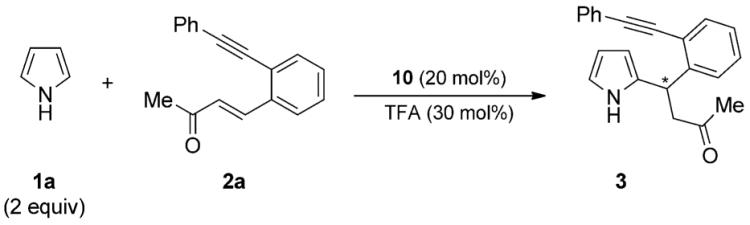
Table 1.
Optimization of the reaction conditions for the Friedel–Crafts Michael-type reaction.[a]
| ||||
|---|---|---|---|---|
| Entry | Solvent [mL] | t [h] | Yield [%][b] | ee [%][c] |
| 1 | CHCl3 (1.5) | 16 | 56 | 91 |
| 2 | CH2Cl2 (1.5) | 15 | 59 | 87 |
| 3 | toluene (1.5) | 21 | 60 | 88 |
| 4 | PhCl (1.5) | 21 | 61 | 87 |
| 5 | toluene (3.0) | 21 | 70 | 91 |
| 6 | CH2Cl2(3.0) | 24 | 68 | 91 |
| 7[d] | CHCl3 (3.0) | 65 | 89 | 93 |
| 8[d] | toluene (3.0) | 65 | 95 | 93 |
General reaction conditions: 2a (0.5 mmol), pyrrole 1a (1.0 mmol), 10 (20 mol %), TFA (30 mol%), rt.
Yield of isolated 3.
Determined by HPLC analysis on a chiral stationary phase.
The reaction was performed at 0 °C.
