Abstract
Natural killer (NK) cells contribute to post-vaccination immune responses following activation by IL-2 from antigen-specific memory T cells or by crosslinking of the low affinity IgG receptor, CD16, by antigen-antibody immune complexes. Sensitivity of NK cells to these signals from the adaptive immune system is heterogeneous and influenced by their stage of differentiation. CD56dimCD57+ NK cells are less responsive to IL-2 and produce less IFN-γ in response to T cell-mediated activation than do CD56bright or CD56dimCD57− NK cells. Conversely, NK cell cytotoxicity - as measured by degranulation - is maintained across the CD56dim subsets. Human cytomegalovirus (HCMV), a highly prevalent herpes virus causing lifelong, usually latent, infections, drives the expansion of the CD56dimCD57+NKG2C+ NK cell population, skewing the NK cell repertoire in favour of cytotoxic responses at the expense of cytokine-driven responses. We hypothesised, therefore, that HCMV seropositivity would be associated with altered NK cell responses to vaccine antigens. In a cross-sectional study of 152 UK adults, with HCMV seroprevalence of 36%, we find that that HCMV seropositivity is associated with lower NK cell IFN-γ production and degranulation following in vitro restimulation with pertussis or H1N1 influenza vaccine antigens. Higher expression of CD57/NKG2C and lower expression of IL-18Rα on NK cells from HCMV seropositive subjects do not fully explain these impaired responses, which are likely the result of multiple receptor-ligand interactions. This study demonstrates, for the first time, that HCMV serostatus influences NK cell contributions to adaptive immunity and raises important questions regarding the impact of HCMV infection on vaccine efficacy.
Introduction
Natural killer (NK) cells are traditionally classified as cells of the innate immune system but can also act as mediators of adaptive immunity. In addition to their well-recognised role in antibody-dependent cytotoxicity, recent research has demonstrated a potential contribution to adaptive responses through their activation by antigen-specific CD4+ T cell-derived IL-2 [1-7]. The heightened IFN-γ response of NK cells in the context of a vaccine recall response suggests that NK cells may play a role in protection from vaccine preventable diseases, particularly as NK cells respond more quickly than T cells and comprise as much as 70% of all IFN-γ producing cells in the first 12-24 hours of the recall response [3].
We have shown, using the individual components of the diphtheria toxoid/tetanus toxoid/whole cell pertussis (DTwP) vaccine, that activation of NK cells following restimulation with vaccine antigens is heterogeneous, with CD56bright and CD56dimCD57− NK cells being most responsive as measured by surface expression of the high affinity IL-2 receptor (CD25) and accumulation of intracellular IFN-γ (CD25+IFN-γ+) [6]. Expression of CD57 by CD56dim NK cells was associated with a reduced capacity to produce IFN-γ, although degranulation responses were maintained [6]. These data are consistent with the accepted model of NK cell maturation whereby acquisition of CD57 is a marker of decreased sensitivity to exogenous cytokine stimulation [8,9].
Human cytomegalovirus (HCMV) infection drives profound changes in the NK cell repertoire. In particular, HCMV infection is strongly associated with preferential expansion of the CD56dimCD57+NKG2C+ NK cell subset [10-12]. This has direct implications for NK cell function as CD56dimCD57+NKG2C+ NK cells degranulate and secrete cytokines such as IFN-γ and TNF-α in response to crosslinking of CD16 (by IgG) or natural cytotoxicity receptors (NCRs; by infected, stressed or transformed cells) but respond poorly to pro-inflammatory cytokines such as IL-12 and IL-18 [12,13].
These observations imply that, in the context of infection or vaccination, NK cells from HCMV seropositive (HCMV+) individuals may effectively mediate antibody-dependent cytotoxicity (ADCC) after crosslinking of CD16 by IgG in immune complexes [11,13,14], but may respond poorly to inflammatory cytokines (reviewed in [15]). Specifically, the expanded CD56dimCD57+NKG2C+ NK cell subset may be less sensitive to IL-2 produced by antigen-specific CD4+ T cells and IL-12/IL-18 from accessory cells, such as dendritic cells and macrophages [3,6]. However, much of the data on skewing of the NK cell repertoire in HCMV+ individuals comes from studies of haematopoietic stem cell or solid organ transplantation [11,16,17] and follow up of these patients over time, in terms of susceptibility to infection or response to vaccination, is lacking. As a result, the true functional significance of HCMV-driven NK cell phenotypic changes is poorly understood. Moreover, previous investigations of the impact of HCMV infection on vaccination have produced rather inconsistent results with some studies reporting impaired vaccine responses in HCMV+ donors [18-23] whereas others find no impact of HCMV infection [24-27]. The impact of HCMV-driven immune differentiation on vaccine responsiveness and efficacy is therefore still unclear.
The aim of this study, therefore, is to compare NK cell responses to antigens previously encountered during immunisation (Bordetella pertussis) or during natural infection (H1N1 influenza virus), in HCMV seronegative (HCMV-) and HCMV+ individuals.
Materials and Methods
Study subjects
Volunteers (n = 152) were recruited from staff and students at LSHTM. All subjects gave written consent and the study was approved by the London School of Hygiene and Tropical Medicine Ethics Committee. Each subject provided a 50ml venous blood sample and reported vaccination history was recorded. Subject characteristics are summarised in Table I.
Table I. Donor characteristics.
Donors were classified as human cytomegalovirus (HCMV) seronegative and HCMV seropositive by anti-HCMV IgG ELISA, using 0.25IU/ml as the cut-off as per manufacturer’s instructions. NKG2C genotype (NKG2C+/+, NKG2C+/−, NKG2C−/−) was determined by PCR. IgG antibody titres against pertussis toxin (PT) and H1N1 were calculated from interpolation of a reference serum or high titre donor standard curve, respectively.
| HCMV seronegative (n = 97) | HCMV seropositive (n = 55) | |
|---|---|---|
| Median Age, Years (range) | 32 (20-70) | 35 (21-77) |
| Female n (%) | 73 (75) | 32 (58) |
| NKG2C Genotype +/+, +/−, −/−n (%) | 67/24/2 (72/26/2) | 35/17/2 (65/31/4) |
| NKG2C− Haplotype Frequency (%) | 15.0 | 19.4 |
| anti-HCMV IgG titre IU/ml median (range) | <0.25 | 394.2 (31.1-4411.6) |
| anti-PT IgG titre IU/ml median (range) | 6.7 (0.5-139.3) | 5.0 (0.8-179.9) |
| anti-H1N1 IgG titre arbitrary ELISA units median (range) | 214.6 (80.7-953.2) | 190.1 (90.2-522.7) |
Antibody detection by ELISA
Plasma was collected from heparinised whole blood and stored at −80°C until use. HCMV infection status was determined by HCMV IgG ELISA (BioKit). IgG antibodies to pertussis toxin (PT; NIBSC) and to formalin-inactivated whole H1N1 influenza virus (influenza A/California/7/2006(H1N1)v(NYMC-X179A); H1N1; NIBSC) were determined using in-house ELISA assays with goat anti-human IgG-peroxidase (Sigma) as the secondary antibody and SIGMAFAST™ OPD (Sigma) as the substrate. IgG concentrations were calculated by interpolation from a standard curve which was produced using anti-pertussis reference serum (NIBSC; IU/ml) or using plasma from a donor with high titres of antibodies to H1N1 influenza (IgG concentration expressed in Arbitrary ELISA Units, AEU) [28]). The pooled AB plasma used for in vitro assays contained 6.8 IU/ml IgG to PT and had an H1N1 titre of 273.8 AEU.
PBMC preparation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinised venous blood on a Ficoll-Hypaque gradient and cryopreserved in liquid nitrogen. Prior to use, PBMC were thawed into complete medium (RPMI 1640 supplemented with 100U/ml penicillin/streptomycin and 20mM L-glutamine [Gibco, Lifesciences] and 10% pooled human AB plasma), washed and rested for 30 minutes before use. For some experiments, AB plasma was IgG-depleted plasma using a protein G Sepharose column (GE Life Sciences).
PBMC were cultured for 18 hours at 37°C at 2×105/well in 96-well U-bottomed plates (Nunc) in complete medium with or without: low concentration of cytokines (LCC; 12.5pg/ml rhIL-12 [PeproTech] plus 10ng/ml rhIL-18 [MBL, Woburn, USA]); high concentration of cytokines (HCC; 5ng/ml rhIL-12 plus 50ng/ml rhIL-18); rat anti-rhIL-2 (3 μg/ml; BD Biosciences); rat IgG2A isotype control (3 μg/ml; BD Biosciences, this was included in wells with medium alone, as well as antigen alone); 1 μg/ml formalin-inactivated whole H1N1 influenza virus (NIBSC, as above); 1 IU/ml killed whole cell B. pertussis (pertussis; NIBSC); or MHC Class I-deficient K562 target cells (E:T ratio 2:1). GolgiStop (containing Monensin, 1/1500 concentration; BD Biosciences) and GolgiPlug (containing Brefeldin A, 1/1000 final concentration; BD Biosciences) were added after 15 hours. Anti-CD107a antibody (A488-conjugated; BD Biosciences) was included in the medium for the entirety of cell culture.
For activation via CD16 crosslinking, 96-well flat-bottomed plates (Nunc) were coated with anti-human CD16 (BD Biosciences) or an isotype-matched control antibody (mIgG1k, BD Biosciences) overnight at 4°C. Wells were rinsed with PBS before addition of 2×105 PBMC/well, which had been incubated overnight at 37°C with 50 IU/ml IL-2 (PeproTech). Anti-CD107a-FITC antibody was added at the beginning of culture and cells were harvested after five hours.
Flow cytometry
PBMCs were stained in 96-well U-bottomed plates as described previously [6]. Briefly, cells were stained with fluorophore-labelled antibodies to cell surface markers, fixed, permeabilised (Cytofix/Cytoperm; BD Biosciences), and stained for intracellular molecules. The following monoclonal antibodies were used: anti-CD3-V500, anti-CD56-PECy7, anti-IFN-γ-APC, anti-CD107a-FITC, anti-CD16-APC-H7, anti-CD25-APC-H7, (all BD Biosciences), anti-CD57-e450, anti-CD25-PerCPCy5.5, anti-CD16-APC, anti-CD25-PE, anti-IL18Rα-PE, anti-IL18Rα-FITC, anti-IFN-γ-APCe780, anti-CD16-APCe780 (all e-Biosciences), anti-NKG2C-APC, anti-NKG2C-PE (both R&D Systems), and anti-NKG2A-FITC (Miltenyi). IL-12Rβ2 antibody was conjugated using EasyLink PE-Cy5 (AbCam). Cells were acquired on an LSRII flow cytometer (BD Biosciences) using FACSDiva® software. Data analysis was performed using FlowJo V10 (Tree Star). FACS gates set on unstimulated cells (medium alone or isotype controls) were applied in standard format across all samples and all conditions.
NKG2C genotyping
DNA was extracted from whole blood using a Wizard genomic DNA extraction kit (Promega). Donors were then genotyped for NKG2C using touch-down PCR (Phusion® High Fidelity PCR kits, New England Biolabs) as described previously [29,30].
Statistical analyses
Statistical analysis of flow cytometry data was performed using Prism 6 (GraphPad), or STATA/IC 13 (StataCorp), as detailed in figure legends. Responses where the gated cell subset contained fewer than 100 cells were excluded. Mann-Whitney U tests were used to compare responses between HCMV− and HCMV+ donors and linear regression was used to adjust for sex and age. Unless otherwise stated, statistical tests were one-sided. **** p≤0.0001, *** p<0.001, ** p<0.01, * p<0.05.
Results
Donor characterisation
Subject characteristics are summarised in Table I. Subjects (n =152) ranged in age from 20-77-years (median = 33-years). Fifty-five subjects (36.2%) were found to be seropositive. Anti-HCMV IgG titre increased significantly with increasing age (R2 = 0.248, p = 0.0001; Figure S1A) but age did not differ significantly between HCMV+ and HCMV− donors (two-tailed Mann-Whitney test, p = 0.561). As the proportion of female and male donors differed between the HCMV− and HCMV+ groups subsequent analyses were adjusted for sex.
Cells from all 152 subjects were analysed for responses to pertussis. The median anti-PT IgG titre was higher among HCMV− donors than among HCMV+ donors, but this difference was not statistically significant (6.7 IU/ml vs 5.0 IU/ml, two-tailed Mann-Whitney, p = 0.078). One hundred and fourteen donors (75.0%) confirmed that they had been vaccinated against pertussis but a minority of donors reported that they had not been vaccinated against pertussis (n = 13; 8.6%) or were unsure of their vaccination status (n = 25; 16.4%). However, the proportions of these individuals did not differ between the HCMV+ and HCMV− groups and their antibody titres did not suggest a difference in vaccination history (data not shown).
All donors analysed for responses to vaccine H1N1 influenza (n = 52) confirmed only natural exposure to H1N1, i.e. no previous seasonal influenza vaccination. Median anti-H1N1 IgG titres were higher among HCMV− donors (204.1 AEU/ml) than among HCMV+ donors (187.2 AEU/ml), although this difference was not statistically significant (two-tailed Mann-Whitney, p = 0.135).
Antibody and antigen-specific IL-2 drive NK cell responses to pertussis and H1N1 influenza virus
PBMC from 100 donors were stimulated overnight with pertussis (Figure 1B-D) and NK cell responses were measured by flow cytometry (Figure 1A). Significant induction of CD25 and IFN-γ (Figure 1B, 1C) and degranulation (CD107a; Figure 1D) was observed in response to pertussis. Analysis of this response by CD56bright and CD56dim subsets reveals that the CD56dim cells respond more robustly to pertussis than do the CD56bright NK cells (and are thus the major contributors to the vaccine response; Figure S2A-C).
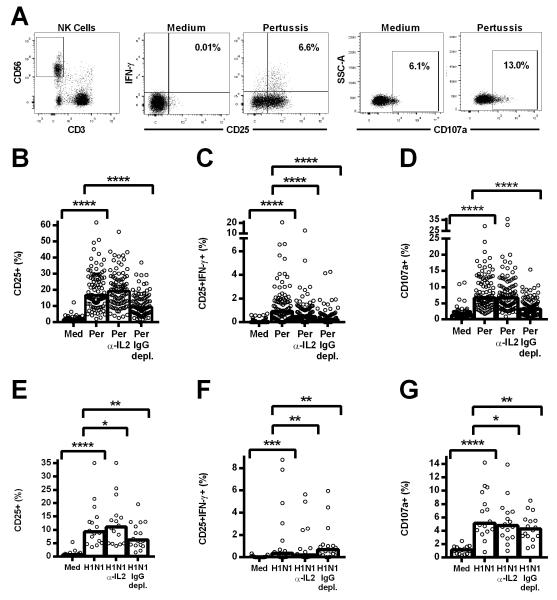
Figure 1. Natural killer (NK) cell responses to pertussis and H1N1 are inhibited by IL-2 neutralisation and IgG depletion.
PBMC were cultured in vitro for 18hr with medium alone, killed whole cell pertussis (Per), inactivated whole H1N1 influenza virus (H1N1), pertussis or H1N1 with blocking antibody to IL-2 (Per α-IL-2, H1N1 α-IL-2) or pertussis or H1N1 in IgG-depleted plasma (Per IgG depl., H1N1 IgG depl.). The isotype control antibody (IgG2A) for the IL-2 blocking antibody was included in the medium, pertussis, and H1N1 wells. Representative flow cytometry plots show gating of CD3−CD56+ NK cells and expression of CD25, IFN-γ, and CD107a (A). Responses to pertussis (B-D) and H1N1 (E-G) were measured by the percentage of NK cells expressing CD25 (B, E), co-expressing CD25/IFN-γ (C, F), and expressing CD107a (D, G). Data were analysed in Prism using paired, one-tailed Wilcoxon signed-rank tests. **** p ≤ 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. Each data point represents one donor, n = 100 (B-D) or n = 16 (E-G), and bar graphs denote medians.
Co-expression of CD25/IFN-γ was markedly attenuated in the presence of a blocking antibody to IL-2 and after depletion of IgG from the plasma used to supplement the culture medium, indicating a role for both memory T cell-derived IL-2 and antigen-specific antibody in the NK cell IFN-γ response. By contrast, the degranulation response (as measured by cell surface expression of the lysosomal marker LAMP-1/CD107a [31]) was dependent upon IgG but not IL-2. The observation that neither anti-IL-2 nor IgG depletion completely abrogated the NK cell IFN-γ response suggests that these two signals may synergise for optimal IFN-γ production.
Cells from a subset of subjects (n = 16) were also analysed for responses to H1N1 influenza in the context of IL-2 blockade or IgG depletion (Figure 1E-G). As observed with pertussis, statistically significant induction of CD25 (Figure 1E), CD25/IFN-γ (Figure 1F) and CD107a (Figure 1G) was observed in response to restimulation with H1N1 antigen, and IL-2 blocking significantly decreased CD25/IFN-γ expression (Figure 1F) whilst IgG depletion inhibited the degranulation (CD107a) response (Figure 1G). Interestingly, and in contrast to the response to pertussis, IgG depletion enhanced IFN-γ production in response to H1N1 and IL-2 blockade slightly decreased degranulation, indicating competition between these pathways for NK cell activation during influenza responses (Figure 1F).
HCMV infection is associated with impaired NK cell responses to pertussis and H1N1 influenza virus
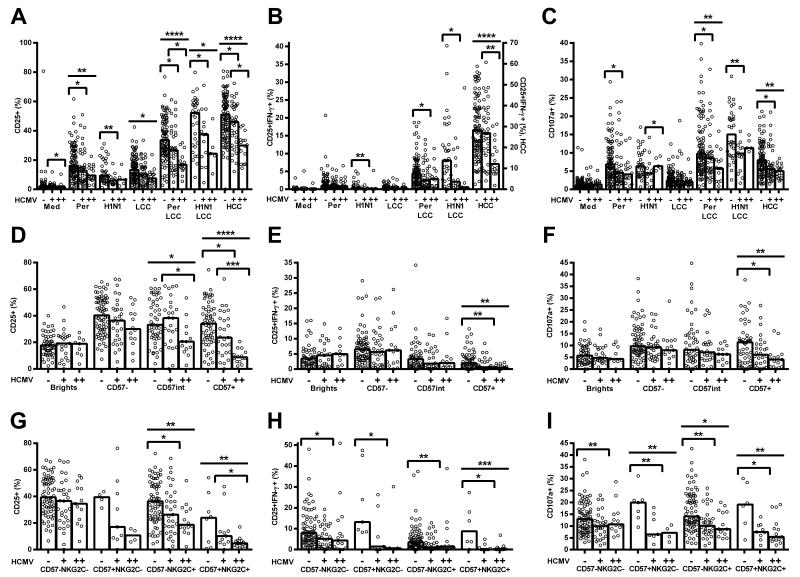
NK cell responses to pertussis (n = 152) and H1N1 (n = 52) were compared between HCMV− and HCMV+ donors (Figure 2). Consistent with prior observations [3,6], responses to pertussis and H1N1 were significantly augmented by low concentrations of cytokines IL-12 and IL-18 LCC (p ≤ 0.0001 for all parameters) indicating that in vitro accessory cell activation and production of IL-12 and IL-18 (which is essential for IL-2-mediated NK cell activation [3,5,32]) were suboptimal.
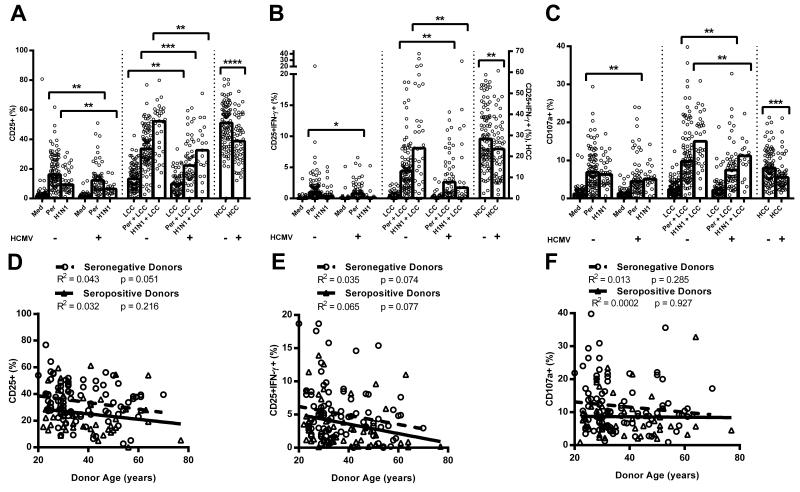
Figure 2. Natural killer (NK) cell responses to vaccine antigen are affected by human cytomegalovirus (HCMV) infection.
PBMC were cultured in vitro for 18hr with medium alone, low concentration of cytokines (LCC), killed whole cell pertussis (Per), inactivated whole H1N1 influenza virus (H1N1), Per + LCC, H1N1 + LCC, or high concentration of cytokines (HCC). Donors were stratified into HCMV seronegative (−) and HCMV seropositive (+) groups. Responses were measured as the percentage of NK cells expressing CD25 (A), co-expressing CD25/IFN-γ (B), or CD107a (C). Data were analysed in Prism using, one-tailed Mann-Whitney tests. **** p ≤ 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. Bivariate regression of age against responses to Per + LCC was performed for the percentage of NK cells expressing CD25 (D) CD25/IFN-γ (E), and CD107a (F). Each data point represents one donor, n = 152, except for H1N1 and H1N1 + LCC where n = 52. Bar graphs denote medians.
NB, all antigen stimulations induced statistically significant increases in expression of CD25, CD25/IFN-γ, and CD107a over background (medium alone for pertussis/H1N1, or LCC for pertussis+LCC/H1N1+LCC; p < 0.05 in all cases), except that H1N1 did not induce a significant increase in CD25+IFN-γ+ NK cells in HCMV+ donors (p = 0.416).
Interestingly, in the absence of LCC, pertussis induces stronger NK cell responses than H1NI whereas in the presence of LCC, H1N1 induces the most robust responses. This may indicate that pertussis induces some IL-12 and IL-18 (such that LCC is redundant in these assays) whereas H1N1 may be a poor inducer of IL-12 and IL-18 but a better inducer of IL-2 or other accessory cytokines. This would be consistent with differences in Toll-like receptor (TLR) signalling by RNA viruses such as influenza (TLR3) and gram-negative bacteria such as pertussis (TLR4) [33-36].
NK cells from both HCMV+ and HCMV− donors responded to pertussis and H1N1 (with or without LCC; Figure 2), however NK cell responses to these two vaccines (whether defined as CD25+, CD25+IFN-γ+, or CD107a+) were significantly lower among HCMV+ donors than among HCMV− donors (Figure 2A, 2B). This was true for both vaccines and all parameters when cells were cultured with LCC, and was also true for the CD25+ and CD25+IFN-γ+ responses to H1N1 and the CD25+ and CD107a+ responses to pertussis in the absence of LCC. Importantly, resting levels of CD25 expression did not differ significantly between HCMV+ and HCMV− donors (Figure 2A), and there was no difference in the potential of T cells from HCMV− and HCMV+ donors to produce IL-2 in response to pertussis antigen (Figure S1B, S1C). Furthermore, there is no intrinsic difference in the ability of NK cells from HCMV+ and HCMV− donors to degranulate in response to CD16 crosslinking or K562 stimulation (Figure S1D, S1E). However, NK cell CD25+, CD25+IFN-γ+ and CD107a+ expression in response to HCC (high concentrations of IL-12 and IL-18) were all significantly higher in HCMV− compared to HCMV+ donors (Figure 2A-C). Analysis of this response by CD56bright and CD56dim subsets reveals that the effect of HCMV status is due entirely to an effect within the CD56dim subset (Figure S2D-F).
In addition to consistently lower NK cell responses to vaccine antigens in HCMV+ individuals, there was a trend for CD25 and CD25/IFN-γ responses to pertussis (with or without LCC) to decline with increasing age (Figure 2D, 2E). This was statistically significant for the cohort as a whole (CD25+ pertussis: R2 = 0.0549, p = 0.0052; CD25+ pertussis + LCC: R2 = 0.0453, p = 0.0122; CD25+IFN-γ+ pertussis: R2 = 0.0379, p = 0.0203; CD25+IFN-γ+ pertussis + LCC: R2 = 0.0478, p = 0.0095) but not when analysed separately for HCMV− and HCMV+ donors due decreased power. There was no effect of age on CD107a expression (pertussis: R2 = 0.00491, p = 0.4089; pertussis + LCC: R2 = 0.00879, p = 0.272; Figure 2F), which is consistent with maturation of the NK cell repertoire, and therefore decreased sensitivity to exogenous cytokines, but maintained cytotoxicity, during normal ageing (reviewed in [37]) and increasing NK cell differentiation [8,9]. Importantly, the effect of HCMV infection on impaired NK cell responses to pertussis and H1N1 is entirely independent of the association between age and NK cell function. In line with this conclusion, adjusting for age by parametric regression did not alter the conclusions of the study (Table II).
Table II. Natural killer (NK) cell responses to vaccine antigens by human cytomegalovirus (HCMV) status after adjusting for sex and age.
A regression analysis was performed in STATA to adjust for sex and age when comparing natural killer (NK) cell responses to pertussis (−/+ LCC), H1N1 (−/+ LCC), and HCC between HCMV seronegative and HCMV seropositive donors. The response was quantified by the percentage of total NK cells expressing CD25, CD25/IFN-γ (CD25+IFN-γ+), and CD107a.
| Stimulus | Parameter (Total NK cells) | Adjusted for sex and age | |
|---|---|---|---|
| Effect (95% CI)1 | p value2 | ||
| Pertussis | CD25+ | −4.4 (−8.3, −0.5) | 0.014 |
| CD25+IFN-γ+ | −0.5 (−1.2, 0.3) | 0.125 | |
| CD107a+ | −1.5 (−3.4, 0.5) | 0.071 | |
| Pertussis + LCC | CD25+ | −8.5 (−13.7, −3.4) | 0.001 |
| CD25+IFN-γ+ | −1.5 (−2.8, −0.1) | 0.020 | |
| CD107a+ | −2.9 (−5.5, −0.3) | 0.016 | |
| H1N1 | CD25+ | −5.4 (−9.5, −1.3) | 0.005 |
| CD25+IFN-γ+ | −0.4 (−1.1, 0.4) | 0.158 | |
| CD107a+ | −1.8 (−3.9, 0.3) | 0.049 | |
| H1N1 + LCC | CD25+ | −12.2 (−22.6, −1.8) | 0.011 |
| CD25+IFN-γ+ | −5.1 (−10.4, 0.1) | 0.027 | |
| CD107a+ | −5.1 (−8.9, −1.5) | 0.004 | |
| HCC | CD25+ | −11.3 (−16.7, −6.0) | <0.0001 |
| CD25+IFN-γ+ | −6.5 (−11.4, −1.7) | 0.005 | |
| CD107a+ | −2.1 (−3.5, −0.6) | 0.004 | |
Effect (coefficient), with 95% confidence interval, represents the change in the mean percentage of NK cells responding in HCMV−seropositive donors as compared to HCMV−seronegative donors.
The p-value refers to the significance of the difference in response between HCMV seronegative and HCMV seropositive donors after adjusting for sex and age.
Overall, NK cell responses did not differ significantly between males and females although there was a trend for median responses to be higher in women than in men and this reached statistical significance (p < 0.05) for the IFN-γ response to pertussis + LCC in HCMV+ donors (data not shown). As the proportion of female subjects differed between the HCMV− and HCMV+ groups (Table I), the data in Figure 2 were reanalysed, adjusting for sex as well as age using parametric regression (Table II). After adjustment, CD25/IFN-γ and CD107a expression in response to vaccine alone (i.e. without LCC) are no longer significantly different between HCMV− and HCMV+ donors, but responses to vaccine with LCC, and responses to HCC, remain significantly lower in HCMV+ compared to HCMV− donors.
Finally, no associations were observed between anti-HCMV titre and any NK cell responses among the HCMV+ subjects, and there was no effect of NKG2C genotype (which may affect NK cell differentiation [30,38,39]) on NK cell responses (data not shown).
NK cell differentiation only partially explains reduced responses to vaccines in HCMV+ donors
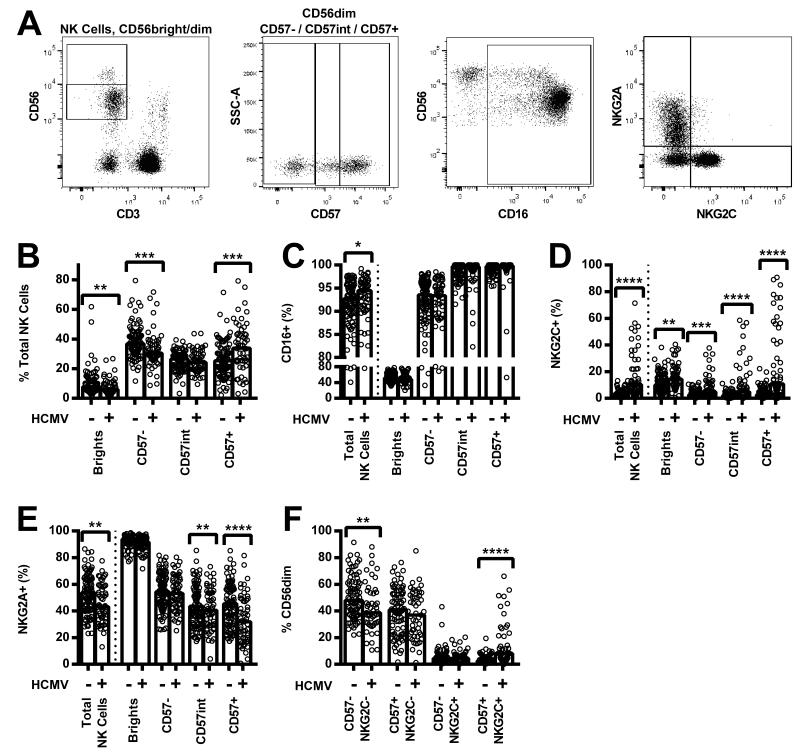
We hypothesised that reduced cytokine-mediated NK cell responses among HCMV+ donors would reflect expansion of the highly differentiated CD56dimCD57+NKG2C+ NK cell subset which is known to be hyporesponsive to cytokines [12]. Indeed, ex vivo analysis confirmed observations from previous studies that HCMV+ donors had lower proportions of CD56dimCD57− NK cells and higher proportions of CD56dimCD57+ NK cells than did HCMV− donors (Figure 3A, 3B); there was no difference between the groups in the proportion of cells with intermediate CD57 expression (CD56dimCD57int, gating shown in Figure 3A). Consistent with previous work [10-12,16,17], HCMV seropositivity was also associated with a higher proportion of CD16+ (Figure 3C) and NKG2C+ (Figure 3D) cells, and a lower proportion of NKG2A+ cells (Figure 3E), within the total NK cell population. Moreover, HCMV seropositivity was correlated with a lower proportion of CD57−NKG2C− cells and a higher proportion of CD57+NKG2C+ cells within the CD56dim NK cell population (Figure 3F).
Figure 3. Comparison of ex vivo expression of natural killer (NK) cell markers and receptors in human cytomegalovirus (HCMV) seronegative and seropositive donors.
PBMC were analysed ex vivo for surface expression of CD56, CD57, CD16, NKG2C, and NKG2A, as shown by representative flow cytometry plots (A). Proportions of total NK cells in the CD56bright, CD56dimCD57−, CD56dimCD57int, and CD56dimCD57+ subsets were compared between HCMV seronegative and HCMV seropositive donors (B), as was expression of CD16 (C), NKG2C (D), NKG2A (E), and CD57/NKG2C (F, CD56dim only) The percentages of cells expressing each marker in HCMV seronegative (−) and HCMV seropositive (+) donors were compared using two-tailed Mann-Whitney tests. **** p ≤ 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. Each data point represents one donor, n = 152, and bar graphs denote medians.
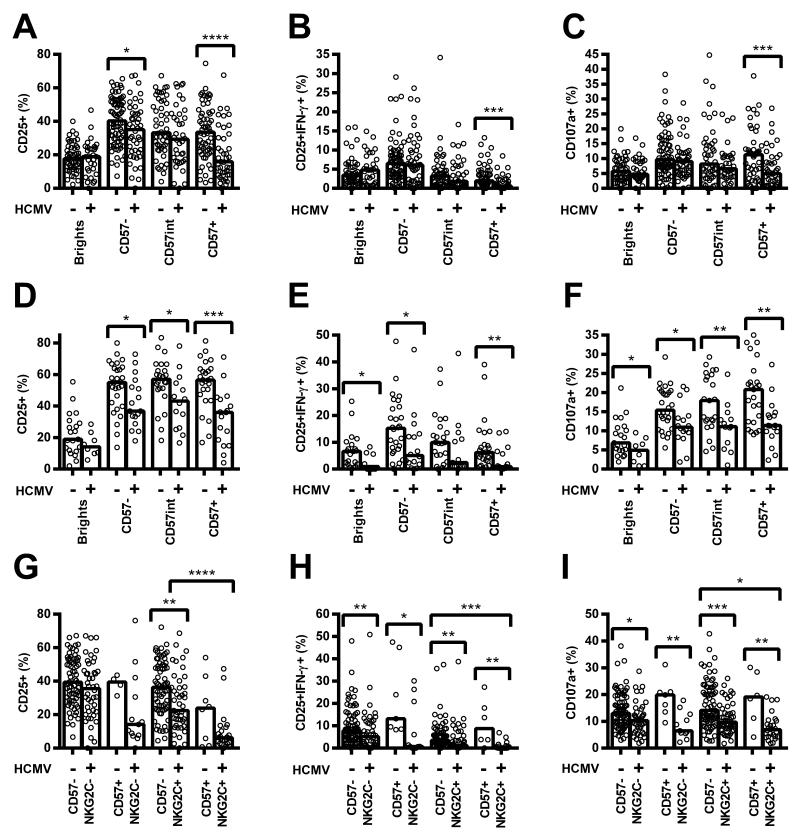
Whilst the increased proportion of CD56dimCD57+ NK cells among HCMV+ donors likely contributes to their reduced responsiveness to cytokines, we also observed significantly reduced CD25, CD25/IFN-γ, and CD107a expression in response to both pertussis and H1N1 within individual NK cell subsets. This was especially evident among CD56dimCD57+ cells and for cultures containing LCC (Figure 4A-F), but was also the case for cultures stimulated with vaccine alone (Figure S3G-I, M-O).
Figure 4. Human cytomegalovirus (HCMV) infection affects vaccine antigen responses of all natural killer (NK) cells, irrespective of their differentiation status.
PBMC were cultured in vitro for 18hr with killed whole cell pertussis with a low concentration of cytokines (pertussis + LCC) (A-C, G-I) or inactivated whole H1N1 influenza virus (H1N1 + LCC) (D-F). Responses were measured as the percentage of cells expressing CD25 (A, D, G), CD25/IFN-γ (B, E, H), and CD107a (C, F, I) by CD56/CD57-defined subsets (A-F), or CD56dim CD57/NKG2C-defined subsets (G-I) and compared between HCMV seronegative (−) and HCMV seropositive donors (+). Data were analysed using one-tailed Mann-Whitney tests. **** p ≤ 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. Each data point represents one donor, n = 152 (A-C, G-I), or n = 52 (D-F), and bar graphs denote medians.
NB, for CD57/NKG2C-defined subsets, CD57int cells were grouped together with CD57− cells.
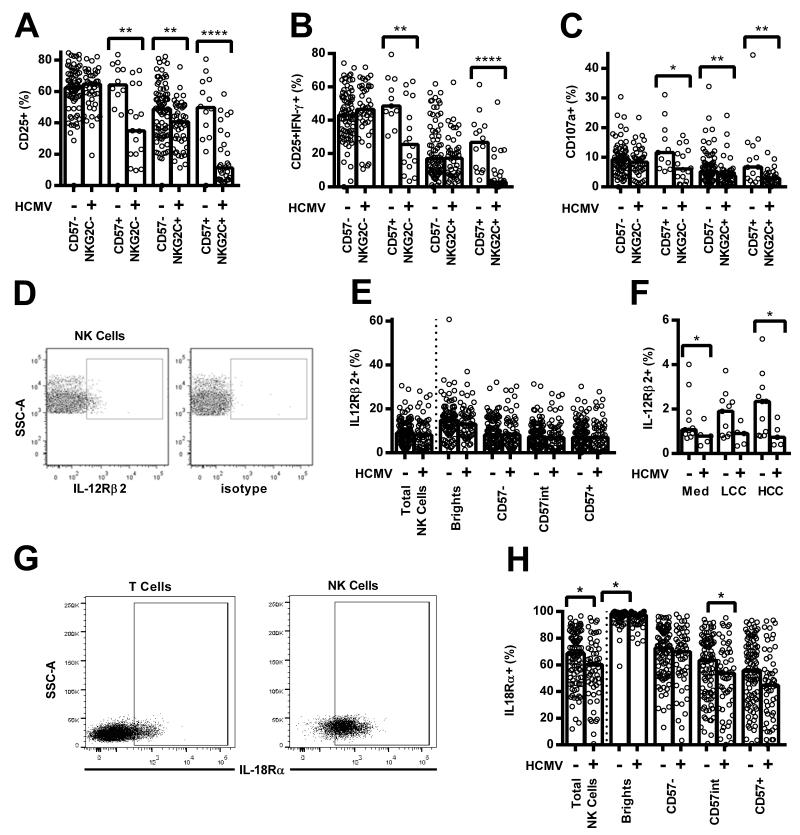
Similarly, when cells were grouped by expression of CD57 and NKG2C we found that responses to pertussis with LCC were lower among NKG2C+ NK cells than among NKG2C− cells (Figure 4G-I). This association was statistically significant for CD57+ NK cells of HCMV+ donors, but evaluation of the HCMV− cohort lacked statistical power as too few donors had sufficient NKG2C+ cells to allow a robust analysis. Interestingly, however, responses of all four subsets were significantly lower among HCMV+ donors than among HCMV− donors (Figure 4G-I), despite minimal differences in responses to LCC alone (Figure S3A-F). These data indicate that the reduced response of HCMV+ donors reflects differences in the intrinsic responsiveness of NK cells within a subset as well as differences in the distribution of these subsets. Although the level of expression (MFI) of both CD57 and NKG2C was higher on CD56dimCD57+ NK cells in HCMV+ donors compared to HCMV− donors (median MFI CD57 13526 vs 10575, p = 0.0032; median MFI NKG2C 141 vs 80.9, p < 0.0001, data not shown), there was no significant association between CD57 and NKG2C expression levels and NK cell responsiveness in HCMV+ donors (data not shown).
Since only some HCMV+ individuals have obvious expansion of the CD56dimCD57+NKG2C+ subset we considered whether NK responses might differ between HCMV+ individuals with and without this expanded population. Sixteen of 55 (29%) HCMV+ donors demonstrated expansion of the CD56dimCD57+NKG2C+ subset (defined as % CD56dimCD57+NKG2C+ cells greater than the mean + 3SD of that in HCMV− donors) and NK cells from these donors tended to respond less robustly than did cells from HCMV+ donors without this expansion (Figure 5). Importantly, there was evidence by trend analysis for decreasing NK cell responsiveness with HCMV infection, and then with HCMV infection plus expansion of the CD56dimCD57+NKG2C+ subset (Figure 5). This confirms that whilst expansion of the CD56dimCD57+NKG2C+ subset is associated with loss of NK cell responsiveness in vaccine recall assays, cells of HCMV+ donors respond less well than do cells of HCMV− donors, irrespective of NKG2C expression.
Figure 5. Natural killer (NK) cell responses of human cytomegalovirus (HCMV) seropositive donors with or without the characteristic CD56dimCD57+NKG2C+ expansion.
PBMC were cultured in vitro for 18hr with medium alone, low concentration of cytokines (LCC), killed whole cell pertussis (Per), inactivated whole H1N1 influenza virus (H1N1), Per + LCC, H1N1 + LCC, or high concentration of cytokines (HCC). Donors were stratified into HCMV seronegative (−), HCMV seropositive without expansion of CD56dimCD57+NKG2C+ cells (+), and HCMV seropositive with expansion of CD56dimCD57+NKG2C+ cells (++). Responses are expressed as the percentage of total NK cells expressing CD25 (A), co-expressing CD25/IFN-γ (B), or expressing CD107a (C). CD57-defined (D-F) or CD57/NKG2C-defined subsets (G-I) were analysed for responses to pertussis with LCC for CD25 (D, G), CD25/IFN-γ (E, H), and CD107a (F, I). Data were analysed in Prism using, one-tailed Mann-Whitney tests to compare responses between HCMV+ donors and either HCMV− donors or HCMV++ donors. Analysis of variance for linear trend (from − to + to ++) was also performed for each functional readout **** p ≤ 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. Each data point represents one donor, n = 152, except for H1N1 and H1N1 + LCC where n = 52. Bar graphs denote medians.
HCMV infection is associated with altered expression of cytokine receptors by NK cells
Although there was a clear role for specific IgG in induction of CD25, CD25/IFN-γ, and CD107a expression (Figure 1), impairment of CD16-mediated signalling seemed an unlikely explanation for reduced NK cell responsiveness since HCMV+ individuals have a higher frequency of CD16+ NK cells (Figure 3C), cells from HCMV+ and HCMV− donors responded equally well to CD16 crosslinking (Figure S1D), and use of pooled AB plasma for in vitro assays ensured that specific IgG concentrations were consistent in all assays.
On the other hand, differences between HCMV+ and HCMV− donors were most marked in cultures containing LCC (Figure 2), and in cultures with high concentrations of the cytokines IL-12 and IL-18 (HCC; Figure 6A-C), suggesting that differences in expression of cytokine receptors might explain our observations. Although there was no difference in resting (ex vivo) expression of IL-12Rβ2 on any NK cell subset (Figure 6D, 6E), IL-12Rβ2 was significantly upregulated on the total NK cell population in HCMV− but not from HCMV+ donors after culture with HCC (Figure 6F). Moreover, and consistent with data showing associations between acquisition of CD57 and increased IL-18Rα expression [6,8,9], resting NK cells from HCMV+ donors were significantly less likely than cells from HCMV− donors to express IL-18Rα and this difference was especially marked in the (expanded) CD56dimCD57+ NK cell subset (Figure 6G, 6H).
Figure 6. Decreased cytokine responsiveness and decreased cytokine receptor expression by NK cells from human cytomegalovirus (HCMV) seropositive donors.
(A-C) PBMC were cultured in vitro for 18hr with a high concentration of cytokines (HCC). Responses were measured as the percentage of CD56dim CD57/NKG2C-defined cells expressing CD25 (A), CD25/IFN-γ (B), and CD107a (C) and compared between HCMV seronegative (−) and HCMV seropositive donors (+). (D-F) NK cells were analysed for surface expression of IL-12Rβ2 using a mIgG1 PECy5-conjugated isotype control to set the gate (D). Total NK cells (E-F) and CD56/CD57-defined subsets (E) were analysed ex vivo (E) and after 18hr culture in vitro with low concentration cytokines (LCC) or HCC (F). (G-H) NK cells were also analysed for IL-18Rα surface expression using the T cell population to set the IL-18Rα gate (G), for total NK cells and CD56/CD57-defined subsets ex vivo (H). HCMV− and HCMV+ donors were compared using one-tailed (A-C) or two-tailed (E-F, H) Mann-Whitney tests. **** p ≤ 0.0001, ** p < 0.01, * p < 0.05. Each point represents one donor, n = 152 (A-C, E, H), or n = 16 (F), and bar graphs denote medians.
Discussion
During secondary immune responses, both CD4+ T cell-derived IL-2 and antigen-antibody immune complexes induce “antigen-specific” NK cell activation, allowing NK cells to act as effectors of the adaptive immune response and to contribute to post-vaccination immunity by secretion of IFN-γ and/or by cytotoxicity [3-6,14]. Here we demonstrate, for the first time, that the contribution of NK cells to adaptive immune responses is affected by HCMV infection: NK cells from HCMV+ donors respond significantly less well than cells from HCMV− donors to killed whole cell pertussis or inactivated whole H1N1 influenza virus. The effect of HCMV infection on NK cell responsiveness is independent of age, sex, or anti-HCMV IgG titre.
Our data also demonstrate, for the first time, that there is an additive effect between the cytokine and the IgG pathways driving NK cell IFN-γ production, as both IgG depletion and IL-2 blockade reduced NK cell IFN-γ responses in response to stimulation of PBMCs with pertussis vaccine. Of particular interest, IgG depletion markedly reduced antigen-induced CD25 expression on NK cells. We propose that CD16 crosslinking by immune complexes upregulates CD25 expression, increasing sensitivity to T cell-derived IL-2 and thereby enhancing IFN-γ production. However, CD16 crosslinking is not essential for upregulation of CD25, as this can be induced by antigen alone, presumably in response to IL-12 and IL-18 produced by APCs [6,40-42]. Release of cytotoxic granules, as measured by upregulation of CD107a on the cell surface, is also inhibited by IgG depletion but is unaffected by IL-2 blockade, suggesting that NK cells could act as effectors of the adaptive response through ADCC in the absence of memory T cells, providing there was sufficient circulating antibody.
However, while IgG depletion also decreased H1N1-induced CD25 expression on NK cells, H1N1 induction of IFN-γ was significantly enhanced in the absence of IgG. We have observed that individual NK cells tend to either produce IFN-γ or degranulate (but not both; unpublished data) suggesting that inhibiting the degranulation response to H1N1 by removing IgG skews the response towards IFN-γ production. However, given the limited effect of IgG depletion on H1N1-induced degranulation, it is unclear why this should be the case. Indeed, expression of CD107a in response to H1N1 seems to be relatively unaffected by either IL-2 blockade or IgG depletion. This suggests that H1N1-driven degranulation may be affected by other stimuli, such as type I interferons [43,44].
We had hypothesised that decreased responses to vaccines in HCMV+ donors would be attributable to a redistribution of the NK cell repertoire. HCMV infection drives the expansion of a CD56dimCD57+NKG2C+ subset of NK cells [11,16,17,45], which display a highly differentiated phenotype, including reduced responsiveness to exogenous cytokine stimulation [8,9] and epigenetic changes at the IFNG locus [46]. These phenotypic and functional changes are similar to those observed during ageing [15,47] and comparisons have been drawn between the effects of HCMV and immunosenescence [48]. As our previous work has indicated that NK cell IFN-γ production after restimulation with vaccine antigens is cytokine-dependent [3], we predicted that fewer NK cells from HCMV+ donors would produce IFN-γ in response to pertussis or influenza antigens due to the reduced capacity of the expanded CD56dimCD57+NKG2C+ subset to respond to cytokines. Ex vivo analyses confirmed that HCMV+ donors had higher proportions of CD56dimCD57+ and CD56dimCD57+NKG2C+ NK cells than did HCMV− donors and functional analysis confirmed that very few of the highly differentiated CD57+ NK cells produced IFN-γ after antigen stimulation. Interestingly, however, our data also show that - irrespective of their CD57/NKG2C surface phenotype - NK cells from HCMV+ are less likely to produce IFN-γ in response to vaccines than are cells from HCMV− donors. In other words, there are pronounced functional differences between HCMV+ and HCMV− donors within NK cell subsets. The reduced NK cell IFN-γ response to vaccine antigens in HCMV+ donors is therefore not simply due to expansion of the CD56dimCD57+NKG2C+ subset. Although acquisition of NKG2C was functionally relevant (associated with reduced IFN-γ and degranulation responses), it was not sufficient to explain the reduced responsiveness of cells from HCMV+ donors.
Whilst further studies are required to define the “within subset” effects of HCMV infection, our data suggest that reduced expression of IL-18Rα or reduced ability to upregulate IL-12Rβ2 among NK cells from HCMV− infected individuals may partially explain their failure to produce IFN-γ. Although decreasing expression of IL-12Rβ2 and IL-18Rα expression have been associated with CD57 expression, this is the first demonstration that there are differences in cytokine receptor expression between HCMV+ and HCMV− donors and it is possible to see how each of these might affect NK cell responses. Higher resting levels of IL-18Rα expression would increase the sensitivity of NK cells to low concentrations of IL-18 being produced by APCs in response to innate receptor ligands in whole cell pertussis or inactivated influenza virus. IL-18 signalling upregulates CD25 [49] thereby increasing sensitivity to IL-2. IL-2 signalling might then upregulate IL-12R2β [50,51] allowing IL-12 to synergise with IL-2 to drive IFN-γ production [3,40,52], whilst also generating a positive feedback loop in which IL-12 signalling upregulates IL-18Rα [53,54], IL-18 signalling and CD25. However, while cytokine receptor expression is likely to play a role in determining NK cell responsiveness to vaccine antigens in HCMV− and HCMV+ donors, the biological relevance of small changes in surface expression on IL-12Rβ2 needs to be demonstrated. Moreover, while we have no evidence to suggest that T cell IL-2 production in response to vaccine antigens is affected by HCMV infection, future studies will need to determine the extent to which concomitant changes in APC function during HCMV infection also affect NK cell responses.
We had initially considered NK cell degranulation during vaccine restimulation to be a result of CD16 crosslinking by IgG immune complexes, as suggested by the IgG depletion data and accepted models of ADCC. The expectation was, therefore, that although IFN-γ responses might be impaired, NK cell degranulation responses would be sustained in HCMV+ donors. Indeed, crosslinking with anti-CD16 antibody induced equivalent levels of CD107a upregulation. It was, therefore, somewhat surprising that degranulation responses to vaccine were lower in HCMV+ donors than in HCMV− donors. However, degranulation responses to HCC were also lower in HCMV+ donors, supporting the notion of synergy between the cytokine and CD16 pathways and adding weight to the suggestion that HCMV infection may affect cytokine receptor expression.
Our findings have potentially important implications. HCMV infection is a known risk factor for all-cause mortality in adults [55] and perinatal HCMV infection is associated with slower growth and increased rates of hospitalisation in African children [56]. The underlying biology of these relationships is unknown but reduced responsiveness to vaccination or reduced resilience in the face of infection are plausible explanations. Distorted T cell and NK cell phenotypes in HCMV+ individuals have been widely reported [15,57-59] giving credence to the possibility that adaptive immune responses may be less effective in infected individuals. Further work will need to address the clinical consequences of altered NK cell responses to infection and vaccination in HCMV− infected individuals.
To our knowledge, this is the first published study of the effect of HCMV infection on NK cell responses to vaccine antigens. When compared to the marked effect of HCMV on cellular immune responses in our adult cohort, the modest phenotype seen in the infant studies raises the intriguing question as to whether the duration of HCMV infection affects vaccine responses. We have previously shown in an African population that, with near universal infant HCMV infection, the characteristic “adult HCMV” NK cell profile is reached by early adolescence [30]. The majority of our donors are of European or North American origin (data not shown) suggesting that they may have been infected in adolescence or adulthood [60,61], potentially explaining some of the heterogeneity in the responses we see within the HCMV+ group. Similarly, there will be variation among our donors in time since vaccination (pertussis) or infection (H1N1), and it is likely that relatively low IFN-γ responses we observe in comparison to earlier studies [3] is due to the much longer interval between primary and secondary exposures to antigen. Future studies will need to assess whether the duration of HCMV infection is a risk factor for altered NK responses and whether this manifests itself as reduced responsiveness to active vaccination and reduced vaccine efficacy.
Supplementary Material
Acknowledgments
We are most grateful to Carolynne Stanley for subject recruitment and blood sample collection, Elizabeth King for assistance with flow cytometry, Adriana Goncalves for assistance with NKG2C genotyping, Steven Smith for advice regarding IL-2 ELISPOT assays, and Brian de Souza, Rebecca Dabbs and Tom Hall for assistance with IgG depletion and serology.
Footnotes
Funding sources: This research is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement (G1000808). Carolyn Nielsen is supported by an MRC PhD Studentship in Vaccine Research (MR/J003999/1) and Ana Rodríguez-Galán is supported by a scholarship from Fundación ‘la Caixa’, Spain.
References
- 1.He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, Arvin AM, Parham P, Greenberg HB. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–1819. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long BR, Michaelsson J, Loo CP, Ballan WM, Vu BA, Hecht FM, Lanier LL, Chapman JM, Nixon DF. Elevated frequency of gamma interferon-producing NK cells in healthy adults vaccinated against influenza virus. Clin Vaccine Immunol. 2008;15:120–130. doi: 10.1128/CVI.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 4.Evans JH, Horowitz A, Mehrabi M, Wise EL, Pease JE, Riley EM, Davis DM. A distinct subset of human NK cells expressing HLA-DR expand in response to IL-2 and can aid immune responses to BCG. Eur J Immunol. 2011;41:1924–1933. doi: 10.1002/eji.201041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P, Cohen J, Vekemans J, Villafana T, Corran PH, Bejon P, Drakeley CJ, von Seidlein L, Riley EM. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J Immunol. 2012;188:5054–5062. doi: 10.4049/jimmunol.1102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White MJ, Nielsen CM, McGregor RH, Riley EH, Goodier MR. Differential activation of CD57-defined natural killer cell subsets during recall responses to vaccine antigens. Immunology. 2014;142:140–150. doi: 10.1111/imm.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramski M, Parsons MS, Stratov I, Kent SJ. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr HIV Res. 2013;11:388–406. doi: 10.2174/1570162x113116660061. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debre P, Bjorkstrom NK, Malmberg KJ, Marcellin P, Vieillard V. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, Schirmbeck R, Mertens T. Human Cytomegalovirus-Induced NKG2Chi CD57hi Natural Killer Cells Are Effectors Dependent on Humoral Antiviral Immunity. J Virol. 2013;87:7717–7725. doi: 10.1128/JVI.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol. 2013;4:422. doi: 10.3389/fimmu.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machala M, Mysliwski A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 19.Moro-Garcia MA, Alonso-Arias R, Lopez-Vazquez A, Suarez-Garcia FM, Solano-Jaurrieta JJ, Baltar J, Lopez-Larrea C. Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age (Dordr) 2012;34:479–495. doi: 10.1007/s11357-011-9240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald A, Selke S, Magaret A, Boeckh M. Impact of human cytomegalovirus (CMV) infection on immune response to pandemic 2009 H1N1 influenza vaccine in healthy adults. J Med Virol. 2013;85:1557–1560. doi: 10.1002/jmv.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derhovanessian E, Theeten H, Hahnel K, Van Damme P, Cools N, Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 2013;31:685–690. doi: 10.1016/j.vaccine.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Turner JE, Campbell JP, Edwards KM, Howarth LJ, Pawelec G, Aldred S, Moss P, Drayson MT, Burns VE, Bosch JA. Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age (Dordr) 2014;36:287–297. doi: 10.1007/s11357-013-9557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrazzini N, Bajwa M, Vita S, Thomas D, Smith H, Vescovini R, Sansoni P, Kern F. Cytomegalovirus infection modulates the phenotype and functional profile of the T-cell immune response to mycobacterial antigens in older life. Exp Gerontol. 2014;54:94–100. doi: 10.1016/j.exger.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miles DJ, Sanneh M, Holder B, Crozier S, Nyamweya S, Touray ES, Palmero MS, Zaman SM, Rowland-Jones S, van der Sande M, Whittle H. Cytomegalovirus infection induces T-cell differentiation without impairing antigen-specific responses in Gambian infants. Immunology. 2008;124:388–400. doi: 10.1111/j.1365-2567.2007.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holder B, Miles DJ, Kaye S, Crozier S, Mohammed NI, Duah NO, Roberts E, Ojuola O, Palmero MS, Touray ES, Waight P, Cotten M, Rowland-Jones S, van der Sande M, Whittle H. Epstein-Barr virus but not cytomegalovirus is associated with reduced vaccine antibody responses in Gambian infants. PLoS One. 2010;5:e14013. doi: 10.1371/journal.pone.0014013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Elzen WP, Vossen AC, Cools HJ, Westendorp RG, Kroes AC, Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29:4869–4874. doi: 10.1016/j.vaccine.2011.03.086. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor D, Truck J, Lazarus R, Clutterbuck EA, Voysey M, Jeffery K, Pollard AJ. The effect of chronic cytomegalovirus infection on pneumococcal vaccine responses. J Infect Dis. 2014;209:1635–1641. doi: 10.1093/infdis/jit673. [DOI] [PubMed] [Google Scholar]
- 28.Lefevre EA, Carr BV, Inman CF, Prentice H, Brown IH, Brookes SM, Garcon F, Hill ML, Iqbal M, Elderfield RA, Barclay WS, Gubbins S, Bailey M, Charleston B, Cosi Immune responses in pigs vaccinated with adjuvanted and non-adjuvanted A(H1N1)pdm/09 influenza vaccines used in human immunization programmes. PLoS One. 2012;7:e32400. doi: 10.1371/journal.pone.0032400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyashita R, Tsuchiya N, Hikami K, Kuroki K, Fukazawa T, Bijl M, Kallenberg CG, Hashimoto H, Yabe T, Tokunaga K. Molecular genetic analyses of human NKG2C (KLRC2) gene deletion. Int Immunol. 2004;16:163–168. doi: 10.1093/intimm/dxh013. [DOI] [PubMed] [Google Scholar]
- 30.Goodier MR, White MJ, Darboe A, Nielsen CM, Goncalves A, Bottomley C, Moore SE, Riley EM. Rapid natural killer cell differentiation in a population with near universal human cytomegalovirus infection is attenuated by NKG2C deletions. Blood. 2014 doi: 10.1182/blood-2014-05-576124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169:2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 33.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 34.Wong JP, Christopher ME, Viswanathan S, Karpoff N, Dai X, Das D, Sun LQ, Wang M, Salazar AM. Activation of toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine. 2009;27:3481–3483. doi: 10.1016/j.vaccine.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee N, Wong CK, Hui DS, Lee SK, Wong RY, Ngai KL, Chan MC, Chu YJ, Ho AW, Lui GC, Wong BC, Wong SH, Yip SP, Chan PK. Role of human Toll-like receptors in naturally occurring influenza A infections. Influenza Other Respir Viruses. 2013;7:666–675. doi: 10.1111/irv.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno G, Errea A, Van Maele L, Roberts R, Leger H, Sirard JC, Benecke A, Rumbo M, Hozbor D. Toll-like receptor 4 orchestrates neutrophil recruitment into airways during the first hours of Bordetella pertussis infection. Microbes Infect. 2013;15:708–718. doi: 10.1016/j.micinf.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Gayoso I, Sanchez-Correa B, Campos C, Alonso C, Pera A, Casado JG, Morgado S, R Tarazona, Solana R. Immunosenescence of human natural killer cells. J Innate Immun. 2011;3:337–343. doi: 10.1159/000328005. [DOI] [PubMed] [Google Scholar]
- 38.Noyola DE, Fortuny C, Muntasell A, Noguera-Julian A, Munoz-Almagro C, Alarcon A, Juncosa T, Moraru M, Vilches C, Lopez-Botet M. Influence of congenital human cytomegalovirus infection and the NKG2C genotype on NK-cell subset distribution in children. Eur J Immunol. 2012;42:3256–3266. doi: 10.1002/eji.201242752. [DOI] [PubMed] [Google Scholar]
- 39.Muntasell A, Lopez-Montanes M, Vera A, Heredia G, Romo N, Penafiel J, Moraru M, Vila J, Vilches C, Lopez-Botet M. NKG2C zygosity influences CD94/NKG2C receptor function and the NK-cell compartment redistribution in response to human cytomegalovirus. Eur J Immunol. 2013;43:3268–3278. doi: 10.1002/eji.201343773. [DOI] [PubMed] [Google Scholar]
- 40.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 41.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, Fragoso MF, Biron CA. Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J Immunol. 2012;189:2712–2716. doi: 10.4049/jimmunol.1201528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71:565–581. [PubMed] [Google Scholar]
- 44.Hansen ML, Woetmann A, Krejsgaard T, Kopp KL, Sokilde R, Litman T, Straten PT, Geisler C, Wasik MA, Odum N, Eriksen KW. IFN-alpha primes T- and NK-cells for IL-15-mediated signaling and cytotoxicity. Mol Immunol. 2011;48:2087–2093. doi: 10.1016/j.molimm.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Gratama JW, Kluin-Nelemans HC, Langelaar RA, den Ottolander GJ, Stijnen T, D’Amaro J, Torensma R, Tanke HJ. Flow cytometric and morphologic studies of HNK1+ (Leu 7+) lymphocytes in relation to cytomegalovirus carrier status. Clin Exp Immunol. 1988;74:190–195. [PMC free article] [PubMed] [Google Scholar]
- 46.Luetke-Eversloh M, Hammer Q, Durek P, Nordstrom K, Gasparoni G, Pink M, Hamann A, Walter J, Chang HD, Dong J, Romagnani C. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014;10:e1004441. doi: 10.1371/journal.ppat.1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debre P, Merle-Beral H, Vieillard V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 48.Campos C, Pera A, Sanchez-Correa B, Alonso C, Lopez-Fernandez I, Morgado S, Tarazona R, Solana R. Effect of age and CMV on NK cell subpopulations. Exp Gerontol. 2014;54:130–137. doi: 10.1016/j.exger.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Son YI, Dallal RM, Mailliard RB, Egawa S, Jonak ZL, Lotze MT. Interleukin-18 (IL-18) synergizes with IL-2 to enhance cytotoxicity, interferon-gamma production, and expansion of natural killer cells. Cancer Res. 2001;61:884–888. [PubMed] [Google Scholar]
- 50.Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000;95:3183–3190. [PubMed] [Google Scholar]
- 51.Wu CY, Gadina M, Wang K, O’Shea J, Seder RA. Cytokine regulation of IL-12 receptor beta2 expression: differential effects on human T and NK cells. Eur J Immunol. 2000;30:1364–1374. doi: 10.1002/(SICI)1521-4141(200005)30:5<1364::AID-IMMU1364>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 52.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014;20:463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunikata T, Torigoe K, Ushio S, Okura T, Ushio C, Yamauchi H, Ikeda M, Ikegami H, Kurimoto M. Constitutive and induced IL-18 receptor expression by various peripheral blood cell subsets as determined by anti-hIL-18R monoclonal antibody. Cell Immunol. 1998;189:135–143. doi: 10.1006/cimm.1998.1376. [DOI] [PubMed] [Google Scholar]
- 54.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6:e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gompels UA, Larke N, Sanz-Ramos M, Bates M, Musonda K, Manno D, Siame J, Monze M, Filteau S, C.S. Group Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clin Infect Dis. 2012;54:434–442. doi: 10.1093/cid/cir837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157:175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 58.O’Hara GA, Welten SP, Klenerman P, Arens R. Memory T cell inflation: understanding cause and effect. Trends Immunol. 2012;33:84–90. doi: 10.1016/j.it.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Muntasell A, Vilches C, Angulo A, Lopez-Botet M. Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction. Eur J Immunol. 2013;43:1133–1141. doi: 10.1002/eji.201243117. [DOI] [PubMed] [Google Scholar]
- 60.Vyse AJ, Hesketh LM, Pebody RG. The burden of infection with cytomegalovirus in England and Wales: how many women are infected in pregnancy? Epidemiol Infect. 2009;137:526–533. doi: 10.1017/S0950268808001258. [DOI] [PubMed] [Google Scholar]
- 61.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.