Abstract
Childhood trauma confers higher risk of adulthood physical and mental illness, however the biological mechanism mediating this association remains largely unknown. Recent research has suggested dysregulation of the immune system as a possible biological mediator. The present paper conducted a meta-analysis in order to establish whether early life adversity contributes to potentially pathogenic pro-inflammatory phenotypes in adult individuals. A systematic search of Pubmed, PsycINFO, EMBASE, Scopus and Medline identified 25 articles for the meta-analysis, including 18 studies encompassing a sample of 16,870 individuals for C-reactive protein (CRP), 15 studies including 3,751 individuals for interleukin-6 (IL-6), and 10 studies including 881 individuals for tumour necrosis factor-α (TNF-α). Random-effects meta-analysis showed that individuals exposed to childhood trauma had significantly elevated baseline peripheral levels of CRP (Fisher’s z = 0.10, 95% confidence interval [CI] = 0.05 – 0.14), IL-6 (z = 0.08, 95% CI = 0.03 - 0.14) and TNF-α (z = 0.23, 95% CI = 0.14 – 0.32). Subgroup analyses for specific types of trauma (sexual, physical or emotional abuse) revealed that these impact differentially the single inflammatory markers. Moreover, meta-regression revealed greater effect sizes in clinical samples for the association between childhood trauma and CRP but not for IL-6 or TNF-α. Age, BMI and gender had no moderating effects. The analysis demonstrates that childhood trauma contributes to a pro-inflammatory state in adulthood, with specific inflammatory profiles depending on the specific type of trauma.
Keywords: childhood trauma, inflammation, cytokine, mental illness, psychiatric disorders
1 Introduction
A large body of studies has so far supported the notion that childhood traumatic experiences, including physical, sexual and emotional abuse, neglect, and separation from caregivers, significantly increase the risk of developing mental and physical illnesses later on in life 1,2, but the biological mechanisms mediating this association remain unclear. More specifically, childhood trauma has been suggested to increase vulnerability to several psychiatric disorders, including depression 3, anxiety 4, psychosis 5 and post-traumatic stress disorder (PTSD) 4, as well as several chronic physical health problems, including rheumatoid arthritis, cardiovascular disease, lung disease, metabolic syndrome and cancer6,2,7. Furthermore, childhood trauma is associated with more unfavourable psychiatric outcomes, such as more recurrent and treatment-resistant depressive disorder 8 and greater risk of suicidal behaviours 9. Studies in the recent decade have implicated the innate immune system in the relationship between childhood trauma and adulthood disease.
The primary purpose of the innate immune system is to provide an initial line of defence against pathogens as well as to contribute to the adaptive induction of sickness-behaviour, a constellation of behavioural changes that facilitate recovery from infection while affecting mood and cognitive function. In humans, these symptoms are exemplified by those experienced by individuals who take pro-inflammatory cytokines, such as interferon-α, for medical indications, and include depression, anxiety, lethargy, fatigue, fragmented sleep, decreased appetite, psychomotor retardation, and cognitive impairment 10,11. Interestingly, elevated levels of inflammatory markers have been increasingly reported in psychiatric disorders 10 as well as in individuals with a history of childhood trauma (see below). Of note, inflammatory signalling pathways are also known to impact on a network of biological systems extensively implicated in depression, including neuroendocrine, monoaminergic, oxidative, nitrosative, and neurotrophic pathways 12,10. In particular, the hypothalamic-pituitary-adrenal (HPA) is frequently dysregulated in physical and mental illnesses, and its altered function has been involved in the development of specific behavioural phenotypes associated with depression, such as early awakening and changes in weight and appetite13. The HPA axis is also a powerful modulator of inflammatory activity and is in turn modulated by inflammatory processes 14-16, as well as being highly responsive to environmental adversities both in childhood and in adulthood 13. Taken together, these lines of evidence point to the activation of the immune system as one of the biological mechanisms underlying the pathogenesis of mental illness, especially in the context of early life stress.
Several previous studies have reported an association between childhood trauma and increased levels of pro-inflammatory markers, most notably of the acute phase protein C-reactive protein (CRP), and of the cytokines interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α)17. However, in light of several non-significant findings as well as a significant amount of heterogeneity in methods, such as in the definition and assessment of childhood trauma, in the sample compositions and in the statistical approaches 17, a meta-analysis of the subject is warranted. Furthermore, whether any immune abnormalities are specific to one or more types of childhood trauma remains unclear.
The present meta-analysis aims to test whether childhood trauma is consistently associated with dysregulation of the inflammatory system in adulthood, thus increasing the vulnerability to health problems in adulthood. Moreover, we assess potential moderating factors in the association between childhood trauma and adulthood inflammation, including compositions of the samples, and types of childhood trauma. We focus on studies examining peripheral levels of three key inflammatory markers, CRP, IL-6 and TNF-α, as these are the ones which have received most attention within the childhood trauma literature 17 and therefore there is a sufficient number of studies to conduct a meta-analysis; these are also the inflammatory markers most frequently examined in psychiatric research 10.
2 Methods
2.1 Search strategy and selection
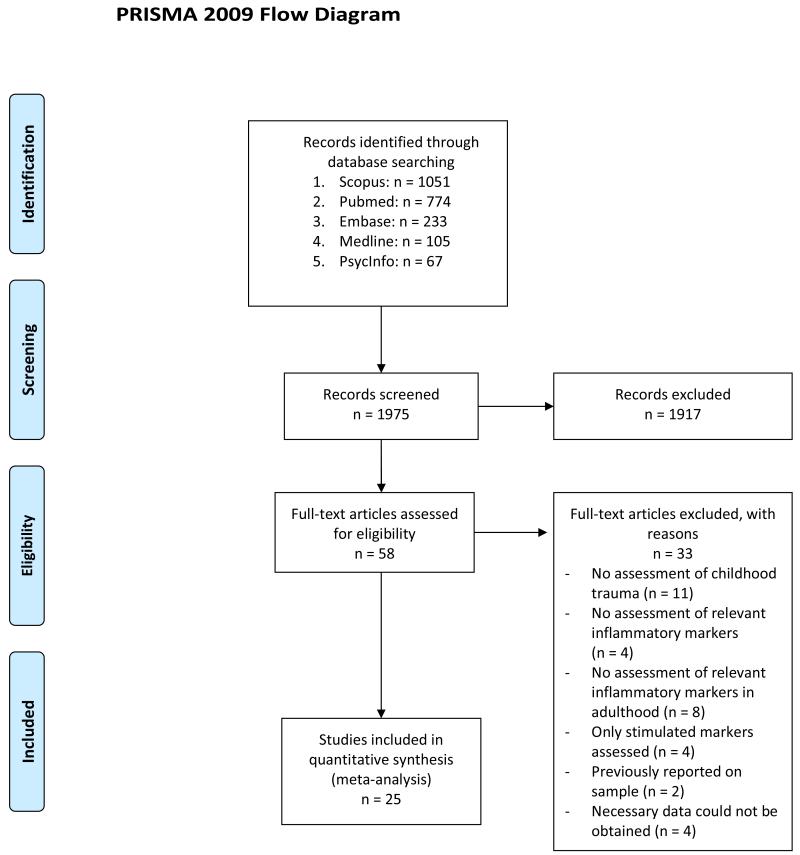
A systematic review of the literature was performed using Pubmed, PsycINFO, EMBASE, Scopus and Medline for the subject headings “Childhood Maltreatment”, “Childhood Trauma”, “Childhood Adversity”, “Early Life Stress”, “Child Abuse” and “Child Neglect” cross-referenced separately with the terms “C-reactive Protein”, “CRP”, “Tumor Necrosis Factor”, “TNF-α”, “Cytokine”, “Interleukin”, “IL-6”, “Inflammatory” and “Inflammation”. The literature review was initially performed between January 1st and March 31st 2014, and updated on February 15th, 2015. Articles were limited to research in human participants, published in English language. The initial search produced 1051 results on Scopus, 774 on Pubmed, 67 on PsycInfo, 233 on Embase and 105 on Medline. Articles were included if they provided original data about the association of any trauma experienced before age 18 (not including adverse socioeconomic status in childhood) with CRP, IL-6 and/or TNF-α levels in adulthood (individuals aged 18 or older). Titles and abstracts were scrutinised for appropriateness to the present objective. Sixty appropriate articles were identified for full-text analysis, of which 25 met criteria for inclusion in the present meta-analysis18-42, including 18 for CRP, 15 for IL-6 and 10 for TNF-α (see Table 1), encompassing a sample of 16870 participants for CRP, 3751 for IL-6 and 881 for TNF-α (see Figure 1).
Table 1.
Systematic overview of articles included in the meta-analysis
| Name | Sample Size* | Inflammatory Markers | Population | Mean Age | Female Proportion | Design | Trauma Measure | Assay |
|---|---|---|---|---|---|---|---|---|
| Archer et al., 2012 | 46 | CRP, IL-6, TNF-a |
Clinical – Cancer patients | 65.64 | 46.2% | Retrospective | CTQ | Multi-spot protocol |
| Bertone-Johnson et al., 2012 | 702 | CRP, IL-6 |
General population | 42.67 | 100.0% | Retrospective | ns | Immunonephelometry |
| ELISA | ||||||||
| Carpenter et al., 2010 | 69 | IL-6 | General population | 26.80 | 60.9% | Retrospective | CTQ | ELISA |
| Carpenter et al., 2012 | 92 | CRP | General population | 30.50 | 51.1% | Retrospective | CTQ | Immunonephelometry |
| Carroll et al., 2013 | 765 | CRP, IL-6 |
General population | 40.00 | 57.3% | Retrospective | RFQ | Immunonephelometry |
| ELISA | ||||||||
| Danese et al., 2009 | 633 | CRP | General population | 32.00 | 48.0% | Prospective | ns | Immunotubodimetry |
| Dennison et al., 2012 | 80 | IL-6, TNF-a |
Clinical – FEP patients vs HC |
37.27 | 53.8% | Retrospective | CTQ | ELISA |
| Di Nicola et al., 2012 | 48 | IL-6, TNF-a |
Clinical - FEP patients vs HC |
27.35 | 35.4% | Retrospective | CECA | ELISA |
| Frodl et al., 2012 | 83 | CRP, IL-6 |
Clinical - MDD patients vs HC |
39.12 | 59.0% | Retrospective | CTQ | ELISA |
| Gouin et al., 2012 | 130 | CRP, IL-6, TNF-a |
General population | 65.13 | 82.3% | Retrospective | CTQ | High Sensitivity Immunoassay |
| Chemiluminescence | ||||||||
| Hartwell et al., 2013 | 38 | CRP, IL-6, TNF-a |
General population | 35.69 | 51.3% | Retrospective | ETI | Multiplex Bead Array |
| Hepgul et al., 2012 | 80 | CRP | Clinical - FEP patients vs HC |
26.69 | 34.4% | Retrospective | CECA | High Sensitivity Immunoassay |
| Kiecolt-Glaser et al., 2011 | 132 | IL-6, TNF-a |
General population | 69.69 | 72.0% | Retrospective | CTQ | High Sensitivity Immunoassay |
| Lacey et al., 2013 | 7462 | CRP | General population | 42.00 | 49.5% | Prospective | ns | Immunonephelometry |
| Lu et al., 2013 | 65 | IL-6, TNF-a |
Clinical - MDD patients vs HC |
29.32 | 55.4% | Retrospective | CTQ | Cytokine Antibody Array |
| Matthews et al., 2013 | 443 | CRP | General population | 45.70 | 100.0% | Retrospective | CTQ | Immunonephelometry |
| McDade et al., 2012 | 1622 | CRP | General population | 20.90 | NA | Prospective | ns | Immunoturbodimetry |
| Rooks et al., 2012 | 482 | CRP, IL-6 |
General population | 55.00 | 0.0% | Retrospective | ETI | Chemiluminscence |
| ELISA | ||||||||
| Runsten et al., 2013 | 116 | CRP | General population | 42.89 | 100.0% | Retrospective | RFQ | ELISA |
| Slopen et al., 2010 | 999 | CRP, IL-6 |
General population | 57.90 | 55.4% | Retrospective | ns | Immunonephelometry |
| ELISA | ||||||||
| Smith et al., 2011 | 177 | TNF-a | Clinical - PTSD patients vs HC |
NA | NA | Retrospective | CTQ | ELISA |
| Taylor et al., 2006 | 3248 | CRP | General population | 40.10 | 54.7% | Retrospective | RFQ | Immunonephelometry |
| Tietjen et al., 2012 | 141 | CRP, IL-6, TNF-a |
Clinical - Migraneurs vs HC | 36.98 | 100.0% | Retrospective | ACEQ | Immunonephelometry |
| Bead-based sandwich immunoassay | ||||||||
| Witek-Janusek et al., 2013 | 40 | IL-6 | Clinical – Breast cancer patients | 55.60 | 100.0% | Retrospective | CTQ | ELISA |
| Zeugmann et al., 2013 | 23 | CRP, TNF-a |
Clinical - MDD patients | 47.80 | 68.0% | Retrospective | CTQ | ELISA |
Abbreviations: FEP – First Episode Psychosis; PTSD – Post-Traumatic Stress Disorder; MDD – Major Depressive Disorder; HC – Healthy Controls; NA – Not Available; CTQ – Childhood Trauma Questionnaire; ACEQ – Adverse Childhood Experiences Questionnaire; RFQ – Risky Families Questionnaire; ETI – Early Trauma Inventory; CECA – Childhood Experiences of Care and Abuse; ns – non-standardised; ELISA – Enzyme-Linked ImmunoSorbent Assay.
Sample sizes may vary depending on individual inflammatory markers.
Figure 1.
PRISMA diagram of the literature search.
2.2 Data extraction and statistical analysis
Two authors (DB & RA) independently extracted data from eligible studies, and inconsistencies were resolved through discussion and consultation with other authors of the paper until consensus was reached. All studies were scored on the Selection Bias subscale of the Quality Assessment Tool 43; modified versions of this scale have previously been used in similar research 44. When data were not available, authors were contacted. All effect sizes were converted to Fisher’s z before being entered into the analysis, to reflect the continuous nature of both levels of inflammatory markers as well as potential degrees of severity of childhood traumatic experiences, whilst decreasing the risk of bias associated with Pearson’s r. As papers often normalise distribution of skewed biomarker data by utilising log-transformation, any raw data was transformed to logarithmic equivalents as described by Higgins, White & Anzures-Cabrera 45.
Since we hypothesise that the true effect sizes would differ depending on sample/exposure variations acting as moderating variables, random-effects models were chosen for the meta-analyses of main effects as well as meta-regressions and subgroup analyses. Samples were characterised for meta-regressions by the proportion of clinical participants (or those assessed for meeting criteria for clinical disorder), grouped into “any clinical disorders” (including physical and mental illnesses), “any psychiatric disorders” and “depressive disorder”. Mean age, body mass index (BMI), and proportion of female participants were recorded for meta-regression. Statistical procedures were carried out using Stata 46, using the metan package for meta-analyses, the metareg package for meta-regressions and the metafunnel and metabias packages for assessment of publication bias. P-values below 0.05 were accepted as being statistically significant, and values below 0.1 were reported as trends.
3 Results
3.1 Main association of childhood trauma with inflammatory markers
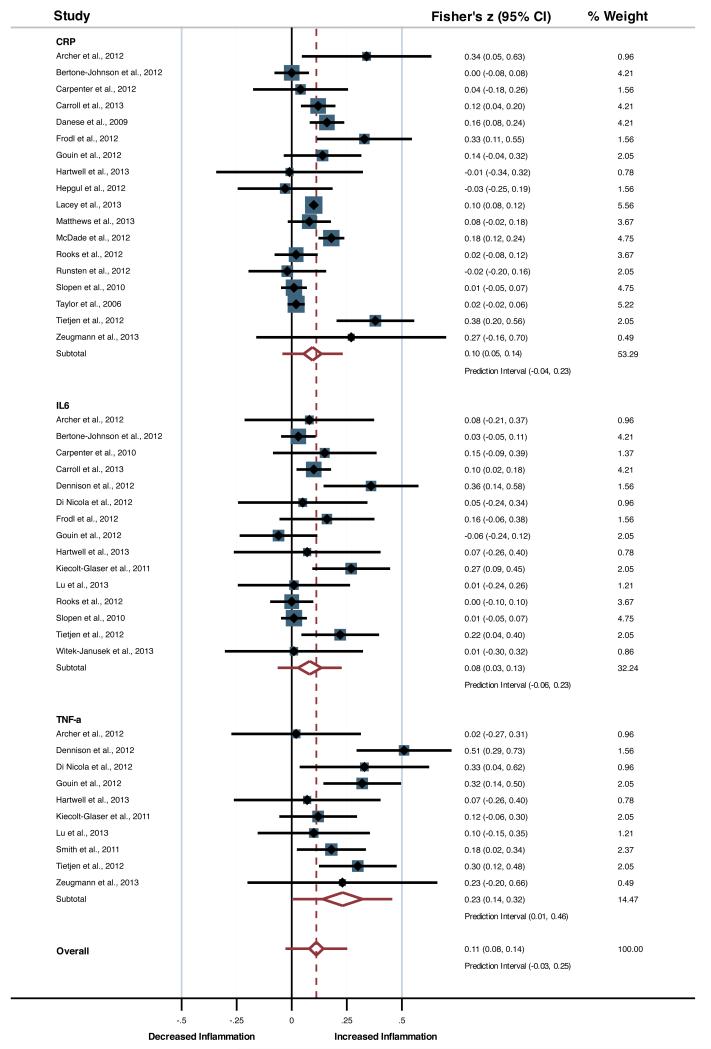
Meta-analysis for the main effects showed consistently elevated levels of inflammation associated with childhood trauma, with small effect sizes, for CRP (Fisher’s z = 0.10, df = 17, p < 0.001, 95%-CI = 0.05 - 0.14, Prediction Interval (PI) = −0.04 - 0.23), IL-6 (z = 0.08, df = 14, p = 0.003, 95%-CI = 0.03 - 0.14, PI = −0.06 - 0.23) and TNF-α (z = 0.23, df = 9, p < 0.001, 95%-CI = 0.14 - 0.32, PI = 0.01 - 0.46; see Figure 2). When grouped into one inflammatory factor, effect sizes were small yet significant (z = 0.11, df = 42, p < 0.001, 95%-CI = 0.08 - 0.14, PI = −.03 - 0.25). High heterogeneity was observed for CRP (p < 0.001, I2 = 72.7%, τ2 = 0.004), while low to moderate heterogeneity scores were observed for IL-6 (p = 0.03, I2 = 44.5%, τ2 = 0.004) and TNF-α (p = 0.11, I2 = 36.8%, τ2 = 0.007), and moderate to high heterogeneity was observed for the overall inflammatory factor (p < 0.001, I2 = 65.6%, τ2 = 0.005).
Figure 2.
Forest plot presenting the main association of childhood trauma with inflammatory markers.
3.2 Type of Trauma
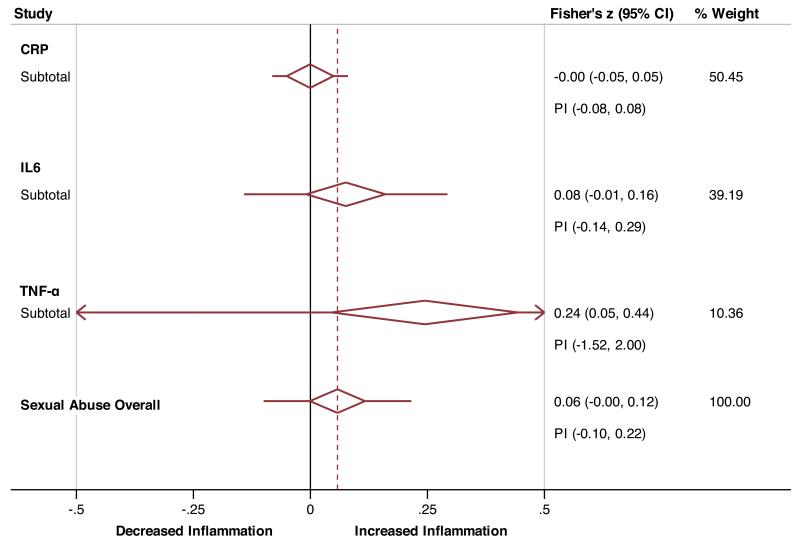
Sub-group analyses for childhood sexual abuse (Figure 3a) showed this trauma type to be significantly associated with a small effect size for TNF-α (z = 0.24, df = 2, p = 0.02, 95%-CI = 0.05 - 0.44) and a trend towards a small effect size for IL-6 (z = 0.08, df = 4, p = 0.08, 95%-CI = −0.01 - 0.16), whilst no such results were found for CRP (z = −0.001, df = 4, p = 0.98, 95%-CI = −0.05 - 0.05). Heterogeneity scores were not significant for estimates of TNF-α (p = 0.25, I2=28.5%, τ2 = 0.009), IL-6 (p = 0.21, I2 = 32.4%, τ2 = 0.003), or CRP (p = 0.62, I2 = 0.0%, τ2 = 0.0).
Figure 3.
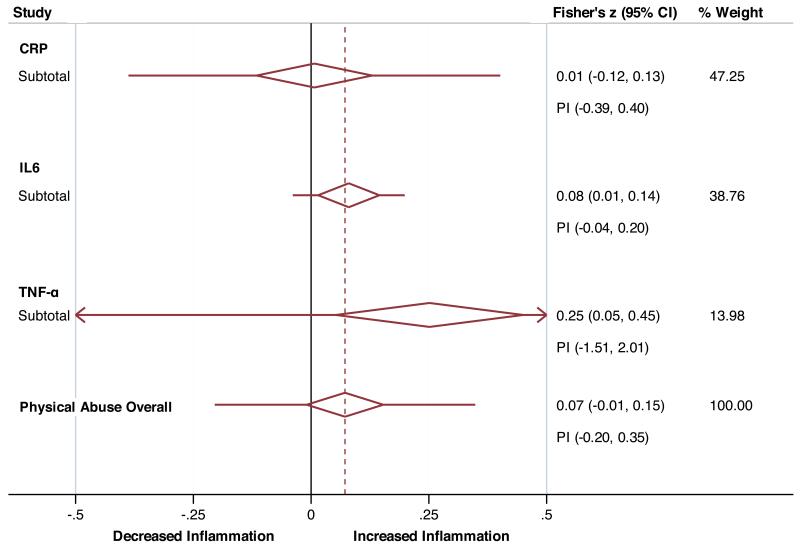
Collapsed forest plots presenting the association of sexual (panel A) and physical (panel B) abuse with inflammatory markers. PI – Prediction Interval.
Sub-group analyses for childhood physical abuse (Figure 3b) similarly showed significant associations with small effect sizes for TNF-α (z = 0.25, df = 2, p = 0.01, 95%-CI = 0.05 - 0.45), and IL-6 (z = 0.08, df = 5, p = 0.02, 95%-CI = 0.02 - 0.15), but not CRP (z = 0.007, df = 5, p = 0.91, 95%-CI = −0.12 - 0.13). Heterogeneity scores were again not significant for estimates of TNF-α (p = 0.25, I2 = 28.5%, τ2 = 0.009), IL-6 (p = 0.35, I2 = 9.8%, τ2 = 0.007), but significant for CRP (p < 0.001, I2 = 79.3%, τ2 = 0.016).
Since only a small number of studies investigated emotional abuse, meta-analysis for this trauma was only possible for CRP and IL-6, but not for TNF-α. Neither CRP (z = 0.03, df = 3, p = 0.31, 95%-CI = −0.03 - 0.10) nor IL-6 (z = −0.008, df = 2, p = 0.87, 95%-CI = −0.10 - 0.08) was significantly associated with childhood emotional abuse. Heterogeneity did not reach significance for CRP (p = 0.81, I2 = 0.0%, τ2 = 0.0) or IL-6 (p = 0.65, I2 = 0.0%, τ2 = 0.0).
Sub-group analyses for parental absence during childhood was only possible for CRP, revealing a significant association with a small effect size (z = 0.11, df = 2, p = 0.001, 95%-CI = 0.02 - 0.19) and significant heterogeneity (p = 0.006, I2 = 80.4%, τ2 = 0.004).
3.3 Moderating effects of sample populations
To investigate whether clinical samples accounted for heterogeneity in findings, meta-regressions were carried out for the proportion of participants with “any clinical disorders” (including physical and mental illnesses), “psychiatric disorders” and “depressive disorder”. Again, random-effects models were chosen as the most conservative model. Whilst meta-regressions showed that samples including any clinical disorders showed augmented trend-level effect sizes for CRP (t = 2.09, p = 0.054, I2res = 70.23%, τ2 = 0.005), explaining 9.81% of the between-sample variance, this was not significant for TNF-α (t = −0.07, p = 0.95, I2res = 43.8%, τ2 = 0.01) and IL-6 (t = 0.48, p = 0.64, I2res = 42.89%, τ2 = 0.006).
The proportion of patients with any psychiatric disorders did not moderate the associations between childhood trauma and CRP (t = 0.02, p = 0.98, I2res = 74.0%, τ2 = 0.007), IL-6 (t = 0.23, p = .82, I2res = 48.5%, τ2 = 0.006) or TNF-α (t = 0.54, p = 0.61, I2res = 42.7%, τ2 = 0.01). Similarly the proportion of depressed patients did not moderate the association between childhood trauma with and CRP (t = 1.07, p = 0.30, I2res = 73.5%, τ2 = 0.006), IL-6 (t = 0.44, p = 0.66, I2res = 47.6%, τ2 = 0.006) or TNF-α (t = 0.22, p = 0.83, I2res = 43.3%, τ2 = 0.01).
No significant results were found for moderating effects for age, BMI or gender, and there was no evidence of publication bias, although significant and trend-level moderating effects of selection bias were found for IL-6 and CRP, respectively. For additional information on sensitivity analyses please see the Supplementary Material.
4 Discussion
4.1 Main findings and proposed mechanism
The present meta-analysis finds a significant association between childhood trauma and the inflammatory markers, with effect sizes being greatest for TNF-α (z = 0.20, 95% CI = 0.10 – 0.29), followed by IL-6 (z = 0.09, 95% CI = 0.04 - 0.15) and then CRP (z = 0.08, 95% CI = 0.04 – 0.11). As such, this provides strong evidence that childhood traumatic events significantly impact on the inflammatory immune system, with trajectories reaching into adulthood, thus offering a potential molecular pathway by which early trauma confers vulnerability to developing psychiatric and physical disorders later in life.
The molecular mechanisms that account for these long-term changes in immune function need to be further explored. Putatively, changes in epigenetic regulation of gene expression may be responsible for this increased immune activation; this appears plausible in view of the considerable evidence that childhood trauma induces modifications of HPA- and neuroplasticity-related methylation patterns 47-49. In particular, early trauma leads to greater methylation of the glucocorticoid receptor (GR) and greater demethylation of FKBP5 48,50-52. The increased methylation of the GR correlates with reduced GR function as shown by impaired negative feedback of the HPA axis 52, while FKBP5 is a heat-shock protein that binds and thereby inhibits the cytosolic GR. As the GR itself is a crucial regulator of inflammatory activity, lower expression and function of the GR due to epigenetic suppression may allow for this exacerbated inflammatory activity. Notably, increased inflammation itself can then maintain and exacerbate the impaired GR function 14-16, thus leading to sustained GR resistance into adulthood. Notably, based on differences in effects sizes found in the present analysis, the association between childhood trauma and adulthood inflammation is stronger for inflammatory pathways related to TNF-α; and, indeed, the GR is crucial in regulating TNF-α signalling and TNF-induced cytokine production, as well as conveying protection against TNF-related tissue damage 53.
The present paper also found evidence that individual types of trauma exposure impact differentially on the inflammatory markers: most interestingly, physical and sexual abuse are associated with significant increased TNF-α and IL-6, but not CRP. Conversely, CRP seemed to be primarily related to parental absence during early development. Interestingly, rodent models have demonstrated that maternal separation is associated with elevated TNF-α levels in the periphery and cerebrospinal fluid 54 as well as in prefrontal and hippocampal brain regions 55. These results stress the need for assessing the potential different effects of each type of trauma in future research. Moreover, this finding raises the question as to why different types of childhood trauma are associated with different aspects of inflammatory dysregulation.
Whilst there is currently no clear answer to this question, several variables associated with individual trauma types, including chronicity and context of the stressor, age of exposure, duration of exposure or relationship to the perpetrator, may offer some insight. For example, it has been shown in adolescent women that episodic stress in the context of high chronic stress leads to reduced GR expression, while GR expression is increased in the absence of chronic stress 56. Moreover, there is evidence that different types of trauma impact differentially on mental health: specific subtypes of anxiety disorders appear to develop depending on whether one is exposed to physical or sexual childhood abuse 4, and childhood sexual abuse is particularly associated with the development of auditory verbal hallucinations in psychosis 5. The effect of early trauma on behaviour in adulthood may be further modulated by other developmental insults: Giovanoli and colleagues 57 showed that prenatal immune viral challenge in mice synergistically interacts with peripubertal stress exposure thus increasing the vulnerability to develop neuropathological behaviours later on in life. Interestingly, these mice show transient neuroimmunological changes, evident during adolescence but not in adulthood, suggesting the presence of sensitive periods of peripubertal brain maturation which might differentially influence behavioural outcomes. Further supporting the role of immune-related molecular pathways in the long-term consequences of early life trauma, previous preclinical studies have shown that early-life stress tends to have a programming effect on neuroimmune functions, leading to a pro-inflammatory state in adulthood, which in turn can trigger an exaggerated cytokine secretion and increase in microglia activity following an immune challenge. In particular, maternal deprivation early in life has been shown to enhance IL-1β responsiveness in adulthood, due to elevated IL-1 receptor levels at the post-synapse of adult hippocampal neurons 58. Moreover, prenatal stress in mice increases expression of IL-1β and TNF-α in the hippocampus during adulthood, and this pro-inflammatory state results in an enhanced activation of microglia and astrocytes in response to an immune challenge 59.
In the case of CRP, the impact of childhood trauma on adulthood inflammation may be characterised by synergistic effects with the presence of “state” (current) ill health, as the effect sizes were significantly greater in clinical samples, including patients with cancer. However, this effect was not observed when focussing exclusively on psychiatric disorders, suggesting that in these individuals the “trait” effects (the history of childhood trauma) may be the main driver of the immune activation. It should be noted that, although the increase in inflammatory activity is not comparable to that of acute systemic inflammatory disorders, it still has clinical relevance. We know that 95% of CRP values in healthy individuals range between 0.07 mg/l and 5.25 mg/l 60, which translates into a mean of 2.66 mg/l and a standard deviation of 4.18 mg/l; this is in line with epidemiological findings, showing mean CRP values in the general population ranging between 1.4mg/l and 2.9mg/l 61. Considering the standardised mean difference of 0.2 (equivalent to a Fisher’s z of 0.1), individuals with a history of childhood trauma would show an average CRP increase of 0.84 mg/l, or a mean CRP value of 3.5 mg/l, which is above the threshold of 3 mg/l acknowledged as risk factor for future heart attack, stroke, and development of diabetes 62. Thus, inflammatory activation as a consequence of childhood trauma is best conceptualized as a subtle effect that is likely to have a significant impact on physical and mental health.
4.2 Limitations
The present meta-analysis found significant yet small effect sizes in the context of particularly high levels of heterogeneity, which remained after subsequent meta-regressions and subgroup analyses. However, this may reflect the fact that the papers reviewed here show great variations in both theoretical and methodological approaches, and that there is considerable variation in the assessment of inflammatory markers (see Table 1). Such variations in measurement instruments may be associated with differences in sensitivity, which, especially in the context of the apparently small changes associated with childhood trauma, may create Type II errors. Sensitivity analysis looking at the different instruments to assess trauma show larger effect sizes for validated instruments when compared with non-standardised assessments (see supplementary material). Notably, sensitivity analysis also shows that prospective studies show greater effect sizes and lower heterogeneity than studies using retrospective trauma assessment (see supplementary material). In light of these various potential sources of heterogeneity the choice of random effects models, albeit more conservative, was indeed the most appropriate for the present purpose. Whilst the lack of evidence for publication bias suggests that the present findings are not an artefact of a distorted literature, the potential impact of selection biases cannot be ruled out given its effect in the results for IL-6 and CRP. Finally, not all the studies included in the meta-analysis explicitly reported acute infection as exclusion criteria for their analyses, and this could have partially affected the findings of those individual studies.
4.3 Implications
The present meta-analysis demonstrates a significant association between childhood trauma and increased immune activation in adulthood, and highlights possible differential effects of different types of trauma as well as heterogeneity in the findings. Future research should further investigate the molecular mechanisms behind this association, and particularly whether inflammatory and neuroendocrine changes actually occur in parallel in the same individuals, and how these changes are embedded from a molecular point of view. Contextualizing these data within a wider array of biological systems may be crucial in identifying why some individuals go on to develop physical or psychiatric disorders, whereas other remain resilient in the face of exposure to trauma.
These findings are also clinically relevant. Besides the potential impact of the increased inflammation on metabolic outcomes and physical illness, as discussed above, the assessment of inflammatory markers may also aid the development of prevention and treatment strategies. For example, a recent meta-analysis has demonstrated that elevations in CRP and IL-6 appear to precede the development of depressive disorders 63, and that patients with increased inflammation are less likely to respond to conventional antidepressants 64, and more likely to respond to adjunctive anti-inflammatory treatment 65. Thus, assessment of childhood trauma in conjunction with that of inflammatory markers may prove crucial in developing more effective prevention strategies and treatments, affecting long-term mental health outcomes.
Supplementary Material
Acknowledgements
This work was supported by the grant “Persistent Fatigue Induced by Interferon-alpha: A New Immunological Model for Chronic Fatigue Syndrome” from the Medical Research Council (UK) MR/J002739/1. Additional support has been offered by the National Institute for Health Research Mental Health Biomedical Research Centre in Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London Dr Artemis Koukounari of the KCL biostatistics department provided independent advice on statistical procedures.
Footnotes
Disclosure
Dr Mondelli and Professor Pariante have received research funding from Johnson & Johnson, a pharmaceutical company interested in the development of anti-inflammatory strategies for depression, but the research described in this paper is unrelated to this funding.
Reference List
- 1.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med. 2004;34:509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- 3.Sachs-Ericsson N, Kendall-Tackett K, Hernandez A. Childhood abuse, chronic pain, and depression in the National Comorbidity Survey. Child Abuse Negl. 2007;31:531–547. doi: 10.1016/j.chiabu.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Cougle JR, Timpano KR, Sachs-Ericsson N, Keough ME, Riccardi CJ. Examining the unique relationships between anxiety disorders and childhood physical and sexual abuse in the National Comorbidity Survey-Replication. Psychiatry Res. 2010;177:150–155. doi: 10.1016/j.psychres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective-and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/289645. doi:10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DW, Anda RF, Felitti VJ, Edwards VJ, Malarcher AM, Croft JB, et al. Adverse childhood experiences are associated with the risk of lung cancer: a prospective cohort study. BMC Public Health. 2010;10:20. doi: 10.1186/1471-2458-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 9.Bebbington PE, Cooper C, Minot S, Brugha TS, Jenkins R, Meltzer H, et al. Suicide attempts, gender, and sexual abuse: Data from the 2000 British psychiatric morbidity survey. Am J Psychiatry. 2009;166:1135–1140. doi: 10.1176/appi.ajp.2009.09030310. [DOI] [PubMed] [Google Scholar]
- 10.Baumeister D, Russell A, Pariante CM, Mondelli V. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social and lifestyle factors. Soc Psychiatry Psychiatr Epidemiol. 2014;49:841–9. doi: 10.1007/s00127-014-0887-z. [DOI] [PubMed] [Google Scholar]
- 11.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, et al. Interleukin-1β: A New Regulator of the Kynurenine Pathway Affecting Human Hippocampal Neurogenesis. Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumeister D, Lightman SL, Pariante CM. The Interface of Stress and the HPA Axis in Behavioural Phenotypes of Mental Illness. 2014. doi:10.1007/7854. [DOI] [PubMed] [Google Scholar]
- 14.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 2011;35:722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain. Behav. Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr Scand. 2014;129:180–92. doi: 10.1111/acps.12217. [DOI] [PubMed] [Google Scholar]
- 18.Archer JA, Hutchison IL, Dorudi S, Stansfeld SA, Korszun A. Interrelationship of depression, stress and inflammation in cancer patients: a preliminary study. J Affect Disord. 2012;143:39–46. doi: 10.1016/j.jad.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med. 2012;43:611–20. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter LL, Gawuga CE, Tyrka AR, Price LH. C-reactive protein, early life stress, and wellbeing in healthy adults. Acta Psychiatr Scand. 2012;126:402–10. doi: 10.1111/j.1600-0447.2012.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–23. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennison U, McKernan D, Cryan J, Dinan T. Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychol Med. 2012;42:1865–71. doi: 10.1017/S0033291712000074. [DOI] [PubMed] [Google Scholar]
- 23.Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, et al. Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun. 2013;31:90–5. doi: 10.1016/j.bbi.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frodl T, Carballedo A, Hughes MM, Saleh K, Fagan A, Skokauskas N, et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry. 2012;2:e88. doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med. 2012;44:287–92. doi: 10.1007/s12160-012-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartwell KJ, Moran-Santa Maria MM, Twal WO, Shaftman S, DeSantis SM, McRae-Clark AL, et al. Association of elevated cytokines with childhood adversity in a sample of healthy adults. J Psychiatr Res. 2013;47:604–10. doi: 10.1016/j.jpsychires.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hepgul N, Pariante CM, Dipasquale S, DiForti M, Taylor H, Marques TR, et al. Childhood maltreatment is associated with increased body mass index and increased C-reactive protein levels in first-episode psychosis patients. Psychol Med. 2012;42:1893–901. doi: 10.1017/S0033291711002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiecolt-Glaser JK, Gouin J-P, Weng N-P, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacey RE, Kumari M, McMunn A. Parental separation in childhood and adult inflammation: the importance of material and psychosocial pathways. Psychoneuroendocrinology. 2013;38:2476–84. doi: 10.1016/j.psyneuen.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, et al. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Compr Psychiatry. 2013;54:953–61. doi: 10.1016/j.comppsych.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Matthews KA, Chang Y-F, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav Immun. 2014;36:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C. Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun. 2013;31:23–30. doi: 10.1016/j.bbi.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Runsten S, Korkeila K, Koskenvuo M, Rautava P, Vainio O, Korkeila J. Can social support alleviate inflammation associated with childhood adversities? Nord J Psychiatry. 2014;68:137–44. doi: 10.3109/08039488.2013.786133. [DOI] [PubMed] [Google Scholar]
- 34.Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert M a, et al. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med. 2010;72:694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:700–8. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tietjen GE, Khubchandani J, Herial NA, Shah K. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache. 2012;52:920–9. doi: 10.1111/j.1526-4610.2012.02165.x. [DOI] [PubMed] [Google Scholar]
- 37.Witek Janusek L, Tell D, Albuquerque K, Mathews HL. Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain Behav Immun. 2013;30(Suppl):S149–62. doi: 10.1016/j.bbi.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeugmann S, Buehrsch N, Bajbouj M, Heuser I, Anghelescu I, Quante A. Childhood maltreatment and adult proinflammatory status in patients with major depression. Psychiatr Danub. 2013;25:227–35. [PubMed] [Google Scholar]
- 39.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse Childhood Experiences and Adult Risk Factors for Age-Related Disease. Arch Pediatr. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews K a, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci U S A. 2013;110:17149–53. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooks C, Veledar E, Goldberg J, Bremner JD, Vaccarino V. Early trauma and inflammation: role of familial factors in a study of twins. Psychosom Med. 2012;74:146–52. doi: 10.1097/PSY.0b013e318240a7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–24. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Wong WCW, Cheung CSK, Hart GJ. Development of a quality assessment tool for systematic reviews of observational studies (QATSO) of HIV prevalence in men having sex with men and associated risk behaviours. Emerg Themes Epidemiol. 2008;5:23. doi: 10.1186/1742-7622-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tursich M, Neufeld RWJ, Frewen PA, Harricharan S, Kibler JL, Rhind SG, et al. Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry. 2014;4:e413. doi: 10.1038/tp.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JPT, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–6092. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.StataCorp. Stata Statistical Software: Release 11. Econ. J. 2009;102 doi:10.2307/2234838. [Google Scholar]
- 47.Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Labonté B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. 2012;69:722–31. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perroud N, Paoloni-Giacobino A, Prada P, Olié E, Salzmann A, Nicastro R, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl. Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030148. doi:10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Bogaert T, De Bosscher K, Libert C. Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine Growth Factor Rev. 2010;21:275–286. doi: 10.1016/j.cytogfr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Réus GZ, dos Santos MAB, Abelaira HM, Ribeiro KF, Petronilho F, Vuolo F, et al. Imipramine reverses alterations in cytokines and BDNF levels induced by maternal deprivation in adult rats. Behav Brain Res. 2013;242:40–46. doi: 10.1016/j.bbr.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 55.Pinheiro RMC, de Lima MNM, Portal BCD, Busato SB, Falavigna L, Ferreira RDP, et al. Long-lasting recognition memory impairment and alterations in brain levels of cytokines and BDNF induced by maternal deprivation: effects of valproic acid and topiramate. J Neural Transm. 2014 doi: 10.1007/s00702-014-1303-2. doi:10.1007/s00702-014-1303-2. [DOI] [PubMed] [Google Scholar]
- 56.Marin TJ, Martin TM, Blackwell E, Stetler C, Miller GE. Differentiating the impact of episodic and chronic stressors on hypothalamic-pituitary-adrenocortical axis regulation in young women. Health Psychol. 2007;26:447–55. doi: 10.1037/0278-6133.26.4.447. [DOI] [PubMed] [Google Scholar]
- 57.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–9. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 58.Viviani B, Boraso M, Valero M, Gardoni F, Marco EM, Llorente R, et al. Early maternal deprivation immunologically primes hippocampal synapses by redistributing interleukin-1 receptor type I in a sex dependent manner. Brain Behav Immun. 2013;35:135–143. doi: 10.1016/j.bbi.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Diz-Chaves Y, Astiz M, Bellini MJ, Garcia-Segura LM. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav Immun. 2013;28:196–206. doi: 10.1016/j.bbi.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Riese H, Vrijkotte T, Meijer P, Kluft C, de Geus E. Diagnostic strategies for C-reactive protein. BMC Cardiovasc Disord. 2002;2:9. doi: 10.1186/1471-2261-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rutter MK, Sattar N, Tajar A, O’Neill TW, Lee DM, Bartfai G, et al. Epidemiological evidence against a role for C-reactive protein causing leptin resistance. Eur J Endocrinol. 2013;168:101–6. doi: 10.1530/EJE-12-0348. [DOI] [PubMed] [Google Scholar]
- 62.Ridker PM. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108:e81–5. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- 63.Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: A systematic review and meta-analysis. Biol. Psychiatry. 2013;73:406–413. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline “predictors” and longitudinal “targets”. Neuropsychopharmacology. 2013;38:377–85. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raison C, Rutherford R. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA …. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.