Abstract
Cancer stem cells can escape therapeutic kill by adopting a quiescent or dormant state. The reversibility of this condition provides the potential for later recurrence or relapse, potentially many years later. We describe the genomics of a rare case of childhood BCR-ABL1 positive, B cell precursor acute lymphoblastic leukaemia (ALL) that relapsed, with an AML immunophenotype, 22 years after the initial diagnosis, sustained remission and presumed cure. The primary and relapsed leukaemias shared the identical BCR-ABL1 fusion genomic sequence and two identical immunoglobulin gene rearrangements, indicating that the relapse was derivative of the founding clone. All other mutational changes (SNV, CNAs) were distinct in diagnostic or relapse samples. These data provide unambiguous evidence that leukaemia-propagating cells, most probably pre-leukaemic stem cells, can remain covert and silent but potentially reactivatable for more than two decades.
INTRODUCTION
Recurrence or relapse of cancer many years 1-3 or occasionally decades 4 after an initial diagnosis has been frequently recorded. These observations raise difficult issues related to presumptions of cure, risk assessment and monitoring of residual disease. A plausible mechanism for persistent, covert cancer cells during and after treatment is provided by the observation that some cancer stem cells can adapt a reversible quiescent or dormant state in which they are relatively resistant to radiation and chemotherapy 5-8. However, the assumption is usually made that late recurring cancer is a derivative of the original clone at diagnosis, evidence for which is very limited, with the exception of some acute leukaemias where physiological rearrangement of immunoglobulin genes (IGH/IGK) provide clone-specific markers 9-11.
MATERIALS AND METHODS
Targeted capture libraries, cloning and sequencing of gene fusions
A cell line, MR-87, was established from the original leukaemic cells of the 4 year old patient and showed the same immunophenotype and karyotype of the diagnostic leukaemic cells. These cells were also shown to express the p190 BCR-ABL1 protein 12. Illumina paired end libraries covering the entire genomic regions of the BCR, ABL1 and IKZF1 genes were prepared from MR87 cell line DNA (diagnosis) using the Agilent SureSelectXT 2 Custom (1kb-499kb) DNA bait library. The custom libraries were sequenced on a HiSeq2500 to a coverage depth of 99×. Casava software (v1.8, Illumina) was used to make base calls and demultiplex the sequencing data and the genomic fusion breakpoints of BCR-ABL1 and IKZF1 were roughly determined using Burrows-Wheeler Aligner (BWA) and Breakdancer software. The BCR-ABL1 breakpoint fusion was predicted based on the location of read pairs that mapped to the fusion partners and the average fragment size of the capture library (320bp). Using GRCh37.p13, the predicted breakpoint region in BCR was at chr22:23533568 – 23533950 (intron 1), and the breakpoint region in ABL1 was expected at chr9:133608500 – 133608811 (intron 1). A large deletion in IKZF1 (~50kb) was observed between regions chr7:50412887 - 50463541. PCR primers were then designed to span the putative breakpoints using Primer3 plus (www.primer3plus.com/). Primers used for cloning the BCR-ABL1 fusion were: 5′-GTCAAAGCATTTTCCCCTGC and 5′-TCTTGATACTGGGTTGGCTGC and for the IKZF1 deletion were: 5′-GTCCTGGGTTTAAGCTTCAGTTCTCTGCCT and 5′-GGGTTGATAAGGAGGGTTTTGTGTCCCAGT, respectively. Patient specific gene fusions were amplified using AccuPrime™ Taq DNA Polymerase High Fidelity (Life Technologies) and PCR products sequenced using BigDye Terminator v1.1 and an ABI-3730xl Genetic Analyzer (Applied Biosystems, UK). Sequences were aligned by BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Screening for immunoglobulin (IG) and T-cell receptor (TCR) gene rearrangements
DNA was extracted from diagnostic (MR87) and relapse cells (PB and BM). PCR amplification of IG heavy chain variable-diversity-joining (IGH V(D)J; complete and incomplete), IG kappa variable-joining (IGK-VJ), IGK V-kappa deleting element (Kde), intron recombination signal sequence (IRSS)-Kde, IG lambda (IGL), TCR beta (TCRB), TCR gamma (TCRG) and TCR delta gene rearrangements were performed using primers and conditions recommended by the BIOMED-2 Consortium 13. The FAM-labelled products were analysed using an ABI 3500 Genetic Analyzer (Applied Biosystems,), clonality was assessed by GeneScan® software (Applied Biosystems) and the results were interpreted in accordance with the EuroClonality/BIOMED-2 guidelines 14. PCR products were cloned into pCR2.1 (Life), sequenced and analysed as above. Junction analyses were performed using IgBLAST (www.ncbi.nlm.nih.gov/igblast/) and the ImMunoGeneTics database (www.imgt.org/).
Genome-wide copy number analysis
SNP array analysis was carried out using Affymetrix SNP 6.0 arrays according to the manufacturer’s instructions on DNA extracted using standard methods from the diagnostic cell line (MR87) and from relapse bone marrow. Genotyping and generation of QC data was performed in Genotyping Console™ v4.1.4 software (Affymetrix). The sample files were scrutinised for copy number alterations by visual inspection and using Partek Genomics Suite 6.6 software (Partek®, USA). Copy number was determined by normalisation to Partek distributed baseline files which comprise 270 Hapmap files using a genomic segmentation algorithm.
Whole exome sequencing (WES)
Exome capture was performed using the Agilent SureSelect Human All Exon V5 kit following manufacturer’s instructions (Agilent) and sequenced by Illumina paired end sequencing (protocol v1.2). Briefly, DNA was sheared by fragmentation (Covaris), purified using Agencourt AMPure XP beads (Beckman Coulter) and the resulting fragments analysed on an Agilent 2100 Bioanalyzer. Fragment ends were repaired and adaptors ligated to the fragments and the library was purified using beads. After amplification and hybridisation with biotinylated RNA baits, bound genomic DNA was purified with streptavidin coated magnetic Dynabeads (Invitrogen) and re-amplified to include barcoding tags before finally pooling for sequencing on an Illumina HiSeq 2000.
Exome analysis was completed in Oxford Gene Technology’s (OGT, UK) exome pipeline. Briefly, reads were aligned to the human genome reference sequence GRCh37 using BWA 0.6.2. 15. Local realignment was performed around indels with the Genome Analysis Toolkit (GATK v1.6) IndelRealigner 16. Optical and PCR duplicates were marked in BAM files using Picard 1.107 (http://picard.sourceforge.net). Original HiSeq base quality scores were recalibrated using GATK TableRecalibration and per-sample variants called with GATK UnifiedGenotyper. Indels and SNVs were hard-filtered according to Broad Institute best-practice guidelines to eliminate false positive calls.
CNV’s, somatic SNV and somatic indels were identified between presentation and relapse samples using VarScan2 17. Variant annotation was performed with a modified version of Ensembl Variant Effect Predictor (VEP) 18.
RESULTS
Clinical and haematological features of case
A brief case report of the patient was already published 19 and is summarised as follows. A 4 year old boy was diagnosed as having precursor B-cell ALL but with a mixed lympho-myeloid phenotype: positive for myeloperoxidase, CD13+, CD10+ and CD19+. Cytogenetics on leukaemic cells showed 46,XY,9p−,t(9q+;22q−) indicating Ph+ pre-B-ALL. RT-PCR confirmed presence of the minor breakpoint (p190) BCR-ABL1 fusion.
The patient was treated with chemotherapy and achieved complete remission. Eight weeks after the diagnosis, he developed a central nervous system relapse which was successfully treated with cranial irradiation and intrathecal drug administration. Three months after the diagnosis, he received a bone marrow transplant (BMT) from his human leucocyte antigen (HLA) identical (non-twin) brother when in the second complete remission (CR). BMT was successful and no major complications were observed.
At the age of 25 years (20 years after BMT) the patient presented with general fatigue. His white blood cell count was 16.7 × 109/l with 7% blasts, and his bone marrow aspirates showed leukaemic cells with a myeloid immunophenotype positive for CD13 and CD33 and negative for CD10 and CD19. The leukaemic cell karyotype was 46,XY,t(9;22)(q34;q11) × 2 plus other complex abnormalities. He was tentatively diagnosed as having a relapse of the initial Ph+ pre-B-ALL and received intensive chemotherapy resulting in complete remission. He underwent the second BMT from an HLA-identical unrelated donor but had bone marrow relapse 35 weeks after the second BMT and subsequently died of the disease.
Diagnostic and late relapse clones share an identical BCR-ABL1 fusion sequence
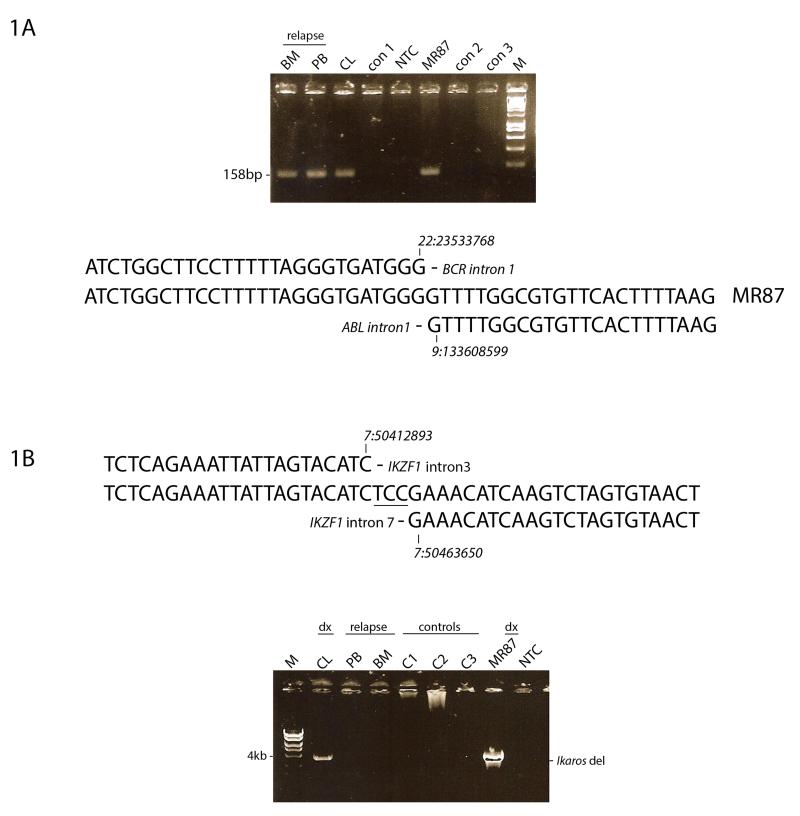
The putative breakpoint regions of the BCR and ABL1 genes were identified by targeted whole genome sequencing of DNA isolated from the cell line (MR87) derived from patient cells at diagnosis. PCR primers were designed 5′ to the putative breakpoint in BCR and 3′ to that in ABL1 and the patient–specific BCR-ABL1 gene fusion was amplified, cloned and sequenced. The breakpoint detected in the BCR gene occurred within intron 1 at GRCh37.p13 position ch22:23533768 and within intron 1 of the ABL1 gene at position ch9:133608599 (Figure 1). The breaks in both BCR and ABL1 are therefore outside the recognised cluster regions described for Ph+ leukaemia 20. The same set of PCR primers were next used to interrogate DNA from PB and BM at relapse and an identical sized fusion product was obtained. Cloning and sequencing of the relapse fusion products proved the BCR-ABL1 fusion sequence to be identical to that present at diagnosis (Figure 1A).
Figure 1. Diagnostic and late relapse clones share an identical BCR-ABL1 fusion sequence with a discordant intra-gene deletion of IKZF1.
A) Upper Panel: PCR primers that span the BCR-ABL1 breakpoint identified in diagnostic material (MR87, CL) were used to interrogate DNA isolated at relapse (BM, PB). An identical product is seen in all patient samples but not in leukaemia controls. Lower Panel: DNA sequence of the BCR-ABL1 fusion and comparison with wild-type BCR and ABL1 gene sequences (GRCh37.p13). The DNA sequence was identical in both diagnostic and relapse samples.
B) Upper Panel: DNA fusion sequence (GRCh37.p13) of the IKZF1 deletion at diagnosis reveals a 50kb deletion between introns 2 and 7. Lower Panel: The deletion/fusion product present at diagnosis (MR87 and CL) is not observed at relapse (BM, PB) or in DNA from leukaemia controls.
Genome-wide copy number analysis
SNP 6.0 analysis on DNA isolated from the diagnostic cell line showed the following recurrent leukaemia CNA: deletion of MTAP, CDKN2A/B, PAX5, 6q14.1 - 6q16.1 and IKZF1. Additionally amplification of MDM2 was noted. Relapse material was discordant for the diagnostic CNA drivers (Table 1), however copy number loss of 9p was demonstrated (including loss of the same genes deleted at diagnosis: CDKN2A/B, MTAP and PAX5) but results clearly demonstrated that this 9p deletion was a re-iterative event with distinct breakpoints to the diagnostic sample (Table S1, S2 and Figures S1A-F, in supplementary material). Potential drivers newly acquired in the relapse material also included deletion of the majority of chromosome 21q, gain of chromosome 20 and deletion of 8p (Table S2).
Table 1. Summary of ‘driver’ CNAs present at diagnosis versus relapse detected by SNP analysis.
| chr | start | end | cytoband | CNA Loss/Gain | Diagnosis | Relapse | Genes (max 3 listed) | |
|---|---|---|---|---|---|---|---|---|
| 1 | chr6 | 78095353 | 93349377 | 6q14.1 - 6q16.1 | Loss | present | absent | IRAK1BP1, PHIP, SYNCRIP |
| 2 | chr7 | 50389071 | 50477011 | 7p12.2 | Loss | present | absent | IKZF1 |
| 3 | chr9 | 16523946 | 21901150 | 9p22.3 - 9p21.3 | Loss | present | absent | MLLT3, MTAP, FOCAD |
| 4 | chr9 | 21901150 | 22347406 | 9p21.3 | Homozygous Loss | present | absent | CDKN2A, CDKN2B |
| 5 | chr9 | 36412035 | 38493465 | 9p13.2 - 9p13.1 | Loss | present | absent | MELK, PAX5, FRMPD1 |
| 6 | chr12 | 68970862 | 69406845 | 12q15 | Gain | present | absent | RAP1B, NUP107, MDM2 |
| 7 | chr 7 | 50389060 | 50486601 | 7p12.2 | Loss | absent | present | IKZF1 |
| 8 | chr8 | 31254 | 43078119 | 8p23.3 - 8p11.21 | Loss | absent | present | ZNF596, FBXO25, DLGAP2 |
| 9 | chr9 | 6988784 | 21926959 | 9p24.1 - 9p21.3 | Loss | absent | present | MPDZ, MLLT3, MTAP |
| 10 | chr9 | 21926959 | 22200408 | 9p21.3 | Heterozygous Loss | absent | present | CDKN2A, CDKN2B |
| 11 | chr9 | 22200408 | 132070825 | 9p21.3 – 9q34.11 | Loss | absent | present | MOB3B, LINGO2, PAX5 |
| 12 | chr20 | 61305 | 62956154 | 20p13-20q13.33 | Gain | absent | present | DEFB125, DEFB126, TOP1 |
| 13 | chr21 | 15939316 | 48096958 | 21q11.2 - 21q22.3 | Loss | absent | present | CHODL,CXADR,NCAM2 |
Based on NCBI37/hg19 Assembly
Given that deletions in the tumour suppressor gene IKZF1 are considered a driving force of leukaemogenesis, we used targeted sequencing of diagnostic DNA to design PCR primers that spanned the putative boundaries of the 50kb IKZF1 intra-gene deletion. Subsequent PCR produced a ~4kb amplification product that was further cloned and sequenced (Figure 1B). The 5′ breakpoint was determined at GRCh37.p13 position 7:50412893 and the 3′ breakpoint at position 7:50463650 with loss of 50,757bp of DNA and the random insertion of 3 nucleotides (Figure 1B). Using the same primer set, we could not detect a deletion in the IKZF1 gene by conventional PCR or sensitive Q-PCR in PB or BM at relapse (Figure 1B and Figure S2). These data indicate that some genes (IKZF1, CDKN2A) were subject to reiterated CNA in diagnosis and relapse but no CNA was preserved from diagnosis to relapse.
Clonality of immunoglobulin gene rearrangements at diagnosis and relapse
Screening for clonal IG and TCR gene rearrangements to assess clonality was performed on both the diagnostic and relapse DNA using multiplex PCR reactions and ABI GeneScan® profiling. Clonal rearrangements were identified in both IGH VDJ (FR 1 and 2) and IGL VJK reactions (Figure 2 and S3) with weaker clonal rearrangements observed in TCRBB/C and in TRG1 (data not shown). A V(N)JK light chain rearrangement was shown to be identical between diagnosis and relapse (Figure 2) and the two major IGH V(N)D(N)J peaks identified at 294 and 335bp at diagnosis were similarly shown to have identical sequences to the respective minor peaks observed at relapse (Figure S3). However, the two major peaks identified in relapse at 330 and 341bp were not detected in diagnostic material by conventional or Q-PCR (Figure S4). One interpretation of these data is that the ‘founder’ IGH rearrangement present in the diagnostic samples underwent further rearrangement in relapse. Taken together, these data further suggest that the diagnostic and relapse clones may have arisen from a pre-leukaemic progenitor cell already partially committed to the B cell lineage. However, the myeloid or AML immunophenotype seen in relapse indicates that the leukaemia was essentially ‘mixed lympho-myeloid’ and may have arisen in a cell with some myeloid differentiation capacity despite the clonal IGH rearrangements present in the bulk cell progeny.
Figure 2. Clonality of immunoglobulin light chain gene rearrangements at diagnosis and relapse.
Upper Panel: PCR amplifications of IGL V(N)J rearrangements at diagnosis and relapse were assessed by GeneScan® software. Lower Panel: DNA sequence analysis of the major V(N)J rearrangement identified at diagnosis shows an identical sequence to that observed at relapse.
N region insertion is shown in bold italics.
WES analysis
We performed WES on patient DNAs isolated at diagnosis, remission (germline) and relapse. All possible cross-comparisons between these three time points were assessed in the data analyses. In terms of somatic alterations, at diagnosis we identified, prior to filtering, a total of 2189 SNVs and 648 insertions and/or deletions. At relapse we identified 7320 SNVs and 1567 indels.
In further analyses, we highlighted relevant functional alterations. In the diagnostic sample, after filtering the data by read depth (between 30-170×), coding areas only and SNVs predicted to alter protein structure and deleterious/possibly damaging at protein level (VEP-Ensembl), we detected 92 somatic SNVs and 59 indels. We selected those genes with functions known to be associated with cancer of which there were twelve: SNVs; NOTCH2, PIK3CG, IL2RB, BAI3, FREM2 and RERE and indels; UTRN, CDHR3, NCOA5, CABYR, HOTAIR and FOLH1 (Table 2). In the relapse sample after a similar filtration, we identified 156 SNVs and 46 indels and identified ten potential ‘driver’ genes: SNVs; THOC6, VANGL2, THBS1, STAT2, ACY1, and indels; NBEAL1, SMG7, TRIM29, FANCG, FAM186A (Table 2). All 22 genes have previously been shown to play a relevant role in tumourigenesis or have potential to be a ‘driver’ of leukaemogenesis. We confirmed selected heterozygous point mutations or indels by Sanger sequencing, i.e. NOTCH2, HOTAIR, STAT2 and FANCG (data not shown). None were shared between the diagnostic and relapse samples and absent in remission (control, constitutive DNA)
Table 2. Principal mutations detected by whole exome sequencing of the patient DNA at diagnosis and relapse.
| Genes | Diagnosis | Relapse | |
|---|---|---|---|
| SNV1 | NOTCH2 | ||
| SNV2 | PIK3CG | ||
| SNV3 | IL2RB | ||
| SNV4 | BAI3 | ||
| SNV5 | FREM2 | ||
| SNV6 | RERE | ||
| Ind1 | UTRN | ||
| Ind2 | CDHR3 | ||
| Ind3 | NCOA5 | ||
| Ind4 | CABYR | ||
| Ind5 | HOTAIR | ||
| Ind6 | FOLH1 | ||
| SV7 | THOC6 | ||
| SV8 | VANGL2 | ||
| SV9 | THBS1 | ||
| SV10 | STAT2 | ||
| SV11 | ACY1 | ||
| Ind7 | NBEAL1 | ||
| Ind8 | SMG7 | ||
| Ind9 | TRIM29 | ||
| Ind10 | FANCG | ||
| Ind11 | FAM186A |
 Somatic SNV
Somatic SNV
 Somatic Inddel
Somatic Inddel
 WT
WT
List of the relevant genes affected by somatic mutations (SNVs and indels) at diagnosis and relapse. The remission (germline) sample was also evaluated and did not harbour any of these mutations thus confirming their somatic/clonal origin. The colours differentiate SNVs (light grey) from indels (dark grey).
DISCUSSION
The identity of shared and clone-specific genotypic sequences in this patient’s diagnostic and very late relapse leukaemia cell population provides unambiguous evidence that the relapse derived, after 22 years, from descendent progeny of the original founder clone (Figure 3). Late relapses derived from the founder diagnostic clones in ALL have been described before 9, 10 but this is the longest dormancy interval recorded with the possible exception of a case relapsing after 34 years in which the genetic evidence was very limited 21. It is striking that although the BCR-ABL1 fusion gene was identical in the paired diagnostic/relapse samples, all other genetic abnormalities detected by SNP arrays as CNA or by exomic sequencing as SNVs were distinctive although the same gene was in some reiteratively mutated (e.g. CDKN2A, IKZF1). Reiterative CNA have been reported before in ALL 22, 23 and the predominant mutational mechanism for these structural changes appears to be driven by the lymphoid recombinases RAG1/2 24. SNV in ALL have a different mutational mechanism involving APOBEC’s 24. It is unclear if the predominance of CNA as recurrent changes in ALL is a reflection of the relative activity of these different mutational mechanisms, the prevalence of different selective pressures or differential functional impacts of CNA versus SNV on cellular fitness.
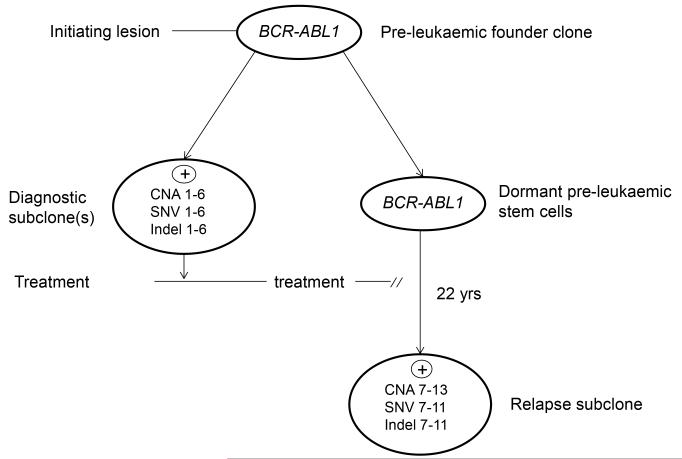
Figure 3. Model for the pre-leukaemic origins of very late relapse.
CNA, SNV and indel numbers – distinctive mutations found in diagnostic and relapse clones (numbers 1-13 refer to aberrations noted in Tables 1 and 2).
ALLs have multiple, genetically distinct stem cells at diagnosis 22. Our interpretation of the genomic data on this patient is that the long term surviving stem cells that spawned very late relapse derived from stem cells of a minor clone at diagnosis and most likely from a pre-leukaemic clone that harboured a founder BCR-ABL1 lesion but not other secondary genetic changes (Figure 3). Evidence for such pre-malignant clones in ALL with BCR-ABL or other founder lesions have been provided by comparative genetics of monozygotic twins with discordant ALL 25-27. Sharing of identical or clonotypic BCR-ABL1 genomic fusions in monozygotic twins with concordant or discordant ALL but discordance of other genetic changes 27 suggests that the BCR-ABL1 fusion in such cases is an early or likely founder or initiation event spawning a pre-leukaemic clone. Limited comparative genetics had previously suggested that some late relapses in ALL might be spawned by persistent pre-leukaemic clones 28, 29. Immunophenotypically or genetically defined pre-leukaemic cells have previously been shown to preferentially survive chemotherapy in ALL 30 and acute myeloblastic leukaemia (AML) 31. Recently, Zhang et al 32 reported a relapse after 17 years in a case of acute promyelocytic leukaemia (APML). The comparative genetics in this case was also compatible with the relapse originating from pre-leukaemic stem cells.
Many mechanisms have been proposed to explain protracted clinical dormancy of cancer including balanced proliferation, cell death, non-angiogenic phenotypes, negative signalling within stromal niches maintaining cells out of cycle and immune-surveillance 6, 7, 33. Whatever the prevailing restraints, a late recurrence derived from the original clone requires that cells with self-renewal or stem cell potential survive to re-establish disease. In this respect, the recognised therapeutic resistance of quiescent cancer stem cells and residence in specialised bone marrow niches 33 provides a basis for their survival in a dormant state as we assume occurred in our patient.
Adopting dormancy as a survival strategy is not unique to cancer stem cells. Normal blood stem cells fluctuate between proliferative and quiescent or out of cycle phases 34. Bacteria ‘hunker down’ or adopt a non-proliferative state when confronted with stressful conditions 35. The capacity of cancer stem cells to avoid lethal therapy by switching to a dormant state can be seen as a legacy of evolutionary programming of protective mechanisms for essential normal stem cells.
The case reported here reflects an extremely rare occurrence and does not conflict with the suggestion that cure in childhood ALL can be operationally defined by remission of four years post cessation of treatment when the risk of relapse is <1% 10, 36.
Although many very late (>20 years) recurrences or relapses of cancer have been recorded, the assumption that this reflects re-awakening of the original clone or one of its subclones requires genetic scrutiny. Provided the diagnostic sample or biopsy is archived, this can be resolved, as in the current case, by comparative genomics.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Leukaemia & Lymphoma Research (MG, AMF, CF), the European Hematology Association - EHA Partner Fellowship #2011/01, Instituto Nacional de Cancer - INCA and the Lady Tata Memorial Trust – LTMT International Award for Research in Leukaemia (MBM), The Kay Kendall Leukaemia Fund (FWvD) and by a Wellcome Trust Strategic Award [105104/Z/14/Z] (to MG). We thank Dr Brian Walker for help with custom library preparation and the members of the ICR Tumour Profiling Unit for providing the targeted sequencing data on the diagnostic material.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Supplementary information is available at Leukemia’s website.
REFERENCES
- 1.Malogolowkin M, Spreafico F, Dome JS, van Tinteren H, Pritchard-Jones K, van den Heuvel-Eibrink MM, et al. Incidence and outcomes of patients with late recurrence of Wilms’ tumor. Pediatr Blood Cancer. 2013 Oct;60(10):1612–1615. doi: 10.1002/pbc.24604. [DOI] [PubMed] [Google Scholar]
- 2.Tsao H, Cosimi AB, Sober AJ. Ultra-late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997 Jun 15;79(12):2361–2370. [PubMed] [Google Scholar]
- 3.Faries MB, Steen S, Ye X, Sim M, Morton DL. Late recurrence in melanoma: clinical implications of lost dormancy. Journal of the American College of Surgeons. 2013 Jul;217(1):27–34. doi: 10.1016/j.jamcollsurg.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mir R, Phillips SL, Schwartz G, Mathur R, Khan A, Kahn LB. Metastatic neuroblastoma after 52 years of dormancy. Cancer. 1987 Nov 15;60(10):2510–2514. doi: 10.1002/1097-0142(19871115)60:10<2510::aid-cncr2820601027>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10:871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- 6.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013 Nov 7;155(4):750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014 Sep;14(9):611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost L, Goodeve A, Wilson G, Peake I, Barker H, Vora A. Clonal stability in late-relapsing childhood lymphoblastic leukaemia. Br J Haematol. 1997;98:992–994. doi: 10.1046/j.1365-2141.1997.3103129.x. [DOI] [PubMed] [Google Scholar]
- 10.Vora A, Frost L, Goodeve A, Wilson G, Ireland RM, Lilleyman J, et al. Late relapsing childhood lymphoblastic leukemia. Blood. 1998;92:2334–2337. [PubMed] [Google Scholar]
- 11.Levasseur M, Maung ZT, Jackson GH, Kernahan J, Proctor SJ, Middleton PG. Relapse of acute lymphoblastic leukaemia 14 years after presentation: use of molecular techniques to confirm true re-emergency. Br J Haematol. 1994;87:437–438. doi: 10.1111/j.1365-2141.1994.tb04942.x. [DOI] [PubMed] [Google Scholar]
- 12.Okamura J, Yamada S, Ishii E, Hara T, Takahira H, Nishimura J, et al. A novel leukemia cell line, MR-87, with positive Philadelphia chromosome and negative breakpoint cluster region rearrangement coexpressing myeloid and early B-cell markers. Blood. 1988 Oct;72(4):1261–1268. [PubMed] [Google Scholar]
- 13.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003 Dec;17(12):2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 14.Langerak AW, Groenen PJ, Bruggemann M, Beldjord K, Bellan C, Bonello L, et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012 Oct;26(10):2159–2171. doi: 10.1038/leu.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009 Jul 15;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010 Sep;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012 Mar;22(3):568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010 Aug 15;26(16):2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodama Y, Okamura J, Fukano R, Nakashima K, Ito N, Nishimura M, et al. Re-emerging Philadelphia chromosome-positive acute leukaemia more than 20 years after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2013 Apr;161(2):286–289. doi: 10.1111/bjh.12212. [DOI] [PubMed] [Google Scholar]
- 20.Chen SJ, Chen Z, Font MP, d’Auriol L, Larsen CJ, Berger R. Structural alterations of the BCR and ABL genes in Ph1 positive acute leukemias with rearrangements in the BCR gene first intron: further evidence implicating Alu sequences in the chromosome translocation. Nuc Acids Res. 1989;17:7631–7642. doi: 10.1093/nar/17.19.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessho F, Takayama N, Fronkova E, Zuna J. Reappearance of acute lymphoblastic leukemia 34 years after initial diagnosis: a case report and study of the origin of the reappeared blasts. Int J Hematol. 2013 Apr;97(4):525–528. doi: 10.1007/s12185-013-1289-9. [DOI] [PubMed] [Google Scholar]
- 22.Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469:356–361. doi: 10.1038/nature09650. [DOI] [PubMed] [Google Scholar]
- 23.Waanders E, Scheijen B, van der Meer LT, van Reijmersdal SV, van Emst L, Kroeze Y, et al. The origin and nature of tightly clustered BTG1 deletions in precursor B-cell acute lymphoblastic leukemia support a model of multiclonal evolution. PLoS Genet. 2012;8(2):e1002533. doi: 10.1371/journal.pgen.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papaemmanuil E, Rapado I, Li Y, Potter NE, Wedge DC, Tubio J, et al. RAG-mediated recombination is the predominant driver of oncogenic rearrangement in ETV6-RUNX1 acute lymphoblastic leukemia. Nat Genet. 2014;46(2):116–125. doi: 10.1038/ng.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong D, Gupta R, Ancliffe P, Atzberger A, Brown J, Soneji S, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008;319:336–339. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- 26.Bateman CM, Colman SM, Chaplin T, Young BD, Eden TO, Bhakta M, et al. Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood. 2010;115(17):3553–3558. doi: 10.1182/blood-2009-10-251413. [DOI] [PubMed] [Google Scholar]
- 27.Cazzaniga G, van Delft FW, Lo Nigro L, Ford AM, Score J, Iacobucci I, et al. Developmental origins and effect of BCR-ABL1 fusion and IKZF1 deletions in monozygotic twins with Ph+ acute lymphoblastic leukemia. Blood. 2011;118(20):5559–5564. doi: 10.1182/blood-2011-07-366542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford AM, Fasching K, Panzer-Grümayer ER, Koenig M, Haas OA, Greaves MF. Origins of “late” relapse in childhood acute lymphoblastic leukemia with TEL-AML1 fusion genes. Blood. 2001;98:558–564. doi: 10.1182/blood.v98.3.558. [DOI] [PubMed] [Google Scholar]
- 29.van Delft FW, Horsley S, Colman S, Anderson K, Bateman C, Kempski H, et al. Clonal origins of relapse in ETV6-RUNX1 acute lymphoblastic leukemia. Blood. 2011;117:6247–6254. doi: 10.1182/blood-2010-10-314674. [DOI] [PubMed] [Google Scholar]
- 30.Lutz C, Woll PS, Hall G, Castor A, Dreau H, Cazzaniga G, et al. Quiescent leukaemic cells account for minimal residual disease in childhood lymphoblastic leukaemia. Leukemia. 2013 Apr;27(5):1204–1207. doi: 10.1038/leu.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014 Feb 20;506(7488):328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Zhang Q, Dahlstrom J, Tran AN, Yang B, Gu Z, et al. Genomic analysis of the clonal origin and evolution of acute promyelocytic leukemia in a unique patient with a very late (17 years) relapse. Leukemia. 2014 Aug;28(8):1751–1754. doi: 10.1038/leu.2014.113. [DOI] [PubMed] [Google Scholar]
- 33.Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007 Dec 13;1(6):607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007 Jan;5(1):48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 36.Pui CH, Pei D, Campana D, Cheng C, Sandlund JT, Bowman WP, et al. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014 Dec;28(12):2336–2343. doi: 10.1038/leu.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.