Abstract
Endothelial nitric oxide (NO) bioavailability, microvascular function, and host oxygen consumption have not been assessed in pediatric malaria. We measured NO-dependent endothelial function by using peripheral artery tonometry to determine the reactive hyperemia index (RHI), and microvascular function and oxygen consumption (VO2) using near infrared resonance spectroscopy in 13 Indonesian children with severe falciparum malaria and 15 with moderately severe falciparum malaria. Compared with 19 controls, children with severe malaria and those with moderately severe malaria had lower RHIs (P = .03); 12% and 8% lower microvascular function, respectively (P = .03); and 29% and 25% higher VO2, respectively. RHIs correlated with microvascular function in all children with malaria (P < .001) and all with severe malaria (P < .001). Children with malaria have decreased endothelial and microvascular function and increased oxygen consumption, likely contributing to the pathogenesis of the disease.
Keywords: severe malaria, cerebral malaria, Plasmodium falciparum, endothelial function, microvascular function, oxygen consumption, tissue hypoxia
The mortality rate in falciparum malaria remains high [1], and delineation of the pathogenic mechanisms are required to develop adjunctive therapies to improve survival. In falciparum malaria, tissue hypoxia results from decreased microcirculatory flow due to parasite sequestration and endothelial dysfunction [2]. Nitric oxide (NO) regulates these pathogenic processes by decreasing expression of endothelial receptors used for parasite binding [3] and increasing capillary flow [4]. In severe falciparum malaria, decreased systemic and organ-specific NO bioavailability is a result of multiple mechanisms, including decreased L-arginine concentrations, the substrate for NO production [5, 6]; reduced NO synthase 2 (NOS2) expression [5, 6]; increased NO quenching [7, 8]; and elevated expression of asymmetric dimethylarginine, an endogenous NOS inhibitor [6, 9]. African children with severe malarial anemia had decreased NO, with increased pulmonary artery pressure, NO quenching and arginase [8]. Clinical trials evaluating inhaled NO as adjunctive therapy in pediatric cerebral malaria have been undertaken, but no studies have measured endothelial NO bioavailability in pediatric falciparum malaria nor the association with disease severity.
Microvascular function measures the capacity to match oxygen delivery to demand [4], with a major mediator being NO [4]. In adult falciparum malaria, microvascular dysfunction is associated with increased mortality [4]. Oxygen demand may also play a role in tissue hypoxia in pediatric malaria, but results of a previous small study in Kenyan children were inconclusive [10].
We measured endothelial NO bioavailability, skeletal muscle microvascular function, and oxygen consumption in Indonesian children with severe and moderately severe malaria and compared these data to those of controls.
METHODS
The study was approved by ethics committees of the National Institute of Health Research and Development, Indonesia, and the Menzies School of Health Research, Australia, and was undertaken at Mitra Masyarakat Hospital, Timika, Papua, Indonesia, in 2008–2009 [4]. Written informed consent was obtained from parents or guardians of participating children.
Children 4–12 years of age with severe or moderately severe Plasmodium falciparum malaria were enrolled. Severe malaria was defined as peripheral parasitemia with ≥1 of the following modified World Health Organization criteria of severity [4]: a value of <11 on the Glasgow coma scale, acute kidney injury (creatinine level, >265 μmol/L), hyperbilirubinemia, blackwater fever, hypoglycemia (glucose level, <2.2 mmol/L), respiratory distress (pathological deep breathing), acidosis (venous bicarbonate level, <15 mmol/L), shock (systolic blood pressure, <70 mm Hg after fluid resuscitation), and hyperparasitemia (parasites in >10% of erythrocytes). Exclusion criteria were hemoglobin levels of <60 g/L or receipt of antimalarial treatment for >18 hours. Moderately severe malaria was defined as a parasite count of >1000/μL that, on the basis of an assessment by nonstudy physicians, required hospital admission, with no warning signs or severe criteria. Control children were nonrelated hospital visitors who were afebrile in the preceding 48 hours and P. falciparum negative by microscopy and rapid diagnostic testing (HRP2, Paracheck, India).
Standardized history and physical examinations were documented, and parasite counts were determined by microscopy. Hemoglobin level, biochemistry characteristics, acid-base parameters, and lactate level were measured with a bedside i-STAT analyzer. Physiological measurements were performed at enrollment and discharge.
Peripheral Arterial Tonometry (PAT) and Near-Infrared Resonance Spectroscopy (NIRS)
Endothelial NO bioavailability was measured noninvasively by use of PAT, as described elsewhere [6]. Briefly, this method measures the change in the pulse wave amplitude in response to an ischemic stress of 50 mm Hg above the systolic blood pressure for 5 minutes to give a reactive hyperemia index (RHI) [6].
NIRS was performed concurrently with PAT to assess microvascular function and tissue oxygen consumption, using the same level of ischemic stress, as described elsewhere [4]; Supplementary Figure 1. Methemoglobin levels were measured concurrently with a CO-oximeter (Masimo, Radical 7) because increased levels could affect NIRS results [4].
Statistical Methods
Statistical analysis was performed using Stata, version 11. Inter-group differences among children with severe or moderately severe malaria and controls were compared using analysis of variance or the Kruskal-Wallis test, where appropriate. An a priori pairwise comparison, using the Sidak method, compared children with severe malaria to those with moderately severe malaria, children with severe malaria to healthy controls, and children with moderately severe malaria to healthy controls. Pearson or Spearman correlation coefficients were determined, as appropriate. Paired analysis was performed using a paired t test or a paired-sample Wilcoxon signed-rank test. A 2-sided P value of <.05 was considered statistically significant.
RESULTS
Forty-seven children were enrolled; 13 had severe malaria (with cerebral malaria in 5 and parasitemia of >10% in 8), 15 had moderately severe malaria, and 19 were healthy controls. The only death involved a child with cerebral malaria. All children with severe malaria were treated with intravenous artesunate, and 4 received antibiotics. All patients with moderately severe malaria received intravenous artesunate initially, switching to oral artemisinin- combination therapy once artesunate therapy was tolerated. Baseline demographic characteristics, clinical features, and hematological and biochemical results are summarized in Table 1.
Table 1. Baseline Demographic Characteristics, Clinical Features, and Results of Hematological and Biochemical Tests Among Patient Groups.
| Characteristic | Healthy Control Group (n = 19) | Moderately Severe Malaria Group (n = 15) | Severe Malaria Group (n = 13) | P a |
|---|---|---|---|---|
| Age, y | 8 (6–12) | 7 (4-12) | 8 (5-11) | |
| Male sex, no. (%) | 14 (73%) | 9 (60%) | 9 (69%) | |
| Fever duration before presentation, d | NA | 2 (1-14) | 3 (1-7) | |
| Temperature, °C, mean (range) | 36.2 (36-36.8) | 37.2 (35.7-39.2) | 38.0 (35.8-39.5) | <.05 |
| Weight, kg | 20 (14-40) | 22 (14-32) | 20 (15-32) | |
| Blood pressure, mm Hg, mean (range) | ||||
| Systolic | 106 (84-128) | 98 (80-126) | 90 (80-139) | <.05 |
| Diastolic | 68 (38-81) | 60 (50-70) | 61 (40-79) | <.05 |
| Heart rate, beats/min, mean (range) | 80 (61-117) | 113 (86-138) | 125 (61-187) | <.05 |
| Respiratory rate, breaths/min | 18 (18-24) | 24 (20-36) | 28 (20-48) | <.05 |
| Pulse O2 saturation, %, mean (range) | 99 (99-100) | 99 (98-100) | 98 (96-100) | |
| Methemoglobin level, % of total hemoglobin | 0.6 (0-1.7) | 0.9 (0.2-1.7) | 0.75 (0-1.5) | |
| White blood cell count, ×103 cells/μL, mean (range) | 8.3 (4.6-12.8) | 7.1 (2-19.7) | 9.8 (3.3-14.9) | <.05 |
| Hemoglobin level, g/dL, mean (range) | 11.3 (8.3-13.7) | 9.8 (6.7-13.3) | 10.5 (7.2-12.4) | <.05 |
| Platelet count, ×109 platelets/L, mean (range) | 188 (132-431) | 89 (21-242) | 99 (34-326) | <.05 |
| Creatinine level, mmol/L, mean (range) | NA | 44 (4-65) | 44 (25-66) | <.05 |
| Lactate level, mmol/L, mean (range) | NA | 1.37 (0.70-1.64) | 1.77 (0.78-4.56) | <.05 |
| Parasite density, parasites/μL, geometric mean (95% CI) | NA | 25 813 (9225-72 231) | 116 023(13 014-1 034 359) | <.05 |
| Reactive hyperemia index | 1.29 (1.05-2.4) | 1.09 (1-1.30) | 1.13 (1.0-1.34) | <.05 |
| Tissue O2 saturation, % at baseline | 81 (76-85) | 81 (80-84) | 87 (77-90) | |
| Tissue hemoglobin index at baseline | 14.4 (13.9-15.7) | 14.3 (13.4-15.4) | 15.5 (13.4-16.3) | |
| StO2, % at end of occlusion | 36 (33-42) | 37 (34-38) | 38 (32-43) | |
| Recovery StO2, % increase/min | 251 (223-266) | 230 (203-242) | 220 (197-242) | <.05 |
| Peak StO2 after release, % | 95 (94-95) | 94 (93-95) | 96 (91 -97) | |
| Difference between peak and baseline StO2, % | 15 (14-17) | 12 (10-14) | 9 (7-13) | <.05 |
| O2 consumption, StO2 %/min | −10.3 (−11.6 to −8) | −13.0 (−13.73 to −10.72) | −13.3 (−13.94 to −11.43) | <.05 |
All results are median (range), unless otherwise indicated.
Abbreviations: CI, confidence interval; NA, not applicable; StO2, tissue O2 saturation.
By the Kruskal-Wallis test, comparing the healthy control group, severe malaria group, and moderately severe malaria group.
Endothelial Function With Clinical Disease and Biomarkers of Severity
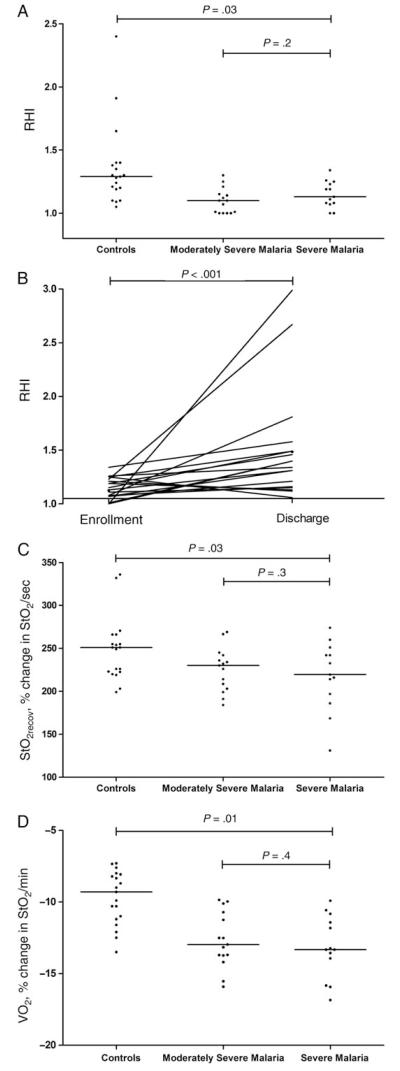
The median RHI was lower for patients with severe malaria (1.13; interquartile range [IQR], 1.08–1.23) and for those with moderately severe malaria (1.09; IQR, 1–1.15), compared with that for health controls (1.29; IQR, 1.1–1.65; P = .03; Table 1 and Figure 1A), with no difference between the malaria groups. There was an increase in RHI between enrollment and discharge among all children with malaria (P < .001; Figure 1B). Only 1 PAT result was recorded for the child who died. For patients with cerebral malaria, patients who were hyperparasitemic, and patients with moderately severe malaria, the median hospital stay was 4 days (range, 2–15 days), 2 days (range, 1–3 days), and 1 day (range, 1–2 days), respectively. No association was found between the RHI and either lactate level or parasite count among all patients, among patients with severe malaria, or among patients with moderately severe malaria.
Figure 1.
A, Reactive hyperemia index (RHI), determined by peripheral arterial tonometry, in healthy controls, children with moderately severe malaria, and children with severe malaria (P = .03, by the Kruskal-Wallis test). B, RHI at enrollment and discharge for patients with severe malaria (P < .001, by the Wilcoxon paired sign ranked test). Horizontal bars represent pairwise comparisons between enrollment and discharge values. C, Skeletal muscle reoxygenation rate (StO2recov; defined as the microvascular function or rate of increase in StO2) after release of ischemic stress in healthy controls, children with moderately severe malaria, and children with severe malaria (P = .03, by the Kruskal-Wallis test). D, Skeletal muscle oxygen consumption (VO2) in healthy controls, children with moderately severe malaria, and children with severe malaria (P < .001, by analysis of variance). Horizontal lines represent the median values for each group. Horizontal bars represent pairwise comparisons between groups.
Tissue Oxygen Saturation (StO2), Microvascular Reactivity, Oxygen Consumption, and Disease Severity
NIRS measurements were performed for all children (Table 1). Methemoglobin levels were all <2%, with no significant difference among groups (Table 1). There were no significant differences in the StO2 at enrollment among disease categories and no change with clinical recovery in patients with either severe or moderately severe malaria. Microvascular function at enrollment was 12% and 8% lower in patients with severe malaria and patients with moderately severe malaria, respectively, compared with healthy controls (P = .03; Table 1, Figure 1C, and Supplementary Figure 1). The median difference between peak and baseline StO2 values was lower (Supplementary Figure 1) in patients with severe malaria (9; IQR, 7–13) and patients with moderately severe malaria (12; IQR, 10–14), compared with the difference for healthy controls (15; IQR, 14–17; P = .007; Table 1). However, pairwise comparisons revealed no difference between children with severe malaria and those with moderately severe malaria. There was no correlation between microvascular function and either lactate level or peripheral parasitemia.
Median oxygen consumption was increased for children with severe malaria (median, −13.3%/min; IQR, −13.94%/min to −11.43%/min) and children with moderately severe malaria (−13%/min; IQR, −13.73%/min to −10.72%/min), compared with the value for healthy controls (−10.3%/min; IQR, −11.6%/min to −8%/min; P = .01; Table 1 and Figure 1D), with no difference between the severe malaria and moderately severe malaria groups. There was no association between micro-vascular function and oxygen consumption in all children or in all children with malaria. There was a correlation between oxygen consumption and peripheral parasitemia in patients with moderately severe malaria (ρ = −0.56; P = .03), but this correlation was absent in all patients with malaria (ρ = −0.33; P = .1) and in patients with severe malaria (ρ = −0.3; P = .3). There was no association between lactate level and oxygen consumption in any of the study groups. There was no significant change seen in either lactate level or oxygen consumption after clinical recovery in all children, in those with severe malaria, or in those with moderately severe malaria.
RHI and Microvascular Reactivity
There was an association between the RHI at enrollment and microvascular function for all patients with malaria (r = 0.64; P < .001) and patients with severe malaria (r = 0.81; P < .001), but the association was not observed for patients with moderately severe malaria (r = 0.47; P = .08) or healthy controls (r = −0.2; P = .4). No longitudinal relationship was found between the RHI and microvascular function in all children, in those with severe malaria, or in those with moderately severe malaria.
DISCUSSION
Children with severe malaria and those with moderately severe malaria had decreased endothelial NO bioavailability, decreased microvascular function, and increased oxygen consumption. The association between endothelial NO and microvascular function in severe malaria and moderately severe malaria suggests that regulation of capillary flow may be NO mediated.
The endothelial NO findings in pediatric patients with severe malaria are similar to those found in adults at the same site, in whom both endothelial and pulmonary NO bioavailability are reduced [6]. The difference between age groups is the comparable impairment in both severe and moderately severe malaria in children, compared with impairment only in severe malaria in adults [6]. The decreased endothelial NO bioavailability in this study is consistent with findings from previous pediatric studies [5, 8]. Tanzanian children with cerebral malaria had hypoargininemia and decreased mononuclear NOS2 expression and NO production, compared with patients with moderately severe malaria and controls [5, 11]. Decreased NO has also been reported in Malian children with severe malaria anemia in association with increased levels of plasma arginase, cell-free hemoglobin, and NO quenching [8].
Impaired microvascular function detected by NIRS is also seen in severe malaria in both children and adults. However, the impairment in adults is proportional to disease severity [4], while impairment in children during moderately severe malaria is comparable to that during severe malaria. NIRS assesses only microcirculatory vessels, the major sites of parasite sequestration in falciparum malaria. Microvascular function reflects the overall capacity to respond to ischemia by increasing capillary flow or recruiting additional capillaries to hypoxic areas and decreasing flow to oxygenated tissue [12]. Microvascular dysfunction shunts or decreases oxygen delivery to hypoxic areas while maintaining or increasing flow to normoxic tissue [12]. While this may reflect physical obstruction due to parasite sequestration in the capillaries and postcapillary venules, it does not account for the similar impairment in severe malaria and moderately severe malaria with their presumed different microvascular parasite biomass. Orthogonal polarizing spectroscopy imaging of rectal capillaries in adults shows an increased proportion of obstructed capillaries in severe malaria, compared with moderately severe malaria [13]; however, similar studies have not been conducted in children. Control of capillary density occurs locally, with NO a major mediator of arteriolar dilatation in response to hypoxia [4], and the correlation between endothelial NO and microvascular dysfunction suggests that this may be an additional mechanism. Our results suggest that microvascular dysfunction may result in an inability to increase capillary density in response to hypoxia or increased oxygen demand, probably by exacerbating microvascular obstruction from parasite sequestration and by decreasing endothelial NO-related arteriolar dilatation.
Similar to malaria in adults, oxygen consumption is increased during malaria in children [4]. A Kenyan study using a metabolic cart found a nonsignificant increase in oxygen consumption in children with severe malarial anemia [10]. In addition, children who received blood transfusions had increased oxygen consumption, which was hypothesized to be repayment of an oxygen debt due to increased oxygen carrying capacity [10]. Our studies in both children and adults have found no association between oxygen supply and consumption. While increased oxygen consumption may seem to suggest an adequate oxygen supply, this is at odds with the increased lactate level indicative of tissue hypoxia in severe malaria. Several possible explanations could explain this discrepant observation. First, animal studies show that skeletal muscle increases oxygen extraction in response to decreased oxygen delivery [12]. Second, microvascular dysfunction in severe falciparum malaria may impair the ability of the microcirculation to match oxygen delivery and consumption, with shunting to areas with adequate oxygenation and no compensatory increase to areas with decreased oxygen tensions. Increased oxygen consumption in falciparum malaria could reduce tissue oxygenation by increasing the mismatch with impaired oxygen delivery. The mechanism of increased oxygen consumption in falciparum malaria remains unclear and may be due to a parasite-induced increase in host metabolism [4]. While there was an association between oxygen consumption and peripheral parasitemia in moderately severe malaria, it is unlikely that the parasite biomass is large enough to itself cause a measurable increase in oxygen consumption. In addition, the lack of a significant difference between severe malaria and moderately severe malaria in oxygen consumption, microvascular reactivity, and endothelial function suggests that additional factors, such as intensity of microvascular sequestration and obstruction, are likely to be important in the pathogenesis of severe disease [1].
Use of CO-oximetry revealed that methemoglobin levels were not elevated, in contrast to finding from an African study, which found an increase during severe malaria and moderately severe malaria by spectrophotometry [14]. The reasons are unclear but may relate to differences in methods, the subjects’ genetic background, and/or the subjects’ age.
Our study had several limitations, the main being that we did not enroll children <4 years, as smaller digits and thenar muscles in younger children did not yield reliable results. With changes to national regulations regarding preexisting material-transfer agreements, we were unable to measure plasma L-arginine, arginase, and cell-free hemoglobin levels and so were unable to delineate the probable mechanisms underlying decreased endothelial NO bioavailability and to assess the downstream effects of endothelial dysfunction.
In conclusion, endothelial NO production and microvascular function are decreased and skeletal muscle oxygen consumption is increased in children with severe malaria and moderately severe malaria. The association between endothelial NO production and microvascular function suggests that NO regulates capillary flow in children with falciparum malaria. Our results show that impaired endothelial NO production and microvascular dysfunction occur in severe falciparum malaria in both children and adults and provide further evidence to support trials of agents to increase endothelial NO bioavailability and improve tissue perfusion during severe malaria in all age groups [6, 15].
Supplementary Material
Acknowledgments
We thank Retno Gitawati, Indri Rooslamiati, Sri Muliati, and Erens Meokbum, for their support; Yohanes Kalvein Mira Mangngi, for nursing assistance; Ferryanto Chalfein, Prayoga, and Mitra Masyarakat Hospital staff, for clinical support; and Mauritz Okeseray, Paulus Sugiarto, Jeanne Rini Poespoprodjo, and Lembaga Pengembangan Masyarakat Amungme Kamoro, for support and assistance.
Financial support. This work was supported by the Australian National Health and Medical Research Council (ICRG ID 283321; grants 605807 and 1037304, fellowship 1042072 to N. M. A., and fellowship 605831 to T. W. Y.), the Wellcome Trust (ICRG GR071614MA and fellowship 091625 to R. N. P.), the National Institutes of Health (grant AI041764 to J. B. W. and grants AI057565 and AI100784 to D. L. G.), the US Veterans Affairs Medical Research Service (to J. B. W. and D. L. G.), and AusAID (to the Timika Malaria Research Facility).
Footnotes
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;383:723–35. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 2.Miller LH, Ackerman HC, Su XZ, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med. 2013;19:156–67. doi: 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothe-lial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–8. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis. 2013;207:528–36. doi: 10.1093/infdis/jis692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo TW, Lampah DA, Tjitra E, et al. Relationship of cell-free hemoglo-bin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis. 2009;200:1522–9. doi: 10.1086/644641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janka JJ, Koita OA, Traore B, et al. Increased pulmonary pressures and myocardial wall stress in children with severe malaria. J Infect Dis. 2010;202:791–800. doi: 10.1086/655225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo TW, Lampah DA, Tjitra E, et al. Increased asymmetric dimethylar-ginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog. 2010;6:e1000868. doi: 10.1371/journal.ppat.1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.English M, Muambi B, Mithwani S, Marsh K. Lactic acidosis and oxygen debt in African children with severe anaemia. Q J Med. 1997;90:563–9. doi: 10.1093/qjmed/90.9.563. [DOI] [PubMed] [Google Scholar]
- 11.Lopansri BK, Anstey NM, Weinberg JB, et al. Low plasma arginine con-centrations in children with cerebral malaria and decreased nitric oxide production. Lancet. 2003;361:676–8. doi: 10.1016/S0140-6736(03)12564-0. [DOI] [PubMed] [Google Scholar]
- 12.Vallet B. Vascular reactivity and tissue oxygenation. Intensive Care Med. 1998;24:3–11. doi: 10.1007/s001340050507. [DOI] [PubMed] [Google Scholar]
- 13.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assess-ment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 14.Anstey NM, Hassanali MY, Mlalasi J, Manyenga D, Mwaikambo ED. Elevated levels of methaemoglobin in Tanzanian children with severe and uncomplicated malaria. Trans R Soc Trop Med Hyg. 1996;90:147–51. doi: 10.1016/s0035-9203(96)90118-2. [DOI] [PubMed] [Google Scholar]
- 15.Yeo TW, Lampah DA, Rooslamiati I, et al. A randomized pilot study of L-arginine infusion in severe falciparum malaria: preliminary safety, efficacy and pharmacokinetics. PLoS One. 2013;8:e69587. doi: 10.1371/journal.pone.0069587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.