Abstract
Mosquitoes have potent innate defense mechanisms that protect them from infection by diverse pathogens. Much remains unknown about how different pathogens are sensed and specific responses triggered. Leucine-Rich repeat IMmune proteins (LRIMs) are a mosquito-specific family of putative innate receptors. Although some LRIMs have been implicated in mosquito immune responses, the function of most family members is largely unknown. We screened Anopheles gambiae LRIMs by RNAi for effects on mosquito infection by rodent malaria and found that LRIM9 is a Plasmodium berghei antagonist with phenotypes distinct from family members LRIM1 and APL1C, which are key components of the mosquito complement-like pathway. LRIM9 transcript and protein levels are significantly increased after blood feeding but are unaffected by Plasmodium or midgut microbiota. Interestingly, LRIM9 in the hemolymph is strongly upregulated by direct injection of the ecdysteroid, 20-hydroxyecdysone. Our data suggest that LRIM9 may define a novel anti-Plasmodium immune defense mechanism triggered by blood feeding and that hormonal changes may alert the mosquito to bolster its defenses in anticipation of exposure to blood-borne pathogens.
Keywords: Mosquito, Malaria, Innate immunity, Ecdysone, Blood feeding
Introduction
Mosquitoes transmit numerous human and animal diseases with devastating consequences worldwide. Malaria is caused by protozoan Plasmodium parasites and transmitted to humans by infected female Anopheles mosquitoes. The mosquito life cycle makes it an ideal disease vector as most adult females must feed on vertebrate blood to acquire nutrients for egg production. However, blood feeding also exposes the mosquito to infection from protozoan parasites, viruses and nematode worms. A further consequence of blood feeding is the dramatic rise in levels of endogenous bacteria in the mosquito midgut [1, 2], which puts the mosquito at risk of systemic infection. Therefore, the mosquito immune system must defend against blood-borne infections and control its midgut bacterial populations [3-5].
The Anopheles gambiae innate immune system is responsible for eliminating the majority of invading Plasmodium ookinetes during the midgut stages of mosquito infection [6]. Two key immune proteins involved in anti-Plasmodium defense are Leucine-Rich repeat IMmune protein 1 (LRIM1) and APL1C, as shown by striking increases in live parasites when these genes are silenced [7-9]. LRIM1 and APL1C are closely related proteins that possess leucine-rich repeat (LRR) domains, which are found in host defense proteins of many phyla, such as vertebrate Toll-like receptors [10]. LRIM1 and APL1C circulate in the hemolymph as a disulfide-linked heterodimeric complex [11, 12]. This complex is involved in parasite killing through its interaction with the complement-like effector protein, TEP1. LRIM1/APL1C binds to proteolytically processed, mature TEP1 (known as TEP1cut), promoting its stabilization, preventing it from reacting with self-tissues and enabling it to opsonize parasites [11, 12]. Direct binding of TEP1cut to the ookinete surface triggers parasite lysis and melanization reactions, resulting in parasite killing and clearance [13]. TEP1, LRIM1 and APL1C are core members of the mosquito complement-like pathway, which plays a broad role in innate immunity including defense against bacteria [14]. These proteins are constitutively present in the hemolymph, bathing the basal labyrinth of the midgut and poised to attack malaria parasites as they emerge through invaded cells. Interestingly, the LRIM1/APL1C complex has also been demonstrated to interact with other TEP family members in vitro including TEP3, TEP4 and TEP9 [15].
Bioinformatic searches discovered a novel mosquito-specific family of proteins related to LRIM1 and APL1C [12, 16]. To date, 24 members of this LRIM family have been identified in An. gambiae. Orthologs of most LRIMs and additional homologous proteins were discovered in the genomes of mosquitoes Aedes aegypti and Culex quinquefasciatus. However, no LRIM-related genes were found in other organisms, including Drosophila. LRIM members share a distinct genomic organization and protein domain architecture, which distinguishes them from the larger superfamily of LRR genes. An archetypal LRIM comprises a signal peptide, LRR motifs, a conserved pattern of cysteines and a coiled-coil domain. The members are divided into four subfamilies based on variations to this core structure. Long LRIMs have 10 to 13 LRR motifs, whereas Short LRIMs have 6 or 7. Transmembrane LRIMs possess a C-terminal transmembrane domain and Coil-less LRIMs lack a coiled-coil domain. Interestingly, several LRIMs are encoded within tight genomic clusters with evidence of local gene shuffling and duplication [16]. A cluster of Short LRIMs found in all three mosquitoes consists of LRIM7, LRIM8, LRIM9 and LRIM10. LRIM8 has duplicated in An. gambiae to give LRIM8A and LRIM8B.
Apart from LRIM1 and APL1C, the other LRIM members are largely uncharacterized to date. Certain LRIMs have been implicated in innate immunity, including defense against Plasmodium and bacteria [17-21]. It is unclear whether the LRIM family represents an adaptation to the hematophagous lifestyle of mosquitoes. With their versatile LRR domains, we hypothesize that the LRIMs are pathogen recognition proteins, and the family has diversified to recognize different microbes that mosquitoes encounter. This paper aimed to broaden our understanding of the LRIM family in An. gambiae by investigating whether any uncharacterized LRIMs are involved in anti-Plasmodium defense. We discovered LRIM9 is a novel antagonist of Plasmodium berghei infections with a striking expression profile. LRIM9 is highly enriched in adult female mosquitoes. Expression of LRIM9 is dramatically induced by blood feeding and regulated by ecdysone signaling. Our data suggest that LRIM9 functions via a unique immune mechanism independent of the known mosquito complement-like pathway. We hypothesize that LRIM9 is involved in an anticipatory immune response triggered by blood feeding, which defends against blood-borne infections such as Plasmodium. This is an original concept in An. gambiae innate immunity.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. The protocols for mosquito blood feeding and for infection of mosquitoes with P. berghei by blood feeding on parasite-infected mice were approved and carried out under the UK Home Office License PLL70/7185 awarded in 2010. The procedures are of mild to moderate severity and the numbers of animals used are minimized by incorporation of the most economical protocols. Opportunities for reduction, refinement and replacement of animal experiments are constantly monitored and new protocols are implemented following approval by the Imperial College Ethical Review Committee.
Mosquito Maintenance, Gene Silencing and Infection
An. gambiae N’gousso and L3–5 strains were maintained, blood fed, and assayed for infection with P. berghei CONGFP strain [22] as described previously [15]. Human blood feeding was performed using an artificial membrane feeding system [23]. Single and double knockdown experiments and parasite counts in dissected midguts were performed as described previously [12]. Primers used for synthesis of double-stranded RNA against LRIM9 are as follows with T7 tags in lower case:
LRIM9 RNAi For: taatacgactcactatagggACTGGCAGAAAAGCTTCCAA;
LRIM9 RNAi Rev: taatacgactcactatagggTGGCATTTTCTCGAACACAG.
Other primers for gene silencing (GFP, LRIM1, TEP1 and CTL4) have been reported elsewhere [23].
RNA Extraction and qRT-PCR
Total RNA was extracted from 10 whole mosquitoes per sample using the TRIzol Reagent (Invitrogen). For the developmental profile, 10 eggs, larvae (2nd or 4th instar) or pupae were used per sample. Total RNA was DNase treated using Turbo DNA-free kit (Ambion) and cDNA was synthesized from 1 μg RNA using SuperScript II kit with oligo(dT)12-18 primers (Invitrogen). Quantitative real-time PCR was performed using the ABI Prism 7500 Fast Real-Time PCR System, as previously described [23]. Ribosomal gene S7 was used as the endogenous reference, and gene expression was quantitated relative to a calibrator control sample (e.g. dsGFP-treated mosquitoes). Primers for S7 and LRIM1 have been reported previously [23]. Primers for LRIM9 are as follows:
LRIM9 qRT-PCR F: TTCAGCATGCACTGGAAAAG;
LRIM9 qRT-PCR R: GTCGGTACCATCGGTTGACT.
Generation of LRIM9 Antibodies
An. gambiae LRIM 9 was cloned into the pIEx-10 (Novagen) expression plasmid in-frame with the plasmid signal peptide for secretion, an N-terminal Strep tag and a C-terminal 10× His tag as follows: first a DNA fragment containing LRIM9 was amplified by PCR from genomic DNA using the primers:
LRIM9 F: TGCAATTTTCGATTCAGTGC;
LRIM9 R: AAAGGACCCACATCTCAACG.
The 1,583-bp product was used as a PCR template using primers containing overhangs for ligase-independent cloning:
LRIM9 LIC F: gacgacgacaagatgGAGATTTCCAGCTCCGTGGTG;
LRIM9 LIC R: gaggagaagcccggtttGGCAGACGGTTCGGACGCCAC.
The resulting expression construct encodes a 445-amino acid fragment of LRIM9 removing its endogenous signal sequence and stop (LRIM9HIS).
A cell line stably expressing LRIM9HIS was selected using G418 by cotransfecting Sf9 cells with pIEx-10-LRIM9 and pIE1-neo (Novagen) plasmids as described previously [24]. LRIM9HIS was affinity purified from 2 l of 0.22 μ M sterile-filtered conditioned medium using a 5 ml HisTrap FF column on an ÄKTA purifier (GE Healthcare). After binding, the column was washed with buffer A (1× phosphate-buffered saline, PBS + 0.1% triton, 40 mm imidazole, pH 8.0) and the captured protein was eluted with buffer B (1× PBS + 500 mm imidazole, pH 8.0) and then concentrated using an Amicon Ultra centrifugal filter (Millipore). Purified LRIM9HIS was analyzed by SDS-PAGE followed by Coomassie staining and quantified by Bradford assay. Approximately 215 μg of LRIM9HIS was used to immunize guinea pigs for antibody production (Eurogentec). The pre-immune and immune sera were evaluated using Western blotting (see below).
Additionally, a rabbit anti-peptide antibody was generated against the C-terminal peptide NH2-CDYARRLEVASEPSAK-COOH (Eurogentec). A second peptide against the internal peptide NH2-DSDGTLLDKSTDGTDC-COOH was unsuccessful. These were used for some initial experiments but were replaced by the more sensitive whole-protein antibody, described above.
Hemolymph Collection, Western Blot and Binding Assays
Hemolymph was collected from groups of individual mosquitoes as previously described [12]. Final sample volume was adjusted to 1 mosquito/μl; 10 mosquitoes per lane were used for SDS-PAGE and Western blot analysis. An exception was the CLIPA8 cleavage experiment, where 1.5 mosquito/μl and 15 mosquitoes per lane were used. Western blotting with SRPN3, TEP1 and APL1C antibodies was performed as previously described [12]. Positive LRIM9 guinea pig serum was used at 1/500 dilution in PBS + 3% milk and 0.05% Tween-20 for 1 h at room temperature. The TEP1 binding assay was performed using transfected Sua4.0 cells, as detailed previously [12]. Cells were transfected with pIEx10-LRIM9HIS, pIEx10-GFPsecretedHIS and pIEx10-APL1CHIS.
Mosquito Fecundity Experiment
Upon eclosion, mosquitoes were given 3 days to mate in a 30-cm3 population cage prior to injection with double-stranded RNA. Three days after injection, females were allowed to feed on an anesthetized mouse. After 48 h, a group of mosquitoes was dissected to examine blood meal digestion and ovary development. After 72 h, the remaining mosquitoes were placed in 50-mm Petri dishes on filter paper saturated with 0.1% saline. Dishes were stored at 27°C in darkness for 24 h to encourage oviposition, after which mosquitoes were removed and eggs were counted by direct observation under a dissecting microscope. Egg dishes were then half-filled with 0.1% saline, dusted with powdered fish food and incubated at 27°C for 3 days to allow hatching. Larvae were counted by direct observation under a dissecting microscope.
Bacterial and Ecdysone Challenge Experiments
Ampicillin-resistant Escherichia coli OP-50 was grown in Luria-Bertani (LB) broth, harvested during logarithmic growth phase, washed with PBS and resuspended in PBS to give an optical density at 600 nm (OD 600) of 0.4 [25]. Female mosquitoes were injected with 69 nl of bacterial suspension. Bacterial viability was confirmed by counting colonies formed overnight at 37°C after plating cells onto LB agar. The bacterial proliferation assay was performed as previously described [25], except with 24 h between bacterial inoculation and sample collection. For survival assays, the number of dead mosquitoes was monitored daily for 10 days after bacterial injection. Challenge with bioparticles was performed as described previously [24]. CLIPA8 cleavage was assayed in hemolymph samples analyzed under reducing conditions, as described previously [26]. Female mosquitoes were injected with 69 nl of a 14.5 μg/μl suspension of 20-hydroxyecydsone (Sigma) in sterile PBS. This dose was shown to elicit a maximal increase in protein synthesis in the fat body in vitro [27].
Antibiotic Treatment
For 4 days prior to blood feeding, newly eclosed mosquitoes were given 10% sucrose supplemented with 10 U/ml penicillin, 10 μg/ml streptomycin and 15 μg/ml gentamicin [3]. Efficacy of antibiotic treatment was assayed by plating homogenates of cohorts of 5 mosquitoes on LB agar and counting the colonies formed after incubation at 27°C for 2 days.
VectorBase Gene Identifiers
VectorBase Gene Identifiers were as follows: LRIM9, AGAP007453; LRIM1, AGAP006348; APL1C, AGAP007033; TEP1, AGAP010815; TEP3, AGAP010816; TEP4, AGAP010812; TEP9, AGAP010830; CTL4, AGAP005335; CLIPA8, AGAP010731; vitellogenin, AGAP004203; lipophorin, AGAP001826; S7, AGAP010592; SRPN3, AGAP006910.
Results
LRIM9 Is a Novel Plasmodium Antagonist
To elucidate whether any uncharacterized An. gambiae LRIMs play a role in anti-Plasmodium defense, the family was screened for a parasite infection phenotype after gene knockdown by RNAi. Parasite numbers were monitored 7 days after susceptible mosquitoes were infected with GFP-expressing P. berghei. The screen identified LRIM9, a Short family member, as a novel antagonist of P. berghei infections. Silencing LRIM9 resulted in a significant 3-fold increase in live oocysts compared to dsGFP-treated controls (fig. 1a; online suppl. table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000365331). However, there was no change in the proportion of infected mosquitoes (prevalence). LRIM9 was efficiently silenced with 84% average reduction in the transcript (online suppl. fig. 1) lasting for at least 7 days (data not shown).
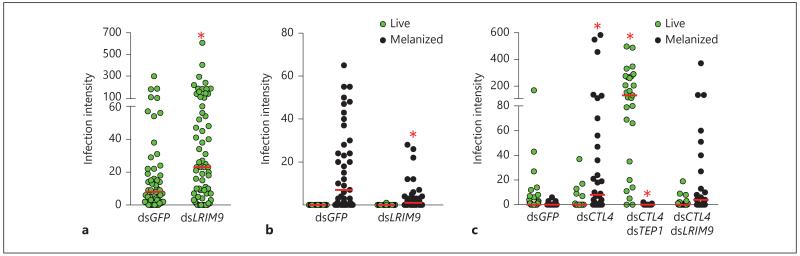
Fig. 1.
LRIM9 is a P. berghei antagonist with involvement in melanization. After gene silencing using RNAi, mosquitoes were infected with fluorescent P. berghei, and parasite load was monitored after 7 days. Live fluorescent oocysts and melanized ookinetes per mosquito midgut are shown. Horizontal lines indicate the median parasite number. a Infection intensity in dsGFP and dsLRIM9-treated susceptible mosquitoes. Data are pooled from 3 independent biological experiments using the N’gousso strain (see online suppl. table 1). Asterisk indicates significance using the Kruskal-Wallis test with Dunn’s post-test (p < 0.05). b Infection intensity in refractory L3–5 mosquitoes after dsGFP and dsLRIM9 injection. These data are representative of 2 independent experiments (see online suppl. table 2). Asterisk indicates significance using Mann-Whitney test (p < 0.05). c Infection intensity in dsGFP-, dsCTL4-, dsCTL4/TEP1- and dsCTL4/LRIM9-injected susceptible mosquitoes. Results shown are representative of 2 independent experiments (see online suppl. table 3). Asterisks indicate significance using the Kruskal-Wallis test with Dunn’s post-test (p < 0.001).
To further investigate the role of LRIM9 in parasite melanization, the gene was silenced in two An. gambiae experimental models that are refractory to P. berghei infection where virtually all of the invading ookinetes are melanized. In L3–5 mosquitoes, a laboratory-selected refractory strain [28], silencing LRIM9 resulted in a significant decrease in the number of melanized parasites but did not produce live oocysts (fig. 1b, online suppl. table 2). In contrast, silencing LRIM9 did not alter the outcome of infection in susceptible mosquitoes following silencing of CTL4 (fig. 1c, online suppl. table 3). In this refractory model, silencing CTL4 alone or in combination with LRIM9 led to the same significant decrease in live oocysts and increase in melanized ookinetes. In comparison, silencing CTL4 in combination with TEP1 completely blocked melanization induced by CTL4 silencing and resulted in a large increase in the number of live oocysts, consistent with previous observations [15]. Silencing efficiency of the LRIM9 transcript in L3–5 and CTL4 knockdown mosquitoes (89 and 76%, respectively) was comparable to the single knockdown in susceptible mosquitoes (online suppl. fig. 1).
LRIM9 Is Adult Female Enriched
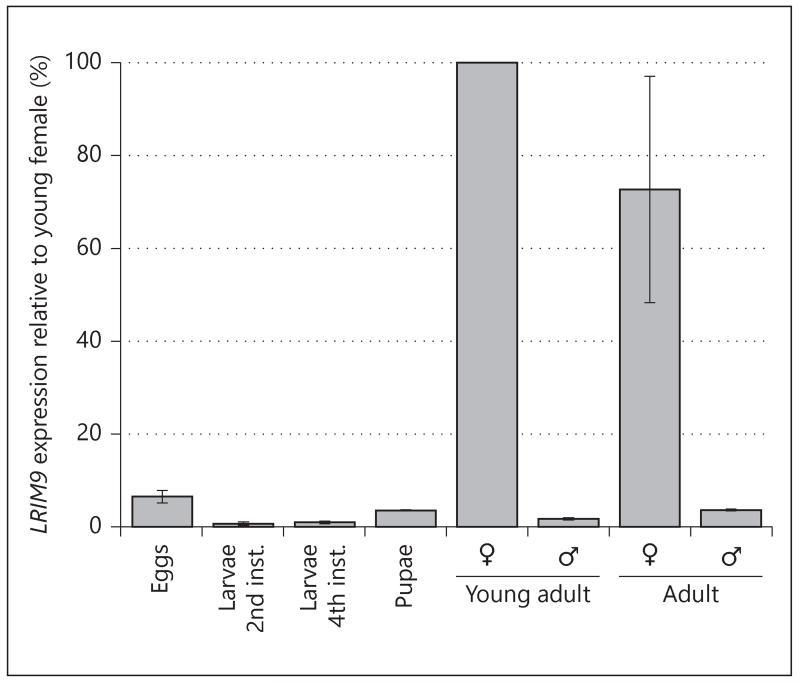
To initially characterize LRIM9, we examined its developmental expression profile by quantitative real-time PCR (qRT-PCR). RNA was collected from eggs, two larval stages (2nd and 4th instar), pupae, newly eclosed, and 4-day-old sugar-fed mosquitoes. The transcript was most abundant in adult female mosquitoes (fig. 2). Compared to adult males, LRIM9 transcript was greater than 20-fold enriched in adult females. The LRIM9 transcript level was also extremely low in eggs, larval and pupal stages. These data suggest that LRIM9 functions primarily in adult female mosquitoes.
Fig. 2.
LRIM9 is enriched in adult female mosquitoes. RNA was extracted from An. gambiae eggs, larvae (2nd and 4th instar), pupae, newly eclosed female and male mosquitoes (‘young adult’), and 4-day-old sugar-fed female and male mosquitoes (‘adult’). Using synthesized cDNA, qRT-PCR determined LRIM9 transcript levels at each developmental stage. LRIM9 expression was normalized to S7 (a constitutively expressed ribosomal gene) and calculated relative to the young adult female. The mean of 2 independent replicates is shown with standard error bars.
LRIM9 Transcript Is Upregulated after Blood Feeding
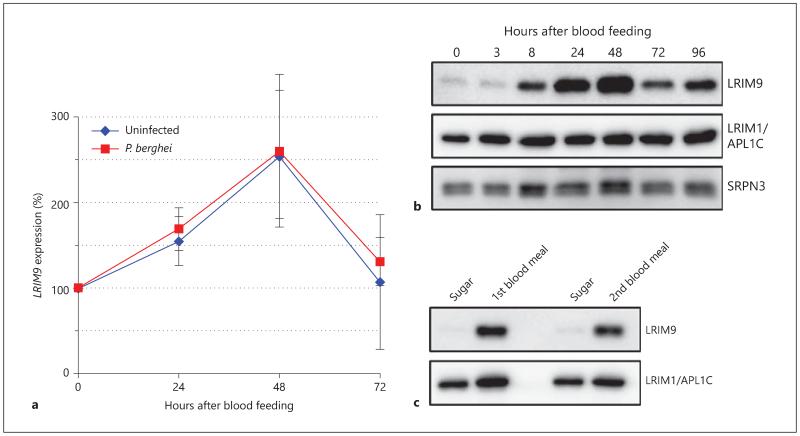
As LRIM9 is female enriched and a P. berghei antagonist, we investigated whether the LRIM9 transcript is induced in response to P. berghei infection. Indeed, qRT-PCR analysis showed that the LRIM9 transcript was robustly upregulated after feeding on infected blood. When maintained at 19°C, the permissive temperature of P. berghei, LRIM9 expression peaked at 48 h after feeding, where the transcript was 2.6-fold higher than sugar-fed controls (fig. 3a). Expression returned to baseline levels by 72 h. Interestingly, the same regulation was observed when mosquitoes fed on uninfected blood revealing that LRIM9 expression is not triggered by parasites, but instead is a consequence of blood feeding.
Fig. 3.
LRIM9 is upregulated after blood feeding at the transcript and protein level. a RNA was extracted from mosquitoes 24, 48 and 72 h after feeding on uninfected or P. berghei- infected murine blood. Sugar-fed mosquitoes were used for the baseline expression (0 h). LRIM9 expression was determined using qRT-PCR, normalizing to ribosomal S7 and calculated relative to sugar-fed mosquitoes. The mean of 4 independent experiments is shown with standard error bars. b Mosquitoes were allowed to feed on uninfected murine blood, and hemolymph was collected after 3, 8, 24, 48, 72 and 96 h. Hemolymph was analyzed by Western blot under nonreducing conditions and probed with antibodies against LRIM9, APL1C (to analyze the LRIM1/APL1C complex) and SRPN3 (as a loading control). c Mosquitoes were given two consecutive murine blood meals 96 h apart (1st and 2nd blood meal) and hemolymph was collected 24 h after each blood meal. Hemolymph was collected from sugar-fed mosquitoes of the same age (but not given either blood meal), for comparison. Samples were analyzed by nonreducing Western blot using antibodies against LRIM9 and APL1C.
LRIM9 Protein Is Present in the Hemolymph and Is Enriched after Blood Feeding
To gain insights into its function in vivo, an antibody was raised against LRIM9 and used to assay hemolymph (online suppl. fig. 2). The specific band identified at 50 kDa is consistent with the predicted size of LRIM9, suggesting that LRIM9, unlike LRIM1, APL1C and LRIM4, does not form covalent dimers in the hemolymph [12, 15].
The antibody was next used to determine the temporal dynamics of the LRIM9 protein following a blood meal. Mosquitoes were given a murine blood meal and maintained at 19°C. Hemolymph was collected 3, 8, 12, 24, and 48 h after feeding and analyzed by Western blot (fig. 3b). Compared to the LRIM1/APL1C complex and SRPN3, which both remained relatively stable across all time points, LRIM9 was massively enriched in hemolymph after blood feeding. The earliest induction was observed at 8 h with a striking peak between 24 and 48 h followed by a rapid decrease by 72 h. However, even at 96 h, the LRIM9 protein was more abundant than it was prior to blood feeding.
Given that LRIM9 protein is so strongly induced by blood feeding, RNAi knockdown was measured in blood-fed mosquitoes (online suppl. fig. 3). Indeed, following knockdown, LRIM9 protein levels were significantly reduced even after blood feeding with only a very faint band detectable. We also found that LRIM9 was upregulated when mosquitoes fed on human blood (online suppl. fig. 4). Protein abundance peaked at 24 h after human blood feeding, which is likely because mosquitoes were kept at 27°C rather than 19°C as in the previous experiment using murine blood.
We next asked whether LRIM9 upregulation occurs after subsequent blood meals or is specific to the first. To test this, mosquitoes were allowed to take two consecutive murine blood meals (separated by 96 h), and hemolymph was collected from cohorts 24 h after their first and second blood meal. Hemolymph from sugar-fed controls, which were the same age as the blood-fed mosquitoes but were not blood fed, was analyzed for comparison. Western blot analysis using the LRIM9 antibody demonstrated that the LRIM9 protein (and transcript, not shown) is highly induced after both the first and second blood meal, with no obvious dampening of the second response (fig. 3c).
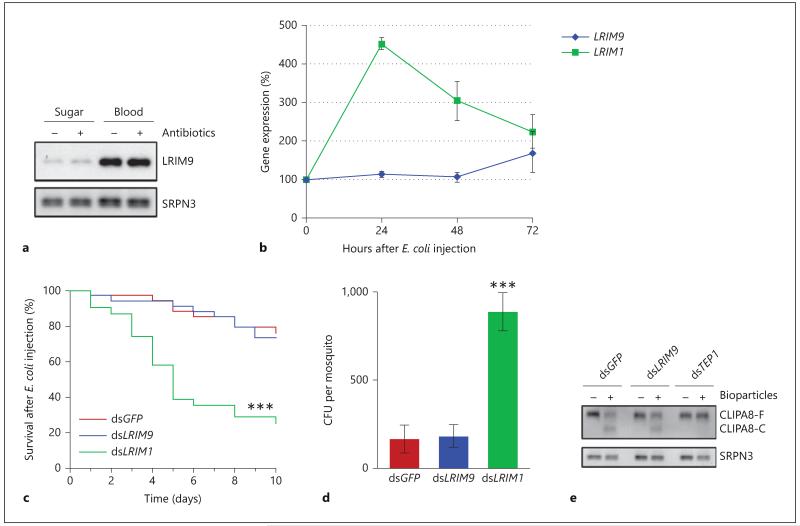
LRIM9 Is Not Regulated by Bacteria
We then investigated whether LRIM9 induction is triggered by the increase in midgut bacteria that occurs after blood feeding [1, 2]. To test this hypothesis, mosquitoes were fed a spectrum of antibiotics to significantly reduce their midgut flora prior to blood feeding [3]. LRIM9 protein levels were unaffected by antibiotic treatment in both sugar-fed and blood-fed mosquitoes (fig. 4a). These findings suggest that LRIM9 upregulation after blood feeding is independent of the endogenous midgut bacteria. To further test whether LRIM9 is bacterial responsive, we performed qRT-PCR after injection of bacteria directly into the hemocoel of sugar-fed mosquitoes. LRIM9 transcript was unaffected 24, 48 and 72 h after Escherichia coli (gram-negative bacteria) injection, whereas LRIM1 was highly induced at 24 and 48 h (fig. 4b). Furthermore, LRIM9 silencing had no effect on the number of colony-forming units or the survival of blood-fed mosquitoes after E. coli injection (fig. 4c, d). In contrast, silencing LRIM1 significantly increased mosquito mortality and bacterial proliferation. Finally, silencing LRIM9 did not affect the bacterial-induced cleavage activation of CLIPA8 (fig. 4e), which is deemed a functional marker of antibacterial defense [26, 29]. Together, these data show that LRIM9 is not regulated by midgut bacteria or infection with exogenous bacteria.
Fig. 4.
LRIM9 is not regulated by bacteria or involved in antibacterial defense. a Newly emerged mosquitoes were fed either sterile sugar solution or a cocktail of antibiotics dissolved in sugar. After 4 days of treatment, some mosquitoes were allowed to feed on uninfected murine blood whilst others were kept on sugar. After 24 h, hemolymph was collected and analyzed by nonreducing Western blot, probing with LRIM9 and SRPN3 antibodies. b RNA was extracted from mosquitoes 24, 48 and 72 h after E. coli injection. LRIM9 and LRIM1 expression was determined by qRT-PCR, normalizing to ribosomal S7. Expression was normalized to injection of sterile PBS at each time point and calculated relative to uninjected (0 h) mosquitoes. The mean of 2 independent experiments is shown with standard error bars. c Mosquitoes were injected with dsGFP, dsLRIM9 and dsLRIM1, blood fed after 3 days and innoculated with live E. coli 24 h later. Mosquito survival was monitored daily for 10 days. Survival was compared to dsGFP using the Kaplan-Meier log-rank test (*** p < 0.0001). d Mosquitoes were injected with dsGFP, dsLRIM9 and dsLRIM1 and after 4 days injected with live ampicillin-resistant E. coli. After 24 h, batches of 10 mosquitoes were surface sterilized, washed and homogenized. The homogenate was plated onto ampicillin LB agar and colony-forming units (CFU) were counted after overnight incubation at 37°C. Mean CFU per mosquito from 3 independent experiments is shown with standard error bars. dsLRIM9 and dsLRIM1 were compared with dsGFP using meta-analysis (*** p < 0.0001). e Four days after injection of dsGFP, dsLRIM9 and dsTEP1, hemolymph was collected from half of these mosquitoes. The other half were injected with Staphylococcus aureus bioparticles, and hemolymph was collected 2 h after challenge. Hemolymph was analyzed by reducing Western blot and probed with antibodies against CLIPA8 and SRPN3 (loading control).
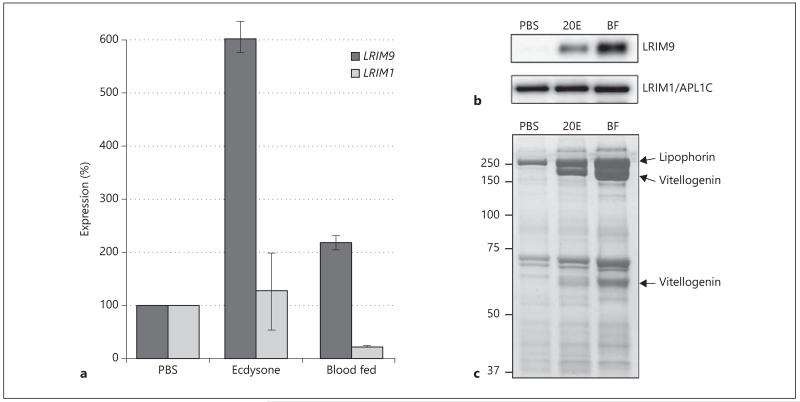
LRIM9 Is Regulated by Ecdysone
As we had excluded bacterial regulation, we investigated whether the hormonal changes that occur after blood feeding influence LRIM9 regulation. In particular, the steroid hormone ecdysone drives the transcription of many key genes for vitellogenesis. Ecdysone is primarily secreted by the ovaries 10–36 h after blood feeding (peaking at 24 to 36 h), and is hydroxylated into 20-hydroxyecdysone (20E) [30]. As LRIM9 is strongly induced by blood feeding, we directly tested whether it is 20E responsive. Using qRT-PCR, the LRIM9 transcript was highly induced 24 h after intrathoracic injection of 20E (fig. 5a). The LRIM1 transcript was relatively unresponsive to both 20E and blood feeding. Furthermore, the LRIM9 protein was strongly enriched in the hemolymph in response to 20E injection (fig. 5b). In contrast to the effect on transcript levels, protein induction was stronger after blood feeding than after 20E injection. This suggests that in addition to regulating the LRIM9 transcript, blood feeding enhances its translation. A similar effect has been shown previously for blood feeding activation of translation of AaGATAa transcripts in the fat body of Ae. aegypti [31]. Again, the LRIM1/APL1C complex was unresponsive to this treatment. Coomassie staining of samples confirmed that vitellogenin and lipophorin, two nutrient transport proteins known to be regulated by 20E, were induced after 20E injection (fig. 5c).
Fig. 5.
LRIM9 is induced after direct injection of 20-hydroxyecdysone. RNA and hemolymph were collected from mosquitoes 24 h after intrathoracic injection of PBS or 20-hydroxyecdsone or a murine blood meal. a LRIM9 and LRIM1 expression in RNA samples was measured by qRT-PCR, normalized to ribosomal S7 and calculated relative to PBS-injected mosquitoes. The mean of 2 independent experiments is shown with standard error bars. b Hemolymph was analyzed by nonreducing Western blot and probed with antibodies against LRIM9 and APL1C (for LRIM1/APL1C). c Hemolymph samples were analyzed by SDS-PAGE and stained with Coomassie.
As An. gambiae female mosquitoes require a blood meal to enable egg development, and the 20E targets vitellogenin and lipophorin play an important role in this process, we hypothesized that, in addition to its role in anti-Plasmodium immunity, LRIM9 may also be involved in mosquito reproduction. To examine this possibility, mated dsGFP- and dsLRIM9-treated female mosquitoes were blood fed and encouraged to lay eggs. Initial microscopic analysis of both treatment groups 72 h after blood feeding revealed no differences in ovarian development or blood meal digestion (data not shown). Furthermore, we found that total eggs laid, mean eggs laid per female, larval hatching and percentage of fertile females were equivalent between dsGFP and dsLRIM9 (table 1). Therefore, LRIM9 silencing had no impact on mosquito reproduction or blood meal digestion.
Table 1.
LRIM9 silencing has no effect on egg laying and larval hatching
| Gene knockdown | Females at start | Females laid eggs | Total eggs laid | Mean eggs per female | Females laid eggs | Hatchability | Fertile females |
|---|---|---|---|---|---|---|---|
| GFP | 46 | 27 | 1,809 | 67 | 59% | 62% | 50% |
| LRIM9 | 46 | 29 | 1,958 | 68 | 63% | 64% | 52% |
Mosquitoes were allowed to mate, treated with dsGFP and dsLRIM9 and then blood fed. After 72 h, individual females were placed in dishes with wet filter paper and encouraged to lay eggs in darkness. The number of eggs laid and larvae hatched were counted. Hatchability is the percentage of eggs that hatched into larvae. Fertile females were defined as those that lay at least one egg and produce at least one larva.
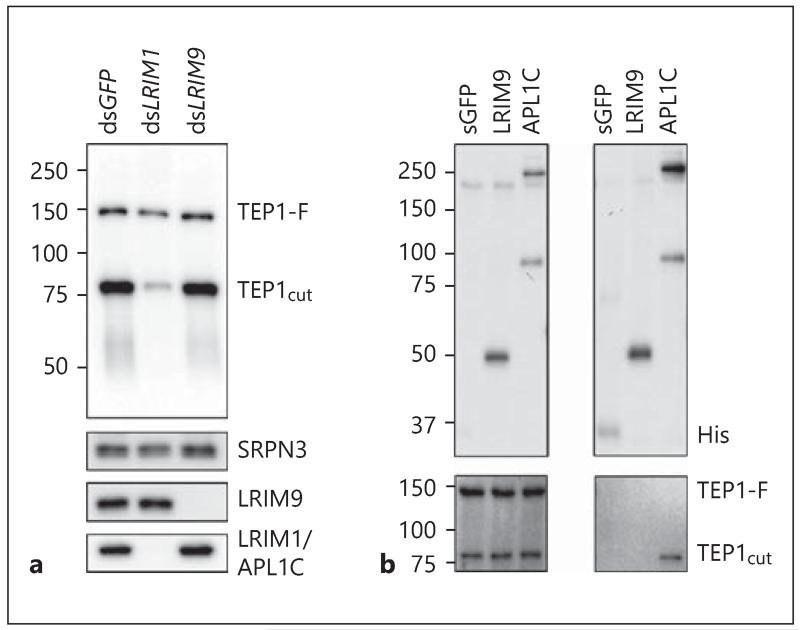
LRIM9 Does Not Interact with TEP1
The mosquito complement-like pathway has a well-established role in parasite killing and melanization. To characterize whether LRIM9 functions in this pathway, we first investigated whether silencing it affects the stability or abundance of LRIM1, APL1C and TEP1. Hemolymph collected from blood-fed mosquitoes following LRIM9 silencing was indistinguishable from the control; there was no effect on the abundance of TEP1-F, TEP1cut, the LRIM1/APL1C complex or the loading control, SRPN3 (fig. 6a). There was also no effect of LRIM1 silencing on LRIM9 abundance or mobility. In contrast, as previously shown, silencing LRIM1 abolishes the LRIM1/APL1C complex and results in loss of TEP1cut from the hemolymph [11, 12]. These results suggest that LRIM9 does not interact with TEP1.
Fig. 6.
LRIM9 does not interact with the known complement-like system. a Hemolymph was collected from dsGFP-, dsLRIM1- and dsLRIM9-injected mosquitoes 24 h after an uninfected murine blood meal. Samples were analyzed by nonreducing Western blot using antibodies against TEP1, SRPN3, LRIM9 and APL1C (for LRIM1/APL1C). b Conditioned medium was collected 3.5 days after transfection of Sua4.0 cells with secreted His-tagged GFP (sGFP), LRIM9 and APL1C. Tagged proteins and interacting partners were captured from the conditioned medium using metal affinity beads. Starting conditioned medium (left panels) and bound material (right panels) were analyzed by nonreducing Western blot. Two blots were probed with a His probe (top panels) and a TEP1 antibody (bottom panels), respectively.
To test this directly, we expressed a recombinant His-tagged LRIM9 in Sua4.0 cells, a mosquito hemocyte-like cell line that naturally secretes endogenous TEP1-F and TEP1cut [12]. LRIM9HIS was affinity purified from the conditioned medium, and the bound material was assayed for the presence of TEP1 (fig. 6b). Interestingly, there was no detectable signal for either TEP1-F or TEP1cut in the bound material, indicating that LRIM9 does not interact with TEP1. Furthermore, because Sua4.0 cells endogenously produce the LRIM1/APL1C/TEP1cut complex, the lack of TEP1 signal in the captured material also reveals that LRIM9 does not interact with the LRIM1/APL1C complex. As a positive control, TEP1cut was robustly present in samples purified from the conditioned medium of APL1CHIS transfected cells. These results indicate that LRIM9 does not directly interact with TEP1. Therefore, LRIM9 acts via an unknown immunity mechanism independent of the known complement system.
Discussion
Here, we have characterized LRIM9, a novel member of the LRIM family in An. gambiae. LRIM9 was found to be a novel antagonist of P. berghei infection. However, the precise function of LRIM9 in defense against Plasmodium remains unclear and requires further study. The characteristic LRR and coiled-coil domains of LRIM9 suggest involvement in pathogen recognition and interactions with other immune proteins [16, 32, 33]. Our data suggest that LRIM9 is not directly involved in the known mosquito complement-like pathway. However, LRIM9 might interact with other TEP family members or could function downstream of TEP1. We currently lack a suitable assay to test the latter. The observation that LRIM9 silencing does not recover live oocysts in refractory L3–5 mosquitoes suggests LRIM9 might function downstream of parasite lysis/killing mechanisms to promote melanization. In Drosophila, melanization has been shown to increase the efficiency of other immune reactions [34] and the same has been suggested in An. gambiae [26]. Melanization has been proposed as a clearance mechanism for dead parasites in refractory L3–5 mosquitoes, whereas in CTL4 knockdown mosquitoes parasites are thought to be directly killed by melanization [29]. As silencing LRIM9 has a phenotype in L3–5 mosquitoes but not in the CTL4 knockdown, we believe that LRIM9 is not a component of the melanization cascade per se but is promoting recognition of dead parasites, which then leads to their melanization. It remains to be determined whether LRIM9 is itself a scavenger receptor of dead parasites.
LRIM9 has a unique expression profile: it is enriched in adult female mosquitoes and strongly induced by blood feeding. Our results correlate well with previous whole-genome transcriptomics analyses [35-37]. Mosquitoes responded similarly to murine and human blood, which indicated that possible causes of upregulation of LRIM9 may include common mammalian blood components, formation of the blood bolus, distension of the gut or signaling occurring after blood feeding.
We have shown that LRIM9 is regulated by 20E, a steroid hormone secreted by the ovaries in response to blood feeding. In agreement with our findings, LRIM9 expression is 3-fold higher 24 h after blood feeding in mosquitoes with ovaries compared to genetically manipulated ovaryless mosquitoes [Magnusson and Crisanti, pers. commun.]. The most parsimonious hypothesis is that LRIM9 is transcriptionally regulated by 20E, and is activated directly by the ecdysone receptor or indirectly via an ecdysone-regulated transcription factor. It would be insightful to determine whether LRIM9 expression is dramatically reduced after 20E injection when the ecdysone receptor is silenced. Alternatively, transcript stability, translational repression or protein turnover could be regulated by 20E [38].
We have determined that neither midgut bacterial flora nor exogenous bacteria influence LRIM9 regulation. However, the nutrient-sensitive target of rapamycin (TOR) pathway, insulin/insulin-like growth factor signaling or microRNAs [39-41] could feasibly contribute to LRIM9 expression. In mosquitoes, steroid hormones and TOR signaling work synergistically to control expression of yolk protein precursors [42].
Regulation of immune genes by 20E has been observed previously in insects. Melanization pathway components, such as PPO, are under 20E regulation in An. gambiae [43, 44] and other blood-feeding insects [45]. Like LRIM9, PPO2, PPO3 and PPO9 are strongly induced after blood feeding [6, 44]. As we demonstrated that LRIM9 can promote melanization, future studies should investigate a possible interaction between LRIM9 and PPO activation. Using the genome-wide expression map available for An. gambiae [46], we found that LRIM9 and vitellogenin are coregulated. Like LRIM9, vitellogenin is regulated by 20E and by multiple blood meals [47-49]. As vitellogenin is produced by the mosquito fat body and ecdysone is hydroxylated into 20E by this tissue [30], we hypothesize that LRIM9 is also produced by the fat body. Indeed, previous microarray analyses demonstrate that LRIM9 expression is significantly higher in the fat body compared to the midgut or ovaries [36, 37]. Furthermore, LRIM9 was not enriched in the transcriptome of circulating An. gambiae hemocytes [50]. However, this warrants further investigation as blood feeding was recently demonstrated to induce hemocyte proliferation and activation [51]. Interestingly, vitellogenin in the hemolymph interferes with TEP1-mediated killing of Plasmodium parasites by reducing the efficiency of TEP1 binding to parasite surfaces [52]. However, we did not observe a link between LRIM9 and mosquito fecundity or TEP1 levels in the hemolymph. Therefore, LRIM9 is likely to function via a novel immunity mechanism.
We hypothesize that LRIM9 is induced in anticipation of blood-borne infections rather than in response to infection, which is an original concept in An. gambiae immunity. In support of this hypothesis, LRIM9 is induced after both uninfected and infected blood meals. Hematophagous insects, like mosquitoes, are at high risk of infection from blood-borne pathogens, including Plasmodium parasites, filarial nematodes and viruses. Our proposed theory of anticipatory immunity in mosquitoes would be a highly important defense mechanism against such infections. By assuming every blood meal is infectious and inducing immune effectors, like LRIM9, in anticipation of such infections, the mosquito does not need to specifically recognise each pathogen but is prepared for imminent danger. It is unknown whether LRIM9 plays a generalized role or is specific to particular blood-borne pathogens, such as Plasmodium. Importantly, it should be determined whether anticipatory immunity and LRIM9 are involved in defense against the human malaria parasite, P. falciparum.
In contrast to LRIM9, LRIM1 and APL1C constitutively circulate at high levels in the hemolymph poised to attack invaders, which has been described as basal immunity [53]. Unlike LRIM9, they are not specific to blood-borne infections because they are also involved in antibacterial defense and phagocytosis [54]. Basal immunity is able to rapidly defend against pathogens that directly enter the hemolymph, such as bacteria and fungi, whereas anticipatory immunity is a slower response best suited to protect against blood-borne pathogens, which take longer to invade the hemolymph. Anticipatory immunity would potentially be less energetically costly than basal immunity, whilst still providing sufficient protection. Anticipatory immunity has not, to our knowledge, been reported in the innate immune response of another organism. Innate immunity is traditionally considered to be poorly specific and nonanticipatory. However, the adaptive immune response of vertebrates has been previously proposed as ‘anticipatory’. In a mechanism distinct from that in mosquitoes, vertebrates generate diverse repertoires of T and B lymphocyte receptors by rearrangement of gene segments to enable recognition of any potential antigen [55].
By coupling the regulation of LRIM9 with hormonal changes that occur after blood feeding, the mosquito enhances its immune system at the most opportune time. The fascinating link between innate immunity and steroid hormones has been widely observed in vertebrates and invertebrates [56-58]. Steroid hormones, like juvenile hormone and 20E, are extremely versatile and able to regulate development, growth, reproduction, ageing and immunity in insects [59-62]. It was recently reported that 20E transferred by male An. gambiae mosquitoes during mating modulates oogenesis in females [63]. Although hormonal regulation of insect development is well understood [64-66], the mechanisms regulating immunity are still being uncovered. In Drosophila, developmental production of 20E regulates the innate immune response via a complex network of transcriptional circuits [67].
20E has been shown to regulate the Drosophila Imd pathway and modulate production of antimicrobial peptides [68]. Furthermore, 20E controls the expression of the pattern recognition receptor, PGRP-LC, which protects the fly against bacterial infection [67]. Therefore, reduced 20E signaling can severely immunocompromise the adult fly. A recent study demonstrated that ecdysone is essential for hemocyte activation in Drosophila larvae and normal immune function [69, 70]. Without this activation, larvae were defective in bacterial phagocytosis and unable to survive bacterial infection [69]. Ecdysone has also been implicated in the control of hemocyte phagocytosis in Rhodius prolixus, the Hemipteran vector of Chagas disease [71]. Importantly, hormone signaling has been implicated in the synchronization of different immune responses [69], which suggests LRIM9 could be involved in more than one immune function. Future research will aim to shed light on the roles of LRIM9 and hormonal regulation in the mosquito’s anticipatory immune response.
The LRIM9 promoter could be favorable for transgenic strategies where adult female-specific expression is required, such as Release of Insects carrying a Dominant Lethal (RIDL) [72]. If the expression profile of LRIM9 in An. gambiae is conserved in other mosquito species, such a strategy could be used to target numerous mosquito-borne diseases, such as arboviruses and filarial worms, as well as malaria. Indeed, using the available data in VectorBase, the LRIM9 ortholog in Ae. aegypti (AAEL001414) is also highly expressed in females and induced 12 h after blood feeding [73]. Furthermore, in all three sequenced mosquito genomes, LRIM9 resides in a genomic cluster with other Short LRIMs (LRIM7, LRIM8A, LRIM8B and LRIM10 in An. gambiae) [12, 16]. Based on extant microarray data, this cluster of Short LRIMs seems to share a similar expression profile (with the exception of LRIM7) in An. gambiae and Ae. aegypti [35, 37, 73]. Future studies should further investigate these Short LRIMs and their relationship with LRIM9 in these and other disease vector mosquitoes where they are present, such as Cx. quinquefasciatus [16].
Supplementary Material
Acknowledgements
This work was funded by the Wellcome Trust (093587/Z/10/Z). L.M.U. was supported by a Biotechnology and Biological Sciences Research Council Doctoral Training Grant. We thank Dr. Lavanya Bhagavatula for generating the stable Sf9 cell line expressing LRIM9HIS. We also wish to thank Katarzyna Sala and Tibebu Habtewold for mosquito production and parasite maintenance.
References
- 1.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meister S, Agianian B, Turlure F, Relogio A, Morlais I, Kafatos FC, Christophides GK. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog. 2009;5:e1000542. doi: 10.1371/journal.ppat.1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yassine H, Kamareddine L, Osta MA. The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog. 2012;8:e1003029. doi: 10.1371/journal.ppat.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- 7.Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- 8.Riehle MM, Markianos K, Niare O, Xu J, Li J, Toure AM, Podiougou B, Oduol F, Diawara S, Diallo M, Coulibaly B, Ouatara A, Kruglyak L, Traore SF, Vernick KD. Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science. 2006;312:577–579. doi: 10.1126/science.1124153. [DOI] [PubMed] [Google Scholar]
- 9.Riehle MM, Xu J, Lazzaro BP, Rottschaefer SM, Coulibaly B, Sacko M, Niare O, Morlais I, Traore SF, Vernick KD. Anopheles gambiae APL1 is a family of variable LRR proteins required for Rel1-mediated protection from the malaria parasite, Plasmodium berghei. PLoS One. 2008;3:e3672. doi: 10.1371/journal.pone.0003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leulier F, Lemaitre B. Toll-like receptors – taking an evolutionary approach. Nat Rev Genet. 2008;9:165–178. doi: 10.1038/nrg2303. [DOI] [PubMed] [Google Scholar]
- 11.Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, Hoffmann JA, Blandin SA, Levashina EA. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 14.Blandin SA, Marois E, Levashina EA. Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway. Cell Host Microbe. 2008;3:364–374. doi: 10.1016/j.chom.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Povelones M, Upton LM, Sala KA, Christophides GK. Structure-function analysis of the Anopheles gambiae LRIM1/APL1C complex and its interaction with complement C3-like protein TEP1. PLoS Pathog. 2011;7:e1002023. doi: 10.1371/journal.ppat.1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterhouse RM, Povelones M, Christophides GK. Sequence-structure-function relations of the mosquito leucine-rich repeat immune proteins. BMC Genomics. 2010;11:531. doi: 10.1186/1471-2164-11-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilar R, Jedlicka AE, Mintz M, Mahairaki V, Scott AL, Dimopoulos G. Global gene expression analysis of Anopheles gambiae responses to microbial challenge. Insect Biochem Mol Biol. 2005;35:709–719. doi: 10.1016/j.ibmb.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong Y, Dimopoulos G. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS Pathog. 2012;8:e1002737. doi: 10.1371/journal.ppat.1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitri C, Jacques JC, Thiery I, Riehle MM, Xu J, Bischoff E, Morlais I, Nsango SE, Vernick KD, Bourgouin C. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 2009;5:e1000576. doi: 10.1371/journal.ppat.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlachou D, Schlegelmilch T, Christophides GK, Kafatos FC. Functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion. Curr Biol. 2005;15:1185–1195. doi: 10.1016/j.cub.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Habtewold T, Povelones M, Blagborough AM, Christophides GK. Transmission blocking immunity in the malaria non-vector mosquito Anopheles quadriannulatus species A. PLoS Pathog. 2008;4:e1000070. doi: 10.1371/journal.ppat.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Povelones M, Bhagavatula L, Yassine H, Tan LA, Upton LM, Osta MA, Christophides GK. The CLIP-domain serine protease homolog SPCLIP1 regulates complement recruitment to microbial surfaces in the malaria mosquito Anopheles gambiae. PLoS Pathog. 2013;9:e1003623. doi: 10.1371/journal.ppat.1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnitger AK, Yassine H, Kafatos FC, Osta MA. Two C-type lectins cooperate to defend Anopheles gambiae against Gram-negative bacteria. J Biol Chem. 2009;284:17616–17624. doi: 10.1074/jbc.M808298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnitger AK, Kafatos FC, Osta MA. The melanization reaction is not required for survival of Anopheles gambiae mosquitoes after bacterial infections. J Biol Chem. 2007;282:21884–21888. doi: 10.1074/jbc.M701635200. [DOI] [PubMed] [Google Scholar]
- 27.Hagedorn HH, O’Connor JD, Fuchs MS, Sage B, Schlaeger DA, Bohm MK. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc Natl Acad Sci USA. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, Collins WE, Campbell CC, Gwadz RW. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 29.Volz J, Muller HM, Zdanowicz A, Kafatos FC, Osta MA. A genetic module regulates the melanization response of Anopheles to Plasmodium. Cell Microbiol. 2006;8:1392–1405. doi: 10.1111/j.1462-5822.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 30.Clements AN. The Biology of Mosquitoes. Chapman & Hall; London: 1992. p. 509. [Google Scholar]
- 31.Park JH, Attardo GM, Hansen IA, Raikhel AS. GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. J Biol Chem. 2006;281:11167–11176. doi: 10.1074/jbc.M601517200. [DOI] [PubMed] [Google Scholar]
- 32.Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528–533. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 33.Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell Mol Life Sci. 2008;65:2307–2333. doi: 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang H, Kambris Z, Lemaitre B, Hashimoto C. Two proteases defining a melanization cascade in the immune system of Drosophila. J Biol Chem. 2006;281:28097–28104. doi: 10.1074/jbc.M601642200. [DOI] [PubMed] [Google Scholar]
- 35.Baker DA, Nolan T, Fischer B, Pinder A, Crisanti A, Russell S. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics. 2011;12:296. doi: 10.1186/1471-2164-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koutsos AC, Blass C, Meister S, Schmidt S, MacCallum RM, Soares MB, Collins FH, Benes V, Zdobnov E, Kafatos FC, Christophides GK. Life cycle transcriptome of the malaria mosquito Anopheles gambiae and comparison with the fruitfly Drosophila melanogaster. Proc Natl Acad Sci USA. 2007;104:11304–11309. doi: 10.1073/pnas.0703988104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinotti O, Nguyen QK, Calvo E, James AA, Ribeiro JM. Microarray analysis of genes showing variable expression following a blood meal in Anopheles gambiae. Insect Mol Biol. 2005;14:365–373. doi: 10.1111/j.1365-2583.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 38.Sempere LF, Sokol NS, Dubrovsky EB, Berger EM, Ambros V. Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-complex gene activity. Dev Biol. 2003;259:9–18. doi: 10.1016/s0012-1606(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 39.Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS One. 2011;6:e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas K, Raikhel AS. Insect microRNAs: biogenesis, expression profiling and biological functions. Insect Biochem Mol Biol. 2013;43:24–38. doi: 10.1016/j.ibmb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S. Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen Comp Endocrinol. 2011;173:303–312. doi: 10.1016/j.ygcen.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2007;37:1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed A, Martin D, Manetti AG, Han SJ, Lee WJ, Mathiopoulos KD, Muller HM, Kafatos FC, Raikhel A, Brey PT. Genomic structure and ecdysone regulation of the prophenoloxidase 1 gene in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 1999;96:14795–14800. doi: 10.1073/pnas.96.26.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller HM, Dimopoulos G, Blass C, Kafatos FC. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem. 1999;274:11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- 45.Genta FA, Souza RS, Garcia ES, Azambuja P. Phenol oxidases from Rhodnius prolixus: temporal and tissue expression pattern and regulation by ecdysone. J Insect Physiol. 2010;56:1253–1259. doi: 10.1016/j.jinsphys.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 46.Maccallum RM, Redmond SN, Christophides GK. An expression map for Anopheles gambiae. BMC Genomics. 2011;12:620. doi: 10.1186/1471-2164-12-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen XG, Marinotti O, Whitman L, Jasinskiene N, James AA, Romans P. The Anopheles gambiae vitellogenin gene (VGT2) promoter directs persistent accumulation of a reporter gene product in transgenic Anopheles stephensi following multiple bloodmeals. Am J Trop Med Hyg. 2007;76:1118–1124. [PubMed] [Google Scholar]
- 48.Martin D, Wang SF, Raikhel AS. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol Cell Endocrinol. 2001;173:75–86. doi: 10.1016/s0303-7207(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 49.Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang SF, Li C, Sun G, Ahmed A, Dittmer N, Attardo G. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem Mol Biol. 2002;32:1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 50.Pinto SB, Lombardo F, Koutsos AC, Waterhouse RM, McKay K, An C, Ramakrishnan C, Kafatos FC, Michel K. Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc Natl Acad Sci USA. 2009;106:21270–21275. doi: 10.1073/pnas.0909463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bryant WB, Michel K. Blood feeding induces hemocyte proliferation and activation in the African malaria mosquito, Anopheles gambiae Giles. J Exp Biol. 2014;217:1238–1245. doi: 10.1242/jeb.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rono MK, Whitten MM, Oulad-Abdelghani M, Levashina EA, Marois E. The major yolk protein vitellogenin interferes with the anti-plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 2010;8:e1000434. doi: 10.1371/journal.pbio.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NF-kappaB- dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–685. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 54.Moita LF, Wang-Sattler R, Michel K, Zimmermann T, Blandin S, Levashina EA, Kafatos FC. In vivo identification of novel regulators and conserved pathways of phagocytosis in A. gambiae. Immunity. 2005;23:65–73. doi: 10.1016/j.immuni.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 56.Meister M, Richards G. Ecdysone and insect immunity: the maturation of the inducibility of the diptericin gene in Drosophila larvae. Insect Biochem Mol Biol. 1996;26:155–160. doi: 10.1016/0965-1748(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 57.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 59.Kozlova T, Thummel CS. Steroid regulation of postembryonic development and reproduction in Drosophila. Trends Endocrinol Metab. 2000;11:276–280. doi: 10.1016/s1043-2760(00)00282-4. [DOI] [PubMed] [Google Scholar]
- 60.Riddiford LM. Hormone receptors and the regulation of insect metamorphosis. Receptor. 1993;3:203–209. [PubMed] [Google Scholar]
- 61.Schwedes CC, Carney GE. Ecdysone signaling in adult Drosophila melanogaster. J Insect Physiol. 2012;58:293–302. doi: 10.1016/j.jinsphys.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Tian L, Guo E, Diao Y, Zhou S, Peng Q, Cao Y, Ling E, Li S. Genome-wide regulation of innate immunity by juvenile hormone and 20-hydroxyecdysone in the Bombyx fat body. BMC Genomics. 2010;11:549. doi: 10.1186/1471-2164-11-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baldini F, Gabrieli P, South A, Valim C, Mancini F, Catteruccia F. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 2013;11:e1001695. doi: 10.1371/journal.pbio.1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karim FD, Guild GM, Thummel CS. The Drosophila Broad-Complex plays a key role in controlling ecdysone-regulated gene expression at the onset of metamorphosis. Development. 1993;118:977–988. doi: 10.1242/dev.118.3.977. [DOI] [PubMed] [Google Scholar]
- 65.King-Jones K, Thummel CS. Nuclear receptors – a perspective from Drosophila. Nat Rev Genet. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- 66.Thummel CS. Flies on steroids – Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 67.Rus F, Flatt T, Tong M, Aggarwal K, Okuda K, Kleino A, Yates E, Tatar M, Silverman N. Ecdysone triggered PGRP-LC expression controls Drosophila innate immunity. EMBO J. 2013;32:1626–1638. doi: 10.1038/emboj.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flatt T, Heyland A, Rus F, Porpiglia E, Sherlock C, Yamamoto R, Garbuzov A, Palli SR, Tatar M, Silverman N. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J Exp Biol. 2008;211:2712–2724. doi: 10.1242/jeb.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Regan JC, Brandao AS, Leitao AB, Mantas Dias AR, Sucena E, Jacinto A, Zaidman-Remy A. Steroid hormone signaling is essential to regulate innate immune cells and fight bacterial infection in Drosophila. PLoS Pathog. 2013;9:e1003720. doi: 10.1371/journal.ppat.1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sampson CJ, Amin U, Couso JP. Activation of Drosophila hemocyte motility by the ecdysone hormone. Biol Open. 2013;2:1412–1420. doi: 10.1242/bio.20136619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figueiredo MB, Castro DP, NF SN, Garcia ES, Azambuja P. Cellular immune response in Rhodnius prolixus: role of ecdysone in hemocyte phagocytosis. J Insect Physiol. 2006;52:711–716. doi: 10.1016/j.jinsphys.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Alphey L, Andreasen M. Dominant lethality and insect population control. Mol Biochem Parasitol. 2002;121:173–178. doi: 10.1016/s0166-6851(02)00040-3. [DOI] [PubMed] [Google Scholar]
- 73.Dissanayake SN, Ribeiro JM, Wang MH, Dunn WA, Yan G, James AA, Marinotti O. aeGEPUCI: a database of gene expression in the dengue vector mosquito, Aedes aegypti. BMC Res Notes. 2010;3:248. doi: 10.1186/1756-0500-3-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.