Abstract
Pathogenic mitochondrial DNA (mtDNA) point mutations are associated with a wide range of clinical phenotypes, often involving multiple organ systems. We report two patients with isolated myopathy due to novel mt-tRNAAla variants. Muscle biopsy revealed extensive histopathological findings including cytochrome c oxidase (COX)-deficient fibres. Pyrosequencing confirmed mtDNA heteroplasmy for both mutations (m.5631G>A and m.5610G>A) whilst single muscle fibre segregation studies (revealing statistically significant higher mutation loads in COX-deficient fibres than in COX-positive fibres), hierarchical mutation segregation within patient tissues and decreased steady-state mt-tRNAAla levels all provide compelling evidence of pathogenicity. Interestingly, both patients showed very high mutation levels in all tissues, inferring that the threshold for impairment of oxidative phosphorylation, as evidenced by COX deficiency, appears to be extremely high for these mt-tRNAAla variants. Previously described mt-tRNAAla mutations are also associated with a pure myopathic phenotype and demonstrate very high mtDNA heteroplasmy thresholds, inferring at least some genotype:phenotype correlation for mutations within this particular mt-tRNA gene.
Keywords: Mitochondrial disease, mitochondrial DNA, myopathy, cytochrome c oxidase, mt-tRNAAla
INTRODUCTION
Mitochondrial DNA disorders are associated with a wide range of different clinical phenotypes, from mild to severe 1. Mutations affecting mitochondrial (mt-) tRNA genes are prevalent amongst adults and usually associated with multi-systemic disease presentations 2; isolated organ involvement is rarely observed. Furthermore, it is unusual for mutations in one specific mt-tRNA gene to associate with a unique clinical phenotype, although several mutations in mt-tRNALeu(UUR) and mt-tRNAIle are linked to MELAS and mitochondrial cardiomyopathy respectively 3.
The overwhelming majority of pathogenic mtDNA mutations are present in a heteroplasmic state, the level of mtDNA mutation within a cell or tissue required to exceed a critical threshold to cause a disease phenotype 4. This threshold level varies for each mutation and tissue and is dependent on several factors including OXPHOS metabolism 5,6. Here we report two patients, both presenting with isolated myopathy, with novel heteroplasmic mutations in the mt-tRNAAla gene exhibiting high thresholds for disease expression.
PATIENTS and METHODS
Patient 1
Patient 1 is a young lady who presented at the age of 29 years with muscle weakness, initially involving her hands but spreading to her arms and legs progressively, resulting in her being non-ambulatory by the age of 40 years. Her mother is healthy with no history of muscle disease, while the patient has no children or siblings. She was noted to have a mild symmetrical ptosis but with no history of myoglobinuria, diabetes or seizures. Neurological examination revealed general floppy tetraparesis (MRC 3-4/5). Muscle tendon reflexes were weak. Sensory examination results, tone and Babinski reflexes were all normal. Electroencephalogram revealed no signs of increased cerebral excitability. Needle electromyogram of the biceps brachii muscle and the vastus medialis muscle revealed distinctive myopathic changes. Nerve conduction studies of the suralis nerve showed normal results. Thigh-MRI showed distinctive fatty degeneration of all muscle groups, equal on both sides. Cerebral MRI, cardiac MRI and audiogram were normal. Creatine kinase was slightly elevated (3.9 μmol/l; normal: <2.4). Resting lactate levels were normal (2.0 mmol/l; normal: <2.8).
Patient 2
This lady presented at the age of 69 years with progressive weakness of the limb girdle muscles including proximal paresis. There is no known muscle disease or muscle weakness in the family. Ptosis, ophthalmoplegia or extramuscular mitochondrial symptoms have not been noticed. Neurological examination revealed dysarthria, a facies myopathica and proximal paresis (MRC 3-4/5). Sensory examination and Babinski reflexes were normal. Muscle tendon reflexes on the upper extremity were weak. Achilles deep tendon reflexes were not obtainable on both sides. Patella deep tendon reflexes were left accentuated obtainable. Needle electromyogram of the deltoideus muscle and the biceps brachii muscle revealed myopathic changes. Creatine kinase was slightly elevated (2.7 μmol/l; normal: <2.4), as was resting lactate levels (2.9 mmol/l; normal: <2.8).
Histopathology and molecular genetic studies
Standard histopathologic analysis of muscle biopsies of both patients was performed and the activities of respiratory chain complexes were determined spectrophotometrically 7. Total DNA from all available tissue (muscle, urinary epithelia, buccal epithelia, hair shafts and blood), including tissue from available maternal relatives, was extracted by standard procedures. Long-range PCR of muscle DNA was undertaken to detect large-scale mtDNA rearrangements 8, followed by sequencing of the entire mitochondrial genome in this tissue 9,10. Analysis of mtDNA heteroplasmy was carried out by quantitative pyrosequencing including segregation studies within individual cytochrome c oxidase (COX)-positive and COX-deficient fibers 11. High resolution northern blotting to assess mt-tRNAAla steady state levels in both patients was performed as described 12.
RESULTS
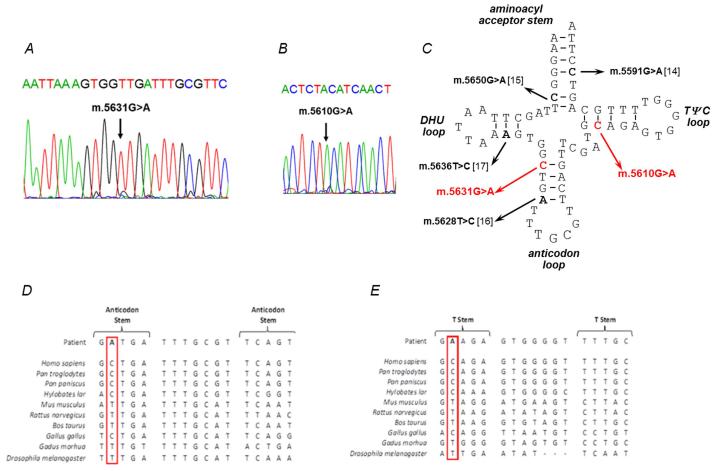
Muscle biopsy analysis revealed numerous COX-deficient (Patient 1: 33%, Patient 2: 40%) and ragged-red-fibres (Patient 1: 2%, Patient 2: 5%) in both patients. Biochemical analysis of muscle from Patient 1 showed decreased activities of respiratory chain complexes I, II/III and IV and a compensatory increase in citrate synthase activity. Decreased activities of respiratory chain complexes I and IV, with a compensatory increase in citrate synthase activity, were noted in Patient 2. Long-range PCR failed to identify large-scale mtDNA deletions, prompting sequencing of the entire mitochondrial genome in muscle which revealed novel mt-tRNAAla (MTTA) gene mutations – m.5631G>A (ClinVar Reference SCV000196082: NC_012920.1) in Patient 1 and m.5610G>A (ClinVar Reference SCV000196083: NC_012920.1) in Patient 2 (Fig. 1C).
Figure 1. Identification of novel mt-tRNAAla variants.
(A) Sequencing electropherogram (reverse sequence) demonstrating the heteroplasmic m.5631G>A transition detected in patient muscle. (B) Sequencing electropherogram demonstrating the heteroplasmic m.5610G>A transition detected in muscle. (C) Schematic representation of the mt-RNAAla cloverleaf structure, illustrating the position of the novel m.5631G>A and m.5610G>A variants and other reported mt-tRNAAla mutations. Phylogenetic conservation of the appropriate regions of the mt-tRNAAla gene sequence for both (D) m.5631G>A and (E) m.5610G>A indicates that both variants affect an evolutionary conserved residue.
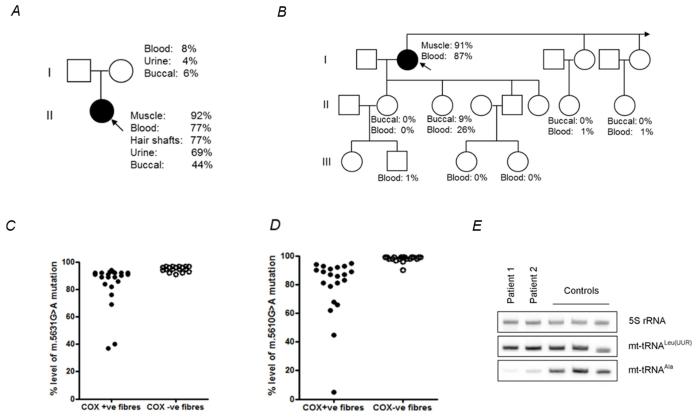
The highest m.5631G>A mutation load in Patient 1 was found in muscle (92% levels of mtDNA heteroplasmy), with lower levels present in blood (77%), hair shafts (77%), urinary epithelial sediment (69%) and buccal epithelial cells (44%). In Patient 2, the highest mutant load was detected also in muscle (91% levels of mtDNA heteroplasmy), followed by blood (87%). Levels of mtDNA heteroplasmy detected in additional family members are shown (Fig. 2A and 2B).
Figure 2. Molecular genetic investigations of patients’ muscle with the novel m.5631G>A and m.5610G>A variants.
(A) Patient 1’s pedigree including mtDNA mutation heteroplasmy levels in the index case and her mother. (B) Family pedigree including mtDNA mutation heteroplasmy levels in the index case (Patient 2) and other family members. (C) Single fibre PCR analysis of the m.5631G>A mutation segregates with a biochemical defect in individual COX-deficient muscle fibres. (D) Single fibre PCR analysis of the m.5610G>A mutation segregates with a biochemical defect in individual COX-deficient muscle fibres. (E) Measurement of mt-tRNAAla steady state levels in muscle showing dramatically-decreased mt-tRNAAla steady-state levels in both patients.
Single muscle fibre analysis of individual COX-positive and COX-deficient fibres of both cases revealed a statistically-significant higher mutation load in COX-deficient fibres than in COX-positive fibres (Patient 1: COX-deficient fibres: 95.1% ± 0.45 (n=18), COX-positive fibres: 83.8% ± 3.38 (n=22), p=0.005; Patient 2: COX-deficient fibres: 98.0% ± 0.46 (n=20), COX-positive fibres: 78.9% ± 4.81 (n=21), p=0.0002) (Fig. 2C and 2D), whilst the muscle from both patients showed dramatically decreased mt-tRNAAla steady state levels compared to normal controls (Fig. 2E).
DISCUSSION
Histopathological findings of diagnostic muscle biopsies in both patients were characterised by numerous COX-deficient fibres and evidence of subsarcolemmal mitochondrial accumulation. This prompted us to thoroughly investigate the mitochondrial genome. The two novel heteroplasmic mt-tRNA point mutations identified, m.5631G>A and m.5610G>A, are both located in mt-tRNAAla and are unequivocally pathogenic according to accepted criteria published by Yarham and colleagues 13.
Additional samples from maternally related members of both patients were available for investigation (Figure 2A and 2B). Interestingly, one daughter of Patient 2 had obviously higher mutation loads in blood and buccal cells than all other investigated family members, although well below the expected disease threshold for the m.5610G>A mutation. She is healthy, with no sign of muscle weakness although she declined detailed investigations such as assessment of CK levels and formal clinical examination.
mtDNA mutations are frequently associated with multisystemic diseases, although isolated organ involvement is rarely observed. Isolated myopathy due to mt-tRNA mutations is a rare clinical presentation. Mutations were detected not only in the mt-tRNAAla gene but single cases are described with mutations in the mt-tRNAAsp, mt-tRNATrp, mt-tRNAPhe, mt-tRNAGln, mt-tRNALeu(CUN), mt-tRNAPro, mt-tRNAIle, mt-tRNASer(UCN) and mt-tRNALys genes. Mutations in the mt-tRNAAla gene all appear to be associated with isolated myopathy in contrast to mutations in these other mt-tRNA genes that are associated with different phenotypes including multisystemic presentations. In addition to the novel mutations presented here, there are four other previously-reported examples of mt-tRNAAla mutations, two of which are associated with myopathy affecting the limbs (m.5591G>A and m.5650G>A) and two mutations (m.5628T>C and m.5636T>C) associated with a myopathy affecting additionally the extraocular and pharyngeal muscles (Table 1 and Fig. 1C). The patient previously-described with a m.5591G>A transition presented with pure myopathy, involving predominantly limb girdle muscles, myalgia, elevated CK levels, and a severe combined respiratory chain enzyme defect 14. Follow-up analysis of urine samples from his two unaffected brothers revealed the presence of the m.5591G>A mutation (67% mutant load) in the younger brother, but not in the older brother 14. Initially, the younger brother presented with asymptomatic elevation of CK, later developing exercised-induced muscle weakness leading to proximal paresis at the age of 51 years. The patient reported with a maternally-inherited m.5650G>A mutation in mt-tRNAAla suffered from an increasingly severe limb-girdle myopathy 15. Two other reported cases showed a preference for skeletal muscle involvement; the m.5628T>C mutation was identified in a patient who presented with proximal paresis, episodic diplopia, external ophthalmoplegia and dysphagia 16, whilst the m.5636T>C mutation was identified in a patient who presented with fatigue, bilateral ptosis, external ophthalmoplegia and mild dysarthria 17.
Table 1. Clinical, histochemical and molecular details of the novel mt-tRNAAla mutations and previously-reported mt-tRNAAla mutations.
| Mutation (patient) | Age of onset (gender) | Phenotype | Histological findings | Level of heteroplasmy in muscle | Threshold single fibre level | CK (normal: <2.4 μmol/l) |
|---|---|---|---|---|---|---|
| m.5631G>A (Patient 1) | 29 (f) | Distal and proximal paresis. Mild symmetrical ptosis, but no external ophthalmoplegia | Numerous COX-deficient fibres (33%) in addition to ragged-red-fibres (2 %) | 92% | approx. 96% | up to 3.9 μmol/l |
| m.5610G>A (Patient 2) | 69 (f) | Proximal Paresis. No ptosis and no external ophthalmoplegia | Mild myopathic changes. Numerous COX-deficient fibres (40%) in addition to ragged-red-fibres (5%) | 91% | approx. 97% | up to 2.7 μmol/l |
| m.5591G>A 14 | 45 (m) | Proximal paresis of arms and legs and distal paresis of both arms. No ptosis and no external ophthalmoplegia | Mild myopathic changes. Marked number (~80%) of COX-deficient fibres with ragged-red fibres (30%) | 98% | approx. 98% | up to 12.31 μmol/l |
| m.5591G>A 14 * | 51 (m) | Proximal paresis. No ptosis and no external ophthalmoplegia | Numerous number of COX-deficient fibres (60%). Small number of ragged-red fibres | not determined in muscle; urine: 67% | Not determined | up to 7.07 μmol/l |
| m.5650G>A 15 | 6 (f) | Proximal paresis. Mild bilateral ptosis, but no external ophthalmoplegia | Marked number of COX-deficient fibres (>50%), marked variation in fibre size, evidence of inflammatory changes | >95% | approx. 99% | up to 6.25 μmol/l |
| m. 5628T>C 16 | 77 (f) | Proximal paresis, episodic diplopia, external ophthalmoplegia and dysphagia. No ptosis | Many COX-deficient fibres (30%) and ragged-red fibres | 40% | Not determined | normal |
| m.5636T>C 17 | 7 (m) | Fatigue syndrome, bilateral ptosis, external ophthalmoplegia, mild dysarthria. No paresis. | Signs of chronic myopathy. Several COX-deficient fibres and ragged-red fibres | 31% | >80% | up to 8.95 μmol/l |
Brother of the patient above who initially presented with asymptomatic elevation of CK levels
It is interesting that the threshold for impairment of oxidative phosphorylation, as evidenced by COX deficiency, appears to be extremely high for both novel mt-tRNAAla mutations. The previously-described cases of mt-tRNAAla mutations associated with a pure myopathic phenotype (m.5591G>A and m.5650G>A) demonstrated similarly high mutation thresholds, inferring at least a genotype:phenotype correlation for mutations within this particular mt-tRNA gene.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Kathleen Zietz, Thekla Wangemann and Gavin Falkous. DL, PRJ, SZ, and MD are members of the German mitoNET funded by the German Ministry of Education and Research. RWT is supported by a Wellcome Trust Strategic Award (096919Z/11/Z), the Medical Research Council (UK) Centre for Translational Muscle Disease Research (G0601943), The Lily Foundation and the UK NHS Specialist Commissioners which funds the “Rare Mitochondrial Disorders of Adults and Children” Diagnostic Service in Newcastle upon Tyne (http://www.mitoresearch.org.uk/).
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nature Reviews Genetics. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakely EL, Yarham JW, Alston CL, et al. Pathogenic mitochondrial tRNA point mutations: nine novel mutations affirm their importance as a cause of mitochondrial disease. Human Mutation. 2013;34:1260–1268. doi: 10.1002/humu.22358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarham JW, Elson JL, Blakely EL, McFarland R, Taylor RW. Mitochondrial tRNA mutations and disease. Wiley Interdisciplinary Reviews. RNA. 2010;1:304–324. doi: 10.1002/wrna.27. [DOI] [PubMed] [Google Scholar]
- 4.DiMauro S, Moraes CT. Mitochondrial encephalomyopathies. Archives of Neurology. 1993;50:1197–1208. doi: 10.1001/archneur.1993.00540110075008. [DOI] [PubMed] [Google Scholar]
- 5.Wong LJ. Diagnostic challenges of mitochondrial DNA disorders. Mitochondrion. 2007;7:45–52. doi: 10.1016/j.mito.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochimica et Biophysica Acta. 2010;1797:113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Gellerich FN, Deschauer M, Chen Y, Muller T, Neudecker S, Zierz S. Mitochondrial respiratory rates and activities of respiratory chain complexes correlate linearly with heteroplasmy of deleted mtDNA without threshold and independently of deletion size. Biochimica et Biophysica Acta. 2002;1556:41–52. doi: 10.1016/s0005-2728(02)00305-5. [DOI] [PubMed] [Google Scholar]
- 8.Deschauer M, Kiefer R, Blakely EL, et al. A novel Twinkle gene mutation in autosomal dominant progressive external ophthalmoplegia. Neuromuscular Disorders. 2003;13:568–572. doi: 10.1016/s0960-8966(03)00071-3. [DOI] [PubMed] [Google Scholar]
- 9.He L, Chinnery PF, Durham SE, et al. Detection and quantification of mitochondrial DNA deletions in individual cells by real-time PCR. Nucleic Acids Research. 2002;30:e68. doi: 10.1093/nar/gnf067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuppen HA, Hogan VE, He L, et al. The p.M292T NDUFS2 mutation causes complex I-deficient Leigh syndrome in multiple families. Brain. 2010;133:2952–2963. doi: 10.1093/brain/awq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White HE, Durston VJ, Seller A, Fratter C, Harvey JF, Cross NC. Accurate detection and quantitation of heteroplasmic mitochondrial point mutations by pyrosequencing. Genetic Testing. 2005;9:190–199. doi: 10.1089/gte.2005.9.190. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RW, Giordano C, Davidson MM, et al. A homoplasmic mitochondrial transfer ribonucleic acid mutation as a cause of maternally inherited hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2003;41:1786–1796. doi: 10.1016/s0735-1097(03)00300-0. [DOI] [PubMed] [Google Scholar]
- 13.Yarham JW, McFarland R, Taylor RW, Elson JL. A proposed consensus panel of organisms for determining evolutionary conservation of mt-tRNA point mutations. Mitochondrion. 2012;12:533–538. doi: 10.1016/j.mito.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swalwell H, Deschauer M, Hartl H, et al. Pure myopathy associated with a novel mitochondrial tRNA gene mutation. Neurology. 2006;66:447–449. doi: 10.1212/01.wnl.0000196490.36349.83. [DOI] [PubMed] [Google Scholar]
- 15.McFarland R, Swalwell H, Blakely EL, et al. The m.5650G>A mitochondrial tRNAAla mutation is pathogenic and causes a phenotype of pure myopathy. Neuromuscular Disorders. 2008;18:63–67. doi: 10.1016/j.nmd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Spagnolo M, Tomelleri G, Vattemi G, Filosto M, Rizzuto N, Tonin P. A new mutation in the mitochondrial tRNAAla gene in a patient with ophthalmoplegia and dysphagia. Neuromuscular Disorders. 2001;11:481–484. doi: 10.1016/s0960-8966(01)00195-x. [DOI] [PubMed] [Google Scholar]
- 17.Pinos T, Marotta M, Gallardo E, et al. A novel mutation in the mitochondrial tRNAAla gene (m.5636T>C) in a patient with progressive external ophthalmoplegia. Mitochondrion. 2011;11:228–233. doi: 10.1016/j.mito.2010.08.008. [DOI] [PubMed] [Google Scholar]