Abstract
Dendritic cells (DCs) are the key initiators of T helper (Th) 2 immune responses against the parasitic helminth Schistosoma mansoni. Although the liver is one of the main sites of Ag deposition during infection with this parasite, it is not yet clear how distinct DC subtypes in this tissue respond to S. mansoni Ags in vivo, or how the liver microenvironment might influence DC function during establishment of the Th2 response. In this study, we show that hepatic DC subsets undergo distinct activation processes in vivo following murine infection with S. mansoni. Conventional DCs (cDCs) from schistosome-infected mice up-regulated expression of the costimulatory molecule CD40 and were capable of priming naïve CD4+ T cells, whereas plasmacytoid DCs (pDCs) up-regulated expression of MHC class II, CD86 and CD40 but were unable to support the expansion of either naïve or effector/memory CD4+ T cells. Importantly, in vivo depletion of pDCs revealed that this subset was dispensable for either maintenance or regulation of the hepatic Th2 effector response during acute S. mansoni infection. Our data provides strong evidence that S. mansoni infection favours the establishment of an immunogenic, rather than tolerogenic, liver microenvironment that conditions cDCs to initiate and maintain Th2 immunity in the context of ongoing Ag exposure.
Introduction
Dendritic cells (DCs) are a heterogeneous population of pathogen-sensing APCs that play a central role in the initiation of immune responses and the polarization of CD4+ T cells.1, 2 Current understanding of the process of DC activation and function is heavily biased towards studies using model Ags or components of pathogens such as bacteria, viruses or protozoan parasites that typically induce T helper (Th) 1/Th17 responses, while the interaction between DCs and Th2-inducing organisms remains less well defined.3-5
Helminth parasites are the most potent natural inducers of Th2 immune responses and murine infection with Schistosoma mansoni is a well-characterized experimental model for studying the development of Th2 immunity in vivo.4, 6 During S. mansoni infection, development of the Th2 response coincides with the onset of egg production by female parasites living in the portal vasculature.7 S. mansoni eggs are metabolically active and highly immunogenic and, while many successfully exit the host by traversing the lumen of the gastrointestinal tract, some eggs are carried by the blood flow into the liver, where they become trapped in the sinusoids and induce inflammation and granuloma formation.4, 8 The liver is therefore one of the main sites of Ag exposure during S. mansoni infection and the Th2-dominated granuloma response, which serves to protect hepatocytes from toxins released by tissue-trapped eggs, is thought to be essential for host survival.9-11 Development of the egg-specific Th2 response begins four to six weeks after infection, with the peak of the ‘acute’ response occurring around week eight, before a combination of regulatory mechanisms and T cell exhaustion combine to dampen down the response during the ‘chronic’ stage from approximately week 12 onwards.4, 7, 12, 13 Although Th2 responses are protective during the initial stages of acute schistosomiasis, prolonged production of IL-4/IL-13 contributes to liver inflammation, fibrosis and immunopathology during chronic infection, and host survival is dependent on mounting a balanced T helper response.4, 14
Tissue-resident DCs can be broadly divided into conventional DC (cDC) and plasmacytoid DC (pDC) populations based on differential use of transcription factors for development, expression of various cell surface markers, and their responses to pathogen molecules.1, 15 While cDCs are highly efficient at priming naïve T cell responses, pDCs are best known for their ability to rapidly produce large amounts of type I interferons in response to viruses, bacteria and certain TLR agonists.16 In the steady-state, the liver is considered an immunosuppressive or tolerogenic microenvironment, and liver-resident DCs have been reported to express lower basal levels of MHC class II and costimulatory molecules than their splenic DC counterparts.17 Importantly, the liver is highly enriched with pDCs, which, in their non-activated state, appear to be immunoregulatory, functioning to suppress immune responses and mediate oral tolerance in vivo.18 Furthermore, pDCs have also been shown to play an anti-inflammatory role in Th2-mediated experimental models of airway inflammation and asthma.19 In this context, pDCs were recruited to the lungs of allergen-challenged mice and their selective depletion enhanced Th2 cytokine production in the draining lymph nodes and exacerbated the degree of immunopathology.19 Whether pDCs also function to down-modulate immune responses in a Th2-infection setting is not known.
We have previously shown that CD11c+ DCs are the key initiators of Th2 immune responses in the liver during acute S. mansoni infection.20 Global depletion of cDC and pDC populations during the priming stage of the Th2 response against parasite eggs (weeks four to six post-infection) dramatically impaired CD4+ T cell production of IL-4, IL-13 and IL-10, but had little effect on the Th1 cytokine IFN-γ.20 While this work established the fundamental importance of DCs in orchestrating Th2 development against S. mansoni infection in vivo, it did not address how hepatic cDCs and pDCs respond to egg Ags or how the liver microenvironment might influence the function of DC populations in terms of their capacity to present Ag to CD4+ T cells. A limited analysis of the activation phenotype of DCs over the course of S. mansoni infection in vivo showed that CD11c+MHC class II+ cells isolated from the spleen displayed only minor up-regulation of expression of conventional activation markers, even at the peak of the Th2 response.21 However, this study did not separate DC populations into cDC and pDC subsets or include a comparison of hepatic DCs isolated directly from the liver effector site.
Here we have characterized the activation status of cDCs and pDCs isolated from the liver during acute S. mansoni infection, both in terms of their numbers and activation state, as well as their ability to present Ag to naïve or effector/memory CD4+ T cells. Our results demonstrate that acute S. mansoni infection is associated with the recruitment of both DC populations to the liver effector site and dramatic transcriptional changes to the liver microenvironment. Importantly, hepatic cDCs displayed increased CD40 expression during S. mansoni infection and were capable of priming naive CD4+ T cell responses ex vivo, suggesting that they are the major DCs responsible for Th2 induction. In contrast, although pDCs isolated from the livers of S. mansoni-infected mice also up-regulated surface markers associated with Ag presentation they were unable to support the proliferation of either naïve or effector/memory CD4+ T cells. Notably, depletion of pDCs during murine S. mansoni infection did not significantly impact hepatic Th2 responses, demonstrating that pDCs neither promote nor suppress Th2 immunity in the effector site. Together, these data extend our previous work20 and indicate that cDCs are likely to be the critical cell population for Th2 effector cell development, function and maintenance during S. mansoni infection.
Results
S. mansoni infection is associated with increased numbers of DCs in the liver effector site and dramatic changes in gene expression
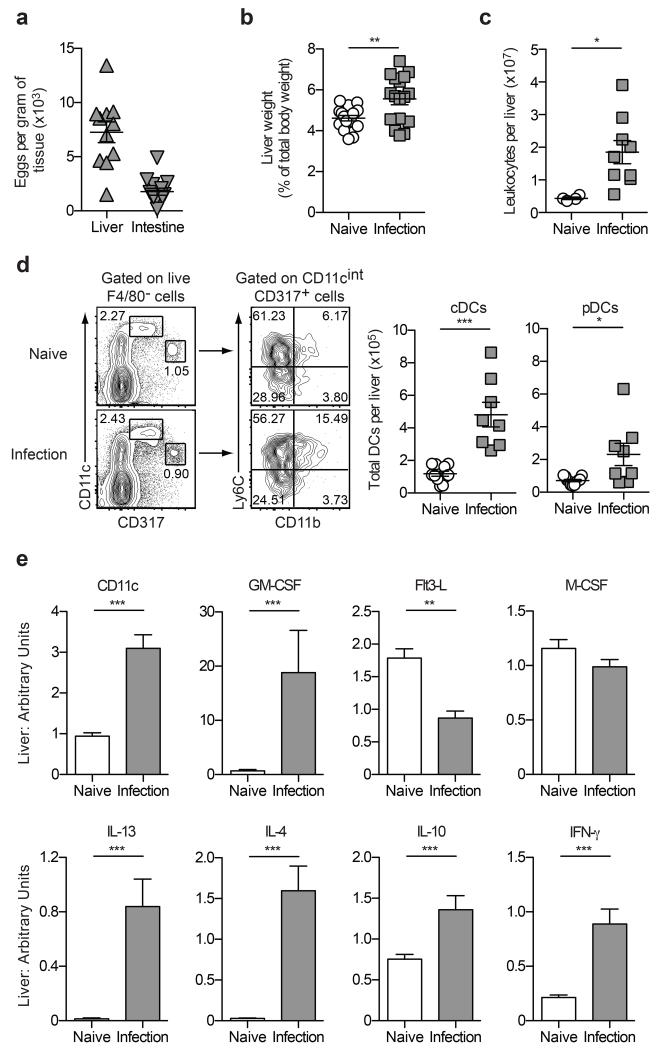
The liver is one of the principal effector sites during S. mansoni infection, with parasite eggs that are carried there by the blood flow becoming trapped in the sinusoids and forming the foci of immune-mediated granulomas.4 To investigate the impact of S. mansoni infection on hepatic DC populations during the acute phase of disease, livers were harvested from mice six weeks after infection for enumeration of DC numbers and characterization of gene expression in the liver microenvironment. This time point was selected based on our previous work, which demonstrated that CD11c+ DCs were critical for Th2 induction at weeks four to six post-infection.20 As expected, S. mansoni eggs were present in both livers and intestines of infected mice, confirming the successful establishment of infection (Figure 1a). Furthermore, acute S. mansoni infection was associated with hepatomegaly, defined as an increase in liver weight as a proportion of overall body weight (Figure 1b), and an increase in the total number of leukocytes present in the liver (Figure 1c). To quantify the total number of DCs in the liver, the number of viable leukocytes was multiplied by the percentage of cDCs (F4/80−CD11chighCD317−) and pDCs (F4/80−CD11cintCD317+Ly6ChighCD11b−) as determined by flow cytometry (Figure 1d). Importantly, while there were no significant differences between the proportion of cDCs (naïve 2.31% ± 0.34 vs infected 2.14% ± 0.38; P = 0.7532) and pDCs (naïve 1.21% ± 0.13 vs infected 0.75% ± 0.20; P = 0.0585) in the liver preparations, there was a significant increase in the total number of both populations (Figure 1d), indicating that DCs are recruited to, or differentiate within, this major effector site during acute S. mansoni infection.
Figure 1. The liver is a major effector site during S. mansoni infection.
(a) The total number of S. mansoni eggs per gram of liver or intestine tissue isolated from mice infected with S. mansoni for six weeks. (b) Liver weights of naïve and infected mice represented as a proportion of total body weight. (c) The total number of leukocytes isolated from the livers of naïve and infected mice. (d) Gating strategy to identify DC populations and quantification of the total number of cDCs and pDCs in the livers of naïve and infected mice. The cDC population was defined as F4/80−CD11chighCD317− cells, while the pDC population was defined as F4/80−CD11cintCD317+Ly6C+CD11b− cells. (e) Quantitative RT-PCR was used to measure mRNA transcripts in whole liver tissue from naïve and infected mice. Data are expressed relative to the housekeeping gene Ubiquitin. Data are pooled from two experiments. Error bars indicate mean ± SEM.
To characterize gene expression in the liver microenvironment at this stage of the response to S. mansoni infection, RNA was purified from whole liver tissue and quantitative RT-PCR was performed to determine transcript levels of key DC and macrophage growth factors and cytokines that drive regulatory/Th1/Th2 immune responses.6, 22 Consistent with the increased number of DCs in the liver at week six post-infection (Figure 1d), gene transcripts for both CD11c and granulocyte/macrophage colony-stimulating factor (GM-CSF) were significantly up-regulated at this time point (Figure 1e), indicating that the liver microenvironment favours the differentiation and proliferation of myeloid-lineage cells23 during establishment of the Th2 response. In contrast, acute S. mansoni infection was associated with a significant down-regulation in transcription of FMS-related tyrosine kinase 3 ligand (Flt3-L), a cytokine that mobilises various DC subsets in vivo and promotes expansion of both cDC and pDC subsets from progenitor cells24-26 (Figure 1e). Importantly, macrophage colony-stimulating factor (M-CSF) gene expression was not altered during acute S. mansoni infection (Figure 1e), consistent with its role in promoting the differentiation and survival of macrophage populations that further the development of liver fibrosis during the later stages of disease.27 Acute S. mansoni infection also had dramatic effects on the Th2 and regulatory cytokine milieu in the liver, with elevated transcription of genes encoding IL-4, IL-13 and IL-10 detected at week six post-infection, as expected (Figure 1e). Similarly, expression of IFN-γ was also elevated in infected livers (Figure 1e), consistent with the characteristic mixed Th1/Th2 immune response induced against the parasites at this time point.4
Hepatic DCs respond to S. mansoni by up-regulating surface markers associated with Ag presentation
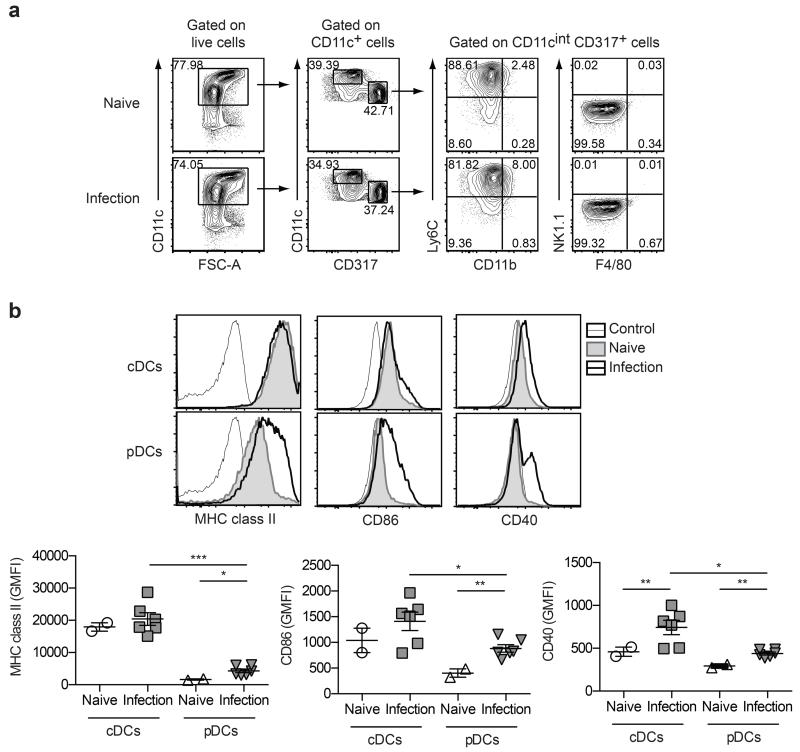
To examine the activation phenotype of the DC subsets recruited to the liver at week six post-infection, CD11c+ DCs were enriched from liver leukocyte preparations, subdivided into cDC and pDC populations based on surface markers CD11c, CD317 (PDCA-1/BST-2), Ly6C, CD11b, NK1.1 and F4/80 (Figure 2a), and then assessed for expression of MHC class II and co-stimulatory molecules CD86 and CD40 by flow cytometry (Figure 2b). While hepatic cDCs did not up-regulate MHC class II or CD86 in response to infection, surface levels of these markers were significantly increased on hepatic pDCs isolated from S. mansoni-infected mice compared to naïve mice (Figure 2b). In contrast, CD40 expression was up-regulated on both cDCs and pDCs isolated from infected livers (Figure 2b), consistent with a requirement for CD40:CD154 interaction for Th2 induction in vivo.21, 28, 29 Importantly, overall marker expression remained significantly higher on cDCs, despite infection-induced activation of the pDC population (Figure 2b).
Figure 2. Hepatic DCs up-regulate markers associated with Ag presentation and T cell costimulation during S. mansoni infection.
(a) Gating strategy to define cDCs and pDCs enriched from the livers of naive mice and mice infected for six weeks with S. mansoni. Following depletion of lineage negative cells, cDCs were defined as CD11chighCD317− cells, while pDCs were defined as CD11cintCD317+Ly6C+CD11b−NK1.1−F4/80− cells. (b) The geometric mean fluorescence intensity (GMFI) values for MHC class II, CD86 and CD40 on cDCs and pDCs enriched from the livers of naïve or infected mice. Data are pooled from two experiments. Error bars indicate mean ± SEM.
As a comparison, DCs were also purified from the spleen and their activation phenotype examined at week six of S. mansoni infection. Splenic cDCs expressed significantly higher levels of CD40 during infection, however, consistent with previous work,21 MHC class II and CD86 expression remained unchanged compared to naïve controls (Supplementary Figure 1). It is likely that our more refined DC enrichment protocol, combined with further separation of CD11c+ cells into cDC and pDC subsets, accounted for our ability to detect the subtle but statistically significant changes in cDC CD40 expression at this time point during S. mansoni infection. MHC class II, CD86 and CD40 expression on splenic pDCs remained unchanged during S. mansoni infection, (Supplementary Figure 1), indicating that the liver is a major site of pDC activation in this infection model. Consistent with the liver data (Figure 2b), the overall levels of expression of these three surface markers were significantly higher on splenic cDCs compared to pDCs (Supplementary Figure 1). Together, these data demonstrate that DC populations have distinct activation profiles in response to S. mansoni depending on their anatomical location. Furthermore, the up-regulation of surface markers associated with Ag presentation and T cell costimulation on pDCs isolated from the liver during S. mansoni infection suggests that this population might play an immunogenic, rather than tolerogenic, role in Th2 infection settings.
Hepatic cDCs isolated from S. mansoni infection are highly efficient professional APCs
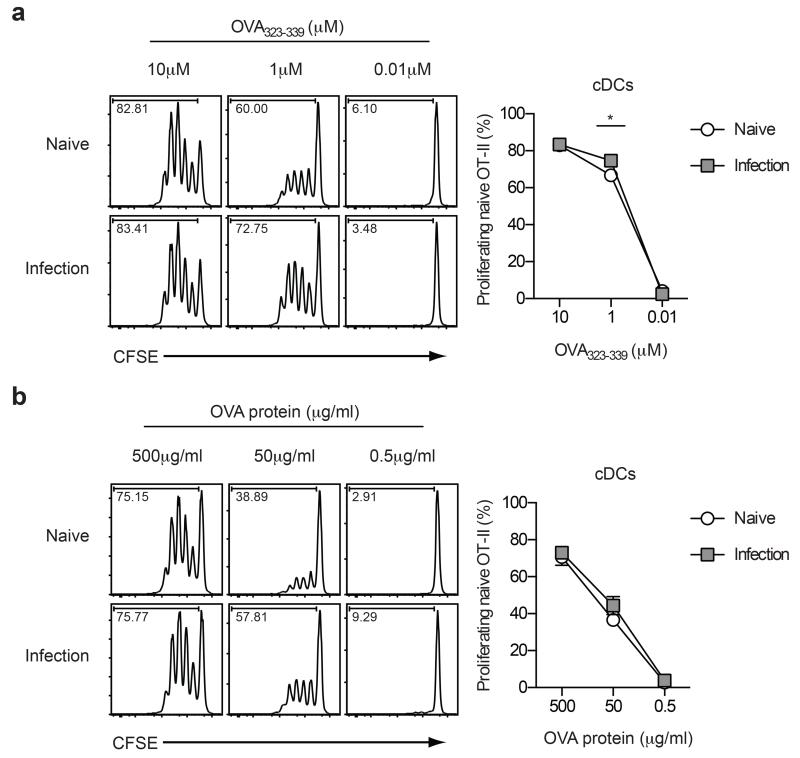
Since hepatic DCs up-regulated MHC and costimulatory molecules associated with Ag presentation during S. mansoni infection (Figure 2), we next investigated their ability to prime naïve CD4+ T cell responses ex vivo. To do this, DCs were purified from the livers of naïve or infected mice, FACS purified into cDC (CD11c+CD317−F4/80−NK1.1−) and pDC (CD11c+CD317+F4/80−NK1.1−) populations (>97% purity), and assessed for their ability to present Ags to naïve CD4+ T cells using a well-described transgenic CD4+ OT-II T cell co-culture system.30-32 This approach was necessary due to a lack of schistosome-specific TCR transgenic mice. Hepatic cDCs from either naïve or S. mansoni-infected mice were highly efficient at inducing naïve OT-II T cell proliferation in vitro in response to unrelated (third-party) peptide and protein Ags, indicating that, despite a relatively ‘muted’ activation phenotype,33-35 the Ag uptake, processing and presenting function of these cDCs was not compromised during S. mansoni infection (Figure 3). In contrast, hepatic pDCs from S. mansoni-infected mice were unable to induce proliferation of naïve OT-II T cells, even at high concentrations of OVA peptide or protein (Supplementary Figure 2a and 2b). This was likely due to the overall lower levels of surface expression of MHC class II and co-stimulatory molecules on pDCs compared to cDCs isolated from infected livers (Figure 2b), and is consistent with previous studies demonstrating that pDCs are poor initiators of naïve T cell proliferation in response to exogenous Ags.16, 30, 36-38
Figure 3. Hepatic cDCs isolated from S. mansoni-infected mice prime naïve CD4+ T cells.
Proliferation of naïve OT-II CD4+ T cells in response to presentation of OVA323-339 peptide (a) or soluble OVA protein (b) by cDCs isolated from the livers of naïve or infected mice (6 weeks post-infection). Data are pooled from three experiments. Error bars indicate mean ± SEM.
Hepatic cDCs isolated from S. mansoni infection support effector/memory CD4+ T cell responses
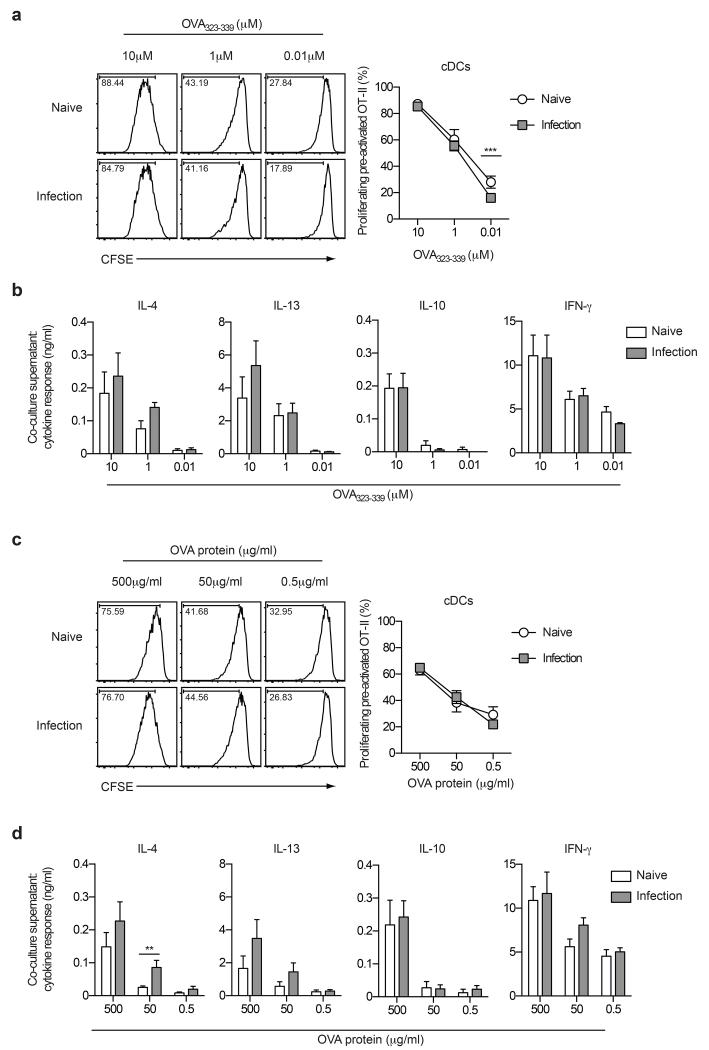
In addition to their unrivalled ability to activate naïve T cells, DCs can also function to direct and maintain effector T cell responses (1, 20). Since the liver acts as a major effector site wherein effector/memory CD4+ T cells are continuously exposed to schistosome eggs and egg Ags,4 we next investigated the ability of hepatic DCs to support effector/memory-like CD4+ T cell responses ex vivo. Purified cDCs and pDCs were sorted from naïve or S. mansoni-infected livers and assessed for their ability to present both peptide and protein Ags to pre-activated effector/memory-like OT-II cells in vitro. Once again, cDCs from S. mansoni-infected mice were fully functional APCs, able to support the expansion of pre-activated OT-II T cells as effectively as cDCs from naïve mice, in response to either peptide or protein Ags (Figure 4a and 4c). Furthermore, pre-activated OT-II cells produced equivalent amounts of IL-4, IL-13, IL-10 and IFN-γ after co-culture with OVA peptide or protein and cDCs isolated from the livers of either naïve or infected mice (Figures 4b and 4d). This indicates that S. mansoni infection does not alter the fundamental ability of hepatic cDCs to process and present Ag to support effector/memory CD4+ T cell responses. In contrast, hepatic pDCs from naïve or S. mansoni-infected mice activated the proliferation of only a small proportion of pre-activated OT-II cells, and only at the highest concentration of peptide tested (10μM) (Supplementary Figure 2c and 2d). Consequently, cytokine production in pDC co-cultures was dramatically reduced compared to that of cDC co-cultures (Supplementary Figure 2e and 2f). Interestingly, however, IL-13 production by pre-activated OT-II cells was significantly enhanced in the presence of pDCs isolated from S. mansoni-infected livers (Supplementary Figure 2e), suggesting that pDCs might play a supporting role in shaping some aspects of the Th2 response during infection.
Figure 4. Hepatic cDCs isolated from S. mansoni-infected mice support effector/memory CD4+ T cell proliferation and cytokine production.
Proliferation and cytokine profiles of pre-activated effector/memory OT-II CD4+ T cells in response to presentation of OVA323-339 peptide (a and b) or soluble OVA protein (c and d) by cDCs isolated from the livers of naïve or infected mice (6 weeks post-infection). Data are pooled from three experiments. Error bars indicate mean ± SEM.
The impact of in vivo pDC depletion on development of Th2 immunity against S. mansoni
To determine whether pDCs influence any aspect of Th2 development in vivo during active S. mansoni infection, we specifically depleted this DC population using two well-established approaches. Firstly, B6 mice were treated with the pDC-depleting mAb 120G8 every 48 hours from day 28 (week four) to day 42 (week six) after S. mansoni infection. Although this treatment regime successfully depleted 60-70% of pDCs from the spleen (Supplementary Figure 3a), as previously reported,39 it was much less effective at depleting pDCs from the liver, where 120G8-treated mice displayed only a 15-20% reduction in hepatic pDCs compared to isotype control-treated mice (Supplementary Figure 3b). Analysis of schistosome-specific (SEA) recall responses revealed that this level of pDC depletion had no impact on the Th2 and regulatory cytokine response by cultured leukocytes from the liver (Supplementary Figure 3c; similar results were obtained for IFN-γ (data not shown)). Intracellular cytokine staining further confirmed that the ability of hepatic CD4+ T cells to produce IL-4, IL-13 or IL-10 in response to PMA and ionomycin stimulation was not altered in 120G8-treated mice (Supplementary Figure 3d).
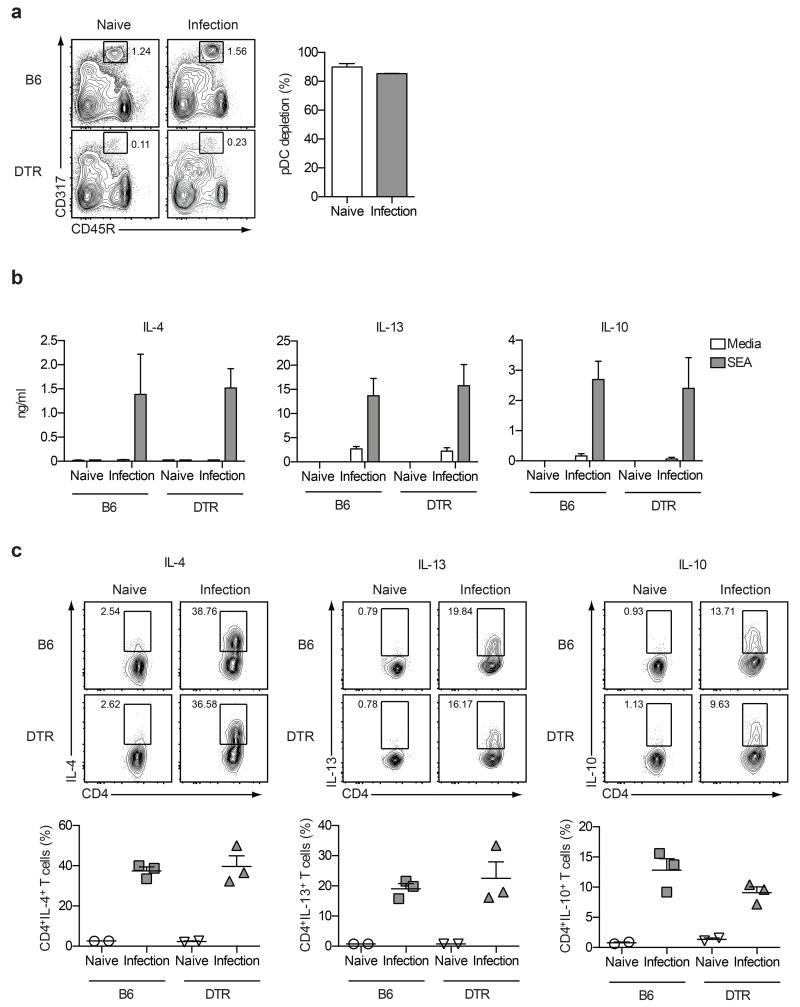
Due to the inefficiency of the 120G8 mAb depletion strategy in the liver effector site (Supplementary Figure 3b), we next used an alternative approach to deplete pDCs using BDCA2-DTR transgenic mice, which express the diphtheria toxin receptor (DTR) under the control of the human pDC gene promoter, blood dendritic cell Ag 2 (BDCA-2).40 Administration of DT every 48 hours from day 32 to day 40 after S. mansoni infection significantly depleted pDCs from the livers of naïve and S. mansoni-infected BDCA2-DTR transgenic mice, with >80% efficacy (Figure 5a). Importantly, DT treatment was highly specific for pDCs and had no measurable impact on cDCs, Ly6Chi monocytes or F4/80+ macrophage populations (Supplementary Figure 4). Additionally, pDC depletion did not affect overall egg burdens, the total number of leukocytes, or CD4+CD25+Foxp3+ regulatory T cells present in the liver (data not shown). Analysis of S. mansoni-specific recall responses showed that the high level of pDC depletion achieved in the BDCA2-DTR transgenic mice had no significant impact on the Th2 cytokine response by cultured liver leukocytes (Figure 5b; similar data were obtained for IFN-γ (data not shown)). These results were confirmed using intracellular cytokine staining to directly assess ex vivo cytokine production by hepatic CD4+ T cells (Figure 5c and data not shown for IFN-γ). Together, these data demonstrate that pDCs do not play a major role in promoting, sustaining or regulating Th2 immunity in the liver between weeks four to six of S. mansoni infection.
Figure 5. pDC-depletion has no impact on Th2 responses in the liver.
(a) DT treatment effectively depleted the CD11cintCD317+CD45R+ pDC population from the livers of BDCA2-DTR mice, when administered every 48 hours from day 32 to day 40 post-infection. (b) Cells isolated from the livers of naïve or infected DT-treated B6 or BDCA2-DTR mice were restimulated with medium alone or SEA and supernatants were analyzed by ELISA for schistosome egg-specific recall responses. (c) Intracellular cytokine staining was used to directly assess liver CD4+ T cell cytokine production. Data represent one of three experiments. Error bars indicate mean ± SEM.
Discussion
S. mansoni parasitic helminths are complex, multicellular pathogens that induce a Th2 immune profile characterized by production of the cytokines IL-4, IL-5, IL-13 and IL-10 by CD4+ T cells. While the precise molecular events and signalling pathways leading to Th2 cell differentiation in vivo remain poorly defined, we and others have demonstrated that the initiation of adaptive Th2 responses against helminths or allergens is dependent on and driven by CD11c+ DCs.20, 41-43 However, much of our knowledge of the interaction between Th2-inducing pathogens such as S. mansoni and DCs is derived from studies using bone marrow-derived DCs generated in vitro, which capably induce Th2 responses despite displaying a non-classical maturation phenotype following exposure to schistosome Ags.33-35 In the present study, we have examined the direct impact of acute S. mansoni infection on the two major functional classes of CD11c+ DCs found in the liver effector site in vivo. We have conducted a comprehensive assessment of the liver microenvironment at week six post-infection, including transcript levels of known DC growth factors and Th1/Th2/regulatory cytokines, enumeration of cDC and pDC numbers, evaluation of their activation states and – most importantly – functional analysis of their ability to act as professional APCs ex vivo and in vivo. Our data suggest that, while the liver is a site of activation of both cDCs and pDCs, it is hepatic cDCs that are likely to play a key role in CD4+ T cell activation and Th2 immunity during acute S. mansoni infection.
At approximately six weeks post S. mansoni infection, mature female worms living in the portal vasculature are releasing eggs, the soluble Ags of which are highly immunogenic and promote Th2 responses.4, 7 Eggs that become trapped in the liver sinusoids cause dramatic changes to the overall liver microenvironment, including the development of granulomas, composed of CD4+ T cells, macrophages, eosinophils and CD11c+ DCs, around the individual eggs.4, 11 In our study, S. mansoni infection was associated with hepatomegaly and an increase in the total number of leukocytes in the liver (Figure 1b and c). As a result of this enhanced immune response, overall numbers of both cDCs and pDCs were also increased in the liver effector site at six weeks post-infection (Figure 1d), indicating expansion or recruitment of these APCs in response to S. mansoni egg Ags. This is likely driven by elevated expression of the growth factor GM-CSF (Figure 1e), which has a known role in recruitment, development and homeostasis of non-lymphoid tissue DCs.41, 44, 45
This is the first study to characterize the activation phenotype of cDC and pDC populations isolated directly ex vivo from the liver during S. mansoni infection. Our data demonstrate that hepatic DC populations display distinct activation profiles following exposure to S. mansoni parasites; while cDCs exhibited increased levels of the costimulatory molecule CD40 during establishment of the Th2 response, pDCs responded to S. mansoni infection by up-regulating surface expression of MHC class II, CD40 and CD86 (Figure 2b). Interestingly, pDCs from the spleen displayed more limited phenotypic activation at the six week time point (Supplementary Figure 1a), indicating that the liver is a major site of pDC activation at this acute stage of S. mansoni infection. Importantly, our data demonstrating that MHC class II expression was not significantly up-regulated on the surface of hepatic or splenic cDCs in response to S. mansoni parasites is consistent with previous work examining the activation status of splenic CD11c+MHC II+ cells over the course of S. mansoni infection.21 Although Straw et al.21 also reported no significant changes in CD40 expression at week six post-infection, our refined DC enrichment protocol and extensive panel of cell surface markers to further subdivide the CD11c+ DC populations revealed a significant increase in CD40 expression on both hepatic and splenic cDCs (Figure 2b and Supplementary Figure 1a). Since the focus of our study was to address the role of hepatic DCs in the early phase of the Th2 immune response to S. mansoni parasites, we concentrated only on the week six time point. However, it is highly likely that DC populations in the liver and spleen will undergo dynamic changes as the infection progresses from acute to chronic stage.
A central role for hepatic cDCs in initiating Th2 immune responses during S. mansoni infection was first supported by our ex vivo studies in which hepatic cDCs, but not pDCs, isolated from infected mice were found to be highly effective professional APCs, efficient at processing and presenting Ag to both naïve and effector/memory CD4+ T cells (Figures 3 and 4). In addition to priming naïve T cells and promoting the expansion of effector/memory T cells, cDCs from S. mansoni-infected livers also effectively supported Th1/Th2 cytokine production by ‘unpolarized’ effector/memory T cells (Figure 4). These data indicate that S. mansoni does not compromise the uptake, processing or presentation of Ag by hepatic cDCs, or their ability to promote adaptive immune responses, during acute infection. Since helminth infections are generally chronic, further studies are required to determine whether soluble proteins released from schistosome eggs trapped in the liver have long-term immunomodulatory effects on the ability of hepatic cDCs to initiate and maintain adaptive immune responses. This will be particularly important for understanding the potential negative impact of helminths on immune responses to vaccines and other major pathogens that coexist in schistosome endemic areas.46
In comparison to hepatic cDCs, pDCs from S. mansoni-infected livers displayed significantly lower absolute levels of expression of MHC II and costimulatory molecules (Figure 2) and, in functional terms, this resulted in poor naïve T cell stimulatory capacity ex vivo (Supplementary Figure 2a and 2b). This can been attributed to the continuous synthesis of MHC II molecules and turnover of MHC II-peptide complexes in activated pDCs, which continues long after activation, rendering this DC population inefficient in the presentation of exogenous Ags but still capable of presenting intracellular Ags in their activated state.38 Our results are also consistent with published studies demonstrating the poor ability of ex vivo-isolated pDCs to present Ags to naïve CD4+ T cells in the context of both Th1 and allergic Th2 immune responses.16, 30, 36-38 Intriguingly, heptic pDCs isolated from naïve or infected mice were equally poor at supporting the expansion of effector/memory CD4+ T cells (Supplementary Figure 2c and 2d), which respond to lower doses of Ag and are less dependent on DC costimulation than naïve CD4+ T cells.47 However, pDCs from S. mansoni-infected mice showed increased ability to support low level IL-13 production by effector/memory OT-II T cells at the highest pDC:OVA323-339 peptide tested (Supplementary Figure 2e). These data imply that schistosome infection may confer on hepatic pDCs a limited ability to support some key facets of Th2 functionality.
Although our ex vivo sorting experiments strongly suggested that hepatic pDCs from schistosome infection did not play a dominant role in antigen processing and presentation to CD4+ T cells, it was important to investigate the contribution of this DC population to the development of Th2 immunity during active infection in vivo. Schistosome-specific Th2 recall responses in the liver were neither reduced nor enhanced in pDC-depleted mice compared to controls (Figure 5). These novel data build upon our previous work showing that global depletion of both cDCs and pDCs dramatically impairs the hepatic Th2 response during murine schistosome infection,20 strongly suggesting that cDCs rather than pDCs are likely to be the major APCs responsible for Th2 induction during S. mansoni infection. Our data are in agreement with a study demonstrating that depletion of lung pDCs had no impact on the priming of naïve CD4+ T cells in an allergic Th2 model of mouse asthma.36 In that setting, where pDCs failed to induce T cell division, they functioned to down-regulate the immune response by suppressing the generation of effector T cells.36 In contrast, in our studies we found no evidence that pDCs from S. mansoni infected livers inhibited the Th2 activating ability of cDCs: firstly, in our DC:OT-II T cell co-culture experiments, CD4+ T cell proliferation was equally efficient in the presence of cDCs alone or following re-addition of pDCs (1:1 ratio; data not shown); secondly, Th2 cytokines were not elevated following pDC depletion of schistosome infected mice (Figure 5). However, we cannot rule out the possibility that pDCs may develop tolerogenic capacity at later stages of S. mansoni infection.
It remains to be determined precisely which cDC subtype(s) are responsible for Th2 initiation during S. mansoni infection. Two recent studies have identified a specialised subset of CD11b+ cDCs that promote Th2 differentiation in the lung (in response to innocuous allergens) or in the skin-draining lymph nodes (in response to the parasitic helminth Nippostrongylus brasiliensis; Nb).48, 49 In these studies, Th2 induction was dependent upon DC-specific expression of the transcription factor interferon regulatory factor 4 (IRF4),48, 49 and further investigation is now required to assess whether S. mansoni-specific Th2 responses are also mediated by an IRF4-dependent population of hepatic cDCs. Connor et al.50 have also recently demonstrated that CD11c+ MHC class II+ DCs from the skin-draining lymph nodes of Nb-treated mice up-regulated expression of IFR4, programmed death ligand 2 and CD301b, and acquired the ability to prime IL-4 responses in vivo without the cooperation of additional cell populations. This data supports the notion that DCs exposed to the appropriate parasite-conditioned environment express all of the signals required to instruct Th2 differentiation.50
In conclusion, this is the first comprehensive study of the activation phenotype and function of hepatic DC populations during infection with a Th2-inducing pathogen. Our data suggest that, despite a low level of phenotypic activation, cDCs are capable of stimulating naïve and effector/memory CD4+ T cell responses and supporting hepatic Th2 immunity during acute S. mansoni infection. Furthermore, our results demonstrate that pDCs neither promote nor regulate hepatic CD4+ T cell responses at this stage of infection. We propose that the liver microenvironment conditions recruited and/or resident cDCs to support the induction and maintenance of both naïve and effector Th2 responses. This would ensure that effective Th2 immunity is generated in the face of persistent and ongoing Ag exposure, which is critical for host survival against this chronic helminth infection.
Methods
Animals, infections and immunizations
C57BL/6 (B6) mice, and B6 background transgenic OT-II and BDCA2-DTR40 mice, were bred and maintained under specific-pathogen-free conditions at the University of Edinburgh, U.K. Experimental mice were infected percutaneously with ~80 S. mansoni cercariae from Biomphalaria glabrata snails. For the first pDC depletion strategy, B6 mice were injected i.p. every 48 h from day 28 to 40 with 200 μg 120G8 or IgG1 control mAbs (courtesy of L. Boon, Bioceros B.V., Utrecht, The Netherlands). For the second pDC depletion strategy, BDCA2-DTR mice were injected i.p. every 48 h from day 32 to 40 with 8 ng/g diphtheria toxin (DT; Sigma Aldrich, St Louis, MO, USA) in PBS. Endotoxin-free soluble egg Ag (SEA) was prepared in-house from S. mansoni eggs harvested from the livers of infected B6 mice as previously described.33 All experiments were approved by Project Licences granted by the Home Office (U.K.) and were conducted in accordance with local guidelines.
S. mansoni egg counts
Livers and intestines from infected mice were digested in 4% potassium hydroxide (15 ml/gram of liver tissue; 7.5 ml/gram of intestine tissue) at 37°C overnight. 100 μl aliquots of the digests were evaluated on gridded petri dishes and the eggs counted at 10x magnification. Each digest was examined in triplicate and the mean results were used to extrapolate the total number of eggs/gram of tissue.
RNA isolation and RT-PCR
Total RNA from liver tissue was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and the RNeasy Mini Kit (Qiagen, Venlo, Limburg, The Netherlands). RNA was translated into cDNA using Superscript III Reverse Transcriptase and Oligo (dT) (Invitrogen). Quantitative RT-PCR was performed using a Light Cycler 480 II Real-Time PCR machine (Roche, Nutley, NJ, USA) and LightCycler-DNA master SYBR Green I (Roche). The relative amounts of mRNA for genes of interest were normalized to Ubiquitin. The following primers were used: Ubiquitin, 5’-TGGCTATTAATTATTCGGTCTGCAT-3’, 5’-GCAAGTGGCTAGAGTGCAGAGTAA-3’; CD11c, 5’-ATGGAGCCTCAAGACAGGAC-3′, 5’-GGATCTGGGATGCTGAAATC-3’; GM-CSF, 5’-GCATGTAGAGGCCATCAAAGA-3’, 5’-CGGGTCTGCACACATGTTA-3’; Flt3-L, 5’-CCTAGGATGCGAGCCTTGT-3’, 5’-TGTTTTGGTTCCCAACTCG-3’; M-CSF, 5’-CAACAGCTTTGCTAAGTGCTCTA-3’, 5’-CACTGCTAGGGGTGGCTTTA-3’; IL-10, 5’-CAGAGCCACATGCTCCTAGA-3’, 5’-TGTCCAGCTGGTCCTTTGTT-3’; IL-4, 5’-GAGAGATCATCGGCATTTTGA-3’, 5’-TCTGTGGTGTTCTTCGTTGC-3’; IL-13, 5’-CCTCTGACCCTTAAGGAGCTTAT-3’, 5’-CCTCTGACCCTTAAGGAGCTTAT-3’; IFN-γ, 5’-GGAGGAACTGGCAAAAGGAT-3’, 5’-TTCAAGACTTCAAAGAGTCTGAGG-3’.
Ex vivo DC enrichment and flow cytometric sorting
Spleen and liver tissues were harvested from citrate saline buffer-perfused mice and digested at 37°C (with tilting and shaking) for 20 min (spleen) or 45 min (liver) with 0.4 U/ml Liberase CI (Roche) and 80 U/ml DNase I type IV (Sigma-Aldrich). Single cell suspensions were then prepared by mechanically disrupting the organs through a 70 μm (spleen) or 100 μm (liver) filter. Low-density cells were enriched from the spleen using NycoPrep™ (1.077 g/ml; Axis-Shield, Oslo, Norway). Liver leukocytes were isolated by centrifugation in 33% Percoll (GE Healthcare, Piscataway, NJ, USA), followed by filtration through a 40 μm cell strainer to remove contaminating S. mansoni eggs before RBC lysis. For characterization of phenotypic activation and DC sorting, non-DC lineage cells were then coated with biotinylated mAbs against murine CD2, CD3ε, CD49b, mIgM and erythrocytes (Ter-119), and depleted using MyOne Streptavidin Dynabeads (Dynabeads Mouse DC Enrichment Kit; Invitrogen). Dead cells were excluded by staining with LIVE/DEAD® Fixable Aqua Dead Cell Stain (Invitrogen). After FcR-block (2.4G2), cells were surface stained with combinations of the following mAbs: F4/80, NK1.1, CD11c, CD317 (PDCA-1/BST-2), Ly6C, CD11b, MHC class II, CD86 and CD40. Live, non-doublet, F4/80−NK1.1− cells that were CD11chighCD317− were gated as cDCs, while pDCs were defined as CD11cintermediate(int)CD317+Ly6ChighCD11b− cells. For CD4+ T cell co-culture experiments, hepatic cDC and pDC populations were sorted from DC-enriched preparations of livers that had been rested overnight at 4°C, using a BD FACS Aria (San Jose, CA, USA). All antibodies for flow cytometry were purchased from BD Biosciences (San Jose, CA, USA), eBioscience (San Diego, CA, USA), Biolegend (San Diego, CA, USA) or Miltenyi Biotech (Bergisch Gladbach, Germany). Samples were acquired on FACS Canto II or LSR flow cytometers using BD FACS Diva Software and analyzed with FlowJo (Tree Star Inc., Ashland, OR, USA).
Restimulation assays and intracellular cytokine staining
Single cell suspensions of liver leukocytes (1 × 106 cells/ml) were cultured in X-vivo 15 medium (Lonza, Walkersville, MD, USA) containing 2 mM L-Glutamine and 50 μM 2-ME (Invitrogen) in 96-well plates at 37°C 5% CO2 with or without 15 μg/ml SEA. After 72 h, supernatants were harvested and analyzed for IL-4, IL-13, IL-10 and IFN-γ using paired capture and detection Abs (produced from hybridomas in-house or purchased from R&D Systems (Minneapolis, MN, USA), BD Biosciences, or eBioscience) and recombinant cytokine standards (Peprotech (Rocky Hill, NJ, USA) or BD Biosciences). For intracellular cytokine staining of liver leukocytes, cells were rested overnight at 4°C and then stimulated with 10 ng/ml PMA and 1 μg/ml Ionomycin (Sigma-Aldrich) for 2 h, followed by treatment with Golgi stop (BD Biosciences) for an additional 3 h. After FcR-block, cells were surface stained with mAbs against CD3 or TCR-β and CD4, fixed with 1% PFA, permeabilized with Perm/Wash buffer (BD Biosciences), and then stained intracellularly with anti-IL-4, anti-IL-13 and anti-IL-10. Identification of cytokine-positive cells was determined using appropriate isotype and Fluorescence Minus One (FMO) controls (data not shown).
Ag presentation assays
Naïve OT-II CD4+ T cells were purified using Dynal Mouse CD4 Cell Negative Isolation Kit (>80% purity) (Invitrogen). Pre-activated ‘effector/memory’ OT-II CD4+ T cells (>90% purity) were generated by culturing OT-II spleen cells in RPMI containing 10% FCS, 200 mM L-Glutamine, 50 μM 2-ME and 1 mg/ml OVA protein (Sigma-Aldrich) at 37°C 5% CO2 for 7 days. On days three and five, cultures were supplemented with 10 ng/ml IL-7, 10 ng/ml IL-15 and 2 ng/ml IL-2 (Peprotech). Cells were examined by flow cytometry for an effector/memory-like phenotype by surface staining with mAbs against CD4, Vα2, CD44 and CD69 (data not shown). For CFSE labelling, purified OT-II CD4+ T cells were labelled with 5 μM CFSE for 15 min at 37°C, and washed three times before use. For presentation of OVA323-339 (OT-II) peptide, FACS sorted liver DC populations were incubated for 45 min with various concentrations of OVA323-339, washed and then 5,000 cDCs or pDCs were co-cultured with 50,000 naïve or pre-activated CFSE-labelled OT-II cells in 96-well V-bottom plates. For presentation of soluble OVA protein, 5,000 cDCs or pDCs were co-cultured with 50,000 naïve or pre-activated CFSE-labelled OT-II cells in the presence of various concentrations of endotoxin-depleted soluble OVA protein in 96-well V-bottom plates. After culture for 60-65 h at 37°C 5% CO2, supernatants were harvested for analysis of cytokine production by ELISA and OT-II cells were surface stained with anti-CD4 and anti-Vα2 for analysis of proliferation by flow cytometry.
Statistical analysis
Statistical analysis was performed using a two-tailed Student’s t test or one-way ANOVA with Bonferroni post hoc test in Prism (GraphPad Software, Inc., La Jolla, CA, USA). For the ex vivo Ag presentation assays, analysis of pooled data from experimental repeats conducted on different days was carried out using a mixed model analysis, labelling the day of the experiment as a random factor (JMP statistical analysis software 11.1.1; SAS Institute Inc., Cary, NC, USA). Differences between groups were determined by ANOVA followed by a Tukey-Kramer HSD multiple comparison test. Asterisks denote statistically significant differences (*, P<0.05; **, P<0.01; ***, P<0.001).
Supplementary Material
Acknowledgements
The authors thank R. Rajan for technical support, the staff of the University of Edinburgh animal facility for animal husbandry, and M. Waterfall for assistance with flow cytometry and cell sorting. S. mansoni infected snails were supplied by the National Institute of Allergy and Infectious Diseases (NIAID) Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD) through Contract N01-AI-30026. This work was supported by the Medical Research Council (MRC) U.K. (G0701437 to ASM) and the Wellcome Trust (L.H.J). RJL is a recipient of a National Health and Medical Research Council of Australia (NHMRC) Early Career Fellowship. The authors gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program, received by the Burnet Institute.
Footnotes
Conflict of Interest
The authors declare no financial or commercial conflicts of interest. The MCCIR is a joint venture between the University of Manchester, AstraZeneka and GSK.
Supplementary information is available at Immunology & Cell Biology’s website.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 5.Perona-Wright G, Jenkins SJ, MacDonald AS. Dendritic cell activation and function in response to Schistosoma mansoni. Int J Parasitol. 2006;36:711–721. doi: 10.1016/j.ijpara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Pearce EJ, C MK, Sun J, J JT, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 7.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunne DW, Hassounah O, Musallam R, Lucas S, Pepys MB, Baltz M, et al. Mechanisms of Schistosoma mansoni egg excretion: parasitological observations in immunosuppressed mice reconstituted with immune serum. Parasite Immunol. 1983;5:47–60. doi: 10.1111/j.1365-3024.1983.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465–466. doi: 10.1016/s0167-5699(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 10.Doenhoff MJ, Pearson S, Dunne DW, Bickle Q, Lucas S, Bain J, et al. Immunological control of hepatotoxicity and parasite egg excretion in Schistosoma mansoni infections: stage specificity of the reactivity of immune serum in T-cell deprived mice. Trans R Soc Trop Med Hyg. 1981;75:41–53. doi: 10.1016/0035-9203(81)90012-2. [DOI] [PubMed] [Google Scholar]
- 11.Dunne DW, Jones FM, Doenhoff MJ. The purification, characterization, serological activity and hepatotoxic properties of two cationic glycoproteins (alpha 1 and omega 1) from Schistosoma mansoni eggs. Parasitology. 1991;103(Pt 2):225–236. doi: 10.1017/s0031182000059503. [DOI] [PubMed] [Google Scholar]
- 12.Caserta S, Nausch N, Sawtell A, Drummond R, Barr T, Macdonald AS, et al. Chronic infection drives expression of the inhibitory receptor CD200R, and its ligand CD200, by mouse and human CD4 T cells. PLoS One. 2012;7:e35466. doi: 10.1371/journal.pone.0035466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J Clin Invest. 2009;119:1019–1028. doi: 10.1172/JCI36534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 15.Colonna M, Pulendran B, Iwasaki A. Dendritic cells at the host-pathogen interface. Nat Immunol. 2006;7:117–120. doi: 10.1038/ni0206-117. [DOI] [PubMed] [Google Scholar]
- 16.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 17.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 18.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol. 2009;183:1074–1082. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 20.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straw AD, MacDonald AS, Denkers EY, Pearce EJ. CD154 plays a central role in regulating dendritic cell activation during infections that induce Th1 or Th2 responses. J Immunol. 2003;170:727–734. doi: 10.4049/jimmunol.170.2.727. [DOI] [PubMed] [Google Scholar]
- 22.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- 25.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, et al. Cutting edge: generation of splenic CD8+ and CD8-dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 27.Barron L, Wynn TA. Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur J Immunol. 2011;41:2509–2514. doi: 10.1002/eji.201141869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald AS, Patton EA, La Flamme AC, Araujo MI, Huxtable CR, Bauman B, et al. Impaired Th2 development and increased mortality during Schistosoma mansoni infection in the absence of CD40/CD154 interaction. J Immunol. 2002;168:4643–4649. doi: 10.4049/jimmunol.168.9.4643. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald AS, Straw AD, Dalton NM, Pearce EJ. Cutting edge: Th2 response induction by dendritic cells: a role for CD40. J Immunol. 2002;168:537–540. doi: 10.4049/jimmunol.168.2.537. [DOI] [PubMed] [Google Scholar]
- 30.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J Immunol. 2004;173:2419–2427. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 32.Kool M, Geurtsvankessel C, Muskens F, Madeira FB, van Nimwegen M, Kuipers H, et al. Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen-presenting potential of plasmacytoid DCs. J Leukoc Biol. 2011;90:1177–1190. doi: 10.1189/jlb.0610342. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8-dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 34.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109:9977–9982. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perona-Wright G, Lundie RJ, Jenkins SJ, Webb LM, Grencis RK, MacDonald AS. Concurrent bacterial stimulation alters the function of helminth-activated dendritic cells, resulting in IL-17 induction. J Immunol. 2012;188:2350–2358. doi: 10.4049/jimmunol.1101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, et al. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 39.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 40.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 43.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pillarisetty VG, Miller G, Shah AB, DeMatteo RP. GM-CSF expands dendritic cells and their progenitors in mouse liver. Hepatology. 2003;37:641–652. doi: 10.1053/jhep.2003.50074. [DOI] [PubMed] [Google Scholar]
- 45.Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundy SK, Lukacs NW. Chronic schistosome infection leads to modulation of granuloma formation and systemic immune suppression. Front Immunol. 2013;4:39. doi: 10.3389/fimmu.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, Nish SA, Jiang R, Hou L, Licona-Limon P, Weinstein JS, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39:722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connor LM, Tang SC, Camberis M, Le Gros G, Ronchese F. Helminth-conditioned dendritic cells prime CD4+ T cells to IL-4 production in vivo. J Immunol. 2014;193:2709–2717. doi: 10.4049/jimmunol.1400374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.