Abstract
Connexin proteins are abundantly present in the digestive system. They primarily form gap junctions, which control the intercellular exchange of critical homeostasis regulators. By doing so, gap junctions drive a plethora of gastrointestinal and hepatic functional features, including gastric and gut motility, gastric acid secretion, intestinal innate immune defense, xenobiotic biotransformation, glycogenolysis, bile secretion, ammonia detoxification and plasma protein synthesis. In the last decade, it has become clear that connexin hemichannels, which are the structural precursors of gap junctions, also provide a pathway for cellular communication, namely between the cytosol and the extracellular environment. Although merely pathological functions have been described, some physiological roles have been attributed to connexin hemichannels, in particular in the modulation of colonic motility. This equally holds true for cellular channels composed of pannexins, connexin-like proteins recently identified in the intestine and the liver, which have become acknowledged key players in inflammatory processes and that have been proposed to control colonic motility, secretion and blood flow.
Keywords: Stomach, Intestine, Liver, Pannexin, Connexin, Physiology
Introduction
Like in all other organs, homeostasis in the digestive system is dictated by the interplay between intracellular, extracellular and intercellular communication networks. Direct intercellular communication is typically governed by gap junctions, composed of 2 hemichannels of neighboring cells, which control the diffusion of small and hydrophilic chemical substances between adjacent cells. This flux is called gap junctional intercellular communication (GJIC) and involves several second messengers, such as adenosine triphosphate (ATP), cyclic adenosine monophosphate (cAMP) and inositol triphosphate (IP3) as well as ions, including calcium and sodium [1-3]. Gap junctions have been first described in 1967 in liver cells [4,5]. In 1974, Goodenough isolated 2 gap junctional proteins from mouse liver and called them connexin (Cx) proteins [6]. The cloning of these first 2 connexins from rat liver was performed in 1986 [7, 8], of which one was simultaneously detected in rat stomach [7], and was the start of almost 3 decades of intensive gap junction research. Over the years, as much as 21 different connexins have been identified in human, all which are named after their molecular weight, namely Cx23, Cx25, Cx26, Cx30, Cx30.2, Cx30.3, Cx31, Cx31.1, Cx31.9, Cx32, Cx36, Cx37, Cx40, Cx40.1, Cx43, Cx45, Cx46, Cx47, Cx50, Cx59 and Cx62 [9]. Connexins share a structure consisting of 4 membrane-spanning domains, 2 extracellular loops, a cytoplasmic loop, a cytoplasmic N-terminal area and a C-terminal region [2, 3] (Fig. 1). Started in 1993 [10, 11], several studies have documented the presence of connexins in the intestine. In 2000, a newcomer entered the connexin arena, namely the so-called pannexin (Panx) protein, which has a topology that is similar to that of connexins [12]. Thus far, 3 pannexin types, namely Panx1, Panx2 and Panx3, have been characterized and they gather in a connexin hemichannel-like configuration, but not as gap junctions, at the cell plasma membrane surface, where they mediate the exchange of chemical messengers between the cytosol and the extracellular environment [13] (Fig. 1). Only in the last few years, however, pannexin expression has been reported in the intestine [14, 15], the stomach [15, 16] and the liver [15-22]. Furthermore, the connexin research field has witnessed the introduction of the controversial concept of functional hemichannels in the last decade. In this view, connexin hemichannels are more than merely structural precursors of gap junctions, as they also provide a pathway for cellular signaling, albeit between the cytosol and the extracellular environment, similar to pannexin channels [23, 24]. The messengers that permeate connexin hemichannels and pannexin channels show great overlap with those involved in GJIC [13, 25]. Over the years, several studies have documented that these single membrane channels are mainly involved in intestinal [14, 26-28] and liver pathology [29, 30]. Nevertheless, a number of physiological roles of connexin hemichannels and pannexin channels have been described in the digestive system, all which will be outlined in the current paper. In the first part, a state-of-the-art overview of connexin and pannexin expression in the stomach, the intestine and the liver is provided. The second part focuses on physiological functions of connexin and pannexin signaling in these organs.
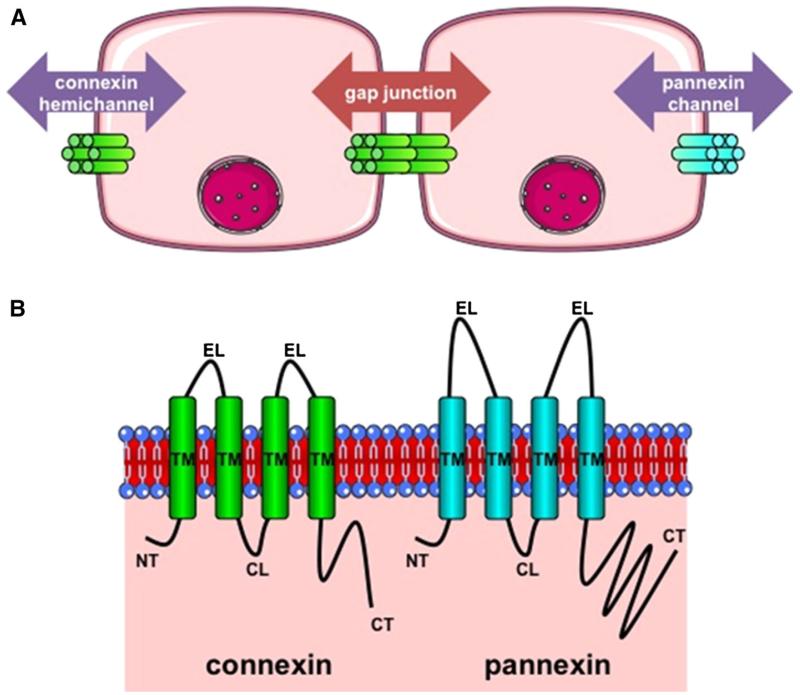
Fig. 1.
a Architecture of connexin and pannexin channels. Gap junctions are formed by the interaction between 2 hemichannels of adjacent cells and mediate intercellular communication (red arrow). Connexin hemichannels and pannexin channels are built up by 6 connexin proteins (green) and 6 pannexin proteins (blue), respectively, and support paracrine communication (purple). b Topology of connexin and pannexin proteins. Connexins (green) and pannexins (blue) all consist of 4 transmembrane domains (TM), 2 extracellular loops (EL), 1 cytoplasmic loop (CL), 1 cytoplasmic carboxy tail (CT) and cytoplasmic amino tail (NT). In comparison with connexins, pannexins have longer EL and CT areas
Connexins and pannexins in the stomach, the intestine and the liver
Gastric connexins and pannexins
Today, 3 connexin isotypes have been identified in the stomach, namely Cx26, Cx32 and Cx43 (Table 1). In particular, Cx26 has been detected in human [31], mouse [32] and rat [33, 34] gastric tissue, where it is only scarcely expressed in the epithelial cells and lamina propria of the fundus [31]. Cx32 is produced in human [35-38], rat [7, 33, 34, 39-42] and equine stomach [43]. In the glandular regions, Cx32 is abundantly expressed in surface and foveolar cells and decreases towards the proliferative zone of the glands, where immature forms of surface epithelial cells are found [35, 40, 41, 43]. Cx43 has been observed in gastric tissue of human [44], mouse [32, 45], rat [46-48], dog [49], rabbit [50] and guinea pig [51-53]. Cx43 is specifically present in circular muscle layers, but not in longitudinal muscle cells, of the antrum and the corpus [44, 45, 49, 51]. In fact, circular muscle cells of the lesser curvature in the corpus of canine stomach are interconnected by numerous gap junctions, whereby each cell has about 200 gap junctions [54]. In human stomach, gap junctions are found in the antrum and in the outermost area of the greater curvature, but are absent in the fundus and in the innermost area of the corporal circular muscle layer [55]. In general, gap junctions are rarely seen at the pylorus [45, 56] and in interstitial cells of Cajal [54], yet in rat, gap junctions are present between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the fundus [48, 57]. A single study showed expression of Cx40 and Cx45 in circular muscle cells of the canine gastric antrum [49]. Panx1 mRNA expression in the rat gastric tissue has been reported [16], while Panx2 protein expression has been described in parietal and epithelial cells of murine stomach [15] (Table 1).
Table 1.
Connexin and pannexin expression in the digestive system
| Species | Tissue | Cell type | References |
|---|---|---|---|
| Cx26 | Stomach (h, m, r) | [31-34] | |
| Small intestine (h) | [59] | ||
| Colon (h) | Epithelial cells (h) | [59, 74, 78, 151] | |
| Muscularis externa cells (h) | [75, 151] | ||
| Liver (h, m, r, gp) | Hepatocytes (m, r) | [33, 152, 153] | |
| Stellate cells (r) | [83] | ||
| Sinusoidal endothelial cells (r) | [83] | ||
| Kupffer cells (r) | [83] | ||
| Cx31 | Small intestine (m) | [64] | |
| Colon (m) | [64] | ||
| Cx31.9 | Colon (h) | [78] | |
| Cx32 | Stomach (h, hs, r) | Foveolar cells (h, hs) | [34, 35, 37-43] |
| Small intestine (h, m) | Epithelial cells (h, m) | [59, 61, 64] | |
| Colon (h) | Epithelial cells (h) | [70, 78, 151] | |
| Liver (h, m, r, gp) | Hepatocytes (h, m, r) | [7, 8, 83, 92, 152-155] | |
| Biliary endothelial cells (r) | [123] | ||
| Sinusoidal endothelial cells (r) | [83] | ||
| Cx36 | Small intestine (m) | Myenteric plexus cells (m) | [60] |
| Colon (h, m) | Myenteric plexus cells (m) | [60, 78] | |
| Cx37 | Small intestine (h, m) | Epithelial cells (h) | [58, 68] |
| Liver (m, r) | Hepatic artery endothelial cells (m, r) | [85-87] | |
| Portal vein endothelial cells (m, r) | [85-87] | ||
| Cx40 | Stomach (d) | [49] | |
| Small intestine (d) | Deep muscular plexus cells (d) | [49] | |
| Myenteric plexus cells (d) | [49] | ||
| Muscularis externa cells (d) | [49] | ||
| Colon (d, r) | Myenteric plexus cells (d) | [49] | |
| Muscularis externa cells (r) | [71] | ||
| Liver (m, r) | Hepatic artery endothelial cells (m, r) | [85-87] | |
| Portal vein endothelial cells (m, r) | [85-87] | ||
| Cx43 | Stomach (h, m, d, r, rb, gp) | [32, 44-53, 156] | |
| Small intestine (h, m, d, r, rm) | Epithelial cells (h, m, rm) | [59, 61, 62, 64, 69] | |
| Deep muscular plexus cells (d, r) | [49, 67] | ||
| Myenteric plexus cells (d) | [49] | ||
| Muscularis externa cells (h, m, d, r) | [11, 44, 49, 65] | ||
| Interstitial cells of Cajal (h) | [66] | ||
| Colon (h, m, d, r, rm) | Epithelial cells (h, m) | [59, 64, 69, 70, 72, 73, 77-79, 151] | |
| Muscularis mucosae cells (h) | [73, 151] | ||
| Deep muscular plexus cells (m, d) | [49, 76] | ||
| Myenteric plexus cells (m, d) | [49, 76] | ||
| Muscularis externa cells (h, r) | [71, 75, 151] | ||
| Liver (h, m, r) | Biliary epithelial cells (r) | [123, 124, 157-161] | |
| Kupffer cells (r) | [82, 83] | ||
| Stellate cells (r) | [83, 85] | ||
| Sinusoidal endothelial cells (r) | [83, 85] | ||
| Hepatic artery endothelial cells (m, r) | [85-87] | ||
| Portal vein endothelial cells (m, r) | [85-87] | ||
| Cx45 | Stomach (d) | [49] | |
| Small intestine (d, r) | Deep muscular plexus cells (d, r) | [49, 65, 67] | |
| Myenteric plexus cells (d) | [49] | ||
| Colon (h, d) | Myenteric plexus cells (d) | [49, 78] | |
| Cx57 | Small intestine (m) | [63] | |
| Panx1 | Colon (h) | Mucosal cells (h) | [14] |
| Muscularis mucosa cells (h) | [14] | ||
| Submucosal cells (h) | [14] | ||
| Muscularis externa cells (h) | [14] | ||
| Liver (m, r) | Hepatocytes (m, r) | [16-20] | |
| Kupffer cells (m) | [22] | ||
| Panx2 | Colon (m) | Epithelial cells (m) | [15] |
| Liver (m, r) | Hepatocytes (m, r) | [15, 21] |
Cx connexin, d dog, gp guinea pig, h human, hs horse, m mouse, Panx pannexin, r rat, rb rabbit, rm rhesus monkey
Intestinal connexins and pannexins
At least 10 different connexin variants have been characterized in the intestinal system. Thus, Cx26, Cx31, Cx32, Cx36, Cx37, Cx40, Cx43, Cx45 and Cx57 have been detected in the small intestine of several species [11, 44, 49, 58-69], while Cx26, Cx31, Cx31.1, Cx32, Cx36, Cx40, Cx43 and Cx45 occur in the colon [49, 59, 60, 64, 70-79] (Table 1). In some reports, the segments of the small intestine in which connexins are produced have been specified. In this regard, Cx31, Cx32 [64], Cx36 [60], Cx43 [49, 62, 64] and Cx45 [49] are present in the ileum, while the duodenum harbors Cx32 [64], Cx37 [58] and Cx43 [64]. Several studies have focussed on the distribution of connexins in the different layers of small intestinal and colonic tissue, in particular the mucosa, submucosa and muscularis externa (Table 1). Furthermore, intestinal connexins, like in other organs, display cell type-specific expression patterns. This might be of importance for delineating physiological compartments with specific functions. Cx32 has been observed in enterochromaffin cells and Paneth cells of small intestinal epithelium in mouse [61]. In rhesus monkeys, Cx43 is exclusively present in crypt epithelial cells, with higher expression in the jejunum and the ileum than in the colon [69]. In the enteric nervous system of the mouse colon, Cx43 is confined to glia [76]. Cx43 is also expressed by circular muscle cells of the small intestine [11, 65], but Cx26, Cx32 and Cx43 immunoreactivity remain absent in the longitudinal muscle layer of the colon [11, 74]. This is linked to the observation that gap junctions occur at specific areas in intestinal tissue. In the mouse intestine, there are no gap junctions between interstitial cells of Cajal of the myenteric plexus and circular or longitudinal muscle cells. With the exception of the latter, however, gap junctions couple these individual cell types among each other. There is another network of interstitial cells of Cajal in the deep muscular plexus, which are connected to circular muscles through gap junctions [67, 80, 81]. These gap junctions may be heteromeric and thus can consist of more than 1 connexin species. Specifically, Cx43 frequently colocalizes with Cx40 and Cx45 in interstitial cells of Cajal in the canine intestine [49]. Besides the gap junctional connexin pool, connexins, in casu Cx26, Cx32 and Cx43, also gather as functional hemichannels, as occurring at the basal pole of human intestinal epithelial cells [59]. In comparison with connexins, much less attention has yet been paid to pannexins in the intestine. One study showed Panx1 expression in all layers of the human colon, including mucosa, muscularis mucosa, submucosa and muscularis externa. Panx1 hereby is mainly found in enteric ganglia, blood vessel endothelium, erythrocytes, epithelial cells and goblet cells [14]. Another report described Panx2 production in epithelial cells of the small intestine and the colon [15] (Table 1).
Hepatic connexins and pannexins
As much as 5 connexin family members are detectable in liver (Table 1), among which Cx32 is the predominant one expressed by hepatocytes [34, 35, 37-43]. Cx43 is the major connexin species produced by nonparenchymal liver cells, including Kupffer cells, stellate cells and sinusoidal endothelial cells [82-85]. The latter 3 as well as hepatocytes also stain positive for Cx26 [83], while endothelial cells of the hepatic arteries and the portal vein express Cx37 and Cx40 [83, 85-87]. Quantitatively, however, most gap junctions are found between hepatocytes. They form so-called plaques that occupy about 3 % of the hepatocyte membrane surface [88]. Unlike Cx32, which is evenly distributed in the liver parenchyma, gap junctions consisting of Cx26 are mainly established by periportal hepatocytes [89, 90]. Hepatocellular Cx32 has many binding partners, including other junctional components, such as the tight junction building stone occludin [91] and mitochondrial proteins [92]. At the transcriptional level, tissue-specific expression of connexins is accomplished by differential promoter usage and tissue-enriched transcription factors [93]. In this light, hepatocellular Cx32 production depends on the binding of hepatocyte nuclear factor 1 alpha at its P1 gene promoter [94]. Epigenetic actions, including DNA methylation and histone modifications, also control connexin expression in liver cells [95, 96]. In addition, Cx43 was found to be regulated by specific microRNAs [97]. At a more downstream level, gap junction opening is controlled by posttranslational connexin changes. Connexin phosphorylation plays a critical role in this so-called gating process. Except for Cx26, all connexins are subject to phosphorylation, which may have a varying outcome [98]. With respect to the liver, Cx32 phosphorylation by protein kinase A enhances GJIC [99], while the same event mediated by protein kinase C results in protection against proteolysis [100]. Pannexin production in liver tissue has been poorly investigated thus far. Only a handful of reports demonstrated Panx1 expression in liver tissue, in particular produced by hepatocytes [16-20] and Kupffer cells [22]. Two studies showed the presence of Panx2 in mouse liver [15] and rat hepatocytes [21].
Connexin and pannexin channels in gastrointestinal and hepatic physiology
Physiological functions of connexin and pannexin signaling in the stomach
Experiments with rats of different age showed that Cx32 presence and gap junction number gradually increase during maturation of surface mucous cells in the stomach. This suggests that GJIC between surface mucous cells is a determinant of gastric cell differentiation and of gastric homeostasis in general [41]. Cx43-based gap junctions are thought to play an important role in the regulation of gastroduodenal motility [52]. Furthermore, gap junctional channels in gastric glands were found to support acid secretion [34]. A recent study in guinea pig revealed that a cAMP-dependent signal propagates intercellularly to induce coordinated secretion in the entire gastric gland [101]. As a matter of fact, gap junction activity in gastric epithelial cells is increased by cAMP [102] and is modulated by the beta adrenergic nervous system [103]. A number of reports have shown a cytoprotective function for Cx32-based gap junctions in the stomach. In this respect, ischemia–reperfusion stress [40] or acid-induced injury [42] combined with perfusion with octanol, a gap junction uncoupler, reduces Cx32-positive spots in rat stomach. This indicates that inhibition of gap junction activity weakens the barrier function of the gastric mucosa in combination with ischemia–reperfusion stress or acid-induced injury. Therefore, facilitation of GJIC may protect the gastric mucosal barrier function by potentiating cellular integrity [40, 42]. Cx32 gradually reappears during healing following ethanol-induced [104] or acetic acid-induced [105] gastric insults in rat. Although pannexins have been identified in gastric tissue, their potential physiological roles in the stomach remain to be established [15, 16].
Physiological functions of connexin and pannexin signaling in the intestine
Cx43-based gap junctions in the gut endoderm are indispensable for the transfer of signals that determine the establishment of left–right asymmetry from the node to the lateral plate mesoderm during embryogenesis [106, 107]. In the adult intestine, Cx26-related GJIC plays a role in maintaining epithelial barrier function by affecting the production of tight junction proteins [108]. Although surrounded by some controversy [80, 109], gap junctions are also believed to be important for intestinal nerve transmission and pacing. This particularly holds true for gap junctions composed of Cx43 in interstitial cells of Cajal and smooth muscle cells, which control intestinal motility [66, 110]. In fact, ablation of Cx43 in smooth muscle cells of the murine intestine results in altered visceromotor responses and muscle contractility, a decrease in gastrointestinal transit time, increased neutrophil infiltration and thickening of the tunica muscularis. This not only points to clear-cut functions for Cx43 in intestinal physiology, but also in its morphology [111]. Recently, Cx43-based hemichannels have been found to mediate calcium responses in enteric glia of mouse colon, which equally is critical for modulating colonic motility and transit [76]. In vitro experiments using cocultures of rat colonic smooth muscle cells and lumbosacral dorsal root ganglion neurons showed the establishment of functional Cx43-based gap junctions between these 2 cell types. Furthermore, mechanistic stimulation of the myocytes increased intracellular calcium concentrations in the neurons, a signal triggered by IP3, which moves between neurons via gap junctions [71]. Cx36 is also involved in intestinal nerve transmission. It is colocalized with nitric oxide synthase in the myenteric plexus of mouse colon, suggesting a role for gap junctions in inhibitory nitrergic enteric neuronal activity. This is further substantiated by the notion that Cx36 knockout mice exhibit modified spontaneous contractility properties and altered responses to electrical field stimulation and cholinergic agonists in the gut [112]. Besides conveying electrical signals, Cx43 represents an important component of the protective innate immune response of the intestinal epithelium. Activation of Toll-like receptor 2 indeed modulates Cx43 expression and increases GJIC in intestinal epithelial cells, thereby controlling their barrier function and restitution during acute and chronic inflammatory damage. This enhances mucosal homeostasis between commensals and hosts [113]. In addition, gap junction activity is critical for the establishment of oral tolerance by antigen-presenting cells in the intestine. Specifically, CX3CR1+ macrophages take up fed antigens in the duodenum, which are subsequently transferred to CD103+ dendritic cells via Cx43-based gap junctions for further antigen presentation [64]. Connexins also seem to stabilize intestinal vasculature, since Cx37 and Cx40 knockout mice exhibit hemorrhages in gastrointestinal tissue with pronounced blood vessel dilatation and congestion [114]. Although solid scientific evidence is currently lacking, channels composed of Panx1 have been proposed to control colonic motility, secretion and blood flow [14].
Physiological functions of connexin and pannexin signaling in the liver
Hepatic connexin expression patterns drastically alter during liver development. Early hepatic progenitor cells express Cx43 and switch to Cx26, but especially to Cx32, during differentiation into hepatocytes [115-117]. Both Cx26 and Cx32 become measurable in the late stages of gestation and culminate 1 week postpartum, with Cx26 being mainly located periportally [90]. This coincides with the establishment of the glucagon receptor zonation pattern, which is particularly present in perivenous hepatocytes [89]. Curiously, glucagon itself is mainly detectable in the periportal area and enhances Cx26 gene transcription [118]. Therefore, the Cx26 zonation pattern in the liver is thought to be controlled at the transcriptional level by hormonal stimuli [90, 118]. With respect to liver vasculature, Cx37 and Cx40 are expressed in early hepatic arteries and portal veins, while Cx43 is detected in portal veins, but not in hepatic veins, during fetal mouse liver development [87].
Gap junctions are indispensable for maintaining the metabolic competence of the adult liver. This has been well exemplified for glycogenolysis. Disintegration of glycogen to glucose is triggered by hormonal and neuronal stimuli and is predominantly performed by periportal hepatocytes [119, 120]. In fact, Cx32-based gap junctions between hepatocytes underlie the propagation of the glycogenolytic response from the periportal to the pericentral acinar pole by controlling the intercellular exchange of IP3. The latter activates calcium release from the endoplasmic reticulum, subsequently causing calcium waves throughout the acinus [119, 121]. In support of this is the finding that Cx32 knockout mice show decreased blood glucose levels upon glycogenolytic stimulation [120, 122]. Similarly, Cx43-containing gap junctions facilitate the spread of calcium waves necessary for ductular secretion from biliary endothelial cells and thus bile formation [123, 124]. Biotransformation capacity also depends on the establishment of gap junctions consisting of Cx32 between hepatocytes. Chemical induction of cytochrome P450 1A1/2 and 2B1/2 in rat parallels the downregulation of pericentral Cx32 protein levels [125-127]. This is believed to reflect a defense mechanism to restrict the intercellular trafficking of reactive intermediates that have been generated in biotransformation reactions [125]. Furthermore, hepatocellular gap junctions govern several other vital functional processes in the liver, such as albumin secretion and ammonia detoxification [128].
During liver regeneration upon partial hepatectomy in rodents, increased GJIC is observed in the G1 phase of the cell cycle, followed by a steep decrease upon initiation of DNA synthesis. These alterations are reflected at the level of Cx32 expression and to a lesser extent in Cx26 production, while Cx43 seems unchanged [129-131]. Although gap junctions are obviously involved in liver cell growth, their role still is a matter of debate. Indeed, inhibition of the p38 mitogen-activated protein kinase pathway prevents reduction in Cx32 expression, but does not affect hepatocyte proliferative activity in the regenerating rat liver [132]. This suggests that downregulation of GJIC may occur independently of liver cell proliferation. Hepatocellular proliferative activity was also found unaltered in the regenerating liver of Cx32 knockout mice, yet the extent of synchronous initiation and termination of DNA synthesis became decreased [131]. Based on this observation, GJIC seems permissive to hepatocyte cell cycling upon mitogenic stimulation, rather than furnishing a decisive liver cell growth signal. Thus, gap junction closure may support the functional segregation of metabolic pools in dividing liver cells from their quiescent neighbors to avoid homeostatic imbalance [129, 133]. In contrast to this is the view that gap junctions fulfill much more determinate functions in liver cell cycling, in particular by controlling the inter-cellular trafficking of critical growth mediators, such as cAMP [134, 135].
The vast majority of research efforts to elucidate the role of connexin signaling in liver cell death thus far have been performed in vitro. During the early stages of experimentally induced apoptosis in cultured liver cells, both Cx43 expression and gap junction activity are promoted. Most likely, transiently enhanced GJIC is necessary for the propagation of apoptotic signals, such as calcium ions. Upon further progression of cell death, gap junction activity deteriorates and disappears between apoptotic bodies. This may serve the reduction of the flux of toxic metabolites, such as nitric oxide and superoxide ions, and hence the protection of living cells [136]. Interestingly, accumulating evidence shows that connexin hemichannels, rather than gap junctions, are involved in liver cell death. Upon induction of Fas-mediated apoptosis in cultured primary hepatocytes, GJIC rapidly declines, which is associated with a decay of the gap junctional Cx32 protein pool. Simultaneously, levels of newly synthesized Cx32 protein increase and gather in a hemichannel configuration. This becomes particularly evident towards the end stages of the cell death process. Subsequent experiments showed that Cx32-based hemichannels support the apoptotic-to-necrotic transition in hepatocytes [29]. Furthermore, Cx43 signaling, also involving hemichannels, was found to facilitate the onset of spontaneous apoptosis in primary hepatocyte cultures [30]. Cx43 hereby interacts with mitochondrial proteins [137], as also described for hepatic Cx32 [92]. As a matter of fact, connexin hemichannels not only occur at the plasma membrane surface, but also reside at other subcellular locations, such as at mitochondria, where their functions have been linked to cell survival [92, 138-140]. In recent years, pannexin channels have been identified as mediators of apoptotic processes [141-143]. Panx1 is known to colocalize with the P2X7 receptor and to form a so-called death receptor complex. Stimulation of the P2X7 receptor, such as mediated by ATP, is believed to trigger the opening of Panx1-based channels [144-146]. Furthermore, Panx1 is a substrate for caspase cleavage, resulting in the formation of an open channel and the release of “find me” signals, including ATP and uridine triphosphate, at the earliest stages of cell death to recruit phagocytes [141, 143]. Although evidence is lacking thus far, there are indications that this also takes place in the liver [18, 20].
Conclusions and perspectives
It has become clear that connexins and pannexins are crucial to maintain digestive homeostasis. GJIC is indispensable during digestive embryogenesis [106, 107] and alterations in connexin expression have been described during gastric cell and hepatocyte differentiation [41], suggesting an important role in cell maturation. Cellular communication through gap junctions is equally a major driver of gastrointestinal homeostasis by controlling processes such as gastroduodenal [52] and gut motility [66, 110], gastric acid secretion [34], gastric cytoprotection [40, 42], intestinal epithelial barrier function [108] and intestinal innate immune defense [113]. Similarly, GJIC underlies critical hepatic functions, including xenobiotic biotransformation [125-127], glycogenolysis [119-122], bile secretion [123, 124], ammonia detoxification and plasma protein synthesis [128]. In contrast to gap junctions, connexin hemichannels and pannexin channels seem to be mainly, but not solely, involved in pathological processes [14, 26-30]. Nonetheless, some physiological functions have been attributed to these channels. In particular, connexin hemichannels are involved in the modulation of colon motility and transit [76], while pannexin channels seem to participate in the control of colonic motility, secretion and blood flow [14]. However, their functional relevance in digestive homeostasis largely remains to be established. A prerequisite to further clarify the role of connexin hemichannels and pannexin channels is the development and use of specific tools to study these particular channel types. In this context, most, if not all, of the presently used inhibitors of connexin hemichannels and pannexin channels also suppress gap junctions [147]. Great expectations now lie with peptides that reproduce sequences of the primary connexin and pannexin protein structure, as they suppress connexin hemichannel and pannexin channel activity, respectively, without affecting the GJIC [148-150]. Further research using such tools will undoubtedly shed more light on the involvement of connexin and pannexin signaling in digestive homeostasis.
Acknowledgments
This work was financially supported by the grants of Agency for Innovation by Science and Technology in Flanders (IWT), the University Hospital of the Vrije Universiteit Brussel-Belgium (“Willy Gepts Fonds” UZ-VUB), the Fund for Scientific Research-Flanders (FWO Grants G009514 N and G010214 N), the European Research Council (ERC Starting Grant 335476), the University of São Paulo-Brazil and the Foundation for Research Support of the State of São Paulo (FAPESP SPEC Grant 2013/50420-6).
Abbreviations
- ATP
Adenosine triphosphate
- cAMP
Cyclic adenosine monophosphate
- CL
Cytoplasmic loop
- CT
Cytoplasmic carboxy tail
- Cx
Connexin
- EL
Extracellular loop
- GJIC
Gap junctional intercellular communication
- IP3
Inositol triphosphate
- NT
Cytoplasmic amino tail
- Panx
Pannexin
- TM
Transmembrane domain
Footnotes
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- 1.Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10(19):2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- 2.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. doi:10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decrock E, Vinken M, De Vuyst E, Krysko DV, D’Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16(4):524–536. doi: 10.1038/cdd.2008.196. doi:10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 4.Loewenstein WR, Kanno Y. Intercellular communication and tissue growth I. Cancerous growth. J Cell Biol. 1967;33(2):225–234. doi: 10.1083/jcb.33.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodenough DA. Bulk isolation of mouse hepatocyte gap junctions. Characterization of the principal protein, connexin. J Cell Biol. 1974;61(2):557–563. doi: 10.1083/jcb.61.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul DL. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar NM, Gilula NB. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986;103(3):767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai D, Wang AH. Extracellular domains play different roles in gap junction formation and docking compatibility. Biochem J. 2014;458(1):1–10. doi: 10.1042/BJ20131162. doi:10.1042/BJ20131162. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Zhou Z, Daniel EE. Expression of gap junction connexin 43 and connexin 43 mRNA in different regional tissues of intestine in dog. Am J Physiol. 1993;265(5):G911–G916. doi: 10.1152/ajpgi.1993.265.5.G911. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen HB, Huizinga JD, Thuneberg L, Rumessen JJ. Immunohistochemical localization of a gap junction protein (connexin43) in the muscularis externa of murine, canine, and human intestine. Cell Tissue Res. 1993;274(2):249–256. doi: 10.1007/BF00318744. [DOI] [PubMed] [Google Scholar]
- 12.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10(13):R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. 2013;1828(1):35–50. doi: 10.1016/j.bbamem.2012.07.002. doi:10.1016/j.bbamem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diezmos EF, Sandow SL, Markus I, Shevy Perera D, Lubowski DZ, King DW, Bertrand PP, Liu L. Expression and localization of pannexin-1 hemichannels in human colon in health and disease. Neurogastroenterol Motil. 2013;25(6):e395–e405. doi: 10.1111/nmo.12130. doi:10.1111/nmo.12130. [DOI] [PubMed] [Google Scholar]
- 15.Le Vasseur M, Lelowski J, Bechberger JF, Sin WC, Naus CC. Pannexin 2 protein expression is not restricted to the CNS. Front Cell Neurosci. 2014;8:392. doi: 10.3389/fncel.2014.00392. doi:10.3389/fncel.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA. 2003;100(23):13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54(1):133–144. doi: 10.1002/hep.24341. doi:10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz M, Csak T, Nath B, Szabo G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol. 2011;17(43):4772–4778. doi: 10.3748/wjg.v17.i43.4772. doi:10.3748/wjg.v17.i43.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HY, Kim SJ, Lee SM. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J. 2015;282(2):259–270. doi: 10.1111/febs.13123. doi:10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- 20.Xiao F, Waldrop SL, Khimji AK, Kilic G. Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. Am J Physiol Cell Physiol. 2012;303(10):C1034–C1044. doi: 10.1152/ajpcell.00175.2012. doi:10.1152/ajpcell.00175.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Cao J, Jin Q, Xie C, He Q, Cao R, Xiong J, Chen P, Wang X, Liang S. A proteomic study reveals the diversified distribution of plasma membrane-associated proteins in rat hepatocytes. J Cell Biochem. 2008;104(3):965–984. doi: 10.1002/jcb.21680. doi:10.1002/jcb.21680. [DOI] [PubMed] [Google Scholar]
- 22.Sáez PJ, Shoji KF, Aguirre A, Sáez JC. Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells. Mediators Inflamm. 2014;2014:742734. doi: 10.1155/2014/742734. doi:10.1155/2014/742734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrasekhar A, Bera AK. Hemichannels: permeants and their effect on development, physiology and death. Cell Biochem Funct. 2012;30(2):89–100. doi: 10.1002/cbf.2794. [DOI] [PubMed] [Google Scholar]
- 24.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54(7):758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 25.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94(1-2):120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. doi:10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guttman JA, Lin AE, Li Y, Bechberger J, Naus CC, Vogl AW, Finlay BB. Gap junction hemichannels contribute to the generation of diarrhoea during infectious enteric disease. Gut. 2010;59(2):218–226. doi: 10.1136/gut.2008.170464. doi:10.1136/gut.2008.170464. [DOI] [PubMed] [Google Scholar]
- 27.Simpson C, Kelsell DP, Marchès O. Connexin 26 facilitates gastrointestinal bacterial infection in vitro. Cell Tissue Res. 2013;351(1):107–116. doi: 10.1007/s00441-012-1502-9. doi:10.1007/s00441-012-1502-9. [DOI] [PubMed] [Google Scholar]
- 28.Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5(8):720–726. doi: 10.1038/ncb1021. doi:10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 29.Vinken M, Decrock E, De Vuyst E, De Bock M, Vandenbroucke RE, De Geest BG, Demeester J, Sanders NN, Vanhaecke T, Leybaert L, Rogiers V. Connexin32 hemichannels contribute to the apoptotic-to-necrotic transition during Fas-mediated hepatocyte cell death. Cell Mol Life Sci. 2010;67(6):907–918. doi: 10.1007/s00018-009-0220-2. doi:10.1007/s00018-009-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinken M, Decrock E, Vanhaecke T, Leybaert L, Rogiers V. Connexin43 signaling contributes to spontaneous apoptosis in cultures of primary hepatocytes. Toxicol Sci. 2012;125(1):175–186. doi: 10.1093/toxsci/kfr277. doi:10.1093/toxsci/kfr277. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Furuya T, Li D, Xu J, Cao X, Li Q, Xu Z, Sasaki K. Connexin 26 expression correlates with less aggressive phenotype of intestinal type-gastric carcinomas. Int J Mol Med. 2010;25(5):709–716. doi: 10.3892/ijmm_00000395. [DOI] [PubMed] [Google Scholar]
- 32.Fiertak A, Semik D, Kilarski WM. Immunohistochemical analysis of connexin26 and 43 expression in the mouse alimentary tract. Folia Biol (Krakow) 1999;47(1):5–11. [PubMed] [Google Scholar]
- 33.Zhang JT, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989;109(6):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radebold K, Horakova E, Gloeckner J, Ortega G, Spray DC, Vieweger H, Siebert K, Manuelidis L, Geibel JP. Gap junctional channels regulate acid secretion in the mammalian gastric gland. J Membr Biol. 2001;183(3):147–153. doi: 10.1007/s00232-001-0062-9. [DOI] [PubMed] [Google Scholar]
- 35.Uchida Y, Matsuda K, Sasahara K, Kawabata H, Nishioka M. Immunohistochemistry of gap junctions in normal and diseased gastric mucosa of humans. Gastroenterology. 1995;109(5):1492–1496. doi: 10.1016/0016-5085(95)90635-5. [DOI] [PubMed] [Google Scholar]
- 36.Mine T, Yusuda H, Kataoka A, Tajima A, Nagasawa J, Takano T. Human chronic gastric ulcer and connexin. J Clin Gastroenterol. 1995;21(Suppl 1):S104–S107. [PubMed] [Google Scholar]
- 37.Ohkusa T, Fujiki K, Tamura Y, Yamamoto M, Kyoi T. Freeze-fracture and immunohistochemical studies of gap junctions in human gastric mucosa with special reference to their relationship to gastric ulcer and gastric carcinoma. Microsc Res Tech. 1995;31(3):226–233. doi: 10.1002/jemt.1070310306. doi:10.1002/jemt.1070310306. [DOI] [PubMed] [Google Scholar]
- 38.Wu J, Zhou HF, Wang CH, Zhang B, Liu D, Wang W, Sui GJ. Decreased expression of Cx32 and Cx43 and their function of gap junction intercellular communication in gastric cancer. Zhonghua Zhong Liu Za Zhi. 2007;29(10):742–747. [PubMed] [Google Scholar]
- 39.Hertzberg EL, Skibbens RV. A protein homologous to the 27,000 dalton liver gap junction protein is present in a wide variety of species and tissues. Cell. 1984;39(1):61–69. doi: 10.1016/0092-8674(84)90191-0. [DOI] [PubMed] [Google Scholar]
- 40.Iwata F, Joh T, Ueda F, Yokoyama Y, Itoh M. Role of gap junctions in inhibiting ischemia-reperfusion injury of rat gastric mucosa. Am J Physiol. 1998;275(5):G883–G888. doi: 10.1152/ajpgi.1998.275.5.G883. [DOI] [PubMed] [Google Scholar]
- 41.Kyoi T, Ueda F, Kimura K, Yamamoto M, Kataoka K. Development of gap junctions between gastric surface mucous cells during cell maturation in rats. Gastroenterology. 1992;102(6):1930–1935. doi: 10.1016/0016-5085(92)90315-p. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi N, Joh T, Yokoyama Y, Seno K, Nomura T, Ohara H, Ueda F, Itoh M. Importance of gap junction in gastric mucosal restitution from acid-induced injury. J Lab Clin Med. 2000;136(2):93–99. doi: 10.1067/mlc.2000.108158. doi:10.1067/mlc.2000.108158. [DOI] [PubMed] [Google Scholar]
- 43.Fink C, Hembes T, Brehm R, Weigel R, Heeb C, Pfarrer C, Bergmann M, Kressin M. Specific localisation of gap junction protein connexin 32 in the gastric mucosa of horses. Histochem Cell Biol. 2006;125(3):307–313. doi: 10.1007/s00418-005-0047-3. doi:10.1007/s00418-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 44.Nishitani A, Hirota S, Nishida T, Isozaki K, Hashimoto K, Nakagomi N, Matsuda H. Differential expression of connexin 43 in gastrointestinal stromal tumours of gastric and small intestinal origin. J Pathol. 2005;206(4):377–382. doi: 10.1002/path.1799. doi:10.1002/path.1799. [DOI] [PubMed] [Google Scholar]
- 45.Seki K, Komuro T. Distribution of interstitial cells of Cajal and gap junction protein, Cx 43 in the stomach of wild-type and W/Wv mutant mice. Anat Embryol (Berl) 2002;206(1):57–65. doi: 10.1007/s00429-002-0279-0. doi:10.1007/s00429-002-0279-0. [DOI] [PubMed] [Google Scholar]
- 46.Dupont E, el Aoumari A, Roustiau-Sévère S, Briand JP, Gros D. Immunological characterization of rat cardiac gap junctions: presence of common antigenic determinants in heart of other vertebrate species and in various organs. J Membr Biol. 1988;104(2):119–128. doi: 10.1007/BF01870924. [DOI] [PubMed] [Google Scholar]
- 47.Kadle R, Zhang JT, Nicholson BJ. Tissue-specific distribution of differentially phosphorylated forms of Cx43. Mol Cell Biol. 1991;11(1):363–369. doi: 10.1128/mcb.11.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitsui R, Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 2002;309(2):219–227. doi: 10.1007/s00441-002-0592-1. doi:10.1007/s00441-002-0592-1. [DOI] [PubMed] [Google Scholar]
- 49.Wang YF, Daniel EE. Gap junctions in gastrointestinal muscle contain multiple connexins. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G533–G543. doi: 10.1152/ajpgi.2001.281.2.G533. [DOI] [PubMed] [Google Scholar]
- 50.Larson DM, Gilbert RJ, Beyer EC. Two-dimensional coupling by gap junctions in cultured gastric smooth muscle monolayers. Am J Physiol. 1992;263(2):G261–G268. doi: 10.1152/ajpgi.1992.263.2.G261. [DOI] [PubMed] [Google Scholar]
- 51.Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550(3):829–844. doi: 10.1113/jphysiol.2003.042176. doi:10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iino S, Asamoto K, Nojyo Y. Heterogeneous distribution of a gap junction protein, connexin43, in the gastroduodenal junction of the guinea pig. Auton Neurosci. 2001;93(1):8–13. doi: 10.1016/S1566-0702(01)00320-4. doi:10.1016/S1566-0702(01)00320-4. [DOI] [PubMed] [Google Scholar]
- 53.Seki K, Zhou DS, Komuro T. Immunohistochemical study of the c-kit expressing cells and connexin 43 in the guinea-pig digestive tract. J Auton Nerv Syst. 1998;68(3):182–187. doi: 10.1016/s0165-1838(97)00134-3. [DOI] [PubMed] [Google Scholar]
- 54.Daniel EE, Sakai Y, Fox JE, Posey-Daniel V. Structural basis for function of circular muscle of canine corpus. Can J Physiol Pharmacol. 1984;62(10):1304–1314. doi: 10.1139/y84-219. [DOI] [PubMed] [Google Scholar]
- 55.Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. J Submicrosc Cytol Pathol. 1989;21(3):439–460. [PubMed] [Google Scholar]
- 56.Daniel EE, Berezin I, Allescher HD, Manaka H, Posey-Daniel V. Morphology of the canine pyloric sphincter in relation to function. Can J Physiol Pharmacol. 1989;67(12):1560–1573. doi: 10.1139/y89-251. [DOI] [PubMed] [Google Scholar]
- 57.Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2008;20(1):69–79. doi: 10.1111/j.1365-2982.2007.00990.x. doi:10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 58.Bracken S, Byrne G, Kelly J, Jackson J, Feighery C. Altered gene expression in highly purified enterocytes from patients with active coeliac disease. BMC Genom. 2008;9:377. doi: 10.1186/1471-2164-9-377. doi:10.1186/1471-2164-9-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clair C, Combettes L, Pierre F, Sansonetti P, Tran Van Nhieu G. Extracellular-loop peptide antibodies reveal a predominant hemichannel organization of connexins in polarized intestinal cells. Exp Cell Res. 2008;314(6):1250–1265. doi: 10.1016/j.yexcr.2007.12.021. doi:10.1016/j.yexcr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Frinchi M, Di Liberto V, Turimella S, D’Antoni F, Theis M, Belluardo N, Mudò G. Connexin36 (Cx36) expression and protein detection in the mouse carotid body and myenteric plexus. Acta Histochem. 2013;115(3):252–256. doi: 10.1016/j.acthis.2012.07.005. doi:10.1016/j.acthis.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Husøy T, Ølstørn HB, Knutsen HK, Løberg EM, Cruciani V, Mikalsen SO, Goverud IL, Alexander J. Truncated mouse adenomatous polyposis coli reduces connexin32 content and increases matrilysin secretion from Paneth cells. Eur J Cancer. 2004;40(10):1599–1603. doi: 10.1016/j.ejca.2004.02.024. doi:10.1016/j.ejca.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 62.Leaphart CL, Qureshi F, Cetin S, Li J, Dubowski T, Baty C, Batey C, Beer-Stolz D, Guo F, Murray SA, Hackam DJ. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132(7):2395–2411. doi: 10.1053/j.gastro.2007.03.029. doi:10.1053/j.gastro.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 63.Manthey D, Bukauskas F, Lee CG, Kozak CA, Willecke K. Molecular cloning and functional expression of the mouse gap junction gene connexin-57 in human HeLa cells. J Biol Chem. 1999;274(21):14716–14723. doi: 10.1074/jbc.274.21.14716. [DOI] [PubMed] [Google Scholar]
- 64.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity. 2014;40(2):248–261. doi: 10.1016/j.immuni.2013.12.012. doi:10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura K, Kuraoka A, Kawabuchi M, Shibata Y. Specific localization of gap junction protein, connexin45, in the deep muscular plexus of dog and rat small intestine. Cell Tissue Res. 1998;292(3):487–494. doi: 10.1007/s004410051077. [DOI] [PubMed] [Google Scholar]
- 66.Nemeth L, Maddur S, Puri P. Immunolocalization of the gap junction protein Connexin43 in the interstitial cells of Cajal in the normal and Hirschsprung’s disease bowel. J Pediatr Surg. 2000;35(6):823–828. doi: 10.1053/jpsu.2000.6851. doi:10.1053/jpsu.2000.6851. [DOI] [PubMed] [Google Scholar]
- 67.Seki K, Komuro T. Immunocytochemical demonstration of the gap junction proteins connexin 43 and connexin 45 in the musculature of the rat small intestine. Cell Tissue Res. 2001;306(3):417–422. doi: 10.1007/s00441-001-0470-2. doi:10.1007/s00441-001-0470-2. [DOI] [PubMed] [Google Scholar]
- 68.Willecke K, Heynkes R, Dahl E, Stutenkemper R, Hennemann H, Jungbluth S, Suchyna T, Nicholson BJ. Mouse connexin37: cloning and functional expression of a gap junction gene highly expressed in lung. J Cell Biol. 1991;114(5):1049–1057. doi: 10.1083/jcb.114.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gumber S, Nusrat A, Villinger F. Immunohistological characterization of intercellular junction proteins in rhesus macaque intestine. Exp Toxicol Pathol. 2014;66(9):437–444. doi: 10.1016/j.etp.2014.07.004. doi:10.1016/j.etp.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dubina MV, Iatckii NA, Popov DE, Vasil’ev SV, Krutovskikh VA. Connexin 43, but not connexin 32, is mutated at advanced stages of human sporadic colon cancer. Oncogene. 2002;21(32):4992–4996. doi: 10.1038/sj.onc.1205630. doi:10.1038/sj.onc.1205630. [DOI] [PubMed] [Google Scholar]
- 71.Ennes HS, Young SH, Goliger JA, Mayer EA. Chemical signaling from colonic smooth muscle cells to DRG neurons in culture. Am J Physiol. 1999;276(3):C602–C610. doi: 10.1152/ajpcell.1999.276.3.C602. [DOI] [PubMed] [Google Scholar]
- 72.Han Y, Zhang PJ, Chen T, Yum SW, Pasha T, Furth EE. Connexin43 expression increases in the epithelium and stroma along the colonic neoplastic progression pathway: implications for its oncogenic role. Gastroenterol Res Pract. 2011;2011:561719. doi: 10.1155/2011/561719. doi:10.1155/2011/561719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ismail R, Rashid R, Andrabi K, Parray FQ, Besina S, Shah MA, Ul Hussain M. Pathological implications of Cx43 downregulation in human colon cancer. Asian Pac J Cancer Prev. 2014;15(7):2987–2991. doi: 10.7314/apjcp.2014.15.7.2987. [DOI] [PubMed] [Google Scholar]
- 74.Kanczuga-Koda L, Sulkowski S, Koda M, Skrzydlewska E, Sulkowska M. Connexin 26 correlates with Bcl-xL and Bax proteins expression in colorectal cancer. World J Gastroenterol. 2005;11(10):1544–1548. doi: 10.3748/wjg.v11.i10.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattii L, Ippolito C, Segnani C, Battolla B, Colucci R, Dolfi A, Bassotti G, Blandizzi C, Bernardini N. Altered expression pattern of molecular factors involved in colonic smooth muscle functions: an immunohistochemical study in patients with diverticular disease. PLoS One. 2013;8(2):e57023. doi: 10.1371/journal.pone.0057023. doi:10.1371/journal.pone.0057023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McClain JL, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146(2):497–507. doi: 10.1053/j.gastro.2013.10.061. doi:10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sedhom MA, Pichery M, Murdoch JR, Foligné B, Ortega N, Normand S, Mertz K, Sanmugalingam D, Brault L, Grandjean T, Lefrancais E, Fallon PG, Quesniaux V, Peyrin-Biroulet L, Cathomas G, Junt T, Chamaillard M, Girard JP, Ryffel B. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62(12):1714–1723. doi: 10.1136/gutjnl-2011-301785. doi:10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sirnes S, Honne H, Ahmed D, Danielsen SA, Rognum TO, Meling GI, Leithe E, Rivedal E, Lothe RA, Lind GE. DNA methylation analyses of the connexin gene family reveal silencing of GJC1 (Connexin45) by promoter hypermethylation in colorectal cancer. Epigenetics. 2011;6(5):602–609. doi: 10.4161/epi.6.5.15237. [DOI] [PubMed] [Google Scholar]
- 79.Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, Rivedal E. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer. 2012;131(3):570–581. doi: 10.1002/ijc.26392. doi:10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- 80.Daniel EE, Thomas J, Ramnarain M, Bowes TJ, Jury J. Do gap junctions couple interstitial cells of Cajal pacing and neurotransmission to gastrointestinal smooth muscle? Neurogastroenterol Motil. 2001;13(4):297–307. doi: 10.1046/j.1365-2982.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 81.Daniel EE, Yazbi AE, Mannarino M, Galante G, Boddy G, Livergant J, Oskouei TE. Do gap junctions play a role in nerve transmissions as well as pacing in mouse intestine? Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G734–G745. doi: 10.1152/ajpgi.00428.2006. doi:10.1152/ajpgi.00428.2006. [DOI] [PubMed] [Google Scholar]
- 82.Eugenin EA, Gonzalez HE, Sanchez HA, Branes MC, Saez JC. Inflammatory conditions induce gap junctional communication between rat Kupffer cells both in vivo and in vitro. Cell Immunol. 2007;247(2):103–110. doi: 10.1016/j.cellimm.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fischer R, Reinehr R, Lu TP, Schonicke A, Warskulat U, Dienes HP, Haussinger D. Intercellular communication via gap junctions in activated rat hepatic stellate cells. Gastroenterology. 2005;128(2):433–448. doi: 10.1053/j.gastro.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 84.Gonzalez HE, Eugenin EA, Garces G, Solis N, Pizarro M, Accatino L, Saez JC. Regulation of hepatic connexins in cholestasis: possible involvement of Kupffer cells and inflammatory mediators. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G991–G1001. doi: 10.1152/ajpgi.00298.2001. [DOI] [PubMed] [Google Scholar]
- 85.Hernández-Guerra M, González-Méndez Y, de Ganzo ZA, Salido E, García-Pagán JC, Abrante B, Malagón AM, Bosch J, Quintero E. Role of gap junctions modulating hepatic vascular tone in cirrhosis. Liver Int. 2014;34(6):859–868. doi: 10.1111/liv.12446. doi:10.1111/liv.12446. [DOI] [PubMed] [Google Scholar]
- 86.Chaytor AT, Martin PE, Edwards DH, Griffith TM. Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am J Physiol Heart Circ Physiol. 2001;280(6):H2441–H2450. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- 87.Shiojiri N, Niwa T, Sugiyama Y, Koike T. Preferential expression of connexin37 and connexin40 in the endothelium of the portal veins during mouse liver development. Cell Tissue Res. 2006;324(3):547–552. doi: 10.1007/s00441-006-0165-9. doi:10.1007/s00441-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 88.Spray DC, Saez JC, Hertzberg EL, Dermietzel R. Gap junctions in liver: composition, function, and regulation. In: Arias IM, Boyer JL, Fausto N, Jakoby DA, Schachter DA, Shaftrits DA, editors. The liver: biology and pathobiology. Raven Press; New York: 1994. pp. 951–967. [Google Scholar]
- 89.Berthoud VM, Iwanij V, Garcia AM, Sáez JC. Connexins and glucagon receptors during development of rat hepatic acinus. Am J Physiol. 1992;263(5):G650–G658. doi: 10.1152/ajpgi.1992.263.5.G650. [DOI] [PubMed] [Google Scholar]
- 90.Iwai M, Harada Y, Muramatsu A, Tanaka S, Mori T, Okanoue T, Katoh F, Ohkusa T, Kashima K. Development of gap junctional channels and intercellular communication in rat liver during ontogenesis. J Hepatol. 2000;32(1):11–18. doi: 10.1016/s0168-8278(00)80184-1. doi:10.1016/s0168-8278(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 91.Kojima T, Kokai Y, Chiba H, Yamamoto M, Mochizuki Y, Sawada N. Cx32 but not Cx26 is associated with tight junctions in primary cultures of rat hepatocytes. Exp Cell Res. 2001;263(2):193–201. doi: 10.1006/excr.2000.5103. doi:10.1006/excr.2000.5103. [DOI] [PubMed] [Google Scholar]
- 92.Fowler SL, Akins M, Zhou H, Figeys D, Bennett SA. The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus. J Proteome Res. 2013;12(6):2597–2610. doi: 10.1021/pr301166p. doi:10.1021/pr301166p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oyamada M, Takebe K, Oyamada Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim Biophys Acta. 2012;1828(1):118–133. doi: 10.1016/j.bbamem.2011.12.031. doi:10.1016/j.bbamem.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 94.Koffler LD, Fernstrom MJ, Akiyama TE, Gonzalez FJ, Ruch RJ. Positive regulation of connexin32 transcription by hepatocyte nuclear factor-1alpha. Arch Biochem Biophys. 2002;407(2):160–167. doi: 10.1016/s0003-9861(02)00488-5. [DOI] [PubMed] [Google Scholar]
- 95.Piechocki MP, Burk RD, Ruch RJ. Regulation of connexin32 and connexin43 gene expression by DNA methylation in rat liver cells. Carcinogenesis. 1999;20(3):401–406. doi: 10.1093/carcin/20.3.401. [DOI] [PubMed] [Google Scholar]
- 96.Vinken M, Henkens T, Vanhaecke T, Papeleu P, Geerts A, Van Rossen E, Chipman JK, Meda P, Rogiers V. Trichostatin a enhances gap junctional intercellular communication in primary cultures of adult rat hepatocytes. Toxicol Sci. 2006;91(2):484–492. doi: 10.1093/toxsci/kfj152. doi:10.1093/toxsci/kfj152. [DOI] [PubMed] [Google Scholar]
- 97.Klotz LO. Posttranscriptional regulation of connexin-43 expression. Arch Biochem Biophys. 2012;524(1):23–29. doi: 10.1016/j.abb.2012.03.012. doi:10.1016/j.abb.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 98.Johnstone SR, Billaud M, Lohman AW, Taddeo EP, Isakson BE. Posttranslational modifications in connexins and pannexins. J Membr Biol. 2012;245(5):319–332. doi: 10.1007/s00232-012-9453-3. doi:10.1007/s00232-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saez JC, Spray DC, Nairn AC, Hertzberg E, Greengard P, Bennett MV. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci USA. 1986;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elvira M, Díez JA, Wang KK, Villalobo A. Phosphorylation of connexin-32 by protein kinase C prevents its proteolysis by mu-calpain and m-calpain. J Biol Chem. 1993;268(19):14294–14300. [PubMed] [Google Scholar]
- 101.Fukushi Y, Sakurai T, Terakawa S. Cell-to-cell propagation of intracellular signals fluorescently visualized with acridine orange in the gastric glands of guinea pigs. Biochem Biophys Res Commun. 2014;447(1):38–43. doi: 10.1016/j.bbrc.2014.03.095. doi:10.1016/j.bbrc.2014.03.095. [DOI] [PubMed] [Google Scholar]
- 102.Ueda F, Kyoi T, Mimura K, Kimura K, Yamamoto M. Intercellular communication in cultured rabbit gastric epithelial cells. Jpn J Pharmacol. 1991;57(3):321–328. doi: 10.1254/jjp.57.321. [DOI] [PubMed] [Google Scholar]
- 103.Ueda F, Kameda Y, Yamamoto O, Shibata Y. Beta-adrenergic regulation of gap-junctional intercellular communication in cultured rabbit gastric epithelial cells. J Pharmacol Exp Ther. 1994;271(1):397–402. [PubMed] [Google Scholar]
- 104.Endo K, Watanabe S, Nagahara A, Hirose M, Sato N. Restoration of gap junctions in the regenerative process of ethanol-induced gastric mucosal injury. J Gastroenterol Hepatol. 1995;10(5):589–594. doi: 10.1111/j.1440-1746.1995.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 105.Mine T, Kushima R, Fujita T. Relationship between healing of acetic acid-induced chronic gastric ulcer and connexin. J Clin Gastroenterol. 1997;25(Suppl 1):S111–S115. doi: 10.1097/00004836-199700001-00019. [DOI] [PubMed] [Google Scholar]
- 106.Saund RS, Kanai-Azuma M, Kanai Y, Kim I, Lucero MT, Saijoh Y. Gut endoderm is involved in the transfer of left-right asymmetry from the node to the lateral plate mesoderm in the mouse embryo. Development. 2012;139(13):2426–2435. doi: 10.1242/dev.079921. doi:10.1242/dev.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Viotti M, Niu L, Shi SH, Hadjantonakis AK. Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. 2012;10(3):e1001276. doi: 10.1371/journal.pbio.1001276. doi:10.1371/journal.pbio.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morita H, Katsuno T, Hoshimoto A, Hirano N, Saito Y, Suzuki Y. Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intestinal epithelial cell monolayers. Exp Cell Res. 2004;298(1):1–8. doi: 10.1016/j.yexcr.2004.03.046. doi:10.1016/j.yexcr.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 109.Sibaev A, Yüce B, Schirra J, Göke B, Allescher HD, Storr M. Are gap junctions truly involved in inhibitory neuromuscular interaction in mouse proximal colon? Clin Exp Pharmacol Physiol. 2006;33(8):740–745. doi: 10.1111/j.1440-1681.2006.04433.x. doi:10.1111/j.1440-1681.2006.04433.x. [DOI] [PubMed] [Google Scholar]
- 110.Daniel EE, Wang YF. Gap junctions in intestinal smooth muscle and interstitial cells of Cajal. Microsc Res Tech. 1999;47(5):309–320. doi: 10.1002/(SICI)1097-0029(19991201)47:5<309::AID-JEMT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 111.Döring B, Pfitzer G, Adam B, Liebregts T, Eckardt D, Holtmann G, Hofmann F, Feil S, Feil R, Willecke K. Ablation of connexin43 in smooth muscle cells of the mouse intestine: functional insights into physiology and morphology. Cell Tissue Res. 2007;327(2):333–342. doi: 10.1007/s00441-006-0281-6. doi:10.1007/s00441-006-0281-6. [DOI] [PubMed] [Google Scholar]
- 112.Nagy JI, Urena-Ramirez V, Ghia JE. Functional alterations in gut contractility after connexin36 ablation and evidence for gap junctions forming electrical synapses between nitrergic enteric neurons. FEBS Lett. 2014;588(8):1480–1490. doi: 10.1016/j.febslet.2014.02.002. doi:10.1016/j.febslet.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem. 2009;284(33):22332–22343. doi: 10.1074/jbc.M901619200. doi:10.1074/jbc.M901619200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251(2):206–220. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- 115.Naves MMV, Silveira ER, Dagli MLZ, Moreno FS. Effects of beta-carotene and vitamin A on oval cell proliferation and connexin 43 expression during hepatic differentiation in the rat. J Nutr Biochem. 2001;12(12):685–692. doi: 10.1016/s0955-2863(01)00187-5. doi:10.1016/s0955-2863(01)00187-5. [DOI] [PubMed] [Google Scholar]
- 116.Neveu MJ, Hully JR, Babcock KL, Vaughan J, Hertzberg EL, Nicholson BJ, Paul DL, Pitot HC. Proliferation-associated differences in the spatial and temporal expression of gap junction genes in rat liver. Hepatology. 1995;22(1):202–212. [PubMed] [Google Scholar]
- 117.Paku S, Nagy P, Kopper L, Thorgeirsson SS. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39(5):1353–1361. doi: 10.1002/hep.20178. doi:10.1002/hep.20178. [DOI] [PubMed] [Google Scholar]
- 118.Kojima T, Mitaka T, Shibata Y, Mochizuki Y. Induction and regulation of connexin26 by glucagon in primary cultures of adult rat hepatocytes. J Cell Sci. 1995;108(8):2771–2780. doi: 10.1242/jcs.108.8.2771. [DOI] [PubMed] [Google Scholar]
- 119.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83(4):1359–1400. doi: 10.1152/physrev.00007.2003. doi:10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 120.Stumpel F, Ott T, Willecke K, Jungermann K. Connexin 32 gap junctions enhance stimulation of glucose output by glucagon and noradrenaline in mouse liver. Hepatology. 1998;28(6):1616–1620. doi: 10.1002/hep.510280622. doi:10.1002/hep.510280622. [DOI] [PubMed] [Google Scholar]
- 121.Clair C, Chalumeau C, Tordjmann T, Poggioli J, Erneux C, Dupont G, Combettes L. Investigation of the roles of Ca(2+) and InsP(3) diffusion in the coordination of Ca(2+) signals between connected hepatocytes. J Cell Sci. 2001;114:1999–2007. doi: 10.1242/jcs.114.11.1999. [DOI] [PubMed] [Google Scholar]
- 122.Nelles E, Butzler C, Jung D, Temme A, Gabriel HD, Dahl U, Traub O, Stumpel F, Jungermann K, Zielasek J, Toyka KV, Dermietzel R, Willecke K. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci USA. 1996;93(18):9565–9570. doi: 10.1073/pnas.93.18.9565. doi:10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bode HP, Wang L, Cassio D, Leite MF, St-Pierre MV, Hirata K, Okazaki K, Sears ML, Meda P, Nathanson MH, Dufour JF. Expression and regulation of gap junctions in rat cholangiocytes. Hepatology. 2002;36(3):631–640. doi: 10.1053/jhep.2002.35274. doi:10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- 124.Nathanson MH, Rios-Velez L, Burgstahler AD, Mennone A. Communication via gap junctions modulates bile secretion in the isolated perfused rat liver. Gastroenterology. 1999;116(5):1176–1183. doi: 10.1016/s0016-5085(99)70021-1. doi:10.1016/s0016-5085(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 125.Neveu MJ, Babcock KL, Hertzberg EL, Paul DL, Nicholson BJ, Pitot HC. Colocalized alterations in connexin32 and cytochrome P450IIB1/2 by phenobarbital and related liver tumor promoters. Cancer Res. 1994;54(12):3145–3152. [PubMed] [Google Scholar]
- 126.Shoda T, Mitsumori K, Onodera H, Toyoda K, Uneyama C, Imazawa T, Hirose M. The relationship between decrease in Cx32 and induction of P450 isozymes in the early phase of clofibrate hepatocarcinogenesis in the rat. Arch Toxicol. 1999;73(7):373–380. doi: 10.1007/s002040050676. [DOI] [PubMed] [Google Scholar]
- 127.Shoda T, Mitsumori K, Onodera H, Toyoda K, Uneyama C, Takada K, Hirose M. Liver tumor-promoting effect of beta-naphthoflavone, a strong CYP 1A1/2 inducer, and the relationship between CYP 1A1/2 induction and Cx32 decrease in its hepatocarcinogenesis in the rat. Toxicol Pathol. 2000;28(4):540–547. doi: 10.1177/019262330002800406. [DOI] [PubMed] [Google Scholar]
- 128.Yang J, Ichikawa A, Tsuchiya T. A novel function of connexin 32: marked enhancement of liver function in a hepatoma cell line. Biochem Biophys Res Commun. 2003;307(1):80–85. doi: 10.1016/s0006-291x(03)01117-3. [DOI] [PubMed] [Google Scholar]
- 129.Fladmark KE, Gjertsen BT, Molven A, Mellgren G, Vintermyr OK, Døskeland SO. Gap junctions and growth control in liver regeneration and in isolated rat hepatocytes. Hepatology. 1997;25(4):847–855. doi: 10.1002/hep.510250411. doi:10.1002/hep.510250411. [DOI] [PubMed] [Google Scholar]
- 130.Kren BT, Kumar NM, Wang SQ, Gilula NB, Steer CJ. Differential regulation of multiple gap junction transcripts and proteins during rat liver regeneration. J Cell Biol. 1993;123(3):707–718. doi: 10.1083/jcb.123.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Temme A, Ott T, Dombrowski F, Willecke K. The extent of synchronous initiation and termination of DNA synthesis in regenerating mouse liver is dependent on connexin32 expressing gap junctions. J Hepatol. 2000;32(4):627–635. doi: 10.1016/s0168-8278(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 132.Kojima T, Yamamoto T, Murata M, Lan M, Takano K, Go M, Ichimiya S, Chiba H, Sawada N. Role of the p38 MAP-kinase signaling pathway for Cx32 and claudin-1 in the rat liver. Cell Commun Adhes. 2003;10(4-6):437–443. doi: 10.1080/cac.10.4-6.437.443. [DOI] [PubMed] [Google Scholar]
- 133.Chipman JK, Mally A, Edwards GO. Disruption of gap junctions in toxicity and carcinogenicity. Toxicol Sci. 2003;71(2):146–153. doi: 10.1093/toxsci/71.2.146. [DOI] [PubMed] [Google Scholar]
- 134.Koffler L, Roshong S, Park IK, Cesen-Cummings K, Thompson DC, Dwyer-Nield LD, Rice P, Mamay C, Malkinson AM, Ruch RJ. Growth inhibition in G(1) and altered expression of cyclin D1 and p27(kip-1) after forced connexin expression in lung and liver carcinoma cells. J Cell Biochem. 2000;79(3):347–354. doi: 10.1002/1097-4644(20001201)79:3<347::aid-jcb10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 135.Yano T, Hernandez-Blazquez FJ, Omori Y, Yamasaki H. Reduction of malignant phenotype of HEPG2 cell is associated with the expression of connexin 26 but not connexin 32. Carcinogenesis. 2001;22(10):1593–1600. doi: 10.1093/carcin/22.10.1593. doi:10.1093/carcin/22.10.1593. [DOI] [PubMed] [Google Scholar]
- 136.Wilson MR, Close TW, Trosko JE. Cell population dynamics (apoptosis, mitosis, and cell-cell communication) during disruption of homeostasis. Exp Cell Res. 2000;254(2):257–268. doi: 10.1006/excr.1999.4771. [DOI] [PubMed] [Google Scholar]
- 137.Vinken M, Maes M, Cavill R, Valkenborg D, Ellis JK, Decrock E, Leybaert L, Staes A, Gevaert K, Oliveira AG, Menezes GB, Cogliati B, Dagli ML, Ebbels TM, Witters E, Keun HC, Vanhaecke T, Rogiers V. Proteomic and metabolomic responses to connexin43 silencing in primary hepatocyte cultures. Arch Toxicol. 2013;87(5):883–894. doi: 10.1007/s00204-012-0994-0. doi:10.1007/s00204-012-0994-0. [DOI] [PubMed] [Google Scholar]
- 138.Azarashvili T, Baburina Y, Grachev D, Krestinina O, Evtodienko Y, Stricker R, Reiser G. Calcium-induced permeability transition in rat brain mitochondria is promoted by carbenoxolone through targeting connexin43. Am J Physiol Cell Physiol. 2011;300(3):C707–C720. doi: 10.1152/ajpcell.00061.2010. doi:10.1152/ajpcell.00061.2010. [DOI] [PubMed] [Google Scholar]
- 139.Goubaeva F, Mikami M, Giardina S, Ding B, Abe J, Yang J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem Biophys Res Commun. 2007;352(1):97–103. doi: 10.1016/j.bbrc.2006.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lu G, Haider HKh, Porollo A, Ashraf M. Mitochondria-specific transgenic overexpression of connexin-43 simulates preconditioning-induced cytoprotection of stem cells. Cardiovasc Res. 2010;88(2):277–286. doi: 10.1093/cvr/cvq293. doi:10.1093/cvr/cvq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467(7317):863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186(11):6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 143.Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated c-terminal autoinhibitory region. J Biol Chem. 2012;287(14):11303–11311. doi: 10.1074/jbc.M111.323378. doi:10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X(7) receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295(3):C752–C760. doi: 10.1152/ajpcell.00228.2008. doi:10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282(4):2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 147.Bodendiek SB, Raman G. Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem. 2010;17(34):4191–4230. doi: 10.2174/092986710793348563. [DOI] [PubMed] [Google Scholar]
- 148.Abudara V, Bechberger J, Freitas-Andrade M, De Bock M, Wang N, Bultynck G, Naus CC, Leybaert L, Giaume C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;8:306. doi: 10.3389/fncel.2014.00306. doi:10.3389/fncel.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Iyyathurai J, Dhondt C, Wang N, De Bock M, Himpens B, Retamal MA, Stehberg J, Leybaert L, Bultynck G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology. 2013;75:491–505. doi: 10.1016/j.neuropharm.2013.04.050. doi:10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 150.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180(11):7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 151.Kanczuga-Koda L, Sulkowski S, Koda M, Sobaniec-Lotowska M, Sulkowska M. Expression of connexins 26, 32 and 43 in the human colon: an immunohistochemical study. Folia Histochem Cytobiol. 2004;42(4):203–207. [PubMed] [Google Scholar]
- 152.Nicholson B, Dermietzel R, Teplow D, Traub O, Willecke K, Revel JP. Two homologous protein components of hepatic gap junctions. Nature. 1987;329(6141):732–734. doi: 10.1038/329732a0. doi:10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- 153.Kuraoka A, Iida H, Hatae T, Shibata Y, Itoh M, Kurita T. Localization of gap junction proteins, connexins 32 and 26, in rat and guinea pig liver as revealed by quick-freeze, deep-etch immunoelectron microscopy. J Histochem Cytochem. 1993;41(7):971–980. doi: 10.1177/41.7.8390496. [DOI] [PubMed] [Google Scholar]
- 154.Nakashima Y, Ono T, Yamanoi A, El-Assal ON, Kohno H, Nagasue N. Expression of gap junction protein connexin32 in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Gastroenterol. 2004;39(8):763–768. doi: 10.1007/s00535-003-1386-2. [DOI] [PubMed] [Google Scholar]
- 155.Temme A, Traub O, Willecke K. Downregulation of connexin32 protein and gap-junctional intercellular communication by cytokine-mediated acute-phase response in immortalized mouse hepatocytes. Cell Tissue Res. 1998;294(2):345–350. doi: 10.1007/s004410051184. [DOI] [PubMed] [Google Scholar]
- 156.Tang B, Peng ZH, Yu PW, Yu G, Qian F, Zeng DZ, Zhao YL, Shi Y, Hao YX, Luo HX. Aberrant expression of Cx43 is associated with the peritoneal metastasis of gastric cancer and Cx43-mediated gap junction enhances gastric cancer cell diapedesis from peritoneal mesothelium. PLoS ONE. 2013;8(9):e74527. doi: 10.1371/journal.pone.0074527. doi:10.1371/journal.pone.0074527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oyamada M, Krutovskikh VA, Mesnil M, Partensky C, Berger F, Yamasaki H. Aberrant expression of gap junction gene in primary human hepatocellular carcinomas: increased expression of cardiac-type gap junction gene connexin 43. Mol Carcinog. 1990;3(5):273–278. doi: 10.1002/mc.2940030507. [DOI] [PubMed] [Google Scholar]
- 158.Wang ZS, Wu LQ, Yi X, Geng C, Li YJ, Yao RY. Connexin-43 can delay early recurrence and metastasis in patients with hepatitis B-related hepatocellular carcinoma and low serum alpha-fetoprotein after radical hepatectomy. BMC Cancer. 2013;13:306. doi: 10.1186/1471-2407-13-306. doi:10.1186/1471-2407-13-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang D, Kaneda M, Nakahama K, Arii S, Morita I. Connexin 43 expression promotes malignancy of HuH7 hepatocellular carcinoma cells via the inhibition of cell-cell communication. Cancer Lett. 2007;252(2):208–215. doi: 10.1016/j.canlet.2006.12.024. doi:10.1016/j.canlet.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 160.Balasubramaniyan V, Dhar DK, Warner AE, Vivien Li WY, Amiri AF, Bright B, Mookerjee RP, Davies NA, Becker DL, Jalan R. Importance of Connexin-43 based gap junction in cirrhosis and acute-on-chronic liver failure. J Hepatol. 2013;58(6):1194–1200. doi: 10.1016/j.jhep.2013.01.023. doi:10.1016/j.jhep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 161.Cogliati B, Da Silva TC, Aloia TP, Chaible LM, Real-Lima MA, Sanches DS, Matsuzaki P, Hernandez-Blazquez FJ, Dagli ML. Morphological and molecular pathology of CCL4-induced hepatic fibrosis in connexin43-deficient mice. Microsc Res Tech. 2011;74(5):421–429. doi: 10.1002/jemt.20926. doi:10.1002/jemt.20926. [DOI] [PubMed] [Google Scholar]