Abstract
Dendritic cell based vaccines offer promise for therapy of ovarian cancer. Previous studies have demonstrated that oxidation of several antigens, including ovarian cancer cells, using hypochlorous acid strongly enhances their immunogenicity and their uptake and presentation by dendritic cells. The response of T cells and dendritic cells to autologous tumour from patients with active disease has not previously been investigated. Monocyte derived dendritic cells were generated from patients with active disease and activated by co-culture with oxidised tumour cells and the TLR agonist poly I:C. The dendritic cells showed an activated phenotype, but secreted high levels of TGFβ. Co-culture of the antigen-loaded dendritic cells with autologous T cells generated a population of effector T cells which showed a low level of specific lytic activity against autologous tumour, as compared to autologous ovarian mesothelium. The presence of neutralising antibody to TGFβ in DC/T cell co-cultures increased the levels of subsequent tumour killing in three samples tested. Co-culture of monocytes from healthy volunteers with the ovarian cell line SKOV-3 prior to differentiation into dendritic cells reduced the ability of dendritic cells to stimulate cytotoxic effector cells. The study suggests that co-culture of dendritic cells with oxidised tumour cells can generate effector cells able to kill autologous tumour, but that the high tumour burden in patients with active disease may compromise dendritic cell and/or T cell function.
Keywords: dendritic cell therapy, ovarian cancer, immunotherapy, hypochlorous acid
Introduction
The need to develop effective immunotherapy approaches for cancer remains acute, particularly in ovarian cancer, which continues to have a poor survival rate. There is increasing evidence to support the hypothesis that ovarian cancers are immunogenic and that anti-tumour responses correlate with improved prognosis (for reviews see [1] and [2]). Whilst this provides hope that immunotherapy could improve outcome in ovarian cancer patients, the obstacles to generating clinically beneficial anti-tumour immunity are considerable. Dendritic cells (DC) are well known as potent activators of T cell responses but as yet the results of DC vaccines have with few exceptions been disappointing. However, a DC-based vaccine has recently been approved by the FDA for use in hormone-refractory prostate cancer justifying further investigations in other tumours [3]. Ovarian cancer is one example where DC therapy has been evaluated, and in a limited number of patients there have been encouraging results [4 7].
A major hurdle to effective immunotherapy is tolerance to self-antigens, and many different approaches are being investigated to enhance the immunogenicity of tumour associated antigens. We have a long-established interest in the adjuvant role of oxidation via hypochlorous acid, which we have hypothesised may be an important link between innate immunity (neutrophil myeloperoxidase activity) and adaptive immunity [8] We have previously exploited this “natural adjuvant” to enhance the immunogenicity of tumour cells [9, 10] We have shown that oxidation of an ovarian tumour cell line, SKOV-3 with hypochlorous acid enhances antigen uptake and processing by DC [9, 10]. DC isolated from healthy volunteers or ovarian cancer patients in remission, and loaded with oxidised tumour cells stimulate specific IFN-γ responses in vitro against tumour associated peptides [9] and autologous primary tumour [11]. Although the mechanism of enhancement is still not completely understood, hypochlorous acid was found to enhance uptake, cross priming and presentation. Oxidation may also generate neo-epitopes which help bypass self-tolerance.
Future Phase 1 clinical trials of DC based immunotherapy are likely to target patients who have relapsed, and have active disease. In this study we therefore establish the oxidised tumour cell/DC/T cell co-culture model using cells from a group of ovarian cancer patients with active disease. Since the patient cohort we used all presented with ascites, we were also able to isolate both autologous tumour cells and non-transformed mesothelial cells at the same time as collecting PBMC. Using this model we tested the hypothesis that DC derived from patients with active disease and loaded with oxidised ovarian tumour cells (a model we have explored in detail previously [9,11]) can stimulate cytotoxic T cells which lyse autologous primary tumour cells, and show selective killing of tumour cells as compared to normal epithelium. Our studies support this hypothesis, but in addition demonstrate that DC from these group of patients show an abnormal phenotype, secreting high levels of TGFβ, and yielding low levels of lysis. This altered DC function may result from the exposure of the monocyte precursors to a high tumour burden.
Materials and Methods
Patients and Samples
Peripheral blood samples were collected by venepuncture from ovarian cancer patients and healthy volunteers. Primary ovarian tumour cells and mesothelial cells were obtained by aseptic, therapeutic drainage of ascitic fluid from in patients in relapse at UCL Hospitals Gynaecological Cancer Centre. Patient details are shown in Table 1. All samples were obtained after informed consent as approved by North East London Ethics committee and stored in accordance with the UK Human Tissue Act 2005.
Table 1. Patient samples used in this study.
Peripheral blood samples were collected by venepuncture from ovarian cancer patients in remission (Rem) or with active disease (AD). Ascitic fluid samples were obtained by aseptic, therapeutic drainage from symptomatic patients, and were used as a source of primary ovarian tumour cells and mesothelial cells. Table 1 shows patient ages, HLA-*0201 status, sample volumes and time since last cycle of chemotherapy.
| Patient | Age | Time since chemotherapy (weeks) | Blood sample (ml) | Ascitic fluid sample (ml) | HLA-*0201 |
|---|---|---|---|---|---|
| AD 1 | 66 | 3 | 40 | 4500 | - |
| AD 2 | 48 | 8 | 100 | 3000 | − |
| AD 3 | 64 | 4 | 100 | 750 | + |
| AD 4 | 50 | 2 | 18 | 50 | − |
| AD 5 | 68 | 4 | − | 85 | − |
| AD 6 | 62 | 2 | 20 | 500 | − |
| AD 7 | 64 | 0 | 20 | 770 | + |
| AD 8 | 69 | 12 | 40 | 75 | − |
| AD 9 | 64 | 16 | 40 | 150 | − |
| AD 10 | 61 | 4 | 40 | 165 | − |
| AD 11 | 85 | 4 | 20 | 1200 | − |
| AD 12 | 76 | 3 | 40 | 500 | − |
| AD 13 | 54 | 8 | − | 250 | + |
| AD 14 | 66 | 1 | 40 | 500 | − |
| Rem 1 | 66 | 8 | 100 | − | + |
| Rem 2 | 58 | 7 | 100 | − | + |
| Rem 3 | 69 | 123 | 40 | − | − |
| Rem 4 | 68 | 24 | 40 | − | − |
| Rem 5 | 62 | 22 | 40 | − | − |
| Rem 6 | 68 | 9 | 40 | − | + |
Cell Culture
The human ovarian carcinoma cell line SKOV-3 expresses the tumour-associated antigens Her-2-neu and MUC-1 and is HLA*0201, HLA*0203+. An HLA*0201 expressing cell line (SKOV-A2) was generated by retroviral transduction (HLA*0201 plasmid was a kind gift from Hans Stauss, UCL). SKOV-3 and SKOV-A2 were cultured in IMDM media (Gibco) supplemented with 10% FBS (Sigma), penicillin G (50 U/ml) and streptomycin (50 mg/ml). The GFP reporter cell line SMAD-GFP (a kind gift from David Escors, UCL) was cultured in RPMI media (Gibco), supplemented with 10% FBS (Sigma), penicillin G (50 U/ml) and streptomycin (50 mg/ml).
PBMCs were purified by Histopaque (Sigma) density gradient separation. CD14+ cells were isolated from PBMCs using CD14 human microbeads (Miltenyi Biotec) as per manufacturer instructions. The negative fraction was retained to use as effector cells. CD14+ cells were cultured in AIM-V media supplemented with IL-4 (50 ng/ml) and GM-CSF (100 ng/ml) for 5 days, after which non-adherent DC were removed and pulsed with oxidised tumour cells. All primary cells were cultured in serum free conditions in AIM-V medium (AIM-V). In some experiments, purified CD3+ effector cells were obtained using CD3 negative selection beads (Miltenyi Biotec).
Oxidation of SKOV-3 ovarian cell line was carried out using HOCL, prepared from NaOCL reagent (Sigma-Aldrich) as previously described [9]. Washed, oxidised SKOV3 cells were added to DC cultures at a ratio of 1:1 at a density of 1 × 106/ml and maturation factors added, including: IFN-γ (10 ng/ml), MPL (200 ng/ml) and poly I:C (PIC) (50 μg/ml). After 24hrs supernatants were collected and frozen for cytokine analysis. DC were washed and analysed for surface markers by flow cytometry or used to set up co-cultures with autologous effector cells.
‘Tumour educated’ DCs were generated as follows. Monocytes from healthy donors were seeded in the bottom of six-well plates, with confluent SKOV-3 cells in trans well inserts above. After 24 hours, the upper chambers containing tumour cells were removed and the monocyte layer given fresh media supplemented with IL-4 and GM-CSF. After 5 days, non-adherent DC were obtained and used as previously described.
DC were co-cultured with effector cells at a ratio of 1:100 for 7 days in AIM-V media (Gibco). In TGFβ blocking experiments, anti-human TGFβ clone 2E6 (Abcam) was added to DCs at a concentration of 20 μg/ml for 30 min prior to the addition of effector cells, and added again on days 3 and 6. Mouse anti-influenza A nuclear antigen (unconjugated, azide free) was used as a control antibody. At the end of the culture period, effector cells were washed and reseeded in fresh media prior to use in chromium release assays.
Primary tumour cells and mesothelium were obtained from ascitic fluid by centrifugation and seeded at 1 × 106 cells/ml in RPMI supplemented with 10% FBS (Sigma), penicillin G (50 U/ml) and streptomycin (50 mg/ml). After one hour at 37 °C, % C02, two distinct populations were visible; strongly adherent mesothelial cells and loose clumps of non-adherent tumour cells. Non adherent tumour cells were removed by gentle washing, and reseeded in fresh media. In some cases, ascitic fluid was heavily blood-stained and required density gradient extraction prior to cells being separated by adherence.
Cytokine Analysis
DC with or without oxidised antigen and maturation factors were seeded at 1 × 106/ml in AIM-V media. 24 hours later supernatants were collected and frozen for cytokine analysis. Primary tumour cells were seeded at 1 × 106/ml in AIM-V media, and supernatants collected 24 hours later for cytokine analysis. Supernatants were analysed for the presence of IL-1β, IL-6, 1L-10, IL-12 and TNF using cytokine flex set bead array (BD) as per the manufacturer’ instructions, in duplicate. TGF-β levels were determined using a TGF-β responsive cell line, SMAD-GFP which was generated by PSIN-SMAD-GFP lentiviral transduction of 293T cells [12]. SMAD-GFP cells were cultured for 24 hours in culture supernatant samples or known concentrations of TGFβ and induced GFP levels determined by FACS analysis.
Flow Cytometry
FACS analysis of cell surface molecules on DC and primary tumour cells was carried using antibodies from Becton Dickinson, Oxford, UK (HLA-DR,DP,DQ-FITC, CD86-FITC, CD80-FITC) or eBioscience (HLA,B,C-PE, CD40-PE, HLA-G-PE and E-cadherin-PCPeFluor, CD44-FITC). Cells were washed and stained with antibody for 30 min at room temperature, in the dark, in the presence of 1% FBS. Matched isotype controls were included for each sample. Flow cytometric analyses were carried out using a FACScalibur cytometer (BD Biosciences). Mean fluorescence values were calculated by subtracting the fluorescence from isotype controls.
Chromium Release Assays
SKOV-A2, primary ovarian cancer cells or mesothelial cells were labelled with 51Chromium (MP Biomedicals) for 1 hour at 37°C, washed and seeded in AIM-V media in 96 well v-bottomed plates. Effector cells were washed, resuspended in fresh AIM-V media and added to the labelled target cells at various effector to target cell ratios, in triplicate. Plates were incubated for 4 hours at 37°C, 5% CO2. 25μl of supernatants were removed and mixed with 175 μl of scintillation fluid prior to counting with a beta counter. % lysis was calculated in relation to total and background levels of lysis.
Statistical Analysis
The data was analysed using Student’s T test, analysis of variance or Pearson correlation (95% confidence interval), as noted in text or Legend to figures.
Results
Primary tumour cells isolated from ascites express high levels of class I MHC, low levels of HLA-G and secrete immunomodulatory cytokines
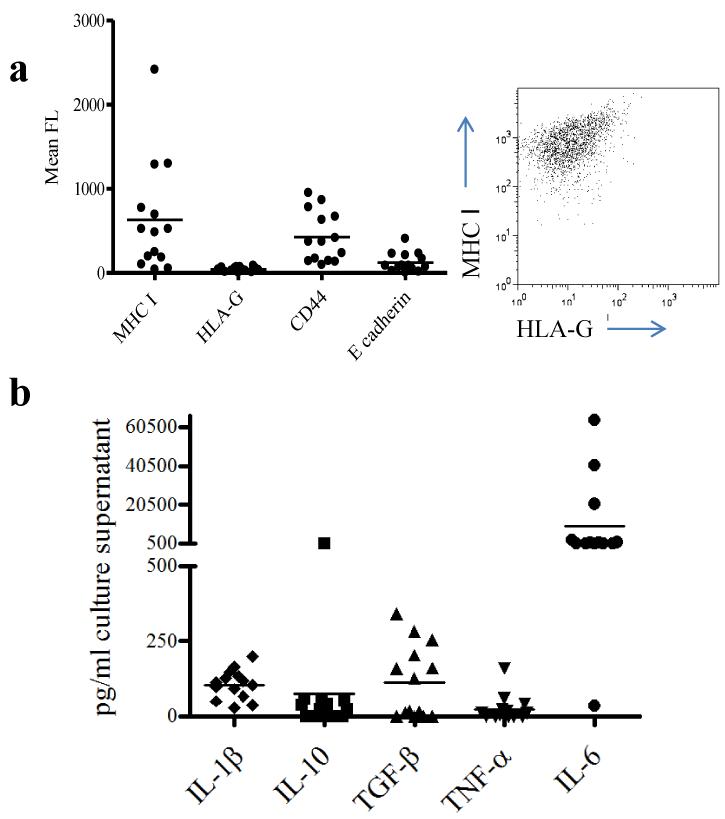
Tumour cells frequently evade detection by cytotoxic T cells either by HLA loss [13] or expression of the non-classical HLA-G molecule [14, 15]. In order to determine whether ovarian cells would act as potential targets for cytotoxic T cells, expression of MHC Class I, and HLA-G on ovarian tumour cells isolated from ascites was measured by flow cytometry (Fig 1a). MHC class I levels were variable, but all samples expressed significant levels of class I molecules (the monomorphic antibody used did not distinguish between class I alleles). In contrast, HLA-G expression was not detected on any sample. The primary tumour cells were also assessed for spontaneous secretion of cytokines (Fig 1b). All samples tested secreted detectable levels of the proinflammatory cytokines IL1β and IL-6. The majority of supernatants also contained detectable levels of the immunosuppressive cytokine TGFβ
Fig.1. Primary ovarian cancer cells isolated from patients with progressive disease express high levels of MHC class I, low levels of HLA-G and high levels of immunosuppressive cytokines.
Primary ovarian cancer cells were obtained from the ascitic fluid of 14 ovarian cancer patients and analysed by FACS for the presence of MHC class I, CD44, E-cadherin and HLA-G. Panel (a) shows mean fluorescence for each sample, together with a representative FACS profile of tumour cells from AD patient 2 co-stained with MHC I and HLA-G. Primary tumour cells were seeded at 1 × 106/ml, cultured in vitro for 48 hours and supernatants collected for cytokine analysis. Panel (b) shows the concentration of IL-1β, IL-10, TGF-β, TNF α and IL-6 in pg/ml in cell culture supernatants as determined by ELISA. Four samples secreted more than 2000 pg/ml IL-6 and are shown as 1,500 pg/ml.
PIC enhances activity of DC loaded with oxidised tumour cells
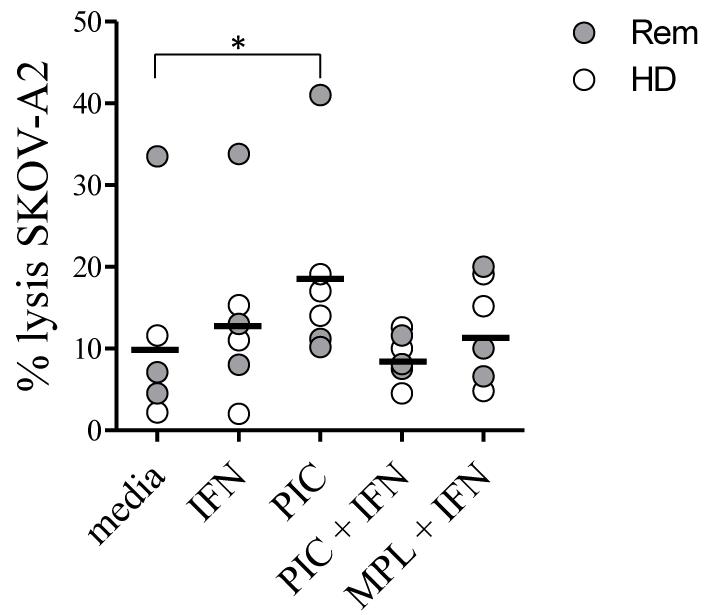
The TLR-3 agonist PIC has been used as an adjuvant in vivo [16]. We first tested whether activation with PIC would enhance the ability of DC to drive the generation of effector T cell activity. Monocyte-derived DC generated from PBMC of HLA*0201+ healthy donors or ovarian cancer patients in remission were incubated with oxidised SKOV-3 cells with or without combinations of PIC, IFN-γ and the TLR4 agonist MPL. After 24 hours, DC were collected, and co-cultured with autologous CD3+ T cells. After 7 days of culture, stimulated T cells (no detectable levels of NK cells were present in the cultures) were tested for their ability to kill SKOV3 cells transfected with HLA-A2 (SKOV A2) (Fig 2). PIC stimulation enhanced the level of killing (p<0.01, n=6), with levels of killing ranging from 10–40%. Addition of IFN-γ or MPL to the PIC gave no additional benefit. All further experiments were therefore carried out using DC cultured with PIC.
Fig.2. PIC enhances activity of DC loaded with oxidised tumour cells.
Dendritic cells were generated from peripheral blood monocytes obtained from HLA*0201+ healthy donors and ovarian cancer patients in remission. The ovarian cancer cell line SKOV-3 was oxidised with HOCL and washed three times prior to use as an antigen source. Immature DCs were incubated with oxidised tumour cells at a ratio of 1:1 and combinations of IFN-γ (10 ng/ml), MPL (200 ng/ml) and P:iC (50 μg/ml) for 24 hours. Pulsed, matured DC were then washed and co-cultured with autologous CD3+ T cells for 7 days. Stimulated T cells were tested in chromium release assays for their ability to lyse SKOV-A2 target cells. Graph shows % lysis of SKOV-A2 achieved with different DC maturation conditions using cells from three patients (Rem) and three healthy donors (HD), at an effector to target ratio of 100:1. The difference between unstimulated DC and PIC stimulated DC was significant, p<0.01, paired T test, n=6.
Differences between DC from patients with active disease and DC generated from healthy donors or patients in remission
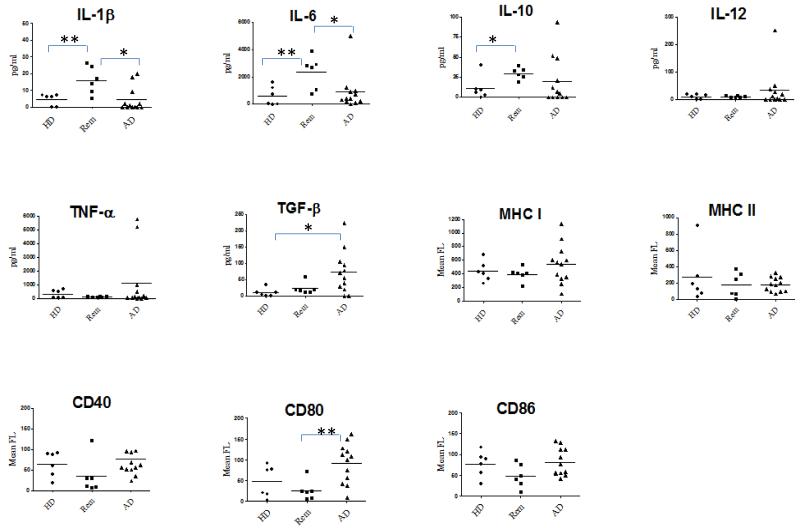
The cytokine secretion profile and surface phenotype of DC loaded with oxidised SKOV3 and activated by PIC isolated from healthy donors, remission patients and patients with active disease was compared (Fig 3). DC from patients in remission secreted more IL-1β, IL-6 and IL-10 than DC generated from the other two sample groups. Interestingly, many of the DC samples cultured from PBMC of patients with active disease also secreted TGFβ, a cytokine which was never detected in supernatants of DC generated from healthy donors and remission patients (P= <0.01, Student’s T-test, n=6-14). DC from patients with active disease expressed comparable levels of MHC and CD40 to DC from the other two groups, but more CD80 (P= <0.01, Student’s T test).
Fig.3. Differences between DC from patients with active disease and DC generated from healthy donors or patients in remission.
Graphs show cytokine secretion (pg/ml in culture supernatants) and surface molecule expression (mean fluorescence) of DC generated from six healthy donors (HD), six patients in remission (Rem) and twelve patients with active disease (AD). Statistically significant differences between groups as calculated by unpaired, two-tailed T test are denoted by one (p<0.05) or two (p<0.01) asterisks *.
DC from patients with active disease stimulate effector T cells showing preferential lysis of primary autologous tumour compared to mesothelium
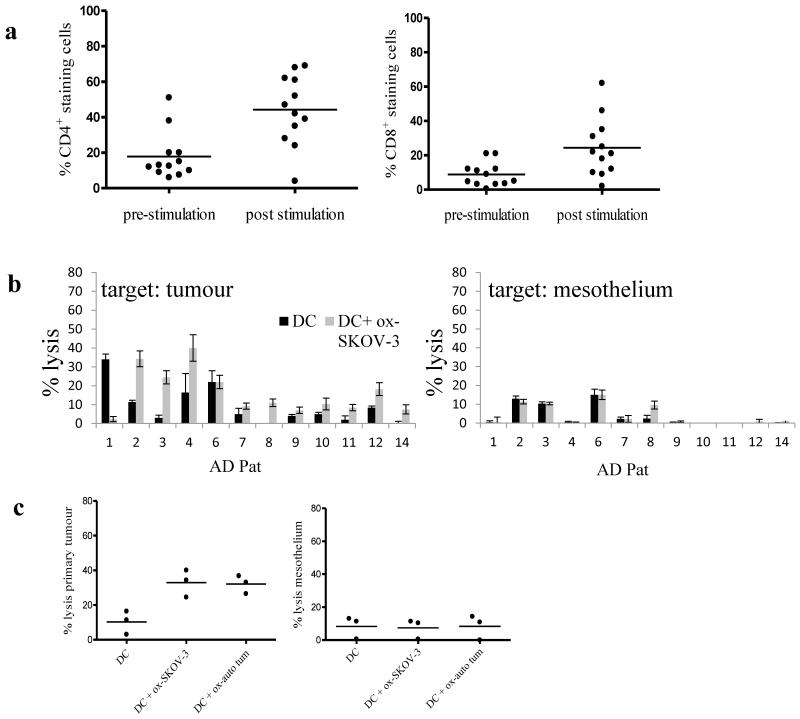
Lymphocytes (CD14+ depleted PBMC) from patients with active disease were co-cultured with autologous DC previously incubated with oxidised SKOV-3 (which we have previously shown can be used as a generic source of ovarian tumour associated antigens [9,11]) and poly I:C. At the end of the culture period, stimulated effector cells were tested in chromium release killing assays. Figure 4a shows the phenotype of the patient effector cells with regard to CD4+ and CD8+ at the beginning and end of the culture period. In almost all experiments, stimulation of lymphocytes with DC loaded with oxidised SKOV-3 increased the percentage of CD4+ and CD8+ cells, while absolute cell numbers in the cultures fell (not shown). Because the number of T cells was often very low, and limiting, no further fractionation of T cells was included. The stimulated effector cells showed variable levels of tumour cell killing which ranged from 1-40%. A degree of killing of mesothelium was apparent in some cases, but was significantly less than tumour killing, and was not dependent on addition of oxidised antigen to DC (Fig 4b, p<0.01, two-way analysis of variance). No correlation was found between tumour lysis and DC phenotype, tumour phenotype or time since the last cycle of chemotherapy. However, an inverse relationship between patient age and tumour killing was statistically significant (p<0.05) by Pearson two-tailed test. In a small subset of patient samples (n=3), DC loaded with autologous oxidised tumour cells were compared to DC loaded with oxidised SKOV-3 in stimulating lytic function in effector cells. Primary cells and the cell line gave comparable results, providing further evidence that SKOV-3 is suitable as a generic source of antigen for ovarian cancer immunotherapy (Fig 4c).
Fig.4. DC generated from patients with active disease stimulate effector cells with activity against primary ovarian cancer cells.
DC were generated from patients with active disease, incubated with or without oxidised SKOV-3 cells and PIC. These DC were then used to stimulate autologous effector cells in vitro. (a) CD4 and CD8 expression on effector cells pre and post culture with DC. (b) After one week of in vitro stimulation, effector cells were tested in chromium release assays for activity against autologous tumour or mesothelium. The data are shown as individual bar charts for each patient representing the mean of triplicate values at an effector to target ratio of 100:1, with error bars showing standard deviation. The killing of tumour cells was significantly greater than of mesothelium (p<0.01, two-way analysis of variance, n=14). (c) Autologous tumour was compared to SKOV-3 as a source of oxidised antigen using cells from AD patients 2,3 and 4 and tested against autologous tumour (left panel c) or autologous mesothelium (right panel c). There were no significant differences between killing of autologous tumour generated by the two antigen sources.
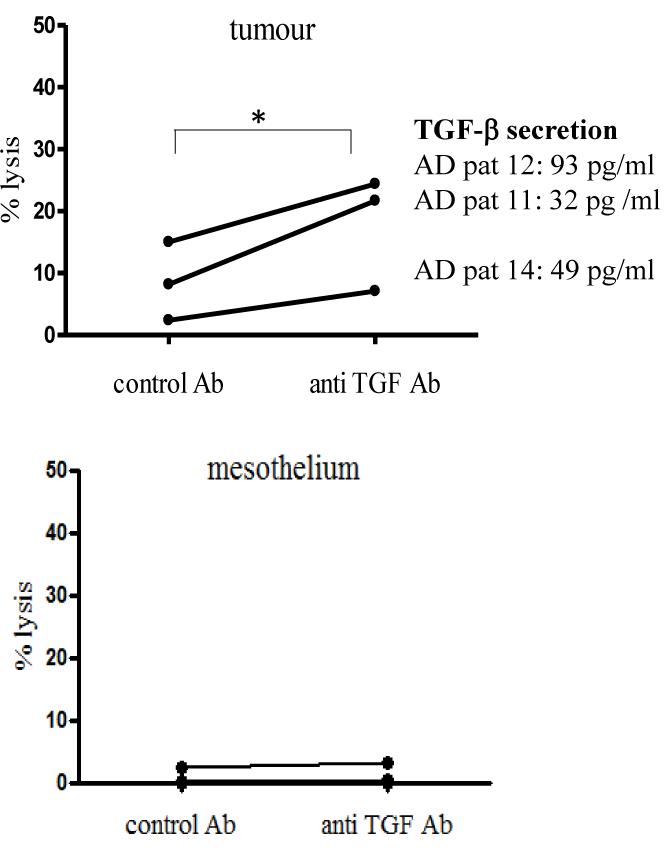
Addition of anti-TGF-β to DC/T cell co-cultures enhances killing of primary autologous tumour
Since a considerable proportion of DC from the patients with active disease secreted TGFβ, we investigated if the presence of TGF-β blocking antibody could enhance the cell mediated tumour killing stimulated by DC from patients with active disease. In all three patient cultures, there was a trend for enhanced lysis of autologous tumour in the presence of anti-TGF-β antibody to the DC-effector cell co-cultures compared to a control antibody (Fig 5).
Fig.5. Addition of anti-TGFβ to DC/T cell co-cultures enhances killing of primary autologous tumour.
DC generated from active disease patients 11, 12 and 14 were incubated with oxidised SKOV-3 and PIC for 24 hours prior to co-culture with autologous effector cells. During the culture period, anti-TGFβ or control antibody (mouse anti-influenza A nuclear antigen) were added to the co-cultures. Stimulated effector cells were then tested in chromium release assays for activity against autologous tumour or mesothelium as previously. Graphs show individual points for each patient (mean of triplicate values). Lysis of primary tumour was significantly higher (p<0.05) by effector cells stimulated in the presence of anti-TGFβ antibody compared to control antibody as determined by one-tailed, paired t-test. Levels of TGF-β (pg/ml) secreted by the different patient’s DC are shown alongside each data set.
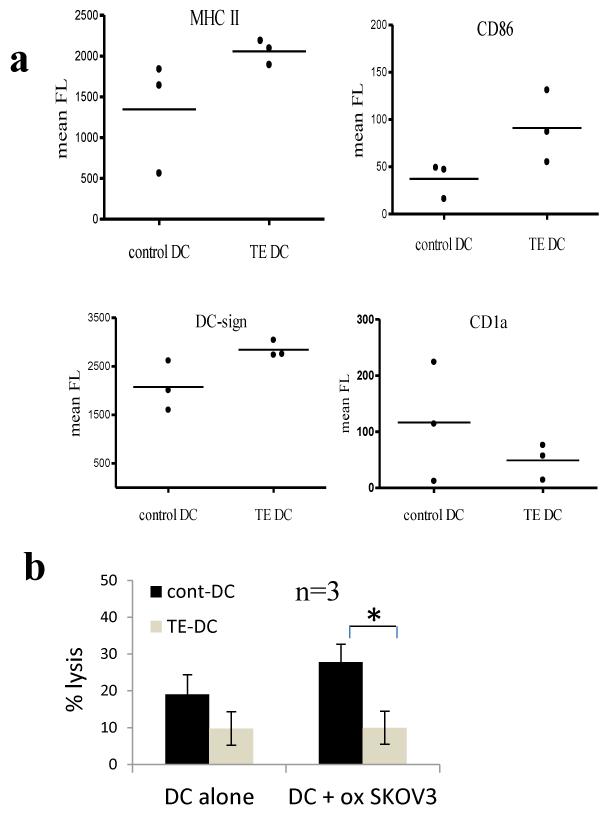
Reduced cytotoxic killing after stimulation with oxidised tumour loaded DC generated from monocytes pre-exposed to SKOV-3 tumour cells
A low level of killing against autologous tumour was observed in this study. One possible factor contributing to this low level of killing was the functional state of the DC which, as shown in fig 3, clearly showed differences from DC prepared from monocytes taken from healthy volunteers or patients in remission. Since in our studies DC are differentiated in vitro, away from any influence of tumour cells, this would suggest that tumour cells can alter the properties of monocytes (the DC precursors in our experiments) in such a way as to influence their ability to respond to GM-CSF/IL4 and hence result in an abnormal DC functional phenotype. Monocytes isolated from the PBMC of patients with active disease have often been exposed to very high tumour load in vivo, since ascites specimens may typically contain around 106 ovarian tumour cells per ml (23). We wished to explore if we could show some evidence for this type of interaction in vitro, by exposing monocytes to the SKOV-3 tumour cells, and then differentiate them in vitro and test them for antigen presenting cell activity as above. For this purpose it was important to use both DC and T cells from healthy individuals, where no prior exposure to tumour had occurred. However, since this obviously precludes the use of autologous tumour cells as targets, we developed an alternative model, in which SK-OV-3 cells could be used both as source of antigen (using oxidised cells as in our previous experiments), and also as a target tumour cell for cytotoxic activity assays. In order to minimise variation due to HLA mismatching, we used SKOV-3 cells transfected with HLA-A2 as targets, and restricted ourselves to DC and T cells from HLA-A2 healthy volunteers. Monocytes were cultured in close proximity (but not in contact) with live SKOV-3 cells for 18 hours using transwells which allowed soluble factors from the tumour cells in the upper chamber to access the monocytes in the lower chamber. The SKOV-3 cells were then removed, and the monocytes cultured in the presence of GM-CSF and IL-4 according to the standard protocol. ‘Tumour-educated DC’ and control DC were then collected, loaded with oxidised SKOV-3 cells and activated with poly I:C, and then compared for their ability to stimulate cytotoxic T cells with activity against SKOV-A2 (Fig 6). Paradoxically, DC from monocytes exposed to SKOV3 showed a trend towards a hyper activated phenotype (higher mean levels of MHC Class II, CD86 and DC-SIGN, but differences did not reach statistical significance), but stimulated less cytotoxic effector activity against SKOV-A2 (Fig 6b, p<0.01, paired T test, n=3).
Fig.6. Reduced cytotoxic killing after stimulation with oxidised tumour loaded DC generated from monocytes pre-exposed to SKOV-3 tumour cells.
Monocytes were isolated from three healthy donor PBMC samples and cultured below transwell inserts containing tumour cells (SKOV-3) or media alone for 24 hours. Transwells were then removed and ‘tumour educated’ and control monocytes were differentiated into DC by culture in GM-CSF and IL-4 for 5 days. The resulting ‘Tumour educated’ (TE DC) and control DC were compared phenotypically and functionally. (a) Mean fluorescence for MHC II, CD86, DC-sign and CD1a. Differences between groups were not significant (p>0.05). (b) ‘Tumour educated’ and ‘control’ DC were co-cultured with autologous CD3+ T cells for 1 week. At the end of the culture period, stimulated T cells were tested in chromium release assays for their ability to lyse SKOV-A2 target cells. The mean values from three donors are shown, with error bars showing standard deviation. The killing by control DC plus oxidised SKOV-3 is significantly higher than by TE-DC plus oxidised SKOV-3 (p<0.01, paired T test, n=3).
Discussion
In this report we describe an in vitro evaluation of DC-based therapy using samples collected from ovarian cancer patients during relapse. The immunosuppressive nature of primary ovarian cancer cells with regard to MHC molecule down-regulation [18, 19], HLA-G expression [15] and secretion of suppressive cytokines [20, 21] is well documented. Furthermore, ascitic fluid from patients with ovarian cancer has been shown to be highly suppressive of T cell function and T cells isolated from ascitic fluid to be unresponsive in vitro[22]. The majority of published data on the phenotype of primary ovarian cancer cells concern samples collected at presentation [2]. In contrast, we have collected samples from patients with progressive disease, often after multiple relapses. These primary ovarian tumour cells showed high levels of MHC I, while HLA-G expression was barely detectable, suggesting that these cells should in principle be sensitive to cytotoxic T cell killing. Escape from cytotoxic T cell killing, at least by these pathways, was not therefore the major driver of tumour progression in these patients.
The tumour cells were, however found to secrete both proinflammatory (IL1β, and IL-6) and anti-inflammatory (TGFβ) cytokines. The close link between inflammation and tumour progression is now well established [23] and IL-6 has been specifically linked to chemoresistance and poor prognosis in ovarian cancer [24]. TGFβ is a well-established immunosuppressive cytokine, with well-defined roles in the differentiation of certain types of regulatory T cell [25, 26]. In addition, TGFβ may play an important direct role in facilitating ovarian cancer metastasis [27].
Choosing the optimal activation/maturation stimuli for preparing DC for human adoptive immunotherapy has been the subject of extensive study [28-30]. We initially examined a variety of maturation factors and found that PIC enhanced DC activity. This is in agreement with other reports on the effectiveness of PIC in activating DC from healthy donors and ovarian cancer patients [31]. Therefore we used PIC in all subsequent experiments.
Interestingly, the DC generated from monocytes isolated from patients with active disease showed differences to DC from either healthy volunteers or patients in remission. In particular, the DC showed significantly elevated levels of surface CD80, and of secreted TGFβ. Although CD80 is a receptor for CD28 on T cells, and is generally regarded as delivering necessary costimulation signals to T cells, a recent report has suggested elevated CD80 may be a characteristic feature of myeloid derived suppressor cells [32]. As discussed above, TGFβ is generally immunosuppressive, and plays a role in differentiation of Tregs [25, 26].
Encouragingly, T cells stimulated by tumour loaded DC showed cytotoxic activity against autologous tumour cells isolated from patient ascites. Furthermore, cytotoxic activity was selective for tumour cells, with little or no specific kill of autologous normal mesothelium. Lower levels of mesothelium lysis could reflect a natural resistance of these cells to cytotoxic T cell lysis or, alternatively, a differential expression of antigens from transformed epithelial cells. Further studies using specific peptides to pulse mesothelium or tumour targets will be required to distinguish these two possibilities. In either case the results suggest that selective killing of tumour cells in vivo may be achievable. However, tumour killing in general was quite low, and highly variable between patients. We believe that this reflects the abnormal phenotype of the DC compared to DC isolated from healthy volunteers or patients in remission (fig 3), as a result of exposure to high tumour burden in vivo. This hypothesis is supported by the in vitro data illustrated in fig 6 as discussed in further detail below. These results therefore suggest that using DC vaccination for poor prognosis patients in temporary disease remission may be a more suitable clinical scenario than using patients with active disease.
However, tumour killing in general was quite low, and highly variable between patients. There was no obvious correlation between any other parameter measured and tumour kill. We obtained optimal killing after a single round of in vitro stimulation, and indeed effector cells from these patients did not culture well and numbers declined rapidly after one week of culture. Recent chemotherapy or the high tumour burden present at the time of blood sampling may have contributed to this poor long term viability. These technical difficulties may account for why the majority of in vitro studies on T cell activity against ovarian cancer cells utilize healthy donor T cells [33], ovarian cancer cell lines [34, 35] or peptide pulsed targets [36]. Hence reports using patient effector cells and primary tumour [37, 38] are limited, especially using samples from patients in relapse.
Since DC from patients with active disease secreted higher levels of the immunosuppressive cytokine TGFβ than healthy donors and remission patients, we examined whether TGFβ blockade could enhance the stimulatory ability of DC from a small subset of patients. The results of these experiments were promising, since the presence of anti-TGFβ antibody in the DC-effector cell co-cultures increased subsequent killing of autologous tumour, although these experiments need to be repeated in a larger group of individuals. TGFβ blockade has already been evaluated in pre-clinical models of cancer and fibrotic disease [39, 40], and the first phase I clinical trial in patients with advanced glomerulosclerosis using the anti-TGF antibody fresolimumab was published recently [41]. Thus dendritic cell immunotherapy under cover of a TGFβ blockade may offer an interesting future strategy.
In addition to TGFβ however, it is likely that other tumour derived suppressive factors may contribute to enhance an immunosuppressive/tolerogenic rather than immunostimulatory phenotype. In the case of relapsed ovarian cancer patients, the PB monocytes which serve as DC precursors are exposed to an enormous tumour burden in vivo. It has previously been reported that monocytes and DC can obtain a suppressive phenotype after exposure to tumour cells [42-44]. We therefore investigated a similar model using DC from healthy volunteers cocultured with SKOV-3 cells. The DC exposed to SKOV-3 via transwell filters showed an activated phenotype including enhanced DC-sign expression [32], but no difference in cytokine secretion in the three donors tested. However, the ability of ‘tumour educated’ DC to stimulate T cell mediated lysis of SKOV-A2 was reduced. While the precise molecular details of this inhibitory activity has still to be resolved, these experiments provide an in vitro model using human, rather than mouse cells which can readily be used for further more detailed mechanistic studies of this important phenomenon. This model uses SKOV-3 cells as both a source of antigen and a target of cytotoxic activity. Thus the model may favour responses which are specific to the SKOV-3 cell line. However previous studies from our laboratory have shown that the SKOV-3 line provides a source of tumour antigens such as MUC-1 and Her2Neu which are shared by many ovarian tumours [9,11]. Future studies using primary tumour cells in place of SKOV-3 will be valuable to judge whether the property of modulating monocytes is general for all ovarian cancer cells.
In conclusion, our study establishes an in vitro model which can be used to evaluate DC immunotherapy protocols, and uses this model to study the responses of DC and T cells to hypochlorous acid treated SKOV-3 cells as a generic source of multiple tumour associated antigens. The model was tested in the context of patients with advanced relapsed ovarian cancer, with ascites, allowing a ready access to autologous tumour targets for cytotoxicity assays. The study showed that this protocol can be used to stimulate cytotoxic effector cells, at least in vitro. Although it is not possible to compare directly with cells from healthy volunteers (since autologous tumour is obviously not available), a number of observations suggest that DC derived from patients with active disease are suboptimal, perhaps a result of being derived from monocytes exposed to a large tumour burden in vivo. Our results suggest that although, partly for ethical reasons, patients with active disease are often used for Phase I trials of novel therapeutics, this group may be suboptimal for attempts at immunotherapy. Instead future trials on patients in remission may offer a more favourable setting for successful immunotherapeutic intervention.
Acknowledgements
This work was supported by a grant from Ovarian Cancer Action. Benny Chain is a member of the MRC Centre for Medical Molecular Virology.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Kandalaft LE, Motz GT, Duraiswamy J, Coukos G. Tumor immune surveillance and ovarian cancer: lessons on immune mediated tumor rejection or tolerance. Cancer Metastasis Rev. 2011;30:141–151. doi: 10.1007/s10555-011-9289-9. [DOI] [PubMed] [Google Scholar]
- 2.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. doi: 10.1111/j.1600-065X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheever MA, Higano C. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA Approved Therapeutic Cancer Vaccine. Clin Cancer Res. 2011;17:3520–6. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 4.Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- 5.Hernando JJ, Park TW, Kubler K, Offergeld R, Schlebusch H, Bauknecht T. Vaccination with autologous tumour antigen-pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunol Immunother. 2002;51:45–52. doi: 10.1007/s00262-001-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 7.Peethambaram PP, Melisko ME, Rinn KJ, Alberts SR, Provost NM, Jones LA, et al. A phase I trial of immunotherapy with lapuleucel-T (APC8024) in patients with refractory metastatic tumors that express HER-2/neu. Clin Cancer Res. 2009;15:5937–5944. doi: 10.1158/1078-0432.CCR-08-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcinkiewicz J. Neutrophil chloramines: missing links between innate and acquired immunity. Immunol Today. 1997;18:577–580. doi: 10.1016/s0167-5699(97)01161-4. [DOI] [PubMed] [Google Scholar]
- 9.Chiang CL, Ledermann JA, Rad AN, Katz DR, Chain BM. Hypochlorous acid enhances immunogenicity and uptake of allogeneic ovarian tumor cells by dendritic cells to cross prime tumor-specific T cells. Cancer Immunol Immunother. 2006;55:1384–1395. doi: 10.1007/s00262-006-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prokopowicz ZM, Arce F, Biedron R, Chiang CL, Ciszek M, Katz DR, et al. Hypochlorous acid: a natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J Immunol. 2010;184:824–835. doi: 10.4049/jimmunol.0902606. [DOI] [PubMed] [Google Scholar]
- 11.Chiang CL, Ledermann JA, Aitkens E, Benjamin E, Katz DR, Chain BM. Oxidation of ovarian epithelial cancer cells by hypochlorous acid enhances immunogenicity and stimulates T cells that recognize autologous primary tumor. Clin Cancer Res. 2008;14:4898–4907. doi: 10.1158/1078-0432.CCR-07-4899. [DOI] [PubMed] [Google Scholar]
- 12.Arce F, Breckpot K, Stephenson H, Karwacz K, Ehrenstein MR, Collins M, et al. Selective ERK activation differentiates mouse and human tolerogenic dendritic cells, expands antigen-specific regulatory T cells, and suppresses experimental inflammatory arthritis. Arthritis Rheum. 2011;63:84–95. doi: 10.1002/art.30099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitale M, Pelusi G, Taroni B, Gobbi G, Micheloni C, Rezzani R, et al. HLA class I antigen down-regulation in primary ovary carcinoma lesions: association with disease stage. Clin Cancer Res. 2005;11:67–72. [PubMed] [Google Scholar]
- 14.Sheu JJ, Shih Ie M. Clinical and biological significance of HLA-G expression in ovarian cancer. Semin Cancer Biol. 2007;17:436–443. doi: 10.1016/j.semcancer.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung YW, Kim YT, Kim SW, Kim S, Kim JH, Cho NH, et al. Correlation of human leukocyte antigen-G (HLA-G) expression and disease progression in epithelial ovarian cancer. Reprod Sci. 2009;16:1103–1111. doi: 10.1177/1933719109342131. [DOI] [PubMed] [Google Scholar]
- 16.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JK, Hamilton CA, Anderson EM, Cheung MK, Baker J, Husain A, et al. A novel technique for the enrichment of primary ovarian cancer cells. Am J Obstet Gynecol. 2007;197:507–e501 505. doi: 10.1016/j.ajog.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Rolland P, Deen S, Scott I, Durrant L, Spendlove I. Human leukocyte antigen class I antigen expression is an independent prognostic factor in ovarian cancer. Clin Cancer Res. 2007;13:3591–3596. doi: 10.1158/1078-0432.CCR-06-2087. [DOI] [PubMed] [Google Scholar]
- 19.Leffers N, Lambeck AJ, de Graeff P, Bijlsma AY, Daemen T, van der Zee AG, et al. Survival of ovarian cancer patients overexpressing the tumour antigen p53 is diminished in case of MHC class I down-regulation. Gynecol Oncol. 2008;110:365–373. doi: 10.1016/j.ygyno.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Nowak M, Glowacka E, Szpakowski M, Szyllo K, Malinowski A, Kulig A, et al. Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett. 2010;31:375–383. [PubMed] [Google Scholar]
- 21.Giuntoli RL, 2nd, Webb TJ, Zoso A, Rogers O, Diaz Montes TP, Bristow RE, et al. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29:2875–2884. [PubMed] [Google Scholar]
- 22.Tran E, Nielsen JS, Wick DA, Ng AV, Johnson LD, Nesslinger NJ, et al. Polyfunctional T-cell responses are disrupted by the ovarian cancer ascites environment and only partially restored by clinically relevant cytokines. PLoS One. 2010;5:e15625. doi: 10.1371/journal.pone.0015625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, et al. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295:110–123. doi: 10.1016/j.canlet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Zhang H, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25− precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 26.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, et al. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez GC, Haisley C, Hurteau J, Moser TL, Whitaker R, Bast RC, Jr., et al. Regulation of invasion of epithelial ovarian cancer by transforming growth factor-beta. Gynecol Oncol. 2001;80:245–253. doi: 10.1006/gyno.2000.6042. [DOI] [PubMed] [Google Scholar]
- 28.Knippertz I, Hesse A, Schunder T, Kampgen E, Brenner MK, Schuler G, et al. Generation of human dendritic cells that simultaneously secrete IL-12 and have migratory capacity by adenoviral gene transfer of hCD40L in combination with IFN-gamma. J Immunother. 2009;32:524–538. doi: 10.1097/CJI.0b013e3181a28422. [DOI] [PubMed] [Google Scholar]
- 29.Kalady MF, Onaitis MW, Emani S, Abdel-Wahab Z, Tyler DS, Pruitt SK. Sequential delivery of maturation stimuli increases human dendritic cell IL-12 production and enhances tumor antigen-specific immunogenicity. J Surg Res. 2004;116:24–31. doi: 10.1016/j.jss.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Osada T, Nagawa H, Takahashi T, Tsuno NH, Kitayama J, Shibata Y. Dendritic cells cultured in anti-CD40 antibody-immobilized plates elicit a highly efficient peptide-specific T-cell response. J Immunother. 2002;25:176–184. doi: 10.1097/00002371-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Navabi H, Jasani B, Reece A, Clayton A, Tabi Z, Donninger C, et al. A clinical grade poly I:C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine. 2009;27:107–115. doi: 10.1016/j.vaccine.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 33.Weng D, Song B, Durfee J, Sugiyama V, Wu Z, Koido S, et al. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. Int J Cancer. 2011;129:1990–2001. doi: 10.1002/ijc.25851. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Zeng Y, Chen X, Larmonier N, Sepassi M, Graner MW, et al. Human ovarian tumour-derived chaperone-rich cell lysate (CRCL) elicits T cell responses in vitro. Clin Exp Immunol. 2007;148:136–145. doi: 10.1111/j.1365-2249.2007.03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L, Kong B, Sheng X, Sheu JJ, Shih Ie M. Dendritic cells transduced with Rsf-1/HBXAP gene generate specific cytotoxic T lymphocytes against ovarian cancer in vitro. Biochem Biophys Res Commun. 2010;394:633–638. doi: 10.1016/j.bbrc.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 36.Bellone S, Anfossi S, O’Brien TJ, Cannon MJ, Silasi DA, Azodi M, et al. Induction of human tumor-associated differentially expressed gene-12 (TADG-12/TMPRSS3)-specific cytotoxic T lymphocytes in human lymphocyte antigen-A2.1-positive healthy donors and patients with advanced ovarian cancer. Cancer. 2009;115:800–811. doi: 10.1002/cncr.24048. [DOI] [PubMed] [Google Scholar]
- 37.Koido S, Nikrui N, Ohana M, Xia J, Tanaka Y, Liu C, et al. Assessment of fusion cells from patient-derived ovarian carcinoma cells and dendritic cells as a vaccine for clinical use. Gynecol Oncol. 2005;99:462–471. doi: 10.1016/j.ygyno.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Gritzapis AD, Perez SA, Baxevanis CN, Papamichail M. Pooled peptides from HER-2/neu-overexpressing primary ovarian tumours induce CTL with potent antitumour responses in vitro and in vivo. Br J Cancer. 2005;92:72–79. doi: 10.1038/sj.bjc.6602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellavance EC, Kohlhapp FJ, Zloza A, O’Sullivan JA, McCracken J, Jagoda MC, et al. Development of Tumor-Infiltrating CD8+ T Cell Memory Precursor Effector Cells and Antimelanoma Memory Responses Are the Result of Vaccination and TGF-{beta} Blockade during the Perioperative Period of Tumor Resection. J Immunol. 2011;186:3309–3316. doi: 10.4049/jimmunol.1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaraj NS, Datta PK. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin Investig Drugs. 2010;19:77–91. doi: 10.1517/13543780903382609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mytar B, Woloszyn M, Szatanek R, Baj-Krzyworzeka M, Siedlar M, Ruggiero I, et al. Tumor cell-induced deactivation of human monocytes. J Leukoc Biol. 2003;74:1094–1101. doi: 10.1189/jlb.0403140. [DOI] [PubMed] [Google Scholar]
- 43.Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol. 2004;172:7341–7349. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- 44.del Fresno C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, et al. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol. 2005;174:3032–3040. doi: 10.4049/jimmunol.174.5.3032. [DOI] [PubMed] [Google Scholar]