Abstract
Much of the biology surrounding macrophage functional specificity has arisen through examining inflammation-induced polarising signals, but this also occurs in homeostasis, requiring tissue-specific environmental triggers that influence macrophage phenotype and function. The TAM receptor family of receptor tyrosine kinases (Tyro3, Axl and MerTK) mediates the non-inflammatory removal of apoptotic cells by phagocytes through the bridging phosphatidylserine-binding molecules Gas6 or Protein S. We show that one such TAM receptor (Axl) is exclusively expressed on mouse airway macrophages, but not interstitial macrophages and other lung leukocytes, under homeostatic conditions and is constitutively ligated to Gas6. Axl expression is potently induced by GM-CSF expressed in the healthy and inflamed airway, and by type I interferon or TLR3 stimulation on human and mouse macrophages, indicating potential involvement of Axl in apoptotic cell removal under inflammatory conditions. Indeed, an absence of Axl does not cause sterile inflammation in health, but leads to exaggerated lung inflammatory disease upon influenza infection. These data imply that Axl allows specific identification of airway macrophages, and that its expression is critical for macrophage functional compartmentalisation in the airspaces or lung interstitium. We propose that this may be a critical feature to prevent excessive inflammation due to secondary necrosis of apoptotic cells that have not been cleared by efferocytosis.
Introduction
Tyro3, Axl and MerTK form the TAM receptor tyrosine kinase family and bind Protein S and growth arrest-specific 6 (Gas6) proteins (Gas6 affinity for Axl>Tyro3>MerTK) whose N-terminal Gla domains bridge TAM receptors to phophatidylserine (PtdSer) on the surface of apoptotic cells, whereas the C-terminal sex hormone-binding globulin-like (SHGB) domain binds and activates the TAM receptor.1-3 TAM receptors are broadly expressed by cells of the vascular, nervous, reproductive and immune systems, with mature immune cell populations predominantly expressing Axl and/or MerTK, but not Tyro3.2 There is some evidence of differential expression of TAM receptors on immune cells in health,4 and dynamic regulation of Axl and MerTK expression on macrophages by pro- and anti-inflammatory factors has recently been characterized.5 In the innate immune system, TAM receptors inhibit inflammation during apoptotic cell efferocytosis via a negative feedback loop involving activation of suppressor of cytokine signaling (SOCS)-1 and SOCS-3 that inhibit cytokine and toll-like receptor (TLR) signalling pathways.6-8 An absence of TAM receptors results in impaired apoptotic cell clearance, the generation of antibodies to self-cellular antigens7, 9 and heightened responses to endotoxin9, 10 and inflammatory cytokines.11
Given a critical role of TAM receptors in suppressing immune responses, it is not surprising that defects in the TAM receptor system have been identified in patients with autoimmune diseases, including multiple sclerosis and systemic lupus erythematosus.7, 12 However, while inhibition of innate inflammation is essential to prevent autoimmunity during apoptotic cell clearance, prolonged engagement of TAM receptors may cause a state of unresponsiveness in antigen presenting cells required to clear pathogenic microorganisms. It is noteworthy that elevated Gas6 plasma levels are observed in patients with severe sepsis13 and MerTK is elevated on monocytes from patients with septic shock compared to trauma patients and healthy controls, and is linked to an adverse outcome.14
The mucosal immune system of the lung requires a fine balance between the ability to mount adequate responses to pathogens entering the respiratory tract, and the control of inflammatory processes that might arise from accumulation of large quantities of structural and infiltrating immune cells that undergo apoptosis.15 Despite this critical regulatory role of efferocytosis in the lung mucosa, little is known about TAM receptors or their ligands in the healthy or inflamed lung. Moreover, in light of recent evidence of an oncogenic function of TAM receptors and initial attempts to block TAM receptor signaling in cancer patients,16 it is of great interest to understand how manipulation of TAM receptor signaling could affect susceptibility to lung infections. We now show that, unlike the reported dominance of MerTK on murine primary macrophages4 and the human macrophage cell line U937,6, 17 Axl was specifically and constitutively expressed on airway, but not interstitial lung macrophages. Inhalation of influenza virus or stimulation of macrophages in vitro with viral PAMPs (pathogen-associated molecular patterns) or type I interferon specifically up-regulated Axl. Finally, we show that Axl−/− mice were unable to resolve influenza-induced inflammation causing an accumulation of apoptotic cells and necrotic cell debris. This study provides clear evidence for a constitutive and critical role for the TAM receptor Axl in lung immune homeostasis and in resolution of viral inflammatory lung disease.
Results
The TAM receptor Axl is exclusively expressed on airway macrophages in the homeostatic lung
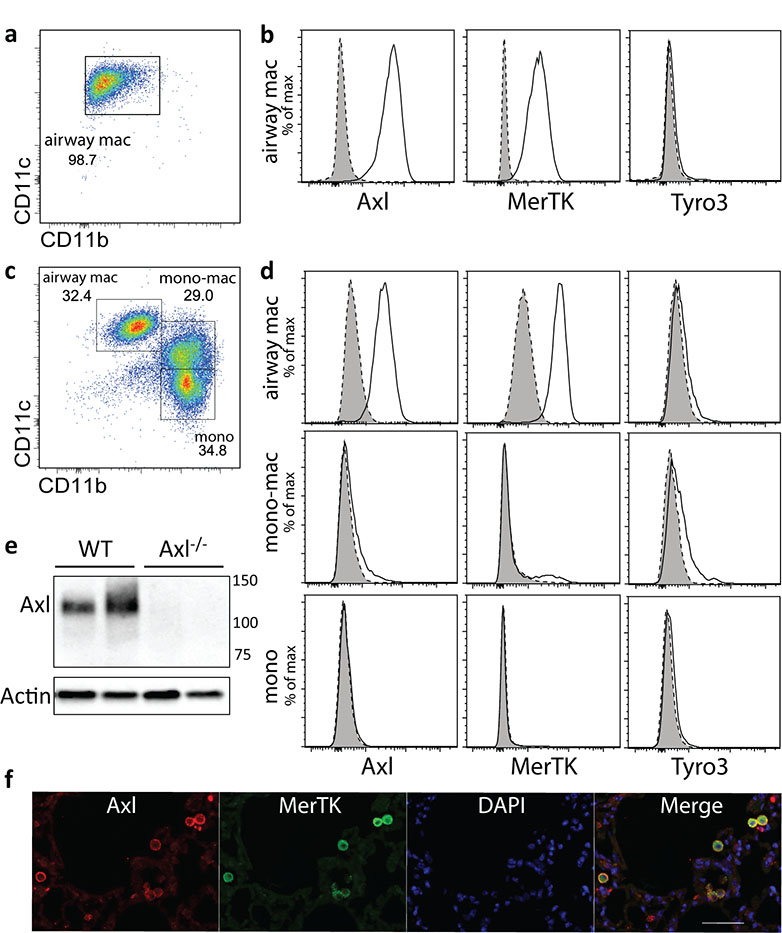
Murine airway macrophages in homeostasis were characterised as CD11bloCD11chiF4/80+Ly6G−, were 95% pure in health (Figure 1a) and expressed high levels of Axl and MerTK, but not Tyro3 (Figure 1b). Airway lavage does not remove all airway macrophages, which can be observed in dissociated lung interstitial tissue. Here, also present were monocyte/macrophages that were CD11bhiCD11cintermediate and monocytes that were CD11bhiCD11clo (Figure 1c). Axl and MerTK were almost exclusively expressed by CD11bloCD11chi airway macrophages at this site, while we did not detect significant levels of Tyro3 on any of the analysed populations (Figure 1d). High Axl protein expression was confirmed by Western blot in purified airway macrophages from wild type but not Axl−/− mice (Figure 1e). The majority of airway macrophages co-expressed both TAM receptors (Figure 1f). Interestingly, airway macrophages were the only immune cell population of the lung expressing high levels of Axl: we failed to detect Axl protein on neutrophils, eosinophils, T cells, NK cells and only a very low level of Axl was detected on dendritic cells residing in the lung under homeostatic conditions (Supplementary Figure S1).
Figure 1.
Axl is primarily expressed on airway macrophages in homeostasis
Flow cytometric analysis of F4/80 positive cells in (a) airway and (c) dissociated interstitial lung tissue by CD11b and CD11c expression. Airway macrophages (airway mac) are defined as CD11bloCD11chi; lung monocyte-macrophages (mono-mac) are defined as CD11bhiCD11cintermediate; lung monocytes (mono) are defined as CD11bhiCD11clo. (b and d) Flow cytometric analysis of Axl, MerTK and Tyro3 expression on airway macrophages, lung monocyte-macrophages, and lung monocytes. Specific staining, solid line/open. Isotype control, dotted line/shaded. (e) Western blot analysis of Axl protein expression by airway macrophages isolated from wild type (WT) or Axl−/− mice. Each lane represents lysates from individual mice. (f) Paraffin embedded lung sections from naïve mice stained with antibodies specific for Axl (red) or MerTK (green), and counter-stained with DAPI (blue). Scale bar: 50 μm. Data are representative of two independent experiments with four to five mice (all except f) or two independent experiments with two mice per group (f, immunohistochemistry).
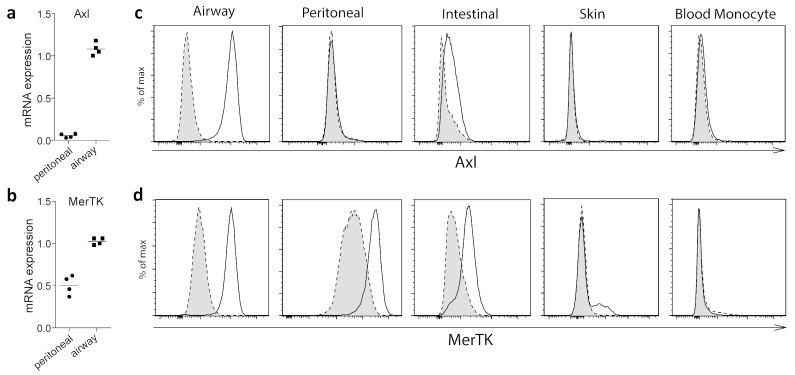
We next compared airway macrophage TAM receptor expression with macrophages in different anatomical locations. Airway macrophages expressed ~20-fold higher levels of Axl mRNA compared to peritoneal macrophages (Figure 2a), while expression of MerTK mRNA was more evenly distributed between these macrophage populations (Figure 2b). Consistently, within the analysed macrophage populations, Axl protein expression at homeostasis was restricted to mucosal macrophages in the intestinal tract and airway, with the most dominant expression on airway macrophages (Figure 2c), whereas MerTK was more widely expressed (Figure 2d), indicating a specific role for Axl in apoptotic cell clearance from the airways.
Figure 2.
Axl expression at homeostasis is restricted to mucosal macrophages
Axl (a) and MerTK (b) relative mRNA expression in purified airway and peritoneal macrophages. Symbols represent data from individual mice and lines represent the mean value. Flow cytometric analysis of (c) Axl and (d) MerTK expression on airway, peritoneal, intestinal and ear skin macrophages and blood monocytes. Specific staining, solid line/open. Isotype control, dotted line/shaded. (c,d) are representative of two independent experiments with five mice, except for the ear skin data; one experiment with five mice.
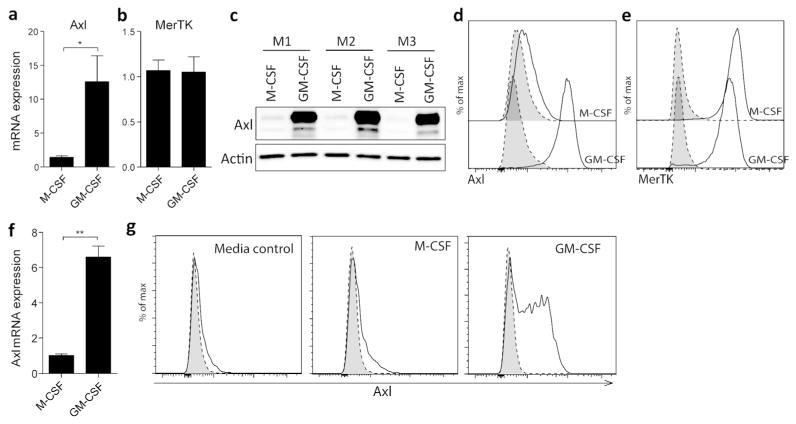
Specific expression of Axl on airway macrophages may reflect constituents of the healthy lung microenvironment. This hypothesis is supported by the exclusive ability of granulocyte-macrophage colony-stimulating factor (GM-CSF), but not macrophage colony-stimulating factor (M-CSF), to induce Axl mRNA (Figure 3a) and protein (Figure 3c) expression in the course of differentiation of bone marrow derived macrophages (BMDMs), an influence clearly visible also by flow cytometry (Figure 3d). High levels of MerTK expression, however, were detected in BMDMs differentiated by either M-CSF or GM-CSF (Figure 3b,e). Furthermore, Axl expression could also be selectively induced by GM-CSF, but not M-CSF, on otherwise Axl-negative terminally differentiated macrophages from the murine peritoneal cavity (Figure 3f,g). Given a critical role of GM-CSF in airway macrophage development,18, 19 this observation indicates that GM-CSF might act as a dominant signal for macrophage expression of Axl in homeostasis.
Figure 3.
GM-CSF drives Axl expression on macrophages
(a) Axl and (b) MerTK relative mRNA expression in M-CSF or GM-CSF-differentiated bone marrow-derived macrophages (BMDMs). (c) Western blot analysis of Axl protein expression in M-CSF- or GM-CSF-differentiated BMDMs. M1-M3 represent lysates of cells obtained from individual mice. Expression of (d) Axl and (e) MerTK in M-CSF or GM-CSF-differentiated BMDMs analysed by flow cytometry. Specific staining, solid line/open. Isotype control, dotted line/shaded. (f) Relative mRNA expression of Axl in peritoneal macrophages measured after either M-CSF (50 ng/ml) or GM-CSF (50 ng/ml) stimulation for 24 h. (g) Flow cytometric analysis of regulation of Axl expression on peritoneal macrophages isolated from naïve mice. Peritoneal macrophages were left untreated or were stimulated for 48 h with M-CSF (50 ng/ml) or GM-CSF (50 ng/ml). Specific staining, solid line/open. Isotype control, dotted line/shaded. Data are representative of two independent experiments with 3-4 mice. qPCR data are expressed as the mean relative gene expression±SEM of 3-4 individual mice (A, B and F). *P<0.05, **P<0.01 versus corresponding group; unpaired t test.
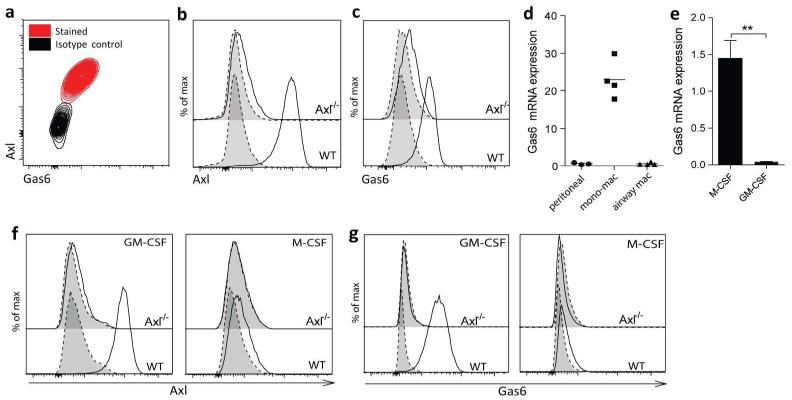
The TAM receptor ligand Gas6 is constitutively bound to Axl
TAM receptors recognise externalised PtdSer on apoptotic cells via the bridging ligands Gas6 or protein S.2 All of the airway macrophages expressing Axl also co-stained with Gas6 by flow cytometry, suggesting constitutive binding in the steady state (Figure 4a). This was confirmed in Axl−/− mice where both Axl (Figure 4b) and Gas6 (Figure 4c) expression was lost. The observation that Gas6 is not detected on Axl−/− airway macrophages despite high MerTK expression on these cells (Figure 1b) also indicated that constitutive binding of Gas6 in the absence of apoptotic cells is restricted to Axl. We next examined whether macrophage populations can also act as a source of Gas6 for Axl-expressing cells by profiling Gas6 mRNA expression. Low levels of Gas6 mRNA were observed in peritoneal or airway macrophages (CD11bloCD11chi), whereas elevated Gas6 expression was observed in lung monocyte-macrophages (CD11bhiCD11clo) (Figure 4d). This suggests that macrophages driven by particular stimuli can produce Gas6, whereas others can bind it in a paracrine fashion. To test this we derived BMDMs using M-CSF or GM-CSF; importantly, only the former expressed substantial levels of Gas6 mRNA (Figure 4e). Upon incubation with exogenous Gas6, only GM-CSF-differentiated BMDMs, which express high levels of Axl (Figure 4f), acquired Gas6 signal on the cell surface (Figure 4g). On the other hand, neither BMDMs from Axl−/− mice, nor Axl-negative M-CSF-differentiated BMDMs from WT mice were able to bind exogenous Gas6 (Figure 4g), confirming the specificity of homeostatic Axl-Gas6 interaction.
Figure 4.
Constitutive binding of Gas6 to the surface of airway macrophages through specific interaction with Axl
(a) Flow cytometric analysis of Axl and Gas6 co-expression on airway macrophages. Flow cytometric analysis of (b) Axl and (c) Gas6 expression on airway macrophages isolated from wild type (WT) or Axl−/− mice. (d) Relative mRNA expression of Gas6 in purified peritoneal macrophages, lung monocyte-macrophages (mono-mac) and airway macrophages. Symbols represent data from individual mice and lines represent the mean value. (e) Relative mRNA expression of Gas6 in M-CSF- or GM-CSF-differentiated BMDMs. (f,g) Analysis of Axl-dependent binding of Gas6 to M-CSF- or GM-CSF-differentiated BMDMs isolated from WT or Axl−/− mice. BMDMs were left untreated or were incubated with recombinant Gas6 for 1 h and then surface levels of (f) Axl and (g) Gas6 were analysed by flow cytometry. (f) Specific staining, solid line/open. Isotype control, dotted line/shaded. (g) +Gas6, solid line/open. −Gas6, dotted line/shaded. Data are representative of at least two independent experiments with four mice.**P<0.01 versus corresponding group; unpaired t test.
Viral triggers and influenza infection induce Axl expression on macrophages
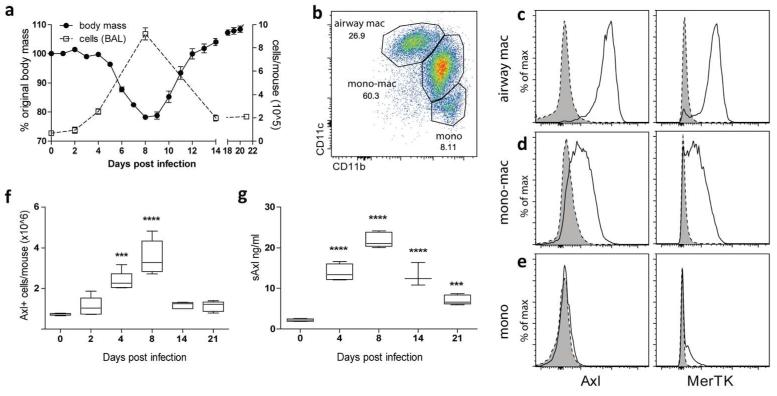
Macrophage populations in the airspaces and lung change dramatically during influenza infection; in wild type mice intranasal influenza H1N1 infection induces a viral titre-dependent weight loss that correlates with the extent of the inflammatory cell infiltrate.20, 21 We used a dose that causes up to 25% weight loss with recovery (Figure 5a). Infection with influenza resulted in monocyte/macrophages recruitment to the airspaces leading to the presence of 3 main populations (Figure 5b). As in health (Figure 1b), all resident airway macrophages (CD11bloCD11chi) were Axl and MerTK-positive (Figure 5c). In contrast to uninfected animals (Figure 1d), following viral infection infiltrating monocyte/macrophages (CD11bhiCD11cintermediate) also expressed Axl and MerTK, albeit to a lower intensity than airway macrophages (Figure 5d). No expression of Axl or MerTK was observed on infiltrating monocytes (Figure 5e). The number of Axl-expressing macrophages and the levels of soluble Axl in airway lavage fluid (Figure 5f,g) increased with infection, peaking at the point of maximal weight loss and cellular infiltrate, then returning to a level above that observed pre-infection. Monocyte/macrophages infiltrating the inflamed airway can therefore increase Axl expression during the course of viral infection.
Figure 5.
Increase in the numbers of Axl-expressing cells during influenza
(a) Change in body mass of wild type mice infected with 7.5pfu influenza (closed symbol) and change in total cell numbers in the BAL (open symbol), assessed at 0-21 days after infection. (b) Flow cytometric analysis of F4/80 positive cells in the BAL from influenza-infected mice (8 days post infection) by CD11b and CD11c expression. Airway macrophages (airway mac), lung monocyte-macrophages (mono-mac), and lung monocytes (mono) are defined as in Fig. 1 A. (c-e) Flow cytometric analysis of Axl and MerTK expression on airway macrophages, monocyte-macrophages, and monocytes in the BAL from mice infected with influenza (8 days post infection). Specific staining, solid line/open. Isotype control, dotted line/shaded. (f) Number of Axl expressing airway macrophages and monocyte-macrophage populations in the total lung during the course of influenza infection (0-21 days post infection). (g) Amount of soluble Axl (sAxl) released into the BAL fluid during the course of influenza infection. Data are representative of two independent experiments with five mice. ***P<0.001, ****P<0.0001 versus naïve group (day 0); one-way ANOVA.
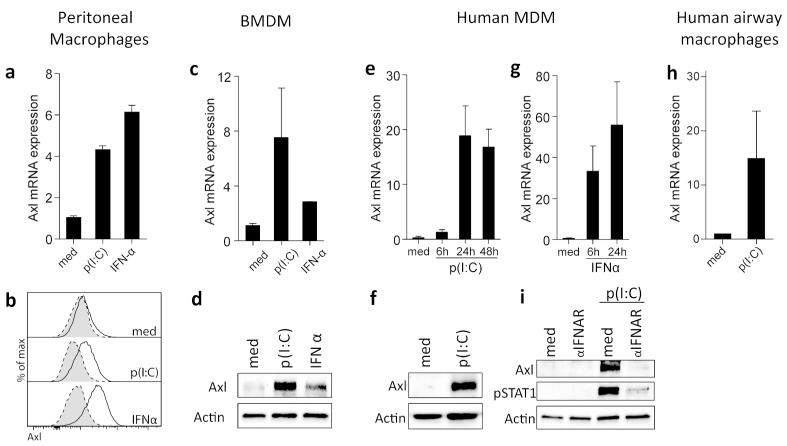
To assess the virally induced factors that might influence macrophage expression of Axl, we stimulated peritoneal macrophages or M-CSF-differentiated BMDMs in vitro with the viral TLR3 agonist polyinosinic:polycytidylic acid (p(I:C)) or with IFNα. Both of these stimuli induced Axl mRNA (Figure 6a,c) and protein expression (Figure 6b,d), demonstrating that both endogenous and exogenous triggers associated with viral infection drive Axl expression. This regulatory mechanism was conserved between mouse and human cells, as p(I:C) also up-regulated Axl mRNA (Figure 6e) and protein expression (Figure 6f) in otherwise Axl-negative human monocyte-derived macrophages (MDMs), as did IFNα (Figure 6g). Interestingly, p(I:C) also up-regulated Axl on human airway macrophages (Figure 6h). As p(I:C) mimics the component of viral genome, stimulation of macrophages with p(I:C) triggers IFNα production. To determine if p(I:C)-mediated Axl up-regulation is directly dependent on the release of IFNα, we treated human MDMs with an anti-type I interferon receptor (IFNAR) neutralizing antibody prior to p(I:C) stimulation and subsequently assessed the expression of Axl. Treating human MDMs with anti-IFNAR antibody blocked the induction of Axl protein expression by p(I:C) in a STAT1 phosphorylation-dependent fashion (Figure 6i), showing that Axl up-regulation by p(I:C) is dependent on IFNα release.
Figure 6.
Axl induction is triggered by immune responses to viruses
(a) Relative mRNA expression of Axl in peritoneal macrophages isolated from naïve mice measured after 24 h stimulation with p(I:C) (10 μg/ml) or IFNα (100 ng/ml). (b) Flow cytometric analysis of Axl expression on peritoneal macrophages stimulated for 48 h with p(I:C) (10 μg/ml) or IFNα (100 ng/ml). Specific staining, solid line/open. Isotype control, dotted line/shaded. (c) Relative mRNA expression of Axl and (d) Western blot analysis of Axl protein expression in M-CSF-differentiated BMDMs stimulated with p(I:C) (10 μg/ml) or IFNα (100 ng/ml) for 24 h. (e) Relative mRNA expression of Axl in human MDMs measured 6, 24 and 48 h after p(I:C) (50 μg/ml) stimulation. (f) Western blot analysis of Axl protein expression in human MDMs stimulated with p(I:C) (50 μg/ml) for 48 h. (g) Relative mRNA expression of Axl in human MDMs after 6 and 24 h stimulation with IFNα (1000 U/ml). (h) Relative mRNA expression of Axl in human airway macrophages stimulated with p(I:C) (1 μg/ml) for 4 h. (i) Western blot analysis of Axl protein expression and STAT1 phosphorylation in human MDMs stimulated with p(I:C) (50 μg/ml) for 48 h in the absence of presence of an anti-IFNAR neutralizing antibody (5 μg/ml). qPCR data are expressed as the mean relative Axl expression±SEM of 3-4 individual mice (a,c) or donors (e,g,h). Protein expression data are representative of 3-4 mice (b,d) or donors (f,i).
Axl is required for resolution of lung inflammatory disease upon influenza infection
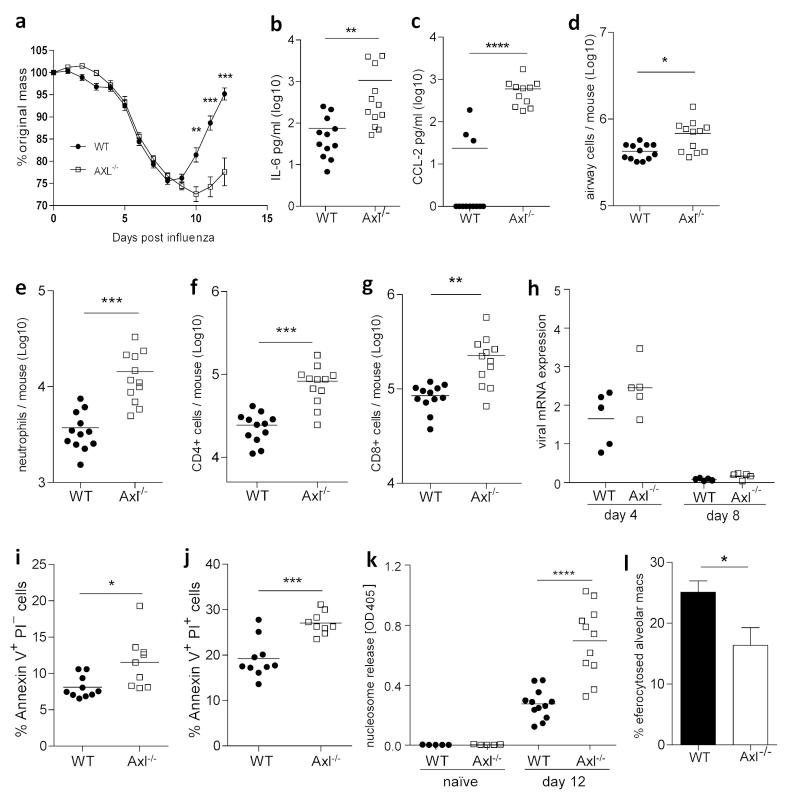
Axl−/− mice did not show any alterations in lung immune cell composition in homeostasis (Supplementary Figure S2), suggesting that the presence of MerTK is sufficient for the clearance of apoptotic cells under homeostatic conditions. However, in light of the high Axl expression on airway macrophages, but not other lung leukocytes, and rapid increases in the numbers of Axl-positive cells in the airways during influenza infection, we hypothesized that Axl plays a distinctive, but complementary role to MerTK during inflammatory lung disease. Indeed, upon influenza infection Axl−/− mice displayed enhanced weight loss, with impaired recovery, requiring the experiment to be terminated (Figure 7a). Exacerbated disease was associated with elevated inflammatory cytokine/chemokine release into the airways (Figure 7b,c). The number of total cells in the airway was also increased in the absence of Axl (Figure 7d), mostly accounted for by increases in neutrophils and CD4+ and CD8+ T cells (Figure 7e-g). Enhanced severity of influenza infection in mice lacking Axl was not due to a delay in viral clearance (Figure 7h) and is likely a result of secondary necrosis of un-efferocytosed apoptotic cells. Indeed, the numbers of early and late apoptotic cells (Figure 7i,j), as well as nucleosome release (Figure 7k) – indicative of necrosis or secondary necrosis of apoptotic cells22 – were elevated in the airway of Axl−/− mice infected with influenza. Finally, airway macrophages from Axl−/− mice displayed reduced uptake of apoptotic cells than those from wild type mice (Figure 7l), indicating that Axl-mediated efferocytosis by airway macrophages is a critical step in the process of resolution of lung inflammatory disease upon viral infection.
Figure 7.
Increased severity of viral lung disease in influenza-infected Axl−/− mice despite efficient clearance of viruses
(a) Change in body mass of wild type (WT) (closed symbol) and Axl−/− (open symbol) mice infected with 7.5pfu influenza. Amount of IL-6 (b) and CCL-2 (c) in the BAL fluid recovered on day 12 post influenza infection from WT and Axl−/− mice. (d) Analysis of viable cells in the BAL from WT and Axl−/− mice recovered on day 12 post influenza infection and counted using trypan blue exclusion. (e-g) Flow cytometric analysis of numbers of (e) neutrophils, (f) CD4+ T cells, and (g) CD8+ T cells in the BAL from WT and Axl−/− mice on day 12 post influenza infection. (h) Influenza genomic mRNA copies recovered from the total lung of WT and Axl−/− mice on days four and eight post influenza infection. (i,j) Flow cytometric analysis of percentage of (i) early (Annexin V+ propidium iodide (PI)−) and (j) late (Annexin V+PI+) apoptotic lymphocytes in the in the BAL from WT and Axl−/− mice on day 10 post influenza infection. (k) Amount of nucleosomes released in the BAL fluid recovered from WT and Axl−/− mice on day zero (naïve) and day 12 post influenza infection. (l) Efficiency of uptake of apoptotic thymocytes by WT (filled bar) and Axl−/− (open bar) airway macrophages measured by flow cytometry. (a-g,k) are representative of two or three independent experiments with 9-12 mice per group. (i,j) show data from one experiment with 10 mice per group. (h,l) are representative of two independent experiments with 4-5 mice per group. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 versus corresponding group; unpaired t test.
Discussion
We have found that under homeostatic conditions the TAM receptor Axl is preferentially expressed on murine airway macrophages and constitutively ligated by Gas6. While constitutive expression of Axl has been reported on certain macrophage populations, such as splenic red pulp macrophages and Kupffer cells in the liver,5, 17 we show that in the healthy lung, airway macrophages are the only population of immune cells expressing high levels of Axl. MerTK on the other hand, is expressed on all mature tissue macrophages.17 Also, despite constitutively binding Gas6, airway macrophages themselves expressed low levels of Gas6, and so may bind it in a paracrine manner after production from other cell types. Bone marrow, endothelial cells and vascular smooth muscle cells express Gas623-25 and we are the first to show differential expression of Gas6 in specific macrophage subsets i.e. CD11bhiCD11cintermediate monocyte/macrophages, but not CD11bloCD11chi airway macrophages. This is likely to result in functional polarisation of macrophages depending on their anatomical location. Though all airway macrophages also express a second TAM receptor, MerTK, Gas6 binding was lost in Axl−/− macrophages, supporting the idea that Axl is the highest affinity receptor for this PtdSer-binding ligand,26 and suggesting a unique and non-redundant role of Axl and MerTK in regulating responses to apoptotic cells. In a recently proposed model, Axl plays a dominant role in apoptotic cell uptake by macrophages under inflammatory conditions, while MerTK mediates macrophage responses to apoptotic cells in homeostasis and during immunosuppression.5 Consistently, we observed induction of Axl expression on macrophages by inflammatory stimuli in vitro and upon viral infection in vivo.
We have also found that GM-CSF induces Axl expression on peritoneal macrophages and BMDMs. GM-CSF is produced by a variety of cells, significantly airway epithelial type II cells,27 and is critical for airway macrophage development18 and the protection of airway epithelial integrity.19 GM-CSF−/− mice lack airway macrophages19 and the presence of GM-CSF autoantibodies or mutations in the GM-CSF receptor α or β chain leads to pulmonary airway proteinosis,28 a condition characterised by insufficient surfactant clearance by airway macrophages. Furthermore, GM-CSF-deficient mice experience severe morbidity to influenza virus infection,19 whereas treatment with GM-CSF increases airway macrophage numbers and protects against a lethal infection.29 Future studies will be needed to elucidate whether GM-CSF drives Axl expression in vivo and to determine which aspects of GM-CSF modulation of airway macrophage function depend on Axl activity.
Influenza infection of mice lacking Axl displayed a very similar pro-inflammatory phenotype to that reported for GM-CSF−/− animals.19 Axl−/− mice experienced excessive weight loss and a few reached the humane endpoint. Although in our experiments we used mice that lack Axl expression globally, recent evidence that airway macrophages are the main cell population responsible for efferocytosis in the respiratory tract supports the idea that the observed phenotype can be attributed to the lack of Axl on airway macrophages.19 Interestingly, viral clearance kinetics was unaffected, despite a number of viruses express PtdSer in their outer envelope30 and are opsonised by the TAM receptor ligands Gas6 or Protein S thereby acting as attachment factors enabling proximity to more specific entry receptors.31 Equivalent viral titres in Axl−/− and wild type mice in our experiments suggest that Axl is not important for control of influenza virus H1N1 infection, or may be compensated for by other PtdSer-recognising receptors. TIM1, for example, promotes productive infection of a wide variety of enveloped viruses and increases their internalisation, though for influenza H7N1 and SARS-CoV virions this does not result in a productive infection.32
Increased morbidity of influenza virus infection in Axl−/− mice is more likely explained by the enhanced pulmonary inflammation and cytokine release, which may have arisen through two different processes. Firstly, a lack of Axl signaling upon engagement of apoptotic cells may prevent induction of SOCS-1 and -3 that inhibit cytokine and TLR signaling,2, 33 and/or the NF-κB repressor, Twist, that binds to the TNF promoter and inhibits NF-κB–dependent transcription.8 Similarly, the process of viral uptake has been shown to subvert antiviral immunity through a process termed “apoptotic mimicry”, where viral envelope-expressed PtdSer sequesters Protein S and Gas6 that activate TAM receptors on bone marrow-derived dendritic cells causing a down-regulation of TLR and interferon signaling pathways.34 An absence of Axl would remove any negative influence of PtdSer-expressing influenza virus on innate inflammation.
Secondly, failure to remove apoptotic cells leads to an increase in their membrane permeability and secondary necrosis that exposes otherwise hidden intracellular danger-associated molecular patterns (DAMPS), including uric acid, high-mobility group box 1, SAP 130, S100 proteins, nucleic acids in mitochondrial DNA, IL-1 and IL-33.35, 36 These DAMPs are recognised by extracellular and intracellular pattern recognition receptors, such as RAGE and ST2,37, 38 that upon engagement produce a state of “sterile inflammation”.36 Clearly timely removal of apoptotic cells is essential to prevent further inflammation and resolve inflammatory disease. The observed increase in nucleosome release in the airways of influenza-infected Axl−/− mice in our study supports the idea of enhanced secondary necrosis, which could explain the observed elevated airway inflammatory cytokines. The airways of mice lacking GM-CSF (which induces Axl expression) following influenza infection are similarly enriched in dead cells and cellular debris indicating impaired clearance of apoptotic cells.19 In line with a pathogenic role of cellular components released from necrotic cells, circulating histones are direct mediators of lung inflammation and damage in patients with trauma-associated lung injury.39
Recently, a beneficial effect of administering an Axl-blocking antibody has been described during influenza virus infection,40 which is the opposite of what would be expected by blocking a receptor that activates anti-inflammatory pathways. The monoclonal antibody used in these studies prevents Gas6 binding to TAM receptors, causes receptor internalisation and inhibits downstream signalling.41 However, we and others5 show that Gas6 is constitutively bound to Axl-expressing airway macrophages; whether this antibody can disrupt this established interaction is not known. TAM receptors are pivotal inhibitory receptors that terminate cytokine receptor signaling.2, 8, 33 Chronic inflammation and systemic autoimmune disorders occur in TAM triple-knockout mice,33 phagocytes lacking TAM receptors display defective phagocytosis and overproduction of proinflammatory cytokines4, 7, 42 and administration of protein S protects against LPS-induced lung injury.43 The beneficial effect of administering intraperitoneally an antibody that blocks Gas6 binding to Axl in virus-infected mice may in fact reflect antibody binding to soluble Axl that we have observed in the peripheral blood (mean concentration±SEM 20.93±0.79 ng/ml, n=12), which would reduce the competition for free Gas6 and promote membrane-bound Axl signalling. The distribution of administered antibody and whether it reached the high intensity of Axl expression on airway macrophages was not tested.40
Targeting Axl for the treatment of Gas6-Axl related diseases is of high current interest.16 A small molecule inhibitor that preferentially targets Axl inhibits breast cancer metastasis and angiogenesis,44 and is currently in phase 1 clinical trials (BGB324 by BergenBio). Complications however, may arise from targeting a receptor that is also cleaved. With regards to the immune system, therapeutics sequestering the soluble form of Axl would be anti-inflammatory as it would remove competition for Gas6 by membrane tethered Axl. Conversely, blockade of Gas6 interaction with membrane-bound Axl or Axl enzymatic activity would be pro-inflammatory and lead to heightened inflammation due to secondary necrosis of apoptotic cells and a loss of anti-inflammatory signalling cascades. Current antibodies do not distinguish between soluble and membrane-bound forms of Axl at present and the drive to target this pathway stems from the known biology of membrane tethered Axl. The selective expression of Axl on airway, but not interstitial macrophages in the homeostatic state and its role in preventing secondary necrosis infers an important function that should not be ignored.
Materials and methods
Detailed information on the experimental materials and methods used in this study can be found in Supplementary Material.
Mouse infection and sampling
8-12 week old female C57BL/6 (Harlan Olac Ltd, Bicester, UK) were maintained in specific pathogen free conditions at Bio Safety Level 2, with a 12 h light-dark cycle, dry food pellets and water ad libitum in accordance with institutional and UK Home Office guidelines. The generation of Axl−/− has been described previously,45 and all Axl−/− mice used in this study have been back-crossed to C57BL/6J background for at least ten generations. Mice were intranasally infected with 7.5 pfu of Influenza A virus, Puerto Rico/8/34(PR8), H1N1 and at specific time points euthanized by i.p. injection of 3 mg pentobarbitone and exsanguination via the femoral artery. Bronchoairway lavage (BAL), lung tissue and samples from distal sites (peritoneum, small intestine, bone marrow and blood) were harvested and dissociated to a single cell suspension using methods described previously.20, 46 Total macrophages from murine ears were extracted by splitting the dermis and epidermis in 0.8% trypsin for 30 min at 37°C. Dermis and epidermis were minced with scissors and then the dermis fraction was further digested in 0.5 Wunch units/ml of collagenase for 30 min at 37°C. Both dermis and epidermis cell suspension were then passed through a 70 μm sieve and combined. Red blood cells were lysed in whole blood and homogenised lung tissue using ammonium-chloride-potassium lysis buffer. BAL fluid was retained for analysis of soluble Axl (R&D systems, Abingdon, UK) or cytokines (e-Bioscience, Hatfield, UK) by ELISA. Viral titre was determined in lung homogenates by qPCR on a QuantStudio 12K Flex PCR system (Life Technologies, Paisley, UK) (fwd: 5′-GGACTGCAGCGTAGACGCTT-3′; rev: 5′-CATCCTGTTGTATATGAGGCCCAT-3′)47 and quantified by the ΔΔCT method using QuantStudio 12K Flex Software v1.1.1 (Life Technologies).
Flow cytometry
2×105 to 1×106 cells were incubated with near IR LIVE/DEAD fixable cell stain kit according to the manufacturer’s instruction (Life Technologies) followed by anti-mouse CD16/32 Fc block (eBioscience) for 20 min at 4°C. After washing with PBA (PBS containing 1% BSA, 0.1% sodium azide), cells were stained with a customised extracellular antibody panel for 30 min at 4°C in PBA and then fixed for 20 min with 2% paraformaldehyde or kept on ice with 5 μM EDTA prior to sorting. Cells were run on a BD FACS Canto II collecting at least 10,000 events of the target population and analysed using FlowJo (Tree Star, Ashland, OR, US). A BD Influx was used for sorting pure airway and peritoneal macrophages for qPCR analysis. Alveolar macrophages were identified as CD11bloCD11chiF4/80hi and highly auto-fluorescent. Peritoneal macrophages were identified as CD11bhighCD11cloF4/80hi. All lineage markers were purchased from eBioscience (Hatfield, UK) or Biolegend (San Diego, CA, USA). Axl (clone 175128), MerTK (polyclonal goat IgG, biotinylated), Tyro3 (clone 109646) and Gas6 (polyclonal goat IgG, biotinylated) antibodies were from R&D Systems (Abingdon, UK). Axl and Tyro3 antibodies were conjugated to Alexa Fluor-647 dye using a labelling kit (Life Technologies) according to the manufacturer’s instruction.
Uptake of apoptotic thymocytes by macrophages
Protocol for measurement of apoptotic thymocyte uptake efficiency was adapted from48 and described in Supplementary Material.
Human alveolar macrophage isolation and culture
Alveolar macrophages were isolated as previously described49 and described in detail in Supplementary Material.
Statistics
GraphPad Prism version 5.04 was used for all statistical calculations. For multiple dataset analysis ANOVA with Bonferroni correction was applied. To compare two datasets paired or unpaired t tests were applied. Data are presented as the mean ± standard error of the mean (SEM). P values<0.05 were considered significant.
Supplementary Material
Acknowledgements
The authors would like to thank Ms. Cecilia Forss and Mr. Thomas Fenton (MCCIR, University of Manchester) for assistance with monocyte isolations, Dr. Amy Saunders (MCCIR, University of Manchester) for extraction of cells from mouse ear skin, and Professor Greg Lemke and Dr. Anna Zagorska (Salk Institute) for critical reading of the manuscript. This research was supported by Manchester Collaborative Centre for Inflammation Research, a joint initiative of The University of Manchester, AstraZeneca and GlaxoSmithKline. TJB was supported by the Wellcome Trust (reference 093612/Z/10/Z).
Footnotes
Disclosure:
The authors declare no conflict of interest.
Supplementary Material is linked to the online version of the paper at http://www.nature.com/mi
References
- 1.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 5.Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol. 2014;15:920–928. doi: 10.1038/ni.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87:869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 7.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharif MN, et al. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 10.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 11.Deng T, Zhang Y, Chen Q, Yan K, Han D. Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology. 2012;135:40–50. doi: 10.1111/j.1365-2567.2011.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder MD, Kilpatrick TJ. TAM receptor signalling and demyelination. Neuro-Signals. 2009;17:277–287. doi: 10.1159/000231894. [DOI] [PubMed] [Google Scholar]
- 13.Ekman C, Linder A, Akesson P, Dahlback B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit Care. 2010;14:R158. doi: 10.1186/cc9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guignant C, et al. Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Med. 2013;39:1556–1564. doi: 10.1007/s00134-013-3006-9. [DOI] [PubMed] [Google Scholar]
- 15.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 16.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 17.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilliams M, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider C, et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10:e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snelgrove RJ, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 21.Didierlaurent A, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit Rev Clin Lab Sci. 2009;46:1–24. doi: 10.1080/10408360802485875. [DOI] [PubMed] [Google Scholar]
- 23.Avanzi GC, et al. GAS6, the ligand of Axl and Rse receptors, is expressed in hematopoietic tissue but lacks mitogenic activity. Exp Hematol. 1997;25:1219–1226. [PubMed] [Google Scholar]
- 24.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The Protein Encoded by a Growth Arrest-Specific Gene (Gas6) Is a New Member of the Vitamin-K-Dependent Proteins Related to Protein-S, a Negative Coregulator in the Blood-Coagulation Cascade. Mol Cell Biol. 1993;13:4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano T, et al. Vascular Smooth Muscle Cell-derived, Gla-containing Growth-potentiating Factor for Ca-mobilizing Growth Factors. J Biol Chem. 1995;270:5702–5705. doi: 10.1074/jbc.270.11.5702. [DOI] [PubMed] [Google Scholar]
- 26.Nagata K, et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 27.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura T, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang FF, et al. GM-CSF in the lung protects against lethal influenza infection. Am J Respir Crit Care Med. 2011;184:259–268. doi: 10.1164/rccm.201012-2036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercer J. Viral apoptotic mimicry party: P.S. Bring your own Gas6. Cell Host Microbe. 2011;9:255–257. doi: 10.1016/j.chom.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Morizono K, et al. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 2011;9:286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jemielity S, et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9:e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharyya S, et al. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rock KL, Lai JJ, Kono H. Innate and adaptive immune responses to cell death. Immunol Rev. 2011;243:191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonilla WV, et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 39.Abrams ST, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata T, et al. Axl receptor blockade ameliorates pulmonary pathology resulting from primary viral infection and viral exacerbation of asthma. J Immunol. 2014;192:3569–3581. doi: 10.4049/jimmunol.1302766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye X, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29:5254–5264. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 42.Cohen PL, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takagi T, et al. Direct effects of protein S in ameliorating acute lung injury. J Thromb Haemost. 2009;7:2053–2063. doi: 10.1111/j.1538-7836.2009.03642.x. [DOI] [PubMed] [Google Scholar]
- 44.Holland SJ, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 45.Lu Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 46.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 47.van Elden LJ, Nijhuis M, Schipper P, Schuurman R, van Loon AM. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miksa M, Komura H, Wu R, Shah KG, Wang P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J Immunol Methods. 2009;342:71–77. doi: 10.1016/j.jim.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kent LM, Fox SM, Farrow SN, Singh D. The effects of dexamethasone on cigarette smoke induced gene expression changes in COPD macrophages. Int Immunopharmacol. 2010;10:57–64. doi: 10.1016/j.intimp.2009.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.