Abstract
Purpose
A number of parameters derived from dynamic contrast enhanced MRI (DCE-MRI) and separate histological features have been identified as potential prognosticators in high-grade glioma. This study evaluates the relationships between DCE-MRI derived parameters and histological features in glioblastoma multiforme (GBM).
Methods
28 patients with newly presenting GBM underwent pre-operative imaging (conventional imaging and T1-DCE-MRI). Parametric maps of IAUC60 (initial area under the contrast agent concentration curve), Ktrans (contrast transfer co-efficient) ve (estimate of volume of the extravascular extracellular space) and vp (estimate of blood plasma volume) were generated and EnF was calculated. Surgical specimens were used to assess subtype and graded (WHO classification system) and assessed for necrosis, cell density, cellular atypia, mitotic activity and overall vascularity scores. Quantitative assessment of endothelial surface area, vascular surface area and a vascular profile count were made using CD34 immunostaining. The relationships between MRI parameters and histo-pathological features were examined.
Results
High values of Ktrans were associated with the presence of frank necrosis (p=0.005). High values of ve were associated with a fibrillary histological pattern (p<0.01) and with increased mitotic activity (p<0.05). No relationship was found between mitotic activity and histological pattern suggesting the correlation between ve and mitotic activity was independent of histological pattern.
Conclusion
A correlation between ve and mitotic activity is reported. Further work is warranted to establish how DCE-MRI parameters relate to more quantitative histological measurements including markers of proliferation and measures of vascular endothelial growth factor (VEGF) expression.
Introduction
Gliomas are the commonest primary cerebral tumour of adulthood. They are histologically classified according to the World Health Organisation (WHO) criteria into tumour grade and subtype (1), which is important for determining appropriate treatment. Within given histological subtypes and tumour grades, a number of additional descriptive histopathological features have been identified as prognosticators including mitotic activity (2-4), microvascular density (MVD) (5,6) and certain vascular patterns (7,8). Both proliferation markers, such as Ki-67 (2-4,7,9,10) and expression of vascular endothelial growth factor (VEGF) (3,5,11-14) have also shown relationships to survival.
Dynamic contrast enhanced-MRI (DCE-MRI) techniques generate a number of parameters that characterize the microvascular environment. The enhancing fraction (EnF) describes the proportion of perfused tumour tissue. CBV and CBF are commonly derived from dynamic susceptibility contrast (DSC-MRI) techniques whilst volume transfer coefficient (Ktrans), ve (fractional volume of the extravascular extracellular space, EES) and vp (fractional blood plasma volume) can be calculated from T1-weighted DCE-MRI (15). In glioma, Ktrans, vp, EnF, CBV and CBF have been shown to relate to histological grade and/or subtype of tumour (16-21). In addition, Ktrans, EnF and CBV have been identified as potentially grade independent prognosticators (22-25). The number of studies examining the relationship between DCE-MRI parameters and more specific histopathological features in glioma is currently small and predominantly focuses on vascular metrics such as blood volume and flow; however, significant correlations have been described between CBV and MVD (26-29), VEGF expression (27,30), cell density (29), endothelial proliferation (31) and mitotic activity (31) and between CBF and endothelial hyperplasia (32).
More recent studies have focused on the potential of ve as an imaging biomarker. In glioma, it has shown value in discriminating between histological grade (33,34), but when compared with other potential candidate biomarkers of the extravascular extracellular space volume (Apparent Diffusion Co-efficient, ADC), on a voxel by voxel basis, no correlation was seen between the two metrics, suggesting that these parameters provide independent information about extravascular extracellular space characteristics (35). Two separate studies of glioma patients of various histological grade have both reported significant correlations between both Ktrans and ve and vascular and microvascular density (33,34), but neither study comments upon the relationship with cellular density or mitotic activity. A high field, 7T MRI study of rat xenografts showed an image matched significant negative correlation between ve and tumor cellularity (36).
We hypothesized that: 1) larger, rapidly growing tumours would show higher mitotic activity as well as high angiogenic activity reflected by Ktrans and EnF; and 2) more proliferative tumours would have a higher cellular density and mitotic activity associated with lower values of ve.
Materials and Methods
Patients
Ethical approval was obtained and all patients gave informed consent. All tumours were histologically confirmed as GBM according to the WHO criteria (1). All imaging was pre-operative and patients received no treatment other than corticosteroids, which were administered for a minimum of 48 hours prior to imaging to allow stabilisation of the effects of steroids on DCE-MRI measures (37). Patients were excluded from the study if they had a history of renal dysfunction or low eGFR (<30 mls/min/1.73m2).
MRI data acquisition
Imaging was performed on a 3 Tesla Philips Achieva system (Philips Healthcare, Best, Netherlands) using a SENSE head coil. DCE-MRI acquisitions were acquired in a sagittal oblique orientation to allow improved definition of the arterial input function (AIF) free from flow related artifact. Three pre-contrast T1-fast field echo (T1-FFE; RF-spoiled gradient echo) series (2°, 5°, 16°) were acquired for calculation of baseline T1 maps (TR 3.5 ms, TE 1.1 ms, slice thickness 4.2 mm, 128 × 128 matrix, FOV 230 mm × 230 mm × 105 mm) in the same geometry. A dynamic, contrast-enhanced acquisition series (TR 3.5 ms, TE 1.1 ms, flip angle 16°, slice thickness 4.2 mm, 128 × 128 matrix, FOV 230 mm × 230 mm × 105 mm) consisting of 100 volumes with temporal spacing of approximately 3.4 seconds followed. A bolus dose of 0.1 mMolkg−1 (body weight) of gadolinium-based contrast agent (Gd-DTPA-BMA; Omniscan, GE Healthcare, Oslo, Norway) was injected at a rate of 3 mls−1, after acquisition of the fifth image volume. Pre and post contrast T1-weighted imaging sequences (TR 9.3 ms, TE 4.6 ms) were acquired in the same sagittal oblique geometry for definition of volume of interest (VOI) of the whole tumour.
MRI data analysis
An experienced neuroradiologist (SJM) manually defined VOIs for each tumour. The VOI corresponded to the enhancing tumour and all non-enhancing tissue contained within it on the post contrast T1-weighted images. This technique has previously shown good interobserver agreement (Intraclass Correlation Coefficient > 0.94)(38). Pharmacokinetic analysis was performed on all pixels within the VOI which showed significant enhancement. Parametric maps of Ktrans, vp, ve and IAUC60 were produced using in-house software (MaDyM – Manchester Dynamic Modelling) and the extended Tofts and Kermode pharmacokinetic model (15). Automated AIFs were generated from an appropriately chosen slice, which included the internal carotid artery (39). Summary statistics for each parameter were generated for enhancing tumour tissue.
For each tumour, EnF (EnFIAUC60>0) and thresholded EnF (EnFIAUC60>2.5) were calculated by dividing the enhancing volume (volume of voxels with IAUC60 > 0 mMol.s for EnFIAUC60>0 and volume of voxels with IAUC60 > 2.5 mMol.s for EnFIAUC60>2.5) by the total volume of the tumour VOI. The cut off threshold of IAUC60 > 2.5 mMol.s for EnFIAUC60>2.5 was previously identified as a optimal threshold for allowing the distinction of high from low grade glioma (40).
Histopathological data analysis
Two experienced neuropathologists (DDP and PP) performed the histopathological analysis. Histological specimens were assessed for the following: necrosis (presence or absence of frank and/or geographic necrosis), cell density (3 point grading score), cell atypia (3 point grading score), mitotic activity (number of mitotic figures seen per 10 high power field units), infiltrates (presence or absence of lymphocytes and/or macrophages), tumour vascular pattern (presence or absence of the following features; endothelial hypertrophy and/or hyperplasia, glomeruloid structures, granulation tissue, large vessel density, thrombosis, sclerosed vessels), an overall vascular density score (3 point grading score) and histological pattern (fibrillar, gemistocytic, oligodendrocytes, sarcomatous, giant cells and small cells) in conjunction with standard histopathological subtyping and grading according to the WHO classification criteria (1). Vascular and histological features were not mutually exclusive, therefore more than one feature could be described in a given tumour specimen.
Quantitative measurement of the endothelial surface area (ESA), the vascular surface area (VSA) and the vascular profile count per mm2 (VPC) was made using CD34 immunostaining and dedicated image analysis software.
Statistical analysis
Statistical analysis was performed using SPSS (version 15.0, SPSS Inc., Chicago, USA) nonparametric statistical tests. Where histological parameters produce a binary classification (necrosis, infiltrates, vascular patterns and histological patterns) Mann Whitney U tests were performed to test the hypothesis that MRI parameter values did not differ between groups. Where histological features produce categorical scores (cell density, cell atypia and overall vascular score) multivariate analysis of variance (ANOVA) was used to test the hypotheses that MRI parameter values did not differ between groups. Spearman's correlation analysis was performed to assess the relationship between quantitative histological measures (mitotic activity, ESA, VSA and VPC) and MRI parameters and to identify correlation between the individual MRI measurements. For Mann Whitney U and ANOVA testing a result was considered to be significantly different if p<0.01, given the number of variables assessed. For Spearman’s correlation analysis significance was taken as <0.05.
Results
28 untreated newly presently GBM were included in the study (10 female, age range 38 - 76 years, mean 60 years). Histological specimens were obtained from 12 biopsies and 16 surgical debulkings. Results of statistical analyses for comparisons of histological and MRI measures are summarized in Table 1.
Table 1.
Summary of statistical comparisons between histopathological and DCE-MRI measures. Mann Whitney U tests were used for histological parameters which produce a binary classification (necrosis, infiltrates, vascular patterns and histological patterns) and multivariate analysis of variance (ANOVA) was used for histological features which produce categorical scores (cell density, cell atypia and overall vascular score). Spearman's correlation analysis was performed to assess the relationship between MR derived parameters and quantitative histological measures (mitotic activity, ESA, VSA and VPC). Significance levels are shown and Spearman’s ρ is shown for correlation analyses.

|
Ktrans (min−1) | ve (unitless) | vp (unitless) | EnFIAUC60>0 (unitless) | EnFIAUC60>2.5 (unitless) | |
|---|---|---|---|---|---|---|
| Necrosis | Frank | 0.005** | 0.694 | 0.694 | 0.264 | 0.088 |
| Geographic | 0.495 | 0.655 | 0.466 | 0.384 | 0.796 | |
| Cell density | 0.392 | 0.165 | 0.953 | 0.455 | 0.169 | |
| Cell atypia | 0.958 | 0.629 | 0.654 | 0.630 | 0.149 | |
| Mitotic activity | 0.400 ρ=0.166 |
0.012* ρ=0.470 |
0.678 ρ=−0.082 |
0.729 ρ=−0.069 |
0.744 ρ=0.065 |
|
| Infiltrates | Lymphocytes | 0.353 | 0.147 | 0.911 | 0.629 | 0.393 |
| Macrophages | 0.629 | 0.738 | 0.683 | 0.629 | 0.970 | |
| Tumour vascular patterns | Hypertrophy | 0.592 | 0.592 | 0.153 | 0.422 | 0.212 |
| Hyperplasia | 0.569 | 0.787 | 0.197 | 0.787 | 0.418 | |
| Glomeruloid | 0.394 | 0.504 | 0.504 | 0.134 | 0.596 | |
| Granulation tissue | 0.325 | 0.896 | 0.896 | 0.015 | 0.076 | |
| Large vessel | 0.164 | 0.164 | 0.137 | 0.848 | 0.502 | |
| Thrombosis | 0.472 | 0.924 | 0.632 | 0.472 | 0.811 | |
| Sclerosed | 0.293 | 0.095 | 0.155 | 0.951 | 0.122 | |
| Histological patterns | Fibrillar | 0.325 | 0.007** | 0.325 | 0.793 | 0.212 |
| Gemistocytic | 0.937 | 0.442 | 0.614 | 0.730 | 0.353 | |
| Oligodendro | 0.155 | 0.353 | 0.757 | 0.155 | 0.155 | |
| Sarcomatous | 0.929 | 0.789 | 0.929 | 0.372 | 0.592 | |
| Giant Cells | 0.697 | 0.697 | 0.976 | 0.787 | 0.610 | |
| Small Cells | 0.970 | 0.683 | 0.911 | 0.738 | 0.738 | |
| Overall vascular score | 0.125 | 0.759 | 0.385 | 0.214 | 0.278 | |
| Vascular measures | ESA Ratio | 0.839 ρ=0.041 |
0.809 ρ=−0.049 |
0.493 ρ=−0.0138 |
0.344 ρ=−0.189 |
0.799 ρ=−0.051 |
| VSA Ratio | 0.919 ρ=0.020 |
0.855 ρ=−0.037 |
0.495 ρ=−0.137 |
0.298 ρ=−0.208 |
0.782 ρ=−0.056 |
|
| VPCmm−2 | 0.224 ρ=0.242 |
0.626 ρ=−0.098 |
0.119 ρ=0.307 |
0.065 ρ=0.360 |
0.071 ρ=0.353 |
|
significance taken as p < 0.05 (Spearman’s correlation analysis)
significance taken as p < 0.01(Mann Whitney U and ANOVA)
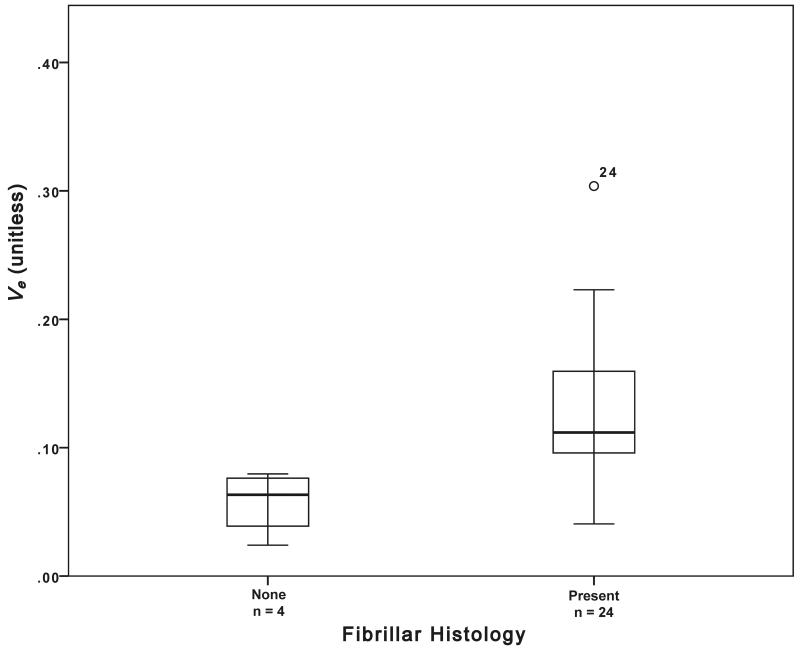
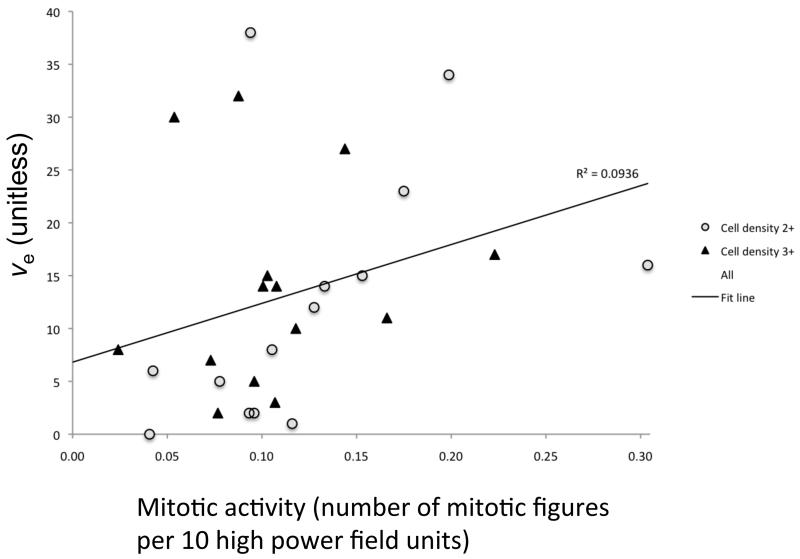
The presence of frank necrosis was associated with significantly higher values of Ktrans (p<0.01, Figure 1). Significantly higher values of ve were seen in the presence fibrillary histology (estimated p<0.01, Figure 1). A positive correlation was found between ve and mitotic activity (p<0.05, ρ =0.470, Figure 2). No relationship was seen between mitotic activity and any of the descriptive or semi-quantitative histology measures, suggesting the relationship between ve and mitotic activity is independent of the relationship between ve and the presence of fibrillar histology. No correlation was observed between ve and cell density.
Figure 1.
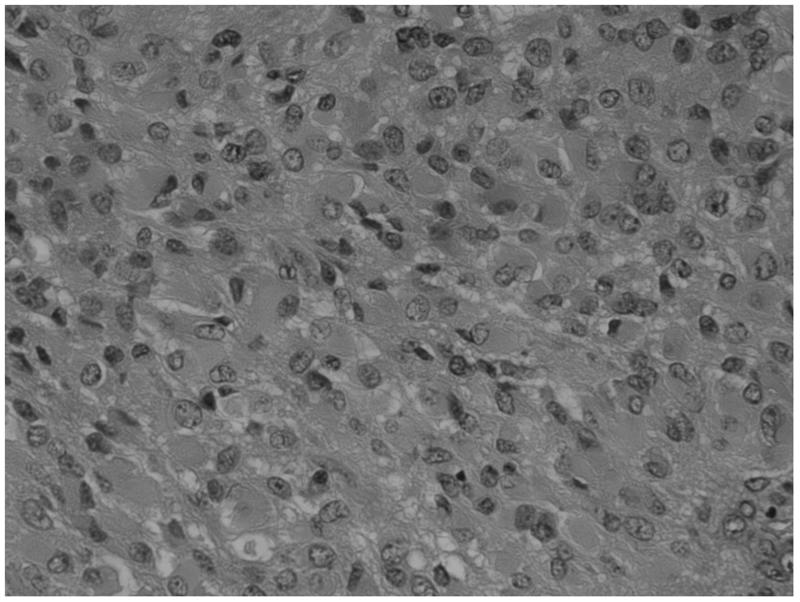
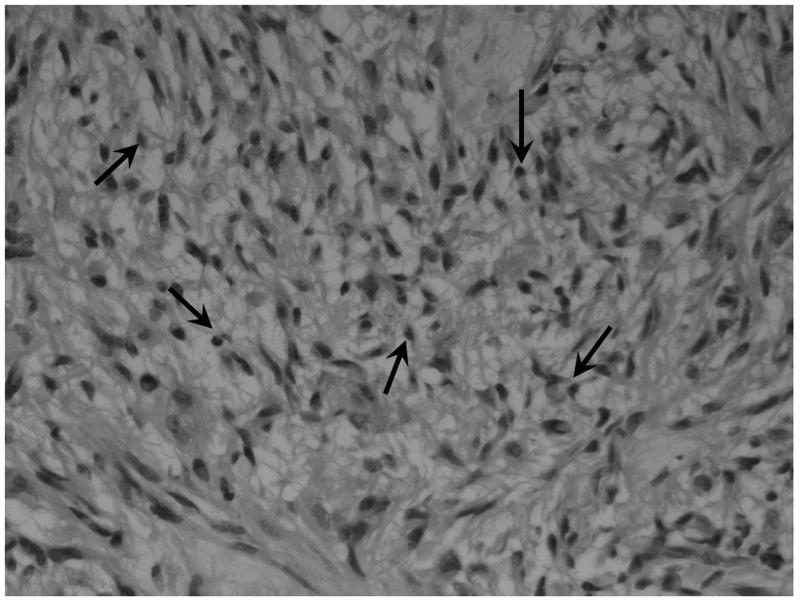
a) Boxplot of fibrillar histology and ve (estimated p = 0.007). b) Example histological specimens showing tumours without and c) with the presence of fibrils (small fibres measuring approximately 1 mm, black arrows). H&E stain × 40 magnification. d) Boxplot of frank necrosis and Ktrans (estimated p = 0.005).
Figure 2.
Scatter plot of mitotic activity versus ve (p = 0.012, r = 0.470), colour coded to demonstrate separate scores of cell density measures.
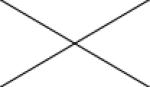
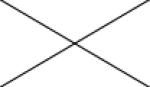
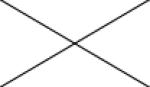
Cross correlations between individual MRI parameters are summarised in Table 2. Positive correlations were found between Ktrans and all other MRI parameters. Significant correlations were present between ve and Ktrans (p<0.05 and ρ=0.450) and between vp and EnF. Mitotic activity did not correlate with any quantitative vascular measure, but cross correlation was seen across all three histological vascular metrics (Table 3). No other significant relationships were identified between histological measures.
Table 2.
Cross correlations of MRI parameters. Significance levels and Spearman’s ρ are shown.
| Untreated GBM (n = 28) | ||||
|---|---|---|---|---|
| v e | v p | EnFIAUC60>0 | EnFIAUC60>2.5 | |
| K trans | 0.016* ρ=0.450 |
0.006** ρ=0.507 |
0.005** ρ=0.513 |
<0.001** ρ=0.784 |
| v e |

|
0.105 ρ=0.313 |
0.888 ρ=0.028 |
0.059 ρ=0.361 |
| v p |
|
|
0.02* ρ=0.437 |
<0.001** ρ=0.620 |
| EnFIAUC60>0 |
|

|
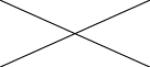
|
<0.001** ρ=0.825 |
significance taken as p < 0.05
significance taken as p < 0.01
Table 3.
Cross correlations of quantitative histological measures. Significance levels and Spearman’s ρ are shown.
| Untreated GBM (n = 28) | |||
|---|---|---|---|
| ESA ratio | VSA ratio | VPC/mm2 | |
| Mitotic activity | 0.098 ρ=0.325 |
0.066 ρ=0.358 |
0.737 ρ=0.068 |
| ESA ratio |
|
<0.001** ρ=0.993 |
0.04* ρ=0.530 |
| VSA ratio |
|
|
0.04* ρ=0.532 |
significance taken as p < 0.05
significance taken as p < 0.01
Discussion
Whilst there has been considerable interest in the development of imaging biomarkers for use in clinical trials, the majority of DCE-MRI studies have focused on identifying correlates of vascularity and angiogenesis (41). This study identified an unexpected positive correlation between ve, a parameter thought to reflect EES volume, and mitotic activity. No relationships were identified between previously described DCE-MRI prognosticators, Ktrans and vp, and mitotic activity, nor between vascular patterns and DCE-MRI measures.
The parameter ve is often overlooked in DCE-MRI studies. In cerebral tumours, it has potential to distinguish intra- from extra-axial tumours (42,43), and exhibits increased values with increasing tumour grade (33,34,42,44). It has also demonstrated sensitivity in identifying changes in response to treatment with corticosteroids, with decreases in ve, presumably reflecting a reduction in interstitial oedema (45,46). In a study of vestibular schwannomas, meningiomas and gliomas, no direct measure of the EES or cell density measure was made but ve values in schwannoma were noted to be significantly larger than in glioma, where cell density is higher (43). In a study comparing ve with ADC in glioma, no relationship was found between the two metrics suggesting that these parameters may provide independent information about the extravascular extracellular space (35). This may reflect the existence of separate extravascular leakage compartments characterised by fast and contrast agent exchange, possibly reflecting the presence of slow leakage / diffusion into and out of necrotic tumour spaces (42,47,48). Previous studies have also reported correlations between MVD and both ve and Ktrans (33,34). We did not find this correlation in our study but there was a cross correlation between the DCE-MRI parameters ve and Ktrans and raising the possibility that the correlation reported in the previous studies reflects the relationship of ve with Ktrans rather than MVD. A previous rat xenograft study has reported correlation between ve and cellular density (36), but our current study found no such relationship. Histological measures of cell density are often a count of the number of cell nuclei per unit area and without quantification of the amount of tissue the cells occupy, thus an increase in histological cell density may reflect an increase in number of cells, but not necessarily a decrease in extra-vascular, extra cellular space if the cells are small in size. It should be noted that there are potential modelling problems associated with the calculation of ve. Clearly it can be estimated only in perfused tissue where there is significant leakage of contrast agent (35). This means that summary statistics presented in this and other studies reflect only perfused tissue with contrast agent leakage, in contrast to diffusion-weighted imaging where high ADC values are seen in association with necrosis. We have specifically excluded non-perfusing tissue from estimates of summary statistics, which could otherwise produce artificially low values of ve. In addition, the relatively low dynamic sampling duration (6 minutes) will affect ve estimates to some degree. First, model fitting errors (assuming the model is correct) will result from undersampling, but modelling studies suggest that these fitting errors are likely to be very small (49). Second, short sampling times will lead to relative under-representation of slow tissue exchange compartments, which would tend to reduce the impact of necrotic tissue on ve estimations as described in (42,47,48). This implies that tumours with greater necrosis would have shown higher ve values if sampling had continued for a longer time and that the measured values of ve in this study are more likely to reflect EES fraction in viable tissue.
The positive correlation between ve and mitotic activity is surprising. Tumours with larger ve values exhibited more mitotic activity, the inverse of what one might expect (and the inverse of our initial hypothesis), whereby more proliferative tumours would be more densely packed with cells. Neither mitotic activity nor ve related to the presence of necrosis, although the lack of relationship between ve and necrosis may in part reflect the relatively low dynamic collection period (vide supra). These observations suggest that the size of the EES in perfused enhancing tumour tissue is truly related to mitotic rate and not simply a reflection of elevated measures of ve due to increased necrosis in rapidly proliferating tumours.
This is initially counter-intuitive. It is well known that in normal developing tissues mitotic activity is higher in areas of low cell packing due to inhibition of proliferation in response to cell to cell contact, a process known as contact inhibition of proliferation (CIP) in developing normal tissue (50). No similar relationship has been described in malignant tissues and loss of contact inhibition of mitosis is one of the hallmarks of the cancer cell (51). This leads to the hypothesis, stated in the introduction, that rapidly proliferating tumours will continue to proliferate leading to increased cell density and decreased size of the EES and consequently of ve. We have no evidence to explain why the observed relationship should exist. One possible explanation may be that tumours with high cellular density still have impaired responses to tumoural growth factors despite loss of CIP (52). Another possible explanation is that tumours with short mitotic cycles are characterised by reduced time for cellular maturation resulting in smaller cells and reduced cell packing. Whatever the underlying biological mechanism this finding appears particularly interesting and requires further study.
The ability to obtain an MRI-based imaging biomarker of mitotic activity and/or tumour cell proliferation is highly desirable. The findings presented here may be tumour specific or reflect an unrecognised phenotype, but the possibility that ve may be a potential marker of mitotic activity merits further evaluation.
The data also demonstrated a positive relationship between tumour cell fibrillarity within the histological specimens and higher ve, although the numbers were very small (n=4). Fibrillar cell processes are cell extensions containing cytoplasm, which are surrounded by cell membranes. These are visible through a microscope and allow tumour astrocytes to be recognised as “fibrillar”. A less cell-dense arrangement due to such cell extensions and/or intercellular oedema facilitate its recognition via light microscopy. Thus, the ability to identify fibrillar cell processes suggests that the tumour cells are more loosely packed or there is localized extracellular oedema. No relationship was identified between fibrillar histology and mitotic activity indicating that the relationships between ve and these measures are independent.
The majority of previous work evaluating the relationship between specific histological features and DCE-MRI has focused predominantly on CBV derived from DSC techniques with significant relationships seen between CBV and MVD (26-29), VEGF expression (27,30), cell density (29), endothelial proliferation (31) and mitotic activity (31). No such relationships were identified between these latter three measures and vp in this study. A study by Ludemann et al compared a variety of MR imaging techniques with H215O-PET for measuring perfusion (53) and found that whilst both DSC and T1-weighted DCE-MRI techniques correlated with the gold standard H215O-PET measure, only borderline correlation was seen between the DSC techniques and the T1-weighted technique, whereby DSC derived blood volumes were generally lower than those derived from the T1-weighted DCE-MRI technique. This may account for the failure of vp to relate to any of the histological measures in this study.
The major limitation of the present work is the lack of stereotactic image matched histological specimens and therefore correlation between the histology and the DCE-MRI measurements at a local level cannot be made. GBMs are notoriously heterogeneous tumours and histological analysis of small tumour specimens may lead to under-grading of a tumour if the sample is not a true reflection of the tumour as a whole. At the time of the study, imaged matched histological samples were not obtained and there was no concurrent post-operative imaging performed which could have helped to identify the site of the histological sample. All histological specimens in this study did confirm the diagnosis of GBM and therefore are considered representative samples. Previous non image registered studies comparing ve with histopathological vascular measures have been reported but these have been performed with a selection of 3-4 small ROIs, taking the maximal value of ve and Ktrans (33,34), which is unlikely to provide a representation of the tumour as a whole. In our current study, for the DCE-MRI measures Ktrans, ve, and vp, median values from whole tumour VOIs were used. Studies using whole tumour VOIs have previously identified significant differences in DCE-MRI parameters between tumour grades (17), and been shown to convey important potential prognostic information (25). Thus a comparison between small histological samples and DCE-MRI parameters from whole tumour VOIs seems reasonable. Further work is required to confirm that the correlations identified in this current study hold true for image matched stereotactic samples. In addition, some histological features such as Ki-67 and VEGF expression, which have previously shown correlation with DSC-MRI derived CBV (20,26-30), were not available.
Conclusion
The DCE-MRI derived measure, ve, has been identified as a potential correlate of mitotic activity in GBM. Whilst this is an interesting result, our understanding of the biological mechanisms responsible for this possible relationship is limited. Further work with the correlation of ve to more precise measures of cell density, additional markers of cellular and vascular proliferation, and measures of VEGF expression is warranted.
Acknowledgments
Funding
This works was funded as part of a CRUK Clinical Research Training Fellowship, ref: C21247/A7473.
Abbreviations
- DCE-MRI
dynamic contrast enhanced MRI
- EES
extravascular extracellular space
- EnF
enhancing fraction
- ESA
endothelial surface area
- 18F-FLT
18F-labeled 3′-deoxy-3′-fluorothymidine
- GBM
glioblastoma multiforme
- IAUC60
initial area under the contrast agent concentration curve
- Ktrans
contrast transfer coefficient
- MVD
microvascular density
- ve
volume of the EES per unit tissue volume
- VEGF
vascular endothelial growth factor
- vp
volume of the blood plasma per unit tissue volume
- VPC
vascular profile count per mm2 (VPC)
- VSA
vascular surface area
- WHO
World Health Organisation
Footnotes
Conflict of Interest
None
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007;1142:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Struikmans H, Rutgers DH, Jansen GH, et al. Prognostic relevance of MIB-1 immunoreactivity, S-phase fraction, 5-bromo-2′-deoxyuridine labeling indices, and mitotic figures in gliomas. Radiat Oncol Investig. 1999;74:243–48. doi: 10.1002/(SICI)1520-6823(1999)7:4<243::AID-ROI6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Korshunov A, Golanov A, Sycheva R. Immunohistochemical markers for prognosis of anaplastic astrocytomas. J. Neurooncol. 2002;583:203–15. doi: 10.1023/a:1016261900413. [DOI] [PubMed] [Google Scholar]
- 4.Scott JN, Rewcastle NB, Brasher PM, et al. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann. Neurol. 1999;462:183–88. [PubMed] [Google Scholar]
- 5.Abdulrauf SI, Edvardsen K, Ho KL, et al. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J. Neurosurg. 1998;883:513–20. doi: 10.3171/jns.1998.88.3.0513. [DOI] [PubMed] [Google Scholar]
- 6.Yao Y, Kubota T, Takeuchi H, et al. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;253:201–6. doi: 10.1111/j.1440-1789.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 7.Preusser M, Gelpi E, Matej R, et al. No prognostic impact of survivin expression in glioblastoma. Acta Neuropathol. 2005;1095:534–38. doi: 10.1007/s00401-005-0992-x. [DOI] [PubMed] [Google Scholar]
- 8.Birner P, Piribauer M, Fischer I, et al. Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: evidence for distinct angiogenic subtypes. Brain Pathol. 2003;132:133–43. doi: 10.1111/j.1750-3639.2003.tb00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colman H, Giannini C, Huang L, et al. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am. J. Surg. Pathol. 2006;305:657–64. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- 10.Coleman KE, Brat DJ, Cotsonis GA, et al. Proliferation (MIB-1 expression) in oligodendrogliomas: assessment of quantitative methods and prognostic significance. Appl. Immunohistochem. Mol. Morphol. 2006;141:109–14. doi: 10.1097/01.pai.0000144557.31747.b9. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JR, Wang L, Gillespie DL, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;1135:1032–42. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karayan-Tapon L, Wager M, Guilhot J, et al. Semaphorin, neuropilin and VEGF expression in glial tumours: SEMA3G, a prognostic marker? Br. J. Cancer. 2008;997:1153–60. doi: 10.1038/sj.bjc.6604641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam D-H, Park K, Suh YL, et al. Expression of VEGF and brain specific angiogenesis inhibitor-1 in glioblastoma: prognostic significance. Oncol. Rep. 2004;114:863–69. [PubMed] [Google Scholar]
- 14.Oehring RD, Miletic M, Valter MM, et al. Vascular endothelial growth factor (VEGF) in astrocytic gliomas--a prognostic factor? J. Neurooncol. 1999;452:117–25. doi: 10.1023/a:1006333005563. [DOI] [PubMed] [Google Scholar]
- 15.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Reson. Imaging. 1997;71:91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 16.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;2410:1989–98. [PMC free article] [PubMed] [Google Scholar]
- 17.Patankar TF, Haroon HA, Mills SJ, et al. Is volume transfer coefficient (K(trans)) related to histologic grade in human gliomas? AJNR Am J Neuroradiol. 2005;2610:2455–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol. 1998;1716:1479–86. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]
- 19.Roberts HC, Roberts TP, Brasch RC, et al. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol. 2000;215:891–99. [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts HC, Roberts TP, Bollen AW, et al. Correlation of microvascular permeability derived from dynamic contrast-enhanced MR imaging with histologic grade and tumor labeling index: a study in human brain tumors. Acad Radiol. 2001;85:384–91. doi: 10.1016/S1076-6332(03)80545-7. [DOI] [PubMed] [Google Scholar]
- 21.Law M, Young R, Babb J, et al. Comparing perfusion metrics obtained from a single compartment versus pharmacokinetic modeling methods using dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2006;279:1975–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected] AJNR Am J Neuroradiol. 2004;252:214–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;2472:490–98. doi: 10.1148/radiol.2472070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Tsien CI, Nagesh V, et al. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected] Int. J. Radiat. Oncol. Biol. Phys. 2006;643:876–85. doi: 10.1016/j.ijrobp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Mills SJ, Patankar TA, Haroon HA, et al. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol. 2006;274:853–58. [PMC free article] [PubMed] [Google Scholar]
- 26.Cha S, Johnson G, Wadghiri YZ, et al. Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn. Reson. Med. 2003;495:848–55. doi: 10.1002/mrm.10446. [DOI] [PubMed] [Google Scholar]
- 27.Haris M, Husain N, Singh A, et al. Dynamic contrast-enhanced derived cerebral blood volume correlates better with leak correction than with no correction for vascular endothelial growth factor, microvascular density, and grading of astrocytoma. J Comput Assist Tomogr. 2008;326:955–65. doi: 10.1097/RCT.0b013e31816200d1. [DOI] [PubMed] [Google Scholar]
- 28.Liao W, Liu Y, Wang X, et al. Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol. 2009;502:217–25. doi: 10.1080/02841850802616752. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghi N, D'Haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am J Neuroradiol. 2008;293:476–82. doi: 10.3174/ajnr.A0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maia ACM, Malheiros SMF, da Rocha AJ, et al. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol. 2005;264:777–83. [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeghi N, Salmon I, Decaestecker C, et al. Stereotactic comparison among cerebral blood volume, methionine uptake, and histopathology in brain glioma. AJNR Am J Neuroradiol. 2007;283:455–61. [PMC free article] [PubMed] [Google Scholar]
- 32.Callot V, Galanaud D, Figarella-Branger D, et al. Correlations between MR and endothelial hyperplasia in low-grade gliomas. J. Magn. Reson. Imaging. 2007;261:52–60. doi: 10.1002/jmri.20995. [DOI] [PubMed] [Google Scholar]
- 33.Jia ZZ, Gu HM, Zhou XJ, et al. The assessment of immature microvascular density in brain gliomas with dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol. 2015;849:1805–9. doi: 10.1016/j.ejrad.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Zhu Y, Kang H, et al. Glioma grading by microvascular permeability parameters derived from dynamic contrast-enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging. 2015;151:4. doi: 10.1186/s40644-015-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills SJ, Soh C, Rose CJ, et al. Candidate biomarkers of extravascular extracellular space: a direct comparison of apparent diffusion coefficient and dynamic contrast-enhanced MR imaging--derived measurement of the volume of the extravascular extracellular space in glioblastoma multiforme. AJNR Am J Neuroradiol. 2010;313:549–53. doi: 10.3174/ajnr.A1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aryal MP, Nagaraja TN, Keenan KA, et al. Dynamic contrast enhanced MRI parameters and tumor cellularity in a rat model of cerebral glioma at 7 T. Magn. Reson. Med. 2014;716:2206–14. doi: 10.1002/mrm.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastin ME, Carpenter TK, Armitage PA, et al. Effects of dexamethasone on cerebral perfusion and water diffusion in patients with high-grade glioma. AJNR Am J Neuroradiol. 2006;272:402–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson G, Cain J, Jackson A, et al. Interobserver agreement for cerebral glioma volumetrics on conventional MR imaging. Conference Proceedings of the 16th Annual Meeting of ISMRM; 2008. [Google Scholar]
- 39.Parker GJ, Jackson A, Waterton JC. Automated arterial input function extraction for T1-weighted DCE-MRI. Conference Proceedings of the 11th Annual Meeting of ISMRM; 2003. [Google Scholar]
- 40.Mills SJ, Soh C, O'Connor JPB, et al. Tumour enhancing fraction (EnF) in glioma: relationship to tumour grade. Eur Radiol. 2009;196:1489–98. doi: 10.1007/s00330-008-1288-z. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor JPB, Jackson A, Parker GJM, et al. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br. J. Cancer. 2007;962:189–95. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lüdemann L, Grieger W, Wurm R, et al. Quantitative measurement of leakage volume and permeability in gliomas, meningiomas and brain metastases with dynamic contrast-enhanced MRI. Magnetic Resonance Imaging. 2005;238:833–41. doi: 10.1016/j.mri.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Zhu XP, Li KL, Kamaly-Asl ID, et al. Quantification of endothelial permeability, leakage space, and blood volume in brain tumors using combined T1 and T2* contrast-enhanced dynamic MR imaging. J. Magn. Reson. Imaging. 2000;116:575–85. doi: 10.1002/1522-2586(200006)11:6<575::aid-jmri2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Lüdemann L, Grieger W, Wurm R, et al. Comparison of dynamic contrast-enhanced MRI with WHO tumor grading for gliomas. Eur Radiol. 2001;117:1231–41. doi: 10.1007/s003300000748. [DOI] [PubMed] [Google Scholar]
- 45.Andersen C, Jensen FT. Differences in blood-tumour-barrier leakage of human intracranial tumours: quantitative monitoring of vasogenic oedema and its response to glucocorticoid treatment. Acta Neurochir (Wien) 1998;1409:919–24. doi: 10.1007/s007010050194. [DOI] [PubMed] [Google Scholar]
- 46.Armitage PA, Schwindack C, Bastin ME, et al. Quantitative assessment of intracranial tumor response to dexamethasone using diffusion, perfusion and permeability magnetic resonance imaging. Magnetic Resonance Imaging. 2007;253:303–10. doi: 10.1016/j.mri.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Fluckiger JU, Loveless ME, Barnes SL, et al. A diffusion-compensated model for the analysis of DCE-MRI data: theory, simulations and experimental results. Phys. Med. Biol. 2013;586:1983–98. doi: 10.1088/0031-9155/58/6/1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lüdemann L, Hamm B, Zimmer C. Pharmacokinetic analysis of glioma compartments with dynamic Gd-DTPA-enhanced magnetic resonance imaging. Magnetic Resonance Imaging. 2000;1810:1201–14. doi: 10.1016/s0730-725x(00)00223-x. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H-LM. Investigation and optimization of parameter accuracy in dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging. 2008;283:736–43. doi: 10.1002/jmri.21489. [DOI] [PubMed] [Google Scholar]
- 50.McClatchey AI, Yap AS. Contact inhibition (of proliferation) redux. Current Opinion in Cell Biology. 2012;245:685–94. doi: 10.1016/j.ceb.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Tumanishvili GD, Salamatina NV. Action of nuclear and cytoplasmic fractions of liver homogenate on liver growth in the chick embryo. J Embryol Exp Morphol. 1968;201:53–71. [PubMed] [Google Scholar]
- 52.Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends in Cell Biology. 2010;206:319–28. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lüdemann L, Warmuth C, Plotkin M, et al. Brain tumor perfusion: comparison of dynamic contrast enhanced magnetic resonance imaging using T1, T2, and T2* contrast, pulsed arterial spin labeling, and H2(15)O positron emission tomography. Eur J Radiol. 2009;703:465–74. doi: 10.1016/j.ejrad.2008.02.012. [DOI] [PubMed] [Google Scholar]