Abstract
Creatine is a principle component of the creatine kinase (CK) phosphagen system common to all vertebrates. It is found in excitable cells, such as cardiomyocytes, where it plays an important role in the buffering and transport of chemical energy to ensure that supply meets the dynamic demands of the heart. Multiple components of the CK system, including intracellular creatine levels, are reduced in heart failure, while ischaemia and hypoxia represent acute crises of energy provision. Elevation of myocardial creatine levels has therefore been suggested as potentially beneficial, however, achieving this goal is not trivial. This mini-review outlines the evidence in support of creatine elevation and critically examines the pharmacological approaches that are currently available. In particular, dietary creatine-supplementation does not sufficiently elevate creatine levels in the heart due to subsequent down-regulation of the plasma membrane creatine transporter (CrT). Attempts to increase passive diffusion and bypass the CrT, e.g. via creatine esters, have yet to be tested in the heart. However, studies in mice with genetic overexpression of the CrT demonstrate proof-of-principle that elevated creatine protects the heart from ischaemia-reperfusion injury. This suggests activation of the CrT as a major unmet pharmacological target. However, translation of this finding to the clinic will require a greater understanding of CrT regulation in health and disease and the development of small molecule activators.
Keywords: Creatine transporter, energetics, heart disease
1. THE ROLE OF CREATINE IN THE HEART
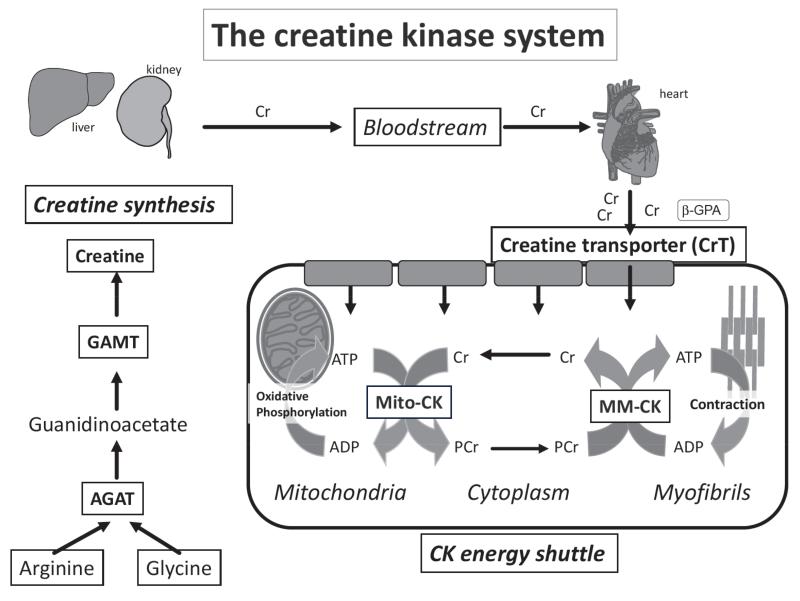
Heart muscle cells (cardiomyocytes) have high and dynamic energy demands that require an exquisite balance between energy production and consumption. Chemical energy in the form of adenosine triphosphate (ATP) is produced from multiple substrates (e.g. fatty acids and glucose) predominantly in the mitochondria via oxidative phosphorylation. Ensuring that the energy within the phosphoryl bonds of ATP is maximised and readily available at the sites of utilisation requires a phosphagen system, which in all vertebrates, consists of the reversible interaction of creatine and ATP under the control of creatine kinase (CK) enzymes [1, 2]: Cr + ATP ↔ Phosphocreatine + ADP + H+ (see Fig. 1). This is the only known metabolic role for creatine, which is taken up into the cardiomyocyte via a specific plasma membrane Creatine Transporter (CrT) [3].
Fig. (1). Creatine biosynthesis and the CK energy shuttle.
Creatine biosynthesis is a two-step process with L-arginine:glycine amidinotransferase (AGAT), mostly in the kidney, and N-guanidinoacetate methyltransferase (GAMT) predominantly in the liver. Creatine is taken-up into cardiomyocytes from the bloodstream by the plasma-membrane creatine transporter (CrT). Mitochondrial creatine kinase (Mt-CK) located in the mitochondrial inter-membrane space catalyses direct transfer of a high-energy phosphoryl group from ATP to creatine to form phosphocreatine (PCr). PCr is small and less polar than ATP and accumulates to high levels in the cytosol acting as a highly mobile, short-term, energy store. The reverse reaction generates ATP and is catalysed by the cystolic CK dimers closely coupled to ATPases. The liberated creatine diffuses back to signal for further ATP production. Compartmentalisation of the reactants without the requirement for ADP and ATP diffusion ensure metabolites are at favourable levels to support forward and reverse reactions and maximise the free energy available from ATP hydrolysis, i.e. low [ATP/ADP] ratio at the mitochondria and high [ATP/ADP] at the ATPases [1, 2, 4, 5]. Created using Servier Medical Art by Servier which is licensed under a Creative Commons Attribution 3.0 Unported License http://www.servier.com/slidekit.
The heart expresses four CK isoenzymes, the most abundant being mitochondrial CK (Mt-CK) and myofibrillar CK (MM-CK), at 10% and 88% of total CK activity in the human heart respectively [6]. The brain isoform (BB-CK) and its dimerized product with M-CK, MB-CK also exists in the heart but at low abundance (~2%) [6]. With approximately two-thirds of the total Cr pool in the phosphorylated (PCr) form, the creatine kinase shuttle thereby acts as an energy buffer, which can provide near instantaneous regeneration of ATP at times of high workload when energy demand outstrips supply [5, 7]. This represents a short-term back-up that seamlessly steps in until ATP production is increased.
2. CREATINE IN HEART DISEASE & THE RATIONALE FOR AUGMENTATION
2.1. Heart Failure
In heart failure there is a gradual loss of myocardial total creatine content and a corresponding reduction in CK activity [1]. This is a remarkably consistent finding across animal models and aetiologies [8-12] and was first described in patients as long ago as 1939 [13], for example, a reduction of 50% in total creatine and 34% in total CK activity was observed in the failing human heart [6]. This creatine loss has been attributed to down-regulation of the creatine transporter (CrT) in the failing heart [14] as a consequence of post-transcriptional modification [9].
These findings come from post-mortem tissue, but 31P-magnetic resonance spectroscopy (MRS) has been used to study high-energy phosphates in vivo. Due to the technical difficulty in obtaining absolute values, the energetic state of the heart is usually expressed as a ratio of PCr/ATP, with the normal value in healthy human heart ~2 [15]. Notably, ATP levels remain near normal until end-stage heart failure due to the buffering capacity of PCr, and the CK equilibrium constant strongly favouring ATP synthesis.
Studies in patients with dilated cardiomyopathy have demonstrated that a low PCr/ATP ratio is associated with more severe heart failure symptoms [15], low contractile function, myocardial structural remodelling [16], and a higher risk of mortality [17]. Impairment in PCr/ATP ratio has been described early in the disease process prior to overt cardiac dysfunction, e.g. in type 2 diabetics [18], obesity [19], hypertension [20], and in patients with cardiac hypertrophy [21]. These observational studies suggest a close association between cardiac energetic status and function, although whether reduced creatine levels have a causal role in driving dysfunction in the failing heart remains a matter of some debate [22]. Regardless, it has long been suggested that preventing the decline in total creatine levels may be of therapeutic benefit in heart failure [1, 23, 24].
2.2. Ischaemia
In the ischaemic heart there is a rapid energy crisis with insufficient oxygen to support ATP production via oxidative phosphorylation, PCr and ATP levels are rapidly depleted (within minutes), and the heart will cease beating. When ischaemia is transient (such as in angina) it seems reasonable to suggest that elevating energy stores in the form of PCr may be therapeutically beneficial, although this remains untested. However, even with prolonged ischaemia resulting in tissue damage (as in myocardial infarction), there may be benefits in terms of re-energising the heart during recovery. Certainly, the converse is true, that hearts from creatine-deficient mice have impaired functional recovery following an ischaemic period [25].
2.3. Anthracycline Cardiotoxicity
Anthracyclines such as doxorubicin are effective chemotherapy agents, but their use is severely limited by dose-dependent adverse effects on cardiac function [26]. These are, in part, thought to be mediated by the generation of reactive oxygen species and subsequent derangement in cardiac energetics [27]. Augmenting creatine levels has been mooted to address both these issues since numerous in vitro studies have ascribed antioxidant effects to creatine, either directly, or by boosting antioxidant defenses [28]. Indeed, a recent in vitro study showed that creatine protected against doxorubicin-induced apoptosis, cell damage and oxidative stress [29]. In contrast, we recently showed that intracellular Cr levels did not influence the functional response upon acute exposure to doxorubicin or hydrogen peroxide, questioning the physiological relevance of earlier observations to the intact beating heart [30]. However, the effect of creatine augmentation on chronic anthracycline toxicity has yet to be established.
3. DETERMINANTS OF CREATINE LEVELS IN CARDIOMYOCYTES
Creatine levels in the heart are tightly controlled and represent the balance between creatine availability, cellular uptake, and degradation / efflux.
3.1. Creatine Biosynthesis
A typical omnivorous Western diet provides one half of daily creatine with the other half synthesised de novo. Creatine synthesis is a two-step metabolic process requiring three amino acids; methionine, glycine and arginine and three enzymes [31]. The first enzyme L-arginine:glycine amidinotransferase (AGAT) transfers the amidino group from arginine to glycine to yield L-ornithine and guanidinoacetic acid (GAA). Methionine adenosyltransferase (MAT) converts methionine into S-adenosylmethionine (SAM). SAM acts as the methyl donor for GAA at the original glycine nitrogen and by action of the third enzyme N-guanidinoacetate methyltransferase (GAMT) yields creatine and S-adenosylhomocysteine (SAH) [2, 31]. Creatine biosynthesis is an inter-organ process as shown in (Fig. 1). Local creatine biosynthesis is not thought to occur within cardiomyocytes, since they do not normally express AGAT or GAMT proteins, confirmed in CrT knockout mice which have low or negligible creatine levels in the heart [32, 33]. Notably, AGAT mRNA may be expressed in the heart under pathological conditions [34], however, the significance of this is open to debate without concomitant expression of GAMT.
3.2. Cellular Creatine Uptake: Creatine Transporter
The CrT (SLC6A8) is the only known mechanism for creatine uptake across the plasma membrane and is expressed in energy-demanding tissues such as skeletal muscle, heart, kidney and brain [2]. Creatine uptake (against a 50-fold concentration gradient) is powered by the trans-membrane Na+ gradient with co-transport of two Na+ and one Cl− for each molecule of creatine [35]. The CrT gene is located on the human X chromosome and encodes for a 635 amino acid, 12 transmembrane domain, protein with several putative phosphorylation and glycosylation sites [36]. It has high specificity for creatine, and is closely related to the γ-aminobutyrate (GABA), taurine and betaine transporters. The crystal structure of the CrT and the creatine-binding sites have yet to be solved, with the closest relative being the bacterial leucine transporter leuT [37].
Several molecular weights have been reported when using tissue samples (as opposed to in vitro), e.g. 58 kDa (native species), 70-80 kDa (a diffuse band due to extensive glycosylation) and 150 kDa (probably a dimer). In part, this is due to inherent problems with the efficient purification of membrane-bound molecules and to the lack of truly specific CrT antibodies [38].
3.3. Cellular Creatine Efflux
The major source of creatine loss is spontaneous, non-enzymatic, degradation to creatinine, which is lost from the cell by diffusion in parallel with low levels of passive creatine efflux [2, 39]. The rate of loss is 1.7% of the total body creatine pool per day and replacement represents a significant metabolic demand [31].
4. STRATEGIES TO ELEVATE MYOCARDIAL CREATINE LEVELS
4.1. Creatine Supplementation
A large body of research has focussed on creatine-supplementation strategies, which usually requires oral dosing with very large quantities (e.g. up to 25g per day in humans). Multiple forms of creatine are available for the public to purchase in the guise of nutraceutical preparations including anhydrous creatine and salt formulations, e.g. pyruvate, phosphate, malate, citrate, magnesium and oroate [40]. This approach is well established within body building and exercise communities, with putative benefits as an ergogenic aid to enhance performance in sport [40]. Claims have also been made for benefits in diseases such as Huntington’s, Parkinson’s, Duchenne muscular dystrophy and other neuromuscular disorders [41], although the quality of evidence is highly variable. In this section, we will focus solely on the effects that exogenous creatine has on the cardiovascular system.
A key consideration is how much creatine actually makes it into the target organ. Not all tissues load creatine to the same extent and the reasons for the variation are unclear [42]. Nonetheless, it is generally accepted that tissues with high starting [Cr] (e.g. heart) have less loading potential than tissues with low [Cr] e.g. liver [42, 43]. For example, rats fed a creatine-supplemented diet had 7-fold higher creatine levels in plasma, but creatine in the heart was not significantly elevated [39]. This is most likely due to substrate inhibition, with high creatine resulting in down-regulation of CrT activity, for example, 6 weeks of Cr supplementation in rats led to suppressed Cr transport Vmax and a subsequent decrease in plasma membrane CrT pool [39]. Studies that fail to measure tissue creatine levels are therefore highly questionable and of little value.
4.1.1. Acute Ischaemia
Hearts excised and perfused in Langendorff mode from rats supplemented with 1% creatine in chow did not alter functional recovery or improve the half-life of ATP during global ischaemia above that of the control group [44]. However, perfusion with PCr at the time of ischaemia or during reperfusion has met with greater success. For example, continuous intravenous administration of PCr before and during coronary artery ligation in rabbits caused a 40% reduction in necrosis [45]. Furthermore, ex vivo rat hearts subjected to ischaemia-reperfusion injury had almost complete recovery of heart function when PCr was included in the perfusion buffer [46]. Cardioplegic solutions supplemented with PCr improved the ability of the heart to perform work following cardiopulmonary bypass in pigs [47], and improved myocardial protection in rat hearts [48]. This apparently translates to humans since significant myocardial protection was reported in patients undergoing coronary artery bypass surgery that received PCr before, during, and after cardioplegic bypass surgery [49]. Furthermore, PCr treated patients had a reduced incidence of both ventricular fibrillation (VF) and ventricular tachycardia (VT) post MI [50]. However, it should be noted that PCr is not a substrate for CrT and so this beneficial effect is unlikely to be due to elevated intracellular levels, instead it has been suggested that PCr promotes plasma membrane stabilization [51].
4.1.2. Heart Failure
Chronic creatine supplementation in rats with heart failure secondary to myocardial infarction did not increase PCr or total [Cr] or improve myocardial energy reserve via CK [42]. Unsurprisingly there was no effect on cardiac function and the decrease in total [Cr] that occurs in the failing heart was not prevented [52]. Supplementation with Cr (20g daily for 10days) in patients with chronic heart failure failed to improve ejection fraction at rest or during exercise; however skeletal muscle strength and performance were improved compared to the placebo group [53]. In line with this study, Cr supplementation also improved skeletal muscle metabolism in congestive heart failure patients [54].
4.1.3. Exercise
The majority of information on the efficacy of Cr supplementation in sport and exercise performance is focused on the beneficial effects in skeletal muscle (reviewed elsewhere in this issue), but little is known about the myocardium. A recent study evaluated the effect of Cr supplementation in swim trained rats, they found no change in basal cardiac function or mitochondrial oxidative capacity and, surprisingly, a decreased tolerance to ischaemia in the exercise-trained Cr supplemented rats [55]. In humans, creatine supplementation for 4 weeks during high intensity interval training improved ventilatory threshold but had no additional benefits on the cardiorespiratory outcomes [56]. Furthermore, short term Cr supplementation improved submaximal cycling efficiency with no detectable negative effect on cardiac structure or function [57].
4.1.4. Hypoxia
Supplementation with creatine in the maternal diet during pregnancy in the Spiny mouse significantly increased total Cr in the placenta and in the foetal vital organs. This enhanced offspring survival, protecting them from the damaging effects of foetal hypoxia during birth [58]. This study suggests that Cr supplementation may have utility in difficult pregnancies, an area yet to be investigated clinically.
4.1.5. Aging
Median healthy life span was prolonged by 9% in mice given creatine in the diet from 1 year of age [59]. Tissue creatine levels were not measured and the extent to which this reflects an improvement in cardiac health is unknown.
4.2. Creatine Analogues and Creatine Loading Strategies
4.2.1. Creatine Methyl Ester and Creatine Metal Complexes
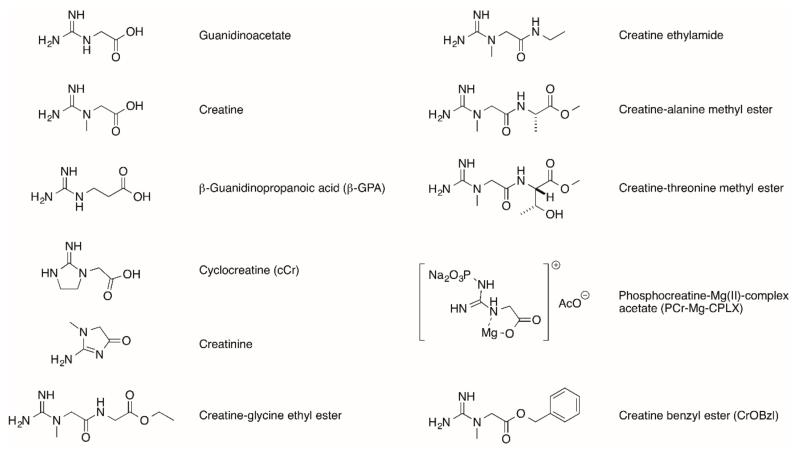
One approach to circumvent the problem of CrT down-regulation is to make chemical modifications to mask the polar or charged functional groups and make creatine more lipophilic, thereby enhancing passive diffusion across the plasma membrane. Ideally the complex will only be cleaved within the intracellular environment to release free creatine and a non-toxic by-product. Examples are the creatine benzyl ester (CrOBzl) or phosphocreatine-Mg(II)-complex acetate (PCr-Mg-CPLX) [60]. Towards the same purpose, later studies analysed amide creatine derivatives such as creatine-glycine ethyl ester, creatine ethylamide in addition to alanine or threonine methyl esters [61] (see Fig. 2 for chemical structures). Of these, the glycine ethyl ester and creatine ethylamide were able to raise PCr levels in brain tissue slices, which is a critical validation step to demonstrate efficacy. This approach is of particular interest for the treatment of patients with creatine-deficiency due to a loss-of-function mutation in the CrT [61]. Whether this approach is capable of loading additional creatine into the heart remains to be established.
Fig. (2).
Structures of precursor GAA, Creatine, β-GPA, creatinine and Cr analogues.
4.2.2. Cyclocreatine (cCr)
Cyclocreatine (cCr) – is an analogue of creatine, which is taken up into cells via the CrT and is a substrate for creatine kinase, but with a significantly lower reaction velocity. This is an unfavourable property during times of maximal energy demand (and therefore represents a trade-off), but in the ischaemic heart it protects against tissue damage by delaying the rate of ATP depletion [62]. Several studies have shown cardioprotection by pre-dosing cCr in animal models [44, 63, 64], for example, I.V. injection of cCr 1 hour before surgery improved post by-pass contractility and cardiac output in a canine model of cold cardioplegic arrest and aortic cross-clamping [65].
4.2.3. β-guanidinopropionic Acid (β-GPA)
β-guanidinopropionic acid (β-GPA) – is a competitive inhibitor of cellular creatine uptake, but is a poor substrate for the CK reaction (~1% of the reaction velocity for creatine). It is therefore a useful pharmacological tool for understanding the effects of creatine depletion [66].
4.3. Increasing Creatine Transporter Activity
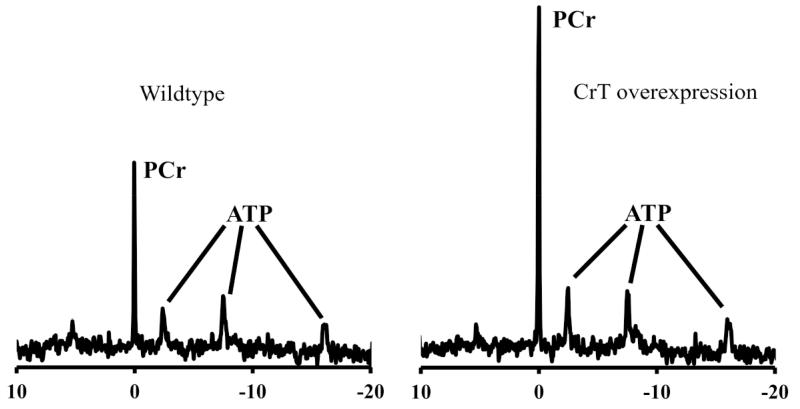
Increasing the activity or expression of the CrT is an attractive strategy for elevating creatine in the heart. In the absence of pharmacological tools, proof-of-principle has relied on transgenic mice. Our laboratory previously created a mouse model constitutively overexpressing CrT specifically in the heart (CrT-OE Oxford) [23]. This resulted in variable levels of total creatine up to 4-fold higher than in wild-type hearts and elevated PCr (Fig. 3). Surprisingly, mice with very high creatine levels (>2-fold elevation) developed cardiac hypertrophy and contractile dysfunction. This was linked to a reduced ratio of PCr/Cr indicating a limit in the capacity of CK to keep the additional creatine pool phosphorylated, with the consequence that less free energy was available from ATP hydrolysis [23]. These mice were also shown to have impaired glycolytic capacity as a result of reduced enolase expression [67]. Recently, a second mouse model of CrT overexpression (CrT-OE Duke) has been described with normal cardiac function and only mild hypertrophy despite creatine levels up to 5.7-fold higher than controls [68]. Notably, PCr levels are elevated at 2 and 4 weeks, but return to normal by 8 weeks of age while creatine levels continue to increase throughout. Furthermore, only 4% of cardiomyocytes appear to express the transgene. Several factors may contribute to these differences, for example, the Oxford mouse over-expresses rabbit CrT using an MLC2v promoter on a C57BL/6J background [23]; whereas the Duke mouse is human CrT using αMHC promoter on an FVB background [68].
Fig. (3). 31P-NMR spectra from ex vivo Langendorff perfused mouse heart.
The left panel is from a wild-type mouse showing a large phosphocreatine (PCr) peak and the three phosphoryl groups of ATP. On the right is a spectrum from a creatine transporter over-expressing mouse (Oxford) heart showing greatly increased PCr peak.
We have subsequently shown that creatine levels elevated up to 2-fold do not have adverse cardiac effects in the CrT-OE Oxford mouse [69] and therefore conducted a series of experiments using mice within this “therapeutic” range. CrT-OE mice were found to be protected against ischaemia/reperfusion injury, exhibiting 27% less myocardial damage compared to control hearts, with the extent of damage negatively correlating with tissue creatine levels. This manifested as improved functional recovery in ex vivo perfused heart experiments. In addition to positive effects on cardiac energetics, in vitro experiments suggested that creatine could also delay mitochondrial commitment to cell death [69].
These mice were also tested in a chronic model of post-MI heart failure and although creatine levels were maintained at supra-physiological levels throughout, there was no benefit in terms of cardiac remodelling and function [69]. Notably, this finding was predicted by an in silico modelling study, which suggested energetic benefits only if pools of creatine, exchangeable phosphates and adenine nucleotides could be elevated simultaneously [70]. It is notable that mice over-expressing M-CK were protected against pressure overload induced heart failure [24] and activation of CK represents another related therapeutic target. It remains to be seen whether a combined substrate plus enzyme approach would be synergistic. The potential for creatine augmentation in heart failure, on its own or in combination with other approaches, requires further study.
5. THE CREATINE TRANSPORTER AS A PHARMACOLOGICAL TARGET
Studies in CrT overexpressing mice have provided the first proof-of-principle evidence that augmenting creatine levels in the heart can be cardioprotective. However, realizing the translational potential will require the development of new pharmacological tools and a much better understanding of CrT regulation in health and disease.
5.1. What is Known About CrT Regulation?
Modulating the CrT and thereby intracellular Cr levels, has been explored in both heart-derived cells and artificial expression systems. Substrate inhibition by creatine itself is the single undisputed, strong regulator of CrT [39, 71, 72] other evidence pertains mostly to signalling molecules that have either direct or indirect effects on CrT function. Most of these reports are not specific to the myocardium, but may provide possible targets for future CrT modulation. However, care should be taken when generalizing between cells types, for example, skeletal and heart muscle cells share the same CrT, yet creatine levels are much higher in skeletal muscle, suggesting differential regulation.
A summary of potential routes of CrT modulation is shown in Table 1. Notably, there is a stronger influence of post-transcriptional and post-translational regulation compared to transcriptional mechanisms. For example, in hearts from creatine-deficient mice the Vmax for creatine uptake kinetics is elevated 7 fold, yet CrT transcript is only increased by 1.4-fold [72].
Table 1. Mechanisms of Creatine transporter modulation.
| Molecule/condition | Aspect of CrT modulation | Effect | System | References |
|---|---|---|---|---|
| Extracellular [Creatine] | Cr uptake | Decrease | L6 myoblasts | [73] |
| Beta-GPA | Activity | Decrease | HL1 cells | [75] |
| mTOR and SGK1 | CrT trafficking | Increase | Xenopus laevis oocytes expressing CrT cRNA | [76, 77] |
| Klotho | Max transport rate and transporter on the plasma membrane | [78] | ||
| SPAK and OSR1 | Decrease | [79] | ||
| PGC1α via ERRα | Transcript | Increase | Skeletal muscle cells | [80] |
| Splice variants SLC6A8C and SLC6A8D | Cr uptake and CrT protein | Increase | 3T3 Swiss fibroblasts | [81] |
| Growth hormone | Transcript | Increase | In vivo in rats post-MI | [82] |
| AICAR via AMPK and substrate availability | Vmax of Cr uptake and CrT on the plasma membrane | Increase in Vmax | HL1 cardiomyocytes and RNCM | [83] |
| Decrease | Kidney proximal tubule cells | [84] | ||
| Phorbol esters | CrT activity | Decrease | X. laevis oocytes expressing CrT | [35] |
| Txnip | Cr uptake CrT protein |
Decrease | Mouse fibroblasts overexpressing CrT | [74] |
| Doxorubicin | Cr uptake and CrT on the plasma membrane | Decrease and removal of CrT from plasma membrane | Rat neonatal cardiomyocytes and HL1 cells | [75] |
| N-glycosylation at Asn192 and Asn197 | CrT function Surface CrT trafficking |
Preserves function | HEK293 cells overexpressing CrT | [85] |
| Phosphorylation at Ser625 Cr supplementation | Cr uptake Phosphorylation at Tyr416 |
Increase Decrease |
Gastrocnemius rat muscle in starvation Gastrocnemius rat muscle in sepsis |
[86, 87] |
Substrate availability is a strong regulator of Cr uptake. β-GPA competes with Cr for uptake by the CrT and decreases Cr entry into the cell. A number of reports describe CrT modulation in X. laevis oocytes that overexpress CrT. There is a role for intracellular kinases such as mammalian target of rapamycin (mTOR), Serum and glucocorticoid-regulated kinase 1 (SGK1), SPS-1 related proline/alanine-rich kinase (SPAK), oxidative stress-responsive kinase 1 (OSR1) and the transmembrane Klotho protein that can also regulate several other transporters. AICAR, via 5′ AMP-activated protein kinase (AMPK) and phorbol esters that activate Protein kinase C can both change CrT activity. Although the above in addition to Doxorubicin and Txnip affect CrT translocation from plasma membrane and/or activity, CrT regulation via its transcript is less likely but possible, via peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha (PGC1α) acting through the Estrogen-related receptor response element (ERRE) in intron 1 of the CrT gene sequence. Post-translational modifications of CrT, such as phosphorylation and N-glycosylation have also been studied both in vitro and in vivo.
It was previously shown that negative feedback regulation by creatine is dependent on de novo protein synthesis, but the identity of this endogenous CrT inhibitor was unknown [73]. Our group used an unbiased global gene array approach to identify Thioredoxin interacting protein (Txnip) as the only gene up-regulated when cells were exposed to saturating creatine levels. Under the same conditions, small interfering RNA against Txnip resulted in increased creatine uptake. This mechanism appears to be relevant in vivo since hearts from CrT overexpressing mice with high intracellular creatine had higher Txnip mRNA and protein compared to wild-type littermates. Conversely creatine-deficient mouse hearts had lower Txnip expression [74]. This evidence is consistent with the role of Txnip as an inhibitor of creatine uptake both in vitro and in vivo. Txnip is an α arrestin with roles in redox regulation via inhibition of the denitrosylating enzyme, thioredoxin. This suggests the potential for regulation of CrT via S-nitrosylation, but further work is required to elucidate the mechanisms of the Txnip-CrT interaction as a potential target for augmenting myocardial creatine levels.
5.2. Screening for Activators of the Creatine Transporter
Current methods for quantifying CrT activity rely on uptake of radio-labelled creatine and are inherently low-throughput [74, 88]. Nevertheless our laboratories have had some limited success in identifying in vitro activators of creatine uptake by using a ligand-based virtual screening method to cherry pick compounds from our in-house 25,000-member screening collection. These types of in silico screening approaches are increasingly employed within discovery programmes, particularly where the structure of the biological target is unknown, or no reliable models are available, and can yield improved hit rates compared to a screen of a randomly diverse compound collection (For a review see: [89, 90]). We use a mouse fibroblast cell line that stably overexpresses the CrT and incubate candidate compounds in 24-well plates spiked with 14C-creatine. After 1 hour, cells are lysed, washed and creatine-uptake calculated by quantification of radiation with comparison to a standard curve. Positive results are then repeated looking for dose-dependency in the more physiologically relevant, cardiomyocyte-derived, HL1 cell line [69]. As a secondary screen, compounds are excluded if they alter membrane potential, since effects on CrT activity may simply reflect changes in the sodium gradient. This can be assessed using cationic dyes such as DiSBAC2(3) [Bis-(1,3-diethylthiobarbituric acid) trimethine oxonol] [91].
Alternatively, transcriptional regulation of CrT can be explored, e.g. using luciferase reporter assays. Evidence for the potential of this approach is demonstrated by the identification of PGC1α-driven modulation of CrT transcript [80]. Using reporter assays, it will be possible to interrogate the ability of different compounds, to alter expression of the CrT promoter [92].
6. SUMMARY AND CONCLUSIONS
There exists a strong rationale for attempting to augment creatine levels in the heart. However, dietary creatine supplementation is not sufficient to achieve this due to down-regulation of the creatine transporter. Mice genetically-modified to overexpress the CrT have provided proof-of-principle evidence that elevated creatine protects the heart against ischaemia/reperfusion injury and has defined a safe working range for elevated creatine. Realising the translational potential of these findings and extending the range of potential indications will require the development of new pharmacological tools aimed at increasing CrT activity. Clearly such agents are also likely to find utility beyond the cardiovascular system.
ACKNOWLEDGEMENTS
Work in our laboratory is supported by the British Heart Foundation programme grant RG/13/8/30266. Funding specifically related to the screening experiments has been received from the Oxford Invention Fund (OIF 507) and the BHF Centre of Research Excellence, Oxford.
Biography

C.A. Lygate
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
REFERENCES
- [1].Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;556(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- [2].Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- [3].Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224(1-2):169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- [4].Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555(Pt 1):1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94(8):1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- [7].Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- [8].Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;105(11):1570–1576. doi: 10.1161/01.cir.103.11.1570. [DOI] [PubMed] [Google Scholar]
- [9].Shen W, Spindler M, Higgins MA, Jin N, Gill RM, Bloem LJ, Ryan TP, Ingwall JS. The fall in creatine levels and creatine kinase isozyme changes in the failing heart are reversible: complex post-transcriptional regulation of the components of the CK system. J Mol Cell Cardiol. 2005;59(3):537–544. doi: 10.1016/j.yjmcc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [10].Neubauer S, Frank M, Hu K, Remkes H, Laser A, Horn M, Ertl G, Lohse MJ. Changes of creatine kinase gene expression in rat heart post-myocardial infarction. J Mol Cell Cardiol. 1998;50(4):803–810. doi: 10.1006/jmcc.1998.0645. [DOI] [PubMed] [Google Scholar]
- [11].Lygate CA, Fischer A, Sebag-Montefiore L, Wallis J, ten Hove M, Neubauer S. The creatine kinase energy transport system in the failing mouse heart. J Mol Cell Cardiol. 2007;42(6):1129–1136. doi: 10.1016/j.yjmcc.2007.03.899. [DOI] [PubMed] [Google Scholar]
- [12].Liao R, Nascimben L, Friedrich J, Gwathmey JK, Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ Res. 1996;78(5):893–902. doi: 10.1161/01.res.78.5.893. [DOI] [PubMed] [Google Scholar]
- [13].Herrman GD, Decherd GM. The chemical nature of heart failure. Annals of Internal Medicine. 1939;12(8):1233–1244. [Google Scholar]
- [14].Neubauer S, Remkes H, Spindler M, Horn M, Wiesmann F, Prestle J, Walzel B, Ertl G, Hasenfuss G, Wallimann T. Downregulation of the Na(+)-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation. 1999;100(18):1847–1850. doi: 10.1161/01.cir.100.18.1847. [DOI] [PubMed] [Google Scholar]
- [15].Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86(6):1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- [16].Neubauer S, Horn M, Pabst T, Godde M, Lubke D, Jilling B, Hahn D, Ertl G. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J. 1995;16(Suppl O):115–118. doi: 10.1093/eurheartj/16.suppl_o.115. [DOI] [PubMed] [Google Scholar]
- [17].Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96(7):2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- [18].Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107(24):3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- [19].Rider OJ, Francis JM, Ali MK, Holloway C, Pegg T, Robson MD, Tyler D, Byrne J, Clarke K, Neubauer S. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation. 2012;125(12):1511–1519. doi: 10.1161/CIRCULATIONAHA.111.069518. [DOI] [PubMed] [Google Scholar]
- [20].Lamb HJ, Beyerbacht HP, van der Laarse A, Stoel BC, Doornbos J, van der Wall EE, de Roos A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation. 1999;99(17):2261–2267. doi: 10.1161/01.cir.99.17.2261. [DOI] [PubMed] [Google Scholar]
- [21].Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114(11):1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lygate CA, Aksentijevic D, Dawson D, ten Hove M, Phillips D, de Bono JP, Medway DJ, Sebag-Montefiore L, Hunyor I, Channon KM, Clarke K, Zervou S, Watkins H, Balaban RS, Neubauer S. Living without creatine: unchanged exercise capacity and response to chronic myocardial infarction in creatine-deficient mice. Circ Res. 2013;112(6):945–955. doi: 10.1161/CIRCRESAHA.112.300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wallis J, Lygate CA, Fischer A, ten Hove M, Schneider JE, Sebag-Montefiore L, Dawson D, Hulbert K, Zhang W, Zhang MH, Watkins H, Clarke K, Neubauer S. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure: insights from creatine transporter-overexpressing transgenic mice. Circulation. 2005;112(20):3131–3139. doi: 10.1161/CIRCULATIONAHA.105.572990. [DOI] [PubMed] [Google Scholar]
- [24].Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, Caceres V, Shi S, Kirk JA, Su J, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122(1):291–302. doi: 10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].ten Hove M, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, Sebag-Montefiore L, Watkins H, Clarke K, Isbrandt D, Wallis J, Neubauer S. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation. 2005;111(19):2477–2485. doi: 10.1161/01.CIR.0000165147.99592.01. [DOI] [PubMed] [Google Scholar]
- [26].Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- [27].Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol. 2006;41(3):389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- [28].Sestili P, Martinelli C, Colombo E, Barbieri E, Potenza L, Sartini S, Fimognari C. Creatine as an antioxidant. Amino Acids. 2011;40(5):1385–1396. doi: 10.1007/s00726-011-0875-5. [DOI] [PubMed] [Google Scholar]
- [29].Santacruz L, Darrabie MD, Mantilla JG, Mishra R, Feger BJ, Jacobs DO. Creatine Supplementation Reduces Doxorubicin-Induced Cardiomyocellular Injury. Cardiovasc Toxicol. 2014 doi: 10.1007/s12012-014-9283-x. [DOI] [PubMed] [Google Scholar]
- [30].Aksentijevic D, Zervou S, Faller KM, McAndrew DJ, Schneider JE, Neubauer S, Lygate CA. Myocardial creatine levels do not influence response to acute oxidative stress in isolated perfused heart. PLoS ONE. 2014;9(10):e109021. doi: 10.1371/journal.pone.0109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids. 2011;40(5):1325–1331. doi: 10.1007/s00726-011-0853-y. [DOI] [PubMed] [Google Scholar]
- [32].Baroncelli L, Alessandri MG, Tola J, Putignano E, Migliore M, Amendola E, Gross C, Leuzzi V, Cioni G, Pizzorusso T. A novel mouse model of creatine transporter deficiency. F1000Res. 2014:3228. doi: 10.12688/f1000research.5369.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Skelton MR, Schaefer TL, Graham DL, Degrauw TJ, Clark JF, Williams MT, Vorhees CV. Creatine Transporter (CrT; Slc6a8) Knockout Mice as a Model of Human CrT Deficiency. PLoS One. 2011;6(1):e16187. doi: 10.1371/journal.pone.0016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cullen ME, Yuen AH, Felkin LE, Smolenski RT, Hall JL, Grindle S, Miller LW, Birks EJ, Yacoub MH, Barton PJ. Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: potential implications for local creatine synthesis. Circulation. 2006;114(1 Suppl):I16–20. doi: 10.1161/CIRCULATIONAHA.105.000448. [DOI] [PubMed] [Google Scholar]
- [35].Dai W, Vinnakota S, Qian X, Kunze DL, Sarkar HK. Molecular characterization of the human CRT-1 creatine transporter expressed in Xenopus oocytes. Arch Biochem Biophys. 1999;561(1):75–84. doi: 10.1006/abbi.1998.0959. [DOI] [PubMed] [Google Scholar]
- [36].Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224(1-2):169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- [37].Kristensen ASAJ, Jørgensen TN, Sørensen L, Eriksen J, Loland CJ, Strømgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63(3):585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- [38].Speer O, Neukomm LJ, Murphy RM, Zanolla E, Schlattner U, Henry H, Snow RJ, Wallimann T. Creatine transporters: a reappraisal. Mol Cell Biochem. 2004;256-257(1-2):407–424. doi: 10.1023/b:mcbi.0000009886.98508.e7. [DOI] [PubMed] [Google Scholar]
- [39].Boehm E, Chan S, Monfared M, Wallimann T, Clarke K, Neubauer S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am J Physiol Endocrinol Metab. 2003;284(2):E399–406. doi: 10.1152/ajpendo.00259.2002. [DOI] [PubMed] [Google Scholar]
- [40].Cooper R, Naclerio F, Allgrove J, Jimenez A. Creatine supplementation with specific view to exercise/sports performance: an update. J Int Soc Sports Nutr. 2012;9(1):33. doi: 10.1186/1550-2783-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 2001;53(2):161–176. [PubMed] [Google Scholar]
- [42].Horn M, Frantz S, Remkes H, Laser A, Urban B, Mettenleiter A, Schnackerz K, Neubauer S. Effects of chronic dietary creatine feeding on cardiac energy metabolism and on creatine content in heart, skeletal muscle, brain, liver and kidney. J Mol Cell Cardiol. 1998;30(2):277–284. doi: 10.1006/jmcc.1997.0590. [DOI] [PubMed] [Google Scholar]
- [43].Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stockler-Ipsiroglu S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 2001;69(15):1805–1815. doi: 10.1016/s0024-3205(01)01268-1. [DOI] [PubMed] [Google Scholar]
- [44].Osbakken M, Ito K, Zhang D, Ponomarenko I, Ivanics T, Jahngen EG, Cohn M. Creatine and cyclocreatine effects on ischemic myocardium: 31P nuclear magnetic resonance evaluation of intact heart. Cardiology. 1992;80(3-4):184–195. doi: 10.1159/000175002. [DOI] [PubMed] [Google Scholar]
- [45].Sharov VG, Afonskaya NI, Ruda MY, Cherpachenko NM, Pozin E, Markosyan RA, Shepeleva II, Samarenko MB, Saks VA. Protection of ischemic myocardium by exogenous phosphocreatine (neoton): pharmacokinetics of phosphocreatine, reduction of infarct size, stabilization of sarcolemma of ischemic cardiomyocytes, and antithrombotic action. Biochem Med Metab Biol. 1986;35(1):101–114. doi: 10.1016/0885-4505(86)90064-2. [DOI] [PubMed] [Google Scholar]
- [46].Sharov VG, Saks VA, Kupriyanov VV, Lakomkin VL, Kapelko VI, Steinschneider A, Javadov SA. Protection of ischemic myocardium by exogenous phosphocreatine. I. Morphologic and phosphorus 31-nuclear magnetic resonance studies. J Thorac Cardiovasc Surg. 1987;94(5):749–761. [PubMed] [Google Scholar]
- [47].Thelin S, Hultman J, Ronquist G, Juhlin C, Hansson HE, Lindgren PG. Improved myocardial protection by creatine phosphate in cardioplegic solution. An in vivo study in the pig during normothermic ischemia. Thorac Cardiovasc Surg. 1987;35(3):137–142. doi: 10.1055/s-2007-1020217. [DOI] [PubMed] [Google Scholar]
- [48].Robinson LA, Braimbridge MV, Hearse DJ. Enhanced myocardial protection with high-energy phosphates in St. Thomas’ Hospital cardioplegic solution. Synergism of adenosine triphosphate and creatine phosphate. J Thorac Cardiovasc Surg. 1987;93(3):415–427. [PubMed] [Google Scholar]
- [49].Cisowski M, Bochenek A, Kucewicz E, Wnuk-Wojnar AM, Morawski W, Skalski J, Grzybek H. The use of exogenous creatine phosphate for myocardial protection in patients undergoing coronary artery bypass surgery. J Cardiovasc Surg (Torino) 1996;37(6 Suppl 1):75–80. [PubMed] [Google Scholar]
- [50].Ruda M, Samarenko MB, Afonskaya NI, Saks VA. Reduction of ventricular arrhythmias by phosphocreatine (Neoton) in patients with acute myocardial infarction. Am Heart J. 1988;116(2 Pt 1):393–397. doi: 10.1016/0002-8703(88)90611-4. [DOI] [PubMed] [Google Scholar]
- [51].Tokarska-Schlattner M, Epand RF, Meiler F, Zandomeneghi G, Neumann D, Widmer HR, Meier BH, Epand RM, Saks V, Wallimann T, Schlattner U. Phosphocreatine interacts with phospholipids, affects membrane properties and exerts membrane-protective effects. PLoS One. 2012;7(8):e43178. doi: 10.1371/journal.pone.0043178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Horn M, Remkes H, Dienesch C, Hu K, Ertl G, Neubauer S. Chronic high-dose creatine feeding does not attenuate left ventricular remodeling in rat hearts post-myocardial infarction. Cardiovasc Res. 1999;43(1):117–124. doi: 10.1016/s0008-6363(99)00075-9. [DOI] [PubMed] [Google Scholar]
- [53].Gordon A, Hultman E, Kaijser L, Kristjansson S, Rolf CJ, Nyquist O, Sylven C. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res. 1995;30(3):413–418. [PubMed] [Google Scholar]
- [54].Andrews R, Greenhaff P, Curtis S, Perry A, Cowley AJ. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J. 1998;19(4):617–622. doi: 10.1053/euhj.1997.0767. [DOI] [PubMed] [Google Scholar]
- [55].Webster I, Du Toit EF, Huisamen B, Lochner A. The effect of creatine supplementation on myocardial function, mitochondrial respiration and susceptibility to ischaemia/reperfusion injury in sedentary and exercised rats. Acta Physiol (Oxf) 2012;206(1):6–19. doi: 10.1111/j.1748-1716.2012.02463.x. [DOI] [PubMed] [Google Scholar]
- [56].Graef JL, Smith AE, Kendall KL, Fukuda DH, Moon JR, Beck TW, Cramer JT, Stout JR. The effects of four weeks of creatine supplementation and high-intensity interval training on cardiorespiratory fitness: a randomized controlled trial. J Int Soc Sports Nutr. 2009;6:18. doi: 10.1186/1550-2783-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Murphy AJ, Watsford ML, Coutts AJ, Richards DA. Effects of creatine supplementation on aerobic power and cardiovascular structure and function. J Sci Med Sport. 2005;8(3):305–313. doi: 10.1016/s1440-2440(05)80041-6. [DOI] [PubMed] [Google Scholar]
- [58].Ireland Z, Dickinson H, Snow R, Walker DW. Maternal creatine: does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am J Obstet Gynecol. 2008;198(4):431 e431–436. doi: 10.1016/j.ajog.2007.10.790. [DOI] [PubMed] [Google Scholar]
- [59].Bender A, Beckers J, Schneider I, Holter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, Quintanilla-Martinez L, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Schmidt J, Klopstock T. Creatine improves health and survival of mice. Neurobiol Aging. 2008;29(9):1404–1411. doi: 10.1016/j.neurobiolaging.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [60].Lunardi G, Parodi A, Perasso L, Pohvozcheva AV, Scarrone S, Adriano E, Florio T, Gandolfo C, Cupello A, Burov SV, Balestrino M. The creatine transporter mediates the uptake of creatine by brain tissue, but not the uptake of two creatine-derived compounds. Neuroscience. 2006;142(4):991–997. doi: 10.1016/j.neuroscience.2006.06.058. [DOI] [PubMed] [Google Scholar]
- [61].Garbati P, Adriano E, Salis A, Ravera S, Damonte G, Millo E, Balestrino M. Effects of amide creatine derivatives in brain hippocampal slices, and their possible usefulness for curing creatine transporter deficiency. Neurochem Res. 2014;39(1):37–45. doi: 10.1007/s11064-013-1188-8. [DOI] [PubMed] [Google Scholar]
- [62].Roberts JJ, Walker JB. Feeding a creatine analogue delays ATP depletion and onset of rigor in ischemic heart. Am J Physiol. 1982;243(6):H911–916. doi: 10.1152/ajpheart.1982.243.6.H911. [DOI] [PubMed] [Google Scholar]
- [63].Jacobstein MD, Gerken TA, Bhat AM, Carlier PG. Myocardial protection during ischemia by prior feeding with the creatine analog: cyclocreatine. J Am Coll Cardiol. 1989;14(1):246–251. doi: 10.1016/0735-1097(89)90081-8. [DOI] [PubMed] [Google Scholar]
- [64].Elgebaly SA, Allam ME, Rossomando EF, Cordis GA, Forouhar F, Farghaly A, Kreutzer DL. Cyclocreatine inhibits the production of neutrophil chemotactic factors from isolated hearts. Am J Pathol. 1990;137(5):1233–1241. [PMC free article] [PubMed] [Google Scholar]
- [65].Houser SL, Elkerm AF, Wei Z, Doyle K, Houser D, Liu XK, Tyles E, Kaddurah-Daouk R, Elgebaly SA. Enhancement of cardiac function by cyclocreatine in models of cardiopulmonary bypass. J Mol Cell Cardiol. 1995;27(4):1065–1073. doi: 10.1016/0022-2828(95)90075-6. [DOI] [PubMed] [Google Scholar]
- [66].Oudman I, Clark JF, Brewster LM. The effect of the creatine analogue beta-guanidinopropionic acid on energy metabolism: a systematic review. PLoS One. 2013;8(1):e52879. doi: 10.1371/journal.pone.0052879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Phillips D, Ten Hove M, Schneider JE, Wu CO, Sebag-Montefiore L, Aponte AM, Lygate CA, Wallis J, Clarke K, Watkins H, Balaban RS, Neubauer S. Mice over-expressing the myocardial creatine transporter develop progressive heart failure and show decreased glycolytic capacity. J Mol Cell Cardiol. 2010;48(4):582–590. doi: 10.1016/j.yjmcc.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Santacruz L, Hernandez A, Nienaber J, Mishra R, Pinilla M, Burchette J, Mao L, Rockman HA, Jacobs DO. Normal cardiac function in mice with supraphysiological cardiac creatine levels. Am J Physiol Heart Circ Physiol. 2014;306(3):H373–H381. doi: 10.1152/ajpheart.00411.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lygate CA, Bohl S, ten Hove M, Faller KM, Ostrowski PJ, Zervou S, Medway DJ, Aksentijevic D, Sebag-Montefiore L, Wallis J, Clarke K, Watkins H, Schneider JE, Neubauer S. Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc Res. 2012;96(3):466–475. doi: 10.1093/cvr/cvs272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu F, Zhang J, Beard DA. Experimentally observed phenomena on cardiac energetics in heart failure emerge from simulations of cardiac metabolism. Proc Natl Acad Sci U S A. 2009;106(17):7143–7148. doi: 10.1073/pnas.0812768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Guerrero-Ontiveros ML, Wallimann T. Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol Cell Biochem. 1998;184(1-2):427–437. [PubMed] [Google Scholar]
- [72].Ten Hove M, Makinen K, Sebag-Montefiore L, Hunyor I, Fischer A, Wallis J, Isbrandt D, Lygate C, Neubauer S. Creatine uptake in mouse hearts with genetically altered creatine levels. J Mol Cell Cardiol. 2008;45(3):453–459. doi: 10.1016/j.yjmcc.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Loike JD, Zalutsky DL, Kaback E, Miranda AF, Silverstein SC. Extracellular creatine regulates creatine transport in rat and human muscle cells. Proc Natl Acad Sci U S A. 1988;85(3):807–811. doi: 10.1073/pnas.85.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zervou S, Ray T, Sahgal N, Sebag-Montefiore L, Cross R, Medway DJ, Ostrowski PJ, Neubauer S, Lygate CA. A role for thioredoxin-interacting protein (Txnip) in cellular creatine homeostasis. Am J Physiol Endocrinol Metab. 2013;505(2):E263–270. doi: 10.1152/ajpendo.00637.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Darrabie MD, Arciniegas AJ, Mantilla JG, Mishra R, Vera MP, Santacruz L, Jacobs DO. Exposing cardiomyocytes to subclinical concentrations of doxorubicin rapidly reduces their creatine transport. Am J Physiol Heart Circ Physiol. 2012;505(5):H539–548. doi: 10.1152/ajpheart.00108.2012. [DOI] [PubMed] [Google Scholar]
- [76].Shojaiefard M, Christie DL, Lang F. Stimulation of the creatine transporter SLC6A8 by the protein kinase mTOR. Biochem Biophys Res Commun. 2006;541(4):945–949. doi: 10.1016/j.bbrc.2006.01.055. [DOI] [PubMed] [Google Scholar]
- [77].Strutz-Seebohm N, Shojaiefard M, Christie D, Tavare J, Seebohm G, Lang F. PIKfyve in the SGK1 mediated regulation of the creatine transporter SLC6A8. Cell Physiol Biochem. 2007;20(6):729–734. doi: 10.1159/000110433. [DOI] [PubMed] [Google Scholar]
- [78].Almilaji A, Sopjani M, Elvira B, Borras J, Dermaku-Sopjani M, Munoz C, Warsi J, Lang UE, Lang F. Upregulation of the Creatine Transporter Slc6A8 by Klotho. Kidney Blood Press Res. 2014;59(6):516–525. doi: 10.1159/000368462. [DOI] [PubMed] [Google Scholar]
- [79].Fezai M, Elvira B, Borras J, Ben-Attia M, Hoseinzadeh Z, Lang F. Negative Regulation of the Creatine Transporter SLC6A8 by SPAK and OSR1. Kidney Blood Press Res. 2014;59(6):546–554. doi: 10.1159/000368465. [DOI] [PubMed] [Google Scholar]
- [80].Brown EL, Snow RJ, Wright CR, Cho Y, Wallace MA, Kralli A, Russell AP. PGC-1alpha and PGC-1beta increase CrT expression and creatine uptake in myotubes via ERRalpha. Biochim Biophys Acta. 2014;1845(12):2937–2943. doi: 10.1016/j.bbamcr.2014.08.010. [DOI] [PubMed] [Google Scholar]
- [81].Ndika JD, Martinez-Munoz C, Anand N, van Dooren SJ, Kanhai W, Smith DE, Jakobs C, Salomons GS. Post-transcriptional regulation of the creatine transporter gene: functional relevance of alternative splicing. Biochim Biophys Acta. 2014;1840(6):2070–2079. doi: 10.1016/j.bbagen.2014.02.012. [DOI] [PubMed] [Google Scholar]
- [82].Omerovic E, Bollano E, Lorentzon M, Walser M, Mattsson-Hulten L, Isgaard J. Growth hormone induces myocardial expression of creatine transporter and decreases plasma levels of IL-1beta in rats during early postinfarct cardiac remodeling. Growth Horm IGF Res. 2003;15(5):239–245. doi: 10.1016/s1096-6374(03)00012-1. [DOI] [PubMed] [Google Scholar]
- [83].Darrabie MD, Arciniegas AJ, Mishra R, Bowles DE, Jacobs DO, Santacruz L. AMPK and substrate availability regulate creatine transport in cultured cardiomyocytes. Am J Physiol Endocrinol Metab. 2011;500(5):E870–876. doi: 10.1152/ajpendo.00554.2010. [DOI] [PubMed] [Google Scholar]
- [84].Li H, Thali RF, Smolak C, Gong F, Alzamora R, Wallimann T, Scholz R, Pastor-Soler NM, Neumann D, Hallows KR. Regulation of the creatine transporter by AMP-activated protein kinase in kidney epithelial cells. Am J Physiol Renal Physiol. 2010;299(1):F167–177. doi: 10.1152/ajprenal.00162.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Straumann N, Wind A, Leuenberger T, Wallimann T. Effects of N-linked glycosylation on the creatine transporter. Biochem J. 2006;595(Pt 2):459–469. doi: 10.1042/BJ20050857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang W, Jobst MA, Bell B, Zhao CR, Shang LH, Jacobs DO. Cr supplementation decreases tyrosine phosphorylation of the CreaT in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab. 2002;282(5):E1046–1054. doi: 10.1152/ajpendo.00506.2001. [DOI] [PubMed] [Google Scholar]
- [87].Zhao CR, Shang L, Wang W, Jacobs DO. Myocellular creatine and creatine transporter serine phosphorylation after starvation. J Surg Res. 2002;105(1):10–16. doi: 10.1006/jsre.2002.6431. [DOI] [PubMed] [Google Scholar]
- [88].Walzel B, Speer O, Boehm E, Kristiansen S, Chan S, Clarke K, Magyar JP, Richter EA, Wallimann T. New creatine transporter assay and identification of distinct creatine transporter isoforms in muscle. Am J Physiol Endocrinol Metab. 2002;285(2):E390–401. doi: 10.1152/ajpendo.00428.2001. [DOI] [PubMed] [Google Scholar]
- [89].Gianella-Borradori M, Christou I, Bataille CJ, Cross RL, Wynne GM, Greaves DR, Russell AJ. Ligand-based virtual screening identifies a family of selective cannabinoid receptor 2 agonists. Bioorg Med Chem. 2015;25(1):241–263. doi: 10.1016/j.bmc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stahura FL, Bajorath J. New methodologies for ligand-based virtual screening. Curr Pharm Des. 2005;11(9):1189–1202. doi: 10.2174/1381612053507549. [DOI] [PubMed] [Google Scholar]
- [91].Freedman JC, Novak TS. Optical measurement of membrane potential in cells, organelles, and vesicles. Methods Enzymol. 1989;172:102–122. doi: 10.1016/s0076-6879(89)72011-5. [DOI] [PubMed] [Google Scholar]
- [92].Ndika JD, Lusink V, Beaubrun C, Kanhai W, Martinez-Munoz C, Jakobs C, Salomons GS. Cloning and characterization of the promoter regions from the parent and paralogous creatine transporter genes. Gene. 2014;533(2):488–493. doi: 10.1016/j.gene.2013.10.008. [DOI] [PubMed] [Google Scholar]