Abstract
BACKGROUND
A growing body of evidence suggests that many downstream pathologies of obesity are amplified or even initiated by molecular changes within white adipose tissue (WAT). Such changes are the result of an excessive expansion of individual white adipocytes and could potentially be ameliorated via an increase in de novo adipocyte recruitment (adipogenesis). Mesoderm specific transcript (MEST) is a protein with a putative yet unidentified enzymatic function and has previously been shown to correlate with adiposity and adipocyte size in mouse.
OBJECTIVES
This study analysed WAT samples and employed a cell model of adipogenesis to characterise MEST expression and function in human.
METHODS AND RESULTS
MEST mRNA and protein levels increased during adipocyte differentiation of human Multipotent Adipose-Derived Stem (hMADS) cells. Further, obese individuals displayed significantly higher MEST levels in WAT compared to normal weight subjects, and MEST was significantly correlated with adipocyte volume. In striking contrast to previous mouse studies, knockdown of MEST enhanced human adipocyte differentiation, most likely via a significant promotion of peroxisome proliferator-activated receptor (PPAR) signaling, glycolysis and fatty acid biosynthesis pathways at early stages. Correspondingly, overexpression of MEST impaired adipogenesis. We further found that silencing of MEST fully substitutes for the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) as inducer of adipogenesis. Accordingly, phosphorylation of the pro-adipogenic transcription factors cAMP response element-binding protein (CREB) and activating transcription factor 1 (ATF1) were highly increased upon MEST knockdown.
CONCLUSIONS
While we found a similar association between MEST and adiposity as previously described for mouse, our functional analyses suggest that MEST acts as an inhibitor of human adipogenesis, contrary to previous murine studies. We have further established a novel link between MEST and CREB/ATF1 that could be of general relevance in regulation of metabolism, particularly obesity-associated diseases.
INTRODUCTION
White adipose tissue (WAT) is the quantitatively most important energy storage in the vast majority of animal species.1 The proper function of its parenchymal cells - the white adipocytes - is a critical determinant for metabolic health. Indeed, many follow-up complications of obesity, most importantly insulin resistance and associated type 2 diabetes, have been shown to be amplified or even caused by white adipocyte dysfunction. Such detrimental changes can be related to the adipocytes’ exhausted capacity for triglyceride storage (resulting in lipotoxicity) and also comprise a pro-inflammatory shift in the WAT secretory profile.2,3 It has further been demonstrated that adipocyte necrosis is highly increased in the obese state4. On the other hand, increasing the number of white adipocytes by pharmacological treatments, e.g. via thiazolidinediones, results in marked amelioration of obesity-associated insulin resistance.5,6
Physiologically, the differentiation of WAT-residing precursor cells (stem cells, preadipocytes) into mature adipocytes is triggered (or restrained) by a large repertoire of hormones and nutritionally derived substances which act on a large array of pro- and anti-adipogenic genes.7 The plethora of molecules generated by enzymes that are involved in lipid and carbohydrate metabolism constitutes a critical regulatory layer in adipogenesis, as for example arachidonic acid, which is implicated in the generation of endogenous ligands for the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ),8 an indispensable factor for adipocyte development,9 or steroids like cortisol10 which acts as a ligand of the glucocorticoid receptor.
Despite the already detailed knowledge about transcriptional networks in adipogenesis11, there are still several genes that are known to be expressed in the adipocyte lineage, that are predicted (based on amino acid sequence and/or secondary structure) to have enzymatic activity, but for which a particular enzymatic reaction has not been identified. One such protein is mesoderm specific transcript (MEST, PEG1), harbouring an α/β hydrolase fold that is present on a large number of enzymes including lipases, esterases, amidases, epoxide hydrolases, dehalogenases, and hydroxynitrile lyases.12 Mest was originally identified as highly abundant in the mesoderm and its derivatives13, and was subsequently found to be an imprinted gene that is expressed solely from the paternal allele in mouse14 and human15, at least in early developmental stages. When comparing gene expression patterns between wild-type and genetically obese (leptin deficient) mice, Soukas et al. for the first time revealed a strong upregulation of Mest in periuterine WAT in the obese state.16 This finding was corroborated and extended by several other studies, implying a potential relevance for Mest in murine WAT physiology. First, Mest was also shown to be increased in parametrial WAT of wild-type mice exposed to a 10-week high-fat diet (HFD).17 Second, Koza et al. profiled inguinal WAT of C57BL/6J mice on a HFD. Despite the animal’s identical genetic background, this study found a substantial variation in body weight gain between individual mice, with Mest being the most dynamically regulated transcript between the groups with highest and lowest weight gains.18 Third, the Mest induction in WAT was found to be promoted by diets high in unsaturated as well as saturated fats, and this induction was prevented when HFD-fed mice were simultaneously exposed to cold stress (4°C, preventing HFD-induced body weight gain).19 Altogether, the levels of Mest in various mouse WAT depots appear to be closely linked to the accumulated fat mass (i.e., adiposity). In line with this observation, transgenic mice overexpressing Mest in the adipogenic lineage display an enlargement of adipocyte size,17 while global Mest knockout (KO) mice exhibit reduced adiposity.19 These effects are likely cell-autonomous, as Mest overexpression promoted, while Mest knockdown impaired adipocyte differentiation of 3T3-L1 preadipocytes.17,20
In the present study, we have aimed to analyse, for the first time, possible functions of MEST in human adipocyte development. As previously described for mouse,17-19,21 MEST expression was upregulated during human adipocyte differentiation, increased in human WAT in the obese state, and significantly correlated with adipocyte volume. However, knockdown of MEST during human adipocyte differentiation evoked an unexpected increase in lipid accumulation and adipocyte marker gene expression, and microarray analysis revealed a significant promotion of PPAR signaling and glycolysis pathways. In line with these results, overexpression of MEST reduced human adipocyte differentiation. Interestingly, knockdown of MEST could fully substitute the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) as inducer of adipogenesis and correspondingly promoted the phosphorylation of cAMP responsive element binding protein 1 (CREB) and activating transcription factor 1 (ATF1), revealing a previously unknown inhibitory function for MEST in the control of these transcriptional regulators.
MATERIAL AND METHODS
Cell culture experiments
Human Multipotent Adipose-Derived Stem (hMADS) cells were established from a 2.6-year-old female donor (umbilical adipose tissue; hMADS-1 cells) and a 4-month-old male donor (prepubic adipose tissue; hMADS-3 cells), respectively.22 hMADS cells were used for experiments between passages 15 and 30. Proliferation medium consisted of Dulbecco’s Modified Eagle’s Medium (DMEM; Lonza, Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS; Lonza), 10 mM HEPES (Life Technologies, Karlsruhe, Germany), 2 mM L-Glutamine (Life Technologies), 100 μg/ml Normocin (InvivoGen, Toulouse, France), and 2.5 ng/ml recombinant human fibroblast growth factor 2 (hFGF2; Sigma, St. Louis, MO, USA). For experiments, cells were seeded and grown to confluence (designated d-2), when the medium was changed to proliferation medium without hFGF2. Adipocyte differentiation was induced two days later (d0) by a chemically defined medium. Unless indicated otherwise, this medium consisted of DMEM/Ham’s F12 (1:1, Lonza), 5 mM HEPES, 2 mM L-Glutamine, 100 μg/ml Normocin, and the pro-adipogenic components Insulin (10 nM), apo-transferrin (10 μg/ml), triiodothyronine (0.2 nM; all Sigma), rosiglitazone (100 nM; Cayman Chemical Technology, Ann Arbor, MI, USA), and for the first 3 days 3-isobutyl-1-methylxantine (IBMX; 100 μM; Sigma) and dexamethasone (Dex; 1 μM; Sigma). Medium was replaced every 2-3 days. Transfection of cells with siRNAs was performed at d-2 using HiPerFect transfection reagent (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions with a final siRNA concentration of 10 nM. Two distinct siRNAs (#1-sense: 5′-UGACUAAGGUUGACAUAAUUU-3′, #1-antisense: 5′-AUUAUGUCAACCUUAGUCAUU-3′; #2-sense: 5′-CAAAAGAGGUCCUGGCCAUUU-3′, #2-antisense: 5′-AUGGCCAGGACCUCUUUUGUU-3′) were designed using the Dharmacon/GE Healthcare siDESIGN center and applied as equimolar pool to silence MEST; both siRNAs targeted all 6 RefSeq mRNA transcript variants. For control transfections, miRIDIAN microRNA Mimic Negative Control #1 (GE Dharmacon, Lafayette, CO, USA, #CN-001000-01) was used. Medium containing transfection solution was replaced after 2 days (d0). MEST overexpression was performed with lentiviral particles manufactured by GeneCopoeia; the construct was based on the pReceiver-Lv105 vector containing the MEST coding sequence (NM_002402.2). A similar vector containing the eGFP coding sequence was used as control. Cells were transduced at d-2 using a multiplicity of infection of 10.
Analysis of lipid accumulation
Staining of intracellular lipids by Oil Red O was performed as described previously.23 To quantify intracellular triglyceride accumulation, cells were washed with PBS (4°C; Life Technologies) and scraped off the surface of 12-well plates using 300 μL PBS per well. Homogenisation was performed by ultrasonication (20 s) using a Sonopuls UW2070 (Bandelin, Berlin, Germany). 15 μL of sample, as well as a dilution series of a glycerol standard (2 mM to 62.5 μM; Roth, Karlsruhe, Germany) were pipetted into wells of a 96-well plate before addition of Infinity Triglycerides Reagent (200 μL/well; Fisher Scientific, Hampton, NH, USA). Subsequently, reactions were incubated at 37°C for 30 min and absorbance at 500 nm was recorded.
Preparation of adipocyte progenitors and mature adipocytes from human adipose tissue samples
The stromal vascular fraction (SVF) and mature adipocytes were prepared from human subcutaneous adipose tissue obtained by liposuctions or plastic surgery as described24. Patients with BMI < 30 were considered as non-obese and patients with BMI ≥30 were considered obese. FACS sorting of SVF was performed as described25. Progenitor cells were sorted as CD45-/CD31-/CD34+ cell population using the following antibodies: CD45 Pacific blue clone T29/33 (DakoCytomation, Glostrup); anti-CD34 clone 8G12 APC and anti-CD31 M89D3, FITC (both from BD biosciences, San Jose, CA). Purity of progenitor fraction was 97.7 ±1.68%. qRT-PCR was performed as described25.
RNA isolation and analysis
Total RNA was obtained using TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. To analyse the expression of particular genes of interest, cDNA synthesis of 0.5-1 μg total RNA was performed using the QuantiTect Reverse Transcription Kit (QIAGEN). Subsequently, quantitative real-time reverse transcription PCR (RT-qPCR) was performed as described previously.26 Primer sequences are provided in Supplementary Table S1. Raw data storage and further analyses were performed with the QPCR application27 using integrated AnalyzerMiner Cq and efficiency calculation algorithms.28 Global gene expression analysis of 15539 distinct RefSeq mRNAs was performed by two-color microarrays; metadata (experimental parameters and detailed procedures), raw data files and final (filtered and normalised) data are accessible via Gene Expression Omnibus (GSE63132).
Western blotting
Harvest of total cellular protein, electrophoresis and blotting were performed as described previously.23 Membranes were blocked for 1 h in tris-buffered saline supplemented with 0.05% Tween 20 (TBS-T) and either 5% non-fat milk (Sigma) or 5% BSA (GE Healthcare, Little Chalfont, UK). Subsequently, membranes were incubated overnight at 4°C with primary antibodies. Detailed information on used antibodies is provided in Supplementary Table S2. After washing (3 × TBS-T for 10 min), membranes were incubated with HRP-conjugated secondary antibody for 2 h at room temperature, followed by washing as described above. Signals were detected by chemiluminescence using ECL Plus (Thermo Scientific) and Lucent Blue X-ray films (advansta, Menlo Park, CA, USA). Stripping of blots was performed using Restore Plus Western Blot Stripping Buffer (Thermo Scientific) according to the manufacturer’s instructions, followed by re-blocking in TBS-T with 5% milk for 1 h. Detection of β-tubulin was performed as loading control. Densitometric analysis of bands was performed using ImageJ.
Glucose measurements
Glucose concentration in supernatants from cells was analysed using a BioProfile 100 Plus device (nova biomedical, Waltham, MA, USA) according to the manufacturer’s instructions.
Enzyme immunoassays
Cells were lysed by incubation in 0.1M HCl (275 μL per well of a 6-well plate) for 20 min, then scraped off the wells and homogenised by repeated pipetting. Lysates were centrifuged (10 min / 1000 × g), and supernatants were used for acetylation and subsequent measurement of intracellular cAMP and cGMP concentrations by EIA (Cayman Chemical Technology) according to the manufacturer’s instructions.
Statistical analysis
Data are presented as means ±SEM and were analysed by Student’s unpaired t-test, unless indicated otherwise. Differences were considered as statistically significant when P<0.05.
RESULTS
Endogenous expression of MEST in human adipocyte differentiation and adipose tissue
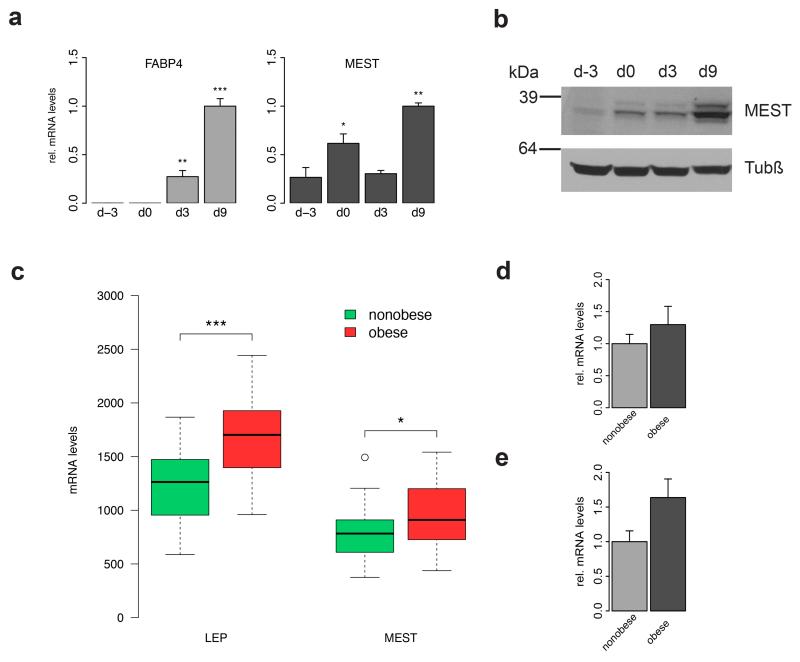
Exposure of two-day post-confluent hMADS cells to a chemically defined adipogenic medium resulted in a strong upregulation of the adipocyte marker fatty acid binding protein 4 (FABP4) within 9 days (Figure 1a, left panel). Similarly, MEST mRNA levels were high at day 9, but already showed a significant increase from day −3 to day 0, i.e. when cells were still cultivated in growth medium (Figure 1a, right panel). This increase was also evident when MEST protein levels were analysed by Western blot, revealing only low amounts of MEST before the confluent stage, but already robust levels at the start of adipocyte differentiation, and a pronounced further increase as differentiation proceeded until day 9 (Figure 1b). To assess whether our in vitro adipogenesis model is reflecting the in vivo situation, we obtained data from a publicly available study in which gene expression profiles of human abdominal scWAT from 30 obese and 26 non-obese females had been compared.29 As expected, mRNA levels of the adipocytokine leptin were significantly higher in obese versus non-obese subjects (Figure 1c). Further, we also observed a significant elevation of MEST expression in the obese state (Figure 1c), thereby confirming previous results from mouse. In line with these results, we also found increased MEST levels in sorted (CD45−/CD31−/CD34+) adipocyte progenitors (Figure 1d) as well as in the mature adipocyte fraction (Figure 1e) of human scWAT. As previous studies have described that MEST overexpression results in enlarged adipocytes, we analyzed the relation of MEST and adipocyte size in human scWAT samples. Indeed, we found in 56 women a significant correlation between MEST mRNA and adipocyte volume (ρ=0.311; p=0.02) measured as described30 (Supplementary Figures S1a and b).
Figure 1.
Endogenous expression of MEST in human adipocyte differentiation and adipose tissue. (a and b) hMADS cells were grown to confluence (d-2) and stimulated to undergo adipocyte differentiation two days later (designated d0). (a) RNA was prepared at indicated time points and subjected to RT-qPCR for FABP4 and MEST. Expression levels were normalized to TBP and are presented relative to d9 (n=3). *P<0.05, **P<0.01, ***P<0.001 versus undifferentiated cells at d-3. (b) Protein lysates were prepared at indicated time points and analysed by Western blot for MEST and β-Tubulin (Tubβ). (c) Abdominal scWAT biopsies from 26 nonobese and 30 obese female donors were analysed by microarray (Gene Expression Omnibus Experiment GSE25401). Expression levels of leptin (LEP) and MEST are shown as boxplots. *P<0.05, ***P<0.001. (d-e) Expression of MEST was analysed by RT-qPCR in (d) adipocyte progenitor cells (CD45−/CD31−/CD34+) from scWAT biopsies of 10 non-obese and 8 obese subjects, and (e) mature adipocytes from scWAT biopsies of 4 non-obese and 6 obese subjects. MEST expression levels were normalised to 18S rRNA.
MEST suppresses human adipocyte differentiation
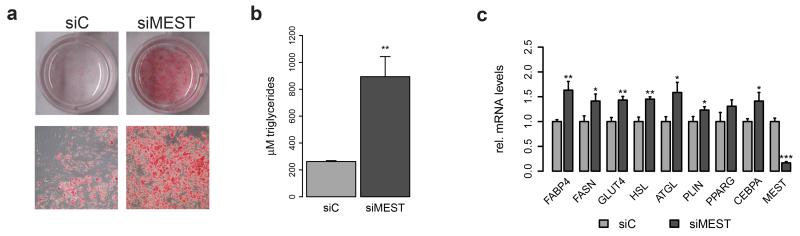
To analyse a possible function for MEST in human adipogenesis, we transfected hMADS cells with siRNAs to silence MEST (siMEST), and subsequently induced adipocyte differentiation. Interestingly, Oil Red O staining (Figure 2a) as well as triglyceride quantification (Figure 2b) revealed a markedly elevated intracellular lipid accumulation upon MEST depletion at day 9 compared to control (siC) transfected cells. RT-qPCR showed corresponding increases in the expression of FABP4, fatty acid synthase (FASN) and the glucose transporter GLUT4 (SLC2A4) (Figure 2c). Further, PPARγ and CCAAT/enhancer binding protein α (CEBPA), both key transcriptional regulators of the adipogenic differentiation program,31,32 were increased by MEST silencing, as were the mRNAs of the lipid droplet-coating protein perilipin (PLIN1), adipose triglyceride lipase (ATGL, PNPL2A) and hormone-sensitive lipase (HSL, LIPE) (Figure 2c). In line with these loss-of-function experiments, MEST overexpression resulted in diminished lipid accumulation and reduced expression of adipocyte markers (Supplementary Figure S2). As hMADS cells can differentiate into brown(-like) adipocytes33, we also analysed uncoupling protein 1 (UCP1); however, UCP1 mRNA levels were very low and not affected by changing the abundance of MEST (data not shown). Collectively, our results argue for a suppressive role of MEST in human adipogenesis.
Figure 2.
Silencing of MEST promotes human adipocyte differentiation. hMADS cells were transfected at confluence with siRNAs to target MEST (siMEST) or a control siRNA (siC) and adipocyte differentiation was induced two days later (d0). Cells were analysed at d9. (a) Triglyceride accumulation was visualized by Oil Red O staining. Upper panels: whole wells; lower panels: representative bright field microscopy images (40× magnification). (b) Quantification of intracellular triglycerides (n=4). (c) RT-qPCR analysis of adipocyte marker gene expression (normalized to TBP, relative to siC, n=3-6). (b and c) *P<0.05, **P<0.01, ***P<0.001 versus siC-transfected cells.
MEST silencing promotes PPAR signaling and glycolysis pathways
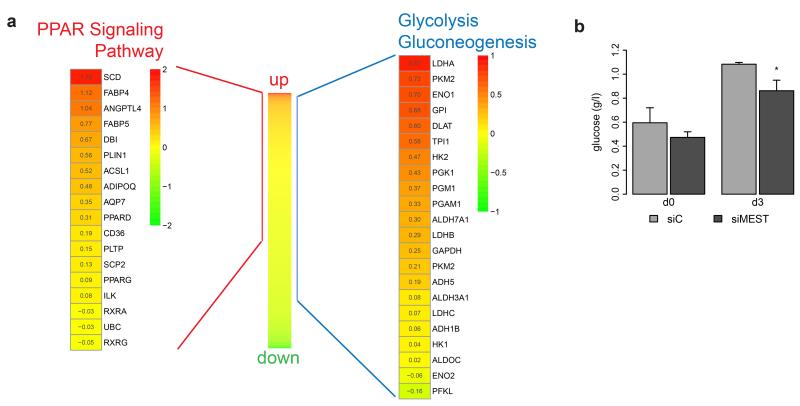
Previous studies in mouse 3T3-L1 preadipocytes have described pro-adipogenic effects of Mest.17,20 Having observed the opposite in human, we decided to investigate the global impact of MEST silencing at the early stage of adipogenesis, i.e. 3 days after induction of differentiation. Microarray analysis yielded a total of 2278 unique RefSeq mRNAs which were robustly detected (Supplementary Table S3). To obtain pathways which might be significantly addressed by MEST knockdown, we subjected this dataset to gene set enrichment analysis (GSEA).34 Interestingly, genes of the PPAR signaling pathway were found to be significantly (FDR q-value < 10−4) enriched among transcripts that were upregulated by MEST silencing (Figure 3a, left panel). The significant (q < 10−4) induction of genes implicated in fatty acid, triacylglycerol, and ketone body metabolism (derived from the reactome database, Supplementary Table S4) was an additional result supporting that adipocyte differentiation and particularly lipid biosynthesis were substantially promoted by MEST depletion even at this early stage of differentiation. Indeed, we found FASN among the most highly upregulated transcripts (>2.5-fold higher abundance in siMEST-transfected versus siC-transfected cells; Supplementary Table S3). Further, genes of the glycolysis / gluconeogenesis pathway were also significantly (q = 1.16 × 10−4) enriched among upregulated transcripts (Figure 3a, right panel). Correspondingly, glucose clearance from the differentiation medium was significantly increased in siMEST-transfected cells at day 3 (Figure 3b). Altogether, this suggests that MEST knockdown strongly promotes de novo triglyceride biosynthesis from glucose.
Figure 3.
MEST depletion induces PPAR signaling and glycolysis pathways. hMADS cells were transfected at confluence (d-2) with siRNAs targeting MEST (siMEST) or a control siRNA (siC). Adipocyte differentiation was induced two days later (d0). (a) RNA from cells at d3 was analysed by microarray to obtain a global view of mRNAs that are responsive to MEST depletion. A total of 2278 unique RefSeq mRNAs was sorted according to differences in expression between siMEST- and siC-transfected cells (middle heat map). Gene set enrichment analysis (GSEA preranked) revealed a significant enrichment of the KEGG pathways “PPAR Signaling” (FDR q-value<0.0001) and “Glycolysis / Gluconeogenesis” (FDR q-value = 0.0001) among upregulated transcripts. Expression of the transcripts assorted to both pathways are shown as heat maps (log2-transformed expression ratios (siMEST/siC). (b) Supernatants of hMADS cells at d0 and d3 were analysed for glucose concentration (n=5,*P<0.05).
MEST knockdown triggers early events in human adipogenesis
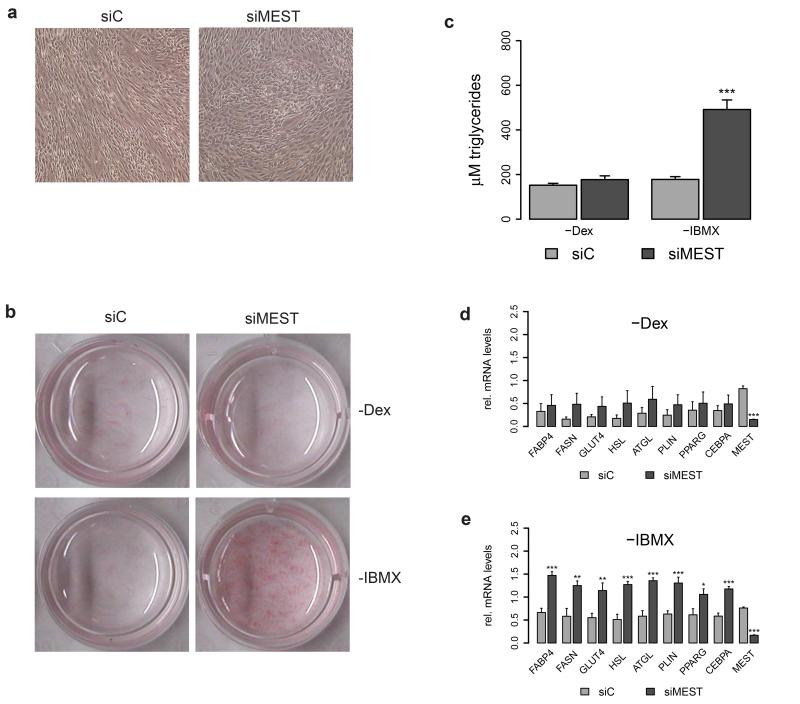
As transcriptomic analyses had revealed that MEST silencing evoked a substantial promotion of lipid biosynthesis pathways already at day 3 of adipocyte differentiation, we reasoned that MEST might be implicated in the early regulatory circuits of adipocyte differentiation. In further support of this hypothesis, the morphology of siMEST-transfected cells (compared to siC-transfected cells) at this time point was indicative of a more advanced differentiation stage, with a higher number of cells having changed their fibroblast-like shape into a more spherical shape (Figure 4a). As for induction of adipocyte differentiation, various in vitro model systems (including hMADS cells) are usually exposed to a cocktail containing Dex and IBMX for the first 2-3 days. We therefore asked whether knockdown of MEST might obviate the cell’s need for one of these compounds. While adipocyte differentiation was clearly suppressed for siC- as well as siMEST-transfected cells that were cultivated without Dex (Figures 4b-d), we observed a strikingly efficient triglyceride accumulation (Figures 4b and c) as well as increased expression of adipocyte marker genes (Figure 4e) for MEST-depleted cells in the absence of IBMX.
Figure 4.
MEST depletion substitutes IBMX as inducer of human adipocyte differentiation. hMADS cells were transfected at confluence (d-2) with siRNAs targeting MEST (siMEST) or a control siRNA (siC). Adipocyte differentiation was induced two days later (d0). (a) Representative bright field microscopy images (40× magnification) of cells at d3. (b-e) hMADS cells were stimulated to undergo adipocyte differentiation in suboptimal differentiation media lacking either dexamethasone (-Dex) or IBMX (-IBMX). (b) Triglyceride accumulation was visualized by Oil Red O staining at d9. (c) Quantification of intracellular triglycerides at d9 (n=4). (d and e) RT-qPCR analysis of adipocyte marker gene expression (d9) for cells differentiated without Dex (d) or without IBMX (e). Gene expression was normalized to TBP and is presented relative to siC-transfected cells that were differentiated in complete differentiation medium (n=4). (c-e) *P<0.05, **P<0.01, ***P<0.001 versus siC-transfected cells exposed to the same differentiation cocktail.
MEST acts on the CREB/ATF1 signaling pathway
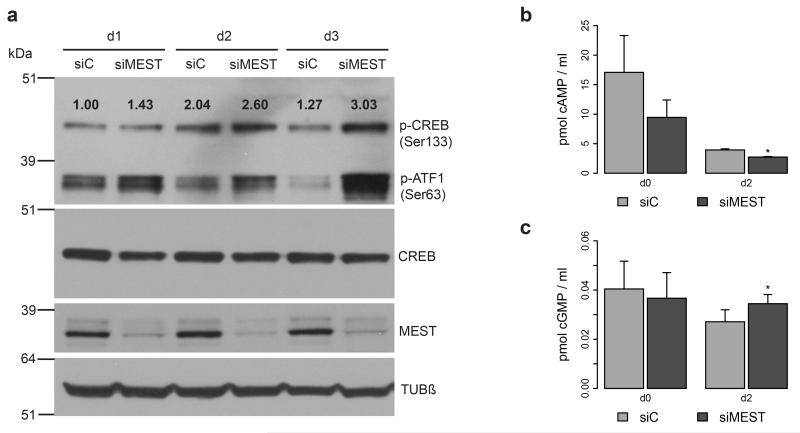
IBMX is a phosphodiesterase inhibitor, preventing the degradation of cAMP and cGMP. Both second messengers have been reported to trigger early events in adipocyte differentiation via activation of protein kinases A (PKA) and G (PKG), respectively, which in turn leads to phosphorylation of the transcription factor CREB. As treatment of hMADS cells with IBMX could be fully substituted by MEST knockdown, we decided to analyse this signaling axis during the early stages of differentiation. Indeed, when exposed to complete differentiation medium, siMEST-transfected cells showed a substantial increase in phosphorylation of CREB and the related protein ATF1 compared to siC-transfected cells at days 1, 2 and 3 after induction of adipogenesis (Figure 5a). A similar, though less pronounced effect was observed when cells were exposed to differentiation medium lacking IBMX (Supplementary Figure S3). We further measured intracellular cAMP and cGMP levels at the start of differentiation, as well as on day 2. Unexpectedly, cAMP levels were reduced due to MEST silencing (Figure 5b). In contrast, cGMP levels were increased by ~25% at day 2 (Figure 5c), yet the absolute levels of cGMP were found to be considerably lower than cAMP levels in differentiating hMADS cells. We thus propose that MEST acts on mediators which lie downstream of these second messengers, or that it affects a distinct signaling pathway which ultimately regulates CREB/ATF1 phosphorylation.
Figure 5.
Effects of MEST depletion on phosphorylation of CREB/ATF1 and intracellular cAMP and cGMP levels. hMADS cells were transfected at confluence (d-2) with siRNAs targeting MEST (siMEST) or a control siRNA (siC). Adipocyte differentiation was induced two days later (d0) using complete differentiation medium. (a) Protein lysates of cells at d1, d2 and d3were analysed by Western blot for phosphorylation of CREB and ATF1 (p-CREB, p-ATF-1), total CREB, MEST and β-Tubulin (Tubβ). Numbers above p-CREB bands reflect the results of densitometric analysis (ratio of p-CREB / CREB) and are presented relative to siC-transfected cells at d1. (b and c) Cells at d0 and d2 were analysed for intracellular levels of cAMP (b) and cGMP (c). Testing for significant differences in second messenger levels was performed by a paired t-test; *P<0.05.
DISCUSSION
Throughout the past decades, excessive weight gain has become a global epidemic. Since 1980, the prevalence of obesity has almost doubled, with ~200 million male and ~300 million female adults worldwide being classified as obese in 2008.35 In contrast to earlier contention, which regarded adipose depots as a rather passive energy storage that expands as obesity develops, adipose tissue is nowadays considered a major endocrine organ with crucial implication in the pathology of obesity-associated diseases.2,3 Indeed, as for the development of insulin resistance and preatherosclerotic changes, an “adipocentric view” has been proposed,36 placing detrimental changes in adipocyte physiology at the beginning of these afflictions. It should be noted that such weight gain-induced changes not only comprise an increase of adipocyte size37 (leading to necrosis at its extreme end4), but also a decrease in adipocyte differentiation.38 Thus, the further elucidation of gene regulatory networks which either promote or inhibit adipogenesis is of utmost importance to better understand the molecular basis of adipose tissue malfunction in the obese state.
Proteins with predicted but not yet identified enzymatic function can be considered as particularly interesting candidates for further investigation. MEST is one such protein exhibiting an intriguingly high correlation with fat mass, which could be indicative of an involvement in lipid storage / mobilisation. In line with previous mouse studies,17-19 we also found MEST to be more abundant in WAT from obese as opposed to normal-weight humans (Figures 1c-e). MEST mRNA levels were further found to be significantly associated with adipocyte size (Supplementary Figure S1), albeit with less distinct correlation as leptin, the classical marker for adipocyte hypertrophy.
While at least three studies in mouse have evolved the concept that Mest is a positive regulator of adipogenesis,17,19,20 our study suggests that MEST might have opposite effects in early steps of adipocyte differentiation in human. Similar to Jung et al.,20 we have employed an RNA interference-based strategy to knock down MEST during adipocyte differentiation, yet have observed a pronounced upregulation of triglyceride accumulation and expression of adipocyte marker genes (Figure 2). These findings were corroborated by pathway analysis of global gene expression data which revealed a significant upregulation of the PPAR signaling pathway and genes involved in fatty acid, triacylglycerol, and ketone body metabolism. Further, overexpression of MEST evoked a corresponding decrease in adipocyte differentiation (Supplementary Figure S2). Based on these results, it can be speculated that an increase of MEST in the obese state might participate in detrimental changes in WAT (and ultimately other tissues) by hampering de novo adipocyte recruitment. As for mouse cells, Jung et al. demonstrated that MEST inhibits the anti-adipogenic Wnt signaling pathway39,40 via blocking maturation of the Wnt co-receptor low density lipoprotein receptor-related protein 6 (LRP6).20 In contrast, although the Wnt signaling pathway was included in a collection of 186 gene sets (derived from the Kyoto Encyclopedia of Genes and Genomes (KEGG)) that we used as input for GSEA, we could not detect a significant upregulation of this pathway due to MEST silencing in differentiating hMADS cells. This was further supported by the fact that we observed a ~2-fold upregulation of the Wnt inhibitor dickkopf homolog 1 (DKK1)41,42 at this early stage of adipocyte differentiation (Supplementary Table S3). Altogether, we propose that species-specific regulatory circuits might be responsible for the diverging impact of MEST in human versus mouse adipogenesis, as for example recently shown for the lim domain only 3 (LMO3) gene.43 Alternatively, we cannot rule out that the effect of MEST is critically dependent on the differentiation stage of adipocyte precursors, as we have employed mesenchymal stem cells that are multipotent at the clonal level, while others have used cells at the preadipocyte stage.
In addition to the pronounced upregulation during adipocyte differentiation, we also noted a preceding increase of MEST mRNA and protein as proliferating hMADS cells (d-3) reached the quiescent stage (d0, Figures 1a and b). In this phase of our experiments, FGF2 was withdrawn from the medium when hMADS cells had grown confluent (d-2). It can thus be hypothesized that MEST is under suppressive control of FGF2. Indeed, a recent study on human dermal fibroblasts (GSE48967) has revealed a significant downregulation of MEST upon treatment with FGF2.44 Alternatively, it can be speculated that growth arrest due to contact inhibition might trigger pathways that unleash enhanced transcription of the MEST gene.
We also observed a significant promotion of the KEGG pathway “Glycolysis and Gluconeogensis” upon MEST knockdown at day 3, concomitant with a depletion of glucose from the medium (Figure 3). In conjunction with the elevated lipid accumulation of hMADS-derived adipocytes at day 9, we propose that MEST silencing triggers de novo triglyceride biosynthesis from glucose. It will be of high interest to test whether the newly discovered relationship between MEST and glycolysis is also existent in other cell types, not least because an increase in glycolytic flux is a frequent characteristic in cancer.45 In this respect, it should be noted that a recent study has described increased CpG island methylation of the MEST promoter region (suggesting downregulation of MEST transcription) in drug-resistant ovarian cancer cell lines.46
Efficient adipocyte differentiation of quiescent human and mouse precursor cells has long been known to be induced by insulin, glucocorticoids, and a cAMP-elevating agent.47,48 As for the latter, it is important to keep in mind that the frequently used phosphodiesterase inhibitor IBMX also acts on cGMP-degrading phosphodiesterases, and that cGMP has pro-adipogenic effects comparable to cAMP.49 Via activation of PKA / PKG, both second messengers trigger the phosphorylation of the downstream transcriptional effector CREB which is necessary for adipocyte differentiation.50 Motivated by the fact that MEST knockdown promoted PPAR signaling and lipid biosynthesis pathways already at day 3 after induction, we decided to repeat experiments in suboptimal differentiation media lacking those pro-adipogenic components which are only present at the very beginning of differentiation (Figure 4). Indeed, this approach revealed that MEST depletion could completely substitute IBMX (but not Dex), thereby implying MEST in the regulatory pathway of cAMP / cGMP, PKA / PKG, and CREB. This hypothesis was principally confirmed as phosphorylation of CREB and the related transcription factor ATF1 was substantially increased by MEST knockdown (Figure 5a). However, we did not detect an elevation, but instead a reduction of cAMP due to MEST silencing in early adipogenesis (Figure 5b). Although cGMP levels were increased at day 2 (Figure 5c), this upregulation is unlikely to be the main cause for the promotion of CREB/ATF1 phosphorylation as (i) cAMP levels are decreased at the same time point, and (ii) overall, cAMP levels were found to be >100-fold higher than cGMP levels in our cell model. We thus propose that invalidation of MEST promotes CREB/ATF1 phosphorylation via one or more distinct routes. For instance, Ras- Mitogen Activated Protein Kinase (MAPK) signaling,51,52 Ca2+ / calmodulin-dependent protein kinase53 and protein kinase C (PKC)54 have been described to phosphorylate CREB. Future studies addressing the responsiveness of these pathways to MEST will therefore be of high interest to further explore the newly discovered link between MEST and CREB/ATF1. Keeping in mind the behavioural abnormalities of Mest KO mice,55 as well as the high importance of CREB in the central nervous system,56 this axis could also be of relevance for brain function.
In summary, we have utilised human samples and cells to confirm the high abundance of MEST in adipocytes and its association with fat mass, findings that had previously been obtained only in mouse experiments. Functional investigations have revealed an unexpected inhibitory role of MEST in human adipogenesis, and for the first time have linked this protein to a pathway that triggers the phosphorylation of CREB, a crucial transcription factor not only in metabolism, but also in cognitive abilities. Altogether, these results have laid a fruitful basis for further research that will ultimately elucidate the precise molecular activity of MEST, and that could eventually lead to the development of novel treatment strategies to ameliorate obesity-associated diseases.
Supplementary Material
Supplementary Figure S1. Mean adipocyte volume (picoliter) from abdominal subcutaneous WAT samples (n=56) was analysed for correlation with (a) MEST and (b) leptin (LEP) mRNA levels that were measured by microarrays (Gene Expression Omnibus Experiment GSE25401). Values above charts denote Spearman’s rank correlation coefficient (ρ) and p-value, respectively.
Supplementary Figure S2. Overexpression of MEST impairs human adipocyte differentiation. hMADS cells were transduced at confluence with lentiviral vectors expressing eGFP or MEST and adipocyte differentiation was induced two days later (d0). Cells were analysed at d9. (a) Triglyceride accumulation was visualised by Oil Red O staining (representative bright field microscopy images; 40x magnification). (b) Quantification of intracellular triglycerides (n=4). (c) RT-qPCR analysis of adipocyte marker gene expression (normalised to TBP, relative to eGFP, n=4). (b and c) #P<0.1, *P<0.05, **P<0.01 versus eGFP-transduced cells.
Supplementary Figure S3. Effects of MEST depletion on phosphorylation of CREB/ATF1 in differentiation medium lacking IBMX. hMADS cells were transfected at confluence (d-2) with siRNAs targeting MEST (siMEST) or a control siRNA (siC). Adipocyte differentiation was induced two days later (d0). (a) Protein lysates of cells at d1, d2 and d3 were analysed by Western blot for phosphorylation of CREB and ATF1 (p-CREB, p-ATF-1), total CREB, MEST and β-Tubulin (Tubβ). Numbers above p-CREB bands reflect the results of densitometric analysis (ratio of p-CREB / CREB) and are presented relative to siC-transfected cells at d1.
ACKNOWLEDGEMENTS
We thank Christian Dani for providing hMADS cells, as well as Daniel Gütl, Lukas Fliedl and Regina Grillari-Voglauer for help with glucose measurements. This work was supported by the Austrian Science Fund (FWF, P25729-B19) and by the EU FP7 project DIABAT (HEALTH-F2-2011-278373).
Footnotes
CONFLICT OF INTEREST
All authors declare no potential conflicts of interest.
Supplementary information is available at International Journal of Obesity’s website (http://www.nature.com/ijo)
REFERENCES
- 1.Gesta S, Tseng Y-H, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Hallakou S, Doaré L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, et al. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46:1393–1399. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 6.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, et al. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 9.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 10.Bujalska IJ, Kumar S, Hewison M, Stewart PM. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:3188–3196. doi: 10.1210/endo.140.7.6868. [DOI] [PubMed] [Google Scholar]
- 11.Siersbæk R, Mandrup S. Transcriptional networks controlling adipocyte differentiation. Cold Spring Harb Symp Quant Biol. 2011;76:247–255. doi: 10.1101/sqb.2011.76.010512. [DOI] [PubMed] [Google Scholar]
- 12.Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 13.Sado T, Nakajima N, Tada M, Takagi N. A Novel Mesoderm-Specific cDNA Isolated from a Mouse Embryonal Carcinoma Cell Line. Develop Growth & Differ. 1993;35:551–560. doi: 10.1111/j.1440-169X.1993.00551.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko-Ishino T, Kuroiwa Y, Miyoshi N, Kohda T, Suzuki R, Yokoyama M, et al. Peg1/Mest imprinted gene on chromosome 6 identified by cDNA subtraction hybridization. Nat Genet. 1995;11:52–59. doi: 10.1038/ng0995-52. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S, Kohda T, Miyoshi N, Kuroiwa Y, Aisaka K, Tsutsumi O, et al. Human PEG1/MEST, an imprinted gene on chromosome 7. Hum Mol Genet. 1997;6:781–786. doi: 10.1093/hmg/6.5.781. [DOI] [PubMed] [Google Scholar]
- 16.Soukas A, Cohen P, Socci ND, Friedman JM. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 2000;14:963–980. [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi M, Kamei Y, Ezaki O. Mest/Peg1 imprinted gene enlarges adipocytes and is a marker of adipocyte size. Am J Physiol Endocrinol Metab. 2005;288:E117–124. doi: 10.1152/ajpendo.00244.2004. [DOI] [PubMed] [Google Scholar]
- 18.Koza RA, Nikonova L, Hogan J, Rim J-S, Mendoza T, Faulk C, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikonova L, Koza RA, Mendoza T, Chao P-M, Curley JP, Kozak LP. Mesoderm-specific transcript is associated with fat mass expansion in response to a positive energy balance. FASEB J. 2008;22:3925–3937. doi: 10.1096/fj.08-108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung H, Lee SK, Jho E. Mest/Peg1 inhibits Wnt signalling through regulation of LRP6 glycosylation. Biochem J. 2011;436:263–269. doi: 10.1042/BJ20101512. [DOI] [PubMed] [Google Scholar]
- 21.Kadota Y, Yanagawa M, Nakaya T, Kawakami T, Sato M, Suzuki S. Gene expression of mesoderm-specific transcript is upregulated as preadipocytes differentiate to adipocytes in vitro. J Physiol Sci. 2012;62:403–411. doi: 10.1007/s12576-012-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez A-M, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne J-Y, Wdziekonski B, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karbiener M, Pisani DF, Frontini A, Oberreiter LM, Lang E, Vegiopoulos A, et al. MicroRNA-26 Family Is Required for Human Adipogenesis and Drives Characteristics of Brown Adipocytes. Stem Cells. 2014;32:1578–1590. doi: 10.1002/stem.1603. [DOI] [PubMed] [Google Scholar]
- 24.Pettersson AML, Stenson BM, Lorente-Cebrián S, Andersson DP, Mejhert N, Krätzel J, et al. LXR is a negative regulator of glucose uptake in human adipocytes. Diabetologia. 2013;56:2044–2054. doi: 10.1007/s00125-013-2954-5. [DOI] [PubMed] [Google Scholar]
- 25.Gao H, Mejhert N, Fretz JA, Arner E, Lorente-Cebrián S, Ehrlund A, et al. Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab. 2014;19:981–992. doi: 10.1016/j.cmet.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karbiener M, Neuhold C, Opriessnig P, Prokesch A, Bogner-Strauss JG, Scheideler M. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol. 2011;8:850–860. doi: 10.4161/rna.8.5.16153. [DOI] [PubMed] [Google Scholar]
- 27.Pabinger S, Thallinger GG, Snajder R, Eichhorn H, Rader R, Trajanoski Z. QPCR: Application for real-time PCR data management and analysis. BMC Bioinformatics. 2009;10:268. doi: 10.1186/1471-2105-10-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arner E, Mejhert N, Kulyté A, Balwierz PJ, Pachkov M, Cormont M, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61:1986–1993. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Löfgren P, Hoffstedt J, Näslund E, Wirén M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia. 2005;48:2334–2342. doi: 10.1007/s00125-005-1961-6. [DOI] [PubMed] [Google Scholar]
- 31.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt SF, Jørgensen M, Chen Y, Nielsen R, Sandelin A, Mandrup S. Cross species comparison of C/EBPα and PPARγ profiles in mouse and human adipocytes reveals interdependent retention of binding sites. BMC Genomics. 2011;12:152. doi: 10.1186/1471-2164-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 2009;27:2753–2760. doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO [accessed 10 Jun2014];Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/#.
- 36.Naukkarinen J, Rissanen A, Kaprio J, Pietiläinen KH. Causes and consequences of obesity: the contribution of recent twin studies. Int J Obes (Lond) 2012;36:1017–1024. doi: 10.1038/ijo.2011.192. [DOI] [PubMed] [Google Scholar]
- 37.Pietiläinen KH, Kannisto K, Korsheninnikova E, Rissanen A, Kaprio J, Ehrenborg E, et al. Acquired obesity increases CD68 and tumor necrosis factor-alpha and decreases adiponectin gene expression in adipose tissue: a study in monozygotic twins. J Clin Endocrinol Metab. 2006;91:2776–2781. doi: 10.1210/jc.2005-2848. [DOI] [PubMed] [Google Scholar]
- 38.Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 2008;5:e51. doi: 10.1371/journal.pmed.0050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 40.Longo KA, Wright WS, Kang S, Gerin I, Chiang S-H, Lucas PC, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 41.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 42.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 43.Lindroos J, Husa J, Mitterer G, Haschemi A, Rauscher S, Haas R, et al. Human but Not Mouse Adipogenesis Is Critically Dependent on LMO3. Cell Metab. 2013;18:62–74. doi: 10.1016/j.cmet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kashpur O, LaPointe D, Ambady S, Ryder EF, Dominko T. FGF2-induced effects on transcriptome associated with regeneration competence in adult human fibroblasts. BMC Genomics. 2013;14:656. doi: 10.1186/1471-2164-14-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akram M. Mini-review on glycolysis and cancer. J Cancer Educ. 2013;28:454–457. doi: 10.1007/s13187-013-0486-9. [DOI] [PubMed] [Google Scholar]
- 46.Zeller C, Dai W, Steele NL, Siddiq A, Walley AJ, Wilhelm-Benartzi CSM, et al. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene. 2012;31:4567–4576. doi: 10.1038/onc.2011.611. [DOI] [PubMed] [Google Scholar]
- 47.Russell TR, Ho R. Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2alpha and 1-methyl-3-isobutyl xanthine. Proc Natl Acad Sci USA. 1976;73:4516–4520. doi: 10.1073/pnas.73.12.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemmrich K, Gummersbach C, Paul NE, Goy D, Suschek CV, Kröncke K-D, et al. Nitric oxide and downstream second messenger cGMP and cAMP enhance adipogenesis in primary human preadipocytes. Cytotherapy. 2010;12:547–553. doi: 10.3109/14653241003695042. [DOI] [PubMed] [Google Scholar]
- 50.Reusch JE, Colton LA, Klemm DJ. CREB activation induces adipogenesis in 3T3-L1 cells. Mol Cell Biol. 2000;20:1008–1020. doi: 10.1128/mcb.20.3.1008-1020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 52.Klemm DJ, Roesler WJ, Boras T, Colton LA, Felder K, Reusch JE. Insulin stimulates cAMP-response element binding protein activity in HepG2 and 3T3-L1 cell lines. J Biol Chem. 1998;273:917–923. doi: 10.1074/jbc.273.2.917. [DOI] [PubMed] [Google Scholar]
- 53.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 54.Guo CM, Kasaraneni N, Sun K, Myatt L. Cross talk between PKC and CREB in the induction of COX-2 by PGF2α in human amnion fibroblasts. Endocrinology. 2012;153:4938–4945. doi: 10.1210/en.2012-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- 56.Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Mean adipocyte volume (picoliter) from abdominal subcutaneous WAT samples (n=56) was analysed for correlation with (a) MEST and (b) leptin (LEP) mRNA levels that were measured by microarrays (Gene Expression Omnibus Experiment GSE25401). Values above charts denote Spearman’s rank correlation coefficient (ρ) and p-value, respectively.
Supplementary Figure S2. Overexpression of MEST impairs human adipocyte differentiation. hMADS cells were transduced at confluence with lentiviral vectors expressing eGFP or MEST and adipocyte differentiation was induced two days later (d0). Cells were analysed at d9. (a) Triglyceride accumulation was visualised by Oil Red O staining (representative bright field microscopy images; 40x magnification). (b) Quantification of intracellular triglycerides (n=4). (c) RT-qPCR analysis of adipocyte marker gene expression (normalised to TBP, relative to eGFP, n=4). (b and c) #P<0.1, *P<0.05, **P<0.01 versus eGFP-transduced cells.
Supplementary Figure S3. Effects of MEST depletion on phosphorylation of CREB/ATF1 in differentiation medium lacking IBMX. hMADS cells were transfected at confluence (d-2) with siRNAs targeting MEST (siMEST) or a control siRNA (siC). Adipocyte differentiation was induced two days later (d0). (a) Protein lysates of cells at d1, d2 and d3 were analysed by Western blot for phosphorylation of CREB and ATF1 (p-CREB, p-ATF-1), total CREB, MEST and β-Tubulin (Tubβ). Numbers above p-CREB bands reflect the results of densitometric analysis (ratio of p-CREB / CREB) and are presented relative to siC-transfected cells at d1.