Summary
Background
We hypothesized that regulatory T cells (Tregs) are involved in the immunological abnormalities seen in patients with polymorphic light eruption (PLE).
Objectives
To investigate the number and suppressive function of peripheral Tregs in patients with PLE compared with healthy controls.
Methods
Blood sampling was done in 30 patients with PLE [seeking or not seeking 311-nm ultraviolet (UV)B photohardening] as well as 19 healthy controls at two time points: TP1, March to June (before phototherapy); and TP2, May to August (after phototherapy). We compared the number of CD4+CD25highCD127−FoxP3+ Tregs by flow cytometry and their function by assessing FoxP3 mRNA levels and effector T cell/Treg suppression assays.
Results
Tregs isolated from healthy controls significantly suppressed the proliferation of effector T cells at TP1 by 68% (P = 0·0156). In contrast, Tregs from patients with PLE entirely lacked the capacity to suppress effector T-cell proliferation at that time point. The medical photohardening seen in 23 patients with PLE resulted in a significant increase in the median percentage of circulating Tregs [both as a proportion of all lymphocytes; 65 6% increase (P = 0·0049), and as a proportion of CD4+ T cells; 32.5% increase (P = 0·0049)]. This was accompanied by an increase in the expression of FoxP3 mRNA (P = 0·0083) and relative immunosuppressive function of Tregs (P = 0·083) comparing the two time points in representative subsets of patients with healthy controls tested. Seven patients with PLE not receiving 311-nm UVB also exhibited an increase in the number of Tregs but this was not statistically significant. No significant differences in Treg numbers were observed in healthy subjects between the two time points.
Conclusions
An impaired Treg function is likely to play a role in PLE pathogenesis. A UV-induced increase in the number of Tregs (either naturally or therapeutically) may be a compensatory mechanism by which the immune system counteracts the susceptibility to PLE.
Polymorphic light eruption (PLE) is a common photosensitivity disorder with a known female preponderance.1–4 The disease is characterized by itchy, self-limiting, nonscarring skin lesions appearing on exposed body sites several hours to days after the first sun exposure of the season. PLE usually appears in early or late spring, but a so-called ‘hardening effect’ arises as summer progresses, resulting in reduced and less intense lesions.5,6 Medical photo(chemo)therapy [including psoralen plus ultraviolet (UV) A, UVB broadband or narrowband 311-nm UVB] simulates this naturally occurring phenomenon of hardening and aims to induce photoadaption with regulated doses of UV radiation (UVR) without inducing the manifestation of the disease.7,8
The immunological events that arise in the skin after UVR lead to activation the of CD4+CD25+CTLA-4+FoxP3+ regulatory T cells (Tregs), controlling inflammation and adaptive immunity by their immunosuppressive capacity.9–13 This may prevent autoreactive, inflammatory responses that could develop against modified lipids, proteins and/or DNA following exposure to UVR.1,2,14 There is now convincing evidence that Tregs play a major protective role in the pathogenesis of a number of inflammatory skin diseases including psoriasis, atopic dermatitis and lupus.15–17 The normal immune suppressive response to UVR is defective in patients with PLE,18–20 which is thought to lead to an inflammatory reaction that results in a recurrent, pruritic skin rash typical of the condition following exposure to sunlight. We hypothesized that patients with PLE might have abnormal Treg levels and/or function.2 In this study we show that successful medical photohardening of patients with PLE resulted in a significant increase in the percentage of FoxP3+ Tregs in the blood, which was also reflected at the mRNA level. Compared with healthy controls, the suppressive function of Tregs from patients with PLE was defective. However, after phototherapy these patients displayed a trend towards an increased suppressive function of Tregs.
Patients and methods
Study setup
This study was conducted at the Photodermatology Unit, Medical University of Graz, Austria to investigate the levels and function of Tregs in patients with PLE and healthy controls (ClinicalTrials.Gov registration number NCT00555178). The following null and alternative hypotheses were tested: H0, < 30% increase and HA, ≥ 30% increase in Treg levels comparing baseline with after-phototherapy treatment. Sample-size calculations were based on the data from a previous study21 and were performed using the Power/Sample Size Calculator (Institute of Medical Statistics, Medical University Vienna, Austria) with an α error of 0·05 and a power of 0·8. By assuming a dropout rate of 10% this resulted in a group size of 23.
The inclusion criteria for the patients with PLE was age above 18 years and good general health status. The diagnosis of PLE had to be confirmed by the patient’s history, histological findings and/or phototesting procedures. Physician-guided patient history leading to diagnosis of PLE included questioning of patients on (i) the typical formation of itchy skin lesions located on sun-exposed body sites such as the V of the neck, the back of the hands, the outside surface of the arms and lower legs, appearing within hours after sun exposure; (ii) resolution of the lesions without scarring within a few days; (iii) and the weakening or entire disappearance of symptoms as spring and summer progress.
Exclusion criteria included the presence or history of malignant skin tumours, dysplastic naevus syndrome, autoimmune diseases, systemic treatment with steroids or other immunosuppressive drugs (ongoing, within the last 6 months or planned during the study period), antinuclear antibodies (such as ds-DNA, Ro, La), specific immunotherapy (i.e. hyposensitization treatment; ongoing, within the last 6 months or planned), and pregnancy or breast-feeding. Patients seeking or not seeking photohardening therapy in spring were recruited. In addition, age-matched healthy control subjects were enrolled during the same period of time. In the initial study protocol, patients with phototherapy-responsive diseases (such as psoriasis and atopic dermatitis) represented another study group. Two additional time points in late summer and fall were also part of the protocol for all patient groups. Due to substantial losses in patient follow-up numbers at those later time points, together with large variability in the group of patients with phototherapy responsiveness, these data have been omitted from the present analysis.
The study was approved by the local Ethical Committee of the Medical University of Graz (No. 18-116 ex 06/07). All patients and controls gave informed consent and the study was conducted according to the Declaration of Helsinki principles.
Study subjects and phototherapy characteristics
Thirty patients with PLE were enrolled in this study between 2008 and 2014. Standard 311-nm UVB photohardening therapy22,23 was started in spring (immediately after the first blood collection; TP1, see below) in 23 of the patients (22 females and one male; mean age 37·4 years, range 18–75) two to three times per week for 4–9 weeks (median, 6 weeks). Seven patients with PLE (all female; mean age 42·0 years, range 21–56) chose not to receive photohardening therapy and represented an additional study group (see Supplementary Table S1 for details about patients and phototherapy). Nineteen healthy subjects (15 females and four males; mean age 38·6 years, range 24–61) without a history of PLE were enrolled and served as controls. One patient with PLE not seeking phototherapy and one healthy volunteer dropped out of the study after first blood drawing, each of them due to personal reasons. Their data are not included in the patient demographics and study analysis (Supplementary Fig. S1).
Blood sample collection and processing
Blood was collected using lithium–heparin tubes (Vacuette®; Greiner Bio-One, Kremsmünster, Austria) before the start of photohardening in March to June and within 48 h of the (pen)ultimate exposure of the photohardening treatment from May to August. The first time point (pre-photohardening) is labelled TP1 and the second time point (post-photohardening) is labelled TP2 in this report and its figures. The average time that elapsed between the first and second blood withdrawal was 48·0 days (photohardened PLE group), 57·7 days (non-photohardened PLE group) and 55·4 days (controls). Blood materials were available from all patients for flow cytometry analysis and from a subset of eight patients and eight healthy controls for suppression assays and mRNA analysis.
Peripheral blood mononuclear cells isolation and flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Lymphoprep™ (Axis-Shield, Heidelberg, Germany) for processing and analysis by flow cytometry, reverse transcriptase–polymerase chain reaction (RT-PCR) and in vitro Treg suppression assays. The following antibodies were used for sorting of PBMCs using a FACSAria IIu cell sorter (BD Biosciences, San Jose, CA, U.S.A.): fluorescein–isothiocyanate (FITC)-conjugated antihuman CD4 (clone RPA-T4), PE-Cy7-conjugated antihuman CD25 (MA251), phycoerythrin (PE)-conjugated antihuman CD127 (clone hIL-7R-M21), all from eBioscience (Vienna, Austria). We used PE-conjugated antihuman CD127 (clone hIL-7R-M21), FITC-conjugated antihuman CD25 (clone 2A3), peridinin chlorophyll protein complex (PerCP)-conjugated antihuman CD4 (clone SK3) (BD Pharmingen, San Diego, CA, U.S.A.) and allophycocyanin (APC)-conjugated antihuman FoxP3 (clone PCH101) from eBioscience for quantification of human PBMCs. Data was acquired on a FACSCalibur flow cytometer and analysed with FLOWJO software (version 7.6.5; TreeStar Inc., Ashland, OR, U.S.A.). All plots were pre-gated on CD4+ lymphocytes. The increase in percentage of Tregs as a proportion of either the CD4 subpopulation or the entire lymphocyte subpopulation was calculated by dividing TP2 by TP1.
Regulatory T-cell suppression assay
Treg suppression assays with blood samples from the subset of eight patients and eight control subjects were performed as described.24 In brief, PBMCs were stimulated with 5 μg mL−1 purified low endotoxin/sodium azide-free mouse antihuman CD3 (clone UCHT1; BD Pharmingen) and 2·5 μg mL−1 antihuman CD28 (Clone 28.2) (BioLegend, London, U.K.) for 96 h. Cellular proliferation was measured with a Wallac 1450 MicroBeta® TriLux (Perkin Elmer, Brunn am Gebirge, Austria) via tritiated thymidine incorporation (1 μCi per [3H]thymidine; Amersham Biosciences, Piscataway, NJ, U.S.A.) added for the final 16 h of the 96 h incubation. For analysis, the 1 : 1 ratio of effector T cells and Tregs was normalized to the proliferative rate of stimulated effector T cells alone (set to 100%).
RNA isolation and quantitative reverse transcriptase–polymerase chain reaction
RNA was isolated from PBMCs of a subset of patients with PLE receiving phototherapy and healthy controls (n = 8 each) using the Qiagen fibrous mini kit (Qiagen, Hilden, Germany) and transcribed using First-Strand cDNA Synthesis kit (Roche, Basel, Switzerland). Quantitative RT-PCR was performed with primers specific for human FoxP3 (forward: GCTCTGCACCT TCCCAAAT; reverse: TCTCTGGAGGAGACATTGTGC) and the GAPDH gene region as a control (forward and reverse primer, PPH00150E-200; Qiagen). The reactions were run on a 7900HT Fast Real-Time PCR System (Life Technologies, Vienna, Austria) using RT2 SYBR Green-qPCR Master Mix (Qiagen). To normalize transcripts to GAPDH and to calibrate the fold change the ΔΔCt method was used. Data are presented as fold change comparing TP2 with TP1.
Statistical analysis
Statistical differences were determined by using the Kruskal–Wallis test, the Wilcoxon signed rank test or the Mann–Whitney U-test, as appropriate for the data. Data presented are expressed as medians with range, medians with 95% confidence intervals (CIs) or box-and-whisker plots (Tukey method). All analyses were performed with Prism 5.0 (Graph-Pad Software, Inc., SD, La Jolla, CA, U.S.A.) or R 3.1.2 (www.r-project.org). Statistical significance was set at P < 0·05.
Results
Regulatory T-cell numbers are increased in patients with polymorphic light eruption after photohardening treatment
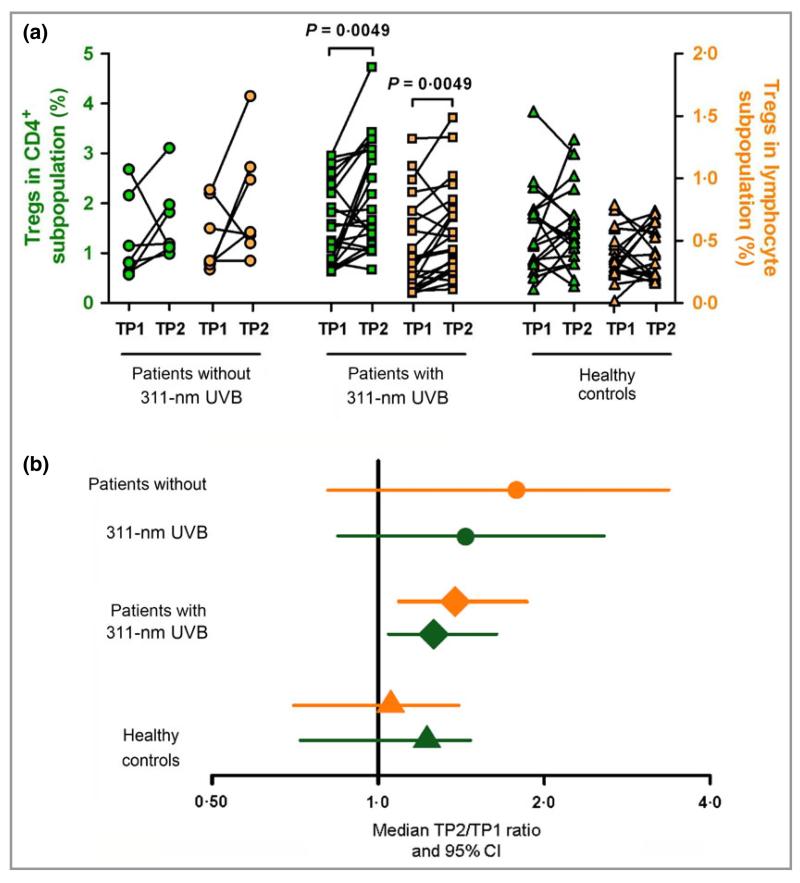
To study the role of Tregs in PLE, we first investigated their numbers by staining PBMCs of patients and controls for CD4, CD25, CD127 and FoxP3 before (Supplementary Fig. S2a) and after (Supplementary Fig. S2b) photohardening treatment. Flow cytometry analysis of PBMC from patients with PLE before (TP1) and after (TP2) medical photohardening revealed a significant increase in the median percentage of CD4+CD25+FoxP3+ Tregs in both the CD4+ subpopulation [1·26% vs. 1·67% (an increase of 32·5%); P = 0·0049] and the total lymphocyte population [0·32% vs. 0·53% (an increase of 65·6%); P = 0·0049] (see Fig. 1a and for descriptive statistics, see Supplementary Table S2). There was no statistically significant change in the number of Tregs in the other two groups when comparing TP1 with TP2 (Fig. 1a and Supplementary Table S2). Changes in Treg numbers for the three different groups are depicted in Figure 1b. These were calculated by plotting the median ratio and 95% CI between TP2 and TP1 for patients with PLE with phototherapy, patients with PLE without phototherapy and healthy control subjects. Any number above 1 is representative of an increase in Tregs during the time course. The only ratio to show a significant increase was that for patients with PLE undergoing photohardening therapy with 1·26 (95% CI, 1·04–1·64; P = 0·0083) as a proportion of CD4+ T cells, and 1·38 (95% CI, 1·09–1·86; P = 0·0015) as a proportion of all lymphocytes. However, a Kruskal–Wallis test revealed no significant differences in Treg levels among the three groups at baseline (TP1) and at TP2. There were no significant differences in total leucocyte or lymphocyte numbers comparing patients with PLE with healthy control subjects at either TP1 or TP2 or between the two time points (data not shown).
Fig 1.
Photohardening treatment increases the number of Tregs in patients with PLE. Peripheral blood mononuclear cells of patients and healthy controls were stained with antibodies for CD4, CD127, CD25 and FoxP3. (a) Percentages of Tregs as a proportion of CD4+ cells (green symbols) or all lymphocytes (orange symbols) in PLE patients with or without 311-nm UVB compared with healthy individuals at TP1 and TP2. (b) Median TP2 : TP1 ratio (± 95% CI) for the percentage of Tregs in CD4+ (green symbols) and lymphocyte (orange symbols) subpopulations from each of the three subject groups. Datasets were logarithmized before calculation of ratios. Patients without 311-nm UVB, n = 7; patients with 311-nm UVB, n = 23; healthy controls, n = 19. P-values were determined by Wilcoxon test. CI, confidence interval; PLE, polymorphic light eruption; TP1, time point 1 (before phototherapy); TP2, time point 2 (after phototherapy); Tregs, regulatory T cells; UVB, ultraviolet B.
Regulatory T-cell function is significantly impaired during spring in patients with polymorphic light eruption
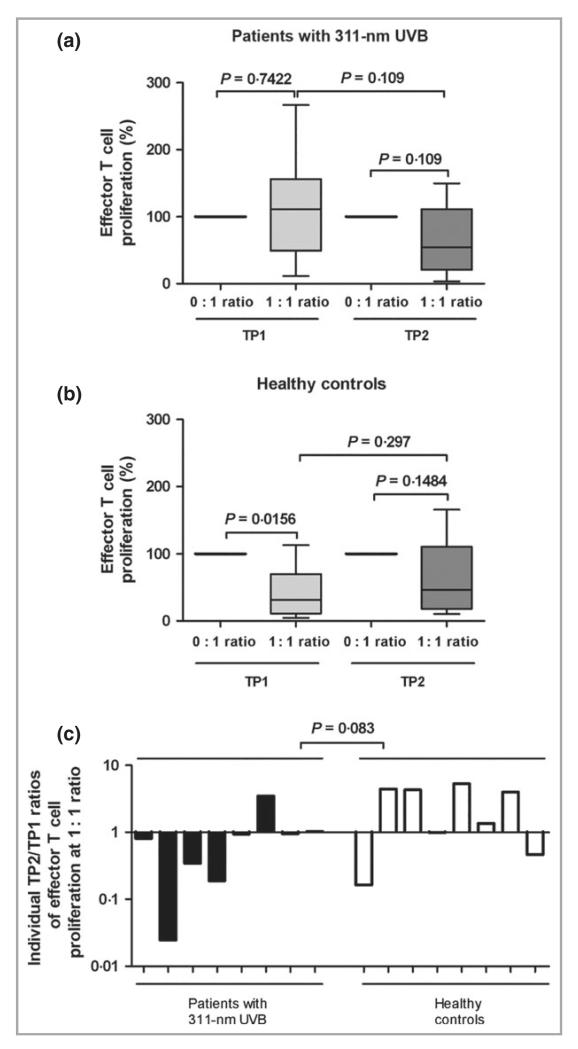
We also investigated the Treg function in a subset of eight patients receiving medical photohardening and eight healthy control subjects (see Supplementary Fig. S1) by measuring their capacity to inhibit T-cell proliferation. This subset of patients and control subjects was representative for the entire patient and control group as similar courses of Treg levels were observed for the subsets as shown for the entire groups in Figure 1a by comparing pre- vs. post-phototherapy determined by flow cytometry (i.e. a similar increase in the subset of patients with PLE and no significant change in the subset of control subjects; data not shown). Tregs from healthy controls significantly suppressed the median proliferation of CD3/CD28-stimulated effector T cells at TP1 by 68% (P = 0·0156) (100% proliferation at 0 : 1 ratio of effector T cells compared with 32% proliferation at a 1 : 1 ratio; Fig. 2b). In contrast, Tregs from patients with PLE lacked any capacity to suppress effector T-cell proliferation at this time point (Fig. 2a). No significant suppression of effector T-cell proliferation was observed at TP2 in any group of subjects. However, when the median suppressive activity over the time course was analysed we observed a change in Treg-mediated suppression in patients with PLE from 111% at TP1 to 54% at TP2. In contrast to healthy controls, where the suppressive activity over the same time course changed from 32% to 46%. For comparison, we expressed this as a ratio of the median Treg suppression at TP2 with that at TP1 (Fig. 2c). Ratios below 1 indicate a rise in suppressive activity whereas ratios above 1 indicate a fall. Analysis of individual ratios confirmed a trend towards a difference between patients receiving 311-nm UVB and healthy controls displaying a TP2/TP1 ratio with a median of 0·87 and 2·67, respectively (P = 0·083; Fig. 2c).
Fig 2.
Tregs from patients with PLE have a reduced immune suppressive capacity. Treg suppression assays from (a) patients with PLE and (b) healthy controls at TP1 and TP2. Data shown are box-and-whisker plots (Tukey method) of proliferation rates, normalized to effector T-cell proliferation alone (0 : 1 ratio = 100%) for patients with PLE and healthy controls. The 1 : 1 ratio represents the results of co-culture of the same number of effector T cells (CD4+CD25−CD127+) and Tregs (CD4+CD25+CD127−). (c) Individual TP2 : TP1 median ratios of T effector cell proliferation., n = 8. P-values were determined by Wilcoxon test (a,b) and Mann–Whitney U-test (c). PLE, polymorphic light eruption; TP1, time point 1 (before phototherapy); TP2, time point 2 (after phototherapy); Tregs, regulatory T cells.
FoxP3 mRNA levels are significantly upregulated in patients with polymorphic light eruption after phototherapy
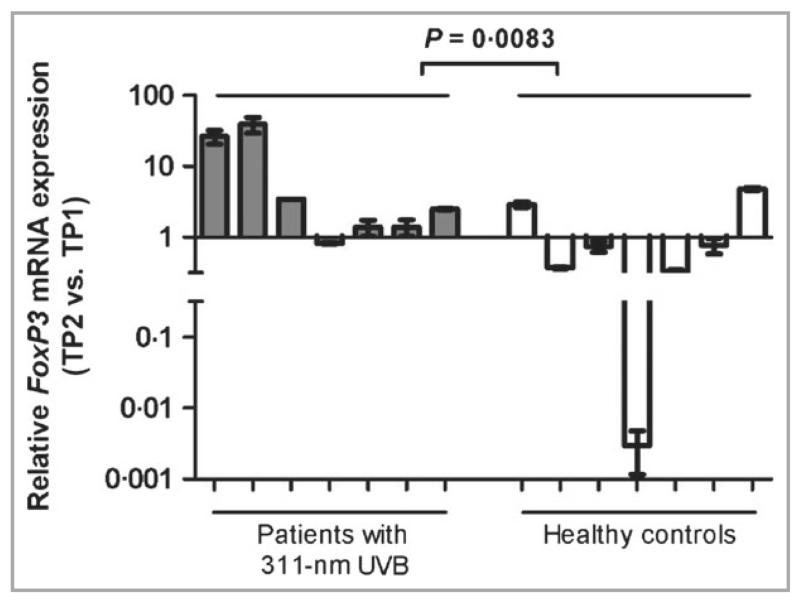
Because higher expression levels of FoxP3 correlate with enhanced Treg suppressive capacity,25 we investigated whether medical photohardening therapy affects FoxP3 mRNA levels in patients with PLE by quantitative RT-PCR. Transcripts were normalized to GAPDH and compared with TP1 for patients with PLE and controls (the same subset of patient and control groups were used as shown in Figure 2c; one patient and one healthy control subject had RNA yields that were too low to be included in this analysis). Relative FoxP3 mRNA levels were upregulated after photohardening in six of seven patients with PLE (Fig. 3). In contrast, the amount of FoxP3 mRNA decreased in five of seven healthy individuals between the two time points. This was a statistically significant difference between the two groups (P = 0·0083).
Fig 3.
311-nm UVB upregulates FoxP3 mRNA in patients with PLE. RT-PCR results from peripheral blood mononuclear cells of patients and healthy controls are shown as fold change of mRNA (TP2–TP1) (median with range). The ΔΔCt method was used to normalize transcripts to GAPDH. Patients with 311-nm UVB, n = 7; healthy controls, n = 7. P-values were determined by Mann–Whitney U-test. PLE, polymorphic light eruption; RT-PCR, reverse transcriptase–polymerase chain reaction; UVB, ultraviolet B.
Discussion
Photohardening therapy given to patients with PLE in spring enables them to tolerate their first high dose of sunlight later in the season with minimal or no eruption. We herewith provide evidence that despite having the same numbers of circulating Tregs as control subjects at the start of spring, Tregs of patients with PLE have an impaired suppressive function (Fig. 2a). This implicates Tregs in the pathogenesis of PLE and potentially explains why these patients are resistant to the normal immune suppressive properties of UVR. Because activation of Tregs is a major way in which UVR suppresses cutaneous immunity, we hypothesized that successful UVR-induced therapeutic hardening was efficacious, in part, by boosting Treg numbers and/or function. Supporting this hypothesis, we have discovered that medical photohardening with 311-nm UVB not only significantly increased the numbers of Tregs (Fig. 1a and Supplementary Table S2), but also increased Treg expression of FoxP3 mRNA, an indicator of increased suppressive function (Fig. 3). A similar increase in the level of Tregs, although not statistically significant, was also observed in patients not receiving medical phototherapy (Fig. 1). This most likely has occurred through hardening by exposure to natural sunlight. Alternatively, a seasonal effect independent of UVR exposure may have caused these changes in both groups of patients compared with healthy control subjects. A limitation of our study is the unequal group size of patients with PLE and further, that the number of enrolled patients without phototherapy was rather low.
The precise reason why patients with PLE have normal numbers of Tregs with suboptimal suppressor capabilities is unknown and the UVR-induced cutaneous signal that normalizes UVR-immune suppression in PLE remains to be determined. One possibility is that genetic factors might play a role.26–30 Genetic regulation might alter the normal response to UVR in patients through modulation of the immune suppressive function of patients directly by affecting Tregs or indirectly by affecting cells or mediators that recruit or activate Tregs.
For instance, glutathione-S-transferase (GST), which is an enzyme that detoxifies reactive oxygen species (ROS), was reported to exert a possible protective effect against PLE because the carrier frequency of the GSTP1 allele was found to be lower in patients than in controls, which would support an involvement of ROS in the pathogenesis of the disease.31 This was in contrast to another study that could not find an association between the GST gene family and PLE.32 Guarrera and Rebora investigated the hydrosoluble antioxidant capacity which included uric acid, bilirubin, vitamin C, thyols and glutathione and found decreased rates in patients with PLE and moreover lower values in female patients and controls compared with males, which increased in diseased women, with age.33 That PLE has a disproportionately higher incidence in young women is known, but the reason is unclear. Sex hormones like 17beta-estradiol prevent UVR-induced suppression of contact hypersensitivities through limiting interleukin (IL)-10 secretion from keratinocytes.34,35 This is substantiated by a study of Widyarini et al.36 who demonstrated a natural photoimmunoprotective role of the oestrogen receptor, as blocking it exacerbated the immune suppression from solar-simulated UVR. Further studies are required to investigate a possible gene link to sex influence which certainly may not be solely responsible for the pathogenesis of PLE, as our control group, which was well matched and mostly composed of women, did exhibit a differential Treg regulation.
Clues from murine models inform us that dermal mast cell density determines one’s susceptibility to UVR immune suppression.37 This in turn is dependent on the migration of mast cells into and away from the skin to activate suppressor cells in the local draining lymph nodes.38 While the precise mechanism by which dermal mast cells mediate UVR-induced immune suppression is not known, their production of immunoregulatory IL-10 following UVR is involved.39,40 Alternatively, mast cells might activate Tregs that are required to maintain peripheral tolerance.41,42 Intriguingly, we recently made the observation that photohardening increased the numbers of mast cells in the papillary dermis of patients with PLE.43 This was accompanied by a recovery of neutrophil responsiveness to the chemoattractants leukotriene B4 and formyl-methionyl-leucyl-phenylalanin.44 That PLE is linked to low numbers of Tregs is supported by a recent observation by Gambichler et al.,45 showing a low Treg infiltration together with a decreased expression of the immunoregulatory factors transforming growth factor-β1, IL-10 and receptor activator of nuclear factor-κB ligand in UVA1-induced skin lesions of patients with PLE. Furthermore, Gruber-Wackernagel et al.46 have shown that lesional skin from patients with PLE contained relatively low numbers of Foxp3+ cells in the dermal infiltrate compared with calcipotriol-pretreated skin, which showed less clinical PLE severity. The observation that 311-nm UVB increased Treg levels in patients with PLE is consistent with the results of a recent study in patients with various skin diseases who showed an increase in peripheral Tregs after treatment with 311-nm UVB.47 The mechanism by which UVR photohardening increases the level and function of Tregs may involve the production of vitamin D. Indeed, its supplementation has been shown to be associated with significantly increased numbers of Tregs in apparently healthy individuals.48,49 That said, a previous study22 from our laboratory has indicated that 311-nm UVB hardening was capable of increasing serum 25-hydroxyvitamin-D3 levels which may be low in patients with photosensitivity and cases of photodermatoses, including PLE.50–52
We conclude that a decreased function of Tregs might play a role in the formation of PLE and an increase in Treg numbers might be a compensatory mechanism by which the immune system intends to counteract the susceptibility to PLE formation.
Supplementary Material
What’s already known about this topic?
Patients with polymorphic light eruption (PLE) display immunological abnormalities.
Previous studies have shown that they are resistant to the immune suppressive effects of sunlight.
What does this study add?
We found that the number and suppressive function of regulatory T cells (Tregs) are crucial in the pathogenesis of PLE.
An increase in Treg levels (after photohardening) might be a compensatory mechanism by which the immune system intends to counteract the susceptibility to PLE formation.
Acknowledgments
The authors thank Dr Heimo Strohmaier, Flow Cytometry Core Facility, Center for Medical Research (ZMF), Medical University of Graz and Katharina Eberhard MA, Office of Biostatistics, Center for Medical Research (ZMF), Medical University of Graz for advice and/or support in this study and Dr Angelika Hofer and Dr Franz J. Legat for help in enrolment and treatment of patients.
Funding sources
This work was supported by the Österreichische Nationalbank Anniversary Fund project no. 13279 and Austrian Science Fund (FWF): project number KLI 132-B00. N.S. and E.R. were supported through the PhD programme of the Medical University of Graz, Graz, Austria.
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s website
Conflicts of interest
None declared.
References
- 1.Gruber-Wackernagel A, Byrne SN, Wolf P. Polymorphous light eruption: clinic aspects and pathogenesis. Dermatol Clin. 2014;32:315–34. doi: 10.1016/j.det.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Wolf P, Byrne SN, Gruber-Wackernagel A. New insights into the mechanisms of polymorphic light eruption: resistance to ultraviolet radiation-induced immune suppression as an aetiological factor. Exp Dermatol. 2009;18:350–6. doi: 10.1111/j.1600-0625.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 3.Stratigos A, Antoniou C, Katsambas A. Polymorphous light eruption. J Eur Acad Dermatol Venereol. 2002;16:193–206. doi: 10.1046/j.1468-3083.2002.00443.x. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes LE, Bock M, Janssens AS, et al. Polymorphic light eruption occurs in 18% of Europeans and does not show higher prevalence with increasing latitude: multicenter survey of 6,895 individuals residing from the Mediterranean to Scandinavia. J Invest Dermatol. 2010;130:626–8. doi: 10.1038/jid.2009.250. [DOI] [PubMed] [Google Scholar]
- 5.Tutrone WD, Spann CT, Scheinfeld N, et al. Polymorphic light eruption. Dermatol Ther. 2003;16:28–39. doi: 10.1046/j.1529-8019.2003.01605.x. [DOI] [PubMed] [Google Scholar]
- 6.Hönigsmann H. Polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2008;24:155–61. doi: 10.1111/j.1600-0781.2008.00343.x. [DOI] [PubMed] [Google Scholar]
- 7.Naleway AL. Polymorphous light eruption. Int J Dermatol. 2002;41:377–83. doi: 10.1046/j.1365-4362.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 8.Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop Report. Br J Dermatol. 2004;151:283–97. doi: 10.1111/j.1365-2133.2004.06128.x. [DOI] [PubMed] [Google Scholar]
- 9.Umetsu DT, Akbari O, Dekruyff RH. Regulatory T cells control the development of allergic disease and asthma. J Allergy Clin Immunol. 2003;112:480–7. [PubMed] [Google Scholar]
- 10.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz A, Navid F, Sparwasser T, et al. In vivo reprogramming of UV radiation-induced regulatory T-cell migration to inhibit the elicitation of contact hypersensitivity. J Allergy Clin Immunol. 2011;128:826–33. doi: 10.1016/j.jaci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz A, Schwarz T. UVR-induced regulatory T cells switch antigen-presenting cells from a stimulatory to a regulatory phenotype. J Invest Dermatol. 2010;130:1914–21. doi: 10.1038/jid.2010.59. [DOI] [PubMed] [Google Scholar]
- 13.Maeda A, Beissert S, Schwarz T, et al. Phenotypic and functional characterization of ultraviolet radiation-induced regulatory T cells. J Immunol. 2008;180:3065–71. doi: 10.4049/jimmunol.180.5.3065. [DOI] [PubMed] [Google Scholar]
- 14.Hofer A, Legat FJ, Gruber-Wackernagel A, et al. Topical liposomal DNA-repair enzymes in polymorphic light eruption. Photochem Photobiol Sci. 2011;10:1118–28. doi: 10.1039/c1pp05009e. [DOI] [PubMed] [Google Scholar]
- 15.Valencia X, Yarboro C, Illei G, et al. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 16.Ou L-S, Goleva E, Hall C, et al. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004;113:756–63. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama H, Gyulai R, Toichi E, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer RA, Friedmann PS. Ultraviolet radiation causes less immunosuppression in patients with polymorphic light eruption than in controls. J Invest Dermatol. 2004;122:291–4. doi: 10.1046/j.0022-202X.2004.22213.x. [DOI] [PubMed] [Google Scholar]
- 19.Van de Pas CB, Kelly DA, Seed PT, et al. Ultraviolet-radiation-induced erythema and suppression of contact hypersensitivity responses in patients with polymorphic light eruption. J Invest Dermatol. 2004;122:295–9. doi: 10.1046/j.0022-202X.2004.22201.x. [DOI] [PubMed] [Google Scholar]
- 20.Koulu LM, Laihia JK, Peltoniemi H-H, et al. UV-induced tolerance to a contact allergen is impaired in polymorphic light eruption. J Invest Dermatol. 2010;130:2578–82. doi: 10.1038/jid.2010.181. [DOI] [PubMed] [Google Scholar]
- 21.Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 22.Gruber-Wackernagel A, Obermayer-Pietsch B, Byrne SN, et al. Patients with polymorphic light eruption have decreased serum levels of 25-hydroxyvitamin-D3 that increase upon 311 nm UVB photohardening. Photochem Photobiol Sci. 2012;11:1831–6. doi: 10.1039/c2pp25188d. [DOI] [PubMed] [Google Scholar]
- 23.Wolf P, Gruber-Wackernagel A, Rinner B, et al. Phototherapeutic hardening modulates systemic cytokine levels in patients with polymorphic light eruption. Photochem Photobiol Sci. 2013;12:166–73. doi: 10.1039/c2pp25187f. [DOI] [PubMed] [Google Scholar]
- 24.Singh TP, Schön MP, Wallbrecht K, et al. 8-methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+ regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder. J Immunol. 2010;184:7257–67. doi: 10.4049/jimmunol.0903719. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 26.Millard TP, Bataille V, Snieder H, et al. The heritability of polymorphic light eruption. J Invest Dermatol. 2000;115:467–70. doi: 10.1046/j.1523-1747.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- 27.McGregor JM, Grabczynska S, Vaughan R, et al. Genetic modeling of abnormal photosensitivity in families with polymorphic light eruption and actinic prurigo. J Invest Dermatol. 2000;115:471–6. doi: 10.1046/j.1523-1747.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 28.Millard TP, Kondeatis E, Vaughan RW, et al. Polymorphic light eruption and the HLA DRB1*0301 extended haplotype are independent risk factors for cutaneous lupus erythematosus. Lupus. 2001;10:473–9. doi: 10.1191/096120301678416024. [DOI] [PubMed] [Google Scholar]
- 29.Millard TP, Kondeatis E, Cox A, et al. A candidate gene analysis of three related photosensitivity disorders: cutaneous lupus erythematosus, polymorphic light eruption and actinic prurigo. Br J Dermatol. 2001;145:229–36. doi: 10.1046/j.1365-2133.2001.04339.x. [DOI] [PubMed] [Google Scholar]
- 30.Millard TP, Lewis CM, Khamashta MA, et al. Familial clustering of polymorphic light eruption in relatives of patients with lupus erythematosus: evidence of a shared pathogenesis. Br J Dermatol. 2001;144:334–8. doi: 10.1046/j.1365-2133.2001.03897.x. [DOI] [PubMed] [Google Scholar]
- 31.Millard TP, Fryer AA, McGregor JM. A protective effect of glutathione-S-transferase GSTP1*Val(105) against polymorphic light eruption. J Invest Dermatol. 2008;128:1901–5. doi: 10.1038/jid.2008.14. [DOI] [PubMed] [Google Scholar]
- 32.Zirbs M, Pürner C, Buters JTM, et al. GSTM1, GSTT1 and GSTP1 gene polymorphism in polymorphous light eruption. J Eur Acad Dermatol Venereol. 2013;27:157–62. doi: 10.1111/j.1468-3083.2011.04431.x. [DOI] [PubMed] [Google Scholar]
- 33.Guarrera M, Rebora A. Serum antioxidant capacity in polymorphic light eruption. Acta Derm Venereol. 2007;87:228–30. doi: 10.2340/00015555-0229. [DOI] [PubMed] [Google Scholar]
- 34.Aubin F. Why is polymorphous light eruption so common in young women? Arch Dermatol Res. 2004;296:240–1. doi: 10.1007/s00403-004-0508-x. [DOI] [PubMed] [Google Scholar]
- 35.Hiramoto K, Tanaka H, Yanagihara N, et al. Effect of 17beta-estradiol on immunosuppression induced by ultraviolet B irradiation. Arch Dermatol Res. 2004;295:307–11. doi: 10.1007/s00403-003-0437-0. [DOI] [PubMed] [Google Scholar]
- 36.Widyarini S, Domanski D, Painter N, et al. Estrogen receptor signaling protects against immune suppression by UV radiation exposure. Proc Natl Acad Sci U S A. 2006;103:12837–42. doi: 10.1073/pnas.0603642103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart PH, Grimbaldeston MA, Swift GJ, et al. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–53. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrne SN, Limón-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–55. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimbaldeston MA, Nakae S, Kalesnikoff J, et al. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 40.Chacón-Salinas R, Limón-Flores AY, Chávez-Blanco AD, et al. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J Immunol. 2011;186:25–31. doi: 10.4049/jimmunol.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L-F, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 42.Schweintzger N, Bambach I, Reginato E, et al. Mast cells are required for phototolerance induction and scratching abatement. Exp Dermatol. 2015;24:491–30. doi: 10.1111/exd.12687. [DOI] [PubMed] [Google Scholar]
- 43.Wolf P, Gruber-Wackernagel A, Bambach I, et al. Photohardening of polymorphic light eruption patients decreases baseline epidermal Langerhans cell density while increasing mast cell numbers in the papillary dermis. Exp Dermatol. 2014;23:428–30. doi: 10.1111/exd.12427. [DOI] [PubMed] [Google Scholar]
- 44.Gruber-Wackernagel A, Heinemann A, Konya V, et al. Photohardening restores the impaired neutrophil responsiveness to chemoattractants leukotriene B4 and formyl-methionyl-leucyl-phenylalanin in patients with polymorphic light eruption. Exp Dermatol. 2011;20:473–6. doi: 10.1111/j.1600-0625.2011.01264.x. [DOI] [PubMed] [Google Scholar]
- 45.Gambichler T, Terras S, Kampilafkos P, et al. T regulatory cells and related immunoregulatory factors in polymorphic light eruption following ultraviolet al challenge. Br J Dermatol. 2013;169:1288–94. doi: 10.1111/bjd.12608. [DOI] [PubMed] [Google Scholar]
- 46.Gruber-Wackernagel A, Bambach I, Legat FJ, et al. Randomized double-blinded placebo-controlled intra-individual trial on topical treatment with a 1,25-dihydroxyvitamin D3 analogue in polymorphic light eruption. Br J Dermatol. 2011;165:152–63. doi: 10.1111/j.1365-2133.2011.10333.x. [DOI] [PubMed] [Google Scholar]
- 47.Milliken SVI, Wassall H, Lewis BJ, et al. Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function. J Allergy Clin Immunol. 2012;129:1554–61. doi: 10.1016/j.jaci.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Prietl B, Pilz S, Wolf M, et al. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J. 2010;12:136–9. [PubMed] [Google Scholar]
- 49.Bock G, Prietl B, Mader JK, et al. The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: a randomized controlled trial. Diabetes Metab Res Rev. 2011;27:942–5. doi: 10.1002/dmrr.1276. [DOI] [PubMed] [Google Scholar]
- 50.Reid SM, Robinson M, Kerr AC, et al. Prevalence and predictors of low vitamin D status in patients referred to a tertiary photodiagnostic service: a retrospective study. Photodermatol Photoimmunol Photomed. 2012;28:91–6. doi: 10.1111/j.1600-0781.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- 51.Rhodes LE, Webb AR, Berry JL, et al. Sunlight exposure behaviour and vitamin D status in photosensitive patients: longitudinal comparative study with healthy individuals at U.K. latitude. Br J Dermatol. 2014;171:1478–86. doi: 10.1111/bjd.13325. [DOI] [PubMed] [Google Scholar]
- 52.Lim HW, Snauwaert JJL. Vitamin D and photodermatoses. Br J Dermatol. 2014;171:1297–8. doi: 10.1111/bjd.13479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.