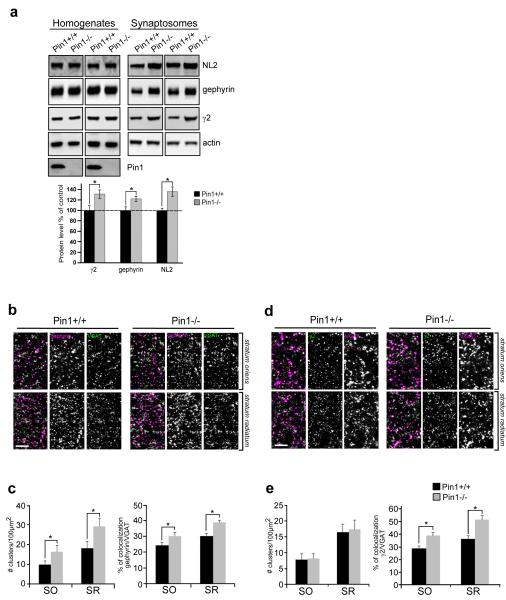
The recruitment of GABAARs at synaptic sites is functionally coupled to NLs expression levels as well as to the gephyrin scaffold6. To assess whether the enhanced NL2/gephyrin complex formation detected at GABAergic synapses similarly affects the distribution of synaptic γ2 subunit-containing GABAARs, we performed a quantitative evaluation of the γ2 subunit present in synaptosome suspensions isolated from the hippocampus of Pin1+/+ and Pin1−/− mice. Quantitative immunoblot analysis was also extended to NL2 and gephyrin to further verify their synaptic enrichment. As shown in Figure 5a, the amount of all three markers investigated was significant increased in Pin1−/− mice as compared to Pin1+/+. The synaptic enrichment (synaptic fraction vs homogenate) was 35 ± 5% for the NL2, 30 ± 6% for the γ2 subunit and 20 ± 4% for gephyrin.
Figure 5. Synaptic enrichment of GABAARs is achieved in Pin1−/−.
(a) Representative immunoblots of NL2, gephyrin and γ2 subunit of GABAA receptor extracted from the hippocampus of Pin1+/+ and Pin1−/− mice (littermates) in two different sets of experiments. Total proteins from the homogenates and synaptosome suspension fractions were analysed by western blotting. Below: quantification of the indicated antigens extracted from hippocampal tissues of Pin1+/+ and Pin1−/− mice. All markers analyzed are enriched at inhibitory synapses. Western blot to actin was done as loading control. Pin1immunoblot indicates hyppocampus from Pin1+/+ and Pin1−/− (n=6 littermate pairs, mean values ± s.d, *p < 0.05, Student’s t-test) Full images of western blots are in Supplementary Fig.5. (b) Representative confocal micrographs of frontal brain sections showing segments of the str. radiatum (SR) and str. oriens (SO) of the CA1 region of the hippocampus from adult Pin1+/+ and Pin1−/− mice immunolabeled for gephyrin (magenta) and VGAT (green). Scale bar: 5μm. (c) Quantification of gephyrin punctum density (normalized to 100 μm2) and their percentage of colocalization with the presynaptic marker VGAT in both mouse genotypes. (d). Confocal micrographs as in (a) immunolabeled for GABAA receptor γ2 subunit (green) and VGAT (magenta). (e) Quantification of γ2 subunit punctum and their percentage of colocalization with VGAT in both mouse genotypes. The number of gephyrin, γ2, gephyrin and VGAT puncta was assessed in at least 8 sections for each genotypes (Pin1+/+ and Pin1−/−), by taking at least 4 images of strata radiatum and oriens of the CA1 region of each hippocampus in each set of experiments (n=3). Mean values ± s.d., *P < 0.05, Student’s t-test. Scale bar: 5μm.
We also examined the number of puncta labeled for gephyrin and γ2 subunit-specific antibodies, as well as their levels of colocalization with the pre-synaptic marker VGAT, in the CA1 region of the hippocampus of both genotypes. The staining pattern of gephyrin in Pin1−/− demonstrated a slight increase in the number of clusters both in the stratum oriens (SO) and stratum radiatum (SR) as compared to Pin1+/+ (SO 16 ±3 clusters/100μm2 and SR 28 ± 3 clusters/100μm2 vs SO 10 ± 2 clusters/100μm2 and SR 19 ± 3 clusters/100μm2; P < 0.05; Fig. 5b and 5c). This increase was paralleled by a small increase (around 6-8%) in gephyrin puncta colocalized with pre-synaptic VGAT (SO 30 ± 2% and SR 39 ± 1.4 % vs SO 24 ± 2% and SR 31 ± 2%; P < 0.05; Fig. 5b and 5c). The average cluster size and intensity were similar in both genotypes (3.6 μm2 ± 0.2 vs 3.5 μm2 ± 0.3 and 61 ± 7 vs 65 ± 4 RFU for cluster size and intensity in Pin1−/− vs Pin1 +/+, respectively).
The γ2 subunit staining pattern exhibited a similar cluster density in the two strata analysed in both genotypes (SO 8 ± 2 and SR 18 ± 2 vs SO 8 ± 1 and SR 17 ± 1.2; P > 0.05; Fig.5d and 5e). A small, although significant, increase in their intensity was evident (120 ± 3 RFU vs 106 ± 2 RFU in Pin1−/− vs Pin1 +/+; P < 0.05) but they were similar in size (4.3μm2 ± 0.5 vs 3.7μm2 ± 0.5). VGAT colocalization was increased by 10-15% in tissue from knock-out animals (SO 38.9 ± 2.7 % and SR 52 ± 3 % vs SO 29 ± 2 %and SR 36 ± 3 %; P < 0.05; Fig. 5d and 5e). The changes in gephyrin and γ2 subunit synaptic fraction are not due to an increase in synapses numbers, the density of inhibitory terminals being unaltered between the two genotypes, as assessed by quantification of VGAT immunolabeling (SO 14 ± 2 % and SR 22 ± 3% vs SO 13 ± 2 % and SR 21 ± 3%; P > 0.05).
Altogether, these data indicate that the enhanced interaction between gephyrin and NL2 observed in the absence of Pin1 is associated with a concomitant increase in the synaptic recruitment of γ2 subunit-containing GABAARs.
Pin1 signaling affects the number of synaptic GABAARs
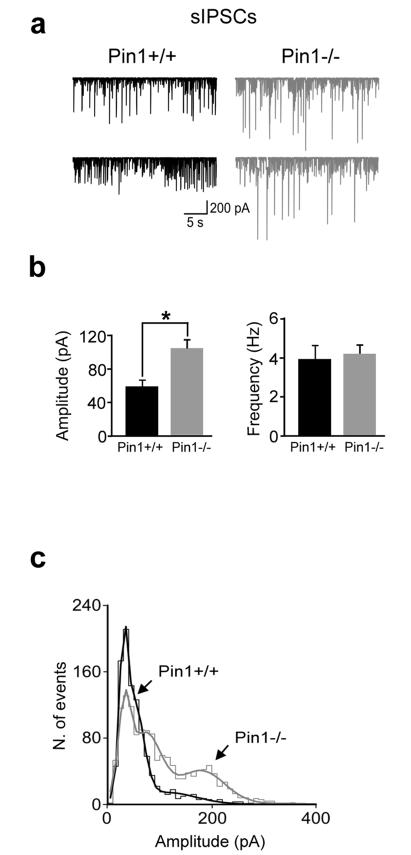
To functionally explore whether the enrichment of γ2 subunit-containing GABAARs in Pin1−/− mice affects GABAergic transmission, whole cell recordings in voltage clamp configuration were performed from CA1 principal cells in hippocampal slices obtained from Pin1+/+ and Pin1−/− mice at postnatal (P) day P10-P13. These neurons presented similar resting membrane potential (Vrest) and input resistance (Rin) values (data not shown) thus indicating that Pin1 does not affect the passive membrane properties of principal cells. Spontaneous GABAA-mediated inhibitory post synaptic currents (sIPSCs) were then recorded from both genotypes in the presence of DNQX (20 μM) to block AMPA-mediated excitatory postsynaptic currents (sEPSCs). As shown in Figure 6a, recordings from Pin1−/− mice exhibited sIPSCs of higher amplitude values compared to control littermates (106 ± 12 pA vs 62 ± 8 pA; P < 0.05), in the absence of any significant change in frequency (4.2 ± 0.5 Hz vs 3.6 ± 0.6 Hz; p>0.05; Fig. 6b). The amplitude distribution histogram of sIPSCs recorded in Pin1−/− unveiled a clear peak at ~ 200 pA (Fig. 6c). The observed effects were selective for sIPSCs since no significant differences in amplitude (22 ± 2 in Pin1−/− mice and pA 27 ± 4 pA in Pin1+/+; n=6 for both genotypes; P > 0.05) or frequency (1.7 ± 0.3 Hz in Pin1−/− mice and 1.3 ± 0.4 Hz in Pin1+/+ mice; P > 0.05) of sEPSCs (recorded in the presence of picrotoxin, PTX, 100 μM) were detected between the two genotypes (Supplementary Fig. 2a and b).
Figure 6. Pin1 affects the amplitude but not the frequency of sIPSCs.
(a) Representative traces of sIPSCs recorded from CA1 principal cells at P11 in hippocampal slices from Pin1+/+ (black) and Pin1−/− mice (gray). Note higher amplitude events in Pin1−/− mice. (b) Each column represents the mean frequency and amplitude values of sIPSCs recorded from Pin1+/+ (black, n= 9) and Pin−/− mice (gray, n= 8). *P < 0.05, Student’s t-test). (c) Amplitude distribution histograms of sIPSCs recorded in Pin1+/+ (1030 events; black) and in Pin1−/− mice (1412 events; gray). Note the appearance of a clear peak at ~200 pA in Pin1−/− mice.
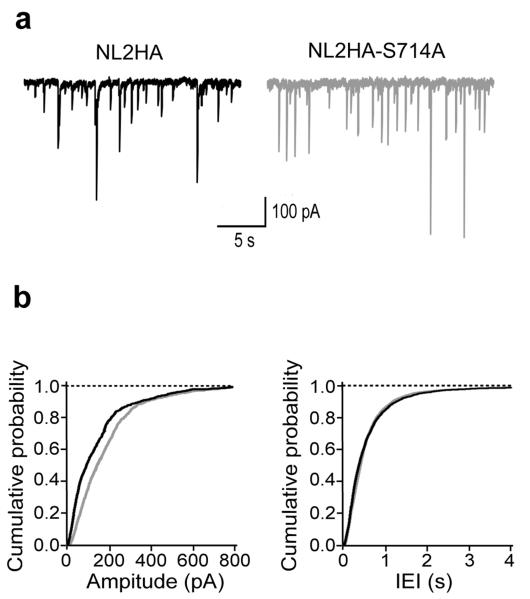
Spontaneous inhibitory events from hippocampal neurons in culture overexpressing the NL2HA-S714A mutation exhibited, compared to NL2HA transfected cells, a significant increase in amplitude (but not frequency), which in part mimicked the phenotype observe in Pin1−/− mice, suggesting that the interaction of Pin1 with NL2 is critical for this effect (Fig. 7a). As shown in the cumulative amplitude plot (Fig. 7b), the curve obtained from NL2HA-S714A transfected cells was shifted to the right as compared to cells expressing NL2HA (P < 0.05).
Figure 7. Changes in amplitude of sIPSCs involve the interaction of Pin1 with NL2.
(a) Samples traces of sIPSCs recorded from hippocampal neurons in culture expressing either the NL2HA or the NL2HA-S714A mutation. (b) Amplitude and inter-event interval (IEI) plots of sIPSCs recorded in cells transfected either with the NL2HA (black; n=7) or the NL2HA-S714A point mutant (gray; n=12). P < 0.05; Kolmogorov-Smirnov test. Note the shift to the right of the cumulative amplitude distribution curve obtained from cells transfected with the mutant as compared to controls
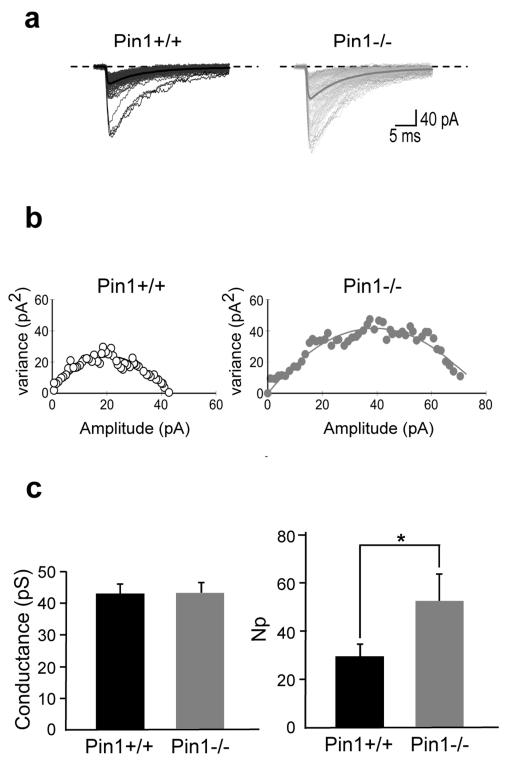
The selective increase in amplitude of sIPSCs detected in Pin1−/− mice suggest a postsynaptic site of action. This may involve an increase in the number of active GABAARs or changes in single receptor channel conductance. To distinguish between these two possibilities, peak scaled non-stationary fluctuations analysis of sIPSCs was performed only on stable recordings with no time-dependent changes in either peak amplitude, 10-90% rise time and decay time (Fig. 8a) (electrotonic filtering was excluded on the basis of no correlation between 10-90% rise time and decay time31). Plotting the mean current amplitude versus variance and fitting individual points with the parabolic equation (equation 2 in the methods; Fig. 8b), allowed estimating single channel conductance and the number of channels open at the peak of spontaneous IPSCs. The single channel conductance was calculated according to equation 3, assuming a reversal potential for chloride equal to 0. Interestingly, while the values of single channel conductance were similar in both genotypes (Fig. 8c) the average number of active channels open at the peak of sIPSCs (Np) was significantly increased in Pin1−/− mice compared to controls (53 ± 11 vs 26 ± 5; P = 0.03; Fig. 8c).
Figure 8. Pin1 controls the number of active receptor channels at GABAergic synapses.
(a) Individual sIPSCs from Pin1+/+ (black) and Pin1−/− mice (gray) are shown with the average currents (thick lines). (b) Current/variance relationships for sIPSCs shown in (a.) (c) Summary plots of weighted mean channel conductance (43 ± 3 pS and 43 ± 3 pS, P = 0.9, Student’s t-test) and number of GABAA receptor channels (Np) in wt (black; n =8) and in Pin1−/− mice (gray; n =5). *P = 0.03, Student’s t-test.
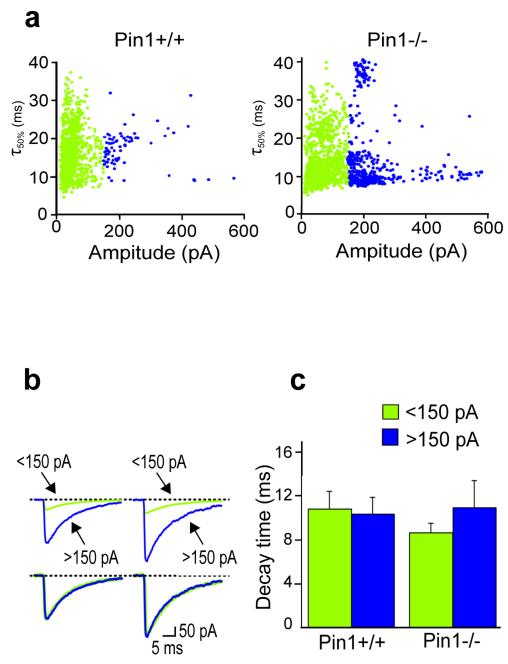
To further evaluate the possibility that higher amplitude inhibitory events recorded in Pin1−/− mice may originate from GABAARs containing different subunits we measured in both genotypes the decay time constants of small and large amplitude events. Spontaneous IPSCs were plotted against their decay half widths and arbitrarily divided in two main classes whose amplitude was < or >150 pA (Fig. 9a, in green and blue, respectively). Notably, larger amplitude events (>150 pA) prevailed in Pin1−/− mice. No differences in decay of sIPSCs < or > 150 pA were observed between Pin1+/+ and Pin1−/−mice, thus excluding the involvement of multiple receptor subtypes with different kinetics (the 90-10% decay (τ) of sIPSCs <150 pA was 9 ± 1 ms in Pin−/− mice and 11 ± 2 ms in Pin1+/+; P > 0.05; τ of sIPSCs >150 pA was 11 ± 2 ms in Pin-/- mice and 10 ± 2 ms in Pin1+/+; P > 0.05. The 90-10 % decay time (τ90-10%) of all sIPSCs was 11 ± 2 ms and 10 ± 2 ms in Pin1+/+ and Pin1−/− mice, respectively; Fig 9 b and c, P > 0.05). These data altogether suggest that the observed increase in amplitude of sIPSCs in Pin1−/− mice is exclusively due a genuine increase in number of GABAARs composed of the same subunits.
Figure 9. Pin1 does not affect the decay kinetics of spontaneous IPSCs.
(a) The peak amplitude of individual sIPSCs <150 pA (green) and >150 pA (blue) is plotted against their decay half-widths (τ50%) in Pin1+/+ and in Pin1−/− mice. (b) In the upper part, average traces of spontaneous IPSCs shown in a. In the lower part, average traces are normalized and superimposed. (c) Each column represents the mean 90-10% decay time constant of spontaneous IPSCs in Pin1+/+ and Pin1−/− mice, <150 pA (green) n=8 and 7 respectively and >150 pA (blue) n=6 and 7 respectively. For all comparisons P > 0.05, Student’s t-test.
GABA release and tonic inhibition are unaltered in Pin1−/−
In a previous study we demonstrated that the functional knock-down of NL2 was accompanied by a reduction in the probability of GABA release32, thus underlying the role of NLs as retrograde regulators of presynaptic function. Therefore, we evaluated here whether Pin1-dependent modulation of NL2-gephyrin interaction could also affect GABA release from presynaptic nerve terminals. To this end, we used 1,2,5,6-tetrahydropyridin-4-yl methylphosphinic acid (TPMPA), a low affinity competitive GABAAR antagonist33. This approach allowed to compare differences in presynaptic GABA transients between Pin1+/+ and Pin1−/− mice. Similar reduction of sIPSCs amplitude in both genotypes (51 ± 6 % vs 54 ± 8 %, P > 0.05, Supplementary Fig. 3a and b) was detected upon bath application of TPMPA (200 μM), thus excluding a trans-synaptic action of Pin1 on GABA release.
Part of GABA released during synaptic activity may escape the cleft and invade the extracellular space to activate extrasynaptic high affinity GABAARs. This feature generates a persistent GABAA-mediated conductance34 that is involved in a number of physiological processes35. To determine whether Pin1 signaling affects extrasynaptic GABAARs, we analyzed the tonic GABAA-mediated conductance in both Pin1+/+ and Pin1−/−mice. The tonic conductance was assessed by the shift of the holding current induced by application of the GABAAR channel blocker PTX (100 μM) (Supplementary Fig. 4a). This drug caused a similar shift in holding current in Pin1−/− and Pin1+/+ mice (Supplementary Fig. 4b and c), indicating that extrasynaptic GABAA receptors are not influenced by Pin1-mediated signaling.
Discussion
The present study shows that NL2 is a newly identified substrate of proline-directed phosphorylation. This post-translational modification, acting on its unique Pin1 consensus motif localized within the cytoplasmic domain (S714-P), modulates the amount of NL2-gephyrin complexes at synaptic sites. This modulation impacts on GABAergic transmission, by selectively affecting the total number of synaptic GABAARs. Based on these findings, post-phosphorylation prolyl-isomerization can play a crucial role in remodeling the GABAergic PSD to sustain plasticity processes.
Protein phosphorylation on serine and threonine residues preceding a proline, the so-called proline-directed phosphorylation, has emerged as a mechanism regulating signaling events through conformational changes that are catalyzed by the phospho-dependent recruitment of the peptidyl-prolyl isomerase Pin1. While the different roles of Pin1 in dividing cells have long been established and characterized19, its function in post-mitotic neurons in general and at synapses in particular is still poorly understood. In a previous study we identified gephyrin, the main scaffolding protein of inhibitory PSD, as a new target of post-phosphorylation prolyl-isomerization22.
Here, by inspecting the protein sequence of NL cytoplasmic domains, we identified S/T-P motifs that may provide Pin1 binding sites if phosphorylated in vivo. In particular, NL2 presents a unique Pin1 consensus site in its cytoplasmic region, S714-P, which is located 15 amino acid apart from the trans-membrane domain. Even though this proximity to the plasma membrane raises doubts about its accessibility by a proline-directed kinase, several lines of evidence suggest that endogenous NL2 can undergo proline-directed phosphorylation. First, this isoform was recognized by the MPM2 antibody upon NL2 immunoprecipitation from mouse brain homogenates. Second, MPM2-mediated NL2 immunoprecipitation was still maintained upon removal of the NL2 gephyrin binding domain, excluding the possibility of an indirect recognition mediated by endogenous gephyrin. Third, such detection was completely lost upon NL2HA-S714A mutagenesis. This phosphorylation event is then able to directly recruit the effector molecule of the signaling cascade Pin1, as shown by co-immunoprecipitation experiments with endogenous neuronal proteins. Also in this case, Pin1 binding to NL2 was still maintained upon removal of the GBD while it was completely abolished by mutating S714 to alanine, thus suggesting that the prolyl-isomerase can be directly recruited by the unique NL2 Pin1 consensus motif in a phosphorylation-dependent manner. These results altogether indicate that NL2 represents a newly identified substrate for proline-directed signaling cascade in vivo.
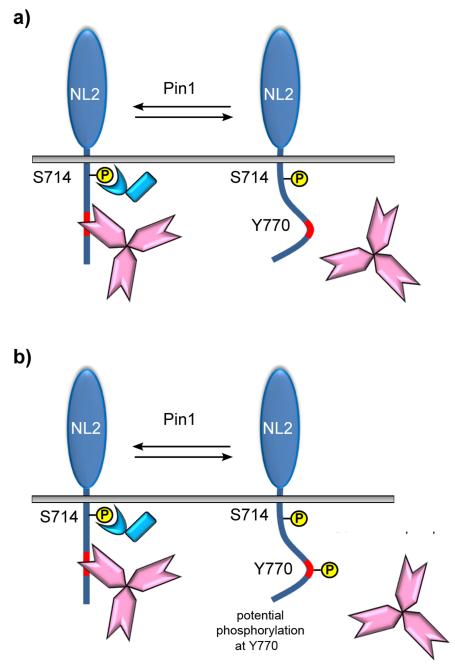
Our biochemical data demonstrate that NL2-gephyrin interaction is negatively regulated by proline-directed phosphorylation. Co-immunoprecipitation experiments on recombinantly expressed gephyrin-FLAG and NL2HA unveiled an enhanced complex formation upon pharmacological inhibition of Pin1 catalytic activity. Similarly, endogenous NL2/gephyrin complexes pulled-down from whole brain or hippocampal tissues of Pin1−/− animals were significantly augmented as compared to the corresponding wild type tissues. These biochemical findings were also validated by immunocytochemistry performed on cultured hippocampal neurons, where we could detect a high number of clusters co-labeled for NL2 and gephryin as well as their increased apposition to presynaptic GABAergic inputs in the absence of Pin1 expression. Interestingly, the NL2 point mutant unable to undergo prolyl-isomerization was capable to recruit gephyrin even more efficiently as compared to the wild type form, whereas gephyrin mutagenesis at two putative Pin1 consensus motifs, S270A and S319A, located within, or close to, the minimal NL2 binding domain, was completely ineffective. The fact that this post-translational modification seems to control the strength of NL2 association with gephyrin by acting mainly on NL2, and not vice versa, further reinforces the emerging idea that cell adhesion molecules are key determinant in regulating synapse function. In a recent study by Giannone and colleagues18, it has been demonstrated that the level of NL1 phosphorylation at a specific tyrosine residue located within the GBD dictates the strength of NL1/gephyrin interaction. In other words, NL1, the isoform enriched at excitatory synapses and therefore mostly associated with PSD95, can potentially recruit gephyrin as well as NL2, but its phosphorylation, promoted by neurexin-adhesion signaling, precludes such interaction while favoring PSD95 binding. Our experimental data indicate that proline-directed phosphorylation is acting similarly to tyrosine phosphorylation signaling. Since NL2 S714 is not positioned within the GBD, but is located just 50 amino acid upstream, it is reasonable to believe that Pin1-driven conformational changes, by affecting the overall folding of the cytoplasmic domain, will induce gephyrin release (Fig. 10 panel a). Alternatively, these conformational changes may promote NL2 tyrosine phosphorylation, an event shown to impede NLs/gephyrin interaction18 (Fig. 10 panel b). Interestingly, tyrosine to alanine mutagenesis on NL2 was shown to completely abolish recombinant gephyrin recruitment by the mutant protein or to strongly reduce its interaction with endogenous gephyrin6. Whether NL2 phosphorylation occurs at tyrosine 770 and whether this event is able to hamper gephyrin binding is still unknown.
Figure 10. Model of the putative cross-talk between proline-directed phosphorylation and tyrosine-phosphorylation.
Phosphorylation of NL2 cytoplasmic domain at S714 by a proline-directed kinase allows the recruitment of the proly-isomerase Pin1. Pin1-driven conformational changes, by altering the folding of the NL2 cytoplasmic domain, may represent the main cause responsible for gephyrin detachment (a). Alternatively, Pin1-mediated structural rearrangement may render the conserved tyrosine residue of the GBD (Y770) susceptible to phosphorylation, an event shown to prevent NL1/gephyrin interaction (b).
The other partner of the complex is represented by gephyrin, a recognized target of Pin122. Gephyrin contains ten consensus motifs mostly concentrated in its C-domain, and all of them found to be phosphorylated in vivo 36,37. This region of the protein is positioned between the N-terminal G- and C-terminal E-domains, which are directly involved in gephyrin multimerization. Conformational changes induced by phosphorylation, possibly followed by prolyl-isomerization, are expected to alter the conformation of the gephyrin C-domain and in turn, regulate specific functional properties of gephyrin, in particular its binding to interacting proteins, including possibly NL2. However, the complexity of the system under investigation makes it very difficult to determine whether and how a specific phosphorylation event can contribute, directly or indirectly, to enhance gephyrin association to NL2. Nevertheless it should be emphasized that gephyrin is robustly phosphorylated at several residues in vivo, thus suggesting that a specific pattern of phosphorylation, rather than a single post-translational modification, is functionally determinant. By contrast, NL2 possesses a unique target for prolyl-isomerization suggesting that it could represent the master switch of the signaling cascade.
Our electrophysiological experiments clearly demonstrate that deletion of Pin1 specifically affects GABAergic transmission, causing a dramatic increase in amplitude, but not in frequency, of sIPSCs due to an increase in the number of GABAARs at post-synaptic sites. Notably, such enhancement was detected upon neuronal over-expression of the NL2 mutant unable to undergo prolyl-isomerization, suggesting a functional link between the signaling cascade strengthening NL2/gephyrin interaction and the increased synaptic recruitment of GABAARs. There is a large body of evidence underlying the key role played by NL2 in promoting clustering and/or stabilization of GABAARs at post-synaptic sites. By employing a heterologous expression system, it was shown that GABAARs are able to co-aggregate with NL2 and only the presence of this isoform can induce strong GABAergic presynaptic differentiation from co-cultured neurons and promote the establishment of fully functional hemi-synapses38. In NL2 deficient mice, the number of functional GABAARs detected in the retina was shown to be drastically reduced39. Furthermore, targeting of GABAARs and gephyrin scaffold appeared severely compromised in the pyramidal cell layer of the CA1 region of the hippocampus, a morphological phenotype accompanied by a strong deficit in synaptic inhibition6.
The increased recruitment of synaptic GABAA receptors in Pin1−/− mice may simply depend on the enhanced gephyrin targeting at synaptic sites. More scaffold deposition should offer a high number of binding sites available for the transient immobilization of GABAARs at inhibitory synapses. In addition, or alternatively, we cannot exclude the possibility that the extracellular domain of NL2 could also participate in GABAARs receptor recruitment. The unique S714-P consensus motif, located very close to the NL2 transmembrane domain, could influence the folding of the extracellular domain of NL2, rendering it incapable to interact in cis with GABAAR subunits. This type of mechanism has been shown to operate at excitatory synapses, where the abundance of NMDARs is controlled by the interaction occurring between the GluN1 subunit with NL1-specific sequences located in its extracellular domain40.
In conclusion, our findings unveil the existence of a new signaling pathway operating at GABAergic synapses to alter the efficacy of GABAergic transmission by modulating NL2/gephyrin interaction. Although a comprehensive understanding of the molecular mechanisms underlying the action of Pin1 on NL2/gephyrin interaction is still lacking, we believe that our study further emphasizes the key role played by NL2 in organizing and stabilizing GABAergic synapses.
Methods
Plasmid constructs
The expression construct for HA-tagged human NL2 in pNice was kindly provided by P. Scheiffele (Biozentrum, Basel). The amino acid sequence ranging from residues 768 to 782 was removed to generate the NL2HA lacking the gephyrin binding domain (pNice-NL2HA-ΔGBD). S714A mutation was also introduced into pNice-NL2HA-ΔGBD to remove the unique Pin1 consensus site (pNice-NL2HA-ΔGBDS714A). All PCR-based mutagenesis were fully sequenced to exclude the possibility of second site mutations. pcDNA3-FLAG-Pin1 wild type and pcDNA3-gephyrin-FLAG have been previously described22. EGFP-tagged gephyrin point mutants (S270A and S319A), the wild type and the truncated version ranging from amino acid 326-736 and 310-736, were PCR-cloned into the XhoI/HindIII sites of pEFP-C1 (Clontech, Mountain View, CA). EGFP-tagged gephyrin GC (1-310) was kindly provided by G. Schwarz (University of Cologne, Germany)41.
Cell cultures and transfections
HEK-293-T cells were cultured at 37°C under a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). They were transiently transfected with various plasmid constructs using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Cells were collected 24–48 h after transfection.
Primary hippocampal neurons from P0 Pin1+/+ and Pin1−/− littermates and rat hippocampal neurons were prepared as previously described42. Being Pin1−/− mice infertile, Pin1+/+ and Pin1−/− littermates for neuronal cultures were routinely obtained by mating heterozygous mice43. Each hippocampus derived from single newborn littermate was processed and plated separately and identified by tail genotyping. Neurons were Lipofectamine transfected after 8 DIV with 1μg of EGFP-gephyrinWT or EGFP-gephyrinS270A and processed for immunofluorescence 2-3 days later. For electrophysiological recordings neurons were co-transfected with 1μg NL2HA/NL2HAS714A and 500 ng of GFP to visualize transfected cells.
PiB treatments
To inhibit Pin1 catalytic activity the chemical inhibitor PiB (diethyl-1,3,6,8-tetrahydro-1,3,6,8-tetraoxobenzol-phenanthroline-2,7-diacetate) was added to the culture medium for 24 hours at a concentration of 2.5μm. PiB was purchased from Calbiochem and resuspended in DMSO.
Immunoprecipitation and chemical cross-linking
Immunoprecipitation for MPM2 experiments was performed using a lysis buffer containing 50 mM Tris–HCl, pH 7.5, 1% Nonidet P-40, 0.5% Triton X-100, 150 mM NaCl, 1 mM Na3VO4, 50 mM NaF and protease inhibitor mixture (Sigma). For NL2HA and gephyrin co-immunoprecipitation, HEK 293 cells overexpressing NL2HA and gephyrin-FLAG were treated 48 h after transfection with 2.5mM PiB or mock treated with DMSO as negative controls. Cells were lysed in 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.1% Tween 20, 10% glycerol, 10 mM EDTA, 2 mM MgCl2, and protease inhibitor mixture and immunoprecipitated by either the anti-FLAG antibody or anti-HA agarose (Pierce).
Coimmunoprecipitation of native gephyrin-NL2 complexes from p15 Pin1+/+ and Pin1−/− mouse brains or hippocampal tissues was performed using a chemical crosslinking approach on postnuclear homogenates as previously described6. Primary antibodies were revealed by HRP-conjugated secondary antibodies (Sigma) followed by ECL (Amersham Biosciences).
Biotinylation assay and analysis on synaptosomes
To examine changes in NL2 transported at the plasma membrane, we performed biotinylation assays on hippocampal neuronal cultures derived from Pin1+/+ and Pin1−/− mice. Neuronal cells were incubated with 0.5 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce) in PBS at 4°C for 30 min. To quench the reaction, cells were washed three times with cold PBS containing 0.1 M Tris-HCl pH 7.4. Cells were then lysed in lysis buffer containing protease inhibitor cocktail followed by centrifugation at 1000 g for 5 min. The collected lysate were incubated with streptavidin cross linked to agarose beads (Pierce) for 2 h at 4°C. The beads were then washed twice with lysis buffer, and eluted with SDS loading buffer. The amount of membrane protein loaded in each experiments was normalized to the amount of the GPI–anchored protein Flotilin1, whose expression levels are identical in both mouse genotypes.
PSD enriched extracts were prepared by using the Syn-PER Synaptic Protein Extraction Reagent (ThermoScientific) following the manufacturer’s instructions. Briefly, a pool of 4 hippocampi derived from the same genotypes were homogenized in the Extraction reagents (10ml of reagent/gram of tissue), centrifuged at 1.200g for 10 minutes. The pellet was discarded while the supernatant (homogenate) was additionally centrifuged at 15.000g for 20 minutes. The cytosolic fraction was discarded and the pellet containing the synaptosomes was resuspended in 400-500μl of reagent and analysed by western blot analysis. The protein concentration of each sample was determined using the Pierce BCA Protein Assay to allow an equal loading of total protein.
Western Blot analysis
Western blot image acquisition was performed using the ECL detection kit and the Alliance 4.7 software (UVITECH, Cambridge). Quantifications were performed using the UVIband imager software (Amersham). The relative amount (Input, 1/20 of the total lysate) of the different antigens considered in this study and the immunoprecipitated fractions were determined by densitometry on the acquired images. The amount of immunoprecipitated and coimmunoprecipitated proteins are first normalized to their corresponding inputs and then the coimmunoprecipated value is additionally normalized on the immunoprecipitated antigen. Full images of western blots are in Supplementary Fig.5.
Antibodies
The following antibodies were used in immunohistochemistry and immunocitochemistry: anti-gephyrin Mab7a (Synaptic System Cat. No 147021), anti-VGAT rabbit or guinea pig (1:1000, Synaptic System Cat. No 131004), anti-NL2 rabbit affinity purified (1:500, Synaptic system Cat, No 129203), guinea pig anti-GABAA γ2 subunit (1:200044), biotinylated anti-guinea pig (1:200, Vector Laboratories, Cat No BA-7000). The following primary antibodies were used in immunoprecipitation and western blot analysis: mouse monoclonal anti-FLAG M2 (Sigma Cat No F1804), mouse monoclonal anti-gephyrin 3B11 (Synaptic System Cat No 147111) and rabbit polyclonal anti-NL2 (Synaptic Systems Cat. No 129202), pS/pT-P (MPM-2, Upstate Biotechnology Cat No 05-368), high affinity rat monoclonal anti-HA 3F10 (Roche), anti-GFP rabbit monoclonal (Life Technology, Cat No G10362). Validation of antibodies used in these assays can be found on the respective manufacturers’ websites.
Immunohistochemistry and immunocytochemistry
Eight-week-old Pin1+/+ and Pin1−/− littermates (for each genotype, n=3) were anesthetized and perfused transcardially with 0.1 M phosphate buffer, pH 7.4 (PB). Brains were quickly remove from the skull and frozen with isopentane cooled to −40°C with liquid nitrogen. Ten to twelve μm thick cryostat sections were collected on Superfrost glass slides and further processed for immunostaining for combined detection of VGAT and GABAA γ2 or VGAT and gephyrin. Briefly, cryostat sections were fixed by immersion in 2% paraformaldehyde, and mildly treated with pepsin as antigen-retrieval procedure, and then incubated for 48 hours with different combination of primary antibodies. Secondary antibody staining was performed for 1 h at room temperature using anti-isotypic fluorophore conjugated antibodies Alexa-488 and Alexa-594 at dilutions of 1:1000 (Molecular Probes).
Hippocampal neurons grown on glass coverslips were fixed with 4% paraformaldehyde and 4% sucrose in PBS. Unspecific binding was blocked by incubation with 10% normal goat serum (NGS) in PBS. Primary and secondary antibodies were diluted in 5% NGS/PBS. Secondary antibodies included anti-isotypic fluorophore conjugated antibodies Alexa-488, Alexa-594 and streptavidin-Alexa 405 at dilutions of 1:1000 (Molecular Probes).
Confocal microscopy and image analysis
Fluorescence images were acquired on a TCS-SP confocal laser scanning microscope (Leica, Bensheim, Germany) with a 40X 1.4 NA or 63X 1.4 NA oil immersion objectives, additionally magnified 5 fold with the pinhole set at 1 Airy unit All the parameters used in confocal microscopy were consistent in each experiment, including the laser excitation power, detector and off-set gains and the pinhole diameter. Stacks of z-sections (12–13 optical sections) with an interval of 0.3 μm were sequentially scanned three times for each emission line to improve the signal/noise ratio. The number of gephyrin, γ2 subunit and VGAT puncta was assessed in at least 8 sections for each genotypes (Pin1+/+ and Pin1−/−), by taking at least 4 images of strata radiatum and oriens of the CA1 region of each hippocampus in each set of experiments (n=3). In the pyramidal cell layer, the high density and elongated shape of VGAT positive terminals precluded the determination of their numbers and their colocalization with the other two antigens investigated.
For immunocitochemistry samples at least 10 cells from at least three independent batches per condition were used for analysis. Images were acquired as a z-stack (6-7 optical sections, 0.25 μm step size). In each image, at least five dendritic segments were outlined and saved as regions of interest (ROIs).
Quantification of immunofluorescence data was performed using the Volocity3D Image Analysis Software (PerkinElmer, London, UK). Gephyrin, NL2, GABAAR γ2 and VGAT clusters were determined after thresholding of images. Thresholds were determined using the ‘voxel spy’ facility of the software and chosen such that all recognizable punctuate structures were included into the analysis (minimal area, 0.1 μm2); colocalization was evaluated based on the determination of thresholded Pearson’s correlation coefficient (PCC > 0,5) for each gephyrin and γ2 cluster previously identified and quantified45. NL2 colocalization with gephyrin puncta was also quantified utilizing the software function “intersect object” that measures size, volume and intensity values of intersecting objects identified by separate protocols in each channel. To determine the degree of apposition of NL2/gephyrin colabeled clusters with the presynaptic marker VGAT, we superimposed the mask of all identified overlapping puncta onto the third channel and count them manually.
Hippocampal slice preparation and drug treatment
All experiments were performed in accordance with the European Community Council Directive of November 24, 1986 (86/609EEC) and were approved by the local authority veterinary service and by SISSA ethical committee. All efforts were made to minimize animal suffering and to reduce the number of animal used. Transverse hippocampal slices (300 μM thick) were obtained from postnatal (P) day P10-P13 mice (male and female) using a standard protocol46. Briefly, after being anesthetized with CO2, animals were decapitated. The brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 130 NaCl, 25 glucose, 3.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 2 CaCl2, and 1.3 MgCl2, saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). Transverse hippocampal slices (300 μm thick) were cut with a vibratome and stored at room temperature (22–24°C) in a holding bath containing the same solution as above. After incubation for at least 45 min, an individual slice was transferred to a submerged recording chamber and continuously superfused at 33–34°C with oxygenated ACSF at a rate of 3–4 ml min−1.
The following drugs were used: 6,7-dinitroquinoxaline-2,3-dione (DNQX), picrotoxin (PTX) and bicuculline, purchased from Ascent Scientific; (1,2,5,6-Tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA) purchased from Tocris Bioscence. DNQX and PTX were dissolved in dimethylsulfoxide (DMSO). The final concentration of DMSO in the bathing solution was 0.1%. At this concentration, DMSO alone did not modify the membrane potential, input resistance, or the firing properties of CA1 pyramidal neurons. Drugs were applied in the bath by gravity via a three-way tap system by changing the superfusion solution to one differing only in its content of drug(s). The ratio of flow rate to bath volume ensured a complete exchange within 2 min.
Electrophysiological recordings
Whole-cell patch-clamp recordings (in voltage clamp configuration) were performed from CA1 pyramidal cells, visualized with an upright microscope equipped with differential interference contrast optics and infrared video camera, using a patch-clamp amplifier (Axopatch 1D amplifier, Molecular Devices, Sunnyvale, CA, USA). Patched electrodes were pulled from borosilicate glass capillaries (Hingelberg, Malsfeld, Germany). They had a resistance of 4-6 MΩ when filled with the intracellular solution containing (in mM): 125 Cs-methanesulphonate, 10 CsCl, 10 HEPES, 0.3 EGTA, 2 MgATP, 0.3 NaGTP, (pH adjusted to ~ 7.3 with CsOH; the osmolarity was adjusted to 290 mOsmol). The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiment. Cells exhibiting >20% changes in series resistance were excluded from the analysis. The series resistance was <25MΩ and was not compensated.
Spontaneous GABAergic (sIPSCs) and glutamatergic (sEPSCs) postsynaptic currents were routinely recorded from a holding potential of −60 mV in the presence of DNQX (20 μM) and picrotoxin (10 μM), respectively. While sEPSCs were recorded using patch pipettes filled with the above mentioned solution, sIPSCs were recorded using an intracellular solution containing (in mM): CsCl 137, Hepes 10, BAPTA 11, MgATP 2, MgCl2 2, CaCl2 1 and 5 QX-314 (pH adjusted to ~ 7.3 with CsOH).
sIPSC were also recorded from cultured hippocampal neurons co-transfected with GFP and NL2HA or NL2HAS714A 24 hours after transfection, at a holding potential of −60 mV in presence of DNQX (20 μM) with the same intracellular solution used for the acute slices experiment. The extracellular solution contained (in mM) 137 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 20 glucose, and 10 HEPES, pH 7.4 (corrected with NaOH).
Data analysis
Data were acquired and digitized with an A/D converter (Digidata 1200, Molecular Device, Sunnyvale, CA, USA) and stored on a computer hard disk. Acquisition and analysis were performed with Clampfit 9 (Molecular Device, Sunnyvale, CA, USA).
Data were acquired at 20 kHz , filtered with a cut-off frequency of 2 kHz and stored on computer hard disk in order to perform off-line analysis. The resting membrane potential (RMP) was measured immediately after break-in and establishing whole-cell recording. The membrane input resistance (Rin) was calculated by measuring the amplitude of voltage responses to steady hyperpolarizing current steps, using the Clampfit 10.0 program (Molecular Device, Sunnyvale, CA, USA).
Spontaneous AMPA and GABAA-mediated postsynaptic currents were analyzed using Clampfit 10.0 (Molecular Device, Sunnyvale, CA, USA). This program uses a detection algorithm based on a sliding template. The template did not induce any bias in the sampling of events because it was moved along the data trace by one point at a time and was optimally scaled to fit the data at each position. The detection criterion was calculated from the template-scaling factor and from how closely the scaled template fitted the data.
Spontaneous GABAergic currents were analyzed with Mini Analysis program (version 6.0.1, Synaptosoft, Leonia, NJ) for their decay time constants. Only events with no deflections in the rising or decaying phases were included in the analysis. Low amplitude (< 5pA) events as well as events whose amplitude correlated with the rising or decaying time constants were discarded from the analysis because they were thought to be affected by dendritic filtering. The decay time of s IPSCs were fitted with a single exponential function as:
| (1) |
where I(t) is the current as a function of time, A is the amplitude at time 0, τ is the time constant.
The Mini Analysis program was used to perform peak scaled non-stationary noise analysis according to Traynelis and co-workers47. Individual, not correlated events, were aligned to the point of steepest rise time. The peak of the mean current response waveform was scaled to the response value at the corresponding point in time of each individual event before subtraction to generate the difference waveforms. The ensemble mean post synaptic current was binned into 50 bins of equal amplitude to assign similar weights to all phases of ensemble mean waveform. Variance was plotted against amplitude and individual points were fitted with the equation:
| (2) |
where i is the unitary single-channel current, I is the mean current, N is the number of channels open at the current peak and σb2 is the variance of the background noise. The single-channel chord conductance (ϒ) was calculated as:
| (3) |
from the holding potential (Em) of −60 mV, assuming a reversal potential (Erev) of 0 mV. Amplitude distribution of sIPSCs amplitude was obtained fitting data with the following Gaussian function:
| (4) |
where Iσis the mean current, ai is the area and σ is the variance.
The amplitude of the tonic current was estimated by the outward shift of the baseline current after the application of the GABAA receptor-channel blocker picrotoxin (100 μM). Only current recordings that exhibited a stable baseline were included in the analysis. Baseline currents were estimated by plotting 4-5 0.5 s periods in all point histograms. These were fitted with a Gaussian function. The peak of the fitted Gaussian was considered as the mean holding current48.
Statistics
Statistical analyses for Co-IP, PSD enriched extracts fractions analyses were performed by using Microsoft Excel. Comparisons were performed by Student’s t-test two tailed distribution unequal variance. Deviation and error bars were calculated using the same software. Statistical significance was defined as p < 0.05.
Statistical analyses of morphological data (NL2 and gephyrin cluster size and density) were performed pair-wise (Pin1−/− versus Pin1+/+) using unpaired, two-tailed Student’s t-test. Bars indicate standard deviation (s.d.).
Statistical analyses for electrophysiological experiments were performed by using pClamp 10 and Microsoft Excel. Comparison were perfomed by Student’s t-test unless otherwise stated. Statistical significance was defined as p < 0.05
Supplementary Material
Acknowledgments
We are grateful to Dr. P Scheiffele (Biozentrum, University of Basel, Basel, Switzerland) for kindly providing us NL2HA cDNA. We thank B Pastore for her excellent technical support with neuronal cultures. We are extremely grateful to L Gasperini, E Meneghetti and F Ruggeri for their help and for critical discussion during experiments and to E Grdina for managing the animal house facility. This work was partially supported by grants from Telethon (GGP11043), Human Brain Project (Neuroantibodies #604102) and MIUR (PRIN 2012) to EC.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Vithlani M, Moss SJ. The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition. Biochem Soc Trans. 2009;37:1355–1358. doi: 10.1042/BST0371355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Südhof TC. Neuroligins and Neurexins Link Synaptic Function to Cognitive Disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levinson JN, Chéry N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- 5.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F. Neuroligin2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 8.Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. Gephyrin, the enigmatic organizer at GABAergic synapses. Front Cell Neurosci. 2012;6:1–16. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- 10.Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maule,t Y, Werner P, Langosch D, Kirsch J, Betz H. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- 11.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 12.Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz G, Schrader N, Mendel RR, Hecht HJ, Schindelin H. Crystal structures of human gephyrin and plant Cnx1 G domains: comparative analysis and functional implications. J Mol Biol. 2001;312:405–418. doi: 10.1006/jmbi.2001.4952. [DOI] [PubMed] [Google Scholar]
- 14.Sola M, Kneussel M, Heck IS, Betz H, Weissenhorn W. X-ray crystal structure of the trimeric N-terminal domain of gephyrin. J Biol Chem. 2001;276:25294–25301. doi: 10.1074/jbc.M101923200. [DOI] [PubMed] [Google Scholar]
- 15.Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I, Saiyed T, O’Sullivan GA, Schmitt B, Betz H, Weissenhorn W. Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J. 2004;23:2510–2519. doi: 10.1038/sj.emboj.7600256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maric HM, Mukherjee J, Tretter V, Moss SJ, Schindelin Gephyrin-mediated GABA(A) and glycine receptor clustering relies on a common binding site. J. Biol. Chem. 2011;286:42105–42114. doi: 10.1074/jbc.M111.303412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalczyk S, Winkelmann A, Smolinsky B, Förstera B, Neundorf I, Schwarz G, Meier JC. Direct binding of GABAA receptor β2 and β3 subunits to gephyrin. Eur J Neurosci. 2013;37:544–554. doi: 10.1111/ejn.12078. [DOI] [PubMed] [Google Scholar]
- 18.Giannone G, Mondin M, Grillo-Bosch D, Tessier B, Saint-Michel E, Czöndör K, Sainlos M, Choquet D, Thoumine O. Neurexin-1β Binding to Neuroligin-1 Triggers the Preferential Recruitment of PSD-95 versus Gephyrin through Tyrosine Phosphorylation of Neuroligin-1. Cell Reports. 2013;3:1996–2007. doi: 10.1016/j.celrep.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signaling and disease. Nature Reviews Molecular Cell Biology. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 20.Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- 21.Shen M, Stukenberg PT, Kirschner MW, Lu KP. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev. 1998;12:706–720. doi: 10.1101/gad.12.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zita MM, Marchionni I, Bottos E, Righi M, Del Sal G, Cherubini E, Zacchi P. Post-phosphorylation prolyl isomerisation of gephyrin represents a mechanism to modulate glycine receptors function. EMBO J. 2007;26:1761–1771. doi: 10.1038/sj.emboj.7601625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signaling and disease. Nature Reviews Molecular Cell Biology. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 25.Lu KP. Pinning down cell signaling, cancer and Alzheimer’s disease. Trends in Biochemical Sciences. 2004;29:200–209. doi: 10.1016/j.tibs.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Uchida T, Takamiya M, Takahashi M, Miyashita H, Ikeda H, Terada T, Matsuo Y, Shirouzu M, Yokoyama S, Fujimori F, Hunter T. Pin1 and Par14 peptidyl prolyl isomerase inhibitors block cell proliferation. Chem Biol. 2003;10:15–24. doi: 10.1016/s1074-5521(02)00310-1. [DOI] [PubMed] [Google Scholar]
- 27.Zacchi P, Antonelli R, Cherubini E. Gephyrin phosphorylation in the functional organization and plasticity of GABAergic synapses. Front Cell Neurosci. 2014;8:103. doi: 10.3389/fncel.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyagarajan SK, Ghosh H, Yévenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU, Gerrits B, Muller D, Fritschy JM. Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc Natl Acad Sci USA. 2011;108:379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levinson JN, Li R, Kang R, Moukhles H, El-Husseini A, Bamji SX. Postsynaptic scaffolding molecules modulate the localization of neuroligins. Neuroscience. 2010;165:782–93. doi: 10.1016/j.neuroscience.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Dumoulin A, Lévi S, Riveau B, Gasnier B. Triller A. Formation of mixed glycine and GABAergic synapses in cultured spinal cord neurons. European Journal of Neuroscience. 2000;12:3883–3892. doi: 10.1046/j.1460-9568.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- 31.Momiyama A, Silver RA, Hausser M, Notomi T, Wu Y, Shigemoto R, Cull-Candy SG. The density of AMPA receptors activated by a transmitter quantum at the climbing fibre-Purkinje cell synapse in immature rats. J Physiol. 2003;549:75–92. doi: 10.1113/jphysiol.2002.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varley ZK, Pizzarelli R, Antonelli R, Stancheva HS, Kneussel M, Cherubini E, Zacchi P. Gephyrin regulates GABAergic and glutamatergic synaptic transmission in hippocampal cell cultures. J Biol Chem. 2011;286:20942–20951. doi: 10.1074/jbc.M111.234641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barberis A, Petrini EM, Cherubini E. Presynaptic source of quantal size variability at GABAergic synapses in rat hippocampal neurons in culture. Eur J Neurosci. 2004;20:1803–1810. doi: 10.1111/j.1460-9568.2004.03624.x. [DOI] [PubMed] [Google Scholar]
- 34.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 35.Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herweg J, Schwarz G. Splice-specific glycine receptor binding, folding, and phosphorylation of the scaffolding protein gephyrin. J Biol Chem. 2012;287:12645–12656. doi: 10.1074/jbc.M112.341826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyagarajan SK, Ghosh H, Yévenes GE, Imanishi SY, Zeilhofer HU, Gerrits B, Fritschy JM. Extracellular signal-regulated kinase and glycogen synthase kinase 3β regulate gephyrin postsynaptic aggregation and GABAergic synaptic function in a calpain-dependent mechanism. J Biol Chem. 2013;288:9634–9647. doi: 10.1074/jbc.M112.442616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong N, Qi J, Chen G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABAA receptors. Mol Cell Neurosci. 2007;35:14–23. doi: 10.1016/j.mcn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Hoon M, Bauer G, Fritschy JM, Moser T, Falkenburger BH, Varoqueaux F. Neuroligin 2 controls the maturation of GABAergic synapses and information processing in the retina. J Neurosci. 2009;29:8039–8050. doi: 10.1523/JNEUROSCI.0534-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budreck EC1, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, Fukazawa Y, Harada H, Tabuchi K, Shigemoto R, Scheiffele P, Kim JH. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc Natl Acad Sci USA. 2013;110:725–730. doi: 10.1073/pnas.1214718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lardi-Studler B, Smolinsky B, Petitjean CM, Koenig F, Sidler C, Meier JC, Fritschy JM, Schwarz G. Vertebrate-specific sequences in the gephyrin E-domain regulate cytosolic aggregation and postsynaptic clustering. J Cell Sci. 2007;120:1371–1382. doi: 10.1242/jcs.003905. [DOI] [PubMed] [Google Scholar]
- 42.Andjus PR1, Stevic-Marinkovic Z, Cherubini E. Immunoglobulins from motoneurone disease patients enhance glutamate release from rat hippocampal neurones in culture. J Physiol. 1997;504:103–112. doi: 10.1111/j.1469-7793.1997.103bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atchison FW, Capel B, Means AR. Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development. 2003;130:3579–3586. doi: 10.1242/dev.00584. [DOI] [PubMed] [Google Scholar]
- 44.Mohler H, Knoflach F, Paysan J, Motejlek K, Benke D, Luscher B, Fritschy JM. Heterogeneity of GABAA-receptors: cell-specific expression, pharmacology, and regulation. Neurochem Res. 1995;20:631–636. doi: 10.1007/BF01694546. [DOI] [PubMed] [Google Scholar]
- 45.Barlow AL, MacLeod A, Noppen S, Sanderson J, Guérin CJ. Colocalization analysis in Fluorescence Micrographs: verification of a more accurate calculation of Pearson’s correlation coefficient. Microsc Microanal. 2010;16:710–724. doi: 10.1017/S143192761009389X. [DOI] [PubMed] [Google Scholar]
- 46.Griguoli M, Maul A, Nguyen C, Giorgetti A, Carloni P, Cherubini E. Nicotine blocks the hyperpolarization-activated current Ih and severely impairs the oscillatory behavior of orienslacunosum moleculare interneurons. J Neurosci. 2010;30:10773–83. doi: 10.1523/JNEUROSCI.2446-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traynelis SF, Silver RA, Cull-Candy SG. Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber-granule cell synapse. Neuron. 1993;11:279–89. doi: 10.1016/0896-6273(93)90184-s. [DOI] [PubMed] [Google Scholar]
- 48.Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.