Abstract
Background
In sub-Saharan Africa, the timing and nature of brain injury and their relation to mortality in neonatal encephalopathy (NE) is unknown. We evaluated cranial ultrasound (cUS) scans from term Ugandan infants with and without NE for evidence of brain injury.
Methods
Infants were recruited from a national referral hospital in Kampala. Cases (184) had NE and controls (100) were systematically selected unaffected term infants. All had cUS scans <36h reported blind to NE status.
Results
Scans were performed at median age 11.5 (IQR 5.2-20.2) and 8.4 (IQR 3.6-13.5) hours, in cases and controls respectively. None had established antepartum injury. Major evolving injury was reported in 21.2% of cases vs 1.0% controls (p<0.001). White matter injury was not significantly associated with bacteraemia in encephalopathic infants (OR 3.06 (95%CI 0.98-9.60). Major cUS abnormality significantly increased the risk of neonatal death (case fatality 53.9% with brain injury vs 25.9% without; OR 3.34(95%CI, 1.61-6.95)).
Conclusions
In this low-resource setting, there was no evidence of established antepartum insult, but a high proportion of encephalopathic infants had evidence of major recent and evolving brain injury on early cUS imaging, suggesting prolonged or severe acute exposure to hypoxia-ischaemia. Early abnormalities were a significant predictor of death.
INTRODUCTION
Neonatal encephalopathy (NE) is a leading cause of neonatal mortality and morbidity worldwide affecting more than one million infants around the world each year.1,2 There is a substantial body of evidence from high-income settings supporting an injury pattern on neuroimaging consistent with hypoxia-ischaemia (HI) that occurs in the immediate perinatal period.3-5 However, the nature and timing of brain injury leading to NE in the term infant is poorly understood, particularly in low-income settings. NE may be considered a multifactorial disease involving a complex causal pathway with the contribution of individual risk factors likely to change according to their prevalence, frequency of exposure, care setting and definition of NE used.6 In low income countries (LICs), difficulties in accurate fetal monitoring and lack of standardized recording of intrapartum complications, mean that measures of intrapartum-related hypoxia, including cause and duration, are difficult to define.1 Delays in identification and referral of women experiencing obstetric and fetal complications may have important effects on the nature and timing of HI insults.7 Pre-clinical study findings suggest that newborn infection may sensitise the immature term brain to subsequent injury,8,9 a potentially important factor in low-resource settings.
Understanding the nature, timing of onset and duration of HI are key to evaluating which neuroprotective strategies may be effective.10 In high-income countries (HICs), magnetic resonance (MR) imaging, including diffusion weighted imaging and MR spectroscopy, has been used to identify the nature and timing of insults and to predict neurodevelopmental outcome,4,11-13 however, MR imaging is not available in the majority of LICs. A decline in brain high energy phosphates seen on MR spectroscopy over the first 8-24h after birth in babies with NE,14 a phenomenon called secondary energy failure, has been observed to occur typically in parallel with the cascade of programmed cell death following a HI insult.15 In keeping with this evolving injury, abnormalities seen on cranial ultrasound (cUS) imaging, such as central grey matter, white matter and cortical echodensities indicative of neuronal death, are frequently not evident on cUS or MR imaging until 24-48 hours after HI.13,16
The aims of this study were: (i) to evaluate cUS scans obtained soon after birth from term Ugandan infants with NE and unaffected term control infants for evidence of established and evolving brain injury, and (ii) to assess the contribution of perinatal infection to the pattern of brain injury.
RESULTS
Study participants
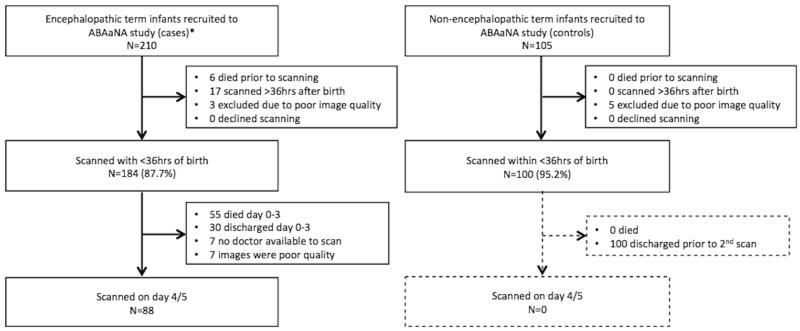
In total, early cUS was performed and assessed for 184 (87.6%) of 210 case infants. Amongst the 409 ABAaNA controls infants, 105 infants were recruited sequentially from the start of the study as long as there was a study doctor available to perform the cUS. Of these 69 were recruited from high-risk labour ward, and 36 recruited from the low risk labour ward. After exclusion for poor image quality, 100 control infants remained (Figure 1). No significant differences were seen in baseline demographic and clinical characteristics between those with and without early scans, in either cohort (data not shown). Good quality images from the second scan (day 4/5) were available for 88 surviving infants with encephalopathy (Figure 1).
Figure 1.
Flow diagram of study participants
Baseline demographic and clinical characteristics of the cohorts are shown in Table 1. The majority of encephalopathic infants were moderately or severely affected (89.7%) (Table 1). A third of case infants died in the neonatal period and half had clinical seizures.
Table 1.
Baseline demographic and clinical characteristics of mothers and babies
| Case N (%) | Control N (%) | P-valueb | |
|---|---|---|---|
| MATERNAL | |||
| Maternal education ≤ primary level | 72/184 (39.1) | 39/100 (39.0) | 0.983 |
| Primiparity | 112/184 (60.9) | 48/100 (48.0) | 0.037 |
| Emergency caesarean section | 43/183 (23.5) | 16/100 (16.0) | 0.138 |
|
| |||
| NEONATAL | |||
| Male sex | 116/184 (63.0) | 51/100 (51.0) | 0.049 |
| Mean birth weight (g) (range) | 3196 (1940-4640) | 3065 1640-4290) | 0.023 |
| 1 minute Apgar <3 | 60/176 (34.1) | 1/100 (1.0) | <0.001 |
| 5 minute Apgar <7 | 118/162 (72.8) | 1/100 (1.0) | <0.001 |
|
| |||
| CLINICAL FEATURES OF ENCEPHALOPATHIC INFANTS | |||
| Grade of encephalopathya | |||
| Mild | 19/184 (10.3) | - | |
| Moderate | 105/184 (57.1) | - | |
| Severe | 60/184 (32.6) | - | |
| Absent suck | 145/183 (79.2) | - | |
| Clinical seizures | 100/184 (54.4) | - | |
| Comatose | 48/184 (26.1) | - | |
| Neonatal Case Fatality | 58/182 (31.9) | - | |
Grade encephalopathy defined according to Sarnat and Sarnat classification18 on the worst day in the first five days after birth
Significance testing used chi-squared test except for mean birth weight where t-test for comparison of means was used
Comparison between cases and controls
Median age at scanning was 11.5h (interquartile range (IQR) 5.2-20.2 hours), and 8.4h (IQR 3.6-13.5 hours) in cases and controls, respectively (p=0.0019). The distribution of BGT and WM scores amongst case and control infants is shown in Table 2.
Table 2.
Distribution of basal ganglia-thalami and white matter scores for case and controls
| Cases (n=184) N (%) | Controls (n=100) N (%) | |
|---|---|---|
| BGT score | ||
| 0 | 100 (54.4) | 72 (72.0) |
| 1 | 62 (33.7) | 27 (27.0) |
|
| ||
| 2 | 19 (10.3) | 1 (1.0) |
| 3 | 3 (1.6) | 0 (0.0) |
|
| ||
| WM score | ||
| 0 | 116 (63.0) | 89 (89.0) |
| 1 | 43 (23.4) | 11 (11.0) |
|
| ||
| 2 | 24 (13.0) | 0 (0.0) |
| 3 | 1 (0.5) | 0 (0.0 |
Abbreviations: BGT, Basal ganglia-thalami; WM, White matter
No abnormality was found in 45.1% (83/184) of cases and 67.0% (67/100) controls (p<0.001). Mild WM changes and mild/unilateral echogenicity in the BGT (score 1 and not indicative of major abnormality) were common amongst cases and controls. More case than control infants had mild WM changes (WM score 1, OR 3.00 (95% CI, 1.46 – 6.15). There was no significant difference in mild BGT scores (BGT score 1, OR 1.65 (95%CI, 0.96 – 2.85). One control had a score of 2 for BGT echogenicity (Table 2); this scan was reviewed post analysis (by FC) and the score was considered marginal between 1 and 2 and the WM and cortex looked normal. Score 3 for WM or BGT was uncommon (Table 2) amongst cases and absent amongst controls.
Overall, scans obtained within 36h of birth showed a major abnormality in one fifth of encephalopathic infants (Table 3). When the analysis was restricted to scans obtained within 18h of birth the high case prevalence of major abnormalities persisted (17.5%) (Table 3).
Table 3.
Proportion of infants with major abnormalities on early cUS imaging
| Age at imaging | Case N (%) | Control N (%) | OR | 95%CI |
|---|---|---|---|---|
| cUS performed <36 hours | 39/184 (21.2) | 1/100 (1.0) | 26.6 | 3.60-197.01 |
| cUS perfomed <18 hours a | 22/126 (17.5) | 1/85 (1.2) | 17.77 | 2.35-134.56 |
Abbreviations: cUS, cranial ultrasound
Analysis restricted to infants scanned at <18 hours of age. For this restricted analysis median age at scanning was 7.8 hours amongst cases (IQR 4.0-11.9) and 7.1 hours (IQR 3.4-11.2) amongst controls, p=0.210)
Pattern and timing of injury in NE
The pattern of injury seen amongst infants with NE is shown in Table 4. All major abnormalities (score ≥2) were of recent onset: no established injury with tissue atrophy, parenchymal cystic change, definite developmental abnormality or large parenchymal haemorrhage was seen in either cases or controls. Subependymal pseudocysts, caudothalamic cysts and choroid plexus cysts were common amongst all infants (12.5% (23/184) in cases vs. 17% (17/100) in controls).
Table 4.
Pattern of major abnormality seen on early cUS amongst encephalopathic (case) infants (N=184)
| Major abnormalitya | N (%) |
|---|---|
| No abnormality | 145(78.8%) |
| BGT abnormality | 22 (12.0%) |
| WM abnormality | 25 (13.6%) |
| Global abnormality b | 8 (4.4%) |
Abbreviations: BGT, Basal ganglia-thalami; WM, White matter
Major abnormality in BGT or WM defined as a score of ≥2 in that area (injury seen in BGT and WM are not mutually exclusive).
Global abnormality was defined as a score ≥2 in both the BGT and WM
Serial scans were performed on day 4/5 in 88 NE infants: 74 had normal early scans, 35 (47.3%) becoming abnormal by day 4/5. In the 14 with abnormal early cUS, 5 normalised and 9 remained abnormal.
Neonatal infection and brain injury
Amongst cases, bacteraemia was detected in 20.0% (5/25) of infants with evidence of WM injury vs 7.6% (12/159) of those without (OR 3.06 (95%CI 0.98-9.60) and in 18.2% (4/22) of cases with major BGT abnormality vs. 8.0% (13/162) of those without (OR 2.55 (95%CI 0.75-8.65)).
Neonatal case fatality
Amongst encephalopathic infants, a major abnormality seen on early cUS (<36h) tripled the odds of neonatal death (case fatality with major abnormality 53.9% (21/39) vs. 25.9% (37/143) with no major abnormality (OR 3.34 (95%CI 1.61-6.95)). A similar pattern of association was seen for those scanned within 18h of birth (case fatality 59.1% (13/22) vs. 29.1% (30/103), respectively (OR 3.51 (95%CI 1.36-9.10)).
DISCUSSION
Our study showed a high prevalence of recent, evolving brain injury amongst encephalopathic infants on early cUS imaging, in this low-resource Ugandan setting. Evidence of established injury, indicative of an antepartum insult or congenital pathology, was not seen. Signs of major evolving injury, suggestive of a relatively recent as opposed to an established antepartum brain insult, affected one in five encephalopathic infants. In infants with encephalopathy, the presence of a major evolving brain injury tripled the likelihood of dying in the neonatal period. There was no significant increase in white matter injury seen amongst encephalopathic infants with neonatal infection.
Benefits of cUS are that it can be performed serially, at the bedside, and in the sickest of infants.17 It allows the early detection of congenital or another non-HI cause of NE and informs the timing of HI injury; as a consequence it continues to be recommended as an important adjunct to MRI scanning for encephalopathic infants with NE in HICs.17,18 Previous studies from HICs have found abnormal cUS to be predictive of outcome,3,17 with good correlation between MRI and cUS findings amongst infants with HIE in some, but not all studies.3,19
The cUS scoring system used by us successfully identified encephalopathic infants with major abnormalities on cUS, and differentiated between abnormalities suggestive of significant brain injury and other changes frequently seen amongst well term infants.20 Studies from HICs have shown unilateral BGT changes, equivalent to our score 1, to be associated with a more benign prognosis,3,21 suggesting that they are poor predictors of adverse neurodevelopmental outcome. This is likely to extend to our Ugandan population where focal unilateral central grey matter changes and focal WM echogenicity were found to be common amongst well term Ugandan newborns.22
This is the first study in a sub-Saharan low-income setting to use cUS to examine the nature and timing of newborn brain injury. A study from South India examined perinatal MRI findings amongst encephalopathic infants.23 That study reported minimal antepartum established injury on MRI performed within 3 weeks of birth. White matter injury was also common in this study (in 91%) and around a quarter had deep grey matter injury.23 Comparison of our results with other cUS studies from HICs is hampered by differing definitions of NE used and inconsistency of brain abnormality definition. In the Netherlands, early changes on cUS (<6h of age) were not found to be predictive of outcome, with most infants with severe encephalopathy developing cUS abnormalities between 24-72 hours of age.19 A good correlation was seen between later cUS abnormalities and histological findings.16
Defining the nature and timing of newborn brain injury in NE is key to understanding the most appropriate prevention and intervention strategies for NE. In this study, one in five encephalopathic infants had features of evolving brain injury on very early (median 11h) scans. Since changes seen on cUS are recognised to take some time to develop after the original brain insult,19 this may imply that the injury pathway begins several hours before delivery, or a very severe acute exposure to hypoxia-ischaemia. It is unclear whether severe injury may appear on cUS more rapidly than moderate injury; experimental studies show that secondary energy failure develops more rapidly in severe HI than moderate HI.24 The accelerated cell death cascade may be reflected in earlier evidence of injury on cUS. As in South India, a high prevalence of WM injury was seen among cases on early imaging. This may be associated with perinatal infection and inflammation or may reflect other exposures, including a less acute and more chronic HI insult. MRI diagnostic techniques could have contributed substantially to defining the nature and timing of brain injury,13,23,25 but unfortunately are not currently available in Uganda.
This study has limitations and challenges. First, all selection, inter-observer and recall bias cannot be excluded completely. The case cohort is likely to have under-represented infants at either end of the spectrum of NE severity since the most severely affected often died prior to recruitment, and mildly affected infants may go undetected. Infants born on the low-risk labour ward were slightly over represented in the control group and cases were a median of 3h older than controls when scanned. However, it seems unlikely that any of these factors contributed substantially to the large differences seen between case and control infants. Scans were read by two external experts blind to study status and all clinical and demographic data, so minimising observer bias but all inter- and intra-observer bias cannot be excluded. Finally, since participants were recruited from a high-risk urban Ugandan referral centre they may not reflect findings amongst infants from other, more rural populations.
Conclusions
In this study, in a Ugandan hospital setting, there was no cUS evidence of established antepartum injury, but a fifth of encephalopathic infants had evidence of major recent and evolving brain injury on early cUS imaging performed at a median age of 11h. Since we assume from studies in HICs that it takes >24h for injury to become apparent on cUS this may suggest a brain insult occurring several hours before delivery or a very severe acute exposure to hypoxia-ischaemia. These early abnormalities were a significant predictor of death in this population.
METHODS
This was a nested study of early brain imaging, conducted within a larger case-control study designed to investigate the aetiology of NE in Uganda (Associations between Birth Asphyxia and infections amongst Newborns in Africa – the ‘ABAaNA study’). Between 11th September 2011 and 3rd October 2012, 210 case and 409 control infants were recruited to the main study. The study was approved by the ethics committees of the London School of Hygiene and Tropical Medicine, Uganda Virus Research Institute and Mulago Hospital, and the Uganda National Council for Science and Technology
Setting
All infants were born and recruited at Mulago Hospital, a National Referral Hospital in Kampala, Uganda’s capital city. Over the 13-month study period, 36,926 deliveries occurred at Mulago Hospital, 23% (8,767) by caesarean section. Women deliver on the low-risk or high-risk labour ward (ratio 1:4). Fetal monitoring uses Pinard stethoscope for intermittent auscultation. Neonatal resuscitation, performed by attending midwives, includes oxygen and bag-mask ventilation. Sick newborns are transferred to the Special Care Unit (SCU). Piped oxygen and simple continuous positive airway pressure support are available, but not intubation and ventilation. Mothers care for well infants on the postnatal wards.
Study participants
Participants were term infants <12h of age with written parental informed consent, taken by trained study staff in the local language. NE case infants had a Thompson score of ≥6.26 Sarnat & Sarnat classification of encephalopathy severity27 was assigned on the most severe day (between days 1-5). Contemporaneously recruited, unmatched controls (Thompson score ≤3) were systematically sampled from the labour ward admissions. For this cUS sub-study, a comparison group of 100 control infants were eligible for inclusion. Information was collected from antenatal and hospital clinical records and structured maternal interviews in the local language. Blood culture and species-specific bacterial real-time PCR assays detected neonatal bacteraemia amongst all cases as previously described.28 Survival outcomes were recorded at 4 weeks.
Cranial Ultrasound scans
Scans were performed using a portable ultrasound machine (z.one ultra-Convertible Ultrasound System; Zonare Medical Systems Inc. Mountain View, California, USA) with the P10-4 Phased Array Transducer (bandwidth 4-10 MHz with tissue and compound harmonics of 8Hx).
Study doctors (KB, KHJ, NN, FN, AT) were trained to perform cUS by CT and FC using methods described previously,22,29,30 Minimally, five coronal, one midline sagittal and two parasagittal views on left and right were taken to visualise general anatomy, basal ganglia-thalami (BGT) and white matter (WM). Scans were performed on recruitment (as soon as a trained study doctor was available) and again on day 4/5 in all surviving infants that remained in-patients. Images were downloaded, anonymised and reported from DICOM formatted images (OsiriX software, Geneva, Switzerland) by FC and CH, blind to case/control status and all clinical data. Abnormalities were scored according to a system adapted from van Wezel-Meijler et al.,17 and used by us previously in Uganda (Table 5).22
Table 5.
Scoring system used for basal ganglia and white matter abnormalities
| Score | Basal ganglia & thalami | White matter | |
|---|---|---|---|
| No abnormality | 0 | No abnormality | No abnormality |
| Minor abnormality | 1 | Mild - swollen and/or small focal unilateral abnormality | Mildly echogenic or patchy |
| Major abnormality | 2 | Clearly demarcated focal bilateral echogencity | More diffuse and moderate/severe echogenicity |
| 3 | Bilateral, severe, extensive abnormalities | Dense echogenicity &/or parenchymal cysts, atrophy, organising haemorrhage |
As previous cUS studies in HICs suggest that changes on neuroimaging become evident >24 after HI,13,16 only infants scanned for the first time within the first 36h after birth were included. Our aim was to assess whether these scans appeared (i) normal (ii) had evidence of longstanding changes consistent with an antepartum insult or a developmental abnormality (parenchymal cysts, calcification, atrophy, organising haemorrhage) or (iii) evidence of a recent event, typical of acute or sub-acute HI injury (extensive echogenicities in the BGT and WM). Signs of haemorrhage and focal arterial territory infarction were also looked for. Isolated cysts in the choroid plexus, subependymal cysts, cysts in the caudo-thalamic notch and lenticulostriate vasculopathy were not considered major abnormalities.19
Examples of image findings and their relationship to scores are shown in Figures 2-5. The presence of a ‘major abnormality’ was defined as a score of 2 or 3 in either BGT, WM or both. Cortex abnormalities were not graded as it was difficult to obtain systematic views on all infants and cortical abnormalities are seldom seen in isolation of BGT and WM changes.
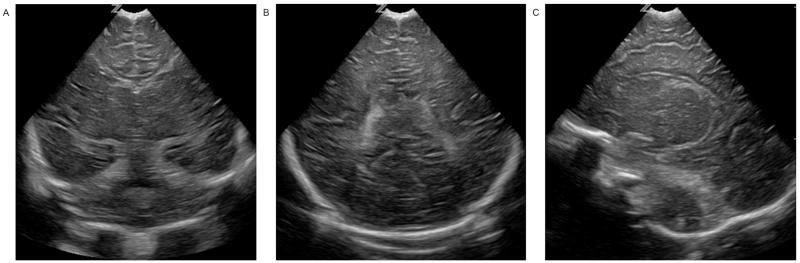
Figure 2.
Normal basal ganglia and white matter seen on coronal (A, B) and parasagittal (C) images consistent with a score of 0
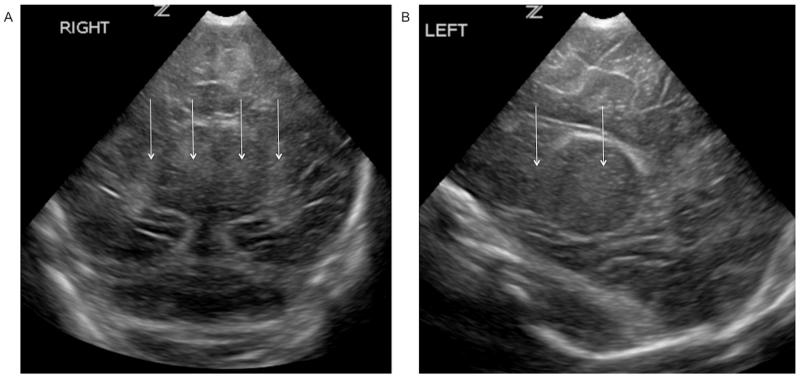
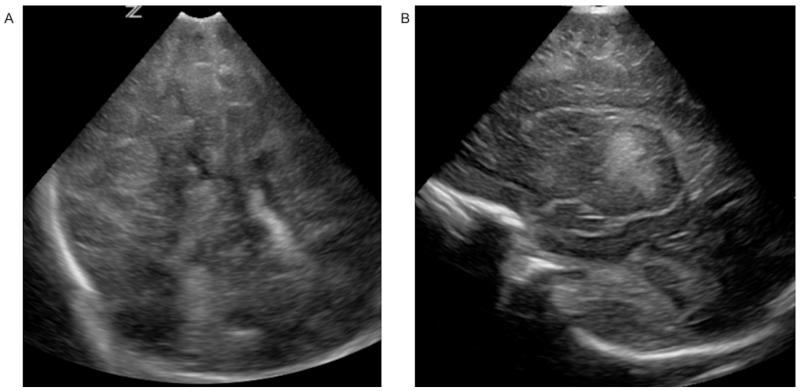
Figure 3.
Bilateral basal ganglia and thalamic echogenicity seen on coronal (A) and parasagittal (B) images consistent with a score of 2
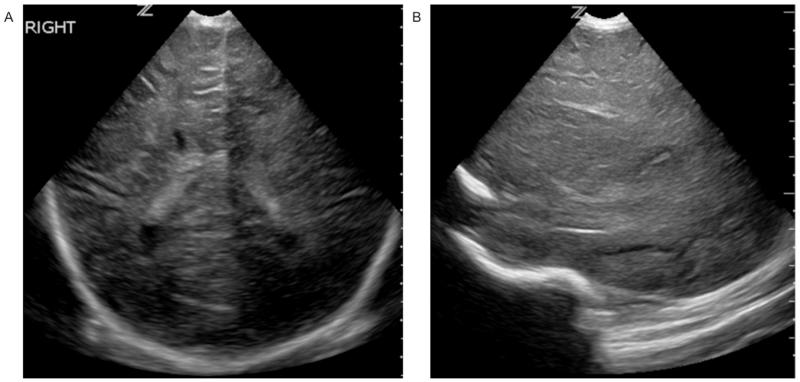
Figure 4.
Diffuse, moderate/severe echogenicity in the white matter seen on coronal (A) and parasagittal (B) images consistent with a score of 2
Figure 5.
Severe dense echogenicity in the basal ganglia and thalami on parasagittal view (A) and global echogenicity and swelling in the white matter and cortex on coronal images (B) consistent with a score of 3.
Statistical analysis
Analysis was performed using Stata version 11.0 (StataCorp, College Station, Texas). Chi-squared test, t-test and Wilcoxon rank sum test were used to compare proportions, means and medians (where data was not normally distributed), respectively. Univariable logistic regression analysis was used to calculate odds ratios (OR) and 95% confidence intervals (CI). P<0.05 was taken as the formal level of statistical significance. Case fatality was defined as a death occurring in the neonatal period (0-28 days) as a proportion of infants at risk.
Acknowledgments
Thanks go to the parents, guardians and children who participated in the study, the ABAaNA study team for their dedicated contribution to data collection and care of study participants; to Jamiir Mugalu, Jolly Nankunda, and all on the Special Care Unit at Mulago Hospital. Thanks go also to the MRC/UVRI Uganda Research Unit on AIDS, the Uganda Women’s Health Initiative and the Wellcome Bloomsbury Centre, LSHTM for their administrative support. We are grateful to Zonare Medical Systems for maintenance and upgrade of the z.one ultrasound system and UCL Institute for Women’s Health for loan of the scanning equipment.
Financial support: The study was funded by a Wellcome Trust Bloomsbury PhD Clinical Fellowship to CT
Footnotes
Disclosure statement: All authors have no financial relationships to disclose or conflicts to resolve.
REFERENCES
- 1.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(Suppl 1):50–72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn JE, Blencowe H, Oza S, et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384:189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 3.Rutherford MA, Pennock JM, Dubowitz LM. Cranial ultrasound and magnetic resonance imaging in hypoxic-ischaemic encephalopathy: a comparison with outcome. Dev Med Child Neurol. 1994;36:813–25. doi: 10.1111/j.1469-8749.1994.tb08191.x. [DOI] [PubMed] [Google Scholar]
- 4.Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–42. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, Mercuri E, Cowan FM. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952–9. doi: 10.1542/peds.2013-0511. [DOI] [PubMed] [Google Scholar]
- 6.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Lawn JE, Lee AC, Kinney M, et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? Int J Gynaecol Obstet. 2009;107(Suppl 1):S5–18. S9. doi: 10.1016/j.ijgo.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58:112–6. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 9.Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–23. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- 10.Soul JS, Robertson RL, Tzika AA, du Plessis AJ, Volpe JJ. Time course of changes in diffusion-weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics. 2001;108:1211–4. doi: 10.1542/peds.108.5.1211. [DOI] [PubMed] [Google Scholar]
- 11.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–95. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 12.Robertson NJ, Thayyil S, Cady EB, Raivich G. Magnetic resonance spectroscopy biomarkers in term perinatal asphyxial encephalopathy: from neuropathological correlates to future clinical applications. Curr Pediatr Rev. 2014;10:37–47. doi: 10.2174/157339631001140408120613. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford MA, Pennock JM, Counsell SJ, et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics. 1998;102:323–8. doi: 10.1542/peds.102.2.323. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt JS, Edwards AD, Azzopardi D, Reynolds EO. Magnetic resonance and near infrared spectroscopy for investigation of perinatal hypoxic-ischaemic brain injury. Arch Dis Child. 1989;64:953–63. doi: 10.1136/adc.64.7_spec_no.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotten CM, Shankaran S. Hypothermia for hypoxic-ischemic encephalopathy. Expert Rev Obstet Gynecol. 2010;5:227–39. doi: 10.1586/eog.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eken P, Jansen GH, Groenendaal F, Rademaker KJ, de Vries LS. Intracranial lesions in the fullterm infant with hypoxic ischaemic encephalopathy: ultrasound and autopsy correlation. Neuropediatrics. 1994;25:301–7. doi: 10.1055/s-2008-1073044. [DOI] [PubMed] [Google Scholar]
- 17.Leijser LM, Vein AA, Liauw L, Strauss T, Veen S, Wezel-Meijler G. Prediction of short-term neurological outcome in full-term neonates with hypoxic-ischaemic encephalopathy based on combined use of electroencephalogram and neuro-imaging. Neuropediatrics. 2007;38:219–27. doi: 10.1055/s-2007-992815. [DOI] [PubMed] [Google Scholar]
- 18.Swarte R, Lequin M, Cherian P, et al. Imaging patterns of brain injury in term-birth asphyxia. Acta Paediatrica. 2009;98:586–592. doi: 10.1111/j.1651-2227.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 19.Eken P, Toet MC, Groenendaal F, de Vries LS. Predictive value of early neuroimaging, pulsed Doppler and neurophysiology in full term infants with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1995;73:F75–80. doi: 10.1136/fn.73.2.f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boo NY, Chandran V, Zulfiqar MA, et al. Early cranial ultrasound changes as predictors of outcome during first year of life in term infants with perinatal asphyxia. J Paediatr Child Health. 2000;36:363–9. doi: 10.1046/j.1440-1754.2000.00518.x. [DOI] [PubMed] [Google Scholar]
- 21.De Vries LS, Smet M, Goemans N, Wilms G, Devlieger H, Casaer P. Unilateral thalamic haemorrhage in the pre-term and full-term newborn. Neuropediatrics. 1992;23:153–6. doi: 10.1055/s-2008-1071332. [DOI] [PubMed] [Google Scholar]
- 22.Hagmann CF, Robertson NJ, Acolet D, et al. Cranial ultrasound findings in well newborn Ugandan infants. Arch Dis Child Fetal Neonatal Ed. 2010;95:F338–44. doi: 10.1136/adc.2009.174607. [DOI] [PubMed] [Google Scholar]
- 23.Lally PJ, Price DL, Pauliah SS, et al. Neonatal encephalopathic cerebral injury in South India assessed by perinatal magnetic resonance biomarkers and early childhood neurodevelopmental outcome. PLoS One. 2014;9:e87874. doi: 10.1371/journal.pone.0087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwata O, Iwata S, Thornton JS, et al. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;1154:173–80. doi: 10.1016/j.brainres.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 25.Barnette AR, Horbar JD, Soll RF, et al. Neuroimaging in the evaluation of neonatal encephalopathy. Pediatrics. 2014;133:e1508–17. doi: 10.1542/peds.2013-4247. [DOI] [PubMed] [Google Scholar]
- 26.Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta paediatr. 1997;86:757–61. doi: 10.1111/j.1651-2227.1997.tb08581.x. (Oslo, Norway : 1992) [DOI] [PubMed] [Google Scholar]
- 27.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 28.Tann CJ, Nkurunziza P, Nakakeeto M, et al. Prevalence of bloodstream pathogens is higher in neonatal encephalopathy cases vs. controls using a novel panel of real-time PCR assays. PLoS One. 2014;9:e97259. doi: 10.1371/journal.pone.0097259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagmann CF, Robertson NJ, Acolet D, et al. Cerebral measurements made using cranial ultrasound in term Ugandan newborns. Early Hum Dev. 2011;87:341–7. doi: 10.1016/j.earlhumdev.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Robertson NJ, Hagmann CF, Acolet D, et al. Pilot randomized trial of therapeutic hypothermia with serial cranial ultrasound and 18-22 month follow-up for neonatal encephalopathy in a low resource hospital setting in Uganda: study protocol. Trials. 2011;12:138. doi: 10.1186/1745-6215-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]