Abstract
Objective
To derive latent classes (longitudinal ‘phenotypes’) of frequency of bedwetting from 4 – 9 years and to examine their association with developmental delay, parental history of bedwetting, length of gestation and birth-weight.
Method
We used data from 8,769 children from the UK ALSPAC cohort. Mothers provided repeated reports on their child’s frequency of bedwetting from 4–9 years. We used longitudinal latent class analysis to derive latent classes of bedwetting and examined their association with gender, developmental level at 18 months, parental history of wetting, birth-weight and gestational length.
Results
We identified five latent classes: (i) ‘normative’ –low probability of bedwetting; (ii) ‘infrequent delayed’ – delayed attainment of nighttime bladder control with bedwetting <twice a week; (iii) ‘frequent delayed’ – delayed attainment of nighttime bladder control with bedwetting >= twice a week; (iv) ‘infrequent persistent’ – persistent bedwetting <twice a week and (v) ‘frequent persistent’ – persistent bedwetting >= twice a week. Male gender (odds ratio= 3.20 [95% confidence interval= 2.36, 4.34]), developmental delay e.g. delayed social skills (1.33 [1.11, 1.58]) and maternal history of wetting (3.91 [2.60, 5.88]) were associated with an increase in the odds of bedwetting at 4–9 years. There was little evidence that low birth weight and shorter gestation period were associated with bedwetting.
Conclusion
We described patterns of development of nighttime bladder control and found evidence for factors that predict continuation of bedwetting at school age. Increased knowledge of risk factors for bedwetting is needed to identify children at risk of future problems attaining and maintaining continence.
Keywords: Bedwetting, risk factors, child development, cohort study, ALSPAC
INTRODUCTION
Bedwetting (referred to as nocturnal enuresis at 5 years or older) is among the most common chronic conditions of childhood and its impact on quality of life is comparable to other chronic paediatric conditions such as asthma and epilepsy.1 Although most children attain nighttime bladder control by 4 – 6 years,2 some experience delays in attaining nighttime dryness, relapses in bedwetting and persistence of bedwetting into late childhood (9 years).3 Persistent bedwetting in childhood has been linked to an increased risk of bedwetting in adolescence and adulthood.4 There is a need to understand factors that predict the prognosis of bedwetting in order to improve the identification of children who are at risk of persistent bedwetting. Previous research identified trajectories of typical and atypical development (pattern of development that falls outside the normal or expected range) of nighttime bladder control using powerful methods for statistical modeling of longitudinal data (longitudinal latent class analysis- LLCA).3 LLCA is a useful tool for researchers examining the developmental trajectories of disorders because it provides an empirical means of summarizing large amounts of data and identifying clusters of individuals (latent classes) with typical and atypical courses of development. LLCA was previously applied to longitudinal data derived from parental reports of bedwetting (presence/absence) at ages 4 – 9 years in almost 11,000 children from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort.3 In this previous study, five latent classes adequately described variability in the development of nighttime bladder control in the ALSPAC cohort: (i) normative (69.9% of the sample) (ii) delayed (8.4%) (iii) severely delayed (9.3%) (iv) persistent (8.6%) and (v) relapse (3.8%). The latent classes for bedwetting were consistent with those reported in an earlier study using data from the 1946 British Birth Cohort.5 Both of these earlier studies are, however, limited because they treated the repeated measures of bedwetting as simple binary variables and ignored information on frequency of bedwetting. There is considerable variability in frequency of bedwetting among children with atypical development of nighttime bladder control. Children with persistent and frequent bedwetting might be identifiable by risk factors in early life.
There is evidence from previous studies that early risk factors including developmental delay,6,7,8,9 parental history of bedwetting,4,10,11 premature birth7 and low birth-weight6,8 are linked to bedwetting. Limitations of earlier studies include a cross sectional design,4,6,10 small sample size,8,9,11 assessment of bedwetting only up to age 4,7 or assessment of bedwetting at a single time point.6 Besides the ALSPAC study, there are only two other longitudinal studies that have examined risk factors for bedwetting. One is a relatively small longitudinal study comprising both healthy and pre-term children that assessed bedwetting only up to age 6 and reported no association between delayed development and age at attainment of nighttime bladder control.12 The other is a prospective cohort study that examined bedwetting up to age 8 and found that the above risk factors (with the exception of gestational length) were linked to later attainment of bladder control.2 However, this earlier prospective study did not take into account the heterogeneity in development of nighttime bladder control through childhood.
In this paper we extend previous work using data from the ALSPAC birth cohort to derive latent classes of nighttime bladder control including information on frequency of bedwetting. We then use the latent classes as outcome variables to examine their association with risk factors including developmental delay, parental history of bedwetting, length of gestation and birth-weight. We examine whether there are specific risk factors that might enable us to distinguish between different atypical patterns of bedwetting e.g. we are interested in whether children who experience a natural resolution of their bedwetting can be distinguished from those with bedwetting that persists into late childhood.
METHODS
Participants
The sample comprised participants from the Avon Longitudinal Study of Parents and Children. Detailed information about ALSPAC is available on the study website (http://www.bristol.ac.uk/alspac), which includes a fully searchable dictionary of available data (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary). Pregnant women resident in the former Avon Health Authority in south-west England, having an estimated date of delivery between 1/4/91 and 31/12/92 were invited to take part, resulting in a cohort of 14,541 pregnancies and 13,973 singletons/twins (7,217 boys and 6,756 girls) alive at 12 months.13 Ethical approval for the study was obtained from the ALSPAC Law and Ethics committee and local research ethics committees. Written informed consent was obtained after the procedure(s) had been fully explained.
Repeated measures of bedwetting
At ages 41/2, 51/2, 61/2, 71/2 & 91/2 years (hereafter referred to as 4 – 9 years) parents were asked “How often usually does your child wet the bed?” and were given the options ‘never’; ‘less than once a week’; ‘about once a week’; ‘2-5 times a week’; ‘nearly every night’; ‘more than once a night’. We derived an ordinal measure by collapsing the bedwetting frequency data into a three-level variable indicating no current bedwetting (coded “0”), infrequent bedwetting (<than once a week or about once a week – coded “1”) and frequent bedwetting (2 – 5 times a week, nearly every night or more than once a night – coded “2”). The latter category corresponds to the frequency of bedwetting required for a DSM-V diagnosis of nocturnal enuresis.
Risk factors
Developmental level
Developmental level at 18 months was assessed using a questionnaire developed by ALSPAC including items from the Denver Developmental Screening Test14 comprising four domains of development (Cronbach’s alphas: fine motor=0.679; gross motor= 0.689; communication= 0.752 and social skills= 0.624). Scores for each domain were adjusted for age of the child when the questionnaire was completed and standardized (using a linear regression model and extracting the residuals) before being reversed so that a high score indicates a low developmental level. Participants who responded more than four weeks either side of the intended age were excluded.
Parental history of incontinence
Prior to the birth of the study child, both parents were asked whether they had experienced bedwetting or daytime wetting beyond the age of five15. We derived binary variables for parental history of incontinence indicating whether mothers or fathers had any versus no history of wetting. We excluded isolated occurrences of maternal bedwetting around the perinatal period.
Length of gestation and birth-weight
ALSPAC staff visited maternity hospitals of study members and recorded information from the obstetric notes on birth weight.13 Gestation was derived from the date of delivery, date of last menstrual period (reported at enrolment) and using ultrasound data where available. We derived binary variables for gestational length (indicating whether length of gestation was less than 37 weeks) and birth weight (indicating whether birth weight was less than 2.5kg) consistent with earlier studies.2,7
Confounders
We adjusted for gender and a range of potential confounders derived from responses to a questionnaire, completed by mothers during the antenatal period, containing items on socioeconomic position and adversity. Binary variables were generated from these questions and each item was scored as 1 if an adversity was present and 0 if not. The items included social class assessed based on the lower of the mother or partner’s occupational social class using the 1991 British Office of Population and Census Statistics (OPCS) classification and dichotomized into non-manual (professional, managerial or skilled professions) and manual (partly or unskilled occupations); early parenthood (< 19 years versus >=19 years), housing adequacy (yes/no - comprising crowding, periods of homelessness, living conditions, major defects/infestation), maternal education (defined as none versus high school qualifications or greater), financial difficulties (yes/no), family size (<3 children versus >=3 children) and the presence of a social network (yes/no – comprising emotional support, practical/financial support).
Statistical modeling
We used longitudinal mixture modelling in Mplus (version 7.2)16 to extract latent classes of bedwetting based on the ordinal measures of bedwetting frequency described above. The relationship between the resulting latent classes and a range of potential risk factors was then examined.
Estimation of trajectories
To establish trajectories of bedwetting using these repeated measures data we employed LLCA. This is simply Latent Class Analysis (LCA) applied to longitudinal data. As with the standard LCA model, there are unconditional probabilities that capture the class sizes in the latent typological classification, and a set of conditional probabilities, for each time-point in each class, that describe the trajectories of bedwetting in each group. Whilst other more parsimonious options exist such as Latent Class Growth Analysis (LCGA), we favour LLCA as it is able to capture a broader range of trajectory shapes such as those observed in the aforementioned 1946 British Birth Cohort.5
Longitudinal mixture modelling methods analyze response strings that describe each child’s longitudinal pattern of frequency of bedwetting (no bedwetting= 0, infrequent bedwetting= 1 or frequent bedwetting= 2) from 4 – 9 years. For instance, the data for a child who attains nighttime bladder control at an early age may be “10000”; a child who experiences delayed attainment might have the response “21000” and the response pattern for a child with persistent and frequent bedwetting may be “22221”. The model assumes that observed heterogeneity in response is due to a latent (unobserved) grouping in the population. Starting with a single class, additional classes are added until the various assessments of model fit reach an acceptable level. Similar to factor analysis, this latent categorical variable should explain the associations within the set of repeated measures such that within each latent class, respondents form a single homogeneous group, and furthermore the repeated measurements are independent of each other, i.e. there is local (conditional) independence.
Multinomial modelling – prediction of class membership
We estimated the association between the risk factors and class membership using a series of univariable multinomial logistic regression models and employing the normative latent class as the baseline category (reference group) for the outcome before re-parameterizing to derive comparisons across the other outcome classes. Parameter estimates were obtained using the “Modal ML” 3-step method proposed by Jeroen Vermunt17 and since implemented in Mplus with the “auxiliary (r3step)” command.
An illustration of the method is shown in Figure A (supplemental digital content). In step one, latent class C is estimated using an unconditional LLCA (i.e. a model in the absence of covariates). This model is used to derive class-assignment probabilities i.e. the probability with which each respondent is believed to belong to each class. Respondents are then assigned to the class for which their probability is greatest creating a non-latent classification C* (step two – not illustrated in figure). Finally in step 3, measurement error inherent in C* is quantified and used to reproduce latent C† using a set of logit constraints. This approach has been shown to produce less-biased estimates than traditional three-step methods such as standard probability weighting or modal assignment, whilst avoiding the problem of covariates impacting on the measurement model itself.18 Models were adjusted for the confounders described above. The analysis examining the effect of low birth weight was additionally adjusted for gestational age.
RESULTS
Complete bedwetting data at all time points from 4 – 9 years were available for 5,852 children (complete case sample); 8,769 had data from at least three time-points (sample with 3 or more measures) and 10,820 had data available on at least one time point. The proportion of children with bedwetting decreased over time and proportions did not change markedly when the sample was restricted to participants with more data (Table 1).
Table 1.
Frequency of bedwetting in the three samples considered for the analysis
| 4.5 years | 5.5 years | 6.5 years | 7.5 years | 9.5 years | |
|---|---|---|---|---|---|
| Complete bedwetting data (n=5,852) | |||||
| No bedwetting | 4075 (69.6%) | 4566 (78.0%) | 4748 (81.1%) | 4993 (85.3%) | 5298 (90.5%) |
| Infrequent bedwetting | 1293 (22.1%) | 989 (16.9%) | 904 (15.5%) | 736 (12.6%) | 476 (8.1%) |
| Frequent bedwetting | 484 (8.3%) | 297 (5.1%) | 200 (3.4%) | 123 (2.1%) | 78 (1.3%) |
|
| |||||
| Sample with 3 or more measures (n=8,769) | |||||
| No bedwetting | 5821 (70.0%) | 6462 (77.9%) | 6560 (80.6%) | 6579 (84.7%) | 6596 (90.4%) |
| Infrequent bedwetting | 1807 (21.7%) | 1351 (16.3%) | 1259 (15.5%) | 986 (12.7%) | 597 (8.2%) |
| Frequent bedwetting | 691 (8.3%) | 479 (5.8%) | 320 (3.9%) | 203 (2.6%) | 104 (1.4%) |
|
| |||||
| Sample with at least one measure (n=10,820) | |||||
| No bedwetting | 6696 (70.0%) | 6954 (77.9%) | 6818 (80.5%) | 6930 (84.6%) | 7002 (90.3%) |
| Infrequent bedwetting | 2068 (21.6%) | 1440 (16.1%) | 1311 (15.5%) | 1041 (12.7%) | 635 (8.2%) |
| Frequent bedwetting | 801 (8.4%) | 532 (6.0%) | 336 (4.0%) | 218 (2.7%) | 116 (1.5%) |
Actual number with available data on each bedwetting measure varies in the two non-complete samples.
Infrequent bedwetting (up to once a week), frequent bedwetting (2+ times a week).
We focused on the sample with three or more measures for further analysis because much of the sample gains made by utilizing the sample with one or more measures were lost following inclusion of the early risk factors. Table 2 provides the model fit statistics for the LLCA models in these three samples.
Table 2.
Model Fit Statistics for LLCA models
| (i) Complete case sample (n = 5,852) | ||||||
| # class | BIC | Entropy | BLRT | LMR | Cond. Ind. | Class distribution |
|
| ||||||
| 2 | 26397.2 | 0.903 | <0.001 | <0.001 | 1789.9 | 80.2%, 19.8% |
| 3 | 25404.8 | 0.900 | <0.001 | <0.001 | 293.8 | 75.7%, 19.6%, 4.7% |
| 4 | 25185.2 | 0.857 | <0.001 | <0.001 | 99.9 | 72.3%, 14.0%, 9.1%, 4.6% |
| 5 | 25134.2 | 0.867 | <0.001 | 0.849 | 21.0 | 71.4%, 14.5%, 9.2%, 2.8%, 2.1% |
| 6 | 25158.3 | 0.865 | <0.001 | 0.815 | 10.7 | 71.9%, 12.8%, 5.6%, 5.0%, 2.6%, 2.1% |
| (ii) Sample with 3 or more measures (n =8,769) | ||||||
| # class | BIC | Entropy | BLRT | LMR | Cond. Ind. | Class distribution |
|
| ||||||
| 2 | 37189.1 | 0.899 | <0.001 | <0.001 | 1789.9 | 80.3%, 19.7% |
| 3 | 35754.4 | 0.889 | <0.001 | <0.001 | 423.9 | 75.8%, 18.9%, 5.3% |
| 4 | 35500.0 | 0.844 | <0.001 | <0.001 | 168.9 | 72.8%, 13.5%, 8.5%, 5.2% |
| 5 | 35409.1 | 0.850 | <0.001 | 0.450 | 30.7 | 71.5%, 14.3%, 8.6%, 3.2%, 2.4% |
| 6 | 35432.5 | 0.851 | <0.001 | 0.904 | 16.6 | 72.0%, 12.5%, 5.2%, 4.9%, 3.1%, 2.3% |
| (iii) Sample with 1 or more measures (n =10,820) | ||||||
| # class | BIC | Entropy | BLRT | LMR | Cond. Ind. | Class distribution |
|
| ||||||
| 2 | 40983.2 | 0.858 | <0.001 | <0.001 | 2991.9 | 80.2%, 19.8% |
| 3 | 39489.3 | 0.851 | <0.001 | <0.001 | 531.0 | 75.7%, 18.8%, 5.5% |
| 4 | 39233.0 | 0.807 | <0.001 | <0.001 | 217.6 | 72.7%, 13.4%, 8.5%, 5.3% |
| 5 | 39139.5 | 0.815 | <0.001 | 0.496 | 38.2 | 71.5%, 14.2%, 8.5%, 3.3%, 2.4% |
| 6 | 39164.7 | 0.816 | <0.001 | 0.882 | 20.9 | 72.0%, 12.3%, 5.3%, 4.9%, 3.2%, 2.4% |
BIC - Bayesian Information Criterion; BLRT - Bootstrap Likelihood Ratio Test; LMR - Lo-Mendell-Rubin; Cond. Ind. - Conditional independence.
Model selection and model fit
To establish the optimal number of latent classes, we used the sample-size adjusted Bayesian Information Criterion (BIC);19 the Bootstrap Likelihood Ratio Test (BLRT) and the Lo-Mendell-Rubin (LMR) test statistics;20 and bivariate model fit information – an assessment of conditional independence. We repeated the estimation procedure whilst varying the amount of missing data and employed multiple random starts to help achieve the optimal maximum likelihood solution. It is rare that all indicators of model fit point to the same solution. The accepted approach is to evaluate the statistical evidence alongside face validity, resemblance to other results in the literature and pragmatic issues such as class size. The BLRT and LMR test statistics both assess change in model fit when adding an additional class. Here a high p-value for a k-class model indicates that the addition of a further class will not substantially improve fit. LMR deems the 5-class model to adequately explain the patterns in the observed data, however, as is often the case, the BLRT is more conservative.
The BIC is the traditional fit statistic for comparing mixture models and will typically decrease and then increase following the incremental additional of classes. Using this statistic the model with the lowest BIC would be deemed optimal.
Conditional independence (Cond. Ind.) is an assessment of the remaining association between each pair of measurements once heterogeneity accounted for by latent C has been removed. There is currently no accepted threshold for this measure, however, it is common to observe marked improvements (reductions) followed by smaller changes. Here these data suggested marked improvements when the 5th class is added to the model.
Entropy is a measure of classification accuracy, and whilst it is generally of little use in determining the optimal model, it indicates the level of bias that one would expect were a standard three-step estimation to be performed. Here entropy is fairly high and above the commonly used threshold of 0.8 for all models.
Inspection of all the model fit statistics indicated that either a 5- or 6-class solution would be acceptable. The 6-class solution did not yield an additional useful class that was considered to have face validity, therefore, we opted for the 5-class model for our analysis. The class distribution indicated classes as small as 2.1% of the sample, however, this was acceptable given our large sample size.
When comparing the five-class solution across the three samples shown in Table 2 there was good agreement, both in the class distribution and also the trajectories exhibited by each class. This was not unexpected given previous work showing little association between bedwetting and questionnaire non-response.3
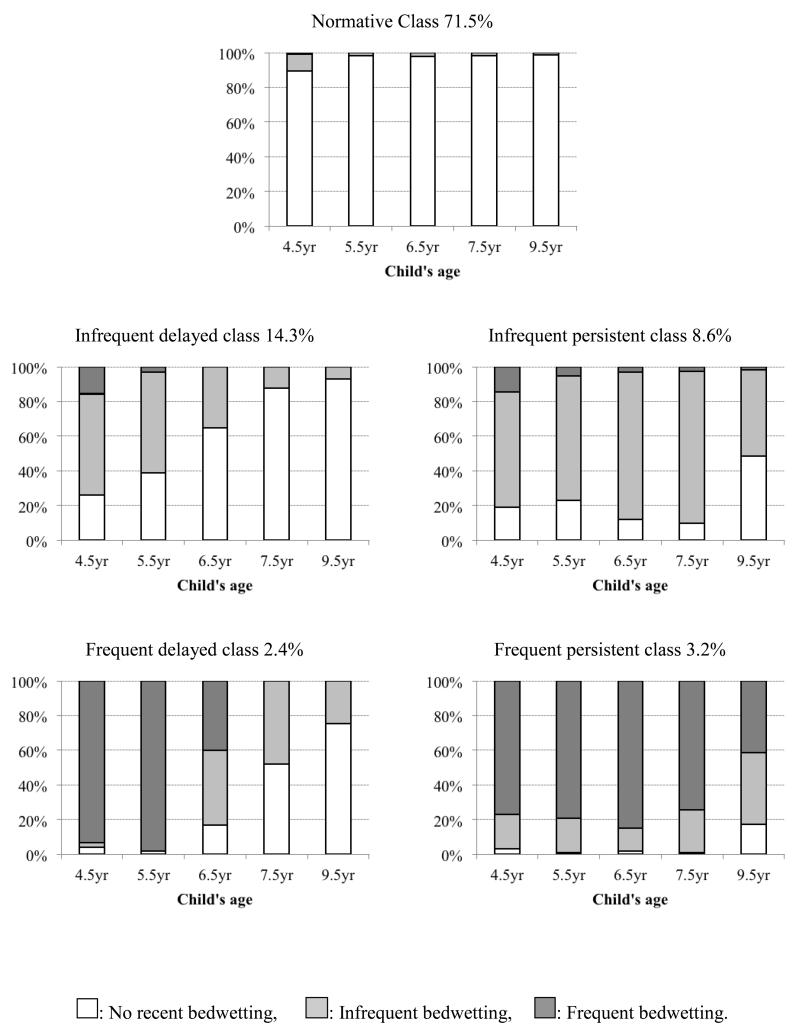
Predicted profiles of behavior within each class are illustrated in Figure 1. Here individual bars indicate the frequency of bedwetting (none, infrequent, frequent) within each of the latent classes at each time point. These results show a ‘normative class’ with low probability of bedwetting at any time point and comprising 71.5% of the sample. The four atypical classes varied according to both frequency and duration of bedwetting: ‘infrequent delayed’ (14.3%) – delayed attainment of nighttime bladder control and decreasing probability of infrequent bedwetting from 4 – 9 years; ‘infrequent persistent’ (8.6%) – relatively high probability of infrequent bedwetting; ‘frequent delayed’ (2.4%) – high probability of frequent bedwetting at age 4 years, which decreased and became more infrequent at 6 – 9 years; ‘frequent persistent’ (3.2%) – relatively high probability of bedwetting at least twice a week from 4 – 9 years.
Figure 1. Frequency of bedwetting from 4 – 9 years in each latent class (n=8769).
The individual bars indicate the frequency of bedwetting (none, infrequent, frequent) within each of the latent classes at each time point.
In view of previous findings showing that more severe bedwetting is often accompanied by daytime wetting,4 we also examined the proportion of daytime wetting within each bedwetting latent class (see Table A, supplemental digital content). The highest percentage of daytime wetting was in the frequent persistent class, followed by the infrequent persistent and frequent delayed classes.
Association between the risk factors and bedwetting latent classes
We present the results for the sample with bedwetting data available on at least three time points. Conclusions were consistent for the samples with bedwetting data available on at least one time point and in the sample with complete bedwetting data (results available on request). Whilst the sample with complete bedwetting data had the lowest rates of socioeconomic disadvantage, there was little variation in the risk factors across samples (Table 3).
Table 3.
Risk factors and confounders in the three samples considered for the analysis
| Sample with complete data (n=5,852) | Sample with 3 or more bedwetting measures (n=8,769) | Sample with at least one bedwetting measure (n=10,820) | |
|---|---|---|---|
| Risk factors | |||
| Male gender | 2960 (50.6%) | 4513 (54.1%) | 5586 (51.6%) |
| Child development at 18 months | |||
| Communication | 7.3 (2.4) | 7.3 (2.4) | 7.3 (2.4) |
| Gross motor skills | 9.4 (1.5) | 9.4 (1.6) | 9.4 (1.6) |
| Social skills | 8.2 (2.2) | 8.2 (2.2) | 8.21(2.2) |
| Fine motor skills | 12.9 (1.7) | 12.8 (1.7) | 12.8 (1.8) |
| Parental history of wetting | |||
| Maternal | 471 (8.6%) | 729 (9.1%) | 924 (9.5%) |
| Paternal | 386 (9.4%) | 572 (9.9%) | 684 (10.0%) |
| Gestational length | |||
| <32 weeks | 42 (0.7%) | 84 (1.0%) | 113 (1.0%) |
| < 37 weeks | 274 (4.7%) | 460 (5.3%) | 614 (5.7%) |
| ≥42 weeks | 430 (7.4%) | 640 (7.3%) | 800 (7.4%) |
| Birth weight | |||
| <1500g | 93 (1.6%) | 153 (1.7%) | 191 (1.8%) |
| <2500g | 309 (5.3%) | 496 (5.7%) | 643 (5.9%) |
| ≥4500g | 105 (1.8%) | 164 (1.9%) | 204 (1.9%) |
| Confounders | |||
| Manual social class | 706 (12.8%) | 1239 (15.4%) | 1663 (15.4%) |
| Early parenthood | 213 (3.6%) | 419 (4.8%) | 648 (6.0%) |
| Housing inadequacy | 408 (7.0%) | 748 (8.6%) | 983 (9.3%) |
| Low maternal education | 580 (10.0%) | 996 (11.6%) | 1343 (12.9%) |
| Financial difficulties | 803 (13.9%) | 1297 (15.1%) | 1653 (16.0%) |
| Family size>=3 | 251 (4.3%) | 424 (4.9%) | 600 (5.8%) |
| Poor social network | 716 (12.3%) | 1130 (13.0%) | 1434 (13.6%) |
n (%) for categorical variable or mean (SD) for continuous variables
Actual number with available data on each risk factor varies in each of the samples shown here.
Manual social class includes manual and part/unskilled.
Table 4 presents the results of the analysis examining the associations between the risk factors and latent class membership in the sample with bedwetting data available on at least three time points. Compared with females, males had between two and three times the odds of belonging to any of the atypical classes.
Table 4.
Odds ratios and 95% confidence intervals for the association between the risk factors and latent class membership (n=8,769)
| Class |
||||||
|---|---|---|---|---|---|---|
| N | Infrequent delayed | Infrequent persistent | Frequent delayed | Frequent persistent | ||
|
|
||||||
| Gender (male) | 8769 | 1.91 [1.58, 2.31] | 2.46 [2.02, 2.99] | 2.21 [1.55,3.14] | 3.20 [2.36, 4.34] | |
| p<0.001 | p<0.001 | p<0.001 | p<0.001 | |||
| Child Development at 18 months (per 1 SD of change) | ||||||
| Communication | Unadjusted | 8217 | 1.26 [1.14, 1.39] | 1.24 [1.13, 1.37] | 1.41 [1.13,1.75] | 1.35 [1.16, 1.57] |
| p<0.001 | p<0.001 | p=0.002 | p<0.001 | |||
| Adjusted | 7508 | 1.15 [1.04, 1.27] | 1.07 [0.97, 1.19] | 1.22 [0.95, 1.55] | 1.21 [1.02, 1.44] | |
| p=0.005 | p=0.167 | p=0.115 | p=0.026 | |||
| Social skills | Unadjusted | 8209 | 1.20 [1.09, 1.32] | 1.18 [1.07, 1.30] | 1.63 [1.30, 2.03] | 1.34 [1.15, 1.55] |
| p<0.001 | p=0.001 | p<0.001 | p<0.001 | |||
| Adjusted | 7502 | 1.22 [1.10, 1.35] | 1.06 [0.95, 1.17] | 1.44 [1.11, 1.88] | 1.33 [1.11, 1.58] | |
| p<0.001 | p=0.140 | p=0.003 | p=0.003 | |||
| Gross motor skills | Unadjusted | 8210 | 1.14 [1.04, 1.26] | 1.13 [1.04, 1.24] | 1.36 [1.19, 1.56] | 1.21 [1.06, 1.37] |
| p=0.005 | p=0.005 | p<0.001 | p=0.005 | |||
| Adjusted | 7501 | 1.13 [1.03, 1.25] | 1.11 [1.01, 1.22] | 1.30 [1.11, 1.52] | 1.23 [1.08, 1.42] | |
| p=0.013 | p=0.021 | p=0.001 | p=0.002 | |||
| Fine motor skills | Unadjusted | 8211 | 1.07 [0.97, 1.19] | 1.15 [1.05, 1.26] | 1.29 [1.08, 1.54] | 1.13 [0.97, 1.31] |
| p=0.183 | p=0.003 | p=0.005 | p=0.121 | |||
| Adjusted | 7503 | 0.99 [0.89, 1.10] | 1.10 [0.99, 1.21] | 1.27 [1.03, 1.56] | 1.06 [0.89, 1.25] | |
| p=0.832 | p=0.076 | p=0.023 | p=0.532 | |||
| Parental history of wetting | ||||||
| Maternal history | Unadjusted | 7649 | 1.68 [1.20 2.36] | 2.97 [2.26, 3.89] | 3.47 [2.17,5.56] | 3.87 [2.67, 5.61] |
| p=0.003 | p<0.001 | p<0.001 | p<0.001 | |||
| Adjusted | 6998 | 1.79 [1.25, 2.58] | 3.23 [2.40, 4.33] | 3.95 [2.39, 6.53] | 3.91 [2.60, 5.88] | |
| p<0.001 | p<0.001 | p<0.001 | p<0.001 | |||
| Paternal history | Unadjusted | 5798 | 1.40 [0.97, 2.02] | 1.35 [0.94, 1.94] | 2.17 [1.24, 3.80] | 2.23 [1.41, 3.54] |
| p=0.070 | p=0.100 | p=0.006 | p=0.001 | |||
| Adjusted | 5407 | 1.53 [1.07, 2.19] | 1.42 [0.987, 2.05] | 2.27 [1.24, 4.18] | 2.26 [1.38, 3.71] | |
| p=0.020 | p=0.065 | p=0.008 | p=0.001 | |||
| Obstetric variables | ||||||
| Gestational length (<37 weeks) | Unadjusted | 8769 | 1.30 [0.88, 1.14] | 1.09 [0.72, 1.64] | 0.93 [0.41, 2.10] | 1.02 [0.55, 1.88] |
| p=0.188 | p=0.686 | p=0.864 | p=0.946 | |||
| Adjusted | 7871 | 1.19 [0.76, 1.85] | 1.02 [0.64, 1.63] | 0.94 [0.39, 2.26] | 0.82 [0.40, 1.70] | |
| p=0.450 | p=0.938 | p=0.888 | p=0.599 | |||
| Birth weight (<2.5kg) | Unadjusted | 8769 | 1.12 [0.68, 1.84] | 0.39 [0.07, 2.04] | 0.81 [0.45, 1.49] | 0.72 [0.29, 1.80] |
| p=0.660 | p=0.265 | p=0.505 | p=0.486 | |||
| Adjusted | 7871 | 0.92 [0.55, 1.53] | 0.97 [0.58, 1.62] | 0.45 [0.15, 1.35] | 1.22 [0.65, 2.28] | |
| p=0.737 | p=0.907 | p=0.154 | p=0.538 | |||
Odds ratios for these analyses were derived in relation to the normative latent class of nighttime bladder control, which was used as the baseline category (reference group).
All models adjusted for gender, social class and family adversity. Birth weight analysis additionally adjusted for length of gestation.
There were increased odds of membership of the atypical latent classes among those with delayed development in communication, social and gross motor domains in the unadjusted models. Adjustment led to some attenuation of these effects, but there was still evidence for associations between delayed development and bedwetting, particularly for the frequent delayed and frequent persistent classes. Gender was the most influential confounder across all development domains.
There was strong evidence for an association between maternal history of wetting and bedwetting in offspring at 4 – 9 years, with increased odds of membership to all atypical latent classes. The odds ratios increased following adjustment, primarily due to gender. Compared with children whose mothers had no history of wetting, those with a maternal history of wetting had an almost four-fold increase in the odds of belonging to the frequent delayed or frequent persistent classes. Paternal history of wetting was also associated with increased odds of membership to the atypical classes (with the exception of ‘infrequent persistent’). Although the odds ratios for maternal history of wetting appeared higher than for paternal history, there was overlap in the confidence intervals.
There was little evidence that gestation of < 37 weeks was associated with increased odds of bedwetting at 4 – 9 years. We conducted additional sensitivity analyses examining gestation <= 32 weeks and included a category for long gestation (>=42 weeks) to examine non-linear effects, but there was little evidence for an association (results available on request). There was little evidence for an effect of low birth weight. We considered a lower threshold (< 1.5kg) and incorporated a category for those with high birth weight to test for non-linear effects (>=4.5kg), but conclusions were unaffected.
Additional comparisons of the bedwetting classes
An additional aim of this study is to examine whether there are specific risk factors that distinguish between different patterns of atypical wetting. To investigate this we re-parameterized our regression models in order to compare the persistent and delayed classes and to compare the frequent and infrequent classes (see tables B & C, supplemental digital content). We found evidence that the association with maternal history of wetting was stronger for the infrequent persistent compared with infrequent delayed class and for the frequent delayed compared with infrequent delayed class. There was some evidence that the associations with social and fine motor skills were stronger for those with frequent, compared with infrequent, bedwetting.
DISCUSSION
It is clinically useful to identify groups of children who show similar patterns in development of continence because it may help to identify risk factors associated with atypical development. Five latent classes adequately captured the variability in frequency of bedwetting from 4 – 9 years in this cohort. These longitudinal ‘phenotypes’ provide an alternative view to previous studies that have examined risk factors associated with bedwetting at specific ages. The latent classes can be ordered in terms of increasing ‘severity’, with persistent bedwetting more severe than delayed attainment of nighttime bladder control, and frequent bedwetting more severe than infrequent. The changing magnitude of the odds ratios for the risk factors was mostly in agreement with a dose response relationship consistent with increasing severity of bedwetting.
Our findings agree with previous reports that bedwetting is more common in boys than girls.2,21 Boys had around a two to threefold increase in odds of belonging to any of the atypical classes. The attainment of nighttime bladder control is an important milestone in child development and, on average boys experience more developmental delays than girls due to sex differences in the rate of brain development.22 Supplementary results show that girls in this cohort were more advanced than boys at 18 months across many areas of development (see Table D, supplemental digital content), thus, providing evidence that delayed acquisition of nighttime bladder control is consistent with developmental delay.
Numerous studies report evidence that developmental delay is linked to problems attaining nighttime bladder control.2,6,7,8,9 We found that children with delayed development at 18 months had increased odds of experiencing bedwetting at school age, with the strongest associations found for the frequent delayed and frequent persistent classes. Bedwetting has been previously described as a genetically determined maturational disorder of the CNS and is believed to be associated with a fundamental deficit in brain maturation.9 This neurological deficit is linked not only to bedwetting, but also to delays in language and motor development.2,7,8,9
Previous studies have repeatedly shown that bedwetting is highly familial.2,4,6,10,11 Consistent with these studies, we found strong evidence that parental history of wetting after age 5 was associated with increased odds of bedwetting in their offspring. Odds ratios were highest again for the frequent delayed and frequent persistent classes e.g. there was an almost four-fold increase in the odds of belonging to one of the atypical classes among children whose mothers had a history of wetting. It is notable that maternal history of wetting distinguished between infrequent delayed and infrequent persistent bedwetting classes and between frequent and infrequent delayed classes. Maternal history of wetting might identify children who are at risk of more severe forms of bedwetting characterised by high frequency and persistence into late childhood. Paternal history of wetting did not distinguish between the atypical classes, but this could be because parental wetting was based on retrospective recall with possible recall biases.15
We found little evidence that gestational age or low birth weight are associated with increased odds of bedwetting. Previous studies investigating these factors have reported inconsistent results.2,7,12
Strengths and limitations
We have extended our previous work through the inclusion of information on frequency of bedwetting, leading to a refinement of our earlier trajectories. For instance, incorporating information on frequency into our models re-classified persistent bedwetting into infrequent and frequent persistent bedwetting. With the exception of some evidence that maternal history of wetting might predict more frequent and persistent forms of bedwetting, there was little evidence for distinct etiologies for the latent classes. This indicates that the risk factors examined are common to all types of atypical development of nighttime bladder control. In particular, we find strong evidence that maturational and familial risk factors are linked to problems attaining continence. It is possible that distinct risk factor pathways would emerge if we further refined our bedwetting typologies by incorporating additional symptom information such as concurrent daytime wetting and indicators of bladder dysfunction.
Previously, a ‘relapse’ class was identified that was characterized by an increase in the probability of bedwetting during mid-childhood3, however a similar pattern was not derived in our updated model. It is important to highlight that LLCA is an empirical tool for simplifying complex data and that the resultant latent classes should be regarded as clusters of individuals following similar trajectories, not literally distinct entities. When comparing our current results with those published earlier we note that the majority (73%) of members of the previously identified ‘relapse’ class were re-classified as ‘infrequent persistent’ in the current model and 24% were re-classified as ‘infrequent delayed’.
An important clinical implication of our results is the identification of the more ‘severe’ and clinically relevant latent classes characterized by frequent (bedwetting twice or more per week) and either delayed attainment of bladder control (frequent delayed) or persistent bedwetting (frequent persistent). These classes are consistent with the DSM-V definition of nocturnal enuresis. Children with these patterns of bedwetting may present to clinicians for treatment. In contrast, the infrequent delayed and infrequent persistent classes would not be considered by DSM-V definition to have a clinical diagnosis of nocturnal enuresis. The infrequent delayed class is consistent with maturational delay in attainment of continence. These children experience a natural resolution of bedwetting that occurs later than in typical developing children. Infrequent persistent bedwetting, whilst not meeting criteria for a diagnosis of nocturnal enuresis, may still have a meaningful impact on functioning due to the persistent nature of bedwetting into late childhood. Those with frequent bedwetting that persists into late childhood and adolescence are believed to have a more refractory form of bedwetting that is often accompanied by underlying bladder dysfunction4. Consistent with studies reporting more daytime wetting in older children who have failed to attain nighttime bladder control4, we found that the percentage of children with daytime wetting at age 9 was highest in the frequent persistent bedwetting class (see Table A, supplemental digital content). This subtype of persistent and frequent bedwetting has been found to be more common among adults with bedwetting that has persisted since childhood.23 It is, therefore, important to identify children at risk of frequent bedwetting that persists into late childhood and prioritise them for treatment because their bedwetting is less likely to resolve with increasing age.4
A potential limitation of this study is the reliance on parental reports of children’s bedwetting frequency. It is unlikely that a child’s bedwetting would go entirely unnoticed by parents and most parents will be able to provide a reasonably accurate estimate of the frequency of bedwetting, due to being woken by these occurrences and having to deal with wet sheets. Errors in maternal reporting of bedwetting frequency are, however, possible, especially if there is an inconsistent pattern of bedwetting. The likely effect of this is to attenuate estimated differences between the classes.
Conclusions
This study examined the role of risk factors in the development of nighttime bladder control and extends previous work by modelling frequency of bedwetting across childhood in a large cohort. Increased understanding of the contributing factors that lead to problems attaining night-time bladder control is essential because a significant proportion of children suffer from bedwetting into their school years and this is associated with adverse impacts on their psychosocial development24. Important areas to consider for future research include extending the range of risk factors examined to see if there is evidence for distinct etiologies between the latent classes of bedwetting and to incorporate additional information to further refine the classes.
Supplementary Material
ACKNOWLEDGEMENTS
This study is based on the Avon Longitudinal Study of Parents and Children (ALSPAC). We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council, the Wellcome Trust (Grant reference: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This research was funded by a grant from the Medical Research Council (Increasing understanding of risk factors and outcomes associated with continence problems in children and adolescents. MRC reference: MR/L007231/1).
Funding: This research was funded by a grant from the Medical Research Council (Increasing understanding of risk factors and outcomes associated with continence problems in children and adolescents. MRC reference: MR/L007231/1).
The UK Medical Research Council, the Wellcome Trust (Grant reference: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC.
Footnotes
The authors have no conflicts of interest relating to this manuscript.
REFERENCES
- 1.Bachmann C, Lehr D, Janhsen E, et al. Health related quality of life of a tertiary referral center population with urinary incontinence using the DCGM-10 questionnaire. J Urol. 2009;182:2000–6. doi: 10.1016/j.juro.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 2.Fergusson DM, Horwood LJ, Shannon FT. Factors related to the age of attainment of nighttime bladder control: an 8-year longitudinal study. Pediatrics. 1986;78(5):884–890. [PubMed] [Google Scholar]
- 3.Joinson C, Heron J, Butler R, et al. Development of nighttime bladder control from 4 – 9 years: association with dimensions of parent rated child maturational level, child temperament and maternal psychopathology. LLCS. 2009;1:73–94. [Google Scholar]
- 4.Yeung CK, Sreedhar B, Sihoe JD, et al. Differences in characteristics of nighttime enuresis between children and adolescents: a critical appraisal from a large epidemiological study. BJU Int. 2006;97(5):1069–1073. doi: 10.1111/j.1464-410X.2006.06074.x. [DOI] [PubMed] [Google Scholar]
- 5.Croudace TJ, Jarvelin MR, Wadsworth ME, et al. Developmental typology of trajectories to nighttime bladder control: epidemiologic application of longitudinal latent class analysis. Am J Epidemiol. 2003;157(9):834–842. doi: 10.1093/aje/kwg049. [DOI] [PubMed] [Google Scholar]
- 6.Järvelin MR, Vikeväinen-Tervonen L, Moilanen I, et al. Enuresis in seven-year-old children. Acta Paediatr Scand. 1988;77:148–153. doi: 10.1111/j.1651-2227.1988.tb10614.x. [DOI] [PubMed] [Google Scholar]
- 7.Touchette E, Petit D, Paquet J, et al. Bed-wetting and its association with developmental milestones in early childhood. Arch Pediatr Adolesc Med. 2005;159(12):1129–1134. doi: 10.1001/archpedi.159.12.1129. [DOI] [PubMed] [Google Scholar]
- 8.Jarvelin MR. Developmental history and neurological findings in enuretic children. Dev Med Child Neurol. 1989;31(6):728–736. doi: 10.1111/j.1469-8749.1989.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 9.von Gontard A, Freitag CM, Seifen S, et al. Neuromotor development in nighttime enuresis. Dev Med Child Neurol. 2006;48(9):744–750. doi: 10.1017/S0012162206001599. [DOI] [PubMed] [Google Scholar]
- 10.Fockema MW, Candy GP, Kruger D, et al. Enuresis in South African children: prevalence, associated factors and parental perception of treatment. BJU Int. 2012;110(11 Pt C):E1114–20. doi: 10.1111/j.1464-410X.2012.11416.x. [DOI] [PubMed] [Google Scholar]
- 11.von Gontard A, Eiberg H, Hollmann E, et al. Molecular genetics of nocturnal enuresis: clinical and genetic heterogeneity. Acta Paediatrica. 1998;87(5):571–578. doi: 10.1080/08035259850158317. [DOI] [PubMed] [Google Scholar]
- 12.Largo RH, Molinari L, von Siebenthal K, et al. Development of bladder and bowel control: significance of prematurity, perinatal risk factors, psychomotor development and gender. European Journal of Pediatrics. 1999;158(2):115–122. doi: 10.1007/s004310051030. [DOI] [PubMed] [Google Scholar]
- 13.Boyd A, Golding J, Macleod J, et al. Cohort Profile: The ‘Children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–27. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankenburg WK, Dodds J, Archer P, et al. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89(1):91–97. [PubMed] [Google Scholar]
- 15.von Gontard A, Heron J, Joinson C. Family history of nocturnal enuresis and urinary incontinence – results from a large epidemiological study. J Urol. 2011;185(6):2303–6. doi: 10.1016/j.juro.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 16.Muthén B, Muthén L. Mplus User’s Guide. Muthén & Muthén; Los Angeles: 2012. [Google Scholar]
- 17.Vermunt JK. Latent Class Modeling with Covariates: Two Improved Three-Step Approaches. Political Analysis. 2010;18:450–469. [Google Scholar]
- 18.Asparouhov T, Muthen BO. [Accessed February 30, 2015];Auxiliary Variables in Mixture Modeling: 3-Step Approaches Using Mplus. 2013 Mplus Web Notes: No 15. Available at: http://www.statmodel.com/examples/webnotes/webnote15.pdf.
- 19.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 20.Nylund KL, Asparouhov T, Muthen BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modelling: A Monte Carlo Simulation Study. Structural Equation Modelling: A Multidisciplinary Journal. 2007;14(4):535–569. [Google Scholar]
- 21.Byrd RS, Weitzman M, Lanphear NE, et al. Bed-wetting in US children: epidemiology and related behavior problems. Pediatrics. 1996;98(3 Pt 1):414–419. [PubMed] [Google Scholar]
- 22.Lim S, Han CE, Uhlhaas PJ, et al. Preferential Detachment During Human Brain Development: Age- and Sex-Specific Structural Connectivity in Diffusion Tensor Imaging (DTI) Data. Cereb Cortex. 2013;15 doi: 10.1093/cercor/bht333. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung CK, Sihoe JD, Sit FK, et al. Urodynamic findings in adults with primary nocturnal enuresis. J Urol. 2004;171(6 Pt 2):2595–8. doi: 10.1097/01.ju.0000112790.72612.0a. [DOI] [PubMed] [Google Scholar]
- 24.Joinson C, Heron J, Emond A, et al. Psychological problems in children with bedwetting and combined (day and night) wetting: A UK population-based study. J Pediatr Psychol. 2007;32:605–16. doi: 10.1093/jpepsy/jsl039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.