Abstract
Tuberculosis (TB) remains a global pandemic and drug resistance is rising. Multicellular granuloma formation is the pathological hallmark of Mycobacterium tuberculosis (Mtb) infection. The membrane-type 1 MMP (MT1-MMP or MMP-14) is a collagenase that is key in leukocyte migration and collagen destruction. In patients with TB, induced sputum MT1-MMP mRNA levels were increased 5.1-fold compared to matched controls and correlated positively with extent of lung infiltration on chest radiographs (r=0.483; p<0.05). Mtb infection of primary human monocytes increased MT1-MMP surface expression 31.7-fold and gene expression 24.5-fold. Mtb-infected monocytes degraded collagen matrix in an MT1-MMP-dependent manner, and MT1-MMP neutralisation decreased collagen degradation by 73%. In human TB granulomas, MT1-MMP immunoreactivity was observed in macrophages throughout the granuloma. Monocyte-monocyte networks caused a 17.5-fold increase in MT1-MMP surface expression dependent on p38 MAP kinase and GPCR-dependent signalling. Monocytes migrating towards agarose beads impregnated with conditioned media from Mtb-infected monocytes expressed MT1-MMP. Neutralization of MT1-MMP activity decreased this Mtb network-dependent monocyte migration by 44%. Taken together, we demonstrate that MT1-MMP is central to two key elements of TB pathogenesis, causing collagen degradation and regulating monocyte migration.
INTRODUCTION
New therapeutic approaches are needed to achieve tuberculosis (TB) control, a disease which caused 9.0 million cases and 1.5 million deaths in 2013 (1). Pathology results from the interaction between Mycobacterium tuberculosis (Mtb) and the host immune response (2). This pathology is particularly critical in cavitatory pulmonary TB, which results in transmission of infection (3, 4), increased drug resistance (5, 6), morbidity and mortality (7-9). Improved understanding host factors that drive lung pathology is required to design novel therapeutic and vaccination strategies (2).
Breakdown of the pulmonary extracellular matrix (ECM) in TB follows inflammatory cellular infiltration and granuloma formation in lung tissue. Secreted matrix metalloproteinases (MMPs) are emerging as key mediators in driving TB pathology. MMPs are zinc-dependent proteolytic enzymes which regulate cellular migration and have the potential to degrade all ECM fibrils (10). MMP-1 causes collagen destruction, while the gelatinase MMP-9 plays a critical role in regulating a chemotactic gradient for monocytes towards TB granulomas (11). Membrane-bound MMPs are important in cell migration in diverse inflammatory conditions and can also act as collagenases (12), but their role in TB is poorly understood.
MT1-MMP was the first membrane-bound MMP to be discovered (13). MT1-MMP degrades collagen in the immediate pericellular environment and is key in cell migration through extracellular matrix (14-16). MT1-MMP gene expression has been found to be upregulated in Mtb-infected monocytes (17) and by unbiased analyses of gene expression in TB (18, 19). However, the functional role of MT1-MMP in TB pathogenesis has not been explored. We hypothesised that MT1-MMP may drive inflammatory cell influx to foci of Mtb infection and also contribute to collagen degradation. We demonstrate that MT1-MMP expression is increased in patients with pulmonary TB and is up-regulated in primary human monocytes by Mtb infection. MT1-MMP is expressed throughout TB granulomas and is also up-regulated by monocyte-monocyte networks. MT1-MMP has dual effects in TB, both causing collagen destruction and also regulating cellular migration, implying a potentially central role in disease pathogenesis.
METHODS
Sputum collection and processing
The clinical study was approved by the University of Cape Town (UCT) Research Ethics Committee (Ref 516/2011). Written informed consent was obtained in all cases. Participants were prospectively recruited from the Ubuntu clinic in the township of Khayelitsha, Cape Town, South Africa. TB patients met at least one inclusion criteria from: sputum smear positive for AFB on microscopy or sputum Gene Xpert positive or sputum culture positive for Mtb or clinical features highly suggestive of TB with diagnostic features on chest radiograph and started on TB treatment by a clinician, conforming to the WHO clinical case definition. Control patients were individuals who were sputum smear and culture negative for Mtb with absent clinical and chest radiograph features of TB. Only HIV negative patients recruited within 72 hours of commencing TB treatment were included. Sputum induction, transportation and mucolysis were performed as described (20). The mucoid layer was filtered by passing through 100μM pore size strainer (BD) and then centrifuged at 500g for 10 mins. The cell pellet was aspirated and 1.5ml cold TRI reagent added before vortexing. Samples were stored at −80°C before proceeding to RT-PCR analysis. The extent of infiltration on chest radiograph was scored on a scale of 0 – 10, according to the number of segments involved, using an established scoring system (20).
Real Time Polymerase Chain Reaction
Sputum was lysed in TRIreagent (Sigma-Aldrich). Total RNA was extracted using phenol:chloroform extraction and the Purelink RNA Mini Kit (Invitrogen) according to manufacturer’s instructions. RNA was reverse transcribed using the Quantitect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions. RT-PCR was performed on a Stratagene Mx3000Pro machine using the synthesized cDNA, Brilliant II Taq polymerase (Agilent Technologies) and primers/probes (Applied Biosystems) for MT1-MMP and the reference genes β-actin and 18S. The cycle thresholds (Ct) for MT1-MMP and β-actin were used to indicate the relative amount of mRNA in each sample.
Monocyte purification and stimulation
Monocytes were isolated from single donor leucocyte cones (NHS Blood and Transfusion, London) or fresh blood from healthy donors by density gradient centrifugation over Ficoll-Paque (GE Healthcare Life Sciences) and adhesion purification. Monocyte purity was >95% by flow cytometry and viability >95% by trypan blue exclusion assay. Monocytes were plated in tissue culture plates or chamber slides. Experiments were commenced immediately using RPMI 1640 supplemented with 2mM glutamine, 10μg/ml ampicillin with 10% heat inactivated FCS (Biowest) in a humidified incubator supplemented with 5% CO2 at 37°C. Stimuli used were direct infection with Mycobacterium tuberculosis (Mtb), strain H37Rv or Conditioned Medium from Mtb Infected Monocytes (CoMTb).
Mtb culture
Mycobacterium tuberculosis (Mtb) H37Rv was cultured in Middlebrook 7H9 medium (BD Biosciences, Oxford, UK). Mtb in mid log growth at an optical density of 0.60 (Biowave cell density meter, WPA) was used for infecting monocytes immediately after adhesion purification at a multiplicity of infection of one.
Preparation of Conditioned Medium from Mtb infected Monocytes (CoMTb)
Monocytes were plated in RPMI 1640 supplemented with 2mM glutamine and 10μg/ml ampicillin and immediately infected with Mtb at an MOI of 1. After 24 hours, the cell culture medium was aspirated and filtered through a 0.2μM Anopore membrane (Whatman) to remove live Mtb and MMPs (21). The resulting cell culture supernatant is termed CoMTb and contains an Mtb induced intercellular network of cytokines, chemokines and growth factors. This was used to stimulate monocytes at 1 in 5 dilution immediately after adhesion purification. Conditioned medium from control monocytes (CoMCont) is the cell culture supernatant from uninfected monocytes after 24 hours incubation.
Flow cytometry
Adhesion purified monocytes were harvested from tissue culture plates using Cell Dissociation Buffer Enzyme Free (Invitrogen) at 37°C for 15 minutes and a cell scraper. Cells were centrifuged at 1200 rpm for 5 minutes for pelleting and blocked with 10% heat inactivated human serum/1% BSA, then stained with a PE-conjugated mouse anti-MT1-MMP antibody (R&D FAB9181P). If cells had been infected with Mtb they were then fixed in 2% paraformaldehyde. Flow cytometry was performed immediately after staining on a FACSCalibur (BD Biosciences). Live cells were gated, confirmed by propidium iodide staining. Harvested monocytes were identified for gating on the forward scatter/side scatter plot. Negative controls were unstained, unstimulated monocytes. Flow Jo software (Version 7.6.2) was used for data analysis.
Western blotting
Monocytes were harvested into SDS lysis buffer (62.5mM Tris, 2% SDS, 10% glycerol, 50mM DDT, 0.01% Bromphenol blue, Sigma-Aldrich), denatured at 90°C, loaded on a NuPAGE® 4-12% Bis-Tris Gel (Invitrogen) and separated by electrophoresis at 200V for 50 minutes in MOPS running buffer (Invitrogen). Proteins were electro-transferred to a nitro-cellulose membrane (GE Healthcare Life Sciences) at 30V for 90 minutes, blocked with 0.1% Tween-20 (Sigma)/5% non-fat milk for 1 hour and then incubated with primary antibody overnight. Detection was by HRP conjugated secondary antibody and chemiluminescent substrate (ECL Plus Western Blotting Detection System, GE Healthcare Life Sciences). Densitometry was analysed using Image J software version 1.44p (NIH, USA). For MT1-MMP western blots, the primary antibodies were rabbit anti-MT1-MMP (Millipore AB 6004) and mouse anti-β-actin (A1978, Sigma-Aldrich). The secondary antibodies were HRP-conjugated goat anti-rabbit (Cell Signalling Technology) and goat anti-mouse (Jackson ImmunoResearch). For phosphowesterns, the primary antibodies were to phosphorylated and total p38/ERK mitogen activated protein kinases (MAPK) (Cell Signalling Technology). The secondary antibody was HRP-conjugated goat anti-rabbit (Cell Signalling Technology).
Immunofluorescent microscopy and fluorescent collagen degradation assay
Monocytes in chamber slides (PAA Laboratories Ltd) were blocked with 5% human serum/1% BSA for 1 hour and stained with mouse anti MT1-MMP (MAB3328, Millipore), goat anti-mouse Alexa647 conjugated secondary antibody (A-21235, Invitrogen) and DAPI nuclear stain (Invitrogen). Permeabalization was performed with 0.5% Triton-X (Merck) and fixation with 2% paraformaldehyde. For the fluorescent collagen degradation assay, chamber slides were coated with FITC conjugated type I collagen from bovine skin (Sigma-Aldrich), prior to adhering the monocytes using a modification of a previously described technique. 0.005% poly-L-lysine (Sigma-Aldrich) was first used to create a substratum to improve cell adherence, then 0.5% glutaraldehyde (BDH) to covalently couple the substratum to the fluorescent collagen. This created a thin two dimensional collagen matrix for detection of pericellular collagen degradation by loss of fluorescent signal. Fluorescent microscopy was performed on a Leica TCS SP5 Confocal with the Leica Application Suite 2.6.2 software. Images were processed and quantified using Image J software version 1.44p (NIH, USA).
MMP-1 and TIMP quantitation
MMP-1 concentrations in cell culture supernatants were measured by Luminex (R and D Systems kit, Biorad Luminex 200 machine). TIMP-1 and-2 concentrations were analyzed by ELISA (R and D Systems).
Lung Immunohistochemistry
Lung tissue from 5 patients with culture proven Mtb was studied. Control tissue was obtained from uninvolved lung parenchyma of patients who underwent to surgical procedure for lung cancer. MT1-MMP immunoreactivity was evaluated using the Mouse monoclonal antibody 114-6G6 (Abcam ab77965). Briefly, sections were rehydrated in graded alcohols and heated in a microwave oven at 900W for 20 min in Citrate buffer at pH6. They were cooled at room temperature before immunostaining. MT1-MMP monoclonal antibody was used at a concentration of 20 μg/ml for 2 hour at room temperature and then processed with Polymer-HRP Kit (BioGenex, San Ramon CA, USA) with Diaminobenzidine development and Mayer haematoxylin counterstaining. Breast and colon tissue was used as a positive external control. Negative controls were obtained by omitting the primary antibody and using competing peptide used to raise the primary antibody. Slides were reviewed with a histopathologist, and images were taken using a Olympus BX51Microscope. This project was approved by the Hammersmith and Queen Charlotte’s research ethics committee (ref 07/H0707/120).
Chemical inhibitors and MT1-MMP inhibitory antibody
For chemical inhibition experiments, monocytes were pre-incubated with the specified inhibitors for 1 hour prior to CoMTb stimulation. The p38 MAPK inhibitor SB203580 (Enzo Life Sciences) and ERK MAPK inhibitor PD98059 (Calbiochem) were used. The G-protein coupled receptor inhibitor Pertussis Toxin (Calbiochem) was used to block chemokine signalling. A mouse anti MT1-MMP antibody (MAB3328, Millipore) was used to inhibit MT1-MMP activity.
Agarose bead assay
This assay was used to investigate monocyte migration towards CoMTb using a modification of a previous assay (22). RPMI 1640 or CoMTb was added to a 0.5% agarose solution at 40°C at a 1:10 dilution. 10μl beads were pipetted on to cover glass bottom chamber slides (PAA-Laboratories) pre-coated with type I collagen as described above. The slide was left at 4°C for 5 minutes for the agarose to set before adhering monocytes to the slide. 0.25 mM manganese was added to the media to activate integrins on the monocytes, which are required for monocyte adhesion to extracellular matrix during migration (23, 24). Images were captured using an Olympus E-620 camera attached to the Olympus CK2 light microscope. Images were processed and quantified using Image J software version 1.44p (NIH, USA). Migration was quantified by measuring 4 radial points per sphere and measuring the diameter of monocyte clusters, and 2 spheres per condition were analyzed.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 by Student’s t-test, Mann Whitney U test or One Way Analysis of Variance (ANOVA) with Tukey’s post hoc analysis as appropriate. P<0.05 was taken as significant.
RESULTS
MT1-MMP expression is increased in sputum of patients with TB and correlates with lung infiltration
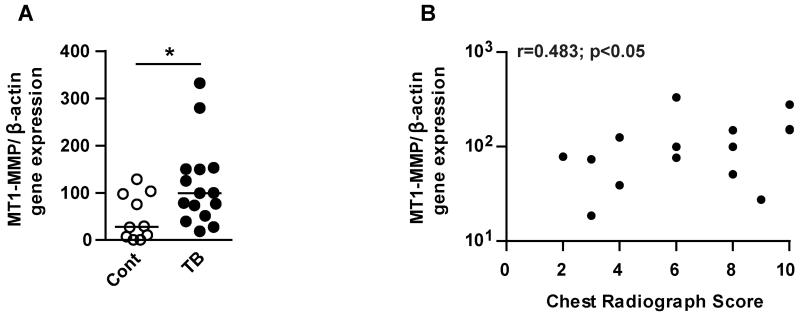
We investigated MT1-MMP gene expression in induced sputum from patients with pulmonary TB (n=15) and controls (n=10). Clinical features of patient cohort are in Table 1. The TB and control group were closely matched for age, but there were a higher proportion of females in the control group, which may reflect a differences in health seeking behaviour (25). TB patients had significantly more symptoms (fever, cough, night sweats, weight loss and breathlessness), a lower body mass index (BMI) and higher number of abnormal respiratory examinations, consistent with the diagnosis of pulmonary TB. All control patients were smear and culture negative for Mtb. Twelve of the TB cases were culture positive and of the 3 remaining cases, one was smear positive. These 3 patients all had clinical and radiological features highly suggestive of TB and were started on TB therapy by their treating clinician. MT1-MMP gene expression normalized to β-actin was 5.1-fold higher in the sputum of patients with TB compared to controls (Fig. 1A p<0.05). MT1-MMP gene expression positively correlated with the extent of lung infiltration on chest radiograph (Fig. 1B, r=0.483; p<0.05).
Table 1. Characteristics of patient cohort.
Significant differences are indicated in the right hand column.
| Patient characteristics | Control | TB | |
|---|---|---|---|
| Total no. | 10 | 15 | |
| Male:Female | 4:6 | 10:5 | |
| Median age (range) years | 31.5 (21-48) | 40 (24-73) | ns |
| Symptoms (duration in days) | |||
| Any | 1 | 15 | |
| Fever | 1 | 11 | |
| Cough | 0 | 14 | |
| Haemoptysis | 0 | 1 | |
| Night sweats | 1 | 12 | |
| Weight loss | 0 | 8 | |
| Breathlessness | 0 | 6 | |
| Pleuritic pain | 1 | 11 | |
| Anorexia | 1 | 3 | |
| Median duration (range) | 0 (0-4) | 30 (7-98) | p<0.0001 |
| Examination | |||
| Median Body Mass Index (range) | 25.4 (17.5 - 55.7) | 21.6 (18.5 – 29.1) | p<0.05 |
| Temp>37.5°C | 0 | 1 | ns |
| Abnormal respiratory examination | 4 | 13 | |
| Smear | |||
| Positive | 0 | 8 | |
| Negative | 10 | 7 | |
| Culture | |||
| Positive | 0 | 12 | |
| Negative | 10 | 3 * |
One of these 3 patients was smear positive. All had clinical features highly suggestive of TB with diagnostic features on chest radiograph, and were commenced on anti-tuberculous therapy by the treating physician.
Figure 1. MT1-MMP gene expression is increased in the sputum of patients with TB and correlates with lung infiltration on chest radiographs.
Induced sputum samples were collected prospectively from healthy controls (n=10) and TB patients (n=15) in Cape Town, South Africa. RNA was extracted from the sputum cell pellets and MT1-MMP and β-actin gene expression were analysed by RT-PCR. A. MT1-MMP gene expression (normalised to β-actin) was increased in the sputum of TB patients compared to healthy controls. Horizontal line indicates median value, *p<0.05 by Mann Whitney U Test. B. The extent of lung infiltration on chest radiograph was scored on a scale of 0-10. Sputum MT1-MMP relative expression positively correlated with lung infiltration by Spearman correlation coefficient (r=0.483; *p<0.05).
Mtb infection of primary human monocytes drives MT1-MMP gene and protein expression
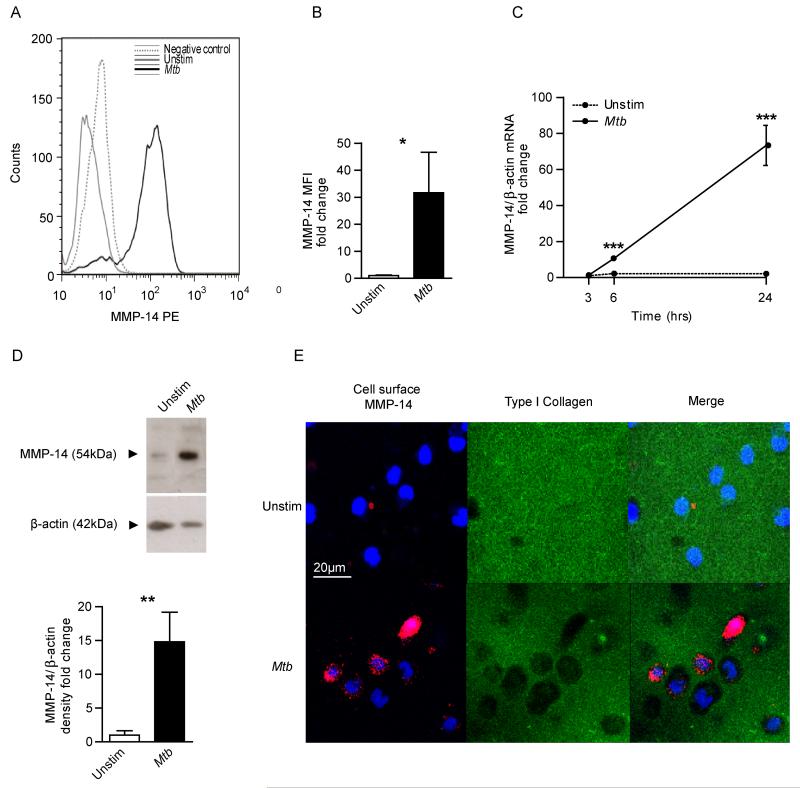
In a primary human cellular model, Mtb infection increased monocyte MT1-MMP surface expression analyzed by flow cytometry compared to uninfected cells at 24 hours (Fig. 2A). Quantification showed a 31.7-fold increase in MT1-MMP surface expression after Mtb infection (Fig. 2B, p<0.05). Next, we analysed the kinetics of MT1-MMP gene expression in Mtb-infected monocytes. Mtb infection caused a non-significant 1.4-fold increase in MT1-MMP gene expression normalized to β-actin at 3 hours, rising to 10.6-fold at 6 hours and 73.4-fold at 24 hours relative to uninfected monocytes (Fig. 2C). Increased MT1-MMP mRNA accumulation was associated with increased MT1-MMP total protein in Mtb infected monocytes analyzed by western blotting (Fig. 2D). Mtb infection of monocytes caused a 14.8-fold increase in MT1-MMP protein in whole cell lysates compared to uninfected monocytes at 48 hours (Fig. 2D, p<0.01). Mtb also increased secretion of MMP-1 into cell culture supernatants analyzed by luminex array (Supplemental fig. 1), although this concentration was below the sensitivity of detection by casein zymography (data not shown).
Figure 2. Mtb infection drives monocyte MT1-MMP expression and collagen degradation.
A. Human monocytes were infected with Mtb (Multiplicity of infection = 1) and MT1-MMP expression was analysed by flow cytometry at 24h. Increased MT1-MMP surface expression is demonstrated by greater median fluorescence intensity (MFI). B. MFI analysis confirms increased MT1-MMP expression. C. Mtb infection causes a progressive increase in MT1-MMP mRNA accumulation infected monocytes (normalised to β-actin). D. Mtb infection increases total cellular MT1-MMP at 48h analyzed by western blotting, confirmed by densitometric analysis. β-actin was probed as a loading control. E. Mtb infection causes collagen degradation around monocytes. Human monocytes were seeded on wells coated with fluorescently conjugated Type I collagen and infected with Mtb. MT1-MMP surface expression and collagen degradation were analysed by immunofluorescent staining and microscopy. Blue is DAPI nuclear stain, Magenta is MT1-MMP and green Type I Collagen. Increased collagen degradation occurred at 24h in Mtb-infected wells, and MT1-MMP surface expression co-localises with the areas of collagen degradation. For panels B, C and D, the mean +/− SD values of experiments performed in triplicate are shown and are representative of a minimum of 2 independent experiments. *p<0.05, **p<0.01, ***p<0.001 by Student’s t-test.
Mtb-dependent MT1-MMP expression causes collagen degradation
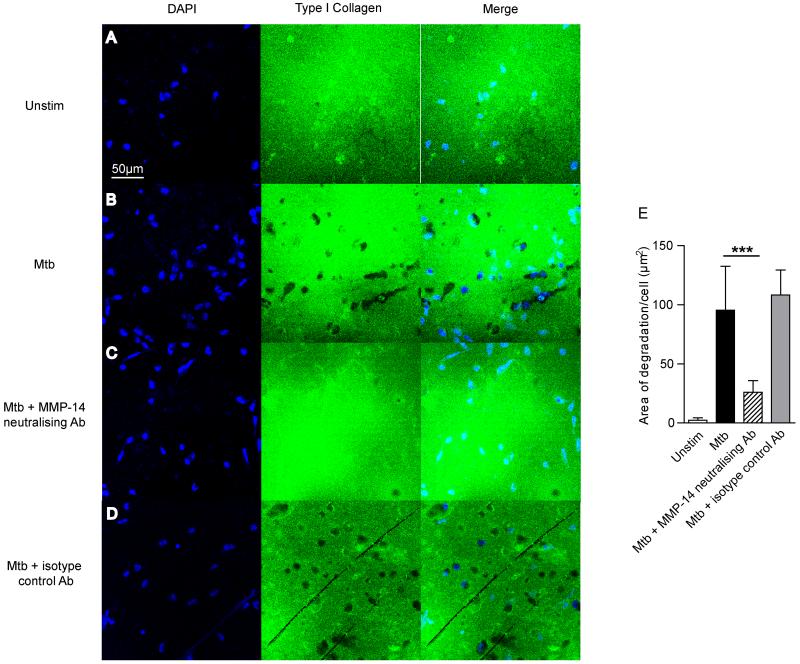
To investigate whether MT1-MMP caused matrix breakdown, we analyzed degradation of fluorescent collagen. Mtb infection increased MT1-MMP surface expression on unpermeabalized monocytes at 24 hours (Fig. 2E). Mtb infection caused collagen degradation, indicated by loss of fluorescence, and this collagen degradation co-localized with MT1-MMP expression (Fig 2E, merged image). MT1-MMP neutralization inhibited such Mtb driven collagen degradation. Zones of collagen breakdown are present around Mtb infected monocytes (Fig. 3B), and incubation with an MT1-MMP inhibitory antibody suppresses this collagen breakdown (Fig. 3C). A matched isotype control antibody did not inhibit collage breakdown (Fig. 3D). Quantification of degraded collagen demonstrated that Mtb infection caused 42.8 times greater collagen degradation by monocytes compared to uninfected cells (Fig. 3E). MT1-MMP inhibition resulted in a 73% reduction in this collagen breakdown (p<0.001).
Figure 3. Mtb-driven collagen degradation is MT1-MMP dependent.
A-B. Human monocytes were seeded on FITC conjugated Type I Collagen and infected with Mtb. Collagen degradation was analysed by immunofluorescent microscopy. Blue: DAPI nuclear stain, Green: Type I Collagen. Mtb infection causes greater collagen degradation. C. MT1-MMP activity was neutralised with an inhibitory anti-MT1-MMP antibody (MAB3328, Millipore) at 10μg/ml. MT1-MMP inhibition inhibited areas of collagen breakdown. D. An isotype control antibody did not inhibit collagen degradation. E. Fluorescence quantitation confirms collagen degradation is MT1-MMP-dependent. Data are from a single experiment and are representative of 3 independent experiments. Mean values +/− SD is shown, ***p<0.001 by One-way ANOVA with Tukey’s post hoc test.
MT1-MMP is expressed in granulomas of patients with TB and is upregulated by Mtb-induced intercellular networks
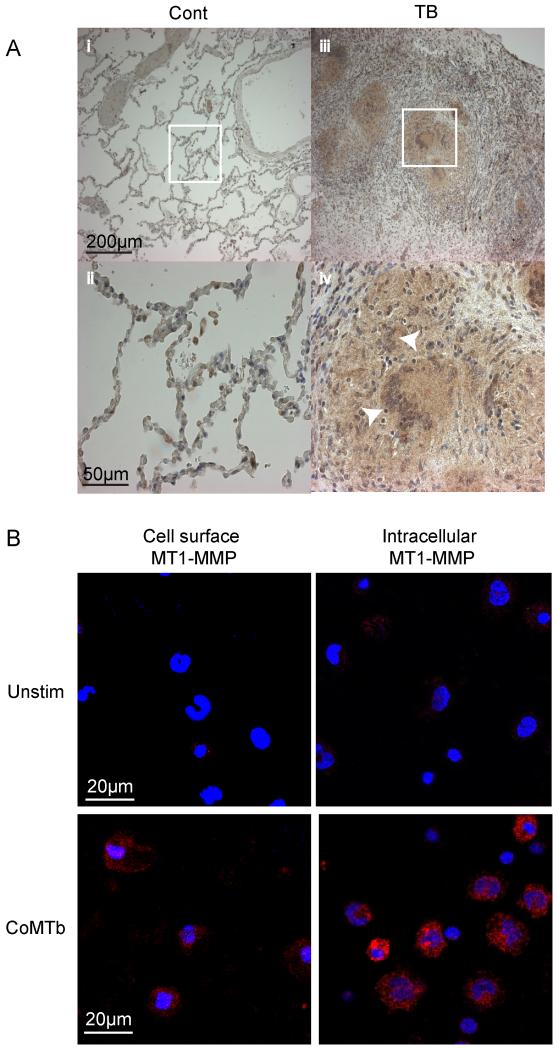
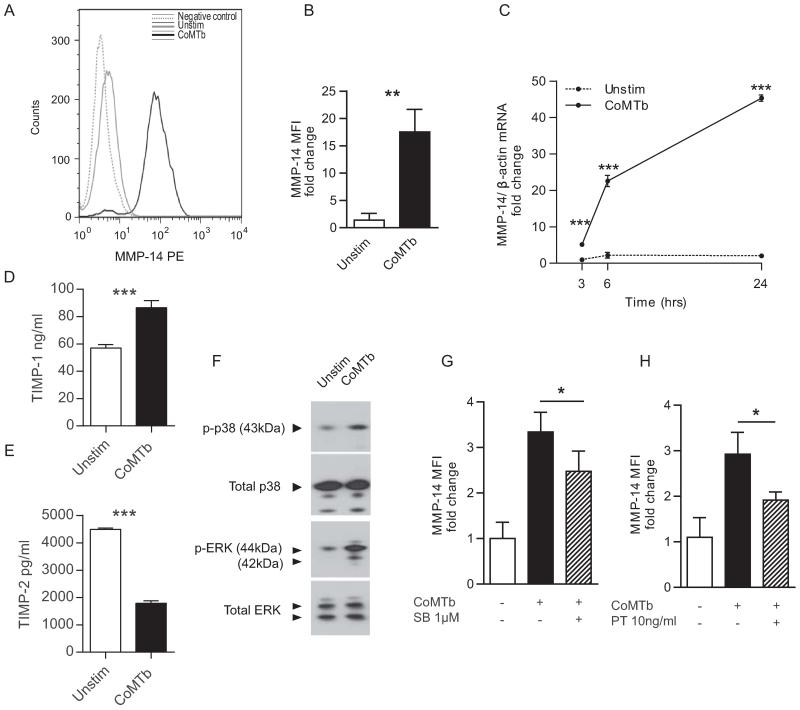
Next, biopsies from patients with active TB and normal lung tissue were immunostained for MT1-MMP. MT1-MMP immunoreactivity was expressed in TB granulomas, including in Langhans multinucleate giant cells and in surrounding epithelioid macrophages (Fig. 4A). In lung tissue of control subjects, MT1-MMP immunoreactivity was only detected in alveolar macrophages. Since MT1-MMP was diffusely expressed in the granuloma, but mycobacteria are relatively sparse within granulomas (26), we investigated whether Mtb-induced intercellular networks increased MT1-MMP expression. We stimulated cells with conditioned media from Mtb-infected monocytes (CoMTb) to model these networks. CoMTb upregulated surface MT1-MMP expression in unpermeabalized and permeabilized monocytes at 24 hours incubation compared to unstimulated cells (Fig. 4B).
Figure 4. MT1-MMP in expressed in TB granulomas from patients and is driven by monocyte-monocyte networks.
A. Lung biopsies from 5 patients with culture proven Mtb infection and control tissue from uninvolved lung parenchyma of patients with lung cancer were immunostained for MT1-MMP. In control biopsies, MT1-MMP immunostaining is present only in alveolar macrophages (i and ii). In TB, MT1-MMP immunoreactivity is present throughout the granuloma, expressed by both giant cells and epithelioid macrophages (iii and iv). White arrows indicate Langerhans multinucleate giant cells, surrounded by epithelioid macrophages. B. Monocytes were stimulated with Conditioned Medium from Mtb infected monocytes (CoMTb) to model intercellular networks. MT1-MMP expression was measured in unpermeabalised and permeabalised monocytes by immunofluorescent staining and microscopy. Blue: DAPI nuclear stain, Magenta: MT1-MMP. CoMTb stimulation increased both surface and intracellular MT1-MMP expression at 24h. Images are representative of 3 independent experiments.
Mtb induced MT1-MMP expression is regulated by p38 mitogen activated protein kinase (MAPK) and chemokine signalling
Flow cytometry analysis demonstrated an increase in MT1-MMP median fluorescence intensity in CoMTb stimulated monocytes compared to unstimulated cells analyzed at 24 hours (Fig. 5A), with a 17.5 fold increase in MT1-MMP surface expression with CoMTb (Fig. 5B, p<0.05). CoMTb stimulation drove a 5.2-fold increase in MT1-MMP gene expression at 3 hours rising to 22.6-fold at 6 hours and 45.4-fold at 24 hours (Fig. 5C). CoMTb may contain and drive the production of inhibitors, so we analyzed TIMP-1 and -2 secretion from monocytes stimulated with CoMTb at 24 hours. TIMP-1 concentrations were upregulated by CoMtb stimulation (Fig. 5D), but TIMP-2, the specific MT1-MMP inhibitor (27), was suppressed (Fig. 5E). MT1-MMP expression may be regulated by the MAPK pathways in response to other stimuli (28). CoMTb stimulation of monocytes caused a 3-fold increase in p38 phosphorylation and a 2.7-fold increase in ERK phosphorylation compared to unstimulated cells at 30 minutes incubation analyzed by western blotting (Fig. 5F). Specific inhibition of the p38 MAPK signalling pathway by SB203580 resulted in reduced CoMTb-driven surface MT1-MMP expression on monocytes at 24 hours (Fig. 5G, p<0.05). In contrast, inhibition of the ERK MAPK pathway with PD98059 10μM had no effect (data not shown).
Figure 5. Mtb-driven monocyte networks upregulate MT1-MMP expression, regulated by p38 mitogen activated protein kinase and GPCR signalling.
A. Human monocytes were stimulated with CoMTb and MT1-MMP expression was analysed at 24h by flow cytometry. CoMTb upregulated MT1-MMP surface expression. B. Analysis of fluorescence from three donors confirms increased monocyte surface MT1-MMP. C. CoMTb causes a progressive increase in MT1-MMP mRNA relative to unstimulated monocytes. D. Monocytes were stimulated with CoMTb and TIMP secretion was analyzed at 24h. CoMTb increased TIMP-1 secretion. E. Monocyte secretion of the specific MMP-14 inhibitor TIMP-2 is suppressed in CoMTb. F. CoMTb stimulation increased p38 and ERK MAPK phosphorylation in monocytes at 30 minutes compared to unstimulated monocytes. Total p38 and ERK were unchanged. G, H. Monocytes were pre-incubated with the p38 MAPK inhibitor SB203580 (SB) or Pertussis Toxin (PT) for 1h prior to stimulation with CoMTb. MT1-MMP surface expression was measured by flow cytometry. Inhibition of p38 MAPK pathway (E) and G-protein coupled receptor signalling (F) downregulated CoMTb driven MT1-MMP expression at 24h. The mean values +/− SD are shown of experiments performed in triplicate on at least 2 occasions.*p<0.05, **p<0.01, ***p<0.001 by Student’s t-test.
Since CC chemokines are critical in monocyte migration to sites of infection, including TB (29), we measured chemokines in CoMTb from 4 different donors by luminex array (Supplementary table 1). Multiple chemokine concentrations including CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CXCL8 (IL-8) and CXCL9 (MIG) were increased. Therefore, we chemically inhibited all chemokine signalling via G-protein coupled receptors with pertussis toxin to investigate if chemokines in CoMTb were driving MT1-MMP expression. Pertussis caused a 35% reduction in CoMTb driven MT1-MMP expression on unpermeabilized monocytes at 24 hours (Fig 5H, p<0.05). Propidium iodide staining showed that pertussis did not increase cell death (data not shown). We stimulated monocytes with CCL-2 (MCP-1), which has previously been shown to upregulate MT1-MMP in endothelial cells (30), but CCL-2 did not increase monocyte MT1-MMP surface expression as a single stimulus.
MT1-MMP is critical to monocyte migration
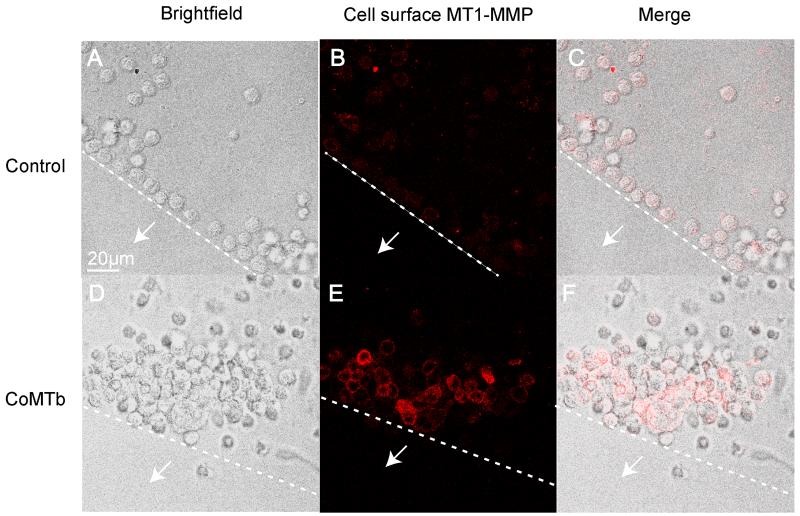
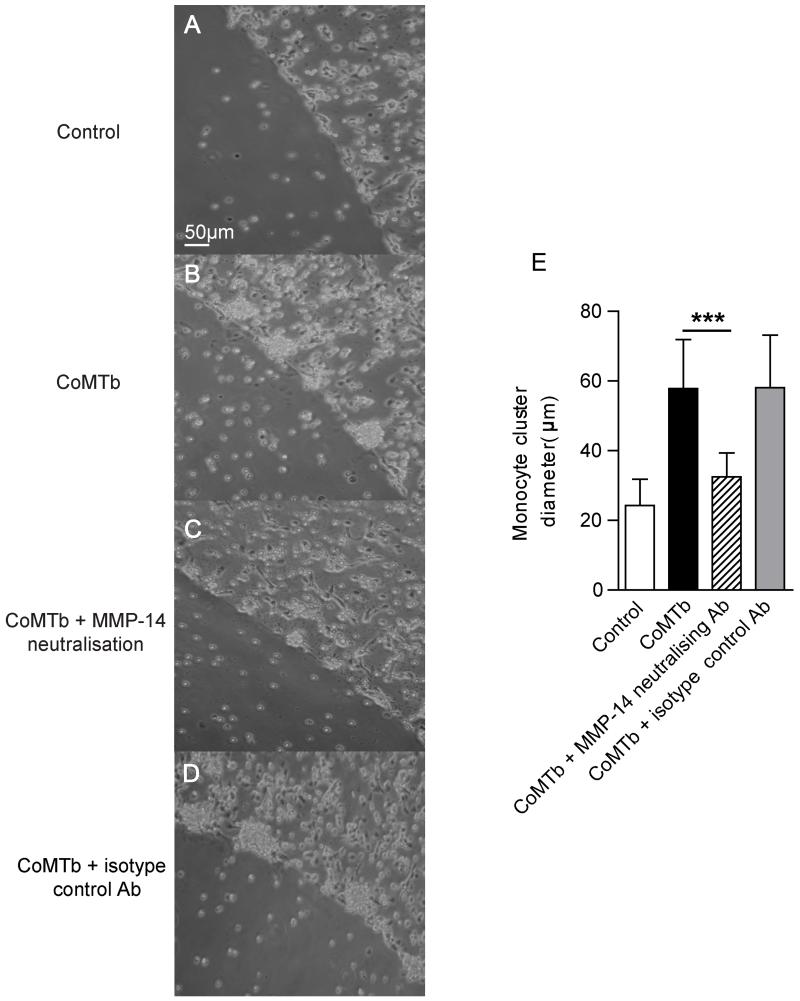
Finally, we specifically investigated the role of MT1-MMP in monocyte migration using a modified agarose drop migration assay (22). Monocytes migrated towards CoMTb-impregnated agarose beads, forming clusters around the edge at 24 hours that were not observed in control beads (Fig. 6A and D). The migrating monocytes expressed increased levels of membrane-associated MT1-MMP than cells around the control bead (Fig. 6B and E). To investigate the functional relevance, we specifically inhibited MT1-MMP activity with a neutralising antibody. MT1-MMP inhibition reduced the size of monocyte clusters around beads compared to untreated cells (Fig. 7B and C). Isotype control antibody did not affect cell migration (Fig. 7D). Migration was quantified using 2 beads per condition, with monocyte migration measured at 4 radial points per sphere. The diameter of the monocyte clusters for CoMTb was twice that for the control bead (p< 0.005), and inhibition of MT1-MMP activity resulted in a 44% reduction in the diameter of the monocyte clusters (Fig. 7E, p< 0.005), while the matched isotype control antibody had no effect on monocyte migration toward CoMTb. Therefore, MT1-MMP is required for monocyte migration in this cellular model of TB.
Figure 6. Mtb-induced intercellular networks stimulate monocyte migration.
Agarose beads impregnated with RPMI (Control) or CoMTb were set on slides coated with Type I collagen. Monocytes were seeded on these slides. White arrows indicate the direction towards the bead centre and dashed lines indicate the edge of the bead. MT1-MMP expression was measured in monocytes by immunofluorescent staining and microscopy. Magenta: MT1-MMP. A, D. Light microscopy images show monocytes migrate towards the CoMTb bead at 24h, forming clusters not observed for the control bead. B, E. Surface MT1-MMP expression on monocytes migrating towards the CoMTb bead is greater than for the monocytes around the control bead. C, F. Merged confocal and fluorescence images. Images shown are representative of 3 independent experiments.
Figure 7. MT1-MMP is necessary for Mtb-driven monocyte migration.
A-B. Light microscopy images show migrating monocytes forming clusters around the CoMTb drop at 24h. C. Neutralisation of MT1-MMP activity with an inhibitory anti-MT1-MMP antibody reduces monocyte migration. D. An isotype control antibody did not affect cellular migration. E. The maximum diameter of the monocyte clusters were analyzed, with images taken at four radial points per bead. Quantification confirms reduced monocyte migration with MT1-MMP neutralisation. Data are mean +/− SD from a single experiment and are representative of 2 independent experiments. ***p<0.001 by one-way ANOVA with Tukey’s post hoc test.
DISCUSSION
We demonstrated in TB patients and in a human cell culture model that MT1-MMP expression in TB regulates both collagen destruction and cellular migration. MT1-MMP mRNA levels are increased in sputum of patients with active pulmonary TB and MT1-MMP is expressed within granulomas of TB patients. Mtb infection and monocyte-dependent networks upregulate primary human monocyte expression of MT1-MMP and cause degradation of type I collagen, the main structural protein of the lung. In addition, monocyte migration in TB is dependent on MT1-MMP, as shown by a novel agarose bead assay. MT1-MMP may play a pivotal role in the immunopathology of human TB by both degrading collagen and regulating cellular migration.
MT1-MMP has previously been implicated in TB pathogenesis by unbiased approaches. Gene expression profiling demonstrates that MT1-MMP is upregulated in Mtb-infected human macrophages (31) and a microarray study of human lung TB granulomas excised at surgery showed MT1-MMP gene expression to be 28.6 fold greater than in normal lung (19). Similarly, whole blood gene expression profiling shows that MT1-MMP expression is higher in patients with active TB than healthy controls (32) and MT1-MMP gene expression is elevated in non-human primate lungs four weeks after TB infection (18). However, the functional significance of MT1-MMP activity has not previously been investigated. Multiple MMPs are emerging as important mediators in TB (11, 17, 20) and different MMPs may regulate specific aspects of immunopathology (33). The role of individual MMPs in TB pathology are likely to be both time and cell-dependent. For example, MMP-9 derived from epithelial cells regulates cellular recruitment to the granuloma by inducing migratory gradients (11) while macrophage-derived MMP-1 is one effector of collagen destruction (17). MT1-MMP neutralization inhibited collagen destruction, suggesting that MT1-MMP is either the dominant collagenase in the immediate pericellular environment early in infection, consistent with other reports (12, 16, 34), or alternatively is activating the pro-form of MMP-1 secreted by monocytes by enzymatic cleavage (27). MT1-MMP may regulate both cell migration and collagen destruction, and these processes may be co-dependent since collagen cleavage may be necessary for cells to migrate through the extracellular matrix.
We demonstrated that MT1-MMP co-localized with collagen destruction and that MT1-MMP neutralisation could abrogate this proteolysis, implying a role in matrix destruction. Consistent with this, MT1-MMP causes matrix breakdown in other destructive pulmonary pathologies. MT1-MMP expression is upregulated in alveolar macrophages of smokers (35) and IL-13 driven emphysema in transgenic mouse models is associated with MT1-MMP upregulation (36). MT1-MMP is expressed in asthma and bronchiectasis, diseases that are characterised by pulmonary matrix remodelling (37). Other infectious conditions can upregulate MT1-MMP. For example in hepatitis B-induced hepatocellular carcinoma, intrahepatic metastasis is driven by HBV protein upregulating MT1-MMP (38). However, the role of MT1-MMP in bacterial infection has not been extensively studied.
We found that MT1-MMP was widely expressed in human TB granulomas, whereas bacilli are very sparse (26), leading us to investigate intercellular networks. Monocyte-monocyte signalling upregulated MT1-MMP expression and this was dependent on p38 MAPK and GPCR signalling. Whilst TIMP-1 was up-regulated by Mtb infection, this does not inhibit MT1-MMP activity (27), whereas the specific inhibitor TIMP-2 was suppressed by monocyte intercellular networks, further skewing the local environment to matrix destruction. Consistent with our findings, p38 MAPK regulates MT1-MMP expression in fibronectin stimulated macrophages (39). Studies in malignant cells have identified a role for ERK in driving constitutive MT1-MMP expression (40, 41), but we did not demonstrate ERK regulation of MT1-MMP in primary monocytes, indicating stimulus and cell-specific regulation. We demonstrated that secretion of multiple chemokines is up-regulated in Mtb-infected monocytes. Chemokines are critical to leukocyte migration to TB granulomas (42), but no single chemokine appears to be dominant due to redundancy within the signalling system. To perform global chemokine inhibition, we studied the effect of pertussis toxin, which inhibits GPCR signalling, and demonstrated that this suppressed CoMTb-driven MT1-MMP upregulation, suggesting a role for cytokines in upregulating MT1-MMP. Consistent with this, CCL-2 stimulation of monocytes transmigrating through endothelium causes MT1-MMP clustering at motility associated protrusions (43), and CCL2 and CXCL8 promote MT1-MMP surface expression, clustering and activity in endothelial cells (30). However, in our system, CCL-2 as a single stimulus did not upregulate total surface expression of MT1-MMP, suggesting that multiple factors may synergise to drive MT1-MMP. These may include either multiple host cytokines (44) or both host and pathogen secreted mediators as described for MMP-9 (11, 45).
While the granuloma has traditionally been viewed as a host strategy to control mycobacterial growth, accumulating evidence suggests that monocyte migration may have deleterious effects to the host. In the M marinum zebrafish model, macrophages migrating into granulomas permit bacterial expansion and can disseminate infection (46). In mice, intranasal administration of polyinosinic-polycytidylic acid, a type I interferon inducer, caused a CCR2-dependent excessive accumulation of monocytes in the lungs of Mtb infected mice, leading to increased bacterial burden and decreased survival (47). However, while the chemotactic gradients driving the cellular recruitment have been dissected, the proteases driving leukocyte migration have not been identified (48). We demonstrate utilising the agarose drop assay that MT1-MMP surface expression is driven by Mtb-induced intercellular networks and monocyte migration is MT1-MMP dependent. Modulating MT1-MMP activity in TB may have diverse effects, for example by affecting intracellular signalling pathways regulated by MT1-MMP such as ERK and PI3K (49, 50). Therefore, investigating the global effect of MT1-MMP inhibition will require careful analysis in appropriate animal model systems.
MT1-MMP is critical to cellular migration in other pathologies (14, 16, 51) and in the agarose bead assay, inhibition suppressed cell migration, suggesting that it is the key collagenase in this process. MT1-MMP may be driving leukocyte migration by multiple mechanisms. First, MT1-MMP cleavages extracellular matrix at the leading edge of the cell, and also modification of cell surface proteins to promote the organised cell adhesion-de-adhesion required for migration (52, 53). Secondly, MT1-MMP dependent cleavage of other cell surface proteins which interact with ECM components, such as CD44, integrins and transglutaminase, are also considered important to migration of malignant cells (54-56). For example, MT1-MMP cleaves CD44 to produce a soluble fragment. Expression of MT1-MMP or CD44 in a breast cancer cell line alone did not stimulate cell migration, but co-expression did. Co-expression of a CD44 deletion mutant, which cannot be cleaved by MT1-MMP, resulted in loss of cell migration (56). Thirdly, it has been postulated that MT1-MMP promotes cell migration by driving ERK MAPK phosphorylation. In a human fibrosarcoma cell line, TIMP-2 binding to MT1-MMP drove ERK signalling, which increased cell migration independently of the direct enzymatic activity of MT1-MMP (57).
In summary, we have demonstrated that MT1-MMP expression is increased in pulmonary TB and is functionally active, contributing to local tissue destruction and leukocyte recruitment to the granuloma. Therefore, MT1-MMP may represent a previously unrecognised regulator of these central immunopathological processes in human TB. Host-targeted therapies are emerging as a novel therapeutic paradigm in TB (58, 59), and the potential effect of such interventions on MMP-dependent cell migration and matrix destruction deserves evaluation.
Supplementary Material
Acknowledgements
We are grateful to Rene Goliath, Ronnett Seldon and all the staff and patients at the Ubuntu HIV/TB clinic in Cape Town, for their assistance with this study.
TS was supported by an MRC Clinical Research Training Fellowship (G0900429) and a Scadding Morriston Travel Award. JSF is grateful for support from the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) funding scheme at Imperial College. PE acknowledges support from the NIH R33AI102239. RJW receives support from the Wellcome Trust (084323, 088316), and Medical Research Council (U1175.02.002.00014.01).
REFERENCES
- 1.WHO . Global tuberculosis report 2014. 2014. [Google Scholar]
- 2.Cooper AM, Torrado E. Protection versus pathology in tuberculosis: recent insights. Curr Opin Immunol. 2012;24:431–437. doi: 10.1016/j.coi.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kline SE, Hedemark LL, Davies SF. Outbreak of tuberculosis among regular patrons of a neighborhood bar. N Engl J Med. 1995;333:222–227. doi: 10.1056/NEJM199507273330404. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigo T, Cayla JA, Garcia de Olalla P, Galdos-Tanguis H, Jansa JM, Miranda P, Brugal T. Characteristics of tuberculosis patients who generate secondary cases. Int J Tuberc Lung Dis. 1997;1:352–357. [PubMed] [Google Scholar]
- 5.Kempker RR, Rabin AS, Nikolaishvili K, Kalandadze I, Gogishvili S, Blumberg HM, Vashakidze S. Additional drug resistance in Mycobacterium tuberculosis isolates from resected cavities among patients with multidrug-resistant or extensively drug-resistant pulmonary tuberculosis. Clin Infect Dis. 2012;54:e51–54. doi: 10.1093/cid/cir904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, Mathema B, Ramaswamy SV, Walther G, Steyn LM, Barry CE, 3rd, Bekker LG. Mycobacterium tuberculosis Growth at the Cavity Surface: a Microenvironment with Failed Immunity. Infect Immun. 2003;71:7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon DS, Kim DH, Kang HS, Hwang SH, Min JH, Kim JH, Sung NM, Carroll MW, Park SK. Survival and predictors of outcomes in non-HIV-infected patients with extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2009;13:594–600. [PubMed] [Google Scholar]
- 8.Erdogan A, Yegin A, Gurses G, Demircan A. Surgical management of tuberculosis-related hemoptysis. The Annals of thoracic surgery. 2005;79:299–302. doi: 10.1016/j.athoracsur.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Rhee CK, Yoo KH, Lee JH, Park MJ, Kim WJ, Park YB, Hwang YI, Kim YS, Jung JY, Moon JY, Rhee YK, Park HK, Lim JH, Park HY, Lee SW, Kim YH, Lee SH, Yoon HK, Kim JW, Kim JS, Kim YK, Oh YM, Lee SD, Kim HJ. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis. 2013;17:67–75. doi: 10.5588/ijtld.12.0351. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol. 2007;213:355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- 11.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 13.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 14.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, Roberts JD, Fay WP, Birkedal-Hansen H, Holmbeck K, Sabeh F, Allen ED, Weiss SJ. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202:663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, Robertson BD, D’Armiento J, Friedland JS. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehra S, Pahar B, Dutta NK, Conerly CN, Philippi-Falkenstein K, Alvarez X, Kaushal D. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS One. 2010;5:e12266. doi: 10.1371/journal.pone.0012266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, Tsenova L, Kaplan G, Russell DG. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2:258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker NF, Clark SO, Oni T, Andreu N, Tezera L, Singh S, Saraiva L, Pedersen B, Kelly DL, Tree JA, D’Armiento JM, Meintjes G, Mauri FA, Williams A, Wilkinson RJ, Friedland JS, Elkington PT. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185:989–997. doi: 10.1164/rccm.201110-1769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkington PT, Green JA, Friedland JS. Filter sterilization of highly infectious samples to prevent false negative analysis of matrix metalloproteinase activity. J Immunol Methods. 2006;309:115–119. doi: 10.1016/j.jim.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Wiggins H, Rappoport J. An agarose spot assay for chemotactic invasion. Biotechniques. 2010;48:121–124. doi: 10.2144/000113353. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari S, Askari JA, Humphries MJ, Bulleid NJ. Divalent cations regulate the folding and activation status of integrins during their intracellular trafficking. J Cell Sci. 2011;124:1672–1680. doi: 10.1242/jcs.084483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garnotel R, Rittie L, Poitevin S, Monboisse JC, Nguyen P, Potron G, Maquart FX, Randoux A, Gillery P. Human blood monocytes interact with type I collagen through alpha × beta 2 integrin (CD11c-CD18, gp150-95) J Immunol. 2000;164:5928–5934. doi: 10.4049/jimmunol.164.11.5928. [DOI] [PubMed] [Google Scholar]
- 25.Mavhu W, Dauya E, Bandason T, Munyati S, Cowan FM, Hart G, Corbett EL, Chikovore J. Chronic cough and its association with TB-HIV co-infection: factors affecting help-seeking behaviour in Harare, Zimbabwe. Trop Med Int Health. 2010;15:574–579. doi: 10.1111/j.1365-3156.2010.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DY, Kim JY, Choi KU, Lee JS, Lee CH, Sol MY, Suh KS. Comparison of polymerase chain reaction with histopathologic features for diagnosis of tuberculosis in formalin-fixed, paraffin-embedded histologic specimens. Arch Pathol Lab Med. 2003;127:326–330. doi: 10.5858/2003-127-0326-COPCRW. [DOI] [PubMed] [Google Scholar]
- 27.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- 28.Munshi HG, Wu YI, Mukhopadhyay S, Ottaviano AJ, Sassano A, Koblinski JE, Platanias LC, Stack MS. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-beta1-induced pericellular collagenolysis. J Biol Chem. 2004;279:39042–39050. doi: 10.1074/jbc.M404958200. [DOI] [PubMed] [Google Scholar]
- 29.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galvez BG, Genis L, Matias-Roman S, Oblander SA, Tryggvason K, Apte SS, Arroyo AG. Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/ccl2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. J Biol Chem. 2005;280:1292–1298. doi: 10.1074/jbc.M408673200. [DOI] [PubMed] [Google Scholar]
- 31.Volpe E, Cappelli G, Grassi M, Martino A, Serafino A, Colizzi V, Sanarico N, Mariani F. Gene expression profiling of human macrophages at late time of infection with Mycobacterium tuberculosis. Immunology. 2006;118:449–460. doi: 10.1111/j.1365-2567.2006.02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maertzdorf J, Weiner J, 3rd, Mollenkopf HJ, Bauer T, Prasse A, Muller-Quernheim J, Kaufmann SH. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A. 2012;109:7853–7858. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190:9–18. doi: 10.1164/rccm.201311-2106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H, Overall CM, McCulloch CA, Sodek J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol Biol Cell. 2006;17:4812–4826. doi: 10.1091/mbc.E06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1(+) smokers with early emphysema. J Leukoc Biol. 2009;86:913–922. doi: 10.1189/jlb.0408240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Jr., Chapman HA, Jr., Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maisi P, Prikk K, Sepper R, Pirila E, Salo T, Hietanen J, Sorsa T. Soluble membrane-type 1 matrix metalloproteinase (MT1-MMP) and gelatinase A (MMP-2) in induced sputum and bronchoalveolar lavage fluid of human bronchial asthma and bronchiectasis. APMIS. 2002;110:771–782. doi: 10.1034/j.1600-0463.2002.1101102.x. [DOI] [PubMed] [Google Scholar]
- 38.Lara-Pezzi E, Gomez-Gaviro MV, Galvez BG, Mira E, Iniguez MA, Fresno M, Martinez AC, Arroyo AG, Lopez-Cabrera M. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J Clin Invest. 2002;110:1831–1838. doi: 10.1172/JCI200215887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duarte S, Shen XD, Fondevila C, Busuttil RW, Coito AJ. Fibronectin-alpha4beta1 interactions in hepatic cold ischemia and reperfusion injury: regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway. Am J Transplant. 2012;12:2689–2699. doi: 10.1111/j.1600-6143.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takino T, Miyamori H, Watanabe Y, Yoshioka K, Seiki M, Sato H. Membrane type 1 matrix metalloproteinase regulates collagen-dependent mitogen-activated protein/extracellular signal-related kinase activation and cell migration. Cancer Res. 2004;64:1044–1049. doi: 10.1158/0008-5472.can-03-1843. [DOI] [PubMed] [Google Scholar]
- 41.Tanimura S, Asato K, Fujishiro SH, Kohno M. Specific blockade of the ERK pathway inhibits the invasiveness of tumor cells: down-regulation of matrix metalloproteinase-3/-9/-14 and CD44. Biochem Biophys Res Commun. 2003;304:801–806. doi: 10.1016/s0006-291x(03)00670-3. [DOI] [PubMed] [Google Scholar]
- 42.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matias-Roman S, Galvez BG, Genis L, Yanez-Mo M, de la Rosa G, Sanchez-Mateos P, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is involved in migration of human monocytes and is regulated through their interaction with fibronectin or endothelium. Blood. 2005;105:3956–3964. doi: 10.1182/blood-2004-06-2382. [DOI] [PubMed] [Google Scholar]
- 44.Richardson VJ. Divergent and synergistic regulation of matrix metalloprotease production by cytokines in combination with C-C chemokines. International journal of immunopathology and pharmacology. 2010;23:715–726. doi: 10.1177/039463201002300305. [DOI] [PubMed] [Google Scholar]
- 45.Elkington PT, Green JA, Emerson JE, Lopez-Pascua LD, Boyle JJ, O’Kane CM, Friedland JS. Synergistic up-regulation of epithelial cell matrix metalloproteinase-9 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2007;37:431–437. doi: 10.1165/rcmb.2007-0011OC. [DOI] [PubMed] [Google Scholar]
- 46.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 49.Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre MP, Annabi B, Beliveau R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP) FEBS Lett. 2001;507:231–236. doi: 10.1016/s0014-5793(01)02985-4. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu-Hirota R, Xiong W, Baxter BT, Kunkel SL, Maillard I, Chen XW, Sabeh F, Liu R, Li XY, Weiss SJ. MT1-MMP regulates the PI3Kdelta.Mi-2/NuRD-dependent control of macrophage immune function. Genes Dev. 2012;26:395–413. doi: 10.1101/gad.178749.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Seminars in cell & developmental biology. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itoh Y. MT1-MMP: a key regulator of cell migration in tissue. IUBMB Life. 2006;58:589–596. doi: 10.1080/15216540600962818. [DOI] [PubMed] [Google Scholar]
- 54.Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- 55.Deryugina EI, Ratnikov BI, Postnova TI, Rozanov DV, Strongin AY. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J Biol Chem. 2002;277:9749–9756. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- 56.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sounni NE, Rozanov DV, Remacle AG, Golubkov VS, Noel A, Strongin AY. Timp-2 binding with cellular MT1-MMP stimulates invasion-promoting MEK/ERK signaling in cancer cells. Int J Cancer. 2010;126:1067–1078. doi: 10.1002/ijc.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawn TR, Matheson AI, Maley SN, Vandal O. Host-directed therapeutics for tuberculosis: can we harness the host? Microbiology and molecular biology reviews: MMBR. 2013;77:608–627. doi: 10.1128/MMBR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE, 3rd, Sher A. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.