Abstract
Influenza-A virus causes considerable morbidity and mortality largely because of a lack of effective anti-viral drugs. Viral neuraminidase inhibitors, which inhibit viral release from the infected cell, are currently the only approved drugs for influenza but have recently been shown to be less effective than previously thought. Growing resistance to therapies that target viral proteins has led to increased urgency in the search for novel anti-influenza compounds. However, discovery and development of new drugs have been restricted because of differences in susceptibility to influenza between animal models and humans, and a lack of translation between cell culture and in vivo measures of efficacy.
In order to circumvent these limitations, we developed an experimental approach based on ex vivo infection of human bronchial tissue explants and optimised a method of flow cytometric analysis to directly quantify infection rates in bronchial epithelial tissues. This allowed testing of the effectiveness of TVB024, a vATPase inhibitor which inhibits viral replication rather than virus release, and to compare efficacy with the current frontline neuraminidase inhibitor, oseltamivir.
The study showed that the vATPase inhibitor completely abrogated epithelial cell infection, virus shedding and the associated induction of pro-inflammatory mediators, whereas oseltamivir was only partially effective at reducing these mediators and ineffective against innate responses. We propose, therefore, that this explant model could be used to predict the efficacy of novel anti-influenza compounds targeting diverse stages of the viral replication cycle, thereby complementing animal models and facilitating progression of new drugs into clinical trials.
Introduction
Influenza has a major impact on global health, especially during seasonal epidemics, resulting in significant mortality, particularly among children and the elderly [1]. It also causes serious complications in patients with chronic respiratory diseases and in immunosuppressed individuals [2]. Despite significant resources spent on preventing and treating influenza, there remains a large unmet need for effective anti-influenza virus therapies. Although recommended by the WHO for at-risk populations [3, 4], vaccination against influenza is not fully effective. Drugs targeting the viral neuraminidases, such as oseltamivir (Tamiflu®) and zanamivir (Relenza®), and M2 ion channel inhibitors, such as Amantadine, are showing increased resistance [5, 6]. Furthermore, their effectiveness has not been definitively proven in patients with chronic airways diseases in whom the impact of influenza on morbidity and mortality is higher than in the general population [7].
The limited size of the viral genome restricts the scope of therapeutic development targeting influenza viral proteins. Recent developments in technology to discover novel host gene targets, such as genome-wide siRNA and homozygous gene perturbation screens [8-13], have identified a large number of genes involved in the replication of the influenza virus which are candidate targets [14]. Progression of therapeutics identified through such screening requires additional proof of efficacy before embarking on clinical trials in human volunteers. Pre-clinical testing of influenza therapeutics has been restricted to a few animal species, such as ferrets, which can be infected by strains that also affect humans [15]; however their use in the development of drugs, especially those targeting human host defences, is limited by inter-species differences in gene sequence, protein structure, and also potential differences in viral-host interactions.
The difference in inflammatory responses to viral infection between therapies which target early and late viral life cycle replication events on has not been fully investigated in humans. This is partly because existing cell models do not produce the wide range of inflammatory mediator responses observed in human infections, and partly because of challenges associated with measuring mediator responses in biofluids derived from in vivo experimental infections of human volunteers.
In order to address the current limitations in development of anti-influenza drugs, we have developed a pre-clinical testing platform where lung tissue samples are infected ex vivo with influenza virus. The extent of infection of lung tissue is then quantified by flow cytometry, and inflammatory responses are assessed by measuring pro-inflammatory mediator production secreted by the infected tissue. We report here on the value of this explant model by comparing the anti-viral efficacy of targeting viral entry mechanisms to inhibit replication using a vATPase inhibitor with that of a neuraminidase inhibitor (oseltamivir) which inhibits viral shedding. We discuss the potential benefits of such a model in determining infection characteristics and therapeutic responses in patients with chronic lung diseases.
Methods
Study design
We firstly optimised the methods for identifying and quantifying influenza infection in cells and tissues by flow cytometry. The lung explant model was then validated by quantifying the extent of epithelial cell infection and viral shedding from bronchial biopsies obtained by bronchoscopy. The dose of infection (MOI) required was then compared with that needed to infect standard monolayer primary bronchial epithelial cell cultures (PBEC). The two culture models were compared further in respect of inflammatory responses by measuring a set of cytokines/chemokines, many of which have been previously shown to be modulated in vivo during human influenza infection [16]. The explant model was then applied to study the anti-viral effects of a vATPase inhibitor, TVB024, which entry into epithelial cells, and Oseltamavir, a neuraminidase inhibitor that inhibits virus release from infected epithelial cells.
Materials
A/H3N2/X31 and A/H3N2/Wisconsin/67/2005 seed stocks were obtained from the National Institute for Biological Standards and Control (NIBSC), UK, propagated in embryonated SPF-free chicken eggs and, subsequently, purified from egg allantoic fluid by sucrose density gradient ultracentrifugation (Virapur LLC, San Diego, USA). Stock viral titre was determined by MDCK plaque assay using standard protocols. Monoclonal anti-influenza NP antibody conjugated to FITC was purchased from BD (Cowley, Oxford, UK). Rabbit polyclonal anti- A/H3N2/Wisconsin/67/2005 antibody was produced from UV inactivated virus by Eurogentec (Seraing, Belgium). Oseltamavir carboxylate and the vATPase inhibitor TVB024 were synthesised in-house and their purity was confirmed to be >99% by NMR and mass spectrometry. Collagenase type I from Clostridium histolyticum was purchased from Sigma (Poole, Dorset, UK). Antibodies used for flow cytometry and immunohistochemistry were purchased from Beckton-Dickinson (Cowley, Oxford, UK), Abcam (Cambridge, UK) and Invitrogen (Paisley, Scotland, UK).
Lung tissue and cell culture
To develop the infection model, lung tissue and cultured primary cell lines were required, including epithelial and endothelial cells, fibroblasts and leukocytes.
Bronchial lung tissue and epithelial cells for ex vivo infection were obtained by fiberoptic bronchoscopy from ten healthy volunteer subjects with no evidence of airways disease, as shown by normal lung function spirometry tests and also no evidence of reversibility to the β2-agonist bronchodilator salbutamol. All subjects were non atopic as shown negative skin prick tests to a panel of common aero-allergens. Standard research protocols were applied [17,18] and up to 10 bronchial biopsy samples and 6 bronchial brushings were taken from the sub-carinae of the proximal bronchi. Pairs of bronchial explants were rested overnight in 500 μl of complete AIM-V medium supplemented with penicillin and amphotericin as previously described [19] and the medium was exchanged prior to infection.
Resected human lung tissue was obtained from patients undergoing surgical lobectomy for lung cancer at Southampton University Hospital. Parenchymal tissue from donors without evidence of obstructive lung disease or asthma, distant from the resection margin and any gross pathology, was dissected And the tissue was then dissected into small cubes (1-3mm3) and transferred to 24-well culture dishes, 3 pieces per well.
Both the primary bronchial epithelial cells (PBECs) and lung tissue explants were cultured in a humidified tissue culture incubator at 37°C, 5% CO2.
Immortal cell line culture
The immortalised type II alveolar cell line A549 (ATCC CCL-185) was cultured in Dulbecco’s modified Eagles’ Medium (DMEM) supplemented with 10% (v/v) heat inactivated foetal calf serum, non-essential amino acids and L-glutamine. Cells were used from logarithmically growing cultures at up to 90% confluence.
Primary cell line culture
A number of primary cell cultures were first established for optimisation of the flow cytometric analysis of bronchial biopsies dispersed after infection (see below).
Primary human lung fibroblasts were isolated by outgrowth from human bronchial biopsies[20]. Cells were maintained in DMEM supplemented with glutamine, penicillin-streptomycin, sodium pyruvate and non-essential amino acids and used within passage 1-2. Human umbilical vein endothelial cells were isolated as previously described [21], and were a kind gift from Dr T. Millar, University of Southampton. They were maintained in DMEM supplemented with 10% human AB serum on gelatin-coated culture surfaces and used within passages 1-2. PBECs were cultured from bronchial brushings as monolayers [22]. They were maintained on collagen I-coated culture surfaces in bronchial epithelial growth medium (BEGM, Lonza) and used within passages 1-2.
All adherent cell cultures were isolated from monolayers using trypsin-EDTA just before use, and re-suspended at 1×106 cells/ml in PBS prior to mixing for flow cytometric analysis. Human peripheral blood mononuclear cells (PBMCs) were obtained from venous blood by density centrifugation using Lymphoprep® as described in the manufacturer’s instructions. PBMC were re-suspended in PBS at a concentration of 1×106/ml.
For infection experiments, PBECs were cultured in 24-well tissue culture plates at a density of 0.5×104 cells/well, and 24 h prior to infection, media were changed to basal growth medium (BEBM) supplemented with insulin, transferrin and selenium solution (Life Technologies, Paisley, UK).
Infection of epithelial cells and lung tissue explants
The A549 cells, PBECs and lung tissue samples were treated with drugs (Oseltamivir or vATPase inhibitor) or 0.05% (v/v) DMSO carrier control (where appropriate) in their respective media for 2 h prior to infection. Influenza virus (A/H2N3/X31 or A/H3N2/Wisconsin/67/2005 as appropriate) was added in the presence of inhibitor or control and incubated for a further 2 h. Virus was then added at MOI of 0.2 to monolayer cell cultures, and at log7.4 infectious unit dose (IUD) to lung explant tissues. Samples were then washed three times by media replacement to remove excess virus and incubated for further time-points in complete AIM-V or BEBM medium for explant and PBEC cultures, respectively, with the addition of study drugs or DMSO control. At appropriate time-points, culture medium samples were collected and centrifuged at 400g, 4°C for 5 min to remove cellular material. Samples were analysed by multiplexed ELISA for a set of cytokines and chemokines (Mesoscale Discovery, Rockville, MD, USA).
Assessment of endosomal acidification using acridine orange assay
The effect of TVB024 on endosomal acidification was assessed by acridine orange staining for determination of endosomal pH. Briefly, A549 cells were pre-incubated with TVB024 for 16 h. The culture media were then replaced with HBSS containing 5μg/ml acridine orange (A1301, Life Technologies) for 15 min at 37°C. The cells were washed three times with HBSS and then incubated with HBSS containing the drug at the same initial concentrations. The culture plates were then immediately assessed for acridine orange staining using a spectrophotometer equipped with a long pass filter set (479/28nm excitation / >500nM emission).
Flow cytometric analysis of infection
After infection with virus, the PBECs were dispersed with trypsin-EDTA, which was subsequently neutralised using 10% (w/v) foetal calf serum and removed by centrifugation at 400 g for 5 min. The bronchial explants were dispersed with 1mg/ml collagenase in basal RPMI culture medium for 90 minutes at 37°C, and the cells were then centrifuged at 400 g for 5 min to remove collagenase. Dispersed explant and PBEC cells were re-suspended in flow cytometry buffer (0.5% w/v BSA, 2 mM EDTA in Dulbecco’s PBS) and treated for 10 min with 5 mg/ml human IgG on ice prior to extracellular staining with fluorochrome-labelled monoclonal antibodies for 30 min on ice. For the analysis of dispersed biopsies antibodies against CD45 (pan-leukocyte marker), CD90/Thy1 (fibroblast marker), CD326/EpCAM (epithelial cell marker) and CD34 (endothelial/haematopoietic progenitor cell marker) were used to demonstrate the specificity of each of the markers for different structural and leukocyte cell populations. Thereafter, a combination of CD326 and CD45 antibodies was used routinely to quantify infection of epithelial cells in bronchial biopsies. The cells were then fixed and permeabilised using BD Fix/Perm reagents prior to staining for intracellular expression of viral nucleoprotein (NP) using monoclonal antibodies conjugated to FITC. Flow cytometry was performed using a FACSAria (BD) with appropriate filters and settings. Data were analysed using FACS Diva software.
Immunohistochemistry
In order to validate the flow cytometric detection of virus infection and localise the infection in the lung tissue, immunohistochemistry was performed using samples fixed in acetone containing protease inhibitors overnight at −20°C. Samples were then embedded in glycol methacrylate as previously described [23]. Two-μm sections were cut and applied to poly-l-lysine coated glass slides. Staining was performed as previously described [24] using the ABC system with peroxidase-linked secondary antibodies against mouse or rabbit primary antibodies. The same antibody clones were used for both immunohistochemistry and flow cytometry, following optimisation of each antibody concentration for the relevant method. HRP signal was detected with DAB stain, and with a haematoxylin counterstain. Images were captured using a Zeiss Axioskop2 microscope fitted with an Axiocam, using Zeiss KS400 v3.0 image analysis software. X31 virus infection was detected using unconjugated HB-65 monoclonal antibody.
Virus release assays
Due to the risk of highly potent anti-viral compounds interfering with standard plaque assay, and the additional presence of infection-inhibitory proteins in the AIM-V culture medium used in explant cultures, a viral immunoassay validated against plaque assay was used to quantify viral shedding. Viral titre quantified by the two methods was log-transformed to normalise the data prior to comparison of the differences in measurements using Bland-Altman analysis (Figure S3A), showing good agreement between both methods, with a mean difference of −0.005 (CI −0.106 to 0.096), well within the limits of agreement of 0.636 to 0.627.
PBEC and explant conditioned media were transferred to nitrocellulose membrane using biodot apparatus (Bio-Rad, Hemel Hempstead, UK). A standard curve of viral protein was generated using viral stocks of known infectious dose (determine by MDCK plaque assay) diluted in the same manner. Immobilised viral proteins were detected using rabbit polyclonal antibodies specific for the influenza virus and visualised by chemiluminscence detection using anti-rabbit detection system (Bio-Rad). Signals were detected and data were interpolated from the standard curves using a Versadoc chemiluminscence reader with QuantityOne software (Bio-Rad).
LDH toxicity assay
25 μl of tissue conditioned medium were diluted in 100 μl of phenol red-free DMEM and then 25 μl of assay buffer (Cytotox non-radioactive LDH assay, Promega, Madison, Wisconsin, USA) were added to each well. Positive controls for each tissue donor were generated by sonicating tissue samples from control wells in their respective media for 3 bursts of 5 seconds at 7 μm on ice using a probe sonicator (Soniprep 150). Following addition of the assay buffer, the colour was left to develop for 2 h and then the plate was analysed by measuring absorbance at 590 nm. Toxicity was measured as the percentage of LDH release relative to positive (100%) controls.
Measurement of cytokines by multiplexed ELISA
Cell-free culture supernatants were analysed by multiplexed ELISA (Mesoscale discoveries) according to the manufacturers’ instructions using a bespoke 8-plex ELISA for IL-6, IL-1β, TNF-α, IFN-γ, and chemokines IP-10, MCP-1, MIP1β and IL-8. Briefly, conditioned media samples were diluted 1:1 in the supplied buffer containing carrier protein and applied to ELISA plates pre-coated with antibodies directed against the eight mediators. A detection antibody solution pre-diluted in blocking buffer was applied and the signal was analysed on a SECTOR 2400 MSD plate reader. Data were analysed using MSD discovery workbench software. Cytokine quantities were interpolated from standard curves generated using mixtures of recombinant proteins.
Data analysis and statistics
Data were analysed using paired non-parametric paired t-test or non-parametric ANOVA with Dunn’s adjustment for multiple comparisons as appropriate to data distribution, using GraphPad Prism software (v6).
Ethics approval
The collection of tissues was undertaken with written informed consent and was approved by and performed in accordance with the ethical standards of the Southampton and South West Hampshire Research Ethics Committee, LREC no: 09/H0504/109.
Results
Identification of structural cells within bronchial explant tissue using flow cytometry
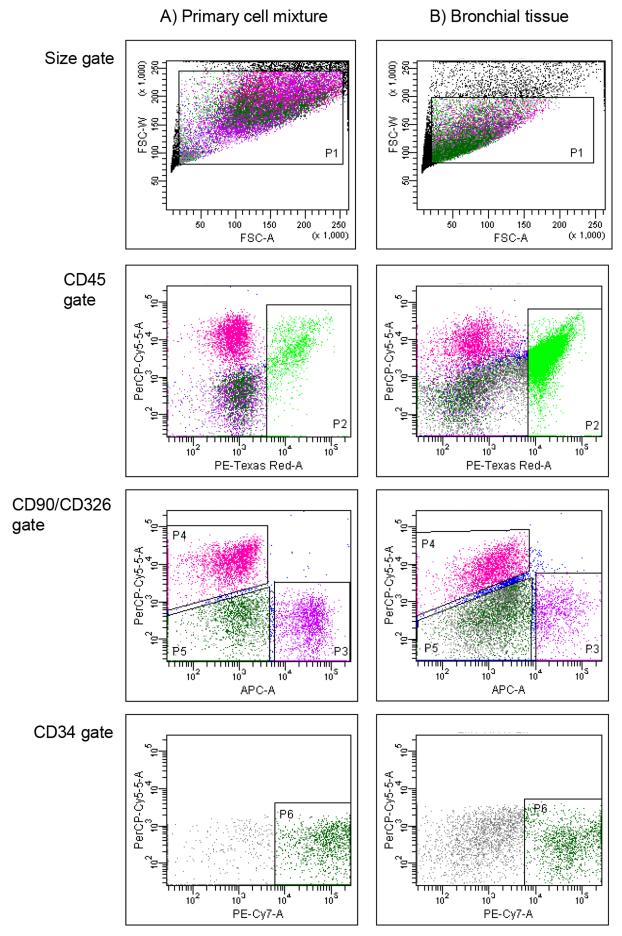
We have previously used flow cytometry to quantify and characterise leukocyte populations within bronchial explant tissues [19, 25-27]. To analyse structural cells (epithelial, fibroblast and endothelial cells) within this tissue type, we optimised their detection using monoclonal antibodies directed against surface markers unique to individual cell types. Specificity of the markers was confirmed firstly by immunohistochemistry (Figure S1). Flow cytometry was then applied to a mixture of primary cultures of cell types known to reside in bronchial tissue (Figure 1A) or to enzymatically dispersed bronchial explant tissue (Figure 1B). Primary cell culture resulted in increased cell volumes when compared to cells freshly dispersed from lung tissues, which reflected in elevated forward scatter properties (P1); however, comparable cell populations of leukocytes, fibroblasts, epithelial and endothelial cells were observed. The results confirmed our ability to identify epithelial cells from within the tissue using flow cytometry using a gating strategy which excluded other cell populations such as leukocytes and fibroblasts.
Figure 1.
Detection of structural cells and leukocytes by flow cytometry. Gating strategy to detect CD45, CD90, CD326 and CD34 positive staining in: i) equal mixtures of primary cultures of human bronchial epithelial cells, human umbilical vein endothelial cells (HUVEC), pulmonary fibroblast cells and peripheral blood mononuclear cells (PBMCs), ii) enzymatically dispersed cells from bronchial tissue using the same gating strategy. Cell suspensions were stained for flow cytometry using antibodies directed against CD90, CD326, CD34 and CD45 labelled with APC, PerCPCy5.5, PE-Cy7 and PE-AF610, respectively, as described in the methods. Controls for each stain were prepared using appropriate isotype antibodies labelled with the same fluorophores. Gating strategy applied: a) size gate to include single cell events, (P1), b) identification of leukocytes using CD45 (P2), c) excluding CD45 positive cells, fibroblasts (CD90+, P3) and epithelial cells (CD326+,P4) were identified. d) excluding all other markers, endothelial cells stained positive for CD34 (P6). Clear populations of leukocytes, epithelial, fibroblast and endothelial cells were observed when stained with antibodies specific for each cell type.
Pattern of influenza infection in bronchial explants
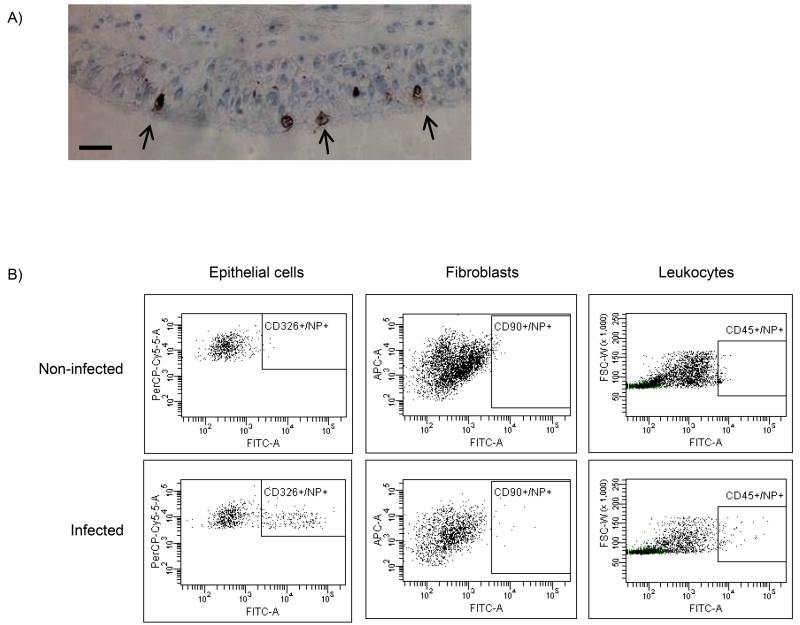
Infection of bronchial explants with influenza virus ex vivo was identified easily by intracellular expression of viral nucleoprotein within the epithelial cell population, both by immunohistochemistry and flow cytometry of cells. Immunohistochemistry showed infection only in the apical epithelial cells (Figure 2A), visible as intense cytoplasmic and membrane staining. Similarly, infection was observed by flow cytometry largely in the epithelial cells and only rarely in leukocytes and fibroblasts (Figure 2B).
Figure 2.
Identification of ex vivo influenza infection. Bronchial explants were infected with log7.4 infectious unit dose (IUD) of A/H2N3/X31 influenza virus for 2 h and further incubated for a total of 24 h. A) Immunohistochemical staining of a bronchial biopsy for viral infection using anti-viral NP protein monoclonal antibody (brown) and haematoxylin counterstain (blue) in bronchial tissues. Arrows indicate infected epithelial cells. Size bar indicates 50μm. B) Flow-cytometric analysis of enzymatically dispersed bronchial biopsies to identify infected cells. Leukocytes, were identified as CD45+ cells, epithelial cells were identified as a CD45−/CD326+ population, and fibroblasts were identified as CD45−/CD90+ population. Influenza infection in each cell type was measured by staining using monoclonal antibodies directed to influenza nucleoprotein (NP), conjugated to FITC. Images shown are representative of three separate experiments. NI: not infected, INF: infected.
Comparison of PBEC and bronchial explant inflammatory responses
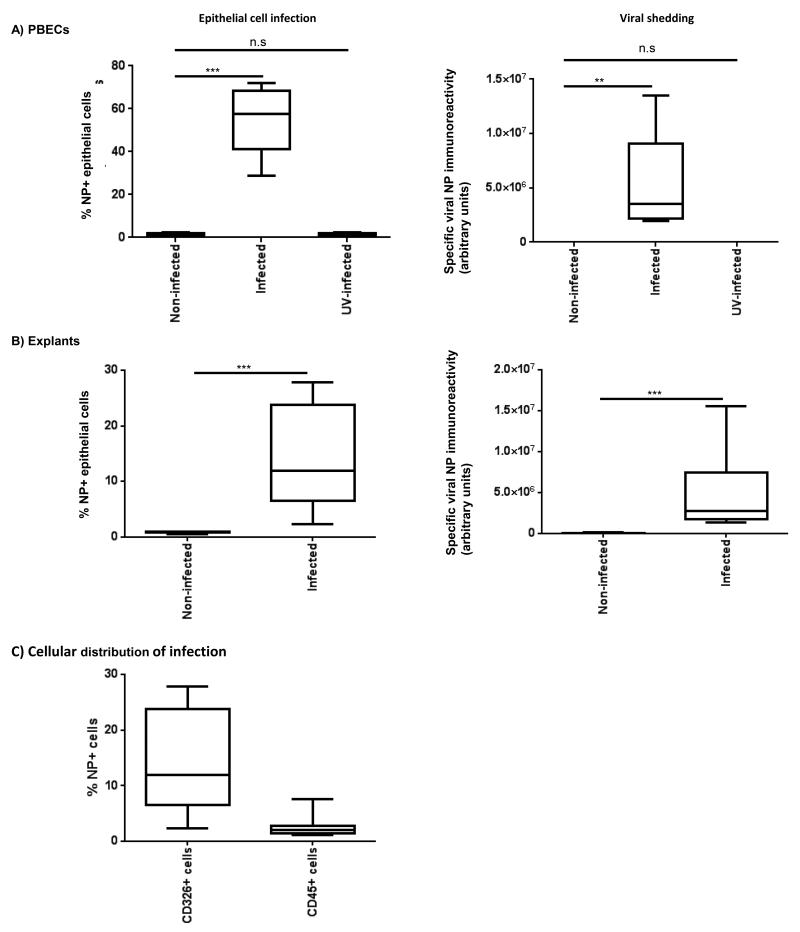
Primary bronchial epithelial cells infected with an MOI of 0.2 resulted in a median infection of 55% after 24 h of culture (Figure 3A). No immunoreactivity was observed in cells exposed to UV-inactivated virus, demonstrating the specificity of this method for actively replicating intracellular virus. The virus dose for bronchial explants was optimised in dose-response experiments using resected lung parenchyma tissue samples, which were more freely available and of similar weight to bronchial explants used in subsequent analyses. The maximum infection rate in explants was 10% of epithelial cells at log 7.4 infectious unit dose in lung parenchyma (data not shown), an infection rate which could not be increased by adding more virus. This dose similarly resulted in an epithelial cell infection rate of 12% in bronchial explants (Figure 3B). As epithelial numbers could only be calculated post-infection, by back calculation we calculated that the application of this maximum virus dose resulted in an approximate MOI of 200 for bronchial biopsies. This difference in MOI demonstrated key differences in viral handling and susceptibility between monolayer cultures and intact tissues. Infection was accompanied by release of viral proteins into the culture medium of both monolayer cells and lung tissues after 24 h of infection (Figure 3A and B), suggesting active viral replication and specific release of virus. No viral shedding was observed in monolayer cells exposed to UV-inactivated virus.
Figure 3.
Quantification of influenza A infection in primary bronchial epithelial cells (PBECs) and bronchial explants. Primary bronchial epithelial cells (PBECs) and bronchial explants were infected with a Multiplicity of Infection (MOI) of 0.2, and log7.4 IUD A/H3N2/Wisconsin/68/2005 influenza virus respectively for 2 h as described in the materials and methods, incubated for a maximum of 48 h, and then enzymatically dispersed. Cells were then stained for viral NP as previously described, and analysed by flow cytometry to identify NP+ epithelial cells. Infection in PBECs (A) and bronchial explants (B) was expressed as % NP+ epithelial cells. Viral shedding into the culture medium from infected PBECs and explants is expressed as arbitrary units of specific immunoreactivity to viral NP (background due to culture medium subtracted from data). C) Influenza infection of human bronchial explants was observed principally in epithelial cells (CD45−/CD326+/NP+), with only limited infection of leukocytes (CD45+/NP+).
Box and whisker plots indicate median and interquartiles +/− range, n=9 PBECs, n=10 bronchial explants. *=p<0.05, **=p<0.01, ***=p<0.001
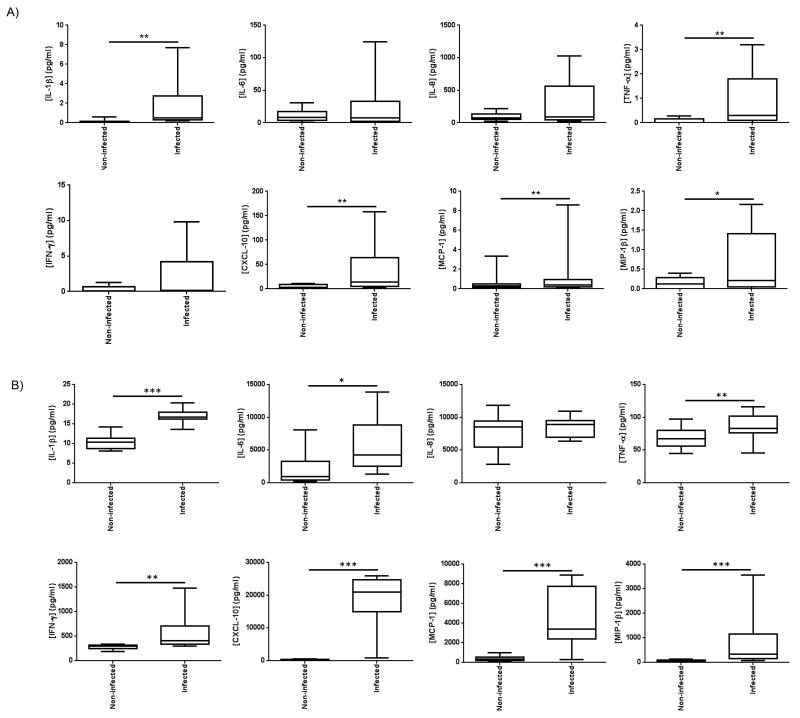
Monolayer PBECs responded to influenza virus infection 24 h post-infection with increased release of Il-1β, CXCL-10, MCP-1, MIP-1β and TNF-α (Figure 4). Bronchial explants additionally released IFN-γ and IL-6. The bronchial explants generally secreted 10-fold greater quantities of mediators upon infection, despite containing approximately 100-fold fewer epithelial cells, suggesting greater responsiveness to infection of explanted tissues as compared to cell monolayers.
Figure 4.
Effect of influenza infection on soluble mediator secretion into the culture medium by monolayer primary epithelial cells and bronchial tissue explants. Primary bronchial epithelial cells (PBEC) and bronchial tissue explants (Bx) were either mock infected with virus diluent, or infected with influenza virus for 2 h, the virus was then removed and the cells/tissues incubated for a further 24 h. The culture supernatants were analysed by multiplexed ELISA for 8 soluble mediators. Data shown are mediator concentrations in pg/ml of conditioned medium supernatant. Box and whisker plots indicate median infection-induced mediator concentration and interquartiles +/− range, n=9 for PBECs, n=10 for bronchial samples. *=p<0.05, **=p<0.01, ***=p<0.001
Development of a small molecule inhibitor of virus infection
Vacuolar ATPase was chosen as a target based on its identification as a mediator of early viral replication events in previous studies [8-11]. A small molecule vATPase inhibitor, TVB024, was custom-synthesised, with a structure based on the indol series of bafilomycin-derived vATPase inhibitors (Figure S2A), but with improved toxicity characteristics [28]. Using acridine orange assay, TVB024 was demonstrated to be an effective vATPase inhibitor, as increasing concentrations of the drug inhibited endosome acidification whilst also reducing influenza infection in a dose-dependent manner (Figure S2B). Its anti-viral and cytotoxic profile was validated in monolayer cultures of A549 cells (Figure S2C) where a 2-log window of therapeutic effectiveness/toxicity could be demonstrated. Data collected in a similar manner using epithelial monolayer and air-liquid interface cultures showed IC50 values for TVB024 of 200 nM and 350 nM respectively (Table 1).
Table I.
Inhibitor profile for TVB024 using primary bronchial epithelial cells (PBEC), airliquid interface cultured PBECs (ALI PBEC) and lung tissue explants. Samples were pretreated with varying doses of TVB024 for 2 h, and then infected with influenza virus in the presence of inhibitors. Infection was measured by quantitation of viral progeny after a further incubation period of up to 22 h in the presence of inhibitors. Dose-dependent inhibition (IC50) calculations were made using GraphPad Prism software by plotting 3-point logistical curves of the percentage of viral release compared to control (uninhibited) in the tissue culture supernatant against the log of the inhibitor concentration.
IC50 and CC50 are half-maximal inhibitor and cytotoxicity concentrations respectively (values shown are μM).
| Assay | IC50 | CC50 |
|---|---|---|
| PBEC | 0.2 | >32 |
| ALI PBEC | 0.35 | >32 |
| Lung tissue explants | 0.88 | >10 |
TVB024 was then compared with oseltamivir carboxylate, the active metabolite of oseltamivir phosphate (a pro-drug form of the influenza A and B neuraminidase inhibitor), an established frontline influenza drug recommended for both post-exposure treatment and prophylaxis in at-risk patients [7]. Optimal doses of 5 μM TVB024 and 100 nM oseltamivir were determined in dose-response experiments of the effects of these inhibitors on viral shedding in lung tissues (Figure S3 Bi-ii). For these experiments, surgical resected lungs were used rather than bronchial biopsies because they provide a large enough number of lung parenchymal samples composed of alveolar tissue and small airways. The effect of TVB024 dose on intracellular viral load was confirmed by flow cytometry (Figure S3C) but this confirmation was not sought for oseltamivir as neuraminidase inhibitors do not affect intracellular viral replication. The optimal doses of TVB024 and oseltamivir showed low levels of toxicity, as measured by LDH release from tissues treated with the compounds (Figure S4).
Demonstration of anti-viral effects of the vATPase inhibitor
Effect on infection and inflammatory responses of treatments applied at optimal doses
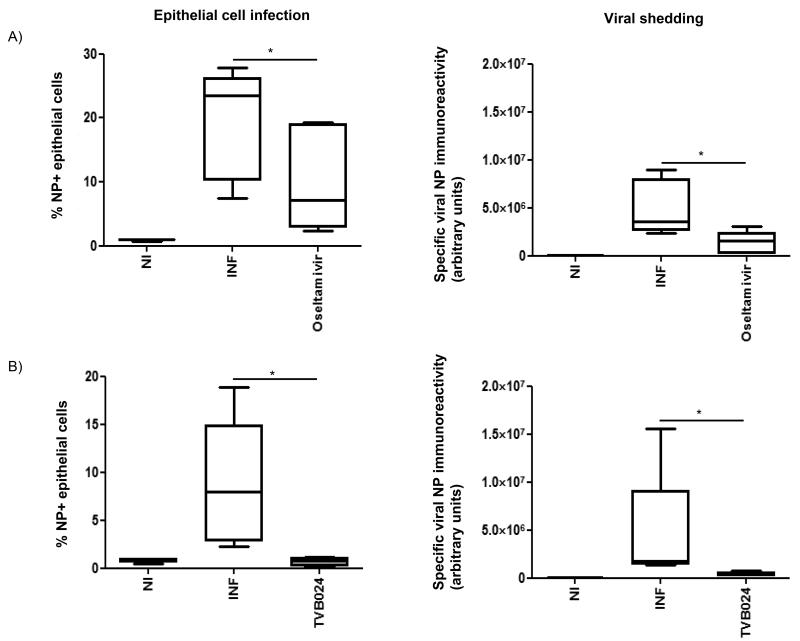
Having established that 5 μM TVB024 and 100 nM oseltamivir had an optimal inhibitory effect on virus shedding and were not toxic, these doses were applied to bronchial biopsy explants from healthy volunteers. When applied prophylactically (2 h prior to infection), Oseltamivir effectively reduced viral shedding but reduced infection only by median 73% (Figure 5A), presumably by limiting re-infection as oseltamivir does not inhibit primary infection at this dose. TVB024 was more effective and inhibited epithelial infection by 100% (Figure 5B) and this was associated with absence of virus shedding.
Figure 5.
Effect of vATPase and neuraminidase inhibition on viral infection and shedding in bronchial explants. Tissue explants were treated with A) Oseltamivir (100 nM) or B) TVB024 (5 μM) for 2 h prior to infection with influenza virus, then incubated for a total of 48 h. Cells dispersed from the tissues were analysed by flow cytometry for epithelial influenza infection and culture media assessed for viral protein immunoreactivity as previously described. NI: not infected, INF: infected. Data shown are mean +/− S.E.M of 5 experiments. *=p<0.05
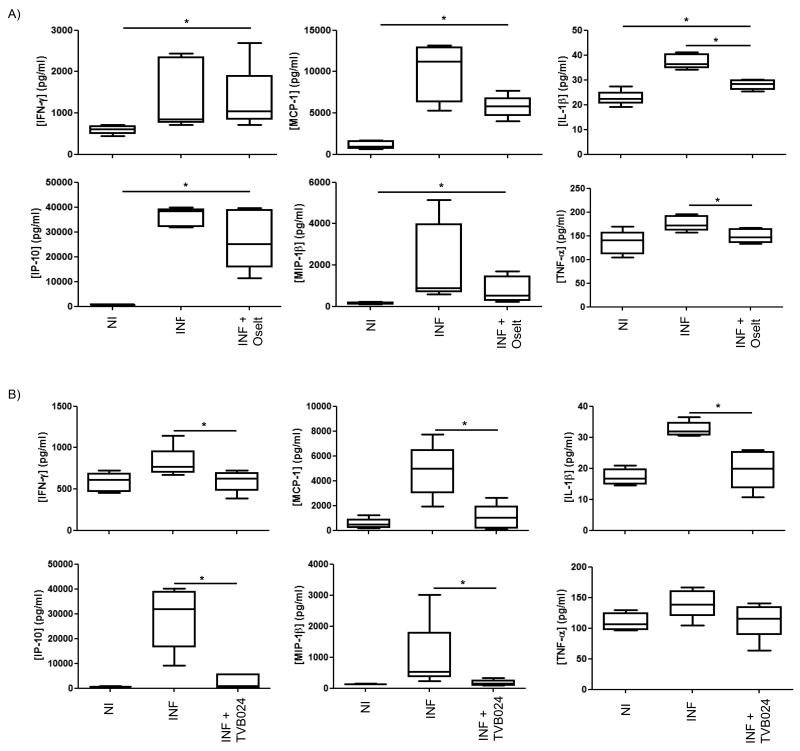
In order to assess how infection-induced inflammatory cytokines and chemokines were affected by these anti-viral compounds, mediator release 48 h following infection and anti-viral compound treatment of bronchial explants was measured. Despite reducing both viral infection and release, Oseltamivir had no effect on the secretion of these mediators with the exception of IL-1β and TNF–α which were reduced by approximately 50% (Figure 6A), and only TNF-α was reduced to its baseline level. In comparison, TVB024 treatment (Figure 6B) significantly reduced five of the mediators, all to baseline levels (IFN-γ, IP-10, IL-1β, MCP-1 and MIP-1β). However, this compound had no significant effect on TNF-α. Neither Oseltamivir nor TVB024 significantly reduced infection-induced IL-6 production.
Figure 6.
Effect of vATPase and neuraminidase inhibition on cytokine/chemokine release. Tissue conditioned media were analysed by multiplexed ELISA to quantify cytokine/chemokine release following prophylactic treatment with (A) neuraminidase inhibitor oseltamivir and (B) vATPase inhibitor TVB024. Treatment controls (Flu) were prophylactically treated with 0.1% (v/v) DMSO carrier prior to infection. Drug treated cells were prophylactically treated for 2 h with either 100nM Oseltamivir carboxylate (Flu + Oselt) or 5 μM TVB024 (Flu + TVB024). Box and whisker plots indicate medians and interquartiles +/− range, n=5. NI: not infected, INF: infected. *=p<0.05
Discussion
Novel anti-viral therapeutics, especially those targeted against host proteins identified by new methods such as siRNA screens, need validation, ideally in a host model. Based on the current study, we propose that bronchial explants offers the opportunity to test new anti-viral compounds safely, compare their efficacy to existing drugs and provide meaningful information on tissue inflammatory responses. To date, epithelial cultures have been used for initial pre-clinical evidence of efficacy; however, although easily infected with lower doses than needed for tissue explants, PBEC cultures lack the immune cells and structural complexity of intact tissues, resulting in limited inflammatory responses, as shown in this study. Tissue explants have the advantage of a complex architecture without the complicating factors of newly recruited cells or significant adaptive immune components, allowing characterisation of primary infection within a complex, yet controlled, system.
Influenza infection of porcine and human lung explants has been demonstrated previously [29, 30], but has relied on immunohistochemical detection of viral nucleoprotein, which poses significant challenges for full quantification. This problem is addressed easily with flow cytometry as applied in our model, enabling robust identification of infection patterns which can be confirmed by immunohistochemistry. A key element of any model is to determine how closely it reflects in vivo processes. We found that influenza virus principally infects epithelial cells in bronchial tissue in line with patterns of infection seen previously in vivo [31].
Influenza-induced inflammation is a significant contributor to morbidity. Studies by others have shown elevation of limited number of inflammatory mediators in blood during influenza infection, including IL-1, IL-6 and IL-8 [32]. In the current study, we demonstrate infection-associated up-regulation of seven chemokines/cytokines in bronchial tissues. Using explant infections, a more complete array of mediators can be observed than in single cell cultures, possibly because of the presence of multiple cell types, including macrophages which are a known source of many of the mediators detected [33].
Previous publications have identified vATPase as a key host gene involved in influenza A infection [8-11] but its role in lung tissue has not been studied to date. To our knowledge, inhibitors of vATPase have not been tested in vivo in humans as anti-viral drugs, despite being proposed as a potential target [34]. This may be because current vATPase inhibitors, such as clarithromycin and bafilomycin, despite proven in vitro anti-influenza effects [35, 36], are relatively expensive and have unfavourable toxicity profiles. Thus, our study provides the first conclusive evidence that vATPase inhibition may be an effective way of inhibiting lung infection with influenza virus and the associated inflammatory responses.
Our study suggests that inhibiting host genes involved early in the viral life cycle considerably reduces viral-induced cytokine release, often to background levels. We demonstrated that TVB024 was an effective vATPase inhibitor at concentrations which were not cytotoxic for the duration of the treatment. The concentration range of efficacy of TVB024 was similar to that observed with bafilomycin and concanamycin A [37] but with the added assurance of using a fully synthetic molecule. TVB024 reduced virus release from infected explants more effectively than Oseltamivir. Oseltamivir has been extensively studied and shown to be clinically beneficial if taken within 2 days of exposure [38, 39]; however, recent meta-analyses have cast its clinical efficacy into doubt. Nevertheless, this study contributes to the understanding of the in vivo mechanisms of Oseltamivir. In addition, the study sheds light on the effects of vATPase inhibition. Following viral uncoating within the endosome of the cell and subsequent initiation of replication, virus is detected by pattern recognition receptors such as NOD- and Toll-like receptors. This results in activation of anti-viral cascades which in turn activate pro-inflammatory cytokine and chemokine release.
The relative efficacies of the two anti-viral compounds observed in reducing inflammatory mediator production probably reflect the known mechanisms of action of oseltamivir and vATPase inhibitors. Neuraminidase inhibition reduces viral particle release but does not affect intracellular viral replication, implying that initial infection rates should not be affected. Thus, neuraminidase inhibition should, theoretically, not have any beneficial effect on pro-inflammatory mediators. This was, indeed, observed in our study, where Oseltamivir was generally poor at inhibiting infection-induced cytokine release. In contrast, treatment with TVB024 completely inhibited viral shedding and infection-induced cytokine release. This effect would also be observed with other inhibitors targeting viral replication rather than release, irrespective of whether they target viral or host infection mechanisms. However, as previously found with Amantidine (a virally-expressed M2 channel inhibitor), viral evasion of this mechanism has been observed, resulting in widespread global resistance [5,6]. Indeed, the influenza strain used in our study is resistant to Amantidine, preventing its use as an early stage inhibitor control.
Although vATPase inhibition is a more effective anti-influenza agent in human tissues than neuraminidases, in terms of reducing viral replication and its associated inflammation, the physiological effect of inhibition of such a ubiquitously expressed protein remains to be determined. Lung explants can effectively model the direct effects of therapeutic agents on target tissues. However, further toxicological investigations would be needed to examine the systemic effects of such inhibitors in vivo. The proof of efficacy in lung tissues should, however, be the primary initial determinant of anti-influenza therapeutic investigations prior to in vivo toxicology which is expensive and generally assessed by administering the drug systemically. Localised lung delivery mechanisms could overcome potential systemic toxicity problems which might be overlooked using standard models.
In addition to acting as a bridge between basic research and high throughput screening, on the one hand, and clinical trials with candidate drugs, on the other, our explant model allows safe investigation of infection mechanisms using more hazardous viruses and testing in samples from individuals with pre-existing chronic lung diseases. The donors of bronchial biopsies in this study were healthy, non-atopic non-smokers under 35 years of age, similar to the volunteer profile in clinical trials, but sampling of older patients selected by disease (e.g. asthma or chronic obstructive pulmonary disease, where morbidity and mortality is greatest) is routinely conducted for research purposes, so it would be easy to extend our study to such at-risk patient populations.
We propose that the lung infection model developed for this proof of concept study complements the use of animal models in the discovery of anti-influenza drugs as it overcomes some of the differences in host-defence mechanisms between humans and other animal species and thereby increases confidence in the efficacy of novel compounds being tested.
In summary, we have provided proof of concept for the use of our ex vivo lung tissue infection model as a clinically relevant, fully human, testing platform for antiviral therapies and have demonstrated the benefits of inhibiting viral replication over viral release in limiting the impact of influenza infections.
Supplementary Material
Acknowledgements
The authors would like to thank the staff of the NIHR Southampton Respiratory Biomedical Research Unit and the NIHR Wellcome Trust Clinical Research Facility, both units of the Southampton Centre for Biomedical Research, for their invaluable assistance. We thank Dr T. Millar for the kind donation of HUVEC cells, R Jewell and C McGuire for assistance with flow cytometry and Dr Borislav Dimitrov for statistical advice. We would also like to thank the nursing and support staff in the cardiothoracic surgical unit and the pathology departments at Southampton University Hospital Foundation trust.
Funding
This study was funded by a collaboration between the University of Southampton and 3V Biosciences. 3V biosciences assisted in the design of the work and the collection, analysis and interpretation of data. The manuscript was conceived and written by the University of Southampton co-authors and was approved by co-authors from 3V Biosciences. Dr T. Hinks was supported by Wellcome Trust Grant no. 088365/z/09/z
References
- 1.Cox NJ, Subbarao K. Global epidemiology of influenza: Past and present. Annu Rev Med. 2000;51:407–21. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson KG, Nguyen-Van-Tam JS, Ahmed AH, et al. Randomised placebo-controlled crossover trial on effect of inactivated influenza vaccine on pulmonary function in asthma. Lancet. 1998;351:326–31. doi: 10.1016/S0140-6736(97)07468-0. [DOI] [PubMed] [Google Scholar]
- 3.Use of influenza A (H1N1) 2009 monovalent vaccine: Recommendations of the advisory committee on immunization practices, 2009. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 58:1–8. [PubMed] [Google Scholar]
- 4.Wesseling G. Occasional review: Influenza in copd: Pathogenesis, prevention, and treatment. Int J Chron Obstruct Pulmon Dis. 2007;2:5–10. doi: 10.2147/copd.2007.2.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark NM, Lynch JP. Influenza: Epidemiology, clinical features, therapy, and prevention. Semin Respir Crit Care Med. 2011;32:373–92. doi: 10.1055/s-0031-1283278. [DOI] [PubMed] [Google Scholar]
- 6.Pizzorno A, Abed Y, Boivin G. Influenza drug resistance. Semin Respir Crit Care Med. 2011;32:409–22. doi: 10.1055/s-0031-1283281. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RJ, Cooper KL, Tappenden P, et al. Oseltamivir, zanamivir and amantadine in the prevention of influenza: A systematic review. J Infect. 2011;62:14–25. doi: 10.1016/j.jinf.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Brass AL, Huang IC, Benita Y, et al. The ifitm proteins mediate cellular resistance to influenza a h1n1 virus, west nile virus, and dengue virus. Cell. 2009;139:1243–54. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao L, Sakurai A, Watanabe T, et al. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–93. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlas A, Machuy N, Shin Y, et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463:818–22. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 11.Konig R, Stertz S, Zhou Y, Inoue A, et al. Human host factors required for influenza virus replication. Nature. 2010;463:813–17. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapira SD, Gat-Viks I, Shum BO, et al. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell. 2009;139:1255–67. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui B, Bamba D, Weng K, et al. The use of random homozygous gene perturbation to identify novel host-oriented targets for influenza. Virology. 2009;387:473–81. doi: 10.1016/j.virol.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T, Watanabe S, Kawaoka Y. Cellular networks involved in the influenza virus life cycle. Cell Host Microbe. 2010;7:427–39. doi: 10.1016/j.chom.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnard DL. Animal models for the study of influenza pathogenesis and therapy. Antiviral Res. 2009;82:A110–22. doi: 10.1016/j.antiviral.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sladkova T, Kostolansky F. The role of cytokines in the immune response to influenza A infection. Acta Virol. 2006;50(3):151–62. [PubMed] [Google Scholar]
- 17.Djukanovic R, Wilson JW, Lai CK, et al. The safety aspects of fiberoptic bronchoscopy, bronchoalveolar lavage, and endobronchial biopsy in asthma. Amer Rev Respir Dis. 1991;143:772–77. doi: 10.1164/ajrccm/143.4_Pt_1.772. [DOI] [PubMed] [Google Scholar]
- 18.Blume C, Swindle EJ, Dennison P, Jayasekara NP, Dudley S, Monk P, Behrendt H, Schmidt-Weber CB, Holgate ST, Howarth PH, Traidl-Hoffmann C, Davies DE. Barrier responses of human bronchial epithelial cells to grass pollen exposure. Eur. Resp. J. 2013;42(1):87–97. doi: 10.1183/09031936.00075612. [DOI] [PubMed] [Google Scholar]
- 19.Vijayanand P, Durkin K, Hartmann G, et al. Chemokine receptor 4 plays a key role in t cell recruitment into the airways of asthmatic patients. J Immunol. 2010;184:4568–74. doi: 10.4049/jimmunol.0901342. [DOI] [PubMed] [Google Scholar]
- 20.Richter A, Puddicombe S, Lordan JL, et al. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am. J. Resp. Cell Mol. Biol. 2001;25(3):385–91. doi: 10.1165/ajrcmb.25.3.4437. [DOI] [PubMed] [Google Scholar]
- 21.Huang ZH, Bates EJ, Ferrante JV, et al. Inhibition of stimulus-induced endothelial cell intercellular adhesion molecule-1, E-selectin, and vascular cellular adhesion molecule-1 expression by arachidonic acid and its hydroxy and hydroperoxy derivatives. Circ. Res. 1997;80(2):149–58. doi: 10.1161/01.res.80.2.149. [DOI] [PubMed] [Google Scholar]
- 22.Lordan JL, Bucchieri F, Richter A, et al. Cooperative effects of Th2 cytokines and allergen on normal and asthmatic bronchial epithelial cells. J. Immunol. 2002;169(1):407–14. doi: 10.4049/jimmunol.169.1.407. [DOI] [PubMed] [Google Scholar]
- 23.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotechnic & histochemistry : official publication of the Biological Stain Commission. 1993;68(5):271–280. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- 24.Puddicombe SM, et al. Increased expression of p21(waf) cyclin-dependent kinase inhibitor in asthmatic bronchial epithelium. Am. J. Respir. Cell. Mol. Biol. 2003;28(1):61–68. doi: 10.1165/rcmb.4715. [DOI] [PubMed] [Google Scholar]
- 25.Hidi R, Riches V, Al-Ali M, et al. Role of b7-CD28/CTLA-4 costimulation and nf-kappa b in allergen-induced t cell chemotaxis by il-16 and rantes. J Immunol. 2000;164:412–18. doi: 10.4049/jimmunol.164.1.412. [DOI] [PubMed] [Google Scholar]
- 26.Dent G, Hosking LA, Lordan JL, et al. Differential roles of il-16 and cd28/b7 costimulation in the generation of t-lymphocyte chemotactic activity in the bronchial mucosa of mild and moderate asthmatic individuals. J Allergy Clin Immunol. 2002;110:906–14. doi: 10.1067/mai.2002.130049. [DOI] [PubMed] [Google Scholar]
- 27.Vijayanand P, Seumois G, Pickard C, et al. Invariant natural killer t cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007;356:1410–22. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 28.Huss M, Wieczorek H. Inhibitors of v-ATPases: Old and new players. J Exp Biol. 2009;212:341–46. doi: 10.1242/jeb.024067. [DOI] [PubMed] [Google Scholar]
- 29.Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J. 2010;7:38. doi: 10.1186/1743-422X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W, Booth JL, Duggan ES, et al. Innate immune response toH3N2 and H1N1 influenza virus infection in a human lung organ culture model. Virology. 2010;396:178–88. doi: 10.1016/j.virol.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng ZM, Chertow DS, Ambroggio X, et al. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci USA. 2011;108:16416–21. doi: 10.1073/pnas.1111179108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ison MG, de Jong MD, Gilligan KJ, et al. End points for testing influenza antiviral treatments for patients at high risk of severe and life-threatening disease. J Inf Dis. 2010;201:1654–62. doi: 10.1086/652498. [DOI] [PubMed] [Google Scholar]
- 33.Deng R, Lu M, Korteweg C, et al. Distinctly different expression of cytokines and chemokines in the lungs of twoH5N1 avian influenza patients. J Pathol. 2008;216:328–36. doi: 10.1002/path.2417. [DOI] [PubMed] [Google Scholar]
- 34.Mehle A, Doudna JA. A host of factors regulating influenza virus replication. Viruses. 2010;2:566–73. doi: 10.3390/v2020566. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaya M, Shinya K, Hatachi Y, et al. Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells. J Pharmacol Exp Ther. 2010;333:81–90. doi: 10.1124/jpet.109.162149. [DOI] [PubMed] [Google Scholar]
- 36.Ochiai H, Sakai S, Hirabayashi T, et al. Inhibitory effect of bafilomycin a1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza a and b viruses in mdck cells. Antiviral Res. 1995;27:425–30. doi: 10.1016/0166-3542(95)00040-s. [DOI] [PubMed] [Google Scholar]
- 37.Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of v-ATPases and p-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Falagas ME, Vouloumanou EK, Baskouta E, et al. Treatment options for 2009 H1N1 influenza: Evaluation of the published evidence. Int J Antimicrob Agents. 2009;35:421–30. doi: 10.1016/j.ijantimicag.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: A randomized controlled trial. US oral neuraminidase study group. JAMA. 2000;283:1016–24. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.